Abstract
Polymer dielectric materials are widely used in electrical and electronic systems, and there have been increasing demands on their dielectric properties at high temperatures. Incorporating inorganic nanoparticles into polymers is an effective approach to improving their dielectric properties. However, the agglomeration of inorganic nanoparticles and the destabilization of the organic-inorganic interface at high temperatures have limited the development of nanocomposites toward large-scale industrial production. In this work, we synthesize metal-organic cage crosslinked nanocomposites by incorporating self-assembled metal-organic cages with amino reaction sites into the polyetherimide matrix. The in-situ crosslinking by self-assembled metal-organic cages not only achieves a homogeneous distribution of inorganic components, but also constructs robust organic-inorganic interfaces, which avoids the interfacial losses of conventional nanocomposites and improves the breakdown strength at elevated temperatures. Ultimately, the developed nanocomposites exhibit exceptionally high energy densities of 7.53 J cm−3 (150 °C) and 4.55 J cm−3 (200 °C) with charge-discharge efficiency of 90%.
Similar content being viewed by others
Introduction
Dielectric capacitors offer significant advantages, including high power density, fast discharging speed, and high reliability, which have found extensive applications in pulse power systems and electronic systems1,2,3,4. With the further miniaturization and integration of electronic devices, the high-temperature stability of dielectric materials has put forward higher requirements5,6,7,8. Currently, the widely utilized dielectric polymer capacitor films are based on biaxially oriented polypropylene (BOPP), which exhibits a discharged energy density of approximately 4 J cm−3 at room temperature7,9,10,11,12. However, its dielectric properties will decline rapidly as the temperature rises, and it can only operate below 105 °C, failing to meet the requirements of robust high-temperature dielectric energy storage capacitors (150 °C~250 °C)5,8,13. Hence, developing dielectric materials with high-temperature resistance and superior energy storage density has gained significant research interest3,14.
Among various polymer dielectrics, polyimide (PI), polyetherimide (PEI), polycarbonate (PC), fluorene polyester (FPE), and polyether ether ketone (PEEK) stand out due to their high glass transition temperatures and excellent thermal stability1,2,15. However, these materials have relatively low energy storage density, prompting researchers to adopt various strategies to enhance their performance. Li et al. obtained nanocomposites by doping boron nitride nanosheets (BNNS) as fillers into the polymer matrix16. Additionally, researchers introduced inorganic nanofillers such as Al2O3, MgO, CaF2, HfO2, TiO2, and SiO2 with wider bandgaps into the polymer matrix, which was used to boost energy storage density17,18,19,20,21. However, the introduction of inorganic nanofillers compromises the flexibility of the polymer material itself, and the enhancement of the energy storage density is relatively limited. Additionally, The intrinsic properties of polymers and inorganic nanoparticles present a significant challenge to achieving homogeneous dispersion of inorganic nanoparticles in polymers. Conversely, the organic-inorganic interface inevitably introduces interfacial losses, which are particularly severe at elevated temperatures. More seriously, the destablization of the organic-inorganic interface at high temperatures not only fails to enhance the breakdown strength but also makes the organic-inorganic interface a vulnerable point for electrical breakdown.
Supramolecular interactions are non-covalent bonding interactions, primarily involving hydrogen bonding, metal coordination, host-guest interactions, and electrostatic interactions22,23. Based on the principles of supramolecular self-assembly, specific components with desired structures and functionalities can be assembled into novel supramolecular compounds in a prescribed manner22,24,25. These newly formed compounds often exhibit unique properties that individual molecules do not possess, offering novel avenues for functional material development24,26,27. Herein, we constructed crosslinking networks using self-assembled metal-organic cages as crosslinking sites (Fig. 1)28,29,30,31,32,33,34,35. This type of crosslinked polymer network utilizes supramolecular metal-organic cages as crosslinking sites, which have been extended from metal-supramolecular polymer systems proposed by Nitschke and Johnson et al. 33,34,35,36,37,38 In specific, a self-assembled metal-organic cage, i.e. Titanium Oxide Cluster (TOC), was chosen. The precise sixteen amine reaction groups exposed on the TOC surface were further connected with PEI polymer via amine-anhydride condensation to afford PEI-g-TOC. This metal-organic cage crosslinked nanocomposite material not only enhances the dielectric constant but also avoids the interface losses associated with traditional polymer/nanofillers composites39. Benefiting from in-situ crosslinking by self-assembled metal-organic cages, the introduction of inorganic components constructs a more robust organic-inorganic interface, which limits the electric field distortion at the interface and also forms deep traps to capture charge carriers. Therefore, the synergistic enhancement of dielectric constant (εr) and breakdown strength (Eb) is achieved in the PEI-g-TOC composites (PEI-g-TOC for short), exhibiting an energy density of 7.53 J cm−3 (150 °C) and 4.55 J cm−3 (200 °C) with efficiency of 90%. Furthermore, PEI-g-TOC demonstrates outstanding charge-discharge cyclability (105) at 300 MV m−1 and 200 °C, making PEI-g-TOC a strong candidate for the next generation of high-temperature dielectric materials.
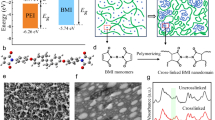
a The single crystal structure of NH2-TOC cage in balls and sticks. b Dynamic light scattering characterization of NH2-TOC. c Schematic structure of traditional nanocomposite: PEI/TiO2. The polydisperse nanoparticles are physically blended in the PEI matrix. d Schematic structure of metal-organic cage crosslinked nanocomposite: PEI-g-TOC. The supramolecular TOC cages with monodisperse size and precise functional groups act as crosslinking sites for the PEI network.
Results
Material synthesis and characterization
As depicted in Supplementary Fig. 1, the PEI-g-TOC composites were synthesized using a one-pot method through the thermal imidization of amino-functionalized titanium oxide clusters (NH2-TOC), bisphenol A diether dianhydride (BPADA), and m-phenylenediamine (MPD). Refer to our previously published article, the NH2-TOC was synthesized via hydrothermal crystallization. The crystal structure of NH2-TOC was resolved by single-crystal X-ray diffraction (XRD), with the size and shape of NH2-TOC directly visualized in crystallographic software (Supplementary Fig. 2)30,31,40. The NH2-TOC shown in Fig. 1a is a supramolecular metal-organic cage in which p-aminobenzoic acid is linked to the titanium-oxygen backbone in the NH2-TOC via supramolecular coordination bonds40,41. The existence of ethylene glycol enhances the compatibility between the titanium oxide cages (TOC) and the polymer, while the presence of amino groups on the surface of NH2-TOC offers an opportunity for additional functionalization. Specifically, the amino groups on the surface of NH2-TOC, along with the amino groups in MPD, serve as reaction sites for the thermal imidization with BPADA. In contrast to the traditional physically blended nanoparticles (e.g. TiO2), this supramolecular TOC cage has distinct advantages, including monodisperse size (2.69 nm), precise functional groups, simple synthesis, low cost, and large surface area. The dynamic light scattering (DLS) characterization in Fig. 1b shows a narrow distribution of NH2-TOC with a maximum probability size of about 3 nm, demonstrating the self-assembly behavior of the NH2-TOC. The metal-organic cage crosslinked nanocomposites were then prepared using the supramolecular TOC cages as the crosslinking sites, with their schematic structures shown in Fig. 1d. Specifically, 4-aminobenzoic acid acts as a bridge for the organic-inorganic hybridization. One end of the bridge is covalently linked to PEI, while the other end forms supramolecular coordination interactions with titanium ions to construct TOC cages. In PEI-g-TOC, a robust organic-inorganic interface was formed due to the presence of supramolecular coordination. The structures of PEI/TiO2 formed by physically mixing TiO2 in the PEI matrix as shown in Fig. 1c. Due to the introduction of TiO2, the mobility of the polymer chains at the organic-inorganic interface is enhanced in PEI/TiO242,43. In addition, we prepared a PEI/TOC composite by simply blending NH2-TOC with PEI. The TOC content was maintained at 2 wt%, consistent with the optimal composition of PEI-g-TOC.
In Fourier transform infrared spectroscopy (FTIR), the peaks at 1776 cm−1, 1717 cm−1, and 1351 cm−1 represent the symmetric and asymmetric C=O stretching vibrations and the C − N stretching vibrations, revealing the imide ring formation in PEI-g-TOC44,45. 1H NMR (Supplementary Fig. 3) also confirms the occurrence of the imidization reaction. Besides, the disappearance of the peak at 3440 cm−1 in FTIR, corresponding to the stretching vibrations of N–H(Supplementary Fig. 4), indicates the successful full conversion of amino group in NH2-TOC to the imide group, which can be further confirmed by the existence of only imide peak in the X-ray photoelectron spectroscopy (XPS) spectra of N 1 s (Supplementary Figs. 5) for PEI-g-TOC46. The O 1 s spectrum of PEI-g-TOC and PEI/TOC presents a new peak, attributed to the O in TOC (Supplementary Fig. 6)47,48. In comparison to the clear solution of PEI dissolved in dimethylacetamide (DMAc), the photographs of PEI-g-TOC suspension in DMAc (Supplementary Fig. 7) visually illustrate the insolubility of this nanocomposite, due to the occurrence of crosslinking by NH2-TOC.
Transmission electron microscopy (TEM) was employed to analyze the size and distribution of TOC in the PEI-g-TOC composites. As shown in Fig. 2a, b and Supplementary Fig. 8, TOC in PEI-g-TOC composite exhibits a more homogeneous dispersion with a size of about 3 nm, indicating that the TOC is present in a monodisperse form in the PEI matrix41. In contrast, there is a noticeable issue of aggregation of TOC with sizes up to about 30 nm in PEI/TOC. The above results demonstrate the achievement of uniform dispersion of TOC within the PEI matrix, which can be attributed to the presence of a covalent linkage between NH2-TOC and PEI. A more substantial interfacial area could thus be expected in PEI-g-TOC when compared with traditional nanocomposites at the same loading. We also investigated PEI/TOC and PEI-g-TOC by atomic force microscopy-based infrared spectroscopy (AFM-IR)49. As shown in Fig. 2c for PEI-g-TOC, the absorbance images for vibrations of C=O at 1724 cm−1 are uniform, while the vibrations of C=O at 1724 cm-1 decrease around the inorganic particle regions in PEI/TOC (Fig. 2d and Supplementary Fig. 9). The above results indicate that a certain degree of chain loosening occurs at the organic-inorganic interface in PEI/TOC, whereas it remains uniform and stable in PEI-g-TOC. In addition, the introduction of NH2-TOC has minimal impact on the surface and cross-sectional morphologies of the material, as confirmed by scanning electron microscopy (SEM) images (Supplementary Figs. 10 and 11).
STEM image of (a) PEI-g-TOC and (b) PEI/TOC, the inset is EDS elemental mapping results of Ti. AFM-IR mapping of IR response with 1724 cm−1 for (c) PEI-g-TOC and (d) PEI/TOC. e XPS Ti 2p spectra of PEI, PEI/TOC and PEI-g-TOC. f Interchain spacing, Tg and Td of PEI, PEI-g-TOC, and PEI/TOC, respectively.
XPS, XRD, differential scanning calorimeter (DSC), and thermogravimetric analysis (TGA) were employed to investigate the effects of TOC integration on the PEI matrix. Figure 2e reveals the Ti 2p spectrum of PEI-g-TOC, which displays two distinct peaks for 2p1/2 (464.48 eV) and 2p3/2 (458.48 eV). These peaks are 0.19 eV lower in binding energy compared with the corresponding peaks observed in the Ti 2p spectra of PEI/TOC50. Based on the comprehensive results of XPS analysis, it can be concluded that the TOC has been successfully incorporated into the PEI matrix. The XRD results (Fig. 2f and Supplementary Figs. 12,13) provide additional confirmation of this fact. The primary peak of PEI-g-TOC exhibits a rightward shift in comparison with pure PEI, while the peak position of PEI/TOC shows a shift to the left. According to Bragg law, the variation in the interchain spacing of the composites can be further analyzed. PEI-g-TOC exhibits a smaller interchain spacing of 4.71 Å, compared to that of 4.84 Å in PEI and 4.91 Å in PEI/TOC. For physically blended PEI/TOC, the introduction of NH2-TOC brings about new organic-inorganic interfaces, and the enhanced molecular chain activity at the interface leads to an increase in the chain spacing42,43. However, PEI-g-TOC is different from the results of conventional organic-inorganic composite systems, which is due to the fact that we constructed a crosslinked network using NH2-TOC as the crosslinkers. Specifically, the crosslinking of polymer matrix would bring the polymer chain closer in space and tighten the chain packing, which has also been observed in other crosslinked polyimide material systems51,52. Meanwhile, the XRD spectra of PEI-g-TOC and PEI/TOC did not exhibit any noticeable TOC crystalline peaks, which can be attributed to the low doping concentration.
The existence of crosslinked metal-organic cages not only affects the structure of composites but also influences their thermal properties. As shown in Fig. 2f and Supplementary Figs. 14-15, the glass transition temperature (Tg) and the thermal decomposition temperatures (Td) of PEI-g-TOC lies between that of pure PEI and PEI/TOC. For PEI/TOC, the introduction of TOC creates an organic-inorganic interface, and the enhanced molecular chain activity at the interface leads to a slight decrease in Tg and Td. This behavior could explain why the Tg in PEI/TOC decreases more than 10 °C compared with pure PEI. The negative effects for Tg and Td due to the creation of an organic-inorganic interface also exist in PEI-g-TOC, resulting in lower Tg and Td values of PEI-g-TOC than pure PEI. However, the positive effect of crosslinking by TOC cage in PEI-g-TOC could compensate the Tg and Td values to some extent, leading to higher Tg and Td values than PEI/TOC but still lower than pure PEI. The results of the thermal stability of PEI/TiO2 are similar to those of PEI/TOC (Supplementary Fig. 16). The above results indicate that the TOC are uniformly dispersed in the PEI matrix with monodisperse size (about 3 nm), and the supramolecular coordination in the metal-organic cage enables PEI-g-TOC to have a structure that differs from both conventional nanocomposites and traditional cross-linked systems.
Dielectric and capacitive performance
The εr and dissipation factor of the PEI-g-TOC were examined as a function of temperature and frequency (Fig. 3a and Supplementary Fig. 17). As seen, the dielectric properties of PEI-g-TOC remain stable at both room temperature and elevated temperatures. As the amount of TOC incorporation increases, the εr of PEI-g-TOC continuously increases (Supplementary Fig. 18). Specifically, the dielectric loss of PEI-g-TOC, within a considerable range of frequencies (100–103 Hz), is lower than that of PEI/TOC and even lower than pure PEI, e.g. 0.00377 for PEI-g-TOC versus 0.00498 for PEI/TOC at 100 Hz and 200 °C. Plus, as the temperature increases, the dielectric loss of PEI/TOC increases dramatically, while the dielectric loss of PEI-g-TOC can still maintain a low value. The results clearly illustrate the effect of the presence of crosslinked metal-organic cages in enhancing εr while reducing the dielectric losses.
a Dielectric properties of PEI, PEI/TOC, and PEI-g-TOC at 200 °C. The inset is temperature dependent of the dielectric properties of these samples at 1 kHz. b Weibull breakdown strengths and Young’s modulus of PEI, PEI/TOC, and PEI-g-TOC. The error bar is the standard deviation. c TSDC curves and (d) Calculated density distribution along the y direction of PEI, PEI/TOC, and PEI-g-TOC. e Energy storage performance of PEI, PEI/TOC, PEI-g-TOC, and PEI/TiO2 at 200 °C and 100 Hz. f Comparison of discharged energy density above 90% efficiency in this work with other typical modification results in PEI matrix at 200 °C.
Two-parameter Weibull statistical analysis was used to determine the Eb of PEI, PEI/TOC, and PEI-g-TOC. As shown in Fig. 3b and Supplementary Fig. 19, PEI-g-TOC exhibits the highest Eb of 553 MV m−1 at 200 °C, which is significantly greater than that of PEI by over 25%. The β value (shape parameter to assess the data scatter) improves to 29.0 for PEI-g-TOC, contrasting to PEI’s 12.8 at 200 °C, signifying the enhanced reliability of PEI-g-TOC. As the amount of TOC incorporation increases, the Eb of PEI-g-TOC follows a pattern of initially increasing and subsequent decrease, with the maximum value observed at an incorporation level of 2 wt% NH2-TOC (Supplementary Fig. 20). The NH2-TOC cage has intrinsic void core and larger surface area, creating a much higher fraction of organic-inorganic interface in PEI-g-TOC. The excess introduction of NH2-TOC would thus result in polymer network defects due to incomplete crosslinking as well as increased free volume for PEI chains. Moreover, the bulky size and cyclic shape of NH2-TOC can impede the tight packing of PEI chains severely at a high fraction of NH2-TOC addition. The particular PEI-g- TOC 2 wt% seems have the tightest chain stacking, the robust organic-inorganic interface, and the best thermal stability, as confirmed by XRD, DSC, and TGA. These results can also be visually reflected by the leakage current measurements (Supplementary Fig. 21). Moreover, PEI/TiO2 exhibits a Eb that is lower than that of PEI-g-TOC, which directly suggests that the supramolecular coordination in PEI-g-TOC plays a significant role in enhancing Eb.
It is well recognized that mechanical strength significantly influences electrical properties, especially in cases of electromechanical breakdown. Variations in breakdown strength between PEI-g-TOC, PEI, and PEI/TOC can be attributed to their different mechanical strengths. We can see that the breakdown strength maintains a consistent trend with the Young’s modulus (Fig. 3b). The results reveal that PEI-g-TOC exhibits the highest Young’s modulus among all the samples, which can be attributed to the cross-linking structure in PEI-g-TOC. Conversely, the incorporation of TOC into PEI through simple blending leads to a decrease in the Young’s modulus of the PEI composite, causing structural damage to pure PEI. Meanwhile, molecular dynamics (MD) simulations further corroborated these results (Supplementary Fig. 22). We calculated the density distribution at the interfaces between TOC and PEI matrix in PEI, PEI/TOC, and PEI-g-TOC. As illustrated in Fig. 3c and Supplementary Fig. 23, the density at the interface in PEI-g-TOC was found to be higher than that of PEI/TOC. This suggests a stronger binding between TOC and PEI in PEI-g-TOC, implying the formation of a more robust interface. Under the influence of electric fields, the crosslinked metal-organic cages have been shown to slow down the electric field distortion at the interface. Moreover, the mean square displacement (MSD) is commonly used to characterize the interfacial interaction between fillers and polymer matrix in composite materials. Its magnitude reflects the strength of molecular chain mobility. The MSD value of PEI/TOC is significantly higher compared with PEI and PEI-g-TOC, indicating a greater migration capacity of PEI chains in PEI/TOC (Supplementary Fig. 24). This finding aligns with previous characterization results, demonstrating that the introduction of the inorganic components into an organic matrix by covalent bonding of surface ligands greatly enhanced their compatibility. As a result, the organic-inorganic interface in PEI-g-TOC is more robust and has higher mechanical properties, which greatly enhances Eb. In contrast, the simple blending method resulted in a sharp density variation at the interface, introducing numerous defects and significantly reducing the Eb. Furthermore, the decrease in leakage current density provides further evidence of the increased Eb (Supplementary Fig. 25). Among the tested samples, PEI-g-TOC exhibits the lowest leakage current density, followed by PEI, and PEI/TOC demonstrated the highest. This is contrary to the observed trend in the variation of Eb. For example, at 200 °C, leakage current density is effectively suppressed from 1.87 × 10−7 A cm−2 for PEI to 8.30 × 10−8 A cm−2 for PEI-g-TOC under 200 MV m−1.
To further analyze the effect of crosslinked metal-organic cages on Eb, thermally stimulated depolarization current (TSDC) test was carried out to reflect the charge-trapping characteristics of PEI composites. As shown in Fig. 3d, the TSDC curves are fitted to two peaks, which are attributed to charge traps due to TOCs (denoted as peak 1, black dotted line) and the injection of electrode charges (denoted as peak 2, black solid line), respectively. Peak 1 can be only be observed in PEI-g-TOC and PEI/TOC, which is attributed to the construction of interfaces through the introduction of TOCs. As for peak 1, higher trap energy level and larger trapped charge quantity are advantageous for trapping charge carriers. In contrast to PEI/TOC, the trap energy level of peak 1 in PEI-g-TOC slightly increases, and the trapped charge quantity increases significantly from 151.74 nC to 341.55 nC (Supplementary Fig. 26 and Table 1). The increase in trapped charge quantity indicates that PEI-g-TOC has more organic-inorganic interfaces compared to PEI/TOC. Specifically, the interface between the supramolecular TOC cage and PEI matrix traps a greater number of charge carriers, ensuring its high energy storage abilities at high temperatures. This is consistent with results from characterization and MD simulations, and further explains the role of crosslinked metal-organic cages in enhancing Eb. The peak 2 corresponding to the injection of electrode charges can be observed in all samples. A larger trap energy level (5.12 eV) and lower trapped charge quantity (11.98 nC) of peak 2 appear in PEI-g-TOC compared to pure PEI (2.75 eV and 14.78 nC). However, in PEI/TOC, the trap energy level (2.48 eV) of peak 2 decreases and the trapped charge quantity (35.92 nC) of peak 2 increases sharply. The above results indicate an increased barrier to charge injection and a decreased number of injected charges, which is consistent with the trend of leakage current density and Eb.
By analyzing the electric displacement-electric field (D–E) loop (Supplementary Figs. 27–31), we assessed the energy storage performance of PEI, PEI/TOC, PEI-g-TOC and PEI/TiO2 at elevated temperature (Fig. 3e and Supplementary Figs. 32). Thanks to the synergistic enhancement of εr and Eb, the energy storage performance of PEI-g-TOC has been greatly improved. The PEI-g-TOC exhibits the discharged energy density (Ud) of 7.53 J cm−3 (150 °C) and 4.55 J cm−3 (200 °C) with efficiency (η) of 90%, which is over 205% and 148% times higher than those of PEI, respectively. The energy storage density of PEI/TiO2, although slightly improved compared to PEI, is not as effective as that of PEI-g-TOC. This provides an obvious illustration of the important role of self-assembled metal-organic cages and supramolecular coordination effects in increasing energy storage density. Due to the significant decrease in Eb of PEI/TOC, its energy storage performance is significantly reduced, even lower than that of pure PEI. To compare the energy storage performance of this work with other reported works in high-temperature environments, we summarize the results of three typical current modification approaches for PEI matrix modification, including surface-modified polymers, all-organic polymers, and polymer nanocomposites. As shown in Fig. 3f for 200 °C and Supplementary Fig. 33 for 150 °C (Supplementary Table 2)9,53,54,55,56,57,58,59,60,61,62, PEI-g-TOC exhibits higher discharge energy densities than other PEI-based high-temperature polymers reported in the literature, and its performance is comparable to the most advanced PEI-based high-temperature polymers reported. In addition, the discharge energy density of PEI-g-TOC is higher than that reported in recent literature based on titanium oxide in polymer matrices (Supplementary Fig. 34)20,54,63.
Reliability and stability assessments
In order to assess the quality of PEI-g-TOC films in different regions, we selected a continuous region and divided it into 5 × 5 lattices to test their alternating current (AC) breakdown strengths separately in units of 50 MV m−1 (Fig. 4a and Supplementary Fig. 35)64. The AC breakdown strengths of each lattice in the PEI-g-TOC film are over 500 MV m−1 and remain uniformly stable over 25 lattices. In the PEI/TOC film, the AC breakdown strengths of the different grids vary considerably, fluctuating between 50 MV m−1 and 450 MV m−1. The stark contrast between these two films illustrates the homogeneous and stable AC breakdown strengths of metal-organic cages crosslinked nanocomposites compared to conventional nanocomposites, suggesting the important role of supramolecular interactions in enhancing the dielectric properties. Cyclic charging-discharging stability is a crucial factor for polymer composites in high-temperature environments. As shown in Fig. 4b, PEI-g-TOC demonstrates stable energy storage performance up to 105 charge-discharge cycles under 200 MV m−1 and 300 MV m−1. Furthermore, we tested the fast discharge capability of PEI-g-TOC (Fig. 4c). The discharge time τ90 was defined as the time at 90% of the maximum of discharged energy density. τ90 of PEI-g-TOC at 200 °C (9.77 μs) is essentially comparable to that of biaxially oriented polypropylene (BOPP) at 70 °C (8.06 μs), demonstrating its excellent stability at high temperatures. Due to its exceptionally high energy storage density, excellent cyclic charging-discharging stability, and fast discharge capability, PEI-g-TOC has the potential to replace commercial BOPP capacitors operating at 200 °C without the need for cumbersome cooling systems.
a Weibull breakdown strengths of PEI/TOC and PEI-g-TOC at 200 °C (in 5 × 5 lattices). The inset is heatmap of AC breakdown strengths for PEI/TOC and PEI-g-TOC. b Discharged energy density and efficiency of the PEI-g-TOC as a function of cycle numbers at 200 °C. c Fast discharge testing of PEI-g-TOC (200 °C) and BOPP (70 °C) at 200 MV m−1 and 20 kΩ.
Discussion
In this study, self-assembled metal-organic cages were introduced into dielectric polymer nanocomposites, and robust interfaces between organic components and inorganic components were successfully constructed in the composites. In contrast to traditional polymer nanocomposites, the metal-organic cages crosslinked nanocomposites increases εr while avoiding the serious interfacial losses at high temperatures. Benefiting from supramolecular coordination in PEI-g-TOC, this crosslinked nanocomposites not only achieved a uniform distribution of small-sized (about 3 nm) inorganic clusters, but also constructed a more robust organic-inorganic interface. At the same time, this organic-inorganic interface has an excellent charge-trapping ability, which effectively improves Eb. Ultimately, the synergistic enhancement of εr and Eb is achieved in the PEI-g-TOC, exhibiting exceptionally Ud of 7.53 J cm−3 (150 °C) and 4.55 J cm−3 (200 °C) with η of 90%. In addition, the PEI-g-TOC composites exhibit exceptional long-term stability at 200 °C and 300 MV m−1 up to 105 cycles. This innovative approach expands the current research scope for high-temperature polymer-based dielectric materials and provides valuable insights for the development of novel dielectric materials.
Methods
Materials
Tetrabutyl titanate (Ti(OBu)4, 99%), 4-aminobenzoic acid, ethylene glycol, and tetrahydrofuran (THF) were purchased from Sinopharm Chemical Reagent Co., Ltd. BPADA and MPD were purchased from ChinaTech (Tianjin) Chemical Co., Ltd. N,N-dimethylacetamide (DMAc) was obtained from China National Chemicals Corporation Ltd. Titanium oxide nanoparticles (TiO2) with average particle sizes of 5–10 nm was purchased from Macklin. Deionized water was used and all the chemicals were used as received without further purification.
Synthesis of NH2-TOC
A 50 mL vial was charged with Ti(OBu)4 (1.7 mL, 5 mmol), 4-aminobenzoic acid (1.03 g, 7.5 mmol) and 40 ml ethylene glycol to form a clear solution. The mixture was put into electric thermostat at 120 °C for 48 h. After cooling down to room temperature, the solvent was removed by centrifugal separation. The yellow solid was washed by THF for two more times. The yellow powder product was obtained and dried.
Preparation of PEI composites
For the preparation of PEI and PEI-g-TOC, the synthesis process can be divided into pre-polymerization and thermal imidization. First, a certain mass of MPD and NH2-TOC were dissolved in extra dry DMAc, then BPADA was added to the mixture, ensuring a 1:1 ratio of anhydride groups to amine groups (the total number of MPD and NH2-TOC). Then, the polycondensation was carried out at room temperature under nitrogen atmosphere for 24 h. The polyamide acid composite was obtained. The solution was cast on a glass substrate, and heated in an oven by a gradient heating method, sequentially holding at 45 °C for 5 h, 80 °C for 1 h, 120 °C for 1 h, and 220 °C for 10 h. Subsequently, the films are detached from the glass substrate following a soaking period in deionised water, and dried in a vacuum oven at 110 °C for 2 h to evaporate the water. For preparation of PEI/TOC and PEI/TiO2 composites, PEI and a certain mass of NH2-TOC or TiO2 were dissolved in DMAc through vigorous stirring at 60 °C for 4 h. The prepared solution was casted on a glass substrate, and placed in a vacuum oven at 45 °C for 12 h, and then at 220 °C for 4 h in order to remove residual solvent. Finally, the film was removed and dried following the steps of PEI-g-TOC. The typical thickness of the films is ~10 μm.
Characterization
Proton Nuclear Magnetic Resonance (1H NMR) spectra were conducted on a JNM-ECZ500R (500 MHz) instrument, and deuterated dimethyl sulfoxide was chosen to dissolve samples using tetramethylsilane as an internal reference. Dynamic Light Scattering (DLS) results were obtained by NanoBrook Omni. Transmission electron microscopic (TEM) images of composites were acquired by a JEOL JEM2010. Scanning transmission electron microscopy (STEM) images and energy-dispersive X-ray spectroscopy (EDS) were obtained using a JEOL JEM-F200 electron microscope operated. Cross-sectional micromorphology of films was characterized by a scanning electron microscope (ZEISS MWRLIN compact). Fourier transform infrared (FT-IR) spectra were conducted in a Thermo. Nicolt Is50 FT-IR spectrometer over a wavenumber range of 400–4000 cm−1. X-rays diffraction (XRD) patterns were measured by a Rigaku D/max-2550. Differential scanning calorimetry (DSC) curves were obtained using a DSC Q2000 (TA Instrument) with a heating rate of 10 °C min−1 under nitrogen atmosphere. Thermogravimetric analysis (TGA) curves were obtained by a TA Discovery instrument. X-ray photoelectron spectroscopy (XPS) were obtained by a Thermo. 250XI spectroscopy. Young’s moduli were measured by a nano-indenter (Keysight Technologies G200). Atomic force microscopy-based infrared spectroscopy (AFM-IR) measurements were conducted using a NanoIR3 (Bruker, USA). The height and IR absorption images were simultaneously collected in the contact-mode using tips with a spring constant between 0.07 and 0.4 N m−1. The absorption AFM-IR maps were recorded at 1724 cm−1.
Copper electrodes with a diameter of 2.5 mm were sputtered on both sides of the films in order to obtain displacement-electric (D-E) field hysteresis loops and leakage current data. Copper electrodes with a diameter of 11.5 mm were sputtered on both sides of the films for dielectric spectra and thermally stimulated depolarization currents. Dielectric spectra were obtained using a Novocontrol broadband dielectric/impedance spectrometer (Novocontrol Technologies GmbH& Co. KG.), which was equipped with a liquid nitrogen cooling system. The spectra were recorded across a frequency range of 1−106 Hz and a temperature range of 25–200 °C. Thermally stimulated depolarization currents were obtained by the Novocontrol broadband dielectric/impedance spectrometer. The samples were polarized under a DC electric field of 40 MV m−1 at 180 °C for 10 min, and then quickly cooled down to −100 °C at a rate of 10 °C min-1 with the electric field maintained. Following the application of the polarization process, the samples were sort-circuited and heated up to 220 °C at a rate of 3 °C min−1, with the associated currents recorded concurrently. Unipolar D-E loop measurements (at 100 Hz) and charge-discharge cycling tests were performed using a polarization loop & dielectric breakdown test system (PolyK Technologies, LLC). Breakdown strength was recorded using a Trek 20/20B instrument with a voltage ramp of 500 V s−1, with 12 data points obtained and analyzed using the two-parameter Weibull statistic for each sample. In the D-E loops, charge-discharge cycling, and breakdown strength tests, the samples are immersed in temperature-resistant silicone oil in order to control the temperature. Leakage currents were obtained with a multiferroic ferroelectric test system (Radiant Technologies, Inc. Premier II). Fast charge-discharge tests were conducted using a PK-DIS20012 system (PolyK), comprising a 20 kΩ load resistor and an internal resistor of 110 Ω integrated into the circuit.
MD simulations
MD simulation was performed on the PEI, NH2-TOC, PEI-g-TOC, and PEI/TOC at ambient temperature (298 K) by using the software packages of Forcite module (Accelrys, Inc., San Diego, CA) with the Material Studio (Version 2018)65,66. All the amorphous cells were checked for space filling regularly when the initial amorphous cells were constructed at the temperature of 298 K. The molecular structures of PEI, NH2-TOC, PEI-g-TOC and PEI/TOC were optimized for geometry and minimized for energy at the end. The amorphous cells were energy minimized by the smart minimizer method for achieving optimized structures, followed by geometric optimization and MD to bring the structures to a final equilibrium, thus the energy and temperatures of the cells were stabilized.
Data availability
All relevant data in the main text or the supplementary materials are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Yang, M., Ren, W., Guo, M. & Shen, Y. High‐energy‐density and high efficiency polymer dielectrics for high temperature electrostatic energy storage: a review. Small 18, 2205247 (2022).
Zhou, L. et al. Research progress and prospect of polymer dielectrics. Appl. Phys. Rev. 10, 031310 (2023).
Feng, Q.-K. et al. Recent progress and future prospects on all-organic polymer dielectrics for energy storage capacitors. Chem. Rev. 122, 3820–3878 (2022).
Liu, X.-J., Zheng, M.-S., Chen, G., Dang, Z.-M. & Zha, J.-W. High-temperature polyimide dielectric materials for energy storage: theory, design, preparation and properties. Energy Environ. Sci. 15, 56–81 (2022).
Li, H. et al. Dielectric polymers for high-temperature capacitive energy storage. Chem. Soc. Rev. 50, 6369–6400 (2021).
Zha, J.-W., Zheng, M.-S., Fan, B.-H. & Dang, Z.-M. Polymer-based dielectrics with high permittivity for electric energy storage: A review. Nano Energy 89, 106438 (2021).
Dang, Z.-M., Zheng, M.-S. & Zha, J.-W. 1D/2D carbon nanomaterial-polymer dielectric composites with high permittivity for power energy storage applications. Small 12, 1688–1701 (2016).
Li, Q. et al. High-temperature dielectric materials for electrical energy storage. Annu. Rev. Mater. Res. 48, 219–243 (2018).
Yuan, C. et al. Polymer/molecular semiconductor all-organic composites for high-temperature dielectric energy storage. Nat. Commun. 11, 3919 (2020).
Li, H. et al. Flexible all-organic nanocomposite films interlayered with in situ synthesized covalent organic frameworks for electrostatic energy storage. Nano Energy 113, 108544 (2023).
Zhu, L. Exploring strategies for high dielectric constant and low loss polymer dielectrics. J. Phys. Chem. Lett. 5, 3677–3687 (2014).
Zhu, L. & Wang, Q. Novel ferroelectric polymers for high energy density and low loss dielectrics. Macromolecules 45, 2937–2954 (2012).
Tan, D. Q. Review of polymer-based nanodielectric exploration and film scale-up for advanced capacitors. Adv. Funct. Mater. 30, 1808567 (2020).
Zha, J.-W. et al. Polymer dielectrics for high-temperature energy storage: Constructing carrier traps. Prog. Mater. Sci. 140, 101208 (2023).
Chen, J. et al. Ladderphane copolymers for high-temperature capacitive energy storage. Nature 615, 62–66 (2023).
Li, Q. et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 523, 576–579 (2015).
Li, H. et al. Enabling high‐energy‐density high‐efficiency ferroelectric polymer nanocomposites with rationally designed nanofillers. Adv. Funct. Mater. 31, 2006739 (2021).
Wang, P. et al. High‐temperature flexible nanocomposites with ultra‐high energy storage density by nanostructured MgO fillers. Adv. Funct. Mater. 32, 2204155 (2022).
Li, L. et al. Wide-bandgap fluorides/polyimide composites with enhanced energy storage properties at high temperatures. Chem. Eng. J. 435, 135059 (2022).
Ai, D. et al. Tuning nanofillers in in situ prepared polyimide nanocomposites for high‐temperature capacitive energy storage. Adv. Energy Mater. 10, 1903881 (2020).
Sun, B. et al. Excellent stability in polyetherimide/SiO2 nanocomposites with ultrahigh energy density and discharge efficiency at high temperature. Small 18, 2202421 (2022).
Yang, L., Tan, X., Wang, Z. & Zhang, X. Supramolecular polymers: historical development, preparation, characterization, and functions. Chem. Rev. 115, 7196–7239 (2015).
Cameron, J. M. et al. Supramolecular assemblies of organo-functionalised hybrid polyoxometalates: from functional building blocks to hierarchical nanomaterials. Chem. Soc. Rev. 51, 293–328 (2022).
Qin, B. et al. Supramolecular polymer chemistry: from structural control to functional assembly. Prog. Polym. Sci. 100, 101167 (2020).
Li, H. et al. Multilaminate energy storage films from entropy-driven self-assembled supramolecular nanocomposites. Adv. Mater. 36, 2401954 (2024).
Périneau, F. et al. Supramolecular design for polymer/titanium oxo-cluster hybrids: an open door to new organic–inorganic dynamers. Polym. Chem. 2, 2785–2788 (2011).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Fang, X. et al. NH2- functionalized carbon-coated Fe3O4 core–shell nanoparticles for in situ preparation of robust polyimide composite films with high dielectric constant, low dielectric loss, and high breakdown strength. RSC Adv. 6, 107533–107541 (2016).
Köytepe, S., Seçkin, T., Kıvrılcım, N. & Adıgüzel, H. İ. Synthesis and dielectric properties of polyimide-titania hybrid composites. J. Inorg. Organomet. Polym. Mater. 18, 222–228 (2008).
Pei, F. et al. Titanium–oxo cluster reinforced gel polymer electrolyte enabling lithium–sulfur batteries with high gravimetric energy densities. Energy Environ. Sci. 14, 975–985 (2021).
Dai, S. et al. S-scheme enhanced photocatalysis on titanium oxide clusters functionalized with soluble perylene diimides. J. Mater. Chem. A 10, 20248–20253 (2022).
Shao, L. et al. Construction of polymeric metal–organic nanocapsule networks via supramolecular coordination-driven self-assembly. J. Am. Chem. Soc. 142, 7270–7275 (2020).
Gu, Y. et al. Photoswitching topology in polymer networks with metal–organic cages as crosslinks. Nature 560, 65–69 (2018).
Zhukhovitskiy, A. V. et al. Polymer structure dependent hierarchy in PolyMOC gels. Macromolecules 49, 6896–6902 (2016).
Zhukhovitskiy, A. V. et al. Highly branched and loop-rich gels via formation of metal–organic cages linked by polymers. Nat. Chem. 8, 33–41 (2016).
Foster, J. A. et al. Differentially addressable cavities within metal–organic cage-cross-linked polymeric hydrogels. J. Am. Chem. Soc. 137, 9722–9729 (2015).
Oldenhuis, N. J. et al. Photoswitchable sol–gel transitions and catalysis mediated by polymer networks with coumarin-decorated Cu24L24 metal–organic cages as junctions. Angew. Chem. Int. Ed. 59, 2784–2792 (2020).
Zhao, J. et al. Polymer networks with cubic, mixed pd(II) and pt(II) M6L12 metal–organic cage junctions: synthesis and stress relaxation behavior. J. Am. Chem. Soc. 145, 21879–21885 (2023).
Liu, H., Zuo, Z., Guo, Y., Li, Y. & Li, Y. Supramolecular interactions at the inorganic–organic interface in hybrid nanomaterials. Angew. Chem. Int. Ed. 49, 2705–2707 (2010).
Zhao, C. et al. Microporous cyclic titanium-oxo clusters with labile surface ligands. Angew. Chem. Int. Ed. 56, 16252–16256 (2017).
Fang, W.-H., Zhang, L. & Zhang, J. A 3.6 nm Ti52-oxo nanocluster with precise atomic structure. J. Am. Chem. Soc. 138, 7480–7483 (2016).
Zhang, T. et al. A highly scalable dielectric metamaterial with superior capacitor performance over a broad temperature. Sci. Adv. 6, eaax6622 (2020).
Thakur, Y. et al. Enhancement of the dielectric response in polymer nanocomposites with low dielectric constant fillers. Nanoscale 9, 10992–10997 (2017).
Li, S. et al. Polyimide resins with superior thermal stability, dielectric properties, and solubility obtained by introducing trifluoromethyl and diphenylpyridine with different bulk pendant groups. Polymer 283, 126245 (2023).
Chae, B., Hong, D. G., Jung, Y. M., Won, J. C. & Lee, S. W. Investigation of phase separated polyimide blend films containing boron nitride using FTIR imaging. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 195, 1–6 (2018).
Gao, L. et al. Intrinsically elastic polymer ferroelectric by precise slight cross-linking. Science 381, 540–544 (2023).
Su, L., Zhang, B., Huang, Y., Fan, Z. & Zhao, Y. Enhanced cellular uptake of iron oxide nanoparticles modified with 1,2-dimyristoyl- sn -glycero-3-phosphocholine. RSC Adv. 7, 38001–38007 (2017).
Qin, Z. et al. Formation of conducting layers on excimer-laser-irradiated polyimide film surfaces. Surf. Interface Anal. 29, 514–518 (2000).
Chen, X. et al. Interfacial origin of dielectric constant enhancement in high-temperature polymer dilute nanocomposites. Appl. Phys. Lett. 122, 212901 (2023).
Xiao, F., Wang, K. & Zhan, M. Atomic oxygen erosion resistance of titania–polyimide hybrid films derived from titanium tetrabutoxide and polyamic acid. J. Appl. Polym. Sci. 123, 143–151 (2012).
Liu, X. et al. Chemically crosslinked polyimide-POSS hybrid: A dielectric material with improved dimensional stability and dielectric properties. Eur. Polym. J. 173, 111315 (2022).
Chen, X. et al. Ultra-selective molecular-sieving gas separation membranes enabled by multi-covalent-crosslinking of microporous polymer blends. Nat. Commun. 12, 6140 (2021).
Azizi, A. et al. High‐performance polymers sandwiched with chemical vapor deposited hexagonal boron nitrides as scalable high‐temperature dielectric materials. Adv. Mater. 29, 1701864 (2017).
Cheng, S. et al. Polymer dielectrics sandwiched by medium-dielectric-constant nanoscale deposition layers for high-temperature capacitive energy storage. Energy Storage Mater. 42, 445–453 (2021).
Yan, C. et al. Improved capacitive energy storage at high temperature via constructing physical cross-link and electron–hole pairs based on p-type semiconductive polymer filler. Adv. Funct. Mater. 34, 2312238 (2024).
Zhang, B. et al. Superior high-temperature energy density in molecular semiconductor/polymer all-organic composites. Adv. Funct. Mater. 33, 2210050 (2023).
Zhou, Y., Zhu, Y., Xu, W. & Wang, Q. Molecular trap engineering enables superior high-temperature capacitive energy storage performance in all-organic composite at 200 °C. Adv. Energy Mater. 13, 2203961 (2023).
Yang, M., Zhou, L., Li, X., Ren, W. & Shen, Y. Polyimides physically crosslinked by aromatic molecules exhibit ultrahigh energy density at 200 °C. Adv. Mater. 35, 2302392 (2023).
Wang, F. et al. Improved capacitive energy storage nanocomposites at high temperature utilizing ultralow loading of bimetallic mof. Small 19, 2300510 (2023).
Yang, M. et al. Sub-nanowires boost superior capacitive energy storage performance of polymer composites at high temperatures. Adv. Funct. Mater. 33, 2214100 (2023).
Yang, M. et al. Roll-to-roll fabricated polymer composites filled with subnanosheets exhibiting high energy density and cyclic stability at 200°C. Nat. Energy 9, 143–153 (2024).
Yang, M. et al. Unifying and suppressing conduction losses of polymer dielectrics for superior high‐temperature capacitive energy storage. Adv. Mater. 36, 2309640 (2024).
Liu, X. et al. Atomic-level matching metal-ion organic hybrid interface to enhance energy storage of polymer-based composite dielectrics. Adv. Mater. 36, 2402239 (2024).
Ju, T. et al. Enhancing breakdown strength and lifetime of multilayer dielectric films by using high temperature polycarbonate skin layers. Energy Storage Mater. 45, 494–503 (2022).
Zhou, T. et al. Concurrently enhanced mechanical properties and capacitive performance in all-organic dielectric polymer blend via phase separation. J. Appl. Phys. 131, 124101 (2022).
Yin, J.-F. et al. Polymer topology reinforced synergistic interactions among nanoscale molecular clusters for impact resistance with facile processability and recoverability. Angew. Chem. Int. Ed. 60, 22212–22218 (2021).
Acknowledgements
This work was supported by the Basic Science Centre Program of Natural Science Foundation of China (Grant No. 52388201 (C.-W.N.)), the National Key Research & Development Program of China (Grant No. 2022YFB3803500 (Y.S.)), and the Natural Science Foundation of China (Grant No. 52027817 (Y.S.)).
Author information
Authors and Affiliations
Contributions
S.Z., W.P., Y.S., and C.-W.N. conceived the idea. S.Z. performed the majority of the experiments. W.P. and S.D. synthesized and characterized the NH2-TOC. L.Z. performed the molecular dynamics simulations. W.R. assisted in the sample preparation and performance tests. E.X. performed the AFM-IR measurements. Y.X. and M.Z. prepared the TEM samples. M.H., Y.S., and C.-W.N. revised the manuscript. All authors discussed the results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiaofan Ji, Junghwan Kim and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, S., Peng, W., Zhou, L. et al. Metal-organic cage crosslinked nanocomposites with enhanced high-temperature capacitive energy storage performance. Nat Commun 16, 769 (2025). https://doi.org/10.1038/s41467-025-56069-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56069-5