Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, COVID-19) continues its significant health and economic impact globally. Despite the success of spike-protein vaccines in preventing severe disease, long-lasting protection against emerging variants and the prevention of breakthrough infections and transmission remain elusive. We generate an intranasal live-attenuated SARS-CoV-2 vaccine, CDO-7N-1, using codon deoptimization. CDO-7N-1 shows highly attenuated replication and minimal or no lung pathology in vivo over multiple passages. It induces robust mucosal and systemic neutralizing antibody and T-cell subset responses, in mice (female K18-hACE2 and male HFH4-hACE2 mice), hamsters, and macaques triggered by a single immunization. Mice and hamsters vaccinated with CDO-7N-1 are protected from challenge with wild-type (WT) SARS-CoV-2 and other variants of concern. Serum from vaccinated animals neutralizes WT SARS-CoV-2, variants of concern (beta and delta), variants of interest (omicron XBB.1.5) and SARS-CoV-1. Antibody responses are sustained and enhanced by repeated immunization or infection with WT SARS-CoV-2. Immunity against all SARS-CoV-2 proteins by CDO-7N-1 should improve efficacy against future SARS-CoV-2 variants.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome virus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19)1,2,3,4, has resulted in over 700 million infections and 7 million deaths worldwide to date5 and continues to be lethal in susceptible individuals. SARS-CoV-2 has a positive-sense, RNA genome with a length of ~30 kb. The genome has six major open reading frames that encode replicase (ORF1a/ORF1b), spike (S), envelope (E), membrane (M) and nucleocapsid protein (N). In addition, 11 ORFs encoding accessory proteins are interspersed between structural genes6. The replicase gene encodes a large polyprotein (pp1a/b) that is proteolytically cleaved into 16 nonstructural proteins involved in viral RNA replication. Although, COVID-19 therapeutic strategies have been developed7,8, vaccination is the primary and most effective way to prevent life-threatening COVID-19 disease. Currently available vaccines are typically designed around the spike protein since the receptor-binding domain (RBD) of this protein is the target of most serum-neutralizing antibodies. The protective effects of these vaccines were noticeably successful against alpha, beta, gamma and delta variants of SARS-CoV-2; however, the protection rate from symptomatic disease has declined sharply as new variants with RBD mutations have emerged. For example, the omicron variant and related strains continues to infect and be transmitted in vaccinated and recovered individuals9,10 and mutations in the S protein can decrease the sensitivity to neutralization by antibodies11. It is highly concerning that SARS-CoV-2 variants can evade neutralization by antibodies elicited by vaccines based on the S protein of the WT SARS-CoV-2 strain. As such, the design of SARS-CoV-2 vaccines targeting multiple antigens is worth exploring. A study has highlighted the challenges in the design and development of vaccines capable of neutralizing emerging SARS-CoV-2 variants12. To control the pandemic effectively, it is important to develop vaccines that elicit broadly neutralizing antibodies that are effective against variants, including those that will arise in the future13.
Live-attenuated vaccines (LAVs) offer several significant advantages over other vaccine approaches. They induce potent and long-lived humoral and cellular immunity, often with just a single immunization. Since a LAV comprises the entire virus, the immune response elicited by vaccination is antigenically broad, in contrast to that of a single antigen (e.g., spike) used in many other vaccine platforms. This is potentially a major advantage in addressing the issue of emerging variants and their escape from vaccine-induced immunity14. Moreover, intranasal administration of LAV can also generate various persistent local responses, such as antibodies, including IgA – which is essential against a respiratory virus such as SARS-CoV-2 and is more likely to prevent infection and transmission15. Based on these potential advantages, we developed a panel of live-attenuated SARS-CoV-2 vaccine candidates using codon deoptimization (CDO). CDO exploits the phenomenon by which multiple codons can code for the same amino acid. Without changing the protein amino acid sequence, recoding synonymous codons affects mRNA GC content and rare tRNA usage, and potentially RNA secondary structure. These changes may affect protein translation efficiency, protein folding, and mRNA stability. It is highly likely that multiple secondary structure elements were also affected by the synonymous mutagenesis used to generate CDO-7N-116. This allows for a precise and well-defined approach to virus attenuation, which is achieved through selectively recoding a large number of synonymous codons in the virus genome to less frequently utilized codons. Codon bias, the uneven use of synonymous codons for encoding the same amino acid, varies across different species due to multiple factors such as the availability of tRNA molecules, GC content of the genome, and natural selection. As a result, each species has its unique pattern of preferred codons, which can impact gene expression, translation efficiency, and protein folding. Therefore, the attenuation design based on codon bias is species-specific. This approach enables the development of viruses capable of replicating in cell culture but with dramatically attenuated replication in vivo and has been used to generate vaccine candidates against a range of viruses17,18,19,20.
In the present study, we report the development of a live attenuated SARS-CoV-2 vaccine candidate using CDO. The CDO-7N-1 vaccine candidate was found to be highly attenuated and stable in vitro and in vivo. A single immunization with CDO-7N-1 induces strong humoral and cellular immunity, including neutralizing antibody production and T-cell responses. Serum from vaccinated animals demonstrated neutralizing activity against the original strain of SARS-CoV-2 (hereafter referred to as wild-type, WT), past and contemporary variants of concern, variants of interest, and SARS-CoV-1. Immunization with CDO-7N-1 protected mice and hamsters from subsequent challenges with most of the major known SARS-CoV-2 variants of concern and interest.
Results
Design and construction of a live-attenuated SARS-CoV-2 vaccine candidate that is genetically stable and does not cause organ pathology
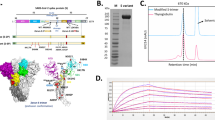
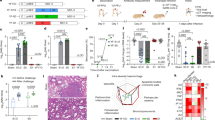
We recently reported an infectious clone of WT SARS-CoV-2 (MT926410) that was assembled from five synthetic DNA fragments21. The second (SanD11534 to PacI8586) and third (PacI8,586 to MluI13,956) fragment were initially chosen for codon deoptimization and generated 31 constructs. However, none of these constructs was rescuable. Then we limited deoptimization to fragment 2 and designed 28 constructs (Fig. 1a). In these constructs, 24 to 546 codons were changed to their rare codons according to Fig. 1b. Sixteen constructs could be rescued as infectious viruses through in vitro transfection and were then assessed for in vitro replication kinetics and plaque morphology. Two highly attenuated candidates (mutations limited to 6982 to 8586) were identified, CDO-SARS-CoV-2-4N-1 (CDO-4N-1) and CDO-SARS-CoV-2-7N-1 (CDO-7N-1), which exhibited smaller plaque sizes than the WT SARS-CoV-2 (Fig. 1c). Vero E6 and Calu-3 cells infected with CDO-4N-1 and CDO-7N-1 showed significantly reduced viral growth kinetics compared to those infected with WT SARS-CoV-2 (Fig. 1d, e). Hamsters infected intranasally (i.n.) with 105 PFU of CDO-4N-1 or CDO-7N-1 exhibited a 0.5 to 1 log reduction in the virus titers in bronchoalveolar lavage fluid (BALF) at day 3 post-infection (p.i), compared to those in hamsters infected with WT SARS-CoV-2 (Fig. 1f). Extensive lung pathology was observed in hamsters infected with WT SARS-CoV-2 at days 5 and 7 p.i. (Fig. 1g, h, Supplementary Fig. 1). Lung pathology in hamsters infected with CDO-4N-1 was much less severe than in hamsters following WT SARS-CoV-2 infection. In contrast, there was little to no observable pathology in the lungs of hamsters infected with CDO-7N-1. Therefore, CDO-7N-1 was selected as the lead vaccine candidate. Additionally, CDO-7N-1 did not cause any acute adverse pathological changes in the brain, olfactory bulbs, nasal turbinate, olfactory nerve, heart, lungs, kidneys, or intestines (duodenum, jejunum, ileum, cecum, colon, and rectum) (Supplementary Figs. 19, 20, Supplementary Tables 2–10).
a Schematic diagram for WT SARS-CoV-2 reverse genetic infectious clone and codon deoptimization design. Examples of mutated nucleotides are indicated in red. CMV, cytomegalovirus promotor; S, spike protein gene; N nucleocapsid gene, E, envelope glycoprotein gene; M membrane glycoprotein gene; UTR, untranslated region. b Homo sapiens codon usage frequency table (DNA HIVE, dnahive.fda.gov). The synonymous codons of each target amino acid (Q, glutamine; I, isoleucine; P, proline; R, arginine; T, threonine; A, alanine; G, glycine) were changed to the corresponding codon with the lowest frequency indicated in red. c–e Plaque morphologies and growth kinetics of WT SARS-CoV-2, CDO-4N-1 and CDO-7N-1. Vero E6 or Calu-3 cells were infected with the respective viruses at an MOI of 0.1. The virus titers in the culture media were determined by plaque assay. Values are expressed as the mean ± standard error of the mean (SEM) from three independent experiments. **P < 0.01 and ***P < 0.001 using two-way ANOVA with Bonferroni’s post hoc test. f Six- to eight-week-old Golden Syrian hamsters were infected i.n. with WT SARS-CoV-2, CDO-4N-1 or CDO-7N-1 at 105 PFU in a volume of 50 µL. BALF was collected at 3 days p.i. for plaque assay analysis. Dots represent individual animals (n = 4). Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. *P < 0.05 and **P < 0.01 using one-way ANOVA with Fisher’s LSD post hoc test. g, h Lung tissue was collected from the infected hamsters at 7 dpi for histopathology analysis using hematoxylin and eosin (H&E) staining. Stained histologic sections of all the tissues collected from all the hamsters were examined using a light microscope (Model-E600, Make-NIKON), and observations were recorded by veterinary pathologist as per the applicable Test Site’s SOPs. Values are expressed as the mean ± SEM from two independent experiments. *P < 0.05, ***P < 0.001, and ****P < 0.0001 using two-way ANOVA with Bonferroni’s post hoc test.
One of the major safety concerns of LAVs is reversion or compensatory mutation to recreate virulent strains. To assess genetic stability, CDO-7N-1 was serially passaged in Vero E6 cells for 15 passages. The plaque morphology of passage 1 (P1), passage 10 (P10), and passage 15 (P15) generation viruses showed that CDO-7N-1 maintained the small-plaque phenotype (Supplementary Fig. 2a). Furthermore, the replication kinetics of the P15 virus in Vero E6 cells were similar to those of the passage 1 virus, indicating that the attenuated replication kinetics of CDO-7N-1 remained stable after multiple passages (Supplementary Fig. 2b). To determine whether the attenuation of CDO-7N-1 is maintained after in vivo passaging, hamsters were infected i.n. with 105 PFU of CDO-7N-1 P15. Three days p.i., the BALF was collected. The virus collected via the first BAL, termed hamster passage 1 (HP1), was further back-passaged in hamsters three times and collected via BALF to inoculate subsequent hamsters and generate hamster passage 2 (HP2), passage 3 (HP3) and passage 4 (HP4) viruses (Supplementary Fig. 2c). Intranasal administration of the back-passaged HP3 virus in hamsters produced very low levels of pulmonary inflammation and pathology, comparable to those observed with passage 1 CDO-7N-1 (Supplementary Fig. 2d) and dramatically less than those seen following administration of WT SARS-CoV-2. Further safety tests on P15-HP3 virus revealed that no genomic viral RNA could be detected in the sub-maxillary salivary glands, duodenum, colon, brain, kidney, spleen, heart, and olfactory bulb. Additionally, there were no signs of inflammation or pathology in these organs (Supplementary Fig. 21, Supplementary Tables 7–10). Sequence analysis by next-generation sequencing (NGS) of viruses from these passages in cells revealed that the CDO region was stable, with no mutations observed. The HP1 and HP2 viruses showed no nucleotide changes in the CDO region, while sequence polymorphism was detected in HP3 and HP4 at position 7330 where, instead of the original G residue, an A residue was detected. However, this does not result in a change of amino acid as it is the third position of Arg codon (GCG and GCA both encode for Arg). This finding underscores the stability of CDO-7N-1, as illustrated in Supplementary Fig. 2e. Thus, the attenuated properties of the CDO-7N-1 are maintained after multiple in vitro or in vivo passages.
