Abstract
We previously developed human CAR macrophages (CAR-M) and demonstrated redirection of macrophage anti-tumor function leading to tumor control in immunodeficient xenograft models. Here, we develop clinically relevant fully immunocompetent syngeneic models to evaluate the potential for CAR-M to remodel the tumor microenvironment (TME), induce T cell anti-tumor immunity, and sensitize solid tumors to PD1/PDL1 checkpoint inhibition. In vivo, anti-HER2 CAR-M significantly reduce tumor burden, prolong survival, remodel the TME, increase intratumoral T cell and natural killer (NK) cell infiltration, and induce antigen spreading. CAR-M therapy protects against antigen-negative relapses in a T cell dependent fashion, confirming long-term anti-tumor immunity. In HER2+ solid tumors with limited sensitivity to anti-PD1 (aPD1) monotherapy, the combination of CAR-M and aPD1 significantly improves tumor growth control, survival, and remodeling of the TME in pre-clinical models. These results demonstrate synergy between CAR-M and T cell checkpoint blockade and provide a strategy to potentially enhance response to aPD1 therapy for patients with non-responsive tumors.
Similar content being viewed by others
Introduction
Chimeric antigen receptor (CAR) T cell therapy has shown remarkable efficacy in hematologic malignancies but responses in solid tumors remain minimal1. We and others have demonstrated pre-clinically that engineering macrophages to express CARs redirects macrophage effector function, including phagocytosis, cytotoxicity, cytokine/chemokine release, and antigen presentation. CAR-M has the potential to transfer the success of CAR-based cell therapy to solid tumors by overcoming barriers associated with trafficking, immunosuppression within the TME, and target antigen heterogeneity2,3,4,5.
We have previously generated human CAR-M and demonstrated the feasibility of redirecting macrophage effector function with CARs harboring a CD3-ζ signaling domain. CAR engagement led to targeted phagocytosis and killing, leading to control of tumor growth in immunodeficient xenograft models4. Innovative advances with genetically engineered macrophages have been explored in preclinical and clinical studies6,7, including, induced pluripotent stem cell (iPSC)-derived macrophages armed with a second-generation CAR3 and direct in vivo delivery of CARs to myeloid cells utilizing LNP/mRNA8. The full mechanism of action (MOA) of CAR-M in relevant immunocompetent settings has not been previously described. Furthermore, the interaction between CAR-M and endogenous anti-tumor T cell immunity has not been evaluated. Here, we developed a fully murine syngeneic CAR-M model using Ad5f35, mirroring the only clinically tested CAR-M therapy for solid tumors – CT-0508 (Adenovirally transduced autologous macrophages engineered to express an anti-human epidermal growth factor receptor 2 (HER2) CAR; ClinicalTrials.gov Identifier: NCT04660929).
Targeting negative checkpoint regulators (NCRs) such as the programmed cell death protein 1 (PD-1) has revolutionized cancer treatment with indications including non-small-cell lung cancer (NSCLC)9, melanoma10, breast cancer11, bladder cancer12, renal cell carcinoma13, and others. Mechanistic studies of PD-1/programmed death-ligand 1 (PD-L1) blockade show increased dendritic cell (DC)14, and T cell infiltration15,16 into the TME and restoration of exhausted CD8+ T cell function17. Importantly, most solid tumor patients do not respond to checkpoint blockade monotherapy due to primary or secondary resistance18, often associated with the presence of immunosuppressive tumor-associated macrophages19.
Several studies have shown that, in patients who do not respond to PD-1 blockade, factors such as low mutational burden, low MHC expression, defective antigen presentation20, low CD8+ T cell infiltration, and minimal Th1 cytokine signatures can negatively impact efficacy21. PD-1 and PD-L1 expression has been reported in HER2+ cancers22. PD1/PDL1 inhibitors are currently indicated for a multitude of solid tumors and hematologic indications9,10,11,12,13,23,24,25,26,27. Their combination with trastuzumab has been reported in esophageal cancer, hepatocellular carcinoma, biliary tract cancer, endometrial carcinoma, tumor mutational burden high cancers, and triple-negative breast cancer26,27,28. Notably, PD1/PDL1 inhibitors are not indicated for HER2 overexpressing breast cancer where responses have been limited. Although durable responses have been demonstrated with pembrolizumab and other T cell checkpoint inhibitors across a wide variety of cancers, only 20% of patients respond to monotherapy.
We have previously shown that the adoptive cell transfer of CAR-M into xenograft models of human cancer controls tumor progression and improves overall survival4. To model the interaction between CAR-M therapy in the TME with the endogenous immune system, we generated two murine tumor cell lines overexpressing HER2: one from CT26 colorectal cancer, and the second from 4T1 breast cancer. These tumor lines were then engrafted into fully immunocompetent syngeneic BALB/c mice. A clinically relevant surrogate murine CAR-M model was generated using Ad5f35 and primary murine bone marrow (BM) derived macrophages (BMDM) which phenotypically and functionally mirror their human counterparts. In vivo, murine CAR-M exerted anti-tumor activity, reprogrammed the TME, primed T cells against tumor-associated antigens, and induced antigen spreading leading to long-term T cell memory with protection from antigen-negative rechallenge.
We hypothesized that co-administration of CAR-M with a PD-1 inhibitor (aPD1) may be advantageous due to CAR-M-driven TME remodeling, T cell recruitment, and enhanced antigen presentation. To assess the synergistic potential of CAR-M + aPD1 combination therapy, in vivo studies were conducted in multiple HER2+ solid tumor models with limited sensitivity to aPD1. Results in these studies showed significant improvement in tumor control, prolonged overall survival, and increased TME remodeling and T cell activation. The data presented herein demonstrate the expanded MOA of CAR-M in immunocompetent hosts and provide proof of concept that CAR-M can sensitize solid tumors to aPD1 therapy. These results led to a phase 1, first-in-human multicenter study of CT-0508 (anti-HER2 CAR-M) and pembrolizumab combination therapy in HER2 overexpressing solid tumors (ClinicalTrials.gov Identifier: NCT04660929).
Results
Development of a syngeneic primary CAR-M model system
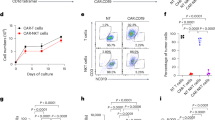
We have previously shown that Ad5f35 overcomes macrophage resistance to genetic manipulation and effectively delivers CARs to primary human macrophages4. Human CAR-M are monocyte-derived-macrophages differentiated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and transduced with Ad5f35. We sought to develop a primary murine CAR-M system to enable syngeneic modeling of a parallel cell product. To evaluate the ability of Ad5f35 to transduce primary murine myeloid cells, we generated BMDM using three different protocols. We isolated monocytes or hematopoietic stem cells (HSCs) from BM and cultured them in macrophage colony-stimulating factor (M-CSF) (both HSCs and monocytes) or GM-CSF (monocytes only) to allow differentiation. Generating BMDM from HSCs yielded significantly more macrophages (Supplementary Fig. 1a), and all protocols led to the derivation of a pure macrophage population (Supplementary Fig. 1b and Supplementary Fig. 5a). Ad5f35 effectively delivered anti-HER2 CD3-ζ CAR (Supplementary Fig. 1c) and generated high amounts of CAR-M (Supplementary Fig. 1d and Supplementary Fig. 5b). To characterize the phenotypic complexity of the different cultures, we performed transcriptomic analysis on the starting cells (HSCs or monocytes), and the fully differentiated cells—untransduced macrophages (UTD-Ms) and CAR-Ms. SingleR prediction analysis confirmed the purity and identity of CAR-M cultures (Supplementary Fig. 1e). Principal component analysis (PCA) (Fig. 1a) clustered HSCs-derived and monocyte-derived, M-CSF-differentiated, CAR-M together, suggesting those cultures are transcriptionally equivalent. By comparing GM-CSF CAR-M (mono) vs M-CSF CAR-M (mono), we showed that GM-CSF generates DC-like CAR-M whereas M-CSF generates macrophage-like CAR-M (Supplementary Fig. 1f). To evaluate effector function, killing assays on the IncuCyte® live-cell imaging system were performed with murine CAR-M cocultured at the effector: target (E:T) ratio 1:1 with HER2 expressing AU565 (human breast carcinoma) and SKOV3 (human ovarian carcinoma) cells modified to express NucLight Green (NLG). Robust killing activity was observed for all CAR-M preparations. Notably, cytotoxicity was higher in M-CSF-derived CAR-Ms compared to the GM-CSF counterpart (Fig. 1b and Supplementary Fig. 1g). Given that adenoviral transduction activates the inflammasome29,30, and our previous observations that Ad5f35 polarizes human macrophages toward a pro-inflammatory phenotype4, we hypothesized that exposure to Ad5f35 would similarly activate murine macrophages30,31. Although GM-CSF, but not M-CSF, is associated with pro-inflammatory macrophage differentiation32, Ad5f35 promoted an inflammatory phenotype across all CAR-M preparations (Supplementary Fig. 1h) as indicated by a proinflammatory macrophage signature comprising 16 genes33. As macrophages derived from HSCs yielded a larger cell number (Supplementary Fig. 1a), and M-CSF-derived CAR-M showed increased functionality (Supplementary Fig. 1g and Fig. 1b) and a pro-inflammatory phenotype (Supplementary Fig. 1h), all further studies were conducted using HSCs-derived, M-CSF-cultured CAR-M designated as M-CSF CAR-M (HSCs).
