Abstract
Anti-epidermal growth factor receptor (EGFR) therapy (cetuximab) shows a limited clinical benefit for patients with locally advanced or recurrent/metastatic head and neck squamous cell carcinoma (HNSCC), due to the frequent occurrence of secondary resistance mechanisms. Here we report that cetuximab-resistant HNSCC cells display a peroxisome proliferator-activated receptor alpha (PPARα)-mediated lipid metabolism reprogramming, with increased fatty acid uptake and oxidation capacities, while glycolysis is not modified. This metabolic shift makes cetuximab-resistant HNSCC cells particularly sensitive to a pharmacological inhibition of either carnitine palmitoyltransferase 1A (CPT1A) or PPARα in 3D spheroids and tumor xenografts in mice. Importantly, the PPARα-related gene signature, in human clinical datasets, correlates with lower response to anti-EGFR therapy and poor survival in HNSCC patients, thereby validating its clinical relevance. This study points out lipid metabolism rewiring as a non-genetic resistance-causing mechanism in HNSCC that may be therapeutically targeted to overcome acquired resistance to anti-EGFR therapy.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) afflicts about 700,000 patients annually and is the sixth most common cancer worldwide1. HNSCC is often diagnosed at late stages, with loco-regionally advanced diseases for which treatment remains a clinical challenge2,3. Indeed, despite aggressive multimodal therapeutic interventions including surgery, radiation therapy with or without concomitant chemotherapy, more than half of HNSCC patients experience loco-regional or distant relapse4. While immunotherapy has recently been implemented for patients with recurrent/metastatic HNSCC exhibiting programmed cell death ligand 1 (PD-L1) expression5, anti-epidermal growth factor receptor (EGFR) therapy remains the standard of care for PD-L1-negative advanced HNSCC as well as one therapeutic option for PD-L1-positive tumors that progress after immunotherapy treatment6. EGFR overexpression is observed in 90% of HNSCC7 and it is associated with poor prognosis and resistance to radiation therapy and chemotherapy8,9. However, the clinical efficacy of anti-EGFR monoclonal antibodies (e.g. cetuximab) is strongly limited by either intrinsic resistance or the development of acquired drug resistance after an initial tumor response, and most patients relapse within few months. Moreover, no validated treatment options exist for the patients who progress after anti-EGFR therapy, indicating an unmet clinical need. Unlike other cancer types (e.g. colorectal cancers with RAS/BRAF mutations or lung cancers with EGFR mutations), alterations in genes from the EGFR-regulated signaling pathways are extremely rare in HNSCC and they have not been clearly associated with the clinical response to anti-EGFR therapy10,11,12. In this context, understanding resistance-supporting non-genetic causes is a critical challenge to improve the outcome of HNSCC patients receiving anti-EGFR targeted therapy. To date, several mechanisms of acquired resistance to cetuximab have already been identified in HNSCC patients13,14. They are very diverse, likely due to the inter-patient heterogeneity as well as the influence of the tumor microenvironment and anatomic location15.
Dysregulated cellular metabolism is a common hallmark of cancer, and it has emerged as an important factor that contributes to disease progression and clinical relapse in cancer patients, including HNSCC16,17. Although it is well established that metabolic preferences in cancer cells can continuously evolve to fulfil their bioenergetic and biosynthetic needs and to support their growth and survival when facing hostile conditions18,19, including therapy-induced stress20, metabolic liabilities that may be therapeutically exploited to improve the response to anti-EGFR therapy have not been clearly identified yet in HNSCC. In this study, we identified major alterations in lipid metabolism, including increased fatty acid (FA) uptake and oxidation, upon acquired resistance to cetuximab in HNSCC cells. We documented that impeding peroxisome proliferator-activated receptor alpha (PPARα)-dependent FA utilization could overcome cetuximab resistance in vitro and in vivo, thereby paving the way for the development of new metabolism-interfering strategies in the clinical management of therapy-refractory HNSCC patients.
Results
Acquired resistance to anti-EGFR therapy in HNSCC cells is associated with major expression changes for metabolism-related genes and proteins
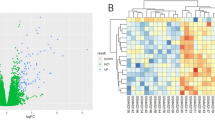
To study the metabolic adaptation of HNSCC cells upon acquired resistance to cetuximab treatment, we used three couples of human HNSCC cell lines (FaDu, SC263 and SCC22b), initially cetuximab-sensitive (denoted as -S), and for which resistant (-R) populations had been previously established upon chronic exposure for several weeks with the drug21,22,23. We first validated the therapy-resistant phenotype for the different cell models in 2D and 3D culture conditions (Fig. 1a, b and Supplementary Fig. 1a–c) upon treatment with 1 µg/mL cetuximab, a drug concentration shown to inhibit efficiently ligand-induced EGFR phosphorylation (Supplementary Fig. 1d) and to induce a maximal growth-inhibitory effect in HNSCC cells (Supplementary Fig. 1e). Importantly, whole exome sequencing (WES) did not reveal any major alteration (i.e. copy number variation or mutation) either for EGFR signaling-related or metabolism-regulating genes in cetuximab-resistant HNSCC cells (Supplementary Data 1–4). Moreover, we observed that cetuximab-resistant HNSCC cells could return to a drug-sensitive state after drug withdrawal for several weeks (Supplementary Fig. 1f–k), thereby supporting the existence of non-genetic reversible mechanisms of acquired resistance to anti-EGFR therapy in HNSCC cells. Instead, transcriptomic and proteomic analyses allowed us to reveal hundreds of genes and proteins, respectively, being differentially expressed between SCC22b and SC263-S versus -R cells (Fig. 1c). Gene set enrichment analysis identified amino acid, nucleotide, glucose and lipid metabolism as some of the most differentially enriched metabolic processes in cetuximab-resistant HNSCC cells (Supplementary Fig. 1l, m). Virtual Inference of Protein-activity by Enriched Regulon (VIPER) analysis was then performed to infer virtual protein-activity (VPA) differences from gene expression data between cetuximab-sensitive and -resistant HNSCC cells. The VIPER algorithm allows computational inference of protein activity, on an individual sample basis, from gene expression profile data24. It uses the expression of genes that are most directly regulated by a given protein, such as the targets of a transcription factor, as an accurate reporter of its activity. VIPER estimates regulon activity by taking into account the regulator mode of action, the regulator-target gene interaction confidence and the pleiotropic nature of each target gene regulation. Target genes that are most directly affected by a protein’s activity are identified by maximization of information transfer over all alternative paths24. In total, 994 proteins were predicted to be differentially activated at a nominal p-value of 10%, among which 26 (3%; primary seeds) displayed consistent VPA and protein expression differences between the HNSCC cell populations (Supplementary Fig. 1n). Moreover, 37 proteins, referred to as metabolic seeds, exhibited significant differences in VPA scores and/or expression levels and were shown to be involved in either FA metabolism, glutamate/glutamine metabolism or glycolysis. Interaction network analysis was performed from the 62 seed proteins (i.e. primary and metabolic seeds) and allowed the identification of an additional set of 161 secondary proteins (OR = 2.722; p < 0.001). The resulting graph was subjected to Louvain clustering and 6 network clusters were identified. Cluster enrichment patterns for primary seeds, metabolic seeds and secondary proteins were investigated. Enrichment analysis revealed that both clusters 1 and 6 were characterized by an increased number of metabolic seed proteins (Fig. 1d). Finally, pathway enrichment analysis confirmed the involvement of cluster 1 in metabolic processes, including glucose, amino acid and lipid metabolism, with a strong enrichment for mitochondrial fatty acid (FA) oxidation (FAO) and electron transport chain activity (Fig. 1e–g), while cluster 6 appeared to be primarily involved in RNA metabolism, thereby suggesting profound changes in the metabolism of cetuximab-resistant HNSCC cells.
Growth of cetuximab-sensitive (-S) and -resistant (-R) SCC22b (a) and SC263 cells (b) after treatment with 1 µg/mL cetuximab (CTX) for 96 h (N = 3, n = 6). c General workflow of the integration of SCC22b-S/-R and SC263-S/-R proteomic analysis with a microarray-DNA data-based inference of protein activity by VIPER algorithm. Created in BioRender. https://BioRender.com/d17s016. d Heatmap showing the –log10 transformed enrichment p–values of different gene sets, indicated along the Y-axis, in each of the six identified network clusters (X-axis). A white color reflects a p-value of 0.05, hence all red colors indicate significant interactions. e Dot plot showing –log10 transformed enrichment p-values (X-axis) of gene sets (Y-axis) in clusters 1 (top panel) and 6 (bottom panel) of the protein-protein interaction network. Each dot is color-coded according to the enrichment odds ratio (OR). Small black dots indicate the enrichment p-value of that gene set in the entire network, in contrast to the cluster specific enrichment. A p-value of 0.05 is indicated using a vertical dashed line. f Minimal spanning tree representation of the protein-protein interaction network of cluster 1. Proteins in red and blue are respectively up- and downregulated in cetuximab-resistant HNSCC cells. The shape of the nodes reflects the protein class (i.e. circles=metabolic seeds, triangles=non-metabolic seeds). Grey nodes are intermediate proteins, that were identified using the shortest-paths algorithm and that are necessary to connect all proteins in the network. g Bar plot showing the node degree distribution of all proteins in the network of cluster 1. The node degree, being the number of connections each protein has in the network, is shown in the X-axis, the different proteins are listed along the Y-axis. Grey bars reflect intermediate proteins. Data are plotted as the means ± SEM (a, b). N indicates the number of independent biological experiments and n indicates the number of technical replicates. Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (a, b). ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
Glycolytic activity is not changed in HNSCC cells upon acquired resistance to anti-EGFR therapy
Since cetuximab was shown to exert its antitumor activity, at least partly, via the inhibition of HIF1-mediated glycolysis in cancer cells25,26,27, we first explored potential changes in glucose metabolism in HNSCC cells upon acquired resistance to anti-EGFR therapy. We incubated cetuximab-sensitive and -resistant HNSCC cells in a culture medium containing 10 mM glucose and assessed extracellular glucose and lactate levels at different timings (0-6-12-18-24 h). Surprisingly, we found that glucose consumption and lactate secretion were not significantly different in cetuximab-sensitive and -resistant HNSCC cells (Fig. 2a, b and Supplementary Fig. 2a). Similarly, we observed that glucose-induced extracellular acidification rate (ECAR), determined by Seahorse-based bioenergetics analysis in HNSCC cells before and after treatment with 10 mM glucose, was not modified upon acquired resistance to cetuximab (Fig. 2c and Supplementary Fig. 2b) Then, we checked the potential growth-inhibitory effects of 10 mM 2-deoxyglucose (2-DG), a concentration found to efficiently inhibit glycolysis in our different HNSCC cell models (Supplementary Fig. 2c–h). After 72 h of treatment with the metabolic inhibitor, a dramatic decrease of viability was observed for all HNSCC cells, without any significant difference between cetuximab-sensitive and -resistant cell populations (Fig. 2d, e and Supplementary Fig. 2i). Finally, injection of uniformly labelled 13C-glucose and subsequent detection of 13C-lactate (Fig. 2f) and 13C-succinate (Supplementary Fig. 2j) by nuclear magnetic resonance in 2D, 3D and ex vivo cell culture models did not reveal any difference between cetuximab-sensitive and -resistant HNSCC cells. Altogether, these data provide evidence that acquired resistance to anti-EGFR therapy is not associated with major changes in glucose metabolism in HNSCC cells.
