Abstract
The chemoselectivity of organic reactions is a fundamental topic in organic chemistry. In the long history of chemical synthesis, achieving chemoselectivity is mainly limited to thermodynamic conditions by an exogenous activation strategy. Here, we design an endogenous activation method, which can be used to control the chemoselectivity of phenol and naphthol through the photo-induced excited-state intramolecular proton transfer (ESIPT). A wavelength-tuned glycosylation is developed to showcase the penitential of this new strategy. Traditionally, an exogenous activator (electrophilic promoters) is essential to induce the cleave of a polar single bond, and this strategy has been extensively studied and used in the glycosylation chemistry, for the formation of oxocarbenium cation intermediate. In our systems, the oxocarbenium cation intermediates can be selectively formed from glycosyl donors bearing tunable chromophoric groups under mild conditions of acid-base free and redox neutrality, which enables continuous synthesis of oligosaccharides.
Similar content being viewed by others
Introduction
The essence of chemical synthesis is the process of bond cleavage and formation, which depends on the in-depth understanding and precise control of chemoselectivity at the molecular scale1. The realization of chemical selectivity can improve synthetic efficiency by directly approaching the target molecules, avoiding adjustment to a circuitous multi-step transformations or using protecting groups. Traditionally, chemoselectivity can be achieved by the thermodynamic conditions (differential reagents, temperature, and chemical environments)2. It’s still a forbidden challenge to distinguish and control the chemoselectivity of multiple similar functional groups such as phenol and naphthol within a complex molecule or a reaction system.
pKa value is a fundamental data to denote the acidity of a molecule, which is widely used to judge its ability to donate protons3,4. Regarding to phenol groups, its pKa value changes along with the electronic properties of substituted groups on the aromatic ring (Fig. 1a), that is difficult to tune their reactivities, with close pKa value under the ground-state conditions. When the photo energy is absorbed by the π-system of phenol, it will dramatically improve its acidity on photochemical excitation by 6∼7 units (ΔpK = pK(S1)–pK(S0))5, and excited-state proton transfer will occur from phenol to other functional groups inter- (ESPT) or intramolecularly (ESIPT) (1 → 2a or 2b, Fig. 1a). This photo phenomenal has been extensively studied and used in the design of fluorescent emission materials6 and fluorescence sensors7, and the excited protons can be well controlled to transfer to hereo-atoms (N, S, O) through ESIPT (Fig. 1a). Yates and coworkers discovered the first example of an ESIPT from phenol to carbon (alkene or alkyne) in the exploration of the photohydrations of aromatic alkenes and alkynes8,9, and carbocations (or o-quinone methide) and vinyl cations were proposed to be the key intermediates in the following water trapping steps. Surprisingly, ESIPT was much less explored in the field of synthetic chemistry, which limited to few reaction developments by Wan10,11,12,13,14,15 and Kutateladze group16,17,18 and synthetic applications by Porco group19,20,21.
We asked whether the similar functional groups, such as phenols, can be distinguished and selectively activated under excited-state by using light of different energies. In order to achieve this goal, there are two critical factors need to be considered. First, a tunable chromophoric group is required to absorb particular wavelengths of light, which could be rationalized according to the Woodward-Fieser rules22,23,24 by tuning the relationship between UV-Visible spectroscopy and its structural properties. Second, energy must be transferred to specific functional groups, which lead to the selective bond cleavage and formation. This rational design will undoubtedly expand the mode of chemoselectivity and provide new ideas for synthetic design in both single-step transformations and cascade reactions. To demonstrate this proposal, we pursued the combination of photo-induced ESIPT with carbocation chemistry, and report herein the wavelength-tuned chemoselective glycosylation.
