Abstract
Carbon-based metal-free catalysts are promising green catalysts for photocatalysis and electrocatalysis due to their low cost and environmental friendliness. A key challenge in utilizing these catalysts is identifying their active sites, given their poor crystallinity and complex structures. Here we demonstrate the key structure of the double-bonded conjugated carbon group as a metal-free active site, enabling efficient O2 photoreduction to H2O2 through a cascaded water oxidation − O2 reduction process. Using ethylenediaminetetraacetic acid as a precursor, we synthesized various carbon-based photocatalysts and analyzed their structural evolution. Under the polymerization conditions of 260 °C to 400 °C, an N-ethyl-2-piperazinone-like structure was formed on the surface of the catalyst, resulting in high photocatalytic H2O2 photoproduction (2884.7 μmol g−1h−1) under visible light. A series of control experiments and theoretical calculations further confirm that the double-bond conjugated carbonyl structure is the key and universal feature of the active site of metal-free photocatalysts.
Similar content being viewed by others
Introduction
The metal-free photoelectrocatalysts are the most popular, green and renewable catalysts for the present critical energy and environmental challenges1. Many recent works have suggested that the carbon-based metal-free catalysts have significant potential for cost reduction and high efficiency and stability1. Additionally, these catalysts have exhibited various advantages, including enhanced electronic conductivity, adjustable structure, abundant availability, and robust tolerance to acidic/alkaline environments2,3. For these metal-free carbon-based catalysts, the porosity, functional groups, specific surface area, π-conjugated and donor-acceptor (D-A) structure, and incorporated heteroatoms (N, B, O, P, S, Cl, Se, Br, and I) significantly impact their catalytic performance4,5,6. On the other hand, hydrogen peroxide (H2O2) is recognized as a sustainable oxidizing agent and is a crucial chemical compound in industry7,8,9,10. The photocatalytic process utilizing solar energy is considered as an alternative method for H2O2 production due to its cost-effectiveness and environmental friendliness2,11,12. Carbon-based photocatalysts such as covalent organic frameworks (COFs)13, graphitic carbon nitride (G-C3N4)14,15, polymer resins5,16, carbon dots (C-Dots)17,18 and graphene oxide (GO)19,20 have been widely studied for photocatalytic H2O2 production due to their appropriate energy band structure, optical excitation characteristics and low price. Through photocatalysis, H2O2 is mainly generated via O2 reduction by the photogenerated electrons on the catalyst surface21,22,23, which is also obtained by the reaction between H2O and the photogenerated holes24,25,26. Many efforts such as the elucidation of reaction mechanisms11,27, including carrier migration patterns28,29, band structure regulation5, and surface modification during catalytic reactions30,31, have been reported. However, there is still no clear understanding of the real structure of their active sites. As a result, the exact mechanisms are contentious, and the advancement of high-performance metal-free catalysts still relies on trial-and-error methods, rendering the investigation of metal-free catalysts for H2O2 photoproduction highly challenging.
Here, we report the synthesis of various carbon-based materials derived from ethylenediaminetetraacetic acid (EDTA) using a one-step thermal polymerization method involving decarboxylation, cracking, and polymerization processes. Specifically, within the synthesized temperature range of 260 °C to 350 °C, a conjugated carbonyl structure, namely, N-ethyl-2-piperazinone, was locally formed and exhibited highly efficient photocatalytic activity for H2O2 production, with the highest rate of 2884.7 μmol g−1 h−1 at 260 °C. Conversely, at synthesized temperatures below 240 °C or above 400 °C, structural analysis indicated the preservation of the original EDTA structure and the formation of an aromatic ring structure, respectively, both of which lack photocatalytic activity for H2O2 production. Notably, in situ characterization and density functional theory (DFT) calculations revealed that the double-bond conjugated carbonyl groups serve as adsorption sites for water molecules, providing active sites for water oxidation reaction (WOR) and subsequent oxygen reduction reaction (ORR). The universal applicability of this conclusion was confirmed through the use of several small molecules as model photocatalysts. Our findings underscore the significance of conjugated carbonyl groups as active sites for visible light-driven H2O2 production by a cascaded WOR−ORR process, namely, a key and universal feature of the active site of metal-free photocatalysts.
Results
Structural characterization of the catalysts
In this work, a carbon-based catalyst, named EA-x, was prepared via EDTA thermal polymerization, where x is the synthesis temperature. To determine the structural information of the carbon skeleton structure and functional group, a series of structural characterization experiments on these samples (EA-x) were first conducted, including the Fourier transform infrared spectroscopy (FTIR), carbon-13 cross polarization/magic angle spinning nuclear magnetic resonance (13C CP/MAS NMR), thermogravimetric analysis (TGA), and time-of-flight secondary ion mass spectrometry (TOF−SIMS).
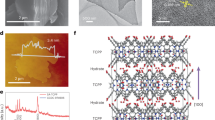
The FTIR spectra of the pristine EDTA and as-prepared catalysts (EA-x, x = 200, 240, 260, 300, 350 and 400) are shown in Fig. 1a and Supplementary Figs. 1 and 2. In Supplementary Fig. 1, the FTIR spectrum of EA-200 (blue line) is consistent with that of pristine EDTA. Specifically, the peak centered at 3000 cm−1 is ascribed to the stretching vibration of C−H, while the peak located at 1700 cm−1 is assigned to the stretching of C=O14. The peaks located at 1413 cm−1 and 1320 cm−1 are ascribed to COO−, and the peaks located at 1213 cm−1 and 1140 cm−1 are attributed to the absorption vibration mode of C−N32. The absorption bands at 1087 cm−1, 1050 cm−1 and 1010 cm−1 are ascribed to C−C stretching vibrations33. For EA-260 (black line in Fig. 1a), an increase in the peak at 3350 cm−1 compared to EA-200 (blue line in Fig. 1a) indicates the formation of −NH, suggesting that the secondary amine was produced by the cleavage reaction3. Simultaneously, the intensity of the C−H peak decreases, accompanied by a redshift to 2941 cm−1. This observation suggested the transformation of methylene group into a double-bond conjugated structure34. Compared with that of EA-200, the C=O characteristic peak of EA-260 shifts from 1700 cm−1 to 1736 cm−1, demonstrating the formation of cycloketone components3. The peak at 1650 cm−1 is attributed to C=N and C=C3. Compared with those of EA-200, the intensities of the −COO−, C−C, and C−N peaks of EA-260 decreased, which indicates the destruction of the carboxyl group and the breakage of the alkane chain. Since the vibrational mode of the acid anhydride was not observed in the FTIR spectrum of EA-260, carboxylic acid polymerization was excluded. When the synthesis temperature reached 400 °C (EA-400, red line in Fig. 1a), almost all the infrared absorption vibrational peaks disappeared, and the peak at 1638 cm−1 was attributed to C=C or C=N on the aromatic ring. The infrared peak is derived in the second order, and the derivative result is integrated to obtain the relative content of the corresponding functional groups. The relative content of the obtained functional groups is shown in Fig. 1b, and it can be intuitively seen that with increasing reaction temperature, the number of unsaturated bonds (C=C, C=N) first increases and then decreases, and the sample synthesized at 260 °C (EA-260) has the maximum content of unsaturated bonds (source data).
