Abstract
Trifluoromethyl arenes (Ar–CF3) are amongst the commonly encountered fluorinated substructures in pharmaceutical, agrochemical, and material sciences. However, predominant methods to access Ar–CF3 possess several limitations, including harsh conditions, lack of availability of substrates, and poor regioselectivity, which combined restrict access to desirable highly functionalized Ar–CF3-containing compounds. To expand the scope of accessible Ar–CF3-based molecules, we present an orthogonal deoxyfluoroalkylation/aromatization approach that exploits readily accessible and programable cyclohexan(en)one substrates, which undergo a reliable 1,2-addition reaction with the Ruppert-Prakash reagent (TMSCF3) followed by aromatization to deliver highly functionalized Ar–CF3 compounds in a one/two-pot sequence. This general strategy enables access to highly substituted Ar–CF3-containing molecules that are difficult, expensive, and/or impossible to access by current synthetic methods.
Similar content being viewed by others
Introduction
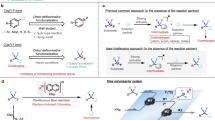
Fluorination of organic molecules perturbs physicochemical properties that are essential for developing therapeutics, biological probes, agricultural chemicals, and materials1,2,3,4,5,6. For these fields, the trifluoromethyl arenes/heteroarenes is the number one fluorine-containing substructure in agrochemicals and second most common fluorine-containing substructure in pharmaceuticals7,8, and as a result, extensive effort has been dedicated over several decades to efficiently deliver this group9,10,11,12,13,14. Common methods to prepare trifluoromethyl arenes (Ar–CF3) typically employ (Fig. 1A): i) Swart’s reaction that requires harsh conditions that destroy many useful functional groups and typically delivers low yield of products15,16, ii) transition metal-promoted trifluoromethylation reactions of aryl electrophiles or nucleophiles that are either limited by the availability of highly functionalized substrates, which limits access to complex products17,18,19,20 or by the use of stoichiometric transition metal21,22, iii) addition of •CF3 into (hetero)arenes controlled by the innate selectivity of the (hetero)arene, which commonly affords mixtures of products and cannot deliver certain regioisomers23,24,25,26, and iv) deoxytrifluoromethylation reactions of carboxylic acid derivatives27,28 that can require harsh gasses and for which many desired substrates might not be readily available. Combined, these limitations in the existing methods restrict access to many desirable highly substituted Ar–CF3 compounds.
A Common methods to access Ar–CF3 substructures are limited by the use of harsh conditions that preclude the presence of many useful functional groups, the application of unselective processes that provide mixtures of products, and/or the availability of readily available substrates. All combined, these limitations restrict access to high-value highly substituted Ar–CF3 substructures. B Deoxytrifluoromethylation to access Ar–CF3 requires multiple steps and produced product in low yield. C A deoxyfluoroalkylation/aromatization strategy enables systematic preparation of countless numbers of highly substituted cyclohexan(en)one-containing substrates prior to conversion to the Ar–CF3 products. The introduction of the CF3 group occurs using a robust and selective 1,2-addition reaction that tolerates many useful functional groups.
As a complementary strategy, deoxytrifluoromethylation/aromatization represents potentially powerful opportunity to access highly substituted Ar–CF3 compounds, addressing the limitations of previously mentioned methods. Such a strategy relies on the conversion of alcohols and ketones – widely found in nature and within commercial catalogs and relatively easy to prepare by many known methods – into valuable functionalized products. Though several groups have explored deoxytrifluoromethylation reactions of sp3-hybridized systems29,30,31,32,33,34,35, extrapolation of these systems to promote reactions of phenol derivatives to deliver Ar–CF3 have not yet been reduced to practice. In some cases, phenols can be converted to the corresponding trifluoromethyl arenes through multistep sequences in low yields (Fig. 1B)36. However, despite the potential for accessing useful Ar–CF3 compounds from simple O-based precursors, complementary deoxytrifluoromethylation reactions to generate aromatic substructures remain unknown.
Herein, we report a deoxytrifluoromethylation/aromatization strategy that exploits readily accessible and programmable cyclohexa(en)none precursors as substrates (Fig. 1C). Using this strategy, a wide array of highly functionalized substrates can be systematically constructed by (a) introduction of desired substituents on cyclohexan(en)one, (b) annulation reactions, or (c) Birch reduction of aromatic ethers. Subsequently, the functionalized cyclohexan(en)one is subjected to a reliable 1,2-addition reaction with the Ruppert-Prakash reagent (TMSCF3) followed by aromatization to deliver highly functionalized Ar–CF3 compounds in a one/two-pot sequence, overall providing an orthogonal strategy relative to known preparations of these valuable products (Fig. 1C). Of note, this proposed strategy would require identification of conditions to promote an unreported oxidation of vinyl trifluoromethane intermediates into trifluoromethyl arenes.
