Abstract
Cerebral white matter lesions prevent cortico-spinal descending inputs from effectively activating spinal motoneurons, leading to loss of motor control. However, in most cases, the damage to cortico-spinal axons is incomplete offering a potential target for therapies aimed at improving volitional muscle activation. Here we hypothesize that, by engaging direct excitatory connections to cortico-spinal motoneurons, stimulation of the motor thalamus could facilitate activation of surviving cortico-spinal fibers thereby immediately potentiating motor output. To test this hypothesis, we identify optimal thalamic targets and stimulation parameters that enhance upper-limb motor-evoked potentials and grip forces in anesthetized monkeys. This potentiation persists after white matter lesions. We replicate these results in humans during intra-operative testing. We then design a stimulation protocol that immediately improves strength and force control in a patient with a chronic white matter lesion. Our results show that electrical stimulation targeting surviving neural pathways can improve motor control after white matter lesions.
Similar content being viewed by others
Introduction
Lesions of the cortico-spinal tract (CST), as a consequence of stroke, traumatic brain injury (TBI), brain tumors, or neurodegenerative disorders, disrupt communication between the cortex and lower motor centers leading to deficits and potential loss of function in face, upper or lower limb muscles1. The consequent upper-limb motor deficits significantly affect the quality of life of patients. It is estimated that approximately 10 million people in the United States alone live with such impairments2,3.
In most cases though, damage to the CST is incomplete. Yet the spared excitatory descending connections are insufficient to fully activate the spinal motoneurons, leading to functional motor deficits1. Facilitation of the activation of the residual cortico-spinal axons could reestablish the missing excitation, restoring voluntary muscle control. We conjectured that this facilitation could be achieved by increasing the excitability of cortico-spinal neurons in the motor cortex, thereby increasing CST output, and consequently enhancing movements of the paretic limb4,5.
Stimulation of subcortical regions could be a viable approach to modulate the excitability of cortico-spinal neurons. Indeed, while certain mechanisms of action of deep brain stimulation (DBS) are still unclear, it is known that stimulation-induced action potentials within the subcortical targets propagate through axon terminals to recipient cortical areas modulating their neural activity6. Therefore, DBS can be used to increase excitability of restricted cortical areas by targeting subcortical regions that preferentially project excitatory axons to those areas.
For example, previous studies have demonstrated that stimulation of thalamic and subthalamic nuclei affects motor cortex excitability. Specifically, motor-evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) were modulated when conditioned by stimulation of subcortical brain areas with direct (ventral thalamus), and indirect (globus pallidus pars interna, GPi, subthalamic nucleus, STN, and lateral cerebral nucleus) projections to the motor cortex7,8,9,10,11,12.
Based on these results and the known anatomical evidence of direct connections to the motor cortex from the thalamic motor nuclei8,13,14,15,16, we hypothesized that stimulation of the motor thalamus could be tailored to immediately facilitate motor cortex and CST neural activity and consequently improve motor deficits after lesions of the CST. In this regard, previous studies provide anecdotally support for our hypothesis by observing that thalamic DBS may lead to some improvements in motor impairments in patients with stroke that were clinically implanted for other conditions, such as pain and dystonia17,18,19. However, these promising observations were limited to the reporting of serendipitous findings and not backed up by investigations of the potential mechanisms of this facilitation20, which leaves open questions as to the underlying mechanisms of action of motor thalamus DBS. Dissection of the mechanisms of action is critical to identifying optimal targets within the motor thalamus and stimulation parameters that would maximize the efficacy of thalamic stimulation for facilitating cortical motor output and improving upper-limb motor deficits.
Here, we built on these previous results and overcame scientific and technological challenges with a translational experimental framework that leveraged experiments in monkeys, i.e., the most relevant animal model for CST anatomy and function1,21,22, to precisely dissect the mechanisms of action and identify optimal stimulation targets, and subsequently tested these findings in human patients. Specifically, we 1) identified the nucleus of the motor thalamus with dense, direct, and preferential excitatory connections to cortico-spinal neurons in primary motor cortex; 2) designed a series of electrophysiology experiments in monkeys and human patients to test whether stimulation of this nucleus could augment excitability of the motor cortex with high specificity to cause increased motor output; and 3) optimized stimulation parameters to maximize these effects. We then built on these findings to demonstrate that targeted stimulation of the motor thalamus increased muscle activation, strength, and force control after lesions of the CST in intraoperative monkeys and human experiments. Finally, we verified that these effects lead to improved functional performance during behavioral tasks in a subject with a chronic lesion of the CST and a DBS system implanted in the motor thalamus. Importantly, improvements were observed immediately when the stimulation was turned ON and vanished when the stimulation was switched OFF.
Overall, we demonstrated the mechanisms of action of motor thalamus stimulation and we optimized it for upper-limb paresis, paving the way towards a potential therapy for motor deficits after CST lesions.
Results
Identification of optimal target of stimulation within the motor thalamus
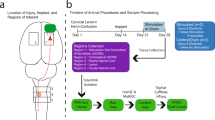
Here we aimed at increasing the excitability of cortico-spinal neurons within the primary motor cortex to potentiate motor output (Fig. 1a). We therefore sought to identify the optimal stimulation target by localizing a subcortical region that has a high number of direct excitatory projections to the motor cortex. Based on previous anatomical evidence, we posited that the motor thalamus could be this optimal target8,13,15,23. In humans, the motor thalamus includes four nuclei that have different preferential projection targets: the ventral caudal (VC), the ventral intermediate (VIM), the ventral oral posterior (VOP), and the ventral oral anterior (VOA) nucleus24,25. Thalamocortical fibers originating in the VC nucleus project preferentially to the somatosensory cortex, whereas those originating in the VIM/VOP project to the motor cortex, and those in the VOA to the pre-motor cortex and supplementary motor area (SMA). We first acquired and analyzed high-resolution diffusion magnetic resonance imaging (MRI) data using high-definition fiber tracking (HDFT) in monkeys (n = 3) to confirm a similar anatomical organization, as previously shown with histological analysis14,15,16, and to identify the optimal target of stimulation. We focused our analysis on the three nuclei of the monkey motor thalamus: the ventral anterolateral (VAL), the ventral laterolateral (VLL), and the ventral posterolateral (VPL) nuclei14,15,16,26,27,28. We reconstructed all the likely axonal pathways between these nuclei and the somatosensory, motor, and premotor cortical areas and quantified the relative strength of these connections by calculating the volume of thalamocortical projections from each nucleus to each cortical region normalized by the total volume of fibers (Fig. 1b). We confirmed a clear functional and somatotopic organization of the primate thalamic nuclei that parallel the organization of the human thalamocortical connections, with axonal projections preferentially projecting towards the somatosensory cortex from the posterior motor thalamus (VPL) and preferentially towards motor and pre-motor cortices from the more anterior nuclei (i.e., VLL and VAL, respectively). Overall these results helped to define the optimal stimulation target. Indeed, using selectivity detection analysis among the three nuclei, we found that the VLL nucleus, which corresponds to the human VIM/VOP nucleus29, had the greatest selectivity of the projections towards M1 (VPL: 0.67, VLL: 0.89, VAL: 0.33). We, therefore, considered the VLL nucleus the optimal target to potentiate motor output.
a Schema of our hypothesis: motor thalamus stimulation potentiates motor output by increasing excitability of the motor cortex. The potentiation persists also after a lesion of the CST highlighted in red. b Top: High definition fiber tracking (HDFT) from VPL, VLL, and VAL nuclei (VPL: ventral posterolateral, VLL: ventral laterolateral, VAL: ventral anterolateral) of monkey motor thalamus to cortical regions (n = 3) (S1: primary somatosensory cortex, M1: primary motor cortex, PMd: dorsal pre-motor cortex, SMA: supplementary motor area). Bottom: Volume of thalamocortical projections (mean ± standard error (SE) over 3 animals) from each nucleus to each cortical region normalized by the total volume of fibers projecting from each nucleus. c Acute experimental setup. First, a cuff electrode was implanted around the motor branch of the radial nerve for stimulation. Animals were then implanted with a DBS electrode in the internal capsule (IC) and one in the VLL using the ROSA robot and intracortical arrays over S1 and M1. An intraspinal probe was implanted at the C6 spinal segment to record spinal local field potentials and EMG needle electrodes were inserted in arm, hand, finger and face muscles. A force transducer was placed in the animal’s hand to measure grip force. Finally, a camera recorded the kinematic of the arm and hand. d Left: Example of VLL electrode implant location localized from post-mortem MRI (Cd: Caudate Nucleus, IC: Internal Capsule, Pt: Putamen). Right: Normalized volume HDFT projections from the area of stimulation to cortical regions (mean ± SE over animals, n = 4). Source data for (b and d) are provided as a Source Data file. a, c Were designed by Isabella Bushko.
Targeted deep brain stimulation of the VLL preferentially excites the motor cortex
To confirm the specificity of the thalamocortical projections from the VLL to the motor cortex, we recorded cortical evoked potentials in the motor and sensory cortex while stimulating the VLL. For this, we implanted a human-grade electrode (Microdeep® SEEG Electrodes, DIXI Medical, Marchaux-Chaudefontaine, France) within the VLL nucleus in anesthetized monkeys (n = 5) using the ROSA ONE® Robot Assistance Platform (ROSA robot, Zimmer Biomet, Warsaw, Indiana, USA; Fig. 1c and Supplementary Fig. 1). High-resolution post-mortem structural MRI confirmed the accurate location of the electrode within the VLL (average distance between the implantation location and the VLL nucleus over animals:1.7 mm ± 0.4 mm (SE), in line with human studies30). Additionally, we used HDFT between the electrode implantation region and the somatosensory, motor, and pre-motor cortical areas and confirmed that the largest volume of fibers within the stimulation area projected to M1 (on average 55% of the fibers) (Fig. 1d). In addition to the electrode within the VLL nucleus, we implanted two 48-channels intracortical arrays (Utah arrays) in the primary motor (M1) and somatosensory (S1) cortices, respectively (Fig. 1c). In n = 4 anesthetized monkeys, we applied continuous stimulation of the VLL at 10 Hz (see Supplementary Table 2 for details on stimulation parameters) while recording local field potentials (Fig. 2a). We then analyzed VLL stimulation triggered averages of evoked potentials (EPs) in M1 and S1 electrodes and observed clear cortical EPs with peak latencies at 5–10 ms post-stimulation (see example Fig. 2b) confirming a direct monosynaptic pathway between the VLL and cortex31,32. Analysis of EPs peak-to-peak amplitude across the entire arrays showed significantly larger responses in M1 as compared to S1 in all monkeys (see histograms Fig. 2b), confirming preferential projections of VLL axons towards M1. As expected, these responses were larger along the pre-central gyrus where the hand and arm representation in M1 are located (see heat maps Fig. 2b).
a Picture (left) and schematic (right) of the implant location of M1 and S1 intracortical arrays. The brain in (a) was created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. b Top: Example heatmap of average peak-to-peak amplitudes of cortical evoked potentials from VLL stimulation at 10 Hz across all channels over S1 and M1 for MK-HS. Center: Example stimulation triggered averages of cortical evoked potentials over S1 and M1 (n = 40 traces). Bottom: Histogram of peak-to-peak amplitudes across all channels for S1 (light blue) and M1 (dark blue) (n = 48 channels per array). c Top: Example baseline corrected spike count heatmaps in S1 and M1 for MK-HS. Bottom: Average spike counts over time across all channels in S1 and M1 array (n = 48 channels per array). d Top: Example traces of antidromic potentials in M1 from IC stimulation without (yellow) and with (blue) conditioning from a burst of VLL stimulation for MK-HS (n = 40 traces). Boxplot of peak-to-peak amplitude of the antidromic potentials when IC stimulation is conditioned by VLL stimulation at various delays (2, 5, 10, and 50 ms). In the boxplot, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. For all (b–d), statistical significance was assessed with two-tail bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001(***). Source data for (b–d) are provided as a Source Data file.
Previous studies revealed that thalamocortical projections like those identified with our HDFT analysis are mostly glutamatergic13,33. We therefore hypothesized that the cortical EPs elicited by VLL stimulation were indicative of an increase in motor cortex excitation. To demonstrate this hypothesis, we measured multi-unit neuronal activity from motor and sensory cortex. Stimulation-evoked spike counts showed an increase in multi- and single-unit firing rates post-stimulation (Fig. 2c), which was significantly higher in M1 as compared to S1 in both monkeys. These results corroborate two important concepts, first the spatial specificity of the EPs towards motor cortex and second that VLL stimulation leads to an increased excitation of the motor cortex.
