Abstract
Our research elucidates the cleavage processes of the RNase III enzyme, DICER, which plays a crucial role in the production of small RNAs, such as microRNAs (miRNAs) and small interfering RNAs (siRNAs). Utilizing high-throughput dicing assays, we expose the bipartite pairing rule that dictates the cleavage sites of DICER. Furthermore, we decode the intricate recognition mechanism of the primary YCR motif and identify an analogous secondary YCR motif that influences DICER's cleavage choices. Collectively, our findings clarify the bipartite pairing rule and enhance our understanding of the role of RNA motifs in modulating DICER's cleavage activity, laying the groundwork for future research on their roles in miRNA biogenesis and gene regulation.
Similar content being viewed by others
Introduction
DICER, a key RNase III enzyme with high evolutionary conservation, is essential to RNA silencing, a critical gene regulatory pathway that influences a myriad of biological1,2,3. Its main role is to process precursor miRNAs (pre-miRNAs) and long double-stranded RNAs (dsRNAs) into microRNAs (miRNAs) and small interfering RNAs (siRNAs), typically 21–25 nucleotides in length4,5,6,7,8,9,10,11,12,13,14,15. These small RNAs are then incorporated into the RNA-induced silencing complex (RISC), targeting specific messenger RNAs (mRNAs) to either degrade them or inhibit their translation, thereby modulating gene expression16,17. Beyond its fundamental role in miRNA generation, DICER is pivotal in short hairpin RNA (shRNA) technology18,19,20. The accuracy of DICER's cleavage is vital for the proper function of miRNAs, as even slight deviations in processing can lead to changes in the targeting specificity of the resulting small RNAs.
DICER possesses two RNase III domains, RIIIDa and RIIIDb, which form an intramolecular dimer, as shown in Supplementary Fig. 1a. This dimerization is critical for DICER's ability to execute double cleavages on precursor RNA molecules such as pre-miRNAs, shRNAs, and pre-siRNAs. The result of these cleavages is the production of small RNA duplexes with lengths typically varying between 20 to 23 base pairs. The specific lengths of these duplexes arise from distinct double cleavage events, referred to as DC20, DC21, DC22, and DC23, with DC21 and DC22 being the most common sites as documented in a wide range of studies4,5,6,7,8,9,10,11,12,13,14,15,21,22.
The precision of DICER's cleavage relies on its recognition of the 5p- and 3p-ends of RNAs, facilitated by specific binding pockets that guide the cleavage to occur approximately 20–23 nucleotides from these ends6,7,8,9,10,12,14,15,21,22, also known as the 5p/3p-counting rule. However, this counting rule alone is not entirely sufficient to explain the exact positioning of the cleavage sites. It is the interplay of this rule with a suite of additional RNA elements that enables DICER to accurately identify and cleave at the precise locations required for the generation of functional small RNAs.
The placement of RNA loops plays a crucial role in directing the cleavage sites of DICER. Previous investigations have demonstrated that loops or bulges, whether they are terminal or internal and situated 2 nucleotides away from the intended cleavage site, can enhance the accuracy of DICER's cleavage23 (Supplementary Fig. 1b). These findings gave rise to the “loop counting rule” model, which posits that DICER determines its cleavage sites by assessing the position of such loops and bulges23. The study delved deeper into the underlying mechanisms and suggested that DICER's helicases are instrumental in recognizing these loops or bulges, thereby reinforcing the 2-nt loop counting principle. To corroborate their theory, researchers introduced variants of shRNAs into human cells and conducted small RNA sequencing. The resulting data were used to infer the nuances of DICER's cleavage activity. However, the study did not include direct in vitro cleavage experiments to confirm these findings, nor did it provide a comprehensive explanation of how helicases assist DICER in measuring distances accurately.
In our recent studies, we employed high-throughput dicing assays with a focus on shRNAs to dissect the intricacies of DICER-mediated cleavage with high precision. These studies have shed light on the additional RNA elements and motifs that dictate the specificity of DICER cleavage sites. Our initial findings revealed that the presence of a bulge on the 3p-strand of shRNAs/pre-miRNAs exerts significant control over the cleavage sites chosen by DICER7. We determined that the optimal position for this bulge to facilitate the DC21 cleavage is 22 nucleotides from the 5p-end of the RNA substrate. Furthermore, our research indicates that the length of the stem in shRNAs/pre-miRNAs influences the choice of DICER cleavage sites. Typically, DICER tends to cleave at DC22 on long-stemmed RNAs and at DC21 on short-stemmed RNAs (Supplementary Fig. 1c)7.
In our subsequent publication, we honed in on the characterization of sequence motifs that dictate DICER cleavage sites, uncovering a two-motif model comprising mWCU and YCR that orchestrates the cleavage process22. Each motif exerts a distinct impact on the selection of DICER cleavage sites, as depicted in Supplementary Fig. 1d. mWCU, where “m” denotes a motif containing a mismatch, “W” represents a weak base pair (A-U or U-A) in the first position, “C” signifies a C-C pair in the second position, and “U” indicates a combination of pairs with a predominant enrichment of U in the third position. YCR, where “Y” stands for a combination containing at least one pyrimidine (C or U), “C” represents a C-G or G-C pair, and “R” denotes purine-pyrimidine pairs (G-C and A-U). The mWCU motif is notably dependent on the interaction with the double-stranded RNA-binding domain (dsRBD) of DICER, whereas the YCR motif functions independently of it. Our research indicates that these motifs can act in concert to enhance the selection of a single cleavage site by DICER. Conversely, when they operate antagonistically, they lead to cleavage at different positions (Supplementary Fig. 1e). In a synergistic scenario, the combination of mWCU and YCR motifs creates a 5-position motif in which the nucleotides in positions 2–4, when read from 5p to 3p on the 3p-strand, are similar to the previously reported GYM motif (Supplementary Fig. 1e)21. While the dsRBD's recognition of the mWCU motif is well-documented, the precise mechanism by which DICER discerns the YCR motif has yet to be elucidated, presenting an intriguing area for further investigation.
In this study, we aimed to elucidate the molecular underpinnings of how elements such as the loop counting rule and YCR motifs influence DICER cleavage sites. Utilizing high-throughput dicing assays, our goal was to delineate a detailed picture of these key RNA features and their impact on DICER's activity. Our findings challenge the previously held notion of the universal applicability of the loop counting rule23. We discovered instances where this rule does not hold and found that helicases do not participate in this rule as previously suggested. We uncovered an alternate model, which we have termed the “bipartite pairing rule,” that provides a more accurate explanation for how the position of loops and the presence of mismatches govern the cleavage sites. Moreover, our research sheds light on how DICER's RIIIDa domain is responsible for recognizing the YCR motif. Through careful mapping of the interactions between RIIIDa and the YCR motif, we identified a secondary YCR recognition site for RIIIDb. Our findings lay the groundwork for a refined model of DICER cleavage, advancing our comprehension of DICER's cleavage dynamics. This research has significant implications for our understanding of miRNA biogenesis and the functional consequences of DICER mutations, particularly those implicated in human diseases, which often affect the RNA-interacting domains critical for miRNA generation.
Results
Reevaluating the role of helicases in the loop counting rule
In a prior investigation, the “loop counting rule” was proposed as a mechanism indicating that a loop or bulge located precisely two nucleotides from DICER's cleavage site improves cleavage accuracy23. The measurement of this exact 2-nt distance was thought to be facilitated by DICER's helicase domains (Supplementary Fig. 1b). This hypothesis was bolstered by miRNA sequencing data from HCT116 cell line containing helicase mutation. The study revealed less precise pre-miRNA processing in the mutant cells compared to wild-type cells, with a significant reduction in cleavage accuracy observed specifically for pre-miRNAs containing the optimal 2-nt distance from DICER's cutting site to the loop.
To further validate these observations, we rescued DICER-knockout (DICER-KO) cells with either wild-type DICER (WT) or DICER∆Helicase mutant. The data indicate that the DICER∆Helicase negatively influenced miRNA production, irrespective of conformity to the 2-nt loop counting rule (Supplementary Fig. 1f). Additionally, a reevaluation of the published data23 with the latest miRNA sequences from miRBase version 22, demonstrated that the helicase mutation impacted accuracy of miRNA processing regardless of the 2-nt loop counting rule adherence (Supplementary Fig. 1g). These findings suggest that the loop counting rule may not be exclusively influenced by the helicases.
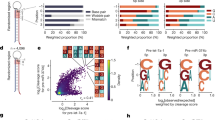
The divergence in the results of past research and our own could be attributed to updates in miRNA datasets and the advancement of RNA folding algorithms. Recognizing this, we embarked on a series of experiments to ascertain the function of helicases. We initiated our investigation by conducting high-throughput dicing assays on DICER∆Helicase. These assays utilized a library of randomized shRNAs with variable loop positions (Fig. 1a and Supplementary Fig. 1h, i). To supplement these assays, we referenced high-throughput dicing data from the same set of shRNAs processed by DICER-WT, as reported in our prior publications7,22. Upon sequencing, we identified 99.98% of expected shRNA variants in the DICER∆Helicase sample, with the median number of barcodes were 157 and 136 for replicate 1 and 2, respectively (Fig. 1b, c). We then computed the cleavage efficiency of DICER∆Helicase and confirmed the reproducibility of two independent experimental repeats (Fig. 1d).
a Schematic of two-loop shRNA featuring primary and secondary loops. A 32-nucleotide randomized sequence (32N) within the secondary loop functioned as a barcode. Green and red arrowheads mark DICER's double cleavage sites, DC21 and DC22, located 21 and 22 nucleotides from the shRNA's 5p-end, respectively. b Detection of shRNA variants in high-throughput dicing assays. Blue bars represent the count of shRNA variants identified per group. c Log2-transformed distribution of barcode counts for shRNA variants found across two separate high-throughput dicing assays for DICERΔHelicase. d Consistency between two high-throughput dicing assays for DICERΔHelicase by calculating double cleavage efficiency for each shRNA variant. Each dot point corresponds to a single shRNA variant, with 'r' denoting Pearson's correlation coefficient. e Comparison of the accuracy between cleavages that are 2 nucleotides from a loop and those that are not in DICER-WT and DICERΔHelicase libraries. The upper panel depicts cleavage occurring 2 nucleotides away from the single-stranded region including terminal loop, internal loop, and bulges. This is designated as “2nt-loop” cleavage. Cleavage events that do not conform to this pattern are classified as “no-2nt-loop” to distinguish them from the “2nt-loop” cleavage process. DC20, DC21, and DC22 refer to double cleavages at the 20, 21, and 22 positions from the shRNA's 5p-end, respectively. Asterisks denote statistical significance (p < 0.05) obtained from two-tailed Mann-Whitney U test. The sample size was indicated in the boxplots. The median value (center line), lower quartile and upper quartile (box edges), and whiskers extended to ±1.5 × IQR (interquartile range) were indicated in each boxplot. f Calculation of DICER's double cleavage accuracy at positions 19–23 in shRNAs with the stem of 21–23 base pairs. Each graph line represents a randomized shRNA variant. g shRNA structures and sequences with green and red arrowheads indicating DC21 and DC22 cleavage sites, respectively. h In vitro DICER cleavage essays for RNAs depicted in (g). i Calculation of the difference in cleavage precision between DC22 and DC21 for DICER-WT and DICERΔHelicase from three repeated assays as shown in (h), the n.s indicate no statistical significant differences from the two-sided t-test. j sh-miR30 structures and sequences. k In vitro DICER cleavage essays for RNAs depicted in (j). l Assessment of cleavage precision at DC21 and DC22 for DICER-WT from three repeated assays as demonstrated in (k). m In vitro DICERΔHelicase cleavage essays for RNAs depicted in (j). n Assessment of cleavage precision at DC21 and DC22 for DICERΔHelicase from three repeated assays as demonstrated in (m). o A model illustrating the loop counting rule's independence from DICER's helicase domains. In the bar plot representing quantitative data from the gel, data values are shown as dots, and the error bars are presented with 95% confidence intervals.