CDO-7N-1 induces potent humoral responses and protects against SARS-CoV-2 challenge in hamsters
To assess antibody responses following CDO-7N-1 immunization, hamsters were immunized i.n. with 105 PFU of CDO-7N-1, and serum was collected on various days post-immunization. In the control group, hamsters were mock-immunized with PBS. CDO-7N-1 induced strong neutralizing antibody responses (PRNT90) after one immunization, maintaining high titers (~1:1000) up to 90 days (Fig. 2a). We measured specific IgG antibody responses against various SARS-CoV-2 proteins, including the spike S1 domain, S2 domain, and RBD and nucleoprotein. Specific antibody responses were detected in vaccinated hamsters over extended periods (Fig. 2b–e). Thus, CDO-7N-1 was immunogenic in the hamster model of infection, inducing strong neutralizing and S and N-binding antibody responses after one immunization.
Six- to eight-week-old Golden Syrian hamsters were inoculated with PBS or 105 PFU CDO-7N-1 i.n. in a volume of 50 µL. Serum was collected at day 12, 20, 40, 75 and 90 post-immunization for (a) PRNT assay (PRNT90) against WT SARS-CoV-2 and ELISAs targeting anti-spike protein S1 (b), S2 (c), RBD (d) and N protein (e) IgG. Dotted horizontal lines indicate the detection limit. Dots represent individual animals (n = 12, 24 and 6 for PBS, 12-day CDO-7N-1 and 20/40/75/90-day CDO-7N-1, respectively). Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from three independent experiments. **P < 0.01, ****P < 0.0001 using two-tailed unpaired Mann‒Whitney U test. At day 28 post-immunization, hamsters were challenged with 105 PFU WT-SARS-CoV-2 i.n in a volume of 50 µL. At day 5 post challenge (33 days post-immunization), nose (f, p = 0.1667, not significant) and BALF (g, p = 0.1667, not significant) were collected for plaque assay analysis. Nasal turbinate and lungs were collected for H&E analysis (h). Scale bar in panel = 100 µm. i Hamsters were inoculated with PBS, or 105 PFU CDO-7N-1 as described in (a). A second and third inoculation of 105 PFU CDO-7N-1 was given at 23 and 33 days post-initial inoculation. Serum was collected at various time points extending over more than one year for PRNT assay (PRNT90) against WT SARS-CoV-2. j–n Hamsters were inoculated intranasally with PBS or 105 PFU CDO-7N-1, and serum samples were collected on day 28 post-immunization (pre-challenge); followed by intranasal challenged with 105 PFU of WT-SARS-CoV-2. Serum samples were then collected on day 5 post-challenge (33 days post-immunization). The samples were used for PRNT assay against WT SARS-CoV-2 (j) and ELISAs targeting anti-spike proteins S1 (k), S2 (l), RBD (m), and N protein (n) IgG. Dotted horizontal lines indicate the detection limit. Dots represent individual animals (panels f, g, n = 5 and 4 for PBS and CDO-7N-1; panel i, n = 24 for PBS, for CDO-7N-1: day 12 n = 21, day 25, n = 23, day 35, n = 22, day 180, n = 10, day 259, n = 18, day 367, n = 9; panels j–n, n = 4). Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from three independent experiments. *P < 0.05; ns, not significant using two-tailed unpaired Mann‒Whitney U test.
To test the protective efficacy of CDO-7N-1, we next determined whether immunized hamsters were protected from disease following challenge with WT SARS-CoV-2. Hamsters were immunized i.n. with 105 PFU of CDO-7N-1. Control hamsters were mock-immunized with PBS. After 28 days, the immunized and control hamsters were challenged with 105 PFU of WT SARS-CoV-2. Mock-immunized hamsters infected with WT SARS-CoV-2 had high virus titers in the nose and BALF (Fig. 2f, g) and showed mild to moderate atrophy of the olfactory epithelium in nasal turbinates and prominent lung inflammation (Fig. 2h). In contrast, CDO-7N-1-immunized hamsters showed no detectable virus, maintained normal structure in the nasal turbinate, and exhibited little or no lung inflammation. In a separate experiment, we investigated whether immunized hamsters were protected from virus challenge and disease after exposure to WT SARS-CoV-2-infected hamsters. Hamsters were immunized i.n. with 105 PFU of CDO-7N-1, while control hamsters received PBS. After 14 days, the immunized and mock-immunized hamsters were separately housed for 24 h with another group of hamsters that had been infected three days earlier with 105 PFU of WT SARS-CoV-2 (Supplementary Fig. 3a). The CDO-7N-1-immunized and control hamsters were maintained in their own cages for an additional 3 and 5 days before sacrifice for assessment of virus titers and lung pathology. Naïve hamsters exposed to infected hamsters for 24 hours exhibited high lung virus titers and significant lung inflammation, while CDO-7N-1-immunized hamsters showed no detectable lung virus titers and minimal or no inflammation (Supplementary Fig. 3b–d). These results indicate that CDO-7N-1 immunization protected hamsters against the development of disease resulting from either direct intranasal challenge with WT SARS CoV-2 or indirect challenge from previously infected hamsters.
We next determined the performance of CDO-7N-1 in models with pre-existing immunity. Hamsters immunized with CDO-7N-1, followed by a booster at 18 days, showed enhanced serum neutralizing activity (Fig. 2i). A third booster of CDO-7N-1 further amplified neutralizing antibody responses, which, although reducing over time, remain detectable up to 367 days post-immunization and by that time not significantly different from those of the first immunization (Fig. 2i). Additionally, a single immunization followed by a WT SARS-CoV-2 challenge at 28 days post-immunization led to increased neutralizing titers and binding antibody responses against various SARS-CoV-2 proteins, observable by 5 days post-challenge (Fig. 2j–n). Our data demonstrate that either a repeat immunization with CDO-7N-1 or a challenge with WT SARS-CoV-2 significantly boosts prior immunity.
CDO-7N-1 induces antibody and T-cell responses in hACE-2 transgenic mice
K18-hACE2 mice were infected i.n. with 103 PFU of CDO-7N-1 or WT SARS-CoV-2, and viral loads in both the lung and nose were assessed on days 3 and 5 p.i. Viral titers in both the lungs and nose at 5 d.p.i. were significantly lower in mice infected with CDO-7N-1 than in those infected with WT SARS-CoV-2 (Fig. 3a, b). In a separate group, mice were immunized i.n. with 103 PFU of CDO-7N-1, and serum and BALF were collected 14 and 21 days after immunization to assess humoral immunity. Control mice were mock immunized with PBS. IgG specific for Spike protein domains (S1 and S2) was detected in serum and BALF samples from immunized mice, whereas no response was detected in mock-immunized mice (Fig. 3c, d). An advantage of intranasal immunization is its ability to induce mucosal immunity and local IgA responses. A high level of IgA specific to the S protein was detected in serum and BALF samples at 14 and 21 days post CDO-7N-1-immunization, which was absent in mock-immunized mice (Fig. 3e, f). Moreover, CDO-7N-1 i.n. immunization elicited potent serum-neutralizing antibody levels, whereas no response was observed in the mice that received PBS (Fig. 3g). In a separate experiment using a different hACE2 transgenic mouse strain (HFH4‐hACE2), a robust SARS-CoV-2-specific serum IgG response was elicited by i.n. immunization of CDO-7N-1 vaccine at day 21 post-immunization (Supplementary Fig. 4a).
Seven-week-old K18-hACE2 female mice were inoculated intranasally (i.n.) with WT SARS-CoV-2 or CDO-7N-1 at 103 PFU per animal in a volume of 20 μL (a–g). Mice were sacrificed at 3 and 5 days p.i., and lungs (a) and noses (b) were collected for plaque assays (n = 4). c–f Mice were inoculated i.n. with CDO-7N-1 at 103 PFU per animal and sacrificed at 14 and 21 days post-immunization. Serum and BALF were collected for measurement of IgG and IgA levels using ELISA, targeting anti-spike (S1 + S2) IgG (c, d) and IgA (e, f). For panel c, n = 10; panel d, n = 8, 9 and 8 for PBS, CDO-7N-1 day 14 and CDO-7N-1 day 21, respectively; panel e and f, n = 7 and 8 for PBS and CDO-7N-1. g Serum from CDO-7N-1-inoculated mice was collected at day 14 post-immunization for the PRNT assay against WT SARS-CoV-2. Dots represent each animal (n = 4). Dotted horizontal lines indicate the detection limit. Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant using two-tailed unpaired Mann-Whitney U test. h–n Antigen specific T cell analysis in immunized mice. Seven-week-old K18-hACE2 mice were inoculated intranasally with CDO-7N-1 at 103 PFU per animal in a volume of 20 μL. At 21 days post-immunization, lung cells were collected and labeled with CFSE. The cells were stimulated with SARS-CoV-2 S protein peptide pool, N protein peptide pool, M protein peptide pool, E protein or CD3e monoclonal antibody as a positive control (data not shown) and labeled with anti-mouse fluorochrome-conjugated monoclonal antibodies against mouse CD45, CD3, CD4 and CD8. CD3 (h, i), CD4 (j, k) and CD8 (l, m) T cells in mock, S, N, M and E protein-stimulated cells were quantified using flow cytometry. (n) Levels of IFN-γ in cell culture of mock, S, N, M and E protein-treated cells was measured by ELISA. Each dot represents pooled samples from three animals. Dotted horizontal lines indicate the detection limit. For panel h–n, n = 7 and 6 for PBS and CDO-7N-1. Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. *P < 0.05; ns, not significant using two-tailed unpaired Mann-Whitney U test.
For analysis of T-cell responses, K18-hACE2 mice were immunized i.n. with 103 PFU of CDO-7N-1. Lungs and spleens were collected on day 21 post-immunization, and cells were isolated for single-cell culture. The cell cultures were stimulated for 3 days with a pool of S, N or M peptides, while recombinant protein was used for the E protein. After exposure to peptide/protein, T-cell proliferation was determined using carboxyfluorescein succinimidyl ester (CFSE) dilution as a measure of specific T-cell responsiveness. There was significant difference in the proliferation among the groups, the total numbers of live CD3+, CD4+ and CD8+ T cells were significantly higher in the antigen-stimulated cultures from immunized mice than in those from mock-immunized mice (Fig. 3h–m, Supplementary Fig. 5), suggesting antigen-specific responses that were detected in the proliferation assay. In addition, IFN-γ-producing CD4+ and CD8+ T-cell responses specific for viral structural proteins (S, N and M) were induced by CDO-7N-1 immunization (Fig. 3n).
CDO-7N-1 protects against SARS-CoV-2 challenge in hACE-2 transgenic mice and is associated with reduced proinflammatory cytokine levels and immune cell infiltration
To assess vaccine-mediated protection, K18-hACE2 mice were immunized i.n. with 103 PFU of CDO-7N-1 or mock immunized with PBS and challenged 21 days later with 104 PFU of WT SARS-CoV-2. Weight loss and disease were pronounced in mock-immunized mice, and these mice were dead by day 7 post-challenge (Fig. 4a, b). In contrast, all immunized mice survived WT SARS-CoV-2 challenge and showed neither weight loss nor signs of clinical disease (Fig. 4a, b). At day 7 post-challenge, the lungs and brain were harvested for measurement of viral titers by plaque assay and viral genome copy number by qRT‒PCR. Live SARS-CoV-2 was undetectable in both organs of mice immunized with CDO-7N-1, whereas high levels of virus replication were detected in mock-immunized mice (Fig. 4c–f). Histological analysis showed extensive lung pathology in challenged mock-immunized mice, with significant pneumonia, immune cell infiltration, vascular congestion, and interstitial edema (Fig. 4g, h). K18-hACE2 mice immunized with CDO-7N-1 had substantially reduced lung inflammation following WT SARS-CoV-2 challenge, with significantly reduced lung infiltrates compared to challenged mock-immunized mice (Fig. 4h). Taken together, these data show marked protection from SARS-CoV-2 infection and disease in K18-hACE2 mice immunized with CDO-7N-1. Similar data were obtained in a separate experiment using HFH4‐hACE2 mice (Supplementary Fig. 4). In this experiment, mock-vaccinated HFH4‐hACE2 mice challenged with WT SARS-CoV-2 all reached endpoint at day 7 post infection and had a high viral load in the lung (~103-4 PFU/organ) and nose (~102 PFU/organ), while all vaccinated mice survived the viral challenge, with no virus detected (Supplementary Fig. 4b–e).
a, b Seven-week-old female K18-hACE2 mice (n = 8, 9, 6 for PBS, PBS + WT SARS-CoV-2, and CDO-7N-1 + WT SARS-CoV-2, respectively) were immunized intranasally with 103 PFU CDO-7N-1 or mock-immunized with PBS in a volume of 20 μL. The mice were challenged with 104 PFU WT SARS-CoV-2 on day 21 post-immunization. Mouse weight (a) and disease (b) were monitored daily. The animal weight is presented as the percentage change relative to day zero post-challenge. The disease development was presented as the percentage of animals showing signs of disease. The severity of the disease was scored numerically in an ascending order from zero to 13. All values represent the mean ± SEM from two independent experiments. **, P < 0.01; ****, P < 0.0001 using two-way ANOVA with Bonferroni’s post hoc test. c–f WT SARS-CoV-2 viral loads in the immunized and challenged mice. The mice were sacrificed at day 7 post-challenge (28 days post-immunization), and lung (c, d) and brain (e, f) tissues were collected to determine virus titers and viral genome copy numbers using plaque assay and probe-based RT‒qPCR. The detection limit of the plaque assay is indicated with dotted horizontal lines. Dots represent individual animals. For panel c, n = 7; panel d, n = 10 and 8 for PBS + WT SARS-CoV-2 and CDO-7N-1 + WT SARS-CoV-2; panel e and f, n = 19 and 15 for PBS + WT SARS-CoV-2 and CDO-7N-1 + WT SARS-CoV-2. Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. ***P < 0.001; ****P < 0.0001 using two-tailed unpaired Mann‒Whitney U test. (g, h) Histopathological changes in the lungs of immunized and challenged mice. The mice were sacrificed at day 7 post-challenge (28 days post-immunization), and lung tissues were collected for H&E staining (g). Four mice in each group (#1-4) were selected to represent the group. Three sections per tissue was used for cell infiltrates statistical analysis using ImageScope (h). For (h), dots represent individual tissue sections (n = 27, 21, and 21 for PBS, PBS + WT SARS-CoV-2, and CDO-7N-1 + WT SARS-CoV-2, respectively). Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. *P < 0.05; ***P < 0.001; ns, not significant using one-way ANOVA with Tukey’s post hoc test.
