Abstract
Younger premenopausal women are more prone to developing ovarian metastases (OM) of gastric cancer (GC) than metastases of other organs; however, the molecular mechanisms remain unclear. Here we perform single-cell RNA sequencing on 45 tumor samples from 18 GC patients with OM. Interestingly, fibroblasts in OM of GC express high levels of estrogen receptor (ER) and midkine (MDK), interacting with tumor cells through activating ER-MDK-LRP1 (low-density lipoprotein receptor-related protein 1) signaling axis. Functional experiments demonstrate that estrogen stimulation induces MDK secretion by ovarian fibroblasts, and binding of MDK to LRP1 increases GC cell migration and invasion. Furthermore, in vivo, estrogen stimulation remarkably augments ovarian engraftment and metastasis of LRP1+ GC cells. Collectively, our findings reveal that ER+ ovarian fibroblasts secrete MDK under estrogen influence, driving OM of GC via the MDK-LRP1 axis. Our study holds the potential to catalyze innovative therapeutic strategies aimed at intercepting and managing OM in GC.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is a prevalent malignancy worldwide and poses a significant threat to human health. Distant metastasis is a major factor contributing to the poor prognosis of GC1. Ovarian metastases (OM), observed in approximately 5–10% of female GC patients, are particularly challenging and associated with a survival of only 7–14 months2,3. GC with OM exhibits distinct characteristics, including aggressive biological behavior and high invasiveness. Notably, OM often continues to progress despite control of the primary tumor.
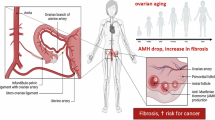
OM of GC can form through multiple mechanisms, including lymph node metastasis, hematogenous metastasis, and seeding metastasis4. Extensive research suggests that ovarian reticular lymphatic tissue is highly vascularized, which provides a potential route for the migration of cancer cells to the pelvic lymph nodes via retroperitoneal lymphatic pathways, facilitating subsequent metastasis to the ovaries5. Hematogenous metastasis is gaining increasing recognition as a significant mechanism of OM formation. The active premenopausal ovaries, characterized by high hormone levels and an abundant blood supply, provide an optimal environment for the growth of metastatic tumors. In addition, following the invasion of serous membranes, gastric cancer cells can enter the abdominal cavity through the split holes formed during ovulation, resulting in seeding metastasis. This mode of metastasis often causes wide dissemination across the peritoneum and is associated with an unfavorable prognosis. The majority of patients develop OM through seeding metastasis, with more than 70% of cases exhibiting a combined peritoneal seeding spread6,7. In particular, young premenopausal patients are more prone to developing OM, suggesting a potential role for age or hormones in the promotion of GC cell targeting to the ovaries, which exhibit elevated estrogen and progesterone levels4; however, the molecular mechanisms underlying OM of GC remain poorly understood, necessitating further investigation.
In this work, we perform single-cell transcriptome sequencing (scRNA-seq), single-cell T-cell receptor (TCR) sequencing (scTCR-seq), and whole exome sequencing (WES) to generate a single-cell atlas of OM in GC. We aim to unravel the molecular features associated with OM in GC, and our results shed light on the reasons why younger premenopausal women are more likely to develop OM of GC than metastases of other organs.
Results
Younger premenopausal women are more prone to develop ovarian metastases
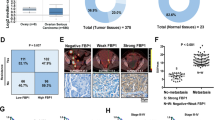
Firstly, we studied the age dependence of OM in GC by evaluating an independent cohort of 1027 patients with metastatic GC from Zhejiang Cancer Hospital (Supplementary Data 1). We found that younger women were more likely to develop OM, among which the median age of patients with only ovarian metastases was 48 (n = 100), the median age of patients with ovarian and other metastases was 47 (n = 207), and the median age of patients with only other metastases was 57 (n = 720) (Fig. 1a). The median age of patients with only ovarian metastases and that of patients with both ovarian and other metastases were similar but significantly lower than that of patients with only other metastases (P < 0.0001). We performed univariate and multivariate logistic regression analysis of the risk factors for OM, among which the variables included age, menstrual state, diabetes, smoking, and alcohol (Fig. 1b). Univariate analysis identified age ≤55 years and premenopausal state as significant risk factors for the development of OM (P < 0.001). Multivariate analysis confirmed that the premenopausal state was the significant OM factor (odd ratio (OR): 7.97, P < 0.001), and the OR of age ≤55 years was 1.01, indicating that younger premenopausal women are more prone to develop OM of GC.
a Violin and box plots showing the median age of patients with only OM (n = 100 patients), patients with ovarian and other metastases (n = 207 patients), and patients with only other metastases (n = 720 patients). The center line of the box represents the median value, the upper and lower limits of the box represent the 25th and 75th percentile points. b Univariate and multivariate logistic regression analysis of the risk factors for the development of OM. Wald test. c Pie chart showing the percentage and median age of premenopausal and postmenopausal patients in our scRNA-seq cohort. d Overview of the workflow and experimental design for scRNA-seq and WES. Mouse model diagram created in BioRender. Hu, C. (2021) BioRender.com/z48q170 released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. e UMAP plot showing the clustering of eight major cell types among 227,836 high-quality single cells. The colors represent the major cell types. f Dot plot showing the highly expressed marker genes in each major cell type. The dot size represents the percentage of cells expressing the marker genes in each major cell type, and the dot color represents the average expression level of the marker genes in each cell type. g Stacked histogram showing the relative percentages of major cell types in each sample. h Bar graph showing the percentage of T cells, myeloid cells, plasma cells, B cells, and mast cells among the total immune cells in different sample types. Each point indicates each sample (n = 50 samples from 22 patients). i Bar graphs showing the percentage of stromal cells, epithelial cells, and endothelial cells among the total non-immune cells in different sample types. Each point indicates each sample (n = 50 samples from 22 patients). In a, h, i, Wilcoxon rank sum test followed by Bonferroni’s multiple-comparison test. In h and i, filtering out non-significant values when displaying significance levels. Source data are provided as a Source Data file.
The single-cell transcriptomic landscape of ovarian metastases of gastric cancer
To investigate the reasons why younger premenopausal women are more likely to develop OM of GC, we created a transcriptomic atlas of 18 GC patients with OM using single-cell RNA sequencing (scRNA-seq) with a view to delineating the related molecular and cellular characteristics. Further clinical information regarding the cohort is provided in Supplementary Data 2. In our single-cell cohort, 12 patients (66.7%) were premenopausal, with a median age of 43.5 years, and only six patients (33.3%) were postmenopausal, with a median age of 65.5 years (Fig. 1c). Among them, 17 patients exhibited both OM and PM, while one patient presented with OM only. Based on Lauren’s classification, 13 primary gastric tumors were classified as diffuse type while five were classified as mixed type. A previous study has precluded the histologic type from a major contributor to OM of GC4. We collected 17 primary gastric tumor samples (PT), 15 OM (14 of which were paired with primaries), five peritoneal metastases (PM), and eight ascites samples (AS) from 18 GC patients with OM (Fig. 1d). Moreover, five adjacent normal (AD) gastric tissue samples were included as controls. A single-cell 5′ reagent kit was coupled with single-cell T-cell receptor (TCR) sequencing (scTCR-seq) to measure transcriptomes using droplet-based scRNA-seq (Supplementary Data 3). Whole exome sequencing (WES) was also performed on 16 samples from five patients.
Following removal of low-quality cells (see Methods), we obtained a total of 227,836 high-quality cells for subsequent analyses, with an average of 4557 cells and 1475 median genes per sample (Supplementary Fig. 1a). After data preprocessing and principal component analysis (PCA) (see Methods), we used graph-based uniform manifold approximation and projection (UMAP) to partition these cells into eight clusters. According to canonical marker genes, we identified stromal cells (COL1A1+COL1A2+DCN+), T cells (CD3E+CD3D+CD3G+), myeloid cells (CD14+MS4A7+FCGR3A+), epithelial cells (EPCAM+KRT8+KRT19+), plasma cells (MZB1+IGKC+DERL3+), B cells (CD79A+MS4A1+CD19+), endothelial cells (VWF+PECAM1+ENG+), and mast cells (TPSAB1+CPA3+MS4A2+)8,9 (Fig. 1e, f). We have effectively eliminated batch effects between samples (see Methods) (Supplementary Fig. 1b–d).
We first compared the cell type proportions between premenopausal and postmenopausal OM samples, and found that there was no significant difference between them (Supplementary Fig. 1e, f). Next, we compared the cellular abundances across different tissue types (Fig. 1g; Supplementary Fig. 1g, h). We observed substantial changes in the proportion of myeloid cells over GC progression with a significant increase from AD to PT and with higher levels in OM and AS (Fig. 1h). In contrast, the cellular abundances of B cells and plasma cells showed gradual decreases, and both populations were largely absent in OM tissues. Mast cells were enriched in PT, while less in other tissues. Among non-immune cells, stromal cells showed gradual increases from AD and PT to metastases and remained at high levels in metastases (Fig. 1i). Endothelial cells were largely absent in AS.
Transcriptional alterations from normal epithelium to gastric cancer to ovarian metastases
To classify malignant and non-malignant epithelial cells, we calculated the malignant and non-malignant scores for each epithelial cell based on differentially expressed genes (DEGs) between paired tumor and normal tissue samples from the TCGA stomach adenocarcinoma (STAD) dataset10, and the non-malignant score was then subtracted from the malignant score for each cell (Fig. 2a). Additionally, we used R package infercnv to infer the copy-number variations (CNVs) in each epithelial cell. Malignant cells are defined as higher malignant scores coupled with apparent CNVs (Supplementary Fig. 2a–c). The CNV patterns for malignant epithelial cells were highly consistent with those generated from paired bulk whole exome sequencing. Finally, we identified 29,592 malignant and 8997 non-malignant epithelial cells (Fig. 2b). PT from endoscopically resected lesions contained 50% malignant and 50% non-malignant epithelial cells, whereas OM, PM, and AS from surgically resected lesions contained almost exclusively malignant cells (Fig. 2c; Supplementary Fig. 2d, e). In contrast to non-malignant epithelial cells that were grouped across patients, malignant epithelial cells exhibited a patient-specific and metastases-specific expression pattern (Fig. 2d; Supplementary Fig. 2a). The types of non-malignant epithelial cells were characterized as surface mucous cells (TFF2+GKN1+GKN2+MUC5AC+), chief cells (LIPF+PGC+PGA4+PGA3), secretory cells (PAX8+KRT7+ESR1+OVGP1+), enteroendocrine cells (CHGA+PROX1+), intestinal metaplastic cells (TFF3+CLDN3+ APOA4+ANPEP+), and ciliated cells (FOXJ1+CCDC17+CCDC78 CAPS+) based on the differential expression of genes from each cluster to known marker genes of various cell types11,12 (Supplementary Fig. 2f, g).