We then performed a second experiment to demonstrate that the cortical EPs elicited from VLL stimulation were indicative of increased excitation of cortico-spinal neurons located in M1. Indeed, if VLL stimulation is increasing excitation of cortico-spinal neurons, then the amplitude of antidromic neural responses elicited in cortical-spinal axons from stimulation of the CST and recorded in M1 should be larger when VLL stimulation is active (because of decreased thresholds in these cortical neurons). To test this hypothesis, a stimulating electrode was implanted into the posterior limb of the internal capsule (IC, Fig. 1c), which contains the cortical-spinal axons originating in the motor cortex and projecting to the spinal cord. We targeted the hand representation of the CST. Direct stimulation of these axons via single pulse stimulation to the IC elicited antidromic action potentials toward the cell body of the pyramidal neurons in M1 at short latencies (5 ms post-stimulation) (Fig. 2d). We then conditioned the single pulse stimulation to the IC by a 100 ms burst at 100 Hz to the VLL nucleus at different delays (2–50 ms). We found that the peak-to-peak amplitude of the antidromic neural responses in M1 was significantly higher when a burst of VLL stimulation preceded IC stimulation (n = 3) (Fig. 2d) confirming that VLL stimulation increases excitability of cortico-spinal neurons in M1. At delays longer than 10 ms, the antidromic potentials returned to amplitudes similar to those of IC stimulation alone.
Overall, these results demonstrate that stimulation of the VLL nucleus increases the excitability of cortico-spinal neurons specifically within M1.
Motor thalamus stimulation potentiates upper-limb motor outputs
We built on previous experiments that reported modulated MEPs from TMS of the motor cortex when conditioned with stimulation of VIM, GPi, or STN7,8,9,10, and we hypothesized that the enhanced cortical excitability observed in our monkeys would lead to larger MEP amplitudes recorded in arm, hand, and finger muscles. To test this, we paired stimulation of the IC at 2 Hz with continuous stimulation of VLL at 50 or 80 Hz (Fig. 3a), i.e., frequencies often used in other neuromodulation applications such as spinal cord stimulation34. Stimulation of the IC at 2 Hz generated short latency (on average 10–15 ms) MEP responses in hand, arm, and finger muscles (Fig. 3b) as well as movements of the limb (Supplementary Fig. 3) suggesting a monosynaptic activation of spinal motoneurons. When 2 Hz IC stimulation was paired with continuous stimulation of the VLL at 50 and 80 Hz, the area under the curve (AUC) of MEPs and the peak-to-peak amplitude of movements of the arm (n = 2), hand (n = 3), and fingers (n = 3) were immediately and significantly increased (p < 0.001, Fig. 3b, Supplementary Fig. 2 and 3, Supplementary Video 1). Interestingly, a dose-response relationship was visible as MEP amplitudes increased proportionally when increasing the VLL stimulation amplitude (Fig. 3a). Additionally, MEPs of face muscles were also significantly larger with stimulation of the VLL at 50 and 80 Hz (Supplementary Fig. 2a).
a Average of binned Flexor Digitorum Minimi (FDM) MEPs generated by IC stimulation at 2 Hz paired with continuous VLL stimulation at 50 Hz with gradual ramp-up of amplitude (0–3 mA; bins: 0–0.6 mA, 0.7–1.2 mA, 1.3–1.8 mA, 1.9–2.4 mA, and 2.5–3 mA, each bin included n = 9 responses). b Left panels: MEPs of one arm muscle (n = 40, Biceps, MK-OP), one hand muscle (n = 40, Extensor Digitorum Communis, MK-HS), and one finger muscle (n = 40, Abductor Pollicus Brevis, MK-HS) with IC stim alone and then paired with VLL stimulation at 50 and 80 Hz. Right panels: percentage of increase of AUC of arm, hand, and finger MEPs between IC stimulation alone and paired with VLL stimulation at 50 and 80 Hz. For each monkey, the percentage of increase was calculated over the medians and averaged over all the muscles. See Supplementary Fig. 2 for boxplots for single muscles. (***), (**), or (*) was placed if muscles in each group showed a significant increase (respective to p-values 0.001, 0.01, and 0.05). c Force transducer experimental setup and stimulation parameters (IC: 45–50 Hz burst, 1 s ON, 2 s OFF; VLL: 50 Hz continuous). Example force traces (n = 20) and boxplots of AUC (IC alone, IC with VLL at 50 Hz, and IC with VLL at 100 Hz). For all boxplots, the whiskers extend to the maximum spread not considering outliers, central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. For all (a–c), statistical significance was assessed with two-tail bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001(***). Source data for (a–c) are provided as a Source Data file.
Finally, in order to demonstrate that VLL stimulation could enhance evoked grasping, we delivered bursts of IC stimulation at about 50 Hz which induced a grasping-like motion producing measurable isometric forces (Fig. 3c). We then paired the 50 Hz IC stimulation with VLL stimulation at 50 or 100 Hz. VLL stimulation at 50 Hz, but not at 100 Hz, immediately and significantly increased the grip force as compared to IC stimulation alone (n = 1) (Fig. 3c, Supplementary Video 2).
In summary, targeted motor thalamus stimulation immediately increased upper-limb motor output as measured by the amplitude of arm and hand muscle MEPs, movement kinematics, and stimulation-induced grip forces.
Control experiments: motor output potentiation is not due to current spreading towards CST axons
We sought to confirm that the enhanced motor output was the result of an increased excitability of the motor cortex and did not result instead from inadvertent activation of descending excitatory tracts due to current spreading from motor thalamus stimulation. We reasoned that if the observed MEP potentiation was occurring within the spinal cord as a result of inadvertent stimulation of descending tracts, then we would expect that 1) MEPs would be present also when stimulating the VLL alone, and 2) peripheral inputs to spinal circuits, such as H-reflexes, would also be potentiated by VLL stimulation35,36. To control for this, we implanted a multi-channel linear probe (NeuroNexus) at the C6-C7 spinal level with dorsal-ventral orientation (Fig. 1c). We observed consistent volleys in the spinal cord in the first 3 ms following VLL stimulation (1.44 ms, 1.73 ms, and 2.13 ms for MK-OP, MK-JC, and MK-HS, respectively, Fig. 4a) with the largest peak to peak amplitude in the intermediate-ventral spinal cord gray zone (Fig. 4b). These evoked potentials could either represent antidromic recruitment of ascending pathways such as spino-thalamic axons, or orthodromic activation of descending excitatory pathways. However, there were no MEPs in either the upper-limb or facial muscles when stimulating the VLL nucleus alone (Fig. 4c, Supplementary Fig. 4a, and Supplementary Video 3) suggesting that the observed spinal responses were not caused by inadvertent stimulation of the CST. Moreover, when we simultaneously stimulated the VLL and the IC, the frequency of the muscle contractions was at 2 Hz (i.e., same frequency of IC stimulation) and not at the frequencies of VLL stimulation (50, 80, or 100 Hz) (Supplementary Fig. 3 and Supplementary Video 1), further demonstrating that the increase in MEPs and movement kinematic were not induced by current spreading to the CST from the VLL. Indeed, if the movements were induced by current spread, the frequency of muscle contractions would be similar to the stimulation frequency of the VLL (i.e., 50, 80, or 100 Hz). It was still possible that the observed spinal responses and the enhanced movements could be carried by other descending tracts that do not have direct spinal motor neuron connections or generate MEPs but that are able to excite the spinal circuits and facilitate movement. However, when we paired continuous VLL stimulation at 50 Hz with stimulation of the radial nerve we observed no significant increase of reflex-mediated responses (Fig. 4d and Supplementary Fig. 4b). These results demonstrate that 1) the observed volleys in the spine are likely the result of antidromic activation of ascending pathways such as spino-thalamic axons; and 2) the observed potentiation of MEPs (Fig. 3) is not occurring inside the spinal cord as a result of current spread to descending axons, but is, instead, a trans-cortically mediated effect.
a Example of antidromic responses in the spinal cord from VLL stimulation at 10 Hz (n = 30 traces) in MK-OP for three different channels (CH1, 16, and 31) along the multi-channel linear spinal probe. The spinal cord section in (a) was created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. b Left: Heatmaps of peak-to-peak amplitude of the antidromic responses in the spinal cord at the C6-C7 spinal level with dorsal-ventral alignments for n = 3 animals. The dashed boxes are highlighting the putative intermediate-ventral zone where we see the greatest responses. Right: Boxplots of the antidromic response latency for each animal (n = 656 MK-OP, n = 600 MK-JC, n = 596 MK-HS). c MEPs of the hand (ECR: Extensor Carpi Radialis, 30 traces for each animal) elicited by VLL stim at 50 Hz. d EMG reflexes and boxplots of the AUC of the EMG reflexes of the ECR muscle elicited by radial nerve stimulation and radial nerve paired with continuous VLL stimulation at 50 Hz (30 example traces each). For all boxplots, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. For (d), statistical significance was assessed with one-tail bootstrapping with Bonferroni correction, however, in all cases the results were not significant. Source data for (b and d) are provided as a Source Data file.
Motor output potentiation occurs through orthodromic thalamocortical pathways
Our results and control experiments suggest that the potentiation of descending CST activity is mediated by the orthodromic recruitment of cortico-spinal neurons in M1 via thalamocortical excitatory synaptic projections. We further confirmed this hypothesis with an experiment. Excitatory synaptic transmission is known to be affected by stimulation frequency via mechanisms such as presynaptic inhibition or homosynaptic neurotransmitter suppression, i.e., stimulation at high frequencies would cause a suppression of the responses37. Accordingly, we explored the effect of multiple frequencies on the efficacy of motor potentiation. We delivered IC stimulation at 2 Hz paired with continuous VLL stimulation at 10, 50, 80, 100, and 200 Hz. We analyzed the MEP responses to the individual IC stimulation pulses over time and observed a variety of modulation patterns (Fig. 5a). We classified these patterns into four distinct categories: (1) no potentiation, as responses were comparable to IC stimulation alone, (2) sustained potentiation, as responses were potentiated, (3) attenuation, as the response amplitudes progressively decreased compared to the initial responses, and (4) suppression, as the responses were completely suppressed after a few pulses. Given that both attenuation and suppression result in decreased MEP responses over time, we combined them in Fig. 5b (see Supplementary Fig. 2d for the separated attenuation and suppression). Generally, the absence of potentiation of the IC stimulation responses was characteristic of low frequencies (10 Hz, observed in > 50% of the muscles across all 3 animals), whereas stimulation frequencies between 50 Hz and 80 Hz consistently potentiated the MEP amplitudes with sustained outputs (Fig. 5b). Importantly, attenuation/suppression was only present in less than 15% of the muscles for 50 Hz and less than 25% of the muscles for 80 Hz. Stimulation at 100 Hz and above, instead, resulted mainly in attenuation and suppression and only in a few instances of sustained potentiation. Therefore, we identified the 50–80 Hz range as optimal to achieve sustained potentiation of MEPs.
a Examples of frequency-dependent modulation of muscular responses. EMG responses were elicited by 2 Hz stimulation of the IC paired with different VLL stimulation frequencies (10, 50, 80, 100, and 200 Hz). b The occurrence of modulation patterns with respect to stimulation frequency. All patterns recorded in all muscles of 3 animals were included in the analysis (n = 24 patterns at 10 Hz, n = 22 patterns at 50 Hz, n = 23 patterns at 80 Hz, n = 43 at 100 Hz, and n = 17 patterns at 200 Hz). c Top: Example of spinal responses in the ventral zone for IC stimulation alone and IC stimulation paired with VLL stimulation at 80 and 100 Hz (n = 30 traces per plot). Bottom: Heatmaps of the AUC calculated from 5 to 10 ms after IC stimulation for all ventral channels (CH 27–32 for MK-HS and CH: 26–31 for MK-JC) for IC stimulation alone and IC stimulation paired with VLL stimulation at 10, 50, 80, and 100 Hz. (*) for significant potentiation and (+) for significant suppression. Regular text represents p < 0.05; whereas bold represents p < 0.001. Statistical significance was tested by comparing IC stimulation alone to all other stimulation conditions for potentiation using one-tailed bootstrapping with Bonferroni correction. d Top: Schematic of the experimental layout for testing frequency dependence within the motor cortex. Bottom: Example traces of the cortical evoked potential responses in the M1 array when stimulating the thalamus at different frequencies (10, 50, 80, and 100 Hz) (n = 30 traces). Boxplots of the peak-to-peak amplitudes of the cortical evoked potentials. Statistical significance for (d) was tested by comparing 50 Hz VLL stimulation to all other stimulation conditions for potentiation using one-tailed bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001(***). For all boxplots, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. Source data for (b–d) are provided as a Source Data file. The spinal cord and the brain in (c, d) were created with BioRender.com released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
We further explored the neural correlates of these effects with intra-cortical and intra-spinal neural recordings. IC stimulation pulses elicited intra-spinal neural responses in the ventral zone38, where spinal motoneurons are located. Consistent with the EMG recordings, the AUC of these responses was significantly larger when the VLL nucleus was stimulated at 50 or 80 Hz (p < 0.05, Fig. 5c). Spinal responses were, instead, suppressed at higher frequencies. Similarly, cortical EPs in M1 when stimulating the VLL alone showed larger peak-to-peak amplitudes with frequencies of stimulation in the 50–80 Hz range as compared to 100 Hz (Fig. 5d). These combined results demonstrate that the effects observed in the MEP and spinal cord responses were a consequence of frequency-dependent excitation of the motor cortex. This frequency-dependent characteristic of excitation could be either the result of presynaptic inhibition or homosynaptic neurotransmitter suppression at the level of the cortico-spinal neurons23,34,37.