K18-hACE2 mice were immunized i.n. with 103 PFU of CDO-7N-1 or mock immunized with PBS and 21 days later challenged with 104 PFU of WT SARS-CoV‐2. At seven days post-challenge, the production of proinflammatory cytokines and chemokines such as IFN-γ, TNF-α, CCL2, CXCL1, and CXCL10 was significantly lower in the lungs of CDO-7N-1-immunized mice than in those of mock-immunized mice (Supplementary Fig. 6). Although the differences were not significant, immunized mice had lower levels of IL-1β, IL-6, IL-10, GM-CSF, IFN-β and CCL5 than mock-immunized mice.
We used mass cytometry to characterize immune cell populations in the BALF, lung and tracheobronchial draining lymph nodes (DLNs) of CDO-7N-1- and mock-immunized K18-hACE2 mice following challenge with WT SARS-CoV-2 at day 7 post challenge. In the BALF and lungs of mock-immunized mice following challenge, there were substantial increases in the proportions of CD4+ and CD8+ T cells and inflammatory monocytes, while proportions of alveolar macrophages and B cells were reduced (Supplementary Fig. 7a, b, Supplementary Fig. 8). In contrast, the population proportions of CD4+ and CD8+ T cells, inflammatory monocytes, B cells and alveolar macrophages in CDO-7N-1-immunized mice following challenge were not significantly different from those in naïve mice. In the DLNs of mock-immunized mice following challenge, there was a reduction in the proportions of CD4+ and CD8+ T cells (Supplementary Fig. 7c, Supplementary Fig. 8), suggesting a potential influx of new cells from the blood and/or migration of these cells from the DLNs into sites of infection such as the lung and airways. In CDO-7N-1-immunized challenged mice, the proportions of CD4+ and CD8+ T cells in the DLNs were similar to those in mock-immunized mice, but higher than those of challenged, mock-immunized mice. We further examined changes in pertinent subsets of CD8+ and CD4+ T cells isolated from lung, BALF, DLN and spleen in more detail. Notably, there were no significant differences between unchallenged groups of mock-immunized and CDO-7N-1-immunized mice in baseline numbers of naïve, Effector (Teff), Regulatory (Treg), Tissue-resident memory (Trm), Central Memory (Tcm) or Effector Memory (Tem) T cell subsets (Supplementary Fig. 9).
Cell numbers in mock-immunized, WT SARS-CoV-2-challenged mice increased most in the lung and BALF. Significant increases were observed in the lung, variously, in CD4+ Treg, Teff, Tcm and Tem subsets and CD8+ Teff, Tcm and Tem subsets, each associated with significant proliferation, as measured by the mean fluorescence intensity (MFI) of Ki67 labeling. Interestingly, although there was no change in numbers of CD4+ and CD8+ naïve T cells in the lung, there was significant proliferation of both (Supplementary Figs. 9 and 10). Furthermore, there was a 75-80% decrease in in the proportions of CD4+ and CD8+ naïve T cells, 70-90% reduction in Trm and ~60% reduction in CD8+ Tcm (Supplementary Fig. 11), with a reciprocal increase in the proportions of CD4+ and CD8+ Teff and CD8+ Tem. In the BALF, there were significant increases in the numbers of CD4+ naïve, Teff, and Tem, as well as all CD8+ subsets, with proliferation of all but the CD8+ Tcm subsets. In the DLN, significant numerical increases occurred in CD4+ Teff, and CD8+ Teff and Tem cells, with proliferation in Treg, as well as Teff, Tcm, and Tem in both CD4+ and CD8+ populations. However, the spleen showed no numerical changes in T cell subsets, despite significant proliferation of Teff and Tem subsets in both CD4+ and CD8+ populations (Supplementary Fig. 10).
Strikingly, in the WT SARS-CoV-2-challenged, CDO-7N-1-immunized mice, there were no significant changes in the numbers of any T cell subset in the lungs or BALF, although the proportion of CD4+ Trm in the lungs in this group was significantly greater than the other groups. In contrast, challenge of either mock-immunized or immunized groups resulted in a significant reduction in the proportion of CD8+ Trm in the lungs. On the other hand, there were significant increases in the numbers of CD4+ Trm in both DLN and spleen, and in CD4+ Tem in the DLN, as well as more substantial increases in CD8+ Teff and Tem cells in the spleen (Supplementary Fig. 11).
CDO-7N-1 protects against SARS-CoV-2 variants of concern and induces SARS-CoV-1 neutralizing antibodies
SARS-CoV-2 variants of concern have been shown to cause disease in K18-hACE2 Tg mice22,23. K18-hACE2 mice were immunized i.n. with 103 PFU of CDO-7N-1 or mock immunized with PBS and challenged 21 days later with 104 PFU of beta (B.1.351), delta (B.1.617.2) or omicron (B.1.1.529) SARS-CoV-2 variants of concern. Mock-immunized mice challenged with the beta or delta variant showed prominent clinical disease signs with significant weight loss (Fig. 5a, b; h, i), high nose, lung and brain virus titers at day 7 post challenge (Fig. 5c–e; j–l) and pronounced lung pathology (Fig. 5f, g; m, n). In contrast, mice immunized with CDO-7N-1 were protected from beta or delta infection, with no weight loss or clinical disease signs, no detectable live virus in the nose, lung or brain and no visible lung pathology (Fig. 5a–n). Omicron is less virulent in K18-hACE2 mice than the beta, delta or WT SARS-CoV-2 strains, and thus no detectable weight loss, clinical disease signs or lung pathology was observed in either mock-immunized or CDO-7N-1-immunized mice following omicron challenge. In mice immunized with CDO-7N-1 and challenged with the omicron variant of concern, virus was not detected in the lung following challenge, while high virus titers were observed in 50% of the lungs of mock-immunized mice at day 6 post-challenge (Fig. 5o). In addition, using serum from CDO-7N-1-immunized K18-hACE2 mice (14-day immunization) or hamsters, we found that virus-specific neutralizing antibodies against the beta, delta, omicron (B.1.1.529, XBB.1.5) and D614G variants of concern were detectable post-immunization (Fig. 5p–r, Supplementary Fig. 4f, g). Importantly, in hamsters, CDO-7N-1 induced the production of cross-reactive neutralizing antibodies against SARS-CoV-1 (Fig. 5s).
Seven-week-old female K18-hACE2 mice were immunized intranasally with 103 PFU CDO-7N-1 or mock-immunized with PBS in a volume of 20 μL. The mice were challenged with 104 PFU beta, delta or omicron variants of SARS-CoV-2 on day 21 post-immunization. (a–g; h–n) Protection against beta and delta variants of SARS-CoV-2. Mouse weight (a, h) and disease (b, i) were monitored daily. The animal weight is presented as the percentage change relative to day zero post-challenge. The disease development was presented as the percentage of animals showing signs of disease. All values represent the mean ± SEM from two independent experiments. ****, P < 0.0001 using two-way ANOVA with Bonferroni’s post hoc test. Nose (c, j), lung (d, k) and brain (e, l) tissues were collected at day 7 post-challenge. Virus titers were determined by plaque assay. Lung tissues from the immunized and challenged mice were collected for H&E staining [f, g (p = 0.9177, not significant), m, n]. Four mice in each group (#1–4) were selected to represent the group. (o, p = 0.1333, not significant) Protection against the omicron variant of SARS-CoV-2 in mice. Viral titers in lung tissue were determined at day 6 post-challenge by plaque assay. (p, q) Seven-week-old K18-hACE2 female mice were inoculated with PBS or CDO-7N-1 as described above. Serum from the inoculated mice was collected at 14 post-immunization for the PRNT assay against beta and delta variants of SARS-CoV-2. (r, s) Six- to eight-year-old Golden Syrian hamsters were inoculated with PBS or 105 PFU CDO-7N-1 intranasally in a volume of 50 µL. Serum was collected at 14 post-immunization for the PRNT assay against SARS-CoV-2-XBB.1.5 (r) or SARS-CoV-1 (s). Dotted lines indicate the detection limit. For panel (c–e, j–l, o–s), dots represent individual animals. For panel (g, n), dots represent individual tissue sections. For panel a, n = 6 and 5 for PBS + Beta and CDO-7N-1 + Beta; panel c, n = 6 and 10 for PBS + Beta and CDO-7N-1 + Beta; panel d and e, n = 5; panel g, n = 11 and 10 for PBS + Beta and CDO-7N-1 + Beta; panel h, n = 6 and 5 for PBS + Delta and CDO-7N-1 + Delta; panel j, n = 6 and 10 for PBS + Delta and CDO-7N-1 + Delta; panel k and l, n = 5; panel n, n = 16; panel o, n = 4 and 6 for PBS + Omicron and CDO-7N-1 + Omicron; panel p and q, n = 4; panel r and s, n = 5. Data are presented as box and whisker ± SD with the median indicated by a line across the box, maximum to minimum points from two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant using two-tailed unpaired Mann‒Whitney U test.
CDO-7N-1 is safe and induces SARS-CoV-2 specific antibody and T-cell responses in cynomolgus macaques
We investigated CDO-7N-1 virus replication, immunogenicity, and clinical and blood parameters in nonhuman primates (NHPs) immunized i.n. with 105 PFU of CDO-7N-1. Immunized animals had detectable (peaked at day 2 post immunization) genomic viral RNA in both tracheal and nasal fluids, suggesting active virus replication (Fig. 6a, b). Very low levels of genomic RNA were detected in BALF, while no RNA was detected in rectal fluids (Fig. 6c, d). At 48 h p.i., 3 macaques were sacrificed. Minimal diffuse lymphocytic pulmonary infiltration could be detected in the vaccinated animal DF918, while another vaccinated animal (DF873) and a control animal (DF977) showed no tissue changes (Supplementary Fig. 22, Supplementary Table 11-12). Both vaccinated animals showed rhinitis. No lesions or tissue changes could be detected in the masseter muscle or facial nerves, suggesting no neuroinflammation at this time point (Fig. 6e). These data suggests that CDO-7N-1 is highly attenuated in NHPs, compared to previous studies showing clear pulmonary disease following SARS-CoV-2 infection24. Clinical and blood parameters remained normal for all animals throughout the study, unaffected by CDO-7N-1 immunization (Supplementary Fig. 12). At 2 days post-immunization, no pathology was observed in any other organ of the vaccinated or control animals, including submaxillary salivary glands, duodenum, colon, brain, kidney, spleen, heart and olfactory bulb. These results suggest a local response and local vaccine-induced lesions with no signs of toxicity in the other organs (Supplementary Fig. 22, Tables 11–12). Pathology was examined in another group of NHPs at 60 days post-immunization: no pathology was observed in the lung, masseter muscle or any other organ or tissue examined (Supplementary Fig. 23, Tables 11–15).
