Abstract
Numerous risk factors for oesophageal cancer are linked to lifestyle habits, but the role of early-life factors in its incidence and mortality is unclear. Using UK Biobank data, we explore the association among breastfeeding, maternal smoking, smoking in offspring, and oesophageal cancer risk in adult offspring via multivariable Cox regression. Here, we show that being breastfed, compared with not being breastfed, is associated with a lower risk of oesophageal cancer incidence (HR: 0.83, 95% CI: 0.70–0.98) and mortality (HR: 0.74, 95% CI: 0.61–0.89) in adult offspring. Additionally, it is associated with a reduced impact of smoking in offspring on oesophageal cancer incidence (HR: 0.79, 95% CI: 0.64-0.96) and mortality (HR: 0.73, 95% CI: 0.59-0.91). We subsequently construct a polygenic risk score for oesophageal cancer to explore the influence of genetic factors. Our findings emphasize the importance of breastfeeding, and smoking cessation to prevent oesophageal cancer.
Similar content being viewed by others
Introduction
Oesophageal cancer is the seventh most common and sixth deadliest cancer worldwide, causing approximately 400,000 deaths annually, accounting for 4.9% of all cancer deaths, with a 5-year survival rate of less than 20%1,2,3. Two major histological types of oesophageal cancer exist: oesophageal squamous cell carcinoma and oesophageal adenocarcinoma4. The causative factors of oesophageal cancer are complex and diverse, with no obvious symptoms in the early stages; most patients are in the middle to late stages at the time of diagnosis5. Therefore, it is particularly important to identify risk factors for the prevention of oesophageal cancer. Previous studies have delved into the multiple environmental risk factors for oesophageal cancer, establishing a complex relationship between them6. Among these, sex, age, and race are notable for influencing the incidence and mortality of oesophageal cancer. For example, the risk of oesophageal adenocarcinoma is significantly greater in men, especially in white men, than in women, and the incidence of oesophageal squamous cell carcinoma is greater in African Americans7,8,9. In addition, the risk of oesophageal cancer increases with age10. These demographic characteristics and genetic backgrounds are nonmodifiable risk factors that may increase the incidence of oesophageal cancer by influencing an individual’s susceptibility to carcinogens11. Furthermore, multiple systematic evaluations and meta-analyses have confirmed that lifestyle factors, such as alcohol consumption, obesity, lack of physical activity, and poor dietary habits, have been extensively studied and recognized as modifiable risk factors. They tend to further increase the risk of oesophageal cancer by interacting with each other6,12,13.
Because cancer progression is usually a decades-long process, the aetiologically relevant period of oesophageal cancer may extend to early childhood14. A growing body of research has suggested that environmental or behavioural factors in early life may have a profound and lasting impact on disease susceptibility in adulthood15. Therefore, early life factors may be potentially modifiable risk factors for oesophageal cancer16.
To date, only a handful of studies have been conducted on the impact of early-life factors on cancer in offspring. A previous prospective cohort study explored the relationships between early life factors and early-onset colorectal cancer and demonstrated no statistically significant associations between the two factors14. In addition, breastfeeding is adversely associated with the risk of neuroblastoma and plays a potentially protective role in childhood Hodgkin’s lymphoma, with attention also being given to the role of breastfeeding in reducing the likelihood of leukaemia in children17,18,19. Nevertheless, there are no specific studies on the relationship between oesophageal cancer and breastfeeding.
The relationship between smoking in adulthood and oesophageal cancer is well established20. The health effects of exposure to tobacco smoke in early life are also profound. Available evidence suggests that early life trajectories, including prenatal, childhood, and adolescence, are critical periods of heightened sensitivity to a variety of environmental factors15. Maternal smoking is another early-life factor that we explored, although its association with oesophageal cancer is unclear. Premature telomere shortening is deleterious to cancer progression, and previous studies have suggested a potential link between smoking during pregnancy and fetal telomere length shortening21. In addition, a HELIX (human early-life exposome) cohort study revealed that both active and secondhand smoke exposure during pregnancy accelerated telomere shortening in children, which may be an important risk factor for oesophageal cancer morbidity and mortality22. However, the sample sizes were too small to provide convincing evidence that maternal smoking was associated with an increased risk23,24,25. Furthermore, regarding the relationship between smoking during pregnancy and cancer in adulthood, a previous study has shown that smoking increases lung cancer incidence and mortality, whereas the risk of oesophageal cancer incidence and mortality in adulthood remains unclear26.
In this work, we hypothesize that early-life factors may have some influence on oesophageal cancer. Prospective cohort studies explore the association between early-life factors and oesophageal cancer, taking into account important life factors and genetic covariates. These factors may play a role in preventing the development of oesophageal cancer and reducing mortality in offspring. Consequently, the aim of our study is to be assessing the influence of maternal smoking, smoking status of offspring, and breastfeeding on oesophageal cancer risk in a large prospective cohort.
Results
Baseline statistical characteristics
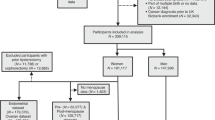
As shown in Supplementary Data 1, we analysed the baseline statistics for the population with oesophageal cancer and the population without oesophageal cancer. Patients who developed oesophageal cancer or died from it were older than those who did not develop or die from it and were more likely to be male or smokers. In addition, these patients are at increased risk due to maternal smoking during pregnancy and have a family history of cancer. In contrast, participants who were breastfed had a lower risk of developing and dying from oesophageal cancer than did those who were not breastfed. Furthermore, participants who typically suffered from insomnia and had lower incomes had a higher risk of developing oesophageal cancer and mortality. Figure 1 shows details of the participant screening process.
502,406 UK Biobank participants were first included. 148 with oesophageal cancer were excluded, leaving 502,258. More exclusions for missing data created subsets: maternal smoking (n = 432,622), offspring smoking (n = 499,313), breastfeeding (n = 383,533), genotyping (n = 487,019). The flowchart details exclusions and retained participants. Source data are provided as a Source Data file.
