Abstract
Potyvirids are the largest group of plant RNA viruses. Pelota, a core component of RNA quality controls (RQC), promotes the degradation of potyvirids’ genomic RNA by recognizing a specific G1-2A6-7 motif. Here we demonstrate that the viral RNA-dependent RNA polymerase, NIb, acts as a SUMOylation decoy to effectively reduce Pelota SUMOylation by competing with SCE1 to inhibit Pelota-mediated RQC. TuMV NIb is comprised of two functional SUMO interacting motif (SIM): SIM2 and SIM3. The former is identified as the key site for NIb’s SUMOylation by SUMO3, whereas the latter is responsible for the interaction with SCE1. These two SIMs are conserved among the majority of potyvirids-encoded NIbs. Thus, virus protein-mediated SUMOylation decoy strategy to suppress host defense may be a common feature in plant virus pathosystems. These findings highlight a dynamic interplay between plant defense mechanism and viral counter-strategy by orchestrating the post-translational modifications of virus and host defense components.
Similar content being viewed by others
Introduction
Plants inhabit environments teeming with abiotic and biotic challenges, including frequent exposure to viral infections. Plants have developed multi-layered defense lines to combat viral infections. Among them, RNA-targeting regulation mechanisms are generally considered an essential line of defense against RNA viruses. These include RNA silencing, RNA decay, and translation-coupled RNA quality control (RQC) mechanisms, targeting and degrading viral RNAs1,2. During the co-evolutionary arms race with their hosts, viruses have evolved sophisticated strategies to counteract, evade, or manipulate host antiviral defenses3,4,5. A dynamic balance between host defense and viral counter defense determines viral pathogenesis and symptom development. This ongoing tug-of-war highlights the adaptive capabilities of the plant host and the infecting virus.
RNA silencing is a well-characterized RNA-targeting defense mechanism that confers resistance to viruses in plants. Almost all viruses encode viral suppressors of RNA silencing (VSR) forged to fine-tune virus-host coexistence. This suggests a plant-virus co-evolution in the molecular arms race between host RNA silencing and virus counter-defense6,7. Due to the paramount roles of VSRs in viral infection, most work has primarily focused on elucidating the molecular mechanisms by which VSRs suppress antiviral RNA silencing in plants8,9,10. The contribution of other viral elements or proteins to the destruction of plant RNA-mediated defense mechanisms may have been underestimated1. Until recently, only a few studies have described the interplay between RQC and viruses in plants11,12,13,14. For instance, nonsense-mediated decay (NMD) has been identified as a viral restriction mechanism against pepino mosaic virus (PepMV) with m6A modification, and against pea enation mosaic virus 2 (PEMV2) with an unusual long 3’ untranslated region (UTR), and potato virus X (PVX) and turnip crinkle virus (TCV) with internally located termination codons (iTCs)11,14. The Pelota-mediated RQC can target and promote the degradation of potyvirids’ RNA, and this host anti-viral strategy is also involved in restricting the infection of the human immunodeficiency virus (HIV, a mammalian virus) and bamboo mosaic virus (BMV, a plant virus)13,15,16. SUMO1-modified Pelota (Dom34 in yeast) forms a heterodimeric complex with GTPase Hbs1 (Hsp70 subfamily B suppressor1) that has an important role in ribosomal rescue, and triggers RNA decay in plants17. Since the RNA recognition mechanisms involved in these antiviral responses are gradually revealed, viruses as successful cellular parasites are expected to evolve counteracting strategies to repress, manipulate or avoid these defenses. Studies have shown that plant viruses can evade NMD through several strategies, including the unstructured region of RNA downstream of the termination codon and the protection of viral transcripts by long-distance movement protein p2618. Several viruses evolved strategies to manipulate the host NMD components through RNA splicing and autophagy pathways19,20. However, the question of whether the plant viruses could suppress or evade the Pelota-mediated RQC pathway has not been investigated. Answers to this question will not only contribute to a better understanding of the roles of RQC in plant-microbe interactions, but also assess if RQC can be targeted for crop improvement with antiviral genetic resistance.
Potyvirids (viruses in the Potyviridae family) constitute the largest group of plant-infecting RNA viruses and cause worldwide crop yield losses21. Most potyvirids are characterized by single-stranded, positive-sense RNA genomes. These genomes encode a large polyprotein, which undergoes processing into nine or ten mature proteins22. A second small ORF, PIPO, is generated by an RNA polymerase slippage mechanism and is expressed as the trans-frame protein P3N-PIPO23,24. These viral proteins have different biological functions and promote virus infection partly by hijacking and manipulating host cellular components. Among the known viral proteins, the nuclear inclusion protein b (NIb) is the RNA-dependent RNA polymerase (RdRP) required for viral genome replication25. Beyond its conventional role in RNA replication, NIb has been implicated in regulating virus-host interactions26. Previous studies highlighted the dual functionality of NIb, as a critical component of the viral replication complexes (VRC) and as a recruiter of host proteins to enhance viral replication efficiency27,28,29. In a few cases, it has been demonstrated or suggested that NIb can subvert host defense responses. The NIb of turnip mosaic virus (TuMV) undergoes SUMOylation by SUMO-CONJUGATING ENZYME 1 (SCE1) and competes with NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1), effectively dampening salicylic acid-mediated immune responses in Arabidopsis30,31.
We have recently demonstrated that the SUMOylated Pelota contributes to the host restriction of potyvirids by the RQC pathway13. In this study, we showed that Pelota’s SUMOylation is suppressed during viral infection. We presented evidence that the NIb of potyvirids competitively interacts with the only SUMO-E2 enzyme, SCE1, leading to the inhibition of Pelota’s SUMOylation. Evolutionary comparisons and molecular experiments revealed the presence of two conserved functional SIMs on NIb: one for SUMO modification and the other for interaction with SCE1 that is responsible for the Pelota modification suppression. These findings shed light on the interplay of posttranslational modifications (PTMs), RQC, and plant-microbe co-evolution.
Results
TuMV infection decreases the Pelota SUMOylation
Our previous studies showed that the SUMOylated Pelota-mediated RQC mechanism by SUMO1 negatively regulates the infection of potyvirids by targeting a conserved G1-2A6-7 motif within the P3 cistron13. Based on the theory of a co-evolutionary arms race between plants and viruses, we speculate that potyvirids might evolve strategies for counter-defending the Pelota-mediated antiviral RQC activity. To test this hypothesis, we first analyzed the Pelota expression pattern under various biotic and abiotic stresses. Wild-type Arabidopsis plants were subjected to wounding (0.5 h and 2 h), cold stress (4 °C for 2 h), salicylic acid (SA) treatment (1 mM for 2 h), or TuMV infection for 14 days. Quantitative RT-PCR (qRT-PCR) revealed a slight upregulation of Pelota after wounding (0.5 h), and cold treatments, and TuMV infected rosette leaves (Fig. 1a, Supplementary Fig. 1a). However, no correlation was observed between the expression of Pelota and several plant virus defense-related systems, including RNA silencing and phytohormone response pathways (Supplementary Fig. 1b, c). These results suggest that the Pelota regulation might be at the level of protein or PTMs.
a qRT-PCR analysis of the Pelota expression level in Arabidopsis rosette and cauline leaves under TuMV infection. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown), and *p = 0.0318, ns means not significant (p = 0.0701) (p values determined by two-tailed Student’s t test). b In vivo SUMOylation analysis of Pelota. Proteins were immunoprecipitated with anti-GFP beads from extracts of Pelota: pelota transgenic plants. SUMOylated Pelota was detected with anti-SUMO1 antibody. TuMV accumulation was detected with anti-CP antibodies. CBB-staining of RbcL was a loading control. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel. c In vivo SUMOylation analysis of Pelota. Pelota-YFP and Myc-SUMO1 co-expressed with a gradient amount of TuMV in N. benthamiana leaves. Samples were treated with MG132 for 6 h before collected at 60 h post-infiltration (hpi). Extracted proteins were immunoprecipitated with anti-GFP beads, and SUMOylated Pelota and total proteins were detected with anti-Myc antibodies. TuMV accumulation was detected with anti-CP antibodies. CBB-staining of RbcL was a loading control. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that gels/blots were processed in parallel. d qRT-PCR analysis of P3 RNA accumulation during expression of Myc-GUS (GUS) or Myc-Pelota (Pelota) in TuMV infected N. benthamiana leaves at 40 hpi. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown), and **p = 0.0005, ns means not significant (p = 0.7360) (p values determined by two-tailed Student’s t test). e Competitive Co-IP assays in vivo. Pelota-YFP and Hbs1-Flag were co-expressed with a gradient amount of TuMV in N. benthamiana leaves. Note: samples were treated with MG132 for 6 h before collection to exclude the variations from protein degradation, and immunoprecipitated with anti-GFP beads. The immunoprecipitated and input proteins were then analyzed with anti-Flag and anti-GFP antibodies. CBB-staining of RbcL was a loading control. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel.
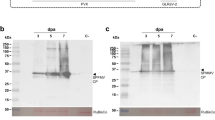
SUMOylation is an emerging PTM that has been shown to fine-tune the biological functions of many intracellular proteins by modulating protein stability, localization, or protein-protein interactions32. Considering that the activity of Pelota in RQC relies on its SUMO modification that determines its interaction with Hbs113, we compared the SUMOylation of Pelota under the conditions described above. Subsequent SUMOylation analysis with polyclonal antibodies against SUMO1 showed that TuMV infection for 14 days significantly reduced the SUMO modification of Pelota in Pelota-YFP: pelota transgenic Arabidopsis plants (Fig. 1b and Supplementary Fig. 1d, e). Further investigations with transient expression in Nicotiana benthamiana, indicated a correlation between the increased TuMV accumulation and the decreased SUMOylation of Pelota (Fig. 1c and Supplementary Fig. 1f). These data demonstrated a significant decrease in the SUMO modification level of Pelota during TuMV infection.
To further validate this observation, we used the YFP-P3 construct carrying G1-2A6-7 motif (a substrate of Pelota) to examine the functional impact of viral infection on eliminating aberrant RNA mediated by Pelota. GUS (used as control) or Pelota together with P19 (a potent RNA silencing suppressor to exclude the effect of RNA silencing) were co-expressed with YFP-P3 during TuMV infection. Subsequent qRT-PCR tests showed that the Pelota-mediated degradation of YFP-P3 transcripts was substantially blocked (Fig. 1d). The SUMO1-modified Pelota is crucial for Pelota recruitment of the GTPase Hbs1, and then triggers RNA decay13,17. Here, we also observed, the decrease in Pelota SUMO modification showed a correlation with a reduced Hbs1 immunoprecipitation (Fig. 1e and Supplementary Fig. 1g). Together, the TuMV infection decreases the SUMOylation level of Pelota and disrupts the Pelota mediated RQC.
NIb is the viral factor that inhibits Pelota SUMOylation
The above results encouraged us to explore this molecular basis driven by TuMV. SCE1 is the only SUMO conjugating enzyme in plants and is responsible for transferring SUMO directly onto Pelota13,33. We then performed a yeast two-hybrid (Y2H) to identify interactions between TuMV proteins and SCE1. Interestingly, the Y2H assay revealed that viral proteins CI, VPg, NIa, NIb, and CP could interact with SCE1 (Supplementary Fig. 2a). Our investigation then focused on which proteins can suppress Pelota SUMOylation. In vivo SUMOylation analysis indicated that, among these SCE1-interacting proteins, only NIb significantly reduced the modification level of Pelota (Fig. 2a). Since NIb is known to interact with SCE1 and SUMO3 in the SUMO-modification pathway30,31, we tested whether NIb could inhibit the global SUMO modification in plants. Of note, the global SUMOylation levels markedly increased under the heat shock condition (37 °C for 30 min), but there were no obvious differences in Col-0 and Myc-NIb transgenic plants to heat shock responses (Supplementary Fig. 2b). We thus speculated that NIb could specifically affect Pelota-mediated RNA degradation. Increased NIb expression was correlated with decreased Pelota SUMOylation, which paralleled the findings in TuMV accumulation (Figs. 1, 2b and Supplementary Fig. 2c). The Pelota-mediated degradation of YFP-P3 transcripts, as observed in TuMV-infected cells, was completely inhibited upon expression of NIb. This functional impact of NIb on the Pelota-mediated RQC activity was further confirmed by an agrobacterium-free transient expression assay performed with the protoplasts of TuMV P3 (the target of Pelota) transgenic Arabidopsis (P3-OE #4) (Fig. 2c–e, Supplementary Fig. 3).
a In vivo SUMOylation of Pelota. Pelota-YFP and Myc-SUMO1 were co-expressed with Flag-tagged TuMV-encoded proteins in N. benthamiana leaves, and Pelota was immunoprecipitated with anti-GFP beads at 60 hpi. Immunoprecipitated proteins were then analyzed with anti-Myc, anti-Flag and anti-GFP antibodies. Stars indicate the Flag-tagged GUS and viral encoded proteins (P1, P3, CI, VPg, NIa, NIb, and CP). This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel. b Pelota-YFP and Myc-SUMO1 were co-expressed with a gradient amount of NIb in N. benthamiana leaves. Note: samples were treated with MG132 for 6 h before collection to exclude the variations from protein degradation, and proteins were immunoprecipitated with anti-GFP beads at 60 hpi. Immunoprecipitated and input proteins were then analyzed with anti-Myc, anti-Flag, and anti-GFP antibodies. CBB-staining of RbcL was a loading control. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel. c Growth phenotype of Col-0 and P3-OE #4 transgenic Arabidopsis plants. d qRT-PCR analysis of P3 RNA accumulation in P3-OE #4 transgenic Arabidopsis protoplasts. GFP, GFP-Pelota or GFP-Pelota with GFP-NIb were co-transformed in P3-OE #4 Arabidopsis protoplasts. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown). p values determined by one-way ANOVA and Dunnett testing, **p = 0.0091, ns means not significant (p = 0.1030). e qRT-PCR analysis of P3 RNA accumulation at 40 hpi during expression of GUS and Pelota in N. benthamiana leaves transfected with buffer and NIb. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown), and **p = 0.0001, ns means not significant (p = 0.8688) (p values determined by two-tailed Student’s t test). f Virus symptoms and GFP fluorescence in TuMV-GFP infected plants after inoculation with GUS (mock), Pelota, Pelota+NIb. Plants were photographed under ambient or UV light at 8 dpi. g Analysis of TuMV-GFP RNA accumulations indicated in (f), systemically infected leaves. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown). p values determined by one-way ANOVA and Dunnett testing, **p = 0.0020, ns means not significant (p = 0.9743).
Next, we assessed the impact of NIb expression on Pelota functionality during TuMV infection. TuMV-GFP infectious clone was co-expressed with a mock control (Flag-GUS), Flag-Pelota, or Flag-Pelota and Flag-NIb in Nicotiana benthamiana by Agrobacterium infiltration. The inoculated plants were maintained to monitor symptom development and analysis of viral RNA accumulation in upper newly emerged leaves. Subsequent qRT-PCR analysis revealed that Pelota-mediated antiviral activity was significantly diminished in NIb transient-expression plants (Fig. 2f, g). This observation aligns with the effects induced by viral infection, suggesting a robust inhibition of the functional role of Pelota due to NIb expression. Therefore, these findings support the idea that TuMV utilizes NIb to inhibit the Pelota-mediated RQC.
NIb and Pelota form an interaction complex in vivo
Next, we tried to dissect the molecular link between NIb and Pelota. A bimolecular fluorescence complementation (BiFC) assay was performed to investigate the interaction between NIb and Pelota. Co-expression of constructs encoding YN-Pelota and YC-NIb resulted in a YFP fluorescence signal in the cytoplasm and the nucleus, but not with the P3N-PIPO, as a negative control (Fig. 3a). Luciferase complementation imaging (LCI) assay was further conducted in N. benthamiana leaves, which revealed luciferase activity when Pelota-NLuc and CLuc-NIb were co-expressed (Fig. 3b). This interaction was further substantiated through a Co-immunoprecipitation (Co-IP) assay, confirming the binding of Pelota with NIb in vivo (Fig. 3c). NIb, SCE1, and Pelota interacted with each other and exhibited similar subcellular localizations in the nucleus, cytoplasm, and endoplasmic reticulum (Fig. 3d, Supplementary Fig. 4a, b).
a BiFC assay showing interactions between Pelota and NIb in RFP-H2B (a nuclear marker, the red fluorescent protein fused to histone 2B) transgenic N. benthamiana leaves at 40 hpi. P3N-PIPO was used as a negative control. Scale bars correspond to 20 μm. This experiment was repeated three times showing similar results. b NIb interactions with Pelota are indicated by a luciferase complementation assay. Pelota-NLuc and CLuc-NIb were transiently co-expressed in N. benthamiana. GUS-NLuc and CLuc were used as negative controls. c Co-IP assays showing binding interactions between Pelota and NIb in vivo. Proteins were extracted from N. benthamiana leaves and immunoprecipitated with anti-GFP beads. The immunoprecipitated proteins were detected with anti-GFP and anti-Flag antibodies. YFP and GUS were used as negative controls. This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel. d BiFC assay showing interactions between SCE1 and NIb, Pelota and NIb, Pelota and SCE1 in N. benthamiana leaves at 40 hpi. mCherry-HDEL is a marker of endoplasmic reticulum. Scale bars correspond to 20 μm. This experiment was repeated three times showing similar results. e Y2H Gold yeast cells harboring the indicated plasmids were co-expressed, subjected to 10-fold serial dilutions and plated on synthetic dextrose (SD)/-Trp, -Leu, -His, -Ade plates at 30 °C and photographed after 3 days. Yeast cells co-expressing AD-T7 and BD-53 or AD-T7 and BD-Lam were used as positive and negative controls, respectively. f Pull-down (PD) assay showing that SCE1 bridges Pelota-NIb interaction in vitro. GST fusion proteins were purified with GST Resin, and the bound proteins were detected with anti-GST, anti-Flag and anti-MBP antibodies. MBP-YFP was used as negative control. This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel.
However, our Y2H and in vitro pull-down assays showed no direct interaction between NIb and Pelota, indicating NIb and Pelota do not physically interact. Since both NIb and Pelota interact directly with SCE1 (Fig. 3e, Supplementary Fig. 4c), we hypothesized that SCE1 might serve as a mediator in this interaction, bridging the interaction between NIb and Pelota. Supporting this, GST-Pelota could pull down NIb in a SCE1-dose dependent manner (Fig. 3f). Furthermore, Y3H analysis also detected the interaction between Pelota and NIb using SCE1 as a bridge (Supplementary Fig. 4d), indicative of a co-existing state of NIb-SCE1-Pelota complex despite that NIb could disrupt the binding of SCE1 and Pelota. These findings collectively imply the formation of an NIb-SCE1-Pelota complex in TuMV-infected plant cells.
NIb disrupts Pelota’s function by competing with SCE1
Furthermore, we found that the NIb expression did not notably alter the subcellular localization of the SCE1-Pelota interaction complex, but it did significantly reduce the BiFC fluorescence intensity of this interaction complex (Fig. 4a, b). Considering that both Pelota and NIb can directly interact with SCE1, we assessed the possibility that NIb interferes with the SCE1/Pelota interaction in a competitive manner. Competitive maltose-binding protein (MBP) pull-down assay showed that the amount of Pelota protein immunoprecipitated by SCE1 was decreased with a gradient amount of NIb increased (Fig. 4c and Supplementary Fig. 5a). The Co-IP assay also demonstrated that NIb could compete with Pelota for binding to SCE1 (Fig. 4d and Supplementary Fig. 5b). In addition, the dissociation of the Pelota-SCE1 complex was also observed with the accumulation of TuMV (Supplementary Fig. 5c). These results indicate that TuMV encoded NIb inhibits the association of SCE1 and Pelota in a competitive manner, leading to a reduction of Pelota’s SUMOylation.
a, b The relationships between the levels of SCE1-Pelota BiFC and NIb-CFP fluorescence intensity in N. benthamiana leaves. Scale bars correspond to 20 μm. Dashed lines separate different levels of NIb fluorescence activity in cells. This experiment was repeated three times showing similar results. Statistical analysis was performed using linear regression to fit the data points. The regression line represents the best fit, and the R² value indicates the goodness of fit. The p value was calculated using a two-sided test, and no adjustments for multiple comparisons were applied. c, Competitive MBP pull-down assays in vitro. MBP-SCE1 protein combined with GST-Pelota was incubated with a gradient amount of His-NIb. The proteins were immunoprecipitated with Amylose Resin. Input and pull-down proteins were analyzed with anti-GST, His, and MBP antibodies. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel. d Competitive Co-IP assays in vivo. SCE1-YFP and Myc-Pelota were co-expressed with a gradient amount of of NIb-Flag in N. benthamiana leaves. Proteins were immunoprecipitated with anti-GFP beads at 40 hpi, and the immunoprecipitated and input proteins were then analyzed with anti-Myc, anti-Flag, and anti-GFP antibodies. CBB-staining of RbcL was a loading control. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel. e Growth phenotype of Col-0, pelota mutant and NIb-OE transgenic Arabidopsis plants. Bar = 5 cm. f The overlap of significantly upregulated genes (log2 fold change > 2, p < 0.001) in transcriptome analysis between NIb overexpressing (in red) and pelota (in blue) plants. g The PCA plot displays the distribution of transcriptome data from wild-type (WT, green dots), NIb transgenic (red dots), and pelota (blue dots) samples. Statistical significance between the two datasets was assessed using a two-sided Mann–Whitney U test. h Gene ontology terms relating to NIb transgenic and pelota samples compared to WT show enrichment across biological processes, cellular components, and molecular functions. The dot size reflects the enrichment score, and the color gradient indicates the −log10 (p-value), indicating the significance of the enrichment. i Functional annotation and network of enriched terms, illustrated by pie charts colored to reflect the proportions of differentially expressed genes within the NIb transgenic (red) and pelota (blue) groups. GO enrichment analysis was performed using a hypergeometric test. P values were adjusted for multiple comparisons using the Benjamini-Hochberg method to control the false discovery rate (FDR).
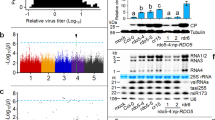
To further verify whether NIb alone is sufficient to inhibit the function of Pelota in plants, we performed RNA-sequencing (RNA-seq) and comparative transcriptome analysis using Col-0, pelota mutant, and NIb transgenic (35S: Myc-NIb, NIb) Arabidopsis seedlings. We found that NIb or pelota mutants significantly affected gene expression in Arabidopsis (Fig. 4e and Supplementary Fig. 6a). Principal component analysis (PCA) showed the Col-0 samples were substantially different from NIb and pelota. At the same time, significant overlap was observed between the NIb and pelota samples. We identified 1001 genes upregulated in pelota mutants with at least a four-fold change and statistical significance (compared to Col-0), categorizing them as potential targets regulated by Pelota. Remarkably, over 86% of these upregulated genes (865 out of 1001) were also affected by the NIb expression in a similar mode (Fig. 4f, g and Supplementary Fig. 6b). MEME motif enrichment analysis showed a A-rich motif with the regulated genes in NIb transgenic and pelota plants (Supplementary Fig. 6c). This A-rich motif is similar with our previously identified Pelota recognition motif on TuMV RNA13. Gene ontology (GO), and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis also indicated a significant overlap between the differentially expressed genes in pelota mutant and NIb transgenic plant. Additionally, the Pelota regulated genes were also extensively overlapped in transcriptome analyses of TuMV-infected plants (Fig. 4h, i and Supplementary Fig. 6d–f). The GSEA (Gene Set Enrichment Analysis) analysis demonstrated a high degree of consistency in the pathways activated or suppressed in NIb and pelota. In particular, the upregulated genes in NIb and pelota were significantly enriched in plant-pathogen interactions and MAPK-related signaling pathways. In contrast, both NIb and pelota significantly inhibited the ribosomal and pyrimidine metabolism pathways (Supplementary Fig. 7). Collectively, these data compellingly demonstrate that the expression of NIb impedes the function of Pelota in plants.
The SIM3 motif in NIb is required for interacting with SCE1
Both NIb and Pelota interact with the SUMO E2 SCE1, leading us to investigate the biological functions and the interaction relationships of these three proteins. We first mapped the potential interaction sites NIb with SCE1. The GPS-SUMO prediction service identified several SUMO interacting motifs (SIMs) within NIb. Among these SIMs, the SIM2 motif, previously established as essential for NIb’s SUMOylation, thereby facilitating its nucleus-cytoplasm transportation31, was also predicted in this study. We next generated NIb sim mutants (NIbsim1, NIbsim2 and NIbsim3), replacing the conserved residues within the SIMs with alanines (A) and assessing their interactions with SCE1 (Fig. 5a and Supplementary Fig. 8a). Y2H assays revealed that NIbsim1 and NIbsim2 mutants retained the ability to interact with SCE1, but the NIbsim3 mutant lacked this capacity (Fig. 5b). Pull-down assay using recombinant proteins expressed in E. coli demonstrated that MBP-SCE1 could interact with NIb, but not MBP-YFP. Intriguingly, NIbsim1 and NIbsim2 mutants maintained SCE1 binding ability, whereas the NIbsim3 mutant exhibited no interaction (Fig. 5c). We further confirmed that the NIbsim3 mutation results in the loss of the ability of NIb to interact with SCE1 in the BiFC and Co-IP assays (Fig. 5d, e and Supplementary Fig. 8b, c). Of note, the vast majority of SUMO interactions involves conserved surfaces of SUMO proteins and SIMs, which are small stretches of hydrophobic amino acids and acidic regions in interacting proteins32. Therefore, SIM-like motifs (SIM3 in this study) might also contribute to the interaction with SCE1. Additional in vivo evidence using Microscale Thermophoresis (MST) demonstrated the interaction strength between Pelota and SCE1 was decreased in the presence of NIb and NIbsim2, but not NIbsim3. Y3H using NIb, NIbsim2, and NIbsim3 as the bridge also showed a similar result. The Pelota-SCE1 LCI experiment showed that NIb and NIbsim2, but not NIbsim3, could obviously disrupt the fluorescence intensity of the interaction signal generated from Pelota and SCE (Supplementary Fig. 8d–f). As NIbsim3 lost its ability to interact with SCE1, it was unable to inhibit the Pelota-SCE1 interaction in competitive experiments and thereby failed to affect the Pelota SUMOylation (Fig. 5e, f and Supplementary Fig. 8g).
a Predicted SUMO interacting motifs (SIMs) on the NIb protein. Replacement of the Valine (V), Isoleucine (I), and Leucine (L) with the Alanine (A) amino acid resulted in SIM mutations. b Identification of the interaction between SCE1 and the NIb (sim mutants) in yeast. Yeast cells co-expressing BD-SCE1 and AD-EV were used as negative control. c MBP-SCE1 protein combined with GST-Pelota was incubated with Flag-NIb (or sim mutants) in vitro. The proteins were immunoprecipitated with Amylose Resin. Input and pull-down proteins were analyzed with anti-GST, Flag, and MBP antibodies. This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel. d BiFC assay showing interactions between Pelota, NIb, NIbsim2 and NIbsim3 in RFP-H2B (a nuclear marker, the red fluorescent protein fused to histone 2B) transgenic N. benthamiana leaves. Scale bars correspond to 20 μm. This experiment was repeated three times showing similar results. e Competitive Co-IP assays in vivo. SCE1-YFP and Myc-Pelota were co-expressed with NIb (or sim mutants)-Flag in N. benthamiana leaves. Note: samples were treated with MG132 for 6 h before collection to exclude the variations from protein degradation, and proteins were immunoprecipitated with anti-GFP beads. The immunoprecipitated and input proteins were then analyzed with anti-Myc, anti-Flag, and anti-GFP antibodies. CBB-staining of RbcL was a loading control. Quantification of protein levels was performed using ImageJ software. Independent experiments were performed three times with similar results. The samples were derived from the same experiment and that blots were processed in parallel. f In vivo SUMOylation assay. Pelota-YFP and Myc-SUMO1 were co-expressed with NIb (or sim mutants)-Flag in N. benthamiana leaves. The proteins were immunoprecipitated with anti-GFP beads at 40 hpi and analyzed with anti-Myc, anti-Flag and anti-GFP antibodies. This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel. g Phenotypes of N. benthamiana plants infected by TuMV, TuMV-NIbsim1, TuMV-NIbsim2 and TuMV-NIbsim3 at 10 dpi. Bar = 5 cm. h Phenotypes of B. napus plants infected by TuMV, TuMV-NIbsim1, TuMV-NIbsim2 and TuMV-NIbsim3 at 15 dpi. Bar = 5 cm. i qRT-PCR analysis of TuMV, TuMV-NIbsim1, TuMV-NIbsim2 and TuMV-NIbsim3 genomic RNA accumulation in (g, h). TuMV-GFP-NIbsim2 genomic RNA accumulation was normalized to 1. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown). p values determined by one-way ANOVA and Tukey testing, ***p < 0.0001 (TuMV or TuMV-NIbsim1 vs TuMV-NIbsim2 or TuMV-NIbsim3). ND means not detected.
To investigate whether the SIM3 motif in NIb is essential for TuMV infection, we created the TuMV mutant clone TuMV-NIbsim3, with a variant of NIb defective in interacting with SCE1. The wild-type clone TuMV, TuMV-NIbsim1, TuMV-NIbsim2 (the SUMOylation-defective form), and TuMV-NIbsim3 were inoculated into N. benthamiana and Brassica napus (B. napus). Plants infected with TuMV and TuMV-NIbsim1 exhibit severe virus symptoms, while plants infected with the TuMV-NIbsim2 or TuMV-NIbsim3 mutants did not show any obvious virus symptoms (Fig. 5g, h). Furthermore, qRT-PCR analysis showed few viral genomic RNA in the newly developed leaves of plants infected with TuMV-NIbsim2 and TuMV-NIbsim3 (Fig. 5i). Of note, here we failed to completely exclude the potential impact of the NIb mutation on its function as a replicase. Despite that, these results together with the following Fig. 6 that mutation of SIM3 of potyvirids’ NIbs abolished their suppression activity on Pelota-mediated RQC suggest that the NIbsim3 mutant lost its capability to inhibit RQC, which might result in ineffective infection to plants. This finding is consistent with the previous report that TuMV systemic infection requires SUMOylated NIb31, indicating that SUMOylation via SCE1 interaction is a pivotal regulatory step during virus infection.
a Comprehensive analysis of NIb sequences from 12 genera in the Potyviridae family, featuring a phylogenetic tree, multi-alignment of conserved sites, and a conservation heatmap. The tree branches depict the genetic divergence, with branch lengths indicating the degree of sequence variation. The multi-alignment of conserved sites showcases three SIM sites across the viruses. The right bubble illustrates the conservation levels of these sites across these virus sequences, with color intensity and bubble size representing the degree of conservation. b BiFC assay showing interactions between SCE1 and PVY, PVMV, and ANRSV NIb (as well as NIbsim mutants) in RFP-H2B (a nuclear marker, the red fluorescent protein fused to histone 2B) transgenic N. benthamiana leaves. Scale bars correspond to 20 μm. This experiment was repeated three times showing similar results. c Analysis of the interactions between SCE1 and PVY, PVMV, and ANRSV NIb (as well as NIbsim mutants) in yeast. d In vivo SUMOylation of Pelota. Pelota-YFP and Myc-SUMO1 were co-expressed with PVY, PVMV, and ANRSV NIb (as well as sim mutants) in N. benthamiana leaves, and Pelota was immunoprecipitated with anti-GFP beads at 40 hpi. Immunoprecipitated proteins were then analyzed with anti-Myc and anti-GFP antibodies. This experiment was repeated three times showing similar results. The samples were derived from the same experiment and that blots were processed in parallel. e qRT-PCR analysis of P3 RNA accumulation at 40 hpi during expression of GUS or Pelota with PVY, PVMV, and ANRSV NIb (as well as NIbsim mutants) in N. benthamiana leaves. The error bars indicate mean ± SD (n = 3 biologically independent samples with averaged technical duplicates shown). p values determined by one-way ANOVA and Dunnett testing, ***p < 0.001.
Counteraction of Pelota defense machinery by NIb SIM3 is conserved amongst potyvirids
To further investigate the functional conservation of NIb proteins, we explored the evolutionary dynamics within this family began with a re-analysis of NCBI RefSeqs of potyvirids, encompassing representative viruses from Arepavirus, Bevemovirus, Brambyvirus, Bymovirus, Celavirus, lpomovirus, Macluravirus, Poacevirus, Potyvirus, Roymovirus, Rymovirus, and Tritimovirus genera. Phylogenetic analysis and multiple sequence alignment, focusing on the NIb proteins, disclosed distinct inter-genus clustering patterns, illustrating the sequence diversity of NIb proteins across various genera (Supplementary Fig. 9a). This sequence diversity in different genera contrasts with the sequence homogeneity observed within each genus, highlighting the close phylogenetic relationships among different virus species (Fig. 6a).
The multiple sequence alignment, with a specific focus on the presence of isoleucine (I), leucine (L), and valine (V), reaffirmed the presence of three previously verified SIMs (SIM1, SIM2, and SIM3). Among these, SIM2 and SIM3 are highly conserved within the viral genome, indicating a potentially conserved role of SUMOylation in the life cycle of potyvirids (Fig. 6a and Supplementary Fig. 9b). Our data above indicated that the TuMV NIb protein disrupts the Pelota-mediated RQC by reducing the Pelota’s SUMOylation through SIM3-SCE1 competitive interaction. Therefore, the sequence conservation of NIb SIM2 and SIM3 implies that SCE1-mediated NIb SUMOylation could be a strategic approach for viral infection within this family.
To investigate whether this phenomenon reflects a general virulence strategy deployed by viruses in this family, the interactions between NIbs from potato Y virus (PVY, Potyvirus), pepper veinal mottle virus (PVMV, Potyvirus) or areca palm necrotic ringspot virus (ANRSV, Arepavirus) and SCE1 were analyzed by Y2H and BiFC. Figure 6b, c illustrates the conserved binding interactions between the NIbs of PVY, PVMV, and ANRSV with SCE1. The NIb SUMO-modification site, SIM2, did not affect the interaction with SCE1, while the mutation in SIM3 lost this ability. Further experiments showed that these NIbs and sim2 mutants (but not SIM3 mutants) expression exhibited decreased levels of Pelota’s SUMOylation (Fig. 6d). Since the RNA accumulation of YFP-P3 can serve as a marker for assessing the Pelota activity, we then tested the impact of NIbs (NIb and the sim mutants) on Pelota function. Consistent with the effect on Pelota SUMO modification, NIbs and sim2 mutants continue to inhibit the Pelota activity in plants. In contrast, these NIbs carrying SIM3 mutation have lost this capability (Fig. 6e). These results together provide compelling evidence for the evolution of a counter-defense mechanism among potyvirids and plants, where viral NIb proteins employ the conserved SIM3 as a decoy tactic to interfere with the Pelota’s SUMO modification and inhibit the Pelota-meditated RQC defense mechanism (Fig. 7).
A diagram illustrating the Pelota-mediated degradation of the viral RNA of potyvirids and the viral NIb counter-defense strategy. In host cells, Pelota is SUMOylated by interacting with the SUMO conjugating enzyme SCE1, which then forms an active Pelota-Hbs1 RNA surveillance complex. The potyvirids conserved G1-2A6-7 motifs are targets of the Pelota-Hbs1 surveillance complex, which triggers RQC to mediate viral RNA decay. The NIb protein from potyvirids contains two conserved SIM sites: SIM2 for SUMO modification and SIM3 for interacting with the SUMO-E2 enzyme, SCE1. NIb-SIM3 interferes with the Pelota-SCE1 interaction as a SUMOylation decoy, suppressing SUMOylation-dependent RQC, which leads to the inhibition of virus transcript degradation and the promotion of viral accumulation. S, SUMOylation. The arrow indicates activation, while the “T” sign represents attenuation or suppression.
Discussion
It is well established that RNA silencing plays a pivotal role in antiviral immunity in eukaryotes1,2,4,7,8. Emerging evidence suggests that RQC is another RNA-targeting mechanism that functions as a critical regulator in plant virus infection1,2,34. Eukaryotes are equipped with RQC systems to monitor the quality of mRNAs during translation. To maximize coding potential, RNA viruses often contain unique structures and coding features, making these viruses ideal targets for RQC. Studies have shown that RQCs play crucial roles in combating viral infections and are recognized as critical virus restriction mechanisms in plants1,2,35. Potyvirids use a genome expression strategy consisting of one or two polyprotein open reading frames (ORFs). Without eliciting signals in the viral genome, potyvirids could evade NMD. However, the conserved G1-2A6-7 motif within their genome has been identified as a target of the Pelota-mediated RQC11,13. Nevertheless, the specific mechanisms by which potyvirids inhibit, manipulate, or evade the reorganization of Pelota have not been methodically explored.
In plants, the spectrum of gene expression alterations observed in pelota mutants is nearly completely covered by the changes induced by the NIb expression (Fig. 4f–i). Consequently, NIb alone can sufficiently compromise Pelota’s RQC function, even without viral infection, indicating that NIb could perturb Pelota’s biological functions. Unlike pathogenic bacteria, fungi, and oomycetes, viruses encode a limited number of viral proteins. No specific effector or paralogous decoy like PsXEG1 or PsXLP1 from Phytophthora sojae is generated from viruses only for counterattacking or evading plant defense responses36. Therefore, viral proteins are multifunctional, a feature thought to be an adaptation to the limited viral genome to maximize their utilization. Our study discovered that the NIb protein of potyvirids acts as a SUMOylation decoy, targeting SCE1, abolishing the SUMOylation of Pelota and thereby facilitating viral infection (Fig. 7). These findings further expand the multiple functionalities of NIb beyond its primary role as an RNA polymerase.
Viruses utilize host cellular components as opportunistic intracellular parasites to facilitate their survival and pathogenicity. SUMO, unlike ubiquitin, can regulate the function (rather than abundance) of target proteins37,38,39. Consequently, many viral proteins undergo PTMs mediated by the host machinery. The SUMOylation of NIb promoted TuMV infection by shifting NIb from the nucleus to the cytoplasm and suppressing host antiviral responses through interactions with the nuclear export protein XPO131,40. The carrot mottle virus (CMoV) movement protein has also been SUMOylated in plant cells, and this modification helps its localization to plasmodesmata, thereby facilitating virus spread in host plants41. The conservation of SUMO sites in viral proteins hints at a broader role for SUMOylation in enabling viruses to establish systemic infection successfully. Nevertheless, the precise mechanism of SUMOylation in plant-microbe interactions remains largely elusive. Our recent data showed SUMO modification acts as a molecular switch of Pelota-dependent RQC in regulating viral infection11. At the same time, this study demonstrated that the viral virulence factors manipulate the SUMO modifications of host defense components to enhance virus infection. These findings suggest orchestrating the PTMs of host defense components and viral proteins, which likely represents a mechanism during the arms race between host and pathogens.
The functional NIb SIM sites (SIM2 and SIM3) were found to be highly conserved across potyvirids, as revealed through homologous sequence comparison (Fig. 6a). NIb and sim2 mutant, but not sim3 mutant, could still interact with SCE1 and reduce the SUMOylation level of Pelota (Figs. 2b, 6b, c). In this study, we identified the NIb SIM3 is responsible for interacting with SCE1. The previous finding has shown the SUMOylation of NIb on SIM2 was required for its virulence31. Both the NIb sim2 and sim3 mutants led to loss of virulence (Fig. 5). However, this result can not exclude the possibility that these motifs of NIb are required for viral RNA replicase activity. Highly conserved SIM2 and SIM3 sites observed at the genomes of potyvirids may underscore the selective pressures operative during virus sequence evolution. Given the evolutionary limitation on viral genomes, we hypothesized that the conservation of these sites ensures the maintenance of crucial biological functions within the host, including the antagonism of the plant defense lines. These two SIM sites, each serving a unique function in the SUMO modification process, collectively enhance viral infectivity. The functional importance of these sites may have pushed them to undergo positive selection dynamics during evolution. This finding provides insights into the functional complexity of the NIb protein and its essential role in the viral life cycle of potyvirids. In addition, this study also demonstrated overexpression of NIb could affect the expression of more plant endogenous genes than knocking out of Pelota (Fig. 4f–i), suggesting that NIb may have additional functions in virus-infected plants beyond interfering with the Pelota-mediated RQC defense. Consistent with this, NIb could significantly repress the ribosomal and pyrimidine metabolism pathways (Supplementary Fig. 7), suggesting the unknown mechanisms regulated by NIb that needs further study. Therefore, clarifying the underlying molecular mechanism in the inhibition of plant defense by NIb in this work is essential for a comprehensive understanding of host-microbe interactions. In conclusion, we propose a model showing a molecular basis in which, through the co-evolutionary arms race, viruses have evolved a virulence strategy that subverts host defense responses by interfering with the SUMO modifications of crucial host defense components (Fig. 7).
Methods
Plant materials and growth conditions
Col-0 was used as a wild type and pelota (SALK_124403) was described previously13. To obtain the TuMV-P3 and TuMV-NIb transgenic plants, P3 and NIb region were cloned into pBA-Flag-Myc4 (Myc tag in the N terminal) gateway destination vector. The constructed plasmids were transformed into Agrobacterium GV3101 and then transformed into Arabidopsis plants using the floral-dip method42. Transgenic RFP-H2B N. benthamiana was a gift from Michael M. Goodin (University of Kentucky, USA). The growth conditions consisted of 60% relative humidity and a day/night regime of 16 h in the light at 22 °C followed by 8 h of darkness at 18 °C.
Plasmid construction
Gene ID and viral sequences used in this study are as follows: Pelota (AT4G27650), Hbs1 (AT5G10630), SUMO1 (AT4G26840), SCE1 (AT3G57870), AtActinII (AT3G18780), NbActin (AY179605), TuMV (NC002509), PVMV (MN082715), and ANRSV (MZ209276). Unless otherwise noted, all plasmids used in this study were constructed using Gateway technology (Invitrogen) according to the manufacturer’s protocol. Firstly, the target coding CDSs were cloned into the pDONR221 entry vector using the BP Clonase reaction mix (Invitrogen) to create the entry clones. After verification by sequencing, each of the clones was mobilized into the corresponding gateway destination vectors (Y2H vectors pGADT7-DEST (prey) or pGBKT7-DEST (bait)), BiFC vectors pEarleygate201-YN or pEarleygate201-YC, or binary destination vectors pEarleyGate101 (YFP in the C terminal), pEarleyGate104 (YFP in the N terminal), pEarleyGate102 (CFP in the C terminal).
For in vitro protein expression, the CDS of candidate genes were cloned into the pGEX-4T-1 (GST), pET-21b (His), pMAL (MBP), respectively. The Flag-NIb sequence from pBFlag vector was cloned into pET-21b to generate Flag-NIb-His recombinant plasmid. The CDS of Pelota and NIb were cloned into the subcellular localization vector pJIT16318, which contains a CaMV 35S promoter and a C-terminal GFP for protoplast transformation. The recombinant viruses TuMV and TuMV-GFP were previously described13. The viral mutants were constructed by overlapping PCR with specific primers. Primers used in this study are listed in Supplementary Table 1.
Agroinfiltration and viral inoculation
Agrobacterium infiltration assays were performed as previously described43. Briefly, the recombinant constructs with target genes were transformed individually into the agrobacterium strain GV3101. Agrobacterium cultures harboring the target gene-expressing vectors were resuspended in infiltration buffer [10 mM MgCl2, 10 mM MES (pH 5.6), 0.1 mM AS] at an OD600 of 0.6 before use.
For TuMV or TuMV-GFP infection analysis, agrobacterium cultures carrying infectious virus clones together with agrobacterium harboring pBA-Flag-Myc4-Pelota or pBA-Flag-Myc4-Pelota together with pBA-Flag-Myc4-NIb expression vectors were co-infiltrated into N. benthamiana leaves. Inoculated plants were photographed with camera at various times after infiltration. Rabbit polyclonal antibodies generated against TuMV-CP produced by HUABIO Co. td (Hangzhou, Zhejiang, China).
Protoplast transformation
Mesophyll protoplasts were isolated and transfected essentially as described previously13. Briefly, mesophyll protoplasts were prepared from 4-week-old Col-0 or P3-OE #4 Arabidopsis leaves. Protoplasts were transfected with pJIT16318-Pelota or pJIT16318-Pelota+ pJIT16318-NIb plasmids in PEG solution (40% w/v PEG 4000 containing 0.2 M mannitol and 100 mM CaCl2) at room temperature for 15 min. The transformed protoplasts were washed and resuspended in W5 buffer [154 mM NaCl, 125 mM CaCl2, 5 mM KCl and 2 mM MES (pH 5.7)]. GFP fluorescence in the transformed protoplasts was imaged using a confocal microscope and then samples were harvested for RNA extraction at 36 hpi.
Yeast two-hybrid (Y2H), BiFC and subcellular localization
Y2H were performed according to the manufacturer’s manual (Clontech). Briefly, Y2H Gold yeast cells harboring the co-transformed plasmids were plated on a selective medium lacking tryptophan and leucine (SD/-Trp-Leu) to confirm the successful transformation, and a high-stringency selective medium SD/-Leu/-Trp, SD/-His/-Leu/-Trp, SD/-Ade/-His/-Leu/-Trp, respectively, to analyze the interactions (Coolaber SD Broth).
BiFC and subcellular localization experiments were performed as described43. Briefly, the indicated constructs were transiently expressed in wild-type or RFP-H2B transgenic N. benthamiana leaves for 36–72 h. Fluorescence signals for each combination were visualized with an inverted confocal microscope (Zeiss LSM 980). CFP was excited at 458 nm and captured at 470–500 nm, YFP was excited at 514 nm and captured at 565–585 nm, GFP was excited at 488 nm and captured at 510–540 nm, and mCherry or RFP was excited at 543 nm and captured at 590–630 nm. The sequential scanning mode was used for co-imaging of different fluorescent proteins to exclude the interferes.
Yeast Three-Hybrid (Y3H) assay
For Y3H assays, the CDS of Pelota was ligated to the pBridge vector, and then the CDS of SCE1, YFP, NIb or NIb sim mutants was ligated into the pBridge vector, constructing the recombination plasmids pBridge-Pelota-SCE1, pBridge-Pelota-YFP, pBridge-Pelota-NIb, pBridge-Pelota-NIbsim2 and pBridge-Pelota-NIbsim3, respectively. Yeast cells harboring the co-transformed plasmids were diluted on selective medium plates and a high-stringency selective medium SD/-Leu/-Trp, SD/-His/-Leu/-Trp, SD/-His/-Leu/-Trp/-Met, SD/-Ade/-His/-Leu/-Trp, SD/-Ade/-His/-Leu/-Trp/-Met, respectively, and incubated at 30 °C for observations and photographs to analyze the interactions (Coolaber SD Broth).
LCI assay
The full length CDS of GUS and Pelota were inserted into pCambia 1300-nLUC and the full length CDS of SCE1 and NIb were inserted into pCambia 1300-cLUC to produce GUS-NLuc, Pelota-NLuc, CLuc-SCE1, CLuc-NIb, respectively. All of the related constructs were transformed into A. tumefaciens strain GV3101. An equal volume of A. tumefaciens harboring pCambia-gene-NLuc and pCambia-CLuc-gene were mixed to a final concentration of OD600 = 0.6 and were infiltrated into N. benthamiana leaves. 0.2 mM luciferin (Solarbio, L7840) was infiltrated into the leaves and measured with a low-light cooled CCD imaging apparatus.
RNA library construction and sequencing
RNA-seq involved three genotypes of Arabidopsis thaliana: the TuMV-NIb transgenic plants, pelota mutant plants, and Col-0 wild-type plants. Healthy, mature plants were selected as experimental samples for each genotype. All plants were grown under controlled conditions with a constant temperature of 22 °C, a photoperiod of 16 h light/8 h dark, and 60% relative humidity. Total RNA was isolated from the Arabidopsis samples using Trizol reagent (Invitrogen, CA, USA), following the manufacturer’s instructions strictly. The quality and quantity of RNA were determined using a Bioanalyzer 2100 and the RNA 6000 Nano LabChip Kit (Agilent, CA, USA), with a requisite RNA Integrity Number (RIN) exceeding 7.0. Approximately 200 μg of total RNA from each sample was subjected to poly-A mRNA isolation using poly-T oligo-attached magnetic beads (Invitrogen). The purified poly-A mRNA fractions were then fragmented into oligonucleotides of ~100 nucleotides in length. Divalent cations facilitated this fragmentation under elevated temperatures. The fragmented mRNA was used as a template for cDNA synthesis. An average insert size of approximately 100 ± 50 bp for library construction was targeted. High-throughput sequencing was then performed on an Illumina Novaseq™ 6000 platform, using a paired-end 2 × 150 bp sequencing protocol as recommended by the manufacturer.
Analysis of the sequencing data
The raw sequencing reads were initially subjected to quality control using FastQC (Version 0.11.9). Low-quality reads and adapter sequences were removed using Trimmomatic (Version 0.39) with the parameters SLIDINGWINDOW:4:15 and MINLEN:36. The cleaned reads were subsequently mapped to the reference genomes of Arabidopsis thaliana (TAIR10) utilizing HISAT2 (Version 2.2.1) with default parameters. Differential expression analysis was conducted using the DESeq2 package (Version 1.28.1) in R, with genes exhibiting an adjusted p < 0.05, as determined by the Benjamini-Hochberg procedure, considered differentially expressed. Gene Ontology (GO) enrichment analysis was performed on differentially expressed genes using the topGO package (Version 2.54.0) in R. Enrichment was determined using a Kolmogorov-Smirnov-like approach, with Fisher’s exact test p < 0.05 considered significant. Pathway analysis was conducted using the KEGG.db package (Version 2.7.1) in R, identifying enriched metabolic and signaling pathways among the DEGs. Pathways with Fisher’s exact test-adjusted p < 0.05 were marked as significantly enriched. The GO network visualization was implemented using Cytoscape (Version 3.10.1) and ClueGO (Version 2.5.10) software.
Immunoprecipitation and immunoblotting analyses
Immunoprecipitation of protein extracts was conducted as described previously43. Briefly, plant samples were treated with 10 μM MG132 (Proteasome inhibitor) for 6 h, then grounded in liquid nitrogen, and homogenized in IP buffer [50 mM Tris, pH 8.0, 0.4% NP40, 5% glycerol, 2 mM EDTA, 150 mM NaCl, 1 mM DTT, and protease inhibitors]. Total protein extracts from 0.4 g samples were incubated with 20 μl GFP-Trap (50% slurry, Chromotek, gta-20) for 2 h at 4 °C. The beads were washed five times at 4 °C for 5 min with IP buffer after incubation, then boiled with SDS loading buffer. Immunoblotting analyses were carried out with anti-GFP, anti-Myc or anti-Flag antibodies.
SUMOylation and Pull-down assays
SUMOylation and Pull-down assays were performed as previously described13. Pelota-YFP and Myc-SUMO1 were co-expressed in N. benthamiana leaves. The proteins were immunoprecipitated with anti-GFP beads and SUMOylated Pelota was detected with anti-Myc antibodies. For in vitro expression of the transformed proteins, E. coli was incubated at 37 °C until the OD600 reached 0.6, followed by adding 0.5 mM IPTG (Sangon Biotech). After an approximately 12 h induction at 18 °C, cells were collected and sonicated in PBS with 0.4% NP40 [Na2HPO4 10 mM, KH2PO4 1.8 mM, 137 mM NaCl, KCl 2.7 mM, 0.4% NP40, PH = 7.4] for protein purification. FLAG-tagged proteins were purified using anti-FLAG M2 affinity gel (Sigma). GST-tagged proteins were purified by ProteinIso GST Resin (TransGen, DP201), and MBP-tagged proteins were purified by Amylose Resin (New England Biolabs). For pull-down assays, amylose resin or ProteinIso GST Resin were used to bind protein complexes in binding buffer [25 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT, protease inhibitor] at 4 °C for 1 h. Then, the beads were collected and washed five times with washing buffer [25 mM Tris-HCl (pH 7.5), 300 mM NaCl, 1 mM DTT 150 mM NaCl, 0.1% Triton X-100]. The beads were collected and boiled in 1× SDS sample-loading buffer for immunoblotting analysis. Equal amount of eluted proteins were analyzed by immunoblotting with anti-Flag from Abmart (1:5000, M20026), anti-MBP from TransGen (1:5000, HT701), anti-His from TransGen (1:5000, HT501), anti-GST from TransGen (1:5000, HT601) and anti-SUMO1 (ABclonal Technology), Goat Anti-Rabbit/Mouse IgG (H + L)-HRP Conjugated (EASYBIO, BE0102/BE0102, 1:10000) antibodies.
Microscale Thermophoresis (MST) assay
MST were performed according to the manufacturer’s manual (Nanotemper). SCE1 proteins were fused with the Green fluorescent protein and used as target. Measurements were performed at 25 °C in buffer containing 40 mM Tris, pH 8.0 and 300 mM NaCl. 16 small micro reaction tubes were labeled from 1 through 16. 20 μL of the highest concentration GST-Pelota in MST buffer was filled into the first micro reaction tube 1, and 10 μL of MST buffer was filled into the micro reaction tubes 2 to 16. 10 μL of tube 1 was transferred to tube 2 and mix well by pipetting up and down several times. 10 μL of GFP-labeled SCE1 proteins were added in the 16 tubes. Binding reactions were measured using a microscale thermophoresis instrument (Nano Temper Technologies GMBH), 20% MST power and 20% LED power. Data analyses were performed using the Nanotemper Analysis software provided by the manufacturer.
Evolutionary analysis of NIb proteins
The NIb protein sequences from viruses in the Potyviridae family were retrieved from the National Center for Biotechnology Information (NCBI) database. Detailed virus species and accession numbers are provided in the supplementary material. The collected NIb protein sequences were aligned using the Multiple Sequence Comparison by Log-Expectation (MUSCLE) tool (Version 3.8.31). The alignment was executed with default parameters, ensuring optimal alignment quality for phylogenetic analysis. The phylogenetic tree of the aligned NIb protein sequences was constructed using the Maximum Likelihood method implemented in MEGA X (Version 10.1.8). The best-fitting model of protein evolution was determined using the MEGA X built-in model selection feature. A bootstrap analysis with 1000 replicates was conducted to assess the robustness of the phylogenetic tree. The final tree was visualized and annotated using ggtree (Version 3.10.0), highlighting significant clades and conserved sites. Color codes: Bootstrap Percentage (BP > = 90 #fcfdfd, 70 < = BP < 90 #bfbfbf, BP < 70 #060101), Genus (Potyvirus #597181, Rymovirus #e07f2b, Brambyvirus #8491B4, Ipomovirus #60b0a1, Roymovirus #4DBBD5, Poacevirus #B09C85, Tritimovirus #F39B7F, Bymovirus #de404a, Macluravirus #00A087, Bevemovirus #3C5488, Arepavirus #FD8CC1, Celavirus #91331 F), Amino acid color (V-I-L #809fef, Others #ffffff), Conservation levels (high #d51214, mid #5cb398, low #5fb79f).
Statistical analysis
Statistical parameters are reported in the Figures and Figure legends. Multiple comparisons were performed using GraphPad Prism with one-way ANOVA followed by Dunnett’s or Tukey multiple comparisons test. Pairwise comparisons were performed by two-tailed Student’s t test. No statistical method was used to predetermine sample size. The quantitative analysis of the specific western blot band is based on the relative intensity to the control band.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw RNA-seq data of Col-0, pelota and NIb-OE generated in this study have been submitted to NCBI under accession PRJNA1109301 [https://www.ncbi.nlm.nih.gov/bioproject/1109301]. Source data are provided with this paper.
References
Li, F. & Wang, A. RNA-targeted antiviral immunity: more than just RNA silencing. Trends Microbiol. 27, 792–805 (2019).
Ge, L., Zhou, X. & Li, F. Plant-virus arms race beyond RNA interference. Trends Plant Sci. 29, 16–19 (2023).
Wang, A. Dissecting the molecular network of virus-plant interactions: the complex roles of host factors. Annu Rev. Phytopathol. 53, 45–66 (2015).
Jin, Y., Zhao, J.-H. & Guo, H.-S. Recent advances in understanding plant antiviral RNAi and viral suppressors of RNAi. Curr. Opin. Virol. 46, 65–72 (2021).
Nelemans, T. & Kikkert, M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses 11, 961 (2019).
Csorba, T., Kontra, L. & Burgyán, J. viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 479-480, 85–103 (2015).
Wu, J. et al. Plant virology in the 21st century in China: recent advances and future directions. J. Integr. Plant Biol. 66, 579–622 (2024).
Guo, Z., Li, Y. & Ding, S.-W. Small RNA-based antimicrobial immunity. Nat. Rev. Immunol. 19, 31–44 (2019).
Baulcombe, D. C. The role of viruses in identifying and analyzing RNA silencing. Annu Rev. Virol. 9, 353–373 (2022).
Li, F., Ge, L., Lozano-Durán, R. & Zhou, X. Antiviral RNAi drives host adaptation to viral infection. Trends Microbiol. 30, 915–917 (2022).
Garcia, D., Garcia, S. & Voinnet, O. Nonsense-mediated decay serves as a general viral restriction mechanism in plants. Cell Host Microbe 16, 391–402 (2014).
May, J. P., Yuan, X., Sawicki, E. & Simon, A. E. RNA virus evasion of nonsense-mediated decay. PLoS Pathog. 14, e1007459 (2018).
Ge, L. et al. SUMOylation-modified Pelota-Hbs1 RNA surveillance complex restricts the infection of potyvirids in plants. Mol. Plant 16, 632–642 (2023).
He, H. et al. m6A modification of plant virus enables host recognition by NMD factors in plants. Sci. China Life Sci. 67, 161–174 (2024).
Gandhi, R., Manzoor, M. & Hudak, K. A. Depurination of Brome mosaic virus RNA3 in vivo results in translation-dependent accelerated degradation of the viral RNA. J. Biol. Chem. 283, 32218–32228 (2008).
Mu, X. et al. HIV-1 exploits the host factor RuvB-like 2 to balance viral protein expression. Cell Host Microbe 18, 233–242 (2015).
Tsuboi, T. et al. Dom34: hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3’ end of aberrant mRNA. Mol. Cell 46, 518–529 (2012).
May, J. P. & Simon, A. E. Targeting of viral RNAs by Upf1-mediated RNA decay pathways. Curr. Opin. Virol. 47, 1–8 (2021).
Chen, Y. et al. A negative feedback loop compromises NMD-Mediated virus restriction by the autophagy pathway in plants. Adv. Sci. 11, e2400978 (2024).
Du, K. et al. Maize splicing-mediated mRNA surveillance impeded by sugarcane mosaic virus-coded pathogenic protein NIa-Pro. Sci. Adv. 10, eadn3010 (2024).
Yang, X., Li, Y. & Wang, A. Research advances in potyviruses: from the laboratory bench to the field. Annu. Rev. Phytopathol. 59, 1–29 (2021).
Cui, H. & Wang, A. The biological impact of the Hypervariable N-Terminal region of potyviral genomes. Annu. Rev. Virol. 6, 255–274 (2019).
Wylie, S. J. et al. ICTV virus taxonomy profile: potyviridae. J. Gen. Virol. 98, 352–354 (2017).
Olspert, A., Carr, J. P. & Firth, A. E. Mutational analysis of the Potyviridae transcriptional slippage site utilized for expression of the P3N-PIPO and P1N-PISPO proteins. Nucleic Acids Res. 44, 7618–7629 (2016).
Li, X. H., Valdez, P., Olvera, R. E. & Carrington, J. C. Functions of the tobacco etch virus RNA polymerase (NIb): subcellular transport and protein-protein interaction with VPg/proteinase (NIa). J. Virol. 71, 1598–1607 (1997).
Shen, W., Shi, Y., Dai, Z. & Wang, A. The RNA-Dependent RNA Polymerase NIb of Potyviruses Plays Multifunctional, Contrasting Roles during Viral Infection. Viruses 12, 77 (2020).
Dufresne, P. J., Ubalijoro, E., Fortin, M. G. & Laliberté, J.-F. Arabidopsis thaliana class II poly(A)-binding proteins are required for efficient multiplication of turnip mosaic virus. J. Gen. Virol. 89, 2339–2348 (2008).
Luan, H. et al. The Potyviral P3 protein targets eukaryotic elongation factor 1A to promote the unfolded protein response and viral pathogenesis. Plant Physiol. 172, 221–234 (2016).
Thivierge, K. et al. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology 377, 216–225 (2008).
Xiong, R. & Wang, A. SCE1, the SUMO-conjugating enzyme in plants that interacts with NIb, the RNA-dependent RNA polymerase of Turnip mosaic virus, is required for viral infection. J. Virol. 87, 4704–4715 (2013).
Cheng, X. et al. Sumoylation of RNA polymerase promotes viral infection by counteracting the host NPR1-mediated immune response. Plant Cell 29, 508–525 (2017).
Lascorz, J., Codina-Fabra, J., Reverter, D. & Torres-Rosell, J. SUMO-SIM interactions: from structure to biological functions. Semin. Cell Dev. Biol. 132, 193–202 (2022).
Tozluoğlu, M., Karaca, E., Nussinov, R. & Haliloğlu, T. A mechanistic view of the role of E3 in sumoylation. PLoS Comput. Biol. 6, e1000913 (2010).
Pouclet, A., Gagliardi, D. & Garcia, D. No-go decay as a novel route to restrict viral infection in plants. Mol. Plant 16, 509–510 (2023).
Balistreri, G. et al. The host nonsense-mediated mRNA decay pathway restricts Mammalian RNA virus replication. Cell Host Microbe 16, 403–411 (2014).
Ma, Z. et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science 355, 710–714 (2017).
Kerscher, O. SUMO junction-what’s your function? New insights through SUMO-interacting motifs. EMBO Rep. 8, 550–555 (2007).
Geiss-Friedlander, R. & Melchior, F. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 (2007).
Mazur, M. J. & van den Burg, H. A. Global SUMO proteome responses guide gene regulation, mRNA biogenesis, and plant stress responses. Front. Plant Sci. 3, 215 (2012).
Zhang, M. et al. Nuclear exportin 1 facilitates turnip mosaic virus infection by exporting the sumoylated viral replicase and by repressing plant immunity. N. Phytol. 232, 1382–1398 (2021).
Jiang, J., Kuo, Y. W., Salem, N., Erickson, A. & Falk, B. W. Carrot mottle virus ORF4 movement protein targets plasmodesmata by interacting with the host cell SUMOylation system. N. Phytol. 231, 382–398 (2021).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Li, F. et al. Beclin1 restricts RNA virus infection in plants through suppression and degradation of the viral polymerase. Nat. Commun. 9, 1268 (2018).
Acknowledgements
We thank Dr. Andrew O. Jackson for valuable suggestions and English editing of the manuscript and Dr. Rosa Lozano-Dura´ n for stimulating discussions and helpful advice. We also thank Dr. Yule Liu (Tsinghua University, China) for providing the TRV VIGS vector; Dr. Jianxiang Wu (Zhejiang University, China) for providing anti-TuMV CP antibodies; and Dr. Yuhai Cui (Agricultural and Agri-Food Canada) for the modified Gateway vectors. The authors acknowledge support from the National Natural Science Foundation of China (32320103010 and 32172385) to F.L., the National Natural Science Foundation of China (32302318) to L.G., and the National Natural Science Foundation of China (32001868) to H.S.
Author information
Authors and Affiliations
Contributions
F.L. and L.G. conceived the project and designed the experiments. L.G. and M.J. performed most of the experiments. H.S., W.G., and L.J. performed the protein interaction experiments. F.L., L.G., H.C., X.C., M.U., X.Z., and A.W. wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dominique Gagliardi, Kristiina Mäkinen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ge, L., Jia, M., Shan, H. et al. Viral RNA polymerase as a SUMOylation decoy inhibits RNA quality control to promote potyvirus infection. Nat Commun 16, 157 (2025). https://doi.org/10.1038/s41467-024-55288-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55288-6