Abstract
Vesicular monoamine transporter 2 (VMAT2) is crucial for packaging monoamine neurotransmitters into synaptic vesicles, with their dysregulation linked to schizophrenia, mood disorders, and Parkinson’s disease. Tetrabenazine (TBZ) and valbenazine (VBZ), both FDA-approved VMAT2 inhibitors, are employed to treat chorea and tardive dyskinesia (TD). Our study presents the structures of VMAT2 bound to substrates serotonin (5-HT) and dopamine (DA), as well as the inhibitors TBZ and VBZ. Utilizing cryo-electron microscopy (cryo-EM), mutagenesis functional assays, and molecular dynamics (MD) simulations, we elucidate the mechanisms of substrate transport and drug inhibition. Our MD simulations indicate potential binding poses of substrate (5-HT) in both cytosol-facing and lumen-facing states, emphasizing the significance of protonation of key acidic residues for substrate release. We demonstrate that TBZ locks VMAT2 in a lumen-facing occluded state, while VBZ stabilizes it in a lumen-facing conformation. These insights enhance our understanding of VMAT2 function and provide valuable insights for the development of novel therapeutic strategies for psychiatric disorders.
Similar content being viewed by others
Introduction
Vesicular amine transporters, also known as solute carrier 18 (SLC18) family members, are responsible for the loading and packaging of amine neurotransmitters into synaptic vesicles1. This family comprises vesicular monoamine transporters 1 and 2 (VMAT1/2, encoded by SLC18A1/2), vesicular acetylcholine transporter (VAChT, encoded by SLC18A3), and vesicular polyamine transporter (VPAT, encoded by SLC18B1) (Supplementary Fig. 1d). These transporters are pivotal for the precise regulation of neurotransmitters packaging, a process closely linked to their subsequent release into the synaptic cleft. VMAT1/2 specifically handles various monoamine neurotransmitters, including 5-HT, DA, norepinephrine (NE), epinephrine, and histamine, all of which play significant roles in physiological processes like emotion, cognition, motor control, and learning2,3,4. VMAT1 is mainly found in the large dense-core vesicles of neuroendocrine cells, while VMAT2 is predominantly expressed in monoaminergic neurons throughout the central, peripheral, and enteric nervous systems5,6. Dysfunction of VMAT2 has been implicated in a variety of neurological and neuropsychiatric disorders, such as schizophrenia, mood disorders, and Parkinson’s disease7.
In the central nervous system, VMAT2 resides on synaptic vesicles and acts as a secondary active transporter. It depends on a proton electrochemical gradient directed toward the cytoplasm, created by the vacuolar-type H+-ATPase (V-ATPase), which maintains a lumen pH of approximately 5.78. The transport of each cytosolic monoamine neurotransmitter into the synaptic vesicle involves the exchange of two luminal protons.
Dopamine dysregulation is closely linked to various movement disorders. Excessive dopaminergic activity can lead to hyperkinetic disorders such as tremors, tics, myoclonus, dystonia, and chorea, while reduced activity is associated with hypokinetic conditions, including Parkinson’s disease9,10,11. Given VMAT2’s critical role in dopamine regulation, inhibiting its activity represents a promising therapeutic strategy. TBZ, the first VMAT2 inhibitor approved by FDA, was sanctioned for the treatment of chorea associated with Huntington’s disease in 2008 and has also been used off-label to manage TD, a severe side effect of antipsychotic medications linked to dopamine receptor hypersensitivity12. However, TBZ has a short serum half-life and is rapidly metabolized into various stereoisomers, some of which may cause off-target side effects like akathisia, somnolence, depression, and suicidality13. In 2017, two TBZ derivatives, deutetrabenazine and VBZ, received FDA approval for TD treatment, both exhibiting a reduced risk of adverse effects14,15. VBZ undergoes unique metabolic conversion to (+)-α-dihydrotetrabenazine (HTBZ) after the cleavage of its valine moiety, circumventing troublesome enantiomers and enabling once-daily dosing due to its longer half-life16.
Despite their clinical relevance, the mechanisms governing VMAT2 transport and inhibition remain inadequately understood, primarily due to the absence of structural data detailing VMAT2 in complex with its substrates or inhibitors. In this study, we present cryo-EM structures of human VMAT2 bound to the substrates 5-HT and DA, alongside the inhibitors TBZ and VBZ. By integrating these structural insights with MD simulations and functional mutagenesis studies, we clarify the substrate release mechanism of VMAT2 and the action mechanisms of drugs targeting TD, contributing to the advancement of neurological therapies centered on VMAT2.
Results
Structure determination
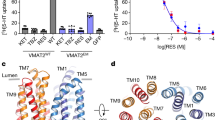
Human VMAT2 is a compact membrane protein that lacks extramembrane features, complicating structure determination via cryo-EM techniques. To address this challenge, we employed a methodology described in the literature17. We fused a BRIL tag to the N-terminus of VMAT212-514 (designated VMAT2EM) and incubated the resulting protein with anti-BRIL Fab and anti-Fab nanobody to enhance particle size (Supplementary Fig. 1a, b). To assess the functionality of this fusion protein, we measured its transport activity for the fluorescent substrate FFN206 in HEK293T cells18. Transfected HEK293T cells effectively allow FFN206 to accumulate in acidic compartments via VMAT2, where V-ATPase provides the necessary proton gradient for transport. The results indicated that the transport activity of VMAT2EM is approximately 60% of that observed with wild-type VMAT2 (VMAT2WT). Additionally, VMAT2EM demonstrated sensitivity to the inhibitors TBZ and VBZ, with FFN206 transport completely inhibited by both compounds, similar to VMAT2WT (Fig. 1a). We also evaluated concentration-dependent inhibition of FFN206 uptake by TBZ in both VMAT2WT and VMAT2EM in HEK293T cells. The IC50 values were comparable: VMAT2WT showed 20.4 ± 4.1 nM, while VMAT2EM had a value of 22.5 ± 6.3 nM (Fig. 1b), aligning well with previous studies19. Therefore, VMAT2EM effectively captures the transport and drug inhibition profiles seen in VMAT2WT.
a Fluorescent substrate FFN206 uptake and its inhibition in wild-type VMAT2 (VMAT2WT) and the cryo-EM construct (VMAT2EM). Data were normalized to VMAT2WT and are presented as mean ± SEM; n = 3 biological replicates. One-way ANOVA; **** P < 0.0001. Source data are provided as a Source Data file. b Concentration-dependent inhibition of FFN206 uptake by TBZ in VMAT2WT and VMAT2EM using HEK293T cells (mean ± SEM, n = 3 biological replicates), showing similar IC50 values for VMAT2WT (20.4 ± 4.1 nM) and VMAT2EM (22.5 ± 6.3 nM). Source data are provided as a Source Data file. c Chemical structures of tetrabenazine (TBZ), valbenazine (VBZ), serotonin (5-HT), and dopamine (DA). The valine moiety in VBZ is highlighted in red circle, distinguishing it from TBZ. d Cryo-EM density maps of VMAT2 in complex with TBZ, VBZ, 5-HT, and DA, showing the transporter in different conformations. e Cartoon representations of VMAT2 bound to TBZ, VBZ, 5-HT, and DA. f Local cryo-EM density maps of TBZ, VBZ, 5-HT, and DA in VMAT2, contoured at 5, 6.5, 5, and 8 σ, respectively.
We successfully solved the structures of VMAT2EM bound to TBZ, VBZ, 5-HT, and DA at 3.28, 3.38, 2.98, and 3.00 Å, respectively (Fig. 1c–e, Supplementary Figs. 2–5 and Supplementary Table 1). Although there were no clear densities for the BRIL, Fab, or nanobody, the cryo-EM densities associated with the compounds were well-defined, allowing for reliable ligand assignments supported by ligand-free docking and MD simulations (Figs. 2a, d, 3a, d, Supplementary Fig. 6 and Supplementary Table 2).
a Root mean square deviation (RMSD) plot of MD simulations for TBZ in 1000 ns for three independent replicas. b Slab view of the electrostatic potential surface of TBZ binding site. TBZ is depicted as sticks. The electrostatic potential surface was calculated using the APBS plug-in in PyMOL (red to blue, −50 kT e−1 to +50 kT e−1). The distance of TBZ relative to the inner leaf of the vesicular bilayer is indicated. c Cartoon representation of VMAT2-TBZ showing the binding site of TBZ in the lumen-facing occluded state of VMAT2. d Root mean square deviation (RMSD) plot of MD simulations for VBZ in 1000 ns for three independent replicas. e Slab view of the electrostatic potential surface of VBZ binding site. VBZ is depicted as sticks. f Cartoon representation of VMAT2-VBZ showing the binding site of VBZ in the lumen-facing state of VMAT2. g Structural superposition of VMAT2-TBZ (lumen-facing occluded, blue) and VMAT2-VBZ (lumen-facing open, green) showing the conformational changes between the two states. W318 is highlighted in red.
a Root mean square deviation (RMSD) plot of MD simulations for 5-HT in 1000 ns for three independent replicas. b Slab view of the electrostatic potential surface of 5-HT binding site. c Cartoon representation of VMAT2-5-HT showing the binding site of 5-HT in the lumen-facing state of VMAT2. d Root mean square deviation (RMSD) plot of MD simulations for DA in 1000 ns for three independent replicas. e Slab view of the electrostatic potential surface of DA binding site. f Cartoon representation of VMAT2-DA showing the binding site of DA in the lumen-facing state of VMAT2. g Superposition of all four ligand-binding sites in VMAT2 in this study. TMs are labeled. TBZ, VBZ, 5-HT, and DA are colored in salmon, yellow, marine, and cyan, respectively. h Fluorescent substrate FFN206 uptake for wild-type VMAT2 and mutants. Data were normalized to VMAT2WT and are presented as mean ± SEM; n = 3 biological replicates. One-way ANOVA; **** P < 0.0001; n.s, P = 0.9538. Source data are provided as a Source Data file.
Structural analyses reveal that VMAT2 adopts a compact major facilitator superfamily (MFS) fold20, characterized by 12 transmembrane helices (TMs) arranged into two pseudo-symmetrical domains: the N-terminal domain (NTD, TMs 1–6) and the C-terminal domain (CTD, TMs 7–12), both of which are oriented toward the cytoplasm. The luminal loop between TM1 and TM2 (LL1-2) exhibited flexibility and remained unresolved (Supplementary Fig. 1c). Notably, VBZ, 5-HT, and DA bind to VMAT2 in a lumen-facing conformation, whereas TBZ appears to trap VMAT2 in a lumen-facing occluded state (Fig. 1d, e). In these structures, the cytoplasmic gate is effectively sealed by two layers of residues: the inner layer consists of M204, M221, M403, and Y422, while the outer layer includes two salt bridges, D214-R357 and R217-D411 (Supplementary Fig. 7d, f). A previous study showed that mutating R218 in rat VMAT2 (corresponding to R217 in humans) enabled reserpine (RES) binding in the absence of a proton gradient, indicating that disruption of this salt bridge results in a conformation facing the cytosol21.
Locking VMAT2 in different conformations by TBZ and VBZ
To elucidate the inhibition mechanisms of TBZ and VBZ on VMAT2, particularly in the context of treatment of TD, we determined the cryo-EM structures VMAT2-TBZ and VMAT2-VBZ complexes. The structures reveal that TBZ traps VMAT2 in a lumen-facing occluded state, rendering it inaccessible from either side of the membrane (Fig. 2b and Supplementary Fig. 7a, c, d). On the luminal side, W318 from the luminal loop connecting TM7 and TM8 (LL7-8), along with a series of hydrophobic residues—V41, P45, F135, and F334—acts as a plug, effectively blocking the luminal gate (Fig. 2g and Supplementary Fig. 7c). TBZ occupies the central cavity formed by TMs 1, 4, 7, and 10. The binding site for TBZ is predominantly composed of non-polar residues from both the NTD and CTD, including L37, I44, L228, V232, and I308, which align with the hydrophobic architecture of TBZ. F135 contributes to π-π stacking with the benzene ring of TBZ. Specifically, the highly conserved residue E312 forms a salt bridge with the positively charged tertiary amine of TBZ, positioned roughly in the center of the molecule, aligning with previous findings that the equivalent residue E313 in rat VMAT2 is critical for both inhibitor recognition and substrate uptake22. R189 forms a polar interaction with one of TBZ’s methoxy groups, further stabilizing its binding (Fig. 2c). Notably, TBZ selectively inhibits VMAT2 rather than VMAT123, likely due to differences in the residues at the binding sites, such as L37, V232, I308, and Y433 in VMAT2 (Supplementary Fig. 1d). The structure indicates that TBZ’s inhibition mechanism may involve inducing an intermediate occluded conformation of VMAT2, disrupting the transport cycle.
As a derivative of TBZ, VBZ is the valine ester of (+)-α-HTBZ, the most active metabolite of TBZ (Fig. 1c)24. Unlike TBZ, VBZ captures VMAT2 in a lumen-facing state (Fig. 2e). The cytoplasmic gate aligns similarly to that of VMAT2-TBZ, while the luminal gate remains open (Fig. 2g and Supplementary Fig. 7a). VBZ binds to the central cavity similarly to TBZ, involving numerous overlapping non-polar residues that contribute to hydrophobic interactions. E312 also forms a salt bridge with VBZ’s positively charged amine. The density reveals that R189 retains weak interaction with VBZ, with additional hydrogen bond interactions between the valine moiety and Y341 and Y433 (Fig. 2f). However, structural superposition indicates that VBZ shifts approximately 1.2 Å towards the lumen compared to TBZ, likely due to the steric effects of its additional valine moiety (Fig. 2g). These differences in binding sites between VBZ and TBZ may explain the distinct conformations observed in VMAT2.
Upon superposing the structures of VMAT2-TBZ and VMAT2-VBZ, we observed a contraction in TMs 2, 8, and 10 in VMAT2-TBZ. Notably, the C-terminus of TM7 transitions from a helical structure to a loop, causing W318 to reposition and block the luminal opening. This transition coincides with a dilation of TM11 and the emergence of luminal loop 1–2, which inserts between LL7-8 and TM11 (Fig. 2g). The distinct conformations induced by VBZ and TBZ suggest different inhibition mechanisms: VBZ stabilizes VMAT2 in a lumen-facing state, while TBZ induces changes leading to a lumen-facing occluded state, obstructing the transport cycle at various stages. Notably, VBZ is metabolized into (+)-α-HTBZ following the cleavage of its valine moiety after oral administration25. This metabolite may further sustain an inhibitory role by promoting a lumen-facing occluded state, in line with VBZ’s longer half-life and the suitability for once-daily dosing.
Substrate binding sites of 5-HT and DA
To investigate the transport mechanism of monoamine neurotransmitters by VMAT2, we analyzed cryo-EM structures of VMAT2 bound to 5-HT and DA. 5-HT was observed in a lumen-facing conformation (Fig. 3b and Supplementary Fig. 7e, f). The cryo-EM density suggests that the amine group of 5-HT interacts with D399 (PoseN-D399) (Fig. 1f), while ligand-free docking indicates coexistence of an alternate pose with the amine group directed at E312 (PoseN-E312). MD simulations confirmed that 5-HT in PoseN-D399 was stable, while PoseN-E312 rapidly converted to PoseN-D399 (Supplementary Fig. 6a, b). Thus, we confidently modeled 5-HT in the lumen-facing state as PoseN-D399, where the positively charged amine forms a salt bridge with D399 and interacts with N305, Y341, and Y433. The hydroxyl group also engages in hydrogen bond with E312, while V232, I308, and F334 contribute to hydrophobic interactions with the indole group of 5-HT (Fig. 3c).
DA similarly binds to VMAT2 in a lumen-facing state (Fig. 3e, f and Supplementary Fig. 7b). The cryo-EM density for DA is well-defined and corroborated by ligand-free docking and MD simulations. Like 5-HT, MD simulations revealed that DA in PoseN-D399 was stable, while PoseN-E312 quickly transitioned to PoseN-D399 (Supplementary Fig. 6c, d). Consequently, we modeled DA in PoseN-D399. Its binding sites closely resemble those of 5-HT, with two hydroxyl groups interacting with E312 and S338, respectively (Fig. 3f).
Cell-based fluorescent uptake assays using FFN206 in HEK293T cells showed that mutations at N305, E312, Y341, and D399 significantly decreased transport activity, underscoring their critical roles in substrate transport (Fig. 3h and Supplementary Figs. 8, 9). These observations align well with previous studies21,22. Despite forming a hydrogen bond with the substrate’s amine, the Y433A mutation exhibited a moderate decrease in transport activity compared to Y341A, likely due to the loss of a cation-π interaction. Given the shared characteristics of substrates for vesicular monoamine transporters, the highly conserved residues E312 and D399 likely play pivotal roles in the transport of monoamine neurotransmitters (Supplementary Fig. 1).
The binding sites of TBZ and VBZ overlap significantly with those of 5-HT and DA. Structural superposition indicates that the isobutyl group of TBZ or VBZ occupies a region similar to the indole group of 5-HT and the benzene ring of DA (Fig. 3g), revealing a steric overlap characteristic of these two inhibitors.
MD simulations for potential protonation sites
The proton gradient toward the cytosol facilitates the transition of VMAT2 from the lumen-facing state to the cytosol-facing state26,27, suggesting potential protonation of acidic residues in the translocation pathway. We calculated the pKa values of acidic amino acids along the substrate pathway in lumen-facing VMAT2, identifying D399, D426, and E312 as possible protonation sites (Fig. 4a).
a Estimated pKa values of acidic residues in the VMAT2-5HT without 5-HT. The cyan and pink bars represent the pKa values of the residues on the lumen and cytosol side, respectively. Dashed lines indicate cytoplasmic (cyan) and luminal (pink) pH values. b–f Root mean square deviation (RMSD) plots of MD simulations and corresponding MD snapshots of the 5-HT-bound lumen-facing VMAT2 structure, with only E312 protonated (b), only D399 protonated (c), both E312 and D399 protonated (d), both D399 and D426 protonated (e), or all E312, D399 and D426 protonated (f). g A summarized diagram depicting the change in substrate pose relative to the protonation site during substrate release. h Fluorescent substrate FFN206 uptake for wild-type VMAT2 and mutants. Data were normalized to VMAT2WT and are presented as mean ± SEM; n = 4 biological replicates. One-way ANOVA; **** P < 0.0001. Source data are provided as a Source Data file. i Schematic illustrating the transport and inhibition mechanisms during VMAT2 transport, using 5-HT as an example.
To further explore the binding of 5-HT under these conditions, we conducted MD simulations for various protonation states: E312, D399, E312-D399, D399-D426, and E312-D399-D426. Our results revealed that protonation of D399 redirects the amine group of 5-HT toward E312, while simultaneous protonation of E312, D399, and D426 facilitates rapid substrate release (Fig. 4b–g). To validate these findings, we performed transport uptake assays with mutagenesis, substituting each amino acid with arginine, alanine, or asparagine/glutamine. All mutants exhibited significantly reduced transport activity, highlighting the critical roles of these residues in substrate transport, consistent with prior studies (Fig. 4h and Supplementary Figs. 8, 9)22.
Based on the established mechanism where one cytosolic transmitter is exchanged for two luminal protons27, previous research indicated that one proton aids in substrate translocation while the other facilitates conformational transition26,27,28. Our results suggest that the protonation of D399, E312, and D426 are linked to rapid substrate release. Given the higher pKa values of D399 and D426 compared to E312, we speculate that the proton from E312 dissociates into the lumen, while those from D399 and D426 are released into the cytosol (Fig. 4i). Although D426 does not directly bind substrates, it participates in a hydrogen bond network that is stronger in the lumen-facing state and weaker in the cytosol-facing state (Supplementary Fig. 10). This suggests that, in addition to its role in substrate release observed in our MD simulations, D426 may also play a crucial role in conformational transitions, aligning with previous studies that proposed a hinge mechanism involving the corresponding residue D427 in rat VMAT222,29.
Discussion
Our study elucidates the cryo-EM structures of human VMAT2 bound to the neurotransmitters 5-HT and DA in a lumen-facing state, as well as the clinical drugs TBZ and VBZ in occluded and lumen-facing states, respectively. While preparing this manuscript, we noted significant advancements in VMAT2 structural studies. Coleman et al. reported30 the structure of VMAT2 bound to TBZ, while Lee et al. resolved31 various states of VMAT2 with ligands such as TBZ, RES, and 5-HT. Jiang et al. published32,33 structures of VMAT2 with multiple ligands, including 5-HT, RES, TBZ, and others. Qu et al. and Iwata et al. detailed34,35 VMAT2 structures with various ligands, and Zhou et al. presented36 structures of frog VMAT2 and engineered sheep VMAT2 adopting a non-canonical fold.
Our structures of 5-HT- and DA-bound VMAT2, along with the TBZ-bound occluded state, align with recent findings, showing similar ligand poses. Notably, we provide the structure of VMAT2 bound to VBZ. Collectively, our findings and existing studies clarify substrate binding and transport mechanisms, highlighting diverse inhibition mechanisms. For instance, RES locks VMAT2 in a cytosol-facing state31,32,34, TBZ traps it in an occluded state30,31,32,34,35, and ketanserin stabilizes VMAT2 in a partially lumen-facing state32. Our study establishes a lumen-facing VBZ-bound state, revealing various inhibition mechanisms that enhance our understanding of drug actions.
Among the recently reported VMAT2 structures, only two cytosol-facing structures bound to substrates—5-HT and NE—have been resolved through site mutations, with their binding poses completely flipped: 5-HT in PoseN-E31231 and NE in PoseN-D39933. To clarify the binding pose of 5-HT in the cytosol-facing state, we conducted MD simulations, indicating that 5-HT flips between poses, suggesting both orientations may coexist (Supplementary Fig. 11). These reported structures, along with our MD results, imply that substrate loading onto VMAT2 from the cytosol may occur in different orientations influenced by substrate type.
Some recent studies on VMAT2 have incorporated MD simulations to explore the protonation impact on transport. Coleman et al. found30 that in the occluded state of DA-bound VMAT2, protonation of D399 directs the nitrogen of DA toward E312, while complete substrate release requires protonation of D33, E312, D399, and D426. Qu et al. conducted34 MD simulations in a lumen-facing state with 5-HT, showing that protonation of D399 causes the nitrogen of 5-HT to orient toward E312, and that protonation of both D399 and E312 facilitates rapid substrate release, differing from our results. Jiang et al. also performed32 MD simulations for VMAT2-5-HT in the lumen-facing state, focusing primarily on the protein backbone. Variations in MD outcomes may arise from different methodologies or subtle conformational changes due to protein modifications. Nonetheless, a common observation is that protonation of D399, which directly interacts with the substrate’s amine group, is likely critical for substrate release, indicating a coupling mechanism between protonation and substrate release. Although the MD simulations in Coleman’s study30 involved a different VMAT2 state, their findings largely align with ours, emphasizing D426 as another key protonation site. Additionally, as noted in Jiang’s work32,33, D33, like D426, is another critical residue that does not participate in substrate binding but is part of the same broad hydrogen bond network, which may be involved in protonation (Supplementary Fig. 10). Further investigations are needed to deepen our understanding of the protonation mechanism of VMAT2.
In addition to our structural analyses of VMAT2 in complex with 5-HT and DA, we present the structure of VMAT2 bound to VBZ and highlight the distinct inhibition mechanisms of the FDA-approved drugs TBZ and VBZ on VMAT2, which have important implications for treating TD. While TBZ primarily acts as a reuptake inhibitor, VBZ’s unique mechanism may offer alternative therapeutic benefits by stabilizing different conformational states of VMAT2. We also investigated the binding poses of 5-HT in both the cytosol-facing and lumen-facing states through comprehensive MD simulations, underscoring the role of protonation on key acidic residues. These findings significantly enhance our understanding of VMAT2 function and offer valuable insights for drug development targeting psychiatric disorders.
Methods
Expression and purification of BRIL-VMAT2
cDNA of human VMAT2 (UniProt ID: Q05940) gene was synthesized and cloned with N-terminal BRIL sequence into a pCAG vector containing C-terminal tandem His and Flag affinity tags (BRIL-VMAT212-514-His-Flag). The plasmid containing BRIL sequence was donated by Z. Zhang group, as described in a previous study37. The detailed protein sequence is provided in the Source Data in this paper. For the expression of VMAT2 protein, Expi293F cells were used and cultured in SMM 293T-II medium at 37 °C under 5% CO2 at 130 rpm in a Multitron-Pro shaker. When the cell density reached approximately 2.0 × 106 cells/mL, Expi293F cells were transiently transfected with the plasmid and polyethylenimines (PEIs) (Shanghai Maokang Biotechnology Co., Ltd.). The total amount of plasmids for transfection of one liter of cell culture is 2 mg and plasmids were pre-mixed with PEIs in 50 mL fresh medium in a 1:2 (w/w) ratio for 20 min before adding the mixture into cell culture.
After transfection for 48 h, cells were harvested by centrifugation at 1200 × g and resuspended in the buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 1.3 μg/mL aprotinin (Amresco), 0.7 μg/mL pepstatin (Amresco), and 5 μg/mL leupeptin (Amresco). The membrane fraction was extracted with 1% (w/v) n-Dodecyl-β-D-Maltopyranoside (DDM, Anatrace) at 4 °C for 2 h. After extraction, the cell lysate underwent centrifugation at 12,000 × g for 1 h (JA 25.50, Beckman Coulter) and supernatant was loaded to anti-Flag M2 affinity resin (GenScript), washed in buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 0.01% LMNG and 0.001% CHS (w/v), and eluted with buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 0.01% LMNG and 0.001% CHS (w/v) and 0.4 mg/mL Flag peptide. The elution was then loaded to nickel affinity resin (Ni-NTA, Cytiva), washed with the wash buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 0.01% LMNG and 0.001% CHS (w/v), 20 mM imidazole. The protein was eluted with the wash buffer plus 300 mM imidazole. The protein sample after elution was collected and concentrated to 5 mg/mL using a 50 kDa molecular weight cutoff concentrator (Millipore).
Expression and purification of anti-BRIL Fab
The plasmid containing anti-BRIL Fab sequence, including heavy chain and light chain, was donated by Z. Zhang group, as described in a previous study37. The detailed protein sequence is provided in the Source Data in this paper. DNA sequences of the heavy and light chains of anti-BRIL Fab were cloned into pCAG expression vectors separately. A His-tag was attached to the C-terminal of the heavy chain. For the expression of Fab, Expi293F cells were cultured and co-transfected by plasmids of heavy chain and light chain under the same conditions as VMAT2. The total amount of heavy chain and light chain plasmids for transfection of one liter of cell culture is 2 mg, 1 mg of each.
Purification of anti-BRIL Fab was conducted as described previously with some modifications37. Briefly, after transfection for 48 h, the cell culture media was centrifuged at 1200 × g for 10 min, and then the supernatant was loaded to nickel affinity resin, washed with the wash buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 50 mM imidazole. The protein was eluted with the wash buffer plus 300 mM imidazole. The protein sample after elution was collected and concentrated to 2 mg/mL using a 50 kDa molecular weight cutoff concentrator (Millipore).
Expression and purification of anti-Fab nanobody
The plasmid containing anti-Fab nanobody sequence was donated by Z. Zhang group, as described in a previous study37. The detailed protein sequence is provided in the Source Data in this paper. The DNA sequence corresponding to the anti-Fab nanobody (Nb) was inserted into the pET24(+) vector, which features an N-terminal PelB signal sequence and a C-terminal 6 × His-tag. This construct allowed for the expression and translocation of Nb to the periplasmic space of Escherichia coli strain BL21. The bacteria were cultured in LB medium at 37 °C until the optical density at 600 nm (OD600) reached approximately 0.8. Subsequently, protein expression was induced by the addition of 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) and continued for 16 h at 16 °C.
For Nb purification, the bacterial cell pellets were lysed by sonication in lysis buffer (25 mM HEPES pH 7.4, 150 mM NaCl, 1 mM PMSF). Cell debris was removed by centrifugation at 33,000 × g for 1 h. Then the supernatant was applied to Ni-NTA, washed with 25 mM HEPES pH 7.4, 150 mM NaCl, 25 mM imidazole and 50 mM imidazole separately. Elution of the protein was performed using an elution buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl and 500 mM imidazole. The protein sample after elution was collected and concentrated to 2 mg/mL using a concentrator with a molecular weight cutoff of 10 kDa (Millipore).
The BRIL-VMAT2/Fab/Nb complex assembly
For complex assembly, the purified BRIL-VMAT2, anti-BRIL Fab, and anti-Fab Nb were incubated on ice for 1 h at the ratio of 1:1.4:2. Then the samples were applied to a Superose 6 Increase 10/300 GL column (Cytiva) equilibrated with buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, 0.01% LMNG and 0.001% CHS (w/v) to remove the excess Fab and Nb components. The peak fractions corresponding to the ternary complex were collected and concentrated to 10 mg/mL.
Cryo-EM sample preparation and data acquisition
The final concentration of protein samples for cryo-EM sample preparation is ∼10 mg/mL. For VMAT2-5-HT/TBZ/VBZ/DA, compounds of serotonin (5-HT, Target-Mol), tetrabenazine (TBZ, Target-Mol), valbenazine (VBZ, MedChemExpress), and dopamine (DA, MedChemExpress) were added to the protein solution at the final concentration of 2.5 mM, 100 μM, 15 μM, and 6.6 mM, individually. 3.5 μL of protein sample with compounds was applied to a glow-discharged Quantifoil R1.2/1.3 400-mesh gold holey carbon grid, blotted for 4.5 s and flash-frozen in liquid ethane cooled by liquid nitrogen with Vitrobot (Mark IV, Thermo Fisher Scientific).
For data acquisition of VMAT2-5-HT/TBZ/VBZ/DA, micrographs were acquired on a Titan Krios microscope (Thermo Fisher Scientific) operated at 300 kV with a Falcon 4i Direct Electron Detector (Thermo Fisher Scientific) and a Selectris X energy filter. EPU was used for automated data collection following standard procedures. A nominal magnification of 130,000 × was used for imaging, yielding a pixel size of 0.932 Å on images. The defocus range was set from −0.9 μm to −1.6 μm. Each micrograph was recorded in a total dose of ∼50 e−/Å2, with total collection of 13,925, 23,115, 13,947, and 19,844 movie stacks, respectively.
Cryo-EM data processing
The data processing pipelines are shown in Supplementary Figs. 2–6. Motion correction was performed using Relion v3.138 and CTF estimations were performed using cryoSPARC v3.3.239. For the data processing of VMAT2-5-HT, after several rounds of 2D classification, an initial model with clear TMs was obtained using cryoSPARC. Heterogeneous Refinement, Local refinement and Local CTF refinement yielded a lumen-facing VMAT2-5-HT at the resolution of 2.98 Å. Similarly, we got an occluded VMAT2-TBZ at the resolution of 3.28 Å, a lumen-facing VMAT2-VBZ at the resolution of 3.38 Å, and a lumen-facing VMAT2-DA at the resolution of 3.00 Å.
Model building
We first built the protein atomic model of VMAT2-5-HT based on the AlphaFold40 predicted model as a starting template. Using UCSF Chimera41 to dock this model into the density maps. Atomic model building based on the density map of VMAT2-5-HT was performed in Coot42, and refined in real space using Phenix.real_space_refine43. Validation tools in Phenix43 and Molprobity44 were used to guide iterative rounds of model adjustment in Coot and refinement in Phenix. For model building of VMAT2-DA/VBZ, atomic model of VMAT2-5-HT was used as initial model, because of the similar conformations. As for VMAT2-TBZ, the AlphaFold predicted model was used as the starting template, and modified subsequently according to the density map.
As regards the model building of the ligands, we conducted ligand-free docking and calculated the binding free energies through the molecular mechanics-generalized born surface area (MM/GBSA) approach by employing the Schrödinger Maestro program (Maestro, Schrödinger, LLC, New York, NY, 2024) for ligand binding pose determination. Consequently, we constructed the ligand models by referencing to the EM density map and the adjacent environment with Coot. These ligand models underwent further refinement with Phenix.real_space_refine and validated by MD simulations. Visualization of all figures was accomplished using PyMOL (www.pymol.org) or UCSF ChimeraX45.
FFN206 uptake assay using HEK293T cells
Uptake assays were performed using fluorescent substrate FFN206 in HEK293T cells as reported previously18, with some modifications. Briefly, HEK293T cells were cultured in 6-well plates until reaching approximately 50–60% confluence, and expression plasmids pCAG-WT or pCAG-mutants were transfected with PEIs following the manufacturer’s instructions. After transfection for 48 h, the medium was discarded, and cells were incubated with 2 mL assay buffer (125 mM sodium gluconate, 4.8 mM potassium gluconate, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 1.3 mM CaCl2, 5.6 mM glucose and 25 mM HEPES pH 7.4) at 37 °C for 15 min. Subsequently, the fluorescent indicator substrate FFN206 (Target-Mol) was added to the cells at a final concentration of 5 μM in assay buffer, and the cells were incubated at 37 °C for 1 h. Uptake was terminated by washing cells three times with 2 mL ice-cold phosphate-buffered saline (PBS). Cells were lysed with PBS containing 1% TritonX-100, and the fluorescence intensity of FFN206 was measured with excitation/emission wavelength at 360 nm/460 nm using a microplate reader (BioTek Synergy2 Microplate Reader). All statistical analyses were performed with one-way ANOVA followed by Dunnett’s multiple comparisons test by comparing WT with mutants using GraphPad Prism v9.3.1. Transport assays were biologically repeated at least three times. The average fluorescent signals of untransfected cells were treated as background correction and the average fluorescent signals of VMAT2WT were defined as 100% uptake.
Inhibition of VMAT2-mediated uptake of FFN206 by compounds of TBZ/VBZ
For the concentration-dependent inhibition of FFN206 uptake assay, HEK293T cells were cultured in 12-well plates, until reaching approximately 50–60% confluence, and expression plasmids pCAG-VMAT2WT or pCAG-VMAT2EM were transfected with PEIs following the manufacturer’s instructions. After transfection for 48 h, the medium was discarded, and cells were treated with assay buffer containing the two compounds in six concentrations of 0.1–10,000 nM for TBZ or DMSO (as control), in the total volume of 1 mL, and incubated at 37 °C for 15 min. Next, cells were incubated for another 1 h in uptake buffer (assay buffer containing FFN206, final concentration 5 μM) at 37 °C and washed three times with 2 mL ice-cold PBS. Cells were lysed using PBS containing 1% TritonX-100 and transformed to a 96-well plate, the fluorescence intensity of FFN206 was measured with excitation/emission wavelength at 360 nm/460 nm using a microplate reader. The concentration-dependent inhibition of FFN206 uptake assays were biologically repeated at least three times. The average fluorescent signals of untransfected cells were treated as background correction and the average fluorescent signals of VMAT2WT or VMAT2EM were defined as 100% uptake separately. Curves were fitted using nonlinear regression with GraphPad Prism v9.3.1.
For the transport inhibition assay, HEK293T cells were cultured in 6-well plates, until reaching approximately 50–60% confluence, and expression plasmids pCAG-VMAT2WT or pCAG-VMAT2EM were transfected with LipofectamineTM 2000 according to the manufacturer’s instructions. After transfection for 24 h, the medium was discarded and cells were divided into 24-well plates. 10 mM sodium butyrate was added, and cells were cultivated at 30 °C for 24 h. Afterward, cells were treated with assay buffer containing 10 mM Glyco-diosgenin (GDN) with inhibitors (TBZ or VBZ) or DMSO (as control), and incubated at 37 °C for 15 min. Next, cells were incubated for another 1 h in uptake buffer (assay buffer containing FFN206, final concentration 5 μM) at 37 °C and washed three times with 2 mL ice-cold PBS. Cells were lysed using PBS containing 1% TritonX-100 and transformed to a 96-well plate, the fluorescence intensity of FFN206 was measured with excitation/emission wavelength at 360 nm/460 nm using a microplate reader (BioTek Synergy2 Microplate Reader). The inhibition assays were biologically repeated at least three times. In this assay, 5 μM TBZ and 5 μM VBZ were applied as inhibitors. The average fluorescent signals of untransfected cells were treated as background correction and the average fluorescent signals of VMAT2WT were defined as 100% uptake.
Cell surface biotinylation and immunoblot analysis
Cell surface biotinylation and immunoblot analysis were performed as reported previously with some modifications46. Briefly, HEK293T cells were cultured in 6-well plates until reaching approximately 70% confluence, and expression plasmids pCAG-WT or pCAG-mutants were transfected with PEIs following the manufacturer’s instructions. After 48 h, the cell culture medium was removed and cells were suspended with 1 mL ice-cold PBS and then treated with 1 mL sulfo-N-hydroxysuccinimide-SS-biotin (1 mg/mL in PBS, Thermo Scientific) at room temperature for 1 h. Afterward, cells were washed three times with 1 mL ice-cold PBS containing 100 mM glycine and incubated at 4 °C for 10 min in the same buffer. Then, cells were lysed with 500 μL lysis buffer (25 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, and 1% Triton X-100, containing protease inhibitors) at 4 °C for 2 h with shaking. After centrifugation at 13,000 × g for 10 min, the supernatants were incubated with 50 μL streptavidin-agarose beads (Thermo Scientific) at room temperature for 1 h. The beads were then centrifuged at 500 g for 1 min, washed three times with ice-cold lysis buffer, and incubated with 100 μL 2 × Laemmli buffer containing 100 mM dithiothreitol at room temperature for 1 h to recover the cell surface proteins. Samples were separated using SDS-polyacrylamide gel electrophoresis followed by western-blot analysis. All proteins were detected using HRP-conjugated DYKDDDDK tag monoclonal antibody (1:5000 dilution; Proteintech; HRP-66008). Then the same membrane was stripped at room temperature for 1 h. Na+/K+-ATPase was used as loading control for normalization and was detected with rabbit anti-Na+/K+-ATPase monoclonal antibody (1:10,000 dilution, ABclonal; A11683) combined with secondary HRP goat anti-rabbit monoclonal antibody (1: 10,000 dilution; ABclonal; AS014).
Total expression levels detected by Western blot
Total expression levels of WT and mutants of VMAT2 were performed as previously described47 with modifications. HEK293T cells were cultured in 6-well plates until reaching approximately 90% confluence, and expression plasmids pCAG-WT or pCAG-mutants were transfected with PEIs following the manufacturer’s instructions. After 48 h, The cell culture medium was removed and cells were suspended with 1 mL ice-cold PBS. Then, after centrifugation and discarding the supernatant, cells were lysed with 150 μL lysis buffer (25 mM HEPES pH 7.4, 150 mM NaCl, 1% DDM, 1.3 μg/mL aprotinin, 0.7 μg/mL pepstatin, and 5 μg/mL leupeptin) at 4 °C for 2 h with shaking. The cell extracts were centrifuged at 16,000 × g for 1 h before the supernatant was taken and mixed with SDS loading buffer. Then the mixtures were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose filter membrane and probed with HRP-conjugated DYKDDDDK tag monoclonal antibody (1:5000 dilution; Proteintech; HRP-66008) or β-Actin rabbit mAb (1:5000 dilution; ABclonal; AC038). The secondary antibodies was used HRP-conjugated goat anti-rabbit IgG (H + L) (1:5000 dilution; ABclonal; AS014). The membrane was visualized with the imaging system (ChemiDoc Touch; Bio-Rad, Hercules, CA, USA) using enhanced chemiluminescence (ECL) (Smart-Lifesciences).
Confocal imaging analysis
HEK293T cells were cultured in 20 mm glass bottom cell culture dishes (NEST, 801001) with 1.0 × 106 cells/mL density overnight, and pCAG-GFP-VMAT2-His-Flag constructs were transfected with PEIs following the manufacturer’s instructions. 24 h post transfection, cells were observed under confocal microscope (Leica SP8).
Molecular dynamics simulations
MD simulations were conducted for VMAT2-5-HT/DA/TBZ/VBZ. The initial proteins include residues 18 to 46 and 117 to 475 for VMAT2-5-HT/VBZ, residues 18 to 46 and 132 to 475 for VMAT2-DA and residues 16 to 50, 117 to 318 and 324 to 476 for VMAT2-TBZ. The pKa values of protein residues were estimated using PROPKA 3.4.048. Charged residues were protonated assuming pH 7.4 unless otherwise specified, and all the N- and C-termini of proteins were capped with acetyl and N-methyl groups, respectively. To build the system, protein-ligand complex was firstly embedded in a POPC bilayer (~302 molecules) and then solvated in a water box (117 × 117 × 90 Å3). Finally, 150 mM NaCl was added to neutralize the system. Details of system composition are listed in Supplementary Table 2.
MD simulations were performed with GROMACS 2021.449. CHARMM36 force field was used for protein, POPC lipid and ions50, and TIP3P model51 was used for water. The force fields of four ligands (5-HT/DA/TBZ/VBZ) were generated by CGenFF program52. After energy minimization by steepest descent algorithm, the systems were simulated in NVT ensemble to relax lipid tail, lipid head and solvent (water and ions) in steps for 1.5 ns. Then, the systems were further relaxed in NPT ensemble. Constraints on (1) lipids and solvent, (2) protein sidechain, (3) ligand, and (4) protein Cα atoms were gradually released each for 2 ns. After pre-equilibrations, 1 μs production simulations were run without any constraint. Periodic boundary condition was applied, and the temperature and pressure were kept at 310 K and 1 atm by Nose-Hoover thermostat53 and Parrinello-Rahman barostat54, respectively. Long range electrostatic interactions were described using the particle mesh Ewald (PME) method55 with a cutoff of 12 Å. Van der Waals interactions were smoothly switched to zero from 10 to 12 Å. All bonds involving hydrogen atoms were constrained by the LINCS algorithm56 to allow a time step of 2 fs. Each system was run for three replicas with different initial velocities and the total simulation time was 39 µs.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Information. Atomic coordinates of VMAT2-5-HT/DA/TBZ/VBZ have been deposited in the Protein Data Bank (http://www.rcsb.org) under the accession codes 8X3K, 9KQE, 9KQA, and 9KQ8. The corresponding electron microscopy maps have been deposited in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/pdbe/emdb/), under the accession codes EMD-38038, EMD-62501, EMD-62498, and EMD-62497. Source data are provided with this paper.
References
Eiden, L. E., Schäfer, M. K. H., Weihe, E. & Schütz, B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pfluegers Arch. 447, 636–640 (2004).
Isingrini, E. et al. Selective genetic disruption of dopaminergic, serotonergic and noradrenergic neurotransmission: insights into motor, emotional and addictive behaviour. J. Psychiatry Neurosci. 41, 169–181 (2016).
Mulvihill, K. G. Presynaptic regulation of dopamine release: role of the DAT and VMAT2 transporters. Neurochem. Int. 122, 94–105 (2019).
Teleanu, R. I. et al. Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 23, 5954 (2022).
Eiden, L. E. & Weihe, E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. 1216, 86–98 (2011).
Weihe, E., Schäfer, M. K., Erickson, J. D. & Eiden, L. E. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J. Mol. Neurosci. 5, 149–164 (1994).
Guillot, T. S. & Miller, G. W. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 39, 149–170 (2009).
Ma, L. et al. Live-cell microscopy and fluorescence-based measurement of luminal pH in intracellular organelles. Front. Cell. Dev. Biol. 5, 71 (2017).
Cepeda, C., Murphy, K. P. S., Parent, M. & Levine, M. S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 211, 235–254 (2014).
Hornykiewicz, O. Biochemical aspects of Parkinson’s disease. Neurology 51, S2–S9 (1998).
Ledonne, A. & Mercuri, N. B. Current concepts on the physiopathological relevance of dopaminergic receptors. Front. Cell. Neurosci. 11, 27 (2017).
Leung, J. G. & Breden, E. L. Tetrabenazine for the treatment of tardive dyskinesia. Ann. Pharmacother. 45, 525–531 (2011).
Paek, S.-M. Synthesis of tetrabenazine and its derivatives, pursuing efficiency and selectivity. Molecules. 25, 1175 (2020).
Canney, D. J., Kung, M. P. & Kung, H. F. Amino- and amido-tetrabenazine derivatives: synthesis and evaluation as potential ligands for the vesicular monoamine transporter. Nucl. Med. Biol. 22, 527–535 (1995).
Kim, E. S. Valbenazine: first global approval. Drugs 77, 1123–1129 (2017).
Davis, M. C. et al. Efficient trial design - FDA approval of valbenazine for tardive dyskinesia. N. Engl. J. Med. 376, 2503–2506 (2017).
Mukherjee, S. et al. Synthetic antibodies against BRIL as universal fiducial marks for single-particle cryoEM structure determination of membrane proteins. Nat. Commun. 11, 1598 (2020).
Hu, G. et al. New fluorescent substrate enables quantitative and high-throughput examination of vesicular monoamine transporter 2 (VMAT2). ACS. Chem. Biol. 8, 1947–1954 (2013).
Black, C. A. et al. Assessing vesicular monoamine transport and toxicity using fluorescent false neurotransmitters. Chem. Res. Toxicol. 34, 1256–1264 (2021).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Yaffe, D., Vergara-Jaque, A., Forrest, L. R. & Schuldiner, S. Emulating proton-induced conformational changes in the vesicular monoamine transporter VMAT2 by mutagenesis. Proc. Natl. Acad. Sci. USA 113, E7390–E7398 (2016).
Yaffe, D. et al. Identification of molecular hinge points mediating alternating access in the vesicular monoamine transporter VMAT2. Proc. Natl. Acad. Sci. USA 110, E1332–E1341 (2013).
Peter, D. et al. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J. Biol. Chem. 269, 7231–7237 (1994).
Grigoriadis, D. E. et al. Pharmacologic characterization of valbenazine (NBI-98854) and its metabolites. J. Pharmacol. Exp. Ther. 361, 454–461 (2017).
Skor, H. et al. Differences in dihydrotetrabenazine isomer concentrations following administration of tetrabenazine and valbenazine. Drugs. R. D. 17, 449–459 (2017).
Parsons, S. M. Transport mechanisms in acetylcholine and monoamine storage. FASEB. J. 14, 2423–2434 (2000).
Schuldiner, S., Shirvan, A. & Linial, M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev. 75, 369–392 (1995).
Henry, J. P., Sagné, C., Bedet, C. & Gasnier, B. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem. Int. 32, 227–246 (1998).
Yaffe, D., Forrest, L. R. & Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 150, 671–682 (2018).
Dalton, M. P., Cheng, M. H., Bahar, I. & Coleman, J. A. Structural mechanisms for VMAT2 inhibition by tetrabenazine. eLife 12, RP91973 (2023).
Pidathala, S. et al. Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2. Nature 623, 1086–1092 (2023).
Wu, D. et al. Transport and inhibition mechanisms of human VMAT2. Nature 626, 427–434 (2024).
Wu, D. et al. Structural snapshots of human VMAT2 reveal insights into substrate recognition and proton coupling mechanism. Cell Res 34, 586–589 (2024).
Wang, Y. et al. Transport and inhibition mechanism for VMAT2-mediated synaptic vesicle loading of monoamines. Cell Res 34, 47–57 (2024).
Im, D. et al. Neurotransmitter recognition by human vesicular monoamine transporter 2. Nat. Commun. 15, 7661 (2024).
Lyu, Y. et al. Engineering of a mammalian VMAT2 for cryo-EM analysis results in non-canonical protein folding. Nat. Commun. 15, 6511 (2024).
Dang, Y. et al. Molecular mechanism of substrate recognition by folate transporter SLC19A1. Cell Discov 8, 141 (2022).
He, S. & Scheres, S. H. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of coot. Acta Crystallogr. Sect. D. Biol. Crystallogr 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D. Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D. Biol. Crystallogr. 66, 12–21 (2010).
Pettersen, E. F. et al. UCSF chimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82 (2021).
Shan, Z. et al. Cryo-EM structures of human organic anion transporting polypeptide OATP1B1. Cell Res. 33, 940–951 (2023).
Nan, J. et al. Cryo-EM structure of the human sodium-chloride cotransporter NCC. Sci. Adv. 8, eadd7176 (2022).
Olsson, M. H. M., Søndergaard, C. R., Rostkowski, M. & Jensen, J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory. Comput. 7, 525–537 (2011).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 114, 7830–7843 (2010).
Mark, P. & Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A. 105, 9954–9960 (2001).
Vanommeslaeghe, K., Raman, E. P. & MacKerell, A. D. Jr Automation of the CHARMM general force field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168 (2012).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A: Gen. Phys. 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys 52, 7182–7190 (1981).
Essmann, U. et al. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Hess, B. P-LINCS: a parallel linear constraint solver for molecular simulation. J. Chem. Theory. Comput. 4, 116–122 (2008).
Acknowledgements
We would like to thank the center of Cryo-Electron Microscopy, Core Facility of Shanghai Medical College, Fudan University. We thank the great support from Prof. Yanhui Xu in Fudan University. We thank Prof. Zhenguo Chen, Hui Zhao, Anqi Dong, Yulei Ren for cryo-EM facility support. We are grateful to Prof. Zhe Zhang from Peking University for kindly providing the plasmids of BRIL, Fab, and nanobody. This work was funded by the National Natural Science Foundation of China (32171196 to Y.Z.), National Key R&D Program of China (2021YFA1300101 to Y.Z.), Start-up funds from Fudan University and Shanghai Fifth People’s Hospital, and Shenzhen Natural Science Foundation Project (GXWD20201231105722002-20200831175432002 to H.L.).
Author information
Authors and Affiliations
Contributions
Y.Z. conceived the project. F.W. and J.W. prepared protein purification. W.Z. and F.W. performed functional transport assay. F.W. performed cryo-EM data acquisition. Y.Z. and F.W. contributed to the cryo-EM data processing and model building. H.L. performed MD simulations. Y.Z. wrote the manuscript. All authors analyzed the data and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information:
Nature Communications thanks Albert Guskov, Harald Sitte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, F., Liu, H., Zhang, W. et al. Drug inhibition and substrate transport mechanisms of human VMAT2. Nat Commun 16, 323 (2025). https://doi.org/10.1038/s41467-024-55361-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55361-0