Abstract
Plasma membrane repair in response to damage is essential for cell viability. The ferlin family protein dysferlin plays a key role in Ca2+-dependent membrane repair in striated muscles. Mutations in dysferlin lead to a spectrum of diseases known as dysferlinopathies. The lack of a structure of dysferlin and other ferlin family members has impeded a mechanistic understanding of membrane repair mechanisms and the development of therapies. Here, we present the cryo-EM structures of the full-length human dysferlin monomer and homodimer at 2.96 Å and 4.65 Å resolution. These structures define the architecture of dysferlin, ferlin family-specific domains, and homodimerization mechanisms essential to function. Furthermore, biophysical and cell biology studies revealed how missense mutations in dysferlin contribute to disease mechanisms. In summary, our study provides a framework for the molecular mechanisms of dysferlin and the broader ferlin family, offering a foundation for the development of therapeutic strategies aimed at treating dysferlinopathies.
Similar content being viewed by others
Introduction
Plasma membrane repair in response to damage is a fundamental and conserved cellular mechanism in eukaryotes1. Skeletal and cardiac muscle cells are highly susceptible to membrane damage owing to their exposure to repetitive mechanical stress2,3,4. Muscle cells possess a conserved protein machinery that actively repairs damaged membranes within seconds to prevent cell death4,5. Disruption of this machinery leads to chronic muscle fiber loss, inflammation, and fibrosis and can cause muscle diseases4,5,6,7,8,9,10,11,12. Dysferlin is a member of the ferlin family of vesicle fusion proteins and was originally identified as the first protein implicated in membrane repair mechanisms in striated muscles7,13,14,15. Dysferlin mediates the Ca2+-dependent vesicle recruitment and fusion during membrane repair to ensure proper muscle function13,16,17,18. Further, a role for dysferlin in stabilizing voltage-induced Ca2+ transients in skeletal muscle has been reported19,20. While the precise mechanisms remain to be established, muscles from patients with dysferlin deficiency and dysferlin-null mice show impaired membrane repair that leads to the accumulation of unfused vesicles and muscular dystrophy12,13. Overexpression of DYSF can rescue the muscular dystrophy phenotype in a Dysf-null mouse model21. Loss-of-function mutations in DYSF cause a loss or reduction of dysferlin and render cells more susceptible to plasma membrane damage and compromise repair mechanisms22,23. This results in progressive forms of dysferlinopathies which include muscular dystrophies such as Limb-Girdle muscular dystrophy type 2B (LGMD2B), Miyoshi myopathy (MMD1), and other myopathies8,24,25,26. Various pre-clinical strategies, including the use of a miniaturized dysferlin, are currently being explored to restore membrane repair in dysferlinopathies22,27,28.
While the importance of understanding dysferlin-mediated muscle membrane repair mechanisms has been widely acknowledged, a significant knowledge gap remains in the absence of a full-length structure to accurately describe the molecular architecture of dysferlin. Dysferlin is a ~237 kDa multidomain protein that comprises seven classical C2 domains (C2A-C2G) separated by Fer and DysF regions. In addition, dysferlin features a single transmembrane domain (Fig. 1a)29,30. Individual domains and regions in dysferlin are separated by long linkers. Prior studies established that all C2 domains contribute to Ca2+-mediated membrane repair mechanisms in muscle20. Specifically, it was shown that the C2A domain mediates the Ca2+-regulated binding of dysferlin to membranes, while C2C-C2G mediate the Ca2+-dependence of the membrane repair20,31,32. In addition, the C2B-C2G domains have been implicated in dysferlin homodimerization23,33. It was also shown that C2 domains of dysferlin bind to other key proteins of the membrane repair machinery such as AHNAK, MG53, caveolin-3, and SNARES34,35,36,37,38,39. The structure and function of the ferlin family specific Fer and DysF regions and interdomain linkers are largely uncharacterized.
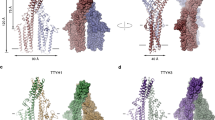
a Domain organization of dysferlin (237 kDa). Individual domains are color-coded. b PageBlue-stained SDS-PAGE gel of purified full-length dysferlin. Three independent experiments were performed. c Representative electron micrograph shows negatively stained dysferlin protein. More than 50 images were collected in two independent sessions. d Cryo-EM micrograph (left) and 2D class average (right) of the dysferlin monomer. e Cryo-EM reconstruction of dysferlin. The front and back views of the cryo-EM maps are shown. Individual domains are color-coded according to panel a. f The front and back view of the molecular model of the dysferlin monomer in cartoon representation. Individual domains are color-coded according to panel a. g Electrostatic potential distribution in the dysferlin monomer. The electrostatic potential of the monomer is calculated using default electrostatic surface potential parameters in ChimeraX, where the acidic surface is shown in red, and the basic surface is in blue. Source data are provided as a Source Data file 2.
Here, we report the cryo-electron microscopy (cryo-EM) structures of the dysferlin monomer and homodimer at ~2.96 Å and 4.65 Å resolution, respectively. The structures revealed how ferlin family proteins arrange their C2 and ferlin-specific regions and allowed us to ascribe specific structural roles to domains and interdomain linkers with historically elusive functions. The structure of the asymmetric dysferlin homodimer reveals how dysferlin concentrates at membrane repair sites. This dimer structure also offers insight into how clinically significant mutations that cause dysferlinopathies are distributed along the buried interface. Together, our findings highlight a detailed structural framework to understand the molecular mechanisms of dysferlin and other related ferlin family proteins. This enhances our understanding of membrane repair mechanisms in health and muscle disorders and will accelerate the design of structure-inspired gene therapies for dysferlinopathies.
Results
Dysferlin forms a compact monomer structure
Full-length dysferlin was produced in the Sf9/baculovirus insect cell expression system and purified to homogeneity via a multistep chromatography approach (Fig. 1b and Supplementary Fig. 1a). The structure of the dysferlin monomer was determined by single-particle cryo-EM to a global resolution of ~2.96 Å (Fig. 1c–f, Supplementary Fig. 1b, c, f, Supplementary Fig. 2a, b, c, and Supplementary Movie 1). This structure is the only core structure of dysferlin and any member of the ferlin protein family. The map shows local resolutions from 2 to 6 Å with clear densities for most side chains and well-defined secondary structure elements (Fig. 1e, f and Supplementary Fig. 2a, b) that allowed us to build a nearly complete model of the dysferlin monomer (Fig. 1e, f). High local resolutions (~2 to 4 Å) were observed in the core of the protein and lower local resolutions were observed at the C-terminus (Supplementary Fig. 2a). The overall structure of dysferlin is carabiner-shaped with a major axis of ~200 Å and a minor axis of ~102 Å (Fig. 1e, f). Analysis of the electrostatic potential and hydrophobicity show that the dysferlin monomer has an overall hydrophilic and highly negative surface charge distribution (Fig. 1g and Supplementary Fig. 3).
Dysferlin contains seven classical type-2 C2 domains (C2A-C2G) that feature a β-sandwich of two antiparallel four-stranded β-sheets connected by surface loops (Fig. 2a and Supplementary Figs. 4a and 5c)40,41. The basic structural organization of the classical C2 domains in dysferlin is diversified by insertions of variable length between the β-sheets (Fig. 2a and Supplementary Fig. 4a). In addition, a composite C2 domain is formed by two antiparallel four-stranded β-sheets located at the N- and C-terminus of the FerA and FerB domain, respectively (Fig. 2b and Supplementary Figs. 4b and 5b, d). The two α-helical segments of the FerA and the FerB domain form a four-helix bundle and emanate from the β4-β5 strands of the composite C2 domain (C2-FerA-FerB-C2) (Fig. 2b and Supplementary Fig. 4b and 5b, d). This basic domain organization is conserved in all human ferlins (Supplementary Figs. 4b and 5b,d). The C2-FerA-FerB-C2 has an overall V-shape (Fig. 2b) and stabilizes, together with the C2B, C2C and C2D (Fig. 2d, e and Supplementary Fig. 6a, b), the DysF region at the tip of the monomer (Figs. 1e, f, 2f and Supplementary Fig. 6c). The spine of the monomer is formed by the C2 domains that emanate from the central Fer and DysF regions (Fig. 1e, f). The absence of the N-terminal C2A domain and the C-terminal single transmembrane domain in our high-resolution structure suggests that they are flexible. In-depth analysis of our cryo-EM data further identified low-resolution densities in one of the 3D classes, where the C2A domain is in proximity to the C2B domain (Supplementary Figs. 1 and 7a).
a Structures of the classical C2 domains (C2B-C2G) of dysferlin. Inserts of variable length extend from the consensus β-sandwich of two antiparallel four-stranded β-sheets. b Structure of the composite C2 domain (C2-FerA-FerB-C2). The topology diagram shows the position and orientation of secondary structures. c Structure of the nested DysF region composed of the DysFN, DysFI, and DysFC. d Residue-level interactions between the C2C insert and the FerB. e Residue-level interactions between the C2C and the composite C2 domain. f Residue-level interactions between the DysF region and the C2-FerA domain.
The central C2 domains form a pentameric ring
The composite C2 domain, together with the C2B, C2C, C2D, and C2E domains, forms a pentameric ring with an inner diameter of ~15 to 18 Å and an outer diameter of ~ 84 to 88 Å (Fig. 3a, b). The pentameric ring is stabilized by interdomain interactions between the C2 domains and the FerA-FerB domains that cross the diameter of the pentameric ring on one side (Fig. 3a, b). The C2B is sandwiched between the C2C, C2E, C2F, and C2-FerA-FerB-C2 domains. The FerA domain interacts with the C2D and C2E while the FerB interacts with the C2C, C2B, and C2E (Fig. 3a–c and Supplementary Fig. 8). The C2-FerA-FerB-C2 interacts with the C2C. The large ~1400 Å2 interface is formed through hydrogen bond and salt bridge interactions (Fig. 3c and Supplementary Fig. 8d, i). The pentameric ring shows the highest local resolution in the monomer structure (Supplementary Fig. 2a), suggesting that it serves as the stable core of dysferlin.
a Two views (front, back) of the pentameric ring formed by the C2B, C2E, C2D, C2C, and composite C2 domain and stabilized by the C2D-C2E linker. Individual domains are color-coded. b Schematic representation of the pentameric ring. Color code according to panel a. c Residue-level interactions at interdomain interfaces in the pentameric ring. d Residue-level interactions of the FerI with the C2B, C2C, and C2E. Color code according to panel a. The electrostatic potential distribution is shown for the C2B, C2C, and C2E (left). e The C2E-C2F (grey) and C2F-C2G (grey) linkers connect the respective domains. The C2E insert interacts with the C2E (yellow), C2F (blue), and C2G (green) domains. f Front view of the electrostatic potential distribution in the pentameric ring. Ca2+ ions are colored in green, and C2 domains are indicated. g Back view of the electrostatic potential distribution in the pentameric ring. Ca2+ ions are colored in green, and C2 domains are indicated. The FerA/B (purple) is shown in cartoon representation. h Residue-level interactions between the DysFN (orange) and DysFC (purple). Side chains are shown as sticks and their van der Waals radii are shown as light grey spheres. i Residue-level interactions in the DysFI (cyan). Side chains are shown as sticks and their van der Waals radii are shown as light grey spheres.
The C2 domains in dysferlin feature unique inserts and are connected by long linkers of unknown function. The monomer structure revealed that the C2B-C2C (FerI), the C2E-C2F, and the C2D-C2E linkers facilitate and stabilize interdomain contacts between C2 domains in the pentameric ring (Fig. 3a, b, d, e). The N- and C-termini of the FerI serve as anchors and interact predominantly with charged residues in the C2B, C2C, and C2E (Fig. 3d and Supplementary Fig. 8). At the N-terminus of the FerI, D348 interacts with H314 of the C2B while E349 interacts with Q1348 of the C2E and R223 of the C2B. At the center of the FerI interface, E353 interacts with T1536 and E1537 of the C2E domain and K355 interacts with E1535 of C2E. In addition, D360 forms a salt bridge with K338 of the C2B domain. The C-terminal anchor includes interactions between FerI hub residues (E359-D363) with residues in the C2B and C2C (Fig. 3d). Thus, the FerI binds to a groove formed by the C2B, C2C, and C2E and stabilizes the pentameric ring, suggesting a role in interdomain communication. The 49-residue long C2D-C2E linker connects the C2D and C2E in the pentameric ring and interacts with the DysF region (Fig. 3a, b and Supplementary Fig. 8a). A large insert between the β6-β7 strands of the C2E wraps around and stabilizes the position of the C2F domain. The position of the C2F in between C2B, C2E, and C2G is stabilized by the C2F-C2G linker (Fig. 3e and Supplementary Movie 2). An insert between the β6-β7 strands of the C2C stabilizes the pentameric ring through interactions with Q750, H747, and K484 of the FerB (Fig. 2d).
Six out of the fifteen reported isoforms of dysferlin (Supplementary Fig. 7b, c) contain a 21 residue long insert (exon 40a) in the C2E. Exon 40a contains a calpain cleavage site that releases mini-dysferlinC72, (C2E fragment together with the C2F-C2G-transmembrane domain) in response to membrane injury that likely plays a specialized role in membrane repair42,43,44,45. While our dysferlin structure lacks exon 40a, mapping of the last (Q1470) and the first residues (E1471) of the exons upstream and downstream in the primary sequence showed that the calpain cleavage site is located on the surface of dysferlin and is thus accessible for proteases (Supplementary Fig. 7d). Cleavage of full-length dysferlin thus separates mini-dysferlinC72 from the dysferlin core, leaving the pentameric ring intact.
Superposition of the published crystal structure of the Ca2+-bound C2A domain (PDB ID: 7JOF46) with the C2 domains in our monomer structure revealed that the putative Ca2+ binding sites of the C2C, C2D, C2E, and the composite C2 are located on the same side of the pentameric ring (Fig. 3f and Supplementary Fig. 9). The Ca2+ binding site of the C2B faces the opposite side (Fig. 3g and Supplementary Fig. 9). Analysis of the electrostatic potential of the C2 domains in the pentameric ring shows a highly positive charge distribution for C2B and C2E while the other C2 domains feature a negative charge distribution (Fig. 3f, g and Supplementary Fig. 9). This observation may explain the reported differences in Ca2+ affinity and functions in Ca2+ signaling of C2 domains in skeletal muscle20,47. Thus, the pentameric ring not only stabilizes the monomer but also serves as the main Ca2+-receptive module in dysferlin.
The DysF region is stabilized by amino-aromatic interactions
A defining feature of type-1 ferlins is the presence of a DysF region (Supplementary Fig. 5a). Our structure shows that the DysF region is located at the tip of the monomer where it connects the composite C2-FerA-FerB-C2 domain with the C2D domain on opposite sides of the molecule (Fig. 1e, f and Supplementary Fig. 8e, i). The DysF is a nested region composed of an N-terminal DysF (DysFN), an inner DysF (DysFI), and a C-terminal DysF (DysFC) domains (Figs. 1a, 2c, 3h, i and Supplementary Fig. 4c). The DysFN and DysFC are each formed by one major two-stranded antiparallel β-sheet that is stabilized by a dense network of interactions including a series of tryptophan (W) and arginine (R) stacks, hydrophobic and electrostatic interactions (Figs. 2c, 3h and Supplementary Fig. 10a). The DysFI is embedded between the DysFN and DysFC by large linkers. The DysFI is composed of two major antiparallel β-strands that are mainly stabilized by extended R/W π−cation stacks (Figs. 2c, 3i and Supplementary Fig. 10b) in agreement with a published X-ray crystallographic structure48. A central tryptophan residue W1094 in the DysFC forms a perpendicular interaction with R1092 and a parallel interaction with K915 in the DysFN (Fig. 3h and Supplementary Fig. 10a). Likewise, the central tryptophan residue W1042 in the DysFI forms a perpendicular interaction with R1040 and a parallel interaction with K983 (Fig. 3i and Supplementary Fig. 10b). Thus, the DysF region at the tip of the dysferlin monomer features amino-aromatic stacks and acts as a stabilizer for the pentameric ring.
Dysferlin forms an asymmetric and parallel homodimer
Our cryo-EM analyses revealed particles and 2D class averages with larger particles reminiscent of a homodimer (Supplementary Fig. 1d, e, f). The presence of homodimers and higher-order oligomers is also evident in native non-denaturing polyacrylamide gel electrophoresis (native PAGE) and size exclusion chromatography with multi-angle light scattering (SEC-MALS) experiments (Fig. 4a, b), supporting the findings of published biochemical and cell biological studies23,33. The cryo-EM structure of the dysferlin homodimer was solved to a global resolution of ~4.65 Å, with local resolutions ranging from 3.5 to 9.5 Å (Supplementary Fig. 2d–f). Using the well-defined monomer structure, we used rigid body docking to fit each protomer in the cryo-EM map to build the structure of the homodimer. The structure shows that dysferlin forms an asymmetric parallel homodimer in which the DysFN domains and the C2G domains of both protomers are located on opposite sides of the molecule (Fig. 4c and Supplementary Fig. 11a). The protomers are rotated by ~64° with respect to each other with a translation of ~7.63 Å along the rotation axis (Supplementary Fig. 11c and Supplementary Movie 3). The C2F and C2G domains at the distal end of the two protomers are rotated by ~60° (Supplementary Fig. 11c). Dysferlin homodimerization occurs via a large ~ 663 Å2 interface along the major axis that is formed by the C2B’, C2D’, C2E’, DysFI’ of protomer 2 and the C2B, C2C, C2-FerA, FerB-C2, DysFC of protomer 1 (Fig. 4c, d and Supplementary Fig. 11b). The interface further comprises the C2C-FerA linker of protomer 1 and the C2B-C2C linkers from both protomers (Fig. 4c, d and Supplementary Movie 3). The DysFI from protomer 2 interfaces with the C2C and C2-FerA-FerB-C2 from protomer 1. The hydrophobic interface is mainly contributed by the DysF regions (Fig. 4d and Supplementary Fig. 11d, e). In addition, each protomer has a largely hydrophilic and negative surface charge distribution that is disrupted by a patch of positive surface charge at the base (Supplementary Fig. 11d, e). The positively charged patch of protomer 1 aids in homodimerization through the formation of electrostatic interactions with the negatively charged surface at the top of protomer 2 (Supplementary Fig. 11d). The putative Ca2+ binding site of the C2D of protomer 2 is sterically occluded by protomer 1 (Supplementary Fig. 11f). The C2A domain is absent in the structure of the dysferlin homodimer, suggesting that it does not contribute to the dimer interface in agreement with published work33.
a Native PAGE of dysferlin shows the presence of monomers, dimers, and higher-order oligomers. M denotes marker. Two independent experiments were performed. b SEC-MALS shows the presence of dysferlin monomers, dimers, and high-order oligomers. The molecular weight of GDN micelles is in the range of ~ 140 to 160 kDa. c Side-view of the asymmetric parallel dysferlin homodimer. The homodimer shows DysF regions on the proximal side and the C2G domains on the distal side of the homodimer. d Close-up view of the dimer interface shows how the two protomers are docked to each other. The homodimer is rotated 180° relative to the overview representation. Individual domains are color coded according to panel c. e Clinically significant dysferlinopathy mutations in the dimer interface. Individual domains are color coded according to panel c. The dimer interface is indicated by a dashed line. f Close-up view of the dimer interface shows the location of select disease-causing mutations. * indicates a terminal codon. Source data are provided as a Source Data file 2.
Structural insights into clinically relevant mutations in dysferlin
We evaluated all known DYSF mutations described in the Leiden muscular dystrophy database and ClinVar to identify key regions in dysferlin that lead to loss of function49,50. 769 unique pathogenic mutations are associated with human dysferlinopathies out of which ~61% are missense mutations. The mutations are distributed throughout the monomer structure, implying that dysferlin is susceptible to structural alterations throughout its entire length (Supplementary Fig. 12). However, mutation frequencies in the C2B-FerI-C2C module and the DysFI are elevated compared to other regions (Supplementary Figs. 12, 13)48,51,52. The three most commonly mutated residues in dysferlin (R959, W999, and R1046) are located in the DysFI and engage in R/W π−cation stacking interactions that contribute to domain stability (Fig. 3i and Supplementary Figs. 10b and 13a, b). Their mutation disrupts π−cation interactions and destabilizes R/W stacks in the DysFI which likely contributes to the reported protein destabilization and aggregation48,53. Indeed, we found that the presence of mutations R959W and W999C in recombinant full-length dysferlin alters the elution profiles during ion exchange chromatography with a fraction of the mutant proteins eluting at 100% NaCl (Supplementary Fig. 14a, b), suggesting structural destabilization. Negative stain electron microscopy and 2D classifications of R959W and W999C revealed classes with altered shapes compared to the native protein (Supplementary Fig. 15a–c). Further, these mutants show different cellular localizations (Supplementary Fig. 15d–f) compared to the native protein, as previously reported54. W999 in the DysFI domain is stabilized by residues W1042, R1044, and K983, whereas R959 in the DysFI domain is stabilized by W965 (Supplementary Fig. 10b). The substitution of W999 with C999, as well as R959 with W959 destabilizes these interactions in the DysF region (Supplementary Figs. 13a, b and 14a, b) and thus are likely to contribute to the observed protein instability and mislocalization (Supplementary Figs. 13a, b and 14c, d). Missense Mutation L344P is located at the junction between C2B and FerI and destabilizes a β-strand which affects the positioning of the FerI linker and the integrity of the pentameric ring (Fig. 3a). In line with this structural interpretation, L344P has been shown to increase protein lability23,55.
A subset of surface-exposed missense mutations are located in the ~ 859 Å2 interprotomer interface with a potential impact on dysferlin homodimer structure and stability. This includes interface mutations in residues of the FerI (V374, R377*), the C2C (D390, D395, Q441, L448, G519, Y522*, L556, E573), the FerA (P731), the FerB (Y739*), the DysFI (R959, W965, Q1010, G1011, Y1014, K1032, P1029), and the C2E (N1351) (Fig. 4e, f). R959 is located in the dimer interface between the C2C of protomer 1 and the DysFI of protomer 2 (Fig. 4e, f). Missense mutations R959W and W999C, the most common mutations in dysferlin, destabilize the structure of the DysFI (Figs. 3i, 4e, f, and Supplementary Fig. 10b). While this does not preclude R959W and W999C from forming homodimers and higher-order oligomers in native PAGE and SEC-MALS experiments (Supplementary Fig. 14c, d, e), it is plausible that the stability of the different oligomeric species differs due to altered interfaces. The observed increased destabilization of the mutant proteins compared to the native protein (Supplementary Fig. 14a, b) may contribute to the reported reduced levels of dysferlin in LGMD2B49,56. LGMD2B mutation P731R is located in the FerA domain49,57 and maps to a loop that connects helices α1 and α2 of the FerA domain in the monomer (Fig. 4e, f). While the mutation is unlikely to change the overall structure of the FerA, it may affect the stabilization of the dimer (Fig. 4c, d).
Discussion
We present the high-resolution cryo-EM structures of the dysferlin monomer and homodimer, providing a foundational understanding of the overall architecture of dysferlin and the structure of ferlin-specific domains. Our study presents a significant milestone in unveiling the structural landscape of members of the ferlin family as a whole, which have been understudied from a structural perspective despite their role in various physiological functions including membrane repair. We show that the dysferlin monomer adopts a compact structure that is stabilized by the complex interaction between its C2 domains. In addition to seven classical C2 domains, we revealed the presence of a composite C2 domain that not only serves as a stabilizing domain for the pentameric ring in the center of the dysferlin structure but also aids in lining the calcium-binding C2 domains. Four C2 domains together with the composite C2 domain form a pentameric ring that represents the structural core of dysferlin. The ferlin-specific Fer and DysF stabilize the pentameric ring. This is in line with previous reports that showed the deletion or mutation within the FerI increased protein lability, decreased half-life, and disrupted cellular function55. Notably, mapping of the known calpain cleavage site of dysferlin isoforms containing exon 40a43 (Supplementary Fig. 7b–d) suggested that cleavage separates a C2E fragment together with the C2F-C2G and transmembrane domain from the intact pentameric ring. The pentameric ring also serves as the main Ca2+ binding module in dysferlin in the monomer and homodimer. Neither the pentameric ring nor the dimer interface includes the C2A that mediates the Ca2+-regulated binding of dysferlin to membranes20,31,32. This implies an architecture with spatially distinct Ca2+ and phospholipid binding modules and likely interaction modules for proteins from the membrane repair complex suggesting complex regulatory mechanisms for membrane repair34,35,36,37,38,39.
This complexity further extends to the formation of dysferlin dimers and higher-order oligomers which likely impacts the ability of dysferlin to effectively respond to membrane damage and to facilitate membrane repair and resealing. Indeed, dimerization and oligomerization of proteins play a central role during membrane repair through the amplification of function36. The structure of the asymmetric dysferlin homodimer shows that homodimerization is driven by electrostatic interactions between both protomers. Ferlin-specific domains including the DysF play a key role in stabilizing the dimer interface. We show that recombinant full-length dysferlin can not only form dimers but also higher-order oligomers, in agreement with previous biochemical and cellular studies23,33. Thus, it is conceivable that dimers serve as building blocks within a pathway that results in the formation of higher-order oligomers. We propose that following membrane damage, dysferlin molecules are recruited to the plasma membrane and trigger the rapid fusion of vesicles with the membrane to seal the damaged sites6. During this process, monomers associate into dimers, which subsequently concentrate into higher-order oligomers by mass action (Fig. 5). This would direct the lipid binding modules of all protomers towards the membrane, thereby creating a hub for Ca2+ and dysferlin binding partner interactions in proximity to the sites of membrane damage (Fig. 5, Supplementary Fig. 11f). Dysregulation of this process caused by missense mutations in dysferlin is likely to change the delicate monomer-oligomer equilibrium and contribute to the reported delay in membrane repair and resealing. This is supported by data from SEC-MALS, native PAGE, and negative stain experiments that show structural alterations in mutant proteins that increase protein instability without changing the functional ability of the mutant proteins to homodimerize and oligomerize (Supplementary Fig. 14). Further, different protein architectures may alter the interaction with binding partners important for the function of dysferlin in membrane repair. While W999C retains membrane resealing activity, its structural instability has been linked to the reduced levels of dysferlin observed in LGMD2B patients58,59.
Upon membrane damage and Ca2+ influx, dysferlin containing vesicles are transported to the sites of membrane repair. These vesicles dock to the membrane. Dysferlin then accumulates in the form of dimers and larger oligomers at the sites of membrane damage, where it, together with binding partners, facilitates repair. The precise structural and molecular mechanisms underlying the interaction between dysferlin, and its binding partners are largely unknown. Schematic not drawn to scale. MG53: Mitsugumin 53, also known as TRIM72; A1: Annexin A1; A2: Annexin A2.
Membrane damage is a common feature of many diseases including muscular dystrophies, heart failure, and neurodegeneration35. Given the significance of understanding the structural mechanisms underlying dysferlinopathies, we also examined the location of clinically relevant mutations in DYSF. The presence of deleterious missense mutations in key domains for monomer stability and the maintenance of the dimer interface explains the reported loss of function and dysferlin instability in dysferlinopathies51,57. Finally, the dysferlin structure can be considered a prototype of the ferlin protein family. It is conceivable that type-1 ferlins myoferlin and Fer1L5 may adopt similar monomer and possibly dimer structures given their structural conservation and domain organization (Supplementary Fig. 5a). The absence of a DysF and C2A in some type-2 ferlins implies broader structural alterations in their architecture compared to dysferlin (Supplementary Fig. 5a).
In conclusion, we describe the overall architecture of the dysferlin monomer and homodimer that define their structural mechanisms. Owing to the key function of dysferlin in Ca2+-mediated membrane repair in striated muscles, our structures provide a framework for a more comprehensive exploration of the functional roles, the characterization of dysferlinopathy-associated mutations, and the assessment of the therapeutic potential of dysferlin and likely other members of the ferlin protein family.
Methods
Cloning of human full-length dysferlin
Human full-length dysferlin (residues 1-2080) was cloned into a customized pFastBac1 plasmid for recombinant production in the baculovirus/Sf9 insect cell system. The codon-optimized dysferlin cDNA (Addgene plasmid # 67878, a gift from Matthew Hirsch)60 was amplified and inserted upstream of a coding sequence for a PreScission protease cleavage site and two strep II tags into a pFastBac1 vector via restriction-fee cloning. For cellular studies, the codon-optimized full-length dysferlin cDNA was amplified and inserted in a pcDNA3 vector containing an N-terminal mVenus tag via restriction-free cloning. Dysferlinopathy mutants R959W and W999C were generated using site-directed mutagenesis (Agilent technologies). All plasmids were sequenced and verified prior to protein production. All primer sequences used in this study are included in Source Data file 1.
Protein production and purification
Native full-length dysferlin and full-length dysferlin mutants R959W and W999C were recombinantly produced in the baculovirus/Sf9 insect cell system and purified using a multi-step chromatography approach. In brief, pelleted cells were resuspended in lysis buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 1% n-dodecyl-ß-D-maltoside (DDM) (Anatrace), 1 mM EGTA, 2 mM DTT supplemented with 1 mM PMSF and EDTA-free protease inhibitor cocktail (Roche)) and incubated for 1 h at 4 °C. The resuspended cells were lysed by sonication and the lysate was clarified by ultracentrifugation (100,000 × g, 30 min, 4 °C). The cleared lysate was loaded onto a Strep-Tactin column (IBA Lifesciences) and eluted with elution buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 0.05% GDN, 0.1 mM EGTA, 50 mM biotin). The eluted protein was dialyzed overnight at 4 °C in buffer A (50 mM HEPES pH 7.4, 50 mM NaCl, 0.01% GDN, 0.1 mM EGTA). The protein was loaded onto a QHP column (Cytiva) equilibrated with buffer A and eluted with buffer B (buffer A + 0.6 M NaCl). The protein was further purified by size exclusion chromatography using a Superose 6 increase 10/300 GL (Cytiva) equilibrated with 20 mM HEPES pH 7.4, 150 mM NaCl, 0.005% GDN, 0.1 mM EGTA, 1 mM TCEP. Fractions containing full-length dysferlin were pooled, concentrated, flash-frozen in liquid nitrogen, and stored at −80 °C.
Western blotting
Purified full-length dysferlin (7.5 µg) was resolved in a 4–12% Bis-Tris SDS-PAGE gel (Thermo Fisher Scientific) and transferred (10 V, 2.5 h) on a 0.45 μm PVDF membrane. The membrane was blocked for 1 h with blocking buffer (1xTBS, 0.1% Tween-20, 5% non-fat milk) and subsequently incubated with anti-dysferlin monoclonal antibody (1:1000; #ab124684, Abcam) in blocking buffer for 1 h at room temperature. The membrane was washed three times with 1X TBS buffer and probed with goat anti-rabbit IgG conjugated to Alexa Fluor™ 680 (1:10,000; #A21109, Invitrogen) in blocking buffer. The membrane was documented with a ChemiDoc MP imaging system (Bio-Rad).
Native PAGE
Dysferlin proteins were supplemented with NativePAGE Sample Buffer (ThermoFisher Scientific) and resolved on NativePAGE 3-12% gels (ThermoFisher Scientific) according to the manufacturer. Gels were stained with PageBlue (ThermoFisher Scientific) and destained in water.
Size exclusion chromatography with multi-angle light scattering (SEC-MALS)
Dysferlin proteins were resolved in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 0.005% GDN, 0.1 mM EGTA, and 1 mM TCEP on a Superose 6 increase 10/300 GL column (Cytiva) connected to an Agilent 1260 Infinity II LC system. The column was coupled to a Heleos 8 light scattering detector (Wyatt Technology) and a refractive index detector Optilab (Wyatt Technology). Data were collected and processed using the ASTRA software v8.2 (Wyatt Technology).
Cell Culture, transfection, and confocal microscopy
U2OS cells were cultured in Dulbecco’s modified Eagle’s medium containing 4.5 g/L glucose and 110 mg/L sodium pyruvate supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco, Thermo Fisher Scientific) at 37 °C and 5% CO2 in a humidified incubator. U2OS cells were transiently transfected with the plasmids of native and mutant full-length human dysferlin as an N-terminal mVenus fusion using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol.
After 24 h, cells were plated on 10 μg/mL fibronectin-coated cover glass and incubated overnight. Cells were fixed with 4% (v/v) paraformaldehyde for 20 min and permeabilized with 0.3% (v/v) Triton X-100 for 10 min. Cells were stained with Vectashield 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. Fluorescence images were collected on a Nikon AXR confocal laser scanning microscope, controlled by NIS Elements software using a 60×, 1.42 NA CFI Plan Apochromat Lambda D oil immersion objective lens (Nikon). Images were further processed using Fiji61. Note that U2OS cells do not contain endogenous dysferlin62.
Screening of proteins using negative stain transmission electron microscopy
Proteins were diluted to 50 nM in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 0.005% GDN, 0.1 mM EGTA, and 1 mM TCEP. Protein samples were applied to freshly glow-discharged (PELCO easiGlow, Ted Pella Inc.) formvar carbon grids (Cu 300-mesh, Electron Microscopy Services). The proteins were incubated for 30 s on the grids and then stained with 1% uranyl acetate. Images were acquired at a magnification of 115,000x on a FEI Tecnai G2 Spirit BioTwin TEM microscope operating at 80 kV. Image processing, particle picking, and 2D class averages were done in cryoSPARC v4.5.163.
Sample preparation and cryo-EM data collection
Protein samples were diluted to 3 µM in buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 0.005% GDN, 0.1 mM EGTA, and 1 mM TCEP. UltrAuFoil 1.2/1.3 Au grids (Quantifoil Micro Tools, Electron Microscopy Services) were glow-discharged at 15 mA and 0.2 mBar for 3 min using a PELCO easiGlow™ (Ted Pella Inc.). The protein sample was applied, and the grids were plunge-frozen in liquid ethane using a Vitrobot™ Mark IV plunger (Thermo Fisher Scientific) and stored in liquid nitrogen. Grids were screened on a Glacios cryo-TEM (Thermo Fisher Scientific) operated at 200 kV and equipped with Falcon 3EC direct electron detector (Thermo Fisher Scientific) with a magnification of 92,000×. Optimal grids, based on the ice thickness and sample distribution, were transferred to a Titan Krios G3i cryo-TEM (Thermo Fisher Scientific) operated at 300 kV and equipped with a K3 direct electron detector (Gatan), a BioQuantum energy filter, and a Cs image corrector for data collection. A total of 7,041 movies with a magnification of 81,000×, corresponding to a pixel size of 0.4455 Å, were collected in super-resolution mode at a defocus range from −0.5 to −2.5 μm, with a total electron dose of 60 e−/Å2 per movie. Data collection was performed using EPU software (Thermo Fisher Scientific). Data collection statistics are shown in Supplementary Table 1.
Data processing and 3D reconstructions
Cryo-EM movies were processed in cryoSPARC v4.1263 as follows. All raw movies were aligned, drift-corrected, and dose-weighed using the Patch Motion Correction wrapper. The defocus and contrast transfer function (CTF) parameters were estimated using the Patch CTF Estimation wrapper. 6,574 movies were curated using the curate exposure module in cryoSPARC based on the CTF resolutions and astigmatism. Particle-picking routines were used for the initial generation of templates for reference-free two-dimensional (2D) classifications and a few well-defined classes were selected for template-based particle picking. Several rounds of 2D classifications were performed iteratively and 876,488 particles were selected for the generation of five ab initio 3D reconstructions. Of these 5 reconstructions, two showed intact dysferlin monomer and dimer shapes. The monomer reconstruction with 453,131 particles was subjected to 3D classification. Two 3D classes showed monomer characteristics: one class with 271,263 particles showed well-aligned, high-resolution particles, and another class with 12,878 particles had an overall lower resolution but featured the C2A domain. We performed iterative non-uniform refinement and local refinements with soft masks with the largest particle-containing class. The mask was extended with a soft edge of six pixels in width to improve the local resolution of the reconstructed map. The final global resolution of the reconstruction was ~2.96 Å. The dimer reconstruction with 162,265 particles was subjected to 3D classification. Analysis of the 3D classes showed a well-defined dimer reconstruction with 74,427 particles that were subsequently subjected to iterative non-uniform refinement and local refinements with soft masks. A soft mask was applied to mask out the flexible C2A region or unknown low-resolution densities and detergent micelles. This mask was extended with a soft edge of five pixels in width to improve the local resolution of the reconstructed map. The final resolution of the dimer reconstruction showed a global resolution of ~4.65 Å. All reported resolutions are based on the gold-standard Fourier Shell Correlation (FSC) at 0.143. 3D maps were post-processed using the DeepEMhancer64. Data collection statistics and image processing summary are shown in Supplementary Table 1 and Supplementary Fig. 1f.
Model building, refinement, and validation
The postprocessed map of the dysferlin monomer reconstruction was used for model building. Since only the C2A domain30,46,65,66 and the DysFI48,67 of dysferlin and other members of ferlin protein family have been structurally characterized, we used the four helical bundles containing the FerA-FerB domain from the dysferlin AlphaFold model as an initial template (Uniprot ID: O75923). Then, we rigid-body docked individual C2 domains of dysferlin from the AlphaFold model using UCSF ChimeraX68. Because of the unambiguous high-resolution of our reconstructions, we manually built the entire model including several linkers connecting the C2 domains and DysF region in Coot69. Iterative model building was performed using real-space refinement in Phenix70, Rosetta71, and Coot to manually build the dysferlin monomer. Using the well-defined monomer structure, we used rigid body docking to dock each protomer in the dimer reconstruction using UCSF ChimeraX. We performed initial model building using real-space refinement in Phenix70 and then fitted and refined the dimer model using Namdinator72. Both monomer and dimer models were refined using post-processed cryo-EM reconstructions and manually verified using Coot. All the refined models were validated using MolProbity73. For analyzing the C2 domain interfaces and protomer interfaces for buried surface area, we used the EBI PISA webserver74. Figures were made with UCSF ChimeraX and PyMOL (https://pymol.org).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Atomic models and EM maps were deposited in the Protein Data Bank (PDB) and the Electron Microscopy Data Bank (EMDB) under accession numbers PDB ID: 9B8K and EMDB ID: EMD-44348 for the dysferlin monomer and PDB ID: 9B8L and EMDB ID: EMD-44349 for the dysferlin homodimer. Source data are provided with this paper.
References
McNeil, P. L., Miyake, K. & Vogel, S. S. The endomembrane requirement for cell surface repair. Proc. Natl. Acad. Sci. USA 100, 4592–4597 (2003).
Clarke, M. S., Caldwell, R. W., Chiao, H., Miyake, K. & McNeil, P. L. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 76, 927–934 (1995).
McNeil, P. L. & Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 140, 1097–1109 (1992).
McNeil, P. L. & Steinhardt, R. A. Plasma membrane disruption: repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 19, 697–731 (2003).
Barthelemy, F., Defour, A., Levy, N., Krahn, M. & Bartoli, M. Muscle cells fix breaches by orchestrating a membrane repair ballet. J. Neuromuscul. Dis. 5, 21–28 (2018).
Bansal, D. & Campbell, K. P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 14, 206–213 (2004).
Cooper, S. T. & McNeil, P. L. Membrane repair: mechanisms and pathophysiology. Physiol. Rev. 95, 1205–1240 (2015).
Lammerding, J. & Lee, R. T. Torn apart: membrane rupture in muscular dystrophies and associated cardiomyopathies. J. Clin. Invest. 117, 1749–1752 (2007).
Fanin, M. & Angelini, C. Muscle pathology in dysferlin deficiency. Neuropathol. Appl. Neurobiol. 28, 461–470 (2002).
Gallardo, E. et al. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology 57, 2136–2138 (2001).
Rawat, R. et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am. J. Pathol. 176, 2891–2900 (2010).
Cenacchi, G., Fanin, M., De Giorgi, L. B. & Angelini, C. Ultrastructural changes in dysferlinopathy support defective membrane repair mechanism. J. Clin. Pathol. 58, 190–195 (2005).
Bansal, D. et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423, 168–172 (2003).
Lek, A., Evesson, F. J., Sutton, R. B., North, K. N. & Cooper, S. T. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic 13, 185–194 (2012).
Lek, A., Lek, M., North, K. N. & Cooper, S. T. Phylogenetic analysis of ferlin genes reveals ancient eukaryotic origins. BMC Evol. Biol. 10, 231 (2010).
Han, R. et al. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J. Clin. Invest. 117, 1805–1813 (2007).
Klinge, L. et al. From T-tubule to sarcolemma: damage-induced dysferlin translocation in early myogenesis. FASEB J. 21, 1768–1776 (2007).
Selcen, D., Stilling, G. & Engel, A. G. The earliest pathologic alterations in dysferlinopathy. Neurology 56, 1472–1481 (2001).
Kerr, J. P. et al. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proc. Natl. Acad. Sci. USA 110, 20831–20836 (2013).
Muriel, J. et al. The C2 domains of dysferlin: roles in membrane localization, Ca(2+) signalling and sarcolemmal repair. J. Physiol. 600, 1953–1968 (2022).
Millay, D. P. et al. Genetic manipulation of dysferlin expression in skeletal muscle: novel insights into muscular dystrophy. Am. J. Pathol. 175, 1817–1823 (2009).
Cooper, S. T. & Head, S. I. Membrane injury and repair in the muscular dystrophies. Neuroscientist 21, 653–668 (2015).
Woolger, N. et al. Limited proteolysis as a tool to probe the tertiary conformation of dysferlin and structural consequences of patient missense variant L344P. J. Biol. Chem. 292, 18577–18591 (2017).
Bashir, R. et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 20, 37–42 (1998).
Liu, J. et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat. Genet. 20, 31–36 (1998).
Weiler, T. et al. Identical mutation in patients with limb girdle muscular dystrophy type 2B or Miyoshi myopathy suggests a role for modifier gene(s). Hum. Mol. Genet. 8, 871–877 (1999).
Krahn, M. et al. A naturally occurring human minidysferlin protein repairs sarcolemmal lesions in a mouse model of dysferlinopathy. Sci. Transl. Med. 2, 50ra69 (2010).
Llanga, T. et al. Structure-based designed nano-dysferlin significantly improves dysferlinopathy in BLA/J mice. Mol. Ther. 25, 2150–2162 (2017).
Aoki, M. et al. Genomic organization of the dysferlin gene and novel mutations in Miyoshi myopathy. Neurology 57, 271–278 (2001).
Fuson, K. et al. Alternate splicing of dysferlin C2A confers Ca(2)(+)-dependent and Ca(2)(+)-independent binding for membrane repair. Structure 22, 104–115 (2014).
Davis, D. B., Doherty, K. R., Delmonte, A. J. & McNally, E. M. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J. Biol. Chem. 277, 22883–22888 (2002).
Kwok, E. et al. The dysferlin C2A domain binds PI(4,5)P2 and penetrates membranes. J. Mol. Biol. 435, 168193 (2023).
Xu, L. et al. Dysferlin forms a dimer mediated by the C2 domains and the transmembrane domain in vitro and in living cells. PLoS ONE 6, e27884 (2011).
Huang, Y. et al. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 21, 732–742 (2007).
Cai, C. et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 11, 56–64 (2009).
Ma, Y., Ding, L., Li, Z. & Zhou, C. Structural basis for TRIM72 oligomerization during membrane damage repair. Nat. Commun. 14, 1555 (2023).
Matsuda, C. et al. The sarcolemmal proteins dysferlin and caveolin-3 interact in skeletal muscle. Hum. Mol. Genet. 10, 1761–1766 (2001).
Matsuda, C. et al. The C2A domain in dysferlin is important for association with MG53 (TRIM72). PLoS Curr. 4, e5035add5038caff5034 (2012).
Codding, S. J., Marty, N., Abdullah, N. & Johnson, C. P. Dysferlin binds SNAREs (Soluble N-Ethylmaleimide-sensitive Factor (NSF) Attachment Protein Receptors) and stimulates membrane fusion in a calcium-sensitive manner. J. Biol. Chem. 291, 14575–14584 (2016).
Rizo, J. & Sudhof, T. C. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 273, 15879–15882 (1998).
Leonard, T. A. in Encyclopedia of Metalloproteins (eds Robert H. Kretsinger, Vladimir N. Uversky, & Eugene A. Permyakov) 309-318 (Springer New York, 2013).
Lek, A. et al. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J. Neurosci. 33, 5085–5094 (2013).
Redpath, G. M. et al. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol. Biol. Cell 25, 3037–3048 (2014).
Ballouhey, O. et al. The dysferlin transcript containing the alternative exon 40a is essential for myocyte functions. Front Cell Dev. Biol. 9, 754555 (2021).
Quinn, C. J., Cartwright, E. J., Trafford, A. W. & Dibb, K. M. On the role of dysferlin in striated muscle: membrane repair, t-tubules and Ca(2+) handling. J. Physiol. 602, 1893–1910 (2024).
Wang, Y. et al. Calcium binds and rigidifies the dysferlin C2A domain in a tightly coupled manner. Biochem. J. 478, 197–215 (2021).
Abdullah, N., Padmanarayana, M., Marty, N. J. & Johnson, C. P. Quantitation of the calcium and membrane binding properties of the C2 domains of dysferlin. Biophys. J. 106, 382–389 (2014).
Sula, A., Cole, A. R., Yeats, C., Orengo, C. & Keep, N. H. Crystal structures of the human Dysferlin inner DysF domain. BMC Struct. Biol. 14, 3 (2014).
Aartsma-Rus, A., Van Deutekom, J. C., Fokkema, I. F., Van Ommen, G. J. & Den Dunnen, J. T. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144 (2006).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Izumi, R. et al. The genetic profile of dysferlinopathy in a cohort of 209 cases: Genotype-phenotype relationship and a hotspot on the inner DysF domain. Hum. Mutat. 41, 1540–1554 (2020).
Zhong, H. et al. Molecular landscape of DYSF mutations in dysferlinopathy: from a Chinese multicenter analysis to a worldwide perspective. Hum Mutat 42, 1615–1623 (2021).
Fujita, E. et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum. Mol. Genet. 16, 618–629 (2007).
Tominaga, K. et al. 4-Phenylbutyrate restores localization and membrane repair to human dysferlin mutations. iScience 25, 103667 (2022).
Evesson, F. J. et al. Reduced plasma membrane expression of dysferlin mutants is attributed to accelerated endocytosis via a syntaxin-4-associated pathway. J. Biol. Chem. 285, 28529–28539 (2010).
Cagliani, R. et al. Molecular analysis of LGMD-2B and MM patients: identification of novel DYSF mutations and possible founder effect in the Italian population. Neuromuscul. Disord. 13, 788–795 (2003).
Krahn, M. et al. Analysis of the DYSF mutational spectrum in a large cohort of patients. Hum. Mutat. 30, E345–375, (2009).
Kokubu, Y. et al. Phenotypic drug screening for dysferlinopathy using patient-derived induced pluripotent stem cells. Stem Cells Transl. Med. 8, 1017–1029 (2019).
Takahashi, T. et al. Clinical features and a mutation with late onset of limb girdle muscular dystrophy 2B. J. Neurol. Neurosurg. Psychiatry 84, 433–440 (2013).
Hirsch, M. L. et al. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol Ther 21, 2205–2216 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
de Morree, A. et al. Proteomic analysis of the dysferlin protein complex unveils its importance for sarcolemmal maintenance and integrity. PLoS ONE 5, e13854 (2010).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Harsini, F. M. et al. Structural basis for the distinct membrane binding activity of the homologous C2A domains of myoferlin and dysferlin. J. Mol. Biol. 431, 2112–2126 (2019).
Helfmann, S. et al. The crystal structure of the C(2)A domain of otoferlin reveals an unconventional top loop region. J. Mol. Biol. 406, 479–490 (2011).
Patel, P. et al. Solution structure of the inner DysF domain of myoferlin and implications for limb girdle muscular dystrophy type 2b. J. Mol. Biol. 379, 981–990 (2008).
Meng, E. C. et al. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods 17, 665–680 (2020).
Kidmose, R. T. et al. Namdinator - automatic molecular dynamics flexible fitting of structural models into cryo-EM and crystallography experimental maps. IUCrJ 6, 526–531 (2019).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Acknowledgements
We thank Dr. Noah Weisleder for discussions. The cryo-EM data were collected at the Center for Electron Microscopy and Analysis (CEMAS) at the Ohio State University. We thank the Ohio Supercomputer Center (OSC) for high-performance computing resources. We acknowledge resources from the Campus Microscopy and Imaging Facility (CMIF), and the OSU Comprehensive Cancer Center (OSUCCC) Microscopy Shared Resource (MSR) at the Ohio State University with NIH S10 OD025008 and NIH NIC P30CA016058. This work was funded by start-up funds from the Ohio State University to S.M.H and K.C.
Author information
Authors and Affiliations
Contributions
H.H. performed cloning of dysferlin in the baculovirus expression vector, expressed and purified the proteins, and performed all the cell biology and SEC-MALS experiments. H.H. and G.G. screened grids and collected cryo-EM data. H.H., S.M.H., and K.C. analyzed the data. K.C. and S.M.H. designed and supervised the work. S.M.H. and K.C. wrote the original draft of the manuscript with inputs from H.H. All authors reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Colin Johnson, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, HL., Grandinetti, G., Heissler, S.M. et al. Cryo-EM structures of the membrane repair protein dysferlin. Nat Commun 15, 9650 (2024). https://doi.org/10.1038/s41467-024-53773-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53773-6
This article is cited by
-
Structural insights into lipid membrane binding by human ferlins
The EMBO Journal (2025)