Abstract
Three-dimensional solar steam evaporators with efficient water purification performance have received increasing attention recently. Herein, elastic polymer covalent organic frameworks (PP-PEG) containing PEG chains with intriguing adaptability to guests are prepared by forming porphyrin rings. PP-PEG foams demonstrate full spectrum absorbance and excellent photothermal conversion properties. Through well-designed thermal management and optimization of the hydrophilicity and PEG chain length, we obtain a highly efficient solar evaporator with an evaporation rate of 4.89 kg m−2 h−1 under 1 sun in self-contained mode. The optimized solar evaporation rate is increased to 18.88 kg m−2 h−1 under 1 sun with a facile truncated cone reflector, exceeding all known solar steam evaporators. This innovative design holds immense promise for desalination and water purification owing to its simple preparation, high efficiency and durability.
Similar content being viewed by others
Introduction
Freshwater resources are the lifeblood of humanity1. However, population growth, economic development, and climate change have caused a significant shortage of freshwater2. Traditional seawater desalination methods like distillation3, reverse osmosis4, and electrodialysis5 have provided viable options to solve this problem in industry. However, these methods still have drawbacks of processing complexity and high cost, etc. To address these challenges, solar steam evaporation technologies have been developed by employing various photothermal materials, such as carbon-based materials6, plasmonic nanoparticles7, biomass-based materials8, and organic polymers9. There are three main factors that predominate the solar steam evaporation performance, including photothermal conversion property, heat management, and water transport management, which have been well designed to enhance solar steam evaporation10. Recently, three-dimensional (3D) solar steam evaporators, like water self-contained aerogels11, hydrogels12,13 and sponges/foams14 featured lightweight, adaptable, and eco-friendly properties have been developed and exhibited superior solar steam evaporation performance compared to typical two-dimensional (2D) solar evaporators15 due to the 3D structure can not only make full use of the incident light and convert it into heat, but also can obtain additional energy from the environment16, which reduces heat radiation and heat conduction loss, achieving solar-to-vapor conversion efficiencies above 100%.
Covalent organic frameworks (COFs)17 as a promising material have drawn increasing interest in the field of solar steam evaporation18,19 because it is convenient to uniformly integrate photothermal function units, such as porphyrin20 and azobenzene21, into the structure for efficient photothermal conversion. As a kind of crystalline material, COFs are typically prepared as powders, microcrystals or some made into membranes21,22,23. 2D solar steam evaporators, including COFs membranes, come into direct contact with bulk water, inevitably resulting in heat loss, which is not conducive to efficient solar steam evaporation. Controlling the synthesis conditions can lead to the formation of COFs gels, such as using a group protection synthesis strategy and the gel exhibited great potential for solar steam evaporation24. For the construction of COFs, rigid building blocks are always preferred over flexible ones because the higher degree of freedom and variable conformations of flexible ligands are not favorable for proper bonding orientation during polymerization and crystallization25,26. COFs constructed from flexible precursors usually suffer from loss of crystallinity or even structural collapse during degassing27,28. Polymer COFs (polyCOFs) are formed by linear polymers as structural building blocks, inheriting the advantages of both COFs and linear polymers which endow polyCOFs with new properties, such as enhanced mechanical properties and stimulation response29. Developing advanced polyCOFs materials with efficient solar-to-vapor conversion, suitable water transport pathways, controllable hydrophilic/hydrophobicity, moldable shape, and enhanced mechanical properties is highly desired for improving solar steam evaporation performance.
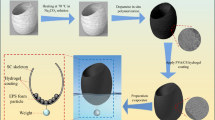
In this work, we design and synthesize elastic porphyrin-based polyCOFs (denoted as PP-PEG) with intriguing adaptability to guests via the reaction between pyrrole and dialdehyde precursors containing polyethylene glycol (PEG) chains and utilize them as solar evaporators in 3D foams for solar steam evaporation (Fig. 1a, b). PP-PEG exhibits full spectrum absorbance and excellent photothermal conversion properties with porphyrin rings as photothermal function units and flexible PEG chains preventing the detrimental aggregation and quenching of porphyrin30. Pristine hydrophobic PP-DEG (PEG of diethylene glycol, denoted as DEG) 3D foam shows an average solar evaporation rate of 1.61 kg m−2 h−1 under 1 sun. We exploit an insulating floater and the capillary action to wick and confine water within foams to isolate evaporators from the bulk water (referred to as self-contained mode) to reduce heat loss (Fig. 1b), which significantly improves solar steam evaporation rate to 2.39 kg m−2 h−1 under 1 sun. Hydrophilic 3D foams are prepared by the in-situ addition of cellulose acetate (CA), which enhances the elasticity of 3D foams and greatly improves the solar evaporation rate to 4.22 kg m−2 h−1 under 1 sun in self-contained mode. A longer PEG chain in PP-PEG-600 (PEG-600, PEG with an average molecular weight of 600) further improves the solar evaporation rate to 4.89 kg m−2 h−1 under 1 sun. Taking advantage of the 3D shape and self-contained water in PP-PEG-600 foam, we designed a facile truncated cone reflector made of aluminum sheets to irradiate the lateral surfaces of the cylindrical foam. The solar evaporation rate under 1 sun is significantly increased to 18.88 kg m−2 h−1, which exceeds the performance of all known solar steam evaporators. The PP-PEG-600 foam utilized for seawater desalination also exhibits excellent performance and durability. The simple preparation, high efficiency, low cost, mechanically robust and durability make PP-PEG foam a promising platform for desalination and water purification.
Results
Preparation and characterization of PP-DEG-Acid
As a typical representative of PP-PEG, PP-DEG is prepared by the reaction of pyrrole and 1,7-bis(4-formylphenyl)−1,4,7-trioxaheptane (DEG-(Ph-CHO)2) in propionic acid via the formation of porphyrin ring (denoted as PP-DEG-Acid, Fig. 2a). The powder is obtained when reacting in a round-bottomed flask with stirring and refluxing, while reacting in an autoclave with static heating results in the formation of a PP-DEG monolith (see details in Methods).
a PP-DEG-Acid is synthesized by cyclic tetramerization of pyrrole with DEG-(Ph-CHO)2 to form porphyrin rings. b N2 and CO2 sorption isotherms of PP-DEG-Acid at 77 K and 273 K, respectively. c FT-IR spectroscopy of PP-DEG-Acid and DEG-(Ph-CHO)2 in the range of 400–2000 cm−1. d Solid-state 13C CP/MAS NMR spectrum of PP-DEG-Acid (the inset image shows the structure with labeled carbons). Digital photo (e), the water contact angle of the surface (f) and SEM image (g) of PP-DEG-Acid foam.
The powder X-ray diffraction (PXRD) pattern (Supplementary Fig. 1) indicates the amorphous nature of PP-DEG-Acid because of multiple rotatable σ-bonds in flexible DEG chains, which also leads to the collapse of the porous structure as revealed by the adsorption isotherm of N2 at 77 K (Fig. 2b). However, PP-DEG-Acid displays a remarkable CO2 adsorption capacity (13 cm3 g−1) at 273 K owing to the interaction between CO2 and porphyrin rings and DEG chains are unfolded at a relatively higher temperature of 273 K to create accessible pores for CO2 adsorption (Fig. 2b). The chemical structure of the PP-DEG-Acid is confirmed by Fourier transform infrared (FT-IR) spectroscopy (Fig. 2c and Supplementary Fig. 2). The C = O stretching vibration at 1687 cm−1 is strongly attenuated, suggesting the absence of carbonyl functional groups from aldehyde group owing to the reaction between pyrrole and aldehyde. The intensive peaks at 1238 and 1120 cm−1 can be attributed to the v (Ph–O–C) and v (C–O–C) bonds of the ether31, which are slightly redshifted compared to DEG-(Ph-CHO)2 (Fig. 2c and Supplementary Fig. 2). The chemical structure of PP-DEG-Acid is further supported by 13C solid-state nuclear magnetic resonance (13C CP-MAS) NMR spectroscopy (Fig. 2d), the significantly eliminated signal at 190 ppm (C = O) also suggests a complete reaction of the aromatic aldehyde molecules with pyrrole for the formation of tetrasubstituted macrocyclic porphyrin32, which corresponds to the characteristic chemical shifts of C1, C2, and C3 in the porphyrin ring (see inset image in Fig. 2d) at 159, 116 and 123 ppm, respectively. The intense and broad peak at 131 ppm corresponds to C5 and C6, and the shoulder at 137 ppm is assigned to C4 and C733,34. The strong resonance at 69 ppm is assigned to the ether carbon31 (C8 and C9) that links the two porphyrin rings, manifesting the formation of porphyrin networks linked by flexible PEG chains. X-ray photoelectron spectroscopy (XPS) measurements (Supplementary Fig. 3) further confirm the success of the synthetic strategy for porphyrin-based polyCOFs, and elemental analysis (Supplementary Table. 1) shows an atomic C/N ratio of PP-DEG-Acid is close to the theoretic value. Combined with the above analyses, the formation of flexible PP-DEG-Acid is confirmed as demonstrated in Fig. 2a. Thermogravimetric analysis (TGA) reveals PP-DEG-Acid is highly thermal stable before about 350 °C (Supplementary Fig. 4). Structural flexibility in porous frameworks refers to their ability to undergo structural transformations when exposed to external stimuli, giving rise to breathing, swelling, linker rotation, and subnetwork displacement at the lattice scale35. To shed light on the structural adaptability of PP-DEG-Acid, dichloromethane response tests are performed on small pieces of PP-DEG-Acid membranes (Supplementary Movie 1), where the swelling properties originated from the elasticity of the flexible DEG chains in PP-DEG-Acid can be clearly observed.
Field-emission scanning electron microscopy (FE-SEM) images (Supplementary Fig. 5) show that PP-DEG-Acid powder is composed of agglomerated sphere-shaped particles. However, when the reaction is conducted in a static condition in an autoclave, hydrophobic PP-DEG-Acid monolith (Fig. 2e) with a contact angle of 120.8° (Fig. 2f) is obtained, and the microscopic morphology changes from spheres in powder to cross-linked chains in monolith (Fig. 2g and Supplementary Fig. 6), indicating that the shearing force plays a crucial role in shaping synthetic products36.
To explore the generality of the approach, we synthesize 1,10-bis(4-formylphenyl)-1,4,7,10-tetraoxadecane (TEG-(Ph-CHO)2) as a replacement for DEG-(Ph-CHO)2 and obtain PP-TEG-Acid under identical reaction conditions, which exhibits a similar structure and characters to PP-DEG-Acid (Supplementary Figs. 7–10 and Table. 1).
Solar evaporation performance of PP-DEG-Acid monolith
The obtained PP-DEG-Acid foam is dark brown and exhibits high and full ultraviolet-visible light-near-infrared (UV-Vis-NIR) absorbance (>98%) owing to the presence of porphyrin units (Fig. 3a). Under 1 sun irradiation (1 kW m−2) and ambient conditions (room temperature ~25 °C, humidity ~60%), the surface temperature of bulk water only reaches 26.9 °C after 10 min (Fig. 3b, c). However, the surface temperature of the foam increases to 80.6 °C within merely 1 min and stabilizes at ca. 94.4 °C after 10 min in the dry state (Fig. 3b, d). The results demonstrate the efficient photothermal conversion properties of PP-DEG-Acid, which can be explained by the excited thermal irradiation process of porphyrin37 and flexible DEG chains preventing the stacking and self-quenching of porphyrin rings30.
a UV-Vis-NIR absorbance spectra of PP-DEG-Acid in the range of 200–2200 nm. b Surface temperature evolution of bulk water and PP-DEG-Acid foam at different modes under 1 sun. Infrared thermography images of the surface temperature of pure water (c) and PP-DEG-Acid foam in dry (d), floating (e) and self-contained mode (f) under 1 sun. g Mass changes of pure water and PP-DEG-Acid evaporator in floating and self-contained modes under 1 sun.
The solar evaporation performance of the PP-DEG-Acid foam is evaluated by directly placing the foam in water (Supplementary Fig. 11a) using a laboratory-made measurement device (Supplementary Fig. 12). The surface temperature of PP-DEG-Acid foam increases to 41.6 °C after 10 min in the floating condition (Fig. 3b, e). It exhibits a water evaporation rate of 1.61 kg m−2 h−1 (Fig. 3g), which is significantly higher than that of bulk water (0.42 kg m-2 h−1). However, the highly hydrophobic nature of PP-DEG-Acid may restrict water from being efficiently transported to the top of the foam during evaporation and thermal diffusion to the bulk water is unavoidable due to the direct contact between the foam and the bulk water, hence resulting in poor water evaporation performance.
The self-contained mode for enhanced solar steam evaporation
To reduce the heat loss to bulk water, three structural components (solar evaporator, water-wick and floater) are integrated as shown in Fig. 1b and Supplementary Fig. 11b, c. We exploit the capillary action of the foam to wick and confine water in the 3D foam. The design not only isolates the solar evaporator from the bulk water which reduces heat conduction loss through the floater, but also provides a continuous water supply through the water-wick. To distinguish it from the evaporation system in direct contact with water (floating mode), the solar evaporation system that contains water and is isolated from bulk water is referred to as the self-contained mode. In the self-contained mode, the surface temperature under 1 sun is almost the same as the floating mode of PP-DEG-Acid foam (Fig. 3b, f), however, the foam in the self-contained mode displays a higher evaporation rate of 2.39 kg m−2 h−1 under 1 sun, representing an increase of 48.4% as compared to the foam in the floating condition (1.61 kg m−2 h−1) as shown in Fig. 3g, illustrating an enhanced and time-extended self-contained solar steam generation. The self-contained mode not only reduces the heat conduction to bulk water but also greatly increases the evaporation area by utilizing the lateral surfaces of the foam to promote the generation of cold steam, both of which improve steam evaporation performance. In addition, the elasticity of the foam helps prevent the foam from collapsing during evaporation, thus maintaining the evaporation performance.
Effect of hydrophilicity on solar steam evaporation
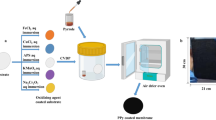
Hydrophobic PP-DEG-Acid foam displays an excellent photothermal conversion but a commonplace evaporation rate. The wettability of the surface plays a vital role in solar evaporation systems, especially for water transport and supply38. Therefore, we endeavor to enhance the hydrophilicity of the foam by in-situ addition of CA (Ac-: 39.8 wt%, -OH: 3.5 wt%) during the preparation. Regrettably, we fail to obtain the intact monolith with propionic acid as the solvent (Supplementary Figs. 13, 14). Therefore, we employ another method to synthesize PP-DEG with anhydrous aluminum chloride as the catalyst and dry N, N-Dimethylformamide (DMF) as the solvent39. Similar to the preparation of PP-DEG-Acid, depending on the reaction condition, we can obtain powder and monolith (Supplementary Fig. 15, Supplementary Movie 2, see Methods for details), denoted as PP-DEG-DMF. The characterizations of PP-DEG-DMF are consistent with that of PP-DEG-Acid (Supplementary Figs. 16, 17). Notably, the morphology of the PP-DEG-DMF powder observed by SEM images (Supplementary Fig. 18) is significantly rougher than that of the smooth spheres of the PP-DEG-Acid (Supplementary Fig. 5), which indicates that solvents with different polarity seriously affect the morphology.
Using DMF as the solvent, we successfully introduce CA into the PP-DEG-DMF and obtain elastic foams. Digital photographs of PP-DEG-DMF foams with different contents of CA are shown in Supplementary Fig. 19. The PP-DEG-DMF-0 wt% CA monolith shrinks to a dense monolithic structure that is unable to store and transport water after the removal of the solvent (Supplementary Fig. 20a) and exhibits an unsatisfactory evaporation rate (Supplementary Fig. 20b). The addition of CA enhances the hydrophilicity and wettability of the foam due to its multiple hydroxyl groups. As shown in Fig. 4a, pristine PP-DEG-DMF shows a contact angle of 102.0°, while adding 0.5 wt% CA decreases the contact angle to 38.9°, and PP-DEG-DMF with higher content of CA (1.5–10 wt%) are highly hydrophilic with contact angles of zero. However, we find that adding more CA (12 wt%) results in the collapse of the foam because excessive amounts of CA would result in a significant gap among the nanospheres of PP-DEG-DMF, whereas pure CA is not apt to form a monolith. As a typical representative of PP-DEG-DMF containing 5 wt% CA, the foam (denoted as PP-DEG-DMF-5 wt% CA) not only maintains the macroscopic cylindrical monolith (Fig. 4b, c) but also exhibits improved elasticity and compressibility (Supplementary Movie 3). SEM images (Fig. 4d, e and Supplementary Fig. 21) reveal a sponge-like architecture with hierarchical meso- and macro-porous of PP-DEG-DMF-5 wt% CA foam, which facilitates water storage, water transportation, and the multiple scattering effects (the incident light is scattered many times after entering the pore, extending the light path length, which is conducive to use light more efficiently). SEM mapping images illustrate the homogeneity (Supplementary Fig. 22). The foam uptakes water immediately and becomes completely wet within a short period of 6 s owing to the wicking effect, demonstrating its excellent water transport capability (Supplementary Fig. 23 and Supplementary Movie 4). Both the 3D porous structure and hydrophilicity enable the foam to rapidly uptake and confine water inside of the foam, ensuring timely water supply to the top surface during continuous evaporation.
a Water contact angles of PP-DEG-DMF foams containing different contents of CA. Digital photographs of the top-view (b), the side-view (c) and SEM images at different scales (d, e) of PP-DEG-DMF-5 wt% CA foam. f UV-Vis-NIR absorbance spectra of PP-DEG-DMF and PP-DEG-DMF-5 wt% CA in the range of 200–2200 nm. Infrared thermography images of the surface temperature of PP-DEG-DMF-5 wt% CA in dry (g) and self-contained mode (h) under 1 sun. i Surface temperature evolution of PP-DEG-DMF-5 wt% CA in the dry and self-contained mode under 1 sun. Mass changes of the bulk water, PP-DEG-DMF evaporators containing different contents of CA (j) and PP-PEG-DMF-5 wt% CA containing different lengths of flexible PEG chains (k) under 1 sun.
The addition of CA exerts a negligible effect on the solar absorbance of PP-DEG-DMF-5 wt% CA, which is almost the same as PP-DEG-DMF and PP-DEG-Acid (Figs. 4f, 3a). The surface temperature of PP-DEG-DMF-5 wt% CA increases to 75.7 °C and 26.0 °C within merely 1 min in dry and self-contained mode under 1 sun, respectively, and maintains at 93.1 °C and 41.3 °C after 30 min of irradiation, respectively (Fig. 4g–i). The infrared image (supplementary Fig. 24) shows thermal localization at the top of the foam, allowing the converted heat to be used more efficiently for evaporation of the thin layer of water on the top surface. In addition, the side temperature of the foam is lower than the ambient temperature, indicating that heat can flow into the foam from the environment (environmental energy input). On the other hand, the water evaporation enthalpy from PP-DEG-DMF-5 wt% CA foam reduced to 2122 J/g with a lower evaporation temperature, which is lower than the measured value of 2338 J/g for pure water (theoretical value: 2450 J/g) and 2298 J/g from PP-DEG-Acid as determined by differential scanning calorimetry (DSC) measurement (Supplementary Fig. 25). PP-DEG-DMF with varying CA contents (0.5, 1.5, 3.0, 5.0, and 10.0 wt%) are used to investigate solar steam evaporation performance in the self-contained mode (Fig. 4j and Supplementary Fig. 26). The evaporation rates are much enhanced in comparison to PP-DEG-Acid (2.39 kg m−2 h−1), except for PP-DEG-DMF-0.5 wt% CA, which is attributed to the fact that the framework is unable to resist shrinkage during solvent removal, and the collapsed foam failed to preserve and transport water (Supplementary Fig. 19). Among them, the PP-DEG-DMF-10 wt% CA exhibits the highest evaporation rate of 4.22 kg m−2 h−1, indicating that the addition of CA that enhances hydrophilicity/wettability is favorable for improving evaporation rates.
Effect of PEG chain length on solar steam evaporation
Since different PEG chains can change the structure of PP-PEG, we further explore the effect of flexible PEG chain lengths on solar steam evaporation. The PP-PEG-DMF foams containing varied PEG chain lengths with CA content fixed at 5 wt% are prepared by the same procedure of PP-DEG-DMF 5 wt% CA (see Methods for details). Digital photographs and SEM images of PP-PEG-DMF-5 wt% CA foams with different PEG chains are shown in Supplementary Figs. 27, 28, where morphologies are similar, featured as the sphere particle with a rough surface. The UV-Vis-NIR absorbance spectra show that all PP-PEG-DMF-5 wt% CA exhibited high full solar absorbance (Supplementary Fig. 29), suggesting that the length of PEG chains will not affect solar absorbance.
As shown in Fig. 4k and Supplementary Fig. 30, PP-PEG-600-DMF-5 wt% CA exhibits the highest evaporation rate of 4.09 kg m−2 h−1. We speculate that the longer PEG chains prevent the aggregation and quenching of porphyrin30 thus holding the intrinsic function of the porphyrin ring as a photothermal conversion unit. The introduction of CA and longer PEG chains favor evaporation. As a result of the optimization, digital photographs, mechanical properties and SEM images of PP-PEG-600-DMF-10 wt% CA foam are presented in Supplementary Figs. 31–33. The foam also exhibits high full solar absorbance (Supplementary Fig. 34) and gives rise to the best performance as a solar evaporator. The evaporation rate reached 4.89 kg m−2 h−1 under 1 sun, 8.92 kg m−2 h−1 under 2 sun, and 11.98 kg m−2 h−1 under 3 sun (Fig. 5a), with excellent stability performance in deionized water (Fig. 5b). This solar evaporation performance surpassed a vast majority of 3D solar steam generators (blue star in Fig. 5c)16,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63. The measured enthalpy of evaporation according to the DSC test was reduced to 2126 J/g (Supplementary Fig. 35). To explain this reduction in vaporization enthalpy, we turned to the water cluster and the intermediate water theory (Supplementary Figs. 36, 37). An energy balance analysis to exhibit energy input and energy loss is provided (Supplementary Note 1 and Supplementary Fig. 38). PP-PEG-600-DMF-10 wt% CA foam is used as the solar evaporator to further demonstrate the desalination of seawater from the Yellow Sea in China. Figure 5d shows the concentrations of four kinds of ions (Na+, K+, Ca2+, and Mg2+) in seawater and collected condensate. After the process of desalination, the concentrations of four main ions in the condensed water are reduced to 2.999, 0.475, 0.461, and 0.294 mg L−1, respectively, significantly lower than the drinking water threshold specified by the World Health Organization. PP-PEG-600-DMF-10 wt% CA foam also shows excellent stability performance in seawater and wastewater with a broad pH region of 1–13 (Fig. 5e and Supplementary Figs. 39, 40).
a Mass changes versus time of PP-PEG-600-DMF-10 wt% CA evaporator under different concentrated sunlight intensities. b The cycling evaporation performance of PP-PEG-600-DMF-10 wt% CA towards deionized water (5 h for each cycle). c Evaporation rates of PP-PEG-600-DMF-10 wt% CA in comparison with the values of 3D solar absorbers reported in literatures. d Ion concentration of Na+, K+, Ca2+, and Mg2+ of seawater before and after desalination. e The stable evaporation performance of PP-PEG-600-DMF-10 wt% CA towards wastewater with a broad pH region of 1–13. f Mass changes versus time of PP-PEG-600-DMF-10 wt% CA evaporator with a truncated cone-shaped reflector made of aluminum sheets (the inset image shows the device design).
Reflector-assisted enhanced solar steam evaporation
Under vertical irradiation, the top surface of the PP-PEG foam shows excellent heat localization, which facilitates surface evaporation. In the self-contained mode using 3D foams as solar evaporators, the lateral surfaces of the foams without irradiation are also exposed to the air, facilitating cold steam evaporation. To speed up the lateral surface evaporation, we design a truncated cone-shaped reflector made of aluminum sheets with different reflection angles to reflect the light to the lateral surfaces of the foam (Supplementary Fig. 41). The 45-degree reflector is the best choice according to the comparison of evaporation rate (Supplementary Fig. 42) because it reflects the light vertically to the lateral surfaces of the foam. When the PP-PEG-600-DMF-10 wt% CA foam is coupled with a 45-degree reflector, the evaporation rate under 1 sun reached 18.88 kg m−2 h−1 (Fig. 5f), which is an increase of 286.1% compared to the evaporation rate of 4.89 kg m−2 h−1 without the reflector. To the best of our knowledge, it is the highest solar evaporation performance reported in the literature so far (pink star in Fig. 5c). In summary, the high solar evaporation performance originated from the high full-spectrum absorbance, superior photothermal conversion properties, high hydrophilicity for efficient water transfer, and spherical microstructure with increased irradiation and evaporation area of porphyrin-based elastic polymer COFs monolith evaporator. On the other hand, the design and structure of the evaporator are also critical to excellent evaporation performance, including self-contained evaporation mode and reflector-assisted evaporation mode which makes full use of not only the top surface of the 3D monolith evaporator, but also the lateral surface for enhanced evaporation. The total material cost of a self-contained solar evaporation system demonstrates its economics and practicality (see Supplementary Note 2). To validate the measurements of indoor simulated solar steam evaporation, outdoor experiments are conducted with the device shown in Supplementary Fig. 43 during the daytime (8:00–18:00) in May. The mass changes of seawater and solar intensity are monitored over time (Supplementary Fig. 44a). A gradual reduction in the mass of water is recorded. The average seawater evaporation rate per 0.5 h based on the PP-PEG-600-DMF-10 wt% CA evaporator varied with light intensity. The average evaporation rates within 10 h for PP-PEG-600-DMF-10 wt% CA without and with reflector are 4.39 kg m−2 h−1 and 7.66 kg m−2 h−1, respectively. The reason for the lower values in comparison to indoor simulated solar measurements is that the actual intensity of outdoor sunlight is generally below 1 kW m−2, especially after 15:30 (about 100–780 W m−2). Impressively, the evaporation rate of PP-PEG-600-DMF-10 wt% CA with the reflector is 74.5% higher than that without the reflector. After about 15:30, when the solar elevation angle drops below 45° (Supplementary Fig. 45a), the reflector starts to shadow the evaporator. As a result, the evaporation rate of solar evaporators with a reflector is lower than that without a reflector during 16:30–18:00 (Supplementary Fig. 44b). However, except for this specific period, the evaporator is not shaded (Supplementary Fig. 45b) and the reflector significantly improves the evaporation performance (Supplementary Fig. 44b). This result further confirms the effectiveness of reflectors in improving evaporation performance. For practical applications, scalability is one of the key factors that will determine the competitiveness of this technology. The local humidity and shading effects are discussed with the conclusion that the system with a reflector performs better overall (Supplementary Fig. 45). Considering that solar energy is a free and natural energy source that does not require artificial inputs, and that the coupling of reflectors significantly enhances the evaporation performance, we believe that this approach would bring inspiration to the researchers in the field of solar evaporation. Note when coupled with airflow, the evaporation rates can be greatly speeded to over 10 kg m−2 h−1, while the evaporation rates of pristine evaporators are only 2.50 and 3.27 kg m−2 h−1 without wind64,65. In the laboratory, airflow requires extra energy input, but reflector-assisted evaporation directly utilizes solar energy from existing xenon lamps. In the actual outdoors, airflow is not always present, but sunlight is almost always existing during the day.
Discussion
In conclusion, we prepare polyCOFs (PP-PEG) by forming porphyrin rings with pyrrole and dialdehyde precursors containing PEG chains. The PP-PEG foams formed by controlling the synthesis condition exhibit full solar spectrum absorbance, excellent photothermal conversion properties and continuous water supply, and hence favor solar steam evaporation. Through well-designed thermal management and optimization of the hydrophilicity and PEG chain length, we successfully obtain a highly efficient 3D solar evaporator with an evaporation rate of 4.89 kg m−2 h−1 under 1 sun in self-contained mode. To further enhance its performance, we designed a simple truncated cone reflector made of aluminum sheets to irradiate the lateral surfaces of the foam. The solar evaporation rate is increased to 18.88 kg m−2 h−1 under 1 sun, respectively, surpassing all the reported solar vapor evaporators, to the best of our knowledge. The elasticity helps to restrict the foam collapse during evaporation and maintain the cycling solar steam evaporation performance. This evaporation system can also be utilized for solar desalination, making it an extremely promising platform for desalination and water purification due to its simple preparation, high efficiency, low cost, mechanically robust and durability. It should be mentioned that in theory, the larger the cold evaporation surface on the side of the evaporator, the more cold steam will be generated, which is conducive to more efficient solar water vapor evaporation, which is worth further research. This work provides a strategy to fabricate elastic polyCOFs containing flexible chains with superior performance for solar steam evaporation and desalination, greatly expanding the potential applications of polyCOFs.
Methods
General
The materials and detailed synthesis procedure of dialdehyde precursors are given in the Supplementary Information. All reagents were purchased from commercial sources and used without further treatments.
Synthesis of PP-DEG-Acid powder and monolith
Direct cyclic tetramerization of pyrrole with aromatic dialdehydes is used to prepare PEG-porphyrin-based polyCOFs. PP-DEG-Acid powder is prepared by facile one-pot polycondensation from DEG-(Ph-CHO)2 (2 mmol, 628.7 mg) with pyrrole (4 mmol, 268.1 mg) in the presence of propionic acid in a round-bottomed flask. The polymerization is carried out by stirring and refluxing at 140 °C for 24 h. After cooling to room temperature, black powder is collected by suction-filtration, followed by washing with MeOH and DCM for 24 h in a Soxhlet apparatus, respectively. Vacuum dry at room temperature is applied to afford the pure PP-DEG-Acid powder. PP-DEG-Acid monolith is synthesized by mixing DEG-(Ph-CHO)2 (2.1 mmol, 660.1 mg) and pyrrole (4.2 mmol, 281.8 mg) in propionic acid (10 mL) in a reactor liner at room temperature for 4 h and maintaining at 140 °C for 24 h. After cooling to room temperature, the intact monolith is obtained followed by washing with MeOH and DCM for 24 h in a Soxhlet apparatus, respectively. Finally, vacuum dry at room temperature is applied to afford the pure PP-DEG-Acid monolith. The size of the monolith is tunable according to the volume of the autoclave and the solid contents of the precursors.
Synthesis of PP-DEG-DMF powder and monolith
PP-DEG-DMF powder is prepared by facile one-pot polycondensation from DEG-(Ph-CHO)2 (2.1 mmol, 660.1 mg), pyrrole (4.2 mmol, 281.8 mg), and anhydrous aluminum chloride (5 mmol, 666.7 mg) as the catalyst in the presence of dry DMF (10 mL) in a round-bottomed flask. The polymerization is carried out by stirring and refluxing at 150 °C for 24 h. After cooling to room temperature, black powder is collected by suction-filtration, followed by washing with MeOH and DCM for 24 h in a Soxhlet apparatus, respectively. Vacuum dry at room temperature is applied to afford the pure PP-DEG-DMF powder. PP-DEG-DMF monolith is synthesized by mixing DEG-(Ph-CHO)2 (2.1 mmol, 660.1 mg), pyrrole (4.2 mmol, 281.8 mg), and anhydrous aluminum chloride (5 mmol, 666.7 mg) as the catalyst in the presence of dry DMF (10 mL) in a reactor liner at room temperature for 4 h and maintaining at 150 °C for 24 h. After cooling to room temperature, the intact monolith is obtained followed by washing with MeOH and DCM for 24 h in a Soxhlet apparatus, respectively. Finally, vacuum dry at room temperature is applied to afford the pure PP-DEG-DMF monolith. The size of the monolith is tunable according to the volume of the autoclave and the solid contents of the precursors.
General procedure for the synthesis of PP-PEG-DMF-CA foam
PP-PEG-DMF-CA foams are synthesized by mixing corresponding PEG dialdehydes precursors (2.1 mmol), pyrrole (4.2 mmol), anhydrous aluminum chloride (5 mmol) as the catalyst, and the corresponding mass percentage of CA in the presence of dry DMF (10 mL) in a reactor liner at room temperature for 4 h and maintained at 150 °C for 24 h. After cooling to room temperature, intact foams are obtained followed by washing with MeOH and DCM for 24 h in a Soxhlet apparatus, respectively. Finally, vacuum dry at room temperature is applied to afford pure PP-PEG-DMF-CA foams. The size of foams is tunable according to the volume of the autoclave and the solid contents of the precursors.
Characterization
Nuclear magnetic resonance (NMR) spectra were recorded on a JNM-ECZ600R spectrometer operating at 600 MHz. Fourier transform infrared (FT-IR) spectra were collected by Nicolet 6700 FT-IR spectrometer (Thermo Scientific, USA) using KBr discs in a range from 4000 to 400 cm−1. The solid phase 13C NMR spectra were obtained on a Bruker AVANCE NEO 600 WB. PXRD measurements were done using a Rigaku MiniFlex 600 X-ray diffractometer with Cu Kα radiation (λ = 1.54178 Å). N2 and CO2 sorption isotherm was measured at 77 K and 273 K, respectively, using a BELsorp-max machine, BEL, Japan. XPS spectra were acquired by Kratos AXIS SUPRA+ high-performance electron spectrometer by using monochromatized Al Kα radiation (hν = 1486.7 eV). Elemental analysis (EA) was determined by Vario EL cube. TGA were done in the range from 25 to 800 °C in the N2 atmosphere. The heating rate was set at 10 °C/min using a TGA Q5000 thermal analyzer. Scanning electron microscopy was carried out with a field emission scanning electron microanalyzer (GeminiSEM 500). The concentrations of Na+, Mg2+, K+, and Ca2+ were measured using an inductively coupled plasma-optical emission spectrometer (ICP-OES) (Thermo Scientific iCAP 7400 ICP-OES). The evaporation enthalpy was measured by DSC Q2000. The absorbance properties in the range of 200–2200 nm were recorded by a SolidSpec-3700 ultraviolet–visible–near–infrared (UV‒Vis–NIR) spectrometer. Contact angle testing was performed with an optical contact angle and interfacial tension meter (Kino SL200KS). The Raman spectra were acquired on a confocal Raman/PL system (LabRamHR Evolution) equipped with a 532 nm laser source. Mechanical properties of the foam were performed using a dynamic mechanical analyzer (Discovery DMA850) in standard stress/strain experiments.
Solar steam generation experiments and evaluation of evaporation performance
Solar evaporation experiments are conducted using a xenon lamp (CEL-HXF300, Beijing China Education AU-Light Technology Co., Ltd.) with an AM 1.5 G optical filter at room temperature of ~25 °C and a humidity of ~60%. The solar flux is calibrated using a thermopile connected to a power meter (VLP-2000, Beijing Ranbond Technology Co., Ltd.). When measuring the incident light intensity, ensure that the absorber and the light intensity measuring instrument are at the same height. Mass changes of water were real-time recorded using a high-precision electronic balance (BSA124S, Sartorius, accuracy: 0.1 mg). The temperature distribution of the systems is monitored by an IR camera (FLIR E8 2.0 L).
The evaporation rate (v) is calculated according to the following Eq. (1):
where m refers to the mass of evaporated water, S is the effective illumination of the evaporator area (for the 3D evaporator, the projected area of the upper surface is used) and t is the time of solar evaporator illuminated. The evaporation of the bulk water and the natural evaporation under dark conditions are subtracted from the calculation of the net evaporation rate of the solar evaporator to ensure that the evaporation rate calculated is only contributed by solar illumination.
Data availability
The authors declare that the main data supporting the findings of this study, including experimental procedures, characterization of materials and products, general methods, and NMR spectra, are available within the main text and its Supplementary Information. All data are available from the corresponding author upon request.
References
D’Odorico, P. et al. The global food-energy-water nexus. Rev. Geophys. 56, 456–531 (2018).
He, C. Y. et al. Future global urban water scarcity and potential solutions. Nat. Commun. 12, 4667 (2021).
Wang, W. B. et al. Simultaneous production of fresh water and electricity via multistage solar photovoltaic membrane distillation. Nat. Commun. 10, 3012 (2019).
Yao, Y. J. et al. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat. Sustain. 4, 138–146 (2021).
Alkhadra, M. A., Conforti, K. M., Gao, T., Tian, H. H. & Bazant, M. Z. Continuous separation of radionuclides from contaminated water by shock electrodialysis. Environ. Sci. Technol. 54, 527–536 (2020).
Toyoda, M. & Inagaki, M. Carbon materials for solar steam-generation. Carbon 214, 118373 (2023).
Wang, Y. et al. Stable, cost-effective Tin-based plasmonic nanocomposites with over 99% solar steam generation efficiency. Adv. Funct. Mater. 33, 2212301 (2023).
Lin, X. L. et al. Fully lignocellulosic biomass-based double-layered porous hydrogel for efficient solar steam generation. Adv. Funct. Mater. 32, 2209262 (2022).
Li, W. G., Li, Z., Bertelsmann, K. & Fan, D. E. Portable low-pressure solar steaming-collection unisystem with polypyrrole origamis. Adv. Mater. 31, 1900720 (2019).
Chen, C., Kuang, Y. & Hu, L. Challenges and opportunities for solar evaporation. Joule 3, 683–718 (2019).
Gu, Y. F. et al. Integrated photothermal aerogels with ultrahigh-performance solar steam generation. Nano Energy 74, 104857 (2020).
Zhao, F. et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotech. 13, 489–495 (2018).
Peng, B. L. et al. Phase-separated polyzwitterionic hydrogels with tunable sponge-like structures for stable solar steam generation. Adv. Funct. Mater. 33, 2214045 (2023).
Zhu, L., Gao, M., Peh, C. K. N., Wang, X. & Ho, G. W. Self-contained monolithic carbon sponges for solar-driven interfacial water evaporation distillation and electricity generation. Adv. Energy Mater. 8, 1702149 (2018).
Wang, J., Kong, Y., Liu, Z. & Wang, H. Solar-driven interfacial evaporation: design and application progress of structural evaporators and functional distillers. Nano Energy 108, 108115 (2023).
Hu, Y. et al. A reconfigurable and magnetically responsive assembly for dynamic solar steam generation. Nat. Commun. 13, 4335 (2022).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Xia, Z., Zhao, Y. & Darling, S. B. Covalent organic frameworks for water treatment. Adv. Mater. Interfaces 8, 2001507 (2021).
Yan, X. et al. Superhydrophilic 2D covalent organic frameworks as broadband absorbers for efficient solar steam generation. Angew. Chem. Int. Ed. 61, e202201900 (2022).
Xia, Z. J. et al. Porphyrin Covalent Organic Framework (POF)-Based interface engineering for solar steam generation. Adv. Mater. Interfaces 6, 1900254 (2019).
Jia, S. et al. Freestanding Hydrophilic/Hydrophobic Janus covalent organic framework membranes for highly efficient solar steam generation. ACS Mater. Lett. 5, 458–465 (2023).
Du, J. C. et al. A 2D soft covalent organic framework membrane prepared via a molecular bridge. Adv. Mater. 35, 2300975 (2023).
Mishra, B. & Tripathi, B. P. Flexible covalent organic framework membranes with linear aliphatic amines for enhanced organic solvent nanofiltration. J. Mater. Chem. A 11, 16321–16333 (2023).
Jia, S. et al. A general group-protection synthesis strategy to fabricate covalent organic framework gels. J. Am. Chem. Soc. 145, 26266–26278 (2023).
Li, X. et al. Construction of covalent organic frameworks with alternating rigid and flexible units and their controlled release of active sites. Chem. Eng. J. 454, 140119 (2023).
Zhou, Z. B., Sun, H. H., Qi, Q. Y. & Zhao, X. Gradually tuning the flexibility of two-dimensional covalent organic frameworks via stepwise structural transformation and their flexibility-dependent properties. Angew. Chem. Int. Ed. 62, e202305131 (2023).
Seth, S. & Jhulki, S. Porous flexible frameworks: origins of flexibility and applications. Mater. Horiz. 8, 700–727 (2021).
Liu, X. et al. A crystalline three-dimensional covalent organic framework with flexible building blocks. J. Am. Chem. Soc. 143, 2123–2129 (2021).
Wang, Z. et al. PolyCOFs: a new class of freestanding responsive covalent organic framework membranes with high mechanical performance. ACS Cent. Sci. 5, 1352–1359 (2019).
Hao, K. et al. Cationic flexible organic framework for combination of photodynamic therapy and genetic immunotherapy against tumors. Small 17, 2008125 (2021).
Lin, E. et al. A class of rigid–flexible coupling crystalline crosslinked polymers as vapomechanical actuators. Angew. Chem. Int. Ed. 61, e202117390 (2022).
Yan, J., Zhang, B., Guo, S. & Wang, Z. Porphyrin-based nanoporous organic polymers for adsorption of carbon dioxide, ethane, and methane. ACS Appl. Nano Mater. 4, 10565–10574 (2021).
Bhunia, S. et al. [2,1,3]-Benzothiadiazole-Spaced Co-Porphyrin-Based covalent organic frameworks for O2 reduction. ACS Nano 17, 3492–3505 (2023).
Modak, A., Nandi, M., Mondal, J. & Bhaumik, A. Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem. Commun. 48, 248–250 (2012).
Ji, C., Kang, C., Patra, B. C. & Zhao, D. Flexible covalent organic frameworks: design, synthesis, and applications. CCS Chem. 6, 856–881 (2024).
Sun, J. K., Sobolev, Y. I., Zhang, W., Zhuang, Q. & Grzybowski, B. A. Enhancing crystal growth using polyelectrolyte solutions and shear flow. Nature 579, 73–79 (2020).
Duan, X. et al. Reusable and near-infrared light-activated Zinc(II) metalated porphyrin with synergetic PDT/PTT for eradicating bacterial pneumonia. Chem. Eng. J. 477, 146937 (2023).
Tao, P. et al. Solar-driven interfacial evaporation. Nat. Energy 3, 1031–1041 (2018).
Cancheng, G., Xinftao, H. & Gangyao, Z. A new synthetic method of TPPH2 and It’s derivatives. Chin. J. Org. Chem. 11, 416–419 (1991).
Wang, C. et al. Superhydrophilic porous carbon foam as a self-desalting monolithic solar steam generation device with high energy efficiency. J. Mater. Chem. A 8, 9528–9535 (2020).
Wu, L. et al. Highly efficient three-dimensional solar evaporator for high salinity desalination by localized crystallization. Nat. Commun. 11, 521 (2020).
Zhou, X., Guo, Y., Zhao, F., Shi, W. & Yu, G. Topology-controlled hydration of polymer network in hydrogels for solar-driven wastewater treatment. Adv. Mater. 32, 2007012 (2020).
Guo, Y. et al. Tailoring surface wetting states for ultrafast solar-driven water evaporation. Energy Environ. Sci. 13, 2087–2095 (2020).
Xu, Z. et al. Ultrahigh-efficiency desalination via a thermally-localized multistage solar still. Energy Environ. Sci. 13, 830–839 (2020).
Zou, M. et al. 3D printing a biomimetic bridge-arch solar evaporator for eliminating salt accumulation with desalination and agricultural applications. Adv. Mater. 33, 2102443 (2021).
Li, N. et al. Solar-driven interfacial evaporation and self-powered water wave detection based on an all‐cellulose monolithic design. Adv. Funct. Mater. 31, 2008681 (2021).
Shi, Y., Ilic, O., Atwater, H. A. & Greer, J. R. All-day fresh water harvesting by microstructured hydrogel membranes. Nat. Commun. 12, 2797 (2021).
Sun, Y. et al. High performance carbonized corncob-based 3D solar vapor steam generator enhanced by environmental energy. Carbon 179, 337–347 (2021).
Peng, Y., Zhao, X. Z. & Liu, C. K. Directional solution transfer of a 3D solar evaporator inhibiting salt crystallization. J. Mater. Chem. A 9, 22472–22480 (2021).
Anukunwithaya, P. et al. A self-regenerating 3D sponge evaporator with a tunable porous structure for efficient solar desalination. J. Mater. Chem. A 10, 15743–15751 (2022).
Bu, Y. M. et al. A bioinspired 3D solar evaporator with balanced water supply and evaporation for highly efficient photothermal steam generation. J. Mater. Chem. A 10, 2856–2866 (2022).
Liu, X. H. et al. 3D hydrogel evaporator with vertical radiant vessels breaking the trade-off between thermal localization and salt resistance for solar desalination of high-salinity. Adv. Mater. 34, 2203137 (2022).
He, J. et al. Efficient solar-powered interfacial evaporation, water remediation, and waste conversion based on a tumbler-inspired, all-cellulose, and monolithic design. Adv. Sustain. Syst. 6, 2200256 (2022).
Wang, Y., Li, W., Wei, Y. & Chen, Q. Recyclable monolithic vitrimer foam for high-efficiency solar-driven interfacial evaporation. ACS Appl. Mater. Interfaces 15, 14379–14387 (2023).
Yang, H. et al. High freshwater flux solar desalination via a 3D plasmonic evaporator with an efficient heat-mass evaporation interface. Adv. Mater. 35, 2304699 (2023).
Liu, H. et al. Bioinspired self-standing, self-floating 3D solar evaporators breaking the trade-off between salt cycle and heat localization for continuous seawater desalination. Adv. Mater. 35, 2301596 (2023).
Li, J. et al. Ultrahigh solar vapor evaporation rate of super-hydrophilic aerogel by introducing environmental energy and convective flow. Chem. Eng. J. 466, 143281 (2023).
Yang, B. et al. Flatband λ-Ti3O5 towards extraordinary solar steam generation. Nature 622, 499–506 (2023).
Zhao, G. R. et al. Engineering high-tortuosity 3D gradient structure and CFD-assisted multifield analysis for solar interfacial evaporation. Small 20, 2305855 (2024).
Lei, Z., Hu, B., Zhu, P., Wang, X. & Xu, B. A multilayer mesh porous 3D-felt fabric evaporator with concave array structures for high-performance solar desalination and electricity generation. Nano Energy 122, 109307 (2024).
He, N. et al. Dual-interface solar evaporator with highly-efficient thermal regulation via suspended multilayer design. Small 20, 2402863 (2024).
Zhong, W., Gao, F., Qu, J., Zang, Y. & Jiao, Z. Quantitative tailoring of water state of sulfonated corncob for boosting solar interfacial evaporation and salt resistance. Carbon 224, 119087 (2024).
Zhao, Z. et al. Tortuosity engineering of water channels to customized water supply for enhancing hydrogel solar evaporation. Small 20, 2402482 (2024).
Li, J. et al. Over 10 kg m−2 h−1 Evaporation rate enabled by a 3D interconnected porous carbon foam. Joule 4, 928–937 (2020).
Wang, H. et al. Over 11 kg m–2 h–1 Evaporation rate achieved by cooling metal-organic framework foam with pine needle-like hierarchical structures to subambient temperature. ACS Appl. Mater. Interfaces 14, 10257–10266 (2022).
Acknowledgements
We acknowledge support from the Chinese Academy of Sciences, University of Science and Technology of China and Hefei National Research Center for Physical Sciences at the Microscale, National Key Research and Development Program of China (2021YFA1500402), National Natural Science Foundation of China (NSFC, 21571167, 51502282 and 22075266), Fundamental Research Funds for the Central Universities (WK2060190053 and WK2060190100).
Author information
Authors and Affiliations
Contributions
B.L. supervised the project and conceived the idea. A.H. designed and carried out the experiments and analyzed the structure. Y.Z. and Q.H. contributed to the experimental design. X.L. and C.C. contributed to some NMR experiments. S.C. took water contact angle images of foams. B.L. and A.H. wrote the manuscript. All authors discussed the results and assisted with the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Fang Yu and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, A., Zhao, Y., Hu, Q. et al. Highly efficient solar steam evaporation via elastic polymer covalent organic frameworks monolith. Nat Commun 15, 9484 (2024). https://doi.org/10.1038/s41467-024-53902-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-53902-1
This article is cited by
-
Interfacial evaporation-induced localized multi-field coupling enables efficient co-recovery of freshwater and nitrates
Nature Communications (2026)
-
Dyson sphere-like evaporators enhanced interfacial solar evaporation via self-generated internal convection
Nature Communications (2025)
-
Reconfigurable 3D Stretchable Fabric Evaporator with Spiral Cone Array for Dynamically Matched Solar Intensity and Efficient Desalination
Advanced Fiber Materials (2025)