a PCA clustering from HSCs, monocytes, and CAR-M cultures (n = 3). b Incucyte-based killing assays of UTD-M or CAR-M generated from monocytes (GM-CSF or M-CSF) and HSCs (M-CSF) cocultured with AU565 NLG at effector:target (E:T) ratio 1:1 for 40 h. Green fluorescence intensity (GFI) values were normalized to the 0 h timepoint, then further normalized to the target cells. Values were transformed to % cytotoxicity (1-GFI)*100 %). Data are representative of three experiments and shows the mean ± SD of n = 4 technical replicates. Statistical analysis was calculated using the ordinary one-way ANOVA test. c Viability, and sustained Ad5f35 CAR expression up to 13 days post-transduction of M-CSF-derived, HSCs-differentiated macrophage. Data are representative of several experiments and show the mean ± SEM of n = 3 technical replicates. d, Representative FACS plots showing CAR expression. e, f Expression of pro-inflammatory (e.g., CD80, CD86 and MHC-II) and anti-inflammatory (e.g., CD206) markers were measured 48 h. after macrophages transduction at the protein level (e, n = 3 technical replicates) by flow cytometry and at the gene level (f, n = 3 biological replicate) by RNA-Seq. Statistical analysis for (e) was calculated with the two-sided paired t test. g Killing assays with UTD-M or CAR-M cocultured with target (MC38-HER2 and CT26-HER2) and parental cell lines (WT, HER2 negative) at E:T 2:1. GFI values were first normalized to the 0 h. timepoint then normalized to the respective target cells. Data is shown as mean ± SEM of n = 2–3 technical replicates. Statistical significance was calculated via two-way RM ANOVA. Graph is representative of three experiments. h, i Cytokine and chemokine production by CAR-M after overnight stimulation with plate-bound recombinant human HER2 protein or recombinant mesothelin (Meso; irrelevant antigen control) was determined by cytokine multiplex assay (n = 3 biological replicate). h Data are represented as the mean ± SD. Statistical analysis was performed using the two-way ANOVA Tukey’s multiple comparisons test. i Heatmap comparing mesothelin- vs HER2-stimulated CAR-M cytokine and chemokine production. Two-sided unpaired Student’s t test was used to calculate significance. j Phagocytosis assay of MC38 cells by UTD-M or CAR-M determined by flow cytometry. Data represents the mean ± SEM of n = 3 technical replicates. Statistical significance was calculated via two-way ANOVA multiple comparisons. Graph is representative of two experiments. k MHC class I expression on MC38WT and MC38-HER2 cells co-cultured with UTD-M or CAR-M. Data show the mean ± SEM of n = 2 technical replicates and representative FACS plots show MHC-I (H2kB) MFI. Graph is representative of two experiments. l Killing kinetics of UTD-M and CAR-M co-cultured with MC38OVA+HER2+ and transgenic CD8+ T cells (OT−1), at E:T:T (Effector:Target:T cells), 2:1:6. GFI values were normalized to the 0 h. timepoint and then, further normalized to the target cells. Data represents the mean ± SEM of n = 3 technical replicates and is representative of two experiments. m UTD-M and CAR-M were co-cultured with MC38OVA+ and transgenic T cells (OT-1), at E:T:T (Effector:Target:T cells), 2:1:6. Flow cytometry was used to assess T cell activation at the 24 h timepoint (n = 3 technical replicates). Fold change was calculated by dividing the % expression of CAR-M by that of UTD-M. For all images: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
We previously showed that CAR expression and activation of human CAR-M is durable and directly related to their functionality4. Flow cytometric analysis showed that Ad5f35 sustained CAR expression for at least 13 days post-transduction in vitro (peak observed on day 6) (Fig. 1c, d and Supplementary Fig. 5b). Furthermore, 48 h post-transduction, (a key timepoint as CAR-M are transduced for 48 h prior to injection), increased expression of activation markers CD80, CD86, and MHC-II, and reduced expression of CD206, an anti-inflammatory phenotype marker, was confirmed at the protein (Fig. 1e and Supplementary Fig. 5b) and transcriptional level (Fig. 1f). Additional analysis showed an upregulation in chemo-attractants genes potentially favoring T cell recruitment (Supplementary Fig. 1i). Pathway analysis confirmed the induction of pro-inflammatory pathways, such as interferon signaling, and inflammatory cytokine signaling. Moreover, antigen-presentation machinery such as co-stimulatory ligands, antigen processing and presentation, and MHC-Class I/II genes were induced (Supplementary Fig. 1j). These findings are consistent with the effect of Ad5f35 on human monocyte-derived-macrophages and confirm the relevance of this murine CAR-M system4.
We then tested the anti-tumor function of CAR-M in vitro against two murine colorectal cancer cell lines (MC38 and CT26) and demonstrated antigen-dependent killing of human HER2-expressing targets (MC38-HER2 and CT26-HER2) but not parental lines (WT, HER2-) (Fig. 1g). Although Ad5f35 transduction induced minor cytokine and chemokine secretion, overnight stimulation of anti-HER2 CAR-M with plate-bound recombinant human HER2 protein resulted in robust, antigen-dependent production of tumor necrosis factor α (TNFα), interleukin (IL)-1β, IL-6, macrophage inflammatory protein-1α (MIP-1α), chemokine (C-C motif) ligand 4 (CCL4), and IFN-γ-inducible protein 10 (IP-10) also referred to as C-X-C motif chemokine ligand 10 (CXCL10). In contrast, recombinant mesothelin (irrelevant antigen control) did not activate CAR-M (Fig. 1h, i and Supplementary Fig. 1k). Collectively, these findings indicate the necessity for redirecting and activating CAR-M against tumor antigen to elicit potent responses like in human CAR-M4.
CAR-M phagocytosed MC38-HER2 cells compared to UTD-M (Fig. 1j and Supplementary Fig. 5c). MC38-HER2 cells co-cultured with CAR-M, but not UTD-M, up-regulated MHC-I (Fig. 1k and Supplementary Fig. 5d), which may facilitate antigen presentation. Next, we assessed the capacity for CAR-M to activate T cells using the OT-1 model (transgenic CD8+ T cells harboring a TCR that recognizes ovalbumin (OVA) in the context of H2Kb. CAR-M or UTD-M were co-cultured with MC38H2Kb engineered to express ovalbumin (MC38OVA+) or co-express ovalbumin and HER2 (MC38OVA+HER2+). CAR-M initially controlled tumor growth in vitro however, MC38OVA+HER2+ tumor cells eventually progressed due to a suboptimal E:T ratio. Addition of T cells to CAR-M + MC38OVA+HER2+ co-cultures, led to a significant increase in anti-tumor activity, compared with CAR-M + MC38OVA+ or UTD-M + MC38OVA+HER2+ (Fig. 1l and Supplementary Fig. 1l). In similar settings, T cell analysis showed an increase in CD69, CD137, and PD1 expression along with CD62L and CD44 modulation in T cells co-cultured with CAR-M + MC38OVA+HER2+, but not with CAR-M + MC38OVA+ (Fig. 1m and Supplementary Fig. 6a). These results indicate that enhanced T cell-mediated killing and activation are dependent on CAR-M/antigen interaction.
CAR-M therapy inhibits tumor growth, improves overall survival and induces long-term anti-tumor immunity
To assess CAR-M anti-tumor activity in vivo, we implanted the immunogenic CT26-hHER234 subcutaneously (SQ) in syngeneic BALB/c mice. Tumors were allowed to grow for 14 days before initiating treatment, and HER2 expression was confirmed by immunohistochemistry (IHC) (Supplementary Fig. 2a). Regional (intratumoral (IT)) administration of CAR-M significantly inhibited tumor progression, whereas tumors treated with UTD-M exhibited progression like untreated mice (Fig. 2a, b). CAR-M treatment yielded a 37.5% complete response (CR) rate and significantly prolonged median survival of mice to 85.5 days, compared to 42 days in the untreated group and 46 days in the UTD-M group (Fig. 2c).
CT26-hHER2 tumor was established SQ in BALB/c mice for 14 days prior to treatment with IT UTD-M (n = 9), CAR-M (n = 8) or left untreated (n = 8) to assess tumor burden. a Tumor growth curves are shown until the first mortality in the respective groups and represent the mean ± SEM. Statistical analysis was performed using the two-way RM ANOVA. b Waterfall plot shows change in tumor volume on day 32 compared to baseline. c Kaplan–Meier survival curve over 118 days. Statistical significance was calculated using the log-rank Mantel–Cox test. Graphs are representative of 2 experiments. d CT26-hHER2 tumor was established SQ in BALB/c and mice were treated for three consecutive days with anti-CD8 (n = 7), anti-CD4 (n = 6) and anti-CD4 and anti-CD8 (n = 7) starting on day 14. IT CAR-M or UTD-M was administered on day 17 (CAR-M n = 6; UTD-M n = 7). Statistical analysis was performed using the two-way RM ANOVA. Data represents the mean ± SEM and the experiment was conducted once. e Schematic of mice post CAR-M (n = 3) therapy rechallenged with HER2-negative CT26WT tumors two months post CR. Created in BioRender. Pierini, S. (2024) https://BioRender.com/s22i371. f Kaplan–Meier survival curve over 40 days post CAR-M therapy rechallenged HER2-negative CT26WT with tumors. Naïve mice were included (n = 9). g Kaplan–Meier survival curve over 42 days post-CAR-M with concomitant anti-CD3 therapy and rechallenged with HER2-negative CT26WT tumors. Naïve mice were included (n = 9). h 4T1-cHER2 tumor was established orthotopically in the MFP of BALB/c mice for 13 days and treated with IV CAR-M. CAR-M (n = 5) controlled tumor growth in 4T1-cHER2 compared to untreated (n = 7) and UTD-M (n = 7). Data represents mean ± SEM. Statistical analysis was performed using the two-way RM ANOVA. i Waterfall plot shows change in tumor volume on day 31 from baseline. Graphs are representative of three experiments. j 5 × 106 of fluorescently labeled (IVISense680) murine CAR-M were IV injected into 4T1-cHER2 tumor-bearing BALB/c mice. Fluorescence was monitored over a period of 12 days and localized in the livers and MFP tumors. Quantification is represented as the mean ± SEM (n = 4). Values are normalized by subtracting the baseline signal from the untreated mouse. The experiment was performed once. For all images: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
Next, mice were treated with anti-CD8 and/or anti-CD4 depleting antibodies prior to IT CAR-M administration. Depletion of CD8+ T cells or both CD4+ and CD8+ T cells abrogated CAR-M-induced anti-tumor responses (Fig. 2d), emphasizing the critical role of endogenous T cells in driving efficacy against established solid tumors. To assess whether CAR-M could prevent antigen-negative relapse—a prominent mechanism of resistance to CAR-T therapy—mice with CT26-hHER2 tumors achieving CRs post-CAR-M therapy were rechallenged with HER2-negative CT26WT tumors two months post-CR (Fig. 2e). All mice from the CAR-M treatment group demonstrated complete tumor rejection and 100% survival post antigen negative rechallenge, suggesting antigen spreading. In contrast, naïve BALB/c mice succumbed to HER2-negative disease within 29 days (Fig. 2f). Furthermore, the surviving mice received a second CT26WT tumor rechallenge concurrently with a T cell-depleting, anti-CD3 antibody (Fig. 2g) and succumbed to disease within 42 days. These data highlight the role of T cells in long-term tumor immunity induced by CAR-M therapy.
