Abstract
Slow progress towards implementation of conventional clinical bacteriology in low resource settings and strong interest in greater speed for antimicrobial susceptibility testing (AST) more generally has focused attention on next-generation rapid AST technologies. In this Review, we systematically synthesize publications and submissions to regulatory agencies describing technologies that provide phenotypic AST faster than conventional methods. We characterize over ninety technologies in terms of underlying technical innovations, technology readiness level, extent of clinical validation, and time-to-results. This work provides a guide for technology developers and clinical microbiologists to understand the rapid phenotypic AST technology landscape, current development pipeline, and AST-specific validation milestones.
Similar content being viewed by others
Introduction
Inadequate access to clinical bacteriology testing in low resource settings (LRS) impedes management of individual patients, detection of antimicrobial resistance (AMR), and implementation of effective antimicrobial stewardship (AMS) interventions1,2,3. This fuels the overuse of empiric antimicrobials driving AMR, now among the greatest threats facing humanity4. AMR disproportionately affects low-income countries, particularly those in sub-Saharan Africa and South Asia4. Unfortunately, only 1.3% of 50,000 medical laboratories in 14 sub-Saharan countries offered any clinical bacteriology testing as of 2019, owing to multiple factors that thwart scale-up of conventional bacteriology5. These include a requirement for specialized infrastructure, a lack of automation, and inadequate local access to a complex supply-chain which is compounded by foreign exchange distortions in many countries3,6. Diagnostic sectors focused on single diseases like HIV, tuberculosis, malaria, or COVID-19 require a comparatively short list of platforms or supplies7,8. In contrast, clinical bacteriology laboratories (CBL) require a complex supply chain, which can involve hundreds of stable-sourced and quality-assured components. Though frequently inexpensive, they are often unavailable in LRS or cannot be purchased without foreign currency, which itself is often inaccessible. Human resource challenges and a relative lack of granular guidance further complicate CBL implementation.
The cornerstone of clinical bacteriology laboratories is diagnosis of bloodstream infections9. This conventionally involves three sequential processes: detection of bacterial growth, taxonomic identification of isolated bacterial colonies, and antimicrobial susceptibility testing (AST). Detection of bacterial growth in blood culture bottles can take up to 5 days, but often occurs within the first 24 h of incubation10,11. Next, bacterial identification typically takes another 24 h. Finally, AST on pure bacterial colonies typically also requires 4–24 h. Nucleic acid amplification tests (NAAT) have been proposed to accelerate this process. Alas, most genotypic methods currently rely on detecting a limited number of targets in a manner that is neither hypothesis-free nor allows for the detection of the diverse AMR mechanisms across clinical settings. For example, a carbapenemase gene is identifiable in fewer than 50% of bacteria found to be phenotypically carbapenem resistant12. Thus, despite considerable contributions of NAAT-based diagnostics in the fields of tuberculosis, HIV, and malaria, few available technologies have emerged so far as a suitable replacement for conventional bacteriology to yield rapid and accurate AST. Lack of progress towards implementation of conventional clinical bacteriology in low resource settings, as well as interest in greater speed and accuracy for AST more generally, has shifted attention toward next-generation, rapid AST technologies.
In this Review, our aim was to understand the current pipeline of AST technologies and what role they may play in bridging the diagnostic gap currently facing LRS. We performed a scoping review of scientific publications and submissions to regulatory agencies describing technologies capable of providing phenotypic AST in a shorter timeframe than conventional methods. We then characterized the pipeline of identified AST technologies in terms of their underlying technical innovations, technology readiness level (TRL), extent of clinical validation, and standardized expected time-to-results from specimen collection.
Methods
Search strategy
We searched PubMed using the MeSH search terms “Microbial Sensitivity Tests” [MeSH]; “Rapid Diagnostic Tests” [MeSH], “Phenotype” [MeSH] and non-MeSH keywords “antimicrobial sensitivity”, “antimicrobial susceptibility”, “rapid”, “phenotyp*”. The search was last updated in July 2024, and articles from 1946 to present were included. No language limit was applied. We first screened titles and abstracts for relevant information and then used a snowball search strategy to screen reference lists to retrieve other relevant articles.
The FDA 510(k) Premarket Notification and FDA Premarket Approval (PMA) databases were searched with the Classification Product Codes “LON”, “JWY” and “LRG” to find tests falling under the categorization of “system, test, automated, antimicrobial susceptibility, short incubation”, “manual antimicrobial susceptibility test systems”, “instrument for auto reader & interpretation of overnight susceptibility systems” respectively. When a device had multiple submissions, the most recent submission that provided details on the organisms included in the study design was used. We also performed Google searches with the terms “rapid phenotypic antimicrobial susceptibility test”. Finally, several content experts in microbiology and bio/biomedical engineering were consulted to probe for lacunes in our search strategy. When we identified a technology missed by our search, we adapted it to ensure it was captured by the above strategy. We considered as “commercialized” any AST technology with any of the following: FDA authorization, approval, or a pre-market notification; European Economic Area CE marking; or authorization by another WHO-Listed Authority (WLA) if specified by authors. All others were considered “non-commercialized”.
Rapid AST platforms were included if they relied on phenotypic antimicrobial susceptibility profiling of bacteria, regardless of the recognition element used. Rapid technologies were defined as those offering a faster time-to-final-result than those possible with conventional clinical microbiology methods. The latter typically require a minimum of 72 h from specimen collection to final susceptibility results and at least 4 h after the isolation of pure bacterial colonies13. Phenotypic tests were defined as those that measure microbial growth or viability in the presence of antimicrobials to determine susceptibility. Hypothesis-free nucleic acid-based tests were defined as those using genomic recognition elements to detect or quantify bacteria in the presence of different antimicrobial conditions without pre-defined targets. Although they represent a subgroup of “phenotypic tests”, we considered methods using nucleic acid-based recognition elements in a distinct category of technologies to facilitate comparison between them. We only included technologies with publications that specifically addressed their application to phenotypic AST. Finally, we only considered technologies used for non-mycobacterial vegetative bacteria routinely isolated in clinical laboratories.
Data extraction
All titles, abstracts, and full texts in PubMed and FDA databases were screened by one reviewer (GR). In addition, an independent microbiologist reviewer (KH) audited all included publications or technologies and extracted data. Data extracted included relevant information on bacterial testing (including number, source, and species of bacterial isolates, as well as species-antimicrobial combinations tested), specimen characteristics (specimen matrix, number of patients, retrospective/prospective collection), performance metrics as outlined by the Clinical and Laboratory Standards Institute (CLSI) (minimum inhibitory concentration [MIC], essential agreement, categorical agreement, minor and major errors), time-to-result, and a description of the technology.
Identified technologies and comparative frameworks
Number of identified platforms
We identified 81 publications describing non-commercialized AST platforms (67 phenotypic and 14 hypothesis-free nucleic acid-based). We also identified 18 commercialized platforms, with 12/18 having FDA 510(k) clearance and CE marking and 6/18 having only CE marking. Eight commercialized platforms were described in 34 publications either pre- or post-commercialization. Nine technologies were identified external to our literature search in PubMed and the FDA databases via consultation with experts and internet searches (PRISMA diagram, Fig. 1).