a UMAP plot showing the malignant minus non-malignant scores of epithelial cells. b UMAP plot showing the distribution of malignant and non-malignant epithelial cells. Purple indicates malignant epithelium, while green indicates non-malignant epithelium. c Stacked histogram showing the relative percentages of malignant and non-malignant epithelial cells among the total epithelial cells in different sample types. d UMAP plots showing the density of epithelial cells in different sample types. e Scatterplot showing the differentially expressed genes (DEGs) between primary malignant and non-malignant cells. The X-axis indicates the log2 (mean+1) in primary non-malignant cells, while the Y-axis indicates the log2 (mean+1) in primary malignant cells. f Scatterplot showing the DEGs between primary and metastatic malignant cells. The X-axis indicates the log2 (mean+1) in metastatic malignant cells, while the Y-axis indicates the log2 (mean+1) in primary malignant cells. g Volcano plot showing the DEGs between primary and OM malignant cells. h Bar graphs showing the significantly enriched pathways of genes highly expressed in OM malignant cells. Hypergeometric test; P < 0.01. i Heatmap showing hierarchical clustering based on the number of genes shared by two programs derived from NMF analysis. Each dot represents one program from an individual sample. Seven highly correlated meta-programs were identified. *two programs share more than 20 genes. j Violin and box plots showing the scores of seven meta-programs in malignant cells from PT, OM, PM, and AS (n = 29,592 cells). The center line of the box represents the median value, the upper and lower limits of the box represent the 25th and 75th percentile points. In e–g and j, Wilcoxon rank sum test followed by Bonferroni’s multiple-comparison test. In c, i and j, AD adjacent normal gastric tissues, PT primary gastric tumors, OM ovarian metastases, PM peritoneal metastases, AS ascites samples.
Analysis of the differential gene expression between primary malignant and non-malignant epithelial cells was performed (Fig. 2e; Supplementary Data 4). Tight junction proteins (encoded by CLDN3 and CLDN4), REG4, TFF3, KRT7, EGFR, and EPCAM were upregulated in primary malignant cells in comparison with primary non-malignant cells. CLDN3 and CLDN4 are highly expressed in human colorectal cancer, esophageal cancer, ovarian cancer, and other malignant tissues, and overexpression of CLDN3 increases the invasive potential of cancer cells13,14,15,16,17,18. Genes upregulated in primary non-malignant cells included MUC6, PGC, GKN1, and LIPF, which are associated with the secretion of gastric mucus and digestive enzymes. These classic chief cell and mucous cell markers are important for maintaining the normality of gastric glandular epithelium.
Analysis of differential gene expression was also performed to explore the difference in transcriptional alterations between malignant cells in primary tumors and those in metastases (OM, PM, and AS). We found that genes upregulated in malignant cells of metastases included insulin-like growth factors (encoded by IGF2), IGF-binding proteins (encoded by IGFBP2), REG1A, REG3A, and CLDN18, and genes upregulated in primary malignant cells included PGC, GKN1, MUC5AC, and CXCL2/3 (Fig. 2f; Supplementary Data 5). Taken together, these results suggest that the loss of gastric glandular epithelium function and increased expression of tight junction proteins, growth factors, and Reg family genes may be key transcriptional alterations accompanying the transition from normal to malignant to metastases in GC.
Individual comparison between malignant cells in primary tumors and ovarian metastases showed that immune-related genes to antigen presentation (CD74, HLA-DRA, and HLA-DRB5) and chemokines CXCL1/2/3 were upregulated in primary tumors, while TFF2, IGFBP2, MUC5B, and CLDN18 were highly expressed in ovarian metastases. Moreover, these upregulated genes were significantly enriched in responses to the estrogen pathway and positive regulation of the inflammatory response, in addition to the senescence-associated secretory phenotype (SASP) (Fig. 2g, h; Supplementary Data 6).
Subsequently, we performed a non-negative matrix factorization (NMF) analysis to explore the intratumor transcriptional diversity and consistency of malignant cells from PT, OM, PM, and AS. Ten programs from each sample with >100 malignant cells were extracted to generate a total of 340 gene programs, revealing high intratumor transcriptional diversity in each sample. The hierarchical clustering of these programs revealed seven meta-programs, which indicated the collective behaviors of malignant cells across the heterogeneous transcriptional spectrum of all tumors. The seven meta-programs represented distinct functional signatures annotated by shared genes, including cell cycle (TUBA1B, MKI67, and TOP2A), oxidative phosphorylation (oxphos) (NDUFB7, ATP5F1D, and COX5B), pEMT (SLC25A6, TXN, and CFL1), interferon (B2M, IFI6, and HLA-B), glandular (TFF1, S100P, and MUC5AC), RNA splicing (SRRM2, CCNL2, and ERN2), and stress (HSPA1B, JUN, and FOSB)19 (Fig. 2i; Supplementary Data 7). We then determined whether these seven meta-programs differed in their expression levels in different tumors by scoring the top 30 signature genes in each meta-program for each malignant cell (Fig. 2j). Compared to PT, OM exhibited lower scores in stress, RNA splicing, interferon and oxphos, and but higher scores in glandular, pEMT and cell cycle.
The cellular state and function of immune cells
To investigate their cellular state and function, CD4+ T cells were re-clustered into sets of naive CD4+ T cells (CD4-Naive; CCR7+), central memory CD4+ T cells (CD4-TCM; IL7R+), effector memory CD4+ T cells (CD4-TEM; IL7R+GZMK+), follicular helper T (Tfh) cells (CD4-Tfh; IL21+BCL6+CXCR5+), regulatory T cells (Tregs) (CD4-Treg; FOXP3+IL2RA+), and proliferating CD4+ T cells (MKI67+TOP2A+) (Fig. 3a; Supplementary Fig. 3a). According to the TCR information, Tregs and proliferating CD4+ T cells showed a relatively high level of clonal expansion (Fig. 3b). We observed a higher relative percentage of Tregs in PT in comparison with AD tissues, but a significantly decreased proportion in OM (P < 0.01, Fig. 3c; Supplementary Fig. 3b).
a UMAP plot showing the distribution of CD4+ T cell subsets. b Bar graphs showing the percentages of TCR expanded clonotypes in the CD4+ T cell subsets. c Bar graphs showing the percentage of Tregs (CD4-Treg) among the total CD4+ T cells in different sample types (n = 50 samples from 22 patients). d UMAP plot showing the distribution of CD8+ T cell subsets. e Dot plot showing the highly expressed marker genes in each CD8+ T cell subset. The dot size represents the percentage of cells expressing the marker genes in each cell type, and the dot color represents the average expression level of the marker genes in each cell type. f Bar graphs showing the percentages of TCR expanded clonotypes in the CD8+ T cell subsets. g Bar graphs showing the percentages of cytotoxic CD8+ T cells (CD8-CTL), precursor exhausted CD8+ T cells (CD8-PEX), and transitory exhausted CD8+ T cells (CD8-TEX) among the total CD8+ T cells in different sample types (n = 50 samples from 22 patients). h Box plots showing the cytotoxic score and exhausted score in CD8+ T cells from different sample types (n = 22,813 cells). i UMAP plot showing the distribution of myeloid cell subsets. j Box plots showing the M1 scores (left) and M2 scores (right) in myeloid cell subsets (n = 42,618 cells). k Bar graphs showing the percentage of Macro_CCL4 among the total myeloid cells in different sample types (n = 50 samples from 22 patients). In h and j, the center line of the box represents the median value, the upper and lower limits of the box represent the 25th and 75th percentile points. In c, g, h and k, Wilcoxon rank sum test followed by Bonferroni’s multiple-comparison test. In c, g, and k, filtering out non-significant values when displaying significance levels. Source data are provided as a Source Data file. In c, h and k, AD adjacent normal gastric tissues, PT primary gastric tumors, OM ovarian metastases, PM peritoneal metastases, AS ascites samples.
We classified CD8+ T cells into nine subsets, including naive CD8+ T cells (CD8-Naive; CCR7+), memory CD8+ T cells (CD8-Memory; IL7R+ZFP36+), central memory CD8+ T cells (CD8-CM; CD27+IL7R+), effector CD8+ T cells (CD8-Effector; GZMK+NKG7+), cytotoxic CD8+ T cells (CD8_CTL; GNLY+PRF1+GZMB+), stress response CD8+ T cells (CD8-Stress; HSPA1A+), and mucosal-associated invariant T (MAIT) cells (CD8-MAIT; KLRB1+TRAV1-2+) (Fig. 3d, e). We also identified two exhaustion-related CD8+ subsets. The precursor exhausted CD8+ T cells (CD8-PEX) displayed high expression of HLA-DRB5, GZMA, GZMH, and low expression of exhaustion-related markers. The transitory exhausted CD8+ T cells (CD8-TEX) were characterized by the highest expression of exhaustion- and proliferation-related markers. CD8-CTL, with the highest cytotoxic score and clonal expansion, were highly abundant in OM (Fig. 3f, g; Supplementary Fig. 3c, d). In addition, CD8-PEX were rarely seen in AS, while CD8-TEX were most abundant in metastatic tumors (OM, PM, and AS). The cytotoxic score was significantly higher than the exhausted score for CD8+ T cells in OM (Fig. 3h; Supplementary Fig. 3d, e). It has previously been reported that the frequencies of terminal exhausted CD8+ T cells are low in GC20.
Next, we explored the heterogeneity and possible functions of myeloid cells, which were divided into monocytes (S100A9+FCN1+), four macrophage subsets (Macro_CCL4, CCL4+; Macro_LYVE1, LYVE1+; Macro_VCAN, VCAN+; and Macro_GPNMB, GPNMB+), cDC1 (CLEC9A+), cDC2 (CD1C+), cDC_LAMP3 (LAMP3+CCR7+), pDC (JCHAIN+IRF7+), and proliferating myeloid cells (Myeloid_MKI67; MKI67+TOP2A+) (Fig. 3i; Supplementary Fig. 3f, g). Macrophages are broadly defined as M1 (antitumor, pro-inflammatory) and M2 (pro-tumor, anti-inflammatory)21. M1 and M2 signature scores were calculated according to previously reported markers22. We found that a LAMP3+ mature cDC subset (cDC_LAMP3) exhibited the highest M1 signature and checkpoint signature scores, which is consistent with previous findings8 (Fig. 3j; Supplementary Fig. 3h). A macrophage subset (Macro_CCL4) with the highest M2 signature score was highly enriched in OM (Fig. 3k), indicating its possible involvement in the facilitation of tumor colonization. Another macrophage subset (Macro_VCAN) with the highest angiogenesis signature score may be important in the promotion of angiogenesis. In comparison with AD tissues, higher M1 and M2 scores were observed in PT, OM, and PM; while the M2 score was the highest in OM, suggesting the presence of myeloid cells with stronger pro-tumor activity in OM (Supplementary Fig. 3i).