Results
Method development
Initial investigation of the deoxytrifluoromethylation/aromatization sequence exploited 4-phenylcyclohexanone as a model substrate, and each individual step (1,2-addition reaction, dehydration, and aromatization) was independently optimized. The 1,2-addition reaction using TMSCF3 as a reagent and catalytic tetra-n-butylammonium fluoride (TBAF) in tetrahydrofuran (THF) delivered a diastereomeric mixture of trifluoromethyl alcohol and trifluoromethyl silyl enol ether intermediate in quantitative yield (Table 1. Entry 1.1)37. From this mixture, a one-pot dehydration and aromatization could presumably deliver the desired Ar–CF3 substructure. Extensive screening established an initial hit using p-toluenesulfonic acid monohydrate (PTSA•H2O) as a dehydrating reagent and 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) as an oxidant (1 equiv. each) at 110 °C in o-xylene (0.1 M), which afforded the desired Ar–CF3 product in 5% yield (Entry 1.2)38. Optimization of the conditions (3 equiv. DDQ, 1 equiv. PTSA•H2O, 0.2 M toluene, reflux) improved the yield of this step to 70% (Entry 1.3). To develop a direct deoxytrifluoromethylation/aromatization sequence, we identified a single solvent that would facilitate all steps in the sequence. Since the 1,2-addition reaction proceeded in THF and the dehydration/aromatization proceeded in toluene, these two solvents were each explored in the one-pot sequence. However, no desired product was obtained in THF, and in toluene, only 4% product was obtained (Entry 1.4 and 1.5). We speculated that the trace amount of THF present in TBAF might react undesirably with DDQ and thus hinder the oxidation step of the sequence. Thus, other TMSCF3 activators were explored for the 1,2-addition step, and the combination of TMSCF3 with 10% CsF in toluene provided a quantitative of yield of a mixture of trifluoromethyl alcohol and trifluoromethyl silyl ether intermediate (Entry 1.6). With no workup, subjection of this crude reaction mixture directly to PTSA•H2O/DDQ increased the yield of the one-pot sequence to 14% (Entry 1.6). Switching the solvent from toluene to o-dichlorobenzene (o-DCB) increased the yield to 62% (Entry 1.7), and finally increasing the reaction temperature to 140 °C provided a quantitative yield for the one-pot sequence (Entry 1.8, Conditions A).
As the model substrate, 4-phenyl cyclohexanone bears a benzylic position that presumably facilitates the final oxidation reaction with DDQ39, and we speculated that this oxidation might not translate to substrates that lack such stabilizing groups. Hence, a non-activated substrate, 4-t-butylcyclohexanone was used for further development of the reaction. Initially, the 1,2-addition reaction proceeded in quantitative yield using a combination of TMSCF3 and catalytic CsF in o-DCB. However, as expected, treatment of the intermediate mixture with PTSA•H2O/DDQ did not promote dehydration and aromatization to yield an Ar–CF3 derived product (Entry 1.9).
Thus, we developed an alternate set of conditions using 4-t-butylcyclohexanone as a substrate. Specifically, 1,2-addition reaction using TMSCF3 and stoichiometric TBAF afforded trifluoromethyl alcohol intermediate in quantitative yield. Dehydration of the trifluoromethyl alcohol intermediate using thionyl chloride (SOCl2) and pyridine proceeded smoothy to deliver a vinyl–CF3 intermediate in quantitative yield (Table 2)40. However, oxidation of the vinyl–CF3 intermediate to the Ar–CF3 did not proceed using a variety of chemical oxidants {N-chlorosuccinimde, (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, azobisisobutyronitrile (AIBN), benzoquinone-derived oxidants, etc.}, or using some Pd-catalyzed oxidation methods41. Ultimately, allylic functionalization and elimination successfully aromatized the vinyl–CF3 intermediate to the target Ar–CF3 product. As an initial hit, allylic bromination and elimination using 1 equiv. N-bromosuccinimde (NBS) and catalytic AIBN afforded 49% of the desired Ar–CF3 product (Entry 2.1)42, and increasing the loading of NBS to 3 equiv. afforded 96% yield of the desired product (Entry 2.2). Attempts to telescope this sequence to a single-pot operation were met with additional challenges, as the 1,2-addition and dehydration steps proceeded smoothly, though aromatization would not occur (Entry 2.3). After the formation of vinyl–CF3 intermediate, removal of THF and switching to o-DCB also did not afford any desired final product (Entry 2.4). To solve this issue, after the dehydration step, filtration of the reaction mixture through a silica plug and subsequent oxidation using NBS/AIBN afforded 82% overall yield (Entry 2.5, Conditions B). This reaction sequence also converted our original model substrate, 4-phenylcyclohexanone, to the anticipated product in 81% overall yield (Entry 2.6). Additionally, using the silica plug filtration step, DDQ could also be used as the oxidant for 4-phenylcyclohexanone affording 92% yield (Entry 2.7, Conditions C). In summary, we established three complimentary sets of conditions for transforming cyclohexanone precursors to Ar–CF3 products (Entries 1.8, 2.5, 2.7), which would become beneficial when encountering new substrates, functional groups, and/or substitution patterns that might not work for a single set of conditions.