In summary, our analysis confirmed a frequency-dependent effect that is consistent with motor cortical potentiation of thalamic afferents to M1 cortex via excitatory synaptic inputs. From these findings, we identified that the optimal stimulation frequencies to increase motor outputs are in the 50–80 Hz range, notably lower than clinical DBS stimulation frequencies (>100 Hz)39.
Potentiation of motor outputs persists after CST lesions
To demonstrate the potential clinical relevance of cortical potentiation, we examined whether electrical stimulation of the VLL nucleus would still augment motor outputs after a partial lesion of the CST. Such a lesion reduces the number of CST fibers projecting to the cervical spinal cord, leading to motor deficits similar to those observed in human patients. To create such a lesion, we implanted a second depth electrode in the posterior limb of the IC, approximately 2 cm ventral/inferior to the IC stimulating electrode. We then generated a controlled and reproducible lesion of the CST fibers by performing thermo-frequency ablations40 through the ventral/inferior electrode (Fig. 6a). As expected, we observed reduced MEP amplitudes when stimulating the dorsal IC electrode after the ventral/inferior CST lesion had been made (Supplementary Fig. 5). Post-mortem HDFT confirmed that the lesion consistently and significantly reduced the volume of CST fibers compared to the intact hemisphere in all tested monkeys (46% reduction) (n = 4) (Fig. 6a). After the CST lesion, the smaller MEPs recorded from the arm, hand and fingers muscles were immediately and significantly increased when the VLL nucleus was simultaneously stimulated at 50 and 80 Hz (p < 0.001, Fig. 6b and Supplementary Fig. 6b). Importantly, IC and VLL stimulation intensity were the same as those used pre-lesion. MEP amplitudes when VLL stimulation was ON were comparable to pre-lesion MEP amplitudes in the same muscles (Supplementary Fig. 5). Furthermore, post-lesion stimulation at 80 Hz resulted in a stronger MEP potentiation as compared to 50 Hz. Similarly, the grip force elicited by IC stimulation bursts immediately and significantly increased with continuous VLL stimulation at 50 Hz and an even greater increase occurred with VLL stimulation at 80 Hz (Fig. 6d, Supplementary Video 4). Finally, face muscle MEPs were also potentiated by VLL stimulation after the CST lesion (Supplementary Fig. 5).
a Top panels: T2-weighted post-mortem MRI of IC lesion and VLL location (axial plane). (Cu: Caudate Nucleus, IC: Internal Capsule, Pt: Putamen). Bottom panels: HDFT of the CST in intact and lesioned hemispheres. Volume of cumulative CST (mean ± SE over animals) for both hemispheres normalized over the volume of the intact hemisphere. b Left panels: Example of post-lesion MEPs of one arm muscle (n = 40, BIC: Biceps, MK-JC), one hand muscle (n = 40, ECR: Extensor Communis Radialis, MK-JC), and one finger muscle (n = 40, FDM: Flexor Digitorum Minimi, MK-JC) with IC stim alone and then paired with VLL stimulation at 50 and 80 Hz. Right panels: percentage of increase of arm, hand, and finger MEPs post-lesion between IC stimulation alone and paired with VLL stimulation at 50 and 80 Hz. For each monkey, the percentage of increase was calculated over the medians and averaged over all the muscles. See Supplementary Fig. 5 for boxplots for single muscles. (***), (**), or (*) was placed if muscles in each group showed a significant increase (respective to p-values 0.001, 0.01, and 0.05). c Left panel: Example of force traces (n = 20). Right panel: boxplot of AUC pre- and post-lesion for IC alone, and IC with VLL 50 Hz and VLL 80 Hz. For all boxplots, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. For all (a–c), statistical significance was assessed with two-tail bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001(***). Source data for (a–c) are provided as a Source Data file.
Overall, these results demonstrate that VLL stimulation can immediately potentiate motor output even in the presence of hemiparesis caused by partial lesions of the CST.
Stimulation of the motor thalamus potentiates motor output in humans
We sought to verify the translational potential of these results in human subjects. For this, after obtaining informed written consent, we performed intraoperative electrophysiological experiments in human subjects (n = 7, 3 males, 4 females) who underwent on-label DBS implants into the motor thalamus (Fig. 7a). Experiments were designed to closely mimic the animal study protocols. The human motor thalamus nuclei comparable to the VLL nucleus are the ventralis intermediate (VIM) and the ventralis oralis posterior (VOP)29. HDFT analysis confirmed preferential anatomical projections from the VIM/VOP to M1 (Fig. 7b). Additionally, we tested the connectivity of these pathways using evoked potential methods. For this, we placed a 6-contact subdural strip electrode (Adtech, Oak Creek, WI, USA) over the arm and hand representation of the primary motor and somatosensory cortices. To confirm electrode placement, we used the validated clinical technique of somatosensory evoked potential (SSEP) phase reversal (PR) mapping41 to locate both the hand representation of S1 (largest amplitude N20/P30 cortical SSEP) and the approximate location of the central sulcus, where the polarity of the SSEP reverses (Fig. 7a) (this is caused by a field effect due to direct depolarization in S1 resulting from electrical stimulation of the median nerve41). We then mapped the upper extremity representation of M1 within the precentral gyrus by direct cortical stimulation (DCS) of the electrode contacts that were anterior to the SSEP PR. MEPs were recorded from six contralateral upper extremity muscle groups (Deltoid, Biceps, Triceps, Flexor carpi, Extensor carpi and APB). The strip electrode position was adjusted to obtain MEPs from at least three muscle groups with the lowest possible stimulation threshold (~3–12 mA).
a Top: Experimental setup for human intraoperative experiments. Enlargement shows a schematic representing the subdural strip electrode placement over M1 and S1 cortices, and the PR to identify the central sulcus. Needle electrodes were inserted in arm, hand, and finger muscles to record MEPs and superficial electrodes were placed over the median nerve for SSEP. The panel (was designed by Isabella Bushko. b Left: HDFT from the VIM/VOP to cortical regions. Right: Normalized volume (mean ± SE over n = 4 subjects S01-S04) of VIM/VOP projections to each cortical region normalized by the total volume of fibers. c Top: Example traces (n = 122) of cortical evoked potentials elicited by VIM/VOP stimulation recorded over an S1 (left) and M1 (right) contact for S04. Bottom: Boxplots of peak-to-peak amplitude of cortical evoked potentials at S1 and M1 contact. From left to right, subjects S01 to S04 are shown (n = 128, n = 585, n = 601, n = 122 trials, respectively). d Example MEP traces (arm, biceps; hand, flexor; n = 60) with VIM/VOP stimulation alone for S03. e Example MEP traces with DCS alone and DCS paired with VIM/VOP stimulation at 50 and 100 Hz (from left to right). Arm is S03 biceps (n = 48 traces), and fingers are S01 abductor pollicis brevis (n = 16 traces). f Boxplots of AUC for MEPs of the arm and fingers (biceps and abductor pollicis brevis respectively; n = 58) with DCS alone and DCS paired with VIM/VOP stimulation at 50 and 100 Hz. g Scatter plots for arm, hand, and finger muscles of subjects S01-S04, representing the percentage of AUC increase calculated over the means, with respect to DCS alone, for all the different VIM/VOP stimulation frequencies (50, 80, and 100 Hz). For all boxplots, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. For all (c, f, g), statistical significance was assessed with two-tail bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001(***). Source data for (b, c, e–g) are provided as a Source Data file.
We then examined the projections from VIM/VOP to S1 and M1 upper extremity representations by recording cortical EPs at each of the 6 electrode contacts in response to low frequency (2 or 10 Hz) stimulation of the clinical DBS electrode (Boston Scientific) implanted in the VIM/VOP nucleus of the same hemisphere at the AC/PC plane (X: 12 mm lateral to the AC/PC line, Y: 6 mm anterior to PC, Z: 0 mm to the AC/PC horizontal plane, Supplementary Fig. 7a). Cortical EPs, occurring within 20 ms of the stimulus onset, were recorded at the M1 contacts (anterior to the PR location) (Fig. 7c) in subjects S01–S04. Importantly, cortical EPs over S1 and M1 were both positive because of a direct depolarization occurring in both regions. This supports orthodromic synaptic transmission through thalamocortical projections from VIM/VOP to the M1 hand/arm representation and to a lesser extent to S1, as was observed in monkeys during stimulation of the VLL. Indeed, in all tested subjects but one, the peak-to-peak amplitude of these evoked responses was significantly larger for precentral contacts than for postcentral contacts confirming preferential anatomical projections of VIM/VOP nucleus to M1.
Next, DCS MEPs generated from stimulating the optimal contact over M1 cortex were recorded from up to six contralateral upper extremity muscles without and with paired VIM/VOP stimulation at 50, 80, or 100 Hz. Similar to the monkey experiments, we observed an immediate and statistically significant increase in the DCS MEP amplitudes across arm, hand, and finger muscles with VIM/VOP stimulation at 50–80 Hz compared to DCS alone (p < 0.001, Fig. 7f). More variable patterns of MEP modulation were, instead, found with VIM/VOP stimulation at higher frequencies. Indeed, stimulation at 100 Hz further amplified or suppressed these responses (Fig. 7g, Supplementary Fig. 8). Interestingly, suppression was predominant for the finger muscles similar to the data in monkeys (Fig. 3b, Supplementary Fig. 2). Importantly, potentiation was maximal during stimulation of the VIM/VOP nucleus. Indeed, when pairing motor cortex DCS with 50 Hz stimulation from thalamic nuclei either anterior or posterior to the VIM/VOP nucleus (subjects S05–S07), the increase in MEP amplitudes was significantly lower than when pairing motor cortex DCS with 50 Hz stimulation of the VIM/VOP nucleus (Supplementary Fig. 9). Finally, we confirmed that electrical stimulation of the VIM/VOP nucleus alone did not produce MEPs in the arm, hand, nor fingers muscles (Fig. 7d), which corroborates our findings in monkeys (Fig. 4 and Supplementary Fig. 4) that show that this potentiation is not occurring via current spreading to nearby passing CST axons.
Overall, these results observed in human subjects were equivalent to those obtained in monkeys, demonstrating the translational potential of the mechanisms of action and of the identified targets and stimulation parameters. Specifically, targeted electrical stimulation of the VIM/VOP nucleus within the motor thalamus enhanced motor cortex and CST excitability at specific stimulation frequencies and immediately potentiated motor output in human subjects.
Stimulation of the motor thalamus increases motor output in a person with CST lesion
We had the opportunity to test our hypothesis in one patient who suffered severe bilateral lesions of the CST (CST01, Fig. 8a) and underwent DBS implants in the motor thalamus to treat tremors (Supplementary Fig., 7b). We quantified damage to the white-matter axons using differential tractography and verified that for both hemispheres the most damaged tract was the CST (57% of damaged tracts in the right hemisphere and 89% in the left hemisphere). Consequently, CST01 suffered from hemiparesis of both upper extremities. Functionally, CST01 required maximal assistance for eating, grooming, bathing, and dressing. Replicating the same human set-up (Fig. 7a), a 6-contact subdural strip electrode was placed over the S1 and M1 cortex of the left hemisphere (i.e., greater CST damage). Consistent with the results in monkeys and human subjects, stimulation of the VIM/VOP nucleus resulted in larger amplitude cortical EPs over motor cortical areas as compared to somatosensory cortex (Fig. 8b). Additionally, DCS MEPs were consistently recorded from the APB and flexor muscles of the hand, even if, because of the lesion, MEPs were smaller for the finger muscles (Fig. 8c, left panels). We then paired DCS of M1 with stimulation of the motor thalamus to assess the effect of VIM/VOP stimulation on the hemiparetic arm. Again, DCS MEPs were immediately and significantly larger with concurrent stimulation of the motor thalamus at 50 Hz and modulated in a frequency-dependent manner (Fig. 8c, right panels). Indeed, stimulation at 100 Hz resulted in suppression of MEPs particularly for the finger muscles, demonstrating the consistency of our findings across multiple monkeys and human subjects, including one patient suffering from a chronic (>6 months) CST lesion.
a Top: Tractography with lesion highlighted in red. Bottom: Amount of lesioned tracts (CBT: corticobulbar tract, DRT: dentatorubrothalamic tract, ML: medial lemniscus). See Supplementary Fig. 10a for lesion segmentation. b Boxplots of peak-to-peak amplitude of cortical EPs over S1 and M1 contact from VIM/VOP stimulation at 10 Hz (number of stimulation pulses: 599). c Left: Example of MEPs with DCS alone and DCS paired with VIM/VOP stimulation at 50 and 100 Hz (number of DCS pulses: 18). Right: boxplots of MEPs AUC with DCS alone and DCS paired with VIM/VOP stimulation. d Schema of the voluntary force control (top) and isometric grip strength (bottom) task. e Bar plot of isometric grip strength without (yellow) and with (blue) VIM/VOP stimulation at 55 Hz (session 1: n = 4, session 2: n = 3). f Left: Example of force traces without (top) and with (bottom) VIM/VOP stimulation. Yellow and blue intensities represent different repetitions. Dashed and dotted lines indicate the ± 5 % of the target force. Right: bar plot of the RMSE of the force (sessions 1 and 2: n = 5, session 3: n = 8). g Left: Example video frames of the weight elevation task for the heavy weight without (top) and with VIM/VOP stimulation (bottom). Right: bar plot of shoulder abduction and elbow extension without and with VIM/VOP stimulation for the three conditions (no and lightweight: n = 10, heavyweight: n = 6). h Bar plot of the arm elevation percentage increase for the three conditions. For all (b, c, e–h), statistical significance was assessed with two-tail bootstrapping with Bonferroni correction: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***). For all bar plots, each bar represents the mean, and error bars represent standard deviation over trials. For all boxplots, the whiskers extend to the maximum spread not considering outliers. Central, top, and bottom lines represent median, 25th, and 75th percentile, respectively. Source data for (a–c and e–h) are provided as a Source Data file.
