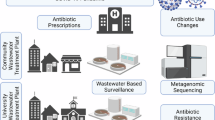
Abstract
Coronavirus Disease 2019 (COVID-19) emerged in December 2019, prompting the implementation of a “zero-COVID” policy in Mainland China. The easing of this policy in December 2022 led to a surge in COVID cases, which was believed to significantly increase antibiotic usage, potentially due to antibiotic misuse or increased coinfections. Our study aimed to compare antibiotic consumption and patterns before and after this policy adjustment. We utilised wastewater-based epidemiology (WBE) to analyse antibiotic levels in samples collected from five wastewater treatment plants in Eastern China during January and February of 2021 and 2023. 27 antibiotics were quantified using ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry (UPLC-MS/MS) and analysed via WBE, with the resulting estimates compared with catchment-specific prescription data. 23 antibiotics were detected in wastewater samples, with a substantial increase in usage in 2023 (ranging from 531% to 3734%), consistent with prescription data. Here, we show a significant rise in antibiotic consumption during the COVID-19 surge and this underscores the need for further investigation into the impacts of inappropriate antibiotic use in China.
Similar content being viewed by others
Introduction
On 12th December 2019, the first case of Coronavirus Disease 2019 (COVID-19) was identified in Wuhan, Hubei province, China1. The World Health Organization (WHO) declared COVID-19 a global pandemic in March 20202. In response to this challenge, the Chinese government implemented a stringent zero-tolerance strategy or “zero-COVID” policy aimed at rapidly reducing infections and minimising transmission through targeted lockdowns and population-wide testing of affected communities together with hospitalisation of all positive cases3. The “zero-COVID” policy was largely relaxed abruptly in December 2022, leading to a surge of COVID-19 cases in Mainland China, with the first wave lasting approximately two months4,5,6. As mass testing and intensive contact tracing were suspended, the daily number of confirmed cases reported by the government ceased to be an accurate reflection of the epidemic, putting great pressure on the healthcare system.
The pandemic witnessed widespread overuse and misuse of antibiotics; a concern underscored by recent studies highlighting antibiotic overuse during its early phase7. Data synthesis from the 118 studies included in a review shows that approximately 82.3% of hospitalised COVID-19 patients were prescribed antibiotics7. In addition, the practice of self-medicating for respiratory and other symptoms using antibiotics purchased without prescription has been widely documented in China. This practice has led to unforeseen consequences for antibiotic resistance (ABR), with low and middle income countries being particularly affected8. To combat antimicrobial resistance, the WHO established the AWaRe classification system, which categorises key antibiotics into three groups: Access, Watch and Reserve. The Access group is recommended for empirical treatment in primary care due to these drugs having a narrow antimicrobial spectrum, low cost, favourable safety profile and reduced risk of resistance. The Watch group includes antibiotics with a higher risk of toxicity or resistance development, while the Reserve group should be used as a last resort in specific clinical situations to preserve their effectiveness. According to WHO, community settings account for 80% of all antibiotics dispensed9. Several factors, such as an increased incidence of bacterial infections coinciding with the lifting of COVID-19 restrictions, the precautionary administration of antibiotics to COVID-19 patients, or shifts in healthcare practices during the pandemic, could contribute to the escalating use of antibiotics during the COVID-19 period10. Whilst antibiotic prescription data can provide usage data, several challenges exist to estimating true consumption, including antibiotics purchased over the counter/online, unconsumed antibiotics, and consumption/excretion of antibiotics in a different catchment from prescription11.
Wastewater-based epidemiology (WBE) is a powerful tool for comprehending community-wide pharmaceutical consumption patterns and monitoring chemical residues12,13,14,15,16,17,18,19,20,21, including antibiotics22,23,24. The core principle of WBE is that chemicals ingested within a community, along with their metabolites, are excreted into the wastewater system23. By measuring these chemical residues in influent wastewater, it is possible to use a back-calculation approach to estimate the level of consumption within the community24,25,26. In our previous study, WBE was applied for the first time in Eastern China for the estimation of community-wide exposure to antibiotics. The results showed similar levels of consumption and usage patterns in summer (August, 2020) and winter (January, 2021)22.
Eastern China, one of the most economically developed regions in the country, is notable for its dense population (approximately 373 million), robust livestock production, and widespread antibiotic use. This makes it as a critical region for addressing issues related to antibiotic use and ABR27,28,29,30,31. In this study, we specifically chose Zhejiang Province (Site A) and Jiangsu Province (Site B) in this region. We measured twenty-seven antibiotics and their metabolites in wastewater before (January, 2021) and after (January to February, 2023) the easing of the “zero-COVID” policy. Our objective was to assess the impact of the relaxation of the “zero-COVID” policy on the patterns and variations of community-wide antibiotic exposure. We hypothesised that there would be a significant change in antibiotic usage following the policy relaxation. In this work, the primary aims of this study were to:
1. Assess the longitudinal community-wide public exposure to antibiotics (public intake vs total environmental burden) using a WBE approach before and after the lifting of “zero-COVID” policy in Eastern China and analyse the impact of easing COVID-19 restrictions on antibiotic usage.
2. Estimate antibiotics intake and variation trends via WBE before and after the easing of COVID-19 restrictions and compare with prescription data in Eastern China.
Results and discussion
Detection frequency, classification and distribution of antibiotics
A total of 23 out of 27 antibiotics were detected in both 2021 and 2023, and their detection frequencies are shown in Fig. 1. Macrolides and sulfonamides showed the highest detection frequencies. Azithromycin, roxithromycin, clarithromycin, erythromycin and sulfamethoxazole were consistently detected in all samples (100%), suggesting widespread usage in Eastern China. In 2021, more antibiotics were detected compared to 2023, with six specific antibiotics, including pefloxacin, danofloxacin, difloxacin, acetylated sulfamethazine, chloramphenicol, and doxycycline, being absent in 2023. This discrepancy might suggest that the surge in caseloads placed a strain on medical resources during a critical period after the lifting of the restrictions in December 2022. Doxycycline was not among the frequently prescribed antibiotics for patients’ therapies in the second phase of the COVID-19 pandemic, which is consistent with the findings from previous studies7,10. Detection frequencies were similar at both Site A and Site B, and the daily numbers of antibiotics detected in all WWTPs are provided in Fig. S2.
The proportional abundances for each drug class across all WWTPs are depicted in Fig. 2A. Quinolones emerged as the dominant category in wastewater samples in both 2021 and 2023, contributing between 18.36% and 96.51% to overall antibiotic composition. Following the WHO AWaRe (Access, Watch, and Reserve) metrics, there is a recommended country-level target of consuming at least 60% of overall antibiotics from the Access group32. Our findings in the studied areas indicated that Access group antibiotics (nine in total) only accounted for 5.84%–22.05% of all detected antibiotics, while Watch group antibiotics (twelve in total) accounted for 77.95%–98.68% (Fig. 2B), with similar results of antibiotic usage in COVID-19 patients in a review study10. There was an even higher percentage of the Watch group than the Access group in 2023. This shift is primarily attributable to the increased contribution of quinolones and macrolides, suggesting potentially inappropriate usage of these drugs following the lifting of COVID-19 restrictions10. In May 2020, WHO recommended against antibiotic use for patients with mild COVID-1933. Several antibiotics on the WHO ‘Watch’ and ‘Reserve’ lists were prescribed frequently in some countries, especially in low- and middle-income countries34.
Concentrations, daily mass loads and PNDLs
The concentration, daily mass load and population-normalised daily load (PNDL) of antibiotics by drug class across all WWTPs are illustrated in Fig. 3 and Table S2. The level of compounds classified by macrolides, quinolones, sulfonamides, phenicols and tetracyclines in WWTPs are provided in the supplementary materials (Fig. S3). The sampling period in 2023 showed higher overall concentration, daily mass load and PNDLs as compared with the period under restrictions in 2021. This increase can be attributed primarily to widespread exposure of antibiotics during the specified timeframe. A longitudinal study that measured various antibiotics in the urine of the general Chinese population also found an increase in antibiotic use during the COVID-19 pandemic35. Additionally, inappropriate antibiotic use and high rates of antibiotic prescribing were found among COVID-19 inpatients in many low- and middle-income countries. These findings are consistent with several other studies36,37,38 that also reported a surge in antibiotic use for COVID-19 patients and widespread use of empirical antibiotic therapy among hospitalised COVID-19 patients39,40.
Results of concentrations classified by the studied compounds are provided in the supplementary material (Fig. S4). Wastewater types included domestic and combined domestic and industrial input. Antibiotic concentrations were significantly higher in 2023 (p < 0.05), with all WWTPs exhibiting concentration increases ranging from 531% to 2757%. In particularly, WWTP-A2, as a domestic WWTP, showed the most substantial increase, underscoring the heightened antibiotic usage during the period of extensive COVID-19 infections. Notably, veterinary antibiotics such as enrofloxacin (WWTP-B5: 2.44 ± 1.32 ng/L in 2021; 56.71 ± 40.16 ng/L in 2023) and florfenicol (WWTP-A2: 3.38 ± 2.95 ng/L in 2021; 193.24 ± 233.69 ng/L in 2023), were also detected in wastewater samples from purely domestic WWTPs. This implies widespread exposure to animal antibiotics within the community, potentially through food consumption or the introduction of animal waste and surface runoff waste into domestic sewage pipelines. Florfenicol, identified in various matrices including food41, drinking water28,42 and urine43, constitutes a major pathway for human exposure to animal antibiotics due to its extensive use in livestock and fishery production in China44.
Several antibiotics namely azithromycin, ciprofloxacin, clarithromycin, florfenicol, norfloxacin, ofloxacin, tilmicosin and trimethoprim, were observed to exceed the Predicted No Effect Concentration (PNEC) derived from Minimal Inhibitory Concentration (MIC) data in 202345. This suggests the potential for antibiotics resistance to develop in the sewer system. Persistent use of these critically important antibiotics can lead to the emergence of multidrug resistant (MDR) strains and a decline in the effectiveness of these drugs. This poses a threat to survival rates for serious infections, neonatal sepsis, and hospital infections, potentially undermining the health benefits of surgery, transplants, and cancer treatment46,47. Widespread resistance to azithromycin and other broad-spectrum antibiotics due to their massive use during the pandemic could result in very few alternative antibiotics available, which are likely to be unaffordable for most patients, especially in low- and middle-income countries10. The widespread usage and continuous introduction of these drugs pose a serious threat of pollution and ABR in the water environment48. Given that antibiotics are used daily in human medicine, as well as in the food and agricultural sectors, a comprehensive and coordinated effort across multiple sectors is imperative to prevent environmental exposure to antibiotics as well as clinically unwarranted use.
Flow rates showed considerable variability corresponding to the capacity of each WWTP, with minimal inter-annual flow fluctuations in WWTPs observed throughout the study period (Fig. S5). In 2021, the overall daily mass loads varied between 3.661 g/day and 553.30 g/day, reflecting the impact of flow variations across different WWTPs. The sampling campaign in 2023 showed higher daily mass loads (133.43 g/day-6099.77 g/day), marking a massive increase of 2348% compared with 2021. WWTP-B4 (mixed WWTP) recorded the highest daily load in the winter of 2023, mainly due to the contribution of ofloxacin (H/VA, 2482.74 ± 474.39 g/day), azithromycin (HA, 888.36 ± 445.74 g/day), roxithromycin (H/VA, 728.49 ± 92.99 g/day) and florfenicol (VA, 190.82 ± 177.72 g/day) (Fig. S3). Notably, tilmicosin was exclusively detected in WWTP-B4 in 2023, representing the most intricate influent compositions involving communities, hospitals and animal husbandry. The high-level load of tilmicosin (VA, 734.32 ± 361.95 g/day), accompanied by a 32% increase in 2023, highlights the concerning extent of antibiotic use in industrial livestock and poultry farming in site B during the sampling campaign of 2023.
The population size served by selected WWTPs was estimated using local census data (Table S1). The surge in PNDLs was apparent between 2021 (471.10 ± 272.51 mg/day/1000inh) and 2023 (9403.31 ± 5349.66 mg/day/1000inh), increasing by 63%–3734% in 2023. WWTP-A3, catering to industrial parks and townships, showed the most significant increase (mixed WWTP: 3734%), followed by WWTP-B5 (domestic WWTP: 3537%) in 2023, which recorded the highest PNDLs at 5094.75 ± 3826.26 mg/day/1000inh. Little variation in population size between 2021 and 2023 is likely, given the country’s strict controls during the period of “zero-COVID” policy. Therefore, there was no reason to believe that the changes of PNDLs of antibiotics were due to population variation. More information about the PNDLs of the studied compounds is presented in Section (Patterns of antibiotics). Additional support to the likelihood that our findings represent a genuine temporal increase in overall antibiotic concentrations and PNDLs in stable populations is provided by our measurement of NH4-N concentration, which can be regarded as a control compound and population size indicator in this context since it is consistently found in wastewater. As shown in Table S1, NH4-N concentrations were maintained a steady level across the whole sampling period in both 2021 and 2023, and no statistically significant difference in NH4-N concentrations was found between two groups.
Patterns of antibiotics
The changes in antibiotic levels between 2021 and 2023 are categorised by drug classes (Fig. 4). In the absence of robust data, there is a possibility that physicians, faced with the absence of effective treatment, may have resorted to combining various antiviral drugs and antibiotics for the management of COVID-19. Some patients with ordinary influenza and other common respiratory tract infections are likely to have self-medicated with the above-mentioned or similar drugs in an attempt to treat suspected COVID-19 infection since many antibiotics are available over the counter (without prescription) in China. Consequently, a discernible increase in the population-normalised daily load of antibiotics in 2023 was observed (p < 0.05).
Macrolides
Azithromycin, detected across all WWTPs, was initially considered as a potential therapeutic option for COVID patients in the early stage of the pandemic49,50. Azithromycin was one of the antibiotics with the highest PNDLs, with a 1253% increase from 161.75 ± 83.73 mg/day/1000inh in 2021 to 2027.91 ± 989.76 mg/day/1000inh in 2023 (p < 0.05). This trend aligns with previous studies reporting a 400% increase in usage in Spain during the COVID-19 pandemic51, and a 36% increase in Greece20.
Roxithromycin (H/VAs), clarithromycin (HAs), and erythromycin (H/VAs) were also detected in 100% of WWTPs with increases of 2332%, 1172% and 2516%, respectively, in 2023 compared with 2021. The elevated PNDLs of azithromycin, roxithromycin, clarithromycin and erythromycin in the sampling period of 2023 suggest that these drugs were preferred choices for symptomatic patients and prophylactic use after exposure during the initial wave of cases following the easing of COVID-19 related restrictions. The fact that these studies presented similar increased trends in antibiotic use in the early stage of the COVID-19 pandemic, provides confidence in our findings and demonstrates that antibiotic usage increased worldwide7,20,51,52,53. However, during the first surge of cases in 2023, the increased rate of macrolides in Eastern China was significantly higher than the historical level in China22.
Quinolones
A previous study has demonstrated that fluoroquinolones, particularly ciprofloxacin, exert a strong capacity for binding to SARS-CoV-2 main protease, suggesting their potential utility as an adjunct treatment in COVID-1954. Ofloxacin, the most widely used antibiotic in quinolones, showed the highest abundance across all WWTPs in both 2021 and 2023, with PNDLs increasing from 109.52 ± 94.45 mg/day/1000inh (2021) to 3968.54 ± 3670.12 mg/day/1000inh (2023). Norfloxacin is the second most prevalent quinolone in wastewater influent in both 2021 (21.43 ± 13.93 mg/day/1000inh) and 2023 (506.62 ± 535.2 mg/day/1000inh), representing a 2264% increase.
Distinctive usage patterns of ciprofloxacin and lomefloxacin were found in the two sampling regions during the COVID-19 pandemic in 2023. While these drugs were not detected at the pure domestic WWTP of Site A (WWTP-A2), they were detected at the pure domestic WWTP of Site B (WWTP-B5), underscoring divergent antibiotic prescribing practices between the two locations. Lomefloxacin, categorised as a Reserve group antibiotic by WHO, is prohibited in food-producing animals and is recommended as a last-line option for human treatments. The high levels of lomefloxacin in WWTP-B5, observed in both 2021 and 2023, suggest potential long-term misuse of this drug at Site B, emphasising the need for vigilant monitoring and regulation.
Sulfonamides
Sulfamethoxazole, primarily used as a human antibiotic in China, was consistently detected in all samples in both 2021 and 2023. There was an increase of 1647% in 2023 (PNDLs: 208.60 ± 150.60 mg/day/1000inh) compared to 2021 (p < 0.01). To verify the potential disposal events in the sewage system, paired parent-metabolite analysis was conducted24. The results showed that the ratio of sulfamethoxazole and its metabolite (SMX/aSMX) was consistent in all WWTPs, ranging from 0.61 to 1.41. The levels and trends of sulfamethoxazole and its metabolite were similar over seven days in wastewater in both sampling campaigns. This suggests that direct disposal of unused sulfamethoxazole was not occurring, as indicated by the stable SMX/aSMX ratio.
Phenicols and tetracyclines
Chloramphenicol and doxycycline, classified as human antibiotics, were only detected in 2021, with the PNDLs being 0.71 ± 1.07 mg/day/1000inh and 36.21 ± 52.53 mg/day/1000inh, respectively. These drugs were not detected in the pure domestic WWTP(B5) in 2021, suggesting that the contributions came from pharmaceutical factories at Site B in that period. Many studies have shown that tetracycline resistance persists for a long period even after discontinuation of drug use55. Despite doxycycline being one of the most widely used antibiotics globally, the results indicate that tetracyclines, including doxycycline, were not commonly used or produced in Eastern China in 2021 to 2023.
Correlations of antibiotics/metabolites: co-prescribing patterns
Correlation analysis of weekly averages in wastewater samples was performed to explore the potential of co-prescribing patterns in both 2021 and 2023 (Fig. 5). Several antibiotics in wastewater samples showed significant correlations (p < 0.05) with more than 50% of all analytes in both periods. Specifically, in 2021, ofloxacin, sulfadiazine, and trimethoprim showed significant correlations, while in 2023, roxithromycin, clarithromycin, erythromycin, norfloxacin, ciprofloxacin, and sulfadiazine displayed such correlations. These findings underscore the popularity of these antibiotics as broad-spectrum options for co-prescribing across various drug classes. Co-prescribing in 2023 showed a greater diversity of drug combinations exhibiting strong correlations (p < 0.05) between macrolides and quinolones, including roxithromycin and norfloxacin (r = 0.71), roxithromycin and ciprofloxacin (r = 0.94), roxithromycin and lomefloxacin (r = 0.84), erythromycin and ciprofloxacin (r = 0.84), clarithromycin and ciprofloxacin (r = 0.67), and erythromycin and lomefloxacin (r = 0.71). Furthermore, a strong correlation between ofloxacin and roxithromycin (r = 0.89, p < 0.01) was observed at Site A in 2023. This co-prescribing pattern was not observed with ofloxacin as a broad-spectrum antibiotic, when co-prescribed with macrolides at Site B, underscoring regional variations in ofloxacin usage between the two areas (Fig. S6).
The relationships between parent compounds and metabolites were investigated further. Sulfamethoxazole and its major metabolite also demonstrated strong positive correlations (r = 0.85, p < 0.05 for 2021; and r = 0.53, p < 0.05 for 2023). Furthermore, correlations between antibiotics commonly prescribed as a combination were also observed. Specifically, sulfamethoxazole, sulfadiazine and trimethoprim, often prescribed as co-trimoxazole (sulfamethoxazole: trimethoprim = 5:1) and potentiated sulfonamides (sulfamethoxazole: sulfadiazine: trimethoprim = 5:5:2), exhibited positive correlations56,57. Positive correlations were observed between acylated sulfamethoxazole and sulfadiazine (r = 0.44 and 0.50 for 2021 and 2023 respectively, both p < 0.05), acylated sulfamethoxazole and trimethoprim (r = 0.52 and 0.73 for 2021 and 2023 respectively, both p < 0.05) and sulfadiazine and trimethoprim (r = 0.80 and 0.75 for 2021 and 2023 respectively, both p < 0.05). Similar PNDLs for sulfadiazine and trimethoprim may be due to concurrent mass production of these two drugs in local manufacturing plants. The ratio of sulfamethoxazole/trimethoprim, 3.19 ± 1.30 for 2021 and 5.33 ± 1.38 for 2023 respectively, suggests that co-trimoxazole was a more common co-prescribing pattern for sulfonamides in Eastern China, compared to potentiated sulfonamides. This insight is crucial as it sheds light on consumption and disposal patterns, akin to the information derived from the ratio of parent antibiotics to metabolites22. Understanding the correlations among co-prescribed antibiotics in wastewater is pivotal for unravelling the dynamics of antibiotic usage and disposal, providing valuable data for informed decision-making and regulatory considerations. Although guidance for treating COVID-19 and prescribing antibiotics has been updated to improve the management of bacterial infections, it remains crucial to develop comprehensive training curricula in Site A and Site B. These curricula are essential for equipping healthcare providers with a thorough understanding of best practices for antibiotic use, as well as risks and health implications associated with unnecessary antibiotic prescribing. In addition, antibiotic stewardship practices need to be extended into the community to address continuing practices of self-medication as well as agricultural antibiotic use.
Community-wide intake calculations of antibiotics
Back-calculations estimating population-normalised daily intake (PNDI) were performed for 12 HAs and H/VAs using correction factors (CFs)58 and PNDLs (Fig. 6). These calculated CF values were used to back-calculate the mass of the drug consumed at the community level. This process involved utilising the quantified number of antibiotics or their respective metabolites in wastewater and considering the proportion by which the target analyte is excreted through urine or faeces.
a PNDIs across the surveyed catchment (Site A and Site B, n = 70 biologically independent samples) community, b PNDIs for Site A (n = 42 biologically independent samples), and c PNDIs for Site B (n = 28 biologically independent samples). The blue boxes represent data from 2021, while the red boxes represent data from 2023. Box plots display median (line within box), 25% and 75% percentiles (box limits), minima and maxima (whiskers). Statistical differences between 2021 and 2023 were evaluated using a two-sided Wilcoxon signed-rank test. Compounds marked with (*) indicate statistical significance with p < 0.05, (**) with p < 0.01, and (***) with p < 0.001.). Source data are provided as a Source Data file.
There was an overall increase in the consumption of antibiotics in 2023 compared to 2021 (p < 0.001, Tables S3 and S4). Upon the application of the appropriate correction factors, an analysis was conducted utilising indices of difference, ratio, and relative increase rate for PNDIs. This analytical approach was employed to investigate the variations in antibiotic consumption between the years 2021 and 2023. Azithromycin had the highest PNDI up to 16,899 mg/day/1000inh in 2023 with a relative increase rate of 1154% (p < 0.001). Azithromycin, as a macrolide antibiotic, gained attention as a possible therapeutic agent for patients with COVID-19 in the early stage of the pandemic and this may explain these results49,50. Ofloxacin was 36 times higher in 2023 (4960.67 mg/day/1000inh) than in 2021 (1347.91 mg/day/1000inh), with an increase of 3524% (p < 0.001), and was the most commonly detected quinolones antibiotic. A previous study has confirmed the efficacy of acetylate sulfamethoxazole in accurately estimating community antibiotic intake of sulfamethoxazole48. Acetylate sulfamethoxazole increased by 1616% in 2023, surpassing consumption levels of antibiotics observed in historical studies conducted in different periods in China59,60,61,62.
A study conducted in Shanghai, China, showed that the concentrations of urinary antibiotics during the COVID-19 pandemic were significantly higher than those recorded both before the pandemic and during its less turbulent phases35. A meta-analysis showed that the prevalence of bacterial infection in the early stage of the COVID-19 pandemic was approximately 8.6%53. Despite this relatively low prevalence, an average of 64% of patients received antibiotics based on an analysis of 154 studies, with 115 conducted in China. The WHO recommends against the empiric use of antibiotics in patients with mild COVID-19. A review of 514 studies found that antibiotics prescriptions in COVID-19 patients overall declined from 82.3% to 39.7% between the first and second phases of the pandemic, but much more markedly in mild and moderate cases10.
Notably, the total PNDIs in 2023 were similar in Sites A and B, while the rate of increase was higher in Site A. PNDIs were in good agreement between two sites in 2023, except for roxithromycin (site A: 179.70 mg/day/1000inh, Site B: 4659.28 mg/day/1000inh), clarithromycin (Site A: 846.78 mg/day/1000inh, Site B: 1548.26 mg/day/1000inh), erythromycin (site A: 223.60 mg/day/1000inh, Site B: 882.90 mg/day/1000inh), ciprofloxacin (Site A: 20.01 mg/day/1000inh, Site B: 517.93 mg/day/1000inh) and sulfadiazine (Site A: 11.89 mg/day/1000inh, Site B: 236.28 mg/day/1000inh). Higher PNDIs in Site B revealed different consumption patterns for these drugs between the two regions, indicating more conservative use of antibiotics in Site A.
Comparison of WBE to prescription data
Catchment prescription data for Site A was compared with PNDIs in wastewater (Table 1). Generally, the level of increase observed via WBE (919.23% ~ 5046.29%) was consistent with prescription data (2484.00% ~ 3944.99%), suggesting a noteworthy increase in prescriptions and antibiotic use following the surge of COVID-19 cases in 2023 after the lifting of restrictions. A previous study demonstrated considerable empirical use of antibiotics for mild and moderate cases of COVID-19, which is not recommended by WHO guidelines10. Increased incidence of bacterial and other viral respiratory infections (such as colds and flu) due to increased transmission and reduced immunity following the end of prolonged restrictions on population movement and mixing due to COVID-19 may also have contributed to the unwarranted use of antibiotics in Site A.
Whilst prescribing data is an invaluable source of information for identifying prescribing trends, it does not necessarily give a true representation of how antibiotics are being used in the community. The increase in prescription rates of Azithromycin and Clarithromycin was higher than the levels detected in wastewater, indicating that patients might not have been fully adherent to their prescribed treatments and might have been stockpiling antibiotics for subsequent use. Conversely, some antibiotics were not recorded as prescribed in the electronic record system at Site A, but were still quantifiable in wastewater. Their presence in wastewater could be due to several factors, including prescriptions outside the catchment area, antibiotics purchased over the counter or online, delayed prescriptions or potential re-use of prescriptions These factors highlight the complexity of estimating community consumption from wastewater and underscore the value of prescription data as a supplementary data source for assessing what is actually being used in the community.
Limitations
Several limitations should be noted in this study. First, the average residence time of wastewater in sewers can vary with population size and distance from the treatment plant, but our calculations assumed uniform loss rates from toilets to WWTPs. Future research should focus on improving understanding of the targets and specific sampling regions. This could include modelling sewer system conditions to account for predicted residence time, as well as incorporating factors such as seasonal flow rates and temperature variations. Secondly, we do not have WBE data on antibiotic levels before COVID-19. Thirdly, in the present study, due to technical limitations, the presence of cephalosporin antibiotics could not be determined. UPLC-MS/MS conditions will be optimised for obtaining highly sensitive and selective analysis of cephalosporin antibiotics in future research. Furthermore, due to the small number of wastewater samples observed and limited medical data available, more extensive analyses are required. In future work, we will analyse antibiotics resistance data from samples that were collected during the same period to evaluate the impact of the “zero-COVID” policy on ABR trends in the surveyed communities.
To the best of our knowledge, data about antibiotic consumption monitoring during the pandemic period, particularly for specific drugs of interest are limited. This study proposed WBE as an effective tool for estimating community-wide exposure to antibiotics, including the analysis of both parent compounds and metabolites. In addition, this study represents the first use of WBE in China to monitor and compare antibiotics consumption and patterns before (2021) and after (2023) a national policy change. Antibiotic usage increased substantially after the easing of the “zero-COVID” policy in 2023. ABR requires ongoing attention and will continue to be an important issue both in China and globally. In 2023, there was an increased proportion of antibiotic use in our sampling sites from the Watch group and unwarranted combination prescribing, primarily driven by the contributions of macrolides and quinolones. This suggests a rise in the empiric and potentially inappropriate use of antibiotics or coinfections following the surge of COVID-19 cases that year. WBE and prescription data showed similar trends in antibiotic use. However, WBE is a superior monitoring tool, particularly during exceptional periods, as it offers a more comprehensive view of antibiotic consumption within the catchment area. Unlike prescription data, which only reflect medications prescribed by qualified healthcare providers that may or may not be consumed within the monitoring catchment, WBE captures antibiotic consumption from all sources including those bought without prescription and those obtained outside the monitoring catchment area.
Methods
Chemicals and materials
The selected twenty seven antibiotics cover a wide range of antibiotic classes including macrolides (azithromycin, roxithromycin, clarithromycin, erythromycin, spiramycin and tilmicosin), quinolones (ofloxacin, norfloxacin, ciprofloxacin, lomefloxacin, pefloxacin, enrofloxacin, danofloxacin, and difloxacin), sulfonamides and metabolites (sulfamethoxazole and acetylated sulfamethoxazole, sulfadiazine, trimethoprim, sulfamethazine and acetylated sulfamethazine), phenicols (chloramphenicol, florfenicol and thiamphenicol) and tetracyclines (doxycycline, tetracycline, oxytetracycline and chlortetracycline). A total of six stable isotope–labelled internal standards (ISTD) were used for the quantification of the target compounds. A full list can be found in Table 2.
Analytical standards and deuterated standards were purchased from Dr. Ehrenstorfer (Germany). HPLC grade methanol (MeOH) and acetonitrile were purchased from Supelco (USA) and formic acid (>95%) was purchased from Aladdin (China), respectively. Stock solutions were prepared in MeOH or acetonitrile at 1.0 mg/mL and mixed working solutions containing all analytes were diluted from the stock solutions to a final concentration of 1.0 mg/L in methanol. Stock and working solutions were stored at −20 °C.
Study areas and sampling methods
Five WWTPs were selected, including three from Zhejiang Province (Site A) and 2 from Jiangsu Province (Site B), (Fig. S1). These WWTPs serve a range of population sizes, from 8730 to 634,000, and manage various types of wastewater, including domestic, industrial, and a combination of both. Detailed information on WWTPs coverage and population sizes is provided in Table S1. The study comprised two sampling campaigns which were carried out over seven consecutive days in January and February of 2021 and 2023, respectively, during which 24-hour flow proportional wastewater samples were collected from the influents of the WWTPs. The sampling approach involved using an auto-sampler (JH-8000D, Qingdao) to collect 10 mL of the influents at regular fifteen-minute time intervals for 24 hour. A total of 70 wastewater samples were collected (Site A: n2021 = 21, n2023 = 21. Site B: n2021 = 14, n2023 = 14.). On the day of sampling, 50 mL of the composite wastewater samples were spiked in duplicate with 50 ng of an internal standard mixture (50 μL of 1 mg/L mix) within 12 hours.
Sample preparation and analysis
A method utilising the isotope dilution ultra-high performance liquid chromatography coupled with triple quadrupole tandem mass spectrometry (UPLC-MS/MS) was used to measure twenty-seven antibiotics in wastewater samples. All samples were pre-treated and analysed immediately after the end of the sampling campaigns in 2021 and in 2023, to avoid chemical degradation. Details on the methods used for sample preparation and analysis can be found elsewhere63,64. The method detection limits (MDLs) ranged from 0.004 to 0.296 ng/L, and the recoveries of 27 antibiotics varied between 70.0 and 120.8% (Table S5).
Briefly, 50 mL of samples were filtered through a GF/F glass fibre filter (0.7 μm, Whatman, USA) and the filtrates were loaded under vacuum onto pre-conditioned Oasis HLB cartridges (60 mg, Waters, USA) at 5 mL/min. The cartridges were conditioned and equilibrated with 2 mL of MeOH, followed by 2 mL of HPLC water under gravity. The retained antibiotics on the SPE cartridge were eluted with 4 mL of MeOH. The eluate was evaporated to dryness at 40 °C under a gentle nitrogen flow (LabTech, China) and reconstituted with 0.5 mL of 4:1 H2O: MeOH before transferring to injection vials (Waters, USA).
UPLC-MS/MS was performed using a ACQUITY UPLC™ system (Acquity TMUltra Performance LC, Waters, USA) coupled to a ABSCIEX QTRAP Mass Spectrometer (QTRAP 6500+, ABSCIEX, USA). A reverse-phase ACQUITY UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm) were used to separate analytes under a 11.5 min mobile phase gradient using 2:3 methanol: acetonitrile (mobile phase A) and 0.2% formic acid water (mobile phase B). Mass spectrometry was performed via fully targeted multiple reaction monitoring (MRM), involving two MRM transitions per analyte and one per ISTD. Detailed information on chromatographic separation as well as mass spectrometry parameters can be found elsewhere54.
Quality control
All analyses of wastewater samples were performed by the same analytical team in the same laboratory. The detection was performed under optimal conditions, and two parallel detections were performed for each sample. Each batch analysed 70 real wastewater samples, eight spiked wastewater samples, and five quality control samples (QC). A QC sample was inserted into every 20 samples for quality control to monitor analyte calibration and physical-chemical behaviour between the varying sample compositions. The accuracies of quality control samples were between 89.2 and 114.7%. The inter-batch relative standard deviations of recoveries ranged from 7.1% to 14.9%. In addition, to check whether there was any potential contamination during the sample preparation stage and instrument analysis, ultrapure water blanks were spiked only with IS and used to check for any possible background concentration of target analytes. None of the target analytes were detected in these spiked blanks, suggesting that there was no contamination during the experiments.
Prescription data
Electronic medical record (EMR) data was obtained from the country health authority with assistance from the Center for Disease Control and Prevention (CDC) in Site A. The EMR data from one community health centre and five township health centres were utilised to analyse total prescription quantities of pharmaceuticals for the WWTP catchments during the study periods of 2021 and 2023. Data on antibiotics purchased over the counter or online as well as from private hospitals were not available. The EMR data tracks daily transactions for healthcare services including the date, amount, location and name of the healthcare facility, as well as inpatient and outpatient visits, diagnoses, examinations, and treatment (including pharmaceuticals). In January 2021, 407 antibiotic prescriptions were obtained from 1st to 31st of January, while in January 2023, 11,150 were recorded. Prescription data were not available for Site B. The study was approved by the Institutional Review Board of the School of Public Health, Fudan University (Ethics approval: IRB#2019-03-0733).
WBE calculations
The daily load (DL, mg/day), population-normalised daily load (PNDL, mg/day/1000 inh) and population-normalised daily intake (PNDI, mg/day/1000 inh) were calculated using the following equations according to previous WBE publications20,22. (1) DLanalyte (mg/day) = Canalyte × F; (2) PNDL (mg/day/1000 inh) = \(\frac{{C}\times \,F}{{PE}\,\times \,{10}^{3}}\); and (3) PNDI (mg/day/1000 inh) = PNDL × CF. In equation (1), Canalyte is the concentration of the analyte (ng/L) in influent wastewater, and F is the flow rate of wastewater (L/day). In equation (2), PE is the population size of the surveyed community. In equation (3), CF is the correction factors used to justify metabolism rate for each analyte.
Statistical analysis
We employed the Wilcoxon test to examine the differences in the levels of antibiotics and metabolites between 2021 and 2023. An analysis of variance (ANOVA) test was used to compare the antibiotic levels across seven consecutive days. Pearson correlation analysis was used to explore the potential relationships between parent compounds/metabolites and co-prescribing patterns. Bonferroni-adjustment for multiple testing was applied where appropriate. A two-sided statistical significance level of was set at p < 0.05. All data collection and analysis were performed using Microsoft Excel version 2019 (Microsoft Office, Redmond, USA), SPSS Statistics version 26.0 (IBM, Armonk, NY, USA), or GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are provided in the article, Source data file and Supplementary Information file. The antibiotic prescribing data and wastewater data from this study are held in coded form at local Centers for Disease Control and Prevention (CDC) in study areas. While legal data sharing agreements between CDCs and theirs superior departments in charge (e.g., Health Commission or local government) prohibit CDCs from making the data publicly available. Access may be granted to those who through a request with specific data needs, analysis plans, and dissemination plans to N.W. (e-mail: na.wang@fudan.edu.cn). The author will give feedback within 30 days. Source data are provided with this paper.
References
Wang, C., Horby, P. W., Hayden, F. G. & Gao, G. F. A novel coronavirus outbreak of global health concern. Lancet 395, 470–473 (2020).
World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19--−11-march-2020 (2020).
Normile, D. ‘Zero COVID’ is getting harder-but China is sticking with it. Science 374, 924 (2021).
Du, Z. et al. Estimate of COVID-19 Deaths, China, December 2022-February 2023. Emerg Infect Dis 29, 2121–2124 (2023).
Lancet, T. The COVID-19 pandemic in 2023: far from over. Lancet 401, 79 (2023).
Lewis, D. China’s COVID wave has probably peaked, model suggests. Nature 613, 424–425 (2023).
Cong, W. et al. Antimicrobial use in COVID-19 patients in the first phase of the SARS-CoV-2 pandemic: a scoping review. Antibiotics 10, 745 (2021).
Ghosh, S., Bornman, C. & Zafer, M. M. Antimicrobial resistance threats in the emerging COVID-19 pandemic: where do we stand?. J. Infect. Public Health 14, 555–560 (2021).
World Health Organization (WHO). PreventionWeb. https://www.preventionweb.net/organization/world-health-organization-who (2007).
Cong, W. et al. Antibiotic use and bacterial infection in COVID-19 patients in the second phase of the SARS-CoV-2 pandemic: a scoping review. Antibiotics 11, 991 (2022).
Kardas, P., Devine, S., Golembesky, A. & Roberts, C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int. J. Antimicrob. Agents 26, 106–113 (2005).
Wen, J. et al. Stability and WBE biomarkers possibility of 17 antiviral drugs in sewage and gravity sewers. Water Res. 238, 120023 (2023).
Boogaerts, T. et al. Current and future perspectives for wastewater-based epidemiology as a monitoring tool for pharmaceutical use. Sci. Total Environ. 789, 148047 (2021).
Huizer, M., Ter Laak, T. L., de Voogt, P. & van Wezel, A. P. Wastewater-based epidemiology for illicit drugs: a critical review on global data. Water Res. 207, 117789 (2021).
Zarei, S. et al. A global systematic review and meta-analysis on illicit drug consumption rate through wastewater-based epidemiology. Environ. Sci. Pollut. Res. Int. 27, 36037–36051 (2020).
Mtetwa, H. N., Amoah, I. D., Kumari, S., Bux, F. & Reddy, P. Surveillance of multidrug-resistant tuberculosis in sub-Saharan Africa through wastewater-based epidemiology. Heliyon 9, e18302 (2023).
Gracia-Lor, E., Zuccato, E., Hernández, F. & Castiglioni, S. Wastewater-based epidemiology for tracking human exposure to mycotoxins. J. Hazard. Mater. 382, 121108 (2020).
Langa, I., Gonçalves, R., Tiritan, M. E. & Ribeiro, C. Wastewater analysis of psychoactive drugs: non-enantioselective vs enantioselective methods for estimation of consumption. Forensic Sci. Int. 325, 110873 (2021).
Zheng, Y. et al. Source tracking and risk assessment of pharmaceutical and personal care products in surface waters of Qingdao, China, with emphasis on influence of animal farming in rural areas. Arch. Environ. Contam. Toxicol. 78, 579–588 (2020).
Galani, A. et al. Patterns of pharmaceuticals use during the first wave of COVID-19 pandemic in Athens, Greece as revealed by wastewater-based epidemiology. Sci. Total Environ. 798, 149014 (2021).
Andrés-Costa, M. J., Escrivá, Ú., Andreu, V. & Picó, Y. Estimation of alcohol consumption during “Fallas” festivity in the wastewater of Valencia city (Spain) using ethyl sulfate as a biomarker. Sci. Total Environ. 541, 616–622 (2016).
Xu, L. et al. Assessment of community-wide antimicrobials usage in Eastern China using wastewater-based epidemiology. Water Res. 222, 118942 (2022).
Holton, E. et al. Quantifying community-wide antibiotic usage via urban water fingerprinting: focus on contrasting resource settings in South Africa. Water Res. 240, 120110 (2023).
Sims, N. et al. Community infectious disease treatment with antimicrobial agents—a longitudinal one year study of antimicrobials in two cities via wastewater-based epidemiology. J. Hazard. Mater. 454, 131461 (2023).
Zuccato, E. et al. Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environ. Health Glob. Access Sci. Source 4, 14 (2005).
Mao, K., Zhang, H., Pan, Y. & Yang, Z. Biosensors for wastewater-based epidemiology for monitoring public health. Water Res. 191, 116787 (2021).
Wang, Y. et al. Estimates of dietary exposure to antibiotics among a community population in East China. Antibiotics 11, 407 (2022).
Wang, Y. et al. Antibiotic residues of drinking-water and its human exposure risk assessment in rural Eastern China. Water Res. 236, 119940 (2023).
Cheng, W., Chen, H., Su, C. & Yan, S. Abundance and persistence of antibiotic resistance genes in livestock farms: a comprehensive investigation in eastern China. Environ. Int. 61, 1–7 (2013).
Chen, Y.-R. et al. Antibiotic resistance genes (ARGs) and their associated environmental factors in the Yangtze Estuary, China: from inlet to outlet. Mar. Pollut. Bull. 158, 111360 (2020).
Cai, S. et al. Impacts of antibiotic residues in the environment on bacterial resistance and human health in Eastern China: an interdisciplinary mixed-methods study protocol. Int. J. Environ. Res. Public Health 19, 8145 (2022).
World Health Organization (WHO). 2021 AWaRe classification. https://www.who.int/publications/i/item/2021-aware-classification (2021).
World Health Organization (WHO). Clinical management of COVID-19. https://www.who.int/publications/i/item/clinical-management-of-covid-19 (2020).
Cong, W. et al. Prevalence of antibiotic prescribing in COVID-19 patients in China and other low- and middle-income countries during the pandemic (December 2019—March 2021): a systematic review and meta-analysis. J. Antimicrob. Chemother. 78, 2787–2794 (2023).
Hu, Y. et al. COVID-19 pandemic impacts on humans taking antibiotics in China. Environ. Sci. Technol. 56, 8338–8349 (2022).
Rawson, T. M. et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71, 2459–2468 (2020).
Russell, C. D. et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2, e354–e365 (2021).
Baghdadi, J. D. et al. Antibiotic use and bacterial infection among inpatients in the first wave of COVID-19: a retrospective cohort study of 64,691 patients. Antimicrob. Agents Chemother. 65, e0134121 (2021).
Goncalves Mendes Neto, A. et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 93, 1489–1495 (2021).
Pettit, N. N. et al. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect. Dis. 21, 516 (2021).
Yang, X. et al. Florfenicol and oxazolidone resistance status in livestock farms revealed by short- and long-read metagenomic sequencing. Front. Microbiol. 13, 1018901 (2022).
Hanna, N. et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: its potential for resistance development and ecological and human risk. Environ. Int. 114, 131–142 (2018).
Wang, H. et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 89–90, 204–211 (2016).
Yang, M. et al. Study of a new matrix certified reference material for accurate measurement of florfenicol in prawn meat powder. Measurement 185, 110011 (2021).
Bengtsson-Palme, J. & Larsson, D. G. J. Concentrations of antibiotics predicted to select for resistant bacteria: proposed limits for environmental regulation. Environ. Int. 86, 140–149 (2016).
Malik, S. S. & Mundra, S. Increasing consumption of antibiotics during the COVID-19 pandemic: implications for patient health and emerging anti-microbial resistance. Antibiotics 12, 45 (2022).
Laxminarayan, R. et al. Access to effective antimicrobials: a worldwide challenge. Lancet 387, 168–175 (2016).
Ang, L., Hernández-Rodríguez, E., Cyriaque, V. & Yin, X. COVID-19’s environmental impacts: challenges and implications for the future. Sci. Total Environ. 899, 165581 (2023).
Miranda, C. et al. Implications of antibiotics use during the COVID-19 pandemic: present and future. J. Antimicrob. Chemother. dkaa350 https://doi.org/10.1093/jac/dkaa350 (2020).
Antonazzo, I. C. et al. Azithromycin use and outcomes in patients with COVID-19: an observational real-world study. Int. J. Infect. Dis. 124, 27–34 (2022).
Gonzalez-Zorn, B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 27, 646–647 (2021).
Chen, X. et al. Occurrence and risk assessment of pharmaceuticals and personal care products (PPCPs) against COVID-19 in lakes and WWTP-river-estuary system in Wuhan, China. Sci. Total Environ. 792, 148352 (2021).
Langford, B. J. et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin. Microbiol. Infect. 27, 520–531 (2021).
Karampela, I. & Dalamaga, M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch. Med. Res. 51, 741–742 (2020).
Narendrakumar, L., Joseph, I. & Thomas, S. Potential effectiveness and adverse implications of repurposing doxycycline in COVID-19 treatment. Expert Rev. Anti Infect. Ther. 19, 1001–1008 (2021).
Khan, S. M. et al. Activity of (1-benzyl-4-triazolyl)-indole-2-carboxamides against Toxoplasma gondii and Cryptosporidium parvum. Int. J. Parasitol. Drugs Drug Resist. 19, 6–20 (2022).
Thiebault, T. Sulfamethoxazole/Trimethoprim ratio as a new marker in raw wastewaters: a critical review. Sci. Total Environ. 715, 136916 (2020).
Holton, E., Sims, N., Jagadeesan, K., Standerwick, R. & Kasprzyk-Hordern, B. Quantifying community-wide antimicrobials usage via wastewater-based epidemiology. J. Hazard. Mater. 436, 129001 (2022).
Jia, A., Wan, Y., Xiao, Y. & Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 46, 387–394 (2012).
Yan, Q. et al. Occurrence and fate of pharmaceutically active compounds in the largest municipal wastewater treatment plant in Southwest China: mass balance analysis and consumption back-calculated model. Chemosphere 99, 160–170 (2014).
Gao, L. et al. Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere 86, 665–671 (2012).
Zhang, Y. et al. Wastewater-based epidemiology in Beijing, China: prevalence of antibiotic use in flu season and association of pharmaceuticals and personal care products with socioeconomic characteristics. Environ. Int. 125, 152–160 (2019).
Wang, N., Zang, J., Fu, C., Jiang, F. & Jiang Q. Ultra high performance liquid chromatography tandem mass spectrometry for rapid analysis of antibiotics in wastewater. Chinese Patent: ZL2022101679989.
Zang, J. et al. Loads and seasonal variation of antibiotics in industrial wastewater in typical small towns of Jiangsu Province and Zhejiang Province. Occup. Health 38, 2388–2395 (2022).
Acknowledgements
The authors would like to thank the managers of WWTPs for their support during the sampling period and the information provided on the wastewater characteristics. Also, this study received support from local Centers for Disease Control and Prevention (CDC). This work was supported by the National Nature Science Foundation of China (81861138050, C.F.) and the Medical Research Council and Newton Fund through a UK-China AMR Partnership Hub Award (MR/S013717/1, H.L.).
Author information
Authors and Affiliations
Contributions
N.W., B.K.H., C.F., Q.J. and H.L. designed the study. J.Z., L.J. and Y.W. collected samples and performed experiments. J.Z., L.J., Y.W. and Y.C. analysed data. J.Z. wrote the manuscript. N.W., B.K.H., Y.C., C.F., Q.J. and H.L. gave technical support and conceptual advice. All authors contributed to the revised manuscript. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zang, J., Jiang, L., Wang, Y. et al. Impact of easing COVID-19 restrictions on antibiotic usage in Eastern China using wastewater-based epidemiology. Nat Commun 15, 10161 (2024). https://doi.org/10.1038/s41467-024-54498-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54498-2