Abstract
Shrub encroachment into grasslands poses a global concern, impacting species biodiversity and ecosystem functioning. Yet, the effect of shrub encroachment on herbaceous diseases and the dependence of that effect on climatic factors remain ambiguous. This study spans over 4,000 km, examining significant variability in temperature and precipitation. Our findings reveal that herbaceous plant species richness diminishes the pathogen load of foliar fungal diseases of herbaceous plants in both shrub and grassland patches. Temperature emerges as the primary driver of variations in herbaceous biomass and pathogen load within herbaceous plant communities. Disparities in herbaceous biomass between shrub and grassland patches elucidate changes in pathogen load. In colder regions, shrub encroachment diminishes herbaceous biomass and pathogen load. Conversely, in warmer regions, shrubs either do not reduce or even amplify pathogen load. These discoveries underscore the necessity for adaptive management strategies tailored to specific shrub encroachment scenarios.
Similar content being viewed by others
Introduction
Shrub encroachment, a prevalent occurrence globally, affects diverse grassland ecosystems, from alpine meadows to temperate steppes1,2,3,4. Recent years have witnessed a steady rise in shrub encroachment, now spanning approximately 0.3%–2% of grassland regions worldwide5. Causal factors include overgrazing, elevated atmospheric CO2, increased precipitation, and climate change2,6,7,8,9. This encroachment significantly impacts species biodiversity, ecosystem function, and the livelihoods of millions dependent on animal husbandry in these areas10,11,12. It alters higher trophic interactions, including plant diseases, affecting plant competition, performance, and ecosystem services13.
Pathogens play a crucial role in maintaining species coexistence, influencing global carbon cycling, primary productivity, and other ecosystem functions essential for human well-being14,15,16,17,18,19,20. Shrub encroachment disrupts plant-pathogen relationships by driving shifts in herbaceous plant community characteristics. Species diversity in grasslands is pivotal in reducing diseases through a dilution effect21,22,23,24,25. Lower herbaceous species aboveground biomass in shrub patches can also reduce foliar fungal disease, aligning with observed “density effects” in agricultural26 and natural ecosystems23,27. The expansion of shrubs into grasslands can decrease herbaceous species richness and biomass due to aboveground light competition and belowground resource competition28, particularly in environmentally suitable areas10,28,29. Consequently, shifts in herbaceous species composition driven by shrub encroachment can alter trophic relationships between plants and pathogens.
Shrubs can additionally impact foliar fungal diseases of herbaceous plants via altering microclimate or soil properties. Shrub encroachment creates a moist microclimate within grasslands, influencing disease dynamics30,31,32,33,34. These environments promote mycelium growth, spore production, and fungal pathogen transmission, leading to increased disease incidence35,36,37,38. Shrubs can also provide shade that cools the herbaceous plants and their pathogens in warmer regions, and benefit foliar fungal pathogens in places where temperatures outside of shrubs are higher than the pathogens’ thermal optima39,40. In addition, changes in soil properties following shrub encroachment41, including decreased soil available nitrogen, organic carbon, and pH levels11, can support pathogen nutrition, enhancing pathogen fitness, particularly for fungal pathogens42.
Despite the well-documented impacts of shrubs on herbaceous assemblages, microclimate, and soil nutrients, which can vary substantially with local climate factors such as precipitation and temperature11,43, the effects of large-scale shrub encroachment on diseases of herbaceous communities beneath the shrub canopy remain elusive. This knowledge gap is pivotal for understanding essential ecosystem functions facilitated by grasslands and the challenges posed by climate change. This study aimed to elucidate (1) how shrubs influence herbaceous plant community characteristics (e.g., aboveground biomass, species diversity, and composition), microclimates (e.g., air temperature and humidity), soil properties, and foliar fungal diseases; (2) whether the impact of shrubs on foliar fungal diseases of herbaceous plants depends on local climate (e.g., mean annual precipitation and temperature); and (3) to what extent shrubs regulate foliar fungal diseases of herbaceous plants via plant community characteristics, microclimates, and soil properties.
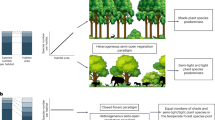
In this work, we conducted our study in the largest contiguous natural grassland on Earth, spanning an area of over 4000 km in China (Fig. 1a). The substantial climatic gradient across these grasslands, with mean annual precipitation (MAP) ranging from 75 mm to 861 mm and mean annual temperature (MAT) ranging from −5.6 °C to 9.9 °C (Fig. 1a), provided an optimal opportunity to examine the climate dependence of the effect of shrub encroachment on herbaceous diseases. Our study focused on the herbaceous communities below the shrub canopy and in adjacent shrub-free grassland patches within landscape mosaics created by shrub encroachment (Fig. 1b). This mosaic enabled the establishment of ideal study plots pairing shrub patches with adjacent grassland patches. At each site, we established four herbaceous plots below the canopy within shrub patches, with each shrub patch plot paired with a corresponding adjoining grassland patch plot of identical size. This design enabled us to effectively examine the impact of shrubs on herbaceous foliar fungal diseases and investigate whether these shrub influences were mediated by climate factors, including precipitation and temperature.
a Inset in the bottom right depicts the spread of mean annual temperature (MAT) and mean annual precipitation (MAP) across these sites. b Schematic diagram of shrub patches spaced with grassland patches in shrub-invaded grasslands. The blue dotted area denotes a shrub patch, and the orange dotted area illustrates an interspaced grassland patch.
Results
Distribution of herbaceous foliar diseases and underlying potential factors following shrub encroachment
Using a dataset collected from 80 sites spread across 4000 km of shrubby grasslands (Fig.1a), comprising a total of 640 plots surveyed (eight 0.25 m2 plots at each site), we observed varying levels of the herbaceous community foliar fungal pathogen load (as % leaf area covered by fungal lesions). In the grassland patches, this load ranged from 0% to 14.8%, whereas under the shrub canopy (i.e., shrub patches), it ranged from 0% to 24.1%. Among the disease symptoms, leaf spots (ranging from 0% to 23.7%), leaf blights (ranging from 0% to 13.4%), and rusts (ranging from 0% to 7.2%) were the most prevalent.