Deoxytrifluoromethylation/aromatization reactions to access a diverse array of products
The three optimized conditions (Fig. 2, Conditions A–C) enabled conversion of a variety of cyclohexan(en)ones to the corresponding Ar–CF3 products bearing many substitution patterns and useful functional groups. Typically, small-scale reactions (0.05–0.1 mmol) were run to assess the suitability of the conditions per substrate, and minor modifications in time, temperature, and reagent equivalents improved the yields for under-performing substrates. The best conditions were then repeated on a higher scale (0.5 mmol). Substrates bearing bulky aryl substituents at the 2-, 3-, and 4- positions of the ketone (1S–3S), as well as alkyl substituents (4S, 5S), were tolerated. A 2,6-disubstited substrate (6S) and one bearing fused saturated rings (7S) both reacted to provide good yields of products. Several important functional groups, cyano (8P), alkynes (9P), nitro (10P), methoxy (11P), and ethoxy (14P) groups, were also tolerated, as well as halogens (12P–13P, and 15P) that provide opportunities for further transformations via transition metal-catalyzed coupling reactions. Additionally, many aromatic and aliphatic heterocycles, were tolerated, including thiophene (16P), indole (17P), and morpholine (18P; for more see Fig. 3). Though methyl and phenyl esters were incompatible with the initial 1,2-addition reaction, the use of a t-butyl ester (19S) allowed the 1,2-addition reaction with CF3TMS to proceed in quantitative yield. Subsequently, subjection of the corresponding t-butyl ester intermediate to thermal/acidic aromatization (Conditions A) promoted aromatization and concurrent release of isobutylene to deliver benzoic acid product (19P1). In contrast, subjecting the intermediate to DDQ alone, without PTSA•H2O, delivered the t-butyl ester product (19P2) in moderate yield. Of note, though many substrates underwent the deoxytrifluoromethylation/aromatization sequence from cyclohexanone-based substrates (1S–5S, 8S, 10S–11S), cyclohexenone-based substrates (6S–7S, 9S, 14S–19S) were also converted to the corresponding Ar–CF3 compounds, which provides a complementary option for certain applications. In a related fashion, the reaction conditions converted tetralone derivatives (12S–13S), which also bear an additional level of unsaturation, to naphthyl trifluoromethane products in good yields (12P–13P). Generally, the aryl-substituted substrates proceeded under all three sets of Conditions A–C, whereas the alkyl-substituted cyclohexanone only proceeded under Conditions B.
All reactions were run in 0.5 mmol batch of substrate. Conditions A: i) TMSCF3 (1.1 equiv.), cat. CsF, o-DCB (0.5 M), rt–50 °C, 4–36 h. ii) PTSA•H2O (0–2 equiv.), DDQ (2–4 equiv.), o-DCB (0.2–0.1 M), 120–140 °C, 14–48 h. Conditions B: i) TMSCF3 (1.1 equiv.), TBAF (1 equiv.), THF (0.5 M), rt, 4–12 h. ii) SOCl2 (3 equiv.), Pyridine (3 equiv.), 10 mol% DMAP, THF (0.5 M), 50 °C, 18–24 h. Then silica filter. iii) NBS (2–4 equiv.), 10 mol% AIBN, o-DCB (0.2 M), 120 °C, 14 h. Conditions C: i) TMSCF3 (1.1 equiv.), TBAF (1 equiv.), THF (0.25 M), rt, 4 h. ii) SOCl2 (3 equiv.), Pyridine (3 equiv.), 10 mol% DMAP, THF (0.25 M), 50 °C, 18 h. Then silica filter. iii) DDQ (2 equiv.), o-DCB (0.2 M), 110 °C, 14 h. a For step ii, PhMe used as solvent at 110 °C. b 19F NMR yield using Conditions B. c For step iii, 1,2-DCE used as solvent at 90 °C. d No PTSA•H2O was used. e Both 19P1 and 19P2 were accessed from same substrate 19S bearing a t-butyl ester.
