Abstract
Aberrant release of mitochondrial reactive oxygen species (mtROS) in response to cellular stress is well known for promoting cancer progression. However, precise molecular mechanism by which mtROS contribute to epithelial cancer progression remains only partially understood. Here, using colorectal cancer (CRC) models, we show that upon sensing excessive mtROS, phosphatase PGAM5, which normally localizes to the mitochondria, undergoes aberrant cleavage by presenilin-associated rhomboid-like protein (PARL), becoming released into the cytoplasm. Cytosolic PGAM5 then directly binds to and dephosphorylates MST3 kinase. This, in turn, prevents STK25-mediated LATS1/2 phosphorylation, leading to YAP activation and CRC progression. Importantly, depletion of MST3 reciprocally promotes accumulation of cytosolic PGAM5 by inducing mitochondrial damage. Taken together, these findings demonstrate how mtROS promotes CRC progression by activating YAP via a post-transcriptional positive feedback loop between PGAM5 and MST3, both of which can serve as potential targets for developing next-generation anti-colon cancer therapeutics.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world, and its incidence and resulting mortality have being on a rise1. Although research on CRC has advanced considerably, it remains the second most common cause of cancer-related death in the world2. Previous studies have reported that mitochondrial dysfunction and, specifically, accumulation of reactive oxygen species (ROS) are involved in the pathogenesis of colorectal tumors3. Mitochondria, as essential organelles in eukaryotic cells, not only play a central role in bioenergetics and biosynthesis, but also produce ROS to regulate tumorigenesis4,5. Mitochondrial dysfunction underlies pathogenesis of numerous cancers, causing increased electron leakage and resulting in high ROS production in tumor cells6. Excessive mitochondrial ROS (mtROS) play dual roles in cancer progression, leading to either ROS-dependent malignant transformation of cells or oxidative stress-induced cell death, depending on the concentration, duration, and distribution of ROS7,8. An increase in mtROS production promotes cancer progression either through direct induction of non-repairable DNA defects, and/or through activation of pro-oncogenic signalling pathways9. For this reason, defects in mtROS scavenging enzymes predispose cells to oncogenic transformation10, and mitochondrial stress-induced aberrant mtROS release is considered to be an essential factor in neoplastic transformation. Thus, understanding the molecular mechanisms by which mitochondrial stress responses drive cancer progression might lead to identification of potential therapeutic targets.
Phosphoglycerate mutase 5 (PGAM5), a mitochondrial serine (Ser)/threonine (Thr) phosphatase, was identified as the key player in mitochondrial homeostasis11,12,13. Several studies have shown that PGAM5 is primarily localized to the mitochondrial inner membrane (MIM), and can shuttle between the inner and outer membranes depending on the mitochondrial membrane potential14,15. Responding to mitochondrial stress, PGAM5 ensures efficient and effective mitophagy, a pro-survival cellular behavior. During this process, PGAM5 is cleaved by Presenilin-associated rhomboid-like protein (PARL), leading to its C-termus release, which includes the phosphatase domain, from mitochondrial membranes15. It has been reported that release of cleaved PGAM5 into cytosol enables its interaction with Axin1 that, in turn, prevents degradation of β-catenin, consequently, activating canonical WNT signalling16,17, that commonly drives growth by epithelial cancer cells, including in the context of CRC. Yet, how cytosolic PGAM5 promotes CRC progression in response to excessive ROS, beyond WNT signalling, remains elusive.
Mammalian sterility 20 (Ste20)-like kinase 3 (MST3) belongs to the highly conserved STE20-like kinase family and mainly localizes to the cytoplasm18. MST3 was identified because it shares 88% amino acid similarity with the oxidative stress response kinase SOK-1 in the kinase domain, suggesting that MST3 might be involved in oxidative stress response18. Additionally, MST3 can trigger cell death by inhibiting JNK survival pathway and by upregulating cytoprotective Heme Oxygenase-1 (HO-1) in H2O2-treated human colon cancer cells, the HCT116 cells19. Besides caspase-mediated apoptosis, MST3 also activates caspase-independent apoptotic pathway in human cervical HeLa cells by promoting nuclear translocation of apoptosis-inducing factors and endonuclease G by disrupting mitochondrial membrane potential20. Taken together, previous works indicate that MST3 might play an important role in regulating mitochondrial stress responses. However, whether MST3 can mediate mtROS signalling upon epithelial cancer development remains unclear.
In this study, we show that mitochondria-localized PGAM5 acts as a sensor for mtROS to regulate cellular homeostasis and malignant transformation. By sensing elevated levels of mtROS, PGAM5 undergoes cleavage and is then released into the cytoplasm, where it dephosphorylates MST3 to activate YAP. Collectively, our data from CRC model reveals a distinct PGAM5-MST3-mediated feedback loop that controls YAP signalling, that promotes CRC development in response to mtROS accumulation.
Results
MST3 functions as tumor suppressor in CRC
To begin understanding the role of MST3 in CRC development, we first analysed 59 pairs of colon adenocarcinoma and adjacent normal tissues from a cohort of patients and found a consistent reduction in MST3 expression in colon tumors (Fig. 1a). Similarly, mRNA assays on colon adenocarcinoma and adjacent normal tissues from 10 additional patients showed low expression of MST3 in colon tumors (Fig. 1b). Analogous to the data from human samples, expression levels of Mst3 decreased in mouse colon tumors from the AOM-DSS (azoxymethane-dextran sulfate sodium)-induced tumor mouse model (Fig. 1c).
a Representative immunohistochemical images of MST3 in human colorectal peri-tumor and tumor tissues from CRC patients. Scores of MST3 expression levels were quantified. n = 59. Scale bar: 50 µm. P = 3.04E-11. b qRT-PCR analysis for MST3 in human peri-tumor and tumor tissues from CRC patients. n = 10. P = 0.03. c Representative immunohistochemical images of Mst3 in mouse colon peri-tumor and tumor tissues from AOM-DSS mouse model. n = 3. Scale bar: 50 µm. d Gross images of AOM-DSS mouse colon tumors from control (Ctrl) and Mst3cKO mice. Tumor area (P = 1.58E-12) and the number of tumors (P = 0.002) per mouse were quantified. Ctrl: n = 50 tumors from 6 mice. Mst3cKO: n = 73 tumors from 5 mice. e Representative histological images of AOM-DSS colon tumors from Ctrl and Mst3cKO mice. n = 3. Scale bar: 2 mm. f Anchorage-independent growth of HCT116 cells transfected with NC or siMST3. Colony formation rate was determined. Colonies grew for 5 weeks. NC, n = 27 fields from 3 independent samples; siMST3, n = 38 fields from 3 independent samples. Scale bar: 10 μm. P = 1.94E-08. g Schematic diagram of constructs of human MST3 kinase-dead mutant plasmid (Lysine at position 53 was mutated to Alanine) and kinase persistent phosphorylation mutant plasmid (Threonine at position 178 was mutated to Glutamate). h HA-labeled pcDNA3.1 empty vector, pcDNA3.1-MST3WT, pcDNA3.1-MST3K53A and pcDNA3.1-MST3T178E were transfected into APKS mouse tumor organoids, respectively. Organoid areas were quantified at the indicated time points. 4 h, n = 50 fields from 3 independent samples; 52 h, n = 53 fields from 3 independent samples. Scale bar: 250 μm. i Schematic diagram of the cecal orthotopic injection mouse model using luciferase-labelled HCT116 cells. j HCT116 cells with stable knockdown of MST3 (shMST3) transfected with pcDNA3.1, pcDNA3.1-MST3WT, pcDNA3.1-MST3K53A or pcDNA3.1-MST3T178E plasmids were injected into the cecum of NOG mice, and bright field images of primary tumor growth and quantification of tumor weight were recorded. n = 3 mice per group. P = 0.032; P = 0.027; P = 0.009. k Representative IVIS luciferase images of CRC liver metastases in NOG mice injected with HCT116 cells into the cecum. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical analysis in panel h was performed with one-way ANOVA followed by Tukey’s test, and the rest were performed with two-tailed unpaired Student’s t-test.
To determine the in vivo role of Mst3 upon CRC development, we generated tissue-specific conditional VillinCre; Mst3fl/fl (Mst3cKO) mice, in which Mst3 was deleted specifically in the intestinal epithelium (Supplementary Fig. 1a–d). Deleting Mst3 did not influence body weight (Supplementary Fig. 1e, f), yet it increased the length of both small intestine and colon (Supplementary Fig. 1g), and also increased height of colonic crypts (Supplementary Fig. 1h). An increase in the number of proliferative intestinal epithelial cells was also observed in Mst3cKO mice (Supplementary Fig. 1i), accompanied by an increase in the number of apoptotic cells at the tips of colonic crypts (Supplementary Fig. 1j), collectively suggesting faster turnover within the intestinal epithelial lineage. Similar phenotypes were also found in the small intestine of Mst3cKO mice (Supplementary Fig. 1k–m).
We next used an AOM-DSS-induced colon tumor mouse model to evaluate the in vivo role of MST3 in tumorigenesis (Supplementary Fig. 2a). Deletion of Mst3 significantly increased the size and the number of tumors (Fig. 1d). Consistently, a significant increase in hyperplastic lesions and proliferative cells was observed upon Mst3 deficiency (Fig. 1e and Supplementary Fig. 2b, c). The results of in vitro assays also showed that knockdown of MST3 promoted proliferation of CRC cells; conversely, MST3 overexpression suppressed it (Supplementary Fig. 2d–h). Furthermore, knockdown of Mst3 significantly promoted growth of mouse tumor organoids harboring mutations in Apc, Tp53, Kras and Smad4, concomitant with an increase in proliferative cells (Supplementary Fig. 2i, j).
The growth of tumor cells in vitro in a non-anchored manner is considered to be a hallmark of malignant transformation21. We found that knockdown of MST3 promoted non-anchored growing capacity of tumor cells (Fig. 1f). Furthermore, transwell and scratch assays showed that knockdown of MST3 promoted migration and invasion potentials of CRC cells (Supplementary Fig. 2k, l). In contrast, MST3 overexpression suppressed migration potential of CRC cells (Supplementary Fig. 2l). Taken together, these in vitro results suggest that MST3 functions as a tumor suppressor in CRC by inhibiting cellular proliferation and metastasis.
To evaluate whether MST3-induced inhibitory effects depend on its kinase activity, we first generated MST3 kinase-dead mutant22 (termed MST3K53A) and a mutant with constitutive kinase activity23 (termed MST3T178E) (Fig. 1g). We next generated stable MST3-knockdown HCT116 cells and APKS tumor organoids to exclude the effect of endogenous MST3 kinase activity (Supplementary Fig. 3a, b), and then treated with MST3WT, MST3K53A or MST3T178E. The total levels of MST3 were comparable in HCT116 cells and APKS organoids overexpressing MST3WT, MST3K53A or MST3T178E (Supplementary Fig. 3c, d). However, MST3K53A downregulated the levels of phosphorylated MST3 both in HCT116 cells and APKS organoids, relative to MST3WT; while MST3T178E upregulated it, relative to MST3WT (Supplementary Fig. 3c, d). These results verified that mutants of kinase-dead and active MST3 are efficient at modulating its activity. Furthermore, we found that sustained activation of MST3 kinase more efficiently suppressed the growth of xenografted tumors compared to wild type MST3 (MST3WT), while kinase-dead MST3 mutant failed to suppress tumor growth (Supplementary Fig. 3e). Consistent with these observations, MST3T178E inhibited growth of mouse tumor organoids, whereas MST3K53A had no such effect (Fig. 1h). Meanwhile, MST3T178E mutant, but not MST3K53A mutant also enhanced MST3’s inhibitory effect on migration and invasion potentials of CRC cells (Supplementary Fig. 3f, g).