We focused on analyzing the impact of the loop counting rule across different DICER cleavage sites, specifically DC20 to DC22, 20 to 22 nucleotides from 5p-end of shRNAs. Our analysis reveal that the presence of a 2nt-loop consistently enhanced the precision of cleavage by both DICER-WT and DICER∆Helicase at DC20, DC21, and DC22 (Fig. 1e, f). These observations suggest that the 2nt-loop contributes to cleavage accuracy independent of the helicase domains. We further compared cleavage by both DICER and DICER∆Helicase using two sets of shRNAs with different stem lengths (Fig. 1g, Supplementary Fig. 1j). The cleavage patterns of both DICER and DICER∆Helicase were similar (Fig. 1h, i and Supplementary Fig. 1k, l), which aligns with the results from the high-throughput dicing assays. Taken together, our data suggest that the helicase domains of DICER do not play a pivotal role in enforcing the 2-nt loop counting rule in our tested shRNA variants.
To solidify our conclusion that helicases do not dictate the 2-nt loop counting rule, we synthesized sh-miR30 variants studied in the previous report23. These variants were designed to exhibit different cleavage sites with various stem lengths (Fig. 1j). Our data reveal that DICER preferentially cleaved sh-miR30-21 at DC21, sh-miR30-22 and sh-miR30-23 at DC21 and DC22 (Fig. 1k, l). These results of sh-miR30-21 at DC21 and sh-miR30-22 at DC22 are in line with the loop counting rule, which posits that the position of the loop 2 nucleotides relative to the cleavage site influences DICER activity. Remarkably, we observed that the DICER∆Helicase cleaved these two shRNAs at the similar respective positions as the DICER-WT (Fig. 1m, n). This finding is unexpected given the previous model's hypothesis about the necessity of helicases for the loop counting rule and suggests that the rule might operate independently of the helicase domains (Fig. 1o).
The bipartite pairing rule
Our summary in Fig. 2a indicates that the loop counting rule can explain the stem of 21 bp, but not 22 or 23 bp (22bp-stem, 23bp-stem in Fig. 1g, sh-miR30-22 and sh-miR-30-23 in Fig. 1j). For shRNAs with a 22bp-stem, DICER cleaves at both DC21 and DC22, even though only DC22 is 2 nucleotides from the loop. In the case of 23bp-stem shRNAs, DICER targets both DC21 and DC22, which are 4 and 3 nucleotides from the loop, respectively, but notably does not cleave at DC23, despite it being 2 nucleotides from the loop. This inconsistency prompted us to explore alternative models that might better elucidate DICER's cleavage site preferences. One such model centers on the importance of base pairing at the position immediately upstream of the cleavage site, designated as the −1 position (Fig. 2a). Our hypothesis suggests that a base-paired nucleotide at the −1 position is a critical determinant for DICER cleavage at either DC21 or DC22. In shRNAs with stems comprising 22 or more base pairs, the −1 position is base-paired for both DC21 and DC22. Consequently, this configuration permits DICER to cleave at both sites. However, in 21bp-stem shRNAs, the −1 base pair for DC22 is absent, which restricts DICER activity exclusively to DC21, where the −1 base pair for DC21 is intact.
a Analysis of shRNA cleavage patterns with 21bp-stem to 23bp-stems according to the 2-nt loop counting rule or the −1mM inhibitory rule. Green and red arrowheads signify DC21 and DC22 cleavages, respectively. b Determination of DICER's double cleavage accuracy at positions 19–23 in shRNAs with the stem of 23 or 24 base pairs, featuring a base pair (bp) or mismatch (mM) at the −1 position relative to DC21 and DC22. Each line on the graph corresponds to a different shRNA variant. c Comparative analysis of double cleavage accuracy between DICER and DICERΔHelicase, in shRNAs with a base pair (bp) or mismatch (mM) at the −1 position relative to DC21 and DC22, with DC21 and DC22 denoting double cleavages at the 21 and 22 nucleotides from the 5p-end of shRNA, respectively. n indicated the sample size. The median value (center line), lower quartile and upper quartile (box edges), and whiskers extended to ±1.5 × IQR (interquartile range) were indicated in each boxplot. d Depiction of shRNA structures and sequences. e In vitro DICER cleavage assays for RNAs shown in (d). f Calculation of DICER-WT cleavage efficiency on various shRNAs from data obtained in three replicate assays as depicted in (e) normalized to 2122-mM RNA. g Evaluation of cleavage accuracy at DC21 and DC22 for DICER-WT based on three repetitive assays as shown in (e). h Assessment of DICER's double cleavage accuracy at positions 19–23 in shRNAs with 22bp-stem, including a base pair (bp) or mismatch (mM) at the −2 position relative to DC21 and DC22. i Comparison of double cleavage accuracy between DICER and DICERΔHelicase in shRNAs with a base pair (bp) or mismatch (mM) at the −2 position relative to DC21 and DC22, with DC21 and DC22 indicating double cleavage at the 21 and 22 nucleotides from the shRNA's 5p-end, respectively. n indicated the sample size. The median value (center line), lower quartile and upper quartile (box edges), and whiskers extended to ±1.5 × IQR (interquartile range) were indicated in each boxplot. j Depiction of shRNA structures and sequences. k In vitro DICER cleavage assays for RNAs shown in (j). l Evaluation of cleavage accuracy at DC21 and DC22 for DICER-WT based on three repetitive assays as shown in (k). m Depiction of pre-mir-339 structures and sequences, highlighting DC21, DC22, and DC23 cleavage sites with green, red, and blue arrowheads, respectively. n In vitro DICER cleavage assays for RNAs shown in (m). o Evaluation of cleavage accuracy at DC21, DC22, and DC23 for DICER-WT based on three repetitive assays as shown in (n). p A model highlighting the bipartite pairing rule, demonstrating the essential roles of the −1 and −2 base pair positions in both DC21 and DC22 cleavages. q The bipartite pairing rule elucidates the cleavage patterns of shRNAs with varying stem lengths or mismatches near the cleavage sites. In the bar plot representing quantitative data from the gel, data values are shown as dots, and the error bars are presented with 95% confidence intervals.
Our model gains support from exhaustive high-throughput dicing assays. These assays have demonstrated that specific mismatches influence DICER cleavage sites: a mismatch at the 22 position, which corresponds to the −1 position relative to DC22, significantly reduced cleavage at DC22 for both DICER-WT and DICER∆Helicase in 24bp-stem shRNAs (Fig. 2b, c). Likewise, a mismatch at the 21 position, the −1 position relative to DC21, led to a comparable inhibition of cleavage at DC21 in 23bp-stem shRNAs for both forms of the enzyme (Fig. 2b, c).
To further substantiate our mechanism, we used shRNAs with a 23bp-stem and a 24bp-stem, incorporating mismatches at positions 21 and 22 (2122-mM), a base pair only at position 21 (21-bp), a base pair only at position 22 (22-bp), or base pairs at both positions 21 and 22 (2122-bp) (Fig. 2d and Supplementary Fig. 2a). The result reinforced our hypothesis: the presence of a mismatch at position 22 and 21 inhibited DC22 and DC21, respectively (Fig. 2e–g and Supplementary Fig. 2b–d). Intriguingly, DICER∆Helicase and DICER-WT cleaved the 23bp-stem shRNA variants in similar manner (Supplementary Fig. 2e–g). This finding confirms that base pairing at positions 21 and 22 is important for enabling cleavage at DC21 and DC22, respectively, and the influence of these base pairs operate independently of the helicase domains. Additionally, we found that the impact of these base pairs also operates independently of a DICER cofactor, TRBP, as DICER-TRBP cleaved 23bp-stem shRNAs as DICER alone performed (Supplementary Fig. 2h–j). This suggests that the bipartite pairing rule also applies to the DICER-TRBP interaction.
Introducing mismatches at both positions 21 and 22 reduced cleavage at DC21 and DC22, with a more pronounced effect on DC22 (Fig. 2e–g and Supplementary Fig. 2b–g). This pattern indicates that a mismatch at position 21, which is 2 position relative to DC22, could have a further negative impact on the cleavage efficiency at DC22. Our high-throughput dicing data unveils that a mismatch at position 20, the 2 position relative to DC21, reduced cleavage efficiency at DC21 for both the DICER-WT and DICER∆Helicase (Fig. 2h, i), though less significantly than a mismatch at the −1 position. To corroborate these findings, we conducted experiments with a 23bp-stem shRNAs featuring a mismatch at position 20 and confirmed that this mismatch indeed impeded DC21 by DICER-WT (Fig. 2j–l), validating our previous observations. They indicate that both −1 and −2 positions relative to the cleavage site contribute to the efficiency of cleavage, though the impact at −2 is less pronounced.
