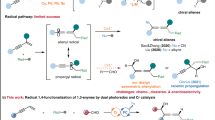
Abstract
Asymmetric multicomponent reactions that aim to control multiple chiral centers with high selectivity in a single step remain an on-gonging challenge. The realm of enantioselective radical-polar crossover transformation achieved through C-H Functionalization has yet to be fully explored. Herein, we present a successful description of a photoredox/Cr-catalyzed enantioselective three-component (hetero)arylalkylation of 1,3-dienes through C-H functionalization. A diverse array of chiral homoallylic alcohols could be obtained in good to excellent yields, accompanied by outstanding enantioselectivity. The asymmetric radical-polar crossover transformation could build two chiral centers simultaneously and demonstrates broad substrate tolerance, accommodating various drug-derived aldehydes, (hetero)aromatics, and 1,3-diene derivatives. Preliminary mechanistic studies indicate the involvement of a radical intermediate, with the chiral allylic chromium species reacting with various aliphatic and aromatic aldehydes through Zimmerman–Traxler transition states enabled by dual photoredox and chiral chromium catalysis.
Similar content being viewed by others
Introduction
Mastering radical transformations with high enantioselectivity, while overcoming rapid, nonselective background reactions, presents a formidable challenge due to the inherent reactivity and unpredictability of highly reactive radical intermediates1,2,3,4,5,6. The radical-metal crossover approach has indeed emerged as one of the most effective strategies for attaining highly selective radical reactions7. This approach involves the integration of radical chemistry with transition metal catalysis, harnessing the reactivity of radicals with the selectivity and tunability of metal catalysts. This integration has propelled the development of enantioselective radical cross-coupling reactions, offering control over the outcome of chemical transformations8,9,10,11,12.
Asymmetric multicomponent reactions (AMCRs) is even more challenging due to the need to control multiple chiral centers with high selectivity in a single step13,14,15,16. Elegant examples regarding difunctionalization of alkenes or 1,3-dienes through radical cross-coupling mechanism have been achieved either through the utilization of chiral copper catalysis17,18,19,20,21,22 or chiral nickel catalysis23,24,25,26,27. Very recently, Xu and co-workers developed an pioneered example of copper-catalyzed enantioselective heteroarylcyanation of alkenes via C-H functionalization under photoelectrochemical conditions20. Alternatively, enantioselective radical reactions that strive to achieve control over multiple chiral centers remain underdeveloped, primarily owing to the scarcity of efficient chiral catalytic systems28,29,30. The Nozaki-Hiyama-Kishi (NHK) reaction31,32 is a significant synthetic method in organic chemistry, particularly renowned for its mild conditions and broad applicability in the construction of homoallylic alcohol motifs and has been widely employed in natural product synthesis33,34,35,36,37. In recent years, the photocatalytic NHK reaction has rising as an attractive topic to form homoallylic alcohols with dual photoredox/chromium catalysis, since the seminal works conducted by Glorius38,39,40 and Kanai41,42 (Fig. 1a). Additionally, low-valent chromium catalysis has also become a powerful catalysis, successfully applied in cross-coupling reactions by Zeng and others43,44,45,46. More recently, elegant photocatalytic three-component NHK reactions to achieve enantioselective dialkylation, utilizing either hantzsch esters39 or alkyl halides47,48 as alkyl radical precursors have also been achieved (Fig. 1b)47,48,49,50,51,52,53,54,55,56,57. These methodologies showcase the versatility of three-component reactions in constructing complex, enantioselective dialkylated products. However, (hetero)arylalkylation enabled by dual photoredox/chromium catalysis remains unknown. Furthermore, radical-polar crossover reaction involving (hetero)arenes via C—H functionalization remains an unsolved problem due to mismatch catalytic cycle and the lack of suitable chiral ligand. Meanwhile, heteroarenes are common motifs in medicinal chemistry and material science58. Developing effective methods for radical (hetero)arylalkylation directly form C- H functionalization would greatly expand the synthetic utility of these compounds, enabling the creation of new molecules with potential applications in drug discovery and advanced materials.
In this work, we envision whether (hetero)aromatics can be oxidized to radical cations by strong oxidizing photocatalysis under visible light condition. These radical cations can then sequentially be trapped by 1,3-dienes59,60 and chiral chromium catalysis to generate chiral allylic chromium intermediates. Finally, chiral homoallylic alcohols can be generated through polar addition via Zimmerman–Traxler transition state (Fig. 1c). This enantioselective three component coupling reaction, initiates from C-H functionalization of (hetero)aromatics, will complement the development of asymmetric radical-polar crossover chemistry.