Glucose and lactate concentrations, at different timings, in the extracellular media from cetuximab-sensitive (-S) and -resistant (-R) FaDu (a) and SC263 (b) cells initially incubated at t0 in a medium containing 10 mM glucose (N = 3, n = 3). c Glucose-dependent extracellular acidification rate (ECAR) in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3, n = 6). Growth of cetuximab-sensitive (-S) and -resistant (-R) FaDu (d) and SC263 cells (e) after treatment with 10 mM 2-deoxyglucose (2-DG) for 72 h (N = 3, n = 3). f 13C-lactate signal, detected by nuclear magnetic resonance, in 2D (N = 4), 3D N = 4) and ex vivo cultures (N = 6) of cetuximab-sensitive (-S) and -resistant (-R) FaDu cells after incubation with U-13C-glucose for 24 h. Data are plotted as the means ± SEM (a–f). N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (c–f). ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
Cetuximab resistance is associated with increased fatty acid uptake and oxidation in HNSCC cells
According to the increasingly recognized contribution of mitochondrial metabolism in therapy resistance for a variety of cancer types28,29, we assessed mitochondrial activity in cetuximab-resistant HNSCC cells. We did not observe any change for neither mitochondrial membrane potential (Δψm) (Supplementary Fig. 3a) nor mitochondrial respiration upon incubation with a substrate-rich medium (i.e. containing 10 mM glucose, 2 mM glutamine and 50 µM BSA-conjugated palmitic acid) (Supplementary Fig. 3b). Instead, when incubating HNSCC cells with single bioenergetic substrates, we revealed that cetuximab-resistant HNSCC cells had increased rates of FAO in glucose- and glutamine-free media (Fig. 3a, b and Supplementary Fig. 3c), while glucose- and glutamine-dependent oxygen consumption rates (OCR) were unchanged between the different cell populations. This was associated with a significantly higher capacity to take up exogenous FA, as shown upon incubation with a fluorescently labelled palmitate (BODIPY-C16) (Fig. 3c and Supplementary Fig. 3d, e) as well as an increased intracellular abundance of FAcyl-CoA (i.e. palmitoyl-CoA, palmitoleyl-CoA, oleyl-CoA and stearoyl-CoA) in cetuximab-resistant HNSCC cells, as detected by LC-MS (Fig. 3d). The scavenger receptor CD36, one of the main FA membrane transporters, was found to be upregulated in cetuximab-resistant HNSCC cells, at both mRNA and protein levels (Fig. 3e, f and Supplementary Fig. 3f, g). Genetic invalidation of CD36, upon transfection with specific siRNA sequences, significantly decreased BODIPY-C16 uptake (Fig. 3g and Supplementary Fig. 3h–j) and reduced FAO rates (Supplementary Fig. 3k, l) in cetuximab-resistant HNSCC cells. Interestingly, the mitochondria-localized carnitine palmitoyltransferase 1A (CPT1A), a key rate-limiting enzyme of FAO, was also overexpressed in cetuximab-resistant HNSCC cells (Fig. 3h, i and Supplementary Fig. 3m, n). Importantly, while CPT1 inactivation, upon either treatment for 3 h with 100 µM etomoxir, an irreversible CPT1 inhibitor, or siRNA-based genetic silencing, significantly impaired FAO in both cetuximab-sensitive and -resistant HNSCC cell populations (Fig. 3j and Supplementary Fig. 3o–s), it only reduced intracellular levels of acetyl-CoA in the latter (Fig. 3k and Supplementary Fig. 3t), thereby highlighting the role of FAO to support bioenergetic demands in HNSCC cells upon acquired resistance to anti-EGFR therapy. We also observed an increase of the inactivating phosphorylation of acetyl-CoA carboxylase (ACC) (Supplementary Fig. 3u), preventing the production of malonyl-CoA, which is involved in FA elongation, but that also acts as a negative regulator of FAO by impeding the transport of fatty acyl-CoA into mitochondria via CPT1 allosteric inhibition. Additionally, although expression of the fatty acid synthase (FASN), the rate-limiting enzyme of the FA synthesis pathway, was not altered in cetuximab-resistant HNSCC cells (Supplementary Fig. 3u), its pharmacological inhibition, with 5-(tetradecyloxy)-2-furoic acid (TOFA), exhibited significantly less toxicity in FaDu-R and SC263-R cells than in the cetuximab-sensitive cell populations (Supplementary Fig. 3v, w), indicating a lower dependency on FA synthesis in the cetuximab-resistant cells. Finally, by exploring the protein-protein interaction network described above to identify potential upstream regulators of CPT1A, ACC, and FASN, that were differentially expressed and/or activated in cetuximab-resistant HNSCC cells, we showed that ACTB, COPB1, CPT1A, HIF1A, IDH1, NDRG1, SREBF1, and TFAM may be master regulators of metabolic shifts during acquisition of cetuximab resistance (Fig. 3l).
Oxygen consumption rate (OCR) of cetuximab-sensitive (-S) and -resistant (-R) FaDu (a) and SC263 cells (b) in media containing either glucose (Glc), glutamine (Gln), or palmitate (PA) (N = 3, n = 6). c Quantification for BODIPY FL C16 uptake in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3). d Intracellular abundance of FAcyl-CoA (i.e. palmitoyl-CoA, palmitoleyl-CoA, oleyl-CoA, stearoyl-CoA) in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3). mRNA expression (e) and representative immunoblotting (f) for CD36 in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3). g Quantification for BODIPY FL C16 uptake in cetuximab-sensitive (-S) and -resistant (-R) FaDu cells upon transfection of siRNA against CD36 gene for 72 h (N = 2). mRNA expression (h) and representative immunoblotting (i) for CPT1A in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3, n = 3). j Palmitate-dependent OCR in cetuximab-sensitive (-s) and -resistant (-R) FaDu cells upon treatment with 100 µM etomoxir for 30 min (N = 3, n = 6). k Intracellular abundance of acetyl-CoA in cetuximab-sensitive (-S) and -resistant (-R) FaDu cells upon treatment with 100 µM etomoxir for 3 h (N = 3). l Heatmap showing the upstream regulators (X-axis) of ACACA, CPT1A and FASN (Y-axis) in the protein-protein interaction network identified in Fig. 1. Upstream regulators directly interact with any of the proteins of interest (i.e. network distance of 1) and are differentially expressed or activated between cetuximab-resistant and -sensitive HNSCC cells. The direction and level of differential activation and/or repression is color-coded as shown in the legend underneath the heatmap. Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (a–e, h and j, k). P-values as indicated or ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
Increased stearoyl-CoA desaturase activity prevents lipotoxicity in cetuximab-resistant HNSCC cells
Gas chromatography-flame ionization detection (GC-FID)-based analysis was then carried out to reveal potential changes in the abundance and in the nature of FA fractions (i.e. neutral lipids (NL), phospholipids (PL) and free fatty acids (FFA)) in HNSCC cells upon acquired resistance to anti-EGFR therapy. No major difference was observed in the abundance of FA, either at the total levels or when considering each fraction (NL, PL and FFA) individually, between cetuximab-sensitive and -resistant HNSCC cells (Fig. 4a and Supplementary Fig. 4a–d). Instead, significant changes were found in the composition of the intracellular pool of FA, with an increased proportion of monounsaturated FA (MUFA), at the detriment of saturated FA (SFA) (Fig. 4b). Upon acute treatment with 50 µM BSA-conjugated palmitic acid for 6 h, we observed a greater increase in the total FA pool in cetuximab-resistant HNSCC cells, mostly reflecting a higher abundance within the NL fraction (Fig. 4c, d), thereby suggesting that part of the exogenous FA, that are taken up, can be stored as neutral lipids in cetuximab-resistant HNSCC cells. In particular, the ratio between palmitoleic acid (C16:1cis9)/oleic acid (C18:1cis9) and palmitic acid (PA; C16:0)/stearic acid (C18:0), denoted as the Δ9 desaturation index, was higher in cetuximab-resistant HNSCC cells (Fig. 4e and Supplementary Fig. 4e). Expression of stearoyl-CoA desaturase (SCD) enzymes SCD1 and SCD5, the two FA Δ9-desaturases reported to convert SFA into MUFA in humans, was upregulated in cetuximab-resistant HNSCC cells at both mRNA and protein levels (Fig. 4f, g and Supplementary Fig. 4f–h). Pharmacological inhibition of SCD1 was found to alter the global FA profile, with a higher proportion of SFA (and PUFA at a lesser extent), at the detriment of MUFA in both cetuximab-sensitive and -resistant HNSCC cells (Fig. 4h, i). This altered FA profile was mostly found in NL and PL fractions while the FFA fraction was barely modified (Fig. 4j, k and Supplementary Fig. 4i–l). Importantly, when SCD1 activity was pharmacologically inhibited, an excess of extracellular PA (100 µM) led to greater toxicity in cetuximab-resistant HNSCC cells cultured either in 2D or 3D conditions (Fig. 4l and Supplementary Fig. 4m, n), thereby suggesting that enhanced FA uptake in cetuximab-resistant HNSCC cells is accompanied by increased Δ9 desaturase activity to prevent further lipotoxicity.
Intracellular abundance of total FA (a) and relative proportion of SFA, MUFA and PUFA (b) in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3). Intracellular FA abundance in neutral lipids (NL), free FA (FFA) and phospholipids (PL) fractions in cetuximab-sensitive (-S) and -resistant (-R) FaDu (c) and SC263 cells (d) upon treatment with 50 µM BSA-conjugated palmitate (PA) for 6 h (N = 3). e Δ9 FA desaturation index in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3). Representative immunoblotting (f) and mRNA expression (g) for SCD1 and SCD5 in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3, n = 3). Relative abundance of saturated FA (SFA), monounsaturated FA (MUFA) and polyunsaturated FA (PUFA) in FaDu and SC263 cells, either at total levels (h, i) or in the NL fraction ( j, k) after treatment with 40 µM A939572 for 24 h (N = 3). l Growth of cetuximab-sensitive (-S) and -resistant (-R) FaDu cells after treatment with 40 µM A939572 (SCD1 inhibitor) with or without the addition of 100 µM PA for 72 h (N = 3, n = 6). Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (a, b, e, and g–l). P-values as indicated or ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
Cetuximab-resistant HNSCC cells rely on the availability and utilization of exogenous FA for their growth in vitro and in vivo
We then assessed the contribution of FA metabolism to sustain the growth of HNSCC cells upon acquired resistance to cetuximab. First, we showed that genetic invalidation of CD36 led to specific growth-inhibitory effects in cetuximab-resistant HNSCC cells (Fig. 5a and Supplementary Fig. 5a). Moreover, incubation for 72 h in a medium supplemented with a delipidated FBS re-sensitized cetuximab-resistant HNSCC cells to anti-EGFR therapy in both 2D and 3D conditions (Fig. 5b, c and Supplementary Fig. 5b). Medium supplementation with a chemically defined lipid concentrate, containing a combination of saturated and unsaturated FA and cholesterol, was able to rescue cell growth under cetuximab treatment (Fig. 5d, e). Interestingly, we showed that addition of bovine serum albumin (BSA)-conjugated oleate and palmitate (50 µM of each FA) was already sufficient to induce similar protective effects against cetuximab in HNSCC cells (Fig. 5d, e). Importantly, we also showed that the absence of lipids in the extracellular medium of cetuximab-sensitive HNSCC cells reduced their capacity to resume growth when chronically exposed to 1 µg/mL cetuximab (Fig. 5f, g and Supplementary Fig. 5c–f), thereby suggesting that the presence of exogenous lipids may facilitate the emergence/selection of HNSCC cell phenotypes prone to resist anti-EGFR therapy. Since CPT1A was found to be upregulated in cetuximab-resistant HNSCC cells (see Fig. 3h, i and Supplementary Fig. 3l, m), we also evaluated the effect of CPT1 inhibition/silencing on HNSCC cell viability. Pharmacological blockade of CPT1 with 100 µM etomoxir showed greater growth-inhibitory effects on cetuximab-resistant HNSCC cells (vs cetuximab-sensitive parental cell counterparts) in 2D and 3D conditions (Fig. 5h, i); similar effects were observed upon siRNA-based CPT1A gene silencing (Supplementary Fig. 5g), thereby minimizing the risk of major off-target effects and data misinterpretation upon etomoxir treatment. Finally, CPT1 inhibition was shown to re-sensitize in vivo tumor xenografts from FaDu-R and SCC22b-R cells to anti-EGFR therapy (Fig. 5j and Supplementary Fig. 5h), as tumor volume was reduced, upon a combinatory treatment with cetuximab and etomoxir, independently from mouse body weight loss (Supplementary Fig. 5i, j).
