Abstract
C–C bond cleavage and recombination provide an efficient strategy for the modification and reconstruction of molecule structures. Herein, we present a method for achieving amidation of aryl C(sp2)–H bond through the cleavage and recombination of C–C triple bond with the involvement of nitrous acid esters. This method marks the instance of precise and controlled stepwise cleavage of C–C triple bond, offering a fresh perspective for the cleavage of such bonds. Nitrous acid ester serves as both a radical source and a hydrogen atom transfer (HAT) reagent to functionalize and utilize the two carbon atoms of the C–C triple bond. The alkoxy radical captures the hydrogen atom from the aryl C(sp2)–H bond or N-hydroxyl to induce the 1,3-oxygen radical migration, which is crucial for the subsequent cleavage of the C–C bond.
Similar content being viewed by others
Introduction
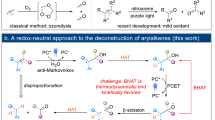
The rapid transformation and reconstruction of molecular structures through the strategies of C–C bond cleavage and recombination have garnered considerable attention from synthetic chemists1,2,3,4,5,6. Compared to synthetic approaches based on functional group chemistry (C–X bond transformation) and non-functional group chemistry (C–H bond transformation) of target molecules, C–C bond cleavage and recombination is virtually irreplaceable4. Not only does this method allows the construction of an entirely new molecular skeleton, but also the simultaneous functionalization of the two terminal carbon atoms3. Currently, this tactic has been extensively implemented in the cleavage and conversion of C–C single bond7,8,9,10,11 and double bonds12,13,14,15,16,17, including the ring-opening of the C–C single bond8, the complex decomposition or ozone oxidation of the C–C double bonds and so on12. Nevertheless, the cleavage, recombination, and transformation of the C–C triple bonds encounter severe challenges, limited to the high bond dissociation energy (>200 kcal/mol)18. Following continuous exploration by synthetic chemists, current protocols for the cleavage and recombination of the C–C triple bonds primarily involve radical19,20,21 and non-radical pathways (Fig. 1a)22,23,24,25. Among them, the radical pathway is categorized into two types based on the position of the substitution in the C–C triple bonds (terminal alkynes and internal alkynes): (1) In terminal alkynes, the bond ends are preferentially coordinated with metals and excited by light, followed by the C–C bond cleavage with the involvement of oxygen and nucleophilic reagents19,20; (2) In internal alkynes, the alkenyl radical is generated by the addition of a radical reagent to the alkyne, which then forms a four-membered cyclic peroxy bond with an oxygen molecule in the transition state to open the ring21. In contrast, the non-radical pathway, primarily relies on transition-metal catalyzed nucleophile addition with two distinct nucleophiles, followed by oxidation using metal species or oxygen to complete the cleavage22,23,24,25. Although significant endeavors have been devoted to the aforementioned approaches, most exhibit limited chemoselectivity, and commonly necessitate the use of precious metal catalysts. Remarkably, only one carbon atom can be functionalized to modify the molecule under the conventional methods of the cleavage and recombination of the C–C triple bonds as is typically done. At the same time, the functionalization of the carbon atom at the other end can be accompanied by ineffective utilization as a leaving group, resulting in low atom economy. Thus, an approach to accomplish the cleavage and recombination of the C–C triple bond is needed to achieve intramolecular difunctionalization by means of a controllable and cost-effective metal catalytic system.
Nitrous acid esters, as cheap, accessible, and practical organic functional reagents, decompose into nitroso and alkoxy radicals upon heating due to the instability of the N–O bond (Fig. 1b)26,27,28. The ntroso radical, or it further oxidized into nitro radical, may couple with other radical reagents or add to unsaturated bonds29,30. The alkoxy radical is extensively known as a hydrogen atom transfer (HAT) reagent for its high activity in capturing active hydrogen, thereby producing a more stable alkyl alcohol31,32. Despite certain progress in the application of nitrous acid esters, most reactions are confined to utilizing only one of the functions. The concurrent use of nitrous acid esters as both nitrogen radical sources and HAT reagents has been rarely reported33,34,35,36,37,38. In addition, nitrous acid esters are currently utilized in the construction of azoxepines33,34,35, nitro compounds36, benzonitriles37 and oximes38, while their role in the synthesis of amides remains unreported. Unlike the conventional amidation, relying on nitrous acid esters to provide a nitrogen source for amidation virtually avoids the problems associated with requiring specific functional group substrates, excessive shrinkage mixture, high by-product formation and low atom economy39,40. Hence, the development of a dual function application of nitrous acid esters, severing both as radical sources and HAT reagents for the construction of amides, is a consistent and imperative pursuit for the synthetic organic community. In light of the role of the α-carbonyl alkyl bromides with the advantages of high reactivity, easy synthesis and good compatibility with sensitive functional groups, it is extensively exploited as an alkyl radical precursor41,42,43. Therefore, we hypothesize that the alkyl radical produced by α-carbonyl alkyl bromine can combine with intramolecular C–C triple bond to form alkenyl radical.