The transformation enables (A) conversion of readily available ketone substrates to Ar–CF3 derivatives, (B) systematic elaboration of simple precursors to highly functionalized Ar–CF3 products, (C) conversion of natural products into Ar–CF3 derivatives, (D) annulation reactions to access highly substituted substrates that convert to Ar–CF3 products, and (E) conversion of heterocyclic ketones to (Het)Ar–CF3. All reactions were run in 0.5 mmol batch of substrate. Conditions A: i) TMSCF3 (1.1 equiv.), 10 mol% CsF, o-DCB (0.5 M), rt–50 °C, 4–48 h. ii) PTSA•H2O (2 equiv.), DDQ (3 equiv.), o-DCB (0.2 M), 120 °C–140 °C, 12–24 h. Conditions B: (i) TMSCF3 (1.1 equiv.), TBAF (1 equiv.), THF (0.5 M), rt, 4 h. (ii) SOCl2 (3 equiv.), Pyridine (3 equiv.), 10 mol% DMAP, THF (0.5 M), 50 °C, 18 h. Then silica filter. (iii) NBS (4 equiv.), 10 mol% AIBN, o-DCB (0.2 M), 120 °C, 18 h. Conditions C: (i) TMSCF3 (1.1 equiv.), TBAF (1 equiv.), THF (0.25–0.5 M), rt–35 °C, 4–12 h. (ii) SOCl2 (3 equiv.), Pyridine (3 equiv.), 10 mol% DMAP, THF (0.25–0.5 M), rt–60 °C, 8–24 h. Then silica filter. (iii) DDQ (2–3 equiv.), o-DCB (0.2 M), 90–120 °C, 12–48 h. a THF was used instead of o-DCB in step 1 followed by basic alumina plug filter and swapping of solvent with o-DCB. b 1,2–DCE was used as a solvent in step 3.
Most significantly, the deoxyfluoroalkylation/aromatization strategy afforded Ar–CF3 compounds that either cannot be accessed by currently available reactions or that are impractical due to limited availability of starting substrates (Fig. 3). For instance, tetralone substrates 20S–22S are commercially available, and can be readily converted to previously unreported trifluoromethyl naphthalene products (20P–22P) in single-pot operations in good yields (Fig. 3A; crystal structure of 22P in SI, CCDC 2366007). However, the corresponding halides or carboxylate derivatives that would be needed for metal-catalyzed transformations are either (a) not commercially available, (b) available for unreasonably high costs (e.g. $1500/mg), or (c) have not previously been reported (except halide precursors for 20P). Additionally, highly substituted cyclohexenone 23S was readily converted to tetrasubstituted arene 23P in good yield (Fig. 3A); though the corresponding aryl halides and aryl carboxylic acid derivatives of 23P are not available commercially and the routes to access them are not known.
Combined with a plethora of classical organic functionalization reactions of cyclohexan(en)ones, the deoxytrifluoromethylation/aromatization sequence provides countless opportunities for delivering highly functionalized Ar–CF3 products, most of which are not readily accessible by other methods due to (a) issues with the presence of functional groups that are prone to decompose under harsh conditions (e.g. SF4, Cl2/SBF4, HF), (b) limitations in commercial availability of aryl halide precursors and/or synthetic challenges associated with preparation of appropriate aryl halides, and/or (c) issues with regioselective preparation of certain isomers. For example, starting from 5-phenyl-1,3-cyclohexanedione, routine halogenation and cross-coupling reactions can systematically program the placement of substituents (23S–26S, Fig. 3A, B), and application of the deoxytrifluoromethylation/aromatization sequence subsequently delivers the corresponding Ar–CF3 derivatives in good yields (23P–26P). With synthetic creativity, the deoxytrifluoromethylation/aromatization sequence can be combined with routine organic transformations to convert cyclohexan(en)one-containing natural products derivatives, such as carvone into custom and highly substituted trifluoromethyl arenes (27P, Fig. 3C)43. In addition to functionalization reactions of cyclohexan(en)ones, complex substrates can also be accessed from natural product derivatives via Birch reduction of aromatic ethers. For instance, Birch reduction of dimethylated estradiol delivered cyclohexenone substrate (28S), which was then subjected to deoxyfluoroalkylation-aromatization sequence)44 – in this case using the Burgess reagent to promote the elimination – to yield valuable and previously inaccessible Ar–CF3 product (28P, Fig. 3C). This strategy demonstrated that aromatic ethers (even in complex natural products) could be reduced to cyclohexenones via a Birch Reduction and be subsequently subjected to deoxyfluoroalkylation/aromatization sequence to access Ar–CF3 products, providing a complimentary approach to cross-coupling chemistry. Considering the abundance of phenols and aromatic ethers in natural products, commercially available building blocks, and therapeutic candidates, many opportunities exist to access fluoroalkyl derivatives of known O-based compounds.