To further investigate the effect of MST3’s kinase activity on the metastatic ability of CRC, we used an orthotopic cecal injection model that better mimics the metastatic process of clinical CRC24. We stably transfected various MST3 mutants into luciferase-expressing HCT116 cells and examined their capacity to generate liver metastases after cell injection into terminal cecum of NOG mice (Fig. 1i). We found that MST3T178E mutant more efficiently inhibited growth of primary cecal tumors and liver metastasis compared to MST3WT, while MST3K53A mutant promoted these effects relative to MST3WT (Fig. 1j, k and Supplementary Fig. 3h). Thus, we conclude that MST3 suppresses CRC growth and metastasis in a kinase activity-dependent manner.
MST3 suppresses YAP through its kinase activity
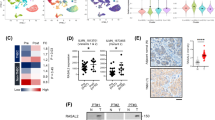
To further investigate the molecular mechanism by which MST3 inhibits CRC proliferation and metastasis, we performed genome-wide transcriptome analysis in MST3-overexpressing CRC cells. We defined differentially expressed genes (DEGs) as genes with P < 0.05 and fold change > 2. This approach yielded 267 downregulated genes and 745 upregulated genes in the experimental group (Supplementary Fig. 4a). KEGG analysis showed that Hippo signalling pathway was enriched in downregulated genes (Supplementary Fig. 4b). Hippo signalling pathway is an evolutionarily conserved kinase cascade, which restricts activity of YAP/TAZ transcriptional coactivator through phosphorylation25,26. When Hippo signal is turned off, YAP/TAZ is dephosphorylated and transported into the nucleus to control the transcription of its target genes27,28. MST3 overexpression resulted in a significant downregulation of many Hippo-related genes, which were validated on qRT-PCR analysis (Fig. 2a and Supplementary Fig. 4c, d). Furthermore, MST3 overexpression suppressed expression of YAP target genes CTGF and CYR61, while knockdown of MST3 upregulated them (Fig. 2b, c). Consistently, MST3 overexpression significantly enhanced phosphorylation of YAP at Ser127 (Fig. 2d), as well as decreased the nuclear localization of YAP (Fig. 2e). In contrast, knockdown of MST3 increased YAP nuclear translocation (Supplementary Fig. 4e). In line with the findings that MST3 suppresses YAP activity and becomes downregulated in CRC tissues, levels of YAP target genes and frequency of nuclear YAP+ cells increased in colorectal tumors, while pYAP/YAP ratio and pMST3 levels became reduced, indicating that YAP is activated in colorectal tumors (Supplementary Fig. 4f–h).
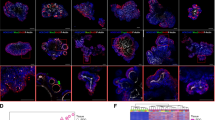
a Heat map depicts altered Hippo-related genes in the transcriptome profile of HCT116 cells transfected with pcDNA3.1 empty vector and pcDNA3.1-MST3 plasmid (OE-MST3). n = 2 independent samples. b qRT-PCR for YAP (P = 0.207), CTGF (P = 0.0008) and CYR61 (P = 0.0001) in HCT116 cells treated with pcDNA3.1 empty vector or pcDNA3.1-MST3 plasmid. n = 3 independent samples. c qRT-PCR for MST3 (P = 0.008), YAP, CTGF (P = 0.008) and CYR61 (P = 0.0005) in HCT116 cells transfected with NC or siMST3. n = 3 independent samples. d Western blotting for MST3, YAP and pYAPS127 in HCT116 cells transfected with pcDNA3.1 or pcDNA3.1-MST3 plasmid. β-Actin was used as the loading control. e Western blotting for YAP and MST3 in cytoplasmic and nuclear fractions of HCT116 cells transfected with pcDNA3.1 or pcDNA3.1-MST3 plasmid. Histone H3 was used as the nuclear positive control, β-Actin was used as the cytoplasmic positive control. f Western blotting for Mst3, Yap and pYapS112 (referred to mouse, which is identical to the phosphorylated site of human pYAPS127) in AOM-DSS tumors from Ctrl and Mst3cKO mice. β-Actin was used as the loading control. g Double immunofluorescence for Yap and β-Catenin in AOM-DSS tumors from Ctrl and Mst3cKO mice. Percentage of nuclear Yap+ cells was quantified. n = 50 fields from 3 mice. Scale bar: 100 µm. P = 6.43E-07. h, i Luciferase assay for TEAD-luciferase reporter gene in HEK293FT cells transfected with flag-YAP and HA-MST3 (h, n = 5 independent samples) or flag-YAP and siMST3 (i, n = 6 independent samples), respectively. j qRT-PCR analysis for CYR61 in HCT116 cells pretreated with shMST3 and transfected with indicated MST3 constructs, with or without flag-YAP plasmid. n = 3 independent samples. k Western blotting for HA, YAP and pYAPS127 in HCT116 cells transfected with HA-MST3WT (WT) and HA-MST3K53A (K53A) plasmids. β-Actin was used as the loading control. l Immunofluorescence for YAP in LOVO cells transfected with HA-MST3WT (WT), HA-MST3K53A (K53A) or HA-MST3T178E (T178E) plasmids. Statistics of YAP subcellular distribution were performed. n = 3 independent samples. Scale bar: 20 μm. m Western blotting for HA, pMST3T178, YAP and pYAPS127 in LOVO cells transfected with HA-MST3WT, HA-MST3K53A and HA-MST3T178E plasmids. β-Actin was used as the loading control. Western blotting images are representative of experiments that were repeated at least three times with similar results. Data are presented as the mean ± SD. **P < 0.01; ***P < 0.001. Statistical analysis in panels h-j was performed with one-way ANOVA followed by Tukey’s test, and the rest were performed with two-tailed unpaired Student’s t-test.
Similar to the in vitro results, Yap target genes were upregulated in Mst3cKO mice (Supplementary Fig. 4i), ratio of pYap/Yap was significantly downregulated in Mst3cKO intestines (Fig. 2f), and frequency of nuclear Yap+ cells increased in colon tumors of Mst3cKO mice (Fig. 2g), collectively indicating elevated Yap activity. To further confirm this finding, we used luciferase-based reporter assay to examine whether MST3 can inhibit YAP-induced TEAD4 transactivation. We found that ectopic expression of YAP induced robust activation of luciferase activity mediated by TEAD4 promoter, whereas MST3 overexpression significantly decreased the reporter activity (Figs. 2h and Supplementary Fig. 4j–l). In contrast, knockdown of MST3 greatly upregulated TEAD4 luciferase activity (Figs. 2i and Supplementary Fig. 4m–o). Finally, knockdown of YAP abolished MST3 knockdown-induced tumor phenotypes (Supplementary Fig. 5a–d). Also, treatment with verteporfin (VP), a compound that disrupts interaction between YAP and TEAD29, abrogated MST3 knockdown-induced tumor growth (Supplementary Fig. 5e). In contrast, MST3 overexpression suppressed growth of xenografted tumors and colorectal tumor organoids, and inhibited invasion and migration of HCT116 cells, while YAP overexpression rescued these inhibitory effects (Supplementary Figs. 6a–c). Taken together, these results suggest that MST3 can inhibit CRC progression by suppressing YAP activity.
To assess whether MST3 regulation of YAP signalling requires its kinase activity, we overexpressed MST3WT and MST3K53A mutant in CRC cells depleted of endogenous MST3. We found that overexpression of MST3WT but not MST353A significantly inhibited YAP-induced expression of CYR61 and TEAD4 luciferase activity (Fig. 2j and Supplementary Figs. 7a–c). Consistent with these observations, overexpression of MST3WT led to phosphorylation of YAP to a level much higher than did MST3K53A mutant (Fig. 2k). In addition, overexpression of MST3K53A mutant promoted nuclear translocation of YAP and decreased the ratio of pYAP/YAP in CRC cells, relative to MST3WT. In contrast, overexpression of MST3T178E mutant led to higher cytoplasmic localization of YAP and increased the ratio of pYAP/YAP, compared to MST3WT (Fig. 2l, m). These results demonstrate that the regulatory effect of MST3 on YAP activity requires its kinase activity.
MST3 suppresses YAP activity via STK25-mediated LATS phosphorylation of YAP
It has been reported that in the Hippo signalling pathway, LATS kinases can be regulated by both GCK II subfamily kinase MST1/2 kinases30,31 and GCK III subfamily kinase STK2532. Since MST3 is a member of GCK III subfamily, we sought to investigate whether it can modulate YAP activity by regulating LATS. In line with this hypothesis, knockdown of MST3 led to the reduction in pLATS in HCT116 cells and APKS tumor organoids, accompanied by decrease in pYAP/YAP ratio (Supplementary Figs. 7d, e). Constitutive activation of MST3 kinase (MST3T178E) promotes phosphorylation of LATS, thereby inhibiting YAP activation. In contrast, MST3 kinase-dead mutant (MST3K53A) attenuated the promoting effect of MST3 on LATS activity, leading to YAP activation (Supplementary Fig. 7f). Furthermore, we found that MST3 overexpression suppressed YAP nuclear translocation and downregulated the expression levels of YAP target genes, while knockdown of LATS enhanced YAP nuclear translocation and expression of YAP target genes (Fig. 3a–c), indicating that MST3 regulation of YAP is dependent on LATS. Next, we performed immunoprecipitation assay using anti-MST3 antibody to determine whether MST3 directly interacts with Hippo pathway kinase components. It showed that MST3 does not directly interact with LATS1/2 kinases, YAP, or its upstream regulators MOB1 and MST1 (Fig. 3d). These findings suggest that the effect of MST3 on LATS1/2 is indirect, which is different from MST1/2 that can directly phosphorylate LATS1/2.