We next examined the base pairs at positions −1 and −2 in two pre-miRNAs, pre-miR-339 and pre-miR-496. DICER cleaved pre-miR-339 at the DC21 and DC23 positions due to the YCR at positions 19 and 21 (Fig. 2m). The introduction of a mismatch at the −2 position of DC21 (pre-miR-339-20mM) or the −1 position of DC21 (pre-miR-339-21mM) significantly inhibited this cleavage, with more inhibition observed with the mismatch at the −1 position. These mismatch introduction mildly affected DC23 cleavage. Interestingly, these mismatches reciprocally enhanced DC22 cleavage as a result of DC21 reduction (Fig. 2m–o). DICER cleaved pre-miR-496 at three positions: DC20, DC21, and DC22 (Supplementary Fig. 2k, l). The mismatch at the 20 position (pre-miR-496-20m), which was the −1 position of DC20 and the −2 position of DC21, resulted in the reduction of both these cleavages, while DC22 cleavage was less affected (Supplementary Fig. 2k–m). These results confirmed the roles of the base pairs at positions −1 and −2 in ensuring DICER cleavage in pre-miRNAs. We also analyzed the miRNA expression from the HCT116 DICER-KO cells rescued with DICER-WT and DICERΔHelicase. We found that both enzymes produced more miRNAs from the cutting sites on pre-miRNAs containing base pairs at the −1 and −2 positions and less miRNAs from the cutting sites on pre-miRNAs containing mismatches at these two positions (Supplementary Fig. 2n). This result demonstrates that base pairing at positions −1 and −2 is crucial for miRNA production by DICER and that the effect of these base pairs is independent of the helicase domains.
Taken together, our data suggests a refined model for understanding DICER's selection of cleavage sites, a “bipartite pairing rule”. This model posits that both base pairs at the −2 and −1 positions relative to a potential cleavage site are critical for directing the cleavage activity of DICER (Fig. 2p). A mismatch at the −1 position significantly inhibits cleavage, while a mismatch at the −2 position also reduces cleavage activity, albeit to a lesser extent. This model explains DICER cleavage at DC21 and DC22 in shRNAs with 22-bp and 23-bp stems, facilitated by base pairing at the −1 and −2 positions relative to each cleavage site (Fig. 2q). For shRNAs featuring a 21-bp stem, DC21 is attributed to the base pairing at the −1 and −2 positions relative to DC21. Mismatch at position −1 relative to DC22 in shRNAs with a 21-bp stem prevents cleavage at this site, resulting in exclusive cleavage at DC21 (Fig. 2q). Furthermore, in the context of longer stems, a mismatch at position 22, which is −1 relative to DC22, inhibits cleavage at DC22, thus favoring cleavage at DC21 (Fig. 2q). Conversely, a mismatch at position 21, which is −1 relative to DC21, impedes cleavage at DC21, thereby promoting cleavage at DC22 (Fig. 2q). Importantly, the mismatch at position 21, which also serves as the −2 position relative to DC22, only weakly inhibits cleavage at DC22. Consequently, even with a mismatch at position 21, DC22 remains a viable cleavage site, as demonstrated in shRNAs with 22-bp, shown in Fig. 2e and f.
Molecular basis of the bipartite pairing rule
We examined DICER/RNA complex structure8 to understand how DICER interacts with the RNA at the −1 and −2 base pair positions near the DC22 cleavage site (Fig. 3a). We found two amino acids, D1709 and S1348, that might interact with the −1 base pair, D1709 with the 5p-strand and S1348 with the 3p-strand (Fig. 3b).
a The arrangement shows RIIIDa in dark blue and RIIIDb in green, interacting with pre-miRNA (PDB: 7XW2). The designated cleavage site on the RNA is at DC22. “R-1” denotes the base pair at the −1 position relative to DC22. Two residues, D1709 and S1348, are positioned in potential interaction with the R-1 base pair. It is proposed that these residues might form hydrogen bonds with the 2-hydroxyl group of the ribose in the nucleotides at the 5p- and 3p-ends, respectively. b In the simplified model of the protein-RNA interaction shown in A, potential hydrogen bonds are depicted as dashed lines. c Anticipated comparison of cleavage accuracy and efficiency between DICER-WT and a DICER variant containing a mutation at the residue interacting with the −1 base pair. d In vitro cleavage assays conducted with DICER-S1348A to assess RNA substrates as detailed in Fig. 2d. e Analysis of cleavage accuracy at sites DC21 and DC22 for DICER-S1348A, using data from three repeated assays illustrated in (d). f Assessment of DICER-S1348A cleavage efficiency across various shRNA constructs, based on results from three replicate assays presented in (d). g In vitro cleavage assays conducted with DICER-D1709A to assess RNA substrates as detailed in Fig. 2d. h Analysis of cleavage accuracy at sites DC21 and DC22 for DICER-D1709A, using data from three repeated assays illustrated in (g). i Assessment of DICER-D1709A cleavage efficiency across various shRNA constructs, based on results from three replicate assays presented in (g). j A schematic representation explaining the influence of the D1709 residue on the bipartite pairing rule during cleavage by DICER. In the bar plot representing quantitative data from the gel, data values are shown as dots, and the error bars are presented with 95% confidence intervals.
To examine these interactions, we performed cleavage assays with purified DICER mutants, D1709A, S1348A, and four shRNA variants, each harboring specific mismatches at nucleotides 21 and/or 22 (Supplementary Fig. 3a, b). We hypothesized that if D1709 or S1348 is pivotal for interactions at the −1 position, mutants at these residues would process a 2122-mM shRNA, which harbors mismatches at positions 21 and 22, in a similar manner to DICER-WT. This similarity would suggest that mutations at D1709 or S1348 disrupt the −1 bp interactions for both DC21 and DC22 or do not interact with −1 mismatch, aligning the mutant's activity with that of DICER-WT. For a 21-bp shRNA with a base pair at the −1 position of DC21, we would expect DICER-WT to cleave predominantly at DC21. However, this base pair at −1 may not induce the mutants to cleave effectively at DC21. In the case of a 22-bp shRNA, which possesses a base pair at the −1 position of DC22, DICER-WT might prefer DC22. Nevertheless, the mutants are not expected to be similarly stimulated by the −1 base pair at DC22 to cleave at this site, especially considering that the -2 position at DC22 is mismatched. Consequently, the mutants are likely to exhibit diminished cleavage at DC22, potentially shifting their cleavage preference towards DC21, where the -2 position is base-paired. Furthermore, with shRNAs of both 21 and 22 base pairs, cleavage at both DC21 and DC22 by the mutants is anticipated to equally reduce. However, the presence of base pairs at positions 21 and 22 should not significantly drive the mutants to cleave at 2122-bp shRNAs, as in DICER-WT (Fig. 3c).
The cleavage assays for the S1348A mutant revealed a cleavage pattern and efficiency akin to those of DICER-WT (the comparison between Figs. 3d–f and 2e–g), suggesting that the S1348 residue may not be crucial for interacting with the −1 base pair position.
D1709A mutation significantly changed DICER's cleavage behavior. D1709, a pivotal metal-binding site in DICER, is integral for processing RNA along the 5p-strands, as established in several studies5,6,8,24,25,26. The mutation leads to a functional loss at this site, causing DICER to exclusively cleave at the 3p-strand. We assumed a corresponding shift in the 5p-strand when observing a shift in the 3p-strand cleavage for this mutant because DICER produces a 2-nt staggered cut on these shRNAs, as also observed in our high-throughput dicing assays (Supplementary Fig. 3c). The D1709A mutant exhibited a cleavage pattern that was noticeably different from that of DICER-WT (Fig. 3g for D1709A, Fig. 2e for WT). This mutant predominantly cleaved both the 2122-mM and 21-bp shRNAs at DC21 (Fig. 3g, h), with only a modest three-fold difference in efficiency between these two substrates (Fig. 3i). This is in sharp contrast to the over tenfold difference in cleavage efficiency observed with DICER-WT, depicted in Fig. 2f, and the S1348A mutant, shown in Fig. 3f. These findings indicate that the D1709A mutation diminishes the stimulatory effect of the −1 base pair at DC21. Furthermore, the D1709A mutant demonstrated a preference for cleaving the 22-bp shRNA at DC21 rather than DC22. This deviates from the cleavage preferences of both DICER-WT and the S1348A mutant, highlighting a reduced sensitivity of the D1709A mutant to the −1 base pair at DC22 (Fig. 3g, h). When processing shRNAs with both 21 and 22 base pairs, the D1709A mutant cleaved at both the DC21 and DC22 sites (Fig. 3g, h). The reason the D1709A mutant still cleaved this shRNA at both sites was that the interaction of DICER with −2 position for both DC21 and DC22 was still retained. However, the efficiency was only six times greater for the 2122-bp shRNAs compared to the 2122-mM shRNAs, as detailed in Fig. 3i. This is a marked departure from the more than tenfold increase in cleavage efficiency exhibited by DICER-WT (Fig. 2f) and the S1348A mutant (Fig. 3f), underscoring the significant role of D1709 in modulating the impact of the −1 base pair on DICER's cleavage specificity.
Here, we proposed two roles for D1709: interacting with the −1 base pair for cleavage site selection, and interacting with a metal ion for the execution of cleavage. D1709A lost both of these functions. We then created the D1709N mutant, where N is similar to D in terms of residue size and RNA interaction function; we anticipated that this residue would lose metal binding but still retain the −1 base pair recognition. As expected, D1709N exhibited the same single cleavage as D1709A, and the cleavage pattern was similar to DICER-WT, particularly still cleaving 22-bp shRNA at DC22 (Supplementary Fig. 3d–f).
Next, we prepared the three plasmids containing shRNA-U (21-bp, 22-bp, and 2122-bp), similar to those used in Fig. 2e, with a modification where the guanine (G) at position 19 on the 3p-strand was changed to uracil (U) (Supplementary Fig. 3g). This alteration ensured that the 5p-ends of both DC21 and DC22 siRNA-3p began with a U, thereby minimizing the impact on Argonaute strand selection. This modification did not alter DICER cleavage site in vitro compared to those used in Fig. 2e (Supplementary Fig. 3h). We sequenced siRNAs resulting from these shRNA-U plasmids in DICER-KO cells expressing either DICER-WT, D1709A, or D1709N variants. With 2122-bp shRNAs, DICER-WT generated both 21-nt and 22-nt siRNAs, with a notably higher proportion of 22-nt compared to 21-nt. In vitro, DICER-WT equally produced 21-nt and 22-nt siRNAs. This variation between in vitro and in vivo findings could be attributed to different influences on DICER cleavage in these environments. In cellular contexts, the final siRNA sequence may be affected by several factors, including DICER cleavage, Argonaute protein selection, and siRNA modification. Consistent with the in vitro observations, DICER-WT predominantly generated 21-nt siRNAs from 21-bp shRNAs and 22-nt siRNAs from 22-bp shRNAs. DICER-D1709A exhibited a similar pattern of 21-nt and 22-nt siRNA production from 2122-bp shRNAs (Supplementary Fig. 3i). However, this mutation significantly reduced the production of 21-nt or 22-nt siRNAs from 21-bp or 22-bp shRNAs due to the lack of interaction with 21-bp or 22-bp from two RNAs. Additionally, DICER-D1709N demonstrated cleavage patterns similar to DICER-WT across all three shRNAs. These findings underscore the role of the D1709 residue in recognizing the −1 base pair, contributing crucially to the specificity of DICER cleavage (Supplementary Fig. 3i).