a FTIR spectra of EA-200, EA-260, and EA-400. b The change trend of the functional group content with synthesis temperature. The peaks of the FTIR spectra of different samples were fitted to obtain the corresponding second derivative spectra. Then, the derived second derivative spectra were used to determine the exact location of the absorption and shoulder peaks in the original spectrum, and the peak area of each subpeak was calculated using the fitted smoothed spectrum of the original spectrum and compared for analysis. 13C CP/MAS NMR spectra of EA-200 (c), EA-260 (d), and (e) EA-400 Inset: Three chemical structures inferred separately from the analysis. All the illuminations were based on the research. f The thermogravimetric curve of EDTA and possible reactions in the corresponding temperature range; ① to ⑤ show the synthesis temperatures of the five catalyst samples. Source data are provided as a Source Data file.
Figure 1c–e and Supplementary Fig. 3 show the 13C CP/MAS NMR spectra of these carbon-based catalysts. The 13C spectra of EA-200 and EA-240 show five signals like those of pristine EDTA, indicating that both the EA-200 and EA-240 still maintain the EDTA structure (Fig. 1c and Supplementary Fig. 3a). Specifically, the peak at 53.2 ppm corresponds to saturated carbon atoms connected to carbon and nitrogen atoms, while the peaks at 58.46 and 61.46 ppm corresponds to saturated carbon atoms connected to carboxyl groups4. The chemical shifts at δ = 168.81 ppm and 174.58 ppm correspond to the carbon on the carboxyl group4. The signals in the 13C NMR spectra of EA-260 and EA-300 can be deconvoluted into nine carbon components, a to i, which can be classified as edge-saturated carbon linkers (methyl or methylene) produced by breaking C−C or C−N bonds (a–d), unsaturated pyrazine conjugated systems (e, f), and C=O or C=N in carbocyclic structures (g, h), respectively (Fig. 1d and Supplementary Fig. 3b)35,36. All these structural features suggested that EDTA undergoes cleavage, decarboxylation and dehydration reactions, producing secondary amines as intermediate substrates for further condensation reactions during thermal polymerization at 260 °C (Supplementary Fig. 4). Ultimately, a pyrazine ring bearing a double-bond conjugated carbonyl structure was synthesized from compound EA-260. The 13C NMR spectrum of EA-400 shows two signals ascribed to the aromatic π-conjugated structure (Fig. 1e). This suggested that at 400 °C, due to further carbonization, the saturated carbon linker and amide structure were destroyed and polymerized to form an aromatic π-conjugated system.
Thermogravimetric analysis (TGA) of EDTA was performed to elucidate the pyrolysis characteristics of EDTA across a temperature range of 50 to 400 °C. As depicted in Fig. 1f and Supplementary Fig. 5, the pyrolysis process can be divided into three stages. The first stage (stage I), between 50 °C and 243 °C, exhibited negligible weight reduction. Subsequently, the following stage (stage II) occurs from 243 °C to 261 °C, during which EDTA undergoes rapid pyrolysis, resulting in a significant mass reduction of 70%. The weight loss process at this stage and the substantial decrease in the number of −COOH groups and C−N bonds illustrated in the results of IR and NMR may be attributed to decarboxylation and cracking reactions during this phase, and the presence of amide structures indicates the potential for dehydration condensation reactions. Beyond 261 °C (stage III), the pyrolysis action becomes weaker, with the total weight loss reaching 83% at 400 °C.
Furthermore, based on the TGA curve, we investigated the various structures of the carbon-based catalysts at five specific temperatures (① to ⑤ for 200 °C, 240 °C, 260 °C, 350 °C, and 400 °C, respectively, in Fig. 1f), and the molecular weight distributions of these samples were determined by gel permeation chromatography (GPC). As shown in Supplementary Fig. 6, with increasing temperature, the molecular weight increases, and the molecular weight distribution becomes wider. Specifically, the molecular weight distribution of EA-260 ranged from 8.2 × 104 g/mol to 5.1 × 106 g/mol, with a peak molecular weight of 8.5 × 104 g/mol, indicating substantial polymerization of EDTA.
The hydrogen component transformation of EDTA during thermal polymerization was elucidated by nuclear magnetic resonance (1H NMR) analysis (Supplementary Fig. 7). The peak at 2.77 ppm observed in the spectrum of EA-200 (black line) corresponds to the methylene proton of ethylenediamine in EDTA. Furthermore, the signal at 3.48 ppm is attributed to the protons of the ethylenediamine methylene grafted to the β ammonium groups37. However, the absence of methylene protons in EA-260, EA-350 and EA-400 indicates complete dehydrogenation or cleavage of the C−N bond at synthesis temperatures exceeding 260 °C. Simultaneously, as the synthesis temperature increases, the number of branched methylene hydrogen molecules in β ammonium shifts toward the low magnetic field region due to the α-carbon electron drawing effect37.
To characterize the structure of EA-x, TOF−SIMS of EA-x (x = 200, 260, 350 and 400) was performed. The TOF−SIMS of EA-200 showed that the largest monomer fragment (positive ion) was identified as EDTA (positive ion) with m/z = 293 (Supplementary Fig. 8). The TOF−SIMS spectra of EA-260 and EA-350 exhibit similar secondary ion fragment compositions; moreover, based on the above analysis, the fragment structure, i.e., m/z = 127, is derived as N-ethyl-2-piperazinone (Supplementary Fig. 9). The characteristic fragment structure at m/z = 159 in the TOF−SIMS spectrum of EA-400 was simulated and defined as 2-methyl-8-hydroxyl-quinoxaline (Supplementary Fig. 10).