a Immunofluorescence for YAP in LOVO cells transfected with siLATS1/2 and/or flag-MST3 plasmids. Ratio of nuclear/cytoplasmic YAP was quantified. Vector+NC, n = 38 fields from 3 independent samples; Flag-MST3 + NC, n = 44 fields from 3 independent samples; Vector+siLATS1/2, n = 44 fields from 3 independent samples; Flag-MST3+siLATS1/2, n = 43 fields from 3 independent samples. Scale bar: 30 µm. P = 0.004. b qRT-PCR for YAP, CTGF (P = 0.04; P = 0.0041) and CYR61 (P = 0.03; P < 0.001) in LOVO cells transfected with siLATS1 and/or flag-MST3. n = 3 independent samples. c qRT-PCR for MST3 (P < 0.001) and LATS1 (P = 0.0053) in LOVO cells transfected with siLATS1 and/or flag-MST3. n = 3 independent samples. d Co-IP assay for endogenous interaction between MST3 and STK25, MST1, MOB1, YAP and LATS1/2 in HCT116 cells after immunoprecipitation with anti-MST3 antibody. e Western blotting for MST3, STK25 and pSTK25T190 in HCT116 cells transfected with flag-pcDNA3.1 or flag-MST3 plasmids. β-Actin was used as the loading control. f Western blotting for MST3, STK25 and pSTK25T190 in HCT116 cells transfected with pcDNA3.1-MST3T178E or pcDNA3.1-MST3K53A plasmids. β-Actin was used as the loading control. g Western blotting for MST3, STK25, pSTK25T190, YAP, pYAPS127, LATS1/2, pLATS1S909 and pLATS1T1079 in HCT116 cells transfected with siSTK25 and/or HA-MST3 plasmids. β-Actin was used as the loading control. h, i Western blotting for YAP in HCT116 cells transfected with NC/siMST3 (h) or pcDNA3.1/pcDNA3.1-MST3 (i), and treated with CHX (cycloheximide, 50 µg/mL) at indicated time points. β-Actin was used as the loading control. Relative gray value of YAP was calculated. j Co-IP analysis for binding between MST3 and 14-3-3. k Ubiquitination of YAP was measured 36 h after transfection with myc-Ub, flag-YAP and HA-MST3 in LOVO cells. l Western blotting for YAP, 14-3-3 and MST3 level in LOVO cells. Cells were transfected with different doses of HA-14-3-3 plasmid after treatment with NC or siMST3. β-Actin was used as the loading control. Western blotting images are representative of experiments that were repeated at least three times with similar results. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical analysis in panels b and c was performed with one-way ANOVA followed by Tukey’s test, and the rest was performed with two-tailed unpaired Student’s t-test.
Next, we investigated whether MST3 regulates LATS kinases through STK25. Indeed, we found that endogenous MST3 protein can interact with endogenous STK25 protein (Fig. 3d). More importantly, MST3 promoted phosphorylation of STK25 (T190) in a manner that depended on kinase activity (Fig. 3e, f). Furthermore, we found that MST3 overexpression led to the increase in phosphorylation of LATS1 at both S909 (activation loop) and T1079 (hydrophobic motif) sites, accompanied by elevated pYAP, while knockdown of STK25 abrogated these effects (Fig. 3g). These findings indicate that MST3 suppresses YAP activity via STK25-mediated LATS phosphorylation.
MST3 promotes YAP ubiquitination and degradation
Given that MST3 overexpression considerably decreased YAP protein level but not its mRNA level (Fig. 2b, d), and that knockout of Mst3 increased Yap protein level without affecting mRNA level (Figs. 2f and Supplementary Fig. 4i), we further investigated whether MST3 affects YAP protein stability. We found that knockdown of MST3 inhibited YAP degradation (Fig. 3h), while MST3 overexpression accelerated it (Fig. 3i). These results further support the notion that MST3 regulates LATS1/2-YAP axis.
Phosphorylation of YAP at S127 facilitates its interaction with 14-3-3 proteins and subsequent ubiquitination and degradation of YAP33,34. In this regard, our mass spectrometry analysis suggested that 14-3-3 protein is a potential binding partner of MST3. The interaction between 14-3-3 and MST3 was further verified by Co-IP assay (Fig. 3j). Moreover, we found that MST3 overexpression promotes YAP ubiquitination (Fig. 3k), and that MST3 knockdown attenuates 14-3-3-mediated YAP degradation (Fig. 3l). These results indicate that MST3 regulates YAP protein stability.
Phosphatase PGAM5 dephosphorylates MST3
To explore upstream regulators of MST3 activity, we performed IP mass spectrometry screening in MST3-overexpressing CRC cells. This approach identified 263 potential MST3-interacting proteins. Among them, we selected PGAM5 (Fig. 4a), a serine (Ser)/threonine (Thr) phosphatase essential for mitochondrial homeostasis12. Our Co-IP assays revealed interaction between endogenous proteins of PGAM5 and MST3 in colon tumor cells (Fig. 4b, c). Moreover, PGAM5 overexpression led to a remarkable decrease in phosphorylated MST3 (Fig. 4d), whereas knockdown of PGAM5 significantly increased it (Fig. 4e). Structural prediction suggested that MST3 might bind to the phosphatase domain of PGAM5 (Fig. 4f). To examine whether PGAM5 phosphatase activity modulates MST3 phosphorylation, we constructed PGAM5 phosphatase-dead mutant35 (Fig. 4g). Overexpression of wild type PGAM5 significantly decreased phosphorylation of MST3, while phosphatase-dead PGAM5H105A mutant failed to do so (Fig. 4h). Furthermore, we established an in vitro dephosphorylation system in which MST3 protein was first allowed to undergo autophosphorylation and then was incubated with purified wild type PGAM5 or PGAM5H105A, respectively (Fig. 4i). Consistently, treatment with wild type PGAM5 but not PGAM5H105A mutant resulted in a prominent reduction in phosphorylated MST3 (Fig. 4j). Taken together, our findings demonstrate that PGAM5 can directly dephosphorylate MST3.
a Immuno-purification and mass spectrometry analysis of HCT116 cells expressing flag-pcDNA3.1 or flag-MST3 on an anti-flag affinity column. b, c Co-IP assay of endogenous interaction between PGAM5 and MST3 in HCT116 cells after immunoprecipitation with either anti-PGAM5 antibody (b) or anti-MST3 antibody (c). d Western blotting for PGAM5, MST3 and pMST3T178 in HCT116 cells transfected with flag-pcDNA3.1 or flag-PGAM5 plasmids. β-Actin was used as the loading control. e Western blotting for PGAM5, MST3 and pMST3T178 proteins in HCT116 cells transfected with NC or siPGAM5. β-Actin was used as the loading control. f AlphaFold predicted binding fragment of MST3 to PGAM5. g Schematic diagram of construction of human PGAM5 phosphatase inactivation mutant plasmid (Histidine at position 105 changed to Alanine). h Western blotting for Flag, MST3 and pMST3T178 proteins in HCT116 cells transfected with NC/siPGAM5 and then transfected with flag-PGAM5WTor flag-PGAM5H105A plasmids. β-Actin was used as the loading control. i Schematic diagram of the in vitro MST3 protein dephosphorylation assay. j Western blotting was used to evaluate the effect of flag-PGAM5WT versus flag-PGAM5H105A on MST3 phosphorylation described in panel (i). k Schematic representation of the sites where PGAM5 protein is cleaved. l Western blotting for Flag, MST3 and pMST3T178 in LOVO cells transfected with PGAM5WT or truncated PGAM5 (25-289). β-Actin was used as the loading control. m GST pull-down assays for interaction with GST-MST3 were performed in the presence or absence of His-SUMO-tagged PGAM5 (25-289). Samples were subjected to Western blotting. GST was used as the negative control. n Purified GST-tagged MST3 was combined with His-SUMO-tagged PGAM5 (25-289) to determine the amount of MST3 phosphorylation remaining by an in vitro dephosphorylation system. o Western blotting for Flag, MST3, pMST3T178 in HCT116 cells with stable knockdown of PGAM5 (shPGAM5) transfected with flag-pcDNA3.1, flag-PGAM5WT or flag-PGAM5△23-29. β-Actin was used as the loading control. Western blotting images are representative of experiments that were repeated at least three times with similar results.
PGAM5 binds to MST3 upon its cytoplasmic release
Next, we elucidated how mitochondrial PGAM5 regulates MST3 kinase activity in the cytoplasm. Upon mitochondrial stress, N-terminal region of PGAM5, including 35 amino acids-long transmembrane α-helix, becomes cleaved36, releasing PGAM5 fragment into the cytoplasm16,37. To determine whether cytoplasmic fragment of PGAM5 binds to MST3, we constructed a plasmid expressing truncated form of PGAM5, that corresponds to its cytoplasmic fragment (amino acids 25 through 289) (Fig. 4k). Strikingly, cytoplasmic fragment of PGAM5 induced dephosphorylation of MST3 even more efficiently than did full-length PGAM5 (Fig. 4l). Consistent with these observations, in vitro pull-down assay showed that cytoplasmic fragment of PGAM5 directly binds to MST3, and induces dephosphorylation of MST3 (Fig. 4m, n). Furthermore, we ablated cleavage site by removing 7 amino acids from 23 to 29. Overexpression of PGAM5△23-29 efficiently prevented PGAM5 cleavage, and failed to dephosphorylate pMST3 (Fig. 4o). These results indicate that cleaved cytosolic PGAM5 directly binds to and dephosphorylates MST3.
Mitochondrial ROS promotes cytoplasmic PGAM5 release
PGAM5 becomes cleaved in response to mitochondrial stress, and mitochondrial dysfunction-related ROS production is known to be elevated in tumor cells38. In agreement with this, we observed high levels of cleaved cytosolic PGAM5 and low levels of MST3 activity in CRC cells, accompanying elevated ROS (Supplementary Fig. 8a-c). To determine whether ROS can promote PGAM5 cleavage, we treated NCM460 intestinal epithelial cells with oxidants, Tert-butyl Hydroperoxide (TBHP) and H2O2. These treatments promoted ROS production and PGAM5 cleavage, accompanied by reduction in pMST3 and pYAP (Supplementary Fig. 8d-f).