Cynomolgus macaques were immunized with 105 PFU CDO-7N-1 intranasally in a volume of 250 µL in each nostril (n = 10). a–d Viral RNA in the nasopharyngeal fluid (a), tracheal fluid (b), BALF (c) and rectal fluid (d) was assessed using RT‒qPCR at days 0, 3, 4, 5, 8, 11, 16 and 32 post-immunization. Dotted horizontal lines indicate the detection limit. e Histopathological changes in the lungs of unimmunized and immunized macaques. Macaques were sacrificed at two days post-immunization. The nasal mucosa, lung and masseter muscle were collected for H&E staining. Scale bar in panel = 50 µm. The images provided are representative of two macaques from the vaccine group and one macaque from the PBS group. f, g Serum samples were collected for ELISA targeting anti-spike and RBD protein IgG at 0, 2, 4 and 6 weeks post-immunization. The data in WHO International Standard (BAU/mL) is included in supplementary information (Supplementary Fig. 16a, b). h Plasma samples were collected at indicated time points for Spike specific neutralizing IgG assay. Dotted horizontal lines indicate the detection limit. Data in µg/mL for monoclonal antibody to SARS-CoV-2 Spike protein is in Supplementary Fig. 16c. i, j Nasopharyngeal fluid was collected for ELISA targeting anti-spike and RBD protein IgA at 0, 7, 15 and 31 days post-immunization. k–m At 0, 7, 15 and 31 days post-immunization, PBMCs were collected and stimulated with spike (k, l) protein and N protein (m) peptide pool (2 μg/mL). IFN-γ producing T cells specific for Spike protein and N protein were quantified with an Automated ELISpot Reader ELR08IFL. Data are presented for individual animals.
Antibody response against SARS-CoV-2 was detected at days 14 and 40 p.i. Elevated levels of neutralizing and binding antibodies in 7 out of 10 animals recognizing the S protein and RBD were observed in the serum at these time points (Fig. 6f–h). The variability in neutralizing antibody levels among individual NHPs and the discrepancy between total IgG and neutralizing antibody levels are discussed in the Supplementary Discussion. Local mucosal immunity was also induced by CDO-7N-1, with high S protein-specific IgA titers observed in most but not all macaques (Fig. 6i, j). T-cell-specific responses were evaluated by stimulating peripheral blood mononuclear cells (PBMCs) collected at day 7 p.i. with a cocktail of peptides from S (S1 pool and S2 pool), the RBD and N. Strong CD4+ and CD8+ T-cell responses were detected in vaccinated animals, accompanied by elevated IFN-γ production (Fig. 6k–m). Cheek muscle and nasal and lung tissue were collected for histopathological analysis at 48 h post-CDO-7N-1 immunization. In nasal tissue, a small amount of localized neutrophilic infiltrate was observed, which is likely directly due to irritation caused during intranasal immunization. The Drazen score was 0, indicating no skin effects (Supplementary Fig. 13a, b). No pathology was evident in the cheek tissue sections (Supplementary Fig. 13c). Another safety concern is the potential spread and replication of the vaccine virus from vaccinated individuals to others. To address this, we immunized two cynomolgus macaques with 105 PFU of CDO-7N-1 and co-housed them with a naïve macaque for up to 48 hours (Supplementary Fig. 14e). We observed substantial increases in subgenomic RNA in the immunized macaques, indicating active virus replication (Supplementary Fig. 14f–i). However, no subgenomic viral RNA was detected in the naïve macaque at either time point, suggesting no replication of the vaccine virus (Supplementary Fig. 14f–i), despite a low genomic signal below the limit of reliable quantification in tracheal fluid, <0.05% of that of the vaccinated animals. Additionally, our hamster studies show that the CDO-7N-1 does not transmit from vaccinated to unvaccinated animals (Supplementary Fig. 14a–d).
Discussion
Despite the application of various successful vaccine platforms, including mRNA, viral vectors, inactivated vaccines and subunit proteins, for COVID-19 management, reports of new infections are still increasing rapidly worldwide. This is a major concern, as it is evident that the current vaccines, while protecting from severe disease, have served only as a temporary solution to the pandemic and do not provide broad, long-lasting protection, particularly against newer variants such as omicron, which shows remarkable immune evasion25. Indeed at the 15th Meeting of the International Health Regulations (2005) Emergency Committee of the World Health Organization, it was acknowledged, despite a determination that it was no longer ‘a public health emergency of international concern’, that COVID-19 was nevertheless an ‘established and ongoing health issue26. Additionally, current COVID vaccines, administered intramuscularly, are reactogenic, with frequent mild/medium side effects27. Here, we report the development and preclinical testing of a strain of SARS-CoV-2, designated CDO-7N-1, using CDO for use as a LAV. CDO-7N-1 is a live attenuated COVID-19 vaccine using codon usage deoptimization and it compares favorably with other LAV candidates28,29,30,31 (discussed further in Supplementary Discussion). LAVs have a successful track record against diseases such as polio and yellow fever among others, as they induce strong mucosal and systemic immunity, mimicking natural infection32. Similarly, immunization with CDO-7N-1 was highly immunogenic, providing strong protection from SARS-CoV-2 infection in mouse and hamster models of COVID-19, with good evidence of immunogenicity in non-human primates (NHP).
The CDO-7N-1 vaccine candidate induced high levels of antibody-neutralizing activity after a single immunization, and a strong T-cell response, including cytotoxic CD8+ T cells. Robust evidence supports the role of neutralizing antibodies in protection from SARS-CoV-2 infection; however, this wanes and is less effective against emerging variants33. In contrast, T-cell epitope responses appear to be longer lived, conserved and shared among variants34. As few as 7% and 3% of identified CD4+ and CD8+ T-cell epitopes, respectively, are evidently affected by mutations in the known variants. T-cell responses seem to have a role in early protection, provide broader protection against COVID-19 less affected by variant emergence, and may reduce severe disease in reinfected and vaccinated individuals35. Our ex vivo antigen stimulation studies suggest robust induction of antigen-specific T cell responses by CDO-7N-1, and although further studies are required to fully characterize these, our data indicates efficient local control of WT SARS-CoV-2 in CDO-7N-1-immunized animals, with systemic induction of effector, effector memory and resident memory T cells in the DLN and spleen.
Current vaccines have exhibited dramatically reduced effectiveness against emerging variants36,37 due to mutations in the S protein, the single antigen of choice for many SARS-CoV-2 vaccines. Thus, the strong current push by major vaccine organizations to develop next-generation COVID-19 vaccines is likely to be achieved by inducing antigenically broader immune responses to vaccination. In the mouse model, CDO-7N-1 elicited similar Spike specific IgA and IgG responses to the mRNA vaccine (Supplementary Fig. 15). However, unlike the mRNA vaccine which targets only the spike protein, CDO-7N-1 induces immunity to all major SARS-CoV-2 proteins and is highly efficacious against all major variants to date. The capacity of our vaccine candidate to elicit cross-neutralizing antibodies against SARS-CoV-1, where no vaccine currently exists, underscores its likely protective capability in future outbreaks. Additionally, CDO-7N-1 can easily be adapted to new variants by replacing the S or N gene, as the codon deoptimization is in the conserved ns-region.
As a LAV, CDO-7N-1 expresses the same spectrum of viral proteins as WT SARS-CoV-2. Following CDO-7N-1 immunization, IgG responses in mice and hamsters against all major SARS-CoV-2 structural proteins were detected in a broad antibody and T cell response, not only against the spike protein, but also against the N, M and E proteins. This antigenically broad immune response is a key advantage of CDO-7N-1 over currently used mRNA or protein subunit vaccines and is likely to be more effective in subsequent infection, especially against variants that escape spike protein-specific immunity.
A significant difference between vaccinated and naturally-infected individuals is the lack of mucosal response due to the intramuscular route of vaccination, as opposed to the intranasal route of infection35. All COVID-19 vaccines currently in use, are administered subcutaneously or intramuscularly. This stimulates strong systemic immunity but is less effective at inducing mucosal immunity38. As SARS-CoV-2 is a respiratory pathogen, mucosal immunity is crucial for preventing infection and its absence may allow the high virus titers in the upper airways during breakthrough infection. The strong cell and humoral immunity induced by natural SARS-CoV-2 infection via the intranasal route may reflect the value of an effective mucosal immune response39. Thus, following intranasal delivery of CDO-7N-1, induction of a strong and long-lasting mucosal IgA response in mice, hamsters and NHP may prevent or significantly reduce breakthrough infection. This is likely to underlie the protection of CDO-7N-1-vaccinated hamsters from WT SARS-CoV-2 challenge by infected littermates.
The safety of LAVs is of utmost importance. The high level of attenuation of CDO-7N-1 in mouse and hamster models while inducing potent, long-lasting protective effect, is a strong indicator of a desirable safety profile to allow progression to human trials. This is further supported by the clinical and immunogenicity profile of CDO-7N-1 observed in NHP. Following intranasal immunization, even at a high dose of 105 PFU, CDO-7N-1 replicated to very low titers in the airways and induced little or no lung pathology. In contrast, WT SARS-CoV-2 replicated to very high titers and induced severe lung pathology in infected mice and hamsters. Following intranasal infection of K18 mice and hamsters with WT SARS-CoV-2, cells of the olfactory bulb are infected3. This is a major safety concern as virus could disseminate to the central nervous system (CNS) via the olfactory nerves40. However, even with high doses, CDO-7N-1 infection was undetectable in the olfactory bulb, again highlighting the safety profile of CDO-7N-1 intranasal immunization. This reassuring feature of CDO-7N-1 is crucial in using the intranasal vaccination route.
For all LAVs, one of the major safety considerations is the potential for reversion to WT virulence. Following multiple in vitro and in vivo passages, sequence analysis demonstrated a high degree of genetic stability, making reversion to WT virulence very unlikely. Another major concern with LAVs for coronaviruses is their propensity for recombination. However, the likelihood of CDO-7N-1 recombining with other variants is low, and potential recombination would likely pose minimal risk (discussed further in Supplementary Discussion).
Following CDO-7N-1 immunization, we characterized immune responses after WT SARS-CoV-2 challenge. A comprehensive assessment of the major components of the cellular immune response was made in lung, BALF, DLNs and spleen, using cytometry by time of flight (CyTOF). In the challenge model, there was a massive reduction in infiltrating immune cells, including T cells and inflammatory monocytes, in the lung and BALF of immunized mice. On the other hand, alveolar macrophages, important for maintaining lung homeostasis, were significantly higher in the BALF and lung in the challenged mice immunized with CDO-7N-1, compared to that in the challenged mock-immunized mice. We also observed a significant increases in B cells in the lungs of immunized challenged mice, compared to mock-immunized challenged mice, consistent with strong B-cell immunity elicited by CDO-7N-1, which is crucial for a rapid response to SARS-CoV-2 virus upon re-exposure41. In these mice, too, we observed increased numbers of CD4+ Trm in the DLN and spleen and CD8+ Teff and Tem in the spleen, emphasizing the systemic immune response to challenge in immunized mice.
Heterologous prime-boost strategies have been shown to be effective against COVID-1942,43. We believe that this vaccine candidate will be a highly effective booster vaccine in individuals that are already vaccinated with currently approved vaccines and/or have had COVID-19. Currently, vaccine responses, irrespective of the vaccine administered, are directed against targeted proteins, making them unsuitable as a booster vaccine, as it allows SARS-CoV-2 mutants to evade antibody neutralization, indeed it may promote mutation, resulting in limited protection against newer variants. In addition to the current repertoire of available vaccines, LAV boosters would induce broader, long-lived immunity in all previous vaccine recipients, mimicking a natural infection and strengthening existing immune responses. In addition, the generation of mucosal immunity following intranasal administration would be a substantial advantage, priming early protective innate cellular responses and a localized adaptive memory response that activates rapidly after subsequent exposure to all current or future emerging SARS-CoV-2 variants32. It is becoming clear that natural SARS-CoV-2 infection following vaccination leads to the affected individual having extremely high levels of immune response, also known as ‘super immunity’44,45. It is very likely that a LAV such as ours will also induce super immunity in previously vaccinated individuals, the same way that natural SARS-CoV-2 infection does. Thus, our vaccine would be particularly effective as a booster and significantly reduce the threat of SARS-CoV-2 dissemination globally32.
Current vaccine development efforts have largely focused on the spike protein. Natural infection with SARS-CoV-2, however, induces strong immunity against numerous virus proteins in addition to spike. The response to natural infection includes a strong T-cell response, with activation of both CD4+ and CD8+ T cells46. The immune response to CDO-7N-1 is likely to be similarly broad in humans. This effect may provide a key advantage in the fight against emerging variants. Vaccines that induce an immune response against a single protein such as the spike will always be vulnerable to the emergence of variants with spike mutations, with consequent immune escape. In contrast, the antigenically broad immune response generated by CDO-7N-1 is likely to prove resistant to new variants, which would need to mutate multiple virus proteins to fully escape vaccine-induced immunity.