The local electronic structure and chemical structure of EA-x were investigated by X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). As depicted in Supplementary Fig. 11, no discernible peaks corresponding to elements other than C, N and O were detected in survey XPS spectra of EA-x (x = 200, 240, 260, 350 and 400). Concurrently, the inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of the EA-x sample revealed an absence of detectable signals associated with metallic elements (The specific elements measured are detailed in Supplementary Table 2). Supplementary Figs. 12a and 13a show the XPS spectra of C 1 s of EA-x (x = 200, 240, 260, 350 and 400), and the peaks of EA-200 centered at 284.8 eV, 286.1 eV, and 288.6 eV are attributed to graphitic carbon (C−C/C=C), C−N/C−O, and C=O, respectively22. As the temperature increased, the intensity of the C−C/C=C peak increased, while the intensities of the C−N/C−O peak and the C=O peak decreased. This suggests that the pyrolysis process destroys the carboxyl group and C−N, providing unsaturated bonds for polymerization. The peak at 284.8 eV observed in the C K-edge XAS spectrum of EA-200 is attributed to the π*, which is a typical out-of-plane C=C bond related to interlayer bonding, and the peak located at 288.1~288.4 eV is attributed to 1 s → σC=O/C−O* (Supplementary Fig. 14)38. The σC=O/C−O* peaks of EA-260 and EA-400 are negatively shifted, which is due to the participation of endogenous oxygen in autooxidation, the formation of carbon oxides by the original carbon species, and the increase in delocalized π bonds in the aromatic carbon layer38. As demonstrated in the N K-edge region of EA-200, the peak located at 407.2 eV is ascribed to 1 s → σN−C* (Supplementary Fig. 15). In the N K-edge XAS spectrum of EA-260, two new signals at 399.4 and 402.5 eV are attributed to the 1 s → π* transitions of heterocyclic aromatic nitrogen atoms (πC=N−C*) and sp3 N−C bridges between triazine groups (πN−C*), respectively38. These results indicate the formation of nitrogen species in prominent triazine-like structures from EA-200 to EA-260, which is beneficial for charge conduction39. From the XPS N 1 s spectrum in Supplementary Figs. 12b and 13b, the transition from C3−N to C=N−C and C−N−H can be observed, which proves the cleavage of C−N bonds on EDTA and the generation of pyrazine structures from EA-200 to EA-400. A negative shift of the peak attributed to C3−N indicates a decrease in the electron density of nitrogen, which may be attributed to the C3−N group being adjacent to the conjugated carbonyl structure. The O K-edge XAS and O 1 s XPS spectra further reveal the changes in surface oxygen species during continuous heat treatment (Supplementary Figs. 12c, 13c and 16). Compared to those of EA-200, no new features are observed in the O K-edge XAS spectra of EA-260 and EA-400 (Supplementary Fig. 16). The peak at 532.7 eV is attributed to the 1 s → π* transition in the carbonyl structure38. In addition, the peak at 539 eV is attributed to the O 1 s → σC−O* transition, which gradually decreases from EA-200 to EA-400. These conclusions are also supported by the XPS O 1 s peak (Supplementary Figs. 12c and 13c).
Next, electron microscopy was used to characterize the morphology and microstructure of the as-synthesized catalysts (EA-200, EA-260 and EA-400). As revealed in Fig. 2a, the transmission electron microscopy (TEM) image of EA-200 shows its block-shaped amorphous morphology. The spherical aberration corrected transmission electron microscopy (AC-TEM) image of EA-200 illustrates that EA-200 has a similar structure to EDTA and a clear edge with 0.193 nm and 0.328 nm crystal lattices, corresponding to the (604) and (−213) planes of the monoclinic structure of EDTA (Fig. 2b)40. From the TEM image of EA-260, a flower-like morphology can be observed (Fig. 2c). The AC-TEM image of EA-260 shows that its surface contains of triazine-like carbocyclic rings, and the lattice spacing measured from the AC-TEM image is 0.26 nm which can be indexed to the (110) of the in-plane pyrazine ring unit (Fig. 2d and Supplementary Fig. 17). The X-ray diffraction (XRD) pattern of EA-260 shows a broad peak at 2θ = 17.5° corresponding to the in-plane structural stacking (100) pattern (Supplementary Fig. 18a)41, which is consistent with the TEM results. As shown in Fig. 2e, the edges of the EA-400 particles are curled. The regular graphene-like aromatic system stacking with a lattice spacing of 0.21 nm, which is related to the (100) graphite-like plane, on the local surface can be clearly observed in the AC-TEM image of EA-400 (Fig. 2f). The HAADF-STEM image of EA-400 also shows a clear edge at 0.33 nm belonging to the (002) plane of graphite (Supplementary Figs. 19 and 20)3.
a HRTEM image of EA-200 showing the blocky morphology; scale: 100 nm. b AC-TEM image of EA-200; scale: 5 Å. The morphology of EA-200 exhibits an EDTA-like atomic arrangement. c HRTEM image of EA-260; scale: 200 nm. d AC-TEM image of EA-260; scale: 2 nm. Inset: Spherical aberration magnified image. EA-260 has a triazine atomic arrangement; scale: 5 Å. e HRTEM image of EA-400; scale: 500 nm. f AC-TEM image of EA-400; scale: 2 nm. Inset: Spherical aberration magnified local image. EA-400 has a graphene-like atomic arrangement; scale: 5 Å.
Cyclic voltammetry (CV) was chosen to qualitatively determine the band configuration for various catalysts6. As illustrated in Supplementary Fig. 21, the redox potential derived from the CV curve decreases as the synthesis temperature increases27. At high synthesis temperatures (above 350 °C), a large loss of the structure of oxygen-containing electron-deficient groups (e.g., acyl, carboxyl) leads to a decrease in the oxidizing capacity of the material. Moreover, the results shown in Fig. 3a reveal that the energy band positions of EA-200, EA-240 and EA-260 satisfy the thermodynamic conditions of the WOR and ORR. Similar results were also acquired from the XPS valence spectra of these samples (Supplementary Fig. 22).
a The band diagram derived from the cyclic voltammetry curves. b Transient photocurrent response curves and c Nyquist plots for the EIS spectra of EA-200, EA-240, EA-260, EA-350, and EA-400. Exact data is provided in the source data. d Transient photovoltage spectra of EA-200, EA-240, EA-260, EA-350 and EA-400. e Comparison of the intensity-time curves obtained by extracting a fixed frequency f = 60 Hz from the wavelet transform graph of the TPV curves.