Since mitochondria are a major source of ROS in mammalian cells39, we examined whether mtROS induces PGAM5 cleavage. Rotenone is mitochondrial complex I inhibitor that induces mtROS production40. Rotenone treatment enhanced cleaved PGAM5, and downregulated pMST3 and pYAP levels (Supplementary Fig. 8g). Furthermore, we employed carbonyl cyanide m-chlorophenyl hydrazone (CCCP), a mitochondrial uncoupling agent that can also induce mtROS production41,42. CCCP treatment significantly promoted PGAM5 cleavage in a dose-dependent and time-dependent manner, concomitant with reduction in MST3 phosphorylation (Figs. 5a and Supplementary Fig. 8h). Furthermore, CCCP treatment led to cytosolic localization of PGAM5 (Fig. 5b) and increased interaction with MST3 (Fig. 5c). Knockdown of PGAM5 attenuated CCCP-induced dephosphorylation of MST3 (Supplementary Fig. 8i), suggesting that CCCP regulates MST3 activity through PGAM5.
a Western blotting for PGAM5, MST3 and pMST3T178 in NCM460 cells after treatment with different concentrations of CCCP (0 µM, 10 µM, 20 µM, 30 µM) for 2 h. β-Actin was used as the loading control. b Western blotting was used to detect cytoplasmic and mitochondrial PGAM5 in NCM460 cells treated with 20 µM CCCP for 2 h. TOM20 was used as the mitochondrial positive control, β-Actin was used as the cytoplasmic positive control. c Co-IP assay of endogenous interaction between PGAM5 and MST3 in NCM460 cells treated with 20 µM CCCP for 2 h. β-Actin was used as the loading control. d Fluorescence intensity of MitoSOX (red) was detected with mitochondrial reactive oxygen species (mtROS) fluorescence probe. NCM460 cells were treated with 20 µM CCCP or 30 µM rotenone with or without 100 µM NAC for 2 h. n = 3 independent samples. Scale bar: 300 µm. e, f NCM460 cells were treated with 20 µM CCCP or 30 µM rotenone with or without 100 µM NAC for 2 h, MitoSOX fluorescence intensity was quantified using a microplate reader. n = 4 independent samples. P < 0.001. g, h Western blotting for PGAM5, MST3, pMST3T178, YAP and pYAPS127 in NCM460 cells treated with 20 µM CCCP (g) or 30 µM rotenone (h) with or without 100 µM NAC for 2 h. β-Actin was used as the loading control. i Immunofluorescence for GFP-Parkin (green) and mitochondrial targeting probe (red) was examined after NCM460 cells were treated with 30 µM rotenone with or without 100 µM NAC for 2 h. n = 3 independent samples. Scale bar: 25 µm. j Western blotting for PGAM5, PARL, MST3, pMST3T178, YAP and pYAPS127 in NCM460 cells transfected with siPARL for 24 h and treated with 30 µM rotenone for 2 h. α-Tubulin was used as the loading control. k Gross images of xenografted tumors 3 weeks after transplantation of siPGAM5 and/or pcDNA3.1-PARL transfected HCT116 cells. Quantification of tumor weight was performed. n = 6 mice. P = 0.0002; P = 0.0001; P < 0.001. l Western blotting for PARL, PGAM5, MST3, pMST3T178, YAP and pYAPS127 in HCT116 cells transfected with siPGAM5 and/or pcDNA3.1-PARL. β-Actin was used as the loading control. Western blotting images are representative of experiments that were repeated at least three times with similar results. Data are presented as the mean ± SD. ***P < 0.001. Statistical analysis in panels e, f and k was performed with one-way ANOVA followed by Tukey’s test.
CCCP- or rotenone-treated cells showed considerable increase in mtROS levels (Fig. 5d–f). To determine whether mtROS induced by CCCP or rotenone are essential for PGAM5 cleavage, we used N-acetylcysteine (NAC), a membrane permeability antioxidant43, to treat CCCP- or rotenone-exposed cells. NAC inhibited CCCP- or rotenone-induced mtROS production, and this significantly decreased PGAM5 cleavage and suppressed dephosphorylation of MST3 and YAP (Fig. 5d–h). Together, these results suggest that increased mtROS promotes PGAM5 cleavage, which then becomes released into the cytoplasm to dephosphorylate MST3, thereby activating YAP.
mtROS regulates PGAM5 cleavage through PARL protein
mtROS causes loss of mitochondrial membrane potential, which depolarizes mitochondria, and activates mitophagy44,45. During mitophagy, mitochondrial cleavage protein PARL cleaves PGAM515. In agreement with previous reports, we found that rotenone and CCCP treatment impaired mitochondrial membrane potential (Supplementary Figs. 8j–l), induced Parkin translocation to mitochondria, and triggered mitophagy (Figs. 5i and Supplementary Fig. 8m). Although adding antioxidant NAC did not restore mitochondrial membrane potential (Supplementary Fig. 8j–l), it attenuated Parkin translocation to mitochondria (Figs. 5i and Supplementary Fig. 8m), indicating that mitophagy was inhibited. Interestingly, knockdown of PARL attenuated mtROS-induced PGAM5 cleavage and increased phosphorylated forms of MST3 and YAP (Fig. 5j and Supplementary Fig. 8n). Furthermore, PARL overexpression promoted growth of xenografted tumors, relative to control, while knockdown of PGAM5 compromised this promoting effect (Fig. 5k). In line with these phenotypes, PARL overexpression downregulated pMST3 and pYAP levels, but did not change MST3 and YAP activity in the context of PGAM5 knockdown (Fig. 5l). These results demonstrate that mitochondrial ROS signalling, in parallel with activating mitophagy, induces PGAM5 cleavage through PARL, which results in PGAM5-mediated dephosphorylation of MST3 and YAP activation.
PGAM5 promotes CRC progression through its phosphatase activity
To further investigate clinical relevance of PGAM5 in CRC, we first analysed its expression in different types of human CRC tissues from the Cancer Genome Atlas (TCGA) database. PGAM5 mRNA levels were found to be significantly higher in colorectal tumor tissues (Fig. 6a, b and Supplementary Fig. 9a, b). Further analyses of colon adenocarcinoma/adjacent normal tissue pairs from CRC patients in our cohort showed marked upregulation of PGAM5 in human colorectal tumors at both protein and RNA levels (Fig. 6c, d), as well as in AOM-DSS-induced mouse colon tumors (Supplementary Fig. 9c). Furthermore, Western blotting showed that cleaved cytosolic PGAM5 levels were higher in human colorectal tumors compared to their paired peri-tumor tissues (Fig. 6e).
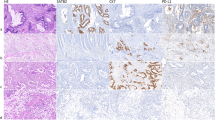
a, b TCGA database analysis showed that PGAM5 expression was upregulated in both COAD (Colon adenocarcinoma, a) and READ (Rectum adenocarcinoma, b). n: sample number of patients per colorectal tumor type. c Representative immunohistochemical images of PGAM5 in human colorectal peri-tumor and tumor tissues from CRC patients. Scores of PGAM5 expression levels were quantified. n = 49. Scale bar: 100 µm. P = 0.0003. d qRT-PCR analysis for PGAM5 in human colorectal peri-tumor (n = 13) and tumor tissues (n = 9) from CRC patients. P = 0.006. e Western blotting for PGAM5 in human colorectal peri-tumor and tumor tissues from CRC patients. β-Actin was used as the loading control. Same blot for β-Actin as shown in Supplementary Fig. 4 g. f Gross images of AOM-DSS tumors and quantification of tumor numbers (P = 0.0008) per mouse and tumor area (P = 0.013) in Ctrl and Pgam5cKO mice. Ctrl: n = 39 tumors from 4 mice; Pgam5cKO: n = 15 tumors from 4 mice. g Double immunofluorescence of Ki67 and β-Catenin in colon tumors from Ctrl and Pgam5cKO mice. n = 50 crypts from 4 mice. Percentage of Ki67+ cells in tumors was quantified. Scale bar: 100 µm. h Gross images of xenografted tumors 4 weeks after transplantation with pcDNA3.1, pcDNA3.1-PGAM5WT or pcDNA3.1-PGAM5H105A–transfected HCT116 cells. Tumor weight was quantified. n = 5 mice. P = 0.0375; P = 0.0001. i Immunofluorescence of EdU in LOVO cells after transfection with pcDNA3.1, pcDNA3.1-PGAM5WT or pcDNA3.1-PGAM5H105A plasmids. The percentage of EdU+ cells was quantified. n = 29 fields from 3 independent samples. Scale bar: 25 µm. P = 0.0001. j Colony formation assay was used to detect the effect of PGAM5 phosphatase activity in LOVO cells after transfection with pcDNA3.1, pcDNA3.1-PGAM5WT or pcDNA3.1-PGAM5H105A plasmids. n = 3 independent samples. k, l Transwell assay was used to detect migration (k, P = 0.0087) and invasion (l, P = 0.0042) of LOVO cells transfected with pcDNA3.1, pcDNA3.1-PGAM5WT or pcDNA3.1-PGAM5H105A plasmids. n = 3 independent samples. Scale bar: 250 µm. Western blotting images are representative of experiments that were repeated at least three times with similar results. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical analysis in panels h, i, k, and l was performed with one-way ANOVA followed by Tukey’s test, and the rest were performed with two-tailed unpaired Student’s t-test.
To assess the in vivo role of PGAM5 in tumorigenesis, we generated VillinCre; Pgam5fl/fl (Pgam5cKO) mice in which Pgam5’s exon 2 was deleted in intestinal epithelium (Supplementary Fig. 9d). Pgam5 deletion significantly decreased the number and size of adenomas in the colon (Fig. 6f). Histological analysis showed significant decrease in neoplastic lesions upon Pgam5 deficiency (Supplementary Fig. 9e), along with decrease in the number of proliferative cells (Fig. 6g and Supplementary Fig. 9f). In contrast, PGAM5 overexpression accelerated growth of subcutaneous xenograft tumors (Fig. 6h), and promoted proliferation (Fig. 6i) and colony formation (Fig. 6j and Supplementary Fig. 9g) in human colon cancer cells. It also significantly promoted growth of APKS tumor organoids (Supplementary Fig. 9h, i). In addition, PGAM5 overexpression significantly promoted migration and invasion of CRC (Fig. 6k, l). However, all of the above promoting effects of PGAM5 could not be achieved by PGAM5H105A mutant, in which phosphatase is inactivated. Furthermore, compared to wild type PGAM5, overexpression of PGAM5△24-29 with disrupted cleavage site, failed to promote growth of xenografted tumors (Supplementary Fig. 9j). Taken together, these findings indicate that cleaved cytosolic PGAM5 promotes CRC progression through its phosphatase activity.
PGAM5 promotes CRC growth by regulating MST3-YAP axis
The above findings demonstrate that released PGAM5 directly dephosphorylates MST3. Next, we sought to test whether MST3 functions as an effective downstream effector, mediating tumor promoting effects of PGAM5 during CRC progression. We generated VillinCre; Mst3fl/fl; Pgam5fl/fl (dKO) mice, in which both Mst3 and Pgam5 were simultaneously deleted in intestinal epithelium, and then induced colon tumors using AOM-DSS protocol. Loss of Pgam5 significantly suppressed tumor growth, while Mst3 deletion reversed this inhibitory effect (Fig. 7a). Consistently, pYAP and pLATS levels were upregulated in tumors from Pgam5cKO mice, while it was reduced in Mst3cKO and dKO mice (Fig. 7b). In agreement with this finding, Ki67+ proliferating cells decreased in tumors from Pgam5cKO mice, while they markedly increased in Mst3cKO and dKO mice, accompanied by an increase in nuclear Yap+ cells (Fig. 7c–e). The results of ex vivo assays showed that knockdown of MST3 partially reversed inhibitory effects on xenografted tumors, growth of APKS tumor organoids and tumor cell proliferation caused by PGAM5 deletion (Fig. 7f, g and Supplementary Fig. 9k, l). Furthermore, silencing PGAM5 led to reduction in YAP activity and nuclear translocation of YAP, while knockdown of MST3 reversed them (Fig. 7h, i and Supplementary Fig. 10a). In contrast, MST3 overexpression inhibited PGAM5 overexpression-induced promoting effects on YAP target genes’ expression and YAP nuclear localization (Figs. 7j, k and Supplementary Fig. 10b). Taken together, these findings indicate that PGAM5 promotes CRC growth by regulating MST3-YAP axis.