Methods
Infectious clone plasmids
The infectious clone of the SARS-CoV-2 strain, WT SARS-CoV-2 (MT926410), was chosen as the parent template for vaccine development21. The fragment spanning SanD11534 to PacI8586 in ORF1a of WT SARS-CoV-2 was designed for the codon deoptimization (CDO) region. The CDO region was obtained as synthetic DNA fragments from GenScript Biotech. The CDO fragment and corresponding wild-type (WT) backbone were constructed using one-step ligation, forming infectious clone plasmids of vaccine candidates. Correct recombinants were identified by restriction analysis and confirmed by sequencing. The obtained plasmids containing the CDO design were transformed into TransforMax™ EPI300™ Chemically Competent E. coli (Lucigen Corporation) for amplification.
Viruses and cells
Viruses - The SARS-CoV-2 strain of MT926410 and CDO SARS-COV-2 vaccine candidates (CDO-4N-1 and CDO-7N-1) were generated by transient transfection of a single plasmid species using Lipofectamine LTX with Plus reagent as previously described47. The SARS-CoV-2 variants of concern, which include beta (B.1.351), delta (B.1.617.2) and omicron (B.1.1.529), were generously provided by the Victorian Infectious Diseases Reference Laboratory (VIDRL). SARS-CoV-2 variant containing only the D614G substitution in the S glycoprotein (SARS-CoV-2 D614G) and the B.1.351 spike (SARS-CoV-2-SA) were generated as previously described48,49.
Cells - The BHK-21 (C-13) and Vero E6 (C1008) cell lines were purchased from Sigma‒Aldrich. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) with 10% fetal calf serum and 2 mM L-glutamine at 37 °C with 5% CO2. Calu-3 epithelial cells were purchased from ATCC (HTB-55) and cultured in Eagle’s Minimum Essential Medium (EMEM; Invitrogen) supplemented with 10% fetal calf serum. All cell lines tested negative for mycoplasma.
Animal ethics statement
Animal experiments in this study were approved by the Animal Ethics Committee of Griffith University (K18-hACE2 mice, MHIQ/08/21/AEC), University of North Carolina ethics (HFH4-hACE2 mice, 18-287.0-C; ratified by Griffith University Animal Ethics Committee, MHIQ/03/21/AEC), and Indian Immunologicals (IIL) ethics (Golden Syrian hamsters, IIL-R&D-SA-14-2020, IIL-R&D-SA-15-2020; ratified by Griffith University Animal Ethics Committee, MHIQ/02/21/AEC). All mice and hamsters were handled in a class 3 biosafety cabinet in a PC3/BSL3 containment facility. All waste generated by the mice are autoclave before being removed from the facility.
All nonhuman primate experimental procedures adhered to the European guidelines for animal care and use for scientific purposes, as outlined in Directive 63-2010 of the “Journal Officiel des Communautés Européennes” (L276, September 22, 2010), and the institutional guidelines of CEA. The NHP studies received approval from the Infectious Diseases Models for Innovative Therapies (IDMIT) Research Center, French Alternative Energies and Atomic Energy Commission (CEA) ethical committee (approval number A20_061) and from the French Administration (APAFIS#28946-2021011312169043 v2; ratified by the Griffith University Animal Ethics Committee, MHIQ/06/21/AEC). All procedures with animals conformed to the National Health and Medical Research Council Australian code for the care and use of animals for scientific purposes.
Mouse studies
The studies used two human ACE2 transgenic mouse models to test a panel of SARS-CoV-2 live attenuated virus vaccines for their efficacy in eliciting protective SARS-CoV-2 immunity. The use of different transgenic mouse strains, driven by distinct promoters that result in varied ACE2 distribution across organs, provides more comprehensive data on the protective effects of the CDO-7N-1 vaccine.
K18-hACE2 mice
K18-hACE2 transgenic mice have been widely used as a mouse model in SARS-CoV-2 research50,51. In these mice, human ACE2 receptor expression is driven by the human keratin 18 (K18) promoter for epithelial cells, making the mice susceptible to SARS-CoV-2 and SARS-CoV. K18-hACE2 mice were obtained from the Animal Resource Centre (Western Australia). The mice were housed in Griffith animal house with food and water provided in the cage. The housing temperature was controlled between 20–25 °C and a 12h light/12h dark cycle was used. Only female K18-hACE2 mice were used in this study (Figs. 3, 4, 5. Supplementary Figs. 5, 9, 10, 11, 15).
HFH4-hACE2 mice
These mice have been shown to be susceptible to SARS-CoV-2 infection52. Due to the nature of the transgenic mice, hACE2 expression is driven by a lung epithelial cell-specific HFH4/FOXJ1 promoter, resulting in limited hACE2 expression in the periphery. HFH4-hACE2 mice were obtained from the University of North Carolina animal facility. The mice were housed in University of North Carolina animal house with food and water provided in the cage. The housing temperature was controlled between 20-25 °C and a 12h light/12h dark cycle was used. Only male HFH4-hACE2 mice were used in this study (Supplementary Fig. 4).
Immunization and challenge of mice
K18-hACE2 mice
Six- to eight-week-old K18-hACE2 mice were anesthetized with ketamine/xylazine (80 mg/kg ketamine combined with 8 mg/kg xylazine, intraperitoneal injection) and inoculated intranasally (i.n.) with 103 PFU of CDO-7N-1 in a volume of 20 µL. Mock-inoculated mice received 20 µL of sterile PBS. Mice were challenged with 104 PFU SARS-CoV-2 clinical isolate strains or mock-challenged by PBS at day 21 post inoculation and then monitored for disease signs daily. Mice were scored using a cumulative and progressive clinical disease matrix. Mice were given a score between 0 and 3 for each of the following five health indicators: eating habit, locomotion, behavior, appearance, and weight loss. A score of 0 was normal. Mice that received an overall score of 3 or more were monitored twice daily. A score of 3 in any one of the 5 categories (e.g., >15% weight loss) or an overall score of >10 led to the mouse being euthanized.
HFH4-hACE2 mice
Six- to ten-week-old HFH4-hACE2 mice were placed under ketamine/xylazine anesthesia and inoculated with 103 PFU of CDO-7N-1. Mock-inoculated mice were inoculated with PBS. Mice were challenged with 105 PFU of wild-type SARS-CoV-2 intranasally at day 21 post inoculation. Mice were monitored daily for virus-induced weight loss, clinical score, and mortality for 4 to 21 days post infection (p.i.). A subset of mice was euthanized at day 4 p.i. to assess challenge virus loads in the lung and nasal tissue.
Plaque assay
K18-hACE2 mice
Animals were sacrificed, and target tissues were collected in reinforced tubes containing 2.8mm ceramic beads (Omni International) and phosphate-buffered saline (PBS) then homogenized using a Bead Ruptor 24 Elite homogenizer (Omni International) according to the manufacturer’s instructions. The supernatant was collected by centrifugation and stored at −80 °C. Vero E6 cells were seeded in 12-well plates at 2.5 × 105 cells per well and cultured in DMEM with 10% FCS overnight. The cells were infected with a dilution series of virus from samples and were incubated at 37 °C with 5% CO2 for 60 minutes with intermittent agitation of the plates. After incubation the cells were overlaid with 1.2% colloidal microcrystalline cellulose (Sigma‒Aldrich) in DMEM with 2% FCS and 1x Penicillin/Streptomycin. The plates were incubated at 37 °C for 72 h and then the overlay was removed and cells washed once with PBS. Cells were then stained and fixed with 0.1% crystal violet with 3.7% formaldehyde. Viral titers were calculated using the following formula: PFU/mL = (average number of plaques/volume [mL] of virus added) × dilution factor and normalized against tissue weight.
HFH4-hACE2 mice
Animal tissues were collected in 0.5 mL of medium (DMEM containing 5% FCS and 1 mM L-glutamine) and stored at −80 °C. To determine the viral titer, the tissue was thawed and homogenized using a Magna Lyser homogenizer (Roche, 6000 rpm for 40 seconds), and debris was pelleted by centrifugation. Vero E6 cells were seeded in 12-well plates at 2 × 105 cells per well and cultured overnight in DMEM with 10% heat inactivated FCS and 1 mM L‐glutamine. The cells were infected with a dilution series of virus from samples and overlaid with 1.25% carboxymethylcellulose (Sigma‒Aldrich) in alpha MEM containing 6% heat inactivated FCS, 2% L-glutamine, 2% Pen Strep and 2% HEPES. The plates were incubated at 37 °C for 96 h and then fixed with 4% paraformaldehyde (PFA), followed by staining with 0.25% crystal violet. Viral titers were calculated using the following formula: PFU/mL = (average number of plaques/volume [mL] of virus added) × dilution factor. For the plaque assay on lung tissue, the superior and middle lobes of the right lung were collected, placed in DMEM with 5% FBS, and processed in a MagNa Lyser (Roche).
Qualitative real-time PCR (qRT‒PCR)
Animals were sacrificed, and target tissues were collected in TRIzol reagent (Invitrogen). The tissues were homogenized using the Bead Ruptor 24 Elite homogenizer, and total RNA was extracted according to the manufacturer’s instructions. The RNA was reverse transcribed into cDNA using reverse transcriptase (Sigma‒Aldrich) with random nonamers (Sigma‒Aldrich). QuantiTect probe real-time PCR was performed on a CFX96 touch real-time PCR system (Bio-Rad) to measure the viral genome copy numbers. The SARS-CoV-2 RdRP gene-targeting primer and probe sequences were 5’-GTGAAATGGTCATGTGTGGCGG-3’ (forward primer), 5’-CAAATGTTAAAAACACTATTAGCATA-3’ (reverse primer), and FAM-CAGGTGGAACCTCATCAGGAGATGC-BHQ (probe), where FAM is 6-carboxyfluorescein and BHQ is a black hole quencher. The QuantiTect probe real-time PCR conditions were set as follows: (i) 1 cycle of 95 °C for 15 minutes and (ii) 40 cycles of 94 °C for 15 s followed by 60 °C for 1 minute. The DNA amplification specificity was evaluated by melting curve analysis. Viral genome copy number was calculated against a standard curve of a viral infectious clone (MT926410)41.
ELISAs
K18-hACE2 mice
Animals were sacrificed, and serum and bronchoalveolar lavage fluid (BALF) were collected for ELISA. The concentrations of anti-Spike IgG and IgA were determined using ELISA kits from Krishgen Biosystems and Xpressbio, respectively. All ELISAs were performed according to the manufacturer’s instructions.
HFH4-hACE2 mice
Plates were coated with 0.8 µg/mL heat-inactivated SARS-CoV-2 virus in carbonate buffer (3.39 g of sodium carbonate (Na2CO3), 5.70 g of sodium bicarbonate (NaHCO3), up to 1 L with water) by adding 100 μL of the coating mixture per well and storing the plates overnight in a humidified container at 4 °C. Unbound antigen was removed from the plates by washing 4 times with EWB (1× PBS containing 0.05% Tween-20). Samples were diluted in EWB containing 5% milk, and 100 µL of each diluted sample was added to a coated well. Plates were placed in a humidified chamber and refrigerated at 4 °C overnight. After washing the plates 4 times with EWB, anti‐IgG‐HRP (in EWB at 1:2000) was added to each well. Two hours later, the secondary antibody was removed, and the plates were washed 5 times with EWB. Substrate (OPD, o- phenylenediamine dihydrochloride, 1 mg/mL in citrate buffer) was prepared immediately before use. Thirty minutes after the addition of activated substrate, plates were read at 450 nm using a spectrophotometer.
PRNT assay
K18-hACE2 mice
Animals were sacrificed, and serum was collected for PRNT assay. The serum samples were heat inactivated at 56 °C for 30 min prior to a twofold series dilution of 10-, 20-, 40-, 80-, 160-, 320-, 640- and 1280-fold. The diluted serum was then combined with the 1000 PFU/ml WT SARS-CoV-2, Beta, Delta, or Omicron strain at a 1 to 1 ratio and incubated at 37 °C for one hour. The plaque numbers of these serum-virus mixtures were enumerated by a plaque assay. The levels of the neutralizing antibody in the serum samples were expressed as the reciprocal of the fold-dilution that reduced 50% (or 90%) of the original virus titer (PRNT50 or PRNT90).