Stimulation of the motor thalamus improves strength and voluntary force control in a person with CST lesion
Finally, to assess whether stimulation of the motor thalamus achieves functional improvements for chronic hemiparesis, we tested the effect of VIM/VOP stimulation in CST01 during several functional tasks using the chronically (>5 months) implanted DBS system. For all tasks, tests were performed without and with bilateral VIM/VOP stimulation at 55 Hz. Importantly, the stimulation was set to 55 Hz only during these tests. We first tested isometric grip strength using a handheld dynamometer during 2 different sessions (Fig. 8d, bottom line). Bilateral VIM/VOP stimulation at 55 Hz led to an immediate statistically significant increase in strength (13% and 78% for session 1 and session 2, respectively), suggesting that DBS can potentiate both arm and hand function (Fig. 8e). We then designed an isometric roadway test to measure voluntary force control. Specifically, CST01 was instructed to match grip force to a time series of thresholds, gradually increasing (2 s), sustaining (4 s), and decreasing (2 s) the force between set percentages of maximum voluntary force levels that were established when stimulation was OFF (Fig. 8d, top line). This experiment mimicked those performed in anesthetized monkeys (Figs. 3c and 6c). We repeated the testing over 3 different sessions to assess robustness of the immediate effects of the stimulation. On all three days, when the stimulation was turned ON at 55 Hz, CST01 was able to reduce the amount of grip force deviation from the requested force compared to when the stimulation was OFF. When the stimulation was ON performances were smoother and more accurate when compared to no stimulation (Fig. 8f, RMSE, Session 1: 0.05 (DBS ON) vs 0.07 (DBS OFF), Session 2: 0.05 (DBS ON) vs 0.14 (DBS OFF), Session 3: 0.013 (DBS ON) vs 0.045 (DBS OFF)). We controlled for the possibility that the improvement in motor performances was due to a reduction in tremor when the stimulation was ON. For this, we estimated the frequency power of the force trace and compared the average power from 6 to 12 Hz (frequency range typical of tremor)42 between stimulation ON and OFF. The average power was not different between stimulation ON and OFF proving that the tremor was consistent with and without stimulation at 55 Hz and demonstrating that the improved voluntary control was not caused by a reduction in the level of tremor (Supplementary Fig. 10b). In order to further demonstrate the functional relevance of these improvements, we asked CST01 to raise his arm above his head without and while lifting two different weights (Fig. 8g). As expected, shoulder abduction (3%, 8%, and 17% increase for no, light, and heavyweight, respectively; Fig. 8g) and arm elevation (4%, 9%, and 27% increase for no, light, and heavyweight, respectively; Fig. 8h) were immediately and significantly increased when the stimulation was ON (Supplementary Video 5). Importantly the effect of motor facilitation at the elbow extension was stronger when CST01 was lifting the heavy weight (36% increase, Fig. 8g), which suggests important functional facilitation of strength. These results demonstrate that VIM/VOP stimulation led to immediate and substantial improvements in strength and overall movement performances of the arm and hand.
Finally, we sought to determine whether these improvements in strength and control translated to improved performances during an activity of daily living. For this, we asked CST01 to reach, grasp, and lift a drinking cup without assistance. Without stimulation, CST01 had severe difficulty in securing the grasp of the cup and especially in releasing it by opening the hand at the end of the trial (Supplementary Video 6). Remarkably, when the stimulation was turned ON, CST01 could smoothly reach for the cup, lift it, bring it to the mouth, and release it. Importantly, during all these functional tasks, when the stimulation was turned ON the patient did not report any side effects.
While preliminary, these results suggest that stimulation of the motor thalamus at optimal stimulation frequencies (50–80 Hz) can immediately and significantly improve strength and volitional force control resulting in substantial functional benefits on motor control with the absence of noticeable side effects in patients with chronic lesions of the CST.
Discussion
In this study we demonstrated with experiments in both monkeys and human subjects that stimulation of the motor thalamus facilitates the recruitment of cortico-spinal neurons within the motor cortex which in turn immediately increases motor output in intact upper extremities and paretic limbs after lesions of the CST.
Importantly, we first investigated the mechanisms of action of motor thalamus stimulation to successfully tailor it to treat upper-limb motor deficits. Specifically, by combining intra-spinal and intra-cortical electrophysiology in monkeys and human subjects, we verified that stimulation of motor thalamus and specifically of the VLL (or VIM/VOP nucleus)29 increased motor output by augmenting the recruitment of cortico-spinal motor neurons within the primary motor cortex via excitatory synaptic inputs from the targeted thalamic nuclei. Because of the short duration of Excitatory Post-Synaptic Potentials on the membrane potential (5–20 ms), synaptic-mediated excitatory inputs necessitate a stimulation frequency sufficiently high (>40 Hz) to induce sustained membrane depolarization43,44. At the same time, synaptic-mediated excitatory inputs are known to be less efficient at high stimulation frequency (>100 Hz) because of presynaptic effects such as depletion of neurotransmitters and presynaptic inhibition23,34,37. For this reason, we explored subclinical DBS frequencies which led us to confirm that the 50–80 Hz range was optimal to sustainably increase motor output. Indeed, when testing 10 Hz we rarely found MEP potentiation, and when trialing commonly used DBS stimulation frequencies (>100 Hz)39 we found frequent suppression of motor output particularly of the fingers muscles, i.e., those innervated by the highest concentration of cortico-spinal tract axons45 and mostly affected by white matter lesions46. In this regard, it is important to highlight that our results, which indicated that 50–80 Hz are optimal stimulation frequencies for motor facilitation, are consistent with a previous work that showed in a single case study that 80 Hz stimulation was facilitating motor output after stroke18. However, these previous experiments did not present any evidence to support an explanation as to why 80 Hz stimulation was better than higher frequencies. In fact, the authors believed that their effects were caused by direct activation of descending motor pathways from the left cerebral peduncle. In our work, instead, we demonstrated, with detailed electrophysiology in monkeys and humans, that the faciliatory motor effects cannot be attributed to direct activation of motor pathways. We provided strong experimental evidence that this facilitation is occurring via synaptic-mediated activity to the motor cortex. Indeed, for stimulation at high frequencies (i.e., >100 Hz) we reduced the stimulation intensity to balance the total charge delivered and verified that the suppression of the responses was indeed due to inhibitory mechanisms and not a confound from a difference in the cumulative charge.
This mechanisms-driven selection of the target and parameters of stimulation resulted in a sustained MEP potentiation with a remarkably high and consistent average increase of +500% in MEP amplitude over all muscles and subjects, which we believe led to enhanced strength and force control. Indeed, stimulation of the motor thalamus availed a patient with severe, chronic bilateral lesions of the CST to volitionally modulate grip force, substantially increase arm range of motion, and enhance object manipulation skills demonstrating that continuous stimulation of the motor thalamus immediately improved voluntary motor control. Previous studies using non-invasive brain stimulation such as TMS or transcranial direct-current stimulation (tDCs) already proposed that enhanced cortical excitability could improve motor functions after CST lesions47,48,49,50,51,52,53,54,55,56,57. However, no studies so far reported average 5-fold increases that we report here. We speculate that these incomparably large effect sizes are necessary to observe the immediate effects on motor control that we measured in our participant when VIM/VOP-DBS was ON. Importantly, we believe that these effect sizes are enabled by the high degree of selectivity of our approach that enables powerful, but selective excitatory drive to motor cortex58,59.
These results offer promising albeit preliminary support for the clinical use of DBS of the VIM/VOP as a therapy to improve motor deficits in people with lesions of the CST. Importantly, in the tested patients we did not find side effects on speech, gait difficulties, or ataxia, which are often clinically observed with VIM/VOP stimulation. Importantly, VIM/VOP stimulation side effects are usually reported with clinical standard frequencies (>100 Hz). We, therefore, believe that the use of lower frequencies (i.e., 50–80 Hz) might limit these secondary effects enabling a safe and effective use of DBS to improve motor control. A potential limiting factor for widespread clinical use of DBS in the context of motor deficits induced by lesion of the CST is surgical safety. In fact, adverse events of DBS implantation are considered low60 and are balanced by the potential benefits. Moreover, surgical risk in patients with a history of cerebral lesions can be minimized by careful pre-operative management of anticoagulants and by delaying the implantation for at least three months following the brain insult61,62. This was recently demonstrated in a study applying DBS to the cerebellar dentate nucleus in n = 12 individuals to treat post-stroke motor deficits63 and in a study testing central lateral nucleus stimulation to improve cognitive functions after TBI in n = 6 patients64. Therefore, the surgical intervention required for DBS implantation does not represent a significant barrier to its clinical translation to treat patients with CST lesions.
While these earlier studies demonstrate the feasibility of using DBS as a safe clinical tool to improve motor control, there is a critical difference with our approach: the goal of improving a network plasticity over time which dictates the different choice of stimulation target. For example, Baker et al., 63 aimed at an increased excitation of a large cortical network that could lead to enhanced plastic cortical reorganization of the entire sensorimotor network to restore motor functions65,66,67; using similar concepts Schiff et al., 64 aimed to improve dysfunction of the large frontostriatal network to improve executive control. In these two cases, the stimulation led to changes that emerged through these targeted large networks over time (weeks/months of stimulation) that are likely indicative of enhanced neuroplasticity. Instead, in our work, we aimed for a restricted, immediate excitation of specific cortico-spinal pathways with the idea to produce strong immediate effects, i.e., effects that can be observed only when the stimulation is turned ON and disappear as soon as the stimulation is turned OFF. Therefore, our work strictly focused on demonstrating potentiation of motor control during stimulation ON. However, the results of these previous studies, together with previous non-invasive stimulation works47,48,49,50,51,52,53,54,55, provide excellent theoretical ground to believe that also our stimulation approach may lead to neuroplastic changes when applied over time and in combination with physical therapy similar to what observed in those works. For this reason, we think that an early feasibility study on a small number of subjects with white matter lesions would be necessary to test these hypotheses and assess the long-term effects of VIM/VOP-DBS. In such as a study, it would also be important to explore whether phasic activation of the thalamus might be more effective than continuous stimulation, as recently demonstrated with direct phasic stimulation of the motor cortex, which showed short-term and long-term benefits on the recovery of hand motor functions68,69.
Importantly, the VIM/VOP target is commonly used as a target for DBS therapy in clinical practice for the treatment of Essential Tremor, and the devices used in this study are commercially available, FDA-approved, and currently implanted in more than 12,000 patients per year for the treatment of different movement-related, epilepsy, and some psychiatric disorders58. DBS has changed the treatment options for millions of patients with movement disorders such as Parkinson’s Disease, Essential Tremor, and Dystonia making the perspective of an application in people with white matter lesions highly promising and appealing for clinicians20. However, while DBS could be a viable approach for increasing CST excitability, non-invasive techniques such as focused ultrasound or transcranial electrical temporal interference could overcome the surgical limitations imposed by DBS potentially serving a larger patient population70,71,72. Additionally, other targets with excitatory projections to the motor cortex, such as the centromedian thalamic nucleus or pre-motor cortex73, could have similar immediate effects like those reported here and would require future investigations.
The most important limitation of this study is that the behavioral tests in humans were performed in only one patient with CST lesions. However, the main goal of this work was to first identify the mechanisms of action of thalamic stimulation to optimize stimulation targets and parameters and quantify the immediate assistive effects. We performed these investigations in several monkeys and human subjects demonstrating the consistency of our findings across species, including one patient suffering from a chronic (>6 months) CST lesion in which we tested the effects of the stimulation in four different functional tasks. This consistency across monkeys and human subjects importantly emphasizes that the observed MEP potentiation is present regardless of etiology and is not due to a change in cortical excitability secondary to the tremor74,75,76 further supporting tests in a larger cohort of patients with CST lesions.