Taken together, our data highlight the crucial role of D1709 in mediating the interaction with the −1 base pair for DICER cleavage accuracy (Fig. 3j). The conservation of a residue that interacts with the −1 base pair in structures of other RNase III enzymes suggests that this mechanism of interaction may be a universal feature among RNase III family members. This conserved interaction points to a fundamental aspect of the RNase III cleavage activity and further emphasizes the significance of the D1709 residue in DICER function (Supplementary Fig. 3j, k)15,27.
YCR recognizing mechanism
Our previous investigation underscored the importance of the YCR motif in dictating the cleavage specificity of DICER22. The other research also identified the GYM motif21, with both studies converging on the significance of a cytosine-guanine (C-G or G-C) base pair (C component in the YCR motif or G component in the GYM motif). Our laboratory’s earlier research22 also highlighted a pronounced enrichment of the C-G pair in the motifs that were most effective in stimulating DICER cleavage, as depicted in Fig. 4a. This enrichment was not only observed in human pre-miRNAs but also across a wide array of species (Supplementary Fig. 4a), reinforcing the essential role of the C-G pair within the YCR motif. To investigate the influence of a single C-G base pair on the preference for cleavage sites, we synthesized two shRNAs that are identical except for a single base pair at position 20, as depicted in Fig. 4b. In agreement with prior research22, we observed that the shRNA containing a C-G pair at position 20 (20-CG) exhibited a greater propensity for cleavage at DC21 compared to the shRNA with an A-U pair at the same position (20-AU), as shown in Fig. 4c.
a Enrichment analysis of C-G and G-C pairs within identified YCR motifs. YCR motifs previously identified were subjected to enrichment analysis using the Logomaker package36 to evaluate the prevalence of nucleotides at specific positions. The graphical representation employs stacks, where the height of each stack signifies the information content of the sequence position. b Representation of shRNA structures and sequences, with green and red arrowheads indicating the DC21 and DC22 cleavage sites, respectively. c In vitro DICER cleavage assays performed on RNAs depicted in (b) using DICER-WT. d In C-G and G-C pairs, G contains an -NH2 group indicated with the red arrow that acts as a hydrogen bond donor toward the minor groove, a feature does not present in C, U, and A. e In vitro DICER cleavage assays on RNAs illustrated in (b) for various DICER mutants. f Assessment of cleavage accuracy at DC21 and DC22 for both DICER-WT and its mutants based on data from three replicate assays as shown in (c and e). “SC” denotes the single cleavage on the 5p-strand of shRNAs. g In vitro DICER cleavage assays conducted on RNAs displayed in (b) for DICER variants with different amino acids at position 1564. h, Assessment of cleavage accuracy at DC21 and DC22 for DICER-E1564Q based on data from three replicate assays as shown in (g). “SC” denotes the single cleavage on the 5p-strand of shRNAs. i A schematic representation illustrates the DICER-RNA complex during the dicing state (PDB: 7XW2). E1564 within RIIIDa and the C-G base pair in the pre-miRNA are depicted as sticks. The residue E1564 identifies the C-G pair situated within the minor groove by establishing a hydrogen bond with the -NH2 group on the guanine base. j Representation of pre-mir-30a structures and sequences, with green and red arrowheads indicating the DC21 and DC22 cleavage sites, respectively. (k, l) In vitro DICER cleavage assays performed on RNAs depicted in (j) using DICER-TRBP (k) or DICER-E1564A-TRBP and DICER-E1564Q-TRBP (l). m Analysis of cleavage accuracy at sites DC21 and DC22 for DICER-TRBP from three replicate assays as shown in (k) using the F2 fragments as indicators for the cleavage site because of the overlapping between F1 and F3 derived from DC21 and DC22. n Analysis of cleavage accuracy at sites DC21 and DC22 for DICER-E1564A-TRBP and DICER-E1564Q-TRBP from three replicate assays as shown in (l) using the SC fragments (F1) as indicators for the cleavage site because the mutant DICER disrupts 3p cleavage generating F1 and F23 fragments. In the bar plot representing quantitative data from the gel, data values are shown as dots, and the error bars are presented with 95% confidence intervals.
To identify potential amino acids interacting with the C-G or G-C base pair, we established specific criteria. The amino acids of interest should be capable of forming a hydrogen bond with the guanine of a C-G or G-C pair through the hydrogen bond donor found in the -NH2 group of guanine, situated within the minor groove (Fig. 4d). This -NH2 group is unique to guanine among the nucleobases28. Therefore, we focused on residues like glutamate (E) or aspartate (D), which possess a carboxyl group known to be one of the strongest hydrogen bond acceptors29. In the available DICER/RNA complex structure, these residues should lie within an appropriate distance from the C-G or G-C pair. Upon analysis of the resolved DICER/RNA structure8, we identified four potential amino acids: E1316, D1561, D1320, and E1564 (Supplementary Fig. 4b). We substituted each of these with alanine (A), as shown in Supplementary Fig. 4c, d, and then assessed the mutant enzymes for changes in cleavage sites in response to the presence of a C-G pair. Of the four mutants, E1316A, D1561A, and D1320A still directed cleavage to DC21 in the presence of a C-G pair, similarly to the DICER-WT, as shown in Fig. 4e, f. In contrast, the E1564A mutant did not exhibit a significant change in the cleavage site compared to DICER-WT when a C-G pair was present (Fig. 4e, f), which supports that E1564 might be the residue interacting with the C-G pair. Turning our focus to the E1316, D1561, D1320, and E1564 residues, their role as a crucial metal binder in DICER's structure and function, particularly in cleaving 3p-strands, is well-supported by extensive research5,6,8,25,30. These residues conservations from bacterial to human RNase III enzymes underscore their importance (Supplementary Fig. 4e). The introduction of these mutations disrupt 3p-strand cutting function, redirecting cleavage activity exclusively to the 5p-strand. Confirmatory gel assays showed this shift clearly. Analyzing these results, we inferred a corresponding reduction in 3p-strand cleavage, a deduction based on the altered 5p-strand activity facilitated by the 2-nt overhang feature of DICER cleavage.
To further validate the role of E1564 in recognizing the C-G pair, we introduced a conservative mutation, substituting glutamic acid (E) with glutamine (Q), a residue of similar size that could potentially recognize and interact with the C-G pair due to its amide group. Both E1564A and E1564Q mutants of DICER produced single cleavage sites, which is consistent with the importance of E1564 in the enzyme's catalytic center. However, in contrast to E1564A, the E1564Q mutant displayed a cleavage pattern akin to that of the DICER-WT in the presence of a C-G pair, as depicted in Fig. 4g, h. This pattern was replicated with the G-C pair, as shown in Supplementary Fig. 4f, g. It is observed that the E1564Q variant solely cleaved a 24bp-stem 20-GC at DC21 (see Supplementary Fig. 4g) but cleaved a 24bp-stem 20-CG (see Fig. 4g) at both DC21 and DC22. This suggests that a G-C pair stimulates the E1564Q variant to cleave at DC21 more effectively than a C-G pair in the same position, or that the Q residue has a stronger affinity for G-C than for C-G. Collectively, these findings lend credence to our hypothesis that the glutamic acid at position 1564 is involved in recognizing the C-G pair through hydrogen bonding, as illustrated in Fig. 4i.
To investigate the role of E1564 in recognizing the C element of the YCR motif within the pre-miRNA context, we conducted experiments using two variants of pre-mir-30a: pre-mir-30a-CG and pre-mir-30a-AU. The presence of a C-G pair at position 21 is hypothesized to facilitate cleavage at DC22 in this pre-miRNA. Subsequently, we assessed the impact of substituting the C-G pair with U-A on pre-miR-30a cleavage by DICER. For these experiments, we used DICER-TRBP, known for enhancing DICER cleavage in this context. We observed that DICER-TRBP predominantly cleaved at the DC22 position in pre-mir-30a-CG, whereas cleavage at this site was significantly reduced in the mutated pre-mir-30a-AU. The DICER-E1564A-TRBP mutant showed a comparable reduction in cleavage efficiency at DC22 for both pre-mir-30a-CG and pre-mir-30a-AU, indicating the crucial role of the E1564 residue in facilitating DC22 cleavage. Furthermore, introducing a mutation converting E1564 to glutamine (E1564Q) altered the cleavage pattern: DICER-E1564Q-TRBP cleaved both pre-mir-30a-CG and pre-mir-30a-AU at DC21. This shift in cleavage preference is attributed to glutamine's enhanced ability to interact with the G-C pair located at position 20 in pre-mir-30a, which improves its binding affinity and promotes cleavage at DC21. The above results using shRNA demonstrated that glutamine has a stronger affinity for G-C than C-G (Supplementary Fig. 4f, g). This experiment underscores the complex interplay between specific nucleotide interactions and DICER cleavage dynamics, highlighting the critical roles of both the C-G pair and the E1564 residue.
Due to the inability to express pre-mir-30a directly in cells, we opted to co-express either pri-mir-30a-CG or pri-mir-30a-AU with DICER-WT, E1564A, or E1564Q variants in DICER-KO cells. We then performed small RNA sequencing to analyze the cleavage sites of DICER within a cellular context (Supplementary Fig. 4h). Initially, we verified that the Microprocessor complex similarly cleaved both pri-miRNAs (Supplementary Fig. 4i) indicating that the presence of C-G or A-U does not affect Microprocessor cleavage. Subsequent analysis of the 5p-ends of miR-30a-5p, which represent the Microprocessor's cleavage sites in cells, showed that they were identical between the expressed pri-mir-30a-CG and pri-mir-30a-AU across all DICER variants (Supplementary Fig. 4j). We then focused on the 5p-ends of miR-30a-3p and the 3p-ends of miR-30a-5p to indicate DICER's cleavage sites. Our results indicated that DICER-WT produced fewer DC22 miR-30a molecules from pri-miR-30a-AU compared to pri-miR-30a-CG. The E1564A variant of DICER resulted in significantly fewer DC22 miR-30a molecules from both pri-miR-30a variants. Conversely, the DICER-E1564Q variant predominantly generated DC21 miR-30a molecules from both pri-miRNA variants (Supplementary Fig. 4k, l). These observations in cellular miRNA expression aligned with our in vitro assay findings, underscoring the impact of the E1566 mutations on the precision and efficiency of DICER's cleavage.