An additional entry to accessing highly substituted Ar–CF3 products involves the use of regioselective annulation strategies to generate highly substituted cyclohexan(en)one substrates that can then undergo deoxyfluoroalkylation/aromatization to produce highly substituted, currently inaccessible products (Fig. 3D). For instance, highly substituted cyclohexan(en)ones were regioselectively prepared using Pd-catalyzed net [4 + 2] cycloaddition (29S)45, Co-catalyzed [5 + 1] annulation (30S)46, and Robinson annulation (31S–32S) reactions47, and subjection of the respective cyclohexan(en)ones to the deoxyfluoroalkylation/aromatization sequence delivered the corresponding highly substituted Ar–CF3 products in good yields (29P–32P), all of which are inaccessible by known routes. Notably, these highly substituted products (20P–23P, 25P–27P, 29P–32P) cannot be synthesized through previously reported methods, due to the aforementioned issues with harsh conditions, control of regiochemistry, and/or lack of functionalized substrates for the transformations (Fig. 1A). Further, in most cases, the corresponding aryl halide precursors required for cross-coupling reactions are not currently known, and accessing such aryl halides would require development of de novo routes that present notable synthetic challenges.
The deoxyfluoroalkylation/aromatization strategy also translates to N-containing heterocycles that are found in a wide variety of biologically active compounds (Fig. 3E). For example, subjection of the FDA-approved drug, ondansetron (33S), which bears imidazole and carbazole rings, and quinolone substrate (34S) effectively afforded the corresponding trifluoromethyl heteroarene products in good yield (33P–34P). Though these examples involve the formation of benzannulated systems, the deoxyfluoroalkylation/aromatization sequence can also convert piperidinones (35S) to trifluoromethyl pyridines (35P), suggesting that a range of trifluoromethyl heterocycles can be generated from this strategy.
Discussion
A deoxytrifluoromethylation/aromatization strategy offers a convenient and efficient one/two-pot sequence for converting readily available cyclohexan(en)one substrates into previously inaccessible highly substituted trifluoromethyl arenes. In contrast to the most widely used classical and modern preparations of Ar–CF3, this strategy functions under orthogonal conditions and demonstrates high functional group compatibility. Critically, the extensive number of opportunities to convergently and/or systematically generate customized substrates enables a countless supply of substrates for the transformation. Moreover, the ability to functionalize drugs, natural products, and heterocycles provides a range of opportunities that are unique for this deoxytrifluoromethylation/aromatization strategy. Ultimately the ability to access highly substituted Ar–CF3 products should benefit a broad spectrum of synthetic chemists in the pharmaceutical, agrochemical, and materials sectors. Ongoing efforts (a) to identify greener and more mild conditions that will improve yields and expand upon the scope of tolerated functional groups, (b) to extend the reaction conditions to other fluoroalkyl groups and heterocyles, and (c) to optimize conditions for promoting reactions on larger scales, are already ongoing in our laboratory, and progress will be reported in due time.
Methods
Conditions A
Step 1: A 20 mL scintillation vial equipped with a magnetic stir bar was charged with cyclohexan(en)one-derived substrate (0.50 mmol). In the N2 filled glove box, CsF (10–50 mol%) was added followed by the addition of o-DCB (1.0 mL) or PhMe (1.0 mL), and the vial was taken outside the glovebox. The procedure can be performed outside a glove box, too, in which the flask containing cyclohexan(en)one-derived substrate (0.50 mmol) and CsF (10–50 mol%) was subjected to evacuation and backfilled with N2 (3x) followed by an addition of o-DCB (1.0 mL). TMSCF3 (1.1 equiv.) was then added, and the reaction was allowed to run for 4–48 h at rt–50 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with fluorobenzene as an internal standard.
Step 2: At completion, the reaction was cooled to rt (if needed), and then PTSA•H2O (0–2 equiv.) and DDQ (2–4 equiv.) were added sequentially. o-DCB (1.5 mL–4 mL) or PhMe (1.5 mL) was then added to maintain the required concentration. The reaction was then allowed to run for 12–48 h at 120 °C–140 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with HFIP as an internal standard.