a Growth of cetuximab-sensitive (-S) and resistant (-R) FaDu cells upon transfection of siRNA against CD36 for 72 h (N = 3, n = 3). Growth of cetuximab-resistant (-R) FaDu (b) and SC263 cells (c) upon treatment with 1 µg/ml cetuximab for 72 h, in a medium supplemented with normal or delipidated FBS (N = 3, n = 3). Growth of FaDu-R (d) and SC263-R cells (e) upon treatment with 1 µg/ml cetuximab for 72 h, in a delipidated FBS-containing medium supplemented with 1% chemically defined lipid concentrate (LC) or a mix of BSA-conjugated palmitate and oleate (50 µM of each FA) (N = 3, n = 6). Growth of cetuximab-sensitive FaDu cells upon incubation up to 6 weeks with 1 µg/mL cetuximab in full or lipid-depleted culture medium (f) and comparison of cell viability at 1 week and 6 weeks of cetuximab treatment (g) (N = 2, n = 3). Growth of cetuximab-sensitive (-S) and -resistant (-R) FaDu cells in 2D (h) and 3D conditions (i) upon treatment with 100 µM etomoxir for 3 or 14 days respectively (N = 3, n = 3). j Volume at day 17 of in vivo tumor xenografts from FaDu-R cells in nude mice daily treated with 40 mg/kg etomoxir (or DMSO as vehicle) alone or in combination with 30 mg/kg cetuximab (or 0.9% NaCl as vehicle) (N = 6). Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (a–e, h and j). P-values as indicated or ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
A PPARα-dependent transcriptional program governs lipid metabolism rewiring in cetuximab-resistant HNSCC cells
Transcriptomic analysis revealed the enrichment of a gene signature associated with the activity of peroxisome proliferator-activated receptors (PPARs) in cetuximab-resistant HNSCC cells (Fig. 6a). By carrying out specific DNA-binding assays on nuclear extracts, we observed that PPARα, but not PPARγ, was more active in cetuximab-resistant HNSCC cells (Fig. 6b and Supplementary Fig. 6a). Furthermore, the nuclear (active) fraction of PPARα was more abundant in cetuximab-resistant HNSCC cells (Fig. 6c), while no change was observed for PPARγ (Supplementary Fig. 6b) as revealed by immunofluorescence. To test the functional relevance of PPARα in the therapy-resistant phenotype of HNSCC cells, we first treated cetuximab-sensitive HNSCC cells with pemafibrate, a selective PPARα agonist. We validated a metabolic switch towards increased FA oxidation (associated with an increased expression of CD36 and CPT1A genes) upon treatment for 48 h with 20 µM pemafibrate in those cells (Fig. 6d, e and Supplementary Fig. 6c–e). Importantly, we also showed that pemafibrate-treated HNSCC cells became less responsive to cetuximab (Fig. 6f, g), thereby suggesting that the availability of exogenous FA and the PPARα-mediated metabolic switch towards enhanced FA utilization may be sufficient to induce resistance to anti-EGFR therapy in HNSCC cells. Oppositely, pharmacological inhibition or genetic invalidation of PPARα transcription factor upon treatment with 10 µM GW6471 for 24 h or transfection of specific siRNA sequences for 72 h respectively, abolished the overexpression of CD36 and CPT1A (Fig. 6h) and reduced the palmitate-dependent mitochondrial respiration in cetuximab-resistant HNSCC cells (Fig. 6i and Supplementary Fig. 6f). Importantly, we showed that PPARα activity blockade induced growth-inhibitory effects in cetuximab-resistant HNSCC cells, while barely impacting the viability of cetuximab-sensitive cells (Fig. 6j, k and Supplementary Fig. 6g). Of note, inhibition of PPARγ isoform, upon treatment with GW9662, reduced the growth of both cell populations to a similar extent (Supplementary Fig. 6h). Again, the specific role of PPARα (vs other isoforms such as PPARγ and PPARδ) to support cetuximab-resistant HNSCC cell viability was validated upon siRNA-based silencing of each gene (Supplementary Fig. 6i–o). Finally, we assessed the therapeutic potential of PPARα targeting in 3D cell cultures and in vivo tumor xenografts of cetuximab-resistant HNSCC cells. We showed, in these experimental conditions, that GW6471 could resensitize cetuximab-resistant HNSCC models to anti-EGFR therapy, while having no or only a slight effect when administered as a monotherapy (Fig. 6l–n). Importantly, no major change of mouse body weight was observed upon treatment with GW6471 (Supplementary Fig. 6p, q), thereby validating the overall safety and therapeutic potential of such PPARα antagonist to overcome cetuximab resistance in HNSCC patients.
a Bar plot showing –log10 transformed enrichment p-values (X-axis) of PPAR-associated gene sets (Y-axis) between cetuximab-sensitive and resistant SCC22b and SC263 cells. A dashed vertical line indicates a p-value of 0.05. PPARα DNA-binding activity (b) and nuclear localization (c) in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 2, n = 2). mRNA expression for CD36 (d) and palmitate-dependent OCR (e) in cetuximab-sensitive (-S) FaDu and SC263 cells upon treatment with 20 µM pemafibrate for 48 h (N = 3, n = 3). Growth of cetuximab-sensitive (-S) FaDu (f) and SC263 cells (g) upon treatment with 20 µM pemafibrate for 48 h and then with 1 µg/mL cetuximab for 72 h (N = 3, n = 6). mRNA expression for CD36 and CPT1A (h) and palmitate-dependent OCR (i) in cetuximab-resistant (-R) FaDu and SC263 cells upon treatment with 10 µM GW6471 for 24 h (N = 3, n = 3). Growth of cetuximab-sensitive (-S) and -resistant (-R) FaDu (j) and SC263 cells (k) upon treatment with 10 µM GW6471 for 72 h (N = 3, n = 6). l Follow-up of spheroid growth from cetuximab-sensitive (-S) and -resistant (-R) FaDu cells upon treatment with 10 µM GW6471 for 14 days (N = 3, n = 6). Volume at days 20 or 25 of in vivo tumor xenografts from cetuximab-resistant (-R) FaDu (m) and SCC22b cells (n), respectively, in nude mice daily treated with 20 mg/kg GW6471 (or DMSO as vehicle) alone or in combination with 30 mg/kg cetuximab (or 0.9% NaCl as vehicle) (N = 6). Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (c–k and m, n). P-values as indicated or ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
An active crosstalk between EGFR signaling and lipid metabolism reprogramming supports acquired resistance to cetuximab in HNSCC
To better understand the role of lipid metabolism reprogramming as a non-genetic mechanism supporting acquired resistance to cetuximab in HNSCC cells, we first tested the potential reversibility of the metabolic phenotype by culturing cetuximab-resistant HNSCC cells in a drug-free medium up to 6 weeks. At the different timings, we assessed the cell viability upon treatment for 72 h with etomoxir and GW6471. We observed a loss of sensitivity to FA metabolism inhibitors upon cetuximab withdrawal (Fig. 7a, b and Supplementary Fig. 7a), and this was associated with a reduced CPT1A protein expression (Fig. 7c). Then, when assessing EGFR expression levels under basal culture conditions, we did not reveal any major change of expression for total EGFR, at both transcript and protein levels, between the different cell lines (Fig. 7d, e and Supplementary Fig. 7b, c). Nevertheless, FACS analyses showed that expression levels of surface-localized EGFR were decreased in cetuximab-resistant HNSCC cells (Fig. 7f, g and Supplementary Fig. 7d, e). Interestingly, FA metabolism inhibition (upon either growth in lipid-depleted medium or treatment with PPARα inhibitor GW6471) was able to rescue EGFR surface levels at a similar extent than in cetuximab-sensitive HNSCC cells (Fig. 7f, g and Supplementary Fig. 7d, e), concomitantly with re-sensitization to EGFR inhibitor treatment (Fig. 7h and Supplementary Fig. 7f). These data suggest that dysregulated lipid metabolism may support resistance to anti-EGFR therapy by triggering EGFR internalization, thereby making the receptor less targetable by cetuximab. Finally, to address whether expression of lipid metabolism-related genes (CD36 and CPT1A) is dependent on EGFR signaling, we performed qRT-PCR analyses on cetuximab-sensitive and -resistant HNSCC cells upon treatment with several pharmacological inhibitors interfering with either EGFR kinase activity (gefitinib, afatinib), PI3K activity (buparlisib) or MEK activity (selumetinib). The analyses revealed that gene expression, for both CPT1A and CD36, was significantly reduced in cetuximab-resistant HNSCC cells upon EGFR signaling inhibition (Fig. 7i, j and Supplementary Fig. 7g, h). More precisely, inhibition of MEK1/2 with selumetinib (2 µM, 24 h) seemed to be more effective than PI3K inhibition (with 2 µM buparlisib for 24 h) to decrease mRNA levels for CPT1A and CD36. Altogether, these data suggest the existence of an active crosstalk between EGFR signaling and lipid metabolism in HNSCC cells upon acquired resistance to cetuximab.
Growth of cetuximab-resistant (-R) FaDu (a) and SC263 cells (b) upon 5 weeks of drug withdrawal (w/d) from the routine culture medium and retreatment with 10 µM GW6471 or 100 µM etomoxir for 72 h. As a comparison, cetuximab-treated parental cell populations (-S) are also indicated (N = 2, n = 3). c Representative immunoblotting for CPT1A in cetuximab-resistant FaDu and SC263 cells upon 5 weeks of drug withdrawal (w/d). mRNA expression (d) and representative immunoblotting (e) for EGFR in cetuximab-sensitive (-S) and -resistant (-R) FaDu and SC263 cells (N = 3, n = 3). Expression levels of surface-localized EGFR in cetuximab-sensitive (-S) and -resistant (-R) FaDu (f) and SC263 cells (g) upon treatment with 10 µM GW6471 or incubation in lipid-depleted medium for 72 h (N = 2). h Growth of cetuximab-resistant FaDu cells, pre-exposed to 10 µM GW6471 or lipid-depleted medium for 72 h, before treatment with 1 µg/mL cetuximab for 72 h (N = 2, n = 3). mRNA expression for CPT1A in cetuximab-sensitive (-S) and -resistant (-R) FaDu (i) and SC263 cells (j) upon treatment with gefitinib, afatinib, selumetinib or buparlisib (2 µM each, for 24 h) (N = 3, n = 3). Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). The samples in c derive from the same experiment but different gels for CPT1A were processed in parallel for the 2 cell lines. The samples in e derive from the same experiment and one gel for EGFR was run. Significance was determined by two-way ANOVA with Sidák’s multiple comparison test (d and i, j). ***P < 0.001; ns not significant. Source data are provided as a Source Data file.