a Representative image of AOM-DSS tumors and quantification of tumor numbers per mouse in Ctrl, Mst3cKO (P = 0.026), Pgam5cKO (P = 0.002) and dKO (P = 0.022) mice. n = 3. b Western blotting for Mst3, pMst3T178, Pgam5, Yap, pYapS112, Lats1, pLats1T1079 in colon tumors from Ctrl, Mst3cKO, Pgam5cKO and dKO mice. β-Actin was used as the loading control. c-e Double immunofluorescence of Ki67/β-Catenin or Yap/E-cadherin in colon tumors of Ctrl, Mst3cKO, Pgam5cKO and dKO mice (e). The percentage of Ki67+ cells (c, n = 31 crypts from 3 mice. P = 0.003; P = 0.0001) and nuclear YAP+ cells (d, n = 37 crypts from 3 mice) in tumors were quantified. Scale bar: 100 µm. f Gross images of xenograft tumors 3 weeks after transplantation of shMST3- and/or shPGAM5-transfected HCT116 cells. n = 6. g Growth of APKS mouse tumor organoids after transfection with siMST3 and/or siPGAM5. Organoid areas were quantified at the indicated time points. 12 h, n = 46 fields from 3 independent samples; 96 h, n = 43 fields from 3 independent samples. Scale bar: 250 µm. P < 0.001; P < 0.001; P = 0.024; P < 0.001. h Western blotting for MST3, PGAM5 and Flag in HEK293FT cells transfected with siMST3 and/or siPGAM5 and/or flag-YAP. β-Actin was used as the loading control. i Luciferase assay for TEAD luciferase reporter genes in HEK293FT cells transfected with flag-YAP, followed by siMST3 and/or siPGAM5, respectively. n = 6 independent samples. j Western blotting for MST3 and PGAM5 in HCT116 cells transfected with pcDNA3.1-MST3 and/or pcDNA3.1-PGAM5 plasmids. β-Actin was used as the loading control. k qRT-PCR for YAP, CTGF (P = 0.002; P = 0.021) and CYR61 (P = 0.018; P = 0.025) in HCT116 cells transfected with pcDNA3.1-MST3 and/or pcDNA3.1-PGAM5 plasmids. n = 3 independent samples. Western blotting images are representative of experiments that were repeated at least three times with similar results. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. Statistical analysis in panels (a, g, and i) was performed with one-way ANOVA followed by Tukey’s test, and the rest were performed with two-tailed unpaired Student’s t-test.
Loss-of-function of MST3 promotes PGAM5 cleavage
Interestingly, transcriptomic analysis revealed that Mst3 depletion resulted in downregulation of oxidative phosphorylation in colon tumors (Supplementary Figs. 11a, b). Conversely, MST3 overexpression induced upregulation of oxidative phosphorylation (Supplementary Fig. 11c). This finding suggestes that MST3 might regulate mitochondrial function. Transmission electron microscopy showed that mitochondria were abnormal with loss of matrix density and disrupted cristae in Mst3cKO intestinal epithelial cells (Supplementary Fig. 11d). Furthermore, MST3 overexpression in tumor cells increased oxygen consumption, which is an indirect indicator of mitochondrial oxidative respiration (Supplementary Fig. 11e). Additionally, knockdown of MST3 decreased the number of active mitochondria, whereas MST3 overexpression increased it (Supplementary Fig. 11f). MST3 deficiency also decreased mitochondrial membrane potential (Supplementary Figs. 11g, h). As low mitochondrial membrane potential can induce PGAM5 cleavage15,46, we examined expression of PGAM5 protein. Knockdown of MST3 promoted increase in cleaved form of PGAM5 (Supplementary Fig. 11i), whereas MST3 overexpression caused opposite effect (Supplementary Fig. 11j). These results suggest that loss-of-function of MST3 promotes PGAM5 cleavage by compromising mitochondrial function. Therefore, it appears that, in response to mitochondrial stress, there exists a positive feedback loop between PGAM5 cleavage and MST3 dephosphorylation to activate YAP signalling, ensuring cancer cell survival.
Discussion
In this study, we demonstrate that mtROS induces cleavage of mitochondrial PGAM5 and its aberrant cytosolic accumulation, that in turn activates YAP signalling by dephosphorylating MST3. Consequently, MST3 inactivation enhances PGAM5 cleavage, thereby promoting CRC progression. Our findings reveal previously unappreciated mechanism whereby elevated mtROS promotes CRC progression by activating YAP signalling through a positive feedback loop between mitochondrial PGAM5 and cytoplasmic MST3 (shown in the schematic diagram, Fig. 8).
In normal intestinal epithelial cells (left panel), PGAM5 resides within mitochondria, leading to increase in pMST3. Elevated pMST3 promotes YAP phosphorylation through STK25-LATS axis, and enhances its subsequent ubiquitination and degradation. In colorectal cancer cells (right panel), PGAM5 undergoes cleavage and become released into the cytoplasm by sensing elevated levels of mtROS. Cytosolic PGAM5 dephosphorylates MST3 to activate YAP signalling by suppressing STK25-mediated LATS phosphorylation, which promotes CRC growth and metastasis. In turn, MST3 inactivation gives rise to reduced mitochondrial membrane potential, that induces PGAM5 cleavage. This reveals a positive feedback loop machinery of PGAM5 cleavage and MST3 inactivation in promoting CRC progression.
PGAM5 has been identified as a key regulator for mitochondrial homeostasis12,13. In response to elevated mtROS, the full-length mitochondrial inner membrane protein PGAM5 is cleaved by the mitochondrial cleavage protease PARL. This process can prevent PARL from cleaving PINK1, promoting recruitment of Parkin to PINK1 and inducing mitophagy47,48, while concomitantly, releasing cleaved PGAM5 into the cytoplasm. Here, we found that mtROS-induced cytosolic PGAM5 interacts with MST3 to activate YAP signalling in a positive feedback manner. Indeed, YAP activation is critical for driving proliferation in numerous cancer cells, as well as cellular migration, metastasis, and resistance to therapeutics, and it can also facilitate metabolic transformation to aerobic glycolysis historically associated with tumorigenesis49,50. Considering importance of YAP activation in promoting CRC progression, positive-feedback interaction between cytosolic PGAM5 and MST3 inactivation might represent an important mechanism underlying pro-tumorigenic effects of mtROS. It has been previously reported that phosphorylated MST3 becomes upregulated in HCT116 upon H2O2 treatment19, which contradicts our findings. We posit that difference could be caused by different doses of H2O2. Here, we treated cells with a relatively high dose of H2O2, because it efficiently induces mitophagy and PGAM5 cleavage.
Our findings show that tumor phenotypes of dKO mice are quite similar to these in Mst3cKO mice. This indicates that MST3 is primarily mediator of PGAM5’s pro-tumorigenic function. However, we cannot rule out other possible mechanisms underlying oncogenic role of PGAM5 in CRC. Indeed, it has been reported that cytosolic PGAM5 can bind Axin1 to activate canonical WNT signalling that enhances stemness and proliferative capacity in various epithelial cancer cell types. Thus, it appears that PGAM5 exerts multimodal pro-tumorigenic effects. In addition to PGAM5, IP-mass spectrometry identified other MST3-binding phosphatases such as PPP1CC/PP1C, PPM1N and PPP4C. Interestingly, it has been reported that PP1 family proteins, such as PP1A, can modulate YAP signalling51,52. However, whether these phosphatases could regulate Hippo pathway by dephosphorylating MST3 merits further investigation.
Another striking finding from our data is that MST3 kinase suppresses YAP activity, functioning as tumor suppressor in CRC. In line with this idea, microRNA-222-mediated MST3 suppression promotes migration and invasion of CRC cells53. Consistent with its inhibitory role in YAP signalling, it has been reported that MST3 is able to phosphorylate NDR protein kinase at Thr444/Thr442 to increase its activity54, and that NDR can phosphorylate YAP at Ser127 to suppress YAP activity55. Thus, our findings together with these previous reports support the notion that MST3 function as tumor suppressor in CRC by inactivating YAP signalling. It has long been known that other mammalian MST kinases, MST1/2, are major components of Hippo pathway, and also function as tumor suppressors in CRC56. Thus, it emerges that MST3 and MST1/2 have similar functions in CRC, although they are regulated by distinct upstream signals. It is worth noting that MST3 potentially plays an oncogenic role in gastric cancer and breast cancer57,58, suggesting that it can exert opposite effects depending on the cancer type.
Here we identified a molecular mechanism behind the inhibitory effect of MST3 on YAP activity. We showed that MST3 directly binds to and phosphorylates GCK-III kinase subfamily member STK25, and then activates pSTK25, phosphorylates LATS1 both at S909 and T1079 sites, thereby suppressing YAP activity. In agreement with our findings, previous report predicted that members of GCK-III kinase subfamily can form a heterodimer59. Thus, our findings support the idea that members of GCK-III kinase subfamily function to regulate YAP activity through heterodimers. Notably, two previously papers reported dual roles of STK25 in regulating YAP activity32,60. Consistent with the report by Lim et al.32, our findings support the notion that STK25 suppresses YAP activity by phosphorylating LATS. Interestingly, the finding that STK25 phosphorylates LATS1 both at S909 and T1079 sites is different from Lim et al.’s report that STK25 kinase only phosphorylate LATS1S909. This difference could be due to the fact that this regulation is context-dependent. However, our findings contradict the report by Bae et al.60 claiming that STK25 functions as a component of the STRIPAK complex to activate YAP signalling. The discrepancy could be due to the fact that in our study on colorectal tumor cells, YAP signalling is aberrantly activated with its dysregulated machineries, while Bae et al. primarily studied in 293 A cell line. It is likely that STK25 can either suppress or activate YAP activity, which depends on its specific interacting proteins under distinct conditions. The exact molecular mechanisms underlying these differences merit further investigation.
To summarize, we identified previously unappreciated mechanism by which mtROS promotes growth and metastasis of CRC. After sensing mtROS signals, mitochondrial phosphatase PGAM5 becomes cleaved in its mitochondrial transmembrane domain and becomes released into the cytoplasm. Subsequently, cytosolic PGAM5 binds to MST3 and promotes its dephosphorylation, resulting in reduction of its activity, which in turn activates YAP signalling. Meanwhile, suppression of MST3 in CRC promotes mitochondrial PGAM5 cleavage and production of cytosolic PGAM5, accelerating malignant transformation upon CRC in a positive-feedback manner. Therefore, PGAM5-MST3 interaction might serve as an important mediator pair of mtROS signalling to the cytoplasm, which plays essential role in regulating CRC proliferation and metastasis. Targeting PGAM5-MST3 axis may thus become a potential therapeutic strategy to control CRC pathogenesis.
Methods
Ethics
All animal experimental procedures and protocols were authorized by Beijing Laboratory Animal Management and were performed in strict accordance with the guidelines of the Institutional Animal Care and Use Committee of China Agricultural University (approval number: AW81212202-3-1).
Mice
VillinCre C57BL/6 mice (stock number: T000142) were purchased from the National Model Mouse Resource Center. Mst3fl/fl C57BL/6 mice were obtained from Zhaocai Zhou Laboratory, Fudan University, Shanghai. Pgam5fl/fl C57BL/6 mice were obtained from Moshi Song Laboratory, Institute of Zoology, Chinese Academy of Sciences. Mice were housed under SPF conditions with 22 °C and 14 h light/10 h dark cycle, and fed regular diet.