Electrochemical tests and transient photovoltage (TPV) analysis were performed to investigate the charge behavior of diverse catalysts. The transient photocurrent response (TPR) curves in Fig. 3b show that EA-260 possesses the best charge separation efficiency among all samples. At the beginning of illumination, the current signal rapidly increases and then gradually decreases to a stable state. This phenomenon indicates that the separation process between electrons and holes is extremely rapid and is accompanied by a redistribution of surface charges on the catalyst. The electrochemical impedance spectroscopy (EIS) curves in Fig. 3c show that EA-260 has the smallest semicircle radius (source data), revealing that it has the lowest charge transfer resistance. The presence of double-bond conjugated carbonyl structures reduce the band width, greatly promoting the generation of charge carriers. Simultaneously, the n-π transition of carbonyl groups promotes carrier separation. TPV was conducted to study the interfacial charge transfer behavior of these five carbon-based catalysts42. The results are presented in Fig. 3d and the Supplementary Table 1, revealing that EA-260 exhibits the highest charge extraction (A), indicating superior surface charge separation efficiency and photogenerated charge. These findings are consistent with those obtained through EIS and TPR analyses. Furthermore, the charge decay constant (τ) of EA-260, which is associated with charge recombination, is much greater than that of EA-350 and EA-400, indicating a longer lifetime for surface photogenerated charges. The results are shown in the Supplementary Table 1 shows the surface effective electron numbers (ne = Aτ/tmax) of the five catalysts, revealing that EA-260 has the highest ne and potential for excellent photocatalytic capacity. The electron transfer dynamics of the catalysts were further studied using fast Fourier transform (FFT) and continuous wavelet transform (CWT) methods27. The FFT and CWT results of the TPV relaxation curve of the EA-x catalysts are shown in Supplementary Figs. 23–26. As shown in Supplementary Fig. 24, the amount of charge at all velocities in EA-260 is greater than that in the other EA-x samples (x = 200, 240, 350 and 400). As shown in Fig. 3e and Supplementary Figs. 25 and 26, the time-intensity spectra of EA-x were compared at seven increasing frequencies. When considering low-speed electrons, the interface charge transfer rate of EA-260 (t1) is much faster than that of the other samples (t2 to t5). When higher-velocity electrons are considered, the surface migration efficiency of all EA-x tends to be consistent. These results indicate that EA-260 can accelerate charge transfer and continuously generate carriers that are delivered to the reaction site owing to the presence of double-bond conjugated carbonyl structures.
Catalytic properties of the catalysts
The photocatalytic performance of EA-x (x = 200, 240, 260, 280, 300, 350 and 400) was evaluated in air using a 420 nm light emitting diode (LED). As depicted in Fig. 4a and Supplementary Fig. 27, none of the samples exhibited the production of H2O2 during the initial 4 h incubation in the dark. During the subsequent 12-hour period of exposure to light, both EA-200 and EA-400 demonstrated a lack of photocatalytic activity in the production of H2O2. The EA catalysts synthesized through calcination at temperatures between 240 °C and 350 °C exhibit the production of H2O2. Among them, EA-260 possesses the greatest amount of photogenerated H2O2, reaching 31.46 ± 2.39 mM after 12 h of light irradiation (source data). A six-day continuous cycle experiment was conducted to evaluate the high-performance retention of EA-260. As depicted in Fig. 4b, the performance of EA-260 reached a H2O2 production of 52.66 ± 2.07 mM after 24 h of illumination on the sixth day, indicating the stability of the catalyst (source data). The apparent quantum efficiency (AQE) versus the UV−Vis absorption diagram revealed that the AQE of EA-260 calculated from the yield of H2O2 was 12.67 ± 2.02 % at 420 nm (source data), while the light absorbance increased with increasing carbonization (Fig. 4c and Supplementary Fig. 28). Supplementary Fig. 29 shows the photocatalytic performance test under different gas atmospheres. The rate of photocatalytic H2O2 production by EA-260 under N2 decreased to 602.2 ± 138.4 μmol g−1 h−1, while the rate of photocatalytic H2O2 production increased to 4001.46 ± 232.6 μmol g−1 h−1 in saturated O2 condition. Thus, O2 is the primary reactant in the H2O2 production process. Using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a radical spin-trapping agent, in situ EPR was performed to investigate the radicals produced through photochemical activation of different reactants. As shown in Fig. 4d (upper panel), the signals attributed to ·OH were observed, indicating that H2O was oxidized to ·OH by photogenerated holes over EA-260. In the methanol solution, ·O2− signals were observed in the in situ EPR spectra, indicating that O2 was reduced by the electrons photogenerated on EA-260 (Fig. 4d, bottom). The WOR transfer electron numbers of EA-200, EA-260 and EA-400 were investigated electrochemically. As shown in Supplementary Fig. 30, the WOR electron transfer numbers of EA-200 and EA-260 are approximately 2.14 and 2.22 (2e− WOR), respectively. However, the WOR electron transfer number of EA-400 is 3.79, suggesting a 4e− WOR route (H2O is converted to O2) on EA-400.
a Photocatalytic H2O2 evolution activity of EA-200, EA-240, EA-260, EA-350, and EA-400; The H2O2 production rates of EA-200 and EA-400 are both zero, and the performance curves of the two samples overlap. b Photocatalytic H2O2 evolution cycles of the EA-260. c AQE versus the UV-vis absorption spectrum of EA-260. Error bars represent the standard deviations of three replicate measurements in a–c. d In situ EPR spectra of the EA-260 photocatalytic system in the dark and after 30 min of visible light irradiation. DMPO-·OH: The test is performed in 200 μL of 1 mg/mL of the EA-260 in H2O, with the addition of 200 μL of DMPO solution at a concentration of 100 mM for ·OH capture. DMPO-·O2−: The test was performed in 200 μL of 1 mg/mL EA-260 in methanol, with the addition of 200 μL of DMPO solution at a concentration of 100 mM for ·O2− capture. Exact data of photocatalytic performance is provided in the source data.