A systematic search strategy encompassed PubMed and the FDA 510(k) Premarket Notification and FDA Premarket Approval (PMA) databases. Iterative internet searches and consultation with content experts in microbiology and bio/biomedical engineering were used to probe for lacunes in our search strategy. Technologies were included if they relied on phenotypic antimicrobial susceptibility profiling of bacteria, regardless of the recognition element used. Rapid technologies were defined as those offering a faster time-to-final-result than those possible with conventional clinical microbiology methods. Phenotypic tests were defined as those that measure microbial growth or viability in the presence of antimicrobials to determine susceptibility. Hypothesis-free nucleic acid-based tests were defined as those using genomic recognition elements to detect or quantify bacteria in the presence of different antimicrobial conditions without pre-defined targets. We considered methods using nucleic acid-based recognition elements in a distinct category of technologies to facilitate comparison between them. We only included technologies with publications that specifically addressed their application to phenotypic AST. Finally, we only considered technologies used for non-mycobacterial vegetative bacteria routinely isolated in clinical laboratories. We considered as “commercialized” any AST technology with any of the following: FDA authorization, approval, or a pre-market notification; European Economic Area CE marking; or authorization by another WHO-Listed Authority (WLA) if specified by authors. All others were considered “non-commercialized”.
Technology Readiness Level for antimicrobial susceptibility testing technologies
To understand how close non-commercialized technologies are to commercialization, we created an AST Technology Readiness Level framework (Table 1) adapted from the general Technology Readiness Level (TRL) frameworks from the Government of Canada14 and US Department of Health and Human Services15. We then described the key elements of each commercial, non-commercial phenotypic, and non-commercial hypothesis-free nucleic-acid based technology and classified all identified non-commercial technologies according to the TRL framework (Tables 2, 3, and 4).
Extent of clinical validation of technologies
While the TRL is designed to broadly stage the development of new technologies, it does not provide a direct understanding of the extent of clinical validation or use-case assessment of a given system16. The FDA provides guidelines on the study design necessary for regulatory approval, but early-phase studies are difficult to compare across publications. In contrast to the case for pharmaceutical therapeutics17, no standardized framework currently classifies the diversity of clinical validation studies of AST platforms. Consequently, we developed a framework to type the full spectrum of AST diagnostic studies, building on previous work for diagnostic studies in general18,19. This framework is meant to help to understand the AST diagnostics landscape and may serve to guide subsequent work. Figure 2 shows the Phase of Clinical Validation framework and how studies identified in by our search strategy map onto it.
This framework differs from the Technology Readiness Levels by focusing on the extent of clinical validation available from diagnostic study designs rather than stages of technological development. When a publication described the requirements for multiple phases, it was put only in the highest-level phase. All papers and FDA submissions were included in this figure. All 3b studies were from FDA submissions. One of these did not specify if the organisms were fresh or stock but referenced the FDA Class II Special Controls Document which requires >50% of organisms to be fresh, so it is assumed this criterion was met.
Evaluation of Turn-Around-Time (TAT) from the time of specimen collection
We defined the time interval from clinical specimen collection (e.g. collecting blood or urine from a patient) to final AST results as the most meaningful metric of a test’s Turn-Around-Time (TAT). This is a key parameter to predict the value of novel rapid AST platforms because delayed administration of effective antimicrobials has been repeatedly associated with increased mortality among patients with severe manifestations of sepsis20,21,22. Reports describing technologies we identified frequently cited time-to-result from a different starting point than specimen collection, opacifying direct comparison of claimed TAT between different technologies.
To standardize TAT reporting and provide a level playing field for inter-technology comparisons, we devised a methodology using evidence-based assumptions regarding the average time from specimen collection to a positive blood culture and from specimen collection to pure colony isolation. Figure 3 depicts a standard clinical microbiology workflow and how the TAT of identified technologies align with it. The assumed delays for each step are in line with the daily workflow used in most clinical microbiology laboratories. However, shorter overall TAT are possible with conventional techniques if a continuous workflow eliminates delays caused by “dead time” (i.e. if each step was initiated at the earliest possible moment for each specimen). In practice, this is possible only with fully robotic automation which is deployed in a small number of laboratories at very high initial cost. Most technologies we identified relied on a combination of innovations to reduce overall TAT rather than a single element (Fig. 4).
The conventional workflow shows a timeline of the standard steps from specimen collection to final results for blood culture specimens (A). Cultures of urine can be assumed to require at least 24 h less than the conventional workflow shown for blood specimens. The time from specimen collection to final AST readout is shown for commercialized phenotypic platforms (B) and non-commercialized platforms (C). For commercialized phenotypic platforms, the shortest time was taken as the shortest time reported by the company and the longest time was taken from the longest time reported by the company or from a paper in our review which evaluated the platform. For non-commercialized tests, when a test reported a range of time-to-results, the shortest and longest time were recorded, otherwise only the shortest time was recorded. For tests that reported a time to result from positive blood culture, an assumption of 24 h was used to estimate the time from sample collection to the start of the test. For tests that reported a time to result from colony isolation, an assumption of 48 h was used to estimate the time from sample collection to the start of the test. When a test was performed directly from specimen collection, no assumptions of extra time were added to the total turnaround time. One assay was performed directly on blood46, while the other direct-from-specimen tests were all performed on urine samples. 41/81 non-commercial phenotypic tests were excluded from this graph because they did not report the time from beginning the test to a minimal inhibitory concentration measurement. Graphics created with BioRender.com.
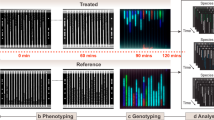
Single-cell bacterial imaging (A) combines optical detection of bacterial growth with small reaction chambers to reduce incubation times, and optimizes geometry for specimen processing. Nano-scale growth chambers are a common feature across several rapid AST technologies. Plasmonic bacterial sensing (B) can enhance the speed and sensitivity of optical readouts. It can be categorized into nanosurface or nanoparticle plasmonic sensing. Nanosurface plasmonic sensing (i) incorporates nano-structured surfaces exhibiting plasmonic structural colours that enhance AST metabolic assay TAT through bright field microscopy. Alternatively, nanosurface plasmonic sensing can utilize surface plasmon resonance (SPR), where a light source illuminates the sensor under-surface through a prism using a wide beam within the range of total internal reflection. The SPR angle shifts in response to changes in the refractive index at the surface of the chip, triggered by the binding of an analyte (e.g. a biomolecule). Nanoparticle plasmonic sensing (ii) employs plasmonic nanoparticles (e.g. gold nanoparticles) for colorimetric AST detection. Changes in the configuration of the nanoparticles (monodispersed or aggregate) yield detectable colour changes (e.g. from red to violet). Different assays integrate plasmonic nanoparticle sensing where the metabolic activity of viable bacterial cells would lead to change of the nanoparticle composition (from monodispersed to aggregate or vice versa) which can be monitored by naked eye or through absorption measurements. Highly-multiplex nucleic acid probe-based bacterial detection (C) uses the incorporation of multiple target-specific probes into replicating bacteria and leverages spectral analysis to assign a quantification and specific identification of bacteria present. Other examples of bacterial probes include the use of isotope labels in Raman-spectroscopy-based methods. Regardless of signal detection method, deep learning is increasingly used for enhanced signal processing. Created with BioRender.com.
Understanding the current rapid AST technology landscape and pipeline
We extend the work of prior narrative reviews by using a rigorous scoping review methodology to perform a comprehensive and reproducible search, encompassing both peer-reviewed scientific literature and submissions to regulatory agencies, to provide an up-to-date understanding of the landscape of available rapid AST technologies and the development pipeline23,24,25. We also expand on the 26 technologies identified in a 2019 landscape analysis on simplified blood culture systems from the Foundation for Innovative New Diagnostics (FIND)26. We identified 120 publications describing over 90 rapid AST technologies promising TAT faster than that currently possible with conventional clinical microbiology methods (81 reporting non-commercialized technologies and 34 describing 9 of the 18 commercially available products). Among non-commercialized technologies, a wide array of phenotypic and hypothesis-free nucleic acid-based AST methods were identified. We proposed AST-adapted frameworks to evaluate and compare Technology Readiness Level and Phase of Clinical Validation for non-commercialized technologies to respectively capture the stage of development of the technology and the extent of published validation with bacterial isolates and clinical specimens. All 18 commercially available technologies were also assessed using the Phase of Clinical Validation framework to characterize the evidence base available for their clinical use.
