Abstract
Hepatocellular carcinoma (HCC) patients with macrovascular invasion (MVI) have dismal prognosis and there are no standard perioperative therapies. This phase 2 trial (ChiCTR2000036385) aimed to investigate the activity and safety of perioperative tislelizumab plus intensity modulated radiotherapy (IMRT) for resectable HCC with MVI. Thirty treatment-naïve patients with MVI received 3 cycles of tislelizumab intravenously (200 mg, every three weeks) and concurrent IMRT (45 Gray in 15 fractions). Primary endpoints were the overall response rate (ORR) and overall survival (OS). Secondary endpoints were the proportion of patients with a complete or major pathological response (pCR or MPR), recurrence-free survival (RFS) and safety. Of patients enrolled, 15 (50%) underwent curative surgery followed by adjuvant tislelizumab. The ORR was 30.0% (90% CI 16.6%-46.5%) and the median OS was 18.7 months. Of the 15 patients underwent surgical resection, 10 (66.7%) achieved pCR or MPR and 8 (53.3%) remained recurrence-free. The median RFS were not reached with a median follow-up of 21.77 months (95% CI 12.50-31.03) post-surgery. 4 (13.3%) patients experienced grade 3 treatment-related adverse events. The most common events were thrombocytopenia, leukopenia, and anemia. The trial has met the pre-specified endpoints, and these results support further studies of perioperative immunotherapy plus radiotherapy in HCC.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer globally and the third leading cause of cancer-related mortality1. HCC displays a distinct ability to invade and grow within the hepatic vasculature and form tumor thrombus in the portal or hepatic vein, which is also called macrovascular invasion (MVI). About 20% of patients have MVI at diagnosis and are designated as advanced disease, with a median survival ranging from 2 to 5 months with best supportive care2. Recently, the combination of atezolizumab and bevacizumab has exhibited substantial antitumor efficacy and was approved for advanced HCC3. However, only about 30% of patients with HCC benefit from immune checkpoint-based therapies4.
Guidelines of Asian-Pacific5 and China6 also recommended local therapies for HCC with MVI. Among which, radiotherapy demonstrated promising clinical efficacy7. Furthermore, combining radiotherapy with immune checkpoint inhibitors (ICIs) is a potentially effective strategy8,9. Two randomized trials have shown that the addition of radiotherapy to immunotherapy improves distal response rates, progression-free survival, and overall survival in patients with metastatic non-small cell lung cancer (NSCLC)10,11. Several clinical studies also reported the promising efficacy of combining ICI and radiotherapy in advanced HCC12,13. On the other hand, surgical resection was also shown to provide survival benefits for patients with MVI14,15. However, the postoperative recurrence rate is high and the long-term survival is poor in these patients. What’s noteworthy is that neoadjuvant radiotherapy can reduce the extent of MVI and improve post-operative survival rate at 12 months16, indicating the efficacy of perioperative radiotherapy in improving the survival of patients with MVI.
Tislelizumab is an monoclonal IgG4 antibody targeting programmed death receptor-1 (PD-1) in humans, has demonstrated antitumor activity in patients with advanced tumors, including HCC17,18. In the RATIONALE 301 trail, in patients with unresectable HCC, first-line tislelizumab monotherapy demonstrated noninferior OS versus sorafenib, with favored results shown in ORR and duration of response19. Positive results of the study has led to the approval of tislelizumab as first-line treatment for unresectable HCC in China.
In this work, we evaluated the efficacy and safety of perioperative tislelizumab plus IMRT in resectable HCC with MVI. Perioperative tislelizumab plus IMRT demonstrated promising efficacy and manageable safety profiles, providing a potential option for HCC with MVI.
Results
Patient characteristics
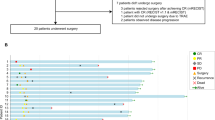
Between October 21, 2020, and February 15, 2023, 31 patients underwent eligibility screening. One patient withdrew consent, and 30 patients were enrolled and received 3 cycles of tislelizumab and concurrent IMRT. After response evaluation, 15 patients with unresectable HCC withdrew from the study. The other 15 patients were considered eligible and underwent curative surgery followed by adjuvant tislelizumab. At the time of data cutoff, 6 patients relapsed within 1 year, 1 patient relapsed after 1 year, and 8 patients had completed the study treatment without recurrence (Fig. 1).
The baseline characteristics of the intention-to-treat population are summarized in Table 1. Most of the patients were male, under age 60 and were classified as Child-Pugh score A. Hepatitis B Virus (HBV) infection was the most common etiology of HCC (96.7%). All participants were treatment-naïve and exhibited tumors with MVI (Supplementary Table 1). The median follow-up was 20.35 months (95% CI 14.17-26.52).
Efficacy
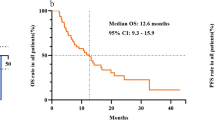
The ORR was 30.0% (90%CI 16.6–46.5%) and the disease control rate (DCR) was 36.6%. Nine patients (30%) achieved PR, 2 (6.7%) patients had SD, and 19 (63.3%) patients had PD (Table 2). Regression in the target lesions was observed in 26 (86%) patients. Among those with PD, 14 patients developed new lesions in the liver and 3 patients developed extrahepatic metastasis (Fig. 2A). The detailed information of response, progression and resectability of patients were listed in Supplementary Table 6. The median OS was 18.7 months. The OS rate at 6-, 12- and 24-month was 79%, 58% and 47%, respectively (Fig. 2B). Patients who underwent surgery after evaluation had a significantly longer median OS compared to those who were considered unresectable (not reached versus 7.0 months, p = 0.025) (Fig. 2C). Furthermore, cox regression also revealed a significant correlation between surgery and OS (Hazard ratio [HR] 0.30 [0.09–0.91]; p = 0.034) (Supplementary Table 2).