To investigate the intermediate species and active sites involved in the photocatalytic H2O2 production process, in situ diffuse reflectance infrared fourier transform spectroscopy (in situ DRIFT spectroscopy) was performed on EA-260. As displayed in Fig. 5a, b and Supplementary Fig. 31, the DRIFTs of EA-260 acquired through the adsorption of O2 and water vapor for 30 minutes in the dark were subjected to background subtraction. To emphasize the spectral evolution trend, noise reduction and differential processing were adopted for in situ DRIFT. After 10 min of light exposure, the downward signal that increases with time appears at approximately 3500 cm−1, suggesting consumption of the adsorbed H2O molecules, and the observation of C−O−H at 1326 cm−1 revealed the conversion of C=O into C−O−H. Additionally, the signal appeared at 1723 cm−1 attributed to the C=O from pyrazinone gradually increased along with increasing illumination (from 10 min to 70 min). Meanwhile, the increase in the downward signal of C=OH+ at 1670 cm−1 confirms the pivotal sites in the photocatalytic process of accessing carbonyl groups6. The peaks at 1629 cm−1, 1580 cm−1 and 1530 cm−1 are attributed to C=N, C=NH*, and N−H vibrations, respectively34,43, indicating that the N atoms may serve as potential water adsorption sites or a vibration modes caused by changes in the distribution of electrons on the C=N functional group due to changes in the surrounding electronic environment. In addition, the formation of intermediate species (*OOH and *HOOH), which occur in the two-step single-electron ORR was observed at 1223 cm−1 and 1285 cm−1, respectively43. As shown in Fig. 5c, from the in situ DRIFT spectroscopy, the above information regarding the intermediates involved in the ORR and WOR was observed, as well as the reaction sites on the catalyst. Specifically, the C=O (1723 cm−1) and C=N (1629 cm−1) functional groups served as adsorption sites (3500 cm−1) for H2O molecules, leading to WOR. It is also possible to adsorb protons directly on C=O or C=N. The ORR occurred subsequent to the formation of C−O−H or C−N−H (1326 cm−1 and 1530 cm−1) species.
a, b In situ DRIFTS spectra of EA-260 under illumination conditions in a flow of H2O and O2 for 70 min; the baseline for DRIFTS is EA-260 with 30 minutes of oxygen and water vapor in the dark, allowing the surface of EA-260 to adsorb reactants first. Local spectrum of in situ DRIFTS (a ranging from 4000 cm−1 to 2800 cm−1 and b ranging from 1900 cm−1 to 1000 cm−1). The spectra of these time intervals were differentially processed (subtracting the infrared spectra measured at 5 minutes from all the infrared spectra measured at that time to highlight the changes in the infrared peaks). c Key reaction sites and intermediate information of the WOR and ORR observed in in situ DRIFTS spectra.
To investigate the influence of H2O on the photocatalytic production of H2O2, the photocatalytic performance of EA-260 was evaluated under various H2O concentrations in acetonitrile, and saturated O2 was introduced into the testing system. As demonstrated in Fig. 6a, no H2O2 was detected in pure acetonitrile after 6 h of illumination. Upon the addition of 1% H2O to the system, the rate of H2O2 production reaches 120.96 ± 16.50 μmol g−1 h−1 (source data). With the increasing volume fraction of H₂O, the yield of H₂O₂ increases proportionally. Therefore, H2O is a necessary reactant for the formation of H2O2. To investigate whether the WOR affects the ORR in the photocatalytic system of EA-260, the ORR electron transfer number was examined under various conditions. As shown in Fig. 6b, the ORR electron transfer number of EA-260 tested in acetonitrile containing 0.0025 g/L H2O is 0.4 (single-electron ORR process). However, the calculated number of transferred electrons of the ORR is close to 2 (Fig. 6c, two-electron ORR process) in acetonitrile containing 0.1 g/L H2O (the detailed calculation is shown in Supplementary Note 1). Therefore, it can be inferred that the WOR reaction should first be performed on EA-260, and the resulting protons should be used for the ORR reaction (Supplementary Fig. 32).
a Catalytic performance of EA-260 in acetonitrile. The H2O content was controlled to test the change in the photocatalytic performance of EA-260 under the condition of trace H2O. Here, 15.0 mg of catalyst was added to 20 mL of acetonitrile. Different volume fractions of H2O (1%, 2%, 3%, 4%, and 5%) and saturated O2 were introduced before illumination of the system, while the other conditions remained unchanged. The amount of H2O2 was measured after 6 h of light exposure. b Rotating ring-disk electrode (RRDE) i-t curves of EA-260 under dark and light conditions (λ ≥ 420 nm) in an O2-saturated 0.1 M TBAP acetonitrile solution (40 mL) containing 2.5 mg/L H2O and c O2-saturated 0.1 M TBAP acetonitrile solution (40 mL) containing 100 mg/L H2O; d free energy diagrams of H2O2 photocatalytic production though cascaded single WOR−1e− ORR pathway (See Supplementary Data 2 for details) and e the models of the molecular structure of the catalyst during the reaction; f free energy diagrams of H2O2 photocatalytic production though cascaded dual WOR−2e− ORR pathway (See Supplementary Data 3 for details); and g models of the molecular structure of the catalyst during the reaction. Source data are provided as a Source Data file.
To verify the detailed reaction mechanism of H2O2 production on the asymmetric units of EA-260, density functional theory (DFT) calculations were conducted. In the first step, the H+ adsorption energies on the N and O atoms of EA-260 were calculated to determine the possible active sites (H2O adsorption sites). As shown in Supplementary Fig. 33, H+ adsorption on the N atom of EA-260 is calculated at 2.21 eV, higher than that of O sites (1.26 eV with an O−H distance of 0.98 Å) (see Supplementary Fig. 33 and Supplementary Data 1). Thus, the WOR mainly occurs on the O atom of EA-260. Based on the experimental results discussed above, DFT calculations of the two proposed reaction mechanisms were conducted (Fig. 6d–g). As shown in the free energy diagram (Fig. 6d, e), the procedure comprises one electron (1e−) transfer mechanism featuring a single ·OH intermediate and H+, followed by an end-on (Pauling type) O2 adsorption configuration and a two-step 1e− ORR (Supplementary Data 2)44. Specifically, C=O groups have the ability to undergo n-π transitions when exposed to light, leading to the transfer of electrons from non-bonding σ orbitals to the π anti-bonding orbitals, resulting in the formation of a triplet biradical. Simultaneously conjugation stabilizes the existence of free radicals (Supplementary Figs. 34 and 35)45,46,47. Subsequently, the H2O molecule is adsorbed onto the O atom of EA-260 (the ΔG of H2O adsorption is calculated to be 0.20 eV), leading to a single-electron WOR with an energy barrier of 1.06 eV (source data), which is the rate-determining step (RDS). The WOR process in this stage is in accordance with the previously discussed first-order reaction (Fig. 6a). Then O2 is adsorbed on the H+ generated from the WOR in the