In conclusion, in this study we explored the parameters and location of stimulation of the motor thalamus that maximized motor improvements, setting the stage towards a therapy for motor-related deficits after white matter lesions. With a series of experiments progressing from monkeys to human subjects, we showed that targeted stimulation of thalamocortical excitatory pathways to cortico-spinal neurons could increase their excitability and restore voluntary motor control. Interestingly, these mechanisms resemble those previously proposed for spinal cord stimulation (SCS). Indeed, we recently showed that SCS of the cervical spinal cord improved upper-limb strength and volitional control by directly activating sensory afferents in the dorsal roots, which form mono- and polysynaptic excitatory connections to spinal motoneurons, thus increasing the responsiveness of the spinal motoneurons to the remaining CST inputs77,78,79. Paralleling these two results, we could speculate a general principle that might apply to other areas of the central nervous system and could guide other neuromodulation applications: direct stimulation of excitatory pathways with strong connectivity to a target area of interest (e.g., motor cortex) improves neural function (e.g., motor output) without disrupting ongoing neural activity. Future studies in patients with cerebral white matter lesions are now necessary to further demonstrate safety and efficacy of our approach for the recovery of voluntary motor control.
Methods
Ethical considerations
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (protocol number IS0017081). All intra-operative procedures in human subjects were approved by the University of Pittsburgh Institutional Review Board (STUDY21040121). The behavioral experiments in human subjects were approved by the University of Pittsburgh Institutional Review Board (STUDY21100020). The study complies in full with the STROBE statement. All the recordings were carried out according to CARE guidelines and in compliance with the Declaration of Helsinki principles. The participants were informed of the procedure and they signed an informed consent, which included the consent for the use of all data collected during the experiment in scientific publications. Additionally, participants provided written consent to the use of photos, videos, and voice recordings acquired during the experiments for research purposes and publications. CSTI01 consented to the publications of Fig. 8 and Supplementary Videos 5 and 6.
Animals
We utilized 4 adult Macaca Fascicularis (3 male: MK-SC 4 y.o. 5.4 kg, MK-SZ 4 y.o. 5.9 kg, MK-JC 6 y.o. 7.5 kg, 1 Female: MK-OP 6 y.o. 6 kg) and 1 adult male Macaca Mulatta (MK-HS 7 y.o. 12 kg). The animals were housed in the Division of laboratory Animal Resources at the University of Pittsburgh. When possible, the animals were pair housed with another animal prior to any surgical procedures. The animals were not water or food restricted and were given daily enrichments (novel food, toys, puzzles). Detailed information on which animals were involved in specific experimental procedures are reported in Supplementary Table 1.
Animal surgical procedure
For each animal, we performed one survival surgical procedure and one terminal surgical procedure. During the survival surgical procedure, we implanted five fiducial titanium screws (1.5 mm × 4 mm, KLS Martin) in the skull at non-coplanar depths. We then performed a computed tomography (CT) scan (250 μm isotropic, Epica Vimago GT30) of the skull with the implanted screws. The procedure was performed using standard aseptic techniques under full anesthesia induced with ketamine (10 mg/km, i.m.) and maintained under isoflurane (1–3%, 2 L/min, inhalant). Over the next 7 days, we injected anti-inflammatory drugs once per day (Rimadyl 4 mg/kg, Dexamethasone 0.4 mg/kg).
During the terminal surgery the following procedures were performed: 1) peripheral nerve and muscle implantation, 2) robotic deep probe implantation, 3) intra-cortical electrodes arrays implantation, 4) spinal probe implantation, and 5) subcortical lesioning. These procedures were performed under full anesthesia induced with ketamine (10 mg/kg, i.m.) and maintained under continuous intravenous infusion of propofol (1.8–5.4 ml/kg/h) and fentanyl (0.2–1.7 ml/kg/h). Anesthesia affects neural responses and local excitability. However, propofol is known to minimize these effects and it is often used in electrophysiology80. Certified neurosurgeons performed these surgical procedures (Drs. Jorge Gonzalez-Martinez, Peter Gerszten, Daryl Fields, Vahagn Karapetyan, UPMC, Pittsburgh, USA). At the end of the experiments, the animals were euthanized with a single injection of pentobarbital (86 mg/kg) and perfused with 4% paraformaldehyde (1 L/kg) for further tissue imaging.
Peripheral nerve and muscle implantation
We dissected from the lateral epicondyle of the arm and implanted a cuff electrode (FNC-2000-V-R-A-30 bipolar nerve-cuff Micro-Leads Neuro, Ann Arbor, MI, USA) around the deep branch of the radial nerve (motor branch). We electrically stimulated two branches of the radial nerve and assessed the EMG response to distinguish the motor branch from the cutaneous branch. We then inserted EMG needle electrodes (disposable single 13 mm Subdermal needle electrode, Rhythmlink) into the extensor carpi radialis (ECR), extensor digitorum communis (EDC), flexor digiti minimi (FDM), flexor carpi radialis (FCR), flexor digitorum communis (FDC), abductor pollicis brevis (APB), and biceps (hand and arm muscles). Additionally, in n = 3 animals (MK-OP, MK-HS, MK-JC), we implanted EMG electrode needles in the Masseter (Mas), Orbicularis Oris (O. oris), and Buccinator (Buc) (face muscles).
Robotic deep probes implantation
We implanted each depth electrode using the ROSA One(R) Robot Assistance Platform (ROSA robot, Supplementary Fig. 1) to allow for highly accurate implantations of the ventral laterolateral (VLL) thalamic nucleus and the hand area of the internal capsule (IC)81. Prior to the terminal surgery, we obtained preoperative T1-weighted magnetic resonance imaging (MRI) scan (400 μm isotropic, TR/TE: 6000/3.7 ms, 7T Siemens whole body human system) and CT imaging to estimate precise targets and trajectories for each electrode. Both imaging procedures were performed with the animals secured in a customized plastic stereotactic frame to facilitate co-registration82. We then co-registered MRI and CT images using ROSA One Brain Application and selected three targets: the hand area of the cortico-spinal tract (CST) within the IC at the AC-PC level (stimulating electrode), a target 2 cm ventral to create a thermal-ablation lesion (lesioning electrode), and the VLL nucleus of the motor thalamus. We planned the trajectories of the probe in the ROSA software, avoiding vasculature and the ventricles. The entrance of the probes was positioned 2 cm in front of the central sulcus to keep the motor and somatosensory cortices intact for the implantation of the intra-cortical electrode arrays. This entrance required an implantation trajectory with a different angle for each probe. The ROSA robot was, therefore, critical to avoid a crossing of the three probes. We then positioned the monkeys prone in a stereotaxic head frame (Kopf, Model 1530, Tujunga, CA, USA) and registered the ROSA robot to the monkey using the implanted fiducial screws (see Supplementary Fig. 1 for precision of the co-registration). The robot-guided the drilling of penetration holes and the precise implant of the fixation bolts. For the two probes within the internal capsule, we inserted and fixed a radiofrequency cannula (S-100 5 mm ActiveTip Straight cannula 22 G, Abbott) and a radiofrequency electrode (RF-SE-10 Reusable Stainless-Steel Electrode, Abbott) at the correct depth into the brain. Correct positions of the probes within the IC were estimated by recording evoked electromyography (EMG) potentials from stimulation of the IC at 2 Hz at amplitudes between 800 uA and 2 mA that should produce mono-synaptic activation of the cervical motoneurons. For the stimulation of the VLL, we inserted a 16 channel Dixi electrode (DIXI Microdeep® SEEG Electrodes) into its fixation bolt. Correct position within the VLL was confirmed by recording evoked potentials in the cortex during electrical stimulation (1 Hz) at amplitudes between 1 and 4.8 mA that elicited clear evoked potentials in the motor cortex, but not in the somatosensory cortex. Detailed information on the amplitudes and pulse durations used for stimulation of the IC and the VLL are reported in Supplementary Table 2.
Intra-cortical electrode array implantation
We performed a 20 mm diameter craniotomy over the central sulcus and removed the dura to expose primary motor and primary somatosensory cortices. Functional motor areas of the arm were identified through anatomical landmarks and intra-surgical micro-stimulation. The position of the primary somatosensory area (S1) was then determined in relation to the hand representation of the primary motor cortex (M1). We then implanted intracortical arrays in M1 (48 channels) and S1 (48 channels) for a total of 96 channels (400 μm pitch, electrode length 1.0 mm Blackrock Microsystems, Salt Lake City, UT, USA). In MK-JC, we implanted a 64-channel array into M1 and a 48-channel array into S1 (total 112 channels, 400μm pitch, electrode length 1.0 mm Blackrock Microsystems, Salt Lake City, UT, USA). The arrays implantation was achieved using a pneumatic compressor system (Impactor System, Blackrock Microsystems).
Spinal probe implantation
After the completion of the cranial surgery, we performed a laminectomy from C3 to T1 vertebrae and exposed the cervical spinal cord. We then implanted a 32-channel linear spinal probe (A1 × 32-15 mm-100-177-CM32 Linear Probe with 32 pin Omnetics Connector, NeuroNexus, Ann Arbor, MI, USA) in the gray matter at the C6-C7 spinal segment to record spinal local field potentials and multi-unit spikes. To implant the probe, we opened the dura mater and placed a small hole in the pia using a surgical needle through which penetration of the probe with micromanipulators was possible. We implanted the probe using Kopf micromanipulators (Kopf, Model 1760, Tujuna, CA, USA).
Subcortical lesioning
We utilized a radiofrequency generator (NeuroTherm NT 1100) to create the lesions40. Time and temperature parameters used for the lesion for each animal are summarized in Supplementary Table 3. At the end of the experiments before perfusion, we also created a small lesion (60 °C for 5 s) in VLL through the Dixi electrode to visualize the implant location post-mortem.
Data acquisition and electrophysiology
Stimulation of the IC, VLL, and radial nerve was provided using an AM stimulator (model 2100 A-M Systems, Sequim, WA, USA). Stimulation was delivered as either single pulses or bursts of cathodal, charge-balanced, symmetric square pulses. The stimulation intensity for the IC and the nerve was set for each animal at the motor threshold (i.e., when movements became visible). Detailed information on the amplitudes and pulse widths used for stimulation of the IC, the VLL, and the radial nerve are reported in Supplementary Table 2. All electrophysiological and neural data was amplified, digitally processed, and recorded using the Ripple Neuro Grapevine and Trellis software at a sampling frequency of 30,000 Hz. Neural events were determined for each channel of the Utah arrays and linear probes by applying a broadband filter between 300 Hz and 3 kHz and setting a voltage threshold of 3 root-mean-square. LFPs were filtered between 0 and 500 Hz and then downsampled to a sample frequency of 1000 Hz.
Grasp force data acquisition
To produce a grasp motion from an anesthetized monkey (MK-JC), we stimulated the IC every two seconds with a 1 s pulse train at 47 Hz and 1 mA. We collected the grasp force data using a 6-axis low-profile force and torque (F/T) sensor (Mini40, ATI Industrial Automation, North Carolina). The sensor was powered using a multi-axis (F/T) transducer system (ATI DAQ F/T) that also calibrated the force data. The sensor was able to measure three degrees of force (±810 N for Fxy, ±2400 N Fz) and torque (±19 Nm Txy, ±20 Nm Tz) simultaneously. A small rod was mounted to the xy-plane of the sensor to be a grip handle for the animal and the system was secure under the animal’s left arm. The force and torque data were digitized and recorded using the Ripple Neuro Grapevine and Trellis software at a sampling frequency of 30000 Hz.
Animal post-mortem high definition fiber tracking (HDFT)
After perfusion, the brain was dissected for post-mortem imaging and soaked with 0.2% gadolinium (1 mmol/mL, Gadavist, Bayer) in 1x PBS for 2 weeks and then with 0.2% gadolinium in pure water for 2 days83. The brain was then placed in a custom 3D-printed holder filled with Fomblin for tissue magnetic susceptibility matching and to prevent specimen dehydration during scanning. We then acquired T2-weighted MRI (125 μm isotropic resolution, TR/TE: 1500/60.57 ms) and diffusion-weighted MRI (0.5 mm isotropic resolution, b-values = 1800, 3400, and 5200 s/mm2, with 30, 40, and 90 diffusion directions, respectively, and 6 A0 images). Image data were acquired using a 9.4 T/31 cm horizontal-bore Bruker AV3 HD animal scanner equipped with a high-performance 12 cm gradient set, capable of 660 mT/m maximum gradient strength, and a 72 mm quadrature birdcage RF coil. Diffusion tensor estimation and tractography were performed using DSI studio (http://dsi-studio.labsolver.org). The accuracy of b-table orientation was examined by comparing fiber orientations with those of a population-averaged template84. The restricted diffusion was quantified using restricted diffusion imaging85. The diffusion data were reconstructed using generalized q-sampling imaging86 with a diffusion sampling length ratio of 0.6.