Discovery of the secondary YCR motif
Given the structural parallels between RIIIDa and RIIIDb domains of DICER and their staggered alignment along double-stranded RNA (dsRNA), it is plausible to posit a similar interaction between RIIIDb and a yet-to-be-identified secondary YCR motif, akin to the interaction observed between E1564 in RIIIDa and the C-G or G-C base pairs within the primary YCR motif (Fig. 5a). To investigate this hypothesis, we commenced assessing whether positioning the YCR motif at hypothetical sites could influence DICER cleavage patterns in the high-throughput dicing assays conducted in our previous study for DICER-WT6. The presence of the secondary YCR motif indeed resulted in expected alterations to DICER cleavage sites, as shown in Fig. 5b. Building on these intriguing findings and predicting a significant influence of the G-C pair on the secondary YCR relative to the primary YCR, we engineered three shRNAs with variations in the presence or absence of a G-C base pair at distinct positions, as shown in Fig. 5c. In line with our expectations, introducing a G-C pair at position 23 resulted in an increase in DC21 cleavage, whereas positioning a G-C pair at position 24 favored DC22 cleavage when compared to the shRNA containing A-U pairs at both positions 23 and 24 (referred to as 2324-UA shRNA), as illustrated in Fig. 5d, e.
a Prediction of the secondary YCR motif. The secondary YCR motif, located at positions 22 and 23, presumably guides DICER to cleave shRNAs at DC21 and DC22, respectively. b Comparison of cleavage accuracy at DC21 and DC22 for between shRNA variants containing the YCR motif at position 22 and 23, respectively, with those that do not in high-throughput DICER-WT libraries. shRNA variants containing mismatches at position 22 were excluded from the analysis due to the violation of bipartite pairing rule for DC22. P-values were obtained from two-tailed Mann-Whitney U test. n indicated the sample size. The median value (center line), lower quartile and upper quartile (box edges), and whiskers extended to ±1.5 × IQR (interquartile range) were indicated in each boxplot. c Illustration of shRNA structures and sequences, with green and red arrowheads marking the DC21 and DC22 cleavage sites, respectively. d In vitro DICER cleavage assays for RNAs shown in (c) using DICER-WT. e Evaluation of cleavage accuracy at DC21 and DC22 for DICER-WT based on results from three repeated assays as depicted in (d). f Superimposition of human DICER RIIIDa (purple color) and RIIIDb (green color). g In vitro DICER cleavage assays on RNAs represented in c for different DICER mutants. (h and i) Analysis of cleavage accuracy at DC21 and DC22 for DICER-E1813A and DICER-E1813Q using data from three replicate assays as shown in (g). j A model illustrating the double YCR (dYCR) motif and the involvement of residues E1564 and E1813 in recognizing the major C-G or G-C pairs within the dYCR. In the bar plot representing quantitative data from the gel, data values are shown as dots, and the error bars are presented with 95% confidence intervals.
Upon examining the structures of RIIIDa and RIIIDb, we identified that the amino acid E1813 in RIIIDb aligns with the amino acid E1564 in RIIIDa (Fig. 5f). To probe the role of E1813 on G-C recognition, we generated and purified E1813A mutant enzyme (Supplementary Fig. 5a, b) and then compared their cleavage activity to that of DICER-WT. In our analysis of the E1813A mutation, we noted that E1813 serves as an essential metal-binding residue, crucial for 5p-strand RNA cleavage by DICER5,6,8,24,25,26. Mutation at this site causes a complete shift of cleavage activity to the 3p-strand. This critical change was confirmed through detailed gel analysis. Furthermore, we utilized the cleavage pattern changes observed on the 3p-strand to infer disruptions in 5p-strand cleavage, applying DICER's mechanism of a 2-nt overhang to explain the observed alterations.
Our results revealed that introducing the G-C pairs at position 23 (23-GC) or 24 (24-GC) did not clearly alter the cleavage sites of the E1813A mutant compared to the 2324-UA shRNA, as shown in Fig. 5g, h. Furthermore, we assessed the influence of the G-C pair on the E1813Q mutant (Supplementary Fig. 5a, b). Interestingly, the cleavage sites of the E1813Q mutant were altered by the G-C pair in a manner similar to DICER-WT (Fig. 5g, i). This supports our hypothesis that E1813 is involved in recognizing the G-C of secondary YCR motif.
The secondary YCR motif is localized in the shRNA backbone at positions 22–24 or 23–25 (Fig. 5a). However, most pre-miRNAs feature a stem shorter than 23 base pairs. Upon analyzing human pre-miRNAs, we identified only a limited number that contained a secondary YCR. Consequently, we investigated the impact of this secondary YCR on DICER's cleavage efficiency in pre-mir-222 and pre-mir-27a. We discovered that replacing the G-C pairs in this motif with U-A or A-U pairs slightly reduced DICER cleavage efficiency (Supplementary Fig. 5c–h).
In summary, our research has led to the identification of double YCR motifs that are recognized by both the RIIIDa and RIIIDb domains of DICER, highlighting a symmetrical mode of substrate recognition by the enzyme (Fig. 5j).
Discussion
A detailed exploration of DICER's dicing model resembles the meticulous process of adjusting a microscope: from coarse calibration to fine-tuning for precision. At the heart of DICER's function is the determination of primary cleavage sites, governed by a dual-binding mechanism that recognizes both the 5p-end and the 3p-end of substrates such as shRNAs and pre-miRNAs (Fig. 6a). This mechanism aligns DICER's catalytic center with the dsRNA region, poised to make double cleavages. The spatial configuration between the binding pockets and the catalytic centers is approximately 21–22 base pairs, forming the basis for what can be likened to a “coarse” measurement in microscopy terms.
a Coarse measurement of DICER by end-binding pockets. The initial interaction between 5p- and 3p-end binding pockets of DICER and the ends of RNA positions the DICER catalytic center approximately 21–22 nucleotides from the RNA ends. Consequently, DICER is predisposed to cleave RNA around the DC21 and DC22 sites. b Fine measurement of DICER is conducted by multiple mechanisms. Bulge model: Bulges on the 3p-strand positioned 22 and 23 nucleotides from the 5p-end of RNAs are known to enhance DICER cleavage at DC21 and DC22, respectively. mWCU model: An mWCU motif located 17 or 18 nucleotides from the 5p-end of RNAs enhances DICER cleavage at DC21 and DC22, respectively. This enhancement is attributed to interactions between the motif and residue R1855 within the dsRBD domain of DICER. Double YCR model: The incorporation of a double YCR motif at the 19 and 20 nucleotides from the 5p-end of RNAs prompts DICER to cleave at DC21 and DC22, respectively. This process involves the participation of residues E1564 and E1813. Bipartite pairing model: The two base pairs located immediately upstream of the cleavage sites, at positions −1 and −2, are integral for cleavage efficiency at DC21 or DC22. Consequently, a mismatch at the −1 position relative to the DC21 site can stimulate cleavage at DC22 by impeding cleavage at DC21, and vice versa.
However, akin to fine adjustments on a microscope, DICER employs a series of “fine measurement” strategies to ascertain the precise cleavage sites, whether at DC21 or DC22 (Fig. 6b). One such fine-tuning mechanism is the recognition of a single nucleotide bulge on the 3p-strand, situated 2 nucleotides away from the cleavage site. The position of this bulge at the 22 or 23 position can sway the cleavage to DC21 or DC22, respectively. Another fine measurement mechanism involves the interaction between mWCU and dsRBD, which can stimulate cleavage at DC21 or DC22 when mWCU being placed at positions 17 (17-mWCU) and 18 (18-mWCU)22, respectively. Additionally, the double YCR (dYCR) motifs interact with RIIIDa and RIIIDb, determining cleavage at DC21 or DC22 depending on whether this motif is at positions 19 (19-dYCR) or 20 (20-dYCR). Furthermore, the bipartite pairing rule involving two base pair integrations with RIIIDb ensures precise cleavage at either DC21 or DC22. The presence of mismatches plays a critical role in determining enzyme cleavage specificity by inhibiting the alternative site: a mismatch at position 21 inhibits DC21, favoring DC22, and vice versa.
Our investigation confronts the prevailing interpretation of the RNA loop's influence, challenging the traditional 2-nt loop counting rule with robust experimental evidence. The prior model proposed that helicases might measure approximately 2 nucleotides from the loop or bulge to determine DICER's cleavage sites. A critical reexamination through the in vitro assays, however, casts doubt on the helicase's pivotal role as proposed by the loop counting rule. Notably, the DICER helicase mutant demonstrated cleavage patterns indistinguishable from the DICER-WT, irrespective of loop positioning. This observation, bolstered by high-throughput dicing assays, suggests a minimal, if any, role of helicases in loop-based measurement. The loop counting rule does not fully account for the cleavage phenomena in shRNAs with 22-bp stems or longer, where DICER consistently prefers cleaving at DC21 and DC22. This observation led us to propose an alternative model, the “bipartite pairing rule”. This paradigm suggests that two base pairs at the −2 and −1 positions relative to the cleavage site are critical for DICER's action. The bipartite pairing rule elucidates the cleavage patterns across various stem lengths and provides insights into the effects of mismatches or loops on DICER's site selection, a nuance unexplained by the loop counting rule. In pursuit of molecular validation, we identified the specific DICER domain that supports the bipartite pairing rule, reinforcing the model's credibility. Our research presents the bipartite pairing rule as a complementary framework to the loop counting rule, offering a more precise and comprehensive understanding of DICER’s cleavage dynamics.
Our investigation has led to the identification of a secondary YCR, a feature previously undetected in both our earlier work and that of other research groups21,22. The discovery of the primary YCR, recognized by the RIIIDa domain, prompted us to explore the potential interaction of a secondary YCR and RIIIDb, which is similar to RIIIDa. This comparative analysis resulted in the identification of an analogous secondary YCR motif. Biochemical assays confirmed that the secondary YCR control DICER cleavage sites are similar to the primary YCR. We also revealed that the G-C and C-G base pairs of the primary and secondary YCRs are engaged by distinct amino acids, identified as E1564 and E1813, respectively. In the dicing state of the current DICER-RNA structural model, these amino acids are positioned at a considerable distance from the YCRs, suggesting the absence of hydrogen bonds. Based on these observations, we propose a dynamic interaction model: these amino acids initially bind to the YCRs during the binding state, aligning the catalytic center that encompasses four critical residues in proximity to the intended cleavage sites. After this initial interaction do Mg2+ ions enter the scene to facilitate the transition to the dicing state, causing the amino acids to retract from the YCRs. A structural analysis of DICER-RNA prior to dicing would be instrumental in validating this hypothesis.