Ovarian fibroblasts express high levels of estrogen receptors
The functions of fibroblasts have been widely described and in particular, cancer-associated fibroblasts (CAFs) have been shown to promote tumor proliferation, invasion, angiogenesis, and metastasis23,24,25. To investigate the function of fibroblasts in metastases of GC, we re-clustered stromal cells into fibroblasts (DCN+COL1A1+COL3A1+COL1A2+), adventitia fibroblasts (C3+FBLN1+), mesothelial cells (KRT8+UPK3B+SLPI+), pericytes (RGS5+NDUFA4L2+PDGFRB+), and smooth muscle cells (ACTA2+MYH11+PLN+)26,27 (Fig. 4a, b; Supplementary Fig. 4a). Fibroblasts in the ovary were characterized by the co-expression of fibroblast markers (DCN, COL1A1), steroidogenic acute regulatory protein (encoded by STAR), and FOXL2, which encodes a critical transcriptional regulator essential for ovary differentiation and maintenance28. Surprisingly, we observed that fibroblasts of OM expressed high levels of estrogen receptors (ER, encoded by ESR1 and ESR2) and progesterone receptors (PR, encoded by PGR), while ER and PR were rarely or barely expressed in fibroblasts from AD, PT, PM, or AS (Fig. 4c). This phenomenon was common across patients and not a characteristic belonging to any particular individual (Supplementary Fig. 4b). The difference in ER expression levels between premenopausal and postmenopausal patients was not as large as the difference among the tissue types (Fig. 4d). ER and PR were predominantly expressed in fibroblasts but not in other stromal cell types such as mesothelial cells and pericytes (Supplementary Fig. 4c). We also checked the expression of ER and PR in malignant cells and other cells, and found that ER and PR expression were absent in malignant cells (except for OM and PM of P03) (Supplementary Fig. 4d). According to the UMAP plots, ER and PR were marginally expressed in plasma cells of PT, but not expressed in other cell types (Supplementary Fig. 4e).
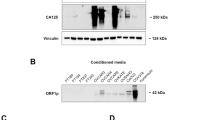
a UMAP plot showing the distribution of stromal cell subsets. b Dot plot showing the highly expressed marker genes in each stromal cell type. The dot size represents the percentage of cells expressing the marker genes in each stromal cell type, and the dot color represents the average expression level of the marker genes in each cell type. c Violin plots showing the expression levels of ESR1, ESR2, and PGR in fibroblasts from different sample types. d Violin plots showing the expression levels of ESR1, ESR2, and PGR in fibroblasts from the premenopausal and postmenopausal groups. Pre-meno, premenopausal groups; Post-meno, postmenopausal groups. e Volcano plot showing the DEGs between fibroblasts of PT and OM. f Dot plots showing the significantly enriched pathways of genes highly expressed in fibroblasts of OM and PT. Hypergeometric test; P < 0.01. g Scatter plot showing Pearson’s correlation between the average RNA levels of MDK and ESR1 in fibroblasts in each sample. Shade represents 95% confidence interval (R = 0.46; 95% CI = 0.19–0.67). Two-sided Student’s t-test. Western blot images (h) and quantitative analysis (i) showing the levels of MDK secreted by human ovarian fibroblasts (n = 3 experiments). Error bars represent mean ± standard deviations. One-way ANOVA followed by Bonferroni’s multiple-comparison test. Without Fulvestrant: Estradiol 0 nM vs. 50 nM: Mean difference (MD) = −104.09230 [95% CI: −165.7119, −42.4727]. Estradiol 0 nM vs. 100 nM: MD = −177.30071 [95% CI: −240.1326,−114.4688]. With Fulvestrant: Estradiol 0 nM vs. 50 nM: MD = 8.91347 [95% CI: −30.0585, 47.8854]. Estradiol 0 nM vs. 100 nM: MD = 4.14143 [95% CI: −34.8305, 43.1134]. j Representative H&E and IHC staining of ER and MDK in OM of GC (n = 9 patients). Scale bar: 200 μm. In c–e, Wilcoxon rank sum test followed by Bonferroni’s multiple-comparison test. In g, h, and i, source data are provided as a Source Data file. In c, f and g, AD adjacent normal gastric tissues, PT primary gastric tumors, OM ovarian metastases, PM peritoneal metastases, AS ascites samples.
We further explored the potential function of fibroblasts in OM by asking the question whether estrogen response in the ovaries is associated with the observed age and menstrual state bias of OM of GC. Analysis of the DEGs between fibroblasts of PT and OM was performed. Genes upregulated in fibroblasts of OM included ESR1, PGR, midkine growth factor (encoded by MDK), and insulin-like growth factors (encoded by IGF1) (Fig. 4e; Supplementary Data 8), which are mainly enriched in the response to hormone pathways and regulation of epithelial cell proliferation (Fig. 4f). MDK is an estrogen-induced gene encoding a protein that promotes cell growth, migration, and angiogenesis, particularly during tumorigenesis29,30. It is of note that in some tumors, such as urinary bladder carcinoma31 and gallbladder cancer32, the expression level of MDK is significantly correlated with malignant progression and poor patient prognosis. Pearson’s correlation analysis demonstrated that the expression levels of ESR1 and MDK were positively correlated with one another (Fig. 4g, cor = 0.46), suggesting that MDK secretion in ovarian fibroblasts may be mediated by estrogen.
Subsequently, we tested this hypothesis in vitro and found that the level of MDK secreted by human ovarian fibroblasts (HOF) was significantly increased in a concentration-dependent manner following treatment with Estradiol, while the addition of Fulvestrant inhibited MDK secretion (P < 0.01, Fig. 4h, i). H&E and immunohistochemical (IHC) staining confirmed that the fibroblasts of OM of GC were ER+ and MDK+ (Fig. 4j). Taken together, these results indicate that fibroblasts express growth factors, such as MDK, in response to estrogen stimulation, which may promote tumor colonization and proliferation.
Fibroblasts of ovarian metastases are transformed into CAFs in OM of GC
We sought to elucidate whether fibroblasts in normal human ovarian tissues and primary ovarian cancer express ER and MDK. To this end, we collected 10X Genomics scRNA-seq data from two cohorts of normal human ovarian tissues33,34 and one cohort of primary ovarian tumors35 from public databases, and then integrated the fibroblasts of OM and PT of GC for further analysis (Supplementary Fig. 5a). Among the integrated fibroblasts, we identified normal fibroblasts (NFs, DCN+) and five CAF types, i.e., inflammatory CAFs (iCAFs, TNFRSF12A+CXCL8+), antigen-presenting CAFs (apCAFs, CD74+HLA − DRA+), and myofibroblastic CAFs (myCAFs: myCAFs_ACTA2, ACTA2+; myCAFs_FAP, ACTA2+FAP+; and myCAFs_ESR1, ACTA2+ESR1+), based on known CAF marker genes25,36 (Fig. 5a; Supplementary Fig. 5b, c).
a UMAP plot showing the types of integrated fibroblasts from the normal ovarian cortex, normal ovarian medulla, primary ovarian tumors, OM of GC, and PT of GC. b Stacked histograms showing the relative percentages of NFs, iCAFs, apCAFs, myCAFs_ACTA2, myCAFs_FAP, and myCAFs_ESR1 in different cohorts. c Violin plots showing the expression levels of ESR1, ESR2 and MDK in fibroblasts from different cohorts. d Venn diagram showing the intersecting genes in the three lists of DEGs. e Bar graphs showing the significantly enriched pathways for the intersecting genes. Hypergeometric test; P < 0.01. f Dot plot showing the presence of MDK–LRP1 and MDK–SORL1 pairs in the interaction between fibroblasts and malignant cells according to CellPhoneDB analysis. Permutation test. g Violin and box plots showing the expression levels of MDK in fibroblasts (n = 27,697 cells) and those of LRP1 in malignant cells (n = 29,592 cells) from different sample types. The center line of the box represents the median value, the upper and lower limits of the box represent the 25th and 75th percentile points. Wilcoxon rank sum test followed by Bonferroni’s multiple-comparison test. h Multiplex immunohistochemistry staining (mIHC) showing the spatial localization of ESR1 and MDK in fibroblasts (DCN+) and that of LRP1 in malignant cells (Pan-CK+) (n = 12 patients). Scale bar: 30 μm. i Quantitative analyses of the proportions of ESR1+ fibroblasts in mIHC of multiple samples (n = 12 patients). MD = −0.02697 [95% CI: −0.05291, −0.00102]. j Quantitative analyses of the proportions of LRP1+ tumor cells in mIHC of multiple samples (n = 12 patients). MD = 0.04241 [95% CI: −0.00879, 0.09361]. In b and c, NOC normal ovarian cortex, NOM normal ovarian medulla, POT pimary ovarian tumor, OM ovarian metastases of GC, PT primary tumor of GC. In f, g, i and j, AD adjacent normal gastric tissues, PT primary gastric tumors, OM ovarian metastases, PM peritoneal metastases, AS ascites samples. In i and j, paired two-sided Student’s t-test. Source data are provided as a Source Data file.
The normal fibroblasts occurred mainly in the normal ovarian cortex and medulla tissues, while iCAFs occurred mainly in normal human ovarian medulla tissues and primary ovarian tumors, and apCAFs occurred mainly in normal human ovarian medulla tissues and OM of GC (Fig. 5b). However, the three subtypes of myCAFs were found mainly in OM of GC, which may be due to fibroblasts being converted into CAFs by tumor cells colonizing the ovary. Previous studies have shown that connective tissue fibroblasts proximal to neoplastic growths can be activated and mesenchymal progenitors can be induced to differentiate into myofibroblasts, known as myCAFs37. Figure 5c showed the expression levels of ESR1, ESR2 and MDK in fibroblasts from different cohorts, and we found that fibroblasts in normal ovarian cortex and medulla tissues also expressed ESR1, ESR2 and MDK. H&E and IHC staining confirmed that the fibroblasts of OM of GC and normal ovarian tissues expressed ER and MDK, while ER was expressed in tumor cells of primary ovarian cancer (Supplementary Fig. 5d–f).
Furthermore, analysis of DEGs was performed between fibroblasts of OM of GC and normal ovarian tissues, primary ovarian tumors, and other metastases of GC. The intersecting genes in the three lists of DEGs included ESR1, PGR, MDK, IGF1, IGFBP7, ACTA2, FOXL2, and COL1A1, which were enriched in epithelial–mesenchymal transition, blood vessel development, response to hormone and growth pathways (Fig. 5d, e).