PPARα-dependent FA metabolism dysregulation correlates with cetuximab resistance in patient-derived HNSCC models
We explored the clinical relevance of our findings by using patient-derived xenograft (PDX) models previously established in our lab from treatment-naive HNSCC patients30,31 (Fig. 8a). UCLHN4 and HNC004 PDX models showed tumor regression and stabilization, for at least 10 weeks, under weekly treatment with 30 mg/kg cetuximab (Fig. 8b and Supplementary Fig. 8a). Resistance to cetuximab was induced in vivo by treating PDX-bearing mice with the drug until tumors reached 200% ±10% of their initial size (Fig. 8c and Supplementary Fig. 8b). Importantly, WES did not identify any reliable resistance-causing genetic alteration in cetuximab-resistant PDX models (Supplementary Data 5, 6). Bulk RNA sequencing was then carried out to identify gene expression changes in treatment-naive and cetuximab-resistant UCLHN4 PDX models. After data filtering to remove murine reads, 268 and 309 genes were found to be up- and down-regulated (FDR < 0.05), respectively, in cetuximab-resistant tumors (Supplementary Fig. 8c). Gene set enrichment analysis revealed an upregulation of several pathways related to lipid metabolism and oxidative metabolism, including a PPAR-mediated gene signature, in cetuximab-resistant UCLHN4 tumors (Fig. 8d, e and Supplementary Fig. 8d, e). Among this signature, the FA transporter CD36 was found to be upregulated at mRNA levels in both cetuximab-resistant UCLHN4 and HNC004 PDX models (Fig. 8f and Supplementary Fig. 8f) as well as at the protein levels, as revealed by immunohistochemical analysis on PDX tissue sections (Fig. 8g, h and Supplementary Fig. 8g, h). Importantly, we also observed that combinatory treatment with etomoxir or GW6471 and cetuximab led to a greater reduction of tumor growth (vs anti-EGFR therapy alone) in cetuximab-resistant UCLHN4 PDX mice (Fig. 8i, j). Altogether these data, obtained in preclinical patient-derived tumor models, highlight the exacerbated PPARα-dependent FA metabolism as a general mechanism of resistance to anti-EGFR therapy in HNSCC and pave the way for the application of PPARα antagonists or FAO inhibitors to overcome cetuximab resistance in HNSCC patients. In order to validate the clinical relevance of our metabolic signature, we first interrogated publicly available human transcriptomic datasets from HNSCC patients. More precisely, we sorted the data of 475 HNSCC patients from a pan-cancer analysis of The Cancer Genome Atlas32 according to the overexpression of genes whose transcription is dependent on PPARα activity, including genes involved in FA uptake and intracellular processing (CD36, ACSL1, ACSL3, ACSL5), mitochondrial and peroxisomal transport and β-oxidation (CPT1A, CRAT, ACADL, ECHDC2, DBI, ACOX1) as well as PDK4 and SCD. Patients with a tumor displaying an increased “PPARα signature” (n = 245 patients) experienced a median overall survival (mOS) of 42.4 months compared to 68.5 months for the other patients (n = 230 patients) (log-rank test p = 0.019) (Supplementary Fig. 8i).
a Schematic representation for the establishment of cetuximab-sensitive and -resistant patient-derived xenograft (PDX) models from treatment-naive HNSCC clinical specimens. Created in BioRender. https://BioRender.com/b56r875. Tumor growth of cetuximab-sensitive (b) and -resistant (c) UCLHN4 PDX in nude mice weekly treated with 30 mg/kg cetuximab or vehicle. Xenografts were considered resistant when tumors reached 200% ±10% of their initial size. d, e Individual GSEA plots of FA metabolism-related pathways in RNA-seq data from cetuximab-sensitive and -resistant UCLHN4 PDX (N = 3 for each condition). f mRNA expression for CD36 in cetuximab-sensitive and -resistant UCLHN4 PDX (N = 3). Representative tumor slides (g) and quantification (h) for anti-CD36 immunohistochemistry on tissue sections from cetuximab-sensitive and -resistant UCLHN4 PDX. Scale bar: 100 µm (N = 4). Follow-up of tumor growth (i) and relative tumor size at day 15 (j) for cetuximab-resistant UCLHN4 PDX in nude mice treated with 30 mg/kg cetuximab in combination with either 40 mg/kg etomoxir or 20 mg/kg GW6471. k Schematic representation for the clinical workflow of the window-of-opportunity study with HNSCC patients treated for 14 days with cetuximab. 18FDG-PET scan was carried out before and after treatment and enabled to discriminate between partial metabolic response (PMR) and stable metabolic disease (SMD). Created in BioRender. https://BioRender.com/o74h220. l GSEA plot of the PPARα-related gene signature in RNA-seq data from cetuximab-treated HNSCC patients (SMD vs PMR) (N = 15). Data are plotted as the means ± SEM. N indicates the number of independent biological experiments and n indicates the number of technical replicates (when >1). Significance was determined by two-tailed unpaired Student’s t-test (h), one-way ANOVA with Dunnett’s multiple comparison test (j) or two-way ANOVA with Sidák’s multiple comparison test (f). P-values as indicated or ***P < 0.001. Source data are provided as a Source Data file.
Finally, to address whether the metabolic gene signature is clinically relevant for anti-EGFR therapy resistance, we exploited RNA-sequencing and 18FDG-PET data collected in a previous window-of-opportunity clinical study in our lab33. In this study, treatment-naive HNSCC patients were treated for 14 days with cetuximab (loading dose of 400 mg/m2 on first administration followed by 250 mg/m2/week for the second and third infusions, if applicable). Analysis of RNA-sequencing and 18FDG-PET data obtained before and after cetuximab treatment allowed us to show an upregulation of the PPARα-dependent gene signature in patients with stable metabolic disease (SMD) but not in patients with partial metabolic response (PMR) (with metabolic response being determined thanks to the delta maximal standardized uptake values (ΔSUVmax) from PET-scan before and after cetuximab treatment) (Fig. 8k, l). Interestingly, by analyzing RNA-sequencing and 18FDG-PET data obtained in a recent window-opportunity clinical study in our lab34, before and after treatment with afatinib (a pan-HER family tyrosine kinase inhibitor; 40 mg 1×/day for 14 days) in treatment-naive HNSCC patients, we also observed an upregulated PPARα-dependent gene signature specifically in post-treatment tumor samples from patients with SMD, but not PMR (Supplementary Fig 8j, k). Altogether, these clinical data strongly suggest that an upregulation of a PPARα-dependent gene signature is tightly associated with resistance to anti-EGFR therapy in HNSCC patients.
Discussion
In the current era of personalized medicine, therapeutic resistance remains a critical hurdle in our ambition for curative cancer treatment35,36. For patients with advanced-stage HNSCC, anti-EGFR targeted therapy can lead to tumor regression, but the effect is often temporary, and the emergence of drug-resistant cells almost invariably leads to clinical relapse, still making the overall prognosis dismal37. Despite a massive amount of data from next-generation sequencing-based genetic profiling of HNSCC38,39,40,41, no reliable genetic alteration has been clearly associated with an acquired resistance to cetuximab and it has not modified yet the routine clinical care of patients with recurrent or metastatic HNSCC42,43,44. In this study, we aimed to tackle the issue of acquired resistance to anti-EGFR therapy in HNSCC through a specific metabolic angle. Indeed, rewiring of cellular metabolism has emerged as an adaptive response to targeted therapy in cancer, thereby creating a new field of investigation for the development of novel anticancer agents with the potential to overcome therapeutic resistance45,46,47. Like most aggressive tumors, HNSCC exhibit a high rate of glycolysis to meet their metabolic demands48,49, with a strong correlation between elevated levels of tumor-derived lactate and high risk of metastatic spread in primary HNSCC50. Here, we showed that, despite some changes of expression for genes involved in glucose metabolism, the overall glycolytic activity was unchanged upon acquired resistance to cetuximab both in vitro and in vivo. These data are in line with previous studies that documented no significant modification of either 2′-deoxy-2′-[18F]fluoro-D-glucose positron emission tomography signal31 or hyperpolarized 13C-pyruvate NMR spectroscopy signal51 in cetuximab-resistant HNSCC PDX models. Instead, we reported a profound dysregulation of FA metabolism in both in vitro and in vivo cetuximab-resistant HNSCC models. These data are reminiscent of other studies documenting that, during acquisition of resistance to targeted agents, metabolic rewiring of cancer cells favors reliance on exogenous FA uptake and oxidation, sometimes over de novo FA synthesis52,53. Importantly, while phosphorylation (inhibition) of ACC was shown in all cetuximab-resistant HNSCC models used in our study, a compensatory increase in total ACC, as reported elsewhere54,55, was only clearly observed in SCC22b-R cells. Moreover, although de novo lipogenesis has not been directly assessed in the current study, we did not show any change in the intracellular FAcyl-CoA abundance, thereby suggesting that increased uptake of exogenous FA could anyway compensate a potential inhibition of FA synthesis in cetuximab-resistant HNSCC cells.
Mitochondrial FAO has been shown to generate a large quantity of acetyl-CoA, which may support gene expression changes epigenetically by driving histone acetylation56. Moreover, a recent study has documented a role for FAO in resistance to chemotherapy-induced apoptosis by promoting mitochondrial activity through upregulation of phospholipid levels in mitochondrial membranes57. Also, palmitoylation of EGFR has been described to influence its cellular distribution and to induce persistent signaling, in acquired tyrosine kinase inhibitor-resistant EGFR mutant non-small cell lung cancer cells58. Finally, a pre-existing CD36+ cell subpopulation has been identified to support metastasis initiation in human oral carcinomas59. These rare pre-existing clones might be selected by anti-EGFR therapy, thereby leading to tumor repopulation and recurrence, as reported in other studies60,61.
Here, our original multi-omics analysis, then validated in patient-derived HNSCC models, revealed a critical role for PPARα and downstream targets CD36 and CPT1 in the development of resistance to EGFR-targeting therapy as well as their potential as therapeutic targets in HNSCC. This is reminiscent of another study reporting the upregulation of a PPAR-related gene signature in metastatic colorectal cancers not responding to cetuximab62, although there was no indication of the isoform being involved in this context. Our data give even more credibility to the clinical use of selective PPARα antagonists, such as TPST-1120 that is currently tested in a phase 1/1b clinical study (NCT03829436) to investigate its safety, tolerability and anti-tumor activity as monotherapy and in combination with immunotherapy, in subjects with advanced solid tumors, including HNSCC63.
In conclusion, our study positions the rewiring of FA metabolism associated with acquired resistance to anti-EGFR in HNSCC cells as a druggable target that may be exploited through novel therapeutic avenues, including dietary interventions64,65, in order to fulfil the current clinical need for patients with advanced HNSCC.