In this work, an alkenyl radical is couple with a nitroso radical, follow by the alkoxy radical as HAT reagents to capture the hydrogen atom of the aryl C(sp2)–H bond or N-hydroxyl. This process occur through two possible pathways, leading to the release of an oxygen radical, which then induces intramolecular difunctionalization by promoting C–C single bond cleavage via a 1,3-O radical migration process (Fig. 1c). This approach provides a method for using nitrous acid esters as a nitrogen source for the directly amidation of aryl C(sp2)–H bond, overcoming the limitations of conventional reactions, which require specific functional group substrates, the addition of condensation agents, and the formation of numerous by-products. Most impressively, the conversion of C–C triple bond into C–C double bond and ultimately C–C single bond under the effect of the dual functionalization of nitrous acid esters has been precisely achieved to gradually realize the cleavage and recombination of the C–C triple bond, thereby opening up a pathway for the cleavage of the C–C triple bond. In particular, a strategy for the cleavage and conversion of the C–C triple bond has been devised to functionalize and utilize the two terminal carbon atoms with the merit of high atom economy. A series of controlled experiments including radical trapping experiments, key intermediate trapping, 18O isotope labeling experiments, and density functional theory (DFT) calculations are employed to reveal and verify the reaction mechanism, the alkoxy radical captures the hydrogen atom from the aryl C(sp2)–H bond or N-hydroxyl inducing the 1,3-oxygen radical migration, which is crucial to the successful cleavage of the subsequent C–C bond.
Results
Reaction optimization
Previous studies guided the initial exploration of optimal conditions using α-carbonyl alkyl bromide and tert-butyl nitrite as model substrates (Table 1)29,44. Comprehensive screening of reaction conditions revealed that alkyne-tethered α-bromocarbonyl (1a, 0.2 mmol), tert-butyl nitrite (2a, 0.4 mmol), CuI (20 mol%), 1,10-phenanthroline (30 mol%), K2CO3 (2.0 equiv.) and H2O (5.0 equiv.), with dimethyl sulfoxide (DMSO, 1.0 mL) as the solvent, yielded the target product 3a in 66% yield at 110 °C under the nitrogen atmosphere for 1 h (Entry 1), and the structure of 3a was definitely confirmed by X-ray crystallography (CCDC 2345226). In the absence of copper/ligand or base, only trace amounts of the target product was obtained, suggesting the indispensable role of the catalyst and base (Entries 2-3). Subsequent assessment of alternative copper catalysts including CuBr, CuCl, Cu2O, CuSO4, Cu(OTf)2, CuBr2, and Cu(OAc)2 based on the preceding experimental findings, demonstrated that none of these catalysts were as effective as CuI (Entries 4-10). From the experimental phenomena, the effect of CuI was found to be superior to that of CuII. Meanwhile, several ligands, including 2,2’-biquinoline, 2,2’-bipyridine, 4,4-di-tert-butyldipyridine, and 2,2’:6’,2”-terpyridine were explored, but none of them had increased the yield of the target product (Entries 11-14). Immediately afterward, the effects of bases were examined in two categories: inorganic bases like Cs2CO3, Na2CO3, and tBuOK, and organic base such as iPr2NEt with the results revealed that the inorganic base K2CO3 was more beneficial, which may be mainly due to the CO32- promotes the reduction of CuII to CuI in this system (Entries 15-18). Additionally, the reaction solvents were also screened, although N,N-dimethylformamide (DMF) performed well, it was still inferior to DMSO (Entries 19-23). Finally, altering the reaction temperature did not enhance conversion efficiency (Entries 24-25). Scale-up experiment was conducted using 1a as the model substrate at a reaction scale of 1.0 mmol, giving product 3a in 57% yield after extending the reaction time to 4 h (Entry 26).
Substrate scope
Throughout the research period, we hypothesized that nitrous acid ester serve as a radical source to provide •NO or •NO2 for the subsequent transformations. With this query, various radical sources 2 were examined under standard reaction conditions (Fig. 2). Strikingly, substituting tert-butyl nitrite with n-butyl nitrite or isoamyl nitrite also yielded product 3a in moderate yields. Nevertheless, only trace amounts of product 3a was detected when Cu(NO3)2 was attempted to be utilized as the •NO source45.
Then, we examined the applicability of α-carbonyl alkyl bromides (Fig. 3). Substrates containing chlorine or methyl in the para- or meta-position substituents of the N-arylacetamide moiety were suitable for the reaction (3b-3d), suggesting that substituent groups on the aryl group had a slight effect on the reaction. Subsequently, the substrates bearing substituents on the nitrogen atom were also compatible with the reaction system, although the efficiency of the reaction varied with diverse substituents (3e-3g). Experimental observations indicated that substrates bearing a benzyl group had poor adaptability with a yield as low as 50%. Conversely, substrates with alkyl substituents like N-allyl and N-isobutyl exhibited good performance, affording products 3f and 3g with yields of 59% and 63%, respectively. At the same time, we further concurrently investigated the adaptability of alkyne-substituted substrates. Various substituents were tested on the aromatic ring, including 4-MeO-C6H4, 4-Me-C6H4, 4-tBu-C6H4, 4-CN-C6H4, and 4-X-C6H4 (X = F, Cl, and Br), all displaying sufficient reaction activity (3h-3r). Electron-withdrawing groups outperformed electron-donating ones in our investigation. Remarkably, the yields decreased with 3-Me-C6H4 and 3-Cl-C6H4 substituents on the alkyne, and the yield of 3o decreased further when the substituent changed to 2-Me-C6H4, indicating a better adaptation of the para-substituent. Moreover, the thiophene group at the terminal alkyne afforded product 3r with a yield of 63%. Unfortunately, only trace amounts of product 3s was detected and the byproduct 1,3-dimethyl-4-pivaloylquinolin-2(1H)-one (3s’) was obtained with a yield of 11% when tert-butyl substituted alkyne was used. This probably attributed to the presence of the tert-butyl group, making the intramolecular cyclization to generate alkenyl radical intermediate more challenging. Subsequently, different α-carbonyl alkyl bromide precursors (R4 = Et, H) were further investigated. The results showed that the target product 3t was obtained with a yield of 60%, while the α-carbonyl secondary alkyl bromide failed to produce the expected product 3u. To our surprise, the substrate of amide linked by pyridine was also suitable for the transformation in 51% yield (3 v). Lastly, the ester derivative of α-carbonyl alkyl bromide compound was attempted without obtaining the product 3w.
[a] Unless otherwise specified, the reactions were performed in the presence of 1 (0.2 mmol), 2a (2.0 equiv.), CuI (20 mol%), 1,10-phen (30 mol%), K2CO3 (2.0 equiv.), H2O (5.0 equiv.) and DMSO (1.0 mL) under N2 at 110 °C for 1 h. [b] 62% of 1s was recovered and 11% of 3s’ (1,3-dimethyl-4-pivaloylquinolin-2(1H)-one) was obtained.
Mechanistic consideration
In order to delve into the possible mechanism of the reaction, a series of experiments were carried out (Fig. 4). As mentioned above, it is conjectured that nitrous acid ester acts as a source of radicals in the system to provide •NO or •NO2. Since •NO was oxidized by oxygen to •NO2, the reaction was studied under different atmospheres (oxygen/air). The results showed a decreased yield of product 3a under air, whereas the yield of the product was trace under oxygen as the oxidation of nitroso to nitro radicals, suggesting that nitrous acid ester offers •NO rather than •NO2 in this reaction (Fig. 4a). Next, the yields of the target products were inhibited to varying degrees by the addition of 2.0 equivalents of radical scavengers such as 2,2,6,6- tetramethylpiperidinyl-1-oxide (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) to the standard reaction, with BHT almost completely inhibiting the formation of target product 3a (Fig. 4b). During the reaction, nitroso radical (4a), tert-butanol oxygen radical (6a), alkenyl radical (7a), and aryl radical (8a) captured by the radical inhibitor were successfully detected by high resolution mass spectrometry (HRMS), implying that the reaction process might proceed via the radical pathway. Notably, the experimental results according to Fig. 4a further confirmed the detection of nitroso radical 4a, while nitro radical 5a remained undetected. Moreover, to demonstrate the mechanism more strongly, 9a or 10a was detected in the basic reaction of 1a and 2a under standard conditions by HRMS (Fig. 4c). Additionally, a cation trapping experiment was undertaken by adding CH3OH under standard conditions and compound 11a was successfully trapped using HRMS (Fig. 4c). Finally, to further elucidate the source of oxygen atoms in the carbonyl and amide functional groups of product 3a, we conducted an isotopic labeling experiment with H218O (Fig. 4d). The HRMS analysis demonstrated that only one oxygen atom in the product was derived from H218O and generated by the amidation. Gas chromatography-mass spectrometry (GC-MS) analysis revealed 45% of 18O, providing further evidence for mechanistic exploration. In addition, another oxygen atom was derived from tert-butyl nitrite.
Based on the experimental results, DFT calculations as well as previous work reported29,31,32,42,46,47,48,49, a reaction mechanism is proposed (Fig. 5, the energy values therein are electronic energy ones with zero-point energy corrections. Please also see the details of Cartesian coordinates in Supplementary Data 1). Firstly, 1a undergoes a bromine atom transfer to CuI via the transition state TS1 (ΔE⧧ = 22.1 kcal/mol) to produce a CuIIBr complex and an α-carbonyl alkyl radical IM1, which readily undergoes (ΔE⧧ = 7.1 kcal/mol) intramolecular cyclization with the C–C triple bond to generate an alkenyl radical intermediate IM242. IM2 was confirmed with radical inhibitor adduction (7a) by HRMS assay. Meanwhile, the consecutive coupling of the •NO radical from tert-butyl nitrite with the radical intermediate IM2 to give the intermediate IM3. The conversion of IM3 to IM6 occurs via two paths. The major path (Path A) is a concerted but nonsynchronous route coupled by a HAT from the aryl ring to the tBuO• with subsequently cyclization of the N = O bond. This path takes an energy advantage of 2.0 kcal/mol (ΔE⧧ = 12.2 kcal/mol (Path A) vs 14.2 kcal/mol (Path B)) to the minor path (Path B), which occurs via stepwise intramolecular cyclization of IM3 to generate a zwitterionic intermediate IM4, and the generation of a neutral intermediate IM5 via an intramolecular proton transfer occur with small activation energies (<15.0 kcal/mol)29. IM3 or IM5 was detected by HRMS. Next, a barrierless HAT from the intermediate IM5 to a tBuO• forms a radical intermediate IM6 (Path B)31,32. The consecutive oxygen shifts from the nitrogen atom of IM6 to the adjacent carbon, and to the carbon close to the benzyl group generate an oxygen-centered radical intermediate IM946. The rate-limiting step of the conversion of IM6 to IM7 holds an activation energy of 42.1 kcal/mol. The cleavage of the C–C bond of IM9 is coupled by an intermolecular electron transfer from IM9 to the CuIIBr complex, which forms a carbonium intermediate IM1047,48. IM10 was confirmed with CH3OH by HRMS assay (11a). The carbonium intermediate IM10 is a robust nucleophile to attack the oxygen of H2O and the target product 3a is formed accompanied by a proton transfer from H2O to the CO32- anion49. Notably, the total exogonic energy of the reaction is up to 104.0 kcal/mol, thus these computational results are in agreement with the experimental observations.
Discussion
In summary, we have developed a strategy to achieve amidation of aryl C(sp2)–H bond through C–C triple bond cleavage and recombination with the involvement of nitrous acid esters. The method accurately achieves C–C triple bond cleavage and recombination with the joint participation of cheap transition metal copper and nitrous acid esters which act as both radical sources and HAT reagents enabling intramolecular carbonylation and amidation. Additionally, this method efficiently avoids the drawbacks of conventional amidations, including the need of condensers and the formation of abundant by-products, as well as modularly synthesis diverse C(sp2)–H bond amidation products under mild reaction conditions. We believe this work will spark the interest of synthetic chemists in C–C bond cleavage and recombination transformations, and inspiring them to further explore new catalytic systems.
Methods
General typical experimental procedures for the synthesis of compounds 3
To a Schlenk tube (10 mL) were added alkyne-tethered α-bromocarbonyls 1 (0.2 mmol), nitrous acid esters 2 (2.0 equiv.), CuI (20 mol%), 1,10-Phen (30 mol%), K2CO3 (2.0 equiv.) and H2O (5.0 equiv.) in DMSO (1.0 mL). The reaction was sealed in N2 atmosphere at 110 °C for 1 h. After the reaction was finished, the mixture was extracted three times with EtOAc. The organic layer was dried over Na2SO4, and then filtration and evaporation of the solvent. And the resulting residue was purified by silica gel column chromatography to obtain the desired products 3.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition number 2345226 (3a). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information file or from the author upon request. For NMR spectra of the compounds in this article, see Supplementary Information.
References
Sivaguru, P., Wang, Z., Zanoni, G. & Bi, X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 48, 2615–2656 (2019).
Murakami, M. & Ishida, N. Cleavage of carbon–carbon σ-bonds of four-membered rings. Chem. Rev. 121, 264–299 (2020).
Liang, Y.-F. et al. Carbon–carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science 361, 171–174 (2018).
Liu, J. et al. Oxygenation of simple olefins through selective allylic C−C bond cleavage: a direct approach to cinnamyl aldehydes. Angew. Chem. Int. Ed. 56, 11940–11944 (2017).
Ge, Y. et al. Palladium-catalyzed cascade carbonylation to α,β-unsaturated piperidones via selective cleavage of carbon–carbon triple bonds. Angew. Chem. Int. Ed. 60, 22393–22400 (2021).
McDonald, T. R., Mills, L. R., West, M. S. & Rousseaux, S. A. L. Selective carbon–carbon bond cleavage of cyclopropanols. Chem. Rev. 121, 3–79 (2020).
Xuan, J., He, X.-K. & Xiao, W.-J. Visible light-promoted ring-opening functionalization of three-membered carbo- and heterocycles. Chem. Soc. Rev. 49, 2546–2556 (2020).
Leonard, D. K., Li, W., Junge, K. & Beller, M. Improved bimetallic cobalt–manganese catalysts for selective oxidative cleavage of morpholine derivatives. ACS Catal. 9, 11125–11129 (2019).
Wang, T. et al. Enantioselective cyanation via radical-mediated C–C single bond cleavage for synthesis of chiral dinitriles. Nat. Commun. 10, 5373–5382 (2019).
Liu, Z.-Q. et al. Transfer vinylation and dienylation via rhodium(I)-catalyzed deketonation of allylic alcohols. ACS Catal. 12, 7030–7036 (2022).
Bailey, P. S. The reactions of ozone with organic compounds. Chem. Rev. 58, 925–1010 (1958).
Park, S., Jeon, W. H., Yong, W.-S. & Lee, P. H. Synthesis of azulen-1-yl ketones via oxidative cleavage of C−C multiple bonds in N-sulfonyl enamides and 1-alkynes under air and natural sunlight. Org. Lett. 17, 5060–5063 (2015).
Li, J., Wei, J., Zhu, B., Wang, T. & Jiao, N. Cu-catalyzed oxygenation of alkene-tethered amides with O2 via unactivated C=C bond cleavage: a direct approach to cyclic imides. Chem. Sci. 10, 9099–9103 (2019).
Kikuchi, T., Yasui, T. & Yamamoto, Y. Cycloaddition of cyclopropenes with alkynes via carbon–carbon double bond cleavage enabled by a ruthenium catalyst: synthesis of cyclopentadienes and cycloheptatrienes. ACS Catal. 13, 9656–9666 (2023).
Song, S., Lai, Y., Tuo, Z., Zhong, J. & Zhou, W. Regio- and chemoselective formal (4+1) carbocyclization of chalcones with internal alkynes via rhodium(III) catalysis. Angew. Chem. Int. Ed. 62, e202305983 (2023).
Xue, W. et al. Direct C−C double bond cleavage of alkenes enabled by highly dispersed cobalt catalyst and hydroxylamine. Angew. Chem. Int. Ed. 62, e202314364 (2023).
Rong, J. et al. Cleavage and reassembly C≡C bonds of ynones to access highly functionalized ketones. ACS Catal. 10, 3664–3669 (2020).
Ragupathi, A., Sagadevan, A., Lin, C.-C., Hwu, J.-R. & Hwang, K. C. Copper(I)-catalysed oxidative C–N coupling of 2-aminopyridine with terminal alkynes featuring a C≡C bond cleavage promoted by visible light. Chem. Commun. 52, 11756–11759 (2016).
Sagadevan, A., Charpe, V. P., Ragupathi, A. & Hwang, K. C. Visible light copper photoredox-catalyzed aerobic oxidative coupling of phenols and terminal alkynes: regioselective synthesis of functionalized ketones via C≡C triple bond cleavage. J. Am. Chem. Soc. 139, 2896–2899 (2017).
Kang, Q.-Q. et al. Copper-catalyzed switchable cyclization of alkyne-tethered α-bromocarbonyls: selective access to quinolin-2-ones and quinoline-2,4-diones. Org. Chem. Front. 9, 6617–6623 (2022).
Asao, N., Nogami, T., Lee, S. & Yamamoto, Y. Lewis acid-catalyzed benzannulation via unprecedented [4+2] cycloaddition of o-alkynyl(oxo)benzenes and enynals with alkynes. J. Am. Chem. Soc. 125, 10921–10925 (2003).
Shimada, T. & Yoshinori, Y. Carbon−carbon bond cleavage of diynes through the hydroamination with transition metal catalysts. J. Am. Chem. Soc. 125, 6646–6647 (2003).
Wang, A. & Jiang, H. Palladium-catalyzed cleavage reaction of carbon−carbon triple bond with molecular oxygen promoted by Lewis acid. J. Am. Chem. Soc. 130, 5030–5031 (2008).
Yan, H. et al. Rhodium-catalyzed C−H annulation of nitrones with alkynes: a regiospecific route to unsymmetrical 2,3-diaryl-substituted indoles. Angew. Chem. Int. Ed. 54, 10613–10617 (2015).
Ma, X. & Song, Q. tert-butyl nitrite mediated synthesis of fluorinated O-alkyloxime ether derivatives. Org. Lett. 21, 7375–7379 (2019).
Huang, T. et al. A Barton nitrite ester-type remote functionalization and cyclization of N-nitrosobenzamides. Org. Chem. Front. 10, 4559–4564 (2023).
Song, W., Liu, Y., Yan, N. & Wan, J.-P. Tunable key [3 + 2] and [2 + 1] cycloaddition of enaminones and α-diazo compounds for the synthesis of isomeric isoxazoles: metal-controlled selectivity. Org. Lett. 25, 2139–2144 (2023).
Liu, Y., Zhang, J.-L., Song, R.-J., Qian, P.-C. & Li, J.-H. Cascade nitration/cyclization of 1,7-enynes with tBuONO and H2O: one-pot self-assembly of pyrrolo[4,3,2-de]quinolinones.Angew. Chem. Int. Ed. 53, 9017–9020 (2014).
Mir, B. A., Singh, S. J., Kumar, R. & Patel, B. K. tert-butyl nitrite mediated different functionalizations of internal alkenes: paths to furoxans and nitroalkenes. Adv. Synth. Catal. 360, 3801–3809 (2018).
Sau, S. & Ma, P. 3-Nitro-coumarin synthesis via nitrative cyclization of aryl alkynoates using tert-butyl nitrite. Chem. Commun. 57, 9228–9231 (2021).
Han, F.-Z., Li, L.-L., Jia, L.-N. & Hu, X.-P. Catalyst-free nitration of the aliphatic C–H bonds of tertiary β-keto esters with tert-butyl nitrite: access to α-quaternary α-amino acid precursors. Tetrahedron Lett. 99, 153844–153848 (2022).
Chen, F. et al. Dehydrogenative N-incorporation: a direct approach to quinoxaline N-oxides under mild conditions. Angew. Chem. Int. Ed. 53, 10495–10499 (2014).
Sau, P., Rakshit, A., Alam, T., Srivastava, H. K. & Patel, B. K. tert-butyl nitrite mediated synthesis of 1,2,4-oxadiazol-5(4H)-ones from terminal aryl alkenes. Org. Lett. 21, 4966–4970 (2019).
Deng, G., Zhong, R., Song, J., Choy, P. Y. & Kwong, F. Y. Assembly of furazan-fused quinolines via an expeditious metal-free [2+2+1] radical tandem cyclization process. Org. Lett. 23, 6520–6524 (2021).
Kilpatrick, B., Heller, M. & Arns, S. Chemoselective nitration of aromatic sulfonamides with tert-butyl nitrite. Chem. Commun. 49, 514–516 (2013).
Wang, F., Zhang, T., Tu, H.-Y. & Zhang, X.-G. Transition-metal-free conversion of trifluoropropanamides into cyanoformamides through C–CF3 bond cleavage and nitrogenation. J. Org. Chem. 82, 5475–5480 (2017).
Li, W., Huang, Z., Zhong, D. & Li, H. Photocatalyst-free activation of sulfamoyl chlorides for regioselective sulfamoyl-oximation of alkenes via hydrogen atom transfer (HAT) and halogen-atom transfer (XAT) relay strategy. Org. Lett. 26, 2062–2067 (2024).
Liu, S., Zhang, Z., Yang, Y. & Huang, J. Recent progress on green methods and technologies for efficient formation of amide bonds. Chin. J. Org. Chem. 44, 409–420 (2024).
Sabatini, M. T., Boulton, L. T., Sneddon, H. F. & Sheppard, T. D. A. Green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2, 10–17 (2019).
Gou, X.-Y. et al. Ruthenium-catalyzed radical cyclization/meta-selective C–H alkylation of arenes via σ-activation strategy. ACS Catal. 11, 4263–4270 (2021).
Li, M. et al. Directed copper-catalyzed tandem radical cyclization reaction of alkyl bromides and unactivated olefins. Org. Lett. 24, 2738–2743 (2022).
Fan, J.-H. et al. Photoredox-catalyzed alkylarylation of N-aryl bicyclobutyl amides with α-carbonyl alkyl bromides: access to 3-spirocyclobutyl oxindoles. Org. Lett. 26, 2073–2078 (2024).
Liu, B., Yu, J.-X., Li, Y., Li, J.-H. & He, D.-L. Copper-catalyzed annulation cascades of alkyne- tethered α-Bromocarbonyls with alkynes: an access to heteropolycycles. Org. Lett. 20, 2129–2132 (2018).
Zhu, Y. et al. From α-keto acids to nitrile oxides enabled by copper nitrate: a facile access to fused isoxazolines. Org. Chem. Front. 9, 676–681 (2022).
Qian, Y.-E. et al. TBN-triggered, manipulable annulations of o-hydroxyarylenaminones for divergent syntheses of oximinochromanones and oximinocoumaranones. Chem. Commun. 57, 12285–12288 (2021).
Keum, H., Ryoo, H., Kim, D. & Chang, S. Amidative β-scission of alcohols enabled by dual catalysis of photoredox proton-coupled electron transfer and inner-sphere Ni-nitrenoid transfer. J. Am. Chem. Soc. 146, 1001–1008 (2023).
Zhang, T.-Y. et al. Iron-catalyzed alkoxyl radical-induced C–C bond cleavage/gem-difluoroalkylation cascade. Org. Lett. 25, 4329–4334 (2023).
Chen, W.-L. et al. Synthesis of 2-aminobenzonitriles through nitrosation reaction and sequential iron (III)-catalyzed C–C bond cleavage of 2-arylindoles. Org. Lett. 20, 3527–3530 (2018).
Acknowledgements
We thank the Natural Science Foundation of Zhejiang Province (No. LY23B020005), the Health Fund of Translational Biomedicine (No. H2024000282), the National 111 Project of China (D 16013), and the National Natural Science Foundation of China (Nos. 22373053 and 42388101) for financial support.
Author information
Authors and Affiliations
Contributions
W.-T. Wei conceived the project and wrote the manuscript. Z.-Y. Wang, N.-N. Dai, Y. Xiao, Y. Zhou, W.-C. Tian performed the experiments. S. Wang, D. Sun, and Y. Wang performed the DFT calculations. Z.-Y. Wang and Q. Li analyzed the data. All authors discussed the results and commented on the manuscript. All authors contributed to the discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Dong-Liang Mo, Yun-Fang Yang and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, ZY., Wang, S., Dai, NN. et al. Carbon-carbon triple bond cleavage and reconstitution to achieve aryl amidation using nitrous acid esters. Nat Commun 16, 993 (2025). https://doi.org/10.1038/s41467-025-56370-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56370-3