Abstract
Glycosylphosphatidylinositol (GPI) acyltransferase is crucial for the synthesis of GPI-anchored proteins. Targeting the fungal glycosylphosphatidylinositol acyltransferase GWT1 by manogepix is a promising antifungal strategy. However, the inhibitory mechanism of manogepix remains unclear. Here, we present cryo-EM structures of yeast GWT1 bound to the substrate (palmitoyl-CoA) and inhibitor (manogepix) at 3.3 Å and 3.5 Å, respectively. GWT1 adopts a unique fold with 13 transmembrane (TM) helixes. The palmitoyl-CoA inserts into the chamber among TM4, 5, 6, 7, and 12. The crucial residues (D145 and K155) located on the loop between TM4 and TM5 potentially bind to the GPI precursor, contributing to substrate recognition and catalysis, respectively. The antifungal drug, manogepix, occupies the hydrophobic cavity of the palmitoyl-CoA binding site, suggesting a competitive inhibitory mechanism. Structural analysis of resistance mutations elucidates the drug specificity and selectivity. These findings pave the way for the development of potent and selective antifungal drugs targeting GWT1.
Similar content being viewed by others
Introduction
Globally, fungal infections are emerging as growing threats to human health and agricultural economics1. Although fungal infections and resistance are becoming more prevalent, only limited classes of antifungal drugs are currently available2,3. For nearly two decades, there has been no innovative class of antifungal drugs approved by the US Food and Drug Administration (FDA)4. This situation underscores the critical need for developing new antifungal agents.
GPI-anchoring is a conserved post-translation modification essential for fungal growth and virulence5,6. During fungal GPI synthesis, GWT1, an ER membrane protein, catalyzes the palmitoylation of GlcN-PI at the 2-position of the inositol ring using palmitoyl-CoA as the donor substrate7,8 (Fig. 1a and Supplementary Fig. 1). Manogepix (also known as E1210 or APX001A), a first-in-class inhibitor of GWT1, offers a promising solution against several fungal pathogens9,10 (Fig. 1a). Manogepix exhibits strong in vitro activity against clinical isolates of Candida and Aspergillus species, even yeast and mold isolates, demonstrating elevated MIC/MEC values compared to other drug classes9,11. As an FDA-designated orphan drug, manogepix has advanced to Phase 3 clinical trials for fungal infections, potentially marking a significant breakthrough in antifungal therapy12. GWT1 is a highly conserved and ubiquitous protein across fungal species (Supplementary Fig. 2). The loss-of-function GWT1 showed lethal phenotype7. Therefore, inhibition of GWT1 represents a therapeutic strategy for combating life-threatening fungal infections.
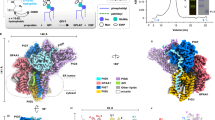
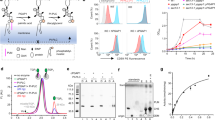
a The cartoon representations of the substrates and products of the GWT1 enzyme are displayed. The chemical structure of manogepix, an inhibitor of GWT1 protein, is demonstrated. The hydroxyl group that accepts the palmitoyl group (yellow) is highlighted in the red circle. b Topology of fGWT1 presents the specific sites of GFP insertion. Through screening, A fusion of GFP at the Y422 position construct exhibited the most homogeneous protein properties. c FACS (fluorescence-activated cell sorting) of HEK293T△PIGW cells expressing fGWT1 (light green) using CD59 as the marker with gating strategies. HEK293TWT cells are highlighted in red, while individual HEK293T△PIGW cells are marked in blue. Those HEK293T△PIGW cells responding to fGWT1 are labeled in light green. The background served as a blank control, referring to HEK293T△PIGW cells not stained with CD59 antibodies. d Fluorescent confocal microscopy analyses were conducted to assess fGWT1 activity, with the construct and cell line indicated alongside the images. The first, second, and third columns illustrate confocal fluorescence microscopy images of CD59, DAPI, and merged views, respectively. A scale bar of 10 μm is included. The confocal microscopy experiment was repeated at least three times.
Here, we determine two cryo-EM structures of yeast GWT1: one bound to its palmitoyl-CoA substrate (3.37 Å) and another bound to its inhibitor, the antifungal drug manogepix (3.55 Å). Manogepix is seen to occupy the hydrophobic cavity of the palmitoyl-CoA binding site, suggesting a competitive inhibitory mechanism. We perform structural analysis of resistance mutations which elucidates the drug specificity and selectivity. Together, these structures provide valuable insights into the inhibitory mechanism of manogepix and the rational design of GWT1-targeting inhibitors.
Results
Characterization of yeast GWT1
We purified the full-length yeast GWT1 using Sf9 cells (Supplementary Fig. 3a). Cryo-EM analysis revealed well-dispersed particles. However, small protein size and lack of distinct features in its soluble domains hindered single-particle processing (Supplementary Fig. 3b, c). To overcome this problem, we employed a green fluorescent protein (GFP)-fusion strategy, systematically fusing GFP to the loops between transmembrane helices of GWT1 (Fig. 1b).
To screen functional GFP-fused GWT1 protein, we developed a fluorescence-based rescue assay in HEK293T cells (Supplementary Fig. 4a and Supplementary Fig. 15). Disruption of PIGW, the homolog of yeast GWT1 in human, led to the loss of GPI-AP CD59 expression on the cell membrane surface in HEK293T cells (Supplementary Fig. 4b). Overexpression of GWT1 restored CD59 surface localization in PIGW knockout cells (Supplementary Fig. 4b). This result supports the view of functional complementation between the yeast and human orthologs, consistent with a previously study13. Based on these in vivo assays, we identified a construct with a fusion GFP at position Y422 (fGWT1) that rescued CD59 expression in PIGW knockout cells (Fig. 1c, d), demonstrating fGWT1’s physiologically relevant activity.
Structure determination
We purified the fGWT1 protein that exhibits homogeneous behavior, making it suitable for further cryo-EM studies (Supplementary Fig. 5a). Initial micrographs revealed well-defined particles with clearly visible GFP moieties in the soluble domains (Supplementary Fig. 5b). Further stabilization with a specific GFP nanobody facilitated a high-quality data acquisition (Supplementary Fig. 5c–e). Following extensive 3D classification and refinement, 238,678 selected particles enabled the generation of a 3D reconstruction with an overall resolution of 3.47 Å (Supplementary Fig. 6a–e). By applying local refinement on the TM region with a mask, we achieved an improved resolution of 3.37 Å (Supplementary Fig. 6c).