Results and discussion
Reaction design and optimization
With this concept in mind, we demonstrated our design by mixing thiophene (1a), butadiene (2a) and aliphatic aldehyde (3a) with catalytic amount of chromium chloride, chiral bisoxazoline (S, R)-L1, and organophotocatalyst (PC1, Mes2Acr-tBu2BF4). This resulted in the formation of desired homoallylic alcohol 4 in 31% isolated yield with a 7:1 diastereomeric ratio and 96% enantiomeric excess (Table 1, entry 1). Firstly, a screening of various chiral bisoxazoline ligands (L2-L10) were conducted, revealing that only the chiral bisoxazoline (S, R)-L1 or (S, R)-L3 were capable of achieving an enantioselectivity of 96% ee for the formation of product 4 (entries 2-10). When the amount of PC1 increased to 10 mol%, the yield of 4 increased to 47% isolated yield without decreasing enantioselectivity (entry 11). Pleasingly, the homoallylic product 4 could be obtained in 79% isolated yield with a 10:1 dr & 98% ee when the mixture of THF and DCE was employed, which remained as the optimized conditions for the current enantioselective radical-polar crossover transformation (entry 12). Different organic solvents (entries 13-14), additives (entries 15-16) and reaction times (entries 17-18) were then screened, but only lower isolated yields of 4 were obtained. Two different chiral bisoxazoline ligands (L11 & L12) were also synthesized, and both the yield and enantioselectivity of 4 decreased when employing either an electron-donating group (entry 19) or an electron-withdrawing group (entry 20). We also investigated several different photocatalysts and clearly showed that PC1 remained the optimal photocatalyst (entries 21-24). Application of the enantiomeric ligand (R, S)-bisoxazoline ligand ent-L1 accordingly gave the enantiomer ent-4 with 79% isolated yield and −97% ee (entry 25). Control experiments, including chiral ligand, chromium catalyst, photocatalyst and light, clearly demonstrated that these elements are necessary to achieve this enantioselective radical-polar crossover transformation (entries 26-29).
Substrate scope
With the optimal condition in hand, we initially investigated a variety of aliphatic aldehydes (Fig. 2, up). Pleasingly, the dual catalytic system tolerates various functional groups such as phenyl (5 & 10), methyl (6), ether (7), imide (8), chloride (9), cyclopropane (11), cyclobutane (12), cyclohexane (13), tetrahydro-2H-pyran (14) and piperidine (15) with excellent yields and high enantiomeric excess. The X-ray-structure (CCDC 2344646) of product 8 unambiguously revealed the absolute configuration as (S, S) by using the (S, R)-L1 ligand and all other compounds were assigned in analogy. Surprisingly, tertiary aldehyde, methacrylaldehyde and 3-phenylpropiolaldehyde could all be tolerated and the desired products 16-18 with good yields and high enantioselectivity. We then investigated various aromatic aldehydes including hydro (19), fluoro (20), chloride (21), bromide (22), methyl (23), nitrile (24), boron (25), trifluoromethyl (26), phenyl (27) and methoxy (28) with excellent yields and very high enantiomeric excess (Fig. 2, down). Based on these results, we also investigated different positions on the aromatic ring, including para (19-28), meta (29) and ortho (30,31) positions, as well as di-substituted (32) and tri-substituted (33) aromatic rings. Then, naphthyl group (34), pyridine (35), benzofuran (36), furan (37), thiophene (38) were all screened, with the desired homoallylic alcohols could also be obtained in good to excellent yield and enantiomeric excess.
Reaction conditions were as follows: in the glovebox, CrCl2 (10 mol%) and (S, R)-L1 (12 mol%) were added to a mixture of THF (0.3 mL) and DCE (1.4 mL). The mixture was stirred for 15 min at room temperature and transferred to another vial containing Mes2Acr-tBu2BF4 (PC1, 10 mol%), 1a (0.4 mmol, 2 equiv.), 2a (0.6 mmol, 3 equiv., 2 M in THF, 0.3 mL) and aliphatic or aromatic aldehyde 3 (0.2 mmol, 1 equiv.). The final reaction mixture was irradiated with 30 W 450 nm blue LEDs with a cooling fan for 17 h outside under N2. Isolated yields. The ee values were determined by HPLC analysis on a chiral stationary phase.