Commercialized rapid phenotypic AST technologies
Most of the 18 commercialized rapid phenotypic AST technologies we identified combine multiple innovations to shorten TAT (Table 2). Nine commercialized technologies or alternative methods produced by standards organizations circumvent the need to isolate bacteria in pure colonies by providing phenotypic results directly from positive blood culture bottles after a necessary incubation period27,28,29,30,31,32,33,34,35,36 (Fig. 3, Table 2). This reduces TAT by at least 24 h compared to conventional practices. Among these, only 5/9 have undergone Phase 4 clinical validation studies as identified in our search, reflecting that such technologies are yet to be widely evaluated in routine clinical use. Notably, one commercialized technology with regulatory approvals and clinical validation data offers direct-from-specimen phenotypic AST without pre-incubation (PA-100 AST System, SYSMEX Europe SE), though only from urine specimens at this time. This is significant because while final AST results for urine cultures are conventionally available within 48 h of collection, the ~ 44 hr reduction in TAT offered by this method opens the possibility of new use cases at the point of need. Nearly all commercialized technologies we identified represent refinements of microbiology processes that must already be implemented in the first place. For low resource settings without conventional bacteriology services, this means that most such technologies may be very useful but do not inherently provide a means of leapfrogging current obstacles to implementing bacteriology in low- and middle-income countries (LMIC). We have highlighted potential advantages and limitations to implementation of each commercialized technology, recognizing that LMIC versus high-income countries may face different barriers.
The pipeline of non-commercialized rapid phenotypic AST technologies
Methods most frequently employed to decrease time-to-AST results by non-commercialized platforms included novel detection elements, single-cell imaging, miniaturization of growth chambers, and microfluidic assay designs, usually in combination with a minimized incubation time (Tables 3 and 4). However, the majority of these rapid phenotypic AST technologies offered only incremental reductions in TAT compared to the modern clinical microbiology laboratory workflow (Fig. 3). Markedly faster non-commercialized technologies were described in six publications reporting phenotypic results directly from positive blood culture bottles—i.e. blood that has been incubated to culture-amplify infecting bacteria until bacterial growth is detected by an automated system—either clinically obtained positive blood culture specimens (n = 1) or sterile blood spiked with bacterial isolates and then incubated for culture-amplification (n = 5)37,38,39,40,41 (Fig. 3, Tables 3 and 4). The technology reporting AST directly from clinically obtained positive blood culture bottles utilizes a microfluidic assay design with integrated antimicrobial content similar to existing commercial technologies but adds an additional centrifugation component to concentrate the specimen for optimal growth and light microscopy-based imaging37. For reference, two recently commercialized technologies capable of direct-from-positive blood culture bottles results (QuickMIC from Gradientech and dRAST from QuantaMatrix) proceeded from pre-commercialization publications to European market approval in 6–7 years42,43,44,45. Another recently published technology provides results for both species identification and AST directly from patient blood specimens without use of culture-amplification in blood culture bottles; this technology uses a novel bacterial isolation step prior to proceeding, demonstrating a significant advancement in managing a more complex specimen matrix46.
We also identified 7 non-commercialized technologies reporting direct-from-specimen phenotypic AST via MIC measurement. One technology describes AST performed directly from a blood specimen, without need for incubation in a blood culture bottle, and incorporates simultaneous species identification46. The other six of these all used a urine matrix, with 1/6 also provided data using a blood matrix47,48,49,50,51,52,53 (Fig. 3, Tables 3 and 4). All were reported in 2020 or later, with advanced TRL and well-described studies using clinical specimens in 3/750,51 and 1/7 having a moderate-sized prospective clinical study (Phase 2b)51. For the moment, a preponderance of data for direct-from-specimen AST platforms reports performance data for only a few antimicrobials against Enterobacterales spp., as would be expected from urinary tract infections. Data on a broader diversity of drug-organism combinations are needed for clinical use. Of note, diverse technologies were employed in this group, including detection of bacterial metabolic activity during exposure to antimicrobials using deuterium labelling and stimulated Raman spectroscopy with single-cell resolution52,53, visualization of bacterial concentration using flow cytometry and a universal fluorescent dye (without use of nucleic-acid based probes)50, and deep-learning enabled detection of bacterial metabolic activity using the change in reflected light spectrum of a microfluidic cassette nanosurface in combination with resazurin reduction51.
Hypothesis-free nucleic-acid based AST platforms
The capacity of metagenomic sequencing to provide hypothesis-free identification of whole-genome sequences from any putative pathogen has transformed our ability to understand their pathogenesis and epidemiology54. Alas, the diversity of evolving antimicrobial resistance mechanisms in the face of continuous global exposure to antimicrobials leads to an imperfect relationship between genotype and drug susceptibility phenotype. This thwarts genomic sequence-based predictions of AST phenotypes that have sufficient accuracy for patient care decisions, for most drug-organism combinations. Thus, the key parameters to understanding how promising nucleic-acid based technologies are for providing rapid automated AST that are robust across time and geography are (i) whether they offer unbiased detection of bacterial targets and (ii) their ability to determine antimicrobial susceptibility by means other than genomic sequence information alone.
We identified 14 publications describing non-commercialized technologies that employed hypothesis-free nucleic acid-based methods to detect and quantify bacteria exposed to different concentrations of antimicrobials without relying on predetermined resistance genes (Table 5). Among phenotypic nucleic-acid based tests, phenotype was characterized either by nucleic acid amplification-based methods (n = 11), or by quantifying physical interactions with a universal DNA or RNA probe (n = 3). One technology reports AST directly from positive blood culture bottles using blood spiked with bacterial isolates55. Seven publications reported technologies designed for AST directly from urine specimens with six reporting results from clinical urine specimens56,57,58,59,60,61,62.
Finally, a number of technologies we identified claim direct-from-specimen AST for specific bug-drug combinations with an output that reports susceptibility versus resistance only. However, because their description did not report the direct measurement of MIC values, and because the interpretation of MIC values as “susceptible” or “resistant” for a given bacterial isolate to a given drug requires knowledge of the bacterial species63, we did not consider these applicable for general clinical bacteriology laboratory work which is the focus of the present manuscript. However, such technologies may have substantial value for specific use cases such a rapid high throughput screening for infection control and prevention and are included in Tables 3 and 4.
Synthesizing the adequacy of clinical diagnostic accuracy studies of new rAST platforms
Figure 2 outlines a proposed classification for diagnostic validation studies of rapid antimicrobial susceptibility testing platforms. Phase 2 study designs are only pertinent for direct-from-specimen technologies. Conversely, all technologies should undergo some version of Phase 3 studies for regulatory submissions. The design and execution of diagnostic studies is a critical determinant of whether their findings are valid and free from bias. Best practices for diagnostic studies in general have been described in detail18,64,65.
For Phase 2-3 studies describing novel AST technologies, the most widely used metric for concordance between two methods of MIC determination is termed essential agreement (EA) and is defined as the proportion of MIC results from an index method that are within one 2-fold dilution of that achieved with a comparator method. Compared to a reference standard such as broth microdilution, complete validation of new methods for AST determination should achieve EA of 90%, categorical agreement (CA) around susceptibility breakpoints of 90%, and “major”/”very major” errors of < 3%66. The precision around these parameters required by regulatory agencies typically requires testing on > 100 isolates for each bacterial species tested67. The origin of specimens evaluated (reference strains versus clinically derived, stored specimens versus prospectively collected ones) is a key determinant of how robust findings are likely to be in real-world use.
Thus, for commercialized rapid AST platforms with FDA authorization, approval, or a pre-market notification, performance metrics can be assumed to meet the basic standards outlined above for common bacterial species and organism-drug combinations. However, assessment of diagnostic accuracy for individual pre-commercialization technologies is complicated by the heterogeneity of the types of data that are reported. Understanding the study designs used for clinical validation (Fig. 2), and how free of bias their results are likely to be, is important for comparison between technologies in early stages of development. Performance metrics may not be directly comparable for data from different study designs or different generations of prototypes. Similarly, even within a given clinical validation phase, testing accuracy may vary significantly between bacterial species, or for a given species against different antimicrobials. For many studies we identified, diagnostic accuracy is reported for only a subset of bacterial species or antimicrobials that would be required for a regulatory submission. The Supplementary Data 1 outlines the reported performance metrics (categorical agreement, essential agreement, and errors compared to a reference standard method) as well as the combinations of bacterial species and antimicrobials tested of non-commercialized rapid phenotypic AST technologies according to phase of clinical validation, to be interpreted weighing the considerations above. Finally, the most robust data are those that are replicated by independent research groups. This is why Phase 4 studies remain important even after technologies are available commercially, as they provide real-world data on how a technology performs across use cases and its effect on clinical or health-economic outcomes.
Specific challenges facing direct-from-specimen rAST platforms
Once a specimen arrives in the laboratory, the greatest contribution to the overall turnaround-time for all currently used phenotypic AST methods—and for most of those in development—is the time required for culture amplification and colony isolation (Fig. 3). These often dwarf the time required to complete AST testing itself once bacterial colonies are isolated. Thus, rapid AST methods capable of being applied directly on clinical specimens have the potential to leapfrog incremental improvements to platforms that require isolated bacterial colonies.
However, several hurdles complicate the development of direct-from-specimen platforms. Probably the greatest technical challenge to achieving standardized MIC measurements directly-from-clinical-specimens is the lack of knowledge of bacterial concentrations (i.e. the inoculum) at the time of loading specimens into the AST device. Conventional methods work from pure isolated bacterial colonies and rely on standardized inoculum concentrations (usually 5 × 105 CFU mL−1) for accurate and reproducible results68. Using these methods, a 100X increase in inoculum may increase the apparent MIC for some antimicrobials, while lower inoculum may artifactually decrease apparent MIC values. Both of these effects are observed especially with beta-lactam antimicrobials69,70,71. Direct-from-specimen AST platforms accordingly must be robust across a range of bacterial inoculums to be usable. Second, 6/7 direct-from-specimen AST methods we identified focused on urinary clinical specimens. This is undoubtedly because urine is a specimen matrix that is relatively free of proteins, cells and other potential inhibitory substances, compared to blood or stool for example. Moreover, clinically significant concentrations of urinary bacteria (generally considered as at least 105 CFUmL−1 from spontaneously voided first morning urine) are well above the expected limit of detection of most detection methods. In contrast to urine, bacterial concentrations in clinical blood specimens are much lower (typically ~ 100 – 102 CFUmL−1). Thus, while blood cultures are the cornerstone of clinical microbiology laboratories, only 1 direct-from-specimen rapid phenotypic AST platform has been validated for blood. For blood specimens, a short pre-incubation may overcome imperfect analytic sensitivity and facilitate signal detection and inoculum standardization. Alternatively, one group circumvents this by showing that bloodborne bacteria at clinically encountered concentrations can be selectively recovered and subjected to phenotypic AST46.
Beyond direct measurement of MIC, translation of MIC values into categorical interpretation for clinicians (i.e. reporting whether bacteria A is “susceptible” or “resistant” to antimicrobial B) is based on susceptibility breakpoints that are designated for individual species of related groups of bacteria. This means that mature direct-from-specimen rapid AST platforms must be combined with a method for bacterial identification if they do not provide it inherently.
Finally, several testing parameters require optimization for application of such methods to the full range of antimicrobials of interest and the spectrum of pathogenic bacteria that may be encountered in clinical use. The testing medium must be shown to support the growth of both common and fastidious organisms (such as Haemophilus sp. Neisseria sp., and Streptococcus pneumoniae). For antimicrobials that require non-standard concentrations of divalent cations (eg daptomycin) or other physicochemical requirements (e.g. dalbavancin)63, dedicated AST cassettes or media are likely required.
Commercialization is only the first step: obstacles to implementing diagnostics where they are needed
Value-chains for global health diagnostics are complex and fragmented72. Many technical and regulatory challenges must be overcome between proof-of-concept and commercialization of tests with acceptable accuracy, costs, and complexity. In addition, pitfalls especially pertinent to low-resource settings include inadequate evaluation in settings of intended use and weak end-user involvement during development stages. Post-commercialization, a second “valley of death” threatens the successful scale-up of useful technologies73. Hazards leading to product failures in the roll-out phase include lack of focus on demand generation, uncertain cost-effectiveness, and weak engagement of country decision-makers and stakeholders (e.g. professional societies and clinical guideline bodies; legislative bodies shaping health priorities; the financial sector for adequate prioritization of foreign exchange allocations). Several guides have been created to navigate this process74, and it is hoped that new initiatives will bridge the current gaps facing the successful dissemination of critical AMR diagnostics to sub-Saharan Africa and Asia where needs are greatest75,76.
Economic models that support successful post-commercialisation scale-up of diagnostic technologies in low resource settings are a multi-sectoral challenge. Strategies to face it have included supranational pooled procurement mechanisms such as those provided by the Global Fund77, product-development partnerships that support the development and commercialization of priority products throughout their lifecycle78, and the provision target product profiles specifying device parameters and ideal costs for products targeting LMIC markets77. This has been done for blood culture systems, for example26. For the new technologies identified in this review, health-economic studies evaluating different implementation models, evolving technology paradigms, dynamic health ecology, and the value placed on prevention will be required for the optimal deployment of new diagnostic technologies aimed at combatting AMR.
New technologies are looked to in the hopes that they will circumvent some of the obstacles to the implementation of conventional bacteriology in low resource settings. However, the imperative of rapidly implementing some type of phenotypic bacteriology AST in LRS should supersede emphasis on any single technological approach – whether new or conventional.
Conclusion
We synthesized the current AST pipeline and highlight necessary milestones yet to be achieved for individual technologies. Overall, a few recent advances promise rapid and accurate phenotypic AST directly from urine specimens. For blood specimens, only one non-commerical technology offers phenotypic AST without prior culture-amplification. However, robust platforms working directly from positive blood culture bottles are themselves a transformative advance. As expected, there is a relative lack of phase 2-3 clinical studies among the non-commercialized technologies we identified, possibly owing to obstacles facing diagnostic technologies on the path to commercialization79 or the desire not to publish results prior to regulatory submissions. We did not systematically assess the suitability of technologies for low resource settings, but several of the rapid phenotypic AST technologies we identified have the potential to bridge a critical diagnostic gap in settings where the AMR crisis is most acute. With adequate prioritization and incentives from the global AMR community, it is plausible that such platforms could be deployed in the next decade.
Data availability
The data supporting the results of this work are available within the paper and its Supplementary Data 1. The raw datasets generated and analysed during the study are available from the corresponding author upon reasonable request.
Change history
21 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-57678-w
References
Petti, C. A., Polage, C. R., Quinn, T. C., Ronald, A. R. & Sande, M. A. Laboratory medicine in Africa: a barrier to effective health care. Clin. Infect. Dis. 42, 377–382 (2006).
Olmsted, S. S. et al. Strengthening laboratory systems in resource-limited settings. Am. J. Clin. Pathol. 134, 374–380 (2010).
Yansouni, C. P. et al. A feasible laboratory-strengthening intervention yielding a sustainable clinical bacteriology sector to support antimicrobial stewardship in a large referral hospital in ethiopia. Front Public Health 8, 258 (2020).
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
African Society for Laboratory Medicine (ASLM). Incomplete Antimicrobial Resistance (AMR) Data in Africa: The Crisis Within The Crisis. 12 https://aslm.org/wp-content/uploads/2022/09/ASLM_MAAP-Policy-Brief_Embargoed-until-15-Sept-6AM-GMT.pdf?x26552 (2022).
Jacobs, J. et al. Diagnostic bacteriology in district hospitals in sub-saharan Africa: at the forefront of the containment of antimicrobial resistance. Front Med. 6, 205 (2019).
Spira, T., Lindegren, M. L., Ferris, R., Habiyambere, V. & Ellerbrock, T. The WHO/PEPFAR collaboration to prepare an operations manual for HIV prevention, care, and treatment at primary health centers in high-prevalence, resource-constrained settings: defining laboratory services. Am. J. Clin. Pathol. 131, 887–894 (2009).
Abimiku, A. G. Building laboratory infrastructure to support scale-up of HIV/AIDS treatment, CARE, and prevention: in-country experience. OUP Academic 131, 875–886 (2009).
Baron, E. J. et al. Cumitech #1c Blood Cultures IV (ASM Press, Washington, D.C., 2005).
Butler-Laporte, G. et al. Real-world time to positivity of 2 widely used commercial blood culture systems in patients with severe manifestations of sepsis: an analysis of the FABLED study. Open Forum Infect. Dis. 7, ofaa371 (2020).
Mirrett, S., Hanson, K. E. & Reller, L. B. Controlled clinical comparison of versaTREK and BacT/ALERT blood culture systems. J. Clin. Microbiol. 45, 299–302 (2007).
Nordmann, P. & Poirel, L. Epidemiology and diagnostics of carbapenem resistance in gram-negative Bacteria. Clin. Infect. Dis. 69, S521–S528 (2019).
Tabak, Y. P. et al. Blood culture turnaround time in U.S. acute care hospitals and implications for laboratory process optimization. J. Clin. Microbiol 56, e00500–e00518 (2018).
Government of Canada. Technology Readiness Levels. https://ised-isde.canada.ca/site/innovation-canada/en/technology-readiness-levels (2018).
US Department of Health and Human Services. Technology Readiness Levels (TRLs) for Medical Countermeasure Products (Diagnostics and Medical Devices). https://medicalcountermeasures.gov/trl/trls-for-medical-devices/. (2024).
Manning, C. Technology Readiness Levels - NASA. https://www.nasa.gov/directorates/somd/space-communications-navigation-program/technology-readiness-levels/ (2023).
Food and Drug Administration. FDA’s Drug Review Process: Continued. https://www.fda.gov/drugs/ (2019).
Boelaert, M. et al. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nat. Rev. Microbiol 5, S31–S39 (2007).
Yansouni, C. P. et al. Rapid diagnostic tests for neurological infections in central Africa. Lancet Infect. Dis. 13, 546–558 (2013).
Kumar, A. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34, 1589–1596 (2006).
Liu, V. X. et al. The timing of early antibiotics and hospital mortality in Sepsis. Am. J. Respir. Crit. Care Med 196, 856–863 (2017).
Seymour, C. W. et al. Time to treatment and mortality during mandated emergency care for Sepsis. N. Engl. J. Med 376, 2235–2244 (2017).
Gajic, I. et al. Antimicrobial susceptibility testing: a comprehensive review of currently used methods. Antibiotics 11, 427 (2022).
van Belkum, A. et al. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol 18, 299–311 (2020).
Vasala, A., Hytönen, V. P. & Laitinen, O. H. Modern tools for rapid diagnostics of antimicrobial resistance. Front Cell Infect. Microbiol 10, 308 (2020).
Dailey, P. & Osborn, J. Blood Culture: Landscape of Simplified and Integrated Systems for Pathogen Identification and Antimicrobial Susceptibility Testing. https://www.finddx.org (2019).
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Methodology - EUCAST Rapid Antimicrobial Susceptibility Testing (RAST) Directly from Positive Blood Culture Bottles. 8 https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/RAST/2023/EUCAST_RAST_methodology_v4.0_final.pdf (2023).
Q-linea. ASTar Instrument. https://qlinea.com/why-astar/astar/astar-instrument/ (2016).
FASTinov. FASTinov – Ultra-rapid Antibiotic Susceptibility Testing. https://www.fastinov.com/ (2022).
Alifax. Laser Light Scattering. https://www.alifax.com/products/laser-light-scattering/ (2024).
QuantaMatrix Inc. dRAST, Direct & Rapid Antimicrobial Susceptibility Test. https://www.quantamatrix.com/drast/ (2017).
Gradientech A. B. QuickMIC® Ultra-rapid Antibiotic Susceptibility Testing. https://gradientech.se/quickmic/ (2023).
Affinity Biosensors. LifeScale: Innovation in Phenotypic Susceptibility Testing. https://affinitybio.com/ (2023).
Resistell A. G. Resistell AG - Developing Leading Rapid AST Solution. https://resistell.com/ (2023).
bioMérieux. VITEK® REVEALTM, Rapid Antimicrobial Susceptibility Testing. https://www.biomerieux.com/corp/en/our-offer/clinical-products/vitek-reveal.html (2024).
Accelerate Diagnostics Inc. Accelerate Pheno® system. https://acceleratediagnostics.com/products/accelerate-pheno-system/ (2024).
Zhu, M. et al. Integrated microfluidic chip for rapid antimicrobial susceptibility testing directly from positive blood cultures. Anal. Chem. 95, 14375–14383 (2023).
Shi, X., Kadiyala, U., VanEpps, J. S. & Yau, S.-T. Culture-free bacterial detection and identification from blood with rapid, phenotypic, antibiotic susceptibility testing. Sci. Rep. 8, 3416 (2018).
Nix, I. D. et al. Detection of methicillin resistance in staphylococcus aureus from agar cultures and directly from positive blood cultures using MALDI-TOF mass spectrometry-based direct-on-target microdroplet growth assay. Front Microbiol. 11, 232 (2020).
Huang, T.-H., Tzeng, Y.-L. & Dickson, R. M. FAST: Rapid determinations of antibiotic susceptibility phenotypes using label-free cytometry. Cytom. A 93, 639–648 (2018).
Bolotsky, A. et al. Organic redox-active crystalline layers for reagent-free electrochemical antibiotic susceptibility testing (ORACLE-AST). Biosens. Bioelectron. 172, 112615 (2021).
Malmberg, C. et al. A novel microfluidic assay for rapid phenotypic antibiotic susceptibility testing of bacteria detected in clinical blood cultures. PLoS One 11, e0167356 (2016).
Choi, J. et al. Direct, rapid antimicrobial susceptibility test from positive blood cultures based on microscopic imaging analysis. Sci. Rep. 7, 1148 (2017).
Gradientech A. B. Gradientech Achieves CE-IVD Approval for the QuickMIC®system. https://gradientech.se/news/gradientech-achieves-ce-ivd-approval-for-the-quickmicsystem/ (2023).
QuantaMatrix Inc. Rapid Antimicrobial Susceptibility Testing for Sepsis I QuantaMatrix—News & Event. https://www.quantamatrix.com/news/page/2/ (2023).
Kim, T. H. et al. Blood culture-free ultra-rapid antimicrobial susceptibility testing. Nature 632, 1893–902 (2024)
Avesar, J. et al. Rapid phenotypic antimicrobial susceptibility testing using nanoliter arrays. Proc. Natl Acad. Sci. USA 114, E5787–E5795 (2017).
Huang, R. et al. Bioinspired plasmonic nanosensor for on-site antimicrobial susceptibility testing in urine samples. ACS Nano 16, 19229–19239 (2022).
Toosky, M. N. et al. A rapid, point-of-care antibiotic susceptibility test for urinary tract infections. J. Med Microbiol. 69, 52–62 (2020).
Velican, A. M. et al. Rapid detection and antibiotic susceptibility of uropathogenic Escherichia coli by flow cytometry. Microorganisms 8, 1233 (2020).
Mahshid, S., Jalali, M., Abdelwahab, T. & Del Real Mata, C. (2022) US20220372556A1, 24 Nov 2022.
Zhang, M. et al. Rapid determination of antimicrobial susceptibility by stimulated raman Scattering imaging of D(2)O metabolic incorporation in a single bacterium. Adv. Sci. (Weinh.) 7, 2001452 (2020).
Zhang, M., Seleem, M. N. & Cheng, J. X. Rapid antimicrobial susceptibility testing by stimulated raman scattering imaging of deuterium incorporation in a single bacterium. J. Vis. Exp. 180, 10.3791/62398 (2022).
Chiu, C. & Miller, S. Next-generation sequencing. In Molecular Microbiology: Diagnostic Principles and Practice, Vol. 835 (ASM Press, Washington, DC, 2016).
Waldeisen, J. R., Wang, T., Mitra, D. & Lee, L. P. A real-time PCR antibiogram for drug-resistant sepsis. PLoS ONE 6, e28528 (2011).
Altobelli, E. et al. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus 3, 293–299 (2017).
Mach, K. E. et al. A biosensor platform for rapid antimicrobial susceptibility testing directly from clinical samples. J. Urol. 185, 148–153 (2011).
Burg, L. et al. Rapid pathogen identification and phenotypic antimicrobial susceptibility directly from urine specimens. Sci. Rep. 12, 18315 (2022).
Feng, L. et al. Multiplexed and rapid AST for escherichia coli infection by simultaneously pyrosequencing multiple barcodes each specific to an antibiotic exposed to a sample. Anal. Chem. 94, 8633–8641 (2022).
Schoepp, N. G. et al. Rapid pathogen-specific phenotypic antibiotic susceptibility testing using digital LAMP quantification in clinical samples. Sci. Transl. Med 9, eaal3693 (2017).
Schoepp, N. G. et al. Differential DNA accessibility to polymerase enables 30-minute phenotypic β-lactam antibiotic susceptibility testing of carbapenem-resistant Enterobacteriaceae. PLoS Biol. 18, e3000652 (2020).
Athamanolap, P. et al. Nanoarray digital polymerase chain reaction with high-resolution melt for enabling broad bacteria identification and pheno-molecular antimicrobial susceptibility test. Anal. Chem. 91, 12784–12792 (2019).
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. https://clsi.org/media/tc4b1paf/m10033_samplepages-1.pdf (2023).
Leeflang, M. M. G. & Allerberger, F. How to: evaluate a diagnostic test. Clin. Microbiol. Infect. 25, 54–59 (2019).
van Griensven, J., Diro, E. & Yansouni, C. P. Hidden sources of bias in diagnostic studies: the example of visceral leishmaniasis in east Africa. Lancet. Infect. Dis. 23, e108-e114 (2022)
International Organisation for Standardisation. International Organisation for Standardisation. ISO 20776: Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. 19 https://www.iso.org/standard/70464.html (2019).
US Department of Health and Human Services. Class II Special Controls Guidance Document: Antimicrobial Susceptibility Test (AST) Systems. https://www.fda.gov/media/88069/download (2009).
Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Thomson, K. S. & Moland, E. S. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 45, 3548–3554 (2001).
Chambers, H. F. Methicillin-resistant staphylococci. Clin. Microbiol Rev. 1, 173–186 (1988).
Granier, S. A. et al. False susceptibility of Klebsiella oxytoca to some extended-spectrum cephalosporins. J. Antimicrob. Chemother. 50, 303–304 (2002).
McNerney, R. Diagnostics for developing countries. Diagnostics (Basel) 5, 200–209 (2015).
World Economic Forum. The Two ‘Valleys of Death’ That Hold Back the Fight Against TB. https://www.weforum.org/agenda/2018/09/tb-is-the-worlds-deadliest-infectious-disease-we-have-one-shot-to-stop-it/ (2018).
U.S. Agency for International Development. Idea to Impact: A Guide to Introduction and Scale | Center for Innovation and Impact. https://www.usaid.gov/cii/guide-introduction-and-scale (2023).
Mfuh, K. O., Abanda, N. N. & Titanji, B. K. Strengthening diagnostic capacity in Africa as a key pillar of public health and pandemic preparedness. PLOS Glob. Public Health 3, e0001998 (2023).
de Oliveira, T. & Baxter, C. Investing in Africa’s scientific future. Science 383, eadn4168 (2024).
WHO. Links to WHO TPPs and PPCs. https://www.who.int/observatories/global-observatory-on-health-research-and-development/analyses-and-syntheses/target-product-profile/links-to-who-tpps-and-ppcs (2024).
AMR test directory. FIND https://www.finddx.org/tools-and-resources/dxconnect/test-directories/amr-test-directory/ (2024).
O’Neill, J. Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics / The Review on Antimicrobial Resistance Chaired by Jim O’Neill. Wellcome Collection https://wellcomecollection.org/works/gcrdafjx/items (2015).
Food and Drug Administration. Antimicrobial Susceptibility Test (AST) Systems - Class II Special Controls Guidance for Industry and FDA. https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/antimicrobial-susceptibility-test-ast-systems-class-ii-special-controls-guidance-industry-and-fda (2023).
Food and Drug Administration. ETEST 510(k) Substantial Equivalence Determination Decision Summary Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K210757.pdf (2023).
Food and Drug Administration. MTS 510(k) Substantial Equivalence Determination Decision Summary Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K211672.pdf (2023).
Food and Drug Administration. ComASP 510(k) Substantial Equivalence Determination Decision Summary Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K230479.pdf (2023).
Food and Drug Administration. VITEK 2 510(k) Substantial Equivalence Determination Decision Memorandum Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K161227.pdf. (2024).
Food and Drug Administration. BD Pheonix 510(k) Substantial Equivalence Determination Decision Summary Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K173252.pdf (2023).
Food and Drug Administration. Sensititre AST Plates 510(k) Substantial Equivalence Determination Decision Summary Assay Only. https://www.accessdata.fda.gov/cdrh_docs/reviews/K232310.pdf (2024).
Food and Drug Administration. DxM MicroScan WalkAway ID/AST System 510(k) Substantial Equivalence Determination Decision Summary. https://www.accessdata.fda.gov/cdrh_docs/reviews/K192355.pdf (2024).
TSA. Antibiogrammes – lutter contre la RAM grâce au diagnostic | TSA. https://www.sysmex.fr/produits/diagnostics/antibiogramme/ (2024).
Food and Drug Administration. Selux Next Generation Phenotyping System. https://www.accessdata.fda.gov/cdrh_docs/pdf21/K211748.pdf (2023).
MacFadden, D. R. et al. Using genetic distance from archived samples for the prediction of antibiotic resistance in Escherichia coli. Antimicrob. Agents Chemother. 64, e02417 (2020).
Pesesky, M. W. et al. Evaluation of machine learning and rules-based approaches for predicting antimicrobial resistance profiles in gram-negative bacilli from whole genome sequence data. Front Microbiol 7, 1887 (2016).
Chen, L. et al. A capillary-based centrifugal indicator equipped with in situ pathogenic bacteria culture for fast antimicrobial susceptibility testing. Analyst 149, 2420–2427 (2024).
Kadlec, M. W., You, D., Liao, J. C. & Wong, P. K. A cell phone-based microphotometric system for rapid antimicrobial susceptibility testing. J. Lab Autom. 19, 258–266 (2014).
Carneiro, M. D. S., Volpato, F. C. Z., Wilhelm, C. M., Wink, P. L. & Barth, A. L. Evaluation of early reading of broth microdilution technique for polymyxin B. Micro. Drug Resist 29, 59–64 (2023).
Sordo, M. et al. Rapid culture-based LNZ test for detection of linezolid susceptibility/resistance in staphylococci and enterococci. Diagn. Microbiol Infect. Dis. 107, 116058 (2023).
Veses-Garcia, M. et al. Rapid phenotypic antibiotic susceptibility testing of uropathogens using optical signal analysis on the nanowell slide. Front Microbiol 9, 1530 (2018).
Nguyen, A. V. et al. Ladder-shaped microfluidic system for rapid antibiotic susceptibility testing. Commun. Eng. 2, 15 (2023).
Sever, E. A., Aybakan, E., Beşli, Y., Karatuna, O. & Kocagoz, T. A novel rapid bioluminescence-based antimicrobial susceptibility testing method based on adenosine triphosphate consumption. Front Microbiol 15, 1357680 (2024).
Kállai, A. et al. MICy: a novel flow cytometric method for rapid determination of minimal inhibitory concentration. Microbiol Spectr. 9, e0090121 (2021).
Sawada, T., Katayama, M., Takatani, S. & Ohiro, Y. Early detection of drug-resistant Streptococcus pneumoniae and Haemophilus influenzae by quantitative flow cytometry. Sci. Rep. 11, 2873 (2021).
Busche, J. F., Möller, S., Stehr, M. & Dietzel, A. Cross-flow filtration of Escherichia coli at a nanofluidic gap for fast immobilization and antibiotic susceptibility testing. Micromachines (Basel) 10, 691 (2019).
Flentie, K. et al. Microplate-based surface area assay for rapid phenotypic antibiotic susceptibility testing. Sci. Rep. 9, 237 (2019).
Kang, W., Sarkar, S., Lin, Z. S., McKenney, S. & Konry, T. Ultrafast parallelized microfluidic platform for antimicrobial susceptibility testing of gram positive and negative bacteria. Anal. Chem. 91, 6242–6249 (2019).
Sun, H. et al. Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid, low-cost antimicrobial susceptibility test. Lab Chip 19, 2915–2924 (2019).
Kaushik, A. M. et al. Accelerating bacterial growth detection and antimicrobial susceptibility assessment in integrated picoliter droplet platform. Biosens. Bioelectron. 97, 260–266 (2017).
Kalashnikov, M., Lee, J. C., Campbell, J., Sharon, A. & Sauer-Budge, A. F. A microfluidic platform for rapid, stress-induced antibiotic susceptibility testing of Staphylococcus aureus. Lab Chip 12, 4523–4532 (2012).
Koskinen, J. O. et al. Development of a rapid assay methodology for antimicrobial susceptibility testing of Staphylococcus aureus. Diagn. Microbiol Infect. Dis. 62, 306–316 (2008).
Bellali, S. et al. Antimicrobial susceptibility testing for gram positive cocci towards vancomycin using scanning electron microscopy. Curr. Res Micro. Sci. 3, 100154 (2022).
Wistrand-Yuen, P. et al. A multiplex fluidic chip for rapid phenotypic antibiotic susceptibility testing. mBio 11, e03109–e03119 (2020).
Mo, M. et al. Rapid antimicrobial susceptibility testing of patient urine samples using large volume free-solution light scattering microscopy. Anal. Chem. 91, 10164–10171 (2019).
Kals, M., Mancini, L., Kotar, J., Donald, A. & Cicuta, P. Multipad agarose plate: a rapid and high-throughput approach for antibiotic susceptibility testing. J. R. Soc. Interface 21, 20230730 (2024).
Li, C. et al. Under-oil open microfluidic systems for rapid phenotypic antimicrobial susceptibility testing. Lab Chip 23, 2005–2015 (2023).
Kandavalli, V., Karempudi, P., Larsson, J. & Elf, J. Rapid antibiotic susceptibility testing and species identification for mixed samples. Nat. Commun. 13, 6215 (2022).
Li, X. et al. Combinatorial screening SlipChip for rapid phenotypic antimicrobial susceptibility testing. Lab Chip 22, 3952–3960 (2022).
Sklavounos, A. A., Nemr, C. R., Kelley, S. O. & Wheeler, A. R. Bacterial classification and antibiotic susceptibility testing on an integrated microfluidic platform. Lab Chip 21, 4208–4222 (2021).
Song, X., Tian, L., Zou, H. & Sun, H. Analysis of the clinical diagnosis data of four experimental detection methods for pediatric syphilis. Minerva Pediatr. 71, 144–149 (2019).
Song, D., Liu, H., Ji, H. & Lei, Y. Whole slide imaging for high-throughput sensing antibiotic resistance at single-bacterium level and its application to rapid antibiotic susceptibility testing. Molecules 24, 2441 (2019).
Cansizoglu, M. F., Tamer, Y. T., Farid, M., Koh, A. Y. & Toprak, E. Rapid ultrasensitive detection platform for antimicrobial susceptibility testing. PLoS Biol. 17, e3000291 (2019).
Idelevich, E. A. et al. Rapid phenotypic detection of microbial resistance in gram-positive bacteria by a real-time laser scattering method. Front Microbiol. 8, 1064 (2017).
Baltekin, Ö., Boucharin, A., Tano, E., Andersson, D. I. & Elf, J. Antibiotic susceptibility testing in less than 30 min using direct single-cell imaging. Proc. Natl Acad. Sci. USA 114, 9170–9175 (2017).
Liu, X. et al. High-throughput screening of antibiotic-resistant bacteria in picodroplets. Lab Chip 16, 1636–1643 (2016).
Syal, K. et al. Antimicrobial susceptibility test with plasmonic imaging and tracking of single bacterial motions on nanometer scale. ACS Nano 10, 845–852 (2016).
Price, C. S., Kon, S. E. & Metzger, S. Rapid antibiotic susceptibility phenotypic characterization of Staphylococcus aureus using automated microscopy of small numbers of cells. J. Microbiol Methods 98, 50–58 (2014).
Burnham, C.-A. D., Frobel, R. A., Herrera, M. L. & Wickes, B. L. Rapid ertapenem susceptibility testing and Klebsiella pneumoniae carbapenemase phenotype detection in Klebsiella pneumoniae isolates by use of automated microscopy of immobilized live bacterial cells. J. Clin. Microbiol 52, 982–986 (2014).
Bennett, I., Pyne, A. L. B. & McKendry, R. A. Cantilever sensors for rapid optical antimicrobial sensitivity testing. ACS Sens 5, 3133–3139 (2020).
Heuer, C. et al. A 3D-printed microfluidic gradient generator with integrated photonic silicon sensors for rapid antimicrobial susceptibility testing. Lab Chip 22, 4950–4961 (2022).
Busche, J. F. et al. Nanofluidic immobilization and growth detection of Escherichia coli in a chip for antibiotic susceptibility testing. Biosens. (Basel) 10, 135 (2020).
Leonard, H., Halachmi, S., Ben-Dov, N., Nativ, O. & Segal, E. Unraveling antimicrobial susceptibility of bacterial networks on micropillar architectures using intrinsic phase-shift spectroscopy. ACS Nano 11, 6167–6177 (2017).
Zhou, K. et al. Dynamic laser speckle imaging meets machine learning to enable rapid antibacterial susceptibility testing (DyRAST). ACS Sens 5, 3140–3149 (2020).
Riester, O., Kaiser, L., Laufer, S. & Deigner, H.-P. Rapid phenotypic antibiotics susceptibility analysis by a 3D printed prototype. Adv. Sci. 11, 2308806 (2024).
Rafiee, Z., Rezaie, M. & Choi, S. Combined electrical-electrochemical phenotypic profiling of antibiotic susceptibility of in vitro biofilm models. Analyst 149, 3224–3235 (2024).
Hannah, S. et al. Development of a rapid, antimicrobial susceptibility test for E. coli based on low-cost, screen-printed electrodes. Biosens. (Basel) 10, 153 (2020).
Yi, X. et al. Development of a fast raman-assisted antibiotic susceptibility test (FRAST) for the antibiotic resistance analysis of clinical urine and blood samples. Anal. Chem. 93, 5098–5106 (2021).
Bauer, D. et al. Heteroresistant bacteria detected by an extended raman-based antibiotic susceptibility test. Anal. Chem. 92, 8722–8731 (2020).
Crane, B. et al. Rapid antibiotic susceptibility testing using resazurin bulk modified screen-printed electrochemical sensing platforms. Analyst 146, 5574–5583 (2021).
Idelevich, E. A., Sparbier, K., Kostrzewa, M. & Becker, K. Rapid detection of antibiotic resistance by MALDI-TOF mass spectrometry using a novel direct-on-target microdroplet growth assay. Clin. Microbiol. Infect. 24, 738–743 (2018)
Fande, S., Amreen, K., Sriram, D., Mateev, V. & Goel, S. Electromicrofluidic device for interference-free rapid antibiotic susceptibility testing of Escherichia coli from real samples. Sens. (Basel) 23, 9314 (2023).
Dadwal, R. et al. Stable isotope labeling as a promising tool for rapid drug susceptibility testing in Neisseria gonorrhoeae. Braz. J. Microbiol 54, 1819–1825 (2023).
Dixon, B., Ahmed, W. M., Mohamed, A. A., Felton, T. & Fowler, S. J. Metabolic phenotyping of acquired ampicillin resistance using microbial volatiles from Escherichia coli cultures. J. Appl Microbiol 133, 2445–2456 (2022).
Wang, J. et al. Rapid antimicrobial susceptibility testing based on a bio-inspired chemiluminescence sensor. Anal. Chem. 94, 17240–17247 (2022).
Verma, T., Annappa, H., Singh, S., Umapathy, S. & Nandi, D. Profiling antibiotic resistance in Escherichia coli strains displaying differential antibiotic susceptibilities using Raman spectroscopy. J. Biophotonics 14, e202000231 (2021).
Idelevich, E. A. et al. Rapid simultaneous testing of multiple antibiotics by the MALDI-TOF MS direct-on-target microdroplet growth assay. Diagnostics (Basel) 11, 1803 (2021).
Thrift, W. J. et al. Deep learning analysis of vibrational spectra of bacterial lysate for rapid antimicrobial susceptibility testing. ACS Nano 14, 15336–15348 (2020).
Wang, R. et al. cAST: Capillary-based platform for real-time phenotypic antimicrobial susceptibility testing. Anal. Chem. 92, 2731–2738 (2020).
Novelli-Rousseau, A. et al. Culture-free antibiotic-susceptibility determination from single-bacterium Raman spectra. Sci. Rep. 8, 3957 (2018).
Entenza, J. M. et al. Rapid detection of Staphylococcus aureus strains with reduced susceptibility to vancomycin by isothermal microcalorimetry. J. Clin. Microbiol 52, 180–186 (2014).
Chen, J. et al. Feasibility and potential significance of rapid in vitro qualitative phenotypic antimicrobial susceptibility testing of gram-negative bacilli with the ProMax system. PLoS One 16, e0249203 (2021).
Schoepp, N. G. et al. Digital quantification of DNA replication and chromosome segregation enables determination of antimicrobial susceptibility after only 15 Min of antibiotic exposure. Angew. Chem. Int Ed. Engl. 55, 9557–9561 (2016).
Tjandra, K. C., Ram-Mohan, N., Abe, R., Wang, T.-H. & Yang, S. Rapid molecular phenotypic antimicrobial susceptibility test for neisseria gonorrhoeae based on propidium monoazide viability PCR. ACS Infect. Dis. 9, 1160–1167 (2023).
Athamanolap, P., Hsieh, K. & Wang, A. T.-H. Integrated bacterial identification and antimicrobial susceptibility testing for polymicrobial infections using digital PCR and digital high-resolution melt in a microfluidic array platform. Annu Int Conf. IEEE Eng. Med. Biol. Soc. 2018, 5346–5349 (2018).
Rolain, J. M., Mallet, M. N., Fournier, P. E. & Raoult, D. Real-time PCR for universal antibiotic susceptibility testing. J. Antimicrob. Chemother. 54, 538–541 (2004).
Mezger, A. et al. A general method for rapid determination of antibiotic susceptibility and species in bacterial infections. J. Clin. Microbiol 53, 425–432 (2015).
Acknowledgements
C.P.Y., M.P.C. and D.N. hold Clinician-Scholar career awards from the Fonds de recherche du Québec—Santé (FRQS). S.M. acknowledges financial support from the Canada Research Chair Program and Pfizer Canada (263259 Pfizer Can/MUHC).
Author information
Authors and Affiliations
Contributions
G.R., K.H. and C.P.Y. conceived the review and wrote the first draft. G.R. and K.H. performed the literature search and performed data extraction. M.P.C., J.P., and D.N. contributed to elaboration of the TRL and clinical validation frameworks. S.M. and T.A. assisted with technology descriptions. R.C., C.C., and the authors above contributed to the iterations of the search strategy, data presentation, figure design, and intellectual content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests. S.M., D.N., and C.P.Y. co-supervised the work in the publication describing the PhenEXA system. C.P.Y. reports being on an Independent Data Monitoring Committees (IDMC) for Medicago Inc. and InventVacc Biologicals Inc., unrelated to the submitted work. M.P.C. reports personal fees from GEn1E Lifesciences (as a member of the scientific advisory board), personal fees from nplex biosciences (as a member of the scientific advisory board), outside the submitted work. He is the co-founder of Kanvas Biosciences and owns equity in the company. In addition, M.P.C. has a patent Methods for detecting tissue damage, graft versus host disease, and infections using cell-free DNA profiling pending, and a patent Methods for assessing the severity and progression of SARS-CoV-2 infections using cell-free DNA pending. J.P. reports grants from MedImmune, Merck; personal fees from Astra-Zeneca and Merck, all outside the submitted work. All other Authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wilbur Lam, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Reszetnik, G., Hammond, K., Mahshid, S. et al. Next-generation rapid phenotypic antimicrobial susceptibility testing. Nat Commun 15, 9719 (2024). https://doi.org/10.1038/s41467-024-53930-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-53930-x
This article is cited by
-
Ultra-rapid nanoplasmonic colorimetry in microfluidics for antimicrobial susceptibility testing directly from specimens
Nature Nanotechnology (2026)
-
Culture-free detection of bacteria from blood for rapid sepsis diagnosis
npj Digital Medicine (2025)
-
Rapid antimicrobial susceptibility tests performed by self-diluting microfluidic chips for drug resistance studies and point-of-care diagnostics
Microsystems & Nanoengineering (2025)
-
Rapid diagnosis of urinary tract infection with miniaturised point-of-care cultivation on a dipstick
European Journal of Clinical Microbiology & Infectious Diseases (2025)
-
Clinical and metagenomic predicted antimicrobial resistance in pediatric critically ill patients with infectious diseases in a single center of Zhejiang
Annals of Clinical Microbiology and Antimicrobials (2024)