Methods
Ethical statement
This research complies with all relevant ethical regulations. Experiments involving mouse xenografts received the approval of the ethics committee from UCLouvain (approval ID 2022/UCL/MD/024 and 2020/UCL/MD/032) and were carried out according to national care regulations.
Cell culture
HPV-negative human HNSCC cell lines SC263 and SCC22b were kindly provided by Prof. Sandra Nuyts (University Hospital Leuven, Leuven, Belgium) and Prof. Olivier De Wever (Laboratory of Experimental Cancer Research, Ghent University Hospital, Ghent, Belgium). Human hypopharyngeal FaDu cells were purchased from ATCC (#HTB-43). Cell lines were stored according to the supplier’s instructions and used within 6 months after resuscitation of frozen aliquots. All cells were cultured in Dulbeccos’s modified Eagle’s medium (DMEM) containing 25 mM glucose and 4 mM GlutaMAX (#61965026, Thermo Fisher Scientific), supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin (Thermo Fisher Scientific) and maintained in exponential growth in 5% CO2/95% air in a humidified incubator at 37 °C. Cetuximab-resistant HNSCC cell populations (FaDu-R, SC263-R and SCC22b-R) were generated by chronically exposing the parental cetuximab-sensitive cell lines to cetuximab until resistance ensued, as previously described21,22,23, and then routinely cultured under continuous treatment with 15-30 µg/mL cetuximab (#L01XC06; Merck). All cell lines were tested for mycoplasma contamination with the PCR-based MycoplasmaCheck service from Eurofins Genomics.
2D cell culture treatment and transfection
Cell treatment was performed in routine culture medium with 2-deoxy-glucose (#D8375; Sigma-Aldrich), 5-(tetradecyloxy)-2-furoic acid (#T6575; Sigma-Aldrich), A939572 (#HY-50709: MedChemExpress), afatinib (#HY-10261; MedChemExpress), buparlisib (#S2247; Selleckchem), etomoxir (E1905; Sigma-Aldrich), gefitinib (#HY-50895; MedChemExpress), GW6471 (G5045; Sigma-Aldrich), GW9662 (M6191; Sigma-Aldrich), pemafibrate (#E2876; Selleckchem), and selumetinib (#HY-50706; MedChemExpress), at different timings and concentrations, as indicated in the figure legends. In some conditions, cells were maintained in a medium supplemented with 10% delipidated FBS (Biowest) in presence or not of 1% chemically defined lipid concentrate (Thermo Fisher Scientific) or BSA-conjugated oleic and palmitic acids (Larodan). Cell growth was assessed either by direct cell counting on a hemocytometer with Trypan Blue exclusion dye or by using the Presto Blue reagent (Thermo Fisher Scientific) according to manufacturer’s instructions. For immunoblotting experiments, HNSCC cells were serum-starved for 4 h before incubation with 1 µg/mL cetuximab for 2 h and 20 nM epidermal growth factor (# 78006.1; STEMCELL Technologies) for 15 min.
Cell transfection with 20 nM of either a non-targeting pool of 4 siRNA sequences (D-001810-10-05) or a pool of 4 siRNA sequences targeting human CD36 (L-010206-00-0005), human CPT1A (L-009749-00-0005), human EGFR (L-003114-00-0005), human PPARA (L-003434-00-0005), human PPARD (L-003435-00-0005), human PPARG (L-003436-00-0005), all from Horizon Discovery, was carried out with Lipofectamine™ RNAiMAX transfection reagent (#13778150; Thermo Fisher Scientific), according to manufacturer’s instructions.
3D spheroid cultures
Spheroids were prepared with cetuximab-sensitive and-resistant FaDu cells by seeding 1000 cells/well in 96-well ultra-low attachment plates (Corning) in DMEM supplemented with 10% heat-inactivated FBS (or 10% delipidated FBS in some specific conditions), 25 mM D-glucose and 2 mM L-glutamine. Spheroids were treated with either 1 µg/mL cetuximab, 100 µM etomoxir, or 10 µM GW6471 3 days after seeding (day 0). Afterwards, the treatment was renewed every 2-3 days. Spheroid growth was monitored using live-cell phase contrast microscope (Axio Observer, Zeiss) and spheroid area was measured by using Fiji software66. Alternatively, cell viability (as an endpoint) was directly assessed by using the Cell Titer Glo 3D reagent (Promega) according to manufacturer’s instructions.
RNA sequencing and data analysis from HNSCC PDX models
Total RNA were extracted from treatment-naive and cetuximab-resistant PDX with phenol/chloroform and purification on RNeasy mini columns (Qiagen), according to manufacturer’s instructions. Libraries were prepared with NEBNext UltraII Directional RNA Library Prep Kit (New England Biolabs) for Illumina protocol according to supplier’s recommendations. Briefly, polyA-containing mRNA molecules were purified using poly-T oligo attached magnetic beads from 1 μg total RNA (with the Magnetic mRNA Isolation Kit; New England Biolabs), before fragmentation using divalent cations (under elevated temperature to obtain approximately 300 bp pieces), double strand cDNA synthesis, and finally Illumina adapter ligation and cDNA library amplification by PCR for sequencing. Sequencing was then carried out on paired-end 100b reads of Illumina NovaSeq. Image analysis and base calling were performed using Illumina Real Time Analysis (3.4.4) with default parameters. All sequencing data were analyzed using the Automated Reproducible MOdular workflow for preprocessing and differential analysis of RNA-seq data (ARMOR) pipeline67. In this pipeline, reads underwent a quality check using FastQC (Babraham Bioinformatics). Quantification and quality control results were summarized in a MultiQC report before being mapped using Salmon68 to the transcriptome index which was built using all Ensembl cDNA sequences obtained in the Homo_sapiens.GRCh38.cdna.all.fasta file. Then, estimated transcript abundances from Salmon were imported into R using the tximeta package69 and analyzed for differential gene expression with edgeR70. Over Representation Analyses (ORA) and Gene Set Enrichment Analyses (GSEA) were performed with the WebGestaltR 0.4.5 Bioconductor package71.
RNA sequencing from HNSCC cell models
Using in-house protocols, total RNA was extracted from cetuximab-sensitive and -resistant SC263 and SCC22b cells, as previously described21. Each cell line represents a biological repeat, and no technical replication was performed. RNA was hybridized onto Illumina Beadchips Human v4 arrays, and raw data were imported into R (v4.2) using the BioC-package beadarray yielding log2-transformed expression values for 48,107 probes vouching for 20,839 unique genes. Redundancy in probes mapping to the same genes was resolved by retaining the probe with the highest standard deviation per gene. Differences in gene expression were analyzed using generalized linear models (BioC-package limma). Genes differentially expressed at a nominal p-value inferior to 5% were considered significant. Vectors of log2-transformed fold-changes were subjected to gene set enrichment analysis (GSEA) using the BioC-package fgsea. Gene sets related to metabolism (N = 208) from Reactome, WikiPathways, KEGG or the Hallmark category of the Molecular Signatures Database (Broad Institute) were analyzed and p-values inferior to 5% were considered significant.
Whole exome sequencing
Total DNA (from HNSCC cell lines and PDX-derived tumor tissues) was extracted from cell pellets using the Wizard Genomic DNA Purification Kit (#A1120; Promega) according to the manufacturer’s protocol and quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). Library preparation and WES were performed at Macrogen Inc. (South Korea) using the Human Core Exome kit and Human RefSeq Panel (Twist Bioscience), on a NovaSeq 6000 sequencing system (Illumina). 2×150 base paired-end reads were generated. Raw data (.fasta files) were aligned to the reference human genome assembly (GRCh38) using BWA 0.7.1572. Aligned sequences (.bam files) were processed using Samtools 1.12 “MarkDup” (for marking duplicates) and GATK 4.2 “BQSR” (for base quality scores recalibration). Variant calling was then performed using GATK 4.2 “Haplotype Caller” (following Broad Institute best practices). The variant calls (.vcf) files generated were annotated, imported and further analyzed on Highlander 17.18 (https://sites.uclouvain.be/highlander/), the in-house bioinformatics framework of the Genomics and Bioinformatics platform of UCLouvain (PGEN: https://www.deduveinstitute.be/research-platform/genomics-core), with a local database currently containing more than 4900 WES categorized by pathology. Highlander provides extensive variant-annotation, filtering and visualization. Filtering was retained for variants that satisfied the following criteria:
-
(1)
pass GATK standard quality-control filters;
-
(2)
read depth >10 and more than 30% of depth for the alternative allele;
-
(3)
<0.3% allele frequency in the population with the maximum allele frequency in GnomAD WES samples (https://gnomad.broadinstitute.org/help/popmax);
-
(4)
detected in <5% samples from individuals with unrelated pathologies (or unaffected controls) in the in-house database of 3650 germline WES and in less than 8 different pathologies;
-
(5)
missense, nonsense, frameshift and changes affecting splicing;
-
(6)
for missense variants, predicted to affect protein function by at least 1 out of 20 prediction tools (DAMAGING in Mutation Taster, FATHMM, FATHMM-XF, Polyphen2 (HDIV), Provean, SIFT4G, Mutation Assessor, MCAP, LRT, Lists2, Deogen, ClinPred, BayesDel (with MaxMAF), PrimateAl and MetaSVM, or a score >20 in CADD phred, >0.5 in VEST, >0.5 in REVEL, >0.75 in MVP, >0.75 in MutPred).
Data analysis from RNA-sequencing and FDG PET-scan in HNSCC patients
To assess the clinical outcome association between the PPARα-related gene signature (including CD36, ACSL1, ACSL3, ACSL5, CPT1A, CRAT, ACADL, ECHDC2, DBI, ACOX1, PDK4, and SCD) and response to anti-EGFR therapy in HNSCC patients, we analyzed the GSE109756 and GSE235910 datasets containing gene expression data from HNSCC patients enrolled in window-of-opportunity clinical studies for treatment with cetuximab33 and afatinib34,73, respectively. Both studies were conducted in accordance with the International Conference on Harmonization Good Clinical Practice standards and the Declaration of Helsinki. Both female and male patients were included in these studies. Patients signed an informed consent, notably for prospective paired biopsies for translational research purposes. Bulk RNA from paired fresh-frozen tumor samples were processed as explained previously from 14 patients (cetuximab study) or 13 patients (afatinib study)34,74. Tumor metabolic response was assessed by the delta-standardized uptake value (SUV) (expressed in %) using the SUVmax, derived from FDG-PET scans carried out in HNSCC patients, before and after treatment with anti-EGFR therapy (cetuximab or afatinib). Tumor metabolic response was defined by the Young’s Criteria published by the EORTC PET study group75. Gene expression data were imported into R v.4.2.1 and analyzed using the limma v.3.54.2 Bioconductor package. To identify genes whose treatment-induced expression variation is associated with metabolic response, we integrated delta SUV values with gene expression data. A linear model incorporating an interaction term between time point (before vs. after treatment) and Delta SUV was constructed for each gene. The resulting interaction effects were then subjected to Gene Set Enrichment Analysis (GSEA) using fGSEA v1.30.0 in order to investigate the PPARα-related gene signature. Gene set variation analysis (GSVA) was also used to compare the enrichment scores of the PPARα-related gene signature in paired tumor samples. For statistical analysis, a unilateral Student’s t-test was performed.