Conditions B
Step 1: A 25 mL round bottom flask equipped with a magnetic stir bar was charged with cyclohexan(en)one-derived substrate (0.50 mmol). The flask was sealed with a rubber septum and evacuated and backfilled with N2 (3x). THF (1–2 mL) was then added followed by the addition of TMSCF3 (1.1 equiv.). TBAF (1.0 equiv.) was then added slowly. The reaction was run for 4–12 h at rt under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with fluorobenzene as an internal standard.
Step 2: Without any work up on previous reaction, DMAP (0.1 equiv.) was then added followed by a slow addition of pyridine (3 equiv.) and SOCl2 (3 equiv.) sequentially. The reaction was then allowed to run for 18–24 h at 50 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with fluorobenzene as an internal standard (further addition of fluorobenzene is not required in this step for a one-pot procedure).
Step 3: The above reaction was cooled to rt and Et2O or DCM was added. The reaction mixture was then filtered through a silica plug and washed with Et2O or DCM in a 20 mL scintillation vial. The solvents were removed in vacuo using a rotary evaporator and a magnetic stir bar was then added. AIBN (0.1 equiv.) and NBS (2–4 equiv.) were then added to the vial. The vial was then evacuated and backfilled with N2 (3x). o-DCB (2.5 mL) or 1,2-DCE (2.5 mL) was then added. The reaction was allowed to run for 12–48 h at 90 °C–120 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with HFIP as an internal standard.
Conditions C
Step 1: A 25 mL round bottom flask equipped with a magnetic stir bar was charged with cyclohexan(en)one-derived substrate (0.50 mmol). The flask was sealed with a rubber septum and evacuated and backfilled with N2 (3x). THF (1–2 mL) was then added followed by the addition of TMSCF3 (1.1 equiv.). TBAF (1.0 equiv.) was then added slowly. The reaction was run for 4–12 h at rt–35 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with fluorobenzene as an internal standard.
Step 2: Without any work up of the previous reaction, DMAP (0.1 equiv.) was then added at rt followed by a slow addition of pyridine (3 equiv.) and SOCl2 (3 equiv.) sequentially. The reaction was then allowed to run for 8–18 h at rt–60 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with fluorobenzene as an internal standard (further addition of fluorobenzene is not required for a one-pot procedure).
Step 3: The above reaction was cooled to rt and Et2O or DCM was added. The reaction mixture was filtered through a silica plug and washed with Et2O or DCM on a 20 mL scintillation vial. The solvents were removed by using a rotary evaporator and a magnetic stir bar was then added. DDQ (1.1–3 equiv.) was then added to the vial, and the vial was then evacuated and backfilled with N2 (3x). o-DCB (2.5 mL) or 1,2-DCE (2.5 mL) was then added. The reaction was allowed to run for 12–14 h at 90–120 °C under an atmosphere of N2. The completion of the reaction was monitored by 19F NMR with HFIP as an internal standard.
In all these conditions, the reaction was cooled to rt, and one of the following workup procedures was followed: (i) the solvents were removed in vacuo using a smart evaporator or a rotary evaporator, and the residue was again filtered through a small plug of silica using Et2O or DCM as an eluent to remove the baseline impurities; (ii) the reaction was quenched with aqueous NaOH solution (6 M, 10 mL), followed by extraction with EtOAc (10 mL x3) The combined organic layer was dried with anhydrous Na2SO4, and concentrated in vacuo with a rotary evaporator. The residue was then purified by flash chromatography to afford the desired product.
Data availability
The authors declare that all the data used in this study that supports the findings of this research are available within the article and its supplementary information. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2366007. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The data can also be obtained by contacting the corresponding author.
References
Dolbier, W. R. Fluorine chemistry at the millennium. J. Fluor. Chem. 126, 157–163 (2005).
Maienfisch, P. & Hall, R. G. The Importance of Fluorine in the Life Science Industry. Chimia 58, 93 (2004).
Richardson, P. Fluorination methods for drug discovery and development. Expert Opin. Drug Discov. 11, 983–999 (2016).
Shah, P. & Westwell, A. D. The role of fluorine in medicinal chemistry. J. Enzym. Inhibition Medicinal Chem. 22, 527–540 (2007).
Jeschke, P. The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem 5, 570–589 (2004).
Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496 (2011).
Inoue, M., Sumii, Y. & Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 5, 10633–10640 (2020).
Ogawa, Y., Tokunaga, E., Kobayashi, O., Hirai, K. & Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 23, 101467 (2020).
Studer, A. A “Renaissance” in Radical Trifluoromethylation. Angew. Chem. Int. Ed. 51, 8950–8958 (2012).
Wu, X., Neumann, H. & Beller, M. Recent Developments on the Trifluoromethylation of (Hetero)Arenes. Chem.– Asian J. 7, 1744–1754 (2012).