HFH4-hACE2 mice
Serum samples from vaccinated mice were evaluated for neutralizing antibody levels using an established SARS-CoV-2 neutralization assay. Briefly, Vero E6 (C1008) cells were plated at 20,000 cells per well the day prior to the assay in clear bottom black‐walled 96-well plates (Corning cat# 3904, Millipore Sigma, USA). Serum samples were heat inactivated at 56 °C for 30 minutes, and serial 3-fold dilutions were made in 96-well plates with up to eight dilution spots. Diluted serum samples were then mixed with equal amounts of diluted icSARS-CoV-2-nLuc (D614G) or icSARS-CoV-2-SA-Spike‐nLuc virus. The virus-sera complexes were incubated at 37 °C with 5% CO2 for 1 h. Following incubation, the growth medium was removed, and virus-sera complexes were added to the cells in duplicate and incubated for another 24 h. As controls, virus‐only controls as well as cell-only controls were included in each neutralization assay plate. At 24 h post incubation, cells were lysed, and luciferase activity was measured via the Nano-Glo Luciferase Assay System (Promega, USA) according to the manufacturer’s protocol. SARS-CoV-2 neutralization titers were defined as the sample dilution at which a 50% reduction in relative light unit (RLU) was observed relative to the average of the virus control wells.
T-cell activation
Animals were sacrificed at designed time points. Spleen and lung tissues were collected and mechanically disrupted in RPMI supplemented with 10% FCS and filtered through a 70 µm cell strainer. The cells were labeled using CellTrace™ CFSE (Thermo Fisher) proliferation dye according to the manufacturer’s instructions. A total of 2 × 106 CFSE-labeled cells were cultured in RPMI supplemented with 10% FCS in 48-well plates. The cells were stimulated with SARS-CoV-2 spike protein peptide pool (0.6 nmol of each peptide/mL, PepTivator® SARS-CoV-2 Prot_S, Miltenyi Biotec), SARS-CoV-2 nucleoprotein protein peptide pool (0.6 nmol of each peptide/mL, PepTivator® SARS-CoV-2 Prot_N, Miltenyi Biotec), SARS-CoV-2 membrane protein [(i) for day 7 collection: 10 µg/mL, MRC PPU & CVR Coronavirus Toolkit; (ii) for day 21 collection: 0.6 nmol of each peptide/mL, PepTivator® SARS-CoV-2 Prot_M, Miltenyi Biotec], SARS-CoV-2 envelope protein (10 µg/mL, MRC PPU & CVR Coronavirus Toolkit) or CD3e monoclonal antibody (5 µg/mL, eBioscience) as a positive control. The cells were monitored daily and collected at day 3 post stimulation. The cell cultures were collected for IFN-γ ELISA using a Mouse IFN-γ ELISA Kit from Thermo Fisher. The cells were resuspended and labeled using anti-mouse fluorochrome-conjugated monoclonal antibodies against mouse CD45 (30-F11, eBiosciences), CD3 (17A2, eBiosciences), CD4 (RM.4-5, eBiosciences) and CD8 (53.6-7, eBiosciences). LIVE/DEAD™ Fixable Near-IR (Thermo Fisher) was used to exclude dead cells. Counting beads (Spherobeads, BD) were added to the samples before acquisition. Cell populations were analyzed on a BD LSRFortessa cell analyzer with BD FACSDiva software, version 6.1.3. Data analysis was performed with FlowJo (TreeStar, Inc.) software, version 9.0 (Supplementary Figs. 17, 18).
Multiplex assay
Animals were sacrificed, and lung tissues were collected and homogenized with a Bead Ruptor 24 Elite homogenizer for cytokine and chemokine analysis using the LEGENDplex™ bead-based immunoassay (BioLegend). IFN-γ, CXCL1, TNF-α, CCL-2, CCL-5, IL-1β, CXCL10, MG-CSF, IL-10, IFN-β, IFN-α and IL-6 were quantified according to the manufacturer’s instructions.
Histological analysis
Animals were sacrificed and perfused with PBS. Lung tissues were collected and fixed in 4% paraformaldehyde (PFA), followed by paraffin embedding. Samples were cut into 5 µm thick sections and stained with hematoxylin and eosin (H&E). Histology slide images were taken using an Aperio AT2 digital whole-slide scanner (Leica). Tissue cellular infiltrates were analyzed using ImageScope software (Algorithm Nuclear v9) using the following threshold settings: image zoom of 0.5, a minimum nuclear size (mm2) of 10, a maximum nuclear size (mm2) of 100, a minimum roundness of 0.4, a minimum compactness of 0.4, and a minimum elongation of 0.2.
Cell labeling for single-cell mass cytometry
Animals were sacrificed, and BALF, lungs, tracheobronchial DLNs and spleen were collected. For cytometric labeling, cells were isolated from these tissues and prepared as live single-cell suspensions by lung lavage (BALF), enzymatic dissociation (lungs) and mechanical dissociation (DLNs and spleen). CD45-bar-coded cells labeled for live/dead cell discrimination with cisplatin were incubated with Fc-Block, washed and then resuspended in a cocktail of metal-conjugated antibodies, incubated for 30 mins at 4 °C, followed by fixation, permeabilization, and intracellular labeling (Supplementary Table 16), according to previously published protocols53. Samples were then washed and fixed in 4% PFA for 30 minutes at room temperature according to approved safety protocols. After incubation with an iridium DNA intercalator, samples were washed in UltraPure water and resuspended in Cell Acquisition Solution (CAS) for mass cytometry.
CyTOF Helios analysis
Computational analysis of acquired data was performed using the Specter R package54, with instructions and source code provided at https://github.com/ImmuneDynamics/specter. Samples were initially prepared in FlowJo, and the population of interest was exported as raw value CSV files. Cells from each tissue type (BALF, lungs, and DLNs) were analyzed independently. Arcsinh transformation was performed on the data in R using a co-factor of 15 to redistribute the data on a linear scale and compress low end values near zero. The dataset was then merged into a single data table, with keywords denoting the sample, group, and other factors added to each row (cell). Sample demultiplexing was performed by clustering on the CD45 sample barcode channels using FlowSOM55 and then visualized using principal component analysis (PCA). Clusters were then assigned to their respective samples. FlowSOM clustering was then run on the merged dataset to cluster the data, where every cell was assigned to a specific cluster and metacluster. Subsequently, the data were downsampled and analyzed by the dimensionality reduction algorithm Fast Interpolation-based t-SNE (FItSNE)56 for cellular visualization.
Hamster studies
Equal number of male and female hamsters have been used in the following experiments.
Genetic stability analysis
CDO-7N-1 at passages P0, P5, P10 and P15 were generated from Vero E6 cells. P15 CDO-7N-1 at passages of P15-HP1, P15-HP2, P15-HP3 and P15-HP4 were generated from hamsters by inoculating hamsters intranasally with the virus. Seventy-two hours post-inoculation, BALF was collected from hamsters (HP1) and further back-passaged twice in hamsters via the intranasal route to generate hamster passage 2 and 3 (HP2, HP3) BALF. The CDO region of the vaccine strain was amplified and sequenced from each passage (Supplementary Fig. 2c). Genome sequencing was performed on the virus isolated from BALF to determine any changes in the sequence of the CDO region.
Immunization, vaccine efficacy, and disease evaluation
Six- to eight-week-old Golden Syrian hamsters were inoculated intranasally with either 104 or 105 PFU CDO-7N-1 in a volume of 50 µL. Mock-inoculated hamsters were inoculated with sterile PBS. Hamsters were euthanized at respective time points to assess viral load, histopathological changes, neutralizing titers and binding antibody. Efficacy of the vaccine was determined by simulating natural infection when co-housing with infected animals. In this experiment, hamsters were immunized using 104 PFU CDO-7N-1 while the control group was given PBS. At 14 days post-immunization, both the immunized and the non-immunized hamsters were co-housed with hamsters that had been previously infected with 105 PFU of WT SARS-CoV-2 three days earlier. After a 24-hours, both the immunized and non-immunized hamsters were moved to a separate cage. At selected time points, hamsters were euthanized to assess virus titers in the BAL fluid and to conduct a histopathological examination of the lungs.
Plaque assay
Animals were euthanized, and tissues were collected in PBS and homogenized. Six-well TC-treated multiple well plates (3516; Corning) were seeded with Vero cells (CCL-81; ATCC) at 0.66 x 106 cells/well and grown at 37 ± 1 °C with 5% CO2 until the cells reached 90% confluence. Tenfold dilutions of the virus were prepared in HMEM (Sigma, Cat No. M1018) supplemented with 1% FBS, and 200 µL of each dilution of samples was added into each well. The plates were incubated at 37 °C with 5% CO2 for 60 minutes with intermittent swirling of the plates every 15 minutes. After incubation, the inoculum was removed, and 2 mL of 1% methyl cellulose (Sigma, Cat. No: M0387) dissolved in HMEM supplemented with 1% FBS and 1× Antibiotic-Antimycotic (15240062; Thermo Scientifics, USA) was added to each well. The plates were incubated at 37 °C with 5% CO2 for 6 days. After 6 days, the contents of the plates were carefully aspirated, the cells were fixed with 2 mL of buffered formalin (10%) for 10-15 minutes at room temperature, and the wells were stained with 2 mL of 0.2% crystal violet (Sigma, Cat. No: C0775) for 30 minutes. Viral titers were calculated using the following formula: PFU/mL = (average number of plaques/volume [mL] of virus added) x dilution factor and normalized against tissue weight.
Histopathology
Histopathological analysis was performed by Aurigene Pharmaceutical Services Ltd. according to the company’s protocol. Briefly, the organs/tissues viz. lungs and nasal turbinates were collected from terminally sacrificed hamsters, preserved in neutral buffered formalin (10%) at the Test Facility (IIL) and submitted to the Test Site (APSL) for further microscopic evaluation. Bony tissues such as nasal cavities were decalcified prior to trimming and processing. The tissues were processed by routine paraffin embedding, and 3–5-micron sections were stained with hematoxylin and eosin (H&E). For histologic evaluation, three sections per tissue from all the groups were examined, which were then subjected to statistical analysis using ImageScope. Stained histologic sections of all the tissues collected from all the hamsters were examined using a light microscope (Model-E600, Make- NIKON), and observations were recorded by a veterinary pathologist as per the applicable Test Site’s SOPs. Histopathology grades were assigned as grade 1 (minimal), grade 2 (mild), grade 3 (moderate), and grade 4 (marked/severe) based on an increasing extent of change, unless otherwise specified.
Antibody assay
The S1 protein from residues 13 to 685 (GenBank: NC_045512.2), the RBD from residues 319 to 541 and the S2 subunit from residues 686 to 1213 were produced recombinantly from mammalian cells. For the expression of these recombinant antigens, the fragments were cloned into a mammalian expression vector, pIRES (631605; Clontech Laboratories), with the human serum albumin secretory signal sequence at the 5’ end and 8x his residues at the 3’ end. The expression vectors were transfected into HEK-293TT cells using PEI MAX (24765: Polysciences, Inc.) at a 1:3 ratio, and the recombinant antigens were purified from the transfected culture supernatant four days post-transfection. The recombinant antigens were purified through Ni-NTA (Ni-NTA Superflow, Qiagen: 30410). The nucleoprotein was purchased from Acro Biosystems, USA (NUN-C5227).
To evaluate the antibody response against individual SARS-CoV-2 antigens, flat-bottom 96-well Immuno Plates (Thermo Scientifics USA: 442404) were coated individually with 100 µL of nucleocapsid protein (50 ng/well), 100 µL of purified S1 protein (100 ng/well), 100 µL of purified RBD protein (50 ng/well) and 100 µL of purified S2 protein (200 ng/well) for 1 hour at 37 °C. After 1 hour, the wells were washed with phosphate-buffered saline supplemented with 0.05% Tween 20 and blocked with 100 µL of blocking buffer (PBST supplemented with 2% skimmed milk) in each well. The plates were incubated for 1 hour at 37 °C. After washing the wells, diluted serum samples (PBST supplemented with 1% skimmed milk) were added, and the plate was incubated at 37 °C for 1 hour. After washing four times, 100 µL of goat anti-hamster-HRPO (SAB3700451, Sigma, USA) or goat anti-rabbit-HRPO (A0545, Sigma, USA) was added at 1:10,000 and incubated at 37 °C for 1 hour. After washing the plates three times with wash buffer, 100 μL/well of freshly prepared TMB substrate solution (T3405, Sigma, USA) was added into each well, and the plates were incubated at room temperature (25 ± 1 °C) for 10 minutes. The reaction was stopped by adding 100 µL of 1.25 M H2SO4. The absorbance was read at 450 nm.