With these satisfied results in hand, we further explored this dual catalytic reaction using relatively complex aldehydes (Fig. 3). For example, the complex aromatic aldehydes that originally derive from Oxaprozin (39), Fenofibrate (40), L-Phenylalanine (41), Ibuprofen (42), Gemfibrozil (43), L-Proline (44), L-Perillaldehyde (45), Estrone (46), D-Fructose (47) were all tolerated in this current dual catalytic system. Remarkably, various functional group such as heterocycles (39), chloride (40), amine (41, 44), ester (39, 40, 42, 43, 46, 47), ether (43), alkene (45), ketone (46), carbohydrates (47) were accommodated.
Reaction conditions were as follows: in the glovebox, CrCl2 (10 mol%) and (S, R)-L1 (12 mol%) were added to a mixture of THF (0.3 mL) and DCE (1.4 mL). The mixture was stirred for 15 min at room temperature and transferred to another vial containing Mes2Acr-tBu2BF4 (PC1, 10 mol%), 1a (0.4 mmol, 2 equiv.), 2a (0.6 mmol, 3 equiv., 2 M in THF, 0.3 mL) and drug derivatized aldehyde 3 (0.2 mmol, 1 equiv.). The final reaction mixture was irradiated with 30 W 450 nm blue LEDs with a cooling fan for 17 h outside under N2. Isolated yields. The ee values were determined by HPLC analysis on a chiral stationary phase.
We then began to explore the substrate scope regarding various heterocycles and dienes (Fig. 4). Firstly, a library of substituted thiophenes was explored, including various functional groups, such as amine (48), ester (49), alkyl (50), ether (51-52), disubstituted methyl (53-54) and amino ester from (S)-thienylalanine (55). This dual catalytic system could also extend to substituted pyridine (56) and electron rich aromatic ring (57). Then, a number of pyrroles containing methyl (58), Boc (59) and benzylic group (60) with good to excellent yields and high enantiomeric excess. In addition to 1,3-butadiene, which is derived from petroleum processing, a diverse range of substituted 1,3-dienes were also investigated, including isoprene (61) and myrcene (62). The desired homoallylic alcohols (61-63) containing an all-carbon quaternary center were obtained in moderate to excellent yields with high enantioselectivity. Furthermore, we explored a variety of 2-substituted 1,3-dienes, including linear alkyl (63), phenyl group (64), methyl (65), fluoro (66 & 67), yielding the desired chiral homoallylic alcohols containing a quaternary carbon center with isolated yields ranging from 72% to 87% smoothly.
Reaction conditions were as follows: in the glovebox, CrCl2 (10 mol%) and (S, R)-L1 (12 mol%) were added to a mixture of THF (0.3 mL for 1,3-Butadiene 2a and 0.6 mL for the rest substituted dienes 2) and DCE (1.4 mL). The mixture was stirred for 15 min at room temperature and transferred to another vial containing Mes2Acr-tBu2BF4 (PC1, 10 mol%), (hetero)arene 1 (0.4 mmol, 2 equiv.), 2a (0.6 mmol, 3 equiv., 2 M in THF, 0.3 mL) or substituted diene 2 (0.6 mmol, 3 equiv.) and aromatic aldehyde 3ab (0.2 mmol, 1 equiv.). The final reaction mixture was irradiated with 30 W 450 nm blue LEDs with a cooling fan for 17 h outside under N2. Isolated yields. The ee values were determined by HPLC analysis on a chiral stationary phase.