Associations of early-life factors with the smoking status of offspring with oesophageal cancer
Over a median follow-up period of 11.7 years, this large prospective cohort study identified oesophageal cancer cases and separately analysed their individual associations with early-life factors and offspring smoking. Maternal smoking, offspring smoking and breastfeeding were associated with the incidence and mortality of oesophageal cancer (Fig. 2). Compared with mothers who did not smoke during pregnancy, participants whose mothers smoked during pregnancy had an 18% increased risk of oesophageal cancer mortality (Fig. 2, HR: 1.18, 95% CI: 1.01–1.37; P = 0.042), whereas no statistically significant associations were found in terms of incidence (Fig. 2, HR: 1.12, 95% CI: 0.97–1.28; P = 0.113). In contrast, breastfeeding was associated with a lower risk of oesophageal cancer incidence (Fig. 2, HR: 0.83, 95% CI: 0.70–0.98; P = 0.027) and mortality (Fig. 2, HR: 0.74, 95% CI: 0.61–0.89; P = 0.001). Additionally, smoking in offspring is associated with a greater risk of oesophageal cancer incidence (Fig. 2, HR: 1.83, 95% CI: 1.61–2.08; P < 0.001) and mortality (Fig. 2, HR: 2.00, 95% CI: 1.72–2.31; P < 0.001) when contrasted with non-smokers.
HR Hazard Ratio, 95%CI 95% confidence intervals, Ref reference. Adjusted for age; sex (Female/Male); race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); Townsend Deprivation index; smoke (Yes/No/Missing); drink (Never/Previous/Current/Missing); body mass index (<25 kg/m2, 25 to 29.9 kg/m2, ≥30 kg/m2); physical activity; family of cancer history (Yes/No); income (<£18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, >£100,000, or missing); insomnia (Never/ Sometimes/ Usually/ Missing). Data are presented as hazard ratios with 95% confidence intervals; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). Source data are provided as a Source Data file.
When we analysed the smoking status of offspring (smokers and nonsmokers) in conjunction with maternal smoking during pregnancy, we found that when both mothers and offspring smoked, the risk of oesophageal cancer development and death was significantly greater than when only the mothers or only the offspring smoked. The p values for the interactions were all less than 0.001, indicating that this combination effect was statistically significant. This implies that maternal smoking may exacerbate the adverse health effects of smoking in offspring and is associated with an even higher risk of oesophageal cancer incidence and mortality. (Fig. 3, HR: 1.91, 95% CI: 1.61–2.38; P < 0.001).
HR Hazard Ratio, 95%CI 95% confidence intervals, Ref reference, FDR false discovery rate. Adjusted for age; sex (Female/Male); race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); Townsend Deprivation index; drink (Never/Previous/Current/Missing); body mass index (<25 kg/m2, 25 to 29.9 kg/m2, ≥30 kg/m2); physical activity; family of cancer history (Yes/No); income (less than £18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, >£100,000, or missing); insomnia (Never/Sometimes/Usually/ Missing). Data are presented as hazard ratios with 95% confidence intervals; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). Source data are provided as a Source Data file.
Combined association of maternal smoking, smoking status of offspring, and breastfeeding with oesophageal cancer in offspring
Immediately thereafter, we analysed the interaction effects of maternal smoking and the smoking status of offspring who were breastfeeding on oesophageal cancer incidence and mortality. The statistically significant results of this study are shown in Fig. 4. Our analysis revealed that breastfeeding had a significant positive effect on both the risk of oesophageal cancer incidence and mortality and that breastfeeding tended to reduce these risks regardless of whether the mother smoked during pregnancy. However, no statistically significant interactions were observed.
HR Hazard Ratio, 95%CI 95% confidence intervals, Ref reference, FDR false discovery rate. Adjusted for age; sex (Female/Male); race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); Townsend Deprivation index; smoke (Yes/No/Missing); drink (Never/Previous/Current/Missing); body mass index (<25 kg/m2, 25 to 29.9 kg/m2, ≥30 kg/m2); physical activity; family of cancer history (Yes/No); income (less than £18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, greater than £100,000, or missing); insomnia (Never/Sometimes/Usually/Missing). Data are presented as hazard ratios with 95% confidence intervals; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). Source data are provided as a Source Data file.
In contrast, when the smoking status of offspring and breastfeeding status of offspring, were analysed, the p value for the interaction effect was <0.05, suggesting that breastfeeding mitigates the adverse effects of smoking to some extent (Fig. 5).
HR Hazard Ratio, 95%CI 95% confidence intervals, Ref reference, FDR false discovery rate. Adjusted for age; sex (female/male); race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); Townsend Deprivation index; smoke (Yes/No/Missing); drink (Never/Previous/Current/Missing); body mass index (<25 kg/m2, 25–29.9 kg/m2, ≥30 kg/m2); physical activity; family of cancer history (Yes/No); income (less than £18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, greater than £100,000, or missing); insomnia (Never/Sometimes/Usually/Missing). Data are presented as hazard ratios with 95% confidence intervals; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). Source data are provided as a Source Data file.
We also analysed the combined association of maternal smoking, the smoking status of offspring, and breastfeeding on the incidence and mortality risk of oesophageal cancer; however, we did not find any significant correlation between the combined associations of these three factors with the risk of oesophageal cancer incidence and mortality (Fig. 6).
HR Hazard Ratio, 95%CI 95% confidence intervals, Ref reference, FDR false discovery rate. Adjusted for age; sex (Female/Male); race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); Townsend Deprivation index; drink (Never /Previous/Current/Missing); body mass index (<25 kg/m2, 25 to 29.9 kg/m2, ≥30 kg/m2); physical activity; family of cancer history (Yes/No); income (less than £18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, greater than £100,000, or missing); insomnia (Never/ Sometimes/ Usually/ Missing). Data are presented as hazard ratios with 95% confidence intervals; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). Source data are provided as a Source Data file.
Mediation analysis
All inflammatory markers except monocyte percentage were significantly associated with breastfeeding while controlling for covariates (Fig. 7A, Monocyte percentage: 95% CI [−0.011, 0.028]; Neutrophil count: 95% CI [−0.109, −0.088]; Monocyte count: 95% CI [−0.007, −0.003]; CRP: 95% CI [−0.156, −0.093]; Leukocyte count: 95% CI [−0.103, −0.073]; Neutrophil percentage: 95% CI [−0.724, −0.598]; Lymphocyte count: 95% CI [0.008, 0.024]; Platelets, 95% CI [−2.583, −1.719]; Lymphocyte percentage: 95% CI [0.573, 0.683]; Except for the monocyte percentage the remaining eight inflammatory markers: P < 0.001). After controlling for covariates and multiple comparisons, strong associations were observed between seven of the nine inflammatory markers (with the exception of lymphocyte levels and monocyte percentage) and oesophageal cancer incidence and mortality. Neutrophils, monocytes, CRP, leukocytes, neutrophil percentage, and platelet levels were positively correlated with oesophageal cancer incidence or mortality. In contrast, the lymphocyte percentage had a protective effect on the risk of oesophageal cancer incidence (Fig. 7B, HR: 0.99; 95% CI: 0.98–0.996; P = 0.008) and mortality (Fig. 7B, HR: 0.98; 95% CI: 0.97–0.99; P = 0.002).