Mass spectrometry-based proteomics
Total proteins were extracted in triplicate from cetuximab-sensitive and -resistant SC263 and SCC22b cells upon cell harvesting with a scraper. More specifically, cell pellets were suspended in 200 μL of RIPA buffer (Thermo Fisher Scientific, #89900) supplemented with protease and phosphatase inhibitor cocktails. (Thermo Fisher Scientific, #78425 and #78428) Pellets were then dissolved, sonicated (3×10 sec at 50% amplitude) and shaken on ice for 15 min before centrifugation (16,000 x g, 10 min, 4 °C) and transfer of the supernatants to new microcentrifuge tubes. This step was repeated. Protein concentration in the supernatants was determined by using bicinchoninic acid-based assay (Thermo Fisher Scientific, #23225). Ten milligrams of each sample were treated with 1% Rapigest (Waters, #186001861) to a final concentration of 0.1% (v/v), and then brought to the same volume with 100 mM tetraethylammonium bromide (TEAB). The samples were then reduced with 10 mM tris-(2-carboxyethyl)phosphine and alkylated with 19 mM 2-iodoacetamide (Thermo Fisher Scientific, #90064). Proteins were precipitated with ice-cold acetone (Acros, #268310010) overnight, at -20 °C. Acetone was removed after centrifugation (8,000 x g, 10 min, 4 °C), and pellets were dissolved in 10 μL of 100 mM TEAB to a concentration of 1 μg/μL. Trypsin Gold (Promega, #V5280) was added to a protease:protein ratio of 1:40. Samples were digested overnight at 37 °C, and then desalted using C18 spin columns (Thermo Fisher Scientific, #89873). Peptides were vacuum-dried and stored at -20 °C. Peptides were dissolved in 12 μL 99:1 water/acetonitrile + 0.1% formic acid and the peptide concentration was measured using the quantitative colorimetric peptide assay kit (Thermo Fisher Scientific, #23275) on the NanoDrop 2000. Each sample (0.5 µg) was loaded on a mPAC™ trapping column and injected on a 200-cm C18 mPAC™ column (Pharmafluidics) connected to a nanoAcquity LC system (Waters). Peptides were eluted with 99:1 water/acetonitrile + 0.1% formic acid (mobile phase A) and 100% acetonitrile + 0.1% formic acid (mobile phase B) at a flow rate of 750 nL/min using the following gradient: 1% B for 4 min, 1%-40% B for 84 min, 40%-100% B for 5 min, 100%-1% B for 3 min, and 1% B for 34 min.
The LC system was coupled to a Q-Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific) programmed to acquire in data-dependent mode. The survey scans were acquired in the Orbitrap mass analyzer operating at 70,000 (FWHM) resolving power at the mass range of 350-1850 m/z, with a target of 3 × 106 ions and 100 ms injection time. Precursors were selected “on the fly” for high energy collision-induced dissociation (HCD) fragmentation with an isolation window of 1.6 amu and a normalized collision energy of 28%. A target of 1.7 × 103 ions and a maximum injection time of 80 ms were used for MS/MS. The method was set to analyze the top 20 most intense ions from the survey scan and dynamic exclusion was enabled for 20 s. Tandem mass spectra were processed using MaxQuant software version 1.6.7.0. Proteins were identified using the Andromeda search engine and using the Homo sapiens proteome reference database (UniProt, release 20200130, 20366 entries). The parameters chosen for the identification include: digestion enzyme used was trypsin, maximum number of allowed missed cleavages was two. Oxidation of methionine, N-terminal acetylation, and phosphorylation of serine, threonine and tyrosine were set as variable modifications, while carbamidomethylation of cysteine was set as a fixed modification. A maximum number of 5 modifications per peptide was set. The precursor mass tolerance was set to 4.5 ppm and the fragmentation mass tolerance to 20 ppm. The peptide-to-spectrum match (PSM) and protein false discovery rates (FDR) were set at 1%. The match-between-runs and the label-free quantification (LFQ) methods were enabled using default parameters. After data treatment using MaxQuant, statistical analysis was done using Perseus software (version 1.6.7.0). The data matrix was filtered by removing the potential contaminants and decoy reverse sequences. The LFQ intensities were then log2-transformed and missing values were replaced by imputation based on the normal distribution. This resulted in the quantification of the expression of 2741 unique proteins. Differences in protein expression were analyzed using generalized linear models with a design matrix set up identically as outlined above. Proteins with a nominal p-value inferior to 5% were considered significant. Vectors of log2-transformed fold-changes were subjected to GSEA for the same metabolism gene sets as described above and p-values inferior to 5% were considered significant.
Integrative analysis of transcriptomics and proteomics data
Gene expression differences between cetuximab-sensitive and -resistant HNSCC cells were first translated into virtual protein activity (VPA) scores using the BioC-package VIPER. The VIPER algorithm infers VPA scores of both transcription factors and signal transduction proteins based on the mRNA expression profiles of their target genes. It considers the mode of action (i.e. activation or suppression), the regulator-target gene interaction confidence and the pleiotropic nature of each target gene regulation. The VIPER algorithm was run without a null model and with lists of target genes for 6054 regulators in a background specific for HNSCC cells (BioC-package aracne.networks). Prior to the VIPER analysis, nominal p-values resulting from differential gene expression analysis (vide supra) were Z-transformed.
Three different sets of seed proteins were then defined: A. primary seeds are proteins for which both the expression and the VPA change significantly in the same direction; B. proteins involved in fatty acid metabolism, glutamate and glutamine metabolism and glycolysis with a significant VPA change between cetuximab-sensitive and -resistant HNSCC cells; and C. proteins involved in the same pathways with a significant expression difference between the conditions of interest. In order to allow a substantial overlap between the different data sets (i.e. transcriptomics and proteomics), significance was considered for nominal p-values inferior to 10%. All identified proteins were used as seeds and were mapped onto the STRING protein-protein interaction (PPI) network that was filtered to contain only interactions with a minimal score of 0.4 and that was downloaded from the website (https://www.string-db.org) and converted into a igraph object for manipulation in R (R-package igraph). Using the STRING PPI network, all shortest paths between each pair of seed proteins were calculated.
The subgraph connecting all pairs of proteins was used to calculate node degree and centrality statistics and was subjected to Louvain clustering to detect communities. For each community, as well as for the full subgraph, overrepresentation analysis (ORA – BioC-package fgsea) was performed for gene sets included in Reactome and WikiPathways. Enrichment in the full subgraph was tested against all proteins in the STRING network, whereas enrichment in the communities was tested against all proteins in the subgraph. In order to reduce redundancy between gene sets, significant results were collapsed. For each of the communities, global activity changes between conditions of interest, based on expression and VPA fold-changes, were also analyzed. Networks are visualized R-packages tidygraph, ggraph, and ggpubr.
Fatty acid quantification
Subconfluent cells were collected in PBS before extraction of total lipids with methanol:chloroform:water (2:2:1.8; v:v:v). Extraction yields and purity of fatty acid fractions (i.e. free fatty acids, phospholipids and neutral lipids) were assessed thanks to the addition, in each sample, of an internal standard composed of nonadecanoic acid, 1,2-dipentadecanoyl-sn-glycero-3-phosphatidylcholine and triheptadecanoin (Larodan). After sample drying (under a stream of nitrogen at 30 °C) and resuspension in chloroform, lipids were loaded on solid phase extraction columns (Bond Elut-NH2, Agilent Technologies). Fractions for neutral lipids, free fatty acids and phospholipids were eluted with chloroform:2-propanol (2:1; v:v); diethyl ether:acetic acid (98:2; v:v) and methanol, respectively. After drying, samples were methylated under alkaline conditions (KOH 0.1 M in methanol at 70 °C for 1 h) and then under acidic conditions (HCl 1.2 M in methanol at 70 °C for 15 min), before extraction of fatty acid methyl esters (FAMEs) with hexane. After addition of methyl-undecanoate (Larodan) as an injection standard, FAMEs were injected in a gas chromatography column (Trace 1310, Thermo Fisher Scientific) equipped with an autosampler TriPlusAS and a RT-2560 capillary column (biscyanopropylpolysiloxane 100 m length, 0.25 mm internal diameter, 0.2 µm film thickness; Restek), under continuous flow with H2 at 200 kPa. Separation was done by using the following temperature program: 80 °C which increased to 175 °C (for 25 min) at 25 °C/min, then to 200 °C (for 20 min) at 10 °C/min, then to 220 °C (for 5 min) at 10 °C/min and finally to 235 °C (for 15 min) at 10 °C/min. Temperature was then decreased to 80 °C at 20 °C/min. FAMEs were detected using a flame ionizing detector kept at 255 °C and flowed by air (350 mL/min), H2 (35 mL/min) and N2 (40 mL/min). Identification and quantification of the (unknown) peaks were done thanks to the use of an external standard composed of 43 pure methyl ester standards (Larodan and Nu-Check Prep), according to the retention time and the known concentrations, respectively. Chromatographs were processed by using ChromQuest 5.0 software (Thermo Fisher Scientific). After data normalization with both internal and injection standards, results are expressed in nmol of FA per million of cells.
Western blot analysis
Subconfluent cancer cells were washed twice with ice-cold PBS and lysed in a RIPA buffer supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and a phosphatase inhibitor cocktail (Roche). Cell lysates were then cleared by centrifugation (6000 x g, 10 min, 4 °C) and stored at −80 °C until analysis. After determination of protein concentration using a bicinchoninic acid-based assay (Thermo Fisher Scientific), samples were denaturated (5 min, 95 °C) with Laemmli sample buffer containing 100 mM dithiothreitol. Samples (20 µg per well) were then separated by SDS-PAGE (8 to 15% acrylamide/bis-acrylamide gels) and transferred to PVDF membranes. Membranes were blocked with 5% bovine serum albumin (BSA) in TBS-0.1% Tween 20 (TTBS) and subsequently immunoblotted overnight at 4 °C with specific primary antibodies against β-actin (clone AC-15, #A5441, Sigma-Aldrich; 1:10,000), phosphorylated (Ser-79; clone D7D11, #11818, Cell Signaling Technology; 1:1000) and total acetyl-CoA carboxylase (clone C83B10, #3676, Cell Signaling Technology; 1:1000), CD36 (#NB400-144, Novus Biologicals; 1:1000), CPT1A (clone D3B3, #12252, Cell Signaling Technology; 1:1000), phosphorylated (Tyr-1068; clone D7A5, #3777, Cell Signaling Technology; 1:1000) and total EGFR (clone D38B1, #4267, Cell Signaling Technology; 1:1000), fatty acid synthase (clone C20G5, #3180, Cell Signaling Technology; 1:1000), HSP90 (clone 68, #610419, BD Biosciences; 1:7500) and SCD1 (clone CD.E10, #ab19862, Abcam, 1:1000). After several washes with TTBS, membranes were then incubated (1 h, room temperature) with horseradish peroxidase (HRP)-conjugated secondary antibodies (#115-035-003 and #111-035-003, Jackson Immunoresearch; 1:10,000) and chemoluminescent signals were revealed by using ECL Western Blotting Detection Kit (GE Healthcare) on X-ray films in a dark chamber. Films were scanned with an Epson V600 scanner and images were processed with ImageJ.
PPAR transcriptional activity assay
Nuclear proteins were isolated from subconfluent cells using the NE-PER extraction kit from Thermo Fisher Scientific (#78833 and #78835), according to manufacturer’s instructions. Cells were suspended in isolation buffer from the kit supplemented with protease and phosphatase inhibitor cocktails. Transcriptional activity for PPAR alpha and gamma was assessed by using specific kits (#ab133107 and #ab133101; Abcam), according to manufacturer’s recommendations.