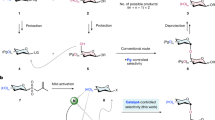
Carbocation, one of the most commonly used reactive species25,26, is generally formed by heterolysis of a covalent bond or electrophilic addition to an unsaturated bond. An exogenous activator (electrophilic promoters such as Lewis or Brønsted acids) is essential to induce the cleave of a polar single bond or addition to a π–bond. This strategy has been extensively studied and used in the glycosylation chemistry, for the formation of oxocarbenium cation intermediate 5 (3 → 4 → 5, Fig. 1b)27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45. Given that the photo-excited protons can quickly migrate to the carbon atoms of ortho–π–system, we were intrigued by the possibilities to utilize this newly-resulted carbocation or its resonance as the precursor for the generation of oxocarbenium. Accordingly, an endogenous activation process was designed, wherein a phenol group was installed as the light-triggered activator in the leaving group of a glycosyl donor (Fig. 1c). Under the irradiation of particular wavelength of light, 6 might undergo ESIPT to form a highly active carbocation 7, which was intramolecularly captured by the carbonyl group of ester, and induce the C–O bond cleavage to form the oxocarbenium cation 5. After reaction with a nucleophilic acceptor, a glycosidic bond was formed and the dissociated fragment 8 was protonated to give 10. This strategy can generate oxocarbenium cations under mild conditions of acid-base free and redox neutrality, which is expected to improve the atomic economy and functional groups tolerance. In principle, the chemoselectivity of this glycosylation can be easily tuned and controlled by the photo properties of the leaving groups. Just changing the wavelength of light, the specific glycosylation could be initiated. This advantage provides a new solution for the preparation of oligosaccharides through a cascade strategy (Fig. 1d). Oligosaccharide, such as 16, could be synthesized through multiple continuous glycosylations from glycosyl donors/acceptors bearing different chromophoric groups, such as 11 and 12, in a one-pot reaction system (11 → 14 → 16, Fig. 1d).
Two fundamental challenges need to be addressed in this photo-induced glycosylation. The first is whether the carbocation center, such as 7, is reactive enough to be captured by the ortho-carbonyl group. Second, how to control the compete reactions between the nucleophilic acceptor and the dissociated leaving fragment, such as 10.
Results
Reaction development
With the concept and synthetic plan in mind, we designed and synthesized five glycosyl donors 17–21 with respect to properties of chromophoric groups and the stability of the carbocation intermediates. Accordingly, phenols (17–20) or naphthol (21) were introduced to initiate ESIPT, and olefins were installed at their ortho-positions as the proton acceptors. Substituents of different electron densities on olefins can also be used to tune the conjugation properties of leaving group. The UV-Vis absorption spectra of 17–20 was first measured, and no significant difference between mono- and disubstituted olefin donors 17 and 18 (λmax ~ 300 nm, Fig. 2a) was observed. When the methyl group in compound 18 was replaced by phenyl or naphthalenyl moiety, 19 and 20 exhibited similar photo properties (Fig. 2b). As expected, when naphthol was introduced, the absorption wavelength of 21 has a significant redshift (λmax ~ 360 nm, Fig. 2c).
a UV-Vis spectrum of glycosyl donors 17 and 18. b UV-Vis spectrum of glycosyl donors 19 and 20. c UV-Vis spectrum of glycosyl donors 18 and 20. d Glycosylations using 17–21 as glycosyl donors and methanol as a glycosyl acceptor. e Model glycosylation reaction using 18 as a glycosyl donor and 1-adamantanol (24) as a glycosyl acceptor. Yields and diastereomeric ratios were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. M: mol/L.
We began to investigate the glycosylation reaction of 17–21 using solvent amount of methanol as acceptor (Fig. 2d). Ultraviolet (UV) irradiation (λmax = 300 nm) of a mixture of methanol/1,2-dichloroethane (1:4) solution of 17 for 2 h led the formation of 17a (d.r. = 1:1.1) in 95% yield instead of the desired methyl glycoside. This suggested that the benzyl cation species or its resonance was generated, which was intermolecularly trapped by methanol rather than the intramolecular trapping by the carbonyl of ester group. Alternatively, the photo-induced glycosylation reactions of 18–20 occurred smoothly and gave a mixture of α- and β-glycoside 18a, as well as the corresponding departure fragments (10, 22 and 23) in reasonable yields. We also tested the glycosyl donor 21 under 366 nm irradiation, and found the target product 18a was produced in a high yield. The β-bias of compound 18a is due to the solvent effect of methanol, and the single crystal of its leaving fragment 13 was obtained to confirm the formation of lactone ring. Interestingly, when 21 was irradiated with a UV light of 300 nm, the reaction rate became slower (18 h) and the yield decreased to 50%. This phenomenal indicated that the reactivity of the substrate can be influenced by the energy of photo source, which was consistent with the recent studies by Sarpong’s group46.
Considering the stability of the naphthol-based donor at room temperature and its ease of preparation, we optimized the reaction conditions using glycosyl donor 18 and acceptor adamantanol 24 as model substrates (Fig. 2e). Anhydrous solvent is crucial to this glycosylation, and 4 Å molecular sieve was found to be a necessary additive to secure a reliable yield (Fig. 2e, entry 1). Without molecular sieve, the product yield reduced to 60% due to the competing nucleophilic addition by water (Fig. 2e, entry 2). When the light source was removed or changed to 366 nm, the glycosylation reaction does not occur (Fig. 2e, entry 3–4). We further optimized photolysis conditions by screening solvents (Fig. 2e, entry 5–6) and reaction concentration (Fig. 2e, entry 7–8) in the presence of 4 Å MS. No better results were achieved and the reaction yields slightly decreased.
Substrate scope of single-step glycosylation
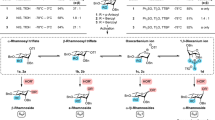
With the optimal conditions for the glycosylation reaction in hand, we then turned out to test the generality of this photo induced glycosylation with respect to: the diversity of glycosyl donors and the properties of acceptors. Using glycosyl 3-hydroxy-2-isopropenylbenzoates (HPB) and glycosyl 4-hydroxy-3-isopropenyl-2-naphthoates (HPN) as donors (Fig. 3), we planned to examine various monosaccharide units bearing different protecting groups, including the D-glucosyl (18, 21, 25–27), D-galactosyl (28–32) and D-mannosyl donors (33–36). We were pleased to observe that a wide range of alcoholic acceptors including primary, secondary, sterically hindered tertiary hydroxyl groups, and sugar alcohols were smoothly coupled with the perbenzyl glucosyl HPB donor 18, affording the desired glycosides (18b-d) and disaccharide (18e) in good yields. As a comparison, photolysis of glucosyl HPN donor 21 in the presence of adamantanol 24 under 366 nm UV light generated 18b with similar yield. Glycosylation of the alcoholic acceptors with 25 proceeded smoothly to give the desired O-glucosides and disaccharides 25a-g in good yields and complete β-selectivity with resort to the anchimeric assistance by O − 2 pivaloyl group. Steroidal alcohol works well as a suitable nucleophile (25e). Taking the monosaccharide, bearing primary and secondary hydroxyls, as the glycosyl acceptor, selective glycosylation occurred with the primary group (25 g). Compared with 25, donor 26 can only produce β-selective product 26a in moderate yield. During the glycosylation of donor 25 and 26, we detected the formation of intermediates through TLC, but only hydrolysates could be isolated. We speculated that orthoester intermediates were first generated during the reaction, and then gradually rearranged into final products in the system. In addition, we investigated the reactivity of nucleoside receptor with donor 26. Under the standard conditions, only 45% of the yield of product 26b could be obtained. We found that pre-silylation of nucleoside receptor with MSTFA can significantly improve the solubility and activity of the reaction, and the reaction yield can reach 65%. It’s worth noting that perbenzoyl donor 27 did not react with amantadanol under ultraviolet light at 300 nm, indicating that this glycosylation protocol is sensitive to the electron deficient substituents on the donors. In addition, we also replaced the anomeric oxygen atom with the more nucleophilic sulfur atom to try to improve its reactivity, but the effect is still limited, and the glycosyl donor gradually decomposed without the formation of the target product. Next, we studied galactosyl donors (28–32), bearing stereo-hindered silyl or cyclic-silyl protecting groups and pivaloyl group. A variety of alcohol acceptors could be glycosylated with good reaction efficiency (28a-c, 30a-g, 30i, 31a-b).
Besides the primary, secondary and tertiary aliphatic alcohols, we were pleased to discover that carboxylic acids (30 h, 31c), phenols (31d-f), N- (31 g, 31 h) or S-acceptors (31i) are competent nucleophiles in this reaction, affording the desired glucosides in good-to-excellent yields. Of note, the stereoselectivity of glycosylation with donors 30 and 31 was well controlled, which produced the target β- and α- glucosides respectively47. The only exception was the glycoside 31c, which was obtained as a mixture of α- and β-configurations. It was speculated that the strong acidity and nucleophilicity of the benzoic acid acceptor lead the SN1 and SN2 nucleophilic substitution proceed simultaneously. Comparing the glycosides 31e and 31 f, we found that the methyl groups close to the hydroxyl group had a significant adverse effect on the reaction yield, indicating that the reaction efficiency was highly dependent on the steric hindrance of nucleophilic substrates. This also provides hints to understand why the leaving fragment, such as 10, 22, 23, did not participate in the glycosylation reaction. Finally, bearing silyl–protecting group (33, 34) and perbenzyl mannosyl donors (35) were also investigated and proved to be viable for the construction glycosides and disaccharides. Tertiary alcohol (33a, 35a) and primary (35b) and secondary (35c) sugar alcohols were good coupling partners to afford the intended products with moderate to good yields. For the diacetonide donor 36, the disaccharide product 36a could be obtained with a yield of 45% under standard reaction conditions, but with almost complete α-selectivity. It is speculated that the conformation of the intermediates by the ions composed of glyoxyonium ions and departing fragment anions can affect the attack direction of the nucleophiles, and thus generate the corresponding glycosylation products.
Multiple continuous glycosylation
Given that the HPB and HPN donors have distinct maximum absorption wavelengths, we were intrigued by the opportunities for the wavelength regulation of glycosyl chemoselectivity and multiple continuous glycosylation in one-pot operation. Competition experiments were firstly conducted with HPN and HPB donors (Fig. 4a). A solution of 34 and 31 in a mixed solvent of methanol and DCE was irradiated under the particular UV light. We found when 366 nm was used as the excitation wavelength, excellent selectivity was achieved for HPN donor 34, producing glycoside 34a in 74% yield, while HPB donor 31 was almost completely recovered. When the same reaction mixture was irradiated with 300 nm light, although the conversion of HPN donor 34 (46%) could not be avoided, the reaction still showed a significant bias to the HPB donor 31 (78% conversion), and the glycoside 31j was obtained in 52% yield.
We then performed the multiple continuous glycosylations to synthesis of oligosaccharides (Fig. 4b). Our synthesis commenced with glycosyl HPN donor 32 and disarmed HPB glycoside 37, under the irradiation of 366 nm light, which produced the coupled α-disaccharide 38 in 61% yield, while the unconverted acceptor 37 was recovered. It was found that the reaction time (18 h) of this step was significantly increased comparing with that in Fig. 3. We attribute the reduced reaction rate to the energy transfer48,49,50 between HPN donor 32 and HPB glycoside 37. In order to avoid the influence of the dissociated naphthol fragment 13 on the HPB donor 38, we performed the second glycosylation with the purified 38 and acceptor 39, under the irradiation at 300 nm, providing the trisaccharide 40 (α:β = 1:1) in 55% yield. Furthermore, trisaccharide 44 (α:β = 1:1) was efficiently prepared, applying the same reaction sequence, from the glycosyl HPN donor 32β (or 32), glucosyl HPB 41 and acceptor 43. Particularly noteworthy is this chemoselectivity is elusive to achieve through thermodynamic conditions, reflecting the uniqueness of wavelength-tuned glycosylation.
Mechanistic insights
To gain a deeper understanding of the reaction mechanism, we firstly performed the control experiments (Fig. 5a). When the HPB donor 31 was directly photolysis without nucleophile, the glycoside 31k, containing the departed chromophoric fragment, could be assembled in 28% yield. Alternatively, only a trace amount of 31k was generated when the glycosylation was performed in the presence of an equimolar quantity of phenol acceptor 45, and 31e was achieved in 65% yield. This result illustrates that the large steric hindrance effect on the ortho-position of the phenol group dramatically influences the nucleophilicity of the leaving fragment, which facilitates the competitive glycosylation reactions with the added acceptors.
We then carried out the deuteration reaction experiments (Fig. 5b). The deuterated product 46 and the departure fragment 47 containing mono-deuterated methyl were obtained from the glycosyl HPB donor by irradiation with 300 nm light for 6 h with the acceptor of deuterated methanol in solvent. Irradiation of mono-substituted olefin glycoside 17 with 300 nm light for 3 h, afforded a deuterated product through the expected ESIPT process as the formation of 17a (Fig. S8 in Supplementary Information). However, irradiation of glycosyl HPN donor 21 in the presence of deuterated methanol, not only yielded the expected deuterated glycoside 46, but also gave the departing species 48 bearing multiple deuterated sites. The two methyl groups of 48 have a deuteration ratio of 75% on average, and the proton on para-position of the phenol group was also partially deuterated. This important information reminded us that multiple and reversable deuterium migrations occurred for donor 21 before the dissociation of the chromophoric fragment. To better understand this photoreaction, we measured the quantum yield of the HPB and HPN donors to 0.11 and 0.039, respectively.
Based on these reactions, we proposed the mechanisms regarding to two types of donors (Fig. 5c, d). For compound 17, ESIPT occurs smoothly under UV irradiation to form the carbocation intermediate 49, which quickly forms quinone methides 50 and 51 through an equilibrium. The steric effect of 51 decreases the degree of its conjugation. We envisioned that the intramolecular trapping of transient species 50 or 51 by ester carbonyl groups becomes inert and incapable due to the orbital prohibition. This provides opportunities for the intermolecular attack by the methanol molecules, which lead the formation of 17a. For HPB and HPN donor, a relatively stable tertiary carbocation intermediate 7 and its resonance 52 were generated through the same ESIPT process. Due to the steric hindrance of gem-dimethyl group, it’s difficult for quinone methide 52 to exist in a form of planar structure, which results the rapid equilibrium inclined to carbocation 7 as the reactive species and captured by ortho-ester carbonyl group intramolecularly. Additionally, the congested chemical environments of the tertiary carbocation and tetrasubstituted olefins also prevents the intermolecular attack by the added nucleophiles. Our studies also revealed that the rate of intramolecular capture of the carbonyl group was relatively slow, the transient intermediates 7 and 52 might return to the ground state HPN donor through the ground state proton transfer (GSPT) or ground state hydrogen transfer (GSHT) process51 according to the results of deuteration experiments. Then, oxocarbenium cation 5 were formed, which faces competitive reaction pathways with anion species 8 or additional nucleophiles. Based on what we observed in the control experiments (Fig. 5a), the coupling reaction with the added acceptor wins the competition to generate the desired glycoside 9.
Discussion
In summary, we developed an endogenous activation strategy to control the chemoselectivity through the photo-induced excited-state intramolecular proton transfer (ESIPT). As an application in synthetic chemistry, the highly active oxocarbenium cation intermediate can be selectively formed from glycosyl donors to construct glycosyl bonds. The ESIPT provides new solutions to distinguish the hydroxyl groups with similar pKa values by introduction tunable chromophoric groups. The glycosylation can easily be initiated through a wavelength-tuned photolysis, without exogenous activator such as metal catalysts or acids. This advantage enables a mild reaction condition, functional group tolerance and broad reaction scope, which was showcased in the single-step (39 cases in Fig. 3) and multiple glycosylations (Fig. 4b). We anticipate that this study will give new life for the ESIPT and promote its developments and applications in the field of synthetic chemistry.
Methods
General procedure for glycosylation reactions
To a quartz tube was added glycosyl donor (0.15 mmol), glycosyl acceptor (0.1 mmol) and a magnetic stirrer, then added activated molecular sieves (200 mg) to the tube in glove box, and sealed with a rubber plug. Then anhydrous 1,2-dichloroethane (2 mL) was added under nitrogen. The solution was stirred and irradiated by a 300 nm or 366 nm LED photoreactor (9 W) at room temperature for 8 h. When the reaction is completed monitored by thin layer chromatography, filtered out the molecular sieves with a sand core funnel. The filtrate was directly removed in vacuo and the residue was purified by silica-gel chromatography (0–20% ethyl acetate – petroleum ether) to afford the desired products.
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2328975 (13) and 2328974 (31). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data discussed in this paper are available in the main text or Supplementary Information. All data are available from the corresponding author upon request.
References
Trost, B. M. Selectivity: A Key to Synthetic Efficiency. Science 219, 245–250 (1983).
Shenvi, R. A., O’Malley, D. P. & Baran, P. S. Chemoselectivity: The Mother of Invention in Total Synthesis. Acc. Chem. Res. 42, 530–541 (2009).
Xue, X.-S., Ji, P., Zhou, B. & Cheng, J.-P. The Essential Role of Bond Energetics in C−H Activation/ Functionalization. Chem. Rev. 117, 8622–8648 (2017).
Yang, J.-D., Ji, P., Xue, X.-S. & Cheng, J.-P. Recent Advances and Advisable Applications of Bond Energetics in Organic Chemistry. J. Am. Chem. Soc. 140, 8611–8623 (2018).
Ireland, J. F. & Wyatt, P. A. H. Acid-Base Properties of Electronically Excited States of Organic Molecules. Adv. Phys. Org. Chem. 12, 131–221 (1976).
Padalkar, V. S. & Seki, S. Excited-state intramolecular proton-transfer (ESIPT)-inspired solid state emitters. Chem. Soc. Rev. 45, 169–202 (2016).
Sedgwick, A. C. et al. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 47, 8842–8880 (2018).
Wan, P., Culshaw, S. & Yates, K. Photohydration of Aromatic Alkenes and Alkynes. J. Am. Chem. Soc. 104, 2509–2515 (1982).
Isaks, M., Yates, K. & Kalanderopoulos, P. Photohydration via Intramolecular Proton Transfer to Carbon in Electronically Excited States. J. Am. Chem. Soc. 106, 2728–2730 (1984).
Diao, L., Yang, C. & Wan, P. Quinone Methide Intermediates from the Photolysis of Hydroxybenzyl Alcohols in Aqueous Solution. J. Am. Chem. Soc. 117, 5369–5370 (1995).
Fischer, M. & Wan, P. m-Quinone Methides from m-Hydroxy-1,1-Diaryl Alkenes via Excited-State (Formal) Intramolecular Proton Transfer Mediated by a Water Trimer. J. Am. Chem. Soc. 120, 2680–2681 (1998).
Foster, K. L., Baker, S., Brousmiche, D. W. & Wan, P. o-Quinone methide formation from excited state intramolecular proton transfer (ESIPT) in an o-hydroxystyrene. J. Photochem. Photobiol. A: Chem. 117, 157–163 (1999).
Lukeman, M. & Wan, P. A New Type of Excited-State Intramolecular Proton Transfer: Proton Transfer from Phenol OH to a Carbon Atom of an Aromatic Ring Observed for 2-Phenylphenol. J. Am. Chem. Soc. 124, 9458–9464 (2002).
Lukeman, M. & Wan, P. Excited-State Intramolecular Proton Transfer in o-Hydroxybiaryls: A New Route to Dihydroaromatic Compounds. J. Am. Chem. Soc. 125, 1164–1165 (2003).
Flegel, M., Lukeman, M., Huck, L. & Wan, P. Photoaddition of Water and Alcohols to the Anthracene Moiety of 9-(2′-Hydroxyphenyl)anthracene via Formal Excited State Intramolecular Proton Transfer. J. Am. Chem. Soc. 126, 7890–7897 (2004).
Mukhina, O. A. et al. Rapid Photoassisted Access to N,O,S-Polyheterocycles with Benzoazocine and Hydroquinoline Cores: Intramolecular Cycloadditions of Photogenerated Azaxylylenes. Angew. Chem. Int. Ed. 50, 9423–9428 (2011).
Mukhina, O. A. & Kutateladze, A. G. Oxazolines as Dual-Function Traceless Chromophores and Chiral Auxiliaries: Enantioselective Photoassisted Synthesis of Polyheterocyclic Ketones. J. Am. Chem. Soc. 138, 2110–2113 (2016).
Kuznetsov, D. M. & Kutateladze, A. G. Step-Economical Photoassisted Diversity-Oriented Synthesis: Sustaining Cascade Photoreactions in Oxalyl Anilides to Access Complex Polyheterocyclic Molecular Architectures. J. Am. Chem. Soc. 139, 16584–16590 (2017).
Gerard, B., Jones, G. II & Porco, J. A. Jr. A Biomimetic Approach to the Rocaglamides Employing Photogeneration of Oxidopyryliums Derived from 3-Hydroxyflavones. J. Am. Chem. Soc. 126, 13620–13621 (2004).
Gerard, B., Cencic, R., Pelletier, J. & Porco, J. A. Jr. Enantioselective Synthesis of the Complex Rocaglate (−)-Silvestrol. Angew. Chem. Int. Ed. 46, 7831–7834 (2007).
Wen, S., Boyce, J. H., Kandappa, S. K., Sivaguru, J. & Porco, J. A. Jr. Regiodivergent Photocyclization of Dearomatized Acylphloroglucinols: Asymmetric Syntheses of (−)-Nemorosone and (−)−6-epi-Garcimultiflorone A. J. Am. Chem. Soc. 141, 11315–11321 (2019).
Woodward, R. B. Structure and the Absorption Spectra of α,β-Unsaturated Ketones. J. Am. Chem. Soc. 63, 1123–1126 (1941).
Liljefors, T. & Allinger, N. L. Conformational Analysis. 128. The Woodward-Fieser rules and α,β-Unsaturated Ketones. J. Am. Chem. Soc. 100, 1068–1073 (1978).
Joung, J. F., Han, M., Jeong, M. & Park, S. Beyond Woodward–Fieser Rules: Design Principles of Property-Oriented Chromophores Based on Explainable Deep Learning Optical Spectroscopy. J. Chem. Inf. Model. 62, 2933–2942 (2022).
Olah, G. A. 100 Years of Carbocations and Their Significance in Chemistry. J. Org. Chem. 66, 5943–5957 (2001).
Aue, D. H. Carbocations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 1, 487–508 (2011).
Demchenko, A. V. Handbook of Chemical Glycosylation. (Wiley, 2008).
Werz, D. B. & Vidal, S. Modern Synthetic Methods in Carbohydrate Chemistry: From Monosaccharides to Complex Glycoconjugates (Wiley, 2014).
Zhao, G. & Wang, T. Angew Stereoselective Synthesis of 2-Deoxyglycosides from Glycals by Visible-Light-Induced Photoacid Catalysis. Angew. Chem. Int. Ed. 57, 6120 (2018).
Zhang, J. et al. Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors. Nat. Commun. 14, 8025 (2023).
Martin, A. et al. Catching elusive glycosyl cations in a condensed phase with HF/SbF5 superacid. Nat. Chem. 8, 186–191 (2016).
Nielsen, M. M. & Pedersen, C. M. Catalytic Glycosylations in Oligosaccharide Synthesis. Chem. Rev. 118, 8285–8358 (2018).
Kulkarni, S. S. et al. One-Pot” Protection, Glycosylation, and Protection−Glycosylation Strategies of Carbohydrates. Chem. Rev. 118, 8025–8104 (2018).
Yang, Y. & Yu, B. Recent Advances in the Chemical Synthesis of C‑Glycosides. Chem. Rev. 117, 12281–12356 (2017).
Li, G. et al. Recent Advances in Protection-Free Glycosylations. Chin. J. Org. Chem. 43, 1991–2001 (2023).
Park, Y. et al. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 355, 162–166 (2017).
Li, Q., Levi, S. M., Wagen, C. C., Wendlandt, A. E. & Jacobsen, E. N. Site-selective, stereocontrolled glycosylation of minimally protected sugars. Nature 608, 74–79 (2022).
Yu, B. Gold(I)-Catalyzed Glycosylation with Glycosyl o‑Alkynylbenzoates as Donors. Acc. Chem. Res. 51, 507–516 (2018).
Yao, W. et al. Automated solution-phase multiplicative synthesis of complex glycans up to a 1,080-mer. Nat. Synth. 1, 854–863 (2022).
Liu, H. et al. ortho-Methoxycarbonylethynylphenyl Thioglycosides (MCEPTs): Versatile Glycosyl Donors Enabled by Electron-Withdrawing Substituents and Catalyzed by Gold(I) or Cu(II) Complexes. J. Am. Chem. Soc. 145, 3682–3695 (2023).
Xiao, X. et al. One-Pot Relay Glycosylation. J. Am. Chem. Soc. 142, 5498–5503 (2020).
Li, P. et al. Glycosyl ortho-(1-phenylvinyl)benzoates versatile glycosyl donors for highly efficient synthesis of both O-glycosides and nucleosides. Nat. Commun. 11, 405 (2020).
Wu, L. et al. Precision native polysaccharides from living polymerization of anhydrosugars. Nat. Chem. 15, 1276–1284 (2023).
Zhang, C. et al. Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation. Nat. Chem. 14, 686–694 (2022).
Ding, H., Lyu, J., Zhang, X.-L., Xiao, X. & Liu, X.-W. Efficient and versatile formation of glycosidic bonds via catalytic strain-release glycosylation with glycosyl ortho−2,2- dimethoxycarbonylcyclopropylbenzoate donors. Nat. Commun. 14, 4010 (2023).
Kim, S. F. et al. Mechanistic Investigation, Wavelength-Dependent Reactivity, and Expanded Reactivity of N–Aryl Azacycle Photomediated Ring Contractions. J. Am. Chem. Soc. 146, 5580–5596 (2024).
Imamura, A. et al. Di-tert-butylsilylene (DTBS) group-directed α-selective galactosylation unaffected by C-2 participating functionalities. Tetrahedron Lett. 44, 6725–6728 (2003).
Sauer, M., Hofkens, J. & Enderlein, J. Excited State Energy Transfer. (Wiley, 2011)
Wu, L. et al. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 49, 5110–5139 (2020).
Großkopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 122, 1626–1653 (2022).
Guo, Y., Li, X., Ma, J. & Phillips, D. L. Reaction Mechanisms of Photoinduced Quinone Methide Intermediates Formed via Excited-State Intramolecular Proton Transfer or Water-Assisted Excited-State Proton Transfer of 4-(2-Hydroxyphenyl) pyridine. J. Phys. Chem. Lett. 12, 11666–11672 (2021).
Acknowledgements
We thank X. Zhao (ECNU) for assisting with the XRD data collection. S.G. is supported by National Natural Science Foundation of China (22225105; 22171089); Program of Shanghai Science and Technology Committee (22JC1401101). M.H. is supported by National Natural Science Foundation of China (22201076); China Postdoctoral Science Foundation (2022M711158).
Author information
Authors and Affiliations
Contributions
S.G. conceived and directed the execution of the study. M.H. and X.J. performed all experiments. G.W. and H.H. contributed to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Qian Wan and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, M., Jing, X., Wen, G. et al. Catalyst-free and wavelength-tuned glycosylation based on excited-state intramolecular proton transfer. Nat Commun 15, 9661 (2024). https://doi.org/10.1038/s41467-024-54020-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54020-8