Patients and clinical specimens
Human colorectal cancer tissue microarrays were purchased from Bioaitech Co., Ltd. (Nos.D100Co01). The detailed information of the microarrays is provided in Supplementary Data 1. Samples of 24 human colorectal cancer tissues and paracancerous tissues and cDNA of 10 human colorectal cancer tissues and paracancerous tissues were collected from the First Affiliated Hospital of Zhengzhou University. Details of the samples are provided in Table S1 and Table S2. Use of clinical samples was approved by the Ethical Committee and Institutional Review Board of The First Affiliated Hospital of Zhengzhou University in compliance with ethical standards and patient confidentiality (approval number: 2021-KY-0918-004). We obtained informed consent from all participants.
Histology, immunohistochemistry, and immunofluorescence
For histological analysis, colon tissues were fixed with 4% paraformaldehyde at room temperature for 24 h, rinsed three times for 15 min each with PBS, and then paraffin-embedded and cut into thin sections (5 µm thick). The sections were baked in an oven at 60 °C, deparaffinized with xylene, and then treated with gradient ethanol. The sections were stained with hematoxylin (Sigma) for 5 min, washed with tap water for 5 min, stained with eosin (Sigma) for another 5 s, dehydrated with gradient ethanol, and finally, sealed.
For immunostaining, after the first gradient ethanol treatment, the samples were kept in distilled water for 5 min, and antigen target repair was performed by heating and boiling in 0.01 M citrate buffer (pH 6.0) for 20 min, followed by cooling to room temperature. Slides were washed three times with 1 × PBS, immersed in 3% H2O2 for 15 min or in PBS-T (PBS, 1% Triton X-100) for 20 min, and then washed three times with 1 × PBS. A blocking solution was used to completely cover the tissue area for 1 h. Primary antibodies were added to the samples and incubated overnight at 4 °C. The primary antibodies were recovered, soaked in PBS-T (PBS, 0.1% Triton X-100) for 5 min, and washed three times using 1 × PBS. Alexa Fluor 488 and 594 goat anti-mouse or anti-rabbit IgG (H + L) were added and incubated for 1 h at room temperature. Tissues were washed three times with 1 × PBS, stained with DAPI for 10 min, and finally covered using an anti-quenching mounting medium. The antibodies used were as follows: anti-MST3 (1:500, Abcam, ab51137, EP1468Y), anti-Ki67 (1:1000, Abcam, ab279653, B56), anti-YAP (1:1000, Cell Signalling Technology, 14074, D8H1X), anti-Cleaved caspase3 (1:500, Cell Signalling Technology, 9661, Asp175), anti-PGAM5 (1:500, Abcam, ab308447, EPR27145-21), anti-β-Catenin (1:250, Abcam, ab32572, E247), anti-β-Catenin (1:500, Santa cruz, sc-53483, 15B8); anti-E-cadherin (1:500, Abcam, ab231303, 4A2) and anti-GFP (1:1000, Abcam, ab290, P42212).
EdU staining was performed using the EdU Cell Proliferation Kit with Alexa Fluor 594 (Beyotime, C0078S), following the manufacturer’s instructions.
Confocal imaging
Cells were grown to a density of about 60% on a circular microscope cover glass (NEST, 801010), the medium was removed, and cells were washed three times with 1 × PBS, and fixed using 4% paraformaldehyde for 30 min. Then, cells were treated with 1% Triton X-100 for 10 min and washed three times with 1 × PBS. Cells were blocked with 5% BSA for 1 h and incubated with primary antibodies overnight at 4 °C. Secondary antibodies were added and incubated for 1 h, DAPI staining was performed for 10 min, and finally cells were blocked using Antifade Mounting Medium (Beyotime, P0126). All images were captured using Leica laser scanning confocal microscope (Leica TCS SP8).
Xenograft tumors
Five-week-old female nude mice (BALB/c nude) were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd., and maintained in pathogen-free conditions. They were randomly assigned to receive a subcutaneous injection of 100 µL of 6.0 × 106 HCT116 cells (1 × PBS as solvent). All mice were sacrificed 3 to 4 weeks after transplantation, and tumor weight and area were measured. All tumors were fixed in 4% paraformaldehyde for paraffin embedding. The maximal tumor size permitted was <20 mm in diameter. Mice were euthanized once tumor size reached 20 mm. In some instances, malignant tumors exhibited explosive growth, potentially exceeding 20 mm. Mice with oversized tumors were euthanized with CO2 immediately upon discovery.
AOM and DSS treatment
8-week-old male mice (C57BL/6) were intraperitoneally injected with AOM (Sigma-Aldrich) at a dose of 10 mg/kg body weight. One week after injection, the mice were administered with 2% DSS (molecular weight: 36,000-50,000, MP Biomedicals) in drinking water for one week, and then, provided normal drinking water for two weeks. Mice were subjected to a total three cycles of DSS. Normal water intake was maintained for 4-6 weeks seven days after the third DSS feeding round. Mice were sacrificed and distal colon tissues were collected to assess the number and area of tumors.
RNA extraction, reverse transcription, and qRT-PCR
Total RNA was extracted from cells, mouse colon tissues, and colon tumors using total RNA extraction reagent TRIzol (Life Technologies). Oligo (dT) primers were used for reverse transcription into cDNA. Then, qRT-PCR (real-time quantitative PCR) was performed using LightCycler 480 SYBR Green I Master Mix, following the manufacturer’s instructions. Relative expression was calculated according to the 2–ΔΔCt method, and Gapdh was used as an internal control. The primers used for qRT-PCR analysis are shown in Table S3.
Cell proliferation assay
Using cell counting plate (ThermoFisher, C10283), cells were seeded in 100 µL of medium at a density of 3000/well in 96-well plates and cultured for the indicated time. Then, 10 µL of luminescent cell viability assay reagent (Beyotime, C0042) was added to each well and incubated at 37 °C for 1 h. The absorbance was measured using a Spark Multimode Microplate Reader (Tecan, Switzerland).
Plasmid construction
The CDS region of full-length MST3 was cloned into the pcDNA3.1 vector with an HA tag at the C-terminus and the pcDNA3.1 vector with a flag tag at the N-terminus. The MST3K53A and MST3T178E plasmids were constructed using the HA-pcDNA3.1 vector and pcDNA3.1 vector. The full-length, truncated (25-289), mutant (H105A) and truncated (△23-29) PGAM5 gene fragments were cloned into the pcDNA3.1 vector with a flag tag. The construct of full-length YAP was cloned into the pcDNA3.1 vector with the flag tag. The bacteria-mediated full-length MST3 protein expression construct was cloned into a modified pGEX-4T-1 vector with a GST tag at the N-terminus. The truncated PGAM5 protein was designed into a pET-30a (+) vector with a C-terminal uncleavable 6 × His tag. shMST3, shPGAM5 and shYAP were subcloned into LV3-GFP vector (Table S5). YAP-TEAD dual luciferase reporter plasmids were donated by the Zhaocai Zhou Laboratory. All mutants were generated by site-directed mutagenesis and validated by DNA sequencing.
Cell culture
HCT116, HEK293FT and LOVO cell lines were purchased from the American Type Culture Collection (ATCC) (Manassas, VA) and cultured in IMDM, DMEM and DMEM/F12 supplemented with 10% Foetal Bovine Serum, respectively. The NCM460 cell line was purchased from Innovative Life Science Solutions (INCELL) (San Antonio, TX), and cultured in 1640 medium supplemented with 10% Foetal Bovine Serum. All cells were grown in 5% CO2 at 37 °C with saturated humidity.
Generation of APKS mouse tumor organoids
The APKS organoids were made by our own laboratory as described in previously published paper61. First, colonic crypts of KrasLSL-G12D mice were isolated and cultured. KrasG12D mutation was then activated by transient transfection of Salk-Cre with pPGK-Puro (Addgene, 11349) plasmid followed by puromycin selection for three days. Then, sgRNAs of APC, P53 and Smad4 were cloned into PX330 plasmid (Addgene, 42230) and transiently transfected into puromycin-selected tumor organoids. One week after transient transfection, tumor-like cells carrying APC, P53, and Smad4 mutations were selected, and each subclone was verified for mutation by PCR and DNA sequencing.
Culture of APKS tumor organoids
Culture medium used for APKS organoids was DMEM/F12 containing 1 mM N-acetyl-cysteine (Sigma, A9165), 1 × B-27 (Gibco, 17504044), 1% Pen Strep (Gibco, 15140122), 1 × N-2 (Gibco, 17502048), 10 mM HEPES (Gibco, 15630080), and 1 × GlutaMAX (Gibco, 35050061). APKS was resuspended in a mixture of medium and Matrigel (Corning, 356231) (1:1), added to a 48-well plate and placed in an incubator at 37 °C for 20 min. After matrigel polymerization, 200 µL of the medium was added to each well. The medium was changed every two days.
Cell transfection
Lipofectamine 2000 reagent (Invitrogen, 11668019) was used following the manufacturer’s protocol. For RNAi-mediated knockdown experiments, 3.5 µL of non-targeting control or targeting siRNAs (Genepharma) were added to one well of a 12-well plate. For plasmid transfection experiments, 1 µg of vector or negative control vector was added to one well in a 12-well plate. Fresh medium was added to the cells 4-6 h after transfection. siRNA sequences are shown in Table S4.
Western blotting analysis
Western blotting analysis was performed according to standard procedures. Fresh tissue was lysed using RIPA buffer (Beyotime Biotechnology, P0013C) with a mixture of protease and phosphatase inhibitor cocktails (Roche). The samples were placed on ice for 30 min, and then, ground with a homogenizer (T10 basic, IKA). Tissue supernatant was collected by high-speed centrifugation, and protein concentration was determined using a BCA protein assay kit (Beyotime Biotechnology, P0009). After cell lysates were denatured by boiling in 5 × SDS loading buffer, total proteins were separated on 6-12% SDS-PAGE gels. This was followed by transfer to PVDF membranes (GE Healthcare), which were blocked with 5% skim milk powder for 1 h at room temperature and incubated overnight at 4 °C with the addition of primary antibodies. Images were acquired using chemiluminescence imaging system (Sagecreation, Beijing). Relative protein band intensities were quantified using the ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). The following antibodies were used: anti-α-Tubulin (1:1000, Beyotime, AF2827), anti-β-Actin (1:1000, Beyotime, AF0003), anti-MST3 (1:5000, Abcam, ab51137, EP1468Y), anti-STK25 (1:2000, Abcam, ab157188, EPR10306), anti-MST3T178 (1:5000, Abcam, ab76579, EP2123Y), anti-YAP (1:1000, Cell Signalling Technology, 14074, D8H1X), anti-pYAPS127 (1:1000, Cell Signalling Technology, 13008, D9W2I), anti-HA-tag (1:1000, Santa Cruz, sc-57592, 12CA5), anti-Flag-tag (1:1000, Cell Signalling Technology, 14793, D6W5B), anti-Myc-tag (1:1000, Immunoway, YN5506), anti-Cyclin D1 (1:2000, Abcam, ab134175, EPR2241), anti-PGAM5 (1:1000, Abcam, ab308447), anti-TOM20 (1:1000, Abcam, ab186735, EPR15581-54), anti-His-tag (1:1000, Abcam, ab18184, HIS.H8), anti-MST1 (1:1000, Cell Signalling Technology, 14946, D8B9Q), anti-MOB1 (1:1000, Cell Signalling Technology, 13730, E1N9D), anti-LATS1 (1:1000, Cell Signalling Technology, 3477, C66B5), anti-LATS2 (1:1000, Cell Signalling Technology, 5888, D83D6), anti-pLATS1T1079 (1:1000, Cell Signalling Technology, 8654, D57D3), anti-pLATS1S909 (1:1000, Cell Signalling Technology, 9157), anti-Histone H3 (1:1000, Cell Signalling, 14269, 1B1B2), and anti-PARL (1:500, Proteintech, 26679-1-AP, 00046721).