end-on (Pauling type) O2 adsorption configuration (Fig. 6e and Supplementary Fig. 36)44,48. O2 is first reduced to ·OOH, with a calculated energy barrier of −1.15 eV, and then further reduced to HOOH, with a calculated energy barrier of −1.66 eV. This result is consistent with the 1e− ORR process observed in our experiments (Fig. 6b). When considering a high H2O content, the second mechanism was illustrated in Fig. 6f, g. As shown in Fig. 6g, two molecules of H2O are adsorbed on the O atoms of two adjacent units of EA-260. The adsorption energy of the first H2O molecule is calculated as 0.20 eV, and that of the second H2O molecule is calculated as 0.03 eV (source data). After WOR (ΔG = 1.96 eV), two H+ remain on the oxygen. O2 is adsorbed onto the H+ generated from the WOR on EA-260, and the side-on (Yeager-type) configuration prevents the cleavage of the O=O bond and facilitates the one-step 2e− ORR (Fig. 6g and Supplementary Data 3)44,48. Sufficient supply of H+ (at a high H2O concentration) promotes the one-step 2e− ORR with an energy barrier of −3.68 eV, which verifies the experimental results (Fig. 6c). The presence of a double-bond conjugated carbonyl group on EA-260 serves as both a water adsorption site and facilitates the generation of protons through the oxidation of water molecules adsorbed under illumination. These protons are safeguarded by the conjugated structure of the molecule. Finally, we evaluated O2 adsorption and direct activation during the initial stage of the ORR, and performed DFT calculations of the ORR on the C atom of EA-260 (Supplementary Data 4). As shown in Supplementary Fig. 37, when adsorbed on the C atom of EA-260, the side-on (Yeager-type) O2 adsorption configuration tends to break the O=O bond, hindering the 2e− ORR44,48. This is consistent with experimental results indicating that the catalytic system produces no H2O2 without water (Fig. 6a). To confirm the proposed reaction mechanism, Fukui function calculations were performed to evaluate the activity of each point reaction. As shown in Supplementary Fig. 38 and Supplementary Table 3, in the simulated monomer structure of EA-260, the electronegativity on the O2 and O3 atoms is relatively higher (E+(O2) = 0.053 e eV, E+(O₃) = 0.03965 e eV), and the CCD values also indicate that the O2 and O3 atoms are more likely to undergo nucleophilic reactions and be reduced (CCD(O₂) = 0.0396 e, CCD(O₂) = 0.0286 e). Hence, in the photocatalytic system of EA-260, the predominant mechanism is the two-step 1e− ORR process under low H2O content conditions, whereas under high H2O content conditions, a one-step, 2e− ORR process is favored.
In the next study, we further confirmed that the double-bond conjugated carbonyl structure is the key and universal feature of the active site of metal-free photocatalysts for H2O2 photoproduction. Five small-molecule organics were selected as model catalysts (Fig. 7a–e), and their catalytic activities for the photocatalytic production of H2O2 were examined under the same conditions. As shown in Fig. 7f, the production of H2O2 in cyclohexanone and 2-phenyl-1,4-benzoquinone was detected under light (H2O2 formation rates of 502 ± 67.6 and 136 ± 34.1 μmol g−1 h−1 for cyclohexanone and 2-phenyl-1,4-benzoquinone catalysts, respectively), but no H2O2 was detected in the inositol, 5-phenyl-1,3-cyclohexadidione and 1,4-cyclohexadidione catalytic systems (source data). Hence, it can be deduced that small molecules containing double-bond conjugated carbonyl structures possess the capability to generate H2O2 through photocatalysis.
Five selected small molecules used as model catalysts: a Cyclohexanone octahydrate, b 1,4-cyclohexanedione, c 2-phenyl-1,4-benzoquinone, d 5-phenyl-1,3-cyclohexanedione, and e inositol. All materials were used directly without further processing. f The H2O2 evolution rate over these five model materials (a–e) under visible light (λ ≥ 420 nm). Error bars represent the standard deviations of three replicate measurements. Source data are provided as a Source Data file.
Discussion
In this work, a series of metal-free photocatalysts were prepared through a one-step thermal polymerization of EDTA. Among them, EA-260 exhibited the highest photocatalytic performance for producing H2O2 at a rate of 2884.7 μmol g−1 h−1 under visible light without any sacrificial agent. Structural analysis, in situ characterization and DFT calculations confirmed that the double-bond conjugated carbonyl groups in the catalyst of EA-260 serve as the catalytic active sites for both H2O adsorption and the cascaded WOR−ORR process. The activity of five small-molecule organics as model catalysts for H2O2 production was investigated, confirming that this type of double-bond conjugated carbonyl structure is the primary and universal characteristic of the active site of metal-free photocatalysts. The main aim of this work was to analyze the structure of small-molecule polymer materials through spectra and to identify specific reaction sites and pathways through a structural perspective in actual photocatalytic reaction systems and theoretical calculations. Moreover, the key structural fragment for the photocatalytic production of H2O2, the double-bond conjugated carbonyl group, was verified. This research is crucial for elucidating how carbon-based materials provide reaction sites and regulate the microenvironment of local reactions in certain photocatalytic reactions, which is pivotal for comprehending the influence of nonmetallic material structures on photocatalytic reactions45. In actual catalytic reaction settings, multiple reaction conditions or factors may yield entirely disparate elucidations and material designs45,49. The findings of this work have the potential to offer perspective on understanding particular reactions and material design from a microscopic vantage point.
Methods
Materials
All commercially available chemicals were not further purified for use, unless otherwise specified. Ethylenediaminetetraacetic acid (99.9%) (EDTA) was purchased from Amresco. KMnO4 titrant (0.02 M) was purchased from Fude Biological Technology. 5-phenyl-1,3-cyclohexanedione and Acetonitrile was purchased from Energy Chemical. Cyclohexanone octahydrate was purchased from J&K Scientific. 1,4-cyclohexanedione was procured from Aladdin. 2-phenyl-1,4-benzoquinone was bought from TCI. Inositol was purchased from Shanghai Yuanye.
Synthesis of EA-x
EDTA (1 g) was placed in a 50 mL ceramic crucible covered with a lid and sealed with high-temperature adhesive. Next, the samples were placed it in a muffle furnace and heated to a specific temperature (i.e., x °C, where x is equal to 200, 240, 260, 280, 300, 350, and 400.) at a rate of 1 °C/min and maintained incubated at x °C for 30 min (the process was completed under an air atmosphere). After naturally cooling to room temperature, the resulting powder was ground to obtain powder. The powder was washed with deionized water and ethanol for 3 times. Finally, the obtained solid was placed in an oven at 70 °C for 24 h and denoted as EA-x (where x corresponds to the temperature during the thermal polymerization).
Instruments
Transmission electron microscopy (TEM) and high−resolution TEM (HRTEM) images were observed by FEI Tecnai F20 transmission electron microscope operating at 200 kV. Spherical Aberration Corrected Transmission Electron Microscope (AC-TEM) was conducted by JEM-ARM300F. The structure of samples was characterized by X−ray powder diffraction (XRD) by using an X’PertProMPD (Holland) D/max−γA X−ray diffractometer with Cu Kα radiation (k = 0.154 nm). X−ray photoelectron spectra (XPS) were obtained by using a Thermo Fisher Nexsa X−ray photoelectron spectrometer with a monochromatised Al Ka X−ray source. Aurora M90 ICP−MS was used to test the element content of Co. X−ray absorption near edge structure (XANES) data were collected X-ray absorption spectroscopy (XAS) experiments were performed at Beamlines MCD-A and MCD-B (Soochow Beamline for Energy Materials) at NSRL. Hyperion spectrometer (Bruker, Germany) was employed to determine Fourier transform infrared (FTIR) spectrum over the scan range of 400–4000 cm−1. In situ FTIR spectrum ranging from 800–4000 cm−1 was conducted with Thermo IS 50. The UV-Vis adsorption spectrum at room temperature was acquired using a UV/visible/NIR spectrophotometer (lambda750, Perkinelmer) with a wavelength range of 300–800 nm. The thermogravimetric test and mass spectrum was obtained on RIGAKU, thermo plus EVO2/ thermo mass photo. Electron spin-resonance spectroscopy (ESR) measurements were performed on Bruker EMXplus-6/1 to analyzed H2O2 evolution process. Electrochemical measurements were conducted on the CHI 760 C workstation (CH Instrument, Shanghai, China). Gel Permeation Chromatography (GPC) was carried out on PL-GPC50. TOF-SIMS was performed on TOF-SIMS 5 iontof. Bruker Avance Neo 400WB was used for the 13 C Nuclear Magnetic Resonance Spectra (13 C NMR). Bruker 400 MHz was applied for the H Nuclear Magnetic Resonance Spectra. (1H NMR).
Photocatalytic performance measurements
The experiments of photocatalytic H2O2 performance was evaluated by a multichannel photocatalytic reaction system (CELLAB200E7, 80 mW/cm2). Unless otherwise specified, 100 mg of photocatalyst was evenly dispersed in 110 mL of ultrapure water evenly by ultrasonication without any introduction of a sacrificial agent or cocatalyst. The photocatalytic reaction was illuminated by visible light (420 nm ≤ λ ≤ 700 nm) under continuous stirring. The suspension was centrifuged and filtered with a 0.22 μm disposable syringe filter to remove the dispersed catalyst. The evolution rate of H2O2 was gauged by potassium permanganate (KMnO4) titration27. Specifically, 5 mL of extraction liquor supplemented with the adjunction of 3 mL of H2SO4 (3 mol/L) was titrated with 0.02 mol/L KMnO4. When the final concentration of the KMnO4 standard solution dropped, the color of the solution changed suddenly and the variation remained for half a minute. At this juncture, the total amount of H2O2 was calculated by the consumption of KMnO4 (Subtract the titration of the solution was subtracted before irradiation to exclude the error caused by the reaction of other substances in the solution with KMnO4).
Data availability
Source data are provided as a Source Data file. Source data are provided in this paper.
References
Liu, X. & Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 1, 16064 (2016).
Sheng, B., Xie, Y., Zhao, Q., Sheng, H. & Zhao, J. Proton reservoirs in polymer photocatalysts for superior H2O2 photosynthesis. Energy Environ. Sci. 16, 4612–4619 (2023).
Lu, S. Y. et al. Chemically exfoliating biomass into a graphene-like porous active carbon with rational pore structure, good conductivity, and large surface area for high-performance supercapacitors. Adv. Energy Mater. 8, 1702545 (2017).
Lan, Z. A. et al. Molecular design of covalent triazine frameworks with anisotropic charge migration for photocatalytic hydrogen production. Small 18, 2200129 (2022).
Shiraishi, Y. et al. Resorcinol-formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 18, 985–993 (2019).
Wu, Q. et al. A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater. Nat. Commun. 12, 483 (2021).
Bawn, C. E. H. Chemistry of hydrogen peroxide. Nature 179, 60–61 (1957).
Artero, V. & Fontecave, M. Solar fuels generation and molecular systems: is it homogeneous or heterogeneous catalysis? Chem. Soc. Rev. 42, 2338–2356 (2013).
Gao, T., Lu, C., Hu, C. & Lyu, L. H2O2 inducing dissolved oxygen activation and electron donation of pollutants over Fe-ZnS quantum dots through surface electron-poor/rich microregion construction for water treatment. J. Hazard. Mater. 420, 126579 (2021).
June, S. L. & Sang, H. J. Practical-scale H2O2 production enabled by paired electrosynthesis. Chem 9, 2051–2062 (2023).
Zhang, Y. et al. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 8, 361–371 (2023).
Xue, Y., Wang, Y., Pan, Z. & Sayama, K. Electrochemical and photoelectrochemical water oxidation for hydrogen peroxide production. Angew. Chem. Int. Ed. 60, 10469–10480 (2021).
Huang, S. et al. Linkage engineering in covalent organic frameworks as metal-free oxygen reduction electrocatalysts for hydrogen peroxide production. Appl. Catal. B 340, 123216 (2024).
Liu, B. et al. Boosting O2 reduction and H2O dehydrogenation kinetics: Surface N-hydroxymethylation of g-C3N4 photocatalysts for the efficient production of H2O2. Adv. Funct. Mater. 32, 2111125 (2021).
Wang, X., Blechert, S. & Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2, 1596–1606 (2012).
Tian, Q. et al. Exceptional photocatalytic hydrogen peroxide production from sandwich-structured graphene interlayered phenolic resins nanosheets with mesoporous channels. Adv. Funct. Mater. 33, 2213173 (2023).
Liu, Y. et al. Charge storage of carbon dot enhances photo-production of H2 and H2O2 over Ni2P/carbon dot catalyst under normal pressure. Chem. Eng. J. 409, 128184 (2021).
Guo, Y. et al. Ultrahigh oxygen-doped carbon quantum dots for highly efficient H2O2 production via two-electron electrochemical oxygen reduction. Energy Environ. Sci. 15, 4167–4174 (2022).
Bai, Y. et al. Facile and efficient photocatalyst for degradation of chlortetracycline promoted by H2O2. Inorg. Chem. Front. 9, 2952–2963 (2022).
Palanivel, B. et al. rGO supported g-C3N4/CoFe2O4 heterojunction: visible-light-active photocatalyst for effective utilization of H2O2 to organic pollutant degradation and OH radicals production. J. Environ. Chem. Eng. 9, 104698 (2021).