PRNT assay
For infected hamsters
Animals were sacrificed, and serum was collected for the PRNT assay. The serum samples were heat inactivated at 56 °C for 30 min prior to a twofold series dilution by 10-, 20-, 40-, 80-, 160-, 320-, 640- and 1280-fold. The diluted serum was then combined with 1000 PFU/ml WT SARS-CoV-2 at a 1 to 1 ratio and incubated at 37 °C for 90 minutes. After incubation, the entire contents of each of the respective dilutions were transferred to individual wells of the Vero cell monolayer. Appropriate virus and cell controls were included in the assay. The plates were incubated at 37 °C with 5% CO2 for 60 minutes with intermittent mixing every 15 minutes. After incubation, the inoculum was removed, and 2 mL of 1% methyl cellulose (Sigma, Cat. No: M0387) dissolved in HMEM supplemented with 1% FBS and 1× Antibiotic-Antimycotic (15240062; Thermo Scientific, USA) was added to each well. The plates were incubated at 37 °C with 5% CO2 for 6 days. After 6 days, the contents of the plates were carefully aspirated, the cells were fixed with 2 mL of buffered formalin (10%) for 10−15 minutes at room temperature, and the wells were stained with 2 mL of 0.2% crystal violet (Sigma, Cat. No: C0775) for 30 minutes. The levels of the neutralizing antibody in the serum samples were expressed as the reciprocal of the fold-dilution that reduced 90% of the original virus titer (PRNT90).
For XBB 1.5 and SARS-CoV-1
The virus neutralization PRNT was performed in duplicate using 24-well tissue culture plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in a biosafety level 3 facility using Vero E6 TMPRSS2 cells57 as previously described58. Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin‒streptomycin. All sera were heat-inactivated at 56 °C for 30 minutes prior to testing. Serial twofold dilutions from 1:10 to 1:320 of each serum sample were incubated with 30–40 plaque-forming units of virus for 1 hour at 37 °C, and the virus–serum mix was added onto preformed cell monolayers and incubated for 1 hour at 37 °C in a 5% CO2 incubator. The virus-antibody inoculum was then removed, and the cell monolayer was overlaid with 1% agarose in cell culture medium. After 3 days of incubation, the plates were fixed with 10% formalin in PBS overnight and stained with 1% crystal violet in ethanol. Antibody titers were defined as the highest serum dilution that resulted in ≥ 50% reduction in the number of virus plaques (PRNT50). The average plaque numbers observed in the duplicate dilution series were used for this computation. Virus back titrations and positive and negative control sera were included in every experiment.
Nonhuman primate studies
Mauritian cynomolgus macaques (Macaca fascicularis) were used in this study. All animals were tested and were not exposed to SARS-CoV-2 prior to this experiment. Five of the macaques had previously been administered 104 PFU of CDO-7N-1 subcutaneously. Interestingly, this route of immunization did not induce any immune responses in these macaques. As a result, we changed the immunization route. These five macaques, along with five other macaques, were then immunized intranasally (i.n.) with 105 PFU of CDO-7N-1. Both male and female animals have been used in this study (Supplementary Fig. 1).
Macaque immunization
Animals received a single intranasal injection of 105 PFU of CDO-7N-1 vaccine on day 0, and a control group received an intranasal PBS injection. The animals were then monitored for 60 days before being euthanized. Trained staff observed the animals every day, documenting weight, temperature, oximetry, heart rate, and respiratory rate at each time point from immunization and challenge. For all procedures (handling procedures, sample collection and inoculation), animals were anesthetized by the intramuscular (i.m.) route with ketamine (Imalgen 1000, 10 mg/kg) combined with medetomidine (Domitor, 0.03 mg/kg) or by the i.m. route with tiletamine chlorohydrate/zolazepam chlorohydrate (Zoletil 100 2.5 mg/kg). An antisedan was administered to reverse the anesthesia (atipamezole, 0.03 mg/kg). The volume of blood sampling did not exceed 7.5% of the total blood volume per week.
Dermal reaction scoring
Dermal scoring was performed at days 0 and 1 post-immunization to assess local skin reaction. A “dermal irritation scoring system” was used for grading erythema, edema, bleeding, scabbing, fissuring, and/or ulceration (0: no swelling, normal color; 1: slight swelling, indistinct border, light pink; 2: defined swelling; distinct border, bright pink/pale red; 3: defined swelling, raised border (<1 mm), bright red; 4: pronounced swelling, raised border (≥1 mm), dark red). If a positive reaction was observed at day 1, the scoring was repeated at day 3 and then every 2 days until the reaction disappeared.
Facial palsy scoring
The facial palsy score was determined at days 36, 43, 50 and 56 post immunization. The facial palsy score (/3 pts) is a yes/no score based on three parameters: a flaccid cheek/lip on one side, no danger response in one eye, and drooling on one side of the mouth.
Procedure for BAL
For performing BAL, the animals were anesthetized by the i.m. route with ketamine (Imalgen 1000, 10 mg/kg) combined with medetomidine (Domitor, 0.03 mg/kg). The animal was placed in left side recumbency, and a laryngoscope was used to visualize the epiglottis and larynx. A spray of 5% lidocaine was applied to the larynx, and after two minutes, the animal was intubated using an endotracheal tube. The intubation was validated by controlling the breath at the end of the tube, and a minimum of 50 mL of 0.9% NaCl was injected into the endotracheal tube with a syringe. A 50 mL conic tube (Falcon®) was placed quickly in front of the endotracheal tube, and the macaque diaphragm was compressed to collect the first part of the lavage. The remaining saline was very quickly aspirated with the 60 mL syringe connected to the endotracheal tube. After completion of the lavage, the animal was extubated and closely monitored until complete recovery.
Sample collection
Whole blood for complete blood count (CBC)
Whole blood was collected in EDTA tubes (K3) for CBC.
Serum collection for antibody response analyzes
The blood collected in dry tubes was centrifuged at 1800 × g for 10 minutes, and the serum was aliquoted (500 μL/aliquot) into a cryotube and stored at −80 °C. The serum sampled after SARS-CoV-2 exposure was heat-inactivated before being stored at −80 °C.
Plasma collection for viral quantification
The blood collected in EDTA tubes was centrifuged at 1800 × g for 10 minutes, and the plasma was aliquoted (75 μL/aliquot) in micronic tubes before being stored at −80 °C for viral load quantification by RT‒qPCR.
Peripheral blood mononuclear cell (PBMC) isolation
Blood was sampled in 8 mL Li-Heparin CPT tubes (#362780, BD Vacutainer®, BD). PBMCs were separated at 1800 × g for 30 minutes at room temperature, and cells were counted using a ViCell counter (Beckman-Coulter) before being used for IFN-γ ELISPOT assays. The remaining cells were cryopreserved by freezing in heat-inactivated FCS/10% DMSO using CoolCell boxes (Biocision) and stored at −80 °C for 24 hours before being stored permanently at −135 °C.
Nasopharyngeal, tracheal and rectal fluids were collected for viral load quantification
Upper respiratory (nasopharyngeal and tracheal) and rectal specimens were collected with swabs (Viral Transport Medium, CDC, DSR-052-01), aliquoted (400 μL/aliquot) and stored at temperatures between 2 °C and 8 °C until analysis. One aliquot was kept at 4 °C in a cryotube for viral quantification (by RT‒PCR and sgRT-qPCR), while the remaining sample was stored in cryotubes at −80 °C.
Collection of BAL supernatant for viral load quantification
Fluids from BAL (using NaCl) were centrifuged at 4 °C at 500× g for 10 minutes, and the supernatant was collected, aliquoted (400 μL/aliquot), and stored at −80 °C for viral load quantification by RT‒qPCR and sgRT-qPCR.
Samples at necropsy
At necropsy, tissues were sampled for the study as described in Annex 2. The lungs were fixed in formol for histology. In case of mortality (unexpected in the protocol), all relevant tissues were sampled at necropsy according to Annex 2. This list of tissues could be adapted according to clinical signs, induced lesions, and cause of death. If any other organ appeared to be affected by the disease, they were also sampled for future analysis.
Full hematology (CBC)
Full hematology, including hemoglobin, red blood cells, platelets, white blood cells, and their subsets, was performed on whole blood in EDTA tubes using a DxH800 ALLIANCE (Beckman Coulter) according to internal procedure (#MO017.02).
IFN-γ ELISpot
The Monkey IFN-γ ELISpot PRO kit (Mabtech Monkey IFN-γ ELISPOT pro, #3421M-2APT) was used following the manufacturer’s instructions. Briefly, 2 × 105 freshly isolated PBMCs were added to each well, and the peptides were added in duplicate to a final concentration of 2 μg/mL. PMA/ionomycin was used as a positive control, and medium alone was used as a negative control. Plates were incubated for 18 hours at 37 °C in an atmosphere containing 5% CO2 and then washed 5 times with PBS. Biotinylated anti-IFN-γ antibody was added, and the plates were incubated for 2 hours at 37 °C before being washed 5 times with PBS. Spots were developed by adding 0.45 μm-filtered ready-to-use BCIP/NBT-plus substrate solution. The spots were counted with an Automated ELISpot Reader ELR08IFL (Autoimmun Diagnostika GmbH, Strassberg, Germany). The antigens used for stimulation were as follows: S pool: peptides derived from a peptide scan through the spike glycoprotein of SARS-CoV-2; N pool: peptides derived from a peptide scan through the nucleoprotein of SARS-CoV-2; P1-specific S pool: peptides derived from a peptide scan through the entire spike glycoprotein of SARS-CoV-2 P (all from Innovative Peptide Solutions).
Viral load quantification
Viral loads were assessed in nasopharyngeal, tracheal, and rectal fluids and in BALF by RT‒qPCR with a plasmid standard concentration range containing an RdRp gene fragment including the RdRp-IP4 RT‒qPCR target sequence. The procedure for the detection of SARS-CoV-2 was followed as described in the protocol available on the WHO website(https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2). SARS-CoV-2 E gene subgenomic mRNA (sgmRNA) was assessed by RT‒qPCR using the following primers and probes: leader-specific primer sgLeadSARSCoV2-F CGATCTCTTGTAGATCTGTTCTC, E-Sarbeco-R primer ATATTGCAGCAGTACGCACACA, and E-Sarbeco probe HEX-ACACTAGCCATCCTTACTGCGCTTCG-BHQ1.
Quantification of binding antibody responses
Neutralizing and binding antibody titers were measured using the Meso Scale Discovery (Rockville USA) MSD COVID-19 Serology Assay kit according to the manufacturer’s instructions. The technique used a MULTI-SPOT® 96-well plate to allow simultaneous detection of IgG binding to nine major SARS-CoV-2 antigens (S, RBD, NTD, and N) from the SARS-CoV-2 virus (MT926410) and from the P1, B1.1.7 and B1.351 variants by chemiluminescence. To measure IgG antibodies, plates were blocked with MSD Blocker A, after which reference standard, controls and samples diluted 1:500 in diluent buffer were added. After incubation, detection antibody was added (MSD SULFO-TAG™ Anti-Human IgG Antibody), MSD GOLD™ Read Buffer B was added, and plates were read using a MESO® SECTOR S 600 Reader. A high chemiluminescence output correlated with a high binding antibody titer. According to the manufacturer’s instructions, to convert MSD assigned concentration arbitrary units (AU/mL) to the WHO International Standard (NIBSC code: 20/136), the following equation is to be used: WHO/NIBSC International Standard Units (BAU/mL) = MSD Units (AU/mL) × conversion factor. As indicated in the manufacturer’s instructions, the conversion factor is 0.00901 for IgG Spike calculation and 0.0272 for IgG RBD calculation.
Quantification of neutralizing antibody responses
Neutralizing and binding antibody titers were measured using the Meso Scale Discovery MSD COVID-19 ACE2 competition assay kit according to the manufacturer’s instructions. In brief, this assay uses chemiluminescence for quantification of antibody-induced ACE-2 binding inhibition. This assay also uses a MULTI-SPOT® 96-well plate reader to measure the activity of antibodies capable of blocking the binding of ACE2 to its cognate receptor, such as the spike protein of SARS-CoV-2. In brief, plates were blocked with MSD Blocker A and washed, and the assay calibrator (COVID-19 neutralizing antibody; monoclonal antibody against S protein; 200 μg/mL), control sera, and test sera samples diluted 1 to 10 in assay diluent were added to the plates. Following incubation of the plates, a 0.25 μg/mL solution of MSD SULFO-TAG™ conjugated ACE-2 was added, after which plates were read using a MESO® SECTOR S 600 Reader. Percentage inhibition was calculated relative to the assay calibrator (maximum 100% inhibition): a low chemiluminescence output correlated with a high neutralizing antibody titer. As stated in the manufacturer’s instructions one arbitrary unit/mL corresponds to neutralizing activity of 1 µg/mL monoclonal antibody to SARS-CoV-2 Spike Protein.