Liu, T. & Shen, Q. Progress in Copper-Mediated Formation of Trifluoromethylated Arenes. Eur. J. Org. Chem. 2012, 6679–6687 (2012).
Tomashenko, O. A. & Grushin, V. V. Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev. 111, 4475–4521 (2011).
Li, G., Zhang, C., Song, C. & Ma, Y. Progress in Copper-Catalyzed Trifluoromethylation. Beilstein J. Org. Chem. 14, 155–181 (2018).
Mandal, D., Maji, S., Pal, T., Sinha, S. K. & Maiti, D. Recent Advances in Transition Metal- Mediated Trifluoromethylation Reactions. Chem. Commun. 58, 10442–10468 (2022).
Swarts, F. Etude Sur Le Fluo Chloroforme. Acad. R. Belgium 24, 474 (1892).
Banks, R. E. & Tatlow, J. C. Synthesis of C–F Bonds: The Pioneering Years, 1835 – 1940. J. Fluor. Chem. 33, 71–108 (1986).
Oishi, M., Kondo, H. & Amii, H. Aromatic Trifluoromethylation Catalytic in Copper. Chem. Commun. 14, 1909–1911 (2009).
Cho, E. J. et al. The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides. Science 328, 1679–1681 (2010).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A Radical Approach to the Copper Oxidative Addition Problem: Trifluoromethylation of Bromoarenes. Science 360, 1010–1014 (2018).
Dubinina, G. G., Furutachi, H. & Vicic, D. A. Active Trifluoromethylating Agents from Well-Defined Copper(I)−CF3 Complexes. J. Am. Chem. Soc. 130, 8600–8601 (2008).
Hu, W., Pan, S., Xu, X., Vicic, D. A. & Qing, F. Nickel‐Mediated Trifluoromethylation of Phenol Derivatives by Aryl C−O Bond Activation. Angew. Chem. Int. Ed. 59, 16076–16082 (2020).
Morimoto, H., Tsubogo, T., Litvinas, N. D. & Hartwig, J. F. A Broadly Applicable Copper Reagent for Trifluoromethylations and Perfluoroalkylations of Aryl Iodides and Bromides. Angew. Chem. Int. Ed. 123, 3877–3882 (2011).
Koike, T. & Akita, M. Trifluoromethylation by Visible-Light-Driven Photoredox Catalysis. Top. Catal. 57, 967–974 (2014).
Ji, Y. et al. Innate C–H Trifluoromethylation of Heterocycles. Proc. Natl Acad. Sci. 108, 14411–14415 (2011).
Nagib, D. A. & Macmillan, D. W. C. Trifluoromethylation of Arenes and Heteroarenes by Means of Photoredox Catalysis. Nature 480, 224–228 (2011).
Campbell, B. M. et al. Electrophotocatalytic perfluoroalkylation by LMCT excitation of Ag(II) perfluoroalkyl carboxylates. Science 383, 279–284 (2024).
Smith, W. C. et al. Fluorination Reactions Of Sulfur Tetrafluoride. J. Am. Chem. Soc. 81, 3165–3166 (1959).
Malapit, C. A., Ichiishi, N. & Sanford, M. S. Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides. Org. Lett. 19, 4142–4145 (2017).
de Azambuja, F., Lovrien, S. M., Ross, P., Ambler, B. R. & Altman, R. A. Catalytic One-Step Deoxytrifluoromethylation of Alcohols. J. Org. Chem. 84, 2061–2071 (2019).
Zhu, L., Liu, S., Douglas, J. T. & Altman, R. A. Copper-Mediated Deoxygenative Trifluoromethylation of Benzylic Xanthates: Generation of a C–CF3 Bond from an O-Based Electrophile. Chem. A Eur. J. 19, 12800–12805 (2013).
Liu, Z.-Y. & Cook, S. P. Interrupting the Barton–McCombie Reaction: Aqueous Deoxygenative Trifluoromethylation of O-Alkyl Thiocarbonates. Org. Lett. 23, 808–813 (2021).
Intermaggio, N. E., Millet, A., Davis, D. L. & MacMillan, D. W. C. Deoxytrifluoromethylation of Alcohols. J. Am. Chem. Soc. 144, 11961–11968 (2022).
Duan, J.-X. & Chen, Q.-Y. Novel synthesis of 2,2,2-Trifluoroethyl Compounds from Homoallylic alcohols: a Copper (I) Iodide-Initiated Trifluoromethyl–Dehydroxylation Process. J. Chem. Soc. Perkin Trans. 1, 725–730 (1994).