Immunoprecipitation (IP)
LOVO cells were co-transfected with plasmids overexpressing HA-14-3-3 and flag-MST3, and cell lysates were incubated with anti-flag antibody (1:50, Cell Signalling Technology, 14793); LOVO cells were co-transfected with Myc-Ub, HA-MST3 and flag-YAP plasmid and cell lysates were incubated with anti-flag antibody (1:50, Cell Signalling Technology, 14793). Endogenous interactions between MST3 and PGAM5 in HCT116 cells or NCM460 cells were detected by anti-MST3 and anti-PGAM5 antibodies. Whole-cell extracts were prepared with a lysis buffer (Beyotime Biotechnology, P0013C), lysed on ice for 10 min, centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was collected. The primary antibodies and protein A/G beads (MedChemExpress, HY-K0202) were added and incubated for 6-8 h and washed five times with washing buffer. Next, 1 × SDS protein denaturant was added, and the mixture was boiled at 98 °C for 10 min. Protein samples were separated on SDS-PAGE gels and examined by Western blotting analysis using the indicated antibodies. The Western blotting images were captured using chemiluminescence imaging system (Sagecreation, Beijing).
Immunoprecipitation-mass spectrometry (IP-MS) assay
HCT116 cells were transfected with either flag-pcDNA3.1 or flag-MST3 for 24 h. After transfection, whole cell extracts were prepared using lysis buffer (Beyotime Biotechnology, P0013C), followed by lysis on ice for 10 min and centrifugation at 4 °C at 12,000 rpm for 10 min to collect the supernatant. 10 µL of Anti-Flag magnetic beads (MedChemExpress, HY-K0207) were added to 500 µL of cell lysate, then incubated overnight at 4 °C. Mixture was placed on a magnetic stand for separation, and supernatant was discarded. 500 µL of washing buffer was added to the magnetic beads obtained from the separation, beads were resuspended thoroughly, separated magnetically, and supernatant was discarded. Washing step was repeated three times until OD280 of the supernatant was less than 0.05. After separation, supernatant was discarded, 50 µL of elution buffer A was added to the magnetic beads, mixed, and incubated at room temperature for 10 min. Magnetic beads were separated, supernatant was collected into a new EP tube, and neutralization buffer was added at a ratio of 25 µL for every 50 µL of elution buffer to adjust the pH of the eluate to neutral.
Protein digestion was performed using FASP method with modifications (Nature Methods 6, 359–362 (2009)). Briefly, 100 µg protein was dissolved with 50 mM ABC (NH4HCO3), reduced with DTT (dithiothreitol) at 56 °C for 45 min, and alkylated with IAM (iodoacetamide) at room temperature for 30 min in the dark. Solution was transferred into a 10 K ultrafiltration tube (Vivacon 500, Satrorius), spinned at 14,000 g for 20 min. 50 mM ABC solution was used to wash the protein for 3 times. 2 µg trypsin in 50 µL 50 mM ABC was added and incubated at 37 °C overnight. Ultrafiltration tube was spinned at 14,000 g for 20 min with a new tube to collect digested peptides. ABC solution was added into ultrafiltration tube to wash digested peptide into the collection tube. Collected solution was diluted with 0.1% FA for nanoLC-MS analysis.
NanoLC separation was achieved with a Waters (Milford, MA, USA) nanoAcquity nanoHPLC. Trap column was Thermo Acclaim PepMap 100 (75 µm × 2 mm, C18, 3 µm). Analytical column was homemade with 100 µm I.D. fused silica capillary (Polymicro) filled with 20 cm of C18 stationary phase (Phenomenex, Aqua 3 µm C18 125 A). Gradient elution program was used, with mobile phase increases linearly from 1% B to 35% B in 65 min. Mobile phase A: 0.1% FA in water, B: 0.1% FA in acetonitrile. Nanospray ESI-MS was performed on a Thermo Q-Exactive high resolution mass spectrometer (Thermo Scientific, Waltham, MA, USA). With 70000 MS scan resolution and 17500 MSMS scan resolution and top-10 MS/MS selection. Raw data from the mass spectrometer were preprocessed with Mascot Distiller 2.7 for peak picking.
RNA-Seq analysis
After AOM-DSS-induced colon adenoma formation in mice, tumor cells were isolated from colon tumors of four Mst3cKO mice and three littermate control mice. Total RNA was isolated from collected intestinal tumor cells using TRIzol reagent following the manufacturer’s instructions. RNA samples were sent to Novogene Co., Ltd. for library preparation and sequencing on the Illumina NovaSeq 6000 platform. All data were statistically analysed online using the NovoMagic data analysis cloud platform (www.magic.novogene.com) or the R software. RNA-Seq data were submitted to the GEO repository under accession number GSE248431.
HCT116 cells were treated with flag-pcDNA3.1 empty vector or flag-pcDNA3.1-MST3 for 24 h, and RNA was extracted following standard procedures. RNA samples were sent to Novogene Co., Ltd. for library preparation and sequencing using the Illumina NovaSeq 6000 platform. All data were statistically analysed online using the NovoMagic data analysis cloud platform (www.magic.novogene.com) or the R software. RNA-Seq data were submitted to the GEO repository under accession number GSE249865.
Anchorage-independent growth
HCT116 cells were used for cell counting, and cell density was adjusted to 1 × 105/mL with IMDM supplemented with 20% FBS. Then, 1.2% agarose and 2 × IMEM medium were mixed at a 1:1 ratio, placed in 6 cm dishes, cooled, and solidified. After mixing 0.7% agarose with 2 × IMEM medium in a 1:1 ratio in a sterile test tube, 0.2 mL of cell suspension was added to the tube, thoroughly mixed, and placed on the dish. After solidification, the cells were placed in a 37 °C incubator containing 5% CO2 for 10 to 14 days.
Mitochondrial protein isolation
CCCP-treated NCM460 cells were washed once with cold PBS, digested with trypsin, centrifuged, and resuspended in cold PBS. Samples were centrifuged at 800 rpm for 6 min. Cytoplasmic and mitochondrial proteins were isolated from harvested cells following manufacturer’s instructions using cytoplasmic and mitochondrial protein extraction kit (Beyotime, C3601). Protein concentration was determined using a BCA kit (Beyotime, P0011).
Luciferase assay for TEAD promoter activity
TEAD-luciferase reporter plasmids were transfected into HEK293FT cells and HCT116 cells. Luciferase activity was measured after 24 h using dual luciferase assay system (Promega).
Nuclear/cytoplasmic protein extraction
Nuclear proteins were isolated from cells using nuclear protein extraction kit (Beyotime, P0027), following manufacturer’s instructions. Briefly, cells were transfected for 24 h and washed once with cold PBS. Samples were centrifuged at 600 V × g for 5 min. Then, they were resuspended in cytosolic protein extraction reagent A and incubated on ice for 15 min to lyse cell membranes. Cytosolic protein extraction reagent B was added, followed by centrifugation at 16,000 × g for 5 min. Supernatant contained cytoplasmic protein. Remaining fraction was resuspended with nucleoprotein extraction reagent, incubated on ice for 30 min, and centrifuged at 16,000 × g for 10 min. Supernatant contained extracted nucleoprotein. Protein concentration was determined using BCA Kit (Beyotime, P0009).
Protein purification
Transformed His-tagged proteins were expressed in Rosetta competent cells, and protein expression was induced by adding 0.2 mM IPTG to LB medium, followed by induction at 18 °C for 12 h at low temperature. Cells were collected by centrifugation at 4,000 rpm for 10 min at 4 °C and resuspended in lysis buffer (20 mM HEPES, pH 7.5, 2 M NaCl, 5 mM β-Me Buffer). Bacterial solution was mixed with 0.1% (v/v) Triton X-100, 1 mM PMSF, and 10 mM imidazole, and bacterial cells were crushed by sonication. Cell debris were removed by centrifugation at 18,300 rpm for 50 min. Supernatant was collected and filtered using a 0.45 µm filter membrane. Filtrate was poured into Ni affinity chromatography column (Thermo Fisher, 25217) with column volume of 2 mL, and filtered fluid was collected. Ni affinity column was rinsed with lysis buffer, eluted with lysis buffer supplemented with 200 mM imidazole, and eluate was collected. For GST-labelled proteins, protein supernatant was poured into GST affinity chromatography column (Glutathione Sepharose 4 Fast Flow) with column volume of 2 mL; the process was repeated thrice, and filtrate was collected.
GST pull-down assay
GST beads (50 µL) were added to a 1.5 mL EP tube to bind to the GST fusion protein, and indicated proteins were added and incubated for 2 h at 4 °C in a buffer containing 20 mM HEPES (pH 7.5), 500 mM NaCl, and 0.1% Triton X-100. Samples were centrifuged at 4,000 g for 5 min at 4 °C to remove supernatant, and process was repeated five times. Supernatant was removed by centrifugation to remove proteins not bound to GST fusion protein. Protein samples were separated on SDS-PAGE gels and detected by Coomassie brilliant blue staining or Western blotting using indicated antibodies.
In vitro dephosphorylation assay
Purified MST3 protein first underwent autophosphorylation. Then, purified PGAM5 protein, PGAM5H105A protein or truncated PGAM5 (25-289) were added, respectively, and mixed with dephosphorylation buffer (20 mM HEPES, pH 7.5, 10 mM MgCl2, 150 mM NaCl, 1 mM DTT, Cocktail). Mixture was incubated at 30 °C for 35 min, and then, MST3 residual phosphorylation was determined by Western blotting analysis using specific antibodies.
Migration assay and invasion assay
Migration and invasion assays were performed using Transwell chamber (Corning, 3422). For migration assays, transfected cells were seeded into the upper chamber with medium containing 1% FBS (2 × 104 cells), while bottom of the chamber contained DMEM medium with 20% FBS. For invasion assays, chamber was coated with Matrigel (Biolead, 356234), and subsequent steps were similar to the migration assay. After cells migrated or invaded for 24 h, they were fixed and stained with crystal violet (Sigma, V5265). Migrated and invaded LOVO cells or HCT116 cells were counted under an inverted light microscope.