For fiber tracking to characterize the thalamocortical projections, we used a tracking threshold of 0, angular threshold of 0, and a step size of 1 mm. Tracks with lengths shorter than 20 mm or longer than 200 mm were discarded87. This ensured that all possible tracts were captured while reducing the amount of tract fragments similar to Ghulam-Jelani et al., 88. A total of 10,000 tracks were placed. Topology-informed pruning89 was applied to the tractography with 2 interactions to remove false connections. We selected each of three regions of the monkey motor thalamus (the ventral anterolateral region (VAL), ventral laterolateral region (VLL), and ventral posterolateral region (VPL)) as a seed to create tracks that projected to the cortical areas of interest: the primary somatosensory cortex (S1), the primary motor cortex (M1), the dorsal premotor cortex (PMd), and the supplementary motor area (SMA). Cortical and subcortical regions were mapped into each animal MRI using the built-in primate CVIM atlas90 and confirmed by certified neurosurgeons (Dr. Jorge Gonzalez-Martinez). For each nucleus (VAL, VLL, and VPL), we calculated the volume of the projections to each cortical region of interest (S1, M1, PMd, and SMA). We then normalized each projection volume by the sum volume of all projections from that thalamic nucleus. We repeated this analysis for all thalamic nuclei individually. This allowed us to estimate the proportion of fibers that project to a cortical area from a single thalamic nucleus and to compare the proportion of projections to each cortical area between the different thalamic nuclei.
To analyze the selectivity of projections to only M1 from each of the motor thalamus nuclei, we computed a receiver operating characteristic (ROC) curve comparing the true positive rate and false positive rate of projections to M1 compared to those not to M1. We counted both rates while thresholding at each unique normalized volume. We then computed the area under the ROC curve for the VPL, VLL, and VAL.
To quantify the projections from the area of stimulation of the depth electrode (i.e., electrode within or in close proximity to the VLL), we manually drew a region, based on the size of the thermal-ablation lesion. The thermal ablation was created at the end of the experiments with the objective to visualize the location of the depth electrode post-mortem. We then mirrored this region to the intact contralateral side (due to disruption of thalamocortical fibers by the lesion) and selected it as the seed. S1, M1, PMd, and SMA were selected as regions of interest. We used the same tracking parameters as above. We then calculated the volume of each tract normalized by the total volume of tracts from the area of stimulation to all cortical areas.
To quantify the damaged cortico-spinal tract (CST), we used a tracking threshold of 0, angular threshold of 0, and a step size of 0 mm. Tracks with lengths shorter than 30 mm or longer than 500 mm were discarded87. This ensured that all possible tracts were captured while reducing the amount of tract fragments similar to Zhang et al., 91. A total of 10,000 tracks were placed. Topology-informed pruning was applied to the tractography with 2 interactions to remove false connections. To reconstruct the CST, we manually drew regions of interest in the left and right cerebral peduncles (confirmed by neurosurgeon, Dr. Jorge Gonzalez-Martinez). We considered the respective left and right sensorimotor cortical areas (M1, S1, PMd, ventral pre-motor cortex—PMv, and SMA) as seed regions. We then calculated the volume of each CST and normalized it by the volume of the intact CST.
Analysis of cortical activity
The broadband cortical data was first bandpass filtered between 10 and 5000 Hz with a 3rd order Butterworth filter and 1 ms blanking was applied over stimulation artifacts. We then extracted a 25 ms window (5 ms before stimulation, 20 ms after stimulation) to capture the entirety of the cortical evoked potential. We then calculated the peak-to-peak amplitude and averaged it across all stimulation trials. We quantified multi-unit activity offline by calculating the average spike count across all trials for each channel. Cortical spikes were detected with a threshold of 3–3.5 (MK-SC: 3, MK-SZ: 3, MK-OP: 3.5, MK-HS: 3, MK-JC: 3) standard deviations above baseline for each channel of the 2 Utah arrays. The average spike counts were baseline corrected and were calculated using a bin size of 2 ms. We blanked 13 ms after stimulation to remove stimulation artifacts.
To analyze antidromic potentials to IC stimulation, we recorded local field potentials in the M1 Utah array when stimulating the IC at 2 Hz. We then sent a 100 ms 100 Hz burst of stimulation to the VLL preceding a single pulse of IC stimulation at various delays (2, 5, 10, and 50 ms). We applied the same filtering to the cortical data as above. We extracted a 45 ms window (5 ms before IC stimulation, 40 ms after IC stimulation) to capture the entire antidromic potential. We then calculated the peak-to-peak amplitude of the antidromic potential and averaged it across all stimulation trials.
To calculate the frequency-dependent cortical responses to VLL, we took the bandpass filtered cortical evoked potentials over M1 and extracted 12 ms windows (2 ms before stimulation, 10 ms after stimulation) for each stimulation pulse at a variety of VLL frequencies (VLL stim 10, 50, 80, and 100 Hz). We then calculated the peak-to-peak amplitude of the evoked potential over the time range of 1.5–10 ms after the simulations and averaged it across all stimulation trials.
Analysis of muscle activity and kinematics
Electromyographic activity was bandpass filtered between 30 and 800 Hz with a 3rd-order Butterworth filter. We then computed the stimulation-triggered averages (window from 5 ms to 25 ms after IC stimulation) of motor-evoked potentials and calculated the area under the curve (AUC) for each pulse of IC stimulation and each muscle.
Kinematics were recorded by a GoPro® Camera and analyzed through Deeplabcut92, a neural network for 2D and 3D markerless pose estimation based on transfer learning. A subset of video frames (40–80 frames) per condition was selected by Deeplabcut to capture diversity of the movement. Next, we manually labeled the thumb, index finger, wrist, and elbow in each of those frames. With the labeled frames, we used Deeplabcut to create a training network by merging all extracted labeled frames. We then used the trained network to analyze all the videos and extract the kinematic traces for each of the labeled body points. We calculated the peak-to-peak amplitude in pixels of each joint for each pulse of IC stimulation.
Analysis of frequency-dependent modulation of muscle responses
For each animal, muscle, and IC paired VLL stimulation (10, 50, 80, 100, and 200 Hz) protocol we concatenated all MEP responses from each IC stimulation pulse (window from 0 ms to 25 ms after IC stimulation). Importantly, stimulation amplitude at 200 Hz was reduced to balance the total charge (see Supplementary Table 2 for stimulation parameters). We then used a 5th-order polynomial regression fit model to classify these MEP traces according to four criteria34: 1) “no potentiation”—the responses are consistent with IC alone stimulation, 2) “sustained potentiation”—the responses are increased as compared to IC alone, 3) “attenuation”—the responses decrease through time, 4) “suppression”—the responses are suppressed through time. The categorization was validated by visual inspection from two independent evaluators. For this, the evaluators were blinded to the stimulation conditions and to the muscle names when inspecting and categorizing the MEP traces. For each unique stimulation protocol, we calculated its probability distribution over all muscles and animals.
Analysis of spinal activity
We applied the same filtering and extraction process to the spinal evoked potentials as we did to the cortical evoked potentials (filter 10–5000 Hz 3rd order Butterworth and 25 ms windows). To identify the antidromic response from the VLL stimulation, we calculated the peak-to-peak amplitude and response onset within the first 5 ms following VLL stimulation across all channels of the intraspinal probe. Using the intraspinal probe map we were able to distinguish the ventral, intermediate, and dorsal areas on the probe. In order to compare the responses across animals, we normalized the peak-to-peak amplitude by the maximum value for each animal independently.
When assessing the frequency-dependent effects of VLL stimulation paired with IC stimulation on the spinal responses, we blanked the IC stimulation artifact between 250 us before stimulation and 500 us after the stimulation pulse ended. We then calculated the area under the curve (AUC) of the spinal evoked potentials for each VLL stimulation protocol (10, 50, 80, and 100 Hz). The AUC was calculated from 5 to 10 ms after IC stimulation to exclude any stimulation artifacts and secondary post-synaptic responses. The AUC was compared against the AUC of the spinal responses from IC stimulation alone for potentiation or attenuation.
Human participants
We performed electrophysiological experiments on n = 7 human subjects (3 males and 4 females) of age 69.7 ± 6.8 (mean ± std) who were undergoing DBS implantation of the VIM/VOP nucleus (ventralis intermediate nucleus/ventralis oralis posterior), which corresponds to the VLL nucleus in monkeys29, to treat medically-intractable asymmetric Essential Tremor (ET) symptoms. We tested the least symptomatic arm to better approximate normal function. Additionally, we tested a traumatic brain injury (CST01) patient (male in his 40s) who had suffered traumatic subarachnoid hemorrhaging from a motor vehicle accident. This hemorrhage led to diffuse axonal injury involving the CST and bilateral edema of the cerebral peduncles and pons (Fig. 8a and Supplementary Fig. 10a, more pronounced in the left than the right hemisphere). Consequently, CST01 suffered from hemiparesis of the right side and left upper extremity muscle weakness and tremors with decreased overall strength, balance, coordination, and endurance. He required maximal assistance for ambulating (taking steps, shifting weight) and daily tasks requiring dexterous control (splint/bandage changes, eating, showering, oral hygiene). He was able to sit without support, stand from a sitting position with moderate assistance, and follow oral commands. The patient was recommended for bilateral DBS electrode implant to treat the post-traumatic tremor.
Human intraoperative data acquisition and electrophysiology
Pairs of needle electrodes (Rhythmlink Columbia, SC) were placed subcutaneously over the deltoid (DELT), biceps brachii (BII), triceps brachii (TRI), flexors (flexor carpi ulnaris, FLEX), extensor (extensor carpi radialis longus, EXT), and abductor pollicis brevis (APB) muscles to record MEPs. Prior to DBS insertion, a subdural strip electrode (6 contact platinum subdural electrode, AD-TECH Medical Instrument Corporation, Oak Creek, WI) was implanted over the cortical surface with verification of positioning provided using median nerve somatosensory evoked potential (SSEP) phase reversal (PR) mapping to locate the hand representation of S1 cortex and the approximate location of the central sulcus41. Specifically, we applied electrical stimulation of the median nerve with surface electrodes (35 mA intensity, 0.3 ms duration, and 2.33 Hz pulse rate). We recorded SSEP from the subdural strip electrode with a 10 ms/division timebase, 10 Hz–1 kHz timebase, 2.5 mV range, and 2 mV reject threshold. Nerve stimulation results in direct depolarization of S1 that creates a synchronized dipole with the negative pole (N20 component) recorded in and posterior to S1 while the positive pole (P20 component) is recorded anterior to the central sulcus (i.e., in M1) where the phase reversal (sink/source relationship) is located (isopotential line). This is caused by a field effect in M1 due to direct depolarization in S1. The subdural strip electrode position was optimized using results from direct cortical stimulation (DCS) MEPs to contralateral upper extremity muscles. We located the contact over the precentral gyrus that generated the largest amplitude MEP in the hand muscle (APB). DCS of the hand representation of M1 was provided using trains of 5 stimulation pulses (0.5 ms) at 400 Hz every two seconds at stimulus intensities up to 15 mA using an intraoperative neurophysiological monitoring system (XLTEK Protektor, Natus Medical, Ontario, Canada). Detailed information on the stimulation parameters used for DCS for each subject is reported in Supplementary Table 4.
For S01–S04, after implantation of the subdural strip electrode, the surgery continued following standard clinical procedures, and DBS electrodes were implanted in the VIM/VOP nucleus bilaterally using subcortical mapping techniques. The electrophysiology experiments were performed following completion of the clinical procedure, with the subject maintained under sedation with propofol. Specifically, DCS MEPs from stimulation of the optimal electrode contact over the hand representation of the primary motor cortex were recorded without and with continuous stimulation of the VIM/VOP nucleus (DBS contacts −1 + 8) at 50, 80, and 100 Hz with pulses of 100us and intensity of 3 mA. MEPs were collected for 100 ms trials following each DCS stimulation burst and recorded using the XLTEK system at a sampling frequency of 6000 Hz. Stimulation of the VIM/VOP was delivered via the Boston Scientific clinician programmer that connects via Bluetooth to an external trial stimulator. The same DBS electrode was used to generate cortical evoked potentials in the subdural strip electrode that were recorded using the Ripple Neuro Grapevine and Trellis software at a sampling frequency of 30,000 Hz.
For S05–S07, instead of stimulating from the clinical DBS electrode once the latter was implanted within the VIM/VOP, we stimulated from the macro contacts (Neuro Omega, Alpha Omega Engineering, Israel) of the microelectrodes that are used clinically to map the motor thalamus and identify the final position of the DBS electrode. Specifically, as part of the standard of care in our institution, DBS implantations are performed using stereotactic techniques, i.e., using the ROSA, Zimmer-Biomet robot that we also used in non-human primates. During the surgery, location of the VIM/VOP for the clinical DBS implantation is confirmed based on electrophysiological microelectrode recordings (MER). Indeed, three microelectrodes are inserted to record neuronal activity and identify the optimal VIM/VOP nucleus, which is usually identified as the target with a strong neuronal response when upper limbs are moved passively to identify “kinesthetic”, “motor” or “somatosensory” functional neurons. Stimulation of the electrodes’ macro contact is, then, used to assess for optimal beneficial clinical effects as well as to identify adverse side effects. In this study, after identification of the VIM/VOP nucleus, we recorded DCS MEPs resulting from stimulation of the optimal subdural electrode contact over the hand representation of the primary motor cortex. The MEPs were recorded without and with continuous stimulation, either from the macro contact of the microelectrode within the VIM/VOP nucleus or with stimulation from the macro contact of electrodes with trajectories that were either more anterior or more posterior to the VIM/VOP trajectory. In all cases, stimulation was performed from the macro contact at 4 mA and 50 Hz. MEPs were collected for 100 ms trials following each DCS stimulation burst and recorded using the XLTEK system at a sampling frequency of 6000 Hz.