Synthetic application and mechanistic studies
In order to explore the mechanistic study, we firstly test the model reaction at the gram-scale level. The chiral homoallylic alcohol 29 could be obtained in 88% isolated yield and 97% ee (Fig. 5a). when the (S, R)-L1 was replaced by (R, S)-L1 under the optimized condition, the chiral alcohol 68 could be obtained in 90% isolated yield with 11:1 dr and −97% ee. Through sequential Mitsunobu reaction and hydrolysis, chiral alcohols 69 and 70 could be obtained smoothly with good yields. The alkene motif of product 29 could be transferred to alkyl (71) through hydrogenation and alcohol (72) through hydroboration–oxidation reaction. The unsaturated lactone 73 could be obtained in 78% isolated yield through acylation and metathesis. When a radical inhibitor (TEMPO) was added to the model reaction, the desired product 4 did not form, suggesting that the reaction may proceed through a radical mechanism (Fig. 5b). In order to further investigate the reaction mechanism, a 1,3-diene containing a three-membered ring 2i was synthesized and subjected the optimal condition, the desired three component coupling product could not be formed, instead, a ring-opening product 74 may be formed based on the result of HRMS (Fig. 5c). In order to exclude the ketyl radical intermediate generated from the corresponding aldehyde, we synthesized the aldehyde 3as containing a three-membered ring and subjected it to our optimized condition. The chiral homoallylic alcohols 75 and 76 were obtained in a total isolated yield of 80%. The products 75 and 76 containing the three membered rings, and we also did not detect any ring-opening product, suggesting that the ketyl radical did not form in the current catalytic system (Fig. 5d). UV-Vis experiment and Stern-Volmer quenching experiment demonstrated that PC1 was the only species capable of absorbing visible light (Fig. 5e), and only thiophene 1a could be quenched by the photoexcited photocatalyst (Fig. 5f). A linear relationship was observed between enantiopurities of (S, R)-L1 and the product 4, which suggests that only a monomeric chromium complex bearing a single chiral ligand is involved in the stereo-determining step (Fig. 5g). The quantum yield of the model reaction was measured (Φ =0.046) and indicated that this dual catalytic system most likely goes through a photocatalytic reaction. According to these mechanistic studies as well as previous reports36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51, a mechanistic catalytic cycle is proposed in Fig. 5h. The thiophene 1a (Ep/2ox = 1.70 V vs SCE)20 could be oxidized to radical cation I by organophotocatalyst (PC1, ERed[PC*/PC−⋅] = 2.0 V vs SCE)61,62,63, after being trapped by 1,3-diene and deprotonated, allylic radical intermediate III is formed; the chiral chromium (II) species will react with intermediate III to generate chiral allylic chromium (III) intermediate IV, which then reacts with both aliphatic and aromatic aldehydes 3 through Zimmerman-Traxler transition state to generate chiral homoallylic alcohols in high to excellent isolated yields and excellent enantiomeric excess with anti-selectivity. Meanwhile, both the chiral chromium catalytic cycle and the photoredox catalytic cycle could undergo turnover under electron transfer conditions (Fig. 5h).
In conclusion, a general, mild enantioselective radical-polar crossover three-component synthetic transformation has been achieved, enabled by dual chiral chromium catalysis and photoredox catalysis. This efficient catalytic platform initiates from the C-H bond of (hetero)aromatics, tolerates various aldehydes and 1,3-dienes with high yields and excellent enantioselectivity. A variety of chiral homoallylic alcohols could be obtained efficiently and scaled up easily. We anticipate that the integration of chiral chromium catalysis with photoredox catalysis will catalyze additional advancements in enantioselective radical-polar crossover chemistry. Furthermore, this combined dual catalytic approach holds significant promise for broadening the applications of such synthetic transformations in drug discovery, materials science, and beyond.
Methods
General method for the photoredox/Cr-catalyzed enantioselective three-component (hetero)arylalkylation of 1,3-dienes
Two oven-dried vials equipped with PTFE-coated stir bar and Teflon® septum were used. In the glovebox, to vial A was added CrCl2 (10 mol%), (S, R)-L1 (12 mol%) and a mixture of THF (0.6 mL, or 0.3 mL if the 2 is 1,3-Butadiene in a 2 M THF solution) and DCE (1.4 mL). The mixture was stirred for 15 min at room temperature in the glovebox. All solid substrates including Mes2Acr-tBu2BF4 (PC1, 10 mol%) and other starting materials in solid forms (scale: 1 (0.4 mmol, 2 equiv.), 2 (0.6 mmol, 3 equiv.) and 3 (0.2 mmol, 1 equiv.)) were added into vial B and the vial was then transferred into the glovebox. The mixture in vial A was transferred into vial B by using a single-channel pipette in the glovebox. Vial B was sealed, moved out of the glovebox and all other starting materials in liquid forms (scale: 1 (0.4 mmol, 2 equiv.), 2 (0.6 mmol, 3 equiv.) and 3 (0.2 mmol, 1 equiv.)) were injected into the vial B by using micro syringes, to form the final reaction mixture with final concentration 0.1 M in a mixture of THF and DCE (3:7). Upon completion, vial B was further sealed by parafilm and was set to stir (500 rpm) and irradiated with four 30 W 450 nm blue LED lamps (approximate 3 cm away, with cooling fan to keep the reaction at room temperature). After 17 h, the reaction mixture was transferred to a 100 mL round-bottom flask and concentrated in vacuo. The crude reaction mixture was purified by flash column chromatography (silica gel), eluting with indicated solvent system to give the desired product.