Through a series of linear mixed-effects models (LMMs), we determined that shrubs led to decreases in herbaceous species richness (F1, 319 = 13.733, P < 0.001), Shannon diversity (F1, 319 = 7.185, P = 0.008), Simpson diversity (F1, 319 = 11.948, P < 0.001), and phylogenetic diversity (i.e., mean phylogenetic pairwise distance; MPD) (F1, 319 = 11.866, P < 0.001). Shrubs also reduced herbaceous plant community aboveground biomass (F1, 319 = 18.495, P < 0.001) and air temperature (F1, 79 = 73.297, P < 0.001) (Supplementary Fig. 1 and Table 1). In contrast, the presence of shrubs increased the first principal component (PC1) of herbaceous plant community (F1, 319 = 5.845, P = 0.016), community weighted mean (CWM) of height (F1, 319 = 587.630, P < 0.001), soil PC1 (F1, 315 = 15.762, P < 0.001) and air humidity (F1, 79 = 29.039, P < 0.001) (Supplementary Fig. 1 and Table 1). However, shrubs did not significantly affect the CWM of specific leaf area (Supplementary Fig. 1 and Table 1).
Furthermore, a permutational multivariate analysis of variance unveiled that mean annual temperature (MAT) (F1, 636 = 18.264, P = 0.0001), mean annual precipitation (MAP) (F1, 636 = 37.255, P = 0.0001) and the presence of shrubs (F1, 636 = 2.498, P = 0.0004) all significantly influenced the composition of the herbaceous plant community (Supplementary Table 2). These findings suggest that shrub encroachment into grassland ecosystems can have profound and multifaceted impacts, altering the diversity, structure, and productivity of the herbaceous plant community and local microclimate.
The temperature-dependent impact of shrubs on herbaceous community foliar fungal diseases
Utilizing a series of zero-inflated generalized linear mixed-effects models, we uncovered associations between higher herbaceous species richness (Z = −5.589, P < 0.001) and greater MPD (Z = −3.223, P = 0.001) with decreased foliar fungal pathogen load in the herbaceous community, suggesting a dilution effect (Supplementary Fig. 2 and Table 3). However, there were marginally significant interactions between species richness and the presence of shrubs (Z = 1.845, P = 0.065), as well as significant interactions between MPD and shrub (Z = 2.180, P = 0.029), on pathogen load (Supplementary Fig. 2 and Table 3). This indicates that the presence of shrubs weakens the (phylogenetic) dilution effect (Supplementary Fig. 2 and Table 3). Moreover, the conditional model revealed that the presence of shrubs directly decreased the foliar fungal pathogen load in the herbaceous community (Z = −3.140, P = 0.002) (Supplementary Fig. 3 and Table 3). Interestingly, there was also an interaction between shrubs and MAT (Z = 2.430, P = 0.015), such that the negative effect of shrubs on herbaceous community foliar fungal pathogen load (conditional model) was greater in regions with lower MAT (Supplementary Fig. 3c and Table 3). These results suggest that while higher herbaceous diversity generally reduces foliar fungal pathogen loads through a dilution effect, the presence of shrubs can diminish this relationship. Furthermore, the negative effect of shrubs on herbaceous community foliar fungal disease is moderated by regional climate conditions, with a stronger suppression of pathogens in cooler environments.
Employing LMMs, we further explored the factors influencing the log-transformed response ratio of the herbaceous community foliar fungal pathogen load between shrub patches and grassland patches (also known as “LRR pathogen load”, where a higher value indicates more disease in shrubs compared to grassland patches). Our analysis uncovered that MAT (F1, 239 = 8.960, P = 0.004; Fig. 2a and Supplementary Table 4) significantly elevated the LRR pathogen load, whereas the mean annual precipitation (MAP) did not have a significant effect (F1, 239 = 2.317, P = 0.132; Fig. 2b and Supplementary Table 4). Specifically, the LRR pathogen load was below 0 in areas with MAT < −3 °C (Supplementary Fig. 4), demonstrating that shrubs typically reduce the foliar fungal pathogen load of the herbaceous community in colder environments. To verify the robustness of these findings, we utilized Bayesian mixed-effects models, which exhibited a positive effect of MAT on LRR pathogen load (estimates = 0.17, 95% CIs, 0.02–0.32; Fig. 3a and Supplementary Table 5), while no significant relationship was detected for MAP (estimates = −0.04, 95% CIs, −0.19 to 0.10; Fig. 3a and Supplementary Table 5). Moreover, employing a variance decomposition approach, we identified that MAT had a greater explanatory power in accounting for the variability in LRR pathogen load than MAP (Fig. 3b and Supplementary Table 6).
The linear mixed-effects models illustrate the effects of a mean annual temperature (MAT) and b mean annual precipitation (MAP) on log-transformed response ratio of herbaceous community foliar fungal pathogen load between shrub patches and grassland patches’ plots (i.e., LRR pathogen load), c MAT and d MAP on log-transformed response ratio of herbaceous plant community aboveground biomass between shrub patches and grassland patches’ plots (i.e., LRR herbaceous biomass), e LRR herbaceous biomass on LRR pathogen load. Parameter significance was assessed by F-tests (two-tailed). Solid lines and shadows indicate significant effects (P < 0.05) and 95% confidence intervals; n = 320 for these linear mixed-effects models. Data points represent the log-transformed response ratios of the original data.
The effects (a) and contributions (b) of log-transformed response ratio (LRR) of soil properties and plant characteristics on herbaceous community foliar fungal pathogen load between shrub patches and grassland patches’ plots (i.e., LRR pathogen load) using Bayesian mixed-effects models. Mean annual temperature (MAT), mean annual precipitation (MAP), the first principal component of soil variables (i.e., LRR soil PC1), herbaceous plant community aboveground biomass (i.e., LRR herbaceous biomass), community weighted mean (CWM) of specific leaf area (i.e., LRR CWM of specific leaf area), CWM of height (LRR CWM of height), species richness (i.e., LRR species richness), mean phylogenetic pairwise distance (i.e., LRR mean phylogenetic pairwise distance) and beta diversity (based on Bray–Curtis distance) of herbaceous community composition between shrub patches and grassland patches are depicted. The distribution and mean, as well as 50% and 95% credible intervals of the parameters are presented; n = 281 for the Bayesian mixed-effects models.
The relative effects of various factors in mediating foliar fungal pathogen load
To discern the effects and relative importance of herbaceous plant community characteristics, microclimates, and soil properties in elucidating how shrubs impact foliar fungal pathogen load, we conducted LMMs and Bayesian mixed-effects models. Our analysis revealed that only the LRR herbaceous biomass between shrub patches and grassland patches positively influenced the LRR pathogen load in both LMMs (F1, 239 = 21.828, P < 0.001; Fig. 2c and Supplementary Table 4) and Bayesian mixed-effects models (estimates = 0.23, 95% CIs, 0.10–0.37; Fig. 3a and Supplementary Table 5). Additionally, MAT positively affected the LRR herbaceous biomass (F1, 239 = 14.644, P < 0.001; Fig. 2d and Supplementary Table 4). Further variance decomposition analysis indicated that the LRR species richness (estimates = 13.91%, 95% CIs, 13.84%–13.99%), MAT (estimates = 8.39%, 95% CIs, 8.31%–8.46%) and LRR herbaceous biomass (estimates = 5.43%, 95% CIs, 5.37%–5.49%) were the three variables with the highest explanatory power (Fig. 3b and Supplementary Table 6). These findings suggest that the temperature-dependence of shrub impact on herbaceous community foliar fungal disease may mainly be explained by MAT and also changes in herbaceous plant community characteristics.
We utilized a structural equation model (SEM) to characterize the relative influence of MAT and MAP on LRR pathogen load, reflecting both their direct and indirect effects through the LRR herbaceous biomass, LRR species richness and LRR CWM of height (Supplementary Fig. 5). The final SEM fit the data well, with a Fisher’s C statistic of = 5.709 (P = 0.058) and AIC = 3205.439. This SEM revealed that MAT (standardized path coefficient β = 0.154, P = 0.031), but not MAP (β = −0.039, P = 0.574) directly increased the LRR pathogen load (Fig. 4a and Supplementary Table 7). Additionally, MAT indirectly increased the LRR pathogen load by enhancing the LRR herbaceous biomass (β = 0.233, P < 0.001) (Fig. 4a and Supplementary Table 7). Moreover, through partial regression analysis, we found that the residuals of MAT increased the residuals of LRR pathogen load (F1, 318 = 2.756, P = 0.098), and the residuals of the LRR herbaceous biomass also increased the residuals of the LRR pathogen load (F1, 318 = 9.799, P = 0.002) (Fig. 4b and Supplementary Table 8). These findings confirm that the temperature-dependent effect of shrubs on the foliar fungal disease in the herbaceous community is indirectly mediated by the LRR herbaceous biomass and directly regulated by MAT. Specifically, shrubs decrease disease by reducing the herbaceous community biomass in cold regions but do not reduce (or even increased) pathogen load in warm regions (Fig. 5).
a SEM illustrates the results of mean annual temperature (MAT), mean annual precipitation (MAP), LRR of herbaceous plant community aboveground biomass (i.e., LRR herbaceous biomass), community weighted mean of height (i.e., LRR CWM of height), and species richness (i.e., LRR species richness) influencing LRR pathogen load. Solid blue arrows denote positive pathways, solid brown arrows indicate evidence for negative pathways, and dotted arrows illustrate insufficient statistical evidence for path coefficients. The strength of the causal relationship is denoted by the width of the arrows. b Partial regression plots illustrate the effects of MAT, MAP, LRR herbaceous biomass, LRR CWM of height, and LRR species richness residuals influencing LRR pathogen load residuals from the SEM. Parameter significance was assessed by F-tests (two-tailed). Dotted lines, solid lines, and shadows represent marginal significant effects (0.05 < P < 0.10), significant effects (P < 0.05), and 95% confidence intervals, respectively; n = 320 for the SEM and these partial regression plots.
To explore the potential effects of herbaceous community composition, shrub species identity, and the history of shrub encroachment, we employed additional LMMs. We discovered that the beta diversity (based on Bray–Curtis distance) of the herbaceous community composition between shrub patches and grassland patches (F1, 239 = 1.516, P = 0.219; Supplementary Fig. 6 and Table 4), and timeline of shrub encroachment (F20, 210 = 0.649, P = 0.854; Supplementary Table 9), did not significantly affect the LRR pathogen load.
Foliar fungal pathogen load in response to future climate change
Given the significant influence of MAT on variations in the LRR pathogen load, we conducted a quantitative assessment of the current distribution of the LRR pathogen load across China’s grasslands (Fig. 6a). Our analysis unveiled higher disease pressures linked to shrub encroachment in the meadow steppe near the Greater Khingan Range, the characteristic steppe in Hulunbuir and Xilingol, and the western and northwestern regions of the Qinghai-Tibet Plateau (Fig. 6a and Supplementary Fig. 7). Furthermore, we employed climate change projections extending to 2040 based on two Intergovernmental Panel on Climate Change (IPCC) Representative Concentration Pathway (RCP) scenarios: the more optimistic RCP2.6 and the more pessimistic RCP8.5. Our findings indicate that the meadow steppe near the Greater Khingan Range and the central parts of the Inner Mongolian Plateau will likely experience an increase in herbaceous diseases due to shrub encroachment under future climate change (Fig. 6b–f and Supplementary Fig. 7).
a The current LRR pathogen load. b The projected distribution of potential LRR pathogen load in 2040 under the ssp1.26 scenario. c The projected distribution of potential LRR pathogen load in 2040 under the ssp5.85 scenario. d The changes in the potential LRR pathogen load between the ssp1.26 and ssp5.85 scenarios in 2040. e The changes in LRR pathogen load between the current and the ssp1.26 scenario in 2040; and f between the current and 5.85 scenario in 2040.
Discussion
Through an in-depth analysis of an expansive dataset covering 4000 km and a variety of grassland environments, we have discerned the pivotal role of mean annual temperature (MAT) in shaping the foliar fungal pathogens within the under-canopy herbaceous community, impacted by shrub encroachment10,11. Shrub presence tends to diminish pathogen load in colder areas while exacerbating it in warmer locales. This observation resonates with previous research, indicating that higher temperatures correlate with increased pathogen load despite concurrent rises in aboveground biomass and declines in species richness10. These divergent ecological effects of shrub encroachment bear significant implications for forage yield and pathogen load in diverse grassland ecosystems under climate change11. Moreover, the cooler regions within our study sites, primarily situated in the Qinghai-Tibet Plateau, exhibit nutrient limitations, notably nitrogen and/or phosphorus scarcity44. Under such circumstances, competition between shrubs and native herbaceous plants may intensify compared to regions with ample nutrients1. This intensified competitive exclusion may result in adverse effects of shrub encroachment28 on aboveground biomass in cold regions, while these detrimental effects are mitigated and even reversed in warmer regions (Fig. 5).
We attribute the directly positive effect of MAT on the log response ratio (LRR) of pathogen load (i.e., LRR pathogen load) to microclimate effects. Overall, shrubs create a moist and cool environment that benefits both herbaceous plants and their pathogens, promoting the occurrence and development of foliar fungal diseases45. Specifically, the thermal tolerance of fungal pathogens, including the growth and reproduction, decrease when temperatures exceed their optimal range40. Thus, shrubs can provide thermal refugia for temperature-sensitive pathogens, particularly in warmer regions39,40. In addition to humidity and temperature, other environmental factors may also play a role in regulating foliar fungal diseases. For instance, shrubs can block ultraviolet (UV) radiation, which benefits UV-sensitive pathogens like powdery mildew46. They may also affect the structure and insulating capacity of the snowpack, improving the survival rate of fungal pathogens during overwintering, especially for rusts47. Overall, in warmer regions, the suitable microclimate afforded by shrubs contributes to the positive effect of MAT on the pathogen load of foliar fungal diseases of herbaceous plants in shrub patches. Notably, the explanatory power of MAT’s effect on LRR pathogen load is relatively low (R2 = 0.047), likely due to the large sample size (n = 320) employed in our study, which tends to reduce explanatory power48,49. Additionally, numerous potential variables, including phylogenetic diversity, trait composition, and herbaceous community variation, may contribute to the residuals not accounted for by MAT.
Increased aboveground biomass in herbaceous plant communities can also lead to greater foliar fungal disease through density effects26. The significant positive association between LRR herbaceous biomass and LRR pathogen load corroborates this relationship. Fungal pathogens are often density-dependent50, with denser communities51 facilitating higher transmission likelihood from diseased individuals to susceptible hosts52. Additionally, increased herbaceous biomass per unit area enhances the probability for herbaceous communities to intercept more airborne inoculum while reducing inoculum loss53. Collectively, these findings align with a previous study that found foliar fungal pathogen biomass is linked to host plant biomass19. In warmer regions, shrubs serve as ecosystem engineers, exhibiting a “fertile island” effect that, influences not only foliar fungal pathogens but also the herbaceous plants that they depend on54. In addition, the more expansive repertoire of resistance genes can make shrub species more resistant to foliar fungal diseases55,56. Of the 13 shrub species found across the 80 study sites, only Dasiphora fruticosa was infected by foliar fungal pathogens (Phragmidium andersoni). Shrubs themselves can physically intercept pathogen spores and reduce pathogen load by an “encounter reduction” mechanism of dilution effect22.
In conclusion, our extensive field survey across China’s natural grasslands offers empirical evidence of the intricate, context-dependent relationships between shrub encroachment, herbaceous plant community dynamics, and foliar fungal disease. Given the importance of shrub encroachment, disease outbreaks, and climate change6,57, understanding these nuanced interactions is crucial for devising adaptive management strategies to alleviate the impacts of global changes on the health of grassland ecosystems worldwide.
Methods
Field survey
From mid-July to mid-August in both 2022 and 2023, we obtained data from 80 sites of shrubby grasslands across the Qinghai-Tibet Plateau and Inner Mongolia Plateau (28.26 °N–49.77 °N and 80.91 °E–121.34 °E; Fig. 1a). The mean annual temperature (°C; MAT) and mean annual precipitation (mm; MAP) in these survey sites ranged from −5.6 to 9.9 °C and from 75 to 861 mm, respectively. These climate variances could produce significant changes in plant community characteristics58. The sampling sites were selected at least 500 m away from settlements and roads to reduce human interference and were representative of local natural vegetation. Employing a randomized block design, we randomly selected four blocks at each site. Each block encompassed two types of patches (grassland patches vs. shrub patches) and shrub patches (at least 10 m apart) paired with adjacent grassland patches, resulting in eight 0.5 m × 0.5 m herbaceous plots at each site. Because most shrubs cover an area of less than 1 m2, and to avoid potential edge effects1,59, we utilized 0.25 m2 plots. Moreover, we recorded geographic information (latitude, longitude, and altitude) to extract bioclimatic variables from WorldClim2 (http://www.worldclim.org/), representing the average for the period of 1970–2000. To limit overfitting, we chose MAT and MAP in the following analyses, given that other climate variables have high collinearity with MAT or MAP (Supplementary Fig. 8).
Herbaceous plant community characteristics
In each plot, we trimmed all aboveground green herbaceous individuals, classified them to the species level, oven-dried all samples at 65 °C to a constant weight, and measured the biomass. The plot (community) level of herbaceous biomass was characterized as the sum of aboveground biomass from all species in each plot. To characterize the herbaceous plant communities, we utilized the diversity function in the vegan package60 to calculate herbaceous plant community species richness, Shannon diversity, and Simpson diversity. We used the rda function in the vegan package to perform principal component analysis (PCA) and extract the first principal component for the herbaceous plant community (plant PC1) (Supplementary Fig. 9), in consideration of the linear distribution for most of the herbaceous species we surveyed. Moreover, we also calculated beta diversity (based on Bray–Curtis distance) of herbaceous community composition between shrub patches and grassland patches.
To calculate the phylogenetic diversity of the herbaceous plant communities, we constructed a phylogenetic tree using a time-calibrated seed plant phylogeny as a phylogenetic backbone61 for the 486 species observed at all sites (Supplementary Fig. 10). For species missed in the phylogeny, we added them as basal polytomies within the corresponding genus or family using V.PhyloMaker in R62. Then, we simultaneously resolved polytomies and adjusted branch lengths based on an evolutionary birth-death model using the PolytomyResolver function and BEAST 1.5.063,64. We ran Markov chain Monte Carlo simulations in BEAST for 106 iterations and sampled phylogenetic trees after every 103 iterations. We discarded the first 25% of phylogenetic trees as burn-in and randomly selected 100 phylogenetic trees for subsequent analyses65. We calculated abundance-weighted mean phylogenetic pairwise distance (MPD) as a measure of the community phylogenetic diversity. MPD represents the average phylogenetic distance between any two species in a focal community and is widely used in community ecology43,66,67. To account for uncertainties in tree topology, the MPD was averaged across 100 phylogenetic trees.
Herbaceous community foliar fungal pathogen load
To characterize herbaceous community-level disease severity, we calculated pathogen load following the methods outlined in ref. 68. Specifically, we recorded species-level disease severity from 25 randomly selected leaves of at least five individuals for each herbaceous plant species in each plot. For species with fewer than 25 leaves, we harvested all available leaves. We recorded species-level disease severity (vi) as a percentage of the leaf area covered by fungal lesions23,69. We calculated the pathogen load (PL), widely employed as an indicator of herbaceous community fungal disease severity throughout disease ecology23,55, as:
where S represents the total number of plant species in a plot; ai is the biomass of host plant species i; and vi represents species-level disease severity of species i.
Finally, we utilized an Olympus CX33 microscope (Shinjuku, Japan) to validate the taxonomy of the identified pathogens according to relevant manuals, including the Fungal Identification Manual70 and prior studies as references71.
Community weighted mean of plant functional traits
For each site, we randomly selected five individuals to measure plant functional traits of each species in two types of patches (grassland patches vs. shrub patches), these data were collected contemporaneously with the herbaceous plant community and foliar fungal pathogen load survey. In addition, our surveys occurred in multiple directions: one team starting from Lanzhou (in the middle of the survey area) northward to Hulunbuir (northernmost), and finally back to Lanzhou. At the same time, the other team starting from Lanzhou, southward to Lijiang (southernmost), and finally back to Lanzhou. Hence, phenology should not bias our results. We examined the plant height (cm) of each selected individual and calculated the mean as the plant height of each species under each type of patch. We then harvested the aboveground parts of all selected individuals, stored them in a cooler box, and took them back to the laboratory. We selected three leaves of each individual to measure the leaf area (mm2, using the LA-S Leaf Area Analysis software, WSeen Detection Technology Co. Ltd., Hangzhou, China) within 24 h, and then dried all the leaves at 65 °C to constant mass and weighed to 0.0001 g to determine specific leaf area (SLA, cm2g−1). We calculated the community weighted mean (CWM) of these plant functional traits (plant height and specific leaf area), as:
where S is the number of species in each plot, ai is the biomass of species i in each plot, traiti is the trait value of species i in the corresponding type of patches.
Soil properties
To characterize the soil properties of each plot, we obtained six upper mineral soil cores (0–10 cm) from random locations within each plot and homogenized them thoroughly to generate a composite sample. All soil samples were stored at 4 °C during transportation. We measured five soil properties, including soil water content (SWC), pH, soil total carbon (mg/g; STC), soil total nitrogen (mg/g; STN), and soil available phosphorus (mg/kg; Soil AP). Soil samples at a depth of 10 cm were collected and stored following sieving through a 2-mm screen to remove visible plant roots and stones. The soil samples were air-dried at 25 °C for determination of soil properties. SWC was determined gravimetrically by drying the soil samples for 48 h at 105 °C. Soil pH was examined using a pH electrode (PHSJ-3F) in 1:5 soil-to-water mixtures. STC and STN were characterized using an elemental analyzer (Elementar vario MACRO cube, Elementar, Germany). Following extraction with 0.5 mol L−1 NaHCO3 at pH 8.5, Soil AP was determined using the molybdenum blue approach (four samples were missing). Due to potential collinearity among the soil variables (Supplementary Fig. 11), we utilized principal component analysis (PCA) on the identified variables and identified the first principal component (soil PC1) utilizing the rda function in the vegan package60 to represent soil properties for subsequent analyses (Supplementary Fig. 12).
Microclimate
At each site, we documented air temperature and humidity by simultaneously placing two temperature and humidity data loggers (Cos-03-0, Renke Control Technology Co., Ltd., Jinan, Shandong, China) at two types of patches (grassland patches vs. shrub patches), respectively. During the field surveys and sampling at each site, the two data loggers were calibrated and placed at each site to record ambient air temperature and humidity of the herbaceous community every 5 s for 20 min. We then calculated the mean air temperature and humidity for each type of patch at each site.
Shrub species identities and encroachment history
At each site, we recorded the species identities of shrubs and obtained a total of 13 shrubs: Ammopiptanthus mongolicus, Calligonum mongolicum, Caragana erinacea, Caragana korshinskii, Caragana versicolor, Hippophae rhamnoides, Krascheninnikovia ceratoides, Lonicera rupicola, Nitraria sibirica, Nitraria tangutorum, Dasiphora fruticosa, Reaumuria soongarica, and Sympegma regelii. According to Flora of China, all these shrubs were native rather than alien species. In addition, we used Google Earth (https://www.google.com/earth/versions/) to extract the satellite imagery of each site from 1985 to 2023. The satellite images at these sites were primarily from the Landsat program and the Copernicus program’s Sentinel satellites. We obtained the timeline of shrub encroachment by comparing the presence of shrubs in the images over different years.
Statistical analysis
To characterize the effect size of shrubs on herbaceous community characteristics (species richness, Shannon diversity, Simpson diversity, mean phylogenetic pairwise distance, herbaceous biomass, CWM of height, and specific leaf area), foliar fungal pathogen load, soil properties (soil PC1) and microclimate (air temperature and humidity), we determined the log-transformed response ratio (LRR) of these indicators between shrub patches and grassland patches’ plots (e.g., LRR pathogen load), as described previously72:
where Xt represents these indicators in the shrub patches, Xc is these indicators in corresponding (adjacent) grassland patches. A value greater than zero indicates shrubs positively influence these indicators and vice versa. Finally, we obtained LRR for 11 indicators, including LRR species richness, LRR Shannon diversity, LRR Simpson diversity, LRR mean phylogenetic pairwise distance, LRR herbaceous biomass, LRR pathogen load, LRR CWM of height, LRR CWM of specific leaf area, LRR soil PC1, LRR air temperature, and LRR air humidity.
To explore the effects of shrub encroachment on herbaceous community characteristics (including species richness, Shannon diversity, Simpson diversity, mean phylogenetic pairwise distance, plant PC1, herbaceous biomass and CWM of height, and specific leaf area), microclimate (air temperature and humidity), and soil properties (soil PC1), we conducted linear mixed-effects models (LMMs) using the lmer function in the lme4 package73, where the block nested in site was treated as a random effect. Prior to model fitting, we checked model residuals for normality and corrected non-normality using mathematical transformation (e.g., log transformation for species richness). We also performed a permutational multivariate analysis of variance (PERMANOVA) using 9999 random permutations on the basis of Bray–Curtis distances to test the effects of shrub, MAT, and MAP on herbaceous community composition.
To examine the effect of herbaceous plant diversity and trait composition on herbaceous community foliar fungal pathogen load and whether these effects were affected by shrub, we used zero-inflated generalized linear mixed-effects models to test effects of shrub, species richness/mean phylogenetic pairwise distance, plant PC1, CWM of height and specific leaf area and their interactions on pathogen load, where the block nested in site was treated as a random effect. We also used zero-inflated generalized linear mixed-effects models to test whether the effect of shrubs on herbaceous community foliar fungal pathogen load was dependent on MAT and MAP, where the block nested in the site was treated as a random effect. These zero-inflated generalized linear mixed-effects models were composed of a zero-inflation model (binary distribution) and conditional model (Beta distributions) and performed using the glmmTMB function in the glmmTMB package74. The zero-inflation model represents the probability of foliar fungal disease, whereas the conditional model indicates the severity of foliar fungal disease.
To further assess how MAT and MAP regulated the effect of shrub on herbaceous community foliar fungal pathogen load, we employed LMMs and general linear model to investigate the effects of climate factors (MAT and MAP) on LRR pathogen load, LRR species richness, LRR mean phylogenetic pairwise distance, LRR herbaceous biomass, LRR CWM of height and SLA, LRR air temperature, LRR air humidity and beta diversity, and the effects of LRR species richness, LRR mean phylogenetic pairwise distance, LRR herbaceous biomass, LRR CWM of height and SLA, LRR air temperature, LRR air humidity and beta diversity on LRR pathogen load using the lmer function in the lme4 package73, where site was established as a random effect. Subsequently, we fit Bayesian mixed-effects models to examine the effects of potential explanatory variables on LRR pathogen load and determined the individual contribution of each selected explanatory variable on LRR pathogen load. We employed the brm function in the brms package to conduct these Bayesian mixed-effects models, where the site was established as a random effect74. Due to the negative values of soil PC1 in calculating LRR soil PC1, 39 points were excluded from this analysis. In Bayesian mixed-effects models, we set posterior summaries of parameters based on four independent chains, each of which included 10,000 samples, with the first 5000 iterations discarded as burn-in. We set the adapt-delta value (the target average proposal acceptance probability) to 0.99, to avoid divergent transitions after warm-up. We monitored the degree of convergence in the Markov chains utilizing trace plots of posterior samples and the Gelman–Rubin convergence statistic (r-hat <1.01)75. Before fitting Bayesian mixed-effects models, we standardized all variables (scaled using their mean and standard deviation) to derive more comparable estimates76 and examined potential collinearity among explanatory variables to avoid overfitting. Moreover, to explore how shrub species identities and the history of shrub encroachment affected LRR pathogen load, we used LMMs to test shrub species identities and timeline of shrub encroachment on LRR pathogen load, where the site was established as a random effect.
According to the piecewise structural equation model (SEM) using the piecewiseSEM package77, we explored how MAT and MAP impact LRR pathogen load directly and indirectly through selected variables with higher explanatory power in Bayesian mixed-effects models (i.e., LRR herbaceous biomass, LRR species richness and LRR CWM of height). We first constructed a full priori model (Supplementary Fig. 5), then simplified the SEM by gradually removing non-significant paths until only significant paths remained78 and/or until model fit stopped increasing (i.e., AIC minimization) with additional path removal79. We incorporated site as a random effect in individual models and assessed SEM using Shipley’s test of d-separation80, which yields a Fisher’s C statistic that is χ2 distributed. Moreover, we performed partial regression to test the effects of explanatory variables residuals (MAT, MAP, LRR herbaceous biomass, LRR species richness, and LRR CWM of height) on LRR pathogen load residuals.
All statistical analyses were conducted in R version 4.2.3 (R Core Team, 2023) and Python version 3.10.9 (Python Software Foundation, 2022).
The current projection for LRR pathogen load
We adopted a space-for-time substitution approach to quantitatively estimate current and future projections for LRR pathogen load across China’s grasslands. As MAT exhibited a dominant role in identifying LRR pathogen load, and the projections of these climate data at fine spatial scales were easily examined under various climate change scenarios, we employed MAT to project LRR pathogen load in the future. Specifically, we estimated the multi-year (2021–2040) average of mean annual temperature using two different climate change scenarios (i.e., ssp1.26 and ssp5.85, assuming sustainable and unsustainable development, respectively)81 according to a spatial resolution of 30 arc-sec of the WorldClim2 database (http://www.worldclim.org/). We acquired the grassland raster data and projected LRR pathogen load based on the correlation coefficient between LRR pathogen load and MAT under various future climate change scenarios. All maps were visualized using ArcGIS 10.4 (ESRI, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All raw data are archived in Figshare at https://doi.org/10.6084/m9.figshare.27643569 and Zenodo at https://doi.org/10.5281/zenodo.14162544. Source data are provided as a Source Data file. Source data are provided with this paper.
Code availability
The R and Python code supporting the findings of this study are available at Figshare: https://doi.org/10.6084/m9.figshare.27643569 and Zenodo: https://doi.org/10.5281/zenodo.14162544.
References
Zhang, A. N. et al. Shrub and precipitation interactions shape functional diversity of nematode communities on the Qinghai–Tibet Plateau. Glob. Change Biol. 29, 2746–2758 (2023).
Heijmans, M. M. P. D. et al. Tundra vegetation change and impacts on permafrost. Nat. Rev. Earth Environ. 3, 68–84 (2022).
Stevens, N., Lehmann, C. E. R., Murphy, B. P. & Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Change Biol. 23, 235–244 (2017).
Maestre, F. T. et al. Biogeography of global drylands. New Phytol 231, 540–558 (2021).
Staver, A. et al. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232 (2011).
Liu, Y. et al. Dispersal and fire limit Arctic shrub expansion. Nat. Commun. 13, 3843 (2022).
Wigley, B. J., Bond, W. J. & Hoffman, M. T. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Change Biol. 16, 964–976 (2010).
Kulmatiski, A. & Beard, K. H. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Chang. 3, 833–837 (2013).
Venter, Z. S., Cramer, M. D. & Hawkins, H. J. Drivers of woody plant encroachment over Africa. Nat. Commun. 9, 2272 (2018).
Knapp, A. K. et al. Shrub encroachment in North American grasslands: shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob. Change Biol. 14, 615–623 (2008).
Eldridge, D. J. et al. Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol. Lett. 14, 709–722 (2011).
Ding, J. Y. & Eldridge, D. J. The success of woody plant removal depends on encroachment stage and plant traits. Nat. Plants 9, 58–67 (2022).
Kohli, M., Henning, J. A., Borer, E. T., Kinkel, L. & Seabloom, E. W. Foliar fungi and plant diversity drive ecosystem carbon fluxes in experimental prairies. Ecol. Lett. 24, 487–497 (2021).
Bradley, D., Gilbert, G. & Martiny, J. Pathogens promote plant diversity through a compensatory response. Ecol. Lett. 11, 461–469 (2008).
Fisher, M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 (2012).
Bagchi, R. et al. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 506, 85–88 (2014).
Packer, A. et al. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 278–281 (2000).
Petermann, J. S., Fergus, A., Turnbull, L. A. & Schmid, B. Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89, 2399–2406 (2008).
Mitchell, C. E. Trophic control of grassland production and biomass by pathogens. Ecol. Lett. 6, 147–155 (2003).
Paseka, R. E. et al. Disease-mediated ecosystem services: pathogens, plants, and people. Trends Ecol. Evol. 35, 731–743 (2020).
Keesing, F., Holt, R. D. & Ostfeld, R. S. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 (2006).
Keesing, F. & Ostfeld, R. S. Dilution effects in disease ecology. Ecol. Lett. 24, 2490–2505 (2021).
Mitchell, C. A., Tilman, D. & Groth, J. V. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 83, 1713–1726 (2002).
Zhang, P., Jiang, H. & Liu, X. Diversity inhibits foliar fungal diseases in grasslands: Potential mechanisms and temperature dependence. Ecol. Lett. 27, e14435 (2024).
Liu, X. et al. Dilution effect of plant diversity on infectious diseases: latitudinal trend and biological context dependence. Oikos 129, 457–465 (2020).
Burdon, J. J. & Chilvers, G. A. Host density as a factor in plant disease ecology. Annu. Rev. Phytopathol. 20, 143–166 (1982).
Liu, X. et al. Spatial Scale-dependent dilution effects of biodiversity on plant diseases in grasslands. Ecology 104, e3944 (2023).
Wieczorkowski, J. D. & Lehmann, C. E. R. Encroachment diminishes herbaceous plant diversity in grassy ecosystems worldwide. Glob. Change Biol. 28, 5532–5546 (2022).
Deng, Y. H., Li, X. Y., Shi, F. Z. & Hu, X. Woody plant encroachment enhanced global vegetation greening and ecosystem water-use efficiency. Glob. Ecol. Biogeogr. 30, 2337–2353 (2021).
Maestre, F. T. et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol. Lett. 12, 930–941 (2009).
Collins, C. G. et al. Belowground impacts of alpine woody encroachment are determined by plant traits, local climate, and soil conditions. Glob. Change Biol. 26, 7112–7127 (2020).
Delgado-Baquerizo, M. et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 4, 210–220 (2020).
Chaloner, T., Gurr, S. & Bebber, D. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Chang. 11, 710–715 (2021).
Romero, F. et al. Humidity and high temperature are important for predicting fungal disease outbreaks worldwide. New Phytol. 234, 1553–1556 (2022).
Qiu, Z. H., Wu, X. L., Zhang, J. X. & Huang, C. Y. High temperature enhances the ability of Trichoderma asperellum to infect Pleurotus ostreatus mycelia. PLoS ONE 12, e0187055 (2017).
Roy, B., Güsewell, S. & Harte, J. Response of plant pathogens and herbivores to warming experiment. Ecology 85, 2570–2581 (2004).
Strengbom, J., Englund, G. & Ericson, L. Experimental scale and precipitation modify effects of nitrogen addition on a plant pathogen. J. Ecol. 94, 227–233 (2006).
Milici, V. R., Dalui, D., Mickley, J. G. & Bagchi, R. Responses of plant-pathogen interactions to precipitation: implications for tropical tree richness in a changing world. J. Ecol. 108, 1800–1809 (2020).
Chaloner, T. M., Gurr, S. J. & Bebber, D. P. Geometry and evolution of the ecological niche in plant-associated microbes. Nat. Commun. 11, 2955 (2020).
Chen, D. V., Slowinski, S. P., Kido, A. K. & Bruns, E. L. High temperatures reduce growth, infection, and transmission of a naturally occurring fungal plant pathogen. Ecology 105, e4373 (2024).
Aguirre-Liguori, J. A., Ramírez-Barahona, S. & Gaut, B. S. The evolutionary genomics of species’ responses to climate change. Nat. Ecol. Evol. 5, 1350–1360 (2021).
Huber, D. M. & Watson, R. D. Nitrogen form and plant disease. Annu. Rev. Phytopathol. 12, 139–165 (1974).
Ratajczak, Z., Nippert, J. & Collins, S. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93, 697–703 (2012).
Niu, K. C., Zhang, S. T. & Lechowicz, M. J. Harsh environmental regimes increase the functional significance of intraspecific variation in plant communities. Funct. Ecol. 34, 1666–1677 (2020).
Rohr, J. R. & Cohen, J. M. Understanding how temperature shifts could impact infectious disease. PLoS Biol. 18, e3000938 (2020).
Fox, Q. N. et al. Effects of microclimate on disease prevalence across an urbanization gradient. Ecology 105, e4313 (2024).
Roy, B. A., Güsewell, S. & Harte, J. Response of plant pathogens and herbivores to a warming experiment. Ecology 85, 2570–2581 (2004).
Shieh, G. Power and sample size calculations for multivariate linear models with random explanatory variables. Psychometrika 70, 347–358 (2005).
Johannesson, E., Ohlson, J. A. & Zhai, S. W. The explanatory power of explanatory variables. Rev. Account 1, 31 (2023).
Agrios, G. Plant Pathology (Academic Press, New York, 2004).
Fitt, B. D. L., Gregory, P. H., Todd, A. D., McCartney, H. A. & Macdonald, O. C. Spore dispersal and plant disease gradients; a comparison between two empirical models. J. Phytopathol. 118, 227–242 (1987).
Carlsson, U. & Elmqvist, T. Epidemiology of anther-smut disease (Microbotryum violaceum) and numeric regulation of populations of Silene dioica. Oecologia 90, 509–517 (1992).
Gilbert, G. S. & Parker, I. M. The Evolutionary Ecology of Plant Disease (Oxford Univ. Press, New York, 2023).
Cai, Y. R. et al. The fertile island effect collapses under extreme overgrazing: evidence from a shrub-encroached grassland. Plant Soil 448, 201–212 (2020).
Saucet, S. B. et al. Resistance to root‐knot nematodes Meloidogyne spp. in woody plants. New Phytol. 211, 41–56 (2016).
Hammond-Kosack, K. E. & Jones, J. D. Plant disease resistance genes. Annu. Rev. Plant Biol. 48, 575–607 (1997).
Baker, R. E. et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 20, 193–205 (2022).
Zhang, J., Wang, J., Chen, W., Li, B. & Zhao, K. Vegetation of Xizang (Tibet) (Science Press, Peking, 1988).
Cuesta, B., Villar-Salvador, P., Puértolas, J., Benayas, J. M. R. & Michalet, R. Facilitation of Quercus ilex in Mediterranean shrubland is explained by both direct and indirect interactions mediated by herbs. J. Ecol. 98, 687–696 (2010).
Oksanen, J. et al. Vegan: community ecology package. R Package Version 2.5–6. https://cran.r-project.org/web/packages/vegan/index.html (2020).
Smith, S. A. & Brown, J. W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018).
Jin, Y. & Qian, H. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359 (2019).
Kuhn, T. S., Mooers, A. Ø. & Thomas, G. H. A simple polytomy resolver for dated phylogenies. Methods Ecol. Evol. 2, 427–436 (2011).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Emer, C. et al. Defaunation precipitates the extinction of evolutionarily distinct interactions in the Anthropocene. Sci. Adv. 5, eaav6699 (2019).
Webb, C. O., Ackerly, D. D. & Kembel, S. W. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24, 2098–2100 (2008).
Webb, C. O., Ackerly, D. D., McPeek, M. A. & Donoghue, M. J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 33, 475–505 (2002).
Liu, X., Lyu, S., Sun, D., Bradshaw, C. J. & Zhou, S. Species decline under nitrogen fertilization increases community-level competence of fungal diseases. Proc. Roy. Soc. B Biol. Sci. 284, 20162621 (2017).
Hantsch, L., Braun, U., Scherer-Lorenzen, M. & Bruelheide, H. Species richness and species identity effects on occurrence of foliar fungal pathogens in a tree diversity experiment. Ecosphere 4, 81 (2013).
Wei, J. C. Fungal Identification Handbook (Shanghai Scientific & Technical Publishers, Shanghai, 1979).
Liu, X. et al. Warming affects foliar fungal diseases more than precipitation in a Tibetan alpine meadow. New Phytol. 221, 1574–1584 (2019).
Hedges, L., Gurevitch, J. & Curtis, P. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Bürkner, P.-C. brms: an R package for Bayesian multilevel models using stan. J. Stat. Softw. 80, 1–28 (2017).
Gelman, A. & Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge Univ. Press, Cambridge, 2006).
Schielzeth, H. Simple means to improve the interpretability of regression coefficients: interpretation of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010).
Lefcheck, J. PIECEWISESEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2015).
Grace, J. B., Anderson, T. M., Olff, H. & Scheiner, S. M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 80, 67–87 (2010).
Lefcheck, J. S. piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016).
Shipley, B. A new inferential test for path models based on directed acyclic graphs. Struct. Equ. Modeling. 7, 206–218 (2000).
Fick, S. & Hijmans, R. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Acknowledgements
The study was funded by the National Natural Science Foundation of China (Grants No. 32422054, 32371611), the National Key Research and Development Program of China (Grants No. 2023YFF0806800), Gansu Provincial Science and Technology Major Projects (Grants No. 24ZD13NA016, 23ZDNA009), Young Talent Development Project of State Key Laboratory of Herbage Improvement and Grassland Agro-ecosystems (Grants No. 202201) and Fundamental Research Funds for the Central Universities (Grants No. lzujbky-2023-ey12) awarded to X.L. We thank Kui Hu, Sirui Zhu, Xiaodan Tan, Zijian Yu, Hongying Jiang, Wenjie Li, and Chenrui Wang for their assistance in the field.
Author information
Authors and Affiliations
Contributions
X.L., B.T., and J.L. conceived and designed the study, Y.D. led the data collection, with help from P.Z., J.K., X.L., M.L., and Y.X. Y.D., and X.L. analyzed the data and wrote the manuscript, with substantial input from P.Z., P.J., J.K., M.L., Y.X., Y.Z., M.L., J.W., J.L., and B.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dang, Y., Zhang, P., Jiang, P. et al. Temperature-dependent variations in under-canopy herbaceous foliar diseases following shrub encroachment in grasslands. Nat Commun 16, 1131 (2025). https://doi.org/10.1038/s41467-025-56439-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56439-z