Ovarian fibroblasts promote the growth of tumor cells through activating MDK/LRP1 signaling axis
We further investigated the mechanism by which ovarian fibroblasts promote the proliferation of tumor cells by analyzing their interactions using CellPhoneDB38. We found the significant presence of ligand–receptor pairs, MDK–LRP1 and MDK–SORL1, in the interactions between fibroblasts and tumor cells, and MDK–LRP1 was only present in OM (Fig. 5f). Low-density lipoprotein (LDL) receptor-related protein 1 (LRP1) is a functional receptor for MDK-mediated anchorage-independent proliferation of cancer cells39. Indeed, the expression levels of MDK in fibroblasts and those of LRP1 in malignant cells were highest in OM, and the primary gastric cancer cells also expressed LRP1 (Fig. 5g). We found that compared with tumor cells in other tissues, genes highly expressed in tumor cells in OM were significantly enriched in the hypoxic pathway (M5891: HALLMARK HYPOXIA) (Supplementary Fig. 5g). Koong et al. have previously reported that LRP1 were induced by hypoxia40. These results indicate that tumor cells in OM are in a more hypoxic environment, resulting in increased LRP1 expression. To demonstrate the interactions between ovarian fibroblasts and tumor cells, we performed multiplex immunohistochemistry (mIHC) staining in tumor tissues. The results show that compared with PT, the proportion of ESR1+ fibroblasts were significantly higher in fibroblasts of OM (P = 0.0429), MDK expression was higher in OM than in primary tumor tissues, and the proportions of LRP1+ tumor cells were higher in tumor cells of OM (Fig. 5h–j). However, there was no significant presence of MDK–LRP1 in the interactions between fibroblasts and tumor cells in primary ovarian tumors (Supplementary Fig. 5h–j). Therefore, we speculated that ER+ fibroblasts of OM may promote tumor cell proliferation via activating ER-MDK-LRP1 signaling axis.
Zhang, Y. et al. have reported that M2 macrophages were predominant in gallbladder cancer with ErbB pathway mutations and increased MDK in these tumors interacted with its receptor LRP1 to promote immunosuppressive (M2) macrophage differentiation32. In our study, there was the significant presence of ligand–receptor pair, MDK–LRP1, in the interactions between fibroblasts and macrophages in OM (Supplementary Fig. 5k), indicating fibroblasts of OM respond to estrogen stimulation that induce expression of MDK which interacted with its receptor LRP1 to promote M2 macrophage differentiation, which leads to tumor immune escape and tumor development.
Estrogen stimulation promotes ovarian implantation and metastasis of LRP1+ GC cells
To evaluate the role of the ER-MDK-LRP1 axis in promoting OM of GC, we utilized Estradiol, Fulvestrant, and MDK inhibitor (iMDK) treatment in our experimental setup, as illustrated in Fig. 6a. Co-culture experiments were conducted using NUGC-3 or MKN-1 GC cells and human ovarian fibroblasts. Transwell assays revealed that treatment with Estradiol enhanced the migration and invasion abilities of NUGC-3 or MKN-1 GC cells, which were inhibited by the addition of Fulvestrant or iMDK (Fig. 6b–e). In addition, we performed CCK-8 assay to measure tumor cell proliferation. These results showed that Estradiol increased proliferation abilities of the tumor cells, while treatment with Fulvestrant or iMDK decreased these abilities (Supplementary Fig. 6a, b).
a Schematic diagram depicting the co-culture strategy for ovarian fibroblasts with GC cells created in BioRender. Hu, C. (2021) BioRender.com/z48q170 released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license. Representative images (b) and quantitation (c) of the Transwell assay showing the migration and invasion abilities of NUGC-3 cells after 72 h of co-culture with ovarian fibroblasts plus Estradiol (100 nM), Fulvestrant (100 nM), or iMDK (100 nM) treatment (n = 3 co-culture experiments). Migration: Control vs. Estradiol: MD = −0.29828 [95% CI: −0.4257, −0.1709]. Control vs. Fulvestrant: MD = 0.34433 [95% CI: 0.2196, 0.4691]. Control vs. Estradiol + Fulvestrant: MD = 0.10304 [95% CI: −0.0839, 0.2900]. Control vs. iMDK: MD = 0.40379 [95% CI: 0.2029, 0.6047]. Invasion: Control vs. Estradiol: MD = −1.16864 [95% CI: −1.9059, −0.4314]. Control vs. Fulvestrant: MD = 0.49216 [95% CI: 0.1354, 0.8489]. Control vs. Estradiol + Fulvestrant: MD = 0.38712 [95% CI: −0.2022, 0.9764]. Control vs. iMDK: MD = 0.52045 [95% CI: 0.0513, 0.9896]. Representative images (d) and quantitation (e) of the Transwell assay showing the migration and invasion abilities of MKN-1 cells after 72 h of co-culture with ovarian fibroblasts plus Estradiol (100 nM), Fulvestrant (100 nM), or iMDK (100 nM) treatment (n = 3 co-culture experiments). Migration: Control vs. Estradiol: MD = −1.13880 [95% CI: −1.3850, −0.8926]. Control vs. Fulvestrant: MD = 0.55276 [95% CI: 0.2492, 0.8563]. Control vs. Estradiol + Fulvestrant: MD = 0.45942 [95% CI: 0.2317, 0.6871]. Control vs. iMDK: MD = 0.60633 [95% CI: 0.2499, 0.9627]. Invasion: Control vs. Estradiol: MD = −0.55892 [95% CI: −1.0038, −0.1140]. Control vs. Fulvestrant: MD = 0.55096 [95% CI: 0.1746, 0.9273]. Control vs. Estradiol + Fulvestrant: MD = 0.47452 [95% CI: 0.1104, 0.8386]. Control vs. iMDK: MD = 0.54618 [95% CI: 0.2378, 0.8546]. Representative images (f) and quantitation (g) of the Transwell assay showing the migration and invasion abilities of NUGC-3 cells (WT/shLRP1/OV-LRP1) after 72 h of co-culture with ovarian fibroblasts (WT/shMDK/OV-MDK) (n = 3 co-culture experiments). Migration: Control vs. shMDK: MD = 0.46386 [95% CI: 0.2720, 0.6557]. Control vs. OV-MDK: MD = −0.32294 [95% CI: −0.5335, −0.1124]. Control vs. shLRP1: MD = 0.22609 [95% CI: 0.0310, 0.4212]. Control vs. OV-LRP1: MD = −0.31610 [95% CI: −0.5176, −0.1146]. Invasion: Control vs. shMDK: MD = 0.40916 [95% CI: 0.1615, 0.6568]. Control vs. OV-MDK: MD = −0.72788 [95% CI: −0.9293, −0.5265]. Control vs. shLRP1: MD = 0.51109 [95% CI: 0.2793, 0.7428]. Control vs. OV-LRP1: MD = −0.86171 [95% CI: −1.4230, −0.3004]. Representative images (h) and quantitation (i) of the Transwell assay showing the migration and invasion abilities of MKN-1 cells (WT/shLRP1/OV-LRP1) after 72 h of co-culture with ovarian fibroblasts (WT/shMDK/OV-MDK) (n = 3 co-culture experiments). Migration: Control vs. shMDK: MD = 0.50487 [95% CI: 0.2123, 0.7974]. Control vs. OV-MDK: MD = −1.17127 [95% CI: −1.8123, −0.5303]. Control vs. shLRP1: MD = 0.52232 [95% CI: 0.2725, 0.7722]. Control vs. OV-LRP1: MD = −1.21510 [95% CI: −2.0497, −0.3805]. Invasion: Control vs. shMDK: MD = 0.49283 [95% CI: 0.0615, 0.9241]. Control vs. OV-MDK: MD = −0.68153 [95% CI: −1.0402, −0.3229]. Control vs. shLRP1: MD = 0.59236 [95% CI: 0.1119, 1.0728]. Control vs. OV-LRP1: MD = −0.91879 [95% CI: −1.3637, −0.4739]. In b, d, f, and h, scale bar: 100 μm. In c, e, g, and i, one-way ANOVA followed by Bonferroni’s multiple-comparison test. Source data are provided as a Source Data file. Error bars represent mean ± standard deviations.
Moreover, we established stable NUGC-3 and MKN-1 GC cell lines with knockdown or overexpression of LRP1, as well as ovarian fibroblasts with knockdown or overexpression of MDK (Supplementary Fig. 6c–e). Transwell assays demonstrated that co-culture of NUGC-3 or MKN-1 GC cells with MDK-knockdown ovarian fibroblasts inhibited the migration and invasion abilities of the GC cells, while co-culture with MDK-overexpressing ovarian fibroblasts increased these abilities (Fig. 6f–i). Notably, co-culture of LRP1-knockdown NUGC-3 or MKN-1 GC cells with ovarian fibroblasts diminished the migration and invasion abilities, while LRP1 overexpression in NUGC-3 or MKN-1 GC cells enhanced these abilities. Interestingly, Estradiol treatment or the overexpression of LRP1 alone did not promote the invasion and migration of NUGC-3 GC cells following independent culture (Supplementary Fig. 6f, g).
Furthermore, we established an ovarian metastasis mouse model to investigate the impact of estrogen stimulation on the promotion of ovarian implantation and metastasis of LRP1+ GC cells. Specifically, NUGC-3 cells expressing the luciferase lentiviral vector (NUGC-3-luc) suspended in Matrigel were injected into the right ovaries of 4-week-old female nude mice to establish OM (Fig. 7a). One week later, mice were randomly divided into four groups and the respective intervention was performed: 0.5 mg/kg/day Estradiol, 0.5 mg/kg/day Fulvestrant, 9 mg/kg/day iMDK, or the equivalent amount of normal saline as the control. The luciferase signals were detected and images obtained using an IVIS imaging system. The results demonstrate that treatment with Estradiol increased the ovarian implantation and metastasis abilities of the tumor cells, while treatment with Fulvestrant or iMDK decreased these abilities (Fig. 7b, c). Additionally, IHC staining revealed an elevated level of MDK expression after Estradiol treatment, which was decreased following treatment with Fulvestrant or iMDK (Fig.7d). In addition, we repeated this mouse experiment using an alternative administration route, in which Estradiol was administered using slow-release pellets and Fulvestrant was delivered intramuscularly. Similarly, mice were subjected to various treatments, which included subcutaneously implanted 0.72 mg estradiol pellets for releasing, intramuscular injection of 0.5 mg/kg/day Fulvestrant, intraperitoneal injection of 9 mg/kg/day iMDK, or an equivalent volume of normal saline as a control. Throughout the experiment, the body weight of the mice and the fluorescence intensity of the tumors were monitored. As shown in Supplementary Fig. 7a–d, we obtained consistent results with our previous findings that treatment with Estradiol increased the ovarian implantation and metastasis abilities of the tumor cells, while treatment with Fulvestrant or iMDK decreased these abilities.