Data availability
Materials and methods, detailed optimization studies, experimental procedures, mechanistic studies and NMR spectra are available in the Supporting Information and from the corresponding authors upon request. CCDC 2344646 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: +44 1223 336033.
References
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3295 (2003).
Mondal, S. et al. Enantioselective Radical Reactions Using Chiral Catalysts. Chem. Rev. 122, 5842–5976 (2022).
Genzink, M. J., Kidd, J. B., Swords, W. B. & Yoon, T. P. Chiral Photocatalyst Structures in Asymmetric Photochemical Synthesis. Chem. Rev. 122, 1654–1716 (2022).
Großkopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 122, 1626–1653 (2022).
Steinlandt, P. S., Zhang, L. & Meggers, E. Metal Stereogenicity in Asymmetric Transition Metal Catalysis. Chem. Rev. 123, 4764–4794 (2023).
Proctor, R. S. J., Colgan, A. C. & Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 12, 990–1004 (2020).
Chan, A. Y. et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 122, 1485–1542 (2022).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 116, 10035–10074 (2016).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-Catalyzed Asymmetric Reactions Involving Radicals. Acc. Chem. Res. 53, 170–181 (2020).
Wang, F., Chen, P. & Liu, G. Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Lipp, A., Badir, S. O. & Molander, G. A. Stereoinduction in Metallaphotoredox Catalysis. Angew. Chem. Int. Ed. 60, 1714–1726 (2021).
de Graaff, C., Ruijter, E. & Orru, R. V. A. A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 41, 3969–4009 (2012).
Zhu, S., Zhao, X., Li, H. & Chu, L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 50, 10836–10856 (2021).
Huang, H., Lin, Y. & Gong, L. Recent Advances in Photochemical Asymmetric Three‐Component Reactions. Chem. Rec. 23, e202300275 (2023).
Lu, F.-D. et al. Recent advances in transition-metal-catalysed asymmetric coupling reactions with light intervention. Chem. Soc. Rev. 50, 12808–12827 (2021).
Li, Z. L., Fang, G. C., Gu, Q. S. & Liu, X. Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 49, 32–48 (2020).
Zhang, G. et al. Asymmetric Coupling of Carbon‐Centered Radicals Adjacent to Nitrogen: Copper‐Catalyzed Cyanation and Etherification of Enamides. Angew. Chem. Int. Ed. 59, 20439–20444 (2020).
Cheng, X.-Y. et al. A Counterion/Ligand-Tuned Chemo- and Enantioselective Copper-Catalyzed Intermolecular Radical 1,2-Carboamination of Alkenes. J. Am. Chem. Soc. 144, 18081–18089 (2022).
Lai, X. L. & Xu, H. C. Photoelectrochemical Asymmetric Catalysis Enables Enantioselective Heteroarylcyanation of Alkenes via C-H Functionalization. J. Am. Chem. Soc. 145, 18753–18759 (2023).
Qian, S., Lazarus, T. M. & Nicewicz, D. A. Enantioselective Amino- and Oxycyanation of Alkenes via Organic Photoredox and Copper Catalysis. J. Am. Chem. Soc. 145, 18247–18252 (2023).
Lu, F. D., Lu, L. Q., He, G. F., Bai, J. C. & Xiao, W. J. Enantioselective Radical Carbocyanation of 1,3-Dienes via Photocatalytic Generation of Allylcopper Complexes. J. Am. Chem. Soc. 143, 4168–4173 (2021).
Dong, Z., Song, L. & Chen, L. A. Enantioselective Ni-Catalyzed Three-Component Dicarbofunctionalization of Alkenes. ChemCatChem 15, 1–9 (2023).
Pan, Q., Ping, Y. & Kong, W. Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings. Acc. Chem. Res. 56, 515–535 (2023).
Li, X. et al. Three-component enantioselective alkenylation of organophosphonates via nickel metallaphotoredox catalysis. Chem 9, 154–169 (2023).
