Abstract
Accidental ingestion of lead (Pb)-contaminated soils represents a major route of Pb exposure for both adults and children, and the development of accessible and cost-effective solutions to reduce Pb poisoning is urgently required. Here, we present an effective and straightforward technique, involving the consumption of cola beverages, for the purpose of lowering blood Pb levels following the ingestion of contaminated soils in animal models. This method facilitated the direct passage of Pb in contaminated soil through the digestive system, enhancing its elimination without absorption into systemic circulation. Our results demonstrated that cola effectively reduced Pb bioaccessibility in 22 contaminated soils by 32.6%–98.8%. In male rats and swine exposed to Pb-contaminated soils, cola treatment decreased blood Pb concentrations by 32.9%–96.0% and 31.5%–81.5%, respectively. This cola-induced reduction in Pb bioaccessibility and bioavailability was attributed to the rich phosphoric acid content in cola, which promoted the formation of insoluble Pb phosphate precipitate (pyromorphite [Pb5(PO4)3Cl]) during the gastric phase. The precipitate was directly excreted in feces, resulting in lower Pb absorption in the blood. These findings suggest that the consumption of cola beverages may be a practical strategy to mitigate the risk of Pb poisoning following the accidental ingestion of contaminated soils. However, the applicability of this approach in humans remains uncertain in the absence of population-based studies. While these findings underscore the potential for cola beverages to reduce Pb absorption following soil ingestion in animal models, further research is necessary to evaluate its safety, efficacy, and possible risks in humans before any such protocols are initiated.
Similar content being viewed by others
Introduction
Lead (Pb) is a potent and pervasive environmental contaminant and neurotoxicant with profound and enduring effects on human health. Children are especially vulnerable to Pb poisoning due to their frequent hand-to-mouth behaviors, higher ingestion rates, and developing neural systems1. According to the United Nations Children’s Fund (2020), Pb poisoning affects one in three children worldwide, amounting to approximately 800 million cases and 900,000 premature deaths per year2. Exposure to Pb also poses a substantial health risk to adults, contributing to an estimated 400,000 deaths annually in the USA, with blood concentrations as low as 5 µg/dL still representing a significant risk factor for mortality, most notably from cardiovascular diseases3. Globally, Pb exposure is estimated to have resulted in 5,545,000 cardiovascular-related deaths in 2019 alone4.
Lead-contaminated soil represents a primary source of Pb exposure for both adults and children, especially after the global phase out of leaded paints and gasoline5,6. Studies have demonstrated strong correlations between blood Pb concentrations and the presence of Pb in contaminated soils7,8, with reductions in surface soil Pb likely contributing to sustained declines in blood Pb concentrations9. Despite ongoing efforts to mitigate Pb contamination, accidental ingestion of contaminated soil particles remains a persistent threat to human health10,11. This risk is particularly pronounced for individuals living near industrial sites, (in)active or remediating smelters, mining operations, lead-acid battery plants, e-waste processing factories, and superfund sites12.
Chelation therapy is widely employed to manage elevated blood Pb levels (BLLs, ≥ 45 μg/dL in general) and mitigate the systemic effects of Pb toxicity13,14. However, according to the US Centers for Disease Control and Prevention, the threshold of 45 μg/dL serves as a guideline rather than a strict criterion, and decisions to initiate chelation are guided by individual clinical assessments15. Chelation relies on the use of chelating agents that bind to Pb in the bloodstream to form chelates, facilitating their excretion14. However, this approach requires Pb to enter systemic circulation before initiation16, and may take up to 60 days to lower BLLs17. Moreover, access to chelation therapy is often limited in low- and middle-income countries (LMICs)13, where populations can exhibit higher mean blood Pb levels (~4.6 μg/dL) compared to those in more affluent nations (~1.3 μg/dL)4,18. These disparities contribute disproportionately to global health impacts, with 95.3% of IQ losses and 90.2% of cardiovascular deaths linked to Pb exposure occurring in LMICs4. Consequently, a cost-effective and readily available solution to reduce Pb poisoning is urgently required.
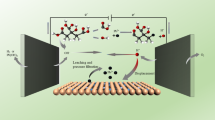
One promising avenue involves preventing Pb absorption into the bloodstream by enabling its direct excretion via the digestive system. Pyromorphite (Pb5(PO4)3Cl), one of the most stable forms of Pb in the environment, is characterized by its insolubility and low bioavailability19. Its rapid formation kinetics and thermodynamic stability allow for the direct conversion of reactive Pb in the presence of phosphorous (P), thereby reducing Pb absorption into the bloodstream20,21. Previous studies have demonstrated that phosphoric acid-containing soft drinks, such as cola, can facilitate the formation of pyromorphite from Pb compounds in a simulated gastrointestinal tract16. Given the wide availability and affordability of cola beverages, this mechanism may serve as a cost-effective method for reducing blood Pb levels in humans. However, direct evidence supporting this hypothesis is currently lacking.
In this study, we investigated the efficacy and underlying mechanisms of cola beverages in reducing the bioaccessibility and bioavailability of Pb from contaminated soils. Based on both in-vitro extraction tests and in-vivo animal models, our results demonstrated that cola consumption may provide a feasible strategy to reduce the risk of Pb poisoning following the ingestion of contaminated soils. Notably, this approach may have relevance for protecting individuals exposed to Pb-contaminated soil particles, especially in resource-limited settings where chelation therapy may not be readily accessible. However, while these findings highlight the potential utility of cola beverages, further investigation is essential to confirm their safety and effectiveness in human populations. Until such evidence is available, caution is warranted when considering cola for this purpose, given the potential for health-related risks.
Results
Cola beverages effectively reduced Pb-bioaccessibility in contaminated soils via physiologically based extraction test (PBET)
To assess the potential applicability of cola as a method to mitigate Pb poisoning from various contaminated soil particles, we initially determined the Pb bioaccessibility in 22 contaminated soils via an in-vitro physiologically based extraction test (PBET). As a frequently employed in-vitro method for evaluating Pb bioaccessibility, PBET is strongly correlated with in-vivo animal bioavailability22 and can simulate the human gastrointestinal tract, including both gastric and intestinal phases. As Pb dissolution in the simulated stomach phase has been shown to be predictive of Pb bioavailability in animal models23, we focused solely on the gastric phase.
Our results revealed significant variations in Pb bioaccessibility across the different soil samples, ranging from 13.7% to 92.3% (Table S1 and Fig. 1a). Notably, however, the addition of cola consistently reduced the bioaccessibility of Pb in all tested soil samples, and the reduction rate of Pb bioaccessibility achieved with cola exposure ranged from 32.6% to 98.8% (Table S1 and Fig. 1a). As cola exhibited efficacy across all 22 tested contaminated soils, the underlying mechanism for its reduction of Pb bioaccessibility is likely not significantly influenced by soil source. Thus, we selected three soil samples (Soils 3, 8, and 20) from shooting range sites with high Pb concentrations for X-ray diffraction (XRD) analysis. As shown in Fig. 1b–d, the predominant form of Pb in the three soils was PbCO3, which was converted into a Pb-phosphate precipitate following PBET extraction in the presence of cola. Cola contains a substantial amount of PO43−-P (~ 152 mg/L)24, which rapidly reacts with Pb to form a stable Pb-phosphate16. Previous work has proved that PO43- can react with heavy metals (Pb, Cu, etc.) to form less soluble phosphate minerals21. The newly formed Pb-phosphate precipitate exhibited characteristic peaks similar to those of pyromorphite [Pb5(PO4)3Cl] (Fig. 1b–d), indicating the formation of pyromorphite during extraction based on the following equations:
Cola beverage greatly reduced Pb bioaccessibility in in-vitro gastric ingestion system (PBET extract) of 22 Pb-contaminated soils (a); XRD patterns of Pb-contaminated Soil 3 (b), Soil 8 (c), and Soil 20 (d) in the presence of PBET extract and cola beverage, showing formation of extremely stable pyromorphite [Pb5(PO4)3Cl], compared to soil itself and 3% pyromorphite in quartz sand standard. Three independent samples from each experiment were performed, with the results from one of these samples shown in the figures (b–d). Source data are provided as a Source Data file.
Pyromorphite is recognized as the least soluble and most stable form of Pb in the soil environment19, with a solubility at least 44 orders of magnitude lower than that of commonly found Pb minerals in contaminated soils, such as galena (PbS), anglesite (PbSO4), cerussite (PbCO3), and litharge (PbO)25,26. Consequently, the transformation of other Pb species (e.g., PbCO3) to pyromorphite has been considered as an effective strategy for the remediation of Pb-contaminated soils via Pb immobilization27,28. To further verify the crucial role of P in cola beverages for the reduction of Pb bioaccessibility, a P-free beverage (i.e., sprite) was included as a negative control. Results indicated that cola exhibited much better effects than sprite in reducing the bioaccessibility of Pb in tested samples (PbCO3 and Soil 22), with no new precipitates formed after sprite treatment (Fig. S1 in the Supporting Information). Therefore, it can be speculated that the formation of pyromorphite with the addition of cola in the gastric system may minimize Pb absorption into the bloodstream, thereby reducing Pb poisoning and exposure in humans.
Cola beverages effectively reduced Pb-bioavailability in contaminated soils in both rat and swine models
After establishing the effectiveness of cola in reducing Pb bioaccessibility in contaminated soils through PBET, we further examined its efficacy in reducing Pb bioavailability in selected soils with different lead concentration gradients using both rat and swine models. Rats, as the most commonly used model animals, are widely applied in clinical research29. The similarity of swine to humans in anatomical size and structure, physiology, immunology, and genome enhances their potential as models for humans30,31. The Pb levels administered in this study were below levels that cause clinical symptoms in rats and swine, and no signs of treatment-induced toxicity were observed. All animals exposed to Pb via the oral route were in good health throughout the study.
Figure 2 shows the changes in Pb concentrations in the blood of rats administered PbCO3, Soil 1 (11, 600 ± 844 mg kg−1 Pb), Soil 3 (22, 030 ± 817 mg kg−1 Pb), or Soil 22 (39,441 ± 1062 mg kg−1 Pb), followed by cola consumption 15 min later. The baseline BLLs of rats in this study were 0.004 ± 0.002 µg/dL. All treated rats experienced a rapid increase in BLLs within 4 h of treatment, which subsequently decreased over time (Fig. 2). The maximum BLLs in rats exposed to PbCO3, Soil 1, Soil 3, and Soil 22 were 31.7 ± 0.80, 17.5 ± 0.24, 28.2 ± 1.42, and 38.9 ± 0.85 µg/dL, which significantly decreased to 3.53 ± 0.10, 1.61 ± 0.07, 3.12 ± 0.20, and 3.01 ± 0.24 µg/dL, respectively, after cola treatment. Cola administration reduced BLLs in rats by 32.9%–92.6%, 87.2%–96.0%, 39.0%–88.9%, and 68.0%–92.3% for PbCO3, Soil 1, Soil 3, and Soil 22, respectively. As a negative control, Sprite showed a very limited effect on reducing the BLLs of rats exposed to any of the soils or compounds tested since the curves of BLLs almost overlapped with those of the blank control. The rat model demonstrated that cola beverages could effectively reduce the BLLs. In addition, the effect of cola dose on the reduction of BLLs in rats exposed to PbCO3 and Soil 22 was further investigated (Fig. S2 in the Supporting Information). The BLLs of Pb-exposed rats showed a gradient decrease after treatment with increasing levels of cola from 0.1 to 4 mL. For rats exposed to PbCO3, the maximum BLLs decreased from 31.7 ± 0.80 µg/dL in blank control to 30.7 ± 1.41 (0.1 mL), 25.6 ± 0.47 (0.5 mL), 17.7 ± 0.48 (1.5 mL), 8.72 ± 0.42 (2.5 mL), 4.28 ± 0.15(3 mL), and 3.53 ± 0.10 (4 mL) µg/L, respectively; While it decreased to 94.1%, 78.7%, 47.0%, 22.3%, 13.2%, and 7.74% of the blank control, respectively, for rats exposed to Soil 22. The BLL reduction in rats exposed to PbCO3 or Soil 22 increased linearly (R2 = 0.95, Fig. S2) with cola dose. These results suggest that higher cola consumption is more favorable for reducing BLL in rats.
The changes in blood Pb concentrations in rats with different treatments (a–d), following the administration of PbCO3, Soil 1 (11,600 ± 844 mg kg−1 Pb), Soil 3 (22,030 ± 817 mg kg−1 Pb), or Soil 22 (39,441 ± 1062 mg kg−1 Pb), showing great reduction (p < 0.001 after 1 h, Table S3) in Pb levels in rat blood with cola beverage treatment, compared to the control and sprite treatment. The concentration of Pb in the blood collected from 9-11-week-old Sprague-Dawley rats (n = 4 per group), mean ± S.D, one-way ANOVA (p-value is shown in Table S3) with Bonferroni test. Source data are provided as a Source Data file.
The swine model also proved the effectiveness of cola beverages in reducing the blood lead levels. Figure 3 shows the changes in Pb concentrations in the blood and urine of swine administered Soil 1 (11,600 ± 844 mg kg−1 Pb) or Soil 3 (22,030 ± 817 mg kg−1 Pb), followed by cola consumption 30 min later. The baseline BLLs and urine Pb-levels were 0.35 ± 0.16 µg/dL and 0.14 ± 0.18 µg/dL, respectively. Like the rats, the treated swine exhibited a rapid increase in blood Pb concentrations at first (within 5 h) and then decreased over time (Fig. 3). Urine Pb levels showed a similar trend, with the highest concentration appearing after 24 h. The administration of cola yielded significant reductions in both blood and urine Pb levels in swine. Notably, in swine exposed to Soil 1 (11, 600 ± 844 mg kg−1 Pb), blood and urine Pb levels decreased by 38.1%–81.5% and 16.0%–59.1%, respectively, while exposure to Soil 3 (22, 030 ± 817 mg kg−1 Pb) led to reductions of 31.5%–33.2% and 44.8%–60.4%, respectively. Relative to sprite, cola demonstrated significantly enhanced efficacy in reducing the BLLs in swine following administration of Pb-contaminated soils or compound (PbCO3), especially within the first 24 h (Fig. S3 in the Supporting Information). The decrease in Pb concentrations in the presence of cola was likely due to the formation of insoluble Pb-phosphate complexes, rendering the Pb in the stomach less soluble and facilitating its excretion in feces. This speculation was supported by scanning electron microscope (SEM)-elemental mapping of fecal samples collected from swine exposed to Soil 3 and cola, which revealed concrete particles associated with P and Pb (Fig. 4), suggesting the precipitation of Pb and P in the gastrointestinal tract, followed by excretion. Based on the in-vitro experiments, these Pb-phosphate precipitates were most likely to be pyromorphite. Thus, our findings suggest that cola may reduce the absorption of Pb into the bloodstream by promoting the formation of Pb-P precipitates in the digestive system, which are subsequently excreted from the body in feces.
Effects of cola beverage on concentrations of Pb in blood (above) and urine (below) samples of swine (a–d) following ingestion of Pb-contaminated Soil 1 (11,600 ± 844 mg kg−1 Pb) and Soil 3 (22,030 ± 817 mg kg−1 Pb), showing significant reduction in Pb levels in swine blood and urine with cola beverage treatment. Data were represented as mean ± S.D. The concentration of Pb in the blood collected from 8−10-week-old Danish Landrace × Yorkshire × Duroc cross-breed swine (n = 4 per group), mean ± S.D, one-way ANOVA (p-value is shown in Table S4) with Bonferroni test. *P < 0.05, **P < 0.01. Source data are provided as a Source Data file.
Mechanisms underlying effective reduction of Pb bioaccessibility and bioavailability by cola beverages
Cola administration demonstrated effectiveness in reducing Pb bioavailability in the animal model and Pb bioaccessibility in PBET extraction, primarily attributed to the reduction in Pb solubility. To further explore the associated mechanism, we conducted pure chemical solution experiments. Results showed that cola effectively reduced soluble Pb in Pb(NO3)2 solution in both the absence and presence of PBET extract. In the absence of PBET extract, cola reduced the concentration of Pb from 80 to 0.31 mg L−1, indicating a remarkable 99.6% reduction in soluble Pb, while in the presence of PBET extract, cola reduced the concentration of Pb by 33.9% to 52.9 mg/L (Fig. S4 in the Supporting Information). X-ray diffraction (XRD) analysis confirmed the formation of pyromorphite in both solutions (Fig. 5a, b). Although the presence of pepsin and organic acid in the PBET extract may competitively complex with Pb2+ to inhibit the formation of insoluble Pb-phosphate precipitates32, thereby lowering the removal of soluble Pb by cola, pyromorphite was still formed in this system, albeit with a more amorphous appearance (Fig. 5b). The atomic force microscope (AFM) images of the mixture solution containing cola and PBET extract in the presence of Pb (Fig. 5d) showed higher average heights than those in the absence of Pb (Fig. 5c), further confirming the formation of Pb precipitates, even in the presence of simulated stomach solution. The TEM images of the precipitates showed rod-shaped pyromorphite crystals ranging in size from tens to hundreds of nanometers (Fig. 5e, f). Collectively, these results suggest that cola beverages can reduce Pb bioavailability by promoting the formation of pyromorphite.
XRD patterns of pyromorphite precipitate after reaction of Pb with cola beverage in absence (a) and presence (b) of in-vitro gastric ingestion system (PBET extract), AFM images of mixed solution of cola beverage and PBET extract in absence (c) and presence (d) of Pb, and TEM images of pyromorphite precipitate after reaction of Pb with cola beverage. ×30,000 (e) and ×50,000 (f). Three independent samples from each experiment were performed, with the results from one of these samples shown in the figures. Source data are provided as a Source Data file.
Discussion
This study provides robust evidence from both in-vitro chemical tests and in-vivo animal assays that cola beverages facilitate the conversion of ingested soil Pb into insoluble pyromorphite in the digestive system, effectively restricting Pb absorption into the systemic circulation and promoting Pb excretion via feces. These findings highlight the potential of cola consumption as an emergency measure or a practical strategy to mitigate Pb poisoning risks associated with accidental ingestion of contaminated soil particles or agents.
While population-level Pb exposure has declined substantially over the past 40 years33, the residual burden is disproportionately concentrated in vulnerable communities, including neighborhoods characterized by older housing, lower socioeconomic status, higher population densities, and greater proportions of minority, immigrant, and refugee residents4,33,34. As such, populations in LMICs generally face higher risks of Pb poisoning compared to those in more affluent nations due to limited access to chelation therapy and ongoing exposure to Pb-contaminated environments. Given the global availability, affordability, and minimal adverse effects of cola drinks compared to those of Pb poisoning, its use may offer an accessible emergency measure for reducing Pb levels in high-risk populations. Furthermore, even after Pb has entered the blood, cola consumption could still reduce the BLLs, which was observed in the rat model (Fig. S5 in the Supporting Information). These results indicated that cola may hold potential as a preventive strategy for occupationally exposed individuals, such as workers in battery manufacturing, painting industries, or shooting ranges. However, human studies are required to validate its safety and efficacy before any recommendations can be made. The potential applicability of cola extends beyond human exposure. In agricultural settings, cola consumption could theoretically modify Pb absorption and distribution in livestock, such as cattle, horses, goats, and chickens, grazing on Pb-contaminated soils. This approach could provide a cost-effective means for mitigating Pb exposure in animals, with implications for food safety and human health.
Despite its promise, this study has several limitations. First, although the mechanism of reducing blood Pb via precipitate formation has been demonstrated in animals, its applicability to humans remains speculative. Epidemiological studies are required to establish a direct relationship between cola consumption and reduced blood Pb levels in human populations. Second, while a dose-dependent relationship between cola consumption and blood Pb reduction in rats was observed, the optimal quantity and consumption method for maximum efficacy remain undefined. Further studies are needed to clarify these parameters. Third, excessive cola consumption carries potential health risks, such as obesity, tooth decay, and gout, which must be weighed against its potential benefits in Pb poisoning scenarios. Fourth, while this study focused on Pb exposure from contaminated soils, dietary sources of Pb, such as cereals and fruit juices, represent a significant exposure pathway in daily life35,36. Whether cola consumption is effective in mitigating Pb absorption from these sources warrants further investigation.
Finally, it is important to emphasize that cola consumption is not a currently recognized therapy for Pb poisoning. In the absence of human population studies to establish its safety and efficacy, the use of cola for this purpose is not recommended based solely on this study, with potential health risks remaining unknown. These findings underscore the need for further research to evaluate the potential of cola beverages as a cost-effective intervention for Pb poisoning while addressing the broader context of environmental and dietary Pb exposure.
Methods
Contaminated soils and cola beverages
Twenty-two Pb-contaminated soils were used in our experiments, and their Pb concentrations were listed in Table S1. Twenty-one samples (Soils 1–21) were collected from eight sites in the USA, including: one sample (Soil 1) from a battery recycling site in Florida; one sample (Soil 2) from a sandblasting site in Georgia; and 19 samples (Soils 3–21) from six shooting ranges in Florida, Connecticut, and New Jersey. One Pb-contaminated soil (Soil 22) was collected from a shooting range site in Fujian, China. Soil samples were air-dried, homogenized, and sieved through nylon mesh (< 250 μm) to obtain the soil fraction likely to be ingested by humans due to these fine particles easily adsorbed on the food, hands, or furniture37. The concentrations of Pb in the 22 soils varied considerably, ranging from 2400 mg kg−1 to 235376 mg kg−1 (Table S1). Classic Coca Cola and Sprite (Table S2) were purchased from a local grocery store, cola is a phosphoric acid-containing soft drink, while sprite is a P-free one.
Effects of cola on Pb-bioaccessibility in contaminated soils based on physiologically based extraction test (PBET)
To evaluate Pb bioaccessibility in contaminated soils, we employed the PBET method22. The PBET extract was prepared by dissolving 1.25 g of pepsin and four common organic acids (0.50 g of citric acid, 0.50 g of DL-malic acid, 420 μL of lactic acid, and 500 μL of acetic acid) into 1.0 L of Millipore water, with fluid pH adjusted to 2.00 ± 0.05 with concentrated HCl22.
The Pb-contaminated soil samples (0.8 g) were weighed into a 100-mL centrifuge tube containing 80 mL of PBET extract (1:100 ratio). To investigate the effects of cola on Pb bioaccessibility, 40 mL of PBET extract was replaced with 40 mL of cola, retaining a total volume of 80 mL. All extractions were conducted with three replicates using a thermostatic shaker at 100 rpm at 37 °C for 1 h. After extraction, the solutions in the tubes were filtered through 0.45-μm membrane filters. The filtrates were acidified with concentrated nitric acid and analyzed using inductively coupled plasma-mass spectrometry (ICP-MS, Agilent 7500a, USA) for Pb concentrations. The solid residues on the filters were washed five times with Milli-Q water to remove soluble solutes and freeze-dried for spectroscopic analysis. For comparison, the effect of sprite on Pb bioaccessibility of Pb compound (PbCO3) or Pb-contaminated soil (Soil 22) was also investigated, and the experimental procedures were similar to those for cola.
Effects of cola on Pb-bioavailability in contaminated soils based on rat and swine models
To determine the effects of cola on Pb-bioavailability in contaminated soils, we used both rat and swine models. These studies were approved by the Animal Ethics Committee of Shanghai Jiao Tong University (Permit Number: A2024252) and South China Agricultural University (Permit Number: 2021F070). All tested animals were individually housed in metabolic cages under controlled environmental conditions at 25 °C.
For the rat model, 60 male Sprague-Dawley rats (9–11 weeks old, 250–270 g) obtained from the Experimental Animal Center of Shanghai Jiao Tong University (Shanghai) were randomly assigned to fifteen groups (four rats per group). After a 3-day acclimation period, rats were fasted for 12 h, and baseline blood samples (0.2 mL, from the orbital venous plexus) were collected. Each group was then orally gavaged with either a dose of 6 mg of Pb compound (Groups 4–6) or 0.2 g of lead-contaminated soil (Groups 7–18) dissolved in 2 mL of Milli-Q water. Specifically, Groups 1–3 served as controls and received 2 mL of Milli-Q water by gavage, while Groups 4–6 were exposed to PbCO3, Groups 7–9 to Soil 1, Groups 10–12 to Soil 3, and Groups 13–15 to Soil 22. Fifteen minutes after exposure, Groups 1, 4, 7, 10, and 13 were given 4 mL of Milli-Q water; Groups 2, 5, 8, 11, and 14 were given 4 mL of cola; and Groups 3, 6, 9, 12, and 15 were given 4 mL of Sprite. The rats resumed their normal diet after treatments. Blood samples were collected at 0.5, 1, 2, 4, 8, 12, and 24 hours after the initial gavage (time point 0) to assess both early and late effects of cola on blood lead levels.
To further determine the effect of the amount of cola on the reduction of blood lead levels in rats, an additional 40 male rats were randomly assigned to ten groups (four rats per group). Groups 1–5 were exposed to PbCO3, while Groups 6–10 were exposed to soil 22. After 15 min, Group 1 and 6 received 0.1 mL cola mixed with 3.9 mL Milli-Q water; Group 2 and 7 received 0.5 mL cola mixed with 3.5 mL Milli-Q water; Group 3 and 8 received 1.5 mL of cola mixed with 2.5 mL of Milli-Q water; Group 4 and 9 received 2.5 mL of cola mixed with 1.5 mL of Milli-Q water; and Group 5 and 10 received 3 mL of cola mixed with 1 mL of Milli-Q water. The total volume of liquid given to the rats was kept at 4 mL. Blood samples were collected at selected intervals within the 24 h.
For the Swine model, 32 Danish Landrace × Yorkshire × Duroc cross-breed male swine (8–10 weeks old, 20–25 kg) obtained from South China Agricultural University (Guangdong, China) were randomly assigned to ten groups (four animals per group). The swine were fed meals at a rate of 100 g kg−1 body weight, consisting of mash containing 45% dry matter three times daily, with water available throughout. Following a 7-day pre-feeding period, the swine were fasted for 12 h, after which blood (5–10 mL, from jugular vein), urine, and fecal samples were collected as a baseline. Each group was subsequently dosed by oral gavage via a gastro-duodenal feeding tube (Servan, Shanghai, China), containing either 1–2 g of Pb compounds (Groups 1–2) or 5–10 g of Pb-contaminated soils (Groups 3–8) dissolved in 150 mL of Milli-Q water. Groups 1 and 2 were exposed to PbCO3, Groups 3 and 4 were exposed to Soil 1, Groups 5 and 6 were exposed to Soil 3, and Groups 7 and 8 were exposed to Soil 22. These selected dosages could substantially increase blood Pb concentrations but without showing obvious treatment-induced toxicity. At 0.5 and 1.5 h after treatment, the swine were administered 250 mL of Milli-Q water (Groups 3, and 5) or 250 mL of cola beverage (Groups 2, 4, 6, 8) or 250 mL of Sprite (Groups 1 and 7) by oral gavage. At 2 and 6 h after administration, blood, urine, and fecal samples were collected. The swine were then fed normal food, with blood, urine, and fecal samples collected at 24, 48, and 72 h. A schematic representation of the experimental design is shown in Fig. S6.
Urine was placed in plastic bottles containing nitric acid and stored at −18 °C until analysis. Blood (1 mL) was mixed with 9.0 mL of a matrix modifier consisting of 0.2% v/v nitric acid, 0.5% v/v Triton X-100, and 0.2% w/v ammonium phosphate in deionized distilled water before analysis. Feces were weighed, homogenized, dried at 65 °C, ground, and stored for spectroscopic analysis. All solution samples were filtered through 0.45-mm membrane filters before ICP-MS analysis. Blanks, spikes, and duplicate analyzes were conducted every 10 samples for quality assurance/control.
At the end of the experiments, animals used in this study were euthanized. The rats were placed in a sealed container and an excess of carbon dioxide was administered to induce asphyxia. After the rats stopped breathing, the carbon dioxide supply was cut off and the cervical dislocation was performed to ensure a painless death. For the swine, they were administered with an overdose of sodium pentobarbital (> 150 mg/kg) by intravenous injection. After the irreversible death was achieved, exsanguination was further performed to ensure a painless death.
Effects of cola on Pb solubility and formation of Pb-P precipitates
To test the effects of cola in reducing Pb solubility, two treatments were included: i.e., 2 mL of 2000 mg L−1 Pb solution prepared from Pb acetate was added to either 48 mL of cola or 43 mL of cola with 5 mL of PBET extract in the centrifuge tube, resulting in 80 mg L−1 Pb. The P concentrations were 146 and 131 mg L−1 in the first and second treatments, respectively, which were more than required to form Pb-P precipitates. After 1 h of reaction, the mixture was filtered through 0.45-μm nitrocellulose membrane filters. The filtrates were acidified with concentrated nitric acid and analyzed by ICP-MS for Pb concentration levels. The solid residues on the filters were washed with Millipore water to remove soluble solutes, then freeze-dried and stored for mineral characterization. All experiments were conducted with three replicates.
Solid characterization
The solid residues on the filters were identified using X-ray diffraction (XRD). The samples were vacuum-dried and manually pulverized to pass a No. 400 US standard sieve. Step-scanned XRD data were collected by a Bruker D8 Advance X-Ray Diffractometer equipped with Cu Kα radiation conducted at 40 kV and 30 mA. The data were collected at 2θ 10° and 50°. The XRD patterns were analyzed using Jade software (v6.0). A scanning electron microscope (SEM, Sirion 200, FEI, USA) coupled with an energy dispersive spectrometer (EDS, INCA X-Act, Oxford, UK) was used to determine the morphology and chemical composition of the fecal samples. The reaction product of the Pb solution with cola was analyzed using an atomic force microscope (AFM) and a high-resolution transmission electron microscopy (HR-TEM, FEI Talos 200X, Thermo Fisher, USA). The AFM images were collected using Si3N4 tips (Bruker, tip model NP-S20) with spring constants of 0.12 N/m and 0.58 N/m and were analyzed using NanoScope (v5.31r1) and WSxM v5.0 Develop v6.4.
Statistics analysis
All data analyzes were conducted using SPSS v24.0 (SPSS, Chicago, USA). Values are expressed as mean ± S.D. Significant differences in Pb concentrations between the control and treatment groups were assessed using one-way analysis of variance (ANOVA) followed by Bonferroni test. A p-value above 0.05 was considered not significantly different. *p < 0.05, **p < 0.01.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All source data supporting the findings of this study are available within the paper and the Supplementary Information files or available from the corresponding author upon request. Source data are provided in this paper.
References
Champion, W. M., Khaliq, M. & Mihelcic, J. R. Advancing knowledge to reduce lead exposure of children in data-poor low- and middle-income countries. Environ. Sci. Technol. Lett. 9, 879–888 (2022).
PureEarth, The toxic truth. https://www.unicef.org/media/73246/file/The-toxic-truth-children%E2%80%99s-exposure-to-lead-pollution-2020.pdf (2022).
Lanphear, B. P., Rauch, S., Auinger, P., Allen, R. W. & Hornung, R. W. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health 3, 177–184 (2018).
Larsen, B. & Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: a health impact and economic modelling analysis. Lancet Planet. Health 7, 831–840 (2023).
Karna, R. R. et al. Bioavailable soil Pb minimized by in situ transformation to plumbojarosite. Proc. Natl. Acad. Sci. USA. 118, e2020315117 (2021).
Zartarian, J., Xue, R., Tornero-Velez, J. & Brown Children’s lead exposure: a multimedia modeling analysis to guide public health decision-making. Environ. Health Perspect. 125, 097009 (2017).
Lanphear, B. P. et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels: a pooled analysis of 12 epidemiologic studies. Environ. Res. 79, 51–68 (1998).
Dixon, S. L. et al. Exposure of U.S. children to residential dust lead, 1999−2004: II. the contribution of lead contaminated dust to children’s blood lead levels. Environ. Health Perspect. 117, 468–474 (2009).
Mielke, H. W. et al. The concurrent decline of soil lead and children’s blood lead in New Orleans. Proc. Natl Acad. Sci. USA 116, 22058–22064 (2019).
Longman, J., Veres, D., Finsinger, W. & Ersek, V. Exceptionally high levels of lead pollution in the Balkans from the Early bronze age to the industrial revolution. Proc. Natl Acad. Sci. USA 115, 5661–5668 (2018).
Mielke, H. W., Gonzales, C. R., Powell, E. T. & Egendorf, S. P. Lead in air, soil, and blood: Pb poisoning in a changing world. Int. J. Environ. Res. Public Health (2022).
Neuwirth, L. & Whigham, K. “Only time will tell”: the underexplored impacts of lead poisoning and COVID-19 on pre-existing ACEs in New York. Youth 3, 1212–1224 (2023).
Meyer, P. A., Brown, M. J. & Falk, H. Global approach to reducing lead exposure and poisoning. Mutat. Res. 659, 166–175 (2008).
Sinicropi, M. S., Amantea, D., Caruso, A. & Saturnino, C. Chemical and biological properties of toxic metals and use of chelating agents for the pharmacological treatment of metal poisoning. Arch. Toxicol. 84, 501–520 (2010).
Tarragó, O. & Brown, M. J. Lead toxicity. US Centers for Disease Control and Prevention (CDC) https://stacks.cdc.gov/view/cdc/61565/cdc_61565_DS1.pdf (2017).
Scheckel, K. G. & Ryan, J. A. In vitro formation of pyromorphite via reaction of Pb sources with soft-drink phosphoric acid. Sci. Total Environ. 302, 253–265 (2003).
Wan, M. M. et al. A safe and efficient strategy for the rapid elimination of blood lead in vivo based on a capture–fix–separate mechanism. Angew. Chem. Int. Ed. 131, 10692–10696 (2019).
Ericson, B. et al. Blood lead levels in low-income and middle-income countries: a systematic review. Lancet Planet. Health 5, e145–e153 (2021).
Scheckel, K. G. & Ryan, J. A. Effects of aging and pH on dissolution kinetics and stability of chloropyromorphite. Environ. Sci. Technol. 36, 2198–2204 (2002).
Cao, X., Ma, L. Q., Chen, M., Singh, S. P. & Harris, W. G. Impacts of phosphate amendments on lead biogeochemistry at a contaminated site. Environ. Sci. Technol. 36, 5296–5304 (2002).
Cao, X., Ma, L. Q., Rhue, D. R. & Appel, C. S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 131, 435–444 (2004).
Ruby, M. V., Davis, A., Schoof, R., Eberle, S. & Sellstone, C. M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 30, 422–430 (1996).
Barnett, M. O. et al. Formation of chloropyromorphite during the physiologically based extraction test: Experimental artifact? Environ. Eng. Sci. 28, 719–724 (2011).
Fan, J. et al. Uptake of vegetable and soft drink affected transformation and bioaccessibility of lead in gastrointestinal track exposed to lead-contaminated soil particles. Ecotoxicol. Environ. Saf. 194, 110411 (2020).
Laperche, V., Traina, S. J., Gaddam, P. & Logan, T. J. Chemical and mineralogical characterizations of Pb in a contaminated soil: reactions with synthetic apatite. Environ. Sci. Technol. 30, 3321–3326 (1996).
Ruby, M. V., Davis, A. & Nicholson, A. In situ formation of lead phosphates in soils as a method to immobilize lead. Environ. Sci. Technol. 28, 646–654 (1994).
Cao, X., Ma, L. Q., Singh, S. P. & Zhou, Q. Phosphate-induced lead immobilization from different lead minerals in soils under varying pH conditions. Environ. Pollut. 152, 184 (2008).
Bosso, S. T., Enzweiler, J. & Angélica, R. S. Lead bioaccessibility in soil and mine wastes after immobilization with phosphate. Water, Air, Soil Pollut. 195, 257–273 (2008).
Homberg, J. R., Wöhr, M. & Alenina, N. Comeback of the rat in biomedical research. ACS Chem. Neurosci. 8, 900–903 (2017).
U.S. Environmental Protection Agency. Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. OSWER 9285, 7–77 (2007).
Li, H. B. et al. Oral bioavailability of As, Pb, and Cd in contaminated soils, dust, and foods based on animal bioassays: A review. Environ. Sci. Technol. 53, 10545–10559 (2019).
Sima, J., Zhao, L., Xu, X., Luo, Q. & Cao, X. Transformation and bioaccessibility of lead during physiologically based extraction test: effects of phosphate amendment and extract fluid components. RSC Adv. 6, 43786–43793 (2016).
Dignam, T., Kaufmann, R. B., LeStourgeon, L. & Brown, M. J. Control of lead sources in the United States, 1970-2017: public health progress and current challenges to eliminating lead exposure. J. Public Health Manag. Pract. 25, 13–22 (2019).
World Health Organization (WHO), Guideline for clinical management of exposure to lead, https://iris.who.int/bitstream/handle/10665/347360/9789240037045-eng.pdf?sequence=1 (2021).
Cabañas, E. et al. Dietary effects of lead as a neurotoxicant. Diet and nutrition in neurological disorders 387–410 (Elsevier, 2023).
Neuwirth, L. S., Cabañas, E., Cadet, P., Zhu, W. & Markowitz, M. E. Cereal and juice, lead and arsenic, our children at risk: a call for the FDA to re-evaluate the allowable limits of lead and arsenic that children may ingest. Int. J. Environ. Res. Public Health 19, 5788 (2022).
Schroder, J. L. et al. In vitro gastrointestinal method to estimate relative bioavailable cadmium in contaminated soil. Environ. Sci. Technol. 37, 1365–1370 (2003).
Acknowledgements
This study was supported by the National Natural Science Foundation of China with (Nos. 42330706, 21377081) awarded to X.C and (No. 42077112) awarded to X.X. and Shanghai Rising-Star Program (No. 23QA1405200) awarded to X.X. We thank Dr. Xuemei Fang from Suzhou College, Zehong Zhang and Xuan-Ang Gong from Shanghai Jiao Tong University, and Prof. Peiqiang Mu from South China Agricultural University for help with swine dosing experiments, Dr. Hongxia Yang and Dr. Wei Liu from the National Research Center of Geoanalysis, and Dr. Nan Sheng from Shanghai Jiao Tong University for analytical support.
Author information
Authors and Affiliations
Contributions
X.C., J.D., and X.X. designed the research; X.X., Q.Q., Y.S., W. Huang, and C.Y. performed the research; X.X., Q.Q., X.C., and L.M. analyzed the data; and X.X., W. Harris, D.H., X.C., and J.D. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Lorenz Neuwirth, Andrés Rodríguez-Seijo and Domen Lestan for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, X., Qian, Q., Shi, Y. et al. Cola beverage reduces risk of lead poisoning from accidental ingestion of contaminated soil particles in rat and swine models. Nat Commun 16, 755 (2025). https://doi.org/10.1038/s41467-025-56138-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56138-9