Dosage of extracellular glucose and lactate
Cancer cells (2 × 105 cells/well; 3 wells/condition) were seeded in 12-well plates with 2 mL of their routine culture medium. After 24 h, medium was replaced by 500 µL of DMEM containing 10 mM D-glucose, 2 mM L-glutamine and supplemented with 10% dialyzed FBS (Sigma-Aldrich). Initial concentrations of glucose and lactate in the experimental medium were also assessed by including control wells containing only cell culture medium (no cells) on each plate. After incubation (for 6, 12, 18 or 24 h), extracellular media were collected and deproteinized by centrifugation (15 min, 6,000 x g, 4 °C) in 10 kDa cut-off filter tubes (VWR). Glucose and lactate concentrations were measured in the samples (50 µL) by using enzymatic assays (CMA Microdialysis AB) and a CMA 600 analyzer (Aurora Borealis). Data analysis was done by calculating the difference in glucose and lactate concentrations between the control wells and the experimental wells. Data were then normalized by the protein content in each well and expressed in µmol/hr/mg protein.
Intracellular metabolite quantification
Cell lines were cultured in 6-well plates and treated or not with 100 µM etomoxir for 2 h before sample processing. Lysates were obtained after quenching of metabolism with liquid nitrogen as described before76. Briefly, after one rapid wash with ice-cold water, culture plates were submerged in liquid nitrogen. Subsequently, 250 µL of a solution consisting of 90 % methanol (Biosolve BV) and 10 % chloroform was added and lysates were transferred into microcentrifuge tubes. After centrifugation for 15 min at 4 °C and 22,000 g, the supernatant was recovered, dried in a SpeedVac vacuum concentrating system (Life Technologies) and resuspended in 40 µL of water before analysis.
LC-MS analysis was performed essentially as previously described77. Briefly, 5 µL of sample were analyzed with an Inertsil 3 μm particle ODS-4 column (150 ×2.1 mm; GL Biosciences) at a constant flow rate of 0.2 mL/min with an Agilent 1290 HPLC system. Mobile phase A consisted of 5 mM hexylamine (Sigma-Aldrich) adjusted to pH 6.3 with acetic acid (Biosolve BV) and phase B of 90% methanol (Biosolve BV)/10 % 10 mM ammonium acetate (Biosolve BV) adjusted to pH 8.5 with ammonia (Merck, Darmstadt, Germany). The mobile phase profile consisted of the following steps and linear gradients: 0 – 2 min at 0 % B; 2 – 6 min from 0 to 20 % B; 6 – 17 min from 20 to 31 %B; 17 – 36 min from 31 to 60 % B; 36 – 41 min from 60 to 100 % B; 41 – 51 min at 100 % B; 51 – 53 min from 100 to 0 % B; 53 – 60 min at 0% B. Analytes were identified with an Agilent 6550 ion funnel mass spectrometer operated in negative mode with an electrospray ionization (ESI) source and the following settings: ESI spray voltage 3500 V, sheath gas 350 °C at 11 l/min, nebulizer pressure 35 psig and drying gas 200 °C at 14 l/min. An m/z range from 70 to 1200 was acquired with a frequency of 1 per second by adding 8122 transients. Compounds were identified based on their exact mass (<5 ppm) and retention time compared to standards (Sigma Aldrich). The areas under the curve of extracted-ion chromatograms of the [M-H]- forms were integrated using MassHunter software (Agilent), and normalized to the mean of the areas obtained for a series of 150 other metabolites (‘total ion current’).
Seahorse analysis
All assays were carried out using a seeding density of 30,000 cells/well in Seahorse Bioanalyzer XFe96 culture plates in non-buffered DMEM, adjusted at pH 7.4 and supplemented with specified metabolic substrates. To evaluate their capacity to oxidize specific bioenergetic substrates, cells were incubated in DMEM containing either 10 mM L-glutamine, 10 mM D-glucose) or 50 µM palmitate-BSA (Agilent). Oxygen consumption rate (OCR) was assessed for 3 cycles (3 min mixing/4 min measuring). Glycolytic function was evaluated in DMEM containing 2 mM L-glutamine and after injection of 10 mM D-glucose; glucose-dependent extracellular acidification rates (ECAR) were calculated by comparing the values before and after addition of the substrate. Data were normalized by the protein content in each well and expressed in mpH/min/µg protein (ECAR) or pmoles/min/µg protein (OCR). OCR and ECAR were measured with Seahorse XFe96 Analyzer (Agilent) and data analysis was performed using Seahorse Wave 2.4 software.
13C-glucose tracing
For 2D cell cultures and 3D spheroids, D-glucose-13C6 tracer (Cambridge Isotope Laboratories; #CLM-1396) was added in the culture medium (final concentration of 10 mM or 25 mM, respectively) whereas, for in vivo experiments, a solution of D-glucose-13C6 (2 g/kg in PBS) was injected intravenously into the tail vein of tumor-bearing nude mice. Twenty-four hours later, cells, spheroids and tumors were collected and intracellular polar metabolites were extracted using dual phase extraction procedure, as described elsewhere78. Metabolites were then analyzed on a high-resolution 600 MHz nuclear magnetic resonance (NMR) spectrometer (Bruker Ascend, NEST platform, UCLouvain). The acquisition time was 0.8 sec with 2,048 repetitions and 10 sec of interpulse delay (1D sequence with inverse gated decoupling using 30° flip angle). The analysis and quantification of obtained spectra were performed using the Mnova v14.3.1 software (Mestrelab). Metabolite quantification was carried out by peak integration relative to internal standards and subsequent correction for protein content (for in vitro experiments) and tumor mass (ex vivo experiments).
RNA extraction and RT-qPCR
Samples (cell pellets and snap-frozen PDX-derived tissues) were resuspended in Tri-Reagent (Molecular Research Center). RNA was recovered after separation in 1-bromo-3-chloropropane and precipitation with isopropanol, washed with 70% ethanol, resuspended in RNase-free water, and then quantified by spectrophotometry (Nanodrop 1000, Thermo Fisher Scientific). After reverse transcription on 1 µg of total RNA with the RevertAid Reverse-Transcriptase, oligo-dT and random hexamers (Thermo Fisher Scientific), quantitative PCR amplification was performed on a ViiA 7 real-time PCR system (Applied Biosystems) using SYBR® select master mix for cfx (Thermo Fisher Scientific) and gene-specific primer sequences (Supplementary Table 1). Data were analyzed according to ddCt method using human GTF2B or ACTB as reference gene.
Flow cytometry
To assess palmitate uptake in cetuximab-sensitive and -resistant cancer cells, the culture medium was removed and the cells were washed with PBS before being incubated with 0.2 µM BODIPY™ FL C16 (#D3821; Thermo Fisher Scientific) for 15 min at room temperature. Cells were washed three times with ice-cold PBS and fixed with 4% PFA for 30 min at room temperature. Cells were then analyzed by flow cytometry using the FL1-FITC channel (BD FACSCanto™ II; BD Biosciences) and data were analyzed with the FlowJo v10.7.1 software.
To evaluate EGFR expression at the cell surface, cancer cells (250,000 cells/condition) were washed twice with PBS, incubated for 30 min at 4 °C with Alexa Fluor® 488-conjugated anti-human EGFR antibody (clone AY13, #352907, BioLegend), at 1 µL/million cells, and 0.5 µM DAPI (#D9542; Sigma-Aldrich) in FACS tubes (#CLS352008; Sigma-Aldrich). Cells were washed twice with PBS and suspended in 200 µL PBS before flow cytometry analysis using the FL1-FITC and FL2- Pacific Blue channel (BD 139 FACSCanto™ II; BD Biosciences).
Immunocytochemistry
For PPARα and PPARγ staining, cells were grown on coverslips, fixed with 4% paraformaldehyde (PFA) for 10 min, and then permeabilized using a PBS-Triton X-100 0.3% (v/v) solution for 10 min at room temperature before saturation with PBS-Tween 0.1% (v/v) with BSA 5% (v/v) for 1 h at room temperature. After overnight incubation with the anti-PPARα (#PA1-822A, Invitrogen; 1:1000) or anti-PPARγ (clone C26H12, #2435, Cell Signaling Technology; 1:200) primary antibody diluted in PBST/BSA 1% (v/v) and several washes, slides were incubated with an anti-rabbit Alexa Fluor 568 secondary antibody (#A-11036, Thermo Fisher Scientific; 1:500). DAPI (1:5000; Sigma-Aldrich) was used for nuclear counterstaining and slides were prepared with a fluorescence mounting medium (Dako). The staining was visualized with a Zeiss LSM800 inverted confocal microscope and the nuclear/cytoplasmic ratio was calculated with Fiji software (v 2.16.0).
Mitochondrial membrane potential assay
The mitochondrial membrane potential (Δψm) was measured using the JC-10 Mitochondrial Membrane Potential Assay Kit (Abcam, #ab112134), according to manufacturer’s recommendations. Cancer cells (2.104 cells/well) were seeded in 96-well plates and Δψm was measured 24 h later, after incubation (1 h, room temperature) with JC-10 dye. Fluorescence intensities were measured at excitation/emission values of 490/525 nm (red) and 540/525 nm (green) using a SpectraMax i3 spectrophotometer (Molecular Devices), and Δψm was determined after calculation of the red-to-green ratio.
In vivo mouse experiments
All mice were housed in the same experimental room with the appropriate 12 h light/12 h dark cycles and controlled for temperature (21–24 °C) and humidity (40-60%), in the facility of the Institut de Recherche Expérimentale et Clinique (IREC) at UCLouvain. Mice were fed ad libitum with complete maintenance diet from SAFE (#U8220G10R). HNC004 PDX model was established in collaboration with Trace, the PDX platform of KU Leuven (https://gbiomed.kuleuven.be/english/research/50488876/54502087/Trace)31. The UCLHN4 model was generated in the Pole of Medical Imaging, Radiotherapy and Oncology (MIRO) at UCLouvain. Both PDX models were derived from patients with treatment-naive HPV-negative HNSCC. The study was approved by the ethics committee from UCLouvain (approval ID UCL/MD/2012/09July/314) in accordance with the principles of the Declaration of Helsinki, and all patients gave their written informed consent. Patient tumor materials were collected in RPMI medium (Gibco) supplemented with 0.4% fungizone (Bristol Myers Squibb), 2.5% penicillin/streptomycin (Sigma-Aldrich) and gentamycin (Braun Medical), and then kept at 4 °C for engraftment within 6 h of resection. Necrotic and supporting tissues were carefully removed using a surgical blade. Some tumor fragments were snap-frozen and stored at −80 °C for genomic profiling, and other fragments were fixed in 4% neutral-buffered formalin and paraffin-embedded for histopathological analysis. The remaining tumor fragments were implanted subcutaneously into the backs of 4- to 6-week-old (22-25 g) athymic nude female mice (NMRI-Foxn1nu/nu, Taconic Biosciences). Successfully engrafted tumor models were then passaged through several generations. Experiments were conducted on the fifth and sixth generations. PDX models were validated as previously described51. Some mice from the initial HNC004 and UCLHN4 models were treated with cetuximab (30 mg/kg, once a week intraperitoneally) until some became resistant to cetuximab. Resistance was defined as continuous tumor growth under cetuximab and an increase in tumor volume of more than 200% compared with baseline. The resistant models were treated continuously with cetuximab without interruption.