Behavioral experimental recordings and analysis
We performed three different motor tasks evaluating strength, voluntary force control, and object manipulation.
Strength
In the first task, CST01 was tasked to grasp a force dynamometer (Pinch/Grip Digital Myometer, MIE Medical Research, Yorkshire, UK), which recorded the force in newtons at a sampling rate of 30,000 Hz, and to produce maximal voluntary force for 5 times (day 1) and 3 times (session 2) consecutively with stimulation ON and OFF. We calculated the average maximum force over the repetitions and compared it between stimulation ON and OFF. We then tasked CST01 to raise his arm above his head without and while lifting different weights (1.4—lightweight—and 3.6 kg—heavyweight—respectively) 10 times. We then calculated shoulder abduction and elbow extension, and arm elevation for each repetition and compared these metrics between stimulation ON and OFF.
Force control
We then measured the level of voluntary force control with the same force dynamometer used for the maximal voluntary force task. For this, the participant was asked to modulate their force between two limits (55–70% of maximum voluntary contraction measured with stimulation OFF) while receiving visual feedback of the target force and the applied force on the screen. Importantly, we fixed the 55–70% interval to the maximum voluntary force recorded when the stimulation was OFF. This was done in order to avoid confounding factors in the results. Indeed, if the stimulation would increase maximum voluntary force, the increase in tracking ability could be explained by the fact that the patient is stronger with stimulation ON and not by the fact that the force control is, indeed, improved. The visual feedback of the target force curve consisted of a white circle that changed in size in relation to the expected target force. The patient had to keep the force within ±5 % of the expected target force (visually displayed as dashed boundaries around the white circle). A second circle represented the force exerted by the patient and it would be green if the force was within the ±5% or red if outside this boundary. The target force curve started at the high force level (70% of MVC) followed by a linear decrease for 2 s, followed by a hold phase at the low force level (55% of MVC) for 4 s, followed by a linear increase for 2 s, and finally a hold phase at the high force level for 4 s. This cycle was repeated 4 times. We calculated the root-mean-squared error (RMSE) between the applied force and the expected force at each time point. We also calculated the frequency power of the force trace to demonstrate that the difference in RMSE was not due to a reduction/increase in tremor with VIM/VOP stimulation ON. For this, we filtered with a band-pass 2nd order Butterworth filter (cut-off frequencies of 2–5000 Hz) and performed a fast Fourier transform of the hold portion of the force task. We then compared the average power from 6 to 12 Hz (frequency range typical of tremor)42 during VIM/VOP stimulation on and off.
Object manipulation
We then asked the participant to perform an object manipulation of daily living. For this, we placed a drinking cup on a table on the side of the participant. The subject was asked to grasp the cup from the side, requiring him to supinate his forearm, lift the cup, bring it to the mouth, and place it back on the table. This task evaluated his ability to reach, grasp, lift, and release a moderately heavy object. The subject was not allowed to use the other arm to assist in grasping the object.
Analysis of intraoperative cortical and muscle activity
Electrocorticographic data were filtered with a band-pass 2nd order Butterworth filter (cut-off frequencies 10–500 Hz) and a 60 Hz notch 2nd order Butterworth filter. The stimulation artifact was blanked. We extracted epochs of 30 ms (10 ms before and 20 ms after the stimulus onset) and computed stimulation-triggered averages. We calculated the peak-to-peak amplitude of each cortical evoked potential as the difference between the maximum and minimum voltage value in the interval 3-15 ms following the stimulus onset.
To quantify DCS MEPs recorded from triggered electromyographic activity, we computed the stimulation-triggered averages (EMG from 10 ms to 75 ms after stimulation) and calculated the AUC for each muscle.
Human volume of activation, HDFT, and lesion segmentation
Volume of activation
After DBS implantation of the VIM/VOP DBS electrode, patients underwent post-implant CT imaging (1.25 mm isotropic, GE Medical Systems) to reconstruct the DBS leads. For S01-S04, with the reconstructed DBS leads, we modeled the volume of tissue activation (VTA) in each patient using the DBS analysis module inside BrainLab Quentry®. Specifically, we visualized a 3D reconstruction of VIM, DBS electrode, and the simulated VTAs from the anode and cathode of the DBS leads. We then aggregated the VTA from the DBS patients (excluding CST01) and overlaid them on axial and coronal T2-weighted MRI images (1.6 mm isotropic, TR/TE = 9690/91 ms, 3 T Siemens Prisma Fit).
For S05–S07, we performed post-implant localization of the microelectrode trajectories. We first reconstructed the DBS leads using the post-implant CT imaging. We then co-registered MRI scans to localize the other microelectrode trajectories based on their position with respect to the VIM/VOP trajectory, i.e., 2 mm anterior or 2 mm posterior to the VIM/VOP trajectory along the AC-PC plane. Finally, we used the BrainLab atlas to identify the VIM nucleus in the MRI and a board-certified neurosurgeon (Dr. Gonzalez-Martinez) manually drew the VOP and VOA nuclei located respectively 2 mm and 4 mm anteriorly to the VIM on the AC-PC plane.
HDFT
The diffusion images were acquired on a SIEMENS Prisma Fit scanner using a diffusion sequence (2 mm isotropic resolution, TE/TR = 99.2 ms/2490 ms, 257 diffusion sampling with maximum b-value 4010 s/mm²). The accuracy of b-table orientation was examined by comparing fiber orientations with those of a population-averaged template84. The tensor metrics were calculated using DWI with b-value lower than 1750 s/mm².
To quantify the projections from the area of stimulation within the VIM/VOP DBS electrode, we manually drew a region at the stimulation contact and considered it as the seed region. S1, M1, PMd, and SMA were the regions of interest. For fiber tracking, we used a tracking threshold of 0, angular threshold of 50, and a step size of 1 mm. Tracks with lengths shorter than 30 mm or longer than 500 mm were discarded. A total of 10,000 tracks were placed. Topology-informed pruning was applied to the tractography with 2 interactions to remove false connections. We then calculated the volume of the tracts between the seed region and each region of interest normalized by the total volume of tracts from the area of stimulation to all cortical areas.
To quantify the white-matter fibers damage in CST01, we used an advanced diffusion MRI technique commonly used in patients with bilateral white-matter damage that allows for quantification of a decrease in anisotropic diffusion by applying a “tracking-the-difference” paradigm when compared to a normal population template: differential tractography93. Differential tractography was performed by comparing the HDFT of CST01 against an age-and-sex-matched template constructed from a group of healthy controls (approximately 40 years old and male) of healthy quantitative anisotropy (QA). To capture neural injury, we highlighted tracks within CST01 that displayed a 20% decrease in QA when compared to the template of healthy controls. We then quantified the composition of lesioned tracts based on a population-averaged atlas of human white matter tracts84 for the main fiber tracts: corticospinal tract, corticobulbar tractdentatorubrothalamic tract, and medial lemniscus.
Lesion segmentation
In CST01, we highlighted areas of lesion. Lesion segmentation was performed by board-certified neurosurgeon (Dr. Jorge Gonzalez-Martinez) from axial T2-flair images acquired by a 3 T Siemens Prisma Fit Scanner (1.6 mm isotropic, TR/TE = 9690/91 ms)s
Statistical procedures
All statistical comparisons of means presented in this manuscript were performed using the bootstrap method, a non-parametric approach which makes no distributional assumptions on the observed data. Instead, bootstrapping uses resampling to construct empirical confidence intervals for quantities of interest. For each comparison, we construct bootstrap samples by drawing a sample with replacement from observed measurements, while preserving the number of measurements in each condition. We construct 10,000 bootstrap samples and, for each, calculate the difference in means of the resampled data. We employed two-tailed bootstrapping with alphas of 0.05 (95% confidence interval), 0.01 (99% confidence interval), or 0.001 (99.9% confidence interval). The null hypothesis of no difference in the mean was rejected if 0 was not included in the confidence interval of the corresponding alpha value. If more than one comparison was being performed at once, we used a Bonferroni correction by dividing the alpha value by the number of pairwise comparisons being performed.
Data analysis software
Data analysis of all kinematics, electromyography, neural data, and functional performance was performed using custom analysis code written in MATLAB R2020, R2021, and R2022. Data from intra-operative electrophysiology were extracted manually from propriety data files using the EPWorks v6.1.2.38 software (Natus). High-definition fiber tracking was performed using DSI-Studio (http://dsi-studio.labsolver.org). The volume of tissue activation was calculated using the DBS analysis module inside BrainLab Quentry. All figures were rendered in Adobe Illustrator CC v26.0—v28.1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper. Preprocessed data can be accessed from the Zenodo repository: https://zenodo.org/records/13208032. Due to the sensitive nature of the dataset, which contains graphic information on monkeys and human patients, raw data, including videos from monkeys are available under restricted access and access can be obtained upon request to the corresponding author (Dr. Elvira Pirondini: elvirap@pitt.edu). Data access will be provided within a month from the request and for up to 6 months once access has been granted. Instead, raw data from human patients are protected and are not available due to data privacy laws. Source data are provided with this paper.
Code availability
Software routines utilized for data analysis are publicly accessible on GitHub: https://github.com/emg27/DBSMotorThalamusPaper_2024/tree/main.
References
Lemon, R. N. Descending pathways in motor control. Annu. Rev. Neurosci. 31, 195–218 (2008).
Skolarus, L., Meurer, W., Burke, J., Bettger, J. P. & Lisabeth, L. Effect of insurance status on postacute care among working age stroke survivors. Neurology 78, 1590–1595 (2012).
Zaloshnja, E., Miller, T., Langlois, J. A. & Selassie, A. W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head. Trauma Rehabil. 23, 394–400 (2008).
Clayton, E., Kinley-Cooper, S., Weber, R. & Adkins, D. Brain stimulation: neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res. 1640, 130–138 (2016).
Simonetta-Moreau, M. Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann. Phys. Rehabil. Med. 57, 530–542 (2014).
Ashkan, K., Rogers, P., Bergman, H. & Ughratdar, I. Insights into the mechanisms of deep brain stimulation. Nat. Rev. Neurol. 13, 548–554 (2017).
Kuriakose, R. et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson’s disease. Cereb. Cortex 20, 1926–1936 (2010).
Molnar, G. et al. Changes in cortical excitability with thalamic deep brain stimulation. Neurology 64, 1913–1919 (2005).
Ni, Z., Udupa, K., Hallett, M. & Chen, R. Effects of deep brain stimulation on the primary motor cortex: insights from transcranial magnetic stimulation studies. Clin. Neurophysiol. 130, 558–567 (2019).
Ni, Z. et al. Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann. Neurol. 83, 352–362 (2018).
Dale, J., Schmidt, S. L., Mitchell, K., Turner, D. A. & Grill, W. M. Evoked potentials generated by deep brain stimulation for Parkinson’s disease. Brain Stimul. 15, 1040–1047 (2022).
Park, H.-J. et al. Modulation of cortical motor evoked potential after stroke during electrical stimulation of the lateral cerebellar nucleus. Brain Stimul. 8, 1043–1048 (2015).
Hooks, B. M. et al. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J. Neurosci. 33, 748–760 (2013).
Rouiller, E. M., Tanne, J., Moret, V. & Boussaoud, D. Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. J. Comp. Neurol. 409, 131–152 (1999).
Holsapple, J. W., Preston, J. B. & Strick, P. L. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J. Neurosci. 11, 2644–2654 (1991).
Strick, P. L. Multiple sources of thalamic input to the primate motor cortex. Brain Res. 88, 372–377 (1975).
Cooper, I., Upton, A. & Amin, I. Reversibility of chronic neurologic deficits. Some effects of electrical stimulation of the thalamus and internal capsule in man. Stereotact. Funct. Neurosurg. 43, 244–258 (1980).
Phillips, N. & Bhakta, B. Affect of deep brain stimulation on limb paresis after stroke. Lancet 356, 222–223 (2000).
Franzini, A., Cordella, R., Nazzi, V. & Broggi, G. Long-term chronic stimulation of internal capsule in poststroke pain and Spasticity. Stereotact. Funct. Neurosurg. 86, 179–183 (2008).
Elias, G. J., Namasivayam, A. A. & Lozano, A. M. Deep brain stimulation for stroke: Current uses and future directions. Brain Stimul. 11, 3–28 (2018).
Goldberg, M. E. The neurology clinic needs monkey research. Proc. Natl Acad. Sci. 116, 26255–26258 (2019).
Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 (2007).