a Schematic diagram and images of luciferase signals in the control, Estradiol, Fulvestrant, and iMDK groups of the OM mouse model. b Macroscopic lesions in the ovaries of mice in different groups. c The tumor mass (determined by the detected photons/sec) of mice in different groups at week 1 (beginning of intervention) and week 5 (end of intervention). One-way ANOVA followed by Bonferroni’s multiple-comparison test. Week 1: log10(tumor mass): Control vs. Estradiol: MD = 0.05772 [95% CI: −0.4858, 0.6013]. Control vs. Fulvestrant: MD = 0.01648 [95% CI: −0.5271, 0.5600]. Control vs. iMDK: MD = −0.09515 [95% CI: −0.6387, 0.4484]. Week 5: log10(tumor mass): Control vs. Estradiol: MD = −0.64320 [95% CI: −1.0341, −0.2523]. Control vs. Fulvestrant: MD = 1.41690 [95% CI: 1.0163, 1.8175]. Control vs. iMDK: MD = 2.04559 [95% CI: 1.6614, 2.4298]. Error bars represent mean ± standard deviations. d Representative H&E and IHC images of MDK expression in mice after different interventions. Scale bar: 100 μm. e Schematic diagram and images of luciferase signals in nude mice injected with GC cells expressing sh-NC or shLRP1. f Macroscopic lesions of bilateral ovarian metastasis in mice at week 4 (end of the experiments). g The tumor mass of bilateral OM (determined by the detected photons/sec) of mice at week 0 (beginning of the construction of the OM model) and at week 4 (end of the experiments). Week 0: log10(tumor mass), MD = 0.05850 [95% CI: −0.18739, 0.30440]. Week 4: log10(tumor mass), MD = −2.04215 [95% CI: −3.44030, −0.64401]. h The tumor weight (g) were measured and documented for the shNC and shLRP1 groups on 4-week-old female nude mice. MD = −0.35840 [95% CI: −0.45218, −0.26462]. In g and h, paired two-sided Student’s t-test. In c, g, and h, n = 5 mice, source data are provided as a Source Data file. In a and e, schematic diagram created in BioRender. Hu, C. (2021) BioRender.com/z48q170 released under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
Subsequently, sh-NC, shLRP1, or OV-LRP1 NUGC-3-luc GC cells suspended in Matrigel were injected into the left and/or right ovaries of 4-week-old female nude mice. As shown in Fig. 7e–h and Supplementary Fig. 7e–h, LRP1 knockdown led to the suppression of ovarian implantation and metastasis of tumor cells, while LRP1 overexpression promoted these events. Collectively, these findings indicate that estrogen stimulation enhances ovarian implantation and metastasis of LRP1+ GC cells by increasing MDK secretion.
Discussion
In the present study, the microenvironment of ovarian metastases (OM) in gastric cancer (GC) was systematically evaluated by single-cell analysis. Our findings delineate a pivotal role for ovarian fibroblasts within OM, primarily driving the expansion of malignant cell populations via the interaction between MDK and LRP1. Subsequent in-depth scrutiny revealed that estrogen stimulation assumes a central position, acting as a prime instigator of heightened MDK secretion by ovarian fibroblasts.
Regarding immune cell state and function, we found that decreased CD4-Tregs and increased CD8-CTL in OM. According to previous studies, CD4-Tregs have an immunosuppressive function41, and CD8-CTL has an anti-tumor function20. So, the decrease of CD4-Tregs and the increase of CD8-CTL in OM may inhibit OM. However, M2 macrophages are enriched in OM, which may support OM, and fibroblasts of OM respond to estrogen stimulation that induce expression of MDK which interacted with its receptor LRP1 to promote M2 macrophage differentiation32, which leads to tumor immune escape and tumor development in OM.
It has been postulated that the invasion of serosa by GC cells serves as a prerequisite for implantation metastasis. However, emerging evidence suggests that OM can be formed even during the early stages of GC, indicating that distant OM occurs through the intricate lymphatic network within the mucosa and submucosa. Notably, our findings reveal that GC patients with OM possess a lower median age in comparison with those who developed other types of metastases and premenopausal state is a significant risk factor for OM, suggesting younger premenopausal women are more prone to develop OM of GC. Several other studies also support our conclusions4,42. It is reasonable to suggest that this phenomenon may be attributed to the heightened ovarian functionality facilitated by increased blood flow and lymphatic reflux in premenopausal women. Moreover, investigations have suggested a potential association between the expression of estrogen-dependent estrogen receptors (ERs) and disease progression in GC patients, with ER expression itself being an independent risk factor for OM of GC43. Our study sheds some light on the potential mechanism.
Fibroblasts play a crucial role in the tumor microenvironment, exerting various tumorigenic functions such as extracellular matrix (ECM) remodeling and the secretion of cytokines, chemokines, and growth factors. By interacting with cancer cells, cancer-associated fibroblasts (CAFs) create a supportive niche for cancer stem cells, leading to the suppression of the tumor immune microenvironment and reprogramming of cancer cell metabolism, which ultimately promotes tumor metastasis and immune evasion44,45. Elyada et al. have previously demonstrated that there were three distinct CAFs subtypes in pancreatic ductal adenocarcinoma, including myofibroblastic CAFs, inflammatory CAFs, and antigen-presenting CAFs36. In our current study, we discovered high expression levels of ERs in fibroblasts of OM, while their expression in fibroblasts of AD, PT, and PM is minimal or negligible, and fibroblasts of OM of GC were transformed into myofibroblastic CAFs. ERs expression in breast CAFs46 and endometrial CAFs47 have been reported to contribute to tumor progression.
A previous study reported an association between estradiol and the expression of midkine (MDK)48. Specifically, estrogen can enhance MDK transcription by inducing the recruitment of ERs to the MDK promoter, leading to increased MDK protein expression. In our study, fibroblasts express growth factor MDK in response to estrogen stimulation. MDK, as a heparin-bound growth factor, can play an important role in cell proliferation, cell migration, angiogenesis, and epithelial–mesenchymal transition49. MDK expression is also strongly induced during tumorigenesis, inflammation, and tissue repair, and in many human cancers, such as breast cancer50, low-grade glioma51, and nasopharyngeal carcinoma52. MDK candidate receptors mainly include anaplastic lymphoma kinase, protein tyrosine phosphatase ζ (PTPζ), integrin, syndecan 1 (SDC1), LRP1, and others30. We demonstrate that the ligand–receptor pair MDK–LRP1 is present in interactions between fibroblasts and malignant cells, but is only significant in OM of GC. LRP1 is involved in MDK endocytosis and the MDK-mediated anchorage-independent proliferation of cancer cells. Following binding to LRP1, MDK is translocated to the nucleus and can regulate cellular proliferation and differentiation53,54.
A previous study has reported that in 152 patients with ovarian metastases from gastric cancer, the positive rate of ER-β (encoded by ESR2) and PR (encoded by PGR) was 44.7% and 31.6%, respectively3. However, ER-α (encoded by ESR1) showed no expression in these patients. In that study, the expression of ER-α, ER-β, and PR in gastric cancer samples were detected by IHC3. In our study, we used scRNA-seq to obtain the expression of ESR1, ESR2, and PGR in 17 primary gastric tumor samples, and we found that only the ovarian and peritoneal metastatic tumor cells from patient P03 expressed ESR1, and none expressed ESR2 or PGR. This discrepancy could be explained by several factors, such as the different detection sensitivity between IHC and scRNA-seq, the limited number of cases, tumor heterogeneity, and inter-patient variability.
Through in vitro and in vivo experiments, we demonstrated that stimulation with estrogen induced the secretion of MDK by ovarian fibroblasts and therefore increased the ovarian implantation and metastasis of the tumor cells, which could be inhibited by the addition of Fulvestrant or MDK inhibitor (iMDK). Fulvestrant and iMDK may be potential treatments for OM of GC with ER–positive ovarian fibroblasts. As we all know, Fulvestrant, a hormone therapy, is commonly used for treating patients with ER–positive breast cancer55. Fulvestrant is an estrogen receptor antagonist that binds to estrogen receptors in breast cancer cells and inhibits the growth of cancer. Meanwhile, studies have reported that midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer and targeting MDK using a specific iMDK broadly reversed IFN-γ-activated EMT, and subsequently abrogated IFN-γ-triggered metastasis56,57.
Our study does have several limitations that should be acknowledged. Firstly, the sample size is limited; therefore, it is imperative to conduct further validation studies involving larger cohorts of GC patients with OM. Secondly, the majority of patients included in our study had already presented with ovarian and other metastases at the time of diagnosis, which rendered it challenging to ascertain the exact timing or to measure the hormone levels at the onset of OM. Thirdly, the underlying mechanism by which ovarian fibroblasts promote MDK secretion in response to estrogen stimulation requires further investigation.
In conclusion, our study successfully generated a single-cell atlas of OM of GC and provides a potential mechanism to explain the age and menstrual state bias. These findings hold promising therapeutic implications for GC patients with OM. Our data may contribute to the development of strategies for the prevention and treatment of OM in GC.
Methods
Ethics approval
This study protocol about human tissue specimens was approved by the Ethics Committee of Zhejiang Cancer Hospital (no. IRB-2020-109), and all patients provided written informed consent. This study adhered to the principles outlined in the Declaration of Helsinki of 1964 and subsequent revisions. Maximum tumor size (1500 mm3) permitted by the ethics committee was not exceeded. The animal subjects were approved by the Ethics Committee of Zhejiang Cancer Hospital (no. zjzlsd-2023-02-114).
Patient cohort, clinical characteristics, and sample collection
A total of 18 gastric cancer (GC) patients with ovarian metastases (OM), who were treated at Zhejiang Cancer Hospital, were included in this study. The diagnosis of primary GC was confirmed in all patients through endoscopic biopsy. OM was confirmed by cytologic examination or laparoscopic exploration, which also enabled the verification of peritoneal metastases (PM), if present. Pathology results were independently reviewed by two experienced gastrointestinal pathologists. Additionally, computed tomography (CT) images of all patients were evaluated by two experienced imaging physicians. According to the Eighth Edition of the American Joint Committee on Cancer Staging Manual, all patients were classified as stage IV. Notably, nine patients had signet-ring cell carcinoma. None of the patients had a history of radical gastrectomy or a previous medical history of gastrointestinal tumors, such as colon cancer or pancreatic cancer. Detailed clinical and histopathological characteristics of the patients are described in Supplementary Data 2. Moreover, four adjacent normal (AD) tissues from GC patients were included as controls.