Wound healing assay
HCT116 cells were transfected with NC/siMST3 or pcDNA3.1/pcDNA3.1-MST3 for 36 h. 1 mL sterile plastic tip was used to create a wound line across cells. Cells were cultured in 1% serum DMEM medium containing mitomycin (1 µg/ml) and placed in humidified 5% CO2 incubator at 37 °C for indicated timepoints, and images were taken by phase contrast microscopy. Each assay was triplicated.
Cecal injection and IVIS imaging
First, 2 × 106 HCT116 cells carrying the luciferase GFP vector were injected into the semitransparent subserosal layer of the 5-week-old female NOG mice cecum. After 5-6 weeks, cecal tumors and liver metastases were detected by tracking the luciferase signal in vivo using an IVIS Luciferase imaging System 200 (Xenogen). Finally, mice were dissected to count the number of liver metastatic tumors.
Statistical analysis
Histological analysis was performed in a blinded fashion. At least three mice were included in each experimental group. For cell experiments, each group contained at least three independent biological replicates. Western blotting images, immunohistochemical staining images, and immunofluorescence images are representative of experiments that were repeated at least three times with similar results. All data are presented as mean ± standard deviation (SD). The statistical significance of differences was evaluated by paired or unpaired two-tailed Student’s t-test or one-way ANOVA followed by Tukey’s test. Asterisks denote statistical significance (*P < 0.05; **P < 0.01; ***P < 0.001).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw fastq files for RNA-seq from this study have been submitted to the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE248431 and GSE249865. Protein mass spectrometry raw data generated in this study have been deposited in the Integrated Proteome Resources database under accession code PXD050513 [https://www.iprox.cn/]. Source data are provided within this article. Remaining data are available within the article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66, 683–691 (2017).
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73, 233–254 (2023).
Song, I. S. et al. FOXM1-induced PRX3 regulates stemness and survival of colon cancer cells via maintenance of mitochondrial function. Gastroenterology 149, 1006–1016 (2015).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Chuang, C. H. et al. Altered mitochondria functionality defines a metastatic cell state in lung cancer and creates in exploitable vulnerability. Cancer Res. 81, 567–579 (2021).
Radisky, D. C. et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 (2005).
Payen, V. L., Zampieri, L. X., Porporato, P. E. & Sonveaux, P. Pro- and antitumor effects of mitochondrial reactive oxygen species. Cancer Metastasis Rev. 38, 189–203 (2019).
Sabharwal, S. S. & Schumacker, P. T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14, 709–721 (2014).
Elchuri, S. et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24, 367–380 (2005).
Wang, Z. G., Jiang, H., Chen, S., Du, F. H. & Wang, X. D. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243 (2012).
Chen, G. et al. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol. Cell 54, 362–377 (2014).
Imai, Y. et al. The loss of PGAM5 suppresses the mitochondrial degeneration caused by inactivation of PINK1 in drosophila. Plos Genet. 6, e1001229 (2010).
Sugo, M. et al. Syntaxin 17 regulates the localization and function of PGAM5 in mitochondrial division and mitophagy. Embo J. 37, e98899 (2018).
Sekine, S. et al. Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J. Biol. Chem. 287, 34635–34645 (2012).
Rauschenberger, V. et al. The phosphatase Pgam5 antagonizes Wnt/beta-catenin signaling in embryonic anterior-posterior axis patterning. Development 144, 2234–2247 (2017).
Denk, D. et al. Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity 55, 2059–2073.e2058 (2022).
Schinkmann, K. & Blenis, J. Cloning and characterization of a human STE20-like protein kinase with unusual cofactor requirements. J. Biol. Chem. 272, 28695–28703 (1997).
Chen, C. B., Ng, J. K., Choo, P. H., Wu, W. & Porter, A. G. Mammalian sterile 20-like kinase 3 (MST3) mediates oxidative-stress-induced cell death by modulating JNK activation. Biosci. Rep. 29, 405–415 (2009).
Lin, C. Y., Wu, H. Y., Wang, P. L. & Yuan, C. J. Mammalian Ste20-like protein kinase 3 induces a caspase-independent apoptotic pathway. Int J. Biochem Cell B 42, 98–105 (2010).
Desgrosellier, J. S. et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med 15, 1163–1169 (2009).
Huang, C. Y. et al. Caspase activation of mammalian sterile 20-like kinase 3 (Mst3). nuclear translocation and induction of apoptosis. J. Biol. Chem. 277, 34367–34374 (2002).
Lu, T. J. et al. Inhibition of cell migration by autophosphorylated mammalian sterile 20-like kinase 3 (MST3) involves paxillin and protein-tyrosine phosphatase-PEST. J. Biol. Chem. 281, 38405–38417 (2006).
Zhang, L. & Bu, P. Generation of an orthotopic mouse model to study colorectal cancer metastasis. STAR Protoc. 2, 100792 (2021).
Dong, J. X. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Lei, Q. Y. et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell Biol. 28, 2426–2436 (2008).
Misra, J. R. & Irvine, K. D. The hippo signaling network and its biological functions. Annu. Rev. Genet. 52, 65–87 (2018).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Perra, A. et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J. Hepatol. 61, 1088–1096 (2014).
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Zheng, Y. & Pan, D. The hippo signaling pathway in development and disease. Dev. Cell 50, 264–282 (2019).
Lim, S. et al. Identification of the kinase STK25 as an upstream activator of LATS signaling. Nat. Commun. 10, 1547 (2019).
Driskill, J. H. & Pan, D. The hippo pathway in liver homeostasis and pathophysiology. Annu Rev. Pathol. 16, 299–322 (2021).
Basu, S., Totty, N. F., Irwin, M. S., Sudol, M. & Downward, J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 (2003).
Takeda, K. et al. Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. P Natl Acad. Sci. USA 106, 12301–12305 (2009).
Lo, S. C. & Hannink, M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp. Cell Res. 314, 1789–1803 (2008).
Yamaguchi, A. et al. Cleaved PGAM5 is released from mitochondria depending on proteasome-mediated rupture of the outer mitochondrial membrane during mitophagy. J. Biochem. 165, 19–25 (2019).
Martinez-Reyes, I. & Chandel, N. S. Cancer metabolism: looking forward. Nat. Rev. Cancer 21, 669–680 (2021).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Li, N. Y. et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525 (2003).
Xiao, B. et al. Superoxide drives progression of Parkin/PINK1-dependent mitophagy following translocation of Parkin to mitochondria. Cell Death Dis. 8, e3097 (2017).
Zhou, B. et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res 28, 1171–1185 (2018).
Halasi, M. et al. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem J. 454, 201–208 (2013).
Zeb, A. et al. A novel role of KEAP1/PGAM5 complex: ROS sensor for inducing mitophagy. Redox Biol. 48, 102186 (2021).
Sun, N. et al. Measuring in vivo mitophagy. Mol. Cell 60, 685–696 (2015).
Wai, T. et al. The membrane scaffold SLP2 anchors a proteolytic hub in mitochondria containing PARL and the i-AAA protease YME1L. EMBO Rep. 17, 1844–1856 (2016).
Deas, E. et al. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum. Mol. Genet 20, 867–879 (2011).
Sekine, S. PINK1 import regulation at a crossroad of mitochondrial fate: the molecular mechanisms of PINK1 import. J. Biochem 167, 217–224 (2020).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015).
Cox, A. G. et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat. Cell Biol. 18, 886–896 (2016).
Wang, P. et al. PP1A-mediated dephosphorylation positively regulates YAP2 activity. Plos One 6, e24288 (2011).
Emami, S. S., Zhang, D. R. & Yang, X. L. Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis. Cancers 12, 2438 (2020).
Luo, F. et al. microRNA-222 promotes colorectal cancer cell migration and invasion by targeting MST3. Febs Open Bio 9, 901–913 (2019).
Stegert, M. R., Hergovich, A., Tamaskovic, R., Bichsel, S. J. & Hemmings, B. A. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol. Cell Biol. 25, 11019–11029 (2005).
Zhang, L. et al. NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr. Biol. 25, 296–305 (2015).
Cheung, P. et al. Regenerative reprogramming of the intestinal stem cell state via hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell 27, 590–604 e599 (2020).
Lee, K. T. et al. The oncogenic role of MST3 in human gastric cancer. Am. J. Cancer Res 8, 2130–2139 (2018).
Cho, C. Y. et al. MST3 promotes proliferation and tumorigenicity through the VAV2/Rac1 signal axis in breast cancer. Oncotarget 7, 14586–14604 (2016).
Ceccarelli, D. F. et al. CCM3/PDCD10 heterodimerizes with germinal center kinase III (GCKIII) proteins using a mechanism analogous to CCM3 homodimerization. J. Biol. Chem. 286, 25056–25064 (2011).
Bae, S. J., Ni, L. & Luo, X. STK25 suppresses Hippo signaling by regulating SAV1-STRIPAK antagonism. Elife 9, e54863 (2020).
Yang, X. et al. Identifying the E2F3-MEX3A-KLF4 signaling axis that sustains cancer cells in undifferentiated and proliferative state. Theranostics 12, 6865–6882 (2022).
Acknowledgements
We thank the members of the laboratory animal center in China Agricultural University for their assistance in animal care. We appreciate the assistance provided by the biological mass spectrometry laboratory from College of Biological Sciences at China Agricultural University on our mass spectrometry experiment. This study was supported by grants from the National Key Research and Development Program of China 2022YFC3602102 (C.L.), 2022YFA1104001 (Z.Y.), 2022YFD1300403 (C.L.), and 2021YFF1000603 (Z.Y.), the National Natural Science Foundation of China 82025006, 82230017, 82341224 (to Z.Y.), 82270588 (to C.L.), 12090052, U24A2014 (to J.S.), the Pinduoduo-China Agricultural University Research Fund PC2023A02006 (Z.Y.), the 2115 Talent Development Program of China Agricultural University (Z.Y.).
Author information
Authors and Affiliations
Contributions
S.W., Z.Y., C.L. and L.Y. designed research; S.W., X.W., W.B., J.X., L.H., G.L., Y.P., H.Z. and M.L. performed research; S.D., M.Z., D.L., S.J., X.S., Y.T., J.S., M.V.P., M.S., Z.Z., L.Y., C.L. and Z.Y. analyzed data; S.W., M.V.P., L.Y., C.L. and Z.Y. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhigao Wang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Wu, X., Bi, W. et al. ROS-induced cytosolic release of mitochondrial PGAM5 promotes colorectal cancer progression by interacting with MST3. Nat Commun 16, 1406 (2025). https://doi.org/10.1038/s41467-025-56444-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56444-2
This article is cited by
-
Mitochondrial metabolic reprogramming in colorectal cancer: mechanisms of resistance and future clinical interventions
Cell Death Discovery (2025)
-
6-Methoxydihydrosanguinarine Suppresses the Proliferation of Non-small Cell Lung Cancer Cells through Elevation of ROS and Activation of IRE1/JNK Signaling
Cell Biochemistry and Biophysics (2025)
-
Constructing a mitochondrial-related genes model based on machine learning for predicting the prognosis and therapeutic effect in colorectal cancer
Discover Oncology (2025)