Takechi, N., Ait-Mohand, S., Medebielle, M. & Dolbier, W. R. Novel Nucleophilic Trifluoromethylation of Vicinal Diol Cyclic Sulfates. Org. Lett. 4, 4671–4672 (2002).
Tan, L., Chen, C., Larsen, R. D., Verhoeven, T. R. & Reider, P. J. An Efficient Asymmetric Synthesis of a Potent COX-2 inhibitor L-784,512. Tetrahedron Lett. 39, 3961–3964 (1998).
Sorrentino, J. P., Ambler, B. R. & Altman, R. A. Late-Stage Conversion of a Metabolically Labile Aryl Methyl Ether-Containing Natural Product to Fluoroalkyl Analogues. J. Org. Chem. 85, 5416–5427 (2020).
Ramaiah, P., Krishnamurti, R. & Surya Prakash, G. K. 1-trifluoromethyl-1-cyclohexanol. Org. Syntheses 72, 232 (1995).
Clive, D. L. J. & Pham, M. P. Conversion of Weinreb Amides into Benzene Rings Incorporating the Amide Carbonyl Carbon. J. Org. Chem. 74, 1685–1690 (2009).
Batista, V. S., Crabtree, R. H., Konezny, S. J., Luca, O. R. & Praetorius, J. M. Oxidative Functionalization of Benzylic C–H Bonds by DDQ. N. J. Chem. 36, 1141 (2012).
Carcenac, Y., Tordeux, M., Wakselman, C. & Diter, P. Convenient Synthesis of Fluorinated Alkanes and Cycloalkanes by Hydrogenation of Perfluoroalkylalkenes Under Ultrasound Irradiation. J. Fluor. Chem. 126, 1347–1355 (2005).
Trost, B. M. & Metzner, P. J. Reaction of Olefins with Palladium Trifluoroacetate. J. Am. Chem. Soc. 102, 3572–3577 (1980).
Boyd, D. R. et al. Chemoenzymatic Synthesis of Trans-Dihydrodiol Derivatives of Monosubstituted Benzenes from the Corresponding cis-Dihydrodiol Isomers. Org. Biomolecular Chem. 5, 514 (2007).
Amongero, M., Visnovezky, D. & Kaufman, T. S. Chiral Auxiliary-Mediated Enantioenrichment of (±)-Ibuprofen, under Steglich Conditions, with Secondary Alcohols Derived from (R)-Carvone. J. Braz. Chem. Soc. 21, 1017–1036 (2010).
Burrows, J., Kamo, S. & Koide, K. Scalable Birch Reduction with Lithium and Ethylenediamine in Tetrahydrofuran. Science 374, 741–746 (2021).
Samser, S., Biswal, P., Meher, S. K. & Venkatasubbaiah, K. Palladium Mediated One-pot Synthesis of 3-Aryl-cyclohexenones and 1,5-Diketones from Allyl Alcohols and Aryl Ketones. Org. Biomolecular Chem. 19, 1386–1394 (2021).
Chen, J. et al. Synthesis of 3,4,5‐Triarylcyclohexanones from Dienones and 2‐Methylquinolines Based on a [5+1] Annulation. ChemistrySelect 6, 10802–10805 (2021).
Tang, L., Luo, Y., Xue, J.-W., He, Y.-H. & Guan, Z. Highly Enantioselective Michael-Aldol-Dehydration Reaction for the Synthesis of chiral 3,5-Diaryl-cyclohexenones Catalyzed by Primary Amine. Tetrahedron 73, 1114–1119 (2017).
Acknowledgements
We gratefully acknowledge the support the National Institutes of Health (GM124661) (R.A.A.) and the donors of the Steve and Lee Ann Taglienti Endowment for supporting this project (R.A.A.). The Purdue Interdepartmental NMR Facility is supported by the Institute for Cancer Research (P30 CA023168), and the X-ray diffractometers by the National Science Foundation (CHE-1625543). We thank Dr. Matthias Zeller for assistance with X-ray crystallography experiments.
Author information
Authors and Affiliations
Contributions
R.A.A. conceived the project. P.B. developed methodology for the project. P.B., M.K., and S.K. contributed to the investigation. Funding was secured by R.A.A. Project administration, and supervision was done by R.A.A. P.B. and R.A.A. wrote original draft, and review and editing were done by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bhattarai, P., Abd El-Gaber, M.K., Koley, S. et al. Deoxytrifluoromethylation/aromatization of cyclohexan(en)ones to access highly substituted trifluoromethyl arenes. Nat Commun 15, 7882 (2024). https://doi.org/10.1038/s41467-024-52035-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52035-9