Chen, L. et al. Simultaneously tuning band structure and oxygen reduction pathway toward high-efficient photocatalytic hydrogen peroxide production using cyano‐rich graphitic carbon nitride. Adv. Funct. Mater. 31, 2105731 (2021).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Zhang, P. et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride. Angew. Chem. Int. Ed. 59, 16209–16217 (2020).
Kondo, Y. et al. Boosting photocatalytic hydrogen peroxide production from oxygen and water using a Hafnium-based metal-organic framework with missing-linker defects and Nickel single atoms. ACS Catal. 12, 14825–14835 (2022).
Zhu, C. et al. Carbon-supported oxygen vacancy-rich Co3O4 for Robust Photocatalytic H2O2 production via coupled water oxidation and oxygen reduction reaction. ACS Appl. Energy Mater. 2, 8737–8746 (2019).
Hu, X. et al. Engineering nonprecious metal oxides electrocatalysts for two-electron water oxidation to H2O2. Adv. Energy Mater. 12, 2201466 (2022).
He, T. et al. A step-by-step design for dual channel metal-free photocatalysts towards high yield H2O2 photo-production from air and water. Chem. Eng. J. 451, 138551 (2023).
Moon, G.-h., Kim, W., Bokare, A. D., Sung, N.-e. & Choi, W. Solar production of H2O2 on reduced graphene oxide-TiO2 hybrid photocatalysts consisting of earth-abundant elements only. Energy Environ. Sci. 7, 4023–4028 (2014).
Moon, B. C., Bayarkhuu, B., Zhang, K. A. I., Lee, D. K. & Byun, J. Solar-driven H2O2 production via cooperative auto- and photocatalytic oxidation in fine-tuned reaction media. Energy Environ. Sci. 15, 5082–5092 (2022).
Kim, K. H. et al. Triphasic metal oxide photocatalyst for reaction site‐specific production of hydrogen peroxide from oxygen reduction and water oxidation. Adv. Energy Mater. 12, 2104052 (2022).
Kato, S., Jung, J., Suenobu, T. & Fukuzumi, S. Production of hydrogen peroxide as a sustainable solar fuel from water and dioxygen. Energy Environ. Sci. 6, 3756 (2013).
Chen, L. et al. Acetylene and diacetylene functionalized covalent triazine frameworks as metal‐free photocatalysts for hydrogen peroxide production: a new two‐electron water oxidation pathway. Adv. Mater. 32, 1904433 (2019).
Zou, W. et al. Metal‐free photocatalytic CO2 reduction to CH4 and H2O2 under non‐sacrificial ambient conditions. Angew. Chem. Int. Ed. 62, e202313392 (2023).
Li, S. et al. Covalent triazine frameworks with unidirectional electron transfer for enhanced photocatalytic oxidation reactions. ACS Catal. 13, 12041–12047 (2023).
Reif, B., Ashbrook, S. E., Emsley, L. & Hong, M. Solid-state NMR spectroscopy. Nat. Rev. Methods Prim. 1, 2 (2021).
Pei, T., Chi-Long, J. & Gerard, S. H. Intercalation complex of proflavine with DNA: structure and dynamics by solid-state NMR. Science 249, 70–72 (1990).
Juan, P., Federico, C. & Bernhard, B. Ex situ NMR in highly homogeneous fields: 1 H spectroscopy. Science 315, 1110–1112 (2007).
Nagasaka, M., Yuzawa, H. & Kosugi, N. Microheterogeneity in aqueous acetonitrile solution probed by soft X-ray absorption spectroscopy. J. Phys. Chem. B 124, 1259–1265 (2020).
Krishnaraj, C. et al. Strongly reducing (diarylamino) benzene-based covalent organic framework for metal-free visible light photocatalytic H2O2 generation. J. Am. Chem. Soc. 142, 20107–20116 (2020).
Janicki, R., Mondry, A. & Starynowicz, P. A new complex of Europium(II) with edta-structure and spectroscopy. Z. Anorg. Allg. Chem. 631, 2475–2477 (2005).
Zhang, G. et al. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem. Int. Ed. 56, 13445–13449 (2017).
Wang, Z. S., Ebadi, F., Carlsen, B., Choy, W. C. H. & Tress, W. Transient photovoltage measurements on perovskite solar cells with varied defect concentrations and inhomogeneous recombination rates. Small Methods 4, 2000290 (2020).
Wang, T. et al. Enhancing oxygen reduction electrocatalysis by tuning interfacial hydrogen bonds. Nat. Catal. 4, 753–762 (2021).
Zhang, X. et al. Developing Ni single-atom sites in carbon nitride for efficient photocatalytic H2O2 production. Nat. Commun. 14, 7115 (2023).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Cohen, S. G. & Green, B. Products and kinetics of photoreduction of acetophenone by amines and alcohols. J. Am. Chem. Soc. 91, 6824–6829 (1969).
Yan, Y. et al. Photoinduced generation of ketyl radicals and application in C–C coupling withoutexternal photocatalyst. Green Chem. 25, 4129–4136 (2023).
Kulkarni, A., Siahrostami, S., Patel, A. & Norskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Rahman, M. Z., Kibria, M. G. & Mullins, C. B. Metal-free photocatalysts for hydrogen evolution. Chem. Soc. Rev. 49, 1887–1931 (2020).
Acknowledgements
This work is supported by the National Key R&D Program of China (2020YFA0406104, 2020YFA0406101), the Natural Science Foundation of Jiangsu Province (BK20220028), Innovative Research Group Project of the National Natural Science Foundation of China (51821002), National Natural Science Foundation of China (52272043, 52271223, 52202107, 52201269), The Science and Technology Development Fund, Macau SAR (0009/2022/ITP), Collaborative Innovation Center of Suzhou Nano Science & Technology, and the 111 Project. We also acknowledge the support from Suzhou Key Laboratory of Functional Nano & Soft Materials and Beamlines MCD-A and MCD-B (Soochow Beamline for Energy Materials) at NSRL.
Author information
Authors and Affiliations
Contributions
T.H. collected the SEM, TEM, HRTEM, and STEM images. H.T. conducted the DFT simulation. T.H., Ji.W., J.Wa., and M.Z. conceived the idea and designed the experiments. T.H. and C.L. performed the EXAFS test and analyzed the data. T.H. and H. H. discussed the results and commented on the paper. T.H. and J.Z. created figures. T.C., Y.L., and Z.K. supervised the whole project and were involved in manuscript preparation and revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, T., Tang, H., Wu, J. et al. A metal-free cascaded process for efficient H2O2 photoproduction using conjugated carbonyl sites. Nat Commun 15, 7833 (2024). https://doi.org/10.1038/s41467-024-52162-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52162-3