Abstract
As bacterial contamination crises escalate, the development of advanced membranes possessing both high flux and antibacterial properties is of paramount significance for enhancing water sterilization efficiency. Herein, an ultrathin layer of TbPa (an imine-linked covalent organic framework) and nanosized Cu2O clusters, sequentially deposited onto polyethersulfone membranes, demonstrate exceptional water flux performance, reaching a permeance level of 16000 LHM bar−1. The deposited TbPa, generating uniformly distributed reduction sites under illumination, facilitates the uniform formation of Cu2O clusters. Furthermore, these anchored Cu2O clusters significantly optimize electron transport within the ultra-thin layer of TbPa, thereby enhancing the performance of the membrane in generating reactive oxygen species (ROS). Consequently, this membrane achieves a flux recovery rate exceeding 98.6% for flux losses caused by bacterial fouling and maintains consistent performance over 10 cycles. This work presents an effective strategy for accessing bactericidal membranes and provides insights into efficient and mild water sterilization.
Similar content being viewed by others
Introduction
Elimination of bacterial contamination in water has become a core safety indicator in multiple fields like healthcare services1,2, food processing3, and livestock farming4, etc. Microfiltration membrane technology offers a pivotal solution for effective and sustainable disinfection, featuring high throughput, low energy consumption, and the complete absence of chemical residues5,6. Nonetheless, a notable challenge in this technology is bacterial fouling, which increases hydraulic resistance and promotes the proliferation of embedded bacteria7. This issue undermines the long-term effectiveness and sustainability of microfiltration as a water treatment solution. Advanced oxidation processes have shown promising potential in bacterial inactivation and biofilm removal, providing solutions to address membrane bacterial fouling8. Consequently, polymeric membranes have been developed by incorporating highly efficient inorganic photocatalysts either as blends or coatings9,10,11. However, compatibility challenges between polymeric substrates and inorganic oxidants cause detachment and wear issues in practical applications12.
Covalent organic frameworks (COFs), which are constructed from organic components linked by robust covalent bonds, exhibit exceptional compatibility with polymeric membranes13,14. Furthermore, their exceptional stability, efficient mass transfer capabilities, and superior charge carrier mobility have garnered significant attention, prompting intensive investigations into their potential to revolutionize photocatalytic systems15. Limited by the high Frenkel exciton binding energy characteristic of COFs, exciton‒exciton annihilation becomes a significant issue, restricting the generation of photo-induced carriers and consequently diminishing the overall efficiency of photocatalytic reactions16,17,18. A promising strategy to address this challenge involves leveraging the robust coordination between metals or oxides and COFs, which has demonstrated the capacity to significantly improve carrier separation and, ultimately, augment catalytic performance19,20,21. However, non-selective metal doping can lead to irregular particle growth, obscuring the active sites and hindering progress towards high-performance COF photocatalysts. Recent studies revealed that photo-generated electrons in COFs accumulate predominantly near imine-N sites22. Thus, researchers have devised a precise in situ reduction method to uniformly photodeposited Pt clusters onto these electron-rich regions, thereby significantly boosting the photocatalytic reaction efficiency. Considering the scarcity and soaring costs of precious metals, it has become imperative to explore cost-effective transition metals as viable alternatives. Notably, Cu2O, distinguished by its abundant reserves, low cost, and suitable energy band, is a promising candidate for depositing clusters on electron-rich regions of COFs, thereby significantly optimising electron transport pathways.
TbPa is an imine-linked two-dimensional COF material with stable π‒π stacking interactions between adjacent layers, making it an ideal scaffold for anchoring metal clusters. Here, we integrated ultra-thin TbPa layers on polyethersulfone (PES) microfiltration membranes via vapor deposition. A photodeposition strategy was subsequently employed to achieve uniform and periodic anchoring of the Cu2O clusters onto the TbPa layers. The resulting Cu2O/TbPa/PES membranes provided an active platform for membrane separation, simultaneously achieving high water flux and bactericidal functions (Fig. 1). Through rigorous examinations involving free radical scavenging tests, electron spin resonance (ESR) measurements and photoelectric performance evaluations, we conducted an in-depth study to elucidate the mechanisms underlying the remarkable anti-bacterial properties of the Cu2O/TbPa/PES membranes. Furthermore, these membranes exhibited exceptional anti-fouling properties during filtration, along with notable stability in their operational performance characteristics. This research achieved the simultaneous integration of high-flux filtration and sterilization, thereby broadening the horizons of membrane applications and fostering the sustainable advancement and exploitation of membrane technology for water treatment applications.
Specifically, the conventional PES membrane experiences a significant decrease in flux due to bacterial fouling. In comparison, Cu2O/TbPa/PES, featuring an ultra-thin Cu2O/TbPa layer, effectively inactivates bacteria and disrupts biofilms under visible light irradiation, thereby providing high flux performance, robust anti-fouling characteristics and sustained operational stability.
Results
Synthesis and characterization of Cu2O/TbPa/PES membranes
TbPa synthesised through the condensation of 1,3,5-triformylbenzene (Tb) and p-phenylenediamine (Pa) features an ideal energy band structure, demonstrating substantial potential in catalysis23 and separation24. Hence, we modified the surface of the PES membranes and explored the performance changes and enhancement mechanisms upon Cu2O cluster anchoring. Figure 2a shows the gas-phase synthesis of the TbPa layer in a tubular furnace. During this process, Tb and Pa are vaporized at elevated temperatures and carried by an Ar flow towards downstream, followed by amino-aldehyde condensation and uniform deposition on the surface of the PES membrane to form the TbPa layer. The temperatures at various locations within the tube were measured using temperature-indicating strips (Supplementary Fig. 1). The results demonstrated that the temperature at the location of the PES membrane ranged from 77 to 82°C, falling within its tolerance temperature range. The entire sequence of heating, deposition and cooling lasts approximately 3 h, culminating in a homogeneously coated TbPa/PES membrane. Afterwards, Cu2O clusters were anchored on the TbPa surface by photodeposition. This two-step synthesis process is shown in Fig. 2b, and the corresponding photographs of the resulting membrane are presented in Fig. 3f.
