Abstract
Recalcitrant bacterial infections can be caused by various types of dormant bacteria, including persisters and viable but nonculturable (VBNC) cells. Despite their clinical importance, we know fairly little about bacterial dormancy development and recovery. Previously, we established a correlation between protein aggregation and dormancy in Escherichia coli. Here, we present further support for a direct relationship between both. Our experiments demonstrate that aggregates progressively sequester proteins involved in energy production, thereby likely causing ATP depletion and dormancy. Furthermore, we demonstrate that structural features of protein aggregates determine the cell’s ability to exit dormancy and resume growth. Proteins were shown to first assemble in liquid-like condensates that solidify over time. This liquid-to-solid phase transition impedes aggregate dissolution, thereby preventing growth resumption. Our data support a model in which aggregate structure, rather than cellular activity, marks the transition from the persister to the VBNC state.
Similar content being viewed by others
Introduction
Bacteria have evolved diverse strategies to survive antibiotic treatment. The most well-known strategy is antibiotic resistance in which genetic changes allow cells to grow in the presence of antibiotics1. Besides resistance, bacteria can endure antibiotic treatment by adopting a tolerant persister state. Persister cells are phenotypic variants in an isogenic population that survive lethal antibiotic exposure. They are characterized by no or slow growth and typically constitute a small fraction of the population. Moreover, persisters can revert to the antibiotic-susceptible, growing state, which allows them to recolonize the environment when the antibiotics are removed2. This temporal tolerance renders persister cells important contributors to treatment failure and infection relapse3,4,5,6,7. In addition to several active mechanisms that allow persisters to survive antibiotic treatment, such as efflux pumps8,9,10,11,12,13, persister cells are generally assumed to be tolerant because they reside in a dormant state14,15,16. Indeed, persister cells exhibit low metabolism8,17, reduced energy levels18,19,20 and no or slow growth8,21. Emerging evidence suggests that persistence is not the end-stage of dormancy. Rather, it is hypothesized that persisters correspond to a shallowly dormant state and can further transition into a deeper dormant viable but nonculturable (VBNC) state from which they cannot easily recover to resume growth22,23,24,25,26. VBNC cells are a subpopulation of nongrowing cells that show metabolic activity, but that cannot restart growth on standard laboratory media that would otherwise support their proliferation. Instead, they need a specific trigger to switch back to the antibiotic-susceptible, growing state27,28. Because of their antibiotic tolerance and regrowth potential, VBNC cells have also been associated with the chronic nature of infections29,30.
Because both persister and VBNC cells have been associated with the recurrence of infections3,4,5,6,29,30, it is important to understand how the transition to these different dormant states occurs. It was demonstrated that dormancy is correlated with protein aggregation19,24,25,31,32,33,34,35,36,37,38 and the hypothesis was put forward that aggregation could drive dormancy development24,25. Aggregation is suggested to do so by sequestering, and thereby depleting, proteins involved in key cellular processes, leading to the shutdown of vital pathways and the induction of dormancy24,25,35,39. This hypothesis is supported by the observation that aggregates contain various proteins involved in essential metabolic processes, energy metabolism, transcription and translation19,24,25,33,37. Nevertheless, no clear link between the aggregation of such proteins and the shutdown of their related cellular pathways has been demonstrated. Besides the limited understanding of the mechanism by which aggregation potentially induces dormancy, it also remains unclear how dormant cells that contain protein aggregates recover and regrow. Previous studies indicate that persister cells remove their aggregates before resuming growth24,25,37,38 and that the chaperones DnaK and ClpB are involved24,38. However, it remains unknown if other chaperones and proteases can stimulate disaggregation and if other factors such as aggregate structure also influence the disaggregation efficiency. While aggregate structure and its relation to functionality has been thoroughly investigated in eukaryotes40,41,42, the structure of aggregates and how it relates to dormancy has not been extensively investigated in bacteria.
Here, we present data that provide support for a role of aggregation in the induction of dormancy in E. coli. First, by perturbing central regulatory and metabolic pathways, we show that the relative timing of aggregation and dormancy is strongly conserved in strains that display different dynamics of dormancy development. Second, we confirm that aggregates are selectively enriched in proteins active in energy production and we demonstrate that the recruitment of these proteins in the aggregates precedes cellular energy depletion and persistence. Third, directly interfering with protein aggregation by manipulating the cellular level of the chaperone DnaK also accelerates or delays dormancy. Fourth, we confirm that aggregates are removed before cells can resume growth. Finally, we show that the ease with which these aggregates are removed—and thus also the regrowth potential of dormant cells—is determined by the structure of the aggregates. We show that, as starvation continues, proteins first assemble into liquid-like condensates that further solidify into aggregates. This solidification impedes disaggregation and thereby restricts regrowth of dormant cells over time. Our results strongly suggest that this aggregate solidification and the corresponding reduced recovery underly the transition from the persister to the VBNC state. This indicates that, contrary to the current hypotheses, VBNC cells are not characterized by lower energy levels compared to persister cells, but rather by aggregate solidification and a decreased recovery potential.
Results
Relative timing of protein aggregation and dormancy development are conserved in different genetic backgrounds
Dormant bacteria often contain protein aggregates19,24,34,37,38. It was demonstrated by multiple studies that dormancy is correlated with protein aggregation19,24,25,31,32,33,34,35,36,37,38. Moreover, it has been suggested that protein aggregation might be the driving force behind dormancy development24,25,39. However, conclusive proof of the causal relationship between aggregation and dormancy is still missing. If there is a causal relationship between both, we expect that aggregation precedes dormancy and that the sequence of these events is conserved in strains with different dormancy dynamics. To test this hypothesis, we compared the timing of dormancy and aggregation in various genotypic backgrounds of E. coli. We chose to delete or overexpress genes that not only alter persister levels, but also have different roles in central regulatory and metabolic processes. These genes include rpoS43,44,45, encoding a general stress response regulator; sdhC46, involved in energy production through both the TCA cycle and the electron transport chain; recA13,43,47,48, important for DNA repair, and obgE25,49, encoding a GTPase involved in ribosome assembly and cell cycle regulation. As a control, we also included a strain overexpressing the Obg-related GTPase Era49 with no effect on persistence. We quantified the number of persister and VBNC cells as described before25 (Fig. 1a, b, Supplementary Figs. 1 and 2). These results demonstrate that different genotypes display different dynamics of persister development. While almost all strains exhibit a similar increase in persistence during the transition from exponential to stationary phase, the timing at which they reach their peak persister level in stationary phase strongly varies. Despite these differences in the timing of reaching peak persister levels, most strains share a comparable maximal persister level. Consequently, the strains, previously identified as low or high persister strains, are therefore better defined as slow or fast persister strains, respectively.
The strains analyzed for their effect on dormancy are the wild-type E. coli strain (WT), E. coli ΔrpoS, ΔsdhC and ΔrecA and E. coli with pBAD33Gm (Vector), pBAD33Gm-obgE (ObgE) and pBAD33Gm-era (Era). a, b The absolute number of persister cells that could grow after ofloxacin treatment was measured at different time points during incubation. Data are represented as averages ± SEM (n ≥ 4) (See Supplementary Table 2 for exact values). Relative values of persisters, VBNC and dead cells are shown in Supplementary Fig. 1. c–f Quantitative microscopy analysis was used to determine the fraction of cells with IbpA (c, d) and Ph (e, f) aggregates at different time points during incubation. A minimum of 50 cells was analyzed for every repeat and every time point. A nonlinear logistic growth model was fitted on the data (solid line). Data are represented as averages ± SEM, (n ≥ 3) (See Supplementary Table 3 and 4 for exact values). g For each strain, the moment of maximal persistence and the start and maximal levels of VBNC cell formation and IbpA and Ph aggregation were determined and visualized. The moment of the start and maximum of IbpA and Ph aggregation were determined by fitting a nonlinear logistic growth model on the aggregation data and calculating the time between reaching 10% and 90% of the maximal value. h Heatmap of the Pearson’s R values that were calculated based on the timing of maximal persistence, the onset of VBNC cell formation and the peak in IbpA and Ph aggregation across the selected strains (n = 7). All correlations were high and significant (P < 0.05) (See Supplementary Table 5 for confidence intervals and p-values). Source data are provided as a Source Data file.
Next, we wanted to verify whether different dynamics of persistence correspond to a different timing of aggregate development. Aggregates can be identified at an early stage using a fluorescent fusion of the small chaperone IbpA to msfGFP50 (IbpA-msfGFP, referred to as IbpA aggregates), while late-stage aggregates can be observed as phase-bright foci (referred to as Ph aggregates)25. The early detection of IbpA aggregates is due to the rapid association of IbpA with the aggregates51 and the higher sensitivity of fluorescence microscopy compared to phase contrast microscopy. We measured the percentage of bacterial cells carrying at least one aggregate for the selected strains using these two different readouts (Fig. 1c–f). To have a more succinct overview of the relative timing of aggregate formation and dormancy, we summarized our data using key timepoints for all processes under investigation (i.e. start and maximal level). Nonlinear logistic growth models were fitted to the data for IbpA and Ph aggregation to determine when aggregation started and when the maximum level was reached (Fig. 1c–f and Supplementary Table 1). We similarly plotted the timing of VBNC cell formation as the period between the start (indicated by the first drop in CFU levels) and the maximal level (indicated by the time when a stable number of CFUs was reached under our conditions) (Fig. 1g). This analysis confirms that both aggregation and dormancy happen progressively25: protein aggregates are first marked by IbpA in an early stage of development and later mature into Ph aggregates, while persistence is followed by the onset of VBNC cell formation. Moreover, our results also demonstrate that the start of aggregation precedes or coincides with the peak in persistence in all tested strains, suggesting that aggregation might induce persistence. Strains with a deletion of rpoS, sdhC and recA and the strain with overexpression of obgE affect the timing of both aggregation and dormancy similarly (Fig. 1g). Specifically, the deletion of rpoS and overexpression of obgE accelerate both dormancy and aggregation, while the deletions of sdhC and recA delay both processes. Importantly, our results demonstrate that the increase in persistence in stationary phase is preceded by, and might therefore be linked to, protein aggregation.
To further verify if the timing of dormancy and aggregation are linked, we calculated the correlation between the time points of maximal persistence, IbpA aggregation and Ph aggregation and the starting point of VBNC cell formation in the different strains (Fig. 1h). This correlation analysis showed that the timing of aggregation and dormancy is significantly and highly correlated. These results support our previous findings that protein aggregation might induce dormancy. It can be noted that the timing of aggregation and persistence is less comparable in the ΔrpoS strain, suggesting that aggregation alone does not necessarily dictate the timing of persistence. Instead, it may involve reaching a specific threshold, potentially defined by the sequestration of proteins involved in energy production, to induce persistence
After establishing that changes in dormancy are associated to similar changes in aggregation, we checked if overexpression of era, which was shown to not affect persistence49, had an effect on the aggregation level in the cells. We observed that overexpression of era indeed did not affect the timing of dormancy. However, to our surprise, it did accelerate aggregation (Fig. 1g). We can thus conclude that although protein aggregation often precedes dormancy, the mere presence of protein aggregates is not sufficient to induce dormancy.
Protein aggregates progressively sequester proteins involved in translation, energy production and metabolism
Our observation that aggregation precedes dormancy in a wide variety of strains suggests that aggregation might induce dormancy in E. coli. However, the specific mechanism by which aggregation might induce dormancy is not known. It is hypothesized that the depletion of important proteins from the cytoplasm causes the shutdown of pathways in which they are involved, thereby inducing dormancy. This hypothesis is supported by the observation that aggregates correlated with dormancy often contain proteins related to transcription, translation, metabolism and energy production19,24,25,33,37. Moreover, reducing transcription52,53, translation52,53 or energy levels54 was demonstrated to induce persistence. Our objective was to explore if and how protein composition drives dormancy development.
To find key proteins that could induce dormancy upon aggregation, we assessed the composition of protein aggregates across the selected genetic backgrounds tested here. By using different strains, we filtered out proteins that only aggregate under specific conditions and that are therefore not generally associated with dormancy development. We grew all strains for 40 hours, to ensure they all exhibited dormancy (Fig. 1a, b and Supplementary Fig. 2), and then isolated their protein aggregates, which correspond to the insoluble fraction that is not resuspended by Triton X-100 and deoxycholate. Protein abundances were quantified by mass spectrometry (MS) analysis. Clusters of Orthologous groups (COG)55 and Kyoto Encyclopedia of Genes and Genomes (KEGG)56 enrichment analyses were performed on the proteins identified from these aggregates to determine which protein classes were overrepresented (Fig. 2 and Supplementary Fig. 3a). These analyses revealed that all the strains contained a wide diversity of proteins (Supplementary Fig. 3a). Moreover, although all aggregates contained a wide diversity of proteins (Fig. 2a, b), they were all enriched in proteins associated with nucleotide transport and metabolism, translation, energy production and different metabolic processes (Fig. 2c, d), confirming previous results19,24,25,33,37. Finally, aggregation occurs similarly in a strain overexpressing era and the vector control, with the exception of Era (Log2 fold change 7.76, p = 0.019).
Aggregate composition was quantified by MS using label-free quantitation values based on spectral counting after 40 hours of incubation for E. coli WT, E. coli ΔrpoS, ΔsdhC and ΔrecA and E. coli with pBAD33Gm (Vector) and pBAD33Gm-obgE (ObgE). a This graph shows the number of unique proteins that were aggregated in all 6 strains, in only one strain, and everything in between. b The number of unique aggregated proteins in each strain is indicated. Bar graphs and error bars represent the mean ± SEM (n = 3, with n the number of samples per strain from which the aggregates were purified). Mean and SEM for WT, ΔrpoS, ΔsdhC, ΔrecA, Vector and ObgE strains are 239.3 ± 65.71, 304.7 ± 10.65, 254 ± 18.81, 265.7 ± 50.34, 259.7 ± 28.59 and 190.7 ± 14.19. c Aggregate composition data of all samples were combined to determine which COG categories were overrepresented using the one-sided Fisher’s exact test with Bonferroni correction for multiple testing. d Aggregate composition data of all the strains were combined to determine which KEGG pathways were enriched using a hypergeometric test with the Benjamini-Hochberg multiple testing correction. Overrepresented categories are shown (P < 0.05). Exact p-values are (top to bottom): 0.0024, 0.0041, 0.0067, 0.00026, 0.0029, 1.01 × 10−8, 0.0046, 0.0057, 1.16 × 10−5, 3.41 × 10−10, 0.0046, 0.0104, 0.00036, 8.72 × 10−5, 0.0096, 1.58 × 10−8, 6.73 × 10−5 and 8.35 × 10−19. The absolute number of aggregated proteins that belong to each category is indicated next to the bars. COG categories represented in c are: A—RNA processing and modification, C—energy production and conversion, D—cell cycle control, cell division, and chromosome partitioning, E—amino acid transport and metabolism, F—nucleotide transport and metabolism, G—carbohydrate transport and metabolism, H—coenzyme transport and metabolism, I—lipid transport and metabolism, J—translation, ribosomal structure, and biogenesis, K—transcription, L—replication, recombination, and repair, M—cell wall/membrane/envelope biogenesis, N—cell motility, O—posttranslational modification, protein turnover, and chaperones, P—inorganic ion transport and metabolism, Q—secondary metabolites biosynthesis, transport, and catabolism, S—function unknown, T—signal transduction mechanisms, U—intracellular trafficking, secretion, and vesicular transport, V—defense mechanisms. Source data are provided as a Source Data file.
Next, we analyzed if and how aggregate composition changes over time. To do so, we selected the strain with overexpression of obgE and the vector control strain for further analysis, as these two strains exhibit the most significant difference in aggregation dynamics. Moreover, extensive physiological and aggregation data are already available for these strains25,49,57,58. We quantified for both strains their aggregate composition at different time points during growth using MS25. In general, the aggregates contained a wide diversity of proteins with an enrichment in proteins involved in metabolism, energy production, nucleotide metabolism and transport, translation and post-translational modification, protein turnover and chaperone functions (Supplementary Fig. 3b–e). To specifically determine which protein classes were increasingly sequestered in the aggregates over time, we used the DESeq2 package59 followed by a COG enrichment analysis55 (Fig. 3). For the vector control strain, it is clear that aggregates sequester increasingly more proteins involved in energy production and translation over time (Fig. 3a). Moreover, in the strain overexpressing obgE, the number of proteins involved in energy production and translation are already high from the first time points sampled (16 and 24 hours) (Fig. 3b). This corresponds to what we expect based on the strong acceleration of dormancy and aggregation in this strain (Fig. 1b, d, f and Supplementary Fig. 2e, f). We can thus conclude that aggregates in dormant cells preferentially sequester proteins important for energy production and translation over time. The sequestration and depletion of these proteins could possibly shut down or reduce the corresponding processes and induce dormancy.
Aggregates were isolated and their composition was quantified for E. coli pBAD33Gm (Vector) (a) after 8, 16, 32, 48 and 72 hours of incubation and for E. coli pBAD33Gm-obgE (ObgE) (b) after 8, 16, 24, 40 and 72 hours of incubation (n = 4 for V32 and n = 5 for all the other samples). For the last 4 sampling points, protein abundances in the aggregate were compared to the abundances in the previous sampling point to identify enriched proteins and their COG categories using the one-sided Fisher’s exact test with Bonferroni correction for multiple testing. Source data are provided as a Source Data file.
Protein aggregation drives dormancy development by triggering ATP depletion, but not by reducing translation
Because aggregates sequester proteins involved in energy production and translation over time, we investigated if the aggregation and depletion of these proteins might result in a lowering of energy production and translation, respectively, that could be linked to dormancy development. We therefore measured ATP depletion and translational activity over time and compared these measurements to the timing of aggregation and dormancy development. Single-cell ATP levels were quantified by ratiometric microscopy using the iATPSnFr1.0 sensor that changes its emission intensity after excitation at specific wavelengths based on ATP binding and therefore reports on the cellular ATP concentration18. At each time point under investigation, we determined the fraction of ATP-depleted cells as those cells that displayed an iATPSnFr1.0 ratio below a certain threshold (Fig. 4a and Supplementary Fig. 4). Additionally, as energy production is linked to membrane potential60, we also measured membrane depolarization over time using bis-(1,3-dibutylbarbituric acid)trimethine oxonol (DiBAC4(3)), a fluorescent dye that exclusively penetrates depolarized cells61 (Fig. 4b). Finally, translation levels were determined using the Click-iT® HPG Alexa Fluor® Protein Synthesis Assay Kit (Thermo Fisher Scientific), which measures translation by monitoring the incorporation of an amino acid analog that can be fluorescently labeled through click chemistry (Fig. 4c). To better compare the timing of different processes, we plotted the moment at which the processes start and reach their maximal level (Fig. 4d). Nonlinear logistic growth models were fitted to the data for IbpA and Ph aggregation, ATP depletion and membrane depolarization to determine both parameters (Figs. 1d, f, 4a, b and Supplementary Tables 5 and 6). The results of these dynamic measurements indicate that cells experience ATP depletion, membrane depolarization and a drop in translation in stationary phase (Fig. 4 and Supplementary Fig. 4). Moreover, we also observed that overexpression of obgE accelerates ATP depletion and membrane depolarization, suggesting a relation between them. In contrast, ObgE did not affect the drop in translation. As overexpression of obgE accelerates aggregation, dormancy, and energy depletion, but it does not decrease translation, we conclude that aggregation and dormancy are linked to a drop in the energy levels, but that there is no detectable relationship between aggregation, dormancy and the reduction in translation in our conditions.
All measurements were performed with E. coli pBAD33Gm (Vector) and pBAD33Gm-obgE (ObgE). a Based on the single-cell ATP measurements and the threshold for ATP depletion indicated in Supplementary Fig. 4, the fraction of cells with ATP depletion was determined at different time points. A nonlinear logistic growth model was fitted onto the data (solid line). b The fraction of cells with membrane depolarization was determined at different time points by measuring the proportion of the bacterial population that stained with DiBAC4(3), a fluorescent dye that penetrates depolarized cells. A nonlinear logistic growth model was fitted onto the data (solid line). c Translation was measured using the Click-iT® HPG Alexa Fluor® Protein Synthesis Assay Kit. As a negative control, the Vector was treated with the translation inhibitor chloramphenicol (Cm). For each repeat and each time point, the sum of the fluorescence intensity in 10,000 bacterial cells is shown. Translation levels were compared between the Vector and ObgE at the different time points by unpaired two-sided t-tests with the Holm-Šídák correction for multiple comparisons (for the comparison after 48 hours of incubation: ****P = 0.000082). Data in graph a–c are represented as averages ± SEM, (n = 4) (See Supplementary Table 7 for exact values). d The timing of dormancy (persistence and VBNC cell formation), energy depletion (ATP depletion and membrane depolarization) and aggregation (IbpA and Ph aggregation) is represented. For persistence, the peak moment is indicated, while for the other processes, both the initiation and the peak moment are shown. Source data are provided as a Source Data file.
Strikingly, when focusing on cellular energy levels, we observed that early-stage IbpA aggregation occurs before ATP depletion and membrane depolarization (Fig. 4d). This indicates that protein aggregation is not induced by ATP depletion and that the reduction in ATP levels is not directly caused by a reduced membrane potential. Furthermore, this suggests that the aggregation of proteins involved in energy production (between 32 and 72 hours for the vector control strain and between 8 and 16 hours for the strain overexpressing obgE (Fig. 3)) might induce ATP depletion (after 40 hours for the vector control strain and after 16 hours for the strain overexpressing obgE) and subsequently dormancy development. Importantly, the difference in timing of ATP depletion between the vector control strain and the strain overexpressing obgE cannot be explained by nutrient depletion alone as both strains reach stationary phase at approximately the same time (Supplementary Fig. 2). Surprisingly, our results also demonstrate that the energy levels in persister cells do not decline further when they transition to the VBNC state. This suggests that the decreased recovery of VBNC cells is not caused by a lower energy level, as suggested before22,23,24,25,26, but that it might be influenced by other factors.
Delaying or accelerating protein aggregation affects dormancy similarly
To further support the hypothesis that protein aggregation induces dormancy, we manipulated protein aggregation by deleting or overexpressing the chaperone DnaK and monitored the effects on aggregation and dormancy development (Fig. 5a–h and Supplementary Fig. 5a–c). We observed that the deletion of dnaK indeed accelerates aggregation and advances the induction of dormancy in the cells (Fig. 5a–d and Supplementary Fig. 5a). Notably, while the deletion of dnaK leads to an earlier peak in persistence, the absolute number of persisters is generally lower in this strain (Fig. 5b). At the same time, there are significantly more VBNC cells, which also form earlier (Fig. 5c, d). These results suggest that the deletion of dnaK accelerates the induction of dormancy, but impedes the recovery and regrowth which is a defining feature of persisters.
Aggregation, persistence and VBNC cell formation were measured for E. coli WT, E. coli ΔdnaK (a-d), E. coli pBAD33Gm (Vector), and E. coli pBAD33Gm-dnaK (DnaK) (e–h). a, e Quantitative analysis of microscopy images shows the fraction of cells containing Ph aggregates. Data are represented as averages ± SEM, (n ≥ 3, > 50 cells analyzed for every repeat) (Supplementary Tables 2-4 and 9-12 for exact values). b, f Persistence was measured at different time points. Data are represented as averages ± SEM, (n ≥ 4) (Supplementary Tables 2-4 and 9-12 for exact values). c, d, g, h The number of CFUs (solid line) and viable cells (dashed line) were measured at different time points. The area between both curves represents the number of VBNC cells. Data are represented as averages ± SEM, (n ≥ 4) (See Supplementary Tables 2-4 and 9-12 for exact values). Note that viable cells and CFUs are measured differently and are therefore not perfectly comparable. The discrepancy observed at some early timepoints suggests that the number of viable cells may be underestimated. i The number of CFUs and viable cells was determined after 72 hours of obgE overexpression in WT and deletion strains. The logarithm of the number of viable and culturable cells was compared between WT and deletion strains using a two-way ANOVA with Dunnett’s correction for multiple comparisons (ΔdnaK: q ratio = 9.771, 42 degrees of freedom, ****P < 0.0001). Bar graphs and error bars represent averages ± SEM, (n ≥ 3) (See Supplementary Table 13 for exact values). j The percentage of proteins that are aggregated after 40 hours of incubation and that are a substrate of DnaK was calculated64. Bar graphs and error bars represent averages ± SEM, (n = 3, with n the number of samples per strain from which the aggregates were purified). For WT, ΔrpoS, ΔsdhC, ΔrecA, Vector and ObgE strains, the mean ± SEM values are 43.56 ± 2.123, 42.27 ± 0.5844, 44.07 ± 1.067, 44.55 ± 1.785, 42.59 ± 0.4752 and 48.01 ± 0.5392. Source data are provided as a Source Data file.
Additionally, overexpression of DnaK is expected to reduce protein aggregation. Accordingly, we observe a significant decrease in Ph aggregation upon dnaK overexpression and a delayed dormancy development (Fig. 5e–h). However, we also detect an increase in IbpA aggregation at earlier time points (Fig. 5e and Supplementary Fig. 5b). This suggests that the IbpA aggregation observed is likely caused by the strong overexpression and partial aggregation of DnaK, which probably does not induce proteome-wide protein aggregation relevant to dormancy induction.
Thus, we conclude that inducing or preventing aggregation by altering cellular levels of the DnaK chaperone advances or delays dormancy development, respectively, further supporting the notion that protein aggregation contributes to the induction of dormancy.
DnaK is important for the recovery and regrowth of dormant cells
After providing strong indications that protein aggregation induces dormancy, we wondered whether aggregates need to be removed before the cells restart growth. We observed that some cells overexpressing obgE spontaneously induce disaggregation and recovery from dormancy in late stationary phase (Fig. 1d, f and Supplementary Fig. 2f) and that this disaggregation precedes the increase in CFUs we observe in late-stationary phase (Supplementary Fig. 5d). We verified that the spontaneous increase in CFUs after overexpression of obgE was due to real recovery and not due to the growth of mutants that have obtained the growth advantage in stationary phase (GASP) phenotype62 (Supplementary Table 8). This implies that cells overexpressing obgE can spontaneously cause disaggregation and thereby recover from the VBNC state, leading to an increase in CFUs. This is consistent with previous observations showing that persister cells remove their aggregates before they restart growth24,25.
As it was previously demonstrated that the chaperone DnaK plays an important role during disaggregation and recovery24,38, we wanted to expand this investigation to other chaperones and proteases. We therefore deleted the most important chaperones and proteases in E. coli and measured their effects on the number of CFUs and viable cells after 72 hours of incubation (Fig. 5i). While none of the deletions significantly alter the number of viable cells, the deletion of dnaK significantly decreases the number of culturable cells (i.e. CFUs), indicating inhibition of recovery due to lower disaggregation. Remarkably, deleting proteases or other chaperones does not significantly affect the number of CFUs after 72 hours, although we did see non-significant decreases for many deletion strains. These data illustrate that, besides DnaK, none of the chaperones or proteases tested play a crucial role individually in disaggregation, even though they may be of minor assistance in the process. These results underscore that cellular regrowth and exit from dormancy is mainly dependent on DnaK. DnaK is known to function in disaggregation and subsequent refolding of disaggregated proteins63, indicating that refolding of liberated proteins might be important for recovery. Indeed, 40 to 50 percent of the aggregated proteins were identified as DnaK interactors (Fig. 5j), while only 25 percent of the cytosolic proteome interacts with DnaK64. The high percentage of DnaK interactors in the aggregates supports the idea that DnaK-dependent refolding is important during recovery and regrowth.
Proteins first assemble into liquid-like condensates, that develop into solid aggregates over time
We provided support for the hypothesis that aggregation induces dormancy by demonstrating that aggregation precedes ATP depletion and dormancy, but also by showing that aggregates are removed before the cells recover from their dormant state. As VBNC cells have a much lower regrowth capacity compared to persister cells, we hypothesized that a difference in disaggregation potential could be responsible. The disaggregation potential of aggregates is strongly affected by the aggregate structure65. Indeed, it was observed previously in eukaryotic cells that proteins often first assemble into liquid-like structures that subsequently progress into a more solid-like end-stage of aggregation which is less easily reversible40,65. The liquid-like structures that remain easily reversible are referred to as biomolecular condensates, whereas the irreversible solid-like structures are termed aggregates66. Therefore, we verified whether the early-stage IbpA aggregates and the late-stage Ph aggregates, which correlate with persisters and VBNC cells, respectively, are indeed true aggregates or if they are biomolecular condensates with liquid-like properties25.
To determine the aggregate structure and solubility, strains overexpressing obgE were grown for 8 or 40 hours, to obtain cells mainly associated with early-stage IbpA foci and cells containing further developed Ph foci, respectively (Fig. 1d, f). The IbpA and Ph foci within these cells were imaged on agar pads containing 1,6-hexanediol (Fig. 6a–c and Supplementary Fig. 6a, b) as this chemical is known to only dissolve liquid-like condensates and not solid-like aggregates67. Quantitative microscopy analysis revealed that the fraction of cells containing IbpA foci after 8 hours of incubation decreased more than 3-fold in the presence of hexanediol (Fig. 6a). Dissolution of IbpA foci was also clear from the fluorescence pattern of IbpA-msfGFP transitioning from distinct foci to a more diffuse distribution (Fig. 6c). Importantly, the small number of Ph foci that were already present after 8 hours of incubation could not be dissolved by hexanediol, suggesting that only early-stage IbpA foci have liquid-like properties (Supplementary Fig. 6a). Indeed, after 40 hours of incubation, most IbpA foci were associated with Ph foci (Fig. 6c) and these Ph foci could not be dissolved by hexanediol (Fig. 6b and Supplementary Fig. 6b). The same was found to be true for a dnaK deletion strain (Fig. 6a, b and Supplementary Fig. 6a, b), indicating that the intrinsic solubility of the aggregates is independent of DnaK. This indicates that early-stage IbpA foci show liquid-like properties, while late-stage Ph structures are more solid-like.
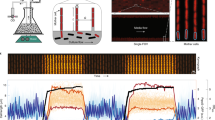
After 8 (a, c) and 40 (b, c) hours of incubation, E. coli ibpA-msfGFP with pBAD33Gm (Vector) or pBAD33Gm-obgE (ObgE) and E. coli ibpA-msfGFP ΔdnaK were imaged with and without 10% 1,6-hexanediol. Quantitative microscopy analysis shows the fraction of cells with IbpA foci in each condition. For the Vector, ObgE and ΔdnaK strains, the fraction of cells with IbpA foci was compared between cells treated with and without hexanediol using a two-way ANOVA with Holm-Šídák correction for multiple comparisons. Bar graphs and error bars represent averages ± SEM, (n = 4, ≥ 50 cells were analyzed for each repeat) (see Supplementary Table 13 for exact values). c Representative microscopy images are shown. Scale bar, 5 µm. FRAP was performed on a small part of the IbpA foci of E. coli ibpA-msfGFP pBAD33Gm-obgE after 8 (d) and 40 (e) hours of incubation. Representative images before and right after bleaching, and after recovery are presented as well as the relative fluorescence compared to the starting point. Scale bar, 1 µm. f The percentage recovery after bleaching is shown. The recovery was compared using a two-sided Wilcoxon signed-rank test. Data are represented as averages ± SEM. E. coli pBAD33Gm-obgE was incubated for 2 (g), 8 (h) and 40 (i) hours and 3 repeats of the samples were imaged using AFM-IR spectroscopy. The results of the first repeat are presented here. The IR absorbance at 1625 cm−1 for the complete sample is shown on the left. Scale bar, 5 µm. Different foci within the sample were selected for which a full IR spectrum was measured (indicated by crosses). The complete IR spectra of these foci are shown on the right. k-means clustering on the second derivative of the amide I band (1580 cm−1–1700 cm−1) was performed to discriminate between spectra with high and low β-sheet signal (red and blue, respectively). j For each complete IR spectrum (Fig. 6g–i and Supplementary Fig. 6d–i), the fraction of β-sheets compared to α-helices was determined. Source data are provided as a Source Data file.
To validate the increased solidification of aggregates over time, we performed FRAP (fluorescence recovery after photobleaching) on a section of IbpA-msfGFP-stained foci in cells that were incubated for 8 (early-stage aggregates) or 40 (late-stage aggregates) hours. In this procedure, a small piece of the aggregate was bleached and recovery of fluorescence inside the aggregate was monitored over time (Fig. 6d–f). Early-stage IbpA foci recovered approximately 35% of their fluorescence intensity after bleaching over a time period of 50 seconds with a half time of 15.4 ± 22.7 seconds, indicating protein mobility, which is a characteristic of liquid-like condensates (Fig. 6d, f and Supplementary Fig. 6c). The low percentage of recovery can probably be attributed to the large fraction of the IbpA focus that was bleached, owing to the small size of the bacteria. However, another explanation might be that the condensates exhibit gel-like properties instead of liquid-like. Since the distinction between liquid-like and gel-like condensates is not yet clear and considering that the low recovery is likely due to extensive bleaching, we still anticipate the condensates to behave as liquid-like. In contrast to the early-stage IbpA foci, late-stage IbpA foci did not display recovery ( < 1%) (Fig. 6e), confirming that liquid-like, early-stage condensates solidify over time into aggregates.
Finally, we investigated whether the transition from liquid-like condensates to solid-like aggregates also involves structural changes in proteins. We therefore investigated the structure of both early- and late-stage aggregates in E. coli cells overexpressing obgE using atomic force microscopy-based infrared spectroscopy (AFM-IR). AFM-IR spectroscopy combines the subwavelength resolution of AFM microscopy with IR spectroscopy, to obtain chemical and structural information on the sample under investigation68. Additionally, analysis of the amide I band in IR spectra offers information about the secondary structure of proteins69. For our analysis, we incubated E. coli pBAD33Gm-obgE for 2, 8 and 40 hours to obtain cells with no, early-stage condensates and late-stage aggregates, respectively (Fig. 1d, f). For each sample, we measured the IR absorbance at 1625 cm−1 to estimate the abundance of β-sheets in the cells69 (Fig. 6g–i and Supplementary Fig. 6d–i). The images obtained after 2 and 8 hours of incubation show a homogeneous absorbance within the cells. However, images measured after 40 hours of incubation show specific regions with a high absorbance at 1625 cm−1, which points at a high β-sheet content and the presence of aggregates. To get more structural information, we also selected different points for which we measured the full IR spectrum with a focus on the amide I (1600-1700 cm−1) and amide II (1500-1600 cm−1) bands (Fig. 6g–i and Supplementary Fig. 6d–i). These results show that the spectra taken after 2 hours of incubation and most of the spectra taken after 8 hours of incubation are all quite similar and do not have a high absorbance at 1625 cm−1, further confirming that these cells are quite homogeneous with a low β-sheet content. However, after 40 hours of incubation and to a lesser extend after 8 hours of incubation, spectra taken at regions with a high 1625 cm−1 absorbance display a shoulder in the amide I band spectrum at 1625 cm−1, which is indicative for the presence of intermolecular β-sheets. Indeed, when we quantify the number of β-sheets relative to α-helices from the different spectra, we see a clear split between points with high and low β-sheet content after 40 hours of incubation, but not so much after 2 and 8 hours of incubation (Fig. 6j). Because cells that were incubated for 8 hours, and that were shown to contain early-stage condensates, show a rather homogeneous absorption of the amide I band within the cells, we can conclude that their condensates do not contain clusters with many β-sheets. However, as a few spectra already show a shoulder in the amide I band spectrum at 1625 cm−1, there seems to be an early transition to the more β-rich aggregates in a few cells at this time point. On the contrary, cells that were incubated for 40 hours, and that are characterized by late-stage aggregates, show a very high abundance of intermolecular β-sheets within their aggregates. Overall, these data demonstrate that the number of intermolecular β-sheets within aggregates increases during their maturation. The increased number of intermolecular interactions agrees with the observation that mature aggregates have a more solid-like structure and a less efficient dissolution. This phase transition from liquid-like condensates to solid-like aggregates over time is likely to hinder disaggregation, thereby explaining why growth resumption in VBNC cells, which are associated with late-stage aggregates, is challenging. This implies that the transition from persistence to the VBNC state is controlled by a reduced recovery due to solidification of protein condensates.
Discussion
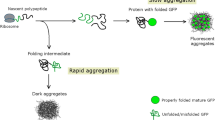
Dormant persister and VBNC cells have both been associated with protein aggregation19,24,25,34,37,38. We delved deeper into the relationship between dormancy and aggregation and provide support for the hypothesis that aggregation might induce dormancy in E. coli. First, we demonstrate that protein aggregation always precedes dormancy development during starvation in a variety of strains displaying diverse dormancy dynamics (accelerated or delayed). Second, we confirm that aggregates are enriched in proteins involved in translation, energy production and metabolism. Moreover, we show that the sequestration of proteins active in energy production precedes—and therefore likely induces—ATP depletion and dormancy. Third, we establish that either delaying or accelerating aggregation by changing the levels of the chaperone DnaK also affects dormancy similarly. Fourth, we confirm that aggregates need to be removed before dormant cells can start regrowth24,25,37,38. Importantly, we also show that the efficiency of aggregate dissolution is dependent on the presence of the chaperone DnaK24,38, but also on the structure of the aggregates. More specifically, persister cells are associated with early-stage condensates that are easily removed because of their liquid-like structure. On the other hand, VBNC cells often contain late-stage aggregates that have matured to obtain a solid-like structure, probably because of their increased number of intermolecular β-sheets, which impedes their disaggregation. Based on these results, we propose a model that explains how aggregation might induce dormancy (Fig. 7). As bacteria experience starvation, they progressively sequester more proteins that are active in energy production into protein aggregates. This aggregation and depletion of protein function shuts down the energy production pathway and causes ATP depletion. This energy depletion then induces the onset of dormancy and antibiotic tolerance. These results correspond to earlier observations that persister cells have lower ATP levels18,19,20. Proteins assemble into liquid-like condensates in the early stages of development, which allows them to be dissolved easily so that dormant cells can quickly recover and restart growth. However, over time these condensates solidify into aggregates. As it is hypothesized that ATP-driven remodeling is needed to keep the condensates in a liquid-like structure70, the low levels of cellular ATP throughout stationary phase might ultimately cause this aggregate solidification. Solidified aggregates significantly hinder disaggregation and this slower disaggregation complicates the recovery from dormancy and pushes the persister cells into the VBNC state. Although solidified protein aggregates cannot be easily dissolved, it has been reported that they can be converted back to the liquid-like state after which dissolution and presumably regrowth can take place65. This conversion from a solid-like to a liquid-like structure was shown to be catalyzed by chaperones when the aggregation-inducing stress was removed65. Although this study focused on the overexpression of firefly luciferase62, which is difficult to refold, we hypothesize that a similar process might also solubilize endogenous proteins from the aggregates and thereby induce recovery and regrowth of VBNC cells generated under our conditions. No conditions were found yet under which the solid-like aggregates in the VBNC cells are solubilised. Additionally, the (apparent) irreversibility of VBNC solid aggregates versus the reversibility of persister states is in agreement with the model of phase separation coupled to percolation71. In this model, irreversible aggregation by percolation is characterized by a networking transition where individual molecules or clusters form a network that spans the entire system once a critical concentration, known as the percolation threshold, is reached (cperc). If the saturation concentration for phase transition is lower than cperc (reversible) phase separation occurs first, and the dense phase itself can subsequently undergo percolation to form a network structure (microgel).
In response to stress such as nutrient exhaustion, bacteria progressively sequester proteins in liquid-like condensates. The condensation of proteins active in energy production precedes—and thereby likely induces—a depletion of cellular ATP and dormancy. The chaperone DnaK is able to dissolve these condensates due to their liquid-like structure. This dissolution allows the recovery and regrowth of the persister cells. Restart of growth is supported by dissoluted and refolded proteins that are released from the biomolecular condensate. Indeed, the condensates in dormant cells contain a variety of proteins involved in translation, energy production and metabolism which could be repurposed for growth. However, if stress persists over an extended period of time, the liquid-like condensates solidify into aggregates by accumulating intermolecular β-sheet structures. This transition complicates their removal by DnaK and consequently the exit from dormancy. The solidification of the aggregates thereby pushes the shallowly dormant persister cells in the deeper dormant VBNC state. Under specific conditions, solidified aggregates from VBNC cells can presumably be converted back to liquid-like structures (dashed arrow), which is thought to allow disaggregation by DnaK and subsequent regrowth.
We here provide support for the hypothesis that aggregation induces dormancy. However, it is important to note that although aggregation precedes dormancy in various E. coli strains, the mere presence of aggregates is not always sufficient to trigger dormancy. We showed that aggregates progressively sequester proteins involved in energy production and that this is followed by ATP depletion and dormancy. This indicates that aggregate composition is an important factor in determining the influence of aggregation on dormancy. We therefore hypothesize that aggregates might only induce dormancy when they sequester a certain threshold of proteins active in energy production. Additionally, we only investigated the relation between aggregation and dormancy in E. coli, which means that these results need to be confirmed in other strains to draw general conclusions about the role of protein aggregation in bacterial dormancy. Results of other groups on the relation between aggregation and dormancy in Staphylococcus aureus suggest that this link may indeed be valid across multiple species19,31.
Furthermore, we provide deeper insight into the difference between persister and VBNC cells. Persister cells were hypothesized to transition to the VBNC state by lowering their energy levels and/or metabolic activity22,23,24,25,26. Our data do not support this hypothesis as we observed that persister and VBNC cells have similar energy levels. Rather our results suggest that the shift from persistence to the VBNC state is primarily the result of aggregate solidification which determines the cell’s potential to recover and restart growth.
When investigating the association between aggregation and dormancy, we discovered that these processes are very dynamic. Persister levels fluctuate significantly over time and persister cells are believed to shift to the VBNC state25. Not measuring the dynamics of these processes in experiments could potentially lead to incorrect conclusions. As an example, the deletion of sdhC was previously claimed to decrease both aggregation and persistence in stationary phase35,37. However, our measurements indicate that this deletion does not decrease these processes, but rather delays them. We therefore urge to change the assays for antibiotic tolerance from a one-point measurement to a dynamic measurement of both persistence and VBNC cell formation.
In addition to persister cells35, many eukaryotic cells have also been associated with liquid-like condensates such as processing bodies, stress granules or aggregates induced by heat or nutrient stress72,73,74. These initially liquid-like condensates are formed upon stress and also solidify over time73,75,76. They serve as storage structures since they sequester numerous mRNA molecules, as well as translation- and metabolism-related proteins, and protect them from degradation77,78,79,80,81. Before regrowth, disaggregation takes place and most of the molecules inside these liquid-like condensates are reused for a rapid restart of growth77,78,79,80,81. The aggregates of dormant bacteria share various similarities with these eukaryotic storage structures. We not only demonstrate that condensates of dormant bacteria are liquid-like and solidify over time into aggregates, but also that they are enriched in proteins important for translation, energy production and metabolism. Additionally, regrowth of dormant cells was shown to strongly depend on DnaK but not on any of the other chaperones and proteases tested (ClpB, IbpA, IbpB, Lon, ClpS, ClpA, ClpP and ClpX). DnaK is known to be important for the refolding of proteins, while the tested proteases help with degrading misfolded proteins after disaggregation63. Moreover, we observed that many of the aggregated proteins interact with DnaK for folding64. These findings are in agreement with the hypothesis that the aggregated proteins are refolded by DnaK and reused instead of being degraded after aggregate dissolution37. We therefore hypothesize that the liquid-like condensates in persister cells also serve as a storage structure to fuel a fast recovery and regrowth (Fig. 7).
Methods
Bacterial strains and growth conditions
All experiments were performed with E. coli BW2511382. To measure protein aggregation, E. coli BW25113 ibpA-msfGFP 25 was used while the E. coli BW25113 iATPSnFr1.0 strain was used for ATP measurements. The E. coli strain with genomic insertion of iATPSnFr1.0 was constructed by first transforming E. coli BW25113 with the pGRG25-iATPSnFr1.0 plasmid18 and then integrating the gene in the attTn7 site83. To obtain the ΔrpoS, ΔsdhC, ΔdnaK and ΔrecA knockout strains, we started from the corresponding deletion strains in the Keio collection82. The kanamycin resistance cassette within these knockout strains was removed using the pCP20 plasmid84.
The plasmids pBAD33Gm-obgE85 and pBAD33Gm-era were used to induce overexpression of obgE and era. The empty vector pBAD33Gm85 served as a control in all these measurements. The pBAD33Gm-era was constructed by replacing the obgE gene in the pBAD33Gm-obgE plasmid by the era gene via Gibson assembly (Bioké). For this Gibson assembly, the plasmid was amplified with the primers SPI13781 and SPI13782 while the era insert was amplified from a wild-type E. coli BW25113 strain with the primers SPI13779 and SPI13780. Verification of the right insert in the final plasmid was conducted by sequencing with primers SPI10499 and SPI10500. Sequences of the primers are SPI10499: CTACTGTTTCTCCATACCCG, SPI10500: TGTTTTATCAGACCGCTTCT, SPI13779: CCGCCAAAACAGCCAAGCTTTTAAAGATCGTCAACGTAACCGAGACT, SPI13780: GAGCTCAGGAGGAATTAACCATGAGCATCGATAAAAGTTACTGCGG, SPI13781: GTTACGTTGACGATCTTTAAAAGCTTGGCTGTTTTGGCG, SPI13782: TAACTTTTATCGATGCTCATGGTTAATTCCTCCTGAGCTCGAAT.
Bacteria were cultivated in lysogeny broth (LB) at 37 °C under orbital shaking. When the strains carried plasmids, the antibiotics to which these plasmids conferred resistance (e.g. 25 µg/ml gentamycin, 40 µg/ml kanamycin, 100 µg/ml ampicillin) were added to the growth medium. Overnight cultures were diluted 100-fold in flasks containing 100 ml LB, the appropriate antibiotics and 0.2% arabinose for strains carrying plasmids. At designated time points, a sample was taken from the flasks and analyzed. For translation measurements, cells were also shortly incubated in M9 minimal medium with 1% casamino acids.
Persister assay
To determine the number of persister cells at a specific time point, 990 µl of culture was transferred from a culture flask to a sterile test tube and treated with 5 µg/ml ofloxacin for 5 hours. Fluoroquinolone antibiotics are regularly used to clear infections of E. coli86,87. Ofloxacin was chosen as it is often used in the persister field13,25,88,89. After the antibiotic treatment, the cells were washed twice with 10 mM MgSO4. 10-fold dilutions were prepared in 10 mM MgSO4 and plated out on non-selective LB plates containing 1.5% agar. The number of CFUs was determined after incubation at 37 °C for 48 hours.
Identifying the number of VBNC cells
The number of VBNC cells was determined by measuring the difference between the number of viable cells and the number of culturable cells at a specific time point, similar to what was described before25. Viable cells were quantified by staining the cells prior to antibiotic treatment with SYTOX Green (Invitrogen), which is a fluorescent and membrane-impermeable dye that can only penetrate dead cells that have lost membrane integrity. Cells were diluted 100- to 1000-fold in 0.5 µM SYTOX Green in filtered phosphate-buffered saline (PBS) and stained for 15 minutes in the dark. Subsequently, cells were measured with the CytoFLEX S flow cytometer (Beckman Coulter Life Sciences) equipped with 405-nm, 488-nm and 561-nm lasers. The forward and side scatter were used to gate bacterial cells (Supplementary Fig. 7). Fluorescence from the SYTOX Green dye was measured for 10,000 bacterial cells at an excitation wavelength of 488 nm and an emission wavelength of 525/40 nm. The number of viable cells per ml was calculated by counting the number of non-fluorescent, living cells per ml.
The number of culturable cells was identified by assessing the number of CFUs per ml of a culture that was not treated with antibiotics. 10-fold dilutions of the cultures were made in 10 mM MgSO4 and plated on LB with 1.5% agar. The CFUs were counted after 24 hours of incubation at 37 °C and the number of CFUs per ml was calculated.
Measuring aggregation
The fraction of cells containing protein aggregates was measured by quantitative microscopy as described before25. For all experiments, a Nikon Ti-E inverted microscope equipped with a Qi2 CMOS camera and temperature-controlled cage incubator was used. Cells were collected at different time points, spotted on pads of 10 mM MgSO4 with 2% agarose and imaged in both the green fluorescent and the phase contrast channel. The measurement of the fraction of cells with Ph aggregates in the E. coli ibpA-msfGFP pBAD33Gm-dnaK strain after 40 hours of incubation was deleted from Fig. 5e because it was an outlier that could be attributed to cells temporarily becoming a little lighter at mid-cell position (Supplementary Fig. 5c). All datapoints of Ph aggregation for the E. coli ibpA-msfGFP pBAD33Gm-dnaK strain and of the E. coli ibpA-msfGFP pBAD33Gm strain are shown. To test the dissolution of aggregates by hexanediol, the same culture was also spotted on pads of 10 mM MgSO4 with 10% 1,6-hexanediol and 2% agarose. IbpA aggregates were determined as foci in the green fluorescent channel, while Ph aggregates appeared as white foci in the phase contrast channel. The ImageJ plug-in MicrobeJ was used for the image analysis90. For each repeat, at least 50 cells were analyzed and the number of IbpA and Ph aggregates was determined in each cell. Based on these results, we calculated the fraction of cells that contained one or more aggregates.
Measuring ATP levels
E. coli BW25113 cells with the genomic iATPSnFr1.0 sensor were used to measure ATP at the single-cell level18. At specific time points, cells were diluted 100- to 1000-fold in filtered PBS and measured with the CytoFLEX S flow cytometer (Beckman Coulter Life Sciences) containing 405-nm, 488-nm and 561-nm lasers. Specific gates based on the forward and side scatter were used to identify the bacterial cells (Supplementary Fig. 7). For 10,000 bacterial cells, the emission at 525/40 nm was measured for both excitation at 488 and at 405 nm and the fraction of the emissions for each excitation was calculated. This fraction (488/405 nm excitation) is a proxy for the ATP level in the cell. For each repeat, the fraction of ATP-depleted cells, with a ratiometric ATP measurement below 0.65, was determined. As the ratiometric ATP levels often display a bimodal distribution pattern, this threshold was chosen to be in the middle of the minimum between the two peaks of the distribution.
Measuring membrane depolarization
Membrane potential was measured by diluting the cells 100- to 1000-fold in PBS solution with 1 µM DIBAC4(3) (Invitrogen), a fluorescent dye that only stains cells that exhibit membrane depolarization58. The fluorescence of 10,000 bacterial cells, gated based on forward and side scatter (Supplementary Fig. 7), was measured at an excitation wavelength of 488 nm and an emission wavelength of 525/40 nm with the CytoFLEX S flow cytometer (Beckman Coulter Life Sciences). The fraction of cells with membrane depolarization was calculated as the fraction of bacterial cells that showed fluorescence.
Measuring translation
Translation was quantified at the single-cell level with the Click-iT® HPG Alexa Fluor® Protein Synthesis Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, cells were washed twice in PBS and incubated for 30 minutes at 37 °C in M9 medium with 1% casamino acids and with a 1000-fold dilution of the Click-it® Homopropargylglycine reagent. Following incubation, cells were washed once with PBS and subjected to permeabilization and fixation. After permeabilization and fixation, cells were incubated for 30 minutes with the Click-iT® reaction cocktail, washed with the Click-iT® reaction rinse buffer and dissolved in filtered PBS. The cells were further diluted 100- to 1000-fold in filtered PBS and the fluorescence of 10,000 bacterial cells, gated based on the forward and side scatter (Supplementary Fig. 7), was measured at an excitation wavelength of 488 nm and an emission wavelength of 525/40 nm using the CytoFLEX S flow cytometer (Beckman Coulter Life Sciences). For each repeat, the sum of the translation fluorescence for the 10,000 bacterial cells was calculated. A negative control was also included in which the E. coli pBAD33Gm strain that was grown for 2, 4 and 8 hours was treated with 35 µg/ml chloramphenicol during the incubation in M9 medium with homopropargylglycine to inhibit translation.
Catalase assay
Catalase activity was measured using the catalase assay on a glass microscopy slide. These experiments were performed for cells from an overnight culture and for cells which were grown overnight, diluted in flasks and grown again for 72 hours. Cells were plated on LB plates with 1.5% agar and incubated for 16 hours at 37 °C. This was done for 3 repeats of each strain. For each repeat, 3 colonies were tested. Each colony was streaked on a glass slide and a drop of freshly prepared 3% hydrogen peroxide was added. The amount of bubbles generated was examined and rated.
FRAP microscopy
For the FRAP measurements, a Nikon TiE A1R microscope with cage incubator was used. E. coli ibpA-msfGFP pBAD33Gm-obgE was grown for 8 and 40 hours in flasks. These experiments were only performed in a strain overexpressing obgE as only these aggregates were big enough to bleach partially25. The cells were diluted 10-fold in MgSO4 and spotted on pads of 10 mM MgSO4 with 2% agarose. The FRAP analysis was performed on 20 and 10 aggregates of cells that were grown for 8 and 40 hours, respectively. A part of the IbpA aggregates was bleached with a 488 nm laser at a power of 100% and a scan speed of 4 frames/second. For the imaging before and after bleaching, the same 488 nm laser was used for excitation, but at a power of 0.5–2% and a scan speed of 1 frame/second, and emission was measured at 500/50 nm. Three images were taken before bleaching at intervals of 4 seconds. The bleaching itself was performed for 4 loops and the recovery after bleaching was monitored every second for a total duration of 50 seconds. For the analysis, the sum of the fluorescence intensity within the bleached area was determined and corrected for fluctuations in fluorescence intensity in the background and in a reference aggregate that remained unbleached. The percentage recovery after bleaching was calculated by dividing the intensity increase after bleaching by the intensity decrease due to the bleaching.
Protein aggregate composition
Aggregate isolation
Aggregate isolation was conducted similar to a previously described protocol91. For each sample, 50 ml of culture was centrifuged for 30 minutes at 4800 x g and the pellets were frozen at −20 °C until further use. Subsequently, the pellets were washed with 10 ml of buffer A (50 mM HEPES (pH 7.5), 300 mM NaCl, 5 mM β-mercaptoethanol and 1 mM EDTA) and centrifuged for 15 minutes at 4000 x g and 4 °C. Afterwards, the pellet was dissolved in 10 ml of buffer B (buffer A with 1 µg/ml leupeptin and 0.1 mg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride). The cells were lysed using the Glen Creston Cell Homogenizer with pressure between 20,000 and 25,000 psi followed by 4 times 30 seconds sonication at 50% amplitude using the Branson Digital sonifier 50/60 Hz. The lysed cells were pelleted by centrifugation for 30 minutes at 11000 x g and 4 °C. The pellet was washed 4 times in 10 ml of buffer D (buffer A with 0.8% (vol/vol) Triton X-100 and 0.1% sodium deoxycholate which are known to solubilize membrane proteins effectively92, thereby facilitating their partitioning into the soluble fraction during the purification process). Finally, the pellet was dissolved in 500 µl of 8 M urea (pH 8).
Coomassie staining
After their isolation, the aggregates were loaded on an SDS gel (Mini-PROTEAN TGX precast protein gels) and stained with Coomassie blue (R250) as described before25. The intensity of the bands was quantified by using Image Lab Software 6.1. The Coomassie intensity for the different strains is represented in Supplementary Fig. 8.
Protein purification for MS analysis
Protein purification was performed as described before91. In short, dithiothreitol was added to the isolated aggregates at a final concentration of 0.02 M and this mixture was incubated for 15 minutes at room temperature. Subsequently, 2-iodoacetamide was added to a final concentration of 0.05 M and the solution was incubated for 30 minutes at room temperature in the dark. Following this, ammonium bicarbonate buffer (0.15 M, pH = 8–8.5) with 0.2 µg/ml trypsin was added to the samples reaching a final concentration of 0.11 M and the mixture was incubated for 16 hours at 37 °C for trypsin digestion. After trypsin digestion, the samples were purified on a C18 spin column (Thermo Fisher Scientific). Finally, the samples were diluted in 10 µl with 5% acetonitrile and 0.1% formic acid for injection into the Q Exactive Orbitrap mass spectrometer (Thermo Scientific, USA).
MS analysis
Peptides were loaded onto a 2 cm C18 trap column (ThermoFisher 164946), which was connected in-line to a 15 cm C18 reverse-phase analytical column (Thermo EasySpray ES904). The separation of the peptides was carried out using 100% Buffer A (0.1% formic acid in water) at 750 bar, with the Thermo EasyLC 1200 HPLC system, and the column temperature maintained at 30 °C. MS analysis was performed following a previously established protocol91 with each condition represented by three biologically independent replicates. Raw data were collected using Xcalibur 3.0.63 software (Thermo Fisher Scientific), and subsequently converted into MGF files using Proteome Discover version 1.4 (Thermo Fisher Scientific).
The converted data were processed using MASCOT version 2.2.06 (Matrix Science) against the Uniprot E. coli database93 using previously described parameters91. MASCOT results were imported to Scaffold version 4 for protein identification (parameters set to retain proteins with 99% confidence and containing at least two identified peptides with confidence level 95%) and label-free quantitation (based on spectrum counting). Finally, protein identities and the total count of unique spectra were used for downstream analyses, including further statistical processing and interpretation.
Analysis of MS data
The proteins present in the aggregates were analyzed in two different ways. To check which proteins were present in the aggregates of the different strains (Fig. 2 and Supplementary Fig. 3), we conducted a qualitative analysis. In this analysis, we tested which types of proteins were enriched in the aggregates compared to the E. coli proteome by using a KEGG56 and COG55 enrichment analysis. To determine which proteins were significantly sequestered in the aggregates over time (Fig. 3), we performed a label-free quantitative analysis based on spectral counting, which is a straightforward and widely used method but comes with certain limitations. These include reduced sensitivity in detecting small differences in protein abundance, and variability between experimental runs. However, in this study, all samples were analyzed in a single run to minimize variability, and comparisons were carefully controlled to minimize these limitations. In this analysis, the quantitative protein abundance data from the MS analysis were compared between different sample groups using the DESeq2 package in R59. To remove proteins with very low abundance, the data underwent pre-filtering, retaining only those proteins with a count higher than 1 in at least 3 samples. Following this pre-filtering step, the DESeq2 package was used to identify proteins that were significantly enriched or depleted between different sample groups based on a Wald test with false discovery rate (FDR)-controlled multiple testing correction. The significance threshold was set at P < 0.05. For each time point, a list with proteins that were significantly enriched in the aggregate was obtained. To see which types of proteins were mainly enriched, this list was also subjected to a KEGG56 and COG55 enrichment analysis. The STRINGdb package in R was used to associate KEGG categories to a list of proteins and to test which categories were enriched94. This enrichment was determined by a hypergeometric test. Multiple testing correction was based on the FDR, which was calculated based on the Benjamini-Hochberg procedure. COG categories were assigned to a list of proteins using the eggNOG tool95. In the same manner as before25, enrichment of COG categories was determined using the one-sided Fisher’s exact tests with a Bonferroni correction for multiple testing.
AFM-IR spectroscopy
E. coli pBAD33Gm-obgE was grown for 2, 8 and 40 hours after dilution and induction. Pellets were harvested, washed twice with 1 ml PBS and fixed in 500 µl of a 2.5% glutaraldehyde solution in 0.1 M cacodylate buffer for 1 hour at room temperature. After washing in 500 µl 0.1 M cacodylate buffer, the samples were fixed in 500 µl of a 1% OsO4 solution in 0.1 M cacodylate buffer for 2 hours at room temperature. The pellets were washed twice in 0.1 M cacodylate buffer and dehydrated in an ethanol series (30, 50, 70, 90, 100, 100, 100%), rotating slowly for 10 minutes at 4 °C. Subsequently, cells were treated twice with 500 µl propylene oxide, rotating for 15 minutes at 4 °C.
The epoxy embedding was done in three stages, first by resuspending in a 1:1 epoxy and propylene oxide mixture, supplementing 27 µl benzyldimethylamine (BDMA) per 1 ml epoxy, and incubating for 1 hour at 4 °C while rotating. Second, the samples were resuspended in a 2:1 mixture (with BDMA) and left to dry overnight. Finally, the samples were incubated in 100% epoxy resin (with BDMA), drying at low vacuum for 4 hours and polymerizing at 60 °C for 2 days. The resin blocks were sectioned to a thickness of 95 nm and the sections were transferred onto silicon wafers (Ted Pella, 16008), which were then glued to a sample disc (Bruker, SD-102).
For the AFM-IR measurements, a cantilever (k = 0.2 N/m and f0 = 13 kHz, PR-EX-nIR2-10, Bruker) was mounted in a nanoIR3 (Bruker) equipped with a MIRcat-QT laser (DRS Daylight Solutions), maximizing the laser sum and optimizing the vertical and lateral deflection to approximately −0.3 V and 0 V respectively. With the laser power set to 1.37%, the pulse length at 160 ns and the pulse rate around 880 kHz, the IR beam was aligned in x and y for each of the QCL chips (at 1730, 1260, 1088, 914 cm−¹). The z position was optimized for 1730 cm−¹. A phase offset was chosen to maximize the IR amplitude and the phase-locked loop (PLL) gains were set to I = 0.1 and P = 1. An incident power spectrum was acquired for every sample. Maps were then collected at 1625 cm−¹ with a field of view of 20x20 µm, resolution 512x512 px, scan rate 0.1 Hz, AFM gains I = 2, P = 1 and PLL gains I = 6, P = 60. Afterwards, the IR focus was re-aligned (only x and y) and a new power spectrum was collected. Spectra were collected on presumptive locations of aggregates, cytoplasma and epoxy matrix, with 5 coaverages and with PLL gains I = 0.1, P = 1 and spectral resolution of 2 cm-¹. To change samples, the sample was moved to its lowest position and replaced with a set of tweezers, taking care not to touch the AFM head. Replicates 2 and 3 were scanned with a different cantilever than replicate 1.
To discriminate between spectra with high and low beta sheet signal and to correlate them with the IR images, we performed k-means clustering on the second derivative of the amide I band (1580 cm−1–1700 cm−1).
Statistics
All measurements were taken from distinct samples. Data were analyzed using GraphPad Prism 9 or R 4.3.096.
A logistic growth model, using the least squares method, was fitted to the data representing the fraction of cells with IbpA and Ph aggregates, with ATP depletion and with membrane depolarization. Outliers and time points where this fraction already started declining were excluded from the analysis to achieve the best possible fit. Based on the fitted logistic growth models, the time required to reach 10 and 90% of the maximal value could be calculated.
Before statistically comparing different groups of data, their normality was assessed with the D’Agostino and Pearson test. To obtain normality, a log transformation was applied to the data describing translation, viable cells and CFUs. Normally distributed data of two or more groups were analyzed with unpaired t-tests or two-way ANOVA, respectively. Non-normally distributed and paired data were analyzed with the Wilcoxon signed-rank test. The appropriate multiple testing corrections were applied when necessary.
A Pearson correlation analysis was conducted to calculate the correlation between the timing of IbpA aggregation, Ph aggregation, persistence and VBNC cell formation. A representation of the raw data and the least square regression line on which the Pearson R values are based is given in Supplementary Fig. 9.
To determine the half-time of recovery after FRAP for each aggregate, a one-phase association model, using least squares regression, was fitted to each repeat of the recovery data.
Data are mainly represented as the mean ± the standard error of the mean (SEM) and every experiment was measured for at least 3 biological repeats. The levels of significance are: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, *****P < 0.00001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Supplementary Information and in the Source Data file which has been deposited, along with MS quantitative counts, in the Zenodo database under accession code 14197261. Mass spectrometry proteomics data have also been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD059817 and PXD059868. Source data are provided with this paper.
References
Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol 21, 280–295 (2023).
Balaban, N. Q. et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17, 441–448 (2019).
Fauvart, M., de Groote, V. N. & Michiels, J. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J Med Microbiol 60, 699–709 (2011).
Mulcahy, L. R., Burns, J. L., Lory, S. & Lewis, K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol 192, 6191–6199 (2010).
Dhar, N. & McKinney, J. D. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc Natl Acad Sci USA 107, 12275–12280 (2010).
Goneau, L. W. et al. Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob Agents Chemother 58, 2089–2097 (2014).
Bollen, C., Louwagie, E., Verstraeten, N., Michiels, J. & Ruelens, P. Environmental, mechanistic and evolutionary landscape of antibiotic persistence. EMBO rep 24, e57309 (2023).
Orman, M. A. & Brynildsen, M. P. Dormancy is not necessary or sufficient for bacterial persistence. Antimicrob Agents Chemother 57, 3230–3239 (2013).
Wu, Y., Vulić, M., Keren, I. & Lewis, K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56, 4922–4926 (2012).
Adams, K. N. et al. Drug tolerance in replicating Mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145, 39–53 (2011).
Stapels, D. A. C. et al. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 362, 1156–1160 (2018).
Nguyen, D. et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986 (2011).
Völzing, K. G. & Brynildsen, M. P. Stationary-phase persisters to ofloxacin sustain DNA damage and require repair systems only during recovery. Mbio 6, e00731–15 (2015).
Wilmaerts, D., Windels, E. M., Verstraeten, N. & Michiels, J. General mechanisms leading to persister formation and awakening. Trends Genet 35, 401–411 (2019).
Keren, I., Shah, D., Spoering, A., Kaldalu, N. & Lewis, K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol 186, 8172–8180 (2004).
Hu, Y. & Coates, A. Nonmultiplying bacteria are profoundly tolerant to antibiotics. In Antibiotic Resistance (ed. Coates, A. R. M.) 99–119 (Springer, Berlin, Heidelberg, 2012).
Amato, S. M. et al. The role of metabolism in bacterial persistence. Front Microbiol 5, 70 (2014).
Manuse, S. et al. Bacterial persisters are a stochastically formed subpopulation of low-energy cells. PLoS Biol 19, e3001194 (2021).
Huemer, M. et al. Molecular reprogramming and phenotype switching in Staphylococcus aureus lead to high antibiotic persistence and affect therapy success. Proc Natl Acad Sci USA 118, e2014920118 (2021).
Quigley, J. & Lewis, K. Noise in a metabolic pathway leads to persister formation in Mycobacterium tuberculosis. Microbiol Spectr 10, e02948–22 (2022).
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004).
Ayrapetyan, M., Williams, T. C. & Oliver, J. D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol 23, 7–13 (2015).
Li, L. et al. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol 5, 1–20 (2014).
Pu, Y. et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol Cell 73, 143–156 (2019).
Dewachter, L. et al. The dynamic transition of persistence toward the viable but nonculturable state during stationary phase is driven by protein aggregation. Mbio 12, e00703–e00721 (2021).
Kim, J.-S., Chowdhury, N., Yamasaki, R. & Wood, T. K. Viable but non-culturable and persistence describe the same bacterial stress state. Environ Microbiol 20, 2038–2048 (2018).
Liu, J., Yang, L., Kjellerup, B. V. & Xu, Z. Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends Microbiol 31, 1013–1023 (2023).
Oliver, J. D. The viable but nonculturable state in bacteria. J Microbiol 43, 93–100 (2005).
Pai, S. R., Actor, J. K., Sepulveda, E., Hunter, R. L. Jr & Jagannath, C. Identification of viable and non-viable Mycobacterium tuberculosis in mouse organs by directed RT-PCR for antigen 85B mRNA. Microb Pathog 28, 335–342 (2000).
Rivers, B. & Steck, T. R. Viable but nonculturable uropathogenic bacteria are present in the mouse urinary tract following urinary tract infection and antibiotic therapy. Urol Res 29, 60–66 (2001).
Peyrusson, F., Nguyen, T. K., Najdovski, T. & Van Bambeke, F. Host cell oxidative stress induces dormant Staphylococcus aureus persisters. Microbiol Spectr 10, e02313–e02321 (2022).
Mordukhova, E. A. & Pan, J. G. Stabilization of homoserine-o-succinyltransferase (MetA) decreases the frequency of persisters in Escherichia coli under stressful conditions. PLoS One 9, e110504 (2014).
Leszczynska, D., Matuszewska, E., Kuczynska-Wisnik, D., Furmanek-Blaszk, B. & Laskowska, E. The formation of persister cells in stationary-phase cultures of Escherichia coli is associated with the aggregation of endogenous proteins. PLoS One 8, e54737 (2013).
Goode, O. et al. Heterologous protein expression favors the formation of protein aggregates in persister and viable but nonculturable bacteria. ACS Infect Dis 7, 1848–1858 (2021).
Jin, X. et al. Membraneless organelles formed by liquid-liquid phase separation increase bacterial fitness. Sci Adv 7, eabh2929 (2021).
Li, Y., Wood, T. K., Zhang, W. & Li, C. Purine metabolism regulates Vibrio splendidus persistence associated with protein aggresome formation and intracellular tetracycline efflux. Front Microbiol 14, 1127018 (2023).
Yu, J., Liu, Y., Yin, H. & Chang, Z. Regrowth-delay body as a bacterial subcellular structure marking multidrug-tolerant persisters. Cell Discov 5, 8 (2019).
Cesar, S., Willis, L. & Huang, K. C. Bacterial respiration during stationary phase induces intracellular damage that leads to delayed regrowth. iScience 25, 103765 (2022).
Bollen, C., Dewachter, L. & Michiels, J. Protein aggregation as a bacterial strategy to survive antibiotic treatment. Front Mol Biosci 8, 669664 (2021).
Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Almeida, Z. L. & Brito, R. M. M. Structure and aggregation mechanisms in amyloids. Molecules 25, 1195 (2020).
Xu, Q. et al. Protein amyloid aggregate: structure and function. Aggregate 4, e333 (2023).
Wu, N. et al. Ranking of persister genes in the same Escherichia coli genetic background demonstrates varying importance of individual persister genes in tolerance to different antibiotics. Front Microbiol 6, 1003 (2015).
Hong, S. H., Wang, X., O’Connor, H. F., Benedik, M. J. & Wood, T. K. Bacterial persistence increases as environmental fitness decreases. Microb Biotechnol 5, 509–522 (2012).
Liu, S. et al. Variable persister gene interactions with (p)ppGpp for persister formation in Escherichia coli. Front Microbiol 8, 1795 (2017).
Orman, M. A. & Brynildsen, M. P. Inhibition of stationary phase respiration impairs persister formation in E. coli. Nat Commun 6, 7983 (2015).
Dörr, T., Lewis, K. & Vulić, M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet 5, e1000760 (2009).
Luidalepp, H., Jõers, A., Kaldalu, N. & Tenson, T. Age of inoculum strongly influences persister frequency and can mask effects of mutations implicated in altered persistence. J Bacteriol 193, 3598–3605 (2011).
Verstraeten, N. et al. Obg and membrane depolarization are part of a microbial bet-hedging strategy that leads to antibiotic tolerance. Mol Cell 59, 9–21 (2015).
Govers, S. K., Mortier, J., Adam, A. & Aertsen, A. Protein aggregates encode epigenetic memory of stressful encounters in individual Escherichia coli cells. PLoS Biol 16, e2003853 (2018).
Janowska, M. K., Baughman, H. E. R., Woods, C. N. & Klevit, R. E. Mechanisms of small heat shock proteins. Cold Spring Harb Perspect. Biol. 11, a034025 (2019).
Kwan, B. W., Valenta, J. A., Benedik, M. J. & Wood, T. K. Arrested protein synthesis increases persister-like cell formation. Antimicrob Agents Chemother 57, 1468–1473 (2013).
McKay, S. L. & Portnoy, D. A. Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother 59, 6992–6999 (2015).
Conlon, B. P. et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1, 16051 (2016).
Tatusov, R. L., Galperin, M. Y., Natale, D. A. & Koonin, E. V. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res 28, 33–36 (2000).
Kanehisa, M., Furumichi, M., Tanabe, M., Sato, Y. & Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45, D353–D361 (2017).
Feng, B. et al. Structural and functional insights into the mode of action of a universally conserved Obg GTPase. PLoS Biol 12, e1001866 (2014).
Verstraeten, N. et al. Biochemical determinants of ObgE-mediated persistence. Mol Microbiol 112, 1593–1608 (2019).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014).
Maloney, P. C., Kashket, E. R. & Wilson, T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci USA 71, 3896–3900 (1974).
Shapiro, H. M. Membrane potential estimation by flow cytometry. Methods 21, 271–279 (2000).
Farrell, M. J. & Finkel, S. E. The growth advantage in stationary-phase phenotype conferred by rpoS mutations is dependent on the pH and nutrient environment. J Bacteriol 185, 7044–7052 (2003).
Dougan, D. A., Mogk, A. & Bukau, B. Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. Cell Mol Life Sci 59, 1607–1616 (2002).
Calloni, G. et al. DnaK functions as a central hub in the E. coli chaperone network. Cell Rep 1, 251–264 (2012).
Tomaszewski, A. et al. Solid-to-liquid phase transition in the dissolution of cytosolic misfolded-protein aggregates. iScience 26, 108334 (2023).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol 22, 196–213 (2021).
Kroschwald, S., Maharana, S. & Simon, A. Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters 3, e201702000010 (2017).
Dazzi, A., Prazeres, R., Glotin, F. & Ortega, J. M. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt Lett 30, 2388–2390 (2005).
Kong, J. & Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39, 549–559 (2007).
Jain, S. et al. ATPase modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Mittag, T. & Pappu, R. V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol Cell 82, 2201–2214 (2022).
Cherkasov, V. et al. Coordination of translational control and protein homeostasis during severe heat stress. Curr Biol 23, 2452–2462 (2013).
Kroschwald, S. et al. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, e06807 (2015).
Zacharogianni, M., Aguilera-Gomez, A., Veenendaal, T., Smout, J. & Rabouille, C. A stress assembly that confers cell viability by preserving ERES components during amino-acid starvation. Elife 3, e04132 (2014).
Lin, Y., Protter, D. S. W., Rosen, M. K. & Parker, R. Formation and maturation of phase separated liquid droplets by RNA binding proteins. Mol Cell 60, 208–219 (2015).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
Buchan, J. R. mRNP granules. RNA Biol 11, 1019–1030 (2014).
Saad, S. et al. Reversible protein aggregation is a protective mechanism to ensure cell cycle restart after stress. Nat Cell Biol 19, 1202–1213 (2017).
Brengues, M., Teixeira, D. & Parker, R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310, 486–489 (2005).
Wallace, E. W. J. et al. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298 (2015).
Narayanaswamy, R. et al. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc Natl Acad Sci USA 106, 10147–10152 (2009).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2, 2006.0008 (2006).
McKenzie, G. J. & Craig, N. L. Fast, easy and efficient: site-specific insertion of transgenes into Enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol 6, 39 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97, 6640–6645 (2000).
Dewachter, L. et al. A mutant isoform of ObgE causes cell death by interfering with cell division. Front. Microbiol. 8, 1193 (2017).
Kim, Y. et al. Fluoroquinolone can be an effective treatment option for acute pyelonephritis when the minimum inhibitory concentration of levofloxacin for the causative Escherichia coli Is ≤16 mg/L. Antibiotics (Basel) 10, 37 (2021).
Collier, R. Escherichia Coli (E Coli) Infections Medication. https://emedicine.medscape.com/article/217485-medication?form=fpf (2023).
Wilmaerts, D. et al. HokB monomerization and membrane repolarization control persister awakening. Mol Cell 75, 1031–1042.e4 (2019).
Goormaghtigh, F. & Van Melderen, L. Single-cell imaging and characterization of Escherichia coli persister cells to ofloxacin in exponential cultures. Sci Adv 5, eaav9462 (2019).
Ducret, A., Quardokus, E. M. & Brun, Y. V. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1, 16077 (2016).
Khodaparast, L. et al. Aggregating sequences that occur in many proteins constitute weak spots of bacterial proteostasis. Nat Commun 9, 866 (2018).
Makino, S., Reynolds, J. A. & Tanford, C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem 248, 4926–4932 (1973).
Perkins, D. N., Pappin, D. J. C., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Szklarczyk, D. et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49, D605–D612 (2021).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res 47, D309–D314 (2019).
R. Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2022).
Acknowledgements
We want to express gratitude to prof. Kim Lewis (Northeastern University) for the pGRG25-iATPSnFr1.0 plasmid and prof. Abram Aertsen (KU Leuven) for the E. coli MG1655 ibpA-msfGFP strain. Additionally, we would like to thank the Proteomics Core at the Biomedical Sciences Group of KU Leuven, SyBioMa, for their help with the analysis of the aggregate composition and the VIB Bioimaging Core in Leuven, with Abril Escamilla Ayala specifically, for their support with the FRAP microscopy. Finally, we thank Natalie Verstraeten and Philip Ruelens for critically reading the manuscript. The work was supported by PhD fellowships from the Research Foundation Flanders (FWO) awarded to C.B. (1126120 N) and W.D. (1128822 N), and by FWO postdoctoral fellowships awarded to Lad. K. (1231021 N) and L.D. (12Q4319N). The Center for Microbiology was funded by FWO projects I011220N, G0B0420N and G0C4322N, FWO-SBO project S001421N, EOS project 40007496, KU Leuven project C16/17/006 and the Flanders Institute for Biotechnology (VIB). The Switch Laboratory was supported by FWO projects G0A6724N and I011220N and by VIB.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: C.B., L.D., J.M.; Investigation: C.B. was involved in all experiments, S.L., F.D. and D.H. performed microscopy, cytometry and persistence/VBNC assays, W.D. conducted AFM-IR, Ladan K. and Laleh K. performed proteomics; Writing of the original draft: C.B.; Review and editing of draft: C.B., S.L., W.D., Ladan K., Laleh K., J.S., F.R., L.D., J.M.; Figures: C.B.; Supervision: L.D., J.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Stephen Fried and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bollen, C., Louwagie, S., Deroover, F. et al. Composition and liquid-to-solid maturation of protein aggregates contribute to bacterial dormancy development and recovery. Nat Commun 16, 1046 (2025). https://doi.org/10.1038/s41467-025-56387-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56387-8
This article is cited by
-
How viable but non-culturable (VBNC) bacteria persist in aquaculture and endanger fish health and water safety
Reviews in Environmental Science and Bio/Technology (2026)
-
Stress changes the material state of a bacterial biomolecular condensate and shifts its function from mRNA decay to storage
Nature Communications (2025)