A Waterfall plot showing the percentage change in target lesions from baseline (n = 30), according to treatment response per RECIST version 1.1. B Kaplan–Meier analysis of overall survival (n = 30). C Kaplan–Meier analysis revealed an improved overall survival in patients with surgery, p-value was compared via a two-sided log-rank test. Source data are provided as a Source Data file. OS, overall survival.
In 15 patients who underwent surgical resection, 10 (66.7%) patients achieved a significant pathological response (pCR or MPR) (Table 2). The median RFS was not reached after a median follow-up of 21.77 months (95% CI 12.50–31.03) post-surgery. The RFS rate at 6-, 12- and 24-month was 67%, 60% and 53%, respectively (Fig. 3A). Univariate analyzes demonstrated that alpha-fetoprotein (AFP) levels at baseline ( ≥ 400 μg/L versus <400 μg/L, HR 9.61 [1.13–81.81]; p = 0.038), AFP reduction ( ≥ 90% or Low [AFP < 400 μg/L at baseline] versus <90%, HR 0.11 [0.01–0.69]; p = 0.019), and significant pathological response (yes versus no, HR 0.18 [0.04–0.88]; p = 0.034) were significantly associated with RFS (Supplementary Table 3). Kaplan–Meier analysis also revealed a remarkably improved RFS in patients with low baseline AFP levels ( < 400 μg/L versus ≥400 μg/L, not reached versus 2.2 months, p = 0.013), AFP reduction ( ≥ 90% or Low versus <90%, not reached versus 2.2 months, p = 0.005), or a significant pathological response (yes versus no, not reached versus 2.2 months, p = 0.02) (Fig. 3B–D). Moreover, we investigated the correlation between baseline characteristics and radiographic and pathological response (Supplementary Table 4 & 5). The findings demonstrated a noteworthy reduction in AFP levels was linked to the significant pathological response (p = 0.022) (Supplementary Table 5).
A Kaplan–Meier analysis of recurrence-free survival (n = 15). B–D Kaplan–Meier analysis revealed a remarkably improved recurrence-free survival in patients with low baseline AFP levels ( < 400 μg/L), significant AFP reduction ( ≥ 90% or Low) and significant pathological response (MPR/pCR), p-value was compared via a two-sided log-rank test. Source data are provided as a Source Data file. AFP, alpha-fetoprotein; RFS, recurrence-free survival.
Safety profile
During this study, 28 (93.3%) patients had at least one treatment related adverse event (TRAE) (Table 3 and Supplementary Table 10). The most common TRAEs of any grade were thrombocytopenia (n = 25, 83.3%), leukopenia (n = 18, 60.0%) and anemia (n = 16, 53.3%). During the preoperative therapy, TRAEs of any grade occurred in 27 patients. The three most frequent TRAEs were thrombocytopenia (n = 22, 73.3%), leukopenia (n = 15, 50%) and anemia (n = 13, 43.3%) (Table 3). Grade 3 TRAEs only occurred in 3 patients (10%), including leukopenia and thrombocytopenia, which resolved after symptomatic treatment without the need for corticosteroid treatment. The postoperative complications were summarized in Supplementary Table 9. Postoperative complications were observed in 8 of 15 (53.3%) patients. Most complications were grade 1, which included ascites (20%), pleural effusion (26.7%) and biliary leakage (6.7%). Whereas only 1 (6.7%) patient had grade 2 complication, which was transient liver dysfunction and was promptly managed. No grade 3 or higher complications occurred. During adjuvant therapy, the three most frequent TRAEs were leukopenia (n = 3, 20.0%), thrombocytopenia (n = 3, 20.0%) and anemia (n = 3, 20.0%) (Table 3). Grade 3 TRAEs only occurred in 1 patient (6.7%), which was ALP increased. All adverse events resolved after symptomatic treatment without the need of corticosteroids treatment.
Subsequent treatments
In addition, subsequent treatments for patients who had PD were listed in Supplementary Table 7. Of the 15 patients who were assessed as unresectable, 8 patients received a combination of tislelizumab plus lenvatinib with or without transarterial chemoembolization (TACE), and 2 patients received lenvatinib or TACE. Of the 7 patients relapsed during adjuvant tislelizumab treatment, 6 received subsequent treatments, including the combination of tislelizumab plus lenvatinib with or without TACE (n = 3), the combination of atezolizumab plus bevacizumab with TACE (n = 1), lenvatinib (n = 1) and HAIC (n = 1).
Discussion
In this trial, perioperative tislelizumab plus IMRT is viable for resectable HCC with MVI, yielded an ORR of 30% and a median OS of 18.7 months. Among the patients enrolled, half of them underwent surgery followed by adjuvant tislelizumab, with 10 (66.7%) patients achieved significant pathological responses and resulting in a remarkably improved RFS in these patients. Grade 3 TRAEs were observed in 13.3% of patients, while there was no grade 4 TRAE or grade 3/4 postoperative complication occurred. The encouraging efficacy and acceptable safety profiles suggested that perioperative tislelizumab plus IMRT could be a potential option for HCC with MVI.
Radiotherapy is important in the treatment of patients with MVI due to the radiosensitivity of MVI cells to irradiation20. Radiotherapy may be a potent immunomodulator that enhances the efficacy of ICIs via multiple mechanisms, including induction of immunogenic cell death with release of neoantigens, upregulation of major histocompatibility complex and enhanced antigen presentation, activation of dendritic cells, and increasing T cell infiltration into the tumor. Furthermore, accumulating evidences suggests that the combination of radiotherapy and immunotherapy has synergistic effects on both local and distant tumor control21,22,23. Synergistic effects between radiotherapy and immunotherapy were also reported in HCC24,25. Moreover, the antitumor effects induced by immunotherapy are more likely to occur when there is a large burden of primary tumor (including rich tumor antigens to be targeted by the immune system) prior to surgery26.
A preclinical study of colon cancer in mice indicated that sequential treatment with anti-PD-L1 mAb 7 days after radiotherapy was ineffective in enhancing OS as compared to concurrent anti-PD-L1 mAb and radiotherapy on day 1. Further analysis suggested that while radiotherapy causes an acute increase in PD-1 expression on T cells, delaying PD-1/PD-L1 blockade after radiotherapy may be ineffective, potentially due to the deletion or anergy of tumor-reactive CD8+ T cells27. In addition, a subgroup analysis of the PACIFIC study suggests that patients with unresectable stage III NSCLC have better outcomes if immunotherapy is given concurrently with, or soon after radiotherapy, compared to starting immunotherapy later after radiotherapy28. Similarly, a multicentric retrospective study for patients with brain metastases of NSCLC also reported that patients with the interval between stereotactic body radiation therapy (SBRT) and immunotherapy ≤7 days had a longer survival compared with the interval >7 days29. In HCC, recent clinical studies also demonstrates that concurrent immunotherapy and radiotherapy is safe and offers promising efficacy in patients25,30,31. In this study, radiotherapy was initiated the day after the first tislelizumab injection. The simultaneous administration of immunotherapy and radiotherapy not only ensured the efficacy and safety but also allowed the patients to be assessed and resected as soon as possible, as all patients enrolled in this study were at high risk of rapid intra- or extrahepatic tumor spread. Indeed, perioperative tislelizumab plus IMRT achieved an ORR of 30% and a median OS of 18.7 months, preliminarily demonstrating the synergistic effects and feasibility of our treatment strategy.
Retrospective studies have demonstrated that surgery can improve survival in selected patients with MVI when compared to nonsurgical treatments32,33,34. Nevertheless, the survival benefits offered by surgery alone remain limited and optimal perioperative therapy to improve survival is still an unsolved issue for patients with MVI. In a phase 2 trial conducted in patients with resectable HCC (14% of patients had stage C disease), it was found that 4 (20%) patients had significant tumor necrosis ( > 70% necrosis) after two cycles of neoadjuvant cemiplimab followed by surgical resection35. Another phase II trial evaluated the efficacy and safety of perioperative camrelizumab in combination with apatinib in patients with intermediate to advanced stage HCC. The findings revealed that out of the 17 patients who underwent surgical resection, 4 (23.5%) patients achieved MPR/pCR36. In a phase 2 trial of patients with early to intermediate stage HCC, perioperative nivolumab alone and nivolumab plus ipilimumab demonstrated a major pathological response ( > 70% necrosis) of 30%37. Another single-arm study with locally advanced HCC (of which 27% had MVI) revealed that neoadjuvant cabozantinib plus nivolumab resulted in MPR/pCR in 5 of 12 patients (41.6%)38. In contrast, in our study, 10 (66.7%) patients achieved MPR/pCR with perioperative ICI plus IMRT. The high rate of pathological response confirmed the potent efficacy of ICI plus IMRT. Additionally, 8 (53.3%) patients remained relapse-free with a median follow-up of 21.77 months post-surgery, further suggesting that perioperative ICI plus IMRT not only effectively induce tumor-cell death, but also elicit durable anti-tumor responses by eliminating potential tumor residual or micro-metastasis.
It is worth noting that our study found no correlation between radiographic response and pathological response (p = 1.0) or RFS (p = 0.870), but patients with pathological responses exhibited notable improved RFS. The discrepancies between standard radiological assessment (RECIST v1.1) and pathological response may be attributable to that early necrosis within the tumor may not lead to significant tumor shrinkage35. Enough or longer treatment duration may result in significant tumor necrosis as well as consistent tumor shrinkage. However, the longer periods of preoperative therapy might increase the likelihood of perioperative toxicity and the risk of losing the opportunity of resection due to progression37. In this study, all patients enrolled were at high risk of intra- or extrahepatic tumor spread. Therefore, the duration needs to ensure that preoperative therapy can take effect while patients can be assessed and resected on schedule. Previously, in a study from our center, resectable HCC with portal vein tumor thrombus (PVTT) were evaluated and underwent surgery 4 weeks after neoadjuvant radiotherapy with an ORR of 20.7%16. In addition, the median time to response of tislelizumab was 2.2 months (about 9 weeks) in patients with advanced HCC19. Thus, 9 weeks of tislelizumab plus IMRT was enough and would induce sufficient tumor response. As expected, after 9 weeks of treatment, among 15 patients who underwent surgery, 10 (66.7%) achieved a significant pathological response, suggesting that it was reasonable to perform response assessment 9 weeks after the initiation of tislelizumab.
Previous experience with colorectal cancer liver metastasis showed that pCR was achieved in approximately 70% of patients who underwent liver resection, and that over half of patients would relapse if the disappeared liver metastases were not resected39. On the other hand, it is challenging to confirm MPR or pCR in tumor until it is surgically removed. Additionally, it remains uncertain whether the remaining viable tumor cells will eventually perish or develop resistance to treatment and give rise to relapse or metastasis in the future. Recent studies on immune-based neoadjuvant or conversion therapy further support the benefits of surgical intervention in patients with advanced HCC34,40. The START-FIT trial with sequential TACE and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for locally advanced unresectable HCC (included 64% patients with MVI) resulted in 55% of patients becoming amenable to curative treatment and achieved an OS of 30.3 months41. Accordingly, following perioperative ICI plus IMRT therapy, half of patients in our study underwent curative resection. Notably, four patients who were still eligible for curative surgery despite a BOR of PD after perioperative treatment were not precluded from curative surgery. Of the four patients, 2 achieved MPR and were still tumor-free for over 20 months. Thus, at current stage, it is necessary to surgically eradicate the tumor after perioperative treatment whenever possible.
The other 2 patients who did not achieve MPR had a RFS of 0.72 months and 2.5 months, an OS of 7.5 months and 5.03 months, respectively. Meanwhile, they were also accompanied with no significant AFP reduction after preoperative treatment, indicating the potential correlation between AFP reduction and postoperative outcome. AFP is one of the most widely used serum biomarker in HCC and elevated AFP levels was considered to be associated with a more aggressive tumor biology and burden42. Alternation in AFP changes ( ≥ 75% decrease or ≤10% increase) after initiating the combination therapy of atezolizumab and bevacizumab has been reported to be associated with prolonged OS and PFS in patients, suggesting that AFP could be a surrogate biomarker for treatment response43. Another recent study reported that early AFP reduction ( > 10%) was the independent predictor of ORR in patients treated with nivolumab or pembrolizumab44. Indeed, in this study, we found a significant correlation between the notable reduction in AFP levels and RFS or pathological response, indicating the value of AFP reduction as a surrogate biomarker for predicting pathological response and postoperative outcome in patients with elevated AFP levels at baseline.
Several studies have reported improved responses for MVI compared to intrahepatic lesions in patients receiving systematic therapies, including tyrosine kinase inhibitors or immunotherapy45,46. Thus, we further defined the response of MVI based on the downstaging (PR) or upstaging (PD) of MVI extent (Supplementary Table 8)16. The results demonstrated no significant correlation of responses between MVI and the primary tumors (p = 0.402), and the ORR for MVI was comparable with that for primary tumors (33.3% versus 30.0%). However, the DCR for MVI was higher than that for intrahepatic tumors (93.3% versus 36.6%), which may be explained by the radiosensitivity of MVI cells to irradiation20, or the higher intrahepatic tumor burden and the unfavorable immunosuppressive microenvironment of the liver47.
Meanwhile, effective adjuvant therapies are urgently needed to prevent recurrence in patients with HCC48,49. Immunotherapy could prevent immune escape via PD-1/PD-L1 pathway and induce necrosis of residual microsatellite lesions in the liver50. Immunotherapy has also been reported to reduce the risk of postoperative recurrence in patients with HCC. In 2015, a systematic review of adjuvant treatment options for HCC also showed that immunotherapy may prevent recurrence of resected HCC51 and was included in National Comprehensive Cancer Network (NCCN) guidelines Version 1.2024. A study conducted on HCC with high-risk of recurrence after curative resection or ablation (IMbrave 050) demonstrated the efficacy of atezolizumab plus bevacizumab in reducing recurrence52. Moreover, in another randomized phase 2 trial, adjuvant treatment with a single PD-1 inhibitor significantly prolonged RFS compared with active monitoring53. Despite of the promising results, there is currently no approved standard adjuvant intervention. The patients enrolled in this study were HCC with MVI and were all at high risk of recurrence49. Furthermore, early recurrence within the first year after resection accounts for more than 60% of HCC recurrences54. Consequently, in the current study, in order to reduce the risk of recurrence, patients were administered with adjuvant tislelizumab for a duration of one year. The findings showed that 1 (6.7%) patient relapsed after 1 year, and 8 (53.3%) patients remained relapse-free with a median follow up of 21.77 months post-surgery, preliminarily demonstrating the efficacy of adjuvant tislelizumab.
The irradiation dose predominantly depends on the tolerable dose for peripheral normal tissues but not tumor burden7,55. In addition, to enable repair of the collateral damage to the normal tissue, radiation is usually given in multiple fractions, usually of 2–5 Gray56,57. Guidelines for the diagnosis and treatment of primary liver cancer of China (2022 Edition) recommended an irradiation dose of 50–75 Gray for conventional fractionation radiotherapy57. Two recent phase II trials which evaluated the efficacy of neoadjuvant IMRT (50–60 Gray in 25–30 fractions) or immunotherapy plus bevacizumab combined with radiotherapy (30-50 Gray in 10 fractions) in HCC all demonstrated promising responses with acceptable safety profiles58,59. Accordingly, the prescribed dose for the planning target volume (PTV) was 45 Gray in 15 fractions over 3 weeks in our study, primarily based on the dose-volume constraints for organs at risk (OARs), while still delivering an effective cumulative dose. As a result, 9 of 30 patients (30.0%) achieved PR, and MPR was achieved in 6 patients (40%) and pCR in 4 patients (26.7%), and grade 3 TRAEs only occurred in 4 patients (13.3%), suggesting that our dose and fractionation ensured both efficacy and safety.
In the combination therapy of atezolizumab plus bevacizumab for unresectable HCC, 43% patients had grade 3/4 TRAEs, and 2% patients had grade 5 TRAEs60. The 6 grade 5 TRAEs were all bleeding events60. In the study of camrelizumab plus apatinib in surgical resectable HCC, Xia et al observed that the proportion of grade 3 or 4 TRAEs was 16.7% before surgery and 38.5% post-surgery, with hypertension being the most common TRAE36. Of note, anti-angiogenesis agents like bevacizumab or apatinib might increase the risk of perioperative bleeding and postoperative complications, and extend the waiting period before surgical resection after the combination therapy. Conversely, TRAEs of perioperative ICI plus IMRT were generally tolerated, and surgery was not delayed or precluded by TRAEs, demonstrating the safety of this combination.
Furthermore, it has been reported that radiotherapy could induce tumor thrombus shrinkage and solidification, leading to the occurrence of spontaneous portal vein embolization (PVE) in some patients with HCC. This additional safety measure enhances the feasibility of subsequent surgical interventions13. Notably, it is worth noting that irradiated solidified MVI induced spontaneous PVE in three patients in this study, and they all underwent successful resection subsequently, further underscoring the potential benefits of IMRT in the perioperative management of certain patients with MVI.
Despite the favorable ORR observed in perioperative treatment, half of patients were still deprived of the opportunity of surgery due to treatment failure. Although 15 patients were assessed surgically unresectable and withdraw from study, their median OS (7.0 months (95% CI 5.2–8.7)) was comparable with previously results reported in patients with PVTT who had undergone surgery (median OS: 6.0-7.8 months)61,62. Moreover, subsequent lines of treatment which included immunotherapy plus tyrosine kinase inhibitors or bevacizumab also did not improve their survival (Supplementary Table 7), indicating the fact that they were not only resistant to immunotherapy plus radiotherapy, but were also probably non-responders to the current main treatment strategies. Biomarkers to identify non-responders and new treatment regimens are urgently needed for these patients.
This study had several limitations. First, it was a single-arm, phase II trial, the effectiveness of the regimen should be further confirmed through comparative control groups, such as the first-line atezolizumab plus bevacizumab group, tislelizumab monotherapy group or IMRT alone group. Secondly, to provide long-term evidence of benefit (OS) in addition to short-term evidence of efficacy (ORR) in this study, OS was also included in the primary study endpoints. However, the sample size was calculated based only on ORR. Thus, the data related to OS in this trial may not be adequately powered. Thirdly, the exact synergistic effect between ICI and IMRT remains unclear, and further mechanism investigations are needed. Also, over 96% of patients in this study had chronic hepatitis B, which may restrict the generalizability of the finding to patients with different etiologies.
In conclusion, perioperative tislelizumab plus IMRT demonstrated promising efficacy for HCC with MVI. However, further investigation, including randomized trials is required before adoption of this approach, and we believe that this treatment strategy could also be a potential option for patients with early and intermediate-stage HCC.
Methods
Study design and participants
This study was a single-arm, phase 2 trial conducted at Eastern Hepatobiliary Surgery Hospital (EHBH) to investigate the efficacy and safety of tislelizumab plus IMRT in patients with resectable locally advanced HCC with macrovascular invasion (MVI). This trial was registered with Chinese Clinical Trial Registry, ChiCTR2000036385, on 22 August 2020, and has met the endpoints. The study was conducted following the Declaration of Helsinki (as revised in 2013) and received approval by the Institutional Ethics Committee of EHBH. Between October 21, 2020, and February 15, 2023, 31 patients underwent eligibility screening. Both male and female patients were eligible for enrollment and sex was self-reported. Data was reported disaggregated for sex and gender. Prior to participation, each patient provided written informed consent. The diagnosis of HCC was based on the clinical criteria of the European Association for the Study of Liver guidelines. The major inclusion criteria were age 18–70 years and having treatment-naïve, surgically resectable HCC with MVI, but no extrahepatic metastasis; with the diagnosis confirmed by radiographic or clinical features, and measurable disease defined by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST version 1.1); ECOG PS 0–1 points; adequate liver function (Total bilirubin (TBIL) ≤ 1.5 times the upper limit of normal value (ULN); aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels ≤1.5 times the ULN; Serum albumin ≥ 30 g/L; international standardized ratio (INR) or activated partial thromboplastin time (APTT) ≤ 1.5×ULN), platelet (Platelet count ≥100 × 109/L) and adequate vital organ function. The complete list of eligibility criteria is provided in the supplementary materials.
Procedures
Eligible patients would receive 3 cycles of tislelizumab (200 mg by intravenous infusion every three weeks). IMRT was conducted during the interval between the first and the second cycle of tislelizumab injection. The total dose of IMRT to the tumor and MVI was 45 Gray, delivered in 3 Gray × 15 fractions over 3 weeks (5 fractions per week). After 3 cycles of tislelizumab and IMRT (9 weeks after the first dose of tislelizumab), the treatment responses and tumor resectability were evaluated, and surgery was scheduled in eligible patients. The response was evaluated per RECIST version 1.1, which includes complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). ORR was calculated by the proportion of patients with CR or PR. Disease control rate (DCR) was calculated by the proportion of patients with CR, PR or SD. Patients were suitable for radical surgery with curative intent would undergo surgical resection. The response of MVI was evaluated based on the downstaging or upstaging of MVI extent. Thus, we further defined the response of MVI via downstaging (PR) or upstaging (PD), while the other was SD16.
The criteria of resectable HCC were as follows: (1) no extrahepatic metastasis; (2) sufficient remnant liver volume after liver resection; (3) adequate liver function (Child-Pugh score: A or B grade); (4) radical surgical resection (R0) can be satisfied: A. the tumor is confined to the ipsilateral liver; the tumor thrombosis were confined to the ipsilateral liver; B. the tumor thrombosis extension to, or beyond, the main portal vein bifurcation, but can be treated with en bloc vascular resection, repair, and reconstruction; C. the tumor thrombosis in the hepatic vein or inferior vena cava, but can be entirely removed in the event of total hepatic blood flow obstruction.
Two weeks post-surgery, patients would be administered with adjuvant tislelizumab every 3 weeks for 1 year, unless PD or intolerable toxic reaction. Participants who were deemed to be unsuitable for curative surgery would withdraw from the study. The participants underwent baseline tumor imaging by contrast-enhanced magnetic resonance imaging (MRI) scans of the liver and positron emission tomography-computed tomography (PET-CT) to exclude extrahepatic metastasis at screening. Tumor imaging including CT scans of the chest and abdomen, and contrast-enhanced MRI scans of the liver were repeated following 3 cycles of tislelizumab and IMRT, one-month post-surgery and subsequently every 2 months unless confirmed PD or patients withdrew from the study. Peripheral bloods samples were collected from patients either at baseline or during the first response evaluation. Surgical resected tumor samples were collected for pathological examinations.
Radiotherapy
IMRT was conducted during the interval between the first and the second dose of tislelizumab. Gross tumor volume (GTV) was defined as the tumor volume enhanced in the arterial phase combined with the PVTT volume, which was shown as a filling defect in the portal venous phase of the computed tomography (CT) scan63. Clinical tumor volume (CTV) was defined as the gross tumor volume plus a 1.0-cm margin. The planning target volume (PTV) was expanded to include a 0.5-cm margin in the anterior-posterior and left-right directions and a 1.0-cm margin in the cranial-caudal direction from the CTV to compensate for internal physiologic movements and variations in size, shape, and position of the CTV64. The Department of Radiation Oncology used Surface Dosage gamma 3.0 for analytical validation, with a pass rate> 95%. IMRT was performed for all patients. The dose-volume histogram (DVH) was used to evaluate the dose. The planned total dose to the PTV was 3 Gray×15 fractions (with a fractional size of 3 Gray at five fractions per week), ensuring that at least 97% of the tumor volume is covered by more than 95% of the prescribed dose, and that dose points greater than 110% of the prescribed dose account for less than 3% of the tumor volume.
In our trial, the dose of IMRT (45 Gray in 15 fractions) was within acceptable ranges for critical organs. During IMRT planning, a minimal number of radiation fields, generally 3 to 4 fields, were selected along with reasonable radiation beam direction to minimize the dose and volume of normal liver tissue irradiated. Mean dose to the normal liver (total liver volume minus gross tumor volume) was limited to ≤24 Gray, and the dose-volume histogram of the normal liver was within the tolerance area: the normal liver volume receiving a dose ≥5 Gray (V5) was <86%; V10 < 68%; V15 < 59%; V20 < 49%; V25 < 35%; V30 < 28%; V35 < 25%; and V40 < 20%. The maximum allowable point dose to the stomach was <54 Gray; V45 < 25%, V50 < 2%. The maximum allowable point dose to the duodenum was <60 Gray; V45 < 33%. The maximum allowable point dose to colon was <55 Gray; V45 < 25%, V50 ≤ 2%. The maximum point dose of spinal cord was <40 Gray. The maximum allowable point dose to the kidney was <20 Gray; V23 < 100%, V30 < 67%, V50 < 33%. To ensure the repeatability of the stomach and duodenum positions, all patients were asked to fast for 4 h before simulation or radiotherapy. Before each treatment session, patients received image-guided radiotherapy with cone-beam CT. Respiratory gating technique was used to reduce dose delivered to healthy tissues and surrounding organs. Abbreviation: V: volume receiving a dose ≥ Gray; Volumes and doses were expressed as percentage (%) and absolute values (Gray).
Macrovascular invasion grading
MVI was confirmed radiographically and was graded based on the MVI grading system from the Liver Cancer Study Group of Japan65. Briefly, MVI in the portal vein (Vp) was divided into five grades: Vp0, no tumor thrombosis in the portal vein; Vp1, tumor thrombosis presence distal to the second-order branches of the portal vein or more peripheral portal branch; Vp2, tumor thrombosis in the second branch of the portal vein; Vp3, tumor thrombosis in the first portal branch; Vp4, tumor thrombosis in the main portal trunk. Hepatic vein tumor thrombosis (Vv) was divided into three grades: Vv1, tumor thrombosis in the peripheral hepatic vein; Vv2, tumor thrombosis in the major hepatic vein; Vv3, tumor thrombosis in the inferior vena cava.
Patients with Vp1, Vp2 or Vp3/Vv1 MVI were classified as grade 1, 2 or 3 MVI, respectively; patients with Vp4, Vv2, Vv3, or both portal (Vp1–4) and hepatic vein invasion (Vv1–3) were classified as grade 4 MVI. Detailed classification of MVI was provided in Supplementary Table 1.
Outcomes
The primary endpoints were ORR according to RECIST version 1.1 after perioperative treatment and OS. OS was defined as the duration between the initiation of tislelizumab treatment and death from any cause. The secondary endpoints were: pathological response which included pathological complete response (pCR), defined as no residual viable tumor cells in the resected tumor and major pathological response (MPR), defined as the percentage of viable tumor cells out of the total tumor area is less than 10%; recurrence-free survival (RFS), defined as the duration from radical resection to the date of the first documented tumor recurrence or death from any cause, whichever occurred first; and safety. TRAEs was evaluated according to Common Terminology Criteria for version 5.0 (National Cancer Institute)66. Postoperative complications were assessed and classified using the Clavien-Dindo classification67. Additionally, the exploratory endpoints included biomarker correlates of radiographic and pathological response which included AFP at baseline or reduction in AFP levels. AFP reduction was measured by comparing AFP levels after perioperative therapy with baseline; the patients whose AFP were <400 μg/L at baseline were denoted as AFP “Low”.
Statistical analysis
The study was designed in 2020. Sorafenib remained one of the standard treatments for advanced HCC in 2020, with an ORR of 5%68,69. Immunotherapy represented by PD-1 monoclonal antibody shows good application prospects in the treatment of advanced HCC, with an ORR of 14.7–18.30%. It was assumed that IMRT plus tislelizumab will improve ORR to 19.5%, with 1-sided α = 0.05 as the threshold of significance. Thus, to achieve a power of 80%, the minimum sample size is 28, which was estimated using the Power Analysis and Sample Size software, version 15.0.5 (NCSS LLC). With the assumed drop-out rate of 5%, this study requires a sample size of 30 cases. Survival was estimated using the Kaplan–Meier method, and the p-values were compared via the log-rank test. The median follow-up was calculated using the reverse Kaplan–Meier method. The percentages and 90% confidence intervals for ORR were estimated using the Clopper-Pearson method. Univariate Cox proportional hazards regression analysis was utilized to compute hazard ratios (HR) and corresponding p-values. Categorical characteristics were compared using the Fisher’s exact test, and two-sided p-values were provided. All statistical analyzes were conducted using SPSS 26.0 software and p < 0.05 was deemed statistically significant. All patients enrolled who have received any study treatment were included in the intention-to-treat population and analyzed for the primary and secondary endpoints.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Clinical datasets are not publicly available due to involving patient privacy but can be requested 1 year after publication from the corresponding authors Ruoyu Wang (E-mail: wangruoyu1213@126.com) or Wen Sun (E-mail: sunwen_sw@aliyun.com) for 3 years. Individual de-identified patient data will be available for clinical study purposes after being reviewed by the institutional review board. Requests for data access will be processed within a timeframe of 3 months, and access will be granted for a duration of 1 year. A study protocol synopsis is available in the Supplementary Information file. The remaining data are available in the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Chan, S. L., Chong, C. C., Chan, A. W., Poon, D. M. & Chok, K. S. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J. Gastroenterol. 22, 7289–7300 (2016).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
Zhou, J. et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer 9, 682–720 (2020).
Bujold, A. et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J. Clin. Oncol. 31, 1631–1639 (2013).
Formenti, S. C. & Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 10, 718–726 (2009).
Daly, M. E., Monjazeb, A. M. & Kelly, K. Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J. Thorac. Oncol. 10, 1685–1693 (2015).
Theelen, W. et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 9, 467–475 (2021).
Theelen, W. et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT Phase 2 randomized clinical trial. JAMA Oncol. 5, 1276–1282 (2019).
Hu, Y. et al. Efficacy and safety of stereotactic body radiotherapy combined with camrelizumab and apatinib in patients with hepatocellular carcinoma with portal vein tumor thrombus. Clin. Cancer Res. 29, 4088–4097 (2023).
Llovet, J. M. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313 (2021).
Cheng, S. et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 Edition). Liver Cancer 9, 28–40 (2020).
Yang, T. et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J. Cancer Res Clin. Oncol. 138, 1121–1129 (2012).
Wei, X. et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J. Clin. Oncol. 37, 2141–2151 (2019).
Lee, A. & Keam, S. J. Tislelizumab: first approval. Drugs 80, 617–624 (2020).
Zhang, T. et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol. Immunother. 67, 1079–1090 (2018).
Qin, S. et al. Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a phase 3 randomized clinical trial. JAMA Oncol. 9, 1651–1659 (2023).
Sharabi, A. B., Lim, M., DeWeese, T. L. & Drake, C. G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 16, e498–e509 (2015).
Hwang, W. L., Pike, L. R. G., Royce, T. J., Mahal, B. A. & Loeffler, J. S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 15, 477–494 (2018).
Jabbour, S. K. et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage iii non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 7, 1–9 (2021).
Sasaki, A. et al. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer 23, 893–903 (2020).
Chiang, C. L., Chan, A. C. Y., Chiu, K. W. H. & Kong, F. S. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 9, 1157 (2019).
Li, Z. et al. Neoadjuvant tislelizumab plus stereotactic body radiotherapy and adjuvant tislelizumab in early-stage resectable hepatocellular carcinoma: the Notable-HCC phase 1b trial. Nat. Commun. 15, 3260 (2024).
Llovet, J. M. et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 21, 294–311 (2024).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 74, 5458–5468 (2014).
Faivre-Finn, C. et al. Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable Stage III NSCLC (PACIFIC). Lung Cancer 151, 30–38 (2021).
Scoccianti, S. et al. Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: results from a multicentric retrospective study on behalf of AIRO. Neuro Oncol. 23, 1750–1764 (2021).
Kim, B. H. et al. Concurrent nivolumab and external beam radiation therapy for hepatocellular carcinoma with macrovascular invasion: a phase II study. JHEP Rep. 6, 100991 (2024).
Su, C. W. et al. Concurrent atezolizumab plus bevacizumab and high-dose external beam radiotherapy for highly advanced hepatocellular carcinoma. Oncologist 29, e922–e931 (2024).
Kokudo, T. et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J. Hepatol. 65, 938–943 (2016).
Peng, Z. W. et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 118, 4725–4736 (2012).
Zhu, X. D. et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer 10, 320–329 (2021).
Marron, T. U. et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 219–229 (2022).
Xia, Y., et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer 10(2022).
Kaseb, A. O. et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 208–218 (2022).
Ho, W. J. et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat. Cancer 2, 891–903 (2021).
Dhir, M. & Sasson, A. R. Surgical management of liver metastases from colorectal cancer. J. Oncol. Pr. 12, 33–39 (2016).
Yi, Y. et al. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: A retrospective study. Front Oncol. 12, 1046584 (2022).
Chiang, C. L. et al. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START-FIT): a single-arm, phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 169–178 (2023).
Di Bisceglie, A. M. Issues in screening and surveillance for hepatocellular carcinoma. Gastroenterology 127, S104–S107 (2004).
Hatanaka, T. et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol. Int 16, 1150–1160 (2022).
Lee, P. C., et al. Predictors of Response and Survival in Immune Checkpoint Inhibitor-Treated Unresectable Hepatocellular Carcinoma. Cancers (Basel) 12 (2020).
Cheon, J. et al. Organ-specific responses to atezolizumab plus bevacizumab in advanced hepatocellular carcinoma: A multicentre, retrospective study. Liver Int 44, 1961–1970 (2024).
Kuo, H. Y. et al. Impact of immune checkpoint inhibitors with or without a combination of tyrosine kinase inhibitors on organ-specific efficacy and macrovascular invasion in advanced hepatocellular carcinoma. Oncol. Res Treat. 43, 211–220 (2020).
Lu, L. C. et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer 8, 480–490 (2019).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
European Association for the Study of the Liver. Electronic address, e.e.e. & European Association for the Study of the, L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Zhu, G. Q. et al. Optimal adjuvant therapy for resected hepatocellular carcinoma: a systematic review with network meta-analysis. Oncotarget 6, 18151–18161 (2015).
Qin, S., et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet (2023).
Wang, K. et al. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: a randomized, controlled, phase 2 trial. Nat. Med. 30, 708–715 (2024).
Imamura, H. et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 38, 200–207 (2003).
Byun, H. K. et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother. Oncol. 133, 1–8 (2019).
Demaria, S. & Formenti, S. C. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2, 153 (2012).
Zhou, J. et al. Guidelines for the diagnosis and treatment of primary liver cancer (2022 Edition). Liver Cancer 12, 405–444 (2023).
Wu, F. et al. Phase 2 evaluation of neoadjuvant intensity-modulated radiotherapy in centrally located hepatocellular carcinoma: a nonrandomized controlled trial. JAMA Surg. 157, 1089–1096 (2022).
Zhu, M., et al. Sintilimab plus bevacizumab combined with radiotherapy as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A multicenter, single-arm, phase 2 study. Hepatology (2024).
Cheng, A. L. et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76, 862–873 (2022).
Wang, K. et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity mathching score analysis. Med. 95, e3015 (2016).
Ye, J. Z. et al. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the Asia-Pacific region beyond the Barcelona Clinic Liver Cancer treatment algorithms: a review and update. Oncotarget 8, 93258–93278 (2017).
Wang, W. et al. Prospective evaluation of microscopic extension using whole-mount preparation in patients with hepatocellular carcinoma: Definition of clinical target volume for radiotherapy. Radiat. Oncol. 5, 73 (2010).
Zhao, Y. T. et al. Observation of different tumor motion magnitude within liver and estimate of internal motion margins in postoperative patients with hepatocellular carcinoma. Cancer Manag Res 9, 839–848 (2017).
The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg 19, 98–129 (1989).
Common terminology criteria for adverse events (CTCAE) v5.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC) 81972777 to RW, 82373300 to WS, and 81972810 to WS, Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR4040 to RW), Program of Science and Technology Commission of Shanghai Municipality (21Y11912600 to RW). The funders had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Author information
Authors and Affiliations
Contributions
LZ, JZ, NS, HM, YL, RJ, WZ and DW contributed to the acquisition or interpretation of data for this article. ZC analyzed the data. HP drafted the manuscript and WS contributed to the manuscript revision. RW and WS conceived and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Landon Chan, Stephen L. Chan, Sze-Huey Tan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pan, H., Zhou, L., Cheng, Z. et al. Perioperative Tislelizumab plus intensity modulated radiotherapy in resectable hepatocellular carcinoma with macrovascular invasion: a phase II trial. Nat Commun 15, 9350 (2024). https://doi.org/10.1038/s41467-024-53704-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-53704-5
This article is cited by
-
KAT2A-driven succinylation of SRSF11 enforces spliceosome-mediated RAD52 splicing to promote homologous recombination and radioresistance in hepatocellular carcinoma
Signal Transduction and Targeted Therapy (2025)