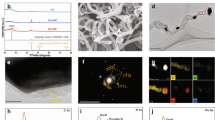
a FT-IR spectra of Tb, Pa, as-synthesized TbPa powders collected in the same tubular furnace as the membrane and Cu2O/TbPa. b XPS survey spectra and c high‐resolution N 1 s spectra of TbPa and Cu2O/TbPa. d Cu LMM Auger TbPa. e Experimental XRD patterns of TbPa powders and simulated XRD patterns of TbPa crystallites. f Photographs of PES, TbPa/PES, and Cu2O/TbPa/PES membranes. g SEM image of Cu2O/TbPa/PES membrane; the inset shows the pore size data fitted using a Gaussian distribution model, yielding the mean and standard deviation of the particle sizes. The scale bar is 1 μm. h Atomic force microscopy (AFM) images (the scale bar is 50 μm) and i corresponding line cuts of the indicated Cu2O/TbPa as-synthesized on a silicon wafer. j HAADF-STEM images of Cu2O/TbPa (Cu2O clusters are marked by yellow circles). The scale bar is 5 nm. k Annular Dark Field (ADF) EDX mapping images of Cu2O/TbPa. The scale bar is 30 nm.
The FTIR analysis (Fig. 3a) of TbPa and Cu2O/TbPa revealed the emergence of a characteristic C=N stretching signal at 1620 cm−1, whereas the N-H stretching signals at 3297 and 3201 cm−1 and the C=O stretching signal at 1696 cm−1 were absent. This finding indicates that the monomers effectively underwent condensation via Schiff base reactions under an Ar atmosphere. The absence of identifiable characteristic peaks of Cu-O in the FT-IR spectra should be attributed to the trace amount of Cu2O anchored on the membrane. As revealed by the broad-scan XPS spectrum (Fig. 3b), a minimal amount of copper is incorporated into TbPa. To further analyse the bonding configuration of copper, we conducted Cu LMM spectroscopy on Cu2O/TbPa (Fig. 3c). The subsequent nonlinear least squares fitting of the results confirmed that Cu is present in its monovalent state, aligning with the prior observation of clustered Cu2O. Figure 3d presents the fine XPS spectra of N 1 s for TbPa and Cu2O/TbPa. The peak deconvolution analysis revealed that both the N–C and N=C bonds significantly increased the binding energy by approximately 0.56 and 0.89 eV, respectively, after Cu2O anchoring. This trend suggests a decrease in the electron cloud density around the N‒C bond, particularly around the N=C bond, indicating a modulation of the electronic properties at the interface of Cu2O and TbPa. Consequently, Cu2O anchoring is anticipated to facilitate the efficient separation of photo-generated carriers during photocatalytic reactions, thereby potentially enhancing the overall photocatalytic performance of the system. TbPa exhibited PXRD signal sets at 4.67°, 8.19°, 9.50° and 12.40°, which were attributed to the (100), (110), (200) and (210) facets, respectively (Fig. 3e). The experimentally obtained curves were closely reproducible through Pawley refinement, indicating that TbPa synthesized by vapor deposition exhibited A–A-eclipsed stacking rather than A–B-staggered stacking (Supplementary Fig. 2).
Figure 3f shows photographs of the PES, TbPa/PES, and Cu2O/TbPa/PES membranes. After TbPa deposition, the membranes transitioned from white to distinctive bright yellow, indicating successful TbPa deposition. Additionally, Cu2O anchoring results in no notable changes, implying minimal Cu2O deposition. We employed SEM to observe the morphology of the membrane surface in detail. As illustrated in Supplementary Fig. 3a, the surface of the PES membrane exhibited a macroporous morphology, with the solid regions among the pores showing a smooth surface texture. After the deposition of TbPa and the anchoring of Cu2O, the average pore size of the membrane remained stable at approximately 0.33 μm (Supplementary Fig. 3b and g). Moreover, high-resolution SEM images indicate that the deposited TbPa presents as a thin film conformally adhered to the PES substrate, and there is no noticeable change in surface morphology after the deposition of Cu2O (Supplementary Fig. 4).
To accurately measure the thickness of the deposited Cu2O/TbPa layer, we substituted porous PES membranes with smooth silicon wafers as the deposition substrate to avoid possible interference. With consistent deposition conditions rigorously maintained, the growth of the TbPa layer on silicon wafers mirrored that on PES membranes, facilitating precise thickness characterization. AFM (Fig. 3h and i) confirmed the uniform deposition of Cu2O/TbPa with an approximate thickness of 10 nm. Given the minimal impact of the ultra-thin deposition on the effective pore size, as evidenced by the SEM images and AFM analysis above, the Cu2O/TbPa/PES membranes are expected to retain the high-flux performance of the pristine PES substrate membranes.
As shown in Fig. 3j, we employed high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) to investigate the morphology of Cu2O. Through the clear contrast variations observed, the Cu2O clusters can be unequivocally distinguished from the TbPa matrix. The visualizations indicate that the Cu2O clusters, measuring approximately 0.25 nm in diameter, exhibited a uniform dispersion pattern on the TbPa surface. This observation was further confirmed by EDX mapping, as illustrated in Fig. 3k. The ordered anchoring mechanism of the clusters is attributed to the localized redox reactions triggered by photoexcitation. These reactions occur specifically at the periodically arranged oxidation and reduction sites within the framework22. The uniform anchoring of Cu2O clusters facilitates the trapping of localized photo-generated electrons on TbPa, effectively suppressing charge recombination during the long-distance electron transfer process. Furthermore, the small Cu2O clusters exposed more active sites to catalyze the surface reaction. The Cu2O clusters within the TbPa framework are expected to significantly increase both the electron transfer efficiency and catalytic performance, making Cu2O/TbPa/PES membranes a highly effective solution for bacterial inactivation and elimination on membrane surfaces.
Photoelectric performance of Cu2O/TbPa/PES membranes
To better understand the precise role and potential enhancement mechanisms associated with the anchoring effect of Cu2O in facilitating photocatalytic processes, we evaluated the photoelectric properties of Cu2O/TbPa. UV−vis diffuse reflectance absorption spectra were employed to quantify the photo-response performance of Cu2O/TbPa. The experimental results (Fig. 4a) revealed that the uniform anchoring of Cu2O clusters on the surface of TbPa significantly induced a red shift in the absorption edge of TbPa, with a magnitude of approximately 100 nm. This broadened visible light response range endows Cu2O/TbPa with ample light energy input, contributing to an efficient catalytic process. Furthermore, based on the UV−vis diffuse reflectance absorption spectra, we constructed a Tauc plot (inset in Fig. 4a), which demonstrates that the bandgap energy of TbPa is approximately 2.52 eV.
a UV−vis diffuse reflectance absorption spectra of TbPa and Cu2O/TbPa (inset: Tauc plots of TbPa and Cu2O/TbPa. b Mott−Schottky plots of TbPa and Cu2O/TbPa. c UPS spectra of TbPa. d Band structure of Cu2O/TbPa. e Photocurrent responses of TbPa and Cu2O/TbPa. f Electrochemical impedance spectroscopy (EIS) data in the form of Nyquist plots. g PL spectra of TbPa and Cu2O/TbPa.
To determine the band structure of TbPa, ultraviolet photoelectron spectroscopy (UPS) measurements were subsequently conducted on TbPa (Fig. 4c). By integrating the UPS outcomes into the formula (Ec = hv − (Ecutoff − EFermi)), we calculated the conduction band position (Ec) of TbPa to be 7.07 eV vs. Vac, equivalent to 2.57 V vs. RHE. Hence, utilizing the relationship between the conduction band position and the bandgap width, we derived the valence band position of TbPa to be 0.07 V vs. RHE. This observation is in concordance with the valence band position of TbPa derived from the Mott−Schottky plot (Fig. 4b), which resulted in a value of −0.53 V vs. Ag/AgCl (0.08 V vs. RHE). Overall, the consistency between the UPS and Mott−Schottky analyses validates the accuracy of the band structure parameters for TbPa. Based on the above results, we illustrated a diagram of the energy band structure of TbPa, as shown in Fig. 4d. This trend shows that the valence band energy of TbPa is high enough to enable the oxidation of water molecules for hydroxyl radical (·OH) production and to catalyze the conversion of dissolved oxygen in water into H2O2. These substances are ROS, which encompass a group of oxygen-containing free radicals and their precursor peroxides, such as superoxide radical (O2−), H2O2, hydroxyl radical (·OH), and singlet oxygen (1O2). These generated ROS play crucial roles in disrupting bacterial cell membranes, thereby achieving bacterial inactivation.
To further verify the effect of Cu2O cluster anchoring on the photo-generated carrier behavior of TbPa, we performed photocurrent tests (Fig. 4e) and electrochemical impedance spectroscopy (EIS) analysis (Fig. 4f). These results indicate that the uniform anchoring of Cu2O clusters significantly promoted the transmission efficiency of the photo-generated electrons and effectively reduced the resistance in the transmission path. This more efficient electron transport channel accelerated the migration of carriers towards the catalytic interface and ultimately facilitated the efficient generation of reactive ROS. A comparison of the photoluminescence (PL) spectra (Fig. 4g) of Cu2O/TbPa and TbPa revealed that Cu2O/TbPa presented a weaker composite luminescence signal, suggesting that the anchoring of Cu2O clusters inhibited the recombination of photo-generated carriers before they arrived at the catalytic sites. This inhibition arose from the optimized charge distribution within TbPa, facilitated by the Cu2O clusters, which facilitated more efficient separation of photo-generated electrons and holes. Subsequently, the separated charges participated in catalytic reactions, enhancing the photocatalytic efficiency of the Cu2O/TbPa composite.
Bactericidal and anti-biofilm properties
Given the exceptional photoelectric properties and optimized energy band structure of Cu2O/TbPa and TbPa, we expect that the Cu2O/TbPa/PES membranes have substantial potential to exhibit efficient bacterial inactivation capabilities. Based on this premise, we systematically evaluated and compared the anti-bacterial efficacy of TbPa/PES membranes and a range of Cu2O/TbPa/PES membranes with varying Cu2O contents in subsequent tests. E. coli and S. aureus served as representative strains for Gram-negative and Gram-positive bacteria, respectively. To investigate whether copper ion leaching impacts bacterial inactivation, we first assayed the copper content within the Cu2O/TbPa/PES membranes. ICP‒MS analysis revealed that the copper content within the Cu2O/TbPa/PES membranes increased within the range of 0.12–1.28 μg g−1 as the concentration of Cu2+ in the photodeposition solution increased (Supplementary Table 1). Notably, the copper content in the Cu2O/TbPa/PES membranes was exceedingly minute, and even if all copper elements from the Cu2O/TbPa/PES membrane were hypothetically fully leached out as Cu2+ into 1 mL of water, the resulting Cu2+ concentration would still be substantially below the sublethal threshold of Cu2+ for bacterial inactivation (50 μg L−1)25. This observation suggests that the anti-bacterial effect of the Cu2O/TbPa/PES membranes is not achieved through the release of copper ions. Thereafter, we evaluated the bacterial inactivation capabilities of the TbPa/PES membranes and a range of Cu2O/TbPa/PES membranes featuring various Cu2O contents under visible light illumination (Fig. 5a and b). The experimental results revealed that the bacterial inactivation efficiency of the composites initially demonstrated a pronounced increasing trend with increasing Cu2O content, indicating a positive influence of Cu2O incorporation on the photocatalytic anti-bacterial properties of TbPa. However, when the content reached 0.74 μg g−1, a further increase in the Cu2O content led to a decrease in the bacterial inactivation efficiency. Notably, the Cu2O/TbPa/PES membranes with a copper content of 0.74 μg g−1 exhibited the most effective bacterial inactivation performance. This trend can be attributed to the uniform distribution of an appropriate amount of Cu2O clusters on the TbPa surface, which facilitated rapid photo-generated electron transport within the composite, prolonged the lifetime of the photo-generated carriers, and enhanced the ROS generation efficiency. Conversely, excessive Cu2O content resulted in the agglomeration of Cu2O clusters into larger particles. This effect not only obscured the original redox-active sites of TbPa, reducing the overall catalytic activity of the material but also hindered the efficient transport of photo-generated carriers, leading to increased electron-hole recombination rates and consequently deteriorating the bacterial inactivation effect. Consequently, the optimization of the Cu2O content to ensure uniform dispersion on the TbPa surface has emerged as a crucial strategy for enhancing the bacterial inactivation performance of Cu2O/TbPa/PES membranes.
Viability survival of (a) E. coli and (b) S. aureus treated with TbPa/PES membranes and different Cu2O anchoring ratios, as determined by bacterial colony counting through gradient dilution and plating on LB agar plates. Data are presented as mean ± SD (n = 3 biologically independent samples). c Survival of E. coli following treatment with Cu2O/TbPa/PES membranes at various illumination durations. d Survival of S. aureus following treatment with Cu2O/TbPa/PES membranes at various illumination durations. e Plate spread photographs of E. coli and S. aureus after treatment with different membranes and their respective control groups. f Representative SEM images showing the morphological changes in E. coli and S. aureus following treatment with PBS and PES, TbPa/PES and Cu2O/TbPa/PES membranes under light illumination (the scale bar is 500 nm). g Representative confocal laser scanning microscopy (CLSM) images of E. coli and S. aureus subjected to various treatments in the presence of SYTO9 and PI, reflecting the integrity of the bacterial cells (green and red represent living and dead cells, respectively) (the scale bar is 20 μm). h Representative 3D CLSM images of biofilms formed by E. coli biofilms treated with PBS and PES, TbPa/PES and Cu2O/TbPa/PES membranes under light illumination (the scale bar is 50 μm).
To comprehensively evaluate the impact of anchoring various metals on the bacterial inactivation performance of TbPa, we further prepared Ag/TbPa/PES and Pt/TbPa/PES membranes as control experimental groups using photodeposition techniques. However, compared with unmodified TbPa or Cu2O/TbPa, the two composite materials did not exhibit the anticipated enhanced bacterial inactivation effect (Supplementary Fig. 5). This finding suggests that the selection and anchoring of metals and metal oxides exhibit a high degree of specificity in enhancing the photocatalytic anti-bacterial properties of TbPa.
In addition, we explored the bacterial inactivation performance of Cu2O/TbPa/PES at different time points. It efficiently inactivates 105 CFU mL−1 of E. coli within 25 min and S. aureus within 30 min at the same concentration (Fig. 5c and d). This variation can be primarily attributed to the inherent structural characteristics of Gram-positive bacteria, specifically their thicker and more compact cell walls, which function as robust protective barriers, thereby contributing to their heightened resistance to attacks. To further visualize the impact of different treatment conditions on bacterial viability, we conducted control experiments, and the results are presented in Fig. 5e. The results of the plating observations demonstrate that TbPa/PES membranes adorned with Cu2O clusters exhibit a superior ability to inactivate bacteria, achieving near-complete eradication of viable bacteria. We carried out a comparative analysis of the synthetic method, bactericidal mechanism, and antibacterial efficacies of various copper-based materials (Supplementary Table 2). Notably, in comparison with other Cu2O-based materials26,27, the Cu2O/TbPa/PES membrane with lower Cu content shows outstanding antibacterial performance by achieving a 5-log reduction against both E. coli and S. aureus within 30 min.
A comparative analysis of the different bacterial treatments was performed to investigate the bacterial inactivation mechanism using SEM and bacterial cell viability staining techniques. The SEM images (Fig. 5f) revealed that bacteria subjected to the TbPa/PES membrane underwent minimal damage, in contrast to those treated with the Cu2O/TbPa/PES membranes, which exhibited significant morphological changes and severe damage to their cell membranes. For further confirmation and quantification of bacterial cell membrane integrity, bacterial cell live/dead staining technology was employed. This technique utilizes fluorescent dyes to differentiate viable from non-viable cells, with live cells stained green (SYTO9) and dead cells emitting red fluorescence (PI). The staining results (Fig. 5g) indicate that the majority of bacterial cells treated with the Cu2O/TbPa/PES membranes exhibited red fluorescence, confirming the disruption of membrane integrity and subsequent loss of viability. In summary, the efficient bactericidal mechanism of the Cu2O/TbPa/PES membranes is attributed primarily to the generation of ROS. These potent ROS effectively permeate and disrupt bacterial cell membranes, thereby impairing cellular functions and swiftly achieving thorough bacterial inactivation.
Based on the above results, we infer that the ROS released by the Cu2O/TbPa/PES membranes not only effectively destroyed free-floating bacteria but were also suitable for the eradication of bacterial biofilms due to their robust oxidation properties. The 3D-CLSM analysis directly revealed significant disruption of the E. coli biofilm in the presence of Cu2O/TbPa/PES. Specifically, a notable reduction in biofilm thickness was observed, accompanied by a near-complete absence of viable bacterial cells within the biofilm matrix (Fig. 5h). The efficacy of biofilm removal by the various groups was subsequently evaluated through crystal violet staining, and photographs of the experimental results are displayed. As illustrated in Supplementary Fig. 6, Cu2O/TbPa/PES exhibited a notable capacity for biofilm removal. Specifically, the Cu2O/TbPa/PES membranes displayed enhanced biofilm removal capabilities, with an improvement of approximately 40% in comparison with the TbPa/PES membrane, which achieved a bacterial biofilm removal efficiency of over 98%. Furthermore, the results depicted in Supplementary Fig. 7 reveal that the Cu2O/TbPa/PES membrane achieves approximately a 1.5-log reduction in Aspergillus niger spore colony counts within 4 h, indicating its promising application in addressing fungal spore contamination in aquatic environments.
The uniform anchoring of Cu2O clusters on the TbPa/PES membrane facilitates the efficient generation of ROS, thereby enabling the Cu2O/TbPa/PES membranes to exhibit highly effective inactivation and removal capabilities towards bacteria and their biofilms. This material, integrated onto a separation membrane and exhibiting exceptional bacterial inactivation and biofilm removal abilities, holds great potential for mitigating the detrimental effects of bacterial fouling on membrane performance, ultimately enhancing the longevity of the membrane.
Mechanistic analysis of bacterial inactivation
A detailed analysis of the Cu2O/TbPa/PES membrane-induced bacterial membrane damage mechanisms is essential for understanding their photocatalytic inactivation benefits for membrane filtration. Initially, radical scavenging experiments were performed to investigate the primary ROS that affect bacterial integrity. This approach entails utilizing targeted radical scavengers to neutralize their activity and subsequently evaluating the efficacy of these scavengers through the examination of alterations in bacterial inactivation. In the experiments, isopropanol, Fe(II) EDTA, sodium oxalate, furfuryl alcohol (FFA), Cr(VI) and 4-hydroxy-2,6,6-tetramethylpiperidinyloxy (TEMPOL) were utilized as scavengers for ·OH, H2O2, photo-generated holes (h+), 1O2, photo-generated electrons (e−) and ·O2−, respectively, to evaluate their individual impacts on attenuating the inactivation of E. coli (Fig. 6a) and S. aureus (Fig. 6b). Eliminating the oxidative effect of ·OH with isopropanol significantly inhibited bacterial inactivation (approximately 3-log reduction in mortality), highlighting the crucial role of ·OH in bacterial inactivation. Moreover, the introduction of Fe(II) EDTA and sodium oxalate as scavengers significantly attenuated the bactericidal effect, indicating that H2O2 and h+ contribute non-negligibly to bacterial inactivation, albeit potentially not as primary factors. The observed complex interplay among the ROS highlights the intricate mechanisms involved in the inactivation process.
ROS scavenger experiments of Cu2O/TbPa/PES membranes in photocatalytic a E. coli inactivation and b S. aureus inactivation systems. ESR spectra for detecting (c) ·OH, d H2O2 and e photo-generated holes (h+). f Relative changes in ATP levels in E. coli under different treatment durations. Data are presented as mean ± SD (n = 3 biologically independent samples). g Schematic illustration of the bacterial inactivation mechanism of the Cu2O/LZU1/PES membrane.
To gain deeper insights into the mechanisms responsible, we employed ESR spectroscopy to investigate the generation of three crucial reactive species (·OH, H2O2 and h+), as depicted in Fig. 6c–e. Within just 5 min of visible light exposure, the spin-trapped radical signals of these reactive species became apparent, validating their swift generation. Additionally, when the illumination duration was extended to 10 min, the signals continued to intensify, demonstrating the stable presence of the reactive species and suggesting potential dynamic changes under extended light exposure conditions. The quantitative data of the radicals derived from the ESR spectrum analysis are presented in Supplementary Table 3, with the concentration order of the radicals being c(h+) > c(·OH) > c(H2O2). This deviation from the outcomes of the radical scavenging experiments can primarily be primarily attributed to the fact that h+ is constrained to exist in a bound state on the membrane surface, thereby limiting their diffusion and reactivity. In contrast, ·OH and H2O2 can dissociate from the bound state and distribute more extensively throughout the system, enabling a thorough interaction with bacterial cells. Thus, ·OH plays a dominant role in the primary process of bacterial inactivation, constituting the principal component responsible for bacterial cell damage and subsequent death.
Figure 6f illustrates the dynamic changes in the intracellular ATP content of bacteria, revealing a marked increase in ATP levels approximately 10 min after exposure to ROS. This initial surge in ATP can be attributed to the intense oxidative stress response of bacteria, as they generate ATP to counter external stimuli. However, with prolonged exposure to ROS, the ATP content gradually decreased, indicating rapid depletion due to the sustained oxidative stress. This decline underscores the dual challenges faced by bacteria under ROS exposure: external disruption coupled with internal energy depletion, ultimately leading to bacterial death. We have summarized the inactivation mechanism of Cu2O/TbPa/PES on bacteria into a schematic diagram, as illustrated in Fig. 6g.
Filtration and anti-fouling performance
Since the deposition of materials on the membrane surface can potentially modify its wettability, water contact angle (WCA) measurements were employed to monitor the changes in the hydrophilicity of the membrane surface. As shown in Supplementary Fig. 8, the PES membrane exhibited a WCA of 49.7°, demonstrating good hydrophilic properties. Following the deposition of TbPa, the WCA increased to 70.5°, indicating a significant decrease in hydrophilicity. This change is attributed to the inherent hydrophobic characteristics of the TbPa material. After the Cu2O clusters were anchored, the water contact angle decreased to 62.5°, indicating partial recovery of hydrophilicity, which is in accordance with previous reports that underscore the inherent hydrophilicity of Cu2O28. Theoretically, alterations in both membrane hydrophilicity and pore size directly impact the separation performance. According to the morphological characterization results, the extremely thin Cu2O/TbPa layer has a negligible effect on the pore size, so the hydrophilicity of the surface predominantly governs the flux change. We employed a dead-end filtration device (with the structure shown in Supplementary Fig. 9) to assess the permeability of the modified membranes, and the results are presented in Fig. 7a. The PES membrane exhibited superior permeability; however, after the TbPa layer deposition, a decrease in permeance was observed, which aligned with the decrease in hydrophilicity. Upon uniform anchoring of the Cu2O clusters, a significant increase in permeance was achieved, approaching levels comparable to those of the PES membrane, with a high permeability level of 16000 LHM bar−1; this conclusively verifies the positive effect of the anchoring of Cu2O clusters on the restoration of permeability.
a Water permeance of PES, TbPa/PES and Cu2O/TbPa/PES membranes. Data are presented as mean ± SD (n = 3 independent samples). b Flux recovery profiles during filtration of E. coli suspensions of PES, TbPa/PES and Cu2O/TbPa/PES membranes. c Long-term anti-biofouling filtration experiment of Cu2O/TbPa/PES membranes with an E. coli suspension. d Permeability, anti-bacterial, and fouling resistance performance comparisons with advanced membranes31,32,33,34,35,36,37. e Schematic diagram of the flux recovery process of Cu2O/TbPa/PES membranes.
Drawing upon the analysis and integration accomplishments from existing research29, it can be rigorously inferred that the pressure differential encountered during the filtration process intensifies the interactive dynamics between bacteria and the Cu2O/TbPa/PES membrane, potentially increasing the contribution of bound h+ and undissociated ·OH and H2O2. This natural complementary trend exhibited by membrane filtration and photocatalytic processes in enhancing mass transfer indicates potential synergy, which ultimately positively influences the overall efficiency of the reaction system.
Thereafter, we conducted filtration tests using an E. coli suspension with a concentration of 105 CFU mL−1 to comprehensively evaluate the anti-biofouling properties of the membranes. As shown in Fig. 7b, under high bacterial load conditions, all the tested membranes, including the PES, TbPa/PES, and Cu2O/TbPa/PES membranes, rapidly became fouled by the bacterial layers, resulting in a significant decrease in permeance. Specifically, the permeance decreased by 93.2%, 93.3%, and 91.2%, respectively. To regenerate these fouled membranes, they were exposed to simulated sunlight for 30 min. The Cu2O/TbPa/PES membrane exhibited near-complete flux recovery, which can be attributed to its efficient photocatalytic properties, and the TbPa/PES membrane attained a flux recovery rate (FRR) of 98.0%. In contrast, the PES membrane demonstrated an FRR of only 21.3%, primarily due to its lack of photocatalytic capability. To evaluate the operational stability of these membranes during repeated filtration processes, four intermittent filtration‒illumination cycles were performed. Notably, the Cu2O/TbPa/PES membranes maintained an exceptionally high FRR of 99.2% even after four cycles, emphasizing their superior resistance to biofouling. In contrast, the TbPa/PES membrane showed a marked decrease in FRR over repeated cycles, indicating its limitations in addressing continuous fouling challenges. Multiple filtration cycles were performed to further substantiate the long-term stability of the Cu2O/TbPa/PES membranes in practical applications. As illustrated in Fig. 7c, the Cu2O/TbPa/PES membranes retained a remarkably high FRR of 98.6% even after undergoing 10 cycles of biofouling contamination, demonstrating their high stability and reliability during prolonged operation.
To elucidate the flux recovery process of the Cu2O/TbPa/PES membrane more clearly, we provided a schematic diagram in Fig. 7e. The membrane efficiently sequesters bacteria via size exclusion, ultimately resulting in bacterial fouling and a subsequent decrease in flux. Upon light stimulation, the Cu2O/TbPa/PES membrane efficiently generates ROS, deeply penetrating the biofilm to disrupt its structure and inactivate bacteria, significantly mitigating bacterial fouling and achieving flux recovery. The applied pressure during the filtration process enhances the intimate attachment of bacteria to the membrane, further shortening the diffusion path of ROS and accelerating the rates of bacterial inactivation and biofilm disruption. By successfully integrating these dual mechanisms, Cu2O/TbPa/PES membranes show potential as filtration materials that offer high flux, anti-fouling properties, and sustainable operation. Specifically, it demonstrates exceptional permeation flux, while also exhibiting significant advantages in antibacterial efficacy and resistance to bacterial fouling (Fig. 7d). By employing a precise anchoring strategy using Cu2O clusters, a high-flux membrane has been developed to address the bottleneck issue of membrane fouling effectively. Furthermore, this innovative approach has demonstrated significant potential in practical applications, providing valuable insights for developing high-flux anti-bacterial membranes.
Discussion
By combining vapor deposition and photodeposition, we synthesized Cu2O/TbPa/PES membranes that exhibit exceptional performance in bacterial inactivation. The uniform in situ photodeposition of Cu2O clusters notably enhances the carrier transport efficiency of TbPa, facilitating the efficient generation of ·OH. Consequently, the bactericidal efficacy of the membrane is significantly elevated, achieving a bacterial removal efficiency of 5-log colony-forming units and enabling the effective removal of over 98% of biofilms. Additionally, the deposition ameliorates the hydrophobic interfacial characteristics of TbPa, thereby facilitating the maintenance of high-flux performance. These optimizations enable the Cu2O/TbPa/PES membranes to achieve complete flux recovery from bacterial fouling and demonstrate stable operational performance over 10 cycles. Our study demonstrates the significant potential of photocatalytic technology in addressing membrane fouling and presents an approach for the utilization of photocatalytic microfiltration membranes in sustainable water sterilization.
Methods
Synthesis of TbPa/PES membranes
Based on the study conducted by Daum et al.30, TbPa was synthesized on PES membranes using vapor deposition. Tb (11.35 mg, 0.07 mmol) was centered, with Pa (7.57 mg, 0.07 mmol) mixed with 20 mg of iron powder placed 100-mm downstream. The membranes were positioned 200–300 mm further. The tube was evacuated and filled with Ar thrice, heated to 220 °C at 2 °C min−1 and held for 5 min under a 10-mL min−1 Ar flow.
Synthesis of Cu2O/TbPa/PES
First, the TbPa/PES membranes were thoroughly wetted in anhydrous ethanol until they flattened. The samples were subsequently immersed in oxygen-free CuSO4 ethanolic solutions (20 mL) at concentrations of 1.25, 3.75, 6.25 and 8.75 mmol L−1. After 5 min of light irradiation (420–680 nm, PLS-SME300E H1 Source), Cu2O/TbPa/PES composite membranes with varying Cu2O loadings were successfully prepared.
Anti-bacterial tests
Staphylococcus aureus (S. aureus, ATCC 29213) and Escherichia coli (E. coli, ATCC 25922) were inoculated into Luria–Bertani (LB) liquid media and cultivated to the logarithmic phase. The bacterial culture medium was removed through multiple centrifugation steps, and the bacterial suspension was adjusted to a concentration of 105 CFU mL−1. The membranes were immersed in the bacterial suspension and subjected to visible light illumination (100 mW cm−2, 420–780 nm, PLS-SME300E H1 Source, Beijing Perfectlight). Afterwards, the samples (100 μL) were collected and spread evenly onto an LB solid medium.
The antimicrobial efficacy of the membranes was assessed through quantification of the bacterial load by gradient dilution and the colony counting method.
The bacterial live/dead staining test and biofilm staining test utilized a live/dead™ BacLight™ bacterial viability kit (L7012), with imaging conducted on a laser scanning confocal microscope imaging system (Leica TCS SP8).
Membrane performance tests
To evaluate the permeation performance, the pure water permeance was measured under a dead-end filtration mode at a pressure of 0.5 bar. Before the test, a pre-pressurization step of 3 min under identical pressure conditions was essentially conducted to ensure a stable filtration state. The water permeance (P, L m−2 h−1 bar−1) was calculated using Eq. (1):
where V represents the volume of permeate (L), A denotes the effective filtration area (m2), t signifies the duration of filtration (h) and Δp represents the pressure applied during filtration (bar).
To evaluate the sieving performance of the membrane, a bacterial suspension was utilized as the test feed, and the bacterial concentration was quantitatively determined through the plate count method. The rejection rate (R, %) was calculated using Eq. (2):
where Cp and Cf are the bacterial concentrations of the permeate and feed, respectively.
Subsequently, multiple filtration tests using bacterial suspensions were conducted to evaluate the flux recovery rate during the filtration study. A bacterial suspension with a concentration of 105 CFU mL−1 was loaded into the filtration setup, and the initial permeability (Pi) was measured. The changes in membrane permeability were recorded every minute based on the weight of the permeate water. Following filtration, the membrane was immersed in water and exposed to light for 30 min before the membrane flux was remeasured to determine the recovered water permeability (Pr). The FRR (%) was then calculated using Eq. (3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare all data supporting the findings of this study are available within the article and supplementary Information file, and additional data are available from the corresponding author upon request. Source data are provided with this paper.
References
Logan-Jackson, A. R. et al. A critical review on the factors that influence opportunistic premise plumbing pathogens: from building entry to fixtures in residences. Environ. Sci. Technol. 57, 6360–6372 (2023).
O’Hara, L. M. et al. Optimizing contact precautions to curb the spread of antibiotic-resistant bacteria in hospitals: a multicenter cohort study to identify patient characteristics and healthcare personnel interactions associated with transmission of methicillin-resistant staphylococcus aureus. Clin. Infect. Dis. 69, S171–S177 (2019).
Thomas, B. Microbial pollution and food safety. AIMS Microbiol 4, 377–396 (2018).
Kahn, L. H. et al. From farm management to bacteriophage therapy: strategies to reduce antibiotic use in animal agriculture. Ann. N. Y Acad. Sci. 1441, 31–39 (2019).
Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat. Rev. Chem. 5, 217–218 (2021).
Zhang, S. et al. Ultrathin membranes for separations: a new era driven by advanced nanotechnology. Adv. Mater. 34, 2108457 (2022).
Zhang, L., Graham, N., Li, G., Zhu, Y. & Yu, W. Excessive ozonation stress triggers severe membrane biofilm accumulation and fouling. Environ. Sci. Technol. 58, 5899–5910 (2024).
Shi, Y., Huang, J., Zeng, G., Cheng, W. & Hu, J. Photocatalytic membrane in water purification: is it stepping closer to be driven by visible light? J. Membr. Sci. 584, 364–392 (2019).
Wu, W. et al. Enhancing the performance of catalytic membranes for simultaneous degradation of dissolved organic phosphonates and phosphorous recovery: A fit-for-purpose loose nanofiltration design. Appl Catal. B: Environ. 354, 124118 (2024).
Ye, J. et al. Intensify mass transfer and molecular oxygen activation by defect-bridged asymmetric catalytic sites toward efficient membrane-based nanoconfined catalysis. Adv. Funct. Mater. 34, 2403964 (2024).
Wang, Z. et al. Oxygen doping cooperated with Co-N-Fe dual-catalytic sites: synergistic mechanism for catalytic water purification within nanoconfined membrane. Adv. Mater. 36, 2404278 (2024).
Sun, J. et al. Improved water flux and separation of polyamide reverse osmosis membranes by trace loading of biomimetic modified carbon nanotubes. ACS Appl Nano Mater. 7, 17840–17854 (2024).
Liu, R. et al. Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 50, 120–242 (2021).
Wang, H. et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 49, 4135–4165 (2020).
Chen, Y. et al. Hierarchical assembly of donor–acceptor covalent organic frameworks for photosynthesis of hydrogen peroxide from water and air. Nat. Synth. 3, 998–1010 (2024).
Zhang, X., Geng, K., Jiang, D. & Scholes, G. D. Exciton diffusion and annihilation in an sp2 carbon-conjugated covalent organic framework. J. Am. Chem. Soc. 144, 16423–16432 (2022).
Wang, H., Jin, S., Zhang, X. & Xie, Y. Excitonic effects in polymeric photocatalysts. Angew. Chem. Int Ed. 59, 22828–22839 (2020).
Cao, Y., Parker, I. D., Yu, G., Zhang, C. & Heeger, A. J. Improved quantum efficiency for electroluminescence in semiconducting polymers. Nature 397, 414–417 (1999).
He, T. et al. Porphyrin-based covalent organic frameworks anchoring au single atoms for photocatalytic nitrogen fixation. J. Am. Chem. Soc. 145, 6057–6066 (2023).
Ming, J. et al. Hot π-electron tunneling of metal–insulator–COF nanostructures for efficient hydrogen production. Angew. Chem. Int Ed. 58, 18290–18294 (2019).
Liu, Y.-H. et al. Self-accelerating H2 evolution activity by in situ transformation on noble-metal-free photocatalyst of covalent organic framework and Cu2O composite. Adv. Funct. Mater. 34, 2316546 (2024).
Li, Y. et al. In situ photodeposition of platinum clusters on a covalent organic framework for photocatalytic hydrogen production. Nat. Commun. 13, 1355 (2022).
Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in suzuki–miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011).
Fan, H., Gu, J., Meng, H., Knebel, A. & Caro, J. High-flux membranes based on the covalent organic framework COF-LZU1 for selective dye separation by nanofiltration. Angew. Chem. Int Ed. 57, 4083–4087 (2018).
Xu, Y., Tan, L., Li, Q., Zheng, X. & Liu, W. Sublethal concentrations of heavy metals Cu2+ and Zn2+ can induce the emergence of bacterial multidrug resistance. Environ. Technol. Inno 27, 102379 (2022).
Singh, A. et al. Antibiofilm and membrane-damaging potential of cuprous oxide nanoparticles against staphylococcus aureus with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 59, 6882–6890 (2015).
Subhadarshini, S., Singh, R., Goswami, D. K., Das, A. K. & Das, N. C. Electrodeposited Cu2O nanopetal architecture as a superhydrophobic and antibacterial surface. Langmuir 35, 17166–17176 (2019).
Zhao, T. et al. Tailoring the catalytic microenvironment of Cu2O with SiO2 to enhance C2+ product selectivity in CO2 electroreduction. ACS Catal. 13, 4444–4453 (2023).
Gao, Y. et al. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@TiO2 nanofibrous membrane: Implication for improving photocatalytic efficiency. Appl Catal. B: Environ. 268, 118737 (2020).
Daum, J. P. et al. Solutions are the problem: ordered two-dimensional covalent organic framework films by chemical vapor deposition. ACS Nano 17, 21411–21419 (2023).
Li, M. et al. Development of an antimicrobial and antifouling PES membrane with ZnO/poly(hexamethylene biguanide) nanocomposites incorporation. Chem. Eng. J. 481, 148744 (2024).
Mamba, P. P., Msagati, T. A. M., Mamba, B. B., Motsa, M. M. & Nkambule, T. T. I. The removal of pathogenic bacteria and dissolved organic matter from freshwater using microporous membranes: insights into biofilm formation and fouling reversibility. Biofouling 40, 245–261 (2024).
Ren, L., Chen, J., Lu, Q., Han, J. & Wu, H. Anti-biofouling nanofiltration membrane constructed by in-situ photo-grafting bactericidal and hydrophilic polymers. J. Membr. Sci. 617, 118658 (2021).
Ghalamchi, L., Aber, S., Vatanpour, V. & Kian, M. Development of an antibacterial and visible photocatalytic nanocomposite microfiltration membrane incorporated by Ag3PO4/CuZnAl NLDH. Sep Purif. Technol. 226, 218–231 (2019).
Mishra, B., Ghosh, J., Dubey, N. C. & Tripathi, B. P. Designing anti(-bio)fouling membranes with synergistic grafting of quaternized and zwitterionic polymers through surface initiated atom transfer radical polymerization. Sep Purif. Technol. 328, 125071 (2024).
Wang, Y., Wang, Z., Han, X., Wang, J. & Wang, S. Improved flux and anti-biofouling performances of reverse osmosis membrane via surface layer-by-layer assembly. J. Membr. Sci. 539, 403–411 (2017).
Ren, L., Chen, J., Lu, Q., Han, J. & Wu, H. Antifouling nanofiltration membrane fabrication via surface assembling light-responsive and regenerable functional layer. ACS Appl Mater. Interfaces 12, 52050–52058 (2020).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2024YFE0112300, Y. W.), the National Natural Science Foundation of China (22438005, Y. W.), the Natural Science Foundation of Jiangsu Province (BK20241331, C. Y.) and China Postdoctoral Science Foundation (2023M740596, C. Y.).
Author information
Authors and Affiliations
Contributions
Y.W. conceived and supervised the project. S.L. carried out the experiments and structural characterizations. J.G. helped to perform structural characterizations. C.Y. and Y.L. provided suggestions for methodology. All authors contributed to the discussions. S.L. and Y.W. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, S., Gao, J., Yin, C. et al. Coupling Cu2O clusters and imine-linked COFs on microfiltration membranes for fast and robust water sterilization. Nat Commun 16, 1114 (2025). https://doi.org/10.1038/s41467-025-56416-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56416-6