Rj:NMRI-Foxn1nu/nu mice (female 7-week-old) were purchased from Janvier Labs and used for subcutaneous injection of HNSCC cell lines. Before injection, FaDu-R and SCC22b-R cells were washed twice with PBS, resuspended in 0.9% NaCl and 2 × 106 cells (in 100 µL) were injected subcutaneously into the right flank of the animals. When tumors (from HNSCC cell lines or UCLHN4 PDX model) reached a mean diameter of 5 mm, mice were treated with cetuximab (30 mg/kg in 0.9% NaCl), GW6471 (20 mg/kg in DMSO) or etomoxir (40 mg/kg in DMSO) by daily intraperitoneal injection (5 days/week). Control groups were treated in parallel with adequate vehicles. Mice were weighed and tumors were measured 3 times a week. None of the tumor volumes of mice in this study exceeded the limits authorized by the local ethical committee. At the end of the experiment, when tumor size reached the ethical endpoint (500 mm³ or visible necrosis), mice were sacrificed and tumors were collected.
Immunohistochemistry
Paraffin-embedded tissue slides prepared from the PDX collected tumors were used for immunohistochemistry. After deparaffinization, rehydration and inactivation of the endogenous peroxidases, heat-induced antigen retrieval was performed with a sodium citrate 10 mM, pH 6.0 buffer for 20 min and slides were rinsed with demineralized water and TBS-Triton X-100 1% (TTBS; Thermo Fisher Scientific). After blocking (45 min, room temperature) in 10% goat serum, slides were incubated (overnight, 4 °C) with the CD36 antibody (clone D8L9T, #14347822 A, Cell Signaling Technology; 1:300). After several washes in TTBS, slides were incubated with a Boost anti-rabbit horseradish peroxidase antibody (45 min, room temperature) (Cell Signaling Technology). Finally, the slides were rinsed 3 times and incubated with 3,3-Diaminobenzidine (DAB) for 3 min (Cell Signaling Technology). Slides were intensively washed with water and nuclear counterstaining was performed with hematoxylin (Dako). Acquisition was performed with a Zeiss AxioScan.Z1 slide scanner and images were analyzed using the open source software QuPath (v.0.5.1)79.
Statistics and reproducibility
Statistical analyses were performed through GraphPad Prism 10 by using Student’s t-test, one-way ANOVA with Tukey’s multiple comparison test, two-way ANOVA with Tukey’s or Sidák’s multiple comparison test when appropriate. Statistical significance is indicated as follows: exact P-values are provided when *P < 0.05 or **P < 0.01, while ***P < 0.001 indicates a threshold value. No statistical method was used to predetermine the sample size. The experimental sample size was chosen based on commonly accepted standards. No data were excluded from the analyses. Mice were randomly assigned to each group, other experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Datasets from RNA-sequencing analyses, generated and/or used during this study, are available at Gene Expression Omnibus: GSE235417 (cetuximab-sensitive and -resistant HNSCC models), GSE109756 (window-of-opportunity clinical study for HNSCC patients treated with cetuximab33) and GSE235910 (window-of-opportunity clinical study for HNSCC patients treated with afatinib34) (https://www.ncbi.nlm.nih.gov/gds). RNA-sequencing data from 475 HNSCC patients, previously generated by The Cancer Genome Atlas Pan-Cancer project32, were analyzed through cBioPortal (https://www.cbioportal.org/). Whole exome sequencing data for HNSCC cell lines are available at European Nucleotide Archive PRJEB80851. Whole exome sequencing data for HNSCC PDX models has been deposited in the European Genome-Phenome Archive (EGA) under the following ID: EGAS50000000800. Proteomics data generated during this study are available in the MassIVE public repository: MSV000096655. All unique reagents generated in this study will be made available on request to C.C. (cyril.corbet@uclouvain.be), with a completed material transfer agreement (MTA). Source data are provided with this paper.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Leemans, C. R., Snijders, P. J. F. & Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 18, 269–282 (2018).
Cramer, J. D., Burtness, B., Le, Q. T. & Ferris, R. L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 16, 669–683 (2019).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92 (2020).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Machiels, J. P. et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31, 1462–1475 (2020).
Kalyankrishna, S. & Grandis, J. R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 24, 2666–2672 (2006).
Ang, K. K. et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 62, 7350–7356 (2002).
Rubin Grandis, J. et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J. Natl Cancer Inst. 90, 824–832 (1998).
Egloff, A. M. & Grandis, J. R. Improving response rates to EGFR-targeted therapies for head and neck squamous cell carcinoma: candidate predictive biomarkers and combination treatment with Src inhibitors. J. Oncol. 2009, 896407 (2009).
Huang, C. et al. Proteogenomic insights into the biology and treatment of HPV-negative head and neck squamous cell carcinoma. Cancer Cell 39, 361–379.e316 (2021).
Kiaris, H., Spandidos, D. A., Jones, A. S., Vaughan, E. D. & Field, J. K. Mutations, expression and genomic instability of the H-ras proto-oncogene in squamous cell carcinomas of the head and neck. Br. J. Cancer 72, 123–128 (1995).
De Pauw, I., Boeckx, C. & Wouters, A. Mechanisms of cetuximab resistance and how to overcome it. in Critical Issues in Head and Neck Oncology (eds Vermorken, J. B., Budach, V., Leemans, C. R., Machiels, J. P., Nicolai, P. & O’Sullivan, B.) (Springer, Cham., 2021).
Zaryouh, H. et al. The role of Akt in acquired cetuximab resistant head and neck squamous cell carcinoma: an in vitro study on a novel combination strategy. Front. Oncol. 11, 697967 (2021).
Li, Q., Tie, Y., Alu, A., Ma, X. & Shi, H. Targeted therapy for head and neck cancer: signaling pathways and clinical studies. Signal Transduct. Target Ther. 8, 31 (2023).
Van den Bossche, V. et al. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: clinical challenges and opportunities for precision medicine. Drug Resist. Updat. 60, 100806 (2022).
Perri, F. et al. Cancer cell metabolism reprogramming and its potential implications on therapy in squamous cell carcinoma of the head and neck: a review. Cancers 14, https://doi.org/10.3390/cancers14153560 (2022).
Corbet, C. & Feron, O. Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer. Curr. Opin. Clin. Nutr. Metab. Care 18, 346–353 (2015).
Corbet, C. & Feron, O. Cancer cell metabolism and mitochondria: nutrient plasticity for TCA cycle fueling. Biochim. Biophys. Acta Rev. Cancer 1868, 7–15 (2017).
Fendt, S. M., Frezza, C. & Erez, A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 10, 1797–1807 (2020).
Boeckx, C. et al. Establishment and characterization of cetuximab resistant head and neck squamous cell carcinoma cell lines: focus on the contribution of the AP-1 transcription factor. Am. J. Cancer Res. 5, 1921–1938 (2015).
De Pauw, I. et al. Simultaneous targeting of EGFR, HER2, and HER4 by afatinib overcomes intrinsic and acquired cetuximab resistance in head and neck squamous cell carcinoma cell lines. Mol. Oncol. 12, 830–854 (2018).
Van Caloen, G. et al. Preclinical evaluation of the association of the cyclin-dependent kinase 4/6 inhibitor, ribociclib, and cetuximab in squamous cell carcinoma of the head and neck. Cancers 13, https://doi.org/10.3390/cancers13061251 (2021).
Alvarez, M. J. et al. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat. Genet. 48, 838–847 (2016).
Li, X. et al. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol. Cancer Ther. 7, 1207–1217 (2008).
Lu, H., Li, X., Luo, Z., Liu, J. & Fan, Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol. Cancer Ther. 12, 2187–2199 (2013).
Luwor, R. B., Lu, Y., Li, X., Mendelsohn, J. & Fan, Z. The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene 24, 4433–4441 (2005).
Morandi, A. & Indraccolo, S. Linking metabolic reprogramming to therapy resistance in cancer. Biochim. Biophys. Acta Rev. Cancer 1868, 1–6 (2017).
Goncalves, A. C. et al. Impact of cancer metabolism on therapy resistance—clinical implications. Drug Resist. Updat. 59, 100797 (2021).
Van Caloen, G. et al. Preclinical activity of ribociclib in squamous cell carcinoma of the head and neck. Mol. Cancer Ther. 19, 777–789 (2020).
Mignion, L. et al. 2’-deoxy-2’-[18F] fluoro-D-glucose positron emission tomography, diffusion-weighted magnetic resonance imaging, and choline spectroscopy to predict the activity of cetuximab in tumor xenografts derived from patients with squamous cell carcinoma of the head and neck. Oncotarget 9, 28572–28585 (2018).
Weinstein, J. N. et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Schmitz, S. et al. Tumour response and safety of cetuximab in a window pre-operative study in patients with squamous cell carcinoma of the head and neck. Ann. Oncol. 24, 2261–2266 (2013).
Beyaert, S. P. et al. Tumor microenvironment modifications induced by afatinib in squamous cell carcinoma of the head and neck: a Window-of-Opportunity Study (EORTC-90111-24111). Clin. Cancer Res. 29, 4076–4087 (2023).
Marine, J. C., Dawson, S. J. & Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 20, 743–756 (2020).
Shi, Z. D. et al. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies. Signal Transduct. Target Ther. 8, 113 (2023).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Stockley, T. L. et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: the Princess Margaret IMPACT/COMPACT trial. Genome Med. 8, 109 (2016).
Meric-Bernstam, F. et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J. Clin. Oncol. 33, 2753–2762 (2015).
Massard, C. et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 7, 586–595 (2017).
Le Tourneau, C. et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 16, 1324–1334 (2015).
Malone, E. & Siu, L. L. Precision medicine in head and neck cancer: myth or reality? Clin. Med. Insights Oncol. 12, 1179554918779581 (2018).
Kaidar-Person, O., Gil, Z. & Billan, S. Precision medicine in head and neck cancer. Drug Resist. Updat. 40, 13–16 (2018).
Ortiz-Cuaran, S., Bouaoud, J., Karabajakian, A., Fayette, J. & Saintigny, P. Precision medicine approaches to overcome resistance to therapy in head and neck cancers. Front. Oncol. 11, 614332 (2021).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Labrie, M., Brugge, J. S., Mills, G. B. & Zervantonakis, I. K. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat. Rev. Cancer 22, 323–339 (2022).
Vander Linden, C. & Corbet, C. Reconciling environment-mediated metabolic heterogeneity with the oncogene-driven cancer paradigm in precision oncology. Semin. Cell Dev. Biol. 98, 202–210 (2020).
Sandulache, V. C. et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer 117, 2926–2938 (2011).
Richtsmeier, W. J., Dauchy, R. & Sauer, L. A. In vivo nutrient uptake by head and neck cancers. Cancer Res. 47, 5230–5233 (1987).
Walenta, S. et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am. J. Pathol. 150, 409–415 (1997).
Mignion, L. et al. Metabolic imaging using hyperpolarized pyruvate-lactate exchange assesses response or resistance to the EGFR inhibitor cetuximab in patient-derived HNSCC xenografts. Clin. Cancer Res. 26, 1932–1943 (2020).
Feng, W. W. et al. CD36-mediated metabolic rewiring of breast cancer cells promotes resistance to HER2-targeted therapies. Cell Rep. 29, 3405–3420.e3405 (2019).
Aloia, A. et al. A fatty acid oxidation-dependent metabolic shift regulates the adaptation of BRAF-mