Abstract
Direct oxidation of KA oil (the mixture of cyclohexanone and cyclohexanol) toward ε-caprolactone is in high demand yet hard to implement in need of juggling the activation of both methyne C-H bond of cyclohexanol and α-C-C bond of cyclohexanone. Here we demonstrate that in situ formed Cu1+δ-Oδ-• active site, which originates from relay reaction at Ni(II) and Cu(I) pairs in a metal-organic framework (known as NiCu-MOF-74) with O2 and benzaldehyde (PhCHO), efficiently oxidizes KA oil toward ɛ-caprolactone along with good stability. Mechanism investigation discloses that the auxiliary Ni(II) site first adsorbs O2 for abstracting formyl hydrogen in PhCHO followed by transfer of PhCO· to react with another O2 over the major Cu(I) site, leading to formation of Cu1+δ-Oδ-• and PhCOOH. This major-auxiliary cooperative strategy will be particularly suitable for multivariate MOFs as next generation catalysts towards complex reactions.
Similar content being viewed by others
Introduction
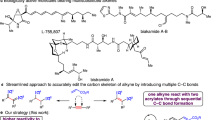
ε-Caprolactone has been widely used as the feedstocks in the preparation of many key polymers, such as polycaprolactone, polyurethane and nylon-61,2. Consequently, the market size of ε-caprolactone has reached $271.3 million by 2022 along with an increasing rate of ~7% in recent years3. The major industrial synthesis of ε-caprolactone is carried out through the Baeyer-Villiger oxidation of cyclohexanone in a homogeneous system with peracid produced from carboxylic acid and hydrogen peroxide (Fig. 1a, the right part)4. And, cyclohexanone is mainly obtained from the industrial KA oil (the mixture of cyclohexanone and cyclohexanol) (Fig. 1a, the left part)5,6,7. Hence, beyond using pure cyclohexanone as the substrate, the preparation of ε-caprolactone directly from KA oil without need of additional separation and purification holds great potential thanks to efficient and cost-saving merits.
a Scheme of industrial synthesis of KA oil and subsequent rectification to obtain cyclohexanone as the source for industrial preparation of ε-caprolactone. b Sequential oxidation of cyclohexanol and cyclohexanone to ε-caprolactone. c Biocatalytic cascade oxidation of cyclohexanol to ε-caprolactone by combining an alcohol dehydrogenase (ADH) with a cyclohexanone monooxygenase (CHMO). d Relay reaction at Ni(II) and Cu(I) pairs in MOF-74 for in situ generating key active species to achieve one-pot cascade oxidation of cyclohexanol to ε-caprolactone.
The oxidation of cyclohexanol toward cyclohexanone experiences dehydrogenation, which is usually realized by metal catalysts under O2 atmosphere (Fig. 1b, Supplementary Table 1)8,9,10. As comparison, the oxidation of cyclohexanone toward ε-caprolactone undergoes nucleophilic addition and elimination in various catalytic systems, i.e., hydrogen peroxide over Lewis acid catalysts or O2/aldehyde over redox catalysts (Fig. 1b, Supplementary Table 2)11,12,13. Hence, these two reactions have been operated separately under varied condition. An exceptional work was reported on cascade oxidation of cyclohexanol toward ε-caprolactone by combining an alcohol dehydrogenase (ADH) with a cyclohexanone monooxygenase (CHMO) at 25 °C (Fig. 1c)14, but it suffered from high cost, poor environmental tolerance and low durability15. In addition, a homogeneous catalyst Ce(NH4)2(NO3)6 was employed for catalytic cascade oxidation of KA oil with a co-catalyst N-hydroxyphthalimide under O2 atmosphere at 45 °C16, while a heterogenous heteropoly acid Cs2.5H0.5PW12O40 was developed for catalytic oxidation of cyclohexanol to ɛ-caprolactone with the use of hydrogen peroxide at 90 °C17; however, both catalysts exhibited the low yields of ɛ-caprolactone. Altogether, one-pot catalytic oxidation of KA oil toward ɛ-caprolactone is rarely reported, and it remains highly challenging for efficiently implementing this tandem oxidation reaction, especially by using heterogeneous catalysts.
The difficulty originates from the fact that the active sites of traditional heterogeneous catalysts are often characteristic of the fixed geometric and electronic structures, and thus their availability is too limited to promote the complex or multi-step reactions18,19. Distinct from those, multivariate metal-organic frameworks (MOFs) are known as a tool library for precisely accommodating polymetallic nodes in individual units while possessing the rich flexibility of adapting the redox behaviors of local metal sites without structural collapse20,21,22. We suppose that the multivariate MOFs are unique for multiple metal synergy to in situ generate transient active sites, which enable catalyzing the challenging reactions23. Among various MOFs, MOF-74 synthesized by reacting divalent metal ions with 2,5-dihydroxyterephthalic acid (DHTA) presents specific advantages including abundant substrate-accessible channels and coordinatively unsaturated metal sites24,25,26. Furthermore, multivariate MOF-74 has been recognized as an ideal system for introducing heterogeneity while maintaining order, for instance, our recent work indicated that Cu(I) as the redox site was incorporated into the MOF-74 framework to modulate the neighboring Co(II) site for enhancing selective aerobic epoxidation of alkenes27. Nevertheless, the intricate correlations between multiple metal sites and complex reactions remain unexplored in multivariate MOFs. In this work, we show utilizing the relay reaction at Cu(I) and Ni(II) pairs in NiCu-MOF-74 with ambient O2 and benzaldehyde (PhCHO) to reconstruct the active Cu(I) sites via the major-auxiliary cooperative strategy, thus facilitating oxidization of KA oil to ɛ-caprolactone (Fig. 1d and Supplementary Fig. 1).
Results
Synthesis and characterization of multivariate NiCu-MOF-74
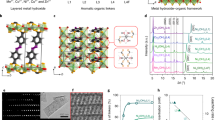
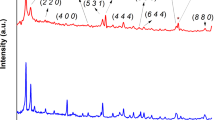
We synthesized multivariate MOF-74 containing Ni and Cu nodes by reacting different feed molar ratios of Ni(NO3)2·6H2O to Cu(NO3)2·3H2O with DHTA in a mixed solution of ethanol, N,N-dimethylformamide (DMF) and deionized water with volume ratio of 1:15: 1 at 105 °C27,28. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) determines that the molar ratios of Ni to Cu are 1:0, 0.9:0.1, 0.7:0.3, 0.5:0.5, 0.3:0.7, 0.1:0.9, and 0:1 for as-synthesized seven samples (Supplementary Table 3), respectively, denoted as Ni-MOF-74, Ni9/10Cu1/10-MOF-74, Ni7/10Cu3/10-MOF-74, Ni1/2Cu1/2-MOF-74, Ni3/10Cu7/10-MOF-74, Ni1/10Cu9/10-MOF-74 and Cu-MOF-74. Transmission electron microscopy (TEM) images display that all the samples are of the aggregated spindle-like shape (Supplementary Figs. 2-8), and the corresponding element mapping imaging manifests that both Ni and Cu elements are uniformly distributed in the whole framework29. Powder X-ray diffraction (XRD) patterns indicate that all the samples possess almost the same crystal phase with the simulated MOF-74 (Fig. 2a and Supplementary Fig. 9)30, but the slight shift of characteristic (110) and (300) peaks is observed28. To further understand the influence of multivariate nodes on the crystalline structure, a full-profile Pawley refinement for each MOF-74 sample is performed (Supplementary Fig. 10 and Supplementary Table 4). Evidently, the experimental data are in consistence with the refined data of MOF-74, belonging to trigonal R-3 space group characteristic of the cell parameters a = b ≠ c, α = β = 90° and γ = 120°. The refined values of cell parameters a, c, and cell volume V0 indicate that with the increasing of Ni/Cu ratio in MOF-74, the value of cell parameter a increases from 25.80 to 26.06 owing to the different ionic radius of Ni and Cu ions31, meanwhile the value of cell parameter c decreases from 6.81 to 6.28 due to the Jahn-Teller distortion effect of Cu(II) with d9 configuration32.
To evaluate the porosity of the MOF-74 samples, the N2 sorption isotherms are conducted (Supplementary Fig. 11 and Supplementary Table 5). The specific surface area is 1061 m2 g−1 for Ni-MOF-74, 1005 m2 g−1 for Ni9/10Cu1/10-MOF-74, 945 m2 g−1 for Ni7/10Cu3/10-MOF-74, 892 m2 g−1 for Ni1/2Cu1/2-MOF-74, 752 m2 g−1 for Ni3/10Cu7/10-MOF-74, 697 m2 g−1 for Ni1/10Cu9/10-MOF-74 and 554 m2 g−1 for Cu-MOF-74, while the average pore size of all the samples is kept at around 1.0 nm33. Note that the average pore size of MOF-74 is larger than the kinetic diameters of 5.39 Å for cyclohexanol, 5.37 Å for cyclohexanone, and 5.63 Å for ε-caprolactone (Supplementary Table 6), thus leading to the facile mass transfer and diffusion of substrates and products during the catalytic oxidation process34.
The existence form of Ni and Cu in multivariate MOF-74 is discerned by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge structure (XANES) analysis. The binding energy of Ni 2p3/2 in XPS spectra is centered at 856.9 eV for all the Ni-containing samples, revealing its divalent nature (Fig. 2b)35. As for Cu 2p spectra of various NiCu-MOF-74, the characteristic peaks at 935.4 eV and 933.4 eV are ascribed to Cu(II) and Cu(I) sites (Fig. 2c)22, respectively. Similar results are obtained with XANES spectra (Supplementary Figs. 12 and 13). Interestingly, the ratio of Ni(II), Cu(II) and Cu(I) is modulated in certain range by altering the content of Ni and Cu in MOF-74 samples (Fig. 2d, Supplementary Fig. 14, and Supplementary Table 7). The coordination state of Ni and Cu is further distinguished by extended X-ray absorption fine structure (EXAFS) survey. The k3-weighted Ni K-edge EXAFS spectra in R space (without phase correction) exhibit the dominant peak at 1.8–2.0 Å (Supplementary Figs. 15−17, Supplementary Table 8), representing the first coordination shell of Ni-O bond36. The corresponding theoretical simulation uncovers 5-coordinated feature, consistent with the theoretical model of Ni sites coordinated with 3 carboxylic O and 2 phenolic O atoms25. Distinctively, the average coordination number of Cu-O in the first shell around Cu sites gradually declines from 5.1 to 4.1 along with the change of Cu(I)/Cu(II) ratio from 0.19 to 1.27 upon increasing the content of Ni in NiCu-MOF-74 (Fig. 2d, Supplementary Figs. 18–20, and Supplementary Table 9).
In addition to the content of Ni and Cu in MOF-74, other possible factors for influencing the molar ratio of Cu(I) to Cu(II) in MOF-74 are explored. First, our recent work disclosed that the formation of Cu(I) in MOF-74 resulted from the partial reduction of Cu(II) to Cu(I) by DMF rather than ethanol and DHTA (Fig. 3a)27. Without use of DMF during synthesis, the obtained Cu-MOF-74 and Ni7/10Cu3/10-MOF-74 samples are characteristic of Cu(II) sites, denoted as CuII-MOF-74 and Ni7/10CuII3/10-MOF-74 (Supplementary Figs. 21–24, Supplementary Table 7). Then, we investigate the influence of the volume ratio of mixed solvents of ethanol, DMF, and water on the molar ratio of Cu(I) to Cu(II) in MOF-74 by choosing a feed-in Ni/Cu ratio of 7:3 as the model at 105 °C. Note that the Ni/Cu ratio of all as-obtained NiCu-MOF-74 samples is close to the feed-in ratios, and meanwhile, the ratio of Cu(I) to Cu(II) is 0.71, 0.94, 1.22 and 0.88 when solvent volume ratio of ethanol, DMF and water is 1:16:0, 1:15.5:0.5, 1:15:1 and 1:14:2 in turn (Supplementary Fig. 25 and Supplementary Table 10). Thus, the solvent volume ratio of 1:15:1 is favorable for generating a higher molar ratio of Cu(I) to Cu(II) in NiCu-MOF-74. Last but not least, we inspect the influence of reaction temperature on the molar ratio of Cu(I) to Cu(II) in MOF-74 under the condition of the feed-in Ni/Cu ratio of 7:3 and the solvent volume ratio of ethanol, DMF, and water of 1:15:1 (Supplementary Fig. 26). At a lower temperature of 95 °C, the crystallinity of the obtained NiCu-MOF-74 sample is very poor, while at a higher temperature of 115 °C, the obtained NiCu-MOF-74 sample is accompanied with metallic Cu. Therefore, the temperature of 105 °C is more suitable for synthesis of crystalline NiCu-MOF-74 without impurities. Altogether, precisely modulating the molar ratio of Cu(I) to Cu(II) in NiCu-MOF-74 correlates with the content of Ni and Cu in MOF-74, the composition of mixed solvents as well as the reaction temperatures.
a Ultraviolet-visible spectroscopy recording possible reduction of Cu(II) ions by ethanol (EtOH), 2,5-dihydroxylterephthalic acid (DHTA) and N,N-dimethylformamide (DMF). FTIR spectra of MOF-74 samples and pristine 2,5-dihydroxyterephthalic acid in the range of b 4000–700 cm−1, c 3530–3050 cm−1, and d 1800−1500 cm−1. Note that ν and δ represent stretching vibration and bending vibration, respectively. e Periodic models of MOF-74 containing Cu(II)-Ni(II) pairs (left part) and Cu(I)-Ni(II) pairs (right part).
Besides, the presence of Cu(I) site in MOF-74 necessitates charge balance with either linker protonation or additional ammonium cations from the decomposition of DMF. To clarify it, the Fourier Transform Infrared (FTIR) spectra of all the MOF-74 samples are collected (Fig. 3b). As for Ni-MOF-74, the peak at 1545 cm−1 corresponds to the vibration of carboxyl C=O groups in MOF-7437, shifting to the lower wavenumber compared with 1650 cm−1 for carboxylic C=O groups in pristine 2,5-dihydroxyterephthalic acid. Meanwhile, the disappearance of the wide peak at 3200–2500 cm−1 indicates that the hydroxyl groups in 2,5-dihydroxyterephthalic acid are deprotonated. Differently, the new peak at 3365 cm−1 and the shoulder peak at 1591 cm−1 are observed (Fig. 3c, d), especially for those with plenty of Cu(I) sites, representing the stretching and bending vibration of remaining phenolic hydroxyl groups38. This result indicates that the proton serves as the cation in the form of phenolic hydroxyl group for balancing the charge when Cu(I) cations are formed in MOF-74. Moreover, the elemental analyses of carbon, hydrogen and nitrogen are conducted for all the synthesized MOF-74 samples (Supplementary Table 11). It is seen that nitrogen element is negligible in each sample, suggesting that ammonium cation does not act as the cation for balancing the charge in the framework. An optimized periodic model of NiCu-MOF-74 demonstrates that through the rotation of one DHTA ligand, the carboxylic group replaces the location of phenolic group, while the phenolic oxygen moves to another side and becomes uncoordinated (Fig. 3e and Supplementary Fig. 27)24. This rotation leads to the declined coordination number of Cu(I) sites.
Cascade oxidation of KA oil to ɛ-caprolactone
Note that cyclohexanone acts as the intermediate product of cascade oxidation of cyclohexanol to ɛ-caprolactone. So, before testing KA oil, the cascade oxidation of cyclohexanol to ɛ-caprolactone was first examined on the NiCu-MOF-74 samples in presence of ambient O2 and PhCHO of 2.5 times equivalent of feed cyclohexanol at 65 °C. Table 1 summarizes the catalytic results by considering carbon balance for each experiment with the use of an internal standard nonane (Supplementary Table 12). Without a catalyst, almost no conversion of cyclohexanol is found (Table 1, entry 10). On the basis of Ni-MOF-74, the conversion ratio of cyclohexanol is 37.0% within 9 h reaction while the yield of ɛ-caprolactone is 18.8% together with 17.4% yield of cyclohexanone (Table 1, entry 1, Supplementary Fig. 28). In contrast, the very low catalytic activity is observed over both Cu-MOF-74 and CuII-MOF-74 samples under the identical condition (Table 1, entries 7 and 8, Supplementary Figs. 29 and 30).
Interestingly, when Cu nodes are introduced into Ni-MOF-74 framework, the catalytic activity is significantly enhanced. With increasing the content of Cu from 10% to 90%, the conversion ratio of cyclohexanol and yield of ɛ-caprolactone first increase and then decrease gradually, but all of them surpass Ni-MOF-74 (Table 1, entries 2-6; Supplementary Figs. 31–35 and Supplementary Note 1). Among them, Ni7/10Cu3/10-MOF-74 displays 98.5% conversion ratio of cyclohexanol and 96.3% yield of ɛ-caprolactone within 9 h reaction (Table 1, entry 3; Supplementary Table 13), much higher than other samples. Besides, all the catalytic results show that there are only minor side products formed during the tandem oxidation process (Table 1). Further, Ni7/10Cu3/10-MOF-74 sample exhibits excellent catalytic stability for cascade oxidation of cyclohexanol to ɛ-caprolactone over successive ten runs (Supplementary Figs. 36 and 37, Supplementary Table 7, Supplementary Note 2).
As the contrast sample, the physical mixture of Ni-MOF-74 and Cu-MOF-74 containing an equal amount of Ni and Cu to Ni7/10Cu3/10-MOF-74 displays only 37.2% conversion ratio of cyclohexanol and 10.1% yield of ɛ-caprolactone (Table 1, entry 9, Supplementary Fig. 38). This comparison highlights that the coupling of Ni and Cu sites in MOF-74 is crucial for efficiently catalyzing the cascade oxidation of cyclohexanol to ɛ-caprolactone.
Besides, the influence of the molar ratio of benzaldehyde to cyclohexanol on cascade oxidation of cyclohexanol is investigated over Ni7/10Cu3/10-MOF-74 at different reaction temperatures. Theoretically, the complete conversion of cyclohexanol to ɛ-caprolactone needs consumption of benzaldehyde of 2.0 times equivalent of feed cyclohexanol. Among various amounts of benzaldehyde and different reaction temperatures, the optimum performance for tandem oxidation of cyclohexanol toward ɛ-caprolactone is achieved over Ni7/10Cu3/10-MOF-74 at 2.5 molar ratio of benzaldehyde/cyclohexanol and 65 °C (Supplementary Table 14 and Supplementary Note 3). In other word, the conversion ratio of cyclohexanol reaches 98.5% accompanying with the 95.2% conversion ratio of benzaldehyde to benzoic acid while the yield of ɛ-caprolactone is 96.3% at 65 °C for 9 h reaction, and correspondingly, the contribution of benzaldehyde on cascade oxidation of cyclohexanol to cyclohexanone and ɛ-caprolactone is up to 81.3%, superior to other contrast catalysts (Supplementary Table 15).
Based on above observations, the oxidation of KA oil was investigated over Ni7/10Cu3/10-MOF-74 at 65 °C. As the K/A ratio ranges from 0.5 to 2, both cyclohexanone and cyclohexanol are almost completely converted to ɛ-caprolactone within 9 h reaction (Table 1, entries 12−14, Supplementary Figs. 39–41, Supplementary Table 15), demonstrating that Ni7/10Cu3/10-MOF-74 has the excellent capability responsible for efficient oxidation of KA oil to ɛ-caprolactone.
During the tandem oxidation of cyclohexanol, benzaldehyde is transformed into benzoic acid (Supplementary Table 16), and thus, it is necessary to consider how to separate it from ɛ-caprolactone. With the addition of NaOH into the post-reaction solution, benzoic acid is converted into insoluble sodium benzoate under ice bath conditions, while ɛ-caprolactone does not react with NaOH (Supplementary Figs. 42 and 43, Supplementary Table 17). Subsequently, as-formed sodium benzoate is separated from ɛ-caprolactone through filtration. Finally, the obtained sodium benzoate is converted into benzoic acid with the treatment of hydrochloric acid under ice bath condition followed by filtration (Supplementary Fig. 44), due to the low solubility of benzoic acid in the NaCl aqueous solution.
Role of Ni(II), Cu(II), and Cu(I) in cascade oxidation of cyclohexanol
Without PhCHO, no catalytic activity is found over Ni7/10Cu3/10-MOF-74, indicating that the simultaneous presence of PhCHO and O2 is imperative for cascade oxidation of cyclohexanol to ɛ-caprolactone. Further, the auto-oxidation of PhCHO to peroxybenzoic acid (PhCOOOH) is observed (Supplementary Figs. 45 and 46)39. Therefore, the cascade oxidation of cyclohexanol to ɛ-caprolactone in presence of PhCHO and O2 is divided into three sequential steps: PhCHO oxidation, cyclohexanol oxidation to cyclohexanone and cyclohexanone oxidation to ɛ-caprolactone.
The oxidation of PhCHO is quantified over different catalysts at 65 °C for 2 h reaction (Fig. 4a). The corresponding conversion ratio is 16.5% without a catalyst, 18.7% over CuII-MOF-74, 72.6% over Ni-MOF-74, 84.1% over Ni7/10Cu3/10-MOF-74 and 71.0% over Ni7/10CuII3/10-MOF-74. Evidently, Ni(II) is mainly responsible for catalyzing the oxidation of PhCHO.
a Benzaldehyde oxidation over different catalysts at 65 °C for 2 h. Condition: 2.5 mmol benzaldehyde, O2 bubble at 10 mL min−1. b Oxidation of cyclohexanol over different catalysts with use of m-chloroperoxybenzoic acid as oxidant at 65 °C for 2 h. Condition: 1 mmol cyclohexanol, 2 mmol m-CPBA. c Cyclohexanone oxidation over different catalysts with use of m-chloroperoxybenzoic acid as oxidant at 65 °C for 15 min. Condition: 50 mg Ni7/10Cu3/10-MOF-74, 36 mg Ni-MOF-74, 14 mg CuII-MOF-74, 50 mg Ni7/10CuII3/10-MOF-74, 10 mL dichloroethane. Condition: 2 mmol cyclohexanone, 2 mmol m-CPBA.
To distinguish the roles of Ni and Cu in the oxidation of cyclohexanol, commercial m-chloroperoxybenzoic acid (m-CPBA) is used to replace PhCHO and O2, since PhCOOOH is very easy to decompose and difficult to be stored. As summarized in Fig. 4b, at 65 °C for 2 h reaction, we obtain 16.2% conversion ratio of cyclohexanol without a catalyst, 25.3% over Ni-MOF-74 and 18.2% over CuII-MOF-74. Notably, Ni7/10Cu3/10-MOF-74 exhibits much higher conversion ratio of 61.8%, whereas the corresponding value of contrast Ni7/10CuII3/10-MOF-74 is only 19.1%. Moreover, in presence of PhCHO and O2, Ni7/10CuII3/10-MOF-74 displays 29.0% conversion ratio of cyclohexanol and 7.0% yield of ɛ-caprolactone at 65 °C for 9 h reaction (Table 1, entry 11, Supplementary Fig. 47), also much lower than 98.5% conversion ratio and 96.3% yield of ɛ-caprolactone over Ni7/10Cu3/10-MOF-74. Obviously, Cu(I) is the key to catalyzing the oxidation of cyclohexanol to cyclohexanone.
Finally, the influence of Ni and Cu on cyclohexanone oxidation is investigated with m-CPBA (Fig. 4c). Auto-oxidation of cyclohexanone to ɛ-caprolactone is detected at 65 °C for 15 min reaction without a catalyst, and the conversion ratio is 21.5%. The similar value is attained with 26.2% over Ni-MOF-74, 22.4% over CuII-MOF-74 and 25.0% over Ni7/10CuII3/10-MOF-74. As comparison, Ni7/10Cu3/10-MOF-74 shows a significantly enhanced conversion ratio of 63.1%. Clearly, Cu(I) plays a dominant role in catalyzing oxidation of cyclohexanone to ɛ-caprolactone.
Reaction pathway and theoretical calculation
To better understand the reaction pathways of three sequential steps, the periodic NiCu-MOF-74 model containing 5-coordinated Ni(II)-O and 4-coordinated Cu(I)-O sites is built for density functional theory (DFT) calculation (Supplementary Data 1). Before calculation, in situ XANES measurements are performed to investigate the interaction of O2 with Ni and Cu sites in Ni7/10Cu3/10-MOF-74. As the temperature increases from 25 °C to 65 °C, the Ni and Cu K-edge absorptions both shift to higher energy (Fig. 5, Supplementary Figs. 48 and 49, Supplementary Note 4), indicating the electron transfer from both Ni(II) and Cu(I) to O2 but weak interaction of O2 with Cu(II) (Supplementary Fig. 50). So, the bi-site adsorption of two O2 molecules for PhCHO oxidation is proposed (Fig. 6a). To better understand the interaction with benzaldehyde, the fluorescence quenching experiments are conducted (Supplementary Fig. 51). The fluorescence spectrum of Ni7/10Cu3/10-MOF-74 under air atmosphere with the excitation wavelength of 360 nm shows the fluorescence peak at 468 nm. Further, with increasing the concentration of benzaldehyde from 20 mM to 100 mM, the fluorescence peak intensity gradually decreases, and the relationship between fluorescence intensity and concentration of benzaldehyde is well in accordance with the Stern-Volmer equation40. This result reveals the activation of benzaldehyde by NiCu-MOF-74 and O2.
a Reaction pathway of PhCHO oxidation over Ni(II) and Cu(I) dual sites to produce Cu1+δ-Oδ-• species. b Energy profile for benzaldehyde oxidation to produce Cu1+δ-Oδ-• species. Periodic DFT calculations were performed using Perdew-Burke-Ernzerhof functional based on generalized gradient approximation and a plane-wave basis set with a kinetic energy cutoff of 400 eV. c Low-temperature EPR spectra of Ni7/10Cu3/10-MOF-74 before and after treating by PhCHO and O2 at 65 °C. d Kinetic isotope effect (KIE) experiments of benzaldehyde oxidation using isotope-labeled PhCDO at 65 °C.
Based on the above experimental results, we set up the calculation model for benzaldehyde oxidation (Supplementary Data 2). In detail, Ni(II) and Cu(I) pairs act as the sites for two O2 adsorption (Fig. 6a, b, S1; Supplementary Fig. 52), in the middle of which one PhCHO is co-adsorbed. One can notice that the adsorption of O2 on Ni(II) is stronger than that on Cu(I) (Supplementary Fig. 53), and the distance between formyl H atom of PhCHO and adsorbed O on Ni(II) is 2.3 Å, shorter than 3.0 Å on Cu(I) (Supplementary Fig. 54). Thus, the formyl H atom of PhCHO is attacked by Ni(II)-OO* to form Ni(II)-OO-H-C(O)Ph (Fig. 6b, S2), and then the C-H bond is broken with a rate-determining energy barrier of 0.73 eV (Fig. 6b, TS1). Interestingly, as-formed PhCO· experiences a relay transfer from Ni(II) to react with another O2 on adjacent Cu(I), giving rise to Cu(I)-OOC(O)Ph/Ni(II)-OOH (Fig. 6b, S3). Subsequently, the H atom from Ni(II)-OOH is transmitted back to produce PhCOOOH on Cu(I) (Fig. 6b, S4 and S5). Afterward, the O-O scission of PhCOOOH on Cu(I) occurs with an energy barrier of 0.36 eV (Fig. 6b, TS2) to produce PhCOOH41, while the Cu(I) site undergoes a redox process to produce active Cu1+δ-Oδ-• species (Fig. 6b, S8)42.
As for the Cu1+δ-Oδ-• species, the Bader charge analysis shows that the charge on Cu element is 0.96 (Supplementary Fig. 55), higher than 0.71 for Cu(I) but lower than 1.11 for Cu(II) in the primary framework. Meanwhile, the charge on O atom in Cu1+δ-Oδ-• species is -0.56, considerably lower than −1.10 for the O atoms in the primary framework, revealing that Cu(I) is partially oxidized to form Cu1+δ-Oδ-• species containing electron-deficient O atom. To experimentally confirm the redox behavior of Cu(I) site during PhCHO oxidation, the low-temperature electron paramagnetic resonance (EPR) test was conducted (Fig. 6c). A set of hyperfine signals of four equally spaced lines with spin Hamiltonian g tensor of 2.342 in the parallel direction and the hyperfine signal with g tensor of 2.076 in the perpendicular direction are attributed to the signal of Cu(II) in Ni7/10Cu3/10-MOF-7443. After treating Ni7/10Cu3/10-MOF-74 by PhCHO and O2 at 65 °C, two new signals with g1 = 2.034 and g2 = 2.006 emerge, denoting the formation of Cu1+δ-Oδ-• species according to the reported literature44.
As uncovered by the above calculation, the C-H dissociation in formyl group of PhCHO behaves as the rate-determining step, which is further evidenced by kinetic isotope effect (KIE) experiments using isotope-labeled PhCDO at 65 °C with the kH/kD value of 5.56 over Ni7/10Cu3/10-MOF-74 (Fig. 6d and Supplementary Fig. 56)45. In addition, two other reaction models for PhCHO oxidation (Step I) are proposed including: (1) the individual Ni(II) for adsorption of O2 and PhCHO (Supplementary Fig. 57)46, and (2) Ni(II) for adsorption of O2 along with Cu(I) for adsorption of PhCHO (Supplementary Fig. 58). One can note that both pathways need to overcome much higher energy barriers for the abstraction of H in formyl group of PhCHO than that through bi-site adsorption of two O2 for PhCHO oxidation.
After PhCHO oxidation reaction (Step I), in situ formed Cu1+δ-Oδ-• species serves as oxidant for cyclohexanol oxidation (Step II) and subsequent cyclohexanone oxidation (Step III). In Step II (Fig. 7a and b, Supplementary Fig. 59), cyclohexanol is first adsorbed on Cu1+δ-Oδ-• species (Fig. 7b, S’1) to dissociate the methyne C-H bond and the H atom is eliminated to form Cu1+δ-OH with an energy barrier of 0.30 eV (Fig. 7b, TS’1). Then, the hydroxyl H atom of cyclohexanol is abstracted by Cu1+δ-OH and eliminated to form H2O with an energy barrier of 0.14 eV (Fig. 7b, TS’2), along with the formation of cyclohexanone and simultaneous reduction of Cu1+δ back to Cu(I) (Fig. 7b, S’3 and S’4).
a Reaction pathway of cyclohexanol oxidation to cyclohexanone catalyzed by in situ formed Cu1+δ-Oδ-• species from PhCHO oxidation. b Energy profile for cyclohexanol oxidation to cyclohexanone. c Reaction pathway of cyclohexanone oxidation to ɛ-caprolactone catalyzed by in situ formed Cu1+δ-Oδ-• species. d Energy profile for cyclohexanone oxidation to ɛ-caprolactone. Periodic DFT calculations were performed using Perdew–Burke–Ernzerhof functional based on generalized gradient approximation and a plane-wave basis set with a kinetic energy cutoff of 400 eV.
In Step III (Fig. 7c, d, Supplementary Fig. 60), cyclohexanone is adsorbed on another Cu1+δ-Oδ-• species (Fig. 7d, S”1) through carbonyl group characteristic with O-Cu1+δ and C-Oδ-•, and then C=O π bond cleavage results in formation of four-member ring Cu-O-C-O (Fig. 7d, S”2). The following α-C-C breakage is an endothermic process with an energy barrier of 0.24 eV (Fig. 7d, TS”1). Thereafter, the broken terminal C atom binds with the O atom in Cu1+δ-Oδ-• to generate a seven-member ring containing C-O-C structure (Fig. 7d, S”3), in sequence of the dissociation of Cu-O coordination in the Cu-O-C-O ring, the electron transfer from O back to Cu to form Cu(I), and the regeneration of carbonyl group to form ɛ-caprolactone (Fig. 7d, S”4).
To further elucidate the unique role of Cu1+δ-Oδ-• species, a couple of comparison calculations are performed. Cyclohexanol oxidation to cyclohexanone (Step II) and cyclohexanone oxidation to ɛ-caprolactone (Step III) by PhCOOOH are evaluated in the absence of metallic sites (Supplementary Data 3)47. Shown by Supplementary Figs. 61 and 62, it needs to overcome a much higher energy barrier of 2.24 eV for methyne C-H bond cleavage in Step II, while an energy barrier of 0.52 eV exists for α-C-C bond cleavage of cyclohexanone in Step III. Then, the model of pure Ni-MOF-74 with 5-coordinated Ni(II)-O is built (Supplementary Data 4), on which the cascade oxidation is inspected (Supplementary Fig. 63, Supplementary Note 5 and Supplementary Data 5). In Step I, PhCHO oxidation toward PhCOOOH occurs over Ni(II) and Ni(II) pairs via relay activation and the energy barrier of formyl C-H bond cleavage in PhCHO is 0.72 eV, but the oxidation reaction hardly occurs on Ni(II) (Supplementary Figs. 64 and 65)48,49. As a result, with use of PhCOOOH on Ni(II), both the methyne C-H bond cleavage in cyclohexanol (Step II) and the α-C-C cleavage in cyclohexanone (Step III) require overcoming the energy barrier of 0.87 and 0.46 eV (Supplementary Figs. 66–69), respectively, much higher than those with Cu1+δ-Oδ-• species over Ni(II) and Cu(I) pairs.
Beyond DFT calculation, other typical contrast samples of Co7/10Cu3/10-MOF-74, Zn7/10Cu3/10-MOF-74, and Ni7/10Zn3/10-MOF-74 are employed for cascade oxidation of cyclohexanol (Supplementary Figs. 70–75, Supplementary Table 3). All of them exhibit much lower catalytic performances than Ni7/10Cu3/10-MOF-74 under identical conditions (Supplementary Table 18). Altogether, modulating Cu(I) site with the adjacent Ni(II) site in Ni7/10Cu3/10-MOF-74 is pivotal for efficiently catalyzing the cascade oxidation of cyclohexanol to ɛ-caprolactone.
Discussion
In summary, a major-auxiliary cooperative strategy is invented to in situ generate highly active Cu1+δ-Oδ-• species via relay reaction at Cu(I) and Ni(II) pairs in multivariate NiCu-MOF-74. As-formed Cu1+δ-Oδ-• species exhibits the outstanding capability for activating both the methyne C-H bond of cyclohexanol and α-C-C bond of cyclohexanone to produce ɛ-caprolactone from KA oil. The universality of the synergy between Cu(I) and Ni(II) pairs is validated by cascade oxidation of other cyclohexanol derivatives, cyclopentanol, and cyclobutanol, and all the corresponding lactones are successfully achieved (Table 1, entries 15–21, Supplementary Figs. 76–82, Supplementary Note 6). We envision that constructing versatile metal pairs of respective major and auxiliary function in single catalysts will endow us with implementing many important but untouched reactions.
Methods
Synthesis of Ni-MOF-74
Ni-MOF-74 was synthesized by using Ni(NO3)2·6H2O and DHTA through solvothermal method50. In a typical procedure, Ni(NO3)2·6H2O (436 mg, 1.5 mmol) and DHTA (100 mg, 0.5 mmol) were dissolved in the mixed solvents containing 10 mL DMF, 0.66 mL ethanol, and 0.66 mL H2O. Subsequently, the solution was added into a 20 mL Teflon-lined autoclave and heated at 120 °C for 24 h. After cooling to room temperature, the solid products were collected by centrifugation at 10,610 × g (10,000 rpm) for 3 min and washed with DMF and methanol for several times, separately. Finally, the obtained Ni-MOF-74 was dried at 80 °C under dynamic vacuum for 12 h.
Synthesis of NiCu-MOF-74
Multivariate MOF-74 was prepared by using DHTA and different molar ratios of Ni(NO3)2·6H2O to Cu(NO3)2·3H2O through solvothermal method. In a typical procedure, 0.5 mmol of DHTA and 1.5 mmol of metal ions (Ni and Cu) were used for synthesis, and the molar ratio of Ni to Cu was varied, i.e., 0.9:0.1, 0.7:0.3, 0.5:0.5, 0.3:0.7 and 0.1:0.9. The mixture of Ni(NO3)2·6H2O, Cu(NO3)2·3H2O and DHTA were dissolved in the mixed solvents containing 15 mL DMF, 1 mL H2O and 1 mL ethanol. Subsequently, the solution was added into a 20 mL Teflon-lined autoclave and heated at 105 °C for 24 h. After cooling to room temperature, the solid products were collected by centrifugation at 10,610 × g (10000 rpm) for 3 min and washed with DMF and methanol for several times, separately. Finally, the obtained NiCu-MOF-74 was dried at 80 °C under dynamic vacuum for 12 h. According to the actual molar ratio of Ni to Cu determined by the ICP-MS, the obtained bimetal MOF-74 samples were denoted as Ni9/10Cu1/10-MOF-74, Ni7/10Cu3/10-MOF-74, Ni1/2Cu1/2-MOF-74, Ni3/10Cu7/10-MOF-74 and Ni1/10Cu9/10-MOF-74, respectively.
Synthesis of Cu-MOF-74
Cu-MOF-74 was synthesized by using Cu(NO3)2·3H2O and DHTA through solvothermal method32. Typically, Cu(NO3)2·3H2O (241 mg, 1.0 mmol) and DHTA (100 mg, 0.5 mmol) were dissolved in 20 mL DMF and 1 mL 2-propanol. The mixture solution was transformed into a 50 mL screw cap bottle and then placed into an oil bath at 80 °C for 24 h. After cooling to room temperature, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with DMF and methanol for several times, and then dried at 80 °C under dynamic vacuum for 12 h.
Synthesis of CuII-MOF-74
CuII-MOF-74 was synthesized by using Cu(CH3COO)2·H2O and DHTA at room temperature with stirring51. Typically, Cu(CH3COO)2·H2O (400 mg, 2.0 mmol) was dissolved in 20 mL methanol, meanwhile, DHTA (200 mg, 1.0 mmol) was dissolved in 10 mL methanol. Then the two solutions were mixed under magnetic stirring and reacted for 24 h at room temperature. After the reaction, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with ethanol several times, and then dried at 80 °C under dynamic vacuum for 12 h.
Synthesis of Ni7/10CuII 3/10-MOF-74
Typically, the mixture of Cu(CH3COO)2·H2O (120 mg, 0.6 mmol) and Ni(CH3COO)2·4H2O (349 mg, 1.4 mmol) was dissolved in 30 mL methanol, meanwhile, DHTA (200 mg, 1.0 mmol) was dissolved in 20 mL methanol. Then the two solutions were mixed under magnetic stirring and reacted for 24 h at room temperature. After the reaction, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with ethanol several times, and then dried at 80 °C under dynamic vacuum for 12 h.
Synthesis of Ni7/10Zn3/10-MOF-74
Ni7/10Zn3/10-MOF-74 was prepared by using Ni(NO3)2·6H2O and Zn(NO3)2·6H2O with a molar ratio of Ni to Zn of 0.7 to 0.3 while maintained the molar ratio of DHTA to metal ions (Ni and Zn) at 1:3. Typically, Ni(NO3)2·6H2O (306 mg, 1.05 mmol), Zn(NO3)2·6H2O (134 mg, 0.45 mmol) and DHTA (100 mg, 0.5 mmol) were completely dissolved in 10 mL DMF and 0.5 mL 2-propanol. Then, 20 mL Teflon-lined autoclave containing the above-mixed solution was placed into an oven and heated to 105 °C and kept at the temperature for 24 h. After cooling to room temperature, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with DMF and methanol for several times, and then dried at 80 °C under dynamic vacuum for 12 h. The actual molar ratio of Ni to Zn was determined by ICP-AES measurement.
Synthesis of Zn7/10Cu3/10-MOF-74
Zn7/10Cu3/10-MOF-74 was synthesized by using Zn(NO3)2·6H2O and Cu(NO3)2·3H2O with DHTA through solvothermal method. The molar ratio of DHTA to the mixed Zn(NO3)2·6H2O and Cu(NO3)2·3H2O was maintained at 1:3, while the molar ratio of Zn(NO3)2·6H2O to Cu(NO3)2·3H2O was 0.7:0.3. Typically, Zn(NO3)2·6H2O (312 mg, 1.05 mmol), Cu(NO3)2·3H2O (108 mg, 0.45 mmol) and DHTA (100 mg, 0.5 mmol) were completely dissolved in 10 mL DMF and 0.5 mL H2O. Then, 20 mL Teflon-lined autoclave containing the above mixed solution was placed into an oven and heated to 100 °C and kept at the temperature for 24 h. After cooling to room temperature, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with DMF and methanol several times, and then dried at 80 °C under dynamic vacuum for 12 h. The actual molar ratio of Zn/Cu was measured by ICP-AES measurement.
Synthesis of Co7/10Cu3/10-MOF-74
Zn7/10Cu3/10-MOF-74 was synthesized by using Co(NO3)2·6H2O and Cu(NO3)2·3H2O with DHTA through solvothermal method. The molar ratio of DHTA to metal ions (Co and Cu) was maintained at 1:3 while the molar ratio of Co to Cu was 0.7:0.3. Typically, Co(NO3)2·6H2O (306 mg, 1.05 mmol), Cu(NO3)2·3H2O (108 mg, 0.45 mmol) and DHTA (100 mg, 0.5 mmol) were completely dissolved in 15 mL DMF, 1 mL ethanol and 1 mL H2O. Then, 50 mL Teflon-lined autoclave containing the above-mixed solution was placed into an oven, heated to 105 °C and kept at the temperature for 24 h. After cooling to room temperature, the sample was separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with DMF and methanol several times, and then dried at 80 °C under dynamic vacuum for 12 h. The actual molar ratio of Co/Cu was measured by the ICP-AES measurement.
Cascade oxidation of cyclohexanol and its derivatives
In a typical experiment, 50 mg catalyst was added to a mixture of 1 mmol cyclic alcohol substrate, 2.5 mmol benzaldehyde and 10 mL DCE. 1 mmol nonane was added as an internal standard. The reaction mixture was stirred continuously in a 50 mL three-neck round-bottom flask equipped with a reflux condenser in oil bath at 65 °C and oxygen was bubbled into the mixture at a flow rate of 10 mL min−1. After reaction, the catalyst was collected by centrifugation at 10,610 × g (10,000 rpm) for 3 min, washed with DCE and ethanol for several times. The resultant solutions were analyzed by gas chromatography (GC, Shimadzu, GC-2010 Plus) with an RTX−1 capillary column and a flame ionization detector, and the temperature of the vaporization chamber and FID detector is controlled to be 220 °C. The temperature of the column is controlled starting at 40 °C for 20 min, then rising to 220 °C with a heating rate of 10 °C min−1 and finally maintaining at 220 °C for 5 min. For cycling test, the catalyst was dried at 80 °C for 3 h under vacuum for regeneration before being subjected to the next catalytic cycle.
Oxidation of benzaldehyde into perbenzoic acid
In a typical experiment, 50 mg catalyst was added to a mixture of 2.5 mmol benzaldehyde and 10 mL DCE. The reaction mixture was stirred continuously in a 50 mL three-neck round-bottom flask equipped with a reflux condenser in oil bath at 65 °C for 2 h, and oxygen was bubbled into the mixture at a flow rate of 10 mL min−1. After the reaction, the catalyst and solution were separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, and the resultant solutions were analyzed by 1H NMR spectra with CDCl3 as the deuterium substituted reagent.
Oxidation of cyclohexanol with m-CPBA
In a typical experiment, 50 mg catalyst was added to a mixture of 1 mmol cyclohexanol, 2 mmol m-CPBA and 10 mL DCE. The reaction mixture was stirred continuously in a 50 mL three-neck round-bottom flask equipped with a reflux condenser in oil bath at 65 °C for 2 h. After reaction, the catalyst and solution were separated by centrifugation at 10,610 × g (10,000 rpm) for 3 min, and the resultant solutions were analyzed by gas chromatography.
Oxidation of cyclohexanone with m-CPBA
In a typical experiment, 50 mg catalyst was added to a mixture of 2 mmol m-CPBA and 10 mL DCE. The reaction mixture was stirred continuously in a 50 mL three-neck round-bottom flask equipped with a reflux condenser in oil bath at 65 °C, and then 2 mmol cyclohexanone was quickly added for reaction in 15 min. After reaction, the resultant system was quickly filtrated, and the solution was analyzed by gas chromatography.
X-ray absorption analysis
The Ni and Cu K-edge X-ray absorption spectra were collected at room temperature in transmission mode at beamline 1W1B of the Beijing Synchrotron Radiation Facility (BSRF), using a Si (111) double-crystal monochromator. To achieve the best signal-to-noise ratio, the powdered samples were uniformly mixed with BN powder and pressed to a pellet to ascertain the edge jump of about 1.0. The energy was calibrated using Ni/Cu foil, and the intensity of the incident and transmitted X-rays was monitored by standard N2-filled ion chambers.
Theoretical calculation
The Vienna Ab-initio Simulation Package (VASP)52 was employed to carry out spin-polarized density functional theory (DFT) calculations using the Perdew–Burke–Ernzerhof (PBE)53 functional based on the generalized gradient approximation (GGA). The ionic cores were described using the Projected Augmented Waves (PAW) pseudopotential54, while the valence electrons were described by a plane wave basis set with a kinetic energy cutoff of 400 eV. A 1 × 1 × 4 Gamma-centered k-point mesh was adopted for the calculations of the MOF systems. The electronic energy converged when the energy change was smaller than 10-5 eV, and geometry optimization was considered convergent when the force on each atom was less than 0.03 eV Å−1. The exchange interaction of Ni 3d and Cu 3d orbits was described by the Hubbard U correction55 with effective U–J values of 3.8 eV56 and 4.0 eV57, respectively. The long-range van der Waals interactions were described by taking the DFT-D2 Grimme method58. The climbing-image nudged elastic band (CI-NEB)59 and the Dimer60 methods were used to locate the transition states.
The definition of adsorption energies (Eads) was as follows:
where Eadsorb., Emol. and EMOF represented the energies of the adsorbate system, the adsorbate, and the MOF substrate, respectively.
The calculation of reaction energies for each reaction step (ΔE) was performed using the following equation:
where EFS and EIS represented the energies of the final state and the initial state of each reaction, respectively.
The homogeneous oxidation of cyclohexanol and cyclohexanone was investigated using Gaussian 09, Revision D. 0161. The molecular geometry was optimized by employing density functional theory (DFT) at the B3LYP62 level, utilizing the 6–31 G (d)63 basis set and incorporating the GD3BJ64 empirical dispersion correction. The energy calculations were performed through single-point calculations with the same functional and basis set, while also considering the influence of the solvent using the SMD65 continuum model with dichloroethane as the solvent. Frequency analysis was carried out at the same theoretical level to verify whether the stationary points corresponded to minima (no imaginary frequencies) or transition states (only one imaginary frequency). Moreover, the intrinsic reaction coordinate (IRC) calculations were executed to pinpoint the transition states66.
The periodic structural models of NiCu-MOF-74 (Supplementary Data 1) and Ni-MOF-74 (Supplementary Data 4) used in theoretical calculations were provided in the form of cif files, in which the lattice parameters, symmetry, and atomic coordinates were included. In addition, the data of atomic coordinates for every state involved in cascade oxidation over NiCu-MOF-74 (Supplementary Data 2), without catalyst (Supplementary Data 3) and over Ni-MOF-74 (Supplementary Data 5), were also provided in the form of txt files.
Data availability
All data supporting the findings of this study are available within the paper, supplementary information files or are available from the corresponding authors upon request.
References
Li, Y. et al. pH-responsive shape memory poly(ethylene glycol)-poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl. Mater. Interfaces 7, 12988–12999 (2015).
Buntara, T. et al. Caprolactam from renewable resources: catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. 50, 7083–7087 (2011).
Jiang, F., Lan, T., Sun, J., Zhao, G. & Lu, Y. Core-shell Cu@SiO2/SiO2 catalyst for 1,6-hexanediol dehydrogenation to ε-caprolactone: High activity and stability from core-shell nanostructure. Nano Res 16, 12270–12280 (2023).
Labet, M. & Thielemans, W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 38, 3484–3504 (2009).
Yang, D. et al. The highly selective aerobic oxidation of cyclohexane to cyclohexanone and cyclohexanol over V2O5@TiO2 under simulated solar light irradiation. Green. Chem. 19, 311–318 (2017).
Li, A., Shen, K., Chen, J., Li, Z. & Li, Y. Highly selective hydrogenation of phenol to cyclohexanol over MOF-derived non-noble Co-Ni@NC catalysts. Chem. Eng. Sci. 166, 66–76 (2017).
Shi, C., Zhu, B., Lin, M., Long, J. & Wang, R. Cyclohexane mild oxidation catalyzed by new titanosilicate with hollow structure. Catal. Today 175, 398–403 (2011).
Gao, Y., Zhang, L., van Hoof, A. J. F., Friedrich, H. & Hensen, E. J. M. A robust Au/ZnCr2O4 catalyst with highly dispersed gold nanoparticles for gas-phase selective oxidation of cyclohexanol to cyclohexanone. ACS Catal. 9, 11104–11115 (2019).
Schrinner, M. et al. Stable bimetallic gold-platinum nanoparticles immobilized on spherical polyelectrolyte brushes: synthesis, characterization, and application for the oxidation of alcohols. Adv. Mater. 20, 1928–1933 (2008).
Yamada, Y. M. A., Arakawa, T., Hocke, H. & Uozumi, Y. A nanoplatinum catalyst for aerobic oxidation of alcohols in water. Angew. Chem. Int. Ed. 46, 704–706 (2007).
Zhang, X. et al. Metal-free mesoporous SiO2 nanorods as a highly efficient catalyst for the Baeyer-Villiger oxidation under mild conditions. ACS Sustain. Chem. Eng. 6, 5868–5876 (2018).
Saikia, P. K. et al. Stabilized Fe3O4 magnetic nanoparticles into nanopores of modified montmorillonite clay: a highly efficient catalyst for the Baeyer-Villiger oxidation under solvent free conditions. Green. Chem. 18, 2843–2850 (2016).
Luo, H. Y., Bui, L., Gunther, W. R., Min, E. & Román-Leshkov, Y. Synthesis and catalytic activity of Sn-MFI nanosheets for the Baeyer-Villiger oxidation of cyclic ketones. ACS Catal. 2, 2695–2699 (2012).
Schmidt, S. et al. An enzyme cascade synthesis of ε-caprolactone and its oligomers. Angew. Chem. Int. Ed. 54, 2784–2787 (2015).
Ma, X., Hortelão, A. C., Patiño, T. & Sánchez, S. Enzyme catalysis to power micro/nanomachines. ACS Nano 10, 9111–9122 (2016).
Du, R., Yuan, H., Yao, J. & Li, H. Oxidation of KA oil to caprolactone with molecular oxygen using N-hydroxyphthalimide-mediated Ce(NH4)2(NO3)6 catalyst. Mol. Catal. 467, 24–29 (2019).
Balbinot, L., Schuchardt, U., Vera, C. & Sepúlveda, J. Oxidation of cyclohexanol to epsilon-caprolactone with aqueous hydrogen peroxide on H3PW12O40 and Cs2.5H0.5PW12O40. Catal. Commun. 9, 1878–1881 (2008).
Huang, Y., Liang, J., Wang, X.-S. & Cao, R. Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions. Chem. Soc. Rev. 46, 126–157 (2017).
Guo, W., Wang, Z., Wang, X. & Wu, Y. General design concept for single-atom catalysts toward heterogeneous catalysis. Adv. Mater. 33, 2004287 (2021).
Liu, X.-Y. et al. Using a multi-shelled hollow metal-organic framework as a host to switch the guest-to-host and guest-to-guest interactions. Angew. Chem. Int. Ed. 57, 2110–2114 (2018).
Zhang, W. et al. Functional porphyrinic metal-organic framework as a new class of heterogeneous halogen-bond-donor catalyst. Angew. Chem. Int. Ed. 60, 24312–24317 (2021).
Zhou, X. et al. Molecular scalpel to chemically cleave metal-organic frameworks for induced phase transition. J. Am. Chem. Soc. 143, 6681–6690 (2021).
Liu, M. et al. Synergetic dual-ion centers boosting metal organic framework alloy catalysts toward efficient two-electron oxygen reduction. Small 18, 2202248 (2022).
Rosi, N. L. et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. J. Am. Chem. Soc. 127, 1504–1518 (2005).
Deng, H. et al. Large-pore apertures in a series of metal-organic frameworks. Science 336, 1018–1023 (2012).
Ji, Z., Li, T. & Yaghi, O. M. Sequencing of metals in multivariate metal-organic frameworks. Science 369, 674–680 (2020).
Liu, H. et al. Modulating charges of dual sites in multivariate metal-organic frameworks for boosting selective aerobic epoxidation of alkenes. J. Am. Chem. Soc. 145, 11085–11096 (2023).
Sun, D., Sun, F., Deng, X. & Li, Z. Mixed-metal strategy on metal-organic frameworks (MOFs) for functionalities expansion: Co substitution induces aerobic oxidation of cyclohexene over inactive Ni-MOF-74. Inorg. Chem. 54, 8639–8643 (2015).
Yuan, K. et al. Bimetal-organic frameworks for functionality optimization: MnFe-MOF-74 as a stable and efficient catalyst for the epoxidation of alkenes with H2O2. Nanoscale 10, 1591–1597 (2018).
Wang, L. J. et al. Synthesis and characterization of metal-organic framework-74 containing 2, 4, 6, 8, and 10 different metals. Inorg. Chem. 53, 5881–5883 (2014).
Molina, M. A., Manjón-Sanz, A. & Sánchez-Sánchez, M. On the contribution of pair distribution function (PDF) to the characterization of nanocrystalline MOFs: the case of M-MOF-74. Micropor. Mesopor. Mater. 319, 110973 (2021).
Sanz, R., Martínez, F., Orcajo, G., Wojtas, L. & Briones, D. Synthesis of a honeycomb-like Cu-based metal-organic framework and its carbon dioxide adsorption behaviour. Dalton Trans. 42, 2392–2398 (2013).
Zurrer, T. et al. Mixed-metal MOF-74 templated catalysts for efficient carbon dioxide capture and methanation. Adv. Funct. Mater. 31, 2007624 (2021).
Jae, J. et al. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 279, 257–268 (2011).
Guo, C., Zhang, Y., Zhang, Y. & Wang, J. An efficient approach for enhancing the catalytic activity of Ni-MOF-74 via a relay catalyst system for the selective oxidation of benzylic C-H bonds under mild conditions. Chem. Commun. 54, 3701–3704 (2018).
Wu, Z.-Y. et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth. 1, 658–667 (2022).
Muratović, S. et al. Low-dimensional magnetism in multivariate copper/zinc MOF-74 materials formed via different mechanochemical methods. Inorg. Chem. 61, 18181–18192 (2022).
Rada, Z. H. et al. Functionalized UiO-66 by single and binary (OH)2 and NO2 groups for uptake of CO2 and CH4. Ind. Eng. Chem. Res. 55, 7924–7932 (2016).
Nabae, Y. et al. Catalysis by carbon materials for the aerobic Baeyer-Villiger oxidation in the presence of aldehydes. ACS Catal. 3, 230–236 (2013).
Liu, Y. et al. Enhanced photoinduced Baeyer–Villiger oxidation of ketones by introducing trinuclear ruthenium clusters into polyoxometalate-based metal-organic frameworks. Chem. Mater. 35, 3941–3950 (2023).
Trammell, R., Rajabimoghadam, K. & Garcia-Bosch, I. Copper-promoted functionalization of organic molecules: from biologically relevant Cu/O2 model systems to organometallic transformations. Chem. Rev. 119, 2954–3031 (2019).
Hong, S., Huber, S. M., Gagliardi, L., Cramer, C. C. & Tolman, W. B. Copper(I)-α-ketocarboxylate complexes: characterization and O2 reactions that yield copper-oxygen intermediates capable of hydroxylating arenes. J. Am. Chem. Soc. 129, 14190–14192 (2007).
Wu, Y. et al. Rate controlling in low-temperature standard NH3-SCR: implications from operando EPR spectroscopy and reaction kinetics. J. Am. Chem. Soc. 144, 9734–9746 (2022).
Kumar, C. P., Gopal, N. O., Wang, T. S., Wong, M.-S. & Chu Ke, S. C. EPR investigation of TiO2 nanoparticles with temperature-dependent properties. J. Phys. Chem. B 110, 5223–5229 (2006).
Baghmar, M. & Sharma, P. K. Kinetics and mechanism of the oxidation of aliphatic aldehydes by tetrabutylammonium tribromide. Int. J. Chem. Kinet. 33, 390–395 (2001).
Wentzel, B. B., Alsters, P. L., Feiters, M. C. & Nolte, R. J. M. Mechanistic studies on the Mukaiyama epoxidation. J. Org. Chem. 69, 3453–3464 (2004).
Yang, N., Su, Z., Feng, X. & Hu, C. Theoretical studies on the asymmetric Baeyer-Villiger oxidation reaction of 4-phenylcyclohexanone with m-chloroperoxobenzoic acid catalyzed by chiral Scandium(III)-N,N’-dioxide complexes. Chem. -Eur. J. 21, 7264–7277 (2015).
Peng, Z. et al. Managing the double-edged sword of Ni3+ in sputter-deposited NiOx by interfacial redox reactions for efficient perovskite solar cells. ACS Appl. Energ. Mater. 6, 1396–1403 (2023).
Zhang, C., Brown, P. J. B. & Hu, Z. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Sci. Total Environ. 621, 948–959 (2018).
Caskey, S. R., Wong-Foy, A. G. & Matzger, A. J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 21, 10870–10871 (2008).
Flores, J. G. et al. Greener synthesis of Cu-MOF-74 and its catalytic use for the generation of vanillin. Dalton Trans. 47, 4639–4645 (2018).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Anisimov, V. V., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Gong, H., Li, Y., Li, H. & Jin, Z. 2D CeO2 and a partially phosphated 2D Ni-based metal-organic framework formed an S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Langmuir 38, 2117–2131 (2022).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA+ U framework. Phys. Rev. B 73, 195107 (2006).
Hamada, I. Van der Waals density functional made accurate. Phys. Rev. B 89, 121103 (2014).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Frisch, M. et al. EM64L-Gaussian 09, Revision D. 01. Gaussian, Inc. (2013).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Rassolov, V. A., Pople, J. A., Ratner, M. A. & Windus, T. L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 109, 1223–1229 (1998).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Maeda, S., Harabuchi, Y., Ono, Y., Taketsugu, T. & Morokuma, K. Intrinsic reaction coordinate: calculation, bifurcation, and automated search. Int. J. Quantum Chem. 115, 258–269 (2014).
Acknowledgements
The authors acknowledge financial support from the National Key Research and Development Program of China (2021YFA1500403, G.D.L.; 2021YFA1200302 and 2022YFA1205400, Z.Y.T.; 2022YFA1203200, T.T.), Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000, Z.Y.T. and G.D.L.), National Natural Science Foundation of China (92356304 and 92056204, Z.Y.T.; 52372079 and 22173024, G.D.L.; 22303021, T.T.), and Youth Innovation Promotion Association CAS (G.D.L.). This work was carried out with the support of 1W1B beamline at the Beijing Synchrotron Radiation Facility.
Author information
Authors and Affiliations
Contributions
Z.T. and G.L. guided and designed the project. G.X. and H.L. performed the synthesis, characterization, and catalytic experiments. W.L. and T.T. performed the theoretical calculation. Z.B. participated in the synthesis and characterization of catalysts. H.L., C.Y., P.A., W.C., and L.Z. performed the XAS experiments and data analyses. Z.T., G.L., H.L., and G.X. drafted the manuscript. All the authors commented on the manuscript. G.X., H.L., and W.L. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xue, G., Liu, H., Liu, W. et al. Major-auxiliary cooperative metal pairs in MOFs enable cascade oxidation of KA oil to ε-caprolactone. Nat Commun 15, 9659 (2024). https://doi.org/10.1038/s41467-024-54064-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54064-w