A Multivariable adjusted linear regression modeling of the relationship between breastfeeding and inflammatory marker levels. The significance of the regression coefficients was assessed using a t-test, and p-values were calculated to evaluate the statistical significance of the association. Neutrophil: P = 4.08 × 10−77; Monocyte: P = 8.04 × 10−6; CRP: P = 7.44 × 10−15; Leukocyte: P = 8.20 × 10−30; Monocyte percentage: P = 0.388; Neutrophil percentage: P = 3.94 × 10−94; Lymphocyte: P = 9.96 × 10−5; Platelet: P = 1.65 × 10−22; Lymphocyte percentage: P = 1.78 × 10−111. B Association of nine inflammatory markers with oesophageal cancer incidence and mortality; Data on these inflammatory indicators were complete for the number of participants: n = 365,377 (leukocytes), n = 364,705 (lymphocytes, neutrophils, monocytes), n = 364,709 (lymphocyte%, monocyte%, neutrophil%), n = 365,378 (platelets), and n = 358,145 (CRP), respectively; Each point shows the point estimate of HR from Cox regression. Bars show 95%CI; HR and 95%CI were estimated by Cox regression (two-sided Wald test). C Exposure-response curves between indicators of inflammation and risk of oesophageal cancer incidence and mortality. The upper left curve represents the exposure-response curve between indicators of inflammation and the risk of death from oesophageal cancer. Bold lines represent HRs, while shaded areas indicate 95% CI; All p-values were calculated using two-sided Wald tests. D Six of the nine inflammatory markers significantly mediated the prospective association between breastfeeding and oesophageal cancer incidence and death. Mediation analysis was conducted using the mediation package in R. The significance of the mediating effects was assessed using 500 bootstrap iterations. NS: not significant. Source data are provided as a Source Data file.
There is evidence of nonlinear associations of leukocytes, platelets, monocytes, percent monocytes, neutrophils and percent neutrophils with increased oesophageal cancer incidence or mortality, and no evidence of nonlinearity was observed for other inflammatory markers (P < 0.05). As shown in Fig. 7C, when these inflammatory markers were in the lower range, they were associated with a lower risk of oesophageal cancer incidence and mortality. However, as the values of these inflammatory markers increase, the risk ratios gradually increase.
Mediation analyses indicated that six of the nine inflammatory markers played a partial but significant mediating role in the prospective association of breastfeeding with oesophageal cancer incidence and mortality. Specifically, the percentage of significant mediating effects ranged from 0.66% (monocyte count) to 6.67% (neutrophil count) (Fig. 7D). These findings suggest that these inflammatory markers play important roles in explaining the association between breastfeeding and oesophageal cancer. Our findings highlight the complex interaction of inflammation between breastfeeding and oesophageal cancer, and further investigation of these mediating mechanisms may help develop targeted interventions to reduce oesophageal cancer incidence and mortality.
Stratification analysis
In this study, the polygenic risk score (PRS) was used as a cumulative genetic risk measure consisting of multiple genetic variants known to be associated with oesophageal cancer. Its potential interaction with these early-life factors was explored. However, no significant PRS interaction effects were observed in our analysis, thus, its role in this study was relatively limited. This further emphasizes the focus of our study, which is the independent role of early life factors in oesophageal cancer risk. Alternatively, larger sample sizes may detect meaningful interactions.
Specifically, we performed an interaction analysis of oesophageal cancer incidence and death according to maternal smoking status, the smoking status of offspring, breastfeeding status, and the PRS. First, we analysed the relationships between the PRS and oesophageal cancer incidence and mortality; our results revealed that a high PRS was significantly associated with a greater risk of death from oesophageal cancer than were low and moderate PRS (Supplementary Table 1, HR: 1.35; 95% CI: 1.01–1.81; P = 0.045). Immediately thereafter, we analysed the interaction effects of the smoking status of offspring and maternal smoking combined with PRS and breastfeeding separately; however, we did not obtain meaningful results. The results suggest the existence of a trend towards a lower risk of oesophageal cancer incidence and death as the genetic risk decreases when offspring do not smoke and when their mothers do not smoke during pregnancy and breastfeeding (Supplementary Table 2–5).
We subsequently stratified the data according to sex, race, and age. In the interaction between maternal smoking and breastfeeding, stratified analyses revealed that breastfeeding was significantly associated with a lower risk of oesophageal cancer among male participants. This protective effect was quite clear in the data, where breastfeeding was associated with lower rates of oesophageal cancer regardless of whether the mother smoked during pregnancy (Supplementary Table 6). Although the effect of breastfeeding did not reach statistical significance in terms of the risk of death from oesophageal cancer, the data still show a trend that breastfeeding may contribute to reducing the risk of death from oesophageal cancer. (Supplementary Table 6, HR: 0.71; 95% CI: 0.49–1.03; P = 0.072). For female participants, the combination of maternal smoking and breastfeeding did not significantly affect oesophageal incidence or mortality. However, the data suggest breastfeeding tends to have a protective effect. Although the exact mechanism is unknown and may involve hormonal differences, this is something that needs to be explored in further studies (Supplementary Table 6). Among nonwhite participants, although breastfeeding appeared to have a potential benefit in reducing the risk of oesophageal cancer, the results failed to reach statistical significance because of the relatively small sample size of nonwhites. This may be due to the majority of white participants in the UK Biobank, resulting in insufficient statistical efficacy in the nonwhite group, which in turn prevents statistically significant conclusions from being drawn (Supplementary Table 7). In addition, breastfeeding was similarly associated with a lower risk of oesophageal cancer among participants aged >50 years (Supplementary Table 8). However, among participants ≤50 years of age, the effect of breastfeeding on oesophageal cancer risk was not significant. Notably, the interaction between maternal smoking and breastfeeding did not show significance in any of the stratified analyses, suggesting that the moderating effects of age, sex, and race on these relationships may be more limited.
The associations between the smoking status of offspring, breastfeeding, and oesophageal cancer incidence and mortality in offspring were consistent with the results of previous analyses. Specifically, in comparison with females, nonwhites, and those aged ≤50 years, the P value for the interaction was less than 0.05 among males, whites, and those aged over 50 years, which implies a significant influence within these specific population subgroups (Supplementary Tables 9–11).