To evaluate intravenously (IV) administered CAR-M in the immunocompetent setting of a clinically relevant syngeneic breast cancer model, we engineered a chimeric HER2 (cHER2) target antigen (murine HER2 with the human trastuzumab epitope) (Supplementary Fig. 2b) and overexpressed it in the poorly immunogenic murine breast cancer cell line 4T1 using lentiviral transduction35. Surface cHER2 expression in this model was confirmed by flow cytometry using murine-specific and human-specific (trastuzumab biosimilar) antibodies (Supplementary Fig. 2c). 4T1-cHER2 cells were orthotopically implanted in the mammary fat pad (MFP) of BALB/c mice to mimic human breast cancer and IHC showed high HER2 expression (Supplementary Fig. 2d). To assess the functionality of IV CAR-M, 4T1-cHER2 was established for 13 days prior to treatment. IV CAR-M treatment significantly reduced tumor growth over time (Fig. 2h and Supplementary Fig. 2e), and three out of five mice in the CAR-M group had a lower tumor burden at day 31 compared to baseline (Fig. 2i). Unlike the results seen in the immunogenic CT26-hHER2 model, CAR-M did not improve survival in the poorly immunogenic 4T1-cHER2 model. Overall, these findings demonstrate potent anti-tumor activity of regionally and systemically administered CAR-M against established solid tumors in immunocompetent hosts.
We have previously demonstrated that human CAR-M injected in NSGS mice persisted at least 62 days and infiltrated multiple xenograft human tumors4. To assess the fate of murine CAR-M, we adoptively transferred fluorescently labeled macrophages in immunocompetent tumor-bearing BALB/c mice. In a longitudinal study, we tracked fluorescence over time and found that CAR-M persisted at least 12 days in BALB/c mice (Fig. 2j and Supplementary Fig. 2f, g). In a separate study, the relative fluorescence in the tumor, liver, spleen, and lung was evaluated in explanted whole-organ tissues on day 2 and 5 post macrophage administration. While the liver and the spleen were the primary organs of CAR-M accumulation, CAR-M was detected in the CT26-hHER2 and 4T1-cHER2 tumors (Supplementary Fig. 2h). We then aimed to reproduce these results using RNAscope in mice that received CAR-M seven days prior to analysis. The CAR transcript was detected within the TME of all 6 tumors analyzed, confirming the ability of primary murine CAR-M to infiltrate and persist within the tumor for at least 7 days (Supplementary Fig. 2i).
Regional CAR-M therapy remodels the tumor microenvironment (TME) and activates tumor-infiltrating lymphocytes (TILs)
To assess the ability of CAR-M to modulate the TME, CT26-hHER2 implanted SQ in immunocompetent BALB/c mice were treated with IT UTD-M or CAR-M. First, we used the IONpath multiplex ion beam imaging (MIBI) technology to perform a 20-marker (Extended Table 1) multiplexed IHC assay for detailed analysis of spatial and functional aspects across 23 distinct cell populations (Extended Table 2), including tumor cells, stromal cells, and immune cells. A 50% reduction in Vimentin+SMA-CD45- cells, a mixture of tumor cells and fibroblasts was observed in the CAR-M group compared to both the untreated and UTD-M groups (Fig. 3a, b). In the CAR-M group, a substantial influx of T cells (23.2%) and pro-inflammatory macrophages (33.3%) were detected in the TME. In contrast, the composition of T cells and macrophages were 8.1% and 17.6% in the UTD-M group and 4.3% and 13% in the untreated group respectively (Fig. 3a, b). Furthermore, CAR-M treatment induced activation of helper T cells (granzyme B+ CD4+ T cells) and proliferation of immune cells (Ki67+ immune cells) (Fig. 3a, c). The MIBI data was validated and further expanded by flow cytometry which confirmed increased tumor infiltration of CD4+ T cells (Fig. 3d and Supplementary Fig. 6b), CD8+ T cells (Fig. 3e and Supplementary Fig. 6b), and NK cells (Fig. 3f and Supplementary Fig. 6b) within tumors of CAR-M-treated mice compared to controls. Moreover, ex vivo stimulation of CD8+TILs with the AH1 peptide, the gp70 immunodominant peptide expressed in CT26 tumors, revealed that TILs from CAR-M-treated mice produced more IFNγ in response to the gp70 (Fig. 3g and Supplementary Fig. 6c). Similarly, using a tetramer assay, we found increased gp70-specific CD8+ T cell infiltration in the CAR-M treated tumors only (Fig. 3h, Supplementary Fig. 2j and Supplementary Fig. 6b). The reactivity against gp70, a secondary tumor-associated antigen (TAA) in response to CAR-M therapy, demonstrated enhanced antigen spreading and CD8+ T cell expansion, which likely protected mice from antigen-negative relapse observed in Fig. 2f. Furthermore, IHC demonstrated higher proportions of intratumoral CD8+ T cells in mice receiving IT CAR-M (CT26-hHER2) (Fig. 3i) and intratumoral CD4+ T cell in mice receiving IV CAR-M (4T1-cHER2) (Fig. 3j), suggesting that the contribution of CD4+ vs CD8+ T cells to the therapy’s efficacy is model-dependent.
CT26-hHER2 tumor was established SQ in BALB/c for 12 days and treated with IT UTD-M or CAR-M. a Representative MIBI multiplexed IHC images showed tissue structure (dsDNA, Beta-tubulin, Vimentin), myeloid (CD11b, CD11c, CD206, F4/80), and lymphocyte (CD3, PAX5, CD49b, Granzyme B, CD4) markers within tumors harvested 25 days post inoculation (13 days post 1st macrophage dose). Untreated mice were included as controls (n = 3/group). b, c Relative percentage of cell populations and cell density by MIBI in UTD-M, CAR-M, and untreated mice. Data represents the mean values of n = 3 and, the experiment was conducted once. d–f Absolute count of CD4+, CD8+, and NK cells by flow cytometry from 1 × 105 digested tumors. g Percentage of IFNγ+ CD8+ T cells determined by intracellular staining (ICS) after overnight stimulation of digested tumor suspension with AH1 peptide. h Absolute count of gp70+ T cells by tetramer assay within 1 × 105 digested tumor suspension. Data in (d–h) represents the mean ± SEM of n = 6 (Untreated and CAR-M) and n = 5 (UTD-M). Statistical analysis was performed using ordinary one-way ANOVA and data is representative of three experiments. i CD8+ T cell density by IHC in tumor sections of CAR-M (n = 6), UTD-M (n = 4) and untreated groups (n = 5). Data represents the mean ± SEM and graph is representative of two experiments. Representative IHC sections are shown at x40 high-power fields per slide. j 4T1-cHER2 tumor was allowed to grow in MFP in BALB/c for 13 days and was treated with IV CAR-M (n = 6), UTD-M (n = 7) or vehicle control (n = 7). Tumors were harvested on day 28 post tumor inoculation (15 days post 1st macrophage dose) and FFPE sections were stained in IHC. The percentage of CD4+ T cells are shown for all treatment groups. Data represents the mean ± SEM and is representative of two experiments. Statistical analysis was performed using ordinary one-way ANOVA. Representative IHC sections are shown at x40 high-power fields per slide. k Quantification of serum IL-6, IP-10 (CXCL10), 6Ckine (CCL21), MIG (CXCL9), IFNγ, and G-CSF levels in CT26-hHER2 tumor-bearing BALB/c mice by multiplexed Luminex assay up to 18 days post treatment with CAR-M and UTD-M (n = 6-7/group). Untreated mice (n = 7) were included. Dotted lines represent macrophage doses. Data represents mean ± SEM and is representative of two experiments. For all images: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
In addition to the TME, we assessed the effect of CAR-M on pro-inflammatory cytokines and chemokines levels in the blood from CT26-hHER2 mice treated with two doses of CAR-M. On day 1 and 4 post first dose, serum analysis showed a moderate increase in the pro-inflammatory cytokine IL-6 in CAR-M-treated mice, which returned to baseline by day 7 (Fig. 3k). These data demonstrate the regional delivery of CAR-M is accompanied by minimal systemic elevation of proinflammatory mediators.
Regional CAR-M therapy in combination with PD1 blockade remodels the TME and drives anti-tumor T cell immunity
To evaluate CAR-M + aPD1 combination therapy, CT26-hHER2 SQ tumors were treated with regional CAR-M either alone or in combination with PD1 blockade. aPD1 initially controlled tumor progression compared to untreated mice (Fig. 4a) with minimal improvement in the CR rate (14.3% aPD1 vs 0% untreated) (Fig. 4b). Conversely, IT CAR-M monotherapy suppressed tumor growth (Fig. 4a) and induced 40% CR rate (Fig. 4b). CAR-M + aPD1 therapy further increased tumor suppression (Fig. 4a) and significantly improved survival, achieving a remarkable 77.8% CR rate (Fig. 4b). Importantly, combination therapy did not impact mouse weight or body condition score, indicative of good tolerability (Supplementary Fig. 3a).
CT26-hHER2 tumor was established SQ in BALB/c and treated with IT CAR-M starting at day 12. aPD1 was administered IP approximately every 3 days, starting at day 12 until day 36. a Tumor growth curves represent the mean ± SEM and are shown until the first mortality was reported in the respective groups. CAR-M (n = 10), aPD1 (n = 7), CAR-M + aPD1 (n = 9), or untreated (n = 9) groups were analyzed. b Tumor volume from individual mice is shown until day 76. Statistical analysis was calculated using the 2-way ANOVA, mixed-effects analysis. The experiment was performed once. To assess TME changes, CT26-hHER2 tumor-bearing BALB/c were treated with IT CAR-M starting on day 12 and aPD1 on day 15. Tumors were harvested 25 days post tumor inoculation. c, d Representative images of cell populations and quantification by relative fractions or cell density from MIBI multiplexed IHC analysis of tumor FFPE specimens from untreated, aPD1, CAR-M, CAR-M + aPD1 groups. Data shows mean values of n = 3. The experiment was conducted once. e,f CD4+ and CD8+ T cell absolute count from 1 × 105 digested tumor cell suspensions. Untreated (n = 6), aPD1 (n = 5), CAR-M (n = 6) and CAR-M + aPD1 (n = 7) groups were evaluated by flow cytometry. Data represents mean ± SEM and is representative of two experiments. Statistical analysis was performed using ordinary one-way ANOVA. g–j Tumor (n = 5/group) and matching peripheral blood (n = 5/group) samples were profiled for TCRB sequence to determine Morisita Index, peripheral T cells fraction, shared clone between subjects and tumor repertoire clonality in all treatment groups. Data is represented as median with wishers indicating the minimum and maximum values. Statistical analysis was calculated using the ordinary one-way ANOVA, multiple comparisons test. Data is representative of two experiments. For all images: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
TME analysis by MIBI technology (Extended Table 2) reported a 6-fold increase in DC population, and a 5-fold increase in other myeloid cells, a mixture of granulocyte, monocyte and myeloid-derived suppressor cells (MDSCs), in the CAR-M + aPD1 group compared to CAR-M alone (Fig. 4c). This finding is crucial as intratumoral DCs present tumor antigens to induce and sustain anti-tumor responses in the immunosuppressive TME36. A reduction in tumor/fibroblasts and tumor-infiltrating macrophages was observed in response to CAR-M + aPD1 (Fig. 4c). Additional MIBI analysis revealed that CAR-M + aPD-1 increased helper T cells density and their activation—based on granzyme B production—(Fig. 4d) compared to either monotherapy. These data were validated and further expanded by flow cytometry analysis showing increased intratumoral CD4+ and CD8+ T cell infiltration (Fig. 4e, f and Supplementary Fig. 6b) in the combination group. Together, these results demonstrate that CAR-M + aPD1 therapy enhances intratumor DC and T cell frequency along with helper T cell activation.
