Abstract
Polyether electrolytes have been widely recognized for their favorable compatibility with lithium-metal, yet they are hampered by intrinsically low oxidation thresholds, limiting their potential for realizing high-energy Li-metal batteries. Here, we report a general approach involving the bridge joints between non-lithium metal ions and ethereal oxygen, which significantly enhances the oxidation stability of various polyether electrolyte systems. To demonstrate the feasibility of the ion-bridging strategy, a Zn2+ ion-bridged polyether electrolyte (Zn-IBPE) with an extending electrochemical stability window of over 5 V is prepared, which enables good cyclability in 4.5 V Li||LiCoO2 batteries. Ampere-hour-level quasi-solid-state batteries of SiO-graphite||LiNi0.8Mn0.1Co0.1O2 (10 Ah, N/P ratio of 1.12, 303 Wh kg−1 at 0.1 C based on the total weight of the pouch cells) and 60 μm-Li||LiNi0.9Mn0.05Co0.05O2 (18 Ah, N/P ratio of 2.5, 452 Wh kg−1 at 0.33 C based on the total weight of the pouch cells) pouch cells with Zn-IBPE present elevated electrochemical performance, benefiting from adequate interfacial stability. Nail penetration tests evidence high battery safety enabled by Zn-IBPE in 4 Ah graphite||LiNi0.8Mn0.1Co0.1O2 pouch cells without combustion or smoke. This work offers a pathway for designing high-voltage polymer electrolytes and a general solution for achieving high-performance quasi-solid-state batteries.
Similar content being viewed by others
Introduction
Lithium-ion batteries (LIBs) have revolutionized the electrified world and will further promote low-carbon and sustainable development1,2. However, the limited progress in pushing the energy-density boundaries has resulted in range anxiety for electric vehicles (EVs) with a range of less than 500 km and limitations for better portable electronic devices3. As a result, Li-metal negative electrodes have been revisited and paired with high-Ni ternary layered oxide (NMC) or high-voltage lithium cobalt oxide (LCO) positive electrodes, enabling a single cell to achieve a specific energy of >400 Wh kg−1 (ref. 4). Despite this advancement, the inherent instability of Li-metal negative electrodes during cycling, including Li-dendrite growth and unstable solid-electrolyte interface (SEI) formation, leads to rapid performance degradation and safety hazards5. To address these challenges and the trade-off between safety concerns and energy density, quasi-solid-state batteries with polymer electrolytes have gained significant attention6,7,8.
Among various polymer electrolytes, polyether-based electrolytes have been extensively studied due to their strong ability to dissociate Li salts and good chemical/electrochemical stability with Li metal9,10,11. Recently, in-situ polymerization strategies have been proposed to prepare in-built polyether electrolytes with high ionic conductivities (around 1 mS cm−1 at room temperature) and low interfacial resistances12,13. However, polyether electrolytes have been long plagued by their narrow electrochemical stability windows (ESWs), limiting their compatibility with high-voltage positive electrodes14,15. Various strategies, including polymer design and cross-linking16,17,18, cathode-electrolyte interphase (CEI) engineering19,20, asymmetry configuration21,22, and introducing inorganic fillers23, have been reported to enhance the oxidative stability of polyether electrolytes24,25. Nevertheless, these methods fail to fundamentally address the electron extraction of ethereal oxygen at high voltages, which weakens the adjacent C–H bond and leads to the subsequent H-transfer from the methylene group to anions of Li salts (Fig. 1a). This results in the formation of conjugated superacids, causing severe degradation of the polyether matrix and catastrophic corrosion of electrodes26.
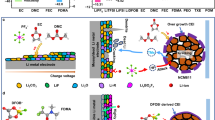
Schematic illustration of batteries with a conventional polyether electrolyte and b Zn-IBPE. c 1H and 13C NMR spectra, d Raman spectra (ν: stretching mode, νs: symmetric stretching, νa: asymmetric stretching, r: rocking mode), e ionic conductivity, f FT-IR spectra, and g TGA curves of various PDOL-based electrolytes. h Digital images of Zn-PDOL and Zn-IBPE electrolytes at 60 °C and 120 °C for certain hours. The normalized intensity of NMR and Raman spectra was shown in arbitrary unit (a.u.).
In this study, we present an ion-bridging approach to extend the oxidation potential of polyether electrolytes (Fig. 1b). A Zn2+ ion-bridged polyether electrolyte (Zn-IBPE) was proposed as an exemplified system. The Zn-IBPE leverages polymer topological entanglement of polydioxolane (PDOL)-cellulose triacetate (CTA) double networks to enhance thermal reliability. At the same time, the Zn2+–O coordination directly stabilizes the lone pairs of ethereal oxygen, thereby fundamentally alleviating the oxidation of polyether electrolytes. The Zn2+ ion-bridging enables stable cycling of Li||LCO batteries at 4.5 V, which exhibits 92% capacity retention over 280 cycles at a current density of 48 mA g−1. Moreover, when tested in the graphite (Gr)||LiNi0.8Mn0.1Co0.1O2 (NMC811) system, the 4 Ah pouch cell with Zn-IBPE maintained a capacity retention of 91.7% over 500 cycles at 0.2 C. It also passed the nail penetration test, demonstrating decent battery safety. Furthermore, 10 Ah SiO-Gr||NMC811 (303 Wh kg−1 at 0.1 C based on the total weight of the pouch cells) and 18 Ah Li||LiNi0.9Mn0.05Co0.05O2 (NMC90) (452 Wh kg−1 at 0.1 C based on the total weight of the pouch cells) pouch cells were assembled using Zn-IBPE electrolytes, exhibiting good capacity retentions of 85.4% after 500 and 95.2% after 105 cycles at 0.33 C, respectively. The successful demonstration of these cells highlights the practicality and versatility of Zn-IBPE in various battery systems.
Results and discussion
Synthesis and physicochemical properties of Zn-IBPE
The Zn-IBPE was developed by in-situ polymerization of DOL triggered by Lewis acid Zn(TFSI)2, and the entire solidification process was completed within 1600 min (Supplementary Fig. 1). The polymerization of DOL monomers occurred within macromolecular CTA frameworks, where CTA was pre-dissolved in the DOL precursor. The resulting PDOL and CTA were interconnected by Zn2+ cross-linking points to form double network structures. For comparison, LiDFOB was used as the initiator for Zn2+-free PDOL electrolytes (Supplementary Note 1). The ring-opening polymerization of the DOL monomer is confirmed by nuclear magnetic resonance (NMR) spectra (Fig. 1c), and the presence of CTA is shown by the 1H NMR peaks at 3.4 and 4.6 ppm (Supplementary Fig. 2). SEM images also illustrate the occurrence of in-situ polymerization, and the as-prepared polymer electrolytes fully incorporated into porous separators (Supplementary Fig. 3), where Al2O3-coated PE separators were used for batteries to enhance the wettability with electrolyte precursors (Supplementary Fig. 4). The S–N–S symmetric stretching vibration is identified by Raman spectra within the 740–760 cm−1 range (Fig. 1d). The Zn-IBPE electrolyte exhibits a larger ratio of free TFSI− ions (42.8%) compared to other electrolytes (Supplementary Fig. 5). This phenomenon could be attributed to the release of TFSI− from the polymer networks after Zn2+ ion-bridging, given that Zn(TFSI)2 also involves the S−N−S stretching mode (Supplementary Fig. 6). Meanwhile, with the addition of the 20 vol% FEC:DEC plasticizer (Supplementary Fig. 7), Zn-IBPE demonstrates a high ionic conductivity of 1.01 mS cm−1 at 20 °C with the lowest activation energy of 0.16 eV among various PDOL-based electrolytes, displaying a favorable Li+ transport characteristic (Fig. 1e and Supplementary Fig. 8). Besides, the Zn-IBPE presents a decent Li+ transference number of 0.57, similar to that of Zn-PDOL (0.55) and PDOL-CTA (0.52, Supplementary Fig. 9 and Supplementary Table 1).
To clarify the Zn2+ ion-bridging effect in the polymer matrix, Fourier transform infrared (FT-IR) spectroscopy was employed on the PDOL-based electrolytes. As shown in Fig. 1f, the red-shifted stretching vibration of the C–O–C bond of Zn-PDOL and Zn-IBPE from PDOL-CTA indicates the coordination between Zn2+ and ethereal oxygen27, and the decrease in intensity of the C=O peak for Zn-IBPE compared with PDOL-CTA suggests the Zn2+ adsorption onto CTA, which is consistent with previous reports28,29. Due to the Zn2+ ion-bridging effect exerted on PDOL-CTA double networks, Zn-IBPE also exhibits interesting thermal properties. Previous studies indicated that PDOL electrolytes melt and degrade into a dark gray oily liquid containing small molecule epoxides when heated above 110 °C30. In contrast, the decomposition of Zn-IBPE was postponed to ~300 °C, as revealed by thermogravimetric analysis (TGA) curves (Fig. 1g). Hence, Zn-IBPE keeps solid-state and thermally stable even at 120 °C for 3 h (Fig. 1h and Supplementary Fig. 10). Zn-IBPE also remains amorphous over a wide temperature range, while both Zn-PDOL and PDOL-CTA show semicrystalline behavior (Supplementary Fig. 11)31. Except for its favorable thermal reliability, Zn-IBPE and the separator infiltrated with Zn-IBPE present a higher elastic modulus (Supplementary Fig. 12) compared with Zn-PDOL and PDOL-CTA, which is beneficial for suppressing the growth of Li dendrites and insulating against detrimental negative/positive electrode contact during nail penetration.
Electrochemical stability with Li metal of Zn-IBPE
The compatibility with Li metal of various PDOL-based electrolytes was examined by Li||Li symmetric cells. Figure 2a displays a swift escalation in the polarization of Li||Li symmetric cells with Zn-PDOL and PDOL-CTA electrolyte after approximately 150 h at 1 mA cm−2 and 1 mAh cm−2, attributable to unstable SEI and ongoing side reactions. On the contrary, the Li||Li symmetric cell utilizing Zn-IBPE exhibits stable cycling and a flat voltage plateau with minimal electrode polarization (~34 mV), spanning approximately 1900 h (Fig. 2b). The influence of LiDFOB addition was also examined. It was found that a small amount of LiDFOB did not impact the Li||Li cycling performance of Zn-containing polymer electrolytes (Supplementary Fig. 13). Besides, the Li||Li cell with Zn-IBPE enjoys good rate performance and presents steady Li plating/stripping profiles even at 10 mA cm−2 (Supplementary Fig. 14). The good electrochemical stability of Zn-IBPE with Li metal is also substantiated by a high Coulombic efficiency (CE) of Li plating/stripping in Li||Cu cells. As depicted in Fig. 2c, the Li||Cu cell incorporating Zn-IBPE delivers an average CE of 98.4% ± 1.07% over 1450 cycles with negligible polarization increase at 0.5 mA cm−2 and 0.5 mAh cm−2 (Fig. 2d). However, the CE of Li||Cu cells with Zn-PDOL and PDOL-CTA precipitously drops to less than 40% by approximately 200 cycles (Supplementary Fig. 15). Even at 1 mA cm−2 and 1 mAh cm−2, Zn-IBPE settles at a long-term CE of 99.6% ± 0.97% over 740 cycles, which is well-placed among previously reported (Supplementary Fig. 16). Scanning electron microscopy (SEM) was applied to examine the morphology of Li deposition on Cu foils. Rounded Li particles are observed in the Li||Cu cell featuring Zn-IBPE, whereas Zn-PDOL results in needle-like Li deposition of uneven size, contributing to a porous structure (Supplementary Fig. 17), which accounts for its lower CE of the Li||Cu cell than that of Zn-IBPE.
a Cycling performance of Li||Li symmetric cells at 1 mA cm−2 and 1 mAh cm−2, and corresponding b plating/stripping profiles at various cycles. c Coulombic efficiency (CE) and d voltage profiles of Li||Cu cells with various PDOL-based electrolytes at 0.5 mA cm−2 and 0.5 mAh cm−2. e XPS F 1s spectra of cycled Li metal with different PDOL-based electrolytes before and after Ar-ion sputtering. f ToF-SIMS corresponding depth profiles of LiF2− and TFSI− for cycled Li metal with Zn-PDOL and Zn-IBPE electrolytes (inset: 3D reconstruction of TFSI− species). The intensity of these spectra was shown in arbitrary unit (a.u.). SEM and digital images of cycled Li metal with g Zn-PDOL, h PDOL-CTA, and i Zn-IBPE electrolytes collected from Li||Li symmetric cells after 100 cycles.
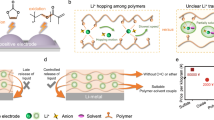
SEI components and distributions, which are key determinants of Li-metal performance, were examined through X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS). Figure 2e presents the F 1s spectra of cycled Li metal with various electrolytes. An intense C–F peak on the top surface of Li metal in Zn-PDOL is attributed to TFSI− accumulation. After 200 s of Ar-ion sputtering, the C–F content of Li metal with Zn-PDOL still accounts for 26.4%. In contrast, both PDOL-CTA and Zn-IBPE exhibit high LiF contents in SEI compositions, construed as a result of the PDOL-CTA double network largely suppressing the decomposition of PDOL at the Li-metal surface. This finding is consistent with depth-profiling ToF-SIMS curves and corresponding 3D reconstruction images, where Li metal in Zn-PDOL displays a low LiF concentration and apparent vertical etching (Fig. 2f). The organic phase, denoted by the C− species in ToF-SIMS results, reveals that Zn-PDOL forms an organic SEI (Supplementary Fig. 18a), while Zn-IBPE facilitates the formation of a LiF-rich SEI. Meanwhile, only a trace amount of Zn elements was detected on the Li-metal surface with both Zn-PDOL and Zn-IBPE, indicating that Zn2+ reduction was trivial and restricted to the top surface of Li metal because of the immobility of the polymer electrolytes (Supplementary Fig. 18). In comparison with PDOL-CTA, Zn-IBPE features higher F and lower C contents, suggesting that the ion-bridged PDOL-CTA networks can effectively inhibit electrolyte decomposition at negative electrode sides. The LiF-dominated SEI also exerts a morphological impact on Li metal. As shown in Fig. 2g–i, Li metal cycled in Zn-PDOL presents clear Li corrosion with a loose and cracked morphology, whereas Zn-IBPE enables a smooth and bulky surface along with slight dullness of metallic luster. Additionally, Li metal in PDOL-CTA also exhibits uneven deposition of both dendritic and bulky Li, accountable for its rapid decay in Li cyclability.
Oxidative stability investigation of Zn-IBPE
The poor oxidation stability of polyether electrolytes has been a long-standing challenge. To evaluate the compatibility of PDOL-based electrolytes with high-voltage positive electrodes, ESWs were initially examined using linear sweep voltammetry (LSV). As depicted in Fig. 3a, both PDOL-CTA and PDOL exhibit a similar oxidative threshold of ~4.2 V, indicating that the sole CTA framework addition is insufficient to prevent PDOL decomposition. In contrast, Zn-IBPE and Zn-PDOL demonstrate high ESWs of over 5 V and 4.7 V, respectively, suggesting that Zn2+ coordination effectively extends the ESW of PDOL-based electrolytes. The different effects of Li+ and Zn2+ on the oxidation stability were also revealed by LSV curves (Supplementary Fig. 19). The results demonstrated that Zn2+ had a pronounced impact on oxidation stability over Li+ at the same concentration, where the oxidation potential exhibited a notable increase from 4.6 V to over 5 V as the Zn2+ concentration exceeded the threshold of [Zn2+]:[DOL] = 1:800 (Supplementary Note 2). Meanwhile, the incorporation of plasticizers or LiDFOB was found to have a negligible impact on the oxidation stability of PDOL-based electrolytes (Supplementary Fig. 20). Though CTA is an important component, CTA alone cannot be used as polymer electrolytes normally, and the oxidation potentials of the CTA electrolyte is only about 3.9 V (Supplementary Fig. 21).
a Linear sweep voltammetry (LSV) curves of Li||stainless steel cells at a scan rate of 0.1 mV s−1, b XPS C 1s spectra, and c electrochemical floating test results of different PDOL-based electrolytes in Li||NMC83 cells. d The plot of the oxidation potentials of Mn+-PDOL-CTA versus BSSE-corrected binding energy of Mn+···PDOL-CTA clusters. e Tested and calculated Eox of structures with and without TFSI− for different PDOL-based electrolytes. Optimized structures and calculated Eox of f PDOL-TFSI− and g Zn2+-PDOL-CTA-TFSI− clusters with H-transfer. h–j Plots of the reduced density gradient (RDG) versus sign(λ2)ρ for different PDOL-based electrolytes. The RDG plots are colored on a scale from blue, green to red, with the value of sign(λ2)ρ ranging from −0.05 to 0.05 a.u., where red indicates steric repulsion and blue indicates attraction interactions. The intensity of all spectra was shown in arbitrary unit (a.u.).
The XPS C 1s spectra reveal a clear down-shifting of C–O–C, C–O, and C=O peaks in Zn-IBPE and Zn-PDOL compared to PDOL-CTA (Fig. 3b). This shift illustrates that Zn2+–O coordination increases the electron density around the carbon atom, thereby impeding the C–H bond cleavage caused by oxidized oxygen under high voltage25,32. Electrochemical floating tests were also conducted to rigorously evaluate the oxidation potentials of PDOL-based electrolytes. In Fig. 3c, all samples, except for Zn-IBPE, become unstable above 4.5 V due to vigorous side reactions between positive electrodes and electrolytes. In contrast, Zn-IBPE remains stable up to 4.8 V with only a minimal rise in leakage currents.
To gain an insight into the effect of different metal ions on the ion-bridging effect, a series of metal cation-coordinated PDOL-CTA polymer electrolytes were synthesized. The oxidation potentials were found to be highly correlated to the binding energy of Mn+···PDOL-CTA clusters (Fig. 3d), presenting a better fitting accuracy than fitting models using electronegativity and Lewis acidity (Supplementary Fig. 22). This suggests the binding energy of Mn+···PDOL-CTA determines the electrochemical stability window of ion-bridged polymer electrolytes. Among various ion-bridging systems, Zn2+···PDOL-CTA has the most negative binding energy and thus the most stable structure. Therefore, we chose Zn2+ as the ion-bridging center in this study. The effectiveness of the ion-bridging approach is further demonstrated by extending the method to other polyether electrolytes, such as polyethylene oxide (PEO). The metal ion-bridged PEO-CTA electrolytes (Mn+-PEO-CTA) exhibit widened ESWs compared with the bare PEO-CTA counterpart (Supplementary Fig. 23).
Quantum chemistry (QC) calculations with the implicit solvent model were employed to investigate the origin of differences in oxidation potentials. We compare the calculated and measured oxidation potentials (Eox vs. Li+/Li) of various PDOL clusters with and without TFSI− anions (Fig. 3e and Supplementary Figs. 24 and 25). In accordance with previous reports33,34, the calculated oxidation potentials of polymer clusters with TFSI− anions provide a more precise estimation of oxidation potentials for polymer electrolytes than using anion-free counterparts35. Although the presence of TFSI− anions significantly reduces the calculated Eox of various structures, the Zn-IBPE cluster with TFSI− still exhibits the highest Eox among all polymer clusters with TFSI− anions. Even considering the H-transfer reaction (Fig. 3f, g), Zn-IBPE shows an extended Eox up to ~6.03 V, much higher than that of PDOL-TFSI− (~4.71 V). Furthermore, non-covalent interaction (NCI)36 analysis was performed to elucidate the effect of Zn2+ ion-bridging on the oxidation resistance of PDOL-based electrolytes (Fig. 3h–j). Although PDOL and PDOL-CTA possess hydrogen bonds in the negative sign(λ2)ρ range from 0 to −0.02 (Supplementary Fig. 26)37, they do not significantly contribute to the stability of PDOL under high voltages. In contrast, the strong Zn2+−O coordination shows the peak at sign(λ2)ρ around −0.06 in Zn-IBPE, which helps mitigate the electron extraction from the ethereal oxygen during the oxidation process, thus raising its oxidation threshold (Supplementary Fig. 27).
Evaluation of high-voltage Li||LCO cells with Zn-IBPE
The electrochemical performance of coin cells with Zn-IBPE was evaluated to assess its compatibility with Li-metal and high-voltage positive electrodes. Remarkably, Zn-IBPE demonstrates decent performance in Li||LFP and Li||NMC83 batteries (Supplementary Fig. 28 and Supplementary Table 2), as well as stable cycling for high-voltage Li||LCO cells (Fig. 4a, b). It maintains 90% initial capacity over 450 cycles at 56 mA g−1 with a cut-off voltage of 4.4 V. Even in the case of the 4.5 V Li|Zn-IBPE|LCO cell, it achieves a specific capacity of 162.1 mAh g−1 (92% capacity retention) after 280 cycles at 48 mA g−1 (Fig. 4c, d). In comparison, the Li||LCO cell with Zn-PDOL loses all reversible capacity within 140 cycles, and Li||LCO cells with PDOL-CTA or PDOL cannot be charged to 4.4 V at the first cycle (Supplementary Fig. 29).
Cycling performance of Li||LCO batteries with a cut-off voltage of a, b 4.4 V and c, d 4.5 V and corresponding voltage profiles with Zn-PDOL and Zn-IBPE. Voltage profiles and in-situ DEMS results of Li||LCO batteries using e Zn-PDOL and f Zn-IBPE electrolytes between 3 and 4.5 V. Focused ion beam (FIB)-SEM images of 4.5 V LCO after 50 cycles with g Zn-PDOL and h Zn-IBPE.
To investigate the gassing behavior of Li||LCO cells, differential electrochemical mass spectrometry (DEMS) was employed with a cut-off voltage of 4.5 V. The analysis reveals that when the voltage exceeds 4.3 V, major signals at m/z = 2 (H2), m/z = 28 (C2H4/CO), and m/z = 44 (CO2) are detected in the Zn-PDOL cell (Fig. 4e), also leading to significant pouch cell swelling (Supplementary Fig. 29b). In contrast, no notable gas evolution is observed in the Li|Zn-IBPE|LCO cell at 4.5 V (Fig. 4f). This successful inhibition of PDOL decomposition can be attributed to the effective Zn2+–O bonding. Meanwhile, evident cracks are observed in LCO particles obtained from Zn-PDOL after cycling (Fig. 4g). Conversely, the LCO particles remain intact after cycling at 4.5 V in Zn-IBPE (Fig. 4h), indicating that Zn-IBPE successfully protects LCO-positive electrodes and enables the formation of an effective and stable CEI layer.
Positive electrode-electrolyte interphase analysis of Zn-IBPE
To gain further insights into the composition of the CEI in PDOL-based electrolytes, post-mortem ToF-SIMS, TEM, and XPS measurements were employed. Figure 5a, d illustrates that LCO-positive electrodes cycled with Zn-PDOL exhibit lower LiF content and a higher ratio of the organic phase, as indicated by the C2HO− signal, compared to LCO electrodes in Zn-IBPE. Furthermore, the LCO electrode cycled with Zn-PDOL exhibits severe electrolyte corrosion and dissolution of transition metals, as evidenced by increased TFSI− and CoF3− species38.
a, b ToF-SIMS depth profiles and c, d 3D reconstruction of LiF2−, TFSI−, CoF3−, and C2HO− fragments of cycled 4.5 V LCO electrodes with Zn-PDOL and Zn-IBPE electrolytes. TEM images and elemental distribution of energy dispersive X-ray spectroscopy (EDS) line scans of 4.5 V cycled LCO particles cycled in e, f Zn-PDOL and g, h Zn-IBPE, respectively. The white dotted lines in TEM images indicate the position and direction of the EDS line scans. i XPS Co 2p spectra of 4.5 V cycled LCO electrodes and j digital images of in vitro experiments for simulating cobalt dissolution in Zn-PDOL and Zn-IBPE. k Schematic illustration of the transition-metal (TM) dissolution and cathode-electrolyte interphase (CEI) in Zn-PDOL and Zn-IBPE. The intensity of all spectra was shown in arbitrary unit (a.u.).
Consistent with the ToF-SIMS analysis, the TEM image of LCO in Zn-PDOL reveals a thick and overgrown CEI (Fig. 5e and Supplementary Fig. 30). Gradual decreases in gradients of Co and O elements in the near-surface region indicate that Zn-PDOL cannot effectively inhibit cobalt dissolution from LCO, resulting in fissure formation on the particle surface (Supplementary Fig. 31) and capacity loss. In contrast, LCO particles collected in Zn-IBPE electrolyte possess a thin CEI shell (around 9 nm) and sharp elemental distribution of Co and O (Fig. 5g, h and Supplementary Fig. 32), indicating effective prevention of Co dissolution and surface corrosion by Zn-IBPE. This effect is further confirmed by XPS Co 2p spectra, where the Co signal is almost undetectable in the LCO electrode cycled with Zn-PDOL, while distinct Co peaks can be observed for the positive electrode cycled with Zn-IBPE (Fig. 5i). In vitro experiments simulating cobalt dissolution also suggest that Co2+ readily dissolves and diffuses in Zn-PDOL, while Co2+ is intercepted by the Zn2+-bridged double network of Zn-IBPE and restricted to the outer region of the bulk electrolyte (Fig. 5j). Similarly, Zn-IBPE can also inhibit Ni dissolution from the high-Ni NMC positive electrode and alleviate the crosstalk of Ni reduction on the Li metal (Supplementary Fig. 33)39. In conclusion, due to its high-voltage stability and ability to prevent transition-metal dissolution, Zn-IBPE facilitates the formation of a thin and robust CEI, leading to promising electrochemical performance (Fig. 5k).
Practicality and versatility of Zn-IBPE for quasi-solid-state batteries
To evaluate the utility of Zn-IBPE, various pouch cells were constructed for performance and safety evaluation. Tests were first conducted on 4 Ah Gr||NMC811 pouch cells (N/P ratio of 1.12) incorporating Zn-PDOL and Zn-IBPE with 2 wt% vinylene carbonate (VC) and 3 wt% 1,3-propane sultone (PS) as additives (Supplementary Table 3). Attributed to the fluidity of liquid monomers, the polymer electrolytes can easily infiltrate into high-loading positive electrodes and porous separators after in-situ polymerization (Supplementary Fig. 34), forming continuous Li+ conductive networks. The introduction of VC/PS additives has been found to further enhance the cycling stability of Li-ion pouch cells with graphite and SiO-graphite-negative electrodes (Supplementary Figs. 35 and 36 and Supplementary Note 3). Though engineered with additives, the Gr||NMC811 pouch cell with Zn-PDOL experiences a rapid decline in capacity within 100 cycles. Conversely, the Gr||NMC811 pouch cell with Zn-IBPE demonstrates stable cycling, maintaining 91.7% of its initial capacity even after 500 cycles without any observable polarization increase (Fig. 6a, b). Meanwhile, apart from enhancing electrochemical performance, thermal-stable Zn-IBPE also offers safety benefits. As illustrated in Fig. 6a, c–e 100% state-of-charge (SOC) Gr|Zn-PDOL|NMC811 pouch cell experienced thermal runaway and critical short circuit during nail penetration (see Supplementary Video 1). In contrast, the pouch cell with Zn-IBPE endured nail penetration without severe short-circuiting owing to its better thermal reliability and mechanical strength than Zn-PDOL (see Supplementary Video 2), avoiding fatal short-circuiting of direct contact between negative and positive electrodes (Supplementary Fig. 37)40.
a Cycling performance of 4 Ah Gr||NMC811 pouch cells with Zn-PDOL and Zn-IBPE electrolytes and b corresponding voltage profiles. Photos of nail penetration tests of 100% SoC Gr||NMC811 pouch cells with c Zn-PDOL and d Zn-IBPE electrolytes with 2 wt% VC and 3 wt% PS, and e voltage-time profiles of pouch cells with Zn-PDOL and Zn-IBPE electrolytes during nail penetration. f Cycling performance and g voltage profiles of 10 Ah SiO-Gr||NMC811 pouch cells using Zn-IBPE electrolytes with 2 wt% VC and 3 wt% PS. h Cycling performance and i voltage profiles of the 18 Ah Li||NMC811 pouch cell using Zn-IBPE electrolytes with 10 vol% TTE between 3 and 4.35 V. (CC constant-current, CV constant voltage).
Beyond traditional lithium-ion batteries with graphite-negative electrodes, high-energy batteries with SiO-graphite (SiO-Gr) and Li-metal electrodes with an N/P ratio of 1:1.12 were utilized to investigate the versatility of Zn-IBPE. Good cycling stability is evident in SiO-Gr||NMC811 with Zn-IBPE with 2 wt% VC and 3 wt% PS additives (Fig. 6f, g), which retains a reversible capacity of 8.58 Ah at 0.33 C over 500 cycles, equating to 85.4% capacity retention (Supplementary Table 4). The SiO-Gr||NMC811 pouch cell with Zn-IBPE also exhibited good rate capability, delivering capacities of 8.5 and 5.5 Ah at discharge rates of 1 C and 2 C, respectively, and recovered almost all initial capacity when the rate returns to 0.1 C (Supplementary Fig. 38). For Li-metal pouch cells with a N/P ratio of 1:2.5, the addition of 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (TTE) additives in Zn2+ ion-bridged polymer electrolytes proved beneficial, further improving cyclability by increasing the F/C atomic ratio in SEI (Supplementary Figs. 39 and 40 and Supplementary Note 4). Consequently, using Zn-IBPE with 10 vol% TTE, the 18 Ah Li-metal pouch cell assembled with 60 μm Li foils and 4.86 mAh cm−2 NMC90 electrodes displayed a high initial Coulombic efficiency of 91.7% (Fig. 6h, i). This Li-metal pouch cell achieved a gravimetric energy density of about 452 Wh kg−1 at 0.33 C based on the total weight of the pouch cells, respectively, and also demonstrated 95.2% capacity retention over 105 cycles ranging between 3 and 4.35 V (Supplementary Table 5). The Zn-IBPE exhibits satisfactory versatility compared with currently mainstream quasi-solid-state and solid-state electrolytes (Supplementary Fig. 41), highlighting its considerable potential for practical applications.
In summary, an ion-bridging strategy has been proposed to address the poor oxidative stability of polyether-based electrolytes. As a demonstration, a Zn2+ ion-bridged PDOL-CTA electrolyte (Zn-IBPE) exhibits an extended electrochemical stable window of over 5 V. The Zn2+–O coordination within Zn-IBPE can directly stabilize the lone pair of oxygen and mitigate the electron extraction from the ethereal oxygen during the oxidation process, thus enabling improved cyclability of 4.5 V Li||LCO cells. Moreover, Zn-IBPE facilitates the formation of LiF-rich SEI and prevents further Li corrosion by electrolytes. On the positive electrode side, Zn-IBPE forms thin and robust CEI and alleviates the transition-metal dissolution/migration from positive to negative electrodes. As a consequence, the 10 Ah SiO-Gr||NMC811 pouch cell with Zn-IBPE presents 85.4% capacity retention after 300 cycles at 0.33 C. The 18 Ah Li|Zn-IBPE|NMC90 pouch cell demonstrates a high energy density of >450 Wh kg−1 and achieves stable cycling (95.2% capacity retention) over 105 cycles at 0.33 C based on the total weight of the pouch cells. These results highlight the practicality and versatility of Zn-IBPE. The ion-bridging strategy also lends creative insights into designing polymer electrolytes for high-energy batteries.
Methods
Preparation of polymer electrolytes
The preparation of electrolytes was conducted in an argon-filled glove box, where both the O2 and H2O content were below 0.1 ppm. For Zn-IBPE, 2 M LiTFSI (99.9%, DodoChem), 5 wt% cellulose triacetate (CTA, Adamas), and 2.5 wt% LiDFOB (99.9%, DodoChem) were first dissolved in DOL (99.9%, DodoChem). Then, the following chemicals were added into the DOL-CTA precursor solution: Zn(TFSI)2 (98%, Aladdin) as an initiator and ion-bridging agent with a molar ratio of [Zn2+]:[DOL] = 1:400, and 20 vol% FEC:DEC (1:1 by volume, DodoChem) as a plasticizer. Similar methods were adopted for the preparation of Zn-PDOL, PDOL-CTA, and PDOL samples without the addition of CTA, Zn(TFSI)2, and both CTA and Zn(TFSI)2, respectively. Various metal ion-bridged PDOL-CTA electrolytes (Mn+-PDOL-CTA) were prepared using different metal trifluoromethanesulfonates (98%, Aladdin) as ion-bridging agents at a molar ratio of [Mn+]:[DOL] = 1:400.
Preparation of coin cells and electrochemical tests
All coin cells were assembled in an argon-filled glove box by sandwiching 16 μm Al2O3-coated polyethylene separators with an average pore size of 0.064 μm and 40% porosity (Semcorp) between 200 μm Li-metal negative electrodes and LiFePO4 (LFP), LiNi0.83Mn0.12Co0.05O2 (NMC83), and LiCoO2 (LCO) positive electrodes, where both the O2 and H2O content were below 0.1 ppm. LFP, NMC83, and LCO-positive electrode slurries were prepared by homogeneously mixing active materials, carbon black, and polyvinylidene difluoride (PVDF, Solef® 5130, Solvay) in a mass ratio of 90:5:5 in n-methyl-2-pyrrolidone (NMP) solvent automatically with a stirring rate of 2000 rpm for 20 min under the ambient condition and then tape-casting on carbon-coated Al foils. The electrodes were obtained by drying at 100 °C overnight in a vacuum condition. All electrodes including LFP, NMC83, and LCO in coin cells possess an active material loading of ~5 mg cm−1. Polymer electrolyte precursors were dropped in the cells. Then, the cells were sealed and waited for the completion of the in-situ polymerization reaction at 25 °C for ~1600 min. The ionic conductivities (σ) of polymer electrolytes were tested by electrochemical impedance spectroscopy (EIS) using stainless steel (SS) symmetric cells separated by polytetrafluoroethylene gaskets with a thickness of 1.25 mm and an inside diameter of 12 mm. The ionic conductivities of polymer electrolytes were calculated by the following equation:
where L, R, and S indicate the thickness, the resistance, and the area of the SS||SS cells. Potentiostatic EIS measurements were carried out on a CHI660E electrochemical working station at an open-circuit voltage in a quasi-stationary condition with a quiet time of 2 s within a frequency range of 100 kHz to 1 Hz and a voltage amplitude of 5 mV for 72 points. The equivalent circuit for fitting impedance spectra was presented in Supplementary Fig. 28e41. Li+ transference numbers (tLi+) were determined by Li||Li symmetric cells using a potentiostatic polarization method, as shown in the following equation42:
where I0 and R0 are the initial current and resistance, Iss and Rss are the steady-state current and resistance after the polarization with an applied potential (ΔV) of 10 mV. The oxidation potentials of the polymer electrolytes were measured by Li||SS cells using the linear sweep voltammetry (LSV) method on a CHI660E at a scan rate of 0.1 mV s−1 at room temperature and determined from the inflection data deviating from the baseline. Electrochemical floating tests for polymer electrolytes were performed on Li||NMC83 cells at voltages ranging from 4.2 to 4.8 V for 10 h each. The galvanostatic charge/discharge tests of coin cells were performed on LAND battery-testing instruments at room temperature (~25 °C). The current densities and cut-off voltages of the Li||LFP, Li||NMC83, and Li||LCO cells were 43 mA g−1/4.0 V, 40 mA g−1/4.3 V, and 56 mA g−1/4.4 V (48 mA g−1/4.5 V), respectively. All measurements were only performed once.
Material characterizations
The 1H and 13C nuclear NMR spectra were obtained using a Bruker AVANCE 400 MHz NMR spectrometer with DMSO-d6 as the deuterated solvent. FT-IR and Raman spectra of various electrolytes were collected from a Nicolet iS50R spectrometer (Thermo Fisher Scientific) and a LabRAM HR800 Raman microscope (HORIBA Scientific), respectively. Plasticizers were excluded from the polymer electrolytes for the above tests to avoid signal interference. TGA tests were carried out on a Pyris 1 TGA analyzer (PerkinElmer) from room temperature to 500 °C under an Ar atmosphere with a heating rate of 10 °C min−1. DSC tests were performed on a DSC 2500 calorimeter (TA Instruments) with a heating rate of 5 °C min−1. XPS was tested by an AXIS Supra+ spectrometer (Kratos Analytical). ToF-SIMS measurements were conducted on a 5-100 instrument (IONTOF GmbH) with a sputtering area of 200 μm × 200 μm. SEM and FIB-SEM images were collected from a Quanta650 FEG (FEI) and Scios 2 HiVac (FEI), respectively. DEMS experiments were investigated by a QAS100 spectrometer (Linglu Instruments) using 4.5 V Li||LCO cells with a LCO loading of ~5 mg cm−2 and a charge/discharge current of ~0.24 mA cm−2. Helium was used as the carrier gas with a constant gas flow rate of 1 ml min−1. Volatile molecules with a molecular mass of 2 (H2), 16 (CH4), 28 (C2H4/CO), 30 (C2H6), 32 (O2), and 44 (CO2) were monitored. TEM images combined with EDS line scans and mapping were obtained by a Talos F200S microscope (Thermo Fisher Scientific). The post-mortem analyses of electrodes were collected from corresponding cells after cycling (Li metal: 100 cycles, LCO and NMC83: 50 cycles, NMC90: 25 cycles), rinsed with 1,2-dimethoxyethane (DME), dried on a hot plate at 45 °C, and sampled from the center of electrodes in an argon-filled glove box. Then, these samples were sealed in sampling tubes and transported from the glove box to the equipment for ex-situ measurements without being exposed to air. Tensile measurements were carried out using the ElectroPuls E1000 test instrument (Instron). X-ray micro-computed tomography (micro-CT) was tested by Xradia 630 Versa (Zeiss). All measurements were only performed once.
Pouch cell assembly and tests
Li-ion and Li-metal pouch cells were assembled in battery dry rooms with dew point temperatures of −20 °C and −45 °C, respectively. The pouch cell design and parameters can be found in Supplementary Tables 3–5. The graphite (Gr) negative electrode slurry was prepared by mixing the active material, carbon black, sodium carboxymethyl cellulose, and styrene butadiene rubber in a weight ratio of 94.5:1:1:3.5 using water as the solvent, while the composition of the SiO-Gr negative electrode slurry was 95.7:0.6:0.92:2.78. The electrode slurries of LiNi0.8Mn0.1Co0.1O2 (NMC811) and LiNi0.9Mn0.05Co0.05O2 (NMC90) electrode materials purchased from Canrd Co., Ltd. were prepared by mixing 96.8 wt% active material, 2 wt% carbon black, and 1.2 wt% PVDF 5130 in NMP automatically with a stirring rate of 5000 rpm for 120 min at a dew point temperature of −20 °C. The negative and positive electrodes were fabricated with slurry coating on Cu and Al foils, respectively, and vacuum dried at 80 °C overnight. Doubled-sided 60 μm Li on 8 μm Cu foils were used for Li-metal pouch cells purchased from China Energy Lithium Co., Ltd. Next, the electrodes were calendered and cut into individual sheets, Z-stacking with Al2O3-coated polyethylene separators, and ultrasonic welded with Al (for positive electrodes) and Ni (for negative electrodes) tabs. Then, the pouch cells were top and side-sealed with packaging Al composite foils, leaving one side unsealed for injecting liquid precursors. After electrolyte filling, pouch cells were vacuum-sealed for subsequent measurements. For Li-ion pouch cells, 2 wt% vinylene carbonate (VC, 99.9%, DodoChem) and 3 wt% 1,3-propane sultone (PS, 99.9%, DodoChem) were added into the polymer electrolyte precursor to help stabilize Gr and SiO-Gr electrodes. Before cycling, Li-ion pouch cells were aged at 45 °C for 24 h, followed by two formation cycles at 0.1 C (Supplementary Fig. 42). The Li-ion pouch cells were tested using a constant-current (CC) constant-voltage (CV) mode with the cut-off voltage and current of 4.25 V and 0.05 C, respectively. For Li-metal pouch cells, 10 vol% 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether (99.9%, DodoChem) were added into the polymer electrolyte precursor to improve the cyclability of Li metal. The galvanostatic charge/discharge tests of pouch cells were performed on NEWARE battery-testing instruments at room temperature (~25 °C). The gravimetric energy densities of pouch cells were calculated based on the total weight of the pouch cells.
Computational details
Quantum chemistry (QC) calculations were performed using the Gaussian 16 package43. An SMD implicit solvation model with DOL parameters was used for all calculations44. The optimization of molecular geometry was carried out using the B3LYP level of theory with the 6-311++G(d) basis set (lanl2DZ basis set only for Ag), and M05-2X/6-31G(d) density functional theory calculations were employed to determine the oxidation potentials of various electrolyte clusters, which were converted from the absolute oxidation potential of Li+/Li by subtracting 1.4 V, as shown in the following equation34:
where G(M) and G(M+) are the free energy of the solvated cluster M and its solvated oxidized form M+ at 295.15 K, and F is the Faraday constant. The atomic charge distributions were calculated from the atomic dipole-corrected Hirshfeld (ADCH) method45. The non-covalent interaction analysis based on reduced density gradient (RDG) and/or interaction region indicator (IRI) methods was implemented by Multiwfn46 and plotted using VMD47. The visualization of molecular geometry was performed by VESTA7. The binding energy between metal cations and PDOL-CTA clusters (Mn+···PDOL-CTA)48 with basis-set superposition-error (BSSE) correction was calculated based on the following equation49:
where E(Mn+···PDOL-CTA), E(PDOL-CTA), E(Mn+), and EBSSE are the energies of Mn+⋯PDOL-CTA, PDOL-CTA, metal ions, and BSSE correction, respectively. The binding energy calculation was conducted at the B3LYP/def2-TZVP theoretical level.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Degen, F., Winter, M., Bendig, D. & Tübke, J. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 8, 1284–1295 (2023).
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021).
Wang, C.-Y. et al. Fast charging of energy-dense lithium-ion batteries. Nature 611, 485–490 (2022).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Zhang, Q.-K. et al. Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries. Nat. Energy 8, 725–735 (2023).
Meng, Y. et al. Designing phosphazene-derivative electrolyte matrices to enable high-voltage lithium metal batteries for extreme working conditions. Nat. Energy 8, 1023–1033 (2023).
Zhang, W. et al. Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries. Nat. Energy 9, 386–400 (2024).
Shi, P. et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 18, 602–610 (2023).
Han, S. et al. Sequencing polymers to enable solid-state lithium batteries. Nat. Mater. 22, 1515–1522 (2023).
Wan, J. et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat. Nanotechnol. 14, 705–711 (2019).
Zhang, Y. et al. Towards better Li metal anodes: challenges and strategies. Mater. Today 33, 56–74 (2020).
Zhao, Q., Liu, X., Stalin, S., Khan, K. & Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019).
Liu, F.-Q. et al. Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci. Adv. 4, eaat5383 (2018).
Huo, S. et al. Challenges of polymer electrolyte with wide electrochemical window for high energy solid-state lithium batteries. InfoMat 5, e12394 (2023).
Cabañero Martínez, M. A. et al. Are polymer-based electrolytes ready for high-voltage lithium battery applications? An overview of degradation mechanisms and battery performance. Adv. Energy Mater. 12, 2201264 (2022).
Su, Y. et al. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 13, 4181 (2022).
Yang, X. et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: main chain or terminal –OH group? Energy Environ. Sci. 13, 1318–1325 (2020).
Mu, K. et al. Hybrid crosslinked solid polymer electrolyte via in-situ solidification enables high-performance solid-state lithium metal batteries. Adv. Mater. 35, 2304686 (2023).
Liang, J.-Y. et al. Enabling a durable electrochemical interface via an artificial amorphous cathode electrolyte interphase for hybrid solid/liquid lithium-metal. Batteries. Angew. Chem. Int. Ed. 59, 6585–6589 (2020).
Li, Z. et al. Interfacial engineering for stabilizing polymer electrolytes with 4 V cathodes in lithium metal batteries at elevated temperature. Nano Energy 72, 104655 (2020).
Zhou, W. et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv. Mater. 31, 1805574 (2019).
Jiang, B. et al. Recent progress of asymmetric solid-state electrolytes for lithium/sodium-metal batteries. EnergyChem 3, 100058 (2021).
Fan, L.-Z., He, H. & Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 6, 1003–1019 (2021).
Yoshida, K. et al. Oxidative-stability enhancement and charge transport mechanism in glyme–lithium salt equimolar complexes. J. Am. Chem. Soc. 133, 13121–13129 (2011).
Nie, K. et al. Increasing poly(ethylene oxide) stability to 4.5 V by surface coating of the cathode. ACS Energy Lett. 5, 826–832 (2020).
Faglioni, F., Merinov, B. V., Goddard, W. A. & Kozinsky, B. Factors affecting cyclic durability of all-solid-state lithium batteries using poly(ethylene oxide)-based polymer electrolytes and recommendations to achieve improved performance. Phys. Chem. Chem. Phys. 20, 26098–26104 (2018).
Cooper, T. E., Carl, D. R., Oomens, J., Steill, J. D. & Armentrout, P. B. Infrared spectroscopy of divalent zinc and cadmium crown ether systems. J. Phys. Chem. A 115, 5408–5422 (2011).
Wang, R. et al. Fabrication of cellulose–graphite foam via ion cross-linking and ambient-drying. Nano Lett. 22, 3931–3938 (2022).
Zhu, C., Soldatov, A. & Mathew, A. P. Advanced microscopy and spectroscopy reveal the adsorption and clustering of Cu(ii) onto TEMPO-oxidized cellulose nanofibers. Nanoscale 9, 7419–7428 (2017).
Li, W. et al. SnF2-catalyzed formation of polymerized dioxolane as solid electrolyte and its thermal decomposition behavior. Angew. Chem. Int. Ed. 61, e202114805 (2022).
Zhao, Q., Liu, X., Stalin, S., & Archer, L. In-built polymer-in-solvent and solvent-in-polymer electrolytes for high-voltage lithium metal batteries. Cell Rep. Phys. Sci. 1, 100146 (2020).
Alabugin, I. V. et al. Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone. Chem. Soc. Rev. 50, 10253–10345 (2021).
Xing, L., Borodin, O., Smith, G. D. & Li, W. Density functional theory study of the role of anions on the oxidative decomposition reaction of propylene carbonate. J. Phys. Chem. A 115, 13896–13905 (2011).
Wang, Y., Xing, L., Li, W. & Bedrov, D. Why do sulfone-based electrolytes show stability at high voltages? Insight from density functional theory. J. Phys. Chem. Lett. 4, 3992–3999 (2013).
Alvarado, J. et al. A carbonate-free, sulfone-based electrolyte for high-voltage Li-ion batteries. Mater. Today 21, 341–353 (2018).
Johnson, E. R. et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010).
Hou, T. et al. Electronegativity-induced single-ion conducting polymer electrolyte for solid-state lithium batteries. Energy Environ. Mater. 6, e12428 (2023).
Chen, Y. et al. Armoring LiNi1/3Co1/3Mn1/3O2 cathode with reliable fluorinated organic–inorganic hybrid interphase layer toward durable high rate battery. Adv. Funct. Mater. 30, 2000396 (2020).
Song, Y. et al. The significance of mitigating crosstalk in lithium-ion batteries: a review. Energy Environ. Sci. 16, 1943–1963 (2023).
Liu, Z. et al. Stretchable separator/current collector composite for superior battery safety. Energy Environ. Sci. 15, 5313–5323 (2022).
Weng, S. et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries. Nat. Commun. 14, 4474 (2023).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Frisch, M. J. et al. Gaussian 16 Rev. A.03. (2016).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Lu, T. & Chen, F.-W. Comparison of computational methods for atomic charges. Acta Phys. Chim. Sin. 28, 1–18 (2012).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Boys, S. F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Acknowledgements
National Key R&D Program of China (2023YFB2503801), the National Natural Science Foundation of China (Grant Nos. 52302253), the Key Program of the National Natural Science Foundation of China (Grant No. 52231009), the Key R&D Program of Hubei Province (2023BAB028). The authors thank the Analytical and Testing Centre of HUST and the State Key Laboratory of Materials Processing and Die & Mold Technology of HUST for NMR, TGA, DSC, SEM, Raman, and XPS measurements.
Author information
Authors and Affiliations
Contributions
T.Y.H., H.H.X., and Y.H.H. conceived and designed the project. T.Y.H., Y.L., and J.K. performed the experiments, material characterizations, electrochemical measurements, and data analysis. D.H.W. assembled the pouch cells and carried out battery performance measurements. T.Y.H. and B.W.J. contributed to the theoretical calculations. T.Y.H., H.H.X., Y.B.H., and Y.H.H. wrote and revised the paper. All authors discussed the results of the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Seung Woo Lee, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hou, T., Wang, D., Jiang, B. et al. Ion bridging enables high-voltage polyether electrolytes for quasi-solid-state batteries. Nat Commun 16, 962 (2025). https://doi.org/10.1038/s41467-025-56324-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56324-9
This article is cited by
-
Anion engineering in a single ether solvent electrolyte enables a 4.7 V high-voltage lithium metal battery
Science China Chemistry (2026)