The −2 position relative to the cleavage site in the bipartite pairing rule corresponds to the C element of the YCR motif and the −1 position to the R element of the YCR motif. We propose a dual-layer regulatory mechanism at these positions. The first layer concerns whether the nucleotides are paired or unpaired, with base pairing generally enhancing DICER cleavage more effectively than mismatches. The second layer of regulation involves specific, sensitive base pairs that boost DICER cleavage. At the −2 position, C-G and G-C pairs are particularly effective, while at the −1 position, A-U and G-C pairs are crucial within the R element of the YCR motif. These findings indicate a sophisticated interplay between base pairing and sequence specificity that dictates DICER's cleavage efficiency at these sites (Supplementary Fig. 6a).
The experimental design featuring a tandem arrangement of YCR motifs, forming a double YCR spanning six base pairs, unveiled a compelling pattern. The double YCR exhibits two pivotal positions reminiscent of those in the DRES (DROSHA dsRNA recognition sites) identified in our previous studies22,31. Specifically, a C-G base pair at position 1 and a G-C base pair at position 4 are shared (Supplementary Fig. 6b, c), hinting at a conserved recognition strategy between these two RNase III family members, which are speculated to have a common evolutionary origin31,32. These insights are not only pivotal for understanding DROSHA's DRES recognition but may also bridge gaps in our knowledge concerning substrate recognition across RNase III enzymes.
Our study employed purified DICER and DICER-TRBP along with synthetic shRNAs and pre-miRNAs to investigate the cleavage mechanisms of DICER. We recognize that miRNA expression in cells is influenced by a variety of factors, including DICER itself, cofactors, post-cleavage miRNA modifications, Ago strand selection, and potential differences in RNA structure between in vitro conditions and cellular environments. Therefore, it is essential to consider all these mechanisms when interpreting miRNA patterns observed in cellular contexts. Understanding the motifs provides us with a better foundation for designing shRNAs. Mismatches and specific sequence motifs near the cleavage sites are crucial in controlling DICER cleavage accuracy and efficiency. We can also integrate the double YCR with the bipartite pairing rule in designing shRNAs. For instance, consider a design with a double YCR targeting DC21 and a mismatch at position 22, which inhibits DC22, thereby facilitating cleavage at DC21. Alternatively, a double YCR targeting DC22 with a mismatch at position 21 inhibits cleavage at DC21, maximizing cleavage at DC22.
Methods
Human DICER expression and purification
We utilized the same pXG-DICER construct as in our prior studies. All mutant plasmids were derived from pXG-DICER by employing the In-Fusion cloning technique; details regarding the primers are available in Supplementary Data 1.
To express DICER wild-type or mutant enzymes, pXG-DICER or the corresponding mutant plasmids were transfected into HEK293E cell cultures (300 mL) using linear polyethylenimine (PEI) and dimethyl sulfoxide (DMSO). The transfected cells were incubated at 37 °C and harvested after 60 h.
Purification followed our established protocol. Briefly, the cell pellet was resuspended in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 500 mM NaCl, 4 mM β-mercaptoethanol (Thermo Fisher Scientific), 0.1 mg/mL RNase A (Thermo Fisher Scientific), and EDTA-free Pierce Protease Inhibitor Mini Tablets (Thermo Fisher Scientific). The suspension underwent sonication and high-speed centrifugation before the lysate was introduced to 0.5 mL of pre-equilibrated Ni-NTA resin (Thermo Fisher Scientific). The resin with bound protein was washed with two buffers: one with 20 mM Tris-HCl (pH 7.5), 4 mM β-mercaptoethanol, and 500 mM NaCl (T500), and the other with 150 mM NaCl (T150), both supplemented with 40 mM imidazole. Proteins were eluted from the Ni-NTA resin using T150 buffer enhanced with 200 mM imidazole and subsequently applied to a Q Sepharose Fast Flow column (GE Healthcare). After washing with T150, proteins were finally eluted with a buffer composed of 20 mM Tris-HCl (pH 7.5), 500 mM NaCl, 10% glycerol, and 2 mM dithiothreitol (DTT) (Sigma-Aldrich).
TRBP protein was purified using the same method as the DICER enzyme. The DICER-TRBP complexes, either wild-type or mutants, were prepared by combining the purified DICER variants with TRBP protein.
Two-loop shRNA synthesis
Supplementary Data 2 contains detailed information on all the oligonucleotides used in this section. We generated 15 shRNA groups using 15 single-stranded DNA (ssDNA) oligos with random nucleotides obtained from Integrated DNA Technologies (IDT). Each ssDNA comprised a 32-nt random barcode sequence, an shRNA-encoding region with a cumulative random nt content across two segments, and a 23-nt sequence complementary to the R-set6 primer (CTG AAG TAT CGG AAT ATG CAT GG).
To create double-stranded DNA (dsDNA), we annealed 100 pmol of each ssDNA oligo with 150 pmol of the R-set6 primer in a 10 µL reaction containing 100 mM NaCl. The annealing process involved heating the mixture to 98 °C for 3 min, maintaining it at 65 °C for 5 min, and then cooling it on ice for 1 min.
Next, we used five units of Klenow fragment (Thermo Scientific) to extend the annealed primer in a 20 µL reaction at 37 °C for 2 h, resulting in complete dsDNA. We amplified the dsDNA using primers F-T7 (TAA TAC GAC TCA CTA TAG GG) and R-set6 to introduce the T7 promoter. The amplified product was then digested with PsiI restriction enzyme (Thermo Scientific) at 37 °C for 2 h using 500–1000 ng of the T7-tagged dsDNA.
Subsequently, we used the digested dsDNA in a 20 µL in vitro transcription reaction with the MEGAscript T7 Kit (Invitrogen) to produce RNA substrates. We purified the transcribed RNA on a gel, measured the concentration using a NanoDrop 2000 spectrophotometer (Thermo Scientific), and stored the samples at −80 °C until needed.
High-throughput shRNA cleavage assays with DICERΔHelicase
In our high-throughput shRNA cleavage assays, we incubated 3 pmol of each shRNA group (numbered 1–15) with 2 pmol of purified DICERΔHelicase. The reactions were carried out in a 10 μL cleavage buffer composed of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 0.2 µg/µL BSA, 1 mM DTT, and 2 mM MgCl2 at 37 °C for 30 min.
To terminate the cleavage reactions, we added 10 μL of 2X-TBE buffer (20 mM Tris-HCl pH 7.5, 20 mM EDTA pH 8.0, and 480 mg/mL urea), followed by incubation with 20 μg of proteinase K (Thermo Fisher Scientific) at 37 °C for 15 min and then at 50 °C for another 15 min. The mixtures were subsequently heated at 95 °C for 5 min.
We resolved the reaction products on a 12% urea-PAGE gel to analyze the cleavage outcomes. The double cleavage (DC) products were gel-purified following electrophoresis.
The RNA cloning and sequencing for high-throughput shRNA cleavage assays
For the cloning and sequencing of RNA from high-throughput shRNA cleavage assays, we initially ligated the original substrates (OS) to the 4N-RA3 adapter using T4 RNA Ligase 2, truncated KQ enzyme (NEB, M0373L). The ligated products, 4N-RA3-OS, were then purified via gel extraction. We proceeded to reverse transcribe the purified RNAs using a cirRTP primer and Superscript IV Reverse Transcriptase (Invitrogen), incubating the mixture for 60 min at 50 °C. To degrade the remaining RNA, 0.1 M NaOH was added to the mixture, which was then heated at 98 °C for 10 min. The resulting cDNAs were purified and circularized using CircLigase ssDNA ligase (Epicentre). The circularized cDNAs were distinguished from linear DNA in an 18% urea-PAGE gel. After gel extraction of the circularized cDNAs, we amplified the OS products by PCR using the RP1 and one of the RPIx primers, part of the TruSeq Illumina primer set.
For the double cleavage (DC) fragments, we ligated them to the 4N-RA3 adapter and resolved the 4N-RA3-ligated DC from the unligated DC and free 4N-RA3 using a 12% urea-PAGE gel. The ligated DC fragments were then gel-purified. We followed by ligating the purified 4N-RA3-ligated DC to the 4N-RA5 primer using T4 RNA Ligase 1. These double-ligated DC fragments were reverse-transcribed with Superscript IV Reverse Transcriptase and the R-RA3 primer. The resulting cDNAs were PCR-amplified using the RP1 and RPIx primers.
The concentration of the resulting DNA libraries was quantified using the Qubit™ dsDNA HS Assay Kit.
In summary, we created two to three DNA libraries for each replicate of the high-throughput dicing assays for a given enzyme. We sequenced all libraries on the Illumina NovaSeq 6000 system employing 150 bp paired-end sequencing.
The sequences of the oligonucleotides used in this process are listed in Supplementary Data 2.
High-throughput sequencing data analysis
Processing of the sequencing reads
In accordance with the methodologies established in our previous research7,22, the analysis of high-throughput sequencing data commenced with the removal of adapter sequences from the raw reads. This step was performed using Cutadapt33 with the following parameters: cutadapt -a TGGAATTCTCGGGTGCCAAGG -m 10 for Read 1, and cutadapt -a GATCGTCGGACTGTAGAACTCTGAAC -m 10 for Read 2. Subsequently, the adapter-trimmed paired-end reads were concatenated using fastq-join34. Following this, low-quality reads were excluded from further analysis, and identical sequencing reads were consolidated using fastq_quality_filter and fastx_collapser from the FASTX-Toolkit (version 0.0.13, available at http://hannonlab.cshl.edu/fastx_toolkit/index.html). The resulting data for OS and DC libraries were then subjected to their respective specialized processing pipelines.
For the processing of OS libraries, the initial step involved trimming the 6-nt barcodes from the 5p-end and 4-nt barcodes from the 3p-end of the sequencing reads. This was accomplished using Cutadapt with the command: cutadapt -u 6 -u -433. Subsequently, Cutadapt was employed once more to divide the reads into two distinct segments, shRNA-OS, and the 32N barcode, employing the parameters: cutadapt -g GCTTGC…GCAAGC -m 32 -M 32 --discard-untrimmed33. This step ensured that shRNA-OS/32N pairs with a 32N barcode associated with more than a single shRNA-OS sequence were excluded from further analysis. The remaining shRNA-OS sequences were then subject to alignment against a reference compendium of 42,496 potential randomized sequences using BWA34. Only those reads that aligned perfectly to the reference were retained for downstream analysis. Finally, the raw counts of each unique shRNA-OS within the shRNA-OS/32N pairings were normalized to reads per million (RPM) to facilitate comparative analysis across samples.