Paek, S. B. et al. Frequency-dependent functional neuromodulatory effects on the motor network by ventral lateral thalamic deep brain stimulation in swine. Neuroimage 105, 181–188 (2015).
Hassler, R. Anatomy of the thalamus. Arch. fur Psychiatr. und Nervenkrankheiten, Ver. mit. Z. fur die Gesamt. Neurologie und Psychiatr. 184, 249–256 (1950).
Shaltenbrand, G. Introduction to stereotaxis with an atlas of the human brain. (Stuttgart, 1959).
Nambu, A., Yoshida, S. & Jinnai, K. Projection on the motor cortex of thalamic neurons with pallidal input in the monkey. Exp. Brain Res. 71, 658–662 (1988).
Krack, P. et al. Surgery of the motor thalamus: problems with the present nomenclatures. Mov. Disord. Off. J. Mov. Disord. Soc. 17, S2–S8 (2002).
Fang, P.-C., Stepniewska, I. & Kaas, J. H. The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti). Neuroscience 143, 987–1020 (2006).
Ilinsky, I. A. & Kultas‐Ilinsky, K. Motor thalamic circuits in primates with emphasis on the area targeted in treatment of movement disorders. Mov. Disord. Off. J. Mov. Disord. Soc. 17, S9–S14 (2002).
Ma, F.-Z. et al. Application of the robot-assisted implantation in deep brain stimulation. Front. Neurorobotics 16, 996685 (2022).
Salt, T., Meier, C., Seno, N., Krucker, T. & Herrling, P. Thalamocortical and corticocortical excitatory postsynaptic potentials mediated by excitatory amino acid receptors in the cat motor cortexin vivo. Neuroscience 64, 433–442 (1995).
Liew, Y. J. et al. Inferring thalamocortical monosynaptic connectivity in vivo. J. Neurophysiol. 125, 2408–2431 (2021).
Kuramoto, E. et al. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex 19, 2065–2077 (2009).
Greiner, N. et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat. Commun. 12, 435 (2021).
Metz, K. et al. Facilitation of sensory transmission to motoneurons during cortical or sensory‐evoked primary afferent depolarization (PAD) in humans. J. Physiol. 601, 1897–1924 (2023).
Metz, K. et al. Post‐activation depression from primary afferent depolarization (PAD) produces extensor H‐reflex suppression following flexor afferent conditioning. J. Physiol. 601, 1925–1956 (2023).
Rudomin, P. & Schmidt, R. F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 129, 1–37 (1999).
Sharma, M. et al. Novel Evoked Synaptic Activity Potentials (ESAPs) Elicited by Spinal Cord Stimulation. eNeuro 10, ENEURO.0429-22.2023 (2023).
Volkmann, J., Herzog, J., Kopper, F. & Deuschl, G. Introduction to the programming of deep brain stimulators. Mov. Disord. Off. J. Mov. Disord. Soc. 17, S181–S187 (2002).
Vinas, F. et al. In vivo and in vitro study of the lesions produced with a computerized radiofrequency system. Stereotact. Funct. Neurosurg. 58, 121–133 (1992).
Cedzich, C., Taniguchi, M., Schäfer, S. & Schramm, J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery 38, 962–970 (1996).
Agarwal, S. & Biagioni, M. C. Essential Tremor. In StatPearls. (StatPearls Publishing, Treasure Island (FL), 2023).
McIntyre, C. C. & Grill, W. M. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 88, 1592–1604 (2002).
Formento, E. et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 21, 1728 (2018).
Sinopoulou, E. et al. Rhesus macaque versus rat divergence in the corticospinal projectome. Neuron 110, 2970–2983 (2022).
Pirondini, E. et al. Poststroke arm and hand paresis: should we target the cervical spinal cord? Trends Neurosci. 45, 568–578 (2022).
Hummel, F. C. & Cohen, L. G. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 5, 708–712 (2006).
Hummel, F. C. et al. Controversy: Noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 1, 370–382 (2008).
Coscia, M. et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain: a J. Neurol. 142, 2182–2197 (2019).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527, 633 (2000).
Triccas, L. T. et al. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin. Neurophysiol. 127, 946–955 (2016).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206 (2014).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92 (2017).
Bahr-Hosseini, M. et al. Transcranial electrical stimulation in stroke EaRly after onset clinical trial (TESSERACT). Brain Stimul. 16, 236 (2023).
Demirtas-Tatlidede, A., Vahabzadeh-Hagh, A. M., Bernabeu, M., Tormos, J. M. & Pascual-Leone, A. Noninvasive brain stimulation in traumatic brain injury. J. Head. Trauma Rehabil. 27, 274 (2012).
Kuo, I.-J. et al. Neurophysiological signatures of hand motor response to dual-transcranial direct current stimulation in subacute stroke: a TMS and MEG study. J. Neuroeng. Rehabil. 17, 1–12 (2020).
Hsu, S.-P. et al. Effects of bihemispheric transcranial direct current stimulation on motor recovery in subacute stroke patients: a double-blind, randomized sham-controlled trial. J. Neuroeng. Rehabil. 20, 27 (2023).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021).
Fenoy, A. J. & Simpson, R. K. Risks of common complications in deep brain stimulation surgery: management and avoidance. J. Neurosurg. 120, 132–139 (2014).
Sharma, D. & Vavilala, M. S. Perioperative management of adult traumatic brain injury. Anesthesiol. Clin. 30, 333–346 (2012).
Mehdi, Z., Birns, J., Partridge, J., Bhalla, A. & Dhesi, J. Perioperative management of adult patients with a history of stroke or transient ischaemic attack undergoing elective non-cardiac surgery. Clin. Med. 16, 535 (2016).
Baker, K. B. et al. Cerebellar deep brain stimulation for chronic post-stroke motor rehabilitation: a phase I trial. Nat. Med. 29, 2366–2374 (2023).
Schiff, N. D. et al. Thalamic deep brain stimulation in traumatic brain injury: a phase 1, randomized feasibility study. Nat. Med. 29, 3162–3174 (2023).
Cooperrider, J. et al. Chronic deep cerebellar stimulation promotes long-term potentiation, microstructural plasticity, and reorganization of perilesional cortical representation in a rodent model. J. Neurosci. 34, 9040–9050 (2014).
Machado, A. G., Baker, K. B., Schuster, D., Butler, R. S. & Rezai, A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 1280, 107–116 (2009).
Machado, A. G. et al. Chronic 30-Hz deep cerebellar stimulation coupled with training enhances post-ischemia motor recovery and peri-infarct synaptophysin expression in rodents. Neurosurgery 73, 344–353 (2013).
Ramanathan, D. S. et al. Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nat. Med. 24, 1257–1267 (2018).
Khanna, P. et al. Low-frequency stimulation enhances ensemble co-firing and dexterity after stroke. Cell 184, 912–930 (2021).
Violante, I. R. et al. Non-invasive temporal interference electrical stimulation of the human hippocampus. Nat. Neurosci. 26, 1994–2004 (2023).
Wessel, M. J. et al. Noninvasive theta-burst stimulation of the human striatum enhances striatal activity and motor skill learning. Nat. Neurosci. 26, 2005–2016 (2023).
Krishna, V., Sammartino, F. & Rezai, A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA Neurol. 75, 246–254 (2018).
Côté, S. L., Hamadjida, A., Quessy, S. & Dancause, N. Contrasting modulatory effects from the dorsal and ventral premotor cortex on primary motor cortex outputs. J. Neurosci. 37, 5960–5973 (2017).
Nicoletti, V. et al. Cerebello-thalamo-cortical network is intrinsically altered in essential tremor: evidence from a resting state functional MRI study. Sci. Rep. 10, 16661 (2020).
Chuang, W., Huang, Y., Lu, C. & Chen, R. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov. Disord. 29, 501–507 (2014).
Buijink, A. W. et al. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 138, 2934–2947 (2015).
Powell, M. P. et al. Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Nat. Med. 29, 689–699 (2023).
Barra, B. et al. Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys. Nat. Neurosci. 25, 924–934 (2022).
Balaguer, J.-M. et al. Supraspinal control of motoneurons after paralysis enabled by spinal cord stimulation. Medrxiv (2023).
Toossi, A. et al. Effect of anesthesia on motor responses evoked by spinal neural prostheses during intraoperative procedures. J. Neural Eng. 16, 036003 (2019).
Ho, J. et al. Robot assisted neurosurgery for high-accuracy, minimally-invasive deep brain electrophysiology in monkeys. in Proc. IEEE Proceedings IEEE Engineering in Medicine & Biology Society (EMBC) (IEEE, 2022).
Liang, L. et al. An open-source MRI compatible frame for multimodal presurgical mapping in macaque and capuchin monkeys. J. Neurosci. Methods 407, 110133 (2024).
Liu, C. et al. A resource for the detailed 3D mapping of white matter pathways in the marmoset brain. Nat. Neurosci. 23, 271–280 (2020).
Yeh, F. C. et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 178, 57–68 (2018).
Yeh, F., Liu, L., Hitchens, T. K. & Wu, Y. L. Mapping immune cell infiltration using restricted diffusion MRI. Magn. Reson. Med. 77, 603–612 (2017).
Yeh, F.-C., Wedeen, V. J. & Tseng, W.-Y. I. Generalized ${q} $-sampling imaging. IEEE Trans. Med. imaging 29, 1626–1635 (2010).
Yeh, F.-C., Verstynen, T. D., Wang, Y., Fernández-Miranda, J. C. & Tseng, W.-Y. I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS one 8, e80713 (2013).
Ghulam‐Jelani, Z. et al. Redundancy circuits of the commissural pathways in human and rhesus macaque brains. Hum. Brain Mapp. 42, 2250–2261 (2021).
Yeh, F.-C. et al. Automatic removal of false connections in diffusion MRI tractography using topology-informed pruning (TIP). Neurotherapeutics 16, 52–58 (2019).
Calabrese, E. et al. A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. Neuroimage 117, 408–416 (2015).
Zhang, H. et al. Diffusion spectrum imaging of corticospinal tracts in idiopathic normal pressure hydrocephalus. Front. Neurol. 12, 636518 (2021).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Yeh, F. C. et al. Differential tractography as a track-based biomarker for neuronal injury. NeuroImage 202, 116131 (2019).
Acknowledgements
We thank Isabella Bushko for the design of fig. elements, Drs. Peter Strick and Aaron Batista for support with monkey housing, Dr. Frank Yeh for providing support with the differential tractography analysis, Dr. Amr Mahrous for providing support during the monkey surgeries, and Christopher Pappas for helping with the VIM/VOP stimulation during the human surgeries. We wish to thank Zimmer Biomet for lending the ROSA robot to use in our surgeries (we declare no conflict of interest). We would like to thank Merek Gourley for providing post-implant reconstructions of the DBS patients. JMB was supported by a scholarship from the Dompé Foundation. The study was executed through the support of internal funding from the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh to E.P. and from the Department of Neurological Surgery at the University of Pittsburgh to M.C. and J.G.M. Additionally, E.P. and M.C. disclose support for the research described in this study from the Walter L. Copeland Foundation and J.G.M. disclose support for the research described in this study from the Hamot Health foundation. Finally, this work was supported by the National Institutes of Health (R01NS131428 to E.P. and J.G.M. and R01NS122927 to J.G.M.).
Author information
Authors and Affiliations
Contributions
E.P. and J.G.M. conceived the study. E.P., J.G.M., and M.C. secured funding. J.H., E.G., L.T., D.C., M.C., J.G.M., and E.P. designed the experiments. J.H., E.G., J.B., L.L., M.C., and E.P. designed and implemented hardware and software for the monkey experiments. J.H. and L.L. performed the image processing for all the monkeys experiments. T.K.H. collected the monkeys imaging data. J.H., L.L., and J.B.M. processed the DTI data for both the humans and the monkeys. J.H. and S.K. processed the deep lab cut data for the kinematic analysis of the monkeys. J.H., L.L., J.G.M., and E.P. designed the neurosurgical approach for the brain implants and J.G.M. performed the surgery. J.H., L.L., J.B., P.G., and M.C. designed the neurosurgical approach for the spinal implants and P.G. performed the surgery. V.K. and D.F. assisted with all monkeys surgeries. T.C. and J.G.M. implemented patient recruitment, eligibility, and monitoring and coordinated study management. J.H., E.G., A.D., and E.P. collected all human and monkey data with the assistance of L.T. for the human data and of J.B., L.L., and M.C. for the monkeys experiments. D.C. performed the subcortical mapping of VIM/VOP for the human DBS implantations. G.M.A. and D.C. collected the electrophysiology human data. J.H., E.G., and A.D. analyzed the data. E.P., J.G.M., M.C., and D.C. helped with data interpretation. E.P., J.G.M., J.H., E.G., and A.D. wrote the paper and all authors contributed to its editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ho, J.C., Grigsby, E.M., Damiani, A. et al. Potentiation of cortico-spinal output via targeted electrical stimulation of the motor thalamus. Nat Commun 15, 8461 (2024). https://doi.org/10.1038/s41467-024-52477-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52477-1