All specimens were collected at Zhejiang Cancer Hospital with the written, informed consent of each patient. PT and AD samples were obtained during endoscopic diagnosis, while ascites (AS) samples were collected during therapeutic paracentesis. OM and PM were collected during laparoscopic oophorectomy. Fresh solid tissues were preserved in tissue storage solution (MACS Media) and dissociated into single-cell suspensions for single-cell RNA sequencing (scRNA-seq). AS samples were centrifuged at 1500 rpm for 10 min, and the pelleted cells were collected and stored in Bambanker™ medium (Nippon Genetics, no.392014681) for scRNA-seq analysis. All samples were processed using the same protocol by the same research assistant.
Furthermore, we conducted a retrospective cohort study, enrolling a total of 1,027 female GC patients with distant metastases who were treated at Zhejiang Cancer Hospital between October 2006 and December 2022. This cohort consisted of 234 patients with OM and 793 patients with metastases in other locations. Medical records of all patients were thoroughly reviewed; and relevant data, including age, gender, menstrual state, smoking state, alcohol state, diabetes state, and metastatic sites were collected.
Sample preparation, tissue dissociation, and cell viability examination
Tissue samples of PT, AD, and metastatic tumors (including OM and PM) were sectioned into approximately 1-mm3 pieces. A tumor dissociation kit (Miltenyi Biotech) was utilized according to the manufacturer’s instructions for the dissociation of all tissue samples. Pelleted cells from AS were resuspended in RPMI-1640 medium supplemented with 5% fetal bovine serum (FBS). The cell suspensions from tissues and AS were sequentially passed through 70-μm and 30-μm cell strainers and then centrifuged at 300 × g for 10 min. The supernatant was discarded, and the pelleted cells were resuspended in red blood cell lysis buffer (Miltenyi Biotech) and incubated on ice for 2 min to lyse any red blood cells. After two washes with 1× phosphate-buffered saline (PBS), the cell pellets were resuspended in RPMI-1640 medium supplemented with 5% FBS. Cell viability was assessed using the Countstar® Rigel S2 cell counter (Countstar). According to the manufacturer’s instructions, cell suspensions were mixed at a 1:1 ratio with AO/PI staining solution (Countstar; 12 μL:12 μL), immediately pipetted into a disposable counting chamber, and analyzed using the Countstar® Rigel S2. Cell viability, cell concentration, and aggregation rate were observed and recorded.
ScRNA-seq library preparation and sequencing
Single-cell RNA sequencing (scRNA-seq) was performed using the 10X Genomics Chromium Platform, which encompassed single-cell separation, complementary DNA (cDNA) amplification, and library construction. Initially, the single-cell suspensions were quantitated using the Countstar® Rigel S2 (Countstar) and then loaded onto a Chromium Controller to generate single-cell gel bead-in-emulsions (GEMs). Subsequently, scRNA-seq libraries were constructed using the Chromium Next GEM Single Cell 5ʹ Library & Gel Bead Kit (PN: 220112, 10X Genomics). The single-cell GEMs facilitated the generation of barcoded cDNA through reverse-transcription PCR, and the cDNA was purified using Dynabeads® MyOne™ SILANE magnetic beads (PN: 2000048, 10x Genomics). cDNA amplification was achieved using the Amplification Master Mix kit (PN: 220125, 10X Genomics), and cDNA was then purified and target-enriched using the Beckman Coulter SPRIselect reagent. The concentration and quality of cDNA libraries were assessed using the Agilent Bioanalyzer High Sensitivity DNA kit. For pooling, 50 ng each sample library in 20 μL was mixed with 30 μL Fragmentation Mix (PN: 220108, 220107/220130, 10X Genomics). The barcoded library was then sequenced on the NovaSeq6000 (Illumina) S2 flow cell (100 cycle kit) using a 26 × 91 run format with an 8-bp index (read 1). To minimize batch effects, all libraries were constructed using the same version of the kit following the same protocol. Subsequently, the libraries were sequenced on the same NovaSeq6000 (Illumina) flow cell and analyzed together.
Raw data processing and quality control of scRNA-seq data
Raw sequencing data from the 10X Genomics platform were converted to fastq format using ‘CellRanger mkfastq’ (v.5.0.1). Next, scRNA-seq reads were aligned to the GRCh38 reference genome using ‘CellRanger count’ (v5.0.1), and scTCR-seq data were aligned to the vdj-GRVh38 reference genome using ‘CellRanger vdj’ (v5.0.1). The CellRanger outputs of multi-sample were combined using ‘CellRanger aggr’ (v5.0.1) for batch correction.
To further analyze aggregation results from the above pipeline using ‘CellRanger aggr,’ we employed the Seurat package (v.4.0.1) in R (v.4.0.3) to visualize the scRNA-seq data58. Our initial dataset contained 366,094 cells. Stringent data quality control was conducted during the downstream analysis. Only genes detected in at least 0.1% of cells were retained. We filtered the cells with the following parameters to exclude outliers: maximum percentage mito = 10%, minimum number of UMIs = 1000, and minimum nGene = 500. Double cell scoring was then performed using the R package scDblFinder (v.1.4.0) to remove cells that were considered double in each sample59.
After removal of poor-quality cells, a total of 227,836 cells were retained for downstream analysis. To normalize the library size effect in each cell, we scaled UMI counts using scale.factor = 10,000. Following log-transformation of the data, other factors, including “percent.mt” and “nCount_RNA,” were corrected for variation regression using the ScaleData function. We performed principal component analysis (PCA) using the top 3000 variably expressed genes. The top 30 principal components were used for subsequent clustering and uniform manifold approximation and projection (UMAP) visualization. Forty initial clusters were identified with the FindClusters function using shared nearest neighbor modularity optimization with the clustering resolution set to 0.5.
Cell type annotation
We first searched for the top differential markers for each identified cluster/sub-cluster using the FindAllMarkers function. The test method used for FindAllMarkers was the Wilcoxon rank sum test followed by Bonferroni’s multiple comparison test. For each cell type, we used multiple cell type-specific/enriched marker genes previously described in the literature to determine cellular identity. These included but were not limited to CD3E, CD3D, and CD3G for T cells60; CD14, MS4A7, FCGR3A, and LYZ for myeloid cells22; EPCAM, KRT8, KRT19, and KRT18 for epithelial cells10; MZB1, IGKC, and DERL3 for plasma cells; CD79A, MS4A1, and CD19 for B cells; VWF, PECAM1, and ENG for endothelial cells; and TPSAB1, CPA3, and MS4A2 for mast cells. Cells expressing double-lineage genes, such as CD3E+CD3D+ and CD79A+MS4A1+, were excluded to eliminate potential doublet-capture bias. We then arranged all the identified cell types into eight major cell sets based on their expression profiles, lineages, and functions.
Definition of malignant and non-malignant scores
DEGs from bulk RNA-seq data for stomach adenocarcinoma were downloaded from a previous article published by Zhang M, et al. (Supplementary Table 1. Top 100 signature genes of tumor samples versus normal samples from TCGA dataset)10. Subsequently, each epithelial cell in our study was simultaneously assigned a malignant score and a non-malignant score. In short, signature genes for malignant scores were selected as the top 50 highly expressed genes (adjusted p < 0.01) in tumor tissues, and signature genes for non-malignant scores were selected as the top 50 highly expressed genes (adjusted p < 0.01) in normal tissues. Malignant scores and non-malignant scores were calculated based on signature genes using the “AddModuleScore” function in the “Seurat” R package. For each cell, the non-malignant score was subtracted from the malignant score.
Copy-number alterations (CNAs)
To unequivocally differentiate malignant from non-malignant epithelia, the large-scale CNAs were inferred from the scRNA-seq data using the R infercnv package (v1.6.0). Epithelial cells from adjacent normal tissues were used as a control. The R package ComplexHeatmap (v2.6.2) was used to visualize the results. In addition, we obtained whole exome sequencing (WES) data from a subset of samples (n = 16) taken from five patients. Paired-end WES reads were aligned to the human genome hg38 (UCSC) using the Burrows–Wheeler Aligner (BWA) (v0.7.17) with default parameters. Samtools (v0.1.19) was used to convert SAM files to compressed BAM files and sort them by chromosomal coordinates61. Then, the Python library CNV kit (v0.9.9) was used for CNA calling and visualization62. Sex chromosomes were excluded from analysis.
Malignant cells were distinguished from non-malignant epithelial cells according to combined information from inferred large-scale CNAs, malignant and non-malignant scores, feature marker gene expression, and cell cluster distribution.
Non-negative matrix factorization (NMF) analysis
To explore the intratumor transcriptional diversity and consistency of malignant cells from PT, OM, PM, and AS, a non-negative matrix factorization (NMF) algorithm documented in the R NMF package (v0.25) was applied to malignant cells from each sample with >100 malignant cells63. For the expression matrix, we excluded mitochondrial and ribosomal genes and filtered genes with a standard deviation of expression <0.5 within each sample. NMF was applied to the relative expression values, with all negative values being replaced with zero.
Gaujoux R and Seoighe C give a formal definition for Nonnegative Matrix Factorization problems, and defines the notations63. Let X be a n×p non-negative matrix, (i.e with xij ≥ 0, denoted X ≥ 0), and r > 0 an integer. NMF consists in finding an approximation X ≈ WH, where W, H are n × r and r × p non-negative matrices, respectively. In practice, the factorization rank r is often chosen such that r ≪ min(n, p). The objective behind this choice is to summarize and split the information contained in X into r factors: the columns of W.
For each tumor sample with >100 malignant cells, non-negative matrix factorization (with the number of factors set to 10) was used to identify variable expression programs, which resulted in a total of 340 programs across the 34 tumors. To investigate common programs among the 340 signatures, the programs were clustered into meta-programs by hierarchical clustering, using overlapping genes (among the 50 top-scoring genes for each program) as a similarity metric. Seven meta-programs (cell cycle, oxidative phosphorylation (oxphos), pEMT, interferon, glandular, RNA splicing, and stress) were identified and further analyzed. For meta-programs, genes shared between at least two programs were defined as meta-signatures. The gene signatures of the seven meta-programs are provided in Supplementary Data 7.
Subclustering of T cells, myeloid cells, and stromal cells
Major cell types (T cells, myeloid cells, and stromal cells) were extracted from the processed dataset first. To eliminate batch effects, the top 3000 variable genes were extracted for run fastMNN based on the mutual nearest neighbors (MNN) method included in Seurat64. We performed PCA using the variably expressed genes for each of the major cell type objects under the “mnn” assay mode. The top 30 PCs were used for subsequent clustering and UMAP visualization. The FindClusters function of the R package Seurat was utilized with a suitable resolution to identify sub-clusters within major cell types. We clustered T cells into sets of CD4+ T cells (CD4+), CD8+ T cells (CD8A+), and natural killer T cells (NKT) (KLRD1+). Subsequently, CD4+ and CD8+ T cells were re-clustered into subtypes of different cell states. Cells expressing double-lineage genes, such as LYZ+ and CD3E+, were excluded to eliminate potential doublet-capture bias.
Scored cell state signature
We scored the signature genes in each meta-program for each malignant cell using the AddModuleScore function of the R package Seurat. The signature genes in meta-program were defined as genes shared between at least two programs. Cytotoxic and exhaustion scores for CD8+ T cells were calculated using the AddModuleScore function. The cytotoxic gene signature consisted of NKG7, PRF1, GZMA, GZMB, GZMK, IFNG, CCL4, CST7, GZMH, GZMM, and GNLY. The exhaustion gene signature consisted of LAG3, PDCD1, TIGIT, HAVCR2, EOMES, ENTPD1, CD38, CD101, CTLA4, CD244, BTLA, and CD160. For macrophages, the M1, M2, angiogenesis, and phagocytosis gene signatures were derived from a previous study22. The checkpoint gene signature consisted of LAIR1, SIRPA, HAVCR2, NECTIN2, PVR, CD274, ADORA2A, IDO1, and BTLA. The module scores were calculated using default parameters. The R package ggplot2 (v3.3.6) was used to visualize the results.
T-cell receptor (TCR) analysis
Single-cell TCR sequencing (scTCR-seq) data were aligned to the vdj-GRCh38 reference genome using ‘cellranger vdj’ (v5.0.1). The cellranger vdj pipeline performs V(D)J sequence assembly and paired cell-by-cell clonotype calling. The outputs of cellranger vdj include the productive nucleotide sequences and translated amino acid sequences of the CDR3 region for TCRs (α and β chains). A clonotype was defined as the identical CDR3 sequences of an α-β TCR pair. In each sample, we defined the clonal expansion type according to the number of cells sharing the clonotype. A clonotype that occurred in only one cell was defined as a unique type, a clonotype shared by two cells was defined as a duplicated type, and a clonotype shared by more than two cells was defined as a clonal type.
Cell–cell interaction analysis
We analyzed the cell–cell interactions of ovarian stromal cells with malignant cells in PT, OM, PM, and AS using the Python module cellPhonedb (v.2.1.8b3)38. The Seurat counts file and cell type annotations were input into ‘cellphonedb method statistical_analysis.’ The average expression values of a receptor in one cell type and a ligand in another cell type were considered the ligand–receptor interaction intensity between the two cell types. A null distribution of the mean average ligand and receptor expression in the interacting clusters was generated by randomly permuting the cluster labels of all cells with 1000 iterations. The P value for the likelihood of cell-type specificity of a given ligand–receptor complex was calculated based on the proportion of the means that were as high as or higher than the actual mean. The significance threshold of cell–cell interactions was P < 0.05. We used the R ggplot2 package (v3.3.6) to visualize the results.
Functional enrichment of differentially expressed genes (DEGs)
Enrichment analysis of DEGs was performed using the Metascape web tool (www.metascape.org)65. The gene sets used for the analysis were obtained from the GO Biological Processes, Reactome Gene Sets, KEGG Pathway, WikiPathways, Canonical Pathways, Hallmark Gene Sets, and PANTHER Pathway. P values were calculated based on the accumulative hypergeometric distribution. Terms with P < 0.01 were collected and grouped into clusters based on their membership similarities. We used the R ggplot2 package (v3.3.6) to visualize the results.
Multiplex immunohistochemistry staining protocol, image acquisition, and data analysis
The tumor samples were formalin-fixed and paraffin-embedded (FFPE). Each FFPE block was evaluated by two pathologists through an examination of H&E-stained slides. Subsequently, 4-μm sections of FFPE tissue were placed on Superfrost™ Plus slides and subjected to deparaffinization and rehydration by sequential immersion in xylene and graded ethanol solutions for multiplex immunohistochemistry staining. The slides were microwaved with the AR9 working solution for 15 min to facilitate antigen retrieval. Following a 10-min incubation with blocking buffer at room temperature, endogenous peroxidase activity was inactivated. The antibodies used in this study included rabbit monoclonal anti-LRP1 (Abcam, ab92544, 1/150), rabbit polyclonal anti-DCN (Proteintech, no.14667-1AP, 1/200), rabbit monoclonal anti-MDK (Abcam, ab52637, 1/300), rabbit monoclonal anti-ESR1 (Abcam, ab108398, 1/200), and rabbit polyclonal anti-Pan-CK (Abcam, no. 26411-1 A, 1/1500). The immunofluorescence signal was visualized using the OPALTM 7-color automation IHC kit (NEL821001KT) with TSA dyes at wavelengths of 480, 570, 620, 690, 780, and TSA-DIG, followed by counterstaining with Spectral DAPI. All slides were imaged on the Vectra Polaris (Akoya Biosciences) imaging system. Color separation, tissue and cell segmentation, and cell phenotyping were performed using the inForm software (Akoya Biosciences) to extract image data.
Cell lines
Human gastric cancer (GC) cell lines NUGC-3 and MKN-1 were obtained from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, China). Authentication of these cell lines was performed using Short Tandem Repeat analysis, and they were regularly tested to ensure the absence of mycoplasma contamination. Human ovarian fibroblasts (HOFs) were obtained from Hangzhou Jenker Biological Technology Co., Ltd (43-year-old female), and their authenticity was confirmed by immunofluorescence analysis.
Gene knockdown or overexpression by lentiviral vector transduction
Lentiviral vectors carrying shRNA targeting LRP1/MDK or LRP1/MDK overexpression constructs, as well as a negative control shRNA, were synthesized by GeneChem Biotechnology Company (Shanghai, China). The sequences of the 21-nucleotide shRNAs targeting LRP1 were GCTCAGACCCGCAAGACTTTA and GCACCTTAACGGGAGCAATGT. The sequences of the 21-nucleotide shRNAs targeting MDK were CGACTGCAAGTACAAGTTTGA, CAAGACCAAAGCAAAGGCCAA, and GCGCTACAATGCTCAGTGCCA. Lentiviral transduction of GC cells was performed following the manufacturer’s instructions. After 72 hours, stable cell lines were selected using 1 µg/mL puromycin. Transfection efficiency was evaluated by western blot analysis.
Assessment of MDK levels
Approximately 2 × 105 human ovarian fibroblasts (HOFs) were seeded on a 24-well plate, allowed to adhere overnight, and then treated with Estradiol (0, 50, 100 nM) alone or in combination with Fulvestrant (100 nM) for 24 h. MDK levels in the supernatant and cell lysates were measured by ELISA (#ab193761), Abcam) according to the supplier’s instructions.
Transwell migration and invasion (Matrigel) experiments
Approximately 2 × 105 HOFs were seeded on a 24-well plate and allowed to adhere overnight. The fibroblasts were then treated with either Estradiol (100 nM), Fulvestrant (100 nM), or iMDK (100 nM) for 24 h, while a control group was left untreated. Following treatment, the media was replaced with serum-containing media. For migration assays, 5 × 104 GC cells in 200 μL serum-free media were seeded in the upper chamber of an insert with an 8-μm pore size (Corning, USA). For invasion assays, 1 × 105 cells in 200 μL serum-free media were seeded in the upper chamber of an insert coated with Matrigel (BD Biosciences, San Diego, CA). After incubation for 72 h, the cells attached to the upper side of the membrane were mechanically removed using a cotton swab. Subsequently, the cells on the bottom surface of the membrane were fixed with 4% paraformaldehyde for 20 min and stained with 0.4% crystal violet for a further 20 min. Images were captured using a Nikon Digital Sight DS-L1 camera.
CCK-8 assay
Approximately 1000 MKN-1/NUGC-3 GC cells were seeded per well in a 96-well plate and allowed to adhere overnight. The conditioned medium from human ovarian fibroblasts treated with Estradiol (100 nM), Fulvestrant (100 nM), or iMDK (100 nM) for 24 h was then added and incubated for 24 h, 48 h, and 72 h. Subsequently, the cells were incubated with CCK-8 reagent for 3 h. Thereafter, a microplate reader (Thermo Varioskan LUX, MA, United States) was used to measure the absorbance (OD) at 450 nm.
Western blot analysis
Cells were lysed in 1× sodium dodecyl sulfate (SDS) lysis buffer. Total protein was quantitated, separated by SDS‒PAGE, and transferred to PVDF membrane (Millipore, MA, USA). The target proteins were probed with antibodies against ESR1 (Abcam, ab108398, 1/500), LRP1 (Abcam, ab92544, 1/500), MDK (Abcam, ab52637, 1/1000), and GAPDH (#60004-1-Ig, ProteinTech). Anti-mouse IgG (#926-6807, Invitrogen) and anti-rabbit IgG (#926-68070, Invitrogen) were used as secondary antibodies. Finally, the protein bands were visualized using enhanced chemiluminescence (ECL; Fdbio Science, Hangzhou, China). The intensity was measured using the ImageJ software.
Ovarian metastasis models
The mice were raised in the laboratory for a week before the experiment. Mice were fed in the Specific Pathogen Free (SPF) barrier center at the animal experimental center of Zhejiang Chinese Medical University, under standard conditions of temperature (25 ± 2 °C) and humility (50 ± 5%) in a 12 h light/12 h dark cycle with normal drink and food.
We have previously performed tumor-forming experiments using lines MKN-1, MKN-45, NUGC-3, NUGC-4 and other gastric cancer cells to understand the rate of tumor growth. During the experiment, we closely observed the status of the mice according to institutional ethical guidelines, and when they showed a 20% weight loss or signs of discomfort (inactivity, wrinkled hair), they were considered in poor condition and killed.
To establish the OM models, 4-week-old female nude mice were injected with 3 × 106 GC cells transfected with the luciferase lentiviral vector (NUGC-3-luc) (sh-NC, shLRP1, or OV-LRP1) mixed with 10 µL PBS and 10 µL Matrigel (BD Biosciences) into the left and/or right ovaries. One week later, mice injected with sh-NC GC cells were randomly divided into four groups. Mice were then subjected to various treatments, which included intraperitoneal injection of 0.5 mg/kg/day Estradiol, 0.5 mg/kg/day Fulvestrant, 9 mg/kg/day iMDK, or an equivalent volume of normal saline as a control. Throughout the experiment, the body weight of the mice and the fluorescence intensity of the tumors were monitored. In vivo, imaging was performed twice a week using a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA) after intraperitoneal administration of fluorescein substrate (150 mg/kg). The tumor inhibition rate was determined using the LT Living Image 4.3 Software.
Statistical analysis
Univariate and multivariate logistic regression analysis were used to analyze the associations between the clinical parameters and OM. The statistical methods and threshold for each analysis are described with the results or detailed in the figure legends or Methods. No data were excluded from the analyses. All values are depicted as the mean ± SEM. A P value less than 0.05 was considered significant. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns no significant difference. Multiple comparisons following one-way ANOVA and the Wilcoxon rank sum test were performed for statistical analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data from the scRNA-seq generated in this study have been deposited in the Genome Sequence Archive of the BIG Data Center at the Beijing Institution of Genomics, Chinese Academy of Science, under accession code HRA004702, which is publicly available. The scRNA-seq data of normal human ovarian medulla tissues used in this study are available in Gene Expression Omnibus (GEO) with accession number GSE118127. The scRNA-seq data of normal human ovarian cortex tissues used in this study are available in the ArrayExpress database at EMBL-EBI and are available under the accession codes ‘E-MTAb−8381’. The scRNA-seq data of primary ovarian tumors used in this study are available in GEO under the accession number GEO: GSE173682. Antibodies and reagents used in this study are listed in Supplementary Data 9. Source data are provided with this paper.
References
Sung, H. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Gao, J. et al. Transcriptomic characterization and construction of M2 macrophage-related prognostic and immunotherapeutic signature in ovarian metastasis of gastric cancer. Cancer Immunol. Immunother. 72, 1121–1138 (2023).
Yu, P. et al. Treatment strategy and prognostic factors for Krukenberg tumors of gastric origin: report of a 10-year single-center experience from China. Oncotarget 8, 82558–82570 (2017).
Namikawa, T. et al. Frequency and therapeutic strategy for patients with ovarian metastasis from gastric cancer. Langenbecks Arch. Surg. 407, 2301–2308 (2022).
Al-Agha, O. M. & Nicastri, A. D. An in-depth look at Krukenberg tumor: an overview. Arch. Pathol. Lab Med 130, 1725–1730 (2006).
Feng, Q., Pei, W., Zheng, Z. X., Bi, J. J. & Yuan, X. H. Clinicopathologic characteristics and prognostic factors of 63 gastric cancer patients with metachronous ovarian metastasis. Cancer Biol. Med 10, 86–91 (2013).
Sodek, K. L., Murphy, K. J., Brown, T. J. & Ringuette, M. J. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 31, 397–414 (2012).
Wang, R. et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell 41, 1407–1426.e9 (2023).
Wang, R. et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat. Med 27, 141–151 (2021).
Zhang, M. et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut 70, 464–475 (2021).
Kim, J. et al. Single-cell analysis of gastric pre-cancerous and cancer lesions reveals cell lineage diversity and intratumoral heterogeneity. NPJ Precis. Oncol. 6, 9 (2022).
Hu, Z. et al. The repertoire of serous ovarian cancer non-genetic heterogeneity revealed by single-cell sequencing of normal fallopian tube epithelial cells. Cancer Cell 37, 226–242.e7 (2020).
Tokuhara, Y. et al. Nuclear expression of claudin-3 in human colorectal adenocarcinoma cell lines and tissues. Oncol. Lett. 15, 99–108 (2018).
Torres, J. B. et al. Radiolabeled cCPE peptides for SPECT imaging of claudin-4 overexpression in pancreatic cancer. J. Nucl. Med 61, 1756–1763 (2020).
Uthayanan, L. & El-Bahrawy, M. Potential roles of claudin-3 and claudin-4 in ovarian cancer management. J. Egypt Natl. Canc. Inst. 34, 24 (2022).
Szade, J. et al. Comparison of claudin-3 and claudin-4 expression in bilateral and unilateral breast cancer. Neoplasma 68, 283–289 (2021).
Montgomery, E. et al. Overexpression of claudin proteins in esophageal adenocarcinoma and its precursor lesions. Appl. Immunohistochem. Mol. Morphol. 14, 24–30 (2006).
de Souza, W. F. et al. Claudin-3 overexpression increases the malignant potential of colorectal cancer cells: Roles of erk1/2 and pi3k-akt as modulators of egfr signaling. PLoS One 8, e74994 (2013).
Barkley, D. et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet 54, 1192–1201 (2022).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Zhang, M. et al. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 7, 19 (2014).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Hu, S. et al. TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma. J. Clin. Invest. 132, e157649 (2022).
Chen, X. & Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18, 99–115 (2019).
Chen, Y. et al. Epithelial cells activate fibroblasts to promote esophageal cancer development. Cancer Cell 41, 903–918.e8 (2023).
Olbrecht, S. et al. High-grade serous tubo-ovarian cancer refined with single-cell RNA sequencing: Specific cell subtypes influence survival and determine molecular subtype classification. Genome Med. 13, 111 (2021).
Xie, Z. et al. Gene set knowledge discovery with enrichr. Curr. Protoc. 1, e90 (2021).
Georges, A. et al. FOXL2: a central transcription factor of the ovary. J. Mol. Endocrinol. 52, R17–R33 (2014).
Tamm-Rosenstein, K., Simm, J., Suhorutshenko, M., Salumets, A. & Metsis, M. Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS One 8, e68907 (2013).
Filippou, P. S., Karagiannis, G. S. & Constantinidou, A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 39, 2040–2054 (2020).
O’Brien, T., Cranston, D., Fuggle, S., Bicknell, R. & Harris, A. L. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 56, 2515–2518 (1996).
Zhang, Y. et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J. Hepatol. 75, 1128–1141 (2021).
Fan, X. et al. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 10, 3164 (2019).
Wagner, M. et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat. Commun. 11, 1147 (2020).
Regner, M. J. et al. A multi-omic single-cell landscape of human gynecologic malignancies. Mol. Cell 81, 4924–4941.e10 (2021).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: Inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Chen, S. et al. Midkine and LDL-receptor-related protein 1 contribute to the anchorage-independent cell growth of cancer cells. J. Cell Sci. 120, 4009–4015 (2007).
Koong, A. C. et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res 60, 883–887 (2000).
Sharma, A. & Rudra, D. Emerging functions of regulatory T cells in tissue homeostasis. Front Immunol. 9, 883 (2018).
Qiu, L., Yang, T., Shan, X. H., Hu, M. B. & Li, Y. Metastatic factors for Krukenberg tumor: a clinical study on 102 cases. Med Oncol. 28, 1514–1519 (2011).
Li, S. Q. et al. Establishment and validation of a nomogram to predict the risk of ovarian metastasis in gastric cancer: Based on a large cohort. World J. Clin. Cases 8, 4331–4341 (2020).
Caligiuri, G. & Tuveson, D. A. Activated fibroblasts in cancer: perspectives and challenges. Cancer Cell 41, 434–449 (2023).
Chen, Y., McAndrews, K. M. & Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 18, 792–804 (2021).
Knower, K. C. et al. Distinct nuclear receptor expression in stroma adjacent to breast tumors. Breast Cancer Res. Treat. 142, 211–223 (2013).
Subramaniam, K. S. et al. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS One 8, e68923 (2013).
Zhao, G. et al. ERbeta-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol. Endocrinol. 26, 1304–1315 (2012).
Cerezo-Wallis, D. et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat. Med 26, 1865–1877 (2020).
Sheng, B., Wei, Z., Wu, X., Li, Y. & Liu, Z. USP12 promotes breast cancer angiogenesis by maintaining midkine stability. Cell Death Dis. 12, 1074 (2021).
Guo, X. et al. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat. Commun. 11, 2177 (2020).
Lu, J. et al. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 37, 147 (2018).
Saikia, M., Cheung, N., Singh, A. K. & Kapoor, V. Role of midkine in cancer drug resistance: regulators of its expression and its molecular targeting. Int. J. Mol. Sci. 24, 8739 (2023).
Kadomatsu, K. & Muramatsu, T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 204, 127–143 (2004).
Slamon, D. J. et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med 382, 514–524 (2020).
Shin, D. H. et al. Midkine is a potential therapeutic target of tumorigenesis, angiogenesis, and metastasis in non-small cell lung cancer. Cancers (Basel) 12, 2402 (2020).
Zheng, L. et al. Targeting MDK abrogates IFN-gamma-elicited metastasis in cancers of various origins. Front Oncol. 12, 885656 (2022).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Germain, P. L., Sonrel, A. & Robinson, M. D. pipeComp, a general framework for the evaluation of computational pipelines, reveals performant single cell RNA-seq preprocessing tools. Genome Biol. 21, 227 (2020).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med 24, 978–985 (2018).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Gaujoux, R. & Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinforma. 11, 367 (2010).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Acknowledgements
We acknowledge all the study participants, patients, and their family members for their contributions and support. This study was supported by the National Key Research and Development Program of China (2021YFA0910100 to X.D.C., 2022YFC2504602 to F.B.), Zhejiang Provincial Research Center for Upper Gastrointestinal Tract Cancer (JBZX-202006 to X.D.C.), Medical Science and Technology Project of Zhejiang Province (WKJ-ZJ-2104 to X.D.C.), Beijing Natural Science Foundation (Z220014 to F.B.), National Natural Science Foundation of China (82074245 to X.D.C., 81973634 to Z.Y.X., 82304946 to C.H., 82241230 to F.B.), National Science Fund for Distinguished Young Scholars (T2125002 to F.B.), Science and Technology Projects of Zhejiang Province (2019C03049 to X.D.C.), and Program of Zhejiang Provincial TCM Sci-tech Plan (2018ZY006 to X.D.C.). Post-doctor innovative Talent Support Program (BX202375 to C.H.)
Author information
Authors and Affiliations
Contributions
X.C., F.B., and Z.X. conceived the study, and directed and supervised the research. F.B. was responsible for bioinformatics investigation. X.C. and Z.X. reviewed the histopathological diagnoses. C.H. and P.Y. contributed to the enrollment of GC patients and collection of patient information. S.H. performed data processing, and bioinformatics analysis and visualization. C.H. and J.X. conducted experiments and interpreted the data. L.Y., Y.Z., J.C., Q.W., S.Z., L.Y., D.S., and Y.D. helped to design the study. Z.L. and H.L. provided advice on data analysis. S.H. and C.H. wrote the manuscript. X.C. and F.B. reviewed and edited the manuscript. All authors provided feedback.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Masanori Terashima and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, S., Hu, C., Xu, J. et al. The estrogen response in fibroblasts promotes ovarian metastases of gastric cancer. Nat Commun 15, 8447 (2024). https://doi.org/10.1038/s41467-024-52615-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52615-9
This article is cited by
-
Single-cell profiling reveals a reduced epithelial defense system, decreased immune responses and the immune regulatory roles of different fibroblast subpopulations in chronic atrophic gastritis
Journal of Translational Medicine (2025)
-
Integrating visceral protein ratios and mid-arm circumference predicts survival and malnutrition in gastric cancer
Scientific Reports (2025)
-
ERO1A-positive tumor epithelial cells in colorectal cancer progression: a multi-omics perspective
Apoptosis (2025)