For the DC libraries, the preprocessing involved the removal of 4-nt barcodes from both ends of the reads using Cutadapt with the command: cutadapt -u 4 -u -433. Similar to the approach taken with the OS reads, the trimmed DC reads were then divided into two segments by Cutadapt: the cleaved shRNA product (shRNA-CP) and a 32N barcode. The command used for this splitting process was cutadapt -g GCTTGC…GCAAGC -m 32 -M 32 --discard-untrimmed. Subsequent to the segmentation, for each shRNA-CP/32N pair, the shRNA-CP segment was correlated with the corresponding shRNA-OS in the shRNA-OS/32N dictionary that had an identical 32N barcode. To accomplish the mapping of shRNA-CP to shRNA-OS, the local alignment mode of the pairwise2 package within Biopython was employed35.
Calculation of cleavage efficiency and accuracy
Using the alignment coordinates returned by the pairwise2 package, denoted as (x, y), the cleavage sites of DICER within the shRNA sequence were determined.
-
1.
Double cleavage with 2-nt overhang at position x (DCx) if 19 ≤ x ≤ 23 and y = 72 – x;
-
2.
Other double cleavages with non-2-nt overhang (Other) if 19 ≤ x ≤ 23 and 68 ≤ y ≤ 72 and y ≠ 72 – x;
For each cleavage site of each shRNA variant, the raw read counts were converted to RPM.
The local cleavage efficiency at the cleavage site x of each variant was calculated by log2(Nx + 0.1) – log2(Ns + 0.1).
The total double cleavage efficiency of each variant was calculated by log2(∑Nx + 0.1) – log2(Ns + 0.1).
The cleavage accuracy score at the cleavage site x of each variant was calculated by Nx/∑Nx.
In the context of assessing DICER cleavage, the normalized count (Nx) for a cleaved product at cleavage site x, expressed in reads per million (RPM), was calculated, as was the normalized count (Ns) for the original shRNA substrate that yielded the cleaved product. To avoid issues with zero counts, a pseudocount of 0.1 was added.
The average cleavage efficiency and accuracy for each shRNA variant were derived from at least two independent experimental replicates. For comparative analyses, the high-throughput (HT) DICER-WT libraries used as controls against the high-throughput DICERΔHelicase libraries created in this study were sourced from two prior publications15,22.
To predict the secondary structures of each shRNA variant, RNAfold from the ViennaRNA Package version 2.4.13 was used32. The length of the shRNA stem was quantified by counting the number of base pairs (bp) and symmetric mismatches on the 5’-strand, starting from the first base pair of the stem and ending at the apical loop. This structural analysis is critical for understanding the properties of shRNA variants, as the structure can influence DICER processing efficiency and accuracy.
Rescue experiment and small RNA library preparation
DICER knockout (DICER-KO) cells, derived from HCT116 and kindly provided by Prof. Narry Kim's lab at Seoul National University, were cultured in 6-well plates. We transfected the cells with 2.5 µg of either plasmid (pXG-DICER or pXG-DICERΔHelicase) using Lipofectamine 3000. At 48 h post-transfection, the cells were harvested and lysed with Trizol to extract total RNA.
To construct the small RNA libraries, we used the NEBNext® Small RNA Library Prep Set for Illumina (NEB, E7330S). We started by separating 10 µg of total RNA on 12% urea-PAGE gels, from which we purified the small RNA fractions through gel extraction and isopropanol precipitation.
The small RNAs were ligated to an adenylated 3′-adapter (rAppAGA TCG GAA GAG CAC ACG TCT-NH2). A reverse complementary oligo was employed to inhibit the excess adapter from participating in downstream reactions. Subsequently, these 3′-ligated RNAs were attached to a 5′-adapter (rGrUrUrCrArGrArGrUrUrCrUrArCrArGrUrCrCrGrArCrGrArUrC). After ligation, the RNAs underwent reverse transcription, and the resulting cDNAs were PCR-amplified using indexed primers to create DNA libraries. All procedures were performed in triplicate.
The DNA libraries were sequenced on an Illumina NovaSeq 6000 system using 150 bp paired-end sequencing.
Co-expression of DICER variants with shRNAs or pri-miRNAs for small RNA library preparation
DICER variants were co-expressed with shRNA or pri-miRNAs, followed by small RNA library preparation. DICER knockout (DICER-KO) cells, derived from HCT116 cells, were cultured in 6-well plates. We transfected the cells with 2 µg of pXG-DICER variant plasmids along with 0.2 µg of pU6-shRNA plasmids (for the D1709 residue experiment) or 0.2 µg of pCDNA3-pri-mir-30a (for the E1564 residue experiment) using Lipofectamine 3000. Details regarding the primers used for cloning shRNAs or pri-miRNAs are provided in Supplementary Data 3.
The process for total RNA isolation and small RNA library preparation was conducted in a manner like that described in the previous rescue experiment.
Small RNA sequencing analysis
For the small RNA libraries generated in this study, adapter sequences were excised from both ends of the sequencing reads using Cutadapt33. The command cutadapt -a AGATCGGAAGAGCACACGTCT was used for Read 1, whereas cutadapt -a GATCGTCGGACTGTAGAACTCTGAAC was employed for Read 2. Following adapter removal, the reads were concatenated, and low-quality sequences were filtered according to the protocols outlined in the high-throughput sequencing data analysis section. In contrast, for small RNA libraries sourced from a prior study23, the 3p-end adapter sequence was removed using cutadapt -a CTGTAGGCACCATCAATC. Subsequent quality filtering was performed using fastq_quality_filter, and then 4-nt barcodes at the 5p-end were trimmed with cutadapt -u 4.
The rescue data and the DICER and RNA co-expression data were processed differently as follows. For the rescue data, the resulting high-quality reads were aligned to the human genome (GRCh38) utilizing Bowtie236. The genomic coordinates for human miRNAs were retrieved from miRBase (v22)37, and annotation of the aligned reads as miRNAs was conducted using bedtools intersect38. Only reads located within a 4-nt window from the annotated miRNA ends, and without modifications at the 5p-end, were retained for downstream analysis. To assess the level of DICER cleavage accuracy alteration, we calculated the variability in the start positions of miRNAs (5p-ends of miRNAs). Only 3p-miRNA species with a minimum of 30 raw reads were considered. Raw counts were normalized to the total read count of each sample to compute reads per million (RPM). The relative abundance of each miRNA isoform (isomiR), denoted by CLi, was determined using the formula N(CLi)/∑N(CLi), where N(CLi) represents the normalized count of that isomiR in RPM. The isomiR with the highest abundance in DICER-WT rescued samples (or in HCT116-WT samples) was labeled as CLx. The position of the 5p-end of isomiR is the start position of the miRNA. Variability (v) was calculated using the formula ∑[|d(CLi – CLx)| × f(CLi)], where |d(CLi – CLx)| is the absolute distance (in nucleotides) from the 5p-end of isomiR CLi to that of CLx, and f(CLi) signifies the abundance of that isomiR. Finally, based on the distance from CLx to the nearest upstream single-stranded region, which may include loops, mismatches, and bulges on either the 3p or 5p-strand, pre-miRNAs were categorized into groups: “2-nt from loop” and “not 2-nt from loop”. This classification helped to elucidate the structural context of miRNA processing and its potential impact on miRNA maturation.
For the DICER and RNA co-expression data, the resulting high-quality reads were mapped to the transfected shRNA or pri-miRNA reference sequences, depending on the corresponding samples, using Bowtie2. The reference sequences for shRNAs include their sequences followed by 10-nt poly-T tails, while the reference sequences for pri-miRNAs contain only their sequences. We collected uniquely mapped reads. To estimate DICER's cleavage accuracy at cleavage site DC21 (or DC22), we calculated the ratio of [the number of cleaved reads at DC21 (or DC22)] to [the sum of cleaved reads from DC20 to DC23]. This ratio serves as an indicator of DICER's cleavage accuracy at DC21 (or DC22); the remaining portion was assigned to the ‘other’ group. Finally, we averaged the cleavage accuracy of DICER at different cleavage sites across two repeated experiments.
Sequence enrichment analysis of DRES and YCR motifs
The catalog of DRES and YCR motifs were sourced from our preceding investigations22,27. For each listed motif, we delineated both the 5p-arm (oriented 5p to 3p) and the 3p-arm (oriented 3′ to 5′). Subsequently, we generated sequence logos for each strand utilizing the Logomaker39. Concisely, the sequence position relative to the 5p cleavage site is denoted along the x-axis, while the y-axis displays the nucleotides' frequency through the character height. The total height of the stacked characters at a given position reflects the sequence's information content at that site40.
To ascertain the nucleotide pair frequency within pre-miRNAs, we compiled all available pre-miRNA sequences across various species from MirGeneDB41. DICER cleavage sites were pinpointed based on the 5p-end of the 3p-miRNA and the 3p-end of the 5p-miRNA, as recorded in MirGeneDB. The pre-miRNAs were then structured using RNAfold (part of the ViennaRNA Package version 2.4.13)42. Within a window spanning positions −1 to +4 relative to DICER's cleavage site and directed towards the pre-miRNA's 5p-end, the frequencies of C-G and G-C base pairs were calculated.
shRNA, pri-/pre-miRNA synthesis
The oligonucleotides utilized in this experiment are detailed in Supplementary Data 4. The procedure for synthesizing double-stranded DNA (dsDNA) for shRNA production is as follows. Single-stranded DNA (ssDNA) oligonucleotides encoding the shRNA/pre-miRNA sequence, along with the complementary sequence for the T7 primer, were designed and synthesized. These ssDNA molecules were annealed with the T7 primer (5′-TAA TAC GAC TCA CTA TAG-3′) in a 50 mM NaCl buffer, forming a partial dsDNA. The Klenow fragment (exo-) enzyme was then employed to extend the partial dsDNA into a complete dsDNA molecule.
To synthesize human pri-/pre-miRNAs, we obtainted the pri-/pre-miRNA sequence from MirGeneDB. dsDNA templates pri-/pre-miRNAs containing a T7 promoter. The G-starting pre-miRNAs were designed as shNRAs, non-G-staring pre-miRNAs include an additional Hammerhead ribozyme sequence after T7 promoter. The full dsDNA sequences were synthesized from PCR using three oligonucleotides as forward reverse primers and PCR template.
Approximately 300 ng of the resultant dsDNA was used as a template for in vitro transcription with the MEGAscript T7 Transcription Kit (Invitrogen). The synthesized shRNAs were subsequently purified using gel electrophoresis, quantified with a NanoDrop 2000 spectrophotometer (Thermo Scientific), and stored at −80 °C for future applications.