Statistical analysis
All data were tested for normality using the D’Agostino-Pearson normality test prior to analysis. Mouse/hamster weight gain, viral growth curve in Vero E6 or Calu-3 cells, and percentage of pathology change in hamster lung caused by infection with WT SARS-CoV-2, CDO-4N-1 or CDO-7N-1 were analyzed using two-way analysis of variance (ANOVA) with the Bonferroni post hoc test. Viral replication (WT SARS-CoV-2, CDO-4N-1 or CDO-7N-1) in hamster BALF was analyzed using one-way ANOVA with Fisher’s LSD post hoc test. PRNT assay, ELISA, virus titer in hamster lung (WT SARS-CoV-2), virus titer in mouse brain/lung/nasal cavity (WT SARS-CoV-2, CDO-7N-1), viral genome copy numbers in mouse brain/lung (WT SARS-CoV-2, CDO-7N-1), and virus titer in HFH4-hACE2 mice were analyzed using unpaired Mann‒Whitney test. Cytokine production and infiltrated cell counts in mouse lungs were analyzed using one-way ANOVA with Tukey’s post hoc test. The overall variance for the CyTOF data were analyzed using one-way ANOVA with Kruskal-Wallis’s post hoc test while pairwise comparison was carried out using Wilcoxon sign test. All statistical analyzes were performed with GraphPad Prism 6.0 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All datasets are provided in this study. Datasets generated and/or analyzed during the current study are provided with the paper or are appended as supplementary data. Source data are provided with this paper.
References
Chan, J. F. et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523 (2020).
Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).
Jeong, G. U. et al. Ocular tropism of SARS-CoV-2 in animal models with retinal inflammation via neuronal invasion following intranasal inoculation. Nat. Commun. 13, 7675 (2022).
Ng, W. H. et al. Comorbidities in SARS-CoV-2 Patients: a Systematic Review and Meta-Analysis. mBio 12, https://doi.org/10.1128/mBio.03647-20 (2021).
WHO. Weekly epidemiological update on COVID-19 – 1 September 2023, <https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-september-2023 (2023).
Chan, J. F. et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9, 221–236 (2020).
Ng, W. H., Tang, P. C. H., Mahalingam, S. & Liu, X. Repurposing of drugs targeting the cytokine storm induced by SARS-CoV-2. Br. J. Pharm. 180, 133–143 (2023).
Robinson, P. C. et al. COVID-19 therapeutics: challenges and directions for the future. Proc. Natl Acad. Sci. USA 119, e2119893119 (2022).
Puhach, O. et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat. Med 28, 1491–1500 (2022).
Pulliam, J. R. C. et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 376, eabn4947 (2022).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644 (2021).
Zhao, F., Zai, X., Zhang, Z., Xu, J. & Chen, W. Challenges and developments in universal vaccine design against SARS-CoV-2 variants. NPJ Vaccines 7, 167 (2022).
Garcia-Beltran, W. F. et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184, 2372–2383.e2379 (2021).
Ng, W. H., Liu, X. & Mahalingam, S. Development of vaccines for SARS-CoV-2. F1000Res 9, https://doi.org/10.12688/f1000research.25998.1 (2020).
Sheikh-Mohamed, S., Sanders, E. C., Gommerman, J. L. & Tal, M. C. Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS-CoV-2 infection. Immunol. Rev. 309, 75–85 (2022).
Yang, S. L. et al. Comprehensive mapping of SARS-CoV-2 interactions in vivo reveals functional virus-host interactions. Nat. Commun. 12, 5113 (2021).
Nogales, A. et al. Influenza A virus attenuation by codon deoptimization of the NS gene for vaccine development. J. Virol. 88, 10525–10540 (2014).
Gimenez-Roig, J., Nunez-Manchon, E., Alemany, R., Villanueva, E. & Fillat, C. Codon usage and adenovirus fitness: implications for vaccine development. Front Microbiol 12, 633946 (2021).
Lorenzo, M. M., Nogales, A., Chiem, K., Blasco, R. & Martinez-Sobrido, L. Vaccinia virus attenuation by codon deoptimization of the a24r gene for vaccine development. Microbiol Spectr. 10, e0027222 (2022).
Konopka-Anstadt, J. L. et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines 5, 26 (2020).
Rihn, S. J. et al. A plasmid DNA-launched SARS-CoV-2 reverse genetics system and coronavirus toolkit for COVID-19 research. PLoS Biol. 19, e3001091 (2021).
Tarres-Freixas, F. et al. Heterogeneous infectivity and pathogenesis of sars-cov-2 variants beta, delta and omicron in transgenic k18-hace2 and wildtype mice.Front. Microbiol. 13, 840757 (2022).
Liu, X. et al. The delta SARS-CoV-2 variant of concern induces distinct pathogenic patterns of respiratory disease in k18-hace2 transgenic mice compared to the ancestral strain from wuhan. mBio 13, e0068322 (2022).
Stammes, M. A. et al. Medical imaging of pulmonary disease in SARS-CoV-2-exposed non-human primates. Trends Mol. Med. 28, 123–142 (2022).
Xia, S., Wang, L., Zhu, Y., Lu, L. & Jiang, S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct. Target Ther. 7, 241 (2022).
WHO. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. <https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (2023).
Beatty, A. L. et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open 4, e2140364 (2021).
Liu, Y. et al. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions. Nat. Commun. 13, 4337 (2022).
Wang, Y. et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl Acad. Sci. USA 118, e2102775118 (2021).
Sanchez-Felipe, L. et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 590, 320–325 (2021).
Zhu, F. et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med 10, 749–760 (2022).
Tang, P. C. H., Ng, W. H., King, N. J. C. & Mahalingam, S. Can live-attenuated SARS-CoV-2 vaccine contribute to stopping the pandemic? PLoS Pathog. 18, e1010821 (2022).
Ramesh, S. et al. Emerging SARS-CoV-2 variants: a review of its mutations, its implications and vaccine efficacy. Vaccines (Basel) 9, https://doi.org/10.3390/vaccines9101195 (2021).
Tarke, A. et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med 2, 100355 (2021).
Goldblatt, D., Alter, G., Crotty, S. & Plotkin, S. A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 310, 6–26 (2022).
Abu-Raddad, L. J. et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in qatar. N. Engl. J. Med 386, 1804–1816 (2022).
Andrews, N. et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med 386, 1532–1546 (2022).
Park, J. H. & Lee, H. K. Delivery Routes for COVID-19 Vaccines. Vaccines (Basel) 9, https://doi.org/10.3390/vaccines9050524 (2021).
Tang, J. et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 7, eadd4853 (2022).
Meinhardt, J. et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 24, 168–175 (2021).
Chen, S. et al. The role of B cells in COVID-19 infection and vaccination. Front Immunol. 13, 988536 (2022).
Lv, J., Wu, H., Xu, J. & Liu, J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: a systematic review. Infect. Dis. Poverty 11, 53 (2022).
Sapkota, B. et al. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel Med. 29, https://doi.org/10.1093/jtm/taab191 (2022)
Bates, T. A. et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 7, eabn8014 (2022).
Bates, T. A. et al. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA 327, 179–181 (2022).
Guo, L. et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet Microbe 3, e348–e356 (2022).
Liu, X. et al. Infectious clones produce SARS-CoV-2 that causes severe pulmonary disease in infected K18-human ACE2 mice. mBio 12, https://doi.org/10.1128/mBio.00819-21 (2021).
Hou, Y. J. et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 370, 1464–1468 (2020).
Martinez, D. R. et al. A broadly neutralizing antibody protects against SARS-CoV, pre-emergent bat CoVs, and SARS-CoV-2 variants in mice. bioRxiv https://doi.org/10.1101/2021.04.27.441655 (2021).
Munoz-Fontela, C. et al. Animal models for COVID-19. Nature 586, 509–515 (2020).
Arce, V. M. & Costoya, J. A. SARS-CoV-2 infection in K18-ACE2 transgenic mice replicates human pulmonary disease in COVID-19. Cell Mol. Immunol. 18, 513–514 (2021).
Jiang, R. D. et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182, 50–58.e58 (2020).
Ashhurst, T. M., Cox, D. A., Smith, A. L. & King, N. J. C. Analysis of the murine bone marrow hematopoietic system using mass and flow cytometry. Methods Mol. Biol. 1989, 159–192 (2019).
Ashhurst, T. M. et al. Integration, exploration, and analysis of high-dimensional single-cell cytometry data using Spectre. Cytom. A 101, 237–253 (2022).
Van Gassen, S. et al. FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytom. A 87, 636–645 (2015).
Linderman, G. C., Rachh, M., Hoskins, J. G., Steinerberger, S. & Kluger, Y. Fast interpolation-based t-SNE for improved visualization of single-cell RNA-seq data. Nat. Methods 16, 243–245 (2019).
Matsuyama, S. et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl Acad. Sci. USA 117, 7001–7003 (2020).
Lau, E. H. et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. EClinicalMedicine 41, 101174 (2021).
Acknowledgements
This study was supported by Indian Immunologicals Ltd. S.M. is the recipient of an NHMRC Senior Research Fellowship (APP1154347). W.H.N. is a recipient of a Griffith University Postgraduate Scholarship. We would like to thank Drs Julian Druce and Leon Caly from the Victorian Infectious Diseases Reference Laboratory (VIDRL) for providing SARS-CoV-2 variant strains. We also appreciate Dr Aroon Supramaniam and Professor Nigel McMillan (both from Griffith University) for kindly cultivating stocks of the omicron variant and providing the virus for our studies. Griffith University contracted COMMISSARIAT A L’ENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES (CEA), Center for Immunology of Viral, Autoimmune, Hematological, and Bacterial Diseases to perform the non-human primate study (research agreement Ref CEA: CJ 2020-0398). We would specifically like to acknowledge CEA staff Drs. Roger Le Grand, Helene Letscher, Marlin Romain, and Quentin Pascal, along with other CEA staff members, for their contributions to the non-human primate data in this study. We would like to acknowledge the contributions of the animal facility staff members from Griffith University. BioRender was used to generate supplementary Fig. 2c and 3a, supplementary Fig. 14a and e and supplementary Fig. 19-23 in this study.
Author information
Authors and Affiliations
Contributions
S.M. and R.L. conceived this study. S.M., R.L., A.M., U.D., P.P., A.K.K., and M.T.H. supervised this study. S.M., X.L., M.T.H., A.M., R.L., and N.J.C.K. designed experiments. W.H.N., X.L., E.Z., J.F., A.Z., A.T., S.W., L.S.L., V.Y., S.M.A., S.G., S.P., R.L.B., P.M., S.A.T-B., E.A., V.K.B., S.S., Z.L.L., and T.M.A. performed experiments. X.L., E.Z., V.Y., S.M.A., S.G., S.P., R.L.B., P.M., S.V.M., S.A.T-B., E.A., V.K.B., S.S., Z.L.L., and T.M.A. analyzed data. R.B., M.T.H., and N.J.C.K. provided essential reagents. S.M., W.H.N., F.B., and X.L. wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Suresh Mahalingam, Xiang Liu, and Andres Merits are inventors of the live-attenuated SARS-CoV-2 vaccine CDO-7N-1, which has been licensed to Indian Immunologicals Ltd for further development. The study was funded by Indian Immunologicals Ltd. Venugopal Yerragunta, Shukra Madhaha Aavula, Sambaiah Gorriparthi, Santhakumar Ponsekaran, Rama Lakshmi Bonda, Priyanka Mani, Sridevi V. Nimmagadda, Priyabrata Pattnaik, Anand Kumar Kanakasapapathy, and Rajendra Lingala are employees of Indian Immunologicals Ltd. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kai Dallmeier, Shibo Jiang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Ng, W.H., Zusinaite, E. et al. A single-dose intranasal live-attenuated codon deoptimized vaccine provides broad protection against SARS-CoV-2 and its variants. Nat Commun 15, 7225 (2024). https://doi.org/10.1038/s41467-024-51535-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51535-y
This article is cited by
-
Silent mutations in coding regions of Hepatitis C virus affect patterns of HCV RNA structures and attenuate viral replication and pathogenesis
Genome Biology (2025)
-
Synthetic biology-inspired development of live attenuated influenza vaccines
npj Vaccines (2025)
-
Codon-deoptimized single-round infectious virus for therapeutic and vaccine applications
Scientific Reports (2025)
-
Live attenuated SARS-CoV-2 vaccine OTS-228 demonstrates efficacy, safety, and stability in preclinical model
npj Vaccines (2025)
-
Needle-free COVID-19 vaccine shows potential to resist new variants
Nature India (2024)