To further understand changes in the adaptive immune response, we characterized the T cell receptor beta chain (TCRB) diversity and clonality by sequencing CT26-hHER2 tumors and matching blood samples. By comparing clones between two samples through the Morisita Index, a significant increase in shared clones between blood and tumor was found in CAR-M + aPD1-treated mice (Fig. 4g), suggesting an increase in tumor-reactive clones in circulation. An increase in the peripheral T cell fraction was also found in the blood of CAR-M + aPD1-treated mice (Fig. 4h), indicative of enhanced adaptive immune engagement and activity. By comparing overlaps between the top 100 clones for all 10 possible pairs of blood samples within treatment groups, the CAR-M + aPD1 group showed the fewest pairwise overlapping clones (Fig. 4i), demonstrating an increase in diversity of tumor antigens. Similarly, a reduction in tumor repertoire clonality was observed in the combination group compared to both monotherapies (Fig. 4j), indicative of a more evenly distributed repertoire and a more diverse immune response.
Finally, we assessed whether an increase in serum cytokines and chemokines in the combination group could be associated with cytokine release syndrome. Except for IL-6 which showed a transient elevation from baseline at a peak of 18.8 pg/mL, no analytes increased over baseline (Supplementary Fig. 3b). To determine the direct impact of aPD1 on CAR-M cytotoxic function, in vitro killing assays were established by coculturing CAR-M with either MC38 or CT26 (Supplementary Fig. 3c, d), in the presence of aPD1. No change was observed with aPD1, indicating that blocking PD1 does not affect macrophages directly.
Systemic CAR-M therapy in combination with PD-1 blockade leads to enhanced anti-tumor activity against established solid tumors
We next evaluated the efficacy of systemic (IV administration) CAR-M + aPD1 combination therapy. First, we used the orthotopic breast cancer 4T1-cHER2 model and showed that IV CAR-M, but not UTD-M, delayed tumor growth like aPD1 monotherapy and that CAR-M + aPD1 exerted an enhanced effect (Fig. 5a). This finding was also confirmed in a separate study (Fig. 5b), and analysis of the TME showed an increase in intratumoral CD4+ T cells in response to CAR-M + aPD1 (Fig. 5c). Subsequently, IV CAR-M was tested in the subcutaneous CT26-hHER2 model which showed that the addition of aPD1 to CAR-M led to significant tumor reduction (Fig. 5d), without affecting body weight or score (Supplementary Fig. 4a). This study was repeated, and the combined survival data from three studies showed a 60.9% CR rate in the CAR-M + aPD1 group, whereas aPD1, UTD-M, CAR-M, and UTD-M + aPD1 groups had 0%, 8.3%, 8.7%, and 13.3% CR rates (Fig. 5e), respectively. These findings highlight the synergistic effect of CAR-M combined with aPD1 compared to either treatment alone. TME analysis showed CD4+, CD8+, and gp70+ CD8+ T cells increased in response to combination therapy (Fig. 5f, g, Supplementary Fig. 4b and Supplementary Fig. 6d), suggesting that regional and systemic administration shared similar MOA leading to tumor inhibition.
a 4T1-cHER2 tumor was established orthotopically and treated with systemic IV CAR-M and IP aPD1, both starting on day 16. Tumor growth curves represent the mean ± SEM of n = 7 and data is representative of two experiments. Statistical analysis was performed using the two-way RM ANOVA. In a separate experiment, 4T1-cHER2 tumor was established orthotopically and treated with systemic IV CAR-M and IP aPD1, both starting at day 14 (n = 7/group except CAR-M n = 6). b Waterfall plot shows changes in tumor volume on day 28 relative to baseline. c, Frequency of CD4+ T cells by IHC in 4T1-cHER2 tumors on day 28. Data represents the mean ± SEM and is representative of two experiments. Statistical analysis was performed using ordinary one-way ANOVA. CT26-hHER2 tumor-bearing mice were treated with systemic IV CAR-M starting on day 10 and IP aPD1 on day 14. d Tumor volume shown as the mean ± SEM of n = 8/group except UTD-M + aPD1 n = 7. Statistical analysis was performed using the two-way ANOVA, mixed model. e Kaplan–Meier survival curve over 98 days shows the combined mice survival from three independent experiments (UTD-M n = 24; CAR-M, aPD1 and CAR-M + aPD1 n = 23, UTD-M + aPD1 n = 15). Statistical significance was calculated using the log-rank Mantel–Cox test. In a separate experiment, CT26-hHER2 tumors were collected on day 29 for TME analysis. f CD4+, CD8+, and gp70+ T cells absolute count from 1 × 106 digested tumor tissue by flow cytometry. Data represents the mean ± SEM (n = 4/group except aPD1 n = 3 and CAR-M + aPD1 n = 5). Statistical analysis was performed using ordinary one-way ANOVA. g CD4 + T cells frequency by IHC. Data represents the mean ± SEM of n = 4. Statistical analysis was performed using ordinary one-way ANOVA. Representative IHC sections at x20 high-power fields per slide. h, i Serum IL-6, IFNγ, MCP-1 (CCL2), IL-16, G-CSF, MCP-5 (CCL12), IP-10 (CXCL10), IL-5, and MIG (CXCL9), 6Ckine (CCL21) levels were quantified by multiplexed Luminex assay up to 20 days post-treatment (n = 2-5/group). Dotted lines represent CAR-M doses. Data represents the mean ± SEM and is representative of two experiments. j Serum SAA-3 level was quantified by ELISA, presented as mean ± SEM of n = 2–4/group, and it is representative of a single experiment. For all images: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Source data are provided as a Source Data file.
To better assess treatment-induced inflammation and tissue damage, we administered two doses of IV CAR-M with and without aPD1 in CT26-hHER2 tumor-bearing BALB/c mice, and collected serum on day −1, 3, 6, 13, and 20 post-first dose as well as key organs at the end of the study on day 26. Serum IL-6, interferon gamma (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), granulocyte colony-stimulating factor (G-CSF) and serum amyloid A3 (SAA-3) levels were increased within the first 6 days, especially in response to the CAR-M + aPD1 therapy, and resolved by day 20 (Fig. 5h–j). An increase in monocyte chemotactic protein-5 (MCP-5), IFN-γ-inducible protein 10 (IP-10), IL-5, monokine induced by interferon-γ (MIG), and chemokine (C-C motif) ligand 21 (CCL21) also referred to as 6Ckine was reported in response to CAR-M monotherapy (Fig. 5i). All analytes returned to baseline levels between days 13 and 20, and no treatment-related abnormalities were observed in the heart, liver, lung, spleen, kidneys, and brain upon histopathological examination (Supplementary Fig. 4c), suggesting good tolerability in response to combination therapy.
Discussion
The data presented herein are the first to establish a fully immunocompetent CAR-M model system and demonstrate CAR-M efficacy against established solid tumors with either regional or systemic administration. While CAR-M can directly kill target cells via phagocytosis, these data support T cell dependence for robust anti-tumor activity and confirm CAR-M capacity for TME remodeling and induction of endogenous T cell immunity. Furthermore, CAR-M reduced tumor growth and improved survival in combination with aPD1 in models where PD1 inhibition alone failed to control tumor growth. These findings support the combination of CAR-M with PD1 blockade for the treatment of established solid tumors.
The development of syngeneic models to evaluate CAR-based therapies has been hampered by the increased immunogenicity from human targets expressed in murine tumors37, which can result in spontaneous tumor regression. Genetically engineered antigens represent a successful strategy to overcome the immunogenicity challenge as shown by the development of a truncated human HER2-expressing 4T1 model which had reduced immunogenicity in vivo38. In our study, we modified the minimally immunogenic 4T1 cell line35 with a chimeric HER2 (cHER2) antigen, which closely resembles the mouse protein but retains the human epitope for CAR binding. 4T1-cHER2 remains poorly immunogenic. Conversely, we overexpressed full-length human HER2 in the CT26 cell line which endogenously expresses the gp70 viral antigen. Although CT26-hHER2 shows elevated immunogenicity34,37, an engrafting clone was identified and enabled tumor implantation in BALB/c mice. It should be noted that in our studies, we exclusively employed implantable BALB/c-derived tumor lines. While this approach provided clear insights into CAR-M efficacy and MOA, it limits variability and may introduce bias by excluding other genetic backgrounds (e.g. C57BL/6) and genetically engineered models (GEMs).
Antigen heterogeneity and hostility to cell recruitment in solid tumors are known to impair anti-tumor immune responses39. As innate immune cells, macrophages have the capacity to induce inflammation, modulate their local environment, recruit T cells, and act as professional antigen-presenting cells40. We showed that CAR-M treatment remodeled the TME and induced both CD8+ and CD4+ T cell infiltration in the CT26-hHER2 colorectal cancer model while only CD4+ T cell infiltration increased in the 4T1-cHER2 breast cancer model41. Importantly, systemic delivery of CAR-M (greater clinical relevance) and intratumoral injection, showed similar TME modulation. We also demonstrated that endogenous CD8+ T cells are required for efficacy as their depletion by CD8a-targeting antibody abrogated CAR-M-induced anti-tumor response. We acknowledge, however, that this approach may also deplete CD8a+ conventional type I dendritic cells (cDC1), suggesting that loss of anti-tumor activity could be attributed to other key immune components beyond CD8+ T cells. Furthermore, induced MHC-I expression on tumor cells by CAR-M therapy may facilitate presentation of tumor derived antigens to CD8+ T cells.
To confirm the ability of CAR-M to induce antigen spreading, mice harboring CT26-hHER2 solid tumors that initially responded to anti-HER2 CAR-M therapy were rechallenged with HER2-negative tumors. Previously CAR-M treated mice were protected against antigen-negative tumor rechallenge in a T cell-dependent manner. Additionally, increased gp70 reactive T cells were found in CAR-M-treated tumors and demonstrated greater activation upon restimulation. While these data confirm antigen spreading in the immunogenic CT26-hHER2, the same phenomenon could not be verified in the poorly immunogenic 4T1-cHER2. This is primarily due to the inability to conduct rechallenge experiments, as there were no complete responses to CAR-M therapy in the 4T1-cHER2 model. Additionally, there is a lack of well-validated tumor-associated antigens (TAA) in the 4T1 model42 required to assess antigen spreading. The discrepancy in the outcome is likely due to the differing immunogenicity between the two models that may affect their response to immunotherapy43.
Given the ability of human CAR-M to persist for more than 62 days in immunocompromised NSGS mice and to infiltrate multiple xenograft solid tumor models4, we assessed the fate of adoptively transferred murine CAR-M in syngeneic BALB/c mice. In contrast to the xenograft system, murine CAR-M persisted for 12 days in BALB/c mice. This difference in persistence may be attributed to the immunodeficient state of NSGS mice resulting in reduced immune rejection, thus prolonged persistence of human CAR-M. Conversely, the functional immune system in BALB/c mice can trigger recognition and clearance of murine CAR-M expressing non-self-protein domains such as the humanized 4D5 scFv, human CD8α hinge and transmembrane domain, and human CD3ζ signaling domain. Additionally, murine BMDM have shorter lifespans than human monocyte-derived-macrophages and produce lower levels of the macrophage survival factor M-CSF44 which may also contribute to the difference in persistence.
Anti PD-1/PDL-1 antibodies are not currently indicated in the HER2+ breast cancer setting, as responses have been minimal. While drugs such as pembrolizumab have led to deep responses in a variety of solid tumors, the majority (~80%) of patients do not respond to monotherapy. Limited efficacy in response to PD-1 blockade include impaired antigen presentation, abundance of pro-tumoral macrophages45, and reduced CD8+ T cell infiltration into the TME46,47. Given the multi-modal CAR-M MOA and their interaction with T cells, we evaluated the combination of CAR-M and PD-1 blockade.
Utilizing syngeneic immunocompetent solid tumor models with limited sensitivity to immune checkpoint blockade, we demonstrate that CAR-M combined with PD-1 blockade was more effective compared with either monotherapy. Assessment of the TME with IONpath MIBI showed an increase in DC and T cells, and a decrease in macrophages in response to CAR-M + aPD1 combination therapy. Multiple studies have identified tumor-infiltrating DCs as a positive prognostic biomarker, including a study in hepatocellular carcinoma which found that PD-1 blockade results in intratumoral DC and CD4+ T helper cells niches which modulate the differentiation of tumor-specific progenitor exhausted CD8+ T cells16. Similarly, in renal cell carcinoma or NSCLC patients treated with atezolizumab, improved overall survival was associated with an enhanced DC gene signature14. Although several studies have shown that macrophage phenotype is context-dependent48,49, monocyte-derived tumor-associated macrophages often contribute to tumor progression and are associated with poor clinical prognosis50,51. Understanding the precise mechanisms behind changes in the TME is beyond the scope of this work; however, our findings suggest that CAR-M + aPD1 treatment leads to sequential migration of immune cells resulting in a favorable immune-stimulating environment.
In CT26 tumor bearing mice, CD8+ cells primarily target gp7052,53 and TILs exhibit an exhausted phenotype54,55. Unbiased analysis of CDR3 regions within the TCRB by deep sequencing in breast cancer and melanoma56 have shown treatment-related differences in shaping TCRB repertoires of TILs. In agreement with those findings and the inverse relationship between TCRB clonality and diversity57, the reduction in clonality in CAR-M + aPD1 treated tumors indicates a broader T-cell response, with fewer dominant clones and a more diverse array of T cell clones. The ability of CAR-M + aPD1 therapy to increase the density of activated T cells in the TME and promote a more diverse TCRB repertoire suggests a mechanism where new TILs are recruited to the TME rather than clonally expanded. Moreover, the increased T cell activation suggests the capacity of the combination therapy to reinvigorate exhausted TIL by restoring their cytotoxic effector function.
Early data from our phase 1, first-in-human multicenter study of CT-0508 in patients with HER2 overexpressing solid tumors (ClinicalTrials.gov Identifier: NCT04660929) demonstrated safety, manufacturing feasibility, and clinical activity. The MOA findings included TME remodeling, increased antigen presentation gene expression, increased tumor inflamed signature (TIS), enhanced T cell clonality, and increased T cell activation after CT-0508 monotherapy58. The pre-clinical data in immunocompetent models in this manuscript aligns with these findings. The synergistic anti-tumor activity between CAR-M and PD-1 blockade presented in this manuscript supports clinical evaluation of CAR-M cell therapies in combination with T cell checkpoint blockade.
While we demonstrated that murine CAR-M infiltrated and persisted within solid tumors for up to 7 days, most of the cells accumulated in liver and spleen. This finding was expected as macrophages are terminally differentiated cells that reside in tissues, do not circulate in the periphery, and accumulate in liver, spleen, tumor, and lung. Murine CAR-M trafficking mirrored that of human CAR-M and demonstrated a low rate of tumor infiltration by RNAscope. Trafficking and persistence are key limiting factors to the adoptive transfer of fully differentiated macrophages and less differentiated monocytes may enhance both parameters. We are currently testing anti-HER2 CAR-Monocytes (CT-0525) in a Phase I safety study for patients with HER2 overexpressing solid tumors (ClinicalTrials.gov Identifier: NCT06254807). CAR-Monocytes are less differentiated, express higher levels of chemokine receptors such as C-C chemokine receptor type 2 (CCR2), and have demonstrated improved trafficking/persistence in pre-clinical models59. As CAR-Monocytes differentiate into CAR-Macrophages in tissues/tumors, the results in this manuscript support the future combination of CAR-Monocytes with T cell checkpoint inhibitors.
Methods
Cell lines
The human cell lines AU565 (CRL-2351) and SKOV3 (HTB-77) and the murine cell lines CT26 (CRL-2638) and 4T1 (CRL-2539) were purchased from the American Type Culture Collection (ATCC). The murine cell line MC38 (CVCL_B288) was purchased under full license from Kerafast (MA, USA). Cells were cultured in RPMI (11875-085, Gibco) or DMEM (10569010, Gibco) (MC38 only) media with 10% FBS (970068-085, Seradigm), 1% Penicillin-Streptomycin (15140-122, Gibco) unless otherwise recommended by the vendor. Some lines were transduced with a lentiviral vector encoding Nuclight Green (NLG) under an EF-1α promoter (4475; Sartorius). The murine cell lines were transduced with a lentiviral vector encoding human HER2, transcript variant 1 (RC212583L1V; Origene) or a chimeric HER2 (cHER2) under a CMV promoter. MC38 cell line was transduced with a lentiviral vector encoding ovalbumin (OVA) and green fluorescent protein (GFP), separated by IRES sequence, under a CMV promoter (Sirion Biotech). Transduced target cell lines were FACS sorted for >95% transgene purity. To establish CT26-hHER2 and 4T1-cHER2 models in vivo, multiple single-cell clones were generated, and injected into BALB/c mice to identify lines that grew successfully and retained high antigen expression in syngeneic host. The phenotype of the cell lines was routinely validated in house by flow cytometry. All cell lines were propagated in 5% CO2 at 37 °C and checked for mycoplasma contamination routinely.
Plasmid construction and virus
Macrophages (CD11b+, CD14+) were transduced with adenoviral vector produced by cloning the anti-HER2 CAR into the pShuttle transfer plasmid using Xba-I and Sal-I, and subsequently, cloning into pAd5f35, a first generation E1/E3 deleted adenoviral backbone, using I-Ceu I and PI-Sce I. All cloning steps were validated by restriction enzyme digest and sequencing. The Ad5F35-CMV-CAR-HER2- ζ vector and the Ad5f35-CMV-CARHer2-P2A-CBG-Luc vector - which expressed both the HER2-CAR and the Click Beetle Green (CBG) Luciferase gene - were generated by transfection, and expansion in either an adherent HEK293 cell line or a suspension serum-free CAP-Ad (Cytiva, Marlborough, MA) cell line. HEK293 cell viral lysates were concentrated and purified using the Adeno-X™ Maxi Purification Kit (Takara Bio, San Jose, CA) by Carisma Therapeutics Inc. CAP-Ad cell adenovirus lysates were purified by a standard clarification, chromatography, and concentration platform. This CAR construct was the same used in CT-0508 studies against the human target and in these studies used in a mouse macrophage. Adenoviral titers were assessed using the Quant-iT PicoGreen Double Stranded Deoxyribonucleic Acid Assay Kit (Thermo Fisher). Ad5f35 was used to transduce macrophages to generate CAR-M. For cancer cell line generation, a chimeric HER2 (cHER2) molecule was designed by replacing Domain IV of murine HER2 (residues 535-653; accession P70424) with Domain IV of human HER2 (residues 534-652; accession P04626), which comprises the trastuzumab epitope60. The cHER2 amino acid composition bore 98.6% pairwise identity with murine HER2. Codon-optimized cHER2 was cloned into a third-generation lentiviral vector using standard molecular biology techniques and expressed under the control of an EF-1α promoter. Lentivirus was packaged in HEK293 cells then purified and concentrated using Lenti-X Concentrator (Takara), following the manufacturer’s protocol.
Animals and in vivo studies
Seven- to 10-week-old female BALB/c, C57BL/6, and C57BL/6-Tg (TcraTcrb)1100Mjb/J (OT-1) (003831) mice were purchased from Jackson Laboratories and housed in microisolator cages under sterile conditions at The Wistar Institute (Philadelphia, PA) or the Charles River Accelerator and Development Lab (CRADL) (Philadelphia, PA). All studies were conducted under Institutional Animal Care and Use Committee (IACUC) approved protocols at The Wistar Institute (Philadelphia, PA) or the Charles River Accelerator and Development Lab (CRADL) (Philadelphia, PA). 0.75-1 × 106 CT26-hHER2 were injected SQ in 100 µL of PBS in the lower back in BALB/c; 2.5 × 104 of 4T1 cells resuspended in PBS and Matrigel (CB-40230C, Corning) at the E:T ratio1:1 was surgically implanted in the MFP of BALB/c mice. Tumors were allowed to growth for 10-16 days prior to starting treatment. Macrophages were dosed approximately every four days for two or three cycles to achieve the target dose, consisting of (5–7) × 106 macrophages IT in SQ CT26-HER2, (7–15) × 106 macrophage IV in SQ CT26-HER2, and 10-15 ×106 macrophage IV in MFP 4T1-cHER2. Mice did not receive lymphodepletion prior macrophage injection. In some experiments, mice received vehicle control (PBS (14190-144, Gibco) + 1% mouse serum (IGMSBCSER10ML, Innovative Research Inc, MI, USA)). Macrophages were diluted in 20 µL (IT) or 200 µL (IV) sterile PBS containing 1% mouse serum. For rechallenge experiments, mice achieving CRs were re-challenged with 1.5 × 105 CT26-WT (HER2-) SQ approximately 2 months after the rejection of the primary tumor. Tumor volumes were recorded by caliper measurements (Tumor volume = length × width × width/2) performed two to three times a week. Mouse weight and body condition score61 were assessed weekly. All animals were euthanized in accordance with IACUC guidelines, which mandate euthanasia if tumors exceed 2000 mm³ in volume or 2.0 cm in any direction (whichever occurs first), if body weight decreases by 20% relative to baseline, or in cases of poor body condition.
Generation of murine CAR-M (Murine Surrogate of CT-0508)
BM was harvested from BALB/c or C57B/L6 mice, and HSCs or monocytes were isolated by magnetic-activated cell sorting (130-110-470 and 130-100-629; Miltenyi Biotec) according to manufacturer instructions. BMDM cultures were established using modified versions of protocols previously described62,63. Briefly, HSCs or monocytes were resuspended in TexMACS™ GMP Medium (170-076-307, Miltenyi Biotec) supplemented with FBS (Seradigm), penicillin, streptomycin, GlutaMAX (35050-061, Gibco), HEPES (15630-080, Gibco), NEAA (11140-050, Gibco), BME (21985-023, Gibco) in the presence of either 10 ng/mL of M-CSF or 10 ng/mL GM-CSF (315-02 or 315-03; Peprotech) and cultured in MACS GMP Cell differentiation Bags (Miltenyi Biotec) for 6 days. BMDM were transduced with Ad5f35 vector at a multiplicity of infection (MOI) of 5000–10,000 viral particles (VP)/cell for 48 h to generate CAR-M. CAR-M phenotype (CD11b and CAR-HER2 expression) was assessed in each preparation along with viability.
In vivo antibodies
For in vivo combination therapy studies, 200 µg/mouse of InVivoPlus anti-mouse PD-1 (CD279) (BP1046, Bio X Cell) or 200 µg/mouse of InVivoMAb anti-mouse PD-1 (BE0146, Bio X Cell) were diluted in PBS, and 200 µL of solution was administered by intraperitoneal (IP) injection every 3–4 days for three or four cycles concomitantly with CAR-M. In one experiment (Fig. 4a, b), anti-PD-1 (aPD1) was administered every 3 days for 8 cycles. For T cell depletion with CAR-M therapy, 250 µg/mouse of InVivoMAb anti-mouse CD8α (BE0117, BioXCell) and/or 250 µg/mouse of InVivoMAb anti-mouse CD4 (BE0003-1, Bio X Cell) were diluted in PBS and 200 µL of the solution was injected IP daily for 3 days prior to starting CAR-M therapy. For T cell depletion during rechallenge, 200 µg/mouse of InVivoMAb anti-mouse CD3 (BE0002, Bio X Cell) was diluted in PBS, and 200 µL of the solution was injected IP every 3 days throughout the rechallenge period.
Flow cytometry
To assess purity and polarization, HSCs, monocytes, UTD-M and CAR-M were preincubated with TruStain Monocyte Blocker (426103, BioLegend) and TruStain FcX™ PLUS (anti-mouse CD16/32) Antibody (156604, BioLegend) followed by staining with combinations of anti-Trastuzumab-AF647 (Anti-Idiotype) (FAB95471R, R&D Systems), CD11b-PE-Cy7 (101216, BioLegend), CD11b-APC-Cy7 (101226, BioLegend), Ly6G-BV421 (127628, BioLegend), CD45-BV605 (103140, BioLegend), CD14-BV785 (123337, Biolegend), CD49b-PE (553858, BD), CD3-PE-Cy7 (100220, BioLegend), CD19-PerCp-Cy5.5 (115534, BioLegend), CD80-FITC (104706, BioLegend), CD86-BV421 (105123, BioLegend), CD206-PerCp-Cy5.5 (141716, BioLegend), and MHCII-PE (107608, BioLegend). HER2 antigen expression was tested using CD340 (erbB2/HER-2)-APC (324408, BioLegend) and Trastuzumab Biosimilar-PE (FAB9589P-100, R&D Systems). For TME analysis, 0.5-1 x 106 single tumor cell suspension were incubated in PBS with 10% TruStain FcX™ PLUS (anti-mouse CD16/32) antibody (156604, BioLegend) for 10 minutes followed by staining with combinations of CD45-APC (103112, BioLegend), CD45-BV605 (103140, BioLegend), CD3-BV711 (100241, BioLegend), CD3-PE-Dazzle (100246, BioLegend), CD8-APC-Cy7 (100714, BioLegend), CD8-FITC (D271-4, MBL), CD4-PerCp-Cy5.5 (100540, BioLegend), CD4-BV421 (100438, BioLegend), Gp70-Tetramer (TB-M521-1, MBL) and CD49b-PE-Cy7 (108922, BioLegend). Viability was confirmed using the Live/Dead Fixable Aqua Dead Cell Staining Kit (L34966, ThermoFisher Scientific). When two or more BD Horizon Brilliant reagents were used, BD Horizon Brilliant Stain Buffer Plus (568264, BD Bioscience) was included in the antibody mix. Appropriate unstained, fluorescence-matched isotype or fluorescence minus one (FMO) controls were used. Samples were acquired on the Attune™ NxT acoustic focusing cytometer autosampler (ThermoFisher Scientific) and analysis was performed using the FlowJo software (FlowJo, LLC).
IFN-γ Intracellular staining (ICS)
4 × 105 of single-cell suspension from digested solid tumors was incubated overnight in V-bottom 96-well plate in media containing 5 µg/mL of AH1 peptide (Gp70 immunodominant peptide) (AS-64798, Anaspec) and 0.7 µL/mL of Golgi Plug (555029, BD)64. Subsequently, cells were stained with CD3-BV711 (100241, BioLegend), CD8-APC-Cy7 (100714, BioLegend), CD45-PerCp-Cy5.5 (103130, BioLegend), and IFN-γ-PE (505808, BioLegend). Cell fixation and permeabilization was performed using the eBioscience FOXP3/transcription factor staining buffer set (00-5523-00, ThermoFisher Scientific). Samples were acquired on the Attune™ NxT acoustic focusing cytometer autosampler (ThermoFisher Scientific) and analysis was performed using the FlowJo software (FlowJo, LLC). Statistical significance was calculated using 2-way ANOVA with multiple comparisons.
T cell phenotyping
CD3+ T cells were isolated by magnetic cell isolation (130-095-130, Miltenyi Biotec) from the spleen of female OT-1 mice. 2 × 104 UTD-M or CAR-M (48 h after transduction) were co-cultured with 1 × 104 MC38OVA+HER2+ and 6 × 104 OT1 T cells in triplicate wells for 24 h. After that, T cell activation and phenotype were interrogated by flow cytometry. Cells were stained with Live/Dead Fix Aqua (L34966A, ThermoFisher Scientific), CD45-Brilliant Violet 605 (103140, Biolegend), CD3-APC-Cy7 (100222, Biolegend), CD8a-Brilliant Violet 650 (100742, Biolegend), CD69-Brilliant Violet 421 (104528, Biolegend), CD137-Brilliant Violet 786 (756687, BD), PD-1-PerCP-Cy5.5 (109120, Biolegend), CD62L-PE-Cy7 (104418, Biolegend), and CD44- Brilliant Violet 711 (103057, Biolegend). Samples were acquired on the Attune™ NxT acoustic focusing cytometer autosampler (ThermoFisher Scientific) and analysis was performed using the FlowJo software (FlowJo, LLC).
FACS-based phagocytosis assay
3 × 104 UTD-M or CAR-M (48 h after transduction) were co-cultured with 3 × 104 MC38-GFP+ or MC38-GFP+HER2+ target cells for 24 h at 37 °C in triplicate wells. After co-culture, cells were harvested with Accutase (Innovative Cell Technologies) and stained with anti-CD11b PE-Cy7 (101216, BioLegend) and Live/Dead Fixable Yellow (L34959, ThermoFisher Scientific). Samples were acquired on the Attune™NxT acoustic focusing cytometer autosampler (ThermoFisher Scientific) and analysis was performed using the FlowJo software (FlowJo, LLC). The percentage of GFP+ events within the live CD11b+ population was plotted as percent phagocytosis. Data are represented as mean ± standard error of the mean (SEM) from triplicate wells. Statistical significance between UTD-M and CAR-M was calculated using 2-way ANOVA with multiple comparisons.
MHC-I expression assay
7.5 × 104 MC38-GFP+ or MC38-GFP+HER2+ target cells were co-cultured overnight in 24-well tissue culture-treated plates. The following day, 2.5 × 105 UTD-M or CAR-M (48 h after transduction) were added on top of the target cells and allowed to interact for 24 h at 37 °C in duplicate wells. Subsequently, cells were harvested and stained with Live/Dead Fixable Aqua Dead Cell Staining Kit (L34966, ThermoFisher Scientific), CD45-APC (103112, BioLegend), and MHC-I (H2kb)-PE-Cy7 (25-5958-82, ThermoFisher Scientific). Samples were acquired on the Attune™ NxT acoustic focusing cytometer autosampler (ThermoFisher Scientific) and analysis was performed using the FlowJo software (FlowJo, LLC). Statistical significance between UTD-M and CAR-M was calculated using 2-way ANOVA with multiple comparisons.
Cytokine secretion assay
Human Her2 (target antigen; 10004-H08H, SinoBiological) or human mesothelin (irrelevant antigen control; 3265-MS, R&D Systems) were resuspended in PBS to a concentration of 28 nM. 100 µL of protein solution was added to wells in a 96 well flat bottom plate and incubated at 4 °C for overnight. PBS control wells were incubated with PBS only. After incubation, wells were washed twice, followed by the addition of 5 × 105 UTD-M or CAR-M (48 h after transduction) and incubated overnight at 37 °C and 5% CO2. Supernatants were collected and stored at −20 °C. Analysis of cytokines and chemokines was performed by Eve Technologies using the Mouse Cytokine 32-Plex Discovery Assay, based on Luminex technology.
In vitro cytotoxicity assay
1 × 104 or 2 × 104 UTD-M or CAR-M (48 h after transduction) were co-cultured with 1 × 104 target cells (AU565 NLG + , SKOV3 NLG + , CT26 NLG + , and MC38 GFP + ) in triplicate wells. In designated experiments, 10 µg/mL of InVivoMAb anti-mouse PD-1 (BE0146, Bio X Cell) or 10 µg/mL InVivoMAb rat IgG2a isotype control (BE0089, Bio X Cell) was added to the wells. In T cell assays, CD3+ T cells were isolated by magnetic cell isolation (130-095-130, Miltenyi Biotec) from the spleen of female OT-1 mice. 2 × 104 UTD-M or CAR-M were co-cultured with 1 × 104 MC38OVA+GFP+ and 6 × 104 T cells in triplicate wells. NLG intensity was measured in real time by Incucyte® Live Cell Analysis System to track tumor cell burden in vitro during the evaluation period. Data is represented as mean ± SEM of triplicate wells.
IVIS-based CAR-M trafficking and persistence
CT26-hHER2 and 4T1-cHER2 cells were implanted into BALB/c mice as described above. 10 days prior to the CAR-M injection, the mice were switched to alfalfa-free diet. CAR-M were stained with IVISense 680 fluorescent cell labeling dye (NEV12000, Revvity) per manufacturer’s protocol. 5 × 106/mouse of fluorescence-labeled CAR-M were injected IV into mice with tumors that had been established for 16 days. Fluorescent imaging was performed using IVIS Spectrum In Vivo 2D Imaging System (Perkin Elmer) at excitation/emission wavelengths of 675/720 nm. The mice were subjected to longitudinal in vivo fluorescent imaging or sacrificed at days 2 and 5 in order to perform ex vivo fluorescent imaging of harvested tumors and organs.
RNAscope
In situ detection of CAR-M transcripts in formalin fixed paraffin embedded (FFPE) samples was performed using Advanced Cell Diagnostics (ACD), USA RNAscope ISH technology. Sequential 4-µm thick sections of tumor tissues from CAR-M and UTD-M treated mice were stained using the Leica Bond RX automated platform. Newly designed probe by ACD specifically detecting CAR-M (pSF-Ad5) and mouse CD11b probe (Mm-Itgam, Cat. N. 311498) were used for detection on the C-1 and C-2 channels, respectively. Detection was performed using RNAscope® 2.5 LS Duplex Reagent Kit-(RED/BROWN) (Cat. N. 322440) and Green Chromogen (Leica, Product Code: DC9913) according to the manufacturer’s protocol. Assay controls used were ACD Bio positive control probe housekeeping mouse (mus musculus) genes PPIB (peptidylprolyl isomerase B [cyclophilin B]) mRNA—(Cat. N 321651) and negative control bacterial gene Polr2A (DNA-directed RNA polymerase II subunit RPB1 (Cat. N 320758). Slides were imaged using Akoya PhenoImager and positive copies were scored on the HALO® Image Analysis Platform (Indica Labs).
Serum cytokine determination
Blood was collected via submandibular bleeding or cardiac puncture depending on the specific experimental design. In CT26-hHER2 mice treated with two doses of CAR-M, serum was obtained from blood on day 1, 4, 7, 11, and 18 (regional CAR-M experiment) or -1, 3, 6, 13, and 20 (systemic CAR-M experiment) post first dose, and stored in aliquots at −80 °C until required. Serum samples were shipped to Eve Technologies and serum biomarkers were determined using the Mouse Cytokine 44-Plex Discovery Assay, based on Luminex technology.
Serum amyloid A3 (SAA-3) quantification
SAA-3 was measured in serum using the Mouse SAA-3 ELISA Kit (EZMSAA3, Millipore). First, a selection of samples was analyzed at multiple dilutions according to the manufacturer’s protocol to optimize SAA-3 detection within the limits of the assay. Then, all serum samples were analyzed at a determined 4-fold dilution and plotted for each treatment group across all timepoints.
Immunohistochemistry
Tumor tissues were fixed in 10% formalin for 24–72 h and shipped in 70% ethanol to HistoWiz (NY, USA) for IHC.FFPE specimens were stained for CD8α (cst98941, Cell Signaling Technology) at 1:600, CD4 (ab183685, Abcam) at 1:500, and HER2 (cst4290, Cell Signaling Technology) at 1:100. Whole slide scanning (40x) was performed on an Aperio AT2 (Leica Biosystems). Cell density (#/mm2) and percentage was determined using the HALO® Image Analysis Platform (Indica Labs).
Multiplexed ion beam imaging
FFPE specimens were shipped to IONpath Inc (CA, USA) for Multiplexed Ion Beam Imaging (MIBI). MIBslide mounted FFPE tissue sections were stained with metal isotope labeled antibodies against the following markers: beta-tubulin, CD11b, CD11c, CD206, CD3, CD31, CD4, CD45, CD49b, CD8, dsDNA, F4/80, FoxP3, Granzyme B, Ki-67, Ly6G, Na-K-ATPase, PAX5, SMA, and Vimentin (Extended Table 1). Each sample was stained in three distinct regions of interest (ROI). MIBIscope was used to acquire ROIs measuring approximately 800 µm per side across all samples and controls at subcellular resolution. Single cell segmentation, cell classification (Extended Table 2), spatial and comparative analysis were performed. Cell count and cell density were reported for each enumerated cell type. Extended Table 1 and Extended Table 2 were provided by IONpath Inc (CA, USA).
TCR-sequencing
To determine T cell diversity and clonality, frozen EDTA-anticoagulated blood and FFPE tissue specimens were sent to Adaptive Biotechnologies (WA, USA) for ImmunoSEQ - T-cell Receptor Sequencing (TCR) sequencing. Extracted genomic DNA was amplified in a bias-controlled multiplex PCR, followed by high-throughput sequencing. Sequences were collapsed and filtered in order to identify and quantitate the absolute abundance of each unique TCRβ CDR3 region for further analysis as previously described65,66,67. Data was analyzed using the immunoSEQ Analyzer software platform. Morisita index is a modified correlation comparing both shared clones and relative abundance of clones between two samples. Values can range from 1 (identical) to 0 (disparate) when comparing repertoires68. Peripheral T cells fraction is calculated as the total T cells divided by an estimate of the total number of nucleated cells based on mass. Higher T cell fraction indicates more recruitment and activation of T cells in peripheral blood. The pairwise comparison of shared clones between subjects assessed the overlap of the top 100 most abundant clones for all 10 possible pairs of blood samples within each treatment group (n = 5), with each pair resulting in a data point representing the number of shared top 100 clones. Dearth of “public clones” may indicate increase in number or diversity of tumor antigens within each subject. Simpson Clonality69 quantifies the extent to which one or few clones dominate the repertoire. It ranges from 0 (even distribution) to 1 (monoclonal) and is robust to sequencing depth. Here Simpson Clonality is defined as the square root of Simpson’s index.
RNA isolation
RNA was isolated from (0.5–1) × 106 HSC, monocytes, UTD-M cultures, and CAR-M cultures using the RNeasy Kit (74004, QIAGEN). RNA was quantified using NanoDrop (ThermoFischer Scientific; MA, USA) and sent to Novogene for bulk RNA sequencing.
RNA sequencing (RNA-Seq)
FASTQ files of reads were aligned to Mouse Genome Assembly GRCm39 using STAR70. The raw gene counts were processed using the DESeq2 package71. Normalization size factors were estimated using the median of ratios method in DESeq272. PCA was performed using the 500 most variable genes across all samples. Variance-stabilized gene expression generated by DESeq2 was used to calculate the Principal Components. DESeq2 was used to identify significant differentially expressed genes. Pathway analysis was performed by Novogene using Fisher’s Exact test to determine if differentially expressed genes were over-represented among pathways.
In order to characterize the differentiation of HSCs and monocytes cultured in M-CSF or GM-CSF, RNA-Seq was performed and cell types were predicted using SingleR73 with the ImmGenData reference dataset from the celldex Bioconductor package74. The average scores resulting from SingleR prediction for each reference cell population were averaged across replicates in the RNA-Seq experiment. The heatmap shows that SingleR prediction applied to the HSC samples produced the greatest score for stem cells. SingleR also correctly assigned the greatest score to the reference monocyte population for the monocyte samples, and SingleR assigned the highest score to the reference macrophage populations for all macrophage samples. Further comparison of the cell differentiation under M-CSF and GM-CSF was characterized using macrophage and DC gene signatures based on previous findings75. An increased proinflammatory phenotype was observed in CAR-M compared to UTD cells using a proinflammatory score computed by applying Gene Set Variation Analysis (GSVA)76 with a 16 gene signature to the RNA-Seq data33.
Pathological examination
BALB/c mice were engrafted with CT26-hHER2 SQ and tumor was allowed to establish for 10 days prior to treatment with IV CAR-M therapy with and without PD1 blockade. At the end of the study, liver, kidney, brain, lung, spleen, and heart were fixed in 10% formalin for 24–72 h and shipped in 70% ethanol to HistoWiz (NY, USA) for hematoxylin and eosin (H&E) staining. Each tissue sample was examined by a certified pathologist at HistoWiz (NY, USA) for evidence of necrosis, inflammation, abnormal development, neoplasia, or other evidence of pathology.
Statistics
Statistical analysis was performed in GraphPad Prism 10 (GraphPad). Each figure legend denotes the statistical test, and the number of replicates used. Mice group sizes were chosen based on pilot experiments and on our experience carrying out similar experiments. No statistical method was used to predetermine sample size. All central tendencies indicate the mean. ANOVA multiple-comparison P values were generated using Tukey’s multiple-comparisons test. All t-tests were two-sided. Unless otherwise specified, “n” refers to the number of biological replicates. No data were excluded from the analyses. The Investigators were not blinded to allocation during experiments and outcome assessment. The Bliss Independence Model was used to evaluate drug synergy. This model assumes that, if two drugs act independently, their combined effect equals the product of their individual effects. The expected combined effect EABexpected was calculated as EA + EB − (EA × EB), where EA and EB represent the effects of drugs A and B, respectively. Synergy is indicated when the observed combined effect EABobserved exceeds the expected effect. For all figures, * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001 and **** indicates P < 0.0001.
Data availability
The RNA sequencing data and the Raw FASTQ files that support the findings of this study were deposited and made publicly in the Gene Expression Omnibus (GEO) - accession number GSE275417[GEO Accession viewer] - and in the NIH’s Sequence Read Archive, respectively. TCR sequencing data was deposited and made public in GEO – accession number GSE285188. Source data are provided with this paper.
Change history
19 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-57496-0
References
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Wang, X. et al. Metabolic Reprogramming via ACOD1 depletion enhances function of human induced pluripotent stem cell-derived CAR-macrophages in solid tumors. Nat. Commun. 14, 5778 (2023).
Lei, A. et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat. Immunol. 25, 102–116 (2024).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Morrissey, M. A. et al. Chimeric antigen receptors that trigger phagocytosis. Elife 7 (2018).
Abdin, S. M., Paasch, D. & Lachmann, N. CAR macrophages on a fast track to solid tumor therapy. Nat. Immunol. 25, 11–12 (2024).
Wang, S. et al. CAR-macrophage: an extensive immune enhancer to fight cancer. EBioMedicine 76, 103873 (2022).
Varghese, B. et al. 1514 In vivo CAR-M: redirecting endogenous myeloid cells with mRNA for cancer immunotherapy. J. Immunother. Cancer 11, A1739–A1739 (2023).
Rizvi, N. A. et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 16, 257–265 (2015).
Robert, C. et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Chen, F. et al. Clinical progress of PD-1/L1 inhibitors in breast cancer immunotherapy. Front. Oncol. 11, 724424 (2021).
Fan, Z. et al. A meta-analysis of the efficacy and safety of PD-1/PD-L1 immune checkpoint inhibitors as treatments for metastatic bladder cancer. Onco Targets Ther. 12, 1791–1801 (2019).
McDermott, D. F. et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 34, 833–842 (2016).
Mayoux, M. et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 12 (2020).
Nagasaki, J. et al. PD-1 blockade therapy promotes infiltration of tumor-attacking exhausted T cell clonotypes. Cell Rep. 38, 110331 (2022).
Magen, A. et al. Intratumoral dendritic cell-CD4(+) T helper cell niches enable CD8(+) T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 29, 1389–1399 (2023).
Balanca, C. C. et al. PD-1 blockade restores helper activity of tumor-infiltrating, exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 6 (2021).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Goswami, S., Anandhan, S., Raychaudhuri, D. & Sharma, P. Myeloid cell-targeted therapies for solid tumours. Nat. Rev. Immunol. 23, 106–120 (2023).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Nowicki, T. S., Hu-Lieskovan, S. & Ribas, A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J. 24, 47–53 (2018).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20, 371–382 (2019).
Colombo, N. et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 385, 1856–1867 (2021).
Migden, M. R. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 379, 341–351 (2018).
Nghiem, P. T. et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma. N. Engl. J. Med 374, 2542–2552 (2016).
Janjigian, Y. Y. et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 402, 2197–2208 (2023).
Janjigian, Y. Y. et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600, 727–730 (2021).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 21, 1283–1295 (2020).
Barlan, A. U., Griffin, T. M., McGuire, K. A. & Wiethoff, C. M. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85, 146–155 (2011).
Lam, E., Stein, S. & Falck-Pedersen, E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J. Virol. 88, 974–981 (2014).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25, 771–784 (2015).
Fleetwood, A. J., Lawrence, T., Hamilton, J. A. & Cook, A. D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245–5252 (2007).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e723 (2021).
Lechner, M. G. et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 36, 477–489 (2013).
Fabian, K. P., Padget, M. R., Fujii, R., Schlom, J. & Hodge, J. W. Differential combination immunotherapy requirements for inflamed (warm) tumors versus T cell excluded (cool) tumors: engage, expand, enable, and evolve. J. Immunother. Cancer 9 (2021).
Liu, Y. & Cao, X. Intratumoral dendritic cells in the anti-tumor immune response. Cell Mol. Immunol. 12, 387–390 (2015).
Nilofar Danishmalik, S., Lee, S. H. & Sin, J. I. Tumor regression is mediated via the induction of HER263-71- specific CD8+ CTL activity in a 4T1.2/HER2 tumor model: no involvement of CD80 in tumor control. Oncotarget 8, 26771–26788 (2017).
Taha, Z. et al. Syngeneic mouse model of human HER2+ metastatic breast cancer for the evaluation of trastuzumab emtansine combined with oncolytic rhabdovirus. Front. Immunol. 14, 1181014 (2023).
Martinez, M., Moon, E. K. & CAR, T. Cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 10, 128 (2019).
Anderson, N. R., Minutolo, N. G., Gill, S. & Klichinsky, M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 81, 1201–1208 (2021).
Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 131 (2021).
Nahar, S. et al. Regression and eradication of triple-negative breast carcinoma in 4T1 mouse model by combination immunotherapies. Cancers (Basel) 15 (2023).
Aaes, T. L. & Vandenabeele, P. The intrinsic immunogenic properties of cancer cell lines, immunogenic cell death, and how these influence host antitumor immune responses. Cell Death Differ. 28, 843–860 (2021).
Kim, A. B. et al. Chimeric antigen receptor macrophages target and resorb amyloid plaques. JCI Insight 9 (2024).
He, Z. & Zhang, S. Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Front. Immunol. 12, 741305 (2021).
Lei, Q., Wang, D., Sun, K., Wang, L. & Zhang, Y. Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev. Biol. 8, 672 (2020).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Jakubzick, C. V., Randolph, G. J. & Henson, P. M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 17, 349–362 (2017).
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G. & Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 30, 36–50 (2019).
Engblom, C., Pfirschke, C. & Pittet, M. J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 16, 447–462 (2016).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Huang, A. Y. et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl Acad. Sci. USA 93, 9730–9735 (1996).
McWilliams, J. A. et al. Age-dependent tolerance to an endogenous tumor-associated antigen. Vaccine 26, 1863–1873 (2008).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Baitsch, L. et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Porciello, N., Franzese, O., D’Ambrosio, L., Palermo, B. & Nistico, P. T-cell repertoire diversity: friend or foe for protective antitumor response? J. Exp. Clin. Cancer Res. 41, 356 (2022).
Reiss, K. et al. 635A phase 1, first in human (FIH) study of autologous macrophages engineered to express an anti-HER2 chimeric antigen receptor (CAR) in participants (pts) with HER2 overexpressing solid tumors. J. Immunother. Cancer 11, A726–A726 (2023).
Blumenthal, D. et al. Abstract 582: pre-clinical development of CAR Monocytes (CAR Mono) for solid tumor immunotherapy. Cancer Res. 82, 582–582 (2022).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Ullman-Cullere, M. H. & Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim. Sci. 49, 319–323 (1999).
Zhang, X., Goncalves, R. & Mosser, D. M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14, 14 11 11–14 11 14 (2008).
Pierini, S. et al. A tumor mitochondria vaccine protects against experimental renal cell carcinoma. J. Immunol. 195, 4020–4027 (2015).
Pierini, S. et al. Combination of vasculature targeting, hypofractionated radiotherapy, and immune checkpoint inhibitor elicits potent antitumor immune response and blocks tumor progression. J. Immunother. Cancer 9 (2021).
Robins, H. S. et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 114, 4099–4107 (2009).
Carlson, C. S. et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat. Commun. 4, 2680 (2013).
Robins, H. et al. Ultra-sensitive detection of rare T cell clones. J. Immunol. Methods 375, 14–19 (2012).
DeWitt, W. S. et al. Dynamics of the cytotoxic T cell response to a model of acute viral infection. J. Virol. 89, 4517–4526 (2015).
Simpson, E. Measurement of diversity. Nature 163 (1949).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Heng, T. S., Painter, M. W. & Immunological Genome Project, C. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Helft, J. et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity 42, 1197–1211 (2015).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7 (2013).
Acknowledgements
Some images were created using BioRender (www.biorender.com) under a full license.
Author information
Authors and Affiliations
Contributions
S.P., R.G., M.C.O.-N., T.C., and M.K. designed the study. S.P., R.G., M.C.O.-N., A.W., S.H., K.N., C.G., L.L., K.R., C.S., M.B., P.S., L.C., D.B., S.C., N.M., K.C., L.S., I.Z., and O.S. performed and generated the key experiments. Y.O., H.L., S.G., B.V., D.C., S.A., T.C., and M.K. provided conceptual guidance. R.Q. and B.S. performed bioinformatics analysis. S.P., S.C.D., and M.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.K. and S.G. are scientific co-founders of Carisma Therapeutics Inc, equity shareholders, and inventors of patents related to this work. S.P., R.G., M.C.O.-N., R.Q., A.W., S.H., K.N., C.G., L.L., Y.O., K.R., C.S., M.B., B.S., P.S., L.C., D.B., S.C., N.M., K.C., L.S., I.Z., D.C., S.C.D, S.A., T.C., and M.K. are employees of Carisma Therapeutics Inc and equity shareholders. H.L and S.G. are Scientific Advisory Board Members and shareholders of Carisma Therapeutics Inc. S.G. has patents related to CAR therapy with royalties paid from Novartis to the University of Pennsylvania. S.G. is a scientific co-founder and holds equity in Interius Biotherapeutics and Carisma Therapeutics Inc. S.G. is a scientific advisor to Carisma, Cartography, Currus, Interius, Kite, NKILT, Mission Bio, and Vor Bio. O.S. declares no competing interests. This work was funded by Carisma Therapeutics Inc which is pursuing the commercial development of related products.
Peer review
Peer review information
Nature Communications thanks Jennifer Guerriero, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pierini, S., Gabbasov, R., Oliveira-Nunes, M.C. et al. Chimeric antigen receptor macrophages (CAR-M) sensitize HER2+ solid tumors to PD1 blockade in pre-clinical models. Nat Commun 16, 706 (2025). https://doi.org/10.1038/s41467-024-55770-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55770-1
This article is cited by
-
Engineering innate immune cells for cancer immunotherapy
Nature Biotechnology (2025)
-
Human CSPG4-targeting CAR-macrophages inhibit melanoma growth
Oncogene (2025)
-
Immune microenvironment in hepatocellular carcinoma: from pathogenesis to immunotherapy
Cellular & Molecular Immunology (2025)