Sensitivity analyses
Sensitivity analyses demonstrated the robustness of the results. First, the included participants included those diagnosed with oesophageal cancer at baseline (Supplementary Tables 12 and 13). Second, we included additional potential confounding factors in our analysis: some studies have reported an association between low birth weight and oesophageal cancer; therefore, we added birth weight to the covariates (Supplementary Tables 14 and 15). Third, we restricted our analyses to participants followed for one year or more to capture events in oesophageal cancer incidence and mortality more comprehensively (Supplementary Tables 16 and 17). Finally, we excluded patients who were diagnosed with cancer at enrolment (Supplementary Tables 18 and 19).
Discussion
The predominant findings of this prospective cohort study can be characterised as follows: maternal smoking and smoking in offspring increase the risk of oesophageal cancer, whereas breastfeeding reduces the adverse effects of these factors on oesophageal cancer risk.
An increasing body of research suggests that early-life factors can profoundly influence susceptibility to disease in adulthood27,28,29,30. Maternal smoking, as an early life factor, is a major cause of childbirth complications and affects the subsequent development of the child31. Few studies have explored the potential associations of maternal smoking with cancer, and only a handful of previous studies have investigated the relationship between maternal smoking during pregnancy and childhood cancer.
Maternal smoking has become a prominent public health concern. In the European region, an estimated 30.6% of pregnant women smoke during pregnancy, a phenomenon that has sparked considerable attention32. Similarly, data from the UK Biobank revealed that 29.2% of participants reported their mothers’ smoking habits around the time of their birth, which was consistent with the aforementioned statistics. These data indicate that the prevalence of smoking during pregnancy poses a serious threat to the health of both mothers and children, highlighting the urgency of implementing effective interventions to reduce smoking behaviour during pregnancy. Although research in this area is still evolving, a number of hypotheses and findings have been proposed. First, smoking during pregnancy releases large amounts of harmful substances, such as polycyclic aromatic hydrocarbons (PAHs), which cross the placenta and affect the integrity of foetal DNA33. These can lead to abnormal cells in the embryo and fetus during growth and development. Second, maternal smoking may affect foetal hormone levels, such as oestrogen and androgen, which can affect embryonic and foetal growth, development, and cellular differentiation, including cell proliferation, differentiation, and organ formation in the embryo. These can lead to poor growth and development of offspring34. Third, maternal smoking causes an inflammatory response in both the mother and fetus and produces high levels of free radicals and oxidative stressors. In turn, this leads to DNA breaks, resulting in reduced telomere length. It can cause oxidative damage to cellular DNA, proteins, and lipids35. Finally, maternal smoking may weaken the immune function of the fetus and reduce its ability to fight off tumour cells36. In summary, maternal smoking may affect embryonic DNA integrity, developmental processes, inflammatory responses, oxidative stress, immune function, and hormone levels, thereby increasing the incidence and mortality of oesophageal cancer in offspring.
To the best of our knowledge, tobacco smoking is a significant contributing factor to the incidence and mortality of oesophageal cancer in China and should not be overlooked37. Many studies have shown that tobacco is a carcinogen and that it harms the human body in multiple ways38,39,40,41,42. Carcinogens in tobacco, such as PAHs, tobacco-specific N-nitrosamines, and aldehydes, may be swallowed into the oesophagus with saliva and absorbed into the oesophageal epithelium, increasing the risk of cancer43. Cigarette smoke and tar have been found to contain a variety of carcinogens, such as epoxides, lactones, peroxides and halogenated ethers, in addition to a variety of nitroso compounds, including nitrosopyrrolidine, dimethylnitrosamine, nitroso desmethylnicotinic acid, or nitroso neonicotinic acid43,44. In addition, large amounts of NO, NO2, and hydrocarbons are produced by the reactions of alkanes and alkoxy radicals, which can directly attack cellular fat proteins, nucleic acids, and other components, resulting in cellular damage. In the long term, the oesophageal mucosa is affected by persistent irritation and damage, and it easily causes oesophageal epithelial tissue damage by carcinoma. Furthermore, long-term smoking weakens the body’s immune and repair abilities, increasing the vulnerability of the oesophageal mucosa to damage and malignant changes45.
Another early-life factor that we are exploring is breastfeeding; however, there is little research on its relationship with cancer in adult offspring. Breastfeeding has previously been reported to reduce the overall incidence of childhood cancers. Previous studies have shown that breastfeeding is negatively associated with the risk of neuroblastoma and another study demonstrated the protective effect of breastfeeding against childhood-onset Hodgkin’s lymphoma18,46. In addition, breastfeeding reduces the risk of leukaemia in children47. However, to the best of our knowledge, studies on the relationship between breastfeeding and cancer in adulthood are still scarce and the association between breastfeeding and oesophageal cancer has not been reported. Our results showed that breastfeeding was associated with a reduced risk of oesophageal cancer-related incidence and mortality in offspring. Currently, the mechanisms by which breastfeeding reduce the risk of oesophageal cancer incidence and death and the adverse effects of smoking in offspring on oesophageal cancer deaths have not been fully elucidated. The potential mechanisms are described below: First, breast milk is rich in antioxidant substances, such as vitamin C, vitamin E, and selenium, which can neutralize free radicals48. These antioxidants are linked to reduce oesophageal damage by reducing oxidative stress to the oesophageal mucosa. Second, breast milk also contains whey proteins and growth factors (e.g., epidermal growth factor), which may reduce the inflammatory response of the oesophageal mucosa, promote cell growth and repair, maintain the integrity of the oesophageal mucosa, and increase resistance to infection and disease49. Although the study by Lemas et al. did not directly address the role of these components in preventing smoking-induced oesophageal damage, we speculate that they may help mitigate damage to oesophageal tissues from chronic inflammation induced by smoking during pregnancy and smoking in future generations, thereby reducing cancer risk. In particular, alpha-lactalbumin plays an antitumour role in inhibiting tumour growth and spread50,51. In addition, breastfeeding has been shown to prevent infants from developing obesity in adulthood, while maintaining a healthy weight reduces the risk of oesophageal cancer52. Third, in addition to nutrients, breast milk contains various non-nutritive bioactive factors; for example, it is enriched with microRNAs that influence epigenetic mechanisms to induce early epigenetic effects, which have long-term effects on gene expression and phenotype53. Furthermore, bioactive substances in breast milk reportedly affect DNA methylation in the human genome, thereby reducing the impact of smoking-related changes in adult offspring and lowering the risk of oesophageal cancer development and death54. On the other hand, breastfeeding helps create an emotional bond between parents and their children, and this closeness may have a positive effect on the mental health of infants, thus indirectly reducing the risk of diseases such as cancer.
In addition, our study provides further evidence for the mechanisms underlying the association between breastfeeding and oesophageal cancer. Independent studies have shown elevated levels of inflammation in the peripheral blood of cancer patients, suggesting inflammatory dysregulation of the immune system55,56. In our prospective study, we included a variety of inflammatory markers, including C-reactive protein (CRP), neutrophils, leukocytes, platelets, and monocytes. More importantly, our study demonstrated that neutrophils, the percentage of lymphocytes, the percentage of neutrophils, leukocytes, CRP, and monocytes were significant mediators in the association of breastfeeding with oesophageal cancer. These findings suggest that the effects of breastfeeding on oesophageal cancer development and mortality may be realized through inflammation-related processes. The immune system plays a key role in tumorigenesis and progression57. The survival and growth of tumour cells, as well as their recognition and clearance by the body, are regulated by the immune system57. Breast milk contains a number of immunoglobulins that provide passive protection against infection and inflammation by delivering antibodies and exerting anti-inflammatory effects. This process is essential for the maturation of the immune system and contributes to the development of a healthy immune system, which in turn reduces the risk of oesophageal cancer development and death. Furthermore, immunoglobulins, lactoferrin and other bioactive components in breast milk inhibit the production of inflammatory factors and reduce the inflammatory response, thereby reducing the likelihood of cancer58. This finding is further supported by our study, which revealed that breastfeeding significantly reduced the levels of inflammatory markers associated with oesophageal cancer. This finding provides strong evidence that breast milk reduces oesophageal cancer risk through its anti-inflammatory properties.
The development of oesophageal cancer is influenced by both genetic and environmental factors59. In an analysis of PRS and oesophageal cancer risk, although statistical significance was not reached with respect to the risk of oesophageal carcinogenesis, the data show a trend that high PRS may be associated with an increased risk of oesophageal carcinogenesis, similar to the association with the risk of death. Although only the risk of death was statistically significant, this trend suggests that the PRS may have a potential impact on both the risk of oesophageal carcinogenesis and the risk of death. We believe that there are several possible reasons for this discrepancy: the development of oesophageal cancer may be influenced by a variety of nongenetic factors, such as diet, smoking, and alcohol consumption. These factors may have different distributions in different populations, masking the influence of genetic factors on pathogenesis. In addition, multiplicity of genetic factors: certain genetic variants may increase the risk of death by affecting a variety of biological processes that may not directly contribute to cancer development. For example, these variants may affect the rate of cancer progression or response to treatment, thereby increasing mortality. The lack of association of PRS with oesophageal cancer development may be because these genes have a lesser impact on the early stages of cancer development and a more significant impact on the advanced stages of the disease or during cancer progression. Third, patients’ lifestyle changes and adherence to treatment after diagnosis may also influence cancer mortality. For example, whether a patient quits smoking, changes their diet, and adheres to regular follow-up visits are all behaviours that may significantly affect disease progression and ultimately outcomes. These factors may not play a significant role in incidence analyses but may be more critical in influencing mortality. Certain genetic factors may increase the risk of death from oesophageal cancer by influencing a patient’s ability to respond to these lifestyle changes, further exacerbating the progression of the disease. Fourth, another possibility is that differences in when cancers are detected may also affect mortality and morbidity. Cancers detected early usually have a better prognosis and a lower mortality rate, whereas cancers detected late tend to have a poorer prognosis and a higher mortality rate. Genetic risk factors may cause the disease to be less detectable in the early stages of certain patients or lead to delays in diagnosis, thereby increasing mortality in these patients. Fifth, some patients may already have other serious health problems or comorbidities at the time of diagnosis, which may accelerate cancer progression and increase the risk of death. These differences in health status may not be adequately accounted for in morbidity analyses but may have a significant impact on mortality analyses. For example, cancer patients with cardiovascular disease or diabetes mellitus may be at greater risk of death due to poorer overall health status. Certain genetic factors may also be associated with these comorbidities, and patients at greater genetic risk are more likely to develop other health problems, which further exacerbates the deterioration of the condition and leads to higher mortality rates. Therefore, PRS may have a greater impact on cancer prognosis than on incidence. Additionally, we analysed the interactions among offspring smoking status, maternal smoking, PRS, and breastfeeding separately but did not obtain meaningful results, possibly for the following reasons: first, the limitation in sample size may have led to insufficient statistical power. When conducting interaction analyses, particularly those involving multiple factors such as offspring smoking status, maternal smoking, PRS, and breastfeeding, the relatively small sample size may not have been sufficient to detect significant interactions among these complex factors. This limitation in sample size could have masked potential interaction effects. Additionally, in our analysis, we also found significant imbalances in the distribution of data between groups, especially in the interactions between different PRS levels, smoking statuses, and breastfeeding. This uneven distribution of data further reduced the sensitivity of the statistical analysis, making it difficult to derive meaningful results. Second, the development of oesophageal cancer is not determined by a single factor but by the synergistic effect of heredity and other external factors such as lifestyle and the environment. Although breastfeeding may prevent oesophageal cancer to some extent, heredity and external factors may play a more important role. Many other lifestyle factors, such as unhealthy habits, diet and external exposure, may also influence oesophageal cancer development and death. In the context of different genetic risks, these factors may overshadow the potential protective effect of breastfeeding against oesophageal cancer.
Several significant strengths of our study validate our conclusions. First, we adjusted for various potential confounders to minimize their impact on the results. Second, we obtained data on oesophageal cancer incidence and deaths from a large cohort and designed a prospective study. We also calculated the PRS for oesophageal cancer to explore the potential influence of genetic factors. Importantly, we explored, for the first time, the associations of breastfeeding in combination with maternal and offspring smoking with the risk of oesophageal cancer. This comprehensive analysis provides public health value for the prevention of oesophageal cancer.
There are several limitations to this study that need to be addressed. First, we recognize that data on maternal smoking and breastfeeding in the UK Biobank are self-reported and may be subject to recall bias, affecting the accuracy of the results. Although this method has been widely used in previous studies, it should be validated in the future in conjunction with objective measures such as biomarkers. Second, as the UK Biobank mainly includes populations of European origin, the generalizability of the findings to other ethnic groups may be limited, and future studies should include more diverse populations to improve applicability. Third, although we controlled for multiple covariates, there may still be residual confounders. In addition, the covariates of the UK Biobank were only measured at baseline, which did not capture dynamics over time and may have affected the accuracy of the results. Therefore, it is recommended that future studies adopt a longitudinal design with regularly updated information on covariates to fully assess their impact on oesophageal cancer risk. Finally, the UK Biobank lacked detailed data on maternal smoking and secondhand smoke exposure during breastfeeding, which limited in-depth analysis. Future studies should collect more comprehensive data to better understand their impact on oesophageal cancer risk and to further investigate the role of these factors as prognostic indicators for oesophageal cancer, particularly with respect to tumour staging at diagnosis.
This study comprehensively explores the interaction of breastfeeding with maternal smoking and the smoking status of offspring and their relationship with oesophageal cancer risk. This view of maternal smoking, breastfeeding, and adulthood smoking as a whole will help us understand their impact on the mechanisms of oesophageal cancer incidence and mortality. This will provide rational prevention strategies for oesophageal cancer in clinical practice, that is, suggesting that exposure to potentially harmful factors (smoking) should be minimized in both mothers and their offspring and that pregnancy (smoking cessation) and the postpartum period (breastfeeding) take protective measures to prevent oesophageal cancer.
Methods
UK biobank and study population
The UK Biobank is an important international cancer resource. To date, UK Biobank participants have recorded over 43,000 cancer cases in national cancer registries60. The UK Biobank data were approved by the North West Multi-centre Research Ethics Committee (MREC) (REC reference: 21/NW/0157) on 29 June 2021. Information on participant compensation states that in accordance with standard UK Biobank practice, participants in UK Biobank studies do not receive compensation for their participation in the study. All the participants provided informed consent for data provision and linkage. In addition, a large amount of data on lifestyle factors, physical assessments, biospecimens, and overall health status have been collected. A total of 502,258 participants who participated in the study between 2006 and 2010 and were aged 37–73 years, were included after screening were included. For the entire UK Biobank cohort, we excluded participants with a prebaseline diagnosis of oesophageal cancer, as well as those with missing data on exposure factors (maternal smoking, the smoking status of offspring, and breastfeeding).
Assessment of exposure
The relevant exposure factors include smoking during pregnancy, the smoking status of offspring, and breastfeeding. Information regarding maternal smoking and breastfeeding during pregnancy was collected via a self-reported touchscreen questionnaire. The participants were asked the following questions: “Did your mother smoke regularly around the time when you were born?” and “Were you breastfed when you were a baby?”. The participants had the following choices: “yes”, “no”, and “don’t know”. Regarding smoking status of offspring, Data-Field 20116 summarised participants’ current/past smoking status, and we defined ever-smokers and current smokers as smokers and never-smokers as nonsmokers. The nine inflammatory markers used in this study were derived from category 100081.These data are available on the UK BioBank website (https://www.ukbiobank.ac.uk/).
Determination of outcomes
Incidence of oesophageal cancer and death were obtained by linking to the National Cancer Registry and the National Death Registry, International Classification of Diseases codes 40001 and 40006, C15, respectively26,60,61,62,63.
Covariates
Additional data were obtained from self-reports or historical diagnoses in the National Hospital Register. To eliminate the effect of potentially confounding factors, we included several key covariates, including demographic characteristics: age, sex (male/female) and race (White, Mixed, Asian or Asian British, Black or Black British, Chinese, Other ethnic group); maternal smoking around birth (yes/no); breastfed as a baby (yes/no); socioeconomic information: Townsend Deprivation Index (TDI), which represents the socioeconomic status of the participant, with higher scores indicating higher levels of poverty; body mass index (BMI) which was categorised as normal (BMI < 25 kg/m2), overweight (BMI 25–29.9 kg/m2), or obesity (BMI ≥ 30 kg/m2) based on criteria set by the World Health Organization; physical activity, which was assessed by calculating metabolic equivalent task (MET) minutes using the International Physical Activity Questionnaire; lifestyle: smoking status (no/yes/missing) and alcohol status (never/past/current/missing); family history of cancer (yes/no); income (less than £18,000, £18,000 to £30,999, £31,000 to £51,999, £52,000 to £100,000, greater than £100,000, or missing); insomnia (never/sometimes/usually/missing); and birth weight (<2 kg and ≥2 kg). The race information was collected via the UK Biobank touchscreen questionnaire (field 21000), where participants self-reported their race background. The available categories for selection were: White: British, Irish, Any other white background.
Mixed: White and Black Caribbean, White and Black African, White and Asian, Any other mixed background; Asian or Asian British: Indian, Pakistani, Bangladeshi, Any other Asian background; Black or Black British: Caribbean, African, Any other Black background; Chinese; Other ethnic group; Do not know; Prefer not to answer. In our study, participants were categorized into six racial groups: White, Mixed, Asian or Asian British, Black or Black British, Chinese, and Other ethnic group. In addition, we conducted a stratified analysis based on race. The participants were classified into two main categories: White and Non-white. ‘White’ includes individuals from White ethnic backgrounds, while ‘Non-white’ encompasses all other ethnic groups, such as Mixed, Asian or Asian British, Black or Black British, Chinese, and Other ethnic groups.
Definition of PRS
To include SNPs in the PRS, we selected the SNPs with the most comprehensive information and the smallest p-values reported across multiple studies of the same ancestry and phenotype64. We employed a genome-wide association study to select single nucleotide polymorphisms (SNPs) that exhibited genome-wide significance and used the multiple imputation (MIP) method to address missing genetic data during the analysis process. The PRS for oesophageal cancer was computed via a weighted method. Based on the number of risk alleles carried, each SNP was categorised as 0, 1, or 2. To calculate the PRS, the formula employed was as follows:
where, n represents the total number of SNPs, and where βk corresponds to the natural logarithm of the odds ratio associated with SNPk and oesophageal cancer, which was estimated via effect size data derived from previous studies65. Based on the distribution of the PRS, participants were categorised into 10 genetic risk groups, with the first risk group defined as low risk, the second through ninth risk groups as medium risk, and the tenth risk group as high risk.
Statistical analysis
SPSS and R software were used for all the statistical analyses. Statistical significance was set at P < 0.05. The baseline characteristics of the participants are displayed according to the status of oesophageal cancer incidence and mortality. The baseline characteristics of the continuous variables are expressed as the means (standard deviation, SDs), and the categorical variables are expressed as numbers (percentages, %). Regarding the treatment of missing covariate values, the mean was used for continuous variables, and categorical variables were used with the indicator method. Multivariate Cox proportional risk models were used to analyse the associations of various inflammatory markers associated with various exposure factors with the incidence and mortality of offspring oesophageal cancer. We used the Schoenfeld residual method to test the proportional risk assumption of the Cox proportional risk model. The data generally met the conditions of the proportional hazards assumption for performing subsequent regression analyses. Linear regression modelling was used to study the correlations between breastfeeding and inflammatory markers. The findings are reported as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Multiple correction of p values was performed via the FDR method. For the mediation analysis, we used the “mediator” package in R to examine the extent to which the associations between breastfeeding and oesophageal cancer incidence and mortality can be mediated by large inflammatory markers66. To accurately describe the relationships between maternal smoking, breastfeeding, the smoking status of offspring, and oesophageal cancer incidence and mortality, we first analysed the associations between each exposure and oesophageal cancer incidence and mortality based on the included covariates, after which we conducted separate interaction analyses with maternal smoking versus no breastfeeding and offspring smoking versus no breastfeeding as reference values.
In addition, we performed a sensitivity analysis and stratification analysis to ensure the stability and comprehensiveness of our results. Initially, we stratified the participants according to sex (male/female), race (white/nonwhite), and age (>50 years/≤50 years) via a Cox proportional risk model, which was consistent with previous studies. Consequent sensitivity analyses were then performed to further validate our results.
Statistics and reproducibility
No statistical methods were used to predetermine the sample size. No randomization of allocation was carried out for the study. Researchers were not blinded to allocation during the study and outcome assessment. To examine the associations between breastfeeding, maternal smoking and the smoking status of offspring and the risk of oesophageal cancer, we excluded participants with a diagnosis of oesophageal cancer at baseline as well as those with missing exposure factors (breastfeeding, smoking during pregnancy, smoking status of offspring). All replication attempts were successful.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
This work was conducted via the UK Biobank Resource. Our study was conducted via the UK Biobank under application number 903547. The UK Biobank is an open-access resource, and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. Due to data privacy laws and participant consent restrictions, the raw data from the UK Biobank cannot be made publicly available. The outcomes of the analyses are included in the Source data file. If any readers wish to access the raw data, they can contact the corresponding author to obtain the relevant Field ID number, then register and download the data. Source data are provided with this paper.
Code availability
Analysis code used for this study is available at: https://github.com/wangaxuer/R-code-analysis. The repository has been made citable via Zenodo, and the DOI is https://doi.org/10.5281/zenodo.14551065.
References
Rogers, J. E., Sewastjanow-Silva, M., Waters, R. E. & Ajani, J. A. Esophageal cancer: emerging therapeutics. Expert Opin. Ther. Targets. 26, 107–117 (2022).
Pather, K. et al. Long-term survival outcomes of esophageal cancer after minimally invasive Ivor Lewis esophagectomy. World J. Surg. Oncol. 20, 50 (2022).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 74, 229–263 (2024).
Lander, S., Lander, E. & Gibson, M. K. Esophageal cancer: overview, risk factors, and reasons for the rise. Curr. Gastroenterol. Rep. 25, 275–279 (2023).
Liu, N. & Xiong, W. Early life exposure to tobacco smoke and lung cancer in adulthood. Am. J. Respir. Crit. Care Med. 207, 370–371 (2023).
Huang, F. L. & Yu, S. J. Esophageal cancer: risk factors, genetic association, and treatment. Asian J. Surg. 41, 210–215 (2018).
Baquet, C. R., Commiskey, P., Mack, K., Meltzer, S. & Mishra, S. I. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J. Natl. Med. Assoc. 97, 1471–1478 (2005).
Wang, S. et al. Global and national trends in the age-specific sex ratio of esophageal cancer and gastric cancer by subtype. Int. J. Cancer. 151, 1447–1461 (2022).
Cook, M. B., Chow, W. H. & Devesa, S. S. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br. J. Cancer. 101, 855–859 (2009).
Enzinger, P. C. & Mayer, R. J. Esophageal cancer. N. Engl. J. Med. 349, 2241–2252 (2003).
Domper, A. M., Ferrandez, A. A. & Lanas, A. A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 21, 7933–7943 (2015).
Seo, J. H., Kim, Y. D., Park, C. S., Han, K. D. & Joo, Y. H. Hypertension is associated with oral, laryngeal, and esophageal cancer: a nationwide population-based study. Sci. Rep. 10, 10291 (2020).
Lu, L., Mullins, C. S., Schafmayer, C., Zeissig, S. & Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 41, 1137–1151 (2021).
Gausman, V., Liang, P. S., O’Connell, K., Kantor, E. D. & Du, M. Evaluation of early-life factors and early-onset colorectal cancer among men and women in the UK biobank. Gastroenterology. 162, 981–983 (2022).
Clarke, M. A. & Joshu, C. E. Early life exposures and adult cancer risk. Epidemiol. Rev. 39, 11–27 (2017).
Bever, A. M. & Song, M. Early-life exposures and adulthood cancer risk: a life course perspective. J. Natl. Cancer Inst. 115, 4–7 (2023).
Su, Q. et al. Breastfeeding and the risk of childhood cancer: a systematic review and dose-response meta-analysis. BMC Med. 19, 90 (2021).
Wang, K. L., Liu, C. L., Zhuang, Y. & Qu, H. Y. Breastfeeding and the risk of childhood Hodgkin lymphoma: a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 14, 4733–4737 (2013).
Amitay, E. L. & Keinan-Boker, L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 169, e151025 (2015).
Abnet, C. C., Arnold, M. & Wei, W. Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 154, 360–373 (2018).
Salihu, H. M. et al. Impact of intrauterine tobacco exposure on fetal telomere length. Am. J. Obstet. Gynecol. 212, 201–205 (2015).
Osorio-Yanez, C. et al. Early life tobacco exposure and children’s telomere length: The HELIX project. Sci. Total Environ. 711, 135028 (2020).
Tettamanti, G., Ljung, R., Mathiesen, T., Schwartzbaum, J. & Feychting, M. Maternal smoking during pregnancy and the risk of childhood brain tumors: results from a Swedish cohort study. Cancer Epidemiol 40, 67–72 (2016).
Heck, J. E. et al. Smoking in pregnancy and risk of cancer among young children: a population-based study. Int. J. Cancer. 139, 613–616 (2016).
Momen, N. C., Olsen, J., Gissler, M. & Li, J. Exposure to maternal smoking during pregnancy and risk of childhood cancer: a study using the Danish national registers. Cancer Causes Control 27, 341–349 (2016).
He, H. et al. In utero and childhood/adolescence exposure to tobacco smoke, genetic risk, and lung cancer incidence and mortality in adulthood. Am. J. Respir. Crit. Care Med. 207, 173–182 (2023).
Gao, X. et al. Early-life risk factors, accelerated biological aging and the late-life risk of mortality and morbidity. QJM 117, 257–268 (2024).
Khalili, H. et al. Early life factors and risk of inflammatory bowel disease in adulthood. Inflamm. Bowel Dis. 19, 542–547 (2013).
Jiang, W. et al. Maternal smoking, nutritional factors at different life stage, and the risk of Incidence type 2 diabetes: a prospective study of the UK Biobank. BMC Med. 22, 50 (2024).
Cupul-Uicab, L. A. et al. In utero exposure to maternal tobacco smoke and subsequent obesity, hypertension, and gestational diabetes among women in the MoBa cohort. Environ. Health Perspect. 120, 355–360 (2012).
Blechter, B. et al. Sub-multiplicative interaction between polygenic risk score and household coal use in relation to lung adenocarcinoma among never-smoking women in Asia. Environ. Int. 147, 105975 (2021).
Lange, S., Probst, C., Rehm, J. & Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob. Health. 6, e769–e776 (2018).
Yuan, J. M. et al. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32, 1366–1371 (2011).
Kapoor, D. & Jones, T. H. Smoking and hormones in health and endocrine disorders. Eur. J. Endocrinol. 152, 491–499 (2005).
Lin, J. & Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 73, 101507 (2022).
McGrath-Morrow, S. A. et al. The effects of nicotine on development. Pediatrics 145, e20191346 (2020).
Wang, Q. L., Xie, S. H., Li, W. T. & Lagergren, J. Smoking cessation and risk of esophageal cancer by histological type: systematic review and meta-analysis. J. Natl. Cancer Inst. 109, (2017).
Yuan, S. et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife 12, e84051 (2023).
Benowitz, N. L. & Liakoni, E. Tobacco use disorder and cardiovascular health. Addiction 117, 1128–1138 (2022).
Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 397, 2337–2360 (2021).
Chen, Z. et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet 386, 1447–1456 (2015).
Chan, K. H. et al. Tobacco smoking and risks of more than 470 diseases in China: a prospective cohort study. Lancet Public Health 7, e1014–e1026 (2022).
Li, Y. & Hecht, S. S. Carcinogenic components of tobacco and tobacco smoke: a 2022 update. Food Chem. Toxicol. 165, 113179 (2022).
Hecht, S. S. & Hatsukami, D. K. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat. Rev. Cancer. 22, 143–155 (2022).
Hecht, S. S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 3, 733–744 (2003).
Martin, R. M., Gunnell, D., Owen, C. G. & Smith, G. D. Breast-feeding and childhood cancer: a systematic review with metaanalysis. Int. J. Cancer. 117, 1020–1031 (2005).
Amitay, E. L., Dubnov, R. G. & Keinan-Boker, L. Breastfeeding, other early life exposures and childhood leukemia and lymphoma. Nutr. Cancer. 68, 968–977 (2016).
Gila-Diaz, A. et al. Influence of maternal age and gestational age on breast milk antioxidants during the first month of lactation. Nutrients 12, 2569 (2020).
Lemas, D. J. et al. Exploring the contribution of maternal antibiotics and breastfeeding to development of the infant microbiome and pediatric obesity. Semin. Fetal Neonatal Med. 21, 406–409 (2016).
Chetta, K. E., Forconi, M., Newton, D. A., Wagner, C. L. & Baatz, J. E. HAMLET in human milk is resistant to digestion and carries essential free long chain polyunsaturated fatty acids and oleic acid. Food Chem. 427, 136752 (2023).
Hallgren, O. et al. Apoptosis and tumor cell death in response to HAMLET (human alpha-lactalbumin made lethal to tumor cells). Adv. Exp. Med. Biol. 606, 217–240 (2008).
Qiao, J., Dai, L. J., Zhang, Q. & Ouyang, Y. Q. A meta-analysis of the association between breastfeeding and early childhood obesity. J. Pediatr. Nurs. 53, 57–66 (2020).
Hatmal, M. M. et al. Immunomodulatory properties of human breast milk: MicroRNA contents and potential epigenetic effects. Biomedicines 10, 1219 (2022).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 10, 295–304 (2009).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Nost, T. H. et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 36, 841–848 (2021).
Xia, L. et al. The cancer metabolic reprogramming and immune response. Mol. Cancer. 20, 28 (2021).
Jiang, X. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer. 18, 10 (2019).
Short, M. W., Burgers, K. G. & Fry, V. T. Esophageal cancer. Am. Fam. Physician. 95, 22–28 (2017).
Conroy, M. C. et al. UK Biobank: a globally important resource for cancer research. Br. J. Cancer. 128, 519–527 (2023).
Li, G. et al. Relationship between glucosamine use and the risk of lung cancer: data from a nationwide prospective cohort study. Eur. Resp. J. 59, 2101399 (2022).
Muller, D. C., Johansson, M. & Brennan, P. Lung cancer risk prediction model incorporating lung function: development and validation in the UK biobank prospective cohort study. J. Clin. Oncol. 35, 861–869 (2017).
Wang, X. et al. Joint association of biological aging and lifestyle with risks of cancer incidence and mortality: a cohort study in the UK biobank. Prev. Med. 182, 107928 (2024).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Lambert, S. A., Abraham, G. & Inouye, M. Towards clinical utility of polygenic risk scores. Hum. Mol. Genet. 28, R133–R142 (2019).
Wang Y, Impact of maternal and offspring smoking and breastfeeding on oesophageal cancer in adult offspring. GitHub Repository. https://doi.org/10.5281/zenodo.14551065 (2024).
Acknowledgements
This work was supported by the Natural Science Foundation of China (82272678 to XY.W.; 82272730 to H.Y.), Haiyan Scientific Research Fund Outstanding Youth Project, Affiliated Cancer Hospital of Harbin Medical University, Harbin, China (JJJQ2024-05 to XY.W.; JJJQ2024-08 to H.Y.) and Young marshal unveiling project of Harbin Medical University (H.Y.).
Author information
Authors and Affiliations
Contributions
Data preparation (G.L., HR.S., YX.W.); study design (XY.W., H.Y., YX.W.); statistical analysis (YX.W.); data interpretation(JX.X., SY.W., SJ.Z., YX.W.); wrote the manuscript (YX.W., HR.S.); critical revisions of the draft (G.L., HR.S., YX.W., JQ.M., TL.Z., TS.H., CH.S.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Sun, H., Li, G. et al. Impact of maternal and offspring smoking and breastfeeding on oesophageal cancer in adult offspring. Nat Commun 16, 938 (2025). https://doi.org/10.1038/s41467-025-56252-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56252-8