Wei, X., Shu, W., García-Domínguez, A., Merino, E. & Nevado, C. Asymmetric Ni-Catalyzed Radical Relayed Reductive Coupling. J. Am. Chem. Soc. 142, 13515–13522 (2020).
Qian, P. et al. Catalytic enantioselective reductive domino alkyl arylation of acrylates via nickel/photoredox catalysis. Nat. Commun. 12, 6613 (2021).
Pitzer, L., Schwarz, J. L. & Glorius, F. Reductive radical-polar crossover: Traditional electrophiles in modern radical reactions. Chem. Sci. 10, 8285–8291 (2019).
Wiles, R. J. & Molander, G. A. Photoredox-Mediated Net-Neutral Radical/Polar Crossover Reactions. Isr. J. Chem. 60, 281–293 (2020).
Sharma, S., Singh, J. & Sharma, A. Visible Light Assisted Radical‐Polar/Polar‐Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal. 363, 3146–3169 (2021).
Okude, Y., Hirano, S., Hiyama, T. & Nozaki, H. Grignard-type carbonyl addition of allyl halides by means of chromous salt. A chemospecific synthesis of homoallyl alcohols. J. Am. Chem. Soc. 99, 3179–3181 (1977).
Namba, K., Wang, J., Cui, S. & Kishi, Y. Surprisingly Efficient Catalytic Cr-Mediated Coupling Reactions. Org. Lett. 7, 5421–5424 (2005).
Fürstner, A. Carbon-Carbon Bond Formations Involving Organochromium(III) Reagents. Chem. Rev. 99, 991–1045 (1999).
Tian, Q. & Zhang, G. Recent Advances in the Asymmetric Nozaki–Hiyama–Kishi Reaction. Synthesis 48, 4038–4049 (2016).
Gil, A., Albericio, F. & Álvarez, M. Role of the Nozaki–Hiyama–Takai–Kishi Reaction in the Synthesis of Natural Products. Chem. Rev. 117, 8420–8446 (2017).
Hargaden, G. C. & Guiry, P. J. The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction. Adv. Synth. Catal. 349, 2407–2424 (2007).
Zeng, X. Recent Advances in Chromium-Catalyzed Organic Transformations. Synlett 31, 205–210 (2020).
Schwarz, J. L., Schäfers, F., Tlahuext-Aca, A., Lückemeier, L. & Glorius, F. Diastereoselective Allylation of Aldehydes by Dual Photoredox and Chromium Catalysis. J. Am. Chem. Soc. 140, 12705–12709 (2018).
Schwarz, J. L., Huang, H., Paulisch, T. O. & Glorius, F. Dialkylation of 1,3-Dienes by Dual Photoredox and Chromium Catalysis. ACS Catal. 10, 1621–1627 (2020).
Schäfers, F. et al. Asymmetric Addition of Allylsilanes to Aldehydes: A Cr/Photoredox Dual Catalytic Approach Complementing the Hosomi–Sakurai Reaction. ACS Catal. 12, 12281–12290 (2022).
Mitsunuma, H., Tanabe, S., Fuse, H., Ohkubo, K. & Kanai, M. Catalytic asymmetric allylation of aldehydes with alkenes through allylic C(sp3)-H functionalization mediated by organophotoredox and chiral chromium hybrid catalysis. Chem. Sci. 10, 3459–3465 (2019).
Tanabe, S., Mitsunuma, H. & Kanai, M. Catalytic Allylation of Aldehydes Using Unactivated Alkenes. J. Am. Chem. Soc. 142, 12374–12381 (2020).
Cong, X. & Zeng, X. Mechanistic Diversity of Low-Valent Chromium Catalysis: Cross-Coupling and Hydrofunctionalization. Acc. Chem. Res. 54, 2014–2026 (2021).
Li, J. & Knochel, P. Chromium-Catalyzed Cross-Couplings and Related Reactions. Synthesis 51, 2100–2106 (2019).
Luo, Z., Zhang, X., Li, Z., Luo, M. & Zeng, X. Mild ketyl radical generation and coupling with alkynes enabled by Cr catalysis: stereoselective access to E-exocyclic allyl alcohols. Chem. Sci. 15, 11428–11434 (2024).
Zeng, X. & Cong, X. Chromium-catalyzed transformations with Grignard reagents-new opportunities for cross-coupling reactions. Org. Chem. Front. 2, 69–72 (2015).
Xiong, Y. & Zhang, G. Enantioselective 1,2-Difunctionalization of 1,3-Butadiene by Sequential Alkylation and Carbonyl Allylation. J. Am. Chem. Soc. 140, 2735–2738 (2018).
Xia, X. & Wang, Z. Cr-Catalyzed Diastereo- and Enantioselective Synthesis of β-Hydroxy Sulfides and Selenides. ACS Catal. 12, 11152–11158 (2022).
Huang, H., Bellotti, P. & Glorius, F. Merging Carbonyl Addition with Photocatalysis. Acc. Chem. Res. 55, 1135–1147 (2022).
Katayama, Y., Mitsunuma, H. & Kanai, M. Recent Progress in Chromium-Mediated Carbonyl Addition Reactions. Synthesis 54, 1684–1694 (2022).
Gualandi, A., Calogero, F., Pinosa, E., Corbisiero, D. & Cozzi, P. G. Developing Organometallic Nucleophilic Reagents Via Photoredox Catalysis. Synthesis 55, 3737–3758 (2023).
Zhang, H., Chen, B. & Zhang, G. Enantioselective 1,2-Alkylhydroxylmethylation of Alkynes via Chromium/Cobalt Cocatalysis. Org. Lett. 22, 656–660 (2020).
Zhang, F.-H., Guo, X., Zeng, X. & Wang, Z. Catalytic Enantioconvergent Allenylation of Aldehydes with Propargyl Halides. Angew. Chem. Int. Ed. 61, e202117114 (2022).
Zhang, F.-H., Guo, X., Zeng, X. & Wang, Z. Asymmetric 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis. Nat. Commun. 13, 5036 (2022).
Guo, X., Shi, Z., Zhang, F.-H. & Wang, Z. Cr-Catalyzed Regio-, Diastereo-, and Enantioselective Reductive Couplings of Ketones and Propargyl Halides. ACS Catal. 13, 3170–3178 (2023).
Shen, H., Zhang, Z., Shi, Z., Gao, K. & Wang, Z. A modular approach to stereoselective homoaldol reaction via photoredox/Cr/Co triple catalysis. Chem 10, 998–1014 (2024).
Lin, S. J. et al. Activation of Chromium Catalysts by Photoexcited Hantzsch Ester for Decarboxylative Allylation of Aldehydes with Butadiene. Org. Lett. 23, 8077–8081 (2021).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Holmes, M., Schwartz, L. A. & Krische, M. J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 118, 6026–6052 (2018).
Xiong, Y., Sun, Y. & Zhang, G. Recent advances on catalytic asymmetric difunctionalization of 1,3-dienes. Tetrahedron Lett. 59, 347–355 (2018).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C-H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Chen, B., Du, Y. & Shu, W. Organophotocatalytic Regioselective C−H Alkylation of Electron‐Rich Arenes Using Activated and Unactivated Alkenes. Angew. Chem. Int. Ed. 61, e202200773 (2022).
Pitzer, L., Sandfort, F., Strieth-Kalthoff, F. & Glorius, F. Carbonyl–Olefin Cross-Metathesis Through a Visible-Light-Induced 1,3-Diol Formation and Fragmentation Sequence. Angew. Chem. Int. Ed. 57, 16219–16223 (2018).
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22201179 & 22471168 to H.-M. H.), the startup funding from ShanghaiTech University (H.-M. H.), Postdoctoral Fellowship Program of CPSF (No. GZC20231674 to S.-Y. T.) and Double First-Class Initiative Fund of ShanghaiTech University (S.-Y. T.). We sincerely thank the great support from Prof. Frank Glorius. We also thank Prof. Chaodan Pu, Zhuo Zhao, Dr. Na Yu, Shu-Ya Wen and Ying Zhang for help with mechanistic study and X-ray analysis.
Author information
Authors and Affiliations
Contributions
H.-M.H. conceived and directed the research; H.-M.H. and S.-Y.T. designed the experiments; S.-Y.T., Z.-J. W., Y.A. and N.W. performed all the experiments and analyzed all the data. H.-M.H. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, SY., Wang, ZJ., Ao, Y. et al. Photoredox/Cr-catalyzed enantioselective radical-polar crossover transformation via C-H functionalization. Nat Commun 16, 1354 (2025). https://doi.org/10.1038/s41467-025-56372-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56372-1