Cryo-EM density for the GWT1 polypeptide is well-resolved (residues 13-490, except for S101-K106), enabling the construction of an accurate model (Supplementary Fig. 7a-c). After most GWT1 amino acids were assigned to the cryo-EM density, an elongated density was observed within the GWT1 tunnel (Fig. 2a). Despite no substrates being added during purification, this density likely corresponds to an endogenous ligand. This apparent density perfectly accommodates a complete palmitoyl-CoA molecule, aligning well with functional studies demonstrating it as the primary substrate of GWT1 in yeast and mammals7,13 (Fig. 2a, b and Supplementary Fig. 7c).
a The density map of the monomeric GWT1 bound to palmitoyl-CoA, front views from within the plane of the membrane (left) and the intracellular side of the membrane (right). The approximate position of the ER membrane is indicated with light blue shading. The elongated density is shown in green. b The structure of the monomeric GWT1 in complex with palmitoyl-CoA monomer is illustrated in cartoon representations, offering perspectives from within the plane of the membrane (left) and the intracellular side of the membrane (right). The approximate position of the ER membrane is highlighted with light blue shading. c. The structural models of GWT1 bound to palmitoyl-CoA monomer are shown in the front view and top view. Helices are shown as cylinders and depicted in rainbow colors. Palmitoyl-CoA is encircled by a transmembrane spiral marked with a blue circle, and a red star indicates palmitoyl-CoA. d, The topology of palmitoyl-CoA-bound GWT1 is represented using colors like in panel (c).
The overall structure of GWT1
The overall structure of GWT1 measures approximately 71 Å × 63 Å × 34 Å (Fig. 2a). The structure of GWT1 unveiled a previously uncharacterized architecture. Spanning the ER membrane with 13 transmembrane helices (TM1-TM13), the enzyme possesses N and C termini facing the luminal and cytosolic sides, respectively (Fig. 2b, c). Notably, only minimal regions extend into the cytosol or ER lumen. Short lateral α-helices (LH1 to LH2) are mostly embedded in the ER membrane and oriented parallel to it. The bound palmitoyl-CoA is surrounded by TM4-7 and TM12. Therefore, these five highly conserved TMs form a “catalytic tunnel” (Fig. 2c, d). TM5 occupied a central position within the GWT1 protein structure. TM12 exhibited notably shorter lengths than other TMs. The N-terminus of GWT1 was positioned on the luminal side of the “catalytic tunnel”, resembling a lid oriented towards the center. TM1-3 and TM13 reside on one side of the catalytic tunnel, while TM8-11 occupy the opposite side, thereby constituting a scaffold domain enveloping the catalytic tunnel (Fig. 2c). Furthermore, TM1 to TM13 are predicted to be evolutionarily conserved among GWT1 proteins from diverse kingdoms. This architecture demonstrates that GWT1 adopts a unique fold distinct from known protein structures.
Comparative structural analysis using Dali server identified heparan-α-glucosaminide N-acetyltransferase (HGSNAT) as the most significant hit to GWT1, despite a low sequence identity of less than 15%14 (Supplementary Fig. 8). Superimposition of GWT1 and HGSNAT yielded a root-mean-square-deviation (RMSD) value of 4.1 Å over backbones of 330 aligned residues, implying a potential evolutionary link (Supplementary Fig. 8a). This unexpected finding contrasts with subsequent structural hits, which lacked significant similarity (RMSD > 5) and belonged to functionally distinct protein families such as lipopolysaccharide transporters and toxin-antitoxin proteins (Supplementary Fig. 8b, c). Moreover, no structural homologs was identified within the MBOAT family. These findings indicate that GWT1 represents a structurally and functionally distinct class of acyltransferases.
Palmitoyl-CoA binding pocket of GWT1
The bound palmitoyl-CoA molecule adopts a distinctive L-shaped conformation positioned in the cytosolic tunnel with the CoA moiety facing the cytosol and the acyl chain lateral inserting into GWT1 (Fig. 3a-c). The orientation observed is in line with the known process of palmitoyl-CoA synthesis taking place at the cytosolic side of the ER membrane15. Palmitoyl-CoA interacts extensively with the TM4, TM5, TM6, TM7, and TM12 of GWT1, burying a substantial surface area of 956.6 Å2.
a Three significant openings leading to the reaction chamber of palmitoyl-CoA include a cytosolic tunnel (highlighted in green), an ER-luminal tunnel (highlighted in blue), and the overall structure of palmitoyl-CoA (highlighted in orange). The electrostatic surface is illustrated. b The structural models of GWT1 bound to palmitoyl-CoA. Helices are represented as cylinders, and the palmitoyl-CoA molecule is depicted as a green ball and stick. The approximate position of the ER membrane is highlighted with light blue shading. c The cut-away view illustrates the binding pocket of the substrate palmitoyl-CoA within the reaction chamber. The regions corresponding to the “head”, “neck”, and “tail” of palmitoyl-CoA are highlighted with orange, purple, and blue frames, respectively. The palmitoyl-CoA molecule is shown as a ball and stick (depicted in green). d–f Amino acids located at the sites surrounding the head (highlighted in orange frame), neck (highlighted in light purple frame), and tail (highlighted in blue frame) of palmitoyl-CoA are shown as sticks with carbon atoms colored in green. g–i Sequence logos were generated using the WebLogo server (https://weblogo.berkeley.edu/logo.cgi) for a region encompassing conserved binding pocket of palmitoyl-CoA. Amino acids are color-coded based on their chemical properties: polar amino acids (G, S, T, Y, C, Q, N) are represented in green; basic amino acids (K, R, H) are depicted in blue; acidic amino acids (D, E) are shown in red; and hydrophobic amino acids (A, V, L, I, P, W, F, M) are illustrated in black.
The cytosolic opening of the palmitoyl-CoA binding site is enriched in basic amino acids, resulting in a positively charged pocket (Fig. 3a, c). This unique composition creates a favorable pocket for binding the 3’, 5’-ADP head group of the palmitoyl-CoA. The adenine ring of palmitoyl-CoA is positioned into the cytosolic pocket of GWT1, stacking between K123 and I126. Hydrogen bonds are formed between the adenine ring of the palmitoyl-CoA and the side chain of T127, as well as with the main chain of K123 (Fig. 3d, Supplementary Fig. 9a). The phosphate groups form a hydrogen bond with R181. Notably, F171 in TM5, F238 and F239 in TM7, and F439 in TM12 contribute to stabilizing the “neck” region of the L-shaped palmitoyl-CoA through hydrophobic interactions (Fig. 3c, e, Supplementary Fig. 9a). The position of the palmitoyl-CoA thioester in the core cavity of GWT1 faces the lumen side. We propose that this cavity might function as the reaction center, where the acylation reaction could cleave the aliphatic chain from this thioester site and transfer it to the substrate GlcN-PI. The hydrophobic tail of palmitoyl-CoA inserts into a binding pocket predominantly constructed by conserved residues from TM4, TM5, and TM11, including T27, Y129, M133, L136, I141, L163, M164, L166, S170, Y400, F404 and Y408 (Fig. 3f–i, Supplementary Fig. 9a). Interestingly, the lipid tail-binding pocket of GWT1 appears wide and deep with minimal restrictions on acyl chain length, allowing it to bind diverse fatty acids such as myristoyl, palmitoyl, stearoyl, and arachidoyl (Supplementary Fig. 10). This observation was largely consistent with prior studies on the acyl-CoA specificity of GWT1 and its homologs7,13.
To assess the functional significance of residues involved in palmitoyl-CoA binding, we performed rescue assays in HEK293T cells. Three classes of GWT1 variations were targeted: (1) 3’, 5’-ADP head group binding site, (2) neck interface, and (3) lipid tail interface. Additionally, two established loss-of-function mutants, D145A and K155A, served as controls16. Notably, all three classes of variations (including T127A, R130A, N175Q, V178A, R181A, N432A for the head group; T137A, F171A, F238A, R386A, F439A for neck interface; Y129A, L136A, I141A, L163A, M164A, S170A, Y400A, N401Q for the tail) had minimal impact on CD59 localization, suggesting limited effects by the single amino acid substitution of GWT1 (Supplementary Fig. 9b, c).
Structural interpretation of luminal access cavity for GlcN-PI
Our structure reveals a luminal cavity designated as the putative GlcN-PI binding pocket (Fig. 3a). Remarkably, additional density is observed within this cavity (Fig. 4a–e). The observed density resides near the palmitoyl-CoA thioester and potentially represents the inositol part of GlcN-PI. Consistently, molecular docking study indicates that GlcN-PI can bind to the proposed luminal access cavity (Supplementary Fig. 11a). This docked pose shows the O2’ atom of GlcN-PI in close proximity to the thioester bond of palmitoyl-CoA (Supplementary Fig. 11a). This spatial arrangement facilitates the transfer of the hydrophobic tail of palmitoyl-CoA to the substrate GlcN-PI. Due to its limited size, we cannot rule out the possibility that the observed density represents a product or detergent molecule.
a, d The cryo-EM map of GWT1 is displayed from both side (a) and top (d) perspectives, with the luminal access cavity highlighted by a blue dashed oval. b, e An additional density within the highly conserved luminal access cavity. c Molecular lipophilicity potential (MLP) of the luminal access cavity. The surfaces are colored by lipophilicity potential calculated by Chimera X. The putative entry route for GlcN-PI is indicated by a dashed blue path. f Comparison of catalytic sites of GWT1 and HGSNAT. The predicted catalytic sites of HGSNAT are N286 and H297, while the putative catalytic sites of GWT1 are D145 and K155. g Expressing fGWT1 in HEK293T△PIGW cells restores CD59 staining in the FACS assay. HEK293T△PIGW cells, where GPI-AP biosynthesis is blocked, serve as the staining control (gray). Loss-of-function mutants are highlighted in brilliant blue. Coordination residue mutants of the luminal access cavity are highlighted in purple.
The luminal access cavity of GWT1, primarily formed by residues from TM4-7 and connecting loops 1-2 (CL1, CL2), is highly conserved among homologous proteins from Candida, Neurospora, and Aspergillus (Fig. 3g–i and Supplementary Fig. 2). The cavity location implies its critical role in catalysis (Fig. 4b, e). Interestingly, the cavity exhibits a lateral opening towards the hydrophobic membrane, potentially serving as the GlcN-PI entry pathway, resembling the entry mechanism observed in DGAT117,18 (Fig. 4c). The luminal surface of the cavity displays high hydrophilicity, possibly favoring the binding of GlcN-PI’s phosphoinositol and deacetylated glucosamine moieties (Fig. 4c). Structural alignment of GWT1 with the HGSNAT revealed that conserved residues I141 and K155 in GWT1 correspond to the proposed catalytic residues N286 and H297 in HGSNAT, occupying similar positions within the protein structure19,20,21 (Fig. 4f).
To investigate the potential role of CL1 and CL2 residues surrounding the GWT1 luminal cavity, we performed fluorescence-based rescue assays. Consistent with previous reports, mutation of K155 to alanine resulted in near-complete abolition of activity16. The D145A mutation led to about a 50% decrease in activity, in agreement with its previously proposed critical role of D145 in GWT1 function (Fig. 4g). While several polar amino acids probably contribute to the architecture of the GlcN-PI binding pocket, individual mutations, including R152A, W159A, R216A, Y225A, E227A, E231A, Y232A, Y308A, N443A and N450A exhibited only moderate decrease in activity (Fig. 4g).
Structural basis of GWT1 inhibition by manogepix
Considering the potential of GWT1 as a target for combating invasive fungal infections, we sought to determine the structure of GWT1 in complex with manogepix, the most potent GWT1 inhibitor identified to date (Supplementary Fig. 12). To promote formation of the inhibitor-enzyme complex, manogepix was incorporated with fGWT1 during protein expression and purification (Supplementary Fig. 5f).
Subsequent cryo-EM analysis yielded a 3.55 Å resolution map of the GWT1-manogepix complex (Supplementary Fig. 12). The map revealed the absence of palmitoyl-CoA density within the catalytic tunnel, suggesting competitive inhibition by manogepix (Fig. 5a). Notably, a strong additional non-protein density was observed within the central cavity and matched the size and shape of manogepix (Fig. 5a, b). The clear density map allowed the accurate building of the manogepix-bound GWT1 structure (Supplementary Table 1). Our molecular docking and molecular dynamics simulations further validated the proposed binding mode (Pose 1, Supplementary Fig. 13). This binding mode outperforms an alternative mode by the ligand density map (Pose 2) in terms of both pose stability and binding interaction energy (Supplementary Fig. 13).
a–b Density map (a) and structure (b) of GWT1 in complex with manogepix. The density map and structure of GWT1 are colored blue, while the density map of manogepix is shown in yellow and its structure is in lilac. c Superposition of the palmitoyl-CoA-bound GWT1 complex and the manogepix-bound GWT1 complex. The palmitoyl-CoA-bound GWT1 complex and the manogepix-bound GWT1 complex are shown as cylinder with colors of pink and blue. d Sliced view of the molecular surface of fGWT1, highlighting the bound manogepix (blue) and palmitoyl-CoA (pink). e–f Close-up views of the manogepix (depicted in lilac), showing cryo-EM density (highlighted in yellow) and contacting GWT1 residues side chain (rendered in white).
Structural comparison of GWT1 in complex with palmitoyl-CoA and manogepix revealed almost identical conformation, with an RMSD of only 0.3 Å over 464 Cα atoms (Fig. 5c). However, manogepix strategically wedged within the palmitoyl-CoA binding pocket, potentially occupying the catalytic center and interfering with lipid tail binding (Fig. 5d). The steric conflict between palmitoyl-CoA and manogepix suggests a competitive inhibition mechanism (Fig. 5d). By occupying the palmitoyl-CoA binding site, manogepix effectively prevents substrate loading into the catalytic center, thereby blocking the initial step of the enzymatic reaction. Meanwhile, the 2-aminopyridine group of manogepix overlaps with the thioester group of palmitoyl-CoA, potentially blocking the cleavage of the lipid tail and interfering with the GWT1 acyltransferase activity (Fig. 5d). The 2-aminopyridine group is positioned near the luminal access cavity, raising the possibility of steric clashes with the secondary substrate GlcN-PI (Fig. 5d). This observation suggests an additional layer of inhibition by restricting substrate access.
Manogepix exhibited potent antifungal properties with a minimum inhibitory concentration (MIC) of 0.01361 µg/ml against yeast (Supplementary Fig. 14a). The structure of the GWT1-manogepix complex offers detailed insights into inhibitor recognition (Fig. 5e, f). Manogepix, like many potent GWT1 inhibitors, adopts an extended conformation and consists of four key components: 2-aminopyridine, isoxazole, para-xylene, and 2-pyridone groups (Supplementary Fig. 14c). This suggests a general binding mode for GWT1 inhibitors. Manogepix is stabilized within a central pocket by an extensive network of residues (Fig. 5e, f; Supplementary Fig. 14g). These residues, including T27, A30, Y129, G132, and others, primarily form hydrophobic interactions with the lipophilic manogepix molecule (logP = 3.2) through van der Waals contacts and some hydrogen bonds. Specifically, residues T137, I141, M164, G167, V168, F171, F238, F404, and F439 form a hydrophobic cavity surrounding the 2-aminopyridine group, while M133/L136 and L163/L166 flank the para-xylene moiety (Fig. 5e, f). Additionally, the 2-pyridone group engages with F404 through a CH-π interaction at a distance of 3.3 Å, with T27, A30, Y129, G132, and Y408 also contributing to its binding (Fig. 5e, f; Supplementary Fig. 14b). Interestingly, molecular lipophilicity potential analysis reveals a spatially distinct hydrophilic and lipophilic nature of the binding pocket, corresponding to the distribution of manogepix moieties (Supplementary Figs. 14d, e). The isoxazole group and the oxygen atom of the 2-pyridone group face the hydrophilic side of the pocket, suggesting polar contacts play a crucial role in manogepix binding. Indeed, Y400 forms a hydrogen bond (3.1 Å) with the 2-pyridone nitrogen. Meanwhile, S170 interacts with its oxygen via another hydrogen bond (Fig. 5e, f). These interaction analyses highlight the combined roles of hydrophobic and hydrogen-bond interactions in GWT1 inhibitor recognition.
Structural basis for drug resistance of GWT1 mutations
The elucidated structure of the GWT1-manogepix complex provides a foundation for examining GWT1 resistance mutations within invasive fungi. Notably, all previously identified GWT1 resistance mutations, including G132R (BIQ), G132W and F238C (G884), S170F (aminopyrifen), and V168A (manogepix), cluster around the manogepix binding pocket8,22 (Fig. 6a). This spatial arrangement strongly suggests a shared binding site for these structurally diverse GWT1 inhibitors. Functional assays validated these findings, with V168A and F238C mutations significantly reducing manogepix inhibitory activity (82.7% and 75.1% CD59 localization, respectively) compared to wild-type GWT1 (41%). Additionally, we identified manogepix resistance mutations in L136A, I141A, F171A, Y400A and Y408L suggesting their roles in reducing manogepix binding (Fig. 6b, c). Meanwhile, T137A and also exhibited weak drug resistance (Fig. 6c). Interestingly, we found that the alanine mutation on some substrate-interacting residues, including Y129, R386, N401, and F439, exhibited increased sensitivity to manogepix (Fig. 6c). Collectively, these findings validate the binding mode revealed by the GWT1-manogepix complex structure and deepen our knowledge of the drug resistance mechanisms. Moreover, these new drug resistance mutations offer valuable insights for clinical diagnosis.
a Previously reported drug resistance sites for GWT1 are shown as spheres and colored in gray-blue. b All drug resistance sites, including those previously reported and newly identified, are shown. c Evaluating the sensitivity of manogepix by staining CD59 in the FACS assay, expressing fGWT1 in HEK293T△PIGW cells. Cells treated with manogepix (orange) or without manogepix (blue) were analyzed by flow cytometry using gating strategies, with negative controls marked in red. All experiments were conducted independently three times (n = 3).
Structural basis of manogepix selectivity for GWT1
Our elucidated GWT1 structure in complex with manogepix sheds light on the selectivity of the inhibitor on GWT1 against PIGW. Consistent with previous report, our cellular experiments confirm the negligible inhibitory effect of manogepix on PIGW at 10 μM, highlighting its selectivity10 (Fig. 6c). Despite sharing 31% sequence identity, superimposing the predicted PIGW AphlaFold structure onto GWT1 revealed high overall structural similarity with an RMSD value of 2.0 over 430 Cα atoms (Supplementary Fig. 2 and Supplementary Fig. 14f). Interestingly, our sequence alignment analysis revealed significant variation in amino acid composition surrounding the P3 and P4 pockets of GWT1 compared to PIGW, while the P1 and P2 pockets remained conserved (Supplementary Fig. 14g and Supplementary Fig. 16). This suggests comparable ligand interactions within the P1-P2 pockets for both enzymes. Notably, within the P3-P4 pocket of GWT1, key residues interacting with manogepix differed significantly from those in PIGW. For example, Y408 (GWT1) corresponds to L388 (PIGW), and similar differences were observed at positions 27, 30, 129, 133, 166, 170, 400 and 404 (Supplementary Fig. 2 and Supplementary Fig. 14g). Changing the GWT1 inhibitor binding pocket residue Y408 to L, resembling those of PIGW, resulted in significant resistance to manogepix, with a fivefold increase in MIC50 (Fig. 6c and Supplementary Fig. 14a). Collectively, our structural observations rationalized the selectivity of manogepix towards GWT1. It is primarily driven by the distinct amino acid composition surrounding the P3-P4 pocket. This knowledge would inspire further drug optimization aiming at enhancing inhibitors’ selectivity.
Structural implication for GWT1 inhibitors
The manogepix-bound GWT1 structure provides a structural basis for interpreting previous structure-activity relationship (SAR) data, illuminating design principles for potent GWT1 inhibitors. First, one study demonstrated that incorporation of the 2-aminopyridine moiety led to notable increases in antifungal efficacy against Candida albicans and Aspergillus fumigatus by approximately 4–8 fold, culminating in the synthesis of benzylthiophene amide derivative 15 with potent activity23. This enhancement likely arises from favorable interactions of the 2-aminopyridine group within the luminal access cavity P1 of GWT1 (Supplementary Fig. 14c, d). Second, substitution of the isobutanoxy group in Gepinacin with a methoxy group resulted in a shift from active to inactive24. Our structure suggests this could arise from weakened hydrophobic interactions between the inhibitor and the P4 cavity, highlighting the importance of the P4 cavity in the inhibitor design (Supplementary Fig. 14c, d). Third, incorporating nitrogen to form a 2-pyridone group significantly boosted the antifungal potency of LCUT-8 against Candida albicans, Candida auris, and Cryptococcus neoformans by 8-32 fold compared to LCUT-925. This is likely due to the introduction of an additional hydrogen bond with Y400 in the P4 pocket, as visualized in the GWT1-manogepix complex. Finally, the crucial hydrogen bond between S170 and the 2-pyridone oxygen in the GWT1-manogepix complex offers a reasonable explanation that the near-ubiquitous presence of an oxygen atom at the P4 position of potent GWT1 inhibitors (Supplementary Fig. 14c). Overall, these findings demonstrate the value of structural insights for guiding GWT1 inhibitor development by interpreting the molecular basis of functional group alterations and informing future optimization strategies.
Discussion
Manogepix, a novel broad-spectrum antifungal drug targeting GWT1 with high efficacy, demonstrates potent in vitro activity against clinically relevant invasive fungal pathogens, including Candida, Aspergillus, and over 200 rare fungi9,11. Notably, it retains activity against strains resistant to prescribed antifungal drugs (azoles, echinocandins, and amphotericin B)9. In this study, we elucidated the structural basis of GWT1 function and inhibition by manogepix.
By determining the cryo-EM structures of yeast GWT1 in complex with its substrate (palmitoyl-CoA) and inhibitor (manogepix), we uncovered a unique fold of 13 transmembrane helices that serves as a structural paradigm for its homologous proteins, including the human PIGW. This knowledge facilitates the interpretation of pathogenic variations in PIGW, which primarily cause hyperphosphatasia with mental retardation syndrome26 (HPMRS, also known as Mabry syndrome). Interestingly, HPMRS variations predominantly cluster around the predicted substrate binding pockets of PIGW (Supplementary Table 2).
Furthermore, the molecular docking study identified the luminal cavity as a potential GlcN-PI binding pocket. Combined with the palmitoyl-CoA bound GWT1 structure, this suggests how the enzyme coordinates two distinct substrates across the membrane for catalysis (Fig. 7a, b). Our GWT1-manogepix structure indicates that manogepix inhibits GWT1 activity by competitively blocking its binding to the essential substrate, palmitoyl-CoA (Fig. 7c). Our work reveals significant structural differences between GWT1 and other known membrane-embedded acyltransferases. Notably, GWT1 functions as a monomer, unlike HGSNAT and MBOAT family members (ACAT1, DGAT1, and LPCAT3), which require dimerization or tetramerization for activity27. Interestingly, while histidine residues play critical roles in HGSNAT (H297) and MBOAT family enzymes (H460 in ACAT1, H415 in DGAT1, H388 in LPCAT3, H356 in MBOAT7, H336 in PORCN, and H379 in HHAT), single and even double mutations of conserved histidine residues (H228 and H235) exhibit negligible effects on GWT1 activity (Supplementary Fig. 9c). These findings suggest a unique catalytic mechanism in GWT1 compared to other known enzymes. With previous studies on HHAT28,29, a sequential reaction sequence appears reasonable based on the structural features of GWT1 (Supplementary Fig. 11b–e). D145 likely functions as a general base, activating the 2’-position of the inositol ring for nucleophilic attack on the thioester group of palmitoyl-CoA, leading to the release of CoA. The conserved K155 likely forms a hydrogen bond with GlcN-PI, thereby facilitating the transfer of the fatty acid tail and the formation of the new ester bond in the final GlcN-(acyl)PI product (Supplementary Fig. 11e). However, the precise catalytic mechanism of GWT1 awaits further structural determination of GWT1 bound to its substrate GlcN-PI and product GlcN-(acyl)PI.
a Hypothetical model for GWT1-catalysed GlcN-(acyl)PI formation. Highlighting the substrate-donor (Palmitoyl-CoA) and acceptor (GlcN-PI) binding pocket. The binding sites for palmitoyl-CoA are colored in blue, while the putative GlcN-PI binding sites are colored in orange. b The putative post-catalytic state contains two products including GlcN-(acyl)PI and free CoA. c The diagram of the inhibition mechanism model. The inhibitor competitively occupies the binding cavity of the lipid tail of palmitoyl-CoA, blocking the palmitoyl-CoA entry.
In conclusion, our comprehensive structural and functional characterization of GWT1 and its interaction with palmitoyl-CoA sheds light on the key acylation step in the fungal GPI biosynthesis pathway. Given the high conservation of GWT1 orthologs among various fungal pathogens, these findings are likely to be broadly applicable. The GWT1-manogepix complex structure provides a valuable framework for understanding drug resistance mechanisms to GWT1 mutations. Moreover, these structure insights pave the way for rational drug design aimed at improving the efficacy and selectivity of GWT1 inhibitors. By elucidating the inhibitory mechanism of GWT1, this work establishes a framework for future research on this promising antifungal target, guiding the development of novel antifungal drugs.
Methods
Minimum inhibitory concentrations (MIC) Measurement
To evaluate the effect of manogepix (3-[3-[[4-(pyridin-2-yloxymethyl)phenyl]methyl]−1,2-oxazol-5-yl]pyridin-2-amine, purity: 99.98%, Selleck) on the growth of yeast (Saccharomyces cerevisiae BY4741, GWT1WT and GWT1Y408L, both containing G418 resistance genes, were generated through homologous recombination and subsequent resistance screening), the half minimum inhibitory concentration (MIC50) was determined over a range of manogepix concentrations (0-10.24 μg/mL). For the assay, a log-phase culture was diluted to 0.05 (OD600) using YPD medium (20 g/L glucose, 10 g/L yeast extract, 20 g/L peptone), as measured in a standard spectrophotometer. Subsequently, the respective concentration of manogepix was added, and the culture was incubated at 30 °C for 12 hours. Finally, 200 µL of the diluted culture was dispensed into the wells of a sterile 96-well assay plate and analyzed using a microplate reader (BioTek Synergy H1) at 600 nm. Each experiment was repeated three times. To determine MIC50 values from the growth rate, the growth rate was calculated as the slope of the exponential growth phase using GraphPad Prism10.
Protein expression and purification
The DNA of yeast GWT1 (NCBI reference sequence NC_001142.9) was cloned into a modified pFastBac1 vector, incorporating an N-terminal Strep tag. For fGWT1, the DNA of GFP (NCBI ID: AAX83124.1) was inserted into GWT1 between positions Y422 and K423. All mutants were generated using a site-directed mutagenesis strategy. The bacmid was produced in DH10Bac cells (Thermo Fisher Scientific) to express recombinant proteins. Baculovirus was generated by transfecting Sf9 cells cultured at 27 °C with the bacmid using the Cellfectin® II Reagent (Gibco). Once the cell density reached 1.5–2.0 × 106 cells/ml, baculovirus was introduced into the culture. For the structural determination of fGWT1-manogepix protein, the inhibitor manogepix (500 nM) was introduced four hours after the addition of the baculovirus. After a 72-h transfection at 27 °C, Sf9 insect cells were harvested.
For the purification of GWT1 and fGWT1, Sf9 cells were harvested and resuspended in lysis buffer (25 mM HEPES pH 7.5, 150 mM NaCl) containing 2% (w/v) n-Dodecyl-β-D-Maltopyranoside (DDM, Anatrace), 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktails (Sangon Biotech) at 4 °C for 1.5 h. Subsequently, the lysate was centrifuged at 18,000 g for 30 minutes, and the resulting supernatant was incubated with Strep-Tactin Sepharose resin (IBA Lifesciences GmbH) at 4 °C for 30 minutes. The resin was thoroughly washed with a wash buffer (25 mM HEPES pH 7.5, 150 mM NaCl, 0.02% DDM) and eluted using a buffer containing 25 mM HEPES pH 7.5, 150 mM NaCl, 60 mM Biotin, and 0.02% DDM. The eluate underwent gel filtration (Superdex 200 Increase 10/300; GE Healthcare) in a buffer containing 25 mM HEPES pH 7.5, 150 mM NaCl and 0.02% DDM; the fractions associated with the peak were concentrated in preparation for subsequent experiments.
Initially, the GFP nanobody (NCBI database protein accession number: BBJ34638.1) cDNA was subcloned into the pET21b vector and expressed in E. coli BL21(DE3) cells for purification. Subsequently, the cells were cultured at 37 °C and induced with 0.2 mM isopropyl β-D-thiogalactoside (IPTG) at 18 °C overnight, initiated once the cell density reached an OD600 of 0.8. After harvesting, the cells were homogenized in a lysis buffer (25 mM Tris-HCl, pH 8.0, 150 mM NaCl) using a high-pressure homogenizer (ATS), and the resultant lysate was subsequently centrifuged at 18,000 g for 30 minutes. The resulting supernatant was applied to Ni-NTA resin (QIAGEN) and then eluted using a buffer consisting of 25 mM Tris-HCl pH 8.0, 150 mM NaCl, and 250 mM imidazole. The eluate underwent ion-exchange chromatography (Source 15Q, GE Healthcare) and gel filtration (Superdex 200 Increase 10/300; GE Healthcare). Finally, nanobodies were eluted in a buffer containing 25 mM HEPES pH 7.5 and 150 mM NaCl.
The concentrated fGWT1 proteins were incubated with nanobodies at a molar ratio of 1:2 at 4 °C for 1 h. The fGWT1-nanobody complex was subjected to size-exclusion chromatography in a buffer containing 25 mM HEPES pH 7.5, 150 mM NaCl, and 0.02% DDM. The fractions corresponding to the peak were combined and concentrated to a final concentration of 11 mg/mL for cryo-EM analysis.
Flow cytometry
HEK293TWT and HEK293T△PIGW (used as a negative control for background staining) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, 100 units/mL of penicillin, and 100 µg/mL streptomycin in a 6-well plate inside a 5% CO2 stationary incubator at 37 °C one day before transfection. The transfection procedure involved mixing 2 μg total plasmid with 4 μL P3000 (Cat. L3000008, Thermo Fisher Scientific) in 100 μL opt-MEM medium. This mixture was then combined with another premix of 2 μL Lipofectamine 3000 in 100 μL opt-MEM medium at room temperature for 15 minutes. The resulting blend was added dropwise to the cell culture in a 6-well plate, and a total of 2 μg plasmid was used for transfection, following the same protocol. For the drug resistance test, we added 10 μM manogepix in the culture medium after transfection. Meanwhile, we added 0.002% DMSO in DMEM as a negative control. After further incubation at 37 °C for 48 h, cells were harvested and washed three times with PBS.
In experiments using CD59 as the GPI-anchored proteins (GPI-AP) reporter, an allophycocyanin (APC) -conjugated anti-CD59 antibody (Sino Biological) was applied at a 100-fold dilution for incubation with the cells for 1 h in the dark. After three washes, cells were suspended in 300 μL of PBS and analyzed using flow cytometry (Beckman CytoFlex LX) with two wavelength pairs (488/525 nm for GWT1 integrated with GFP, 645/660 nm for APC). FACS (fluorescence-activated cell sorting) experiments were conducted following specific gating strategies. In each FACS run, cells were initially gated to select living cells and single cells. Subsequently, the surface expression of CD59 was assessed by gating the population expressing GFP-fused GWT1/GWT1 mutants based on GFP fluorescence (488/525 nm). Finally, this identified population was further analyzed for allophycocyanin (APC) positivity (645/660 nm) as an indicator of cell surface CD59 staining using its antibody. FlowJo v10 was employed for the analysis of the FACS data.
Fluorescence confocal microscopy
We cultured HEK293TWT and HEK293TΔPIGW cells on Circle Microscope Glass (NEST) in a 24-well plate. We transfected them with 500 ng of the pCAG-GWT1-GFP plasmid or the pCAG plasmid with an empty insertion using the Lipofectamine 3000 transfection kit following the manufacturer’s instructions. After 48 h of transfection, cells were stained with a 1/200 diluted CD59 antibody (APC fluorescence) at room temperature for 1 h. Subsequently, cells were washed three times with PBS. Following the staining, cells were treated with 4% Paraformaldehyde Fix Solution (Beyotime, P0099) for 20 minutes at room temperature and washed three times with PBS. Finally, the cells were stained with DAPI staining solution (C1005 Beyotime) for 10 minutes at room temperature and washed three times with PBS. To prepare for imaging, 5 μL of Antifade Mounting Medium was added to the microscope slide, and the Circle Microscope Glass with the cell face was covered on the slide for sealing. Images of these cell samples were captured using a confocal microscope.
Cryo-EM sample preparation and data acquisition
The purified GWT1, either alone or co-purified with manogepix, was concentrated to a final concentration of 11 mg/ml for cryo-EM sample preparation. Each protein sample (3.5 μL) was applied to glow-discharged holey carbon grids (Quantifoil Au R1.2/1.3, 200 mesh, 15 mA, 1 min), respectively. After blotting for 1.5 s, the grids were flash-frozen in liquid ethane using a Vitrobot (Mark IV, Thermo Fisher Scientific) at 4 ̊C with 100% humidity. Subsequently, the cryo-EM grids were transferred to a 300 kV Titan Krios microscope equipped with a Gatan K2 Summit detector for data collection. The defocus values of micrographs ranged from −1.2 to −1.6 μm, and each stack of 30 frames was exposed for 6 s. The total dose for each stack was 50 e-/Å2 for GWT1 and 51.64 e-/Å2 for GWT1 co-purified with manogepix. Data collection was conducted using the EPU program.
Data processing
For the GWT1 in complex with palmitoyl-CoA (PDB ligand ID: PKZ) data set, 3,638 movies were collected and processed in CryoSPARC 4.1.230. 1,085,436 particles were auto-picked without reference, extracted with a box size of 256 pixels, and rescaled to 128 pixels (bin2). After a round of 2D classification, six 2D class averages were selected as templates for following particle picking. 2,484,079 particles were auto-picked using a template picker, and bin2 was extracted. After preliminary 2D classification, 3D classification and non-uniform refinement, 350,754 good particles were selected as “seed”. The 2,484,079 raw particles were evenly divided into 4 subgroups and combined with the “seed” particles, respectively. A round of seed-facilitated 3D classification was performed. After duplicate particles were removed, 861,943 high-quality particles were finally selected. These particles were unbinning and extracted with a box size of 256 pixels. After several rounds of heterogeneous refinement, CTF refinement, and non-uniform refinement, 238,678 particles were selected, resulting in a 3.47 Å map. However, the tiny swing of the fused GFP tag may affect the alignment of the particles and hinder the improvement of the density quality of the TM region. Therefore, a round of local refinement with a mask focus on the TM region of GWT1 was performed, yielding the final map at the resolution of 3.37 Å.
For the fGWT1-manogepix data set, 5,778 movies were collected and processed in CryoSPARC 4.4.1. A similar procedure was applied, and 252,957 good particles were selected, yielding the final map at a resolution of 3.55 Å.
Model building and refinement
We employed AlphaFold to generate a starting model for GWT1 (https://alphafold.ebi.ac.uk/entry/P47026). The model was initially fit into the cryo-EM density map using ChimeraX31. Iterative rounds of manual adjustment in Coot 0.9.8.132 were followed by real-space refinement using Phenix 1.20.133 and ISOLDE 1.634. Model validation was performed with PHENIX-1.20.133. Root-mean-square deviations (RMSDs) were calculated for Cα atoms using ChimeraX Matchmaker. Structural figures were generated with ChimeraX31, and sequence alignments were performed using ClustalW35.
Reactant system building
We prepared the palmitoyl-CoA-bound GWT1 complex by Maestro (Maestro; Schrödinger: New York 2018.), including assigning bond orders, adding hydrogens, and correcting orientation of polar residues contributing hydrogen bonds. The initial poses were sampled using the docking software Smina36, a fork of Autodock Vina with improved flexibility37. The docking site was defined by a 40 × 40 × 40 Å cubic grid box centered on the lumen entrance of GWT1. This relatively large grid box ensures sufficient space for accommodating GlcN-PI during the docking simulations. The “exhaustiveness” parameter was set to 100 to enhance configurational sampling of binding poses. All other parameters were kept at their default settings. Twenty docking poses were generated for further analysis. We added non-polar hydrogens once again to the docking poses in Meastro, and ultimately selected the pose of GlcN-PI that positions the O2’ atom near the thioester bond of palmitoyl-CoA (docking score: −6.0 kcal/mol).
Pose generation of manogepix in the GWT1-manogepix complex
We manually built the pose of manogepix based on its electron density and interaction with the protein. However, due to an incomplete density map, we considered an alternative pose that is opposite to the initially built one. To determine the better pose, we used the following in-silico protocol.
Docking poses were generated using the same software as in the previous section. The docking site was defined by a 30 × 30 × 30 Å cubic grid box centered on the manually placed pose of Manogepix, guided by its electron density. The “exhaustiveness” parameter was set to 100 to ensure sufficient configurational sampling of the ligand. Twenty docking poses were generated for further analysis.
The best-scoring pose (−9.5 kcal/mol) closely resembled the experimental pose (RMSD: 1.1 Å), leading us to select this experimental pose for further MD simulations. The alternative pose, ranked 14th (−8.7 kcal/mol), was oriented in the opposite direction to the experimental pose (RMSD: 9.6 Å). We chose this pose for subsequent MD simulations as it was the best-scoring among the poses with the opposite orientation.
System preparing for MD simulations
Before the MD simulations, the missing loops of GWT1 were modeled using SWISS-MODEL38. Each of the two poses, along with the fixed GWT1 structure, was solvated in a 99 Å rhombic dodecahedron TIP3P water box39, ensuring a 10 Å buffer distance between protein atoms and the water box boundary. To neutralize the protein-ligand system and mimic physiological conditions, 0.15 M of sodium chloride was added to the water box. The CHARMM36m force field40 was used to describe the GWT1 protein, and Manogepix was parameterized using the CGenFF force field41. Notably, to simplify the simulations, an explicit membrane model was not used to encapsulate GWT1. This approach is reasonable because the entire protein’s backbone was restrained during the simulations. Our main purpose is to check the stability of the poses.
Restrained MD simulations
Each simulation system was initially minimized for 10,000 steps under a series of restraints and constraints on the solute atoms to resolve bad contacts and poor geometry. The minimized structure was then heated to 300 K and equilibrated under NVT conditions (constant volume and temperature). This was followed by further equilibration under NPT conditions (constant pressure and temperature). All equilibration phases lasted for 1 ns, using the CHARMM program (version 42b2)42. Production runs of 30 ns each were conducted under NPT conditions using the NAMD program (version 2.14)43.
Pressure was controlled by the Nosé–Hoover Langevin piston method with a 200 ps piston period and a 100 ps piston decay time44,45. Temperature was maintained at 300 K using the Langevin thermostat with a 5 ps friction coefficient. The integration time step was set to 2 fs by constraining all bonds involving hydrogen atoms using the SHAKE algorithm. Van der Waals (vdW) energies were calculated using a switching function with a switching distance from 10 to 12 Å, and electrostatic interactions were evaluated using the particle mesh Ewald summation (PME) method46. The Lennard–Jones long-range correction47 was enabled.
The entire protein backbone was harmonically restrained with a spring constant of 0.01 kcal/mol/Ų during the production MD simulations, keeping the backbone in its original coordinates while allowing the side chains and ligand to move freely. Five independent runs with random initial velocities were carried out for each pose, totaling 150 ns. MD snapshots were saved every 20 ps along the MD trajectories for further analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The coordinates for the model GWT1-palmitoyl-CoA and GWT1- manogepix generated in this study have been deposited in the PDB under accession numbers 8XIJ and 8XIK, respectively. The cryo-EM density maps for GWT1-palmitoyl-CoA and GWT1- manogepix generated in this study have been deposited in the Electron Microscopy Data Bank with accession codes EMD-38374 and EMD-38375. All other data are available in the main text or the supplementary materials. Source data are provided with this paper.
References
Lockhart, S. R., Chowdhary, A. & Gold, J. A. W. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 21, 818–832 (2023).
Perfect, J. R. The antifungal pipeline: a reality check. Nat. Rev. Drug Discov. 16, 603–616 (2017).
Fisher, M. C., Hawkins, N. J., Sanglard, D. & Gurr, S. J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360, 739–742 (2018).
Parums, D. V. Editorial: The World Health Organization (WHO) fungal priority pathogens list in response to emerging fungal pathogens during the COVID-19 pandemic. Med. Sci. Monit. 28, e939088 (2022).
Vogt, M. S., Schmitz, G. F., Varon Silva, D., Mosch, H. U. & Essen, L. O. Structural base for the transfer of GPI-anchored glycoproteins into fungal cell walls. Proc. Natl Acad. Sci. Usa. 117, 22061–22067 (2020).
Orlean, P. Architecture and biosynthesis of the saccharomyces cerevisiae cell wall. Genetics 192, 775 (2012).
Umemura, M. et al. GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J. Biol. Chem. 278, 23639–23647 (2003).
Tsukahara, K. et al. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol. Microbiol. 48, 1029–1042 (2003).
Miyazaki, M. et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55, 4652–4658 (2011).
Watanabe, N. A. et al. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents Chemother. 56, 960–971 (2012).
Pfaller, M. et al. In vitro activity of manogepix and comparators against infrequently encountered yeast and mold isolates from the SENTRY Surveillance Program (2017-2022). Antimicrob Agents Chemother, e0113223, https://doi.org/10.1128/aac.01132-23 (2024).
Hodges, M. R. et al. Safety and pharmacokinetics of intravenous and oral fosmanogepix, a first-in-class antifungal agent, in healthy volunteers. Antimicrob. Agents Chemother. 67, e0162322 (2023).
Murakami, Y. et al. PIG-W is critical for inositol acylation but not for flipping of glycosylphosphatidylinositol-anchor. Mol. Biol. Cell 14, 4285–4295 (2003).
Holm, L., Laiho, A., Toronen, P. & Salgado, M. DALI shines a light on remote homologs: one hundred discoveries. Protein Sci. 32, e4519 (2023).
Grevengoed, T. J., Klett, E. L. & Coleman, R. A. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 34, 1–30 (2014).
Sagane, K. et al. Analysis of membrane topology and identification of essential residues for the yeast endoplasmic reticulum inositol acyltransferase Gwt1p. J. Biol. Chem. 286, 14649–14658 (2011).
Wang, L. et al. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature 581, 329–332 (2020).
Sui, X. W. et al. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature 581, 323 (2020).
Navratna, V., Kumar, A. & Mosalaganti, S. Structure of the human heparan-α-glucosaminide N-acetyltransferase (HGSNAT). Elife 13, https://doi.org/10.7554/eLife.93510.1 (2024).
Zhao, B. et al. Structural and mechanistic insights into a lysosomal membrane enzyme HGSNAT involved in Sanfilippo syndrome. Nat. Commun. 15, 5388 (2024).
Xu, R. et al. Structure and mechanism of lysosome transmembrane acetylation by HGSNAT. Nat. Struct. Mol. Biol., https://doi.org/10.1038/s41594-024-01315-5 (2024).
Kapoor, M., Moloney, M., Soltow, Q. A., Pillar, C. M. & Shaw, K. J. Evaluation of resistance development to the Gwt1 inhibitor manogepix (APX001A) in Candida species. Antimicrob. Agents Chemother. 64, https://doi.org/10.1128/AAC.01387-19 (2019).
Nakamoto, K. et al. Synthesis and evaluation of novel antifungal agents-quinoline and pyridine amide derivatives. Bioorg. Med. Chem. Lett. 20, 4624–4626 (2010).
McLellan, C. A. et al. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem. Biol. 7, 1520–1528 (2012).
Liston, S. D. et al. Antifungal activity of gepinacin scaffold glycosylphosphatidylinositol anchor biosynthesis inhibitors with improved metabolic stability. Antimicrob. Agents Chemother. 64, https://doi.org/10.1128/AAC.00899-20 (2020).
Chiyonobu, T., Inoue, N., Morimoto, M., Kinoshita, T. & Murakami, Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with West syndrome and hyperphosphatasia with mental retardation syndrome. J. Med. Genet. 51, 203–207 (2014).
Pierce, M. R. & Hougland, J. L. A rising tide lifts all MBOATs: recent progress in structural and functional understanding of membrane bound O-acyltransferases. Front. Physiol. 14, 1167873 (2023).
Jiang, Y., Benz, T. L. & Long, S. B. Substrate and product complexes reveal mechanisms of Hedgehog acylation by HHAT. Science 372, 1215–1219 (2021).
Coupland, C. E. et al. Structure, mechanism, and inhibition of Hedgehog acyltransferase. Mol. Cell 81, 5025–5038.e5010 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Croll, T. I. : a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr D. 74, 519–530 (2018).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. Clustal-W - improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Koes, D. R., Baumgartner, M. P. & Camacho, C. J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 53, 1893–1904 (2013).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Huang, J. & MacKerell, A. D. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Martyna, G. J., Tobias, D. J. & Klein, M. L. Constant-pressure molecular-dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).
Feller, S. E., Zhang, Y. H., Pastor, R. W. & Brooks, B. R. Constant-pressure molecular-dynamics simulation - the langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Shirts, M. R., Mobley, D. L., Chodera, J. D. & Pande, V. S. Accurate and efficient corrections for missing dispersion interactions in molecular Simulations. J. Phys. Chem. B 111, 13052–13063 (2007).
Acknowledgements
Cryo-EM data were acquired at the SKLB West China Cryo-EM Center of Sichuan University and the Beijing Frontier Research Center for Biological Structure (FRCBS) of Tsinghua University. Special appreciation goes to Professor Morihisa Fujita from Gifu University for generously donating the PIGW knockout cell line. We sincerely thank the public technology platform at West China Hospital, Sichuan University, for granting us access to the Beckman CytoFLEX for FACS experiments. We also acknowledge Dr. Hua He at the NHC Key Laboratory of Chronobiology, Sichuan University, for providing support with the confocal microscope. We thank the Swedish National Infrastructure for Computing (SNIC) at the High-Performance Computing Center North (HPC2N) for providing the computational resources for the molecular simulations. This work was supported by the National Natural Science Foundation of China (32471264 and 32100979 to X.W.; 31971132 and 32171211 to D.D.). H.C. was funded by the Sichuan Science and Technology Program, grant number 2024NSFSC0723. Y.Z.L. was supported by an International Postdoc Grant funded by the Swedish Research Council (grant VR 2019-00608).
Author information
Authors and Affiliations
Contributions
D.D., H.M.L, and X.W. designed the experimental protocols. X.L.D purified complexes and conducted FACS assays, while J.L.L. collected and processed cryo-EM data with assistance from C.Y.Y. X.Z.L. conducted molecular cloning and protein expression experiments. Y.Z.L. carried out the molecular simulation experiments in this work. The manuscript was written by X.W, with valuable input from X.L.D., X.Z.L, H.C., and J.L.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, X., Liu, X., Li, J. et al. Structural insights into the inhibition mechanism of fungal GWT1 by manogepix. Nat Commun 15, 9194 (2024). https://doi.org/10.1038/s41467-024-53512-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53512-x