The in vitro shRNA/pri-/pre-miRNA cleavage assays
Three pmol of each RNA substrate were combined in a 10 μL reaction mixture consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 1 mM DTT, and 2 mM MgCl2. Purified DICER enzyme was then added in varying amounts as specified in the corresponding figure legends. The reactions were incubated at 37 °C for 30 min before termination by adding 10 μL of 2X TBE buffer and 10 μg of Proteinase K (Thermo Fisher Scientific). This mixture was further incubated at 50 °C for 15 min and subsequently denatured at 95 °C for 5 min. The mixtures (10 μL each) were then loaded onto a pre-run 12% urea-PAGE and stained with SYBR™ Green II RNA gel stain (Invitrogen). Gel images were captured using the Bio-Rad Gel Doc XR+ system.
To calculate the cleavage efficiency for each specific cleavage event, such as DC21 or DC22, the ratio of the cleavage product bands (F1 and F3) to the bands of the RNA substrates (shRNAs/pre-miRNAs) was determined.
The overall cleavage efficiency, encompassing both cleavage events, was quantified as the ratio of the cleavage product bands (F1 and F3) resulting from all cleavage events to the bands of the RNA substrates (shRNAs/pre-miRNAs).
Cleavage accuracy for each specific cleavage, like DC21 or DC22, was calculated as the ratio of the cleavage product bands (F1 and F3) resulting from that specific cleavage to the product bands resulting from all cleavage events.
The cleavage efficiency and accuracy were calculated based on data obtained from three repeated experiments.
The pri-miRNA cleavage assays with Microprocessor were conducted similarly as DICER assay. The reaction was incubated at 37 °C for 2 h and stopped by adding 10 μL of 2X TBE buffer and 10 μg of Proteinase K. The denaturing and gel running program were conducted similarly to DICER assay. The detail for Microprocessor purification was described in our previous studies31,43,44.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing and processed data from this study have been deposited to the Gene Expression Omnibus (GEO) with the accession number GSE252435. Small RNA sequencing data for wild-type and DICER mutant HCT116 cells come from an earlier study by Gu et al. (2012), found in GEO under accession number GSE41292. Furthermore, we utilized high-throughput sequencing data for DICER-WT from Nguyen et al. 7 and Le et al. (2024), available under GEO accession numbers GSE182700 and GSE247119, respectively. All the structures cited in this study are available on the RCSB Protein Data Bank (RCSB PDB) by accession codes 7XW2, 2NUG, and 8DFV. Structure analysis figures were illustrated by Chimera 1.17.3 and PyMol-2.5.4 (Schrödinger). The source data underlying Figs. 1h–i, k–n, 2e–g, k–l, n–o, 3d–i, 4c, e–h, k–n, 3d–e, g–i and Supplementary Figs. 1h, k–l, 2b–j, l–m, 3b, 3d–f, 3h–i, 4d, g, i, j–l, 5b, d–e, g–h are provided as a Source Data file. All other data are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
Source codes for data analysis have been deposited in Zenodo (https://doi.org/10.5281/zenodo.10459219).
References
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Bartel, D. P. Metazoan MicroRNAs. Cell 173, 20–51 (2018).
Shang, R., Lee, S., Senavirathne, G. & Lai, E. C. microRNAs in action: biogenesis, function and regulation. Nat. Rev. Genet. 24, 816–833 (2023).
Zhang, H., Kolb, F. A., Brondani, V., Billy, E. & Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885 (2002).
Zhang, H., Kolb, F. A., Jaskiewicz, L., Westhof, E. & Filipowicz, W. Single processing center models for human Dicer and bacterial RNase III. Cell 118, 57–68 (2004).
Liu, Z. et al. Cryo-EM structure of human Dicer and its complexes with a Pre-miRNA substrate. Cell 173, 1191–1203.e12 (2018).
Nguyen, T. D., Trinh, T. A., Bao, S. & Nguyen, T. A. Secondary structure RNA elements control the cleavage activity of DICER. Nat. Commun. 13, 2138 (2022).
Lee, Y., Lee, H., Kim, H., Kim, V. N. & Roh, S. Structure of the human DICER—pre-miRNA complex in a dicing state. 615, 331–338 (2023).
Zapletal, D. et al. Structural and functional basis of mammalian microRNA biogenesis by Dicer. Mol. Cell 82, 4064–4079.e13 (2022).
MacRae, I. J. et al. Structural basis for double-stranded RNA processing by Dicer. Science 311, 195–198 (2006).
Macrae, I. J., Zhou, K. & Doudna, J. A. Structural determinants of RNA recognition and cleavage by Dicer. Nat. Struct. Mol. Biol. 14, 934–940 (2007).
Park, J. et al. Dicer recognizes the 5′ end of RNA for efficient and accurate processing. Nature 1–7 (2011) https://doi.org/10.1038/nature10198.
Tsutsumi, A., Kawamata, T., Izumi, N., Seitz, H. & Tomari, Y. Recognition of the pre-miRNA structure by Drosophila Dicer-1. Nat. Struct. Mol. Biol. 18, 1153–1158 (2011).
Tian, Y. et al. A phosphate-binding pocket within the platform-PAZ-connector helix cassette of human Dicer. Mol. Cell 53, 606–616 (2014).
Jouravleva, K. et al. Structural basis of MicroRNA biogenesis by Dicer-1 and its partner protein Loqs-PB. Mol. Cell https://doi.org/10.1016/j.molcel.2022.09.002 (2022).
Jonas, S. & Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 16, 421–433 (2015).
Iwakawa, H. & Tomari, Y. Life of RISC: formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 82, 30–43 (2022).
Paddison, P. J., Caudy, A. A., Bernstein, E., Hannon, G. J. & Conklin, D. S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16, 948–958 (2002).
Siolas, D. et al. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 23, 227–231 (2005).
Ros, X. B. & Gu, S. Guidelines for the optimal design of miRNA-based shRNAs. Methods 103, 157–166 (2023).
Lee, Y., Kim, H. & Kim, V. N. Sequence determinant of small RNA production by DICER. https://doi.org/10.1038/s41586-023-05722-4 (2023).
Le, C. T., Nguyen, T. D. & Nguyen, T. A. Two-motif model illuminates DICER cleavage preferences. 1–18 (2024).
Gu, S. et al. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell 151, 900–911 (2012).
Heravi-Moussavi, A. et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N. Engl. J. Med. 366, 234–242 (2012).
Foulkes, W. D., Priest, J. R. & Duchaine, T. F. DICER1: mutations, microRNAs and mechanisms. Nat. Rev. Cancer 14, 662–672 (2014).
Urbanek-Trzeciak, M. O. et al. Pan-cancer analysis of somatic mutations in miRNA genes. EBioMedicine 61, 103051 (2020).
Gan, J. et al. A stepwise model for double-stranded RNA processing by ribonuclease III. Mol. Microbiol 67, 143–154 (2008).
Masliah, G., Barraud, P. & Allain, F. H. T. RNA recognition by double-stranded RNA binding domains: A matter of shape and sequence. Cell. Mol. Life Sci. 70, 1875–1895 (2013).
Pike, S. J., Hutchinson, J. J. & Hunter, C. A. H-bond acceptor parameters for anions. J. Am. Chem. Soc. 6706, 1–7 (2017).
Vedanayagam, J. et al. Cancer-associated mutations in DICER1 RNase IIIa and IIIb domains exert similar effects on miRNA biogenesis. Nat. Commun. 10, 3682 (2019).
Nguyen, T. L., Nguyen, T. D., Ngo, M. K., Le, T. N. Y. & Nguyen, T. A. Noncanonical processing by animal Microprocessor. Mol. Cell 83, 1810–1826.e8 (2023).
Kwon, S. C. et al. Structure of human DROSHA. Cell 164, 81–90 (2016).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011). pp.
Aronesty, E. Comparison of sequencing utility programs. Open Bioinform. J. 7, 1–8 (2013).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kozomara, A., Birgaoanu, M. & Griffiths-jones, S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 47, 155–162 (2019).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26, 841–842 (2010).
Tareen, A. & Kinney, J. B. Sequence analysis Logomaker: beautiful sequence logos in Python. 36, 2272–2274 (2020).
Schneider, T. D. & Stephens, R. M. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 18, 6097–6100 (1990).
Fromm, B. et al. MirGeneDB 2. 1: toward a complete sampling of all major animal phyla. Nucleic Acids Res. 50, 204–210 (2022).
Lorenz, R. et al. ViennaRNA Package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Li, S., Nguyen, T. L. T. A. T. D., Nguyen, T. L. T. A. T. D. & Nguyen, T. L. T. A. T. D. Mismatched and wobble base pairs govern primary microRNA processing by human Microprocessor. Nat. Commun. 11, 1926 (2020).
Nguyen, T. L. T. A. T. D., Nguyen, T. L. T. A. T. D., Bao, S., Li, S. & Nguyen, T. L. T. A. T. D. The internal loops in the lower stem of primary microRNA transcripts facilitate single cleavage of human Microprocessor. Nucleic Acids Res. 48, 2579–2593 (2020).
Acknowledgements
We extend our sincere thanks to our laboratory colleagues, particularly Dr. Trung Duc Nguyen at HKUST, for their invaluable technical assistance, engaging discussions, and insightful critiques of the manuscript. We would like to thank the Biosciences Central Research Facility at HKUST (Clear Water Bay) for their high-quality instruments and services. Special gratitude is owed to Dr. Narry Kim at Seoul National University for generously providing the expression plasmids and cell lines. C.T.L. is grateful to be a recipient of the Hong Kong PhD Fellowship Scheme from the Research Grants Council. This research has been funded by the Research Grants Council of Hong Kong under grant number 16103023.
Author information
Authors and Affiliations
Contributions
T.N.Y.L., C.T.L., and T.A.N. formulated the concept and design of the study. T.N.Y.L. carried out the biochemical assays. Both T.N.Y.L. and C.T.L. were involved in the analysis of the data and the creation of figures. All authors played a role in analyzing data, interpreting results, and drafting the manuscript. T.A.N. oversaw the entire research project and was instrumental in obtaining the necessary funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jundong Zhuang and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Le, T.NY., Le, C.T. & Nguyen, T.A. Determinants of selectivity in the dicing mechanism. Nat Commun 15, 8989 (2024). https://doi.org/10.1038/s41467-024-53322-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53322-1
This article is cited by
-
Key RNA elements influencing DCL1 cleavage in plant microRNA biogenesis
Nature Plants (2025)
-
A conserved RNA motif guides DCL1-mediated cleavage in plant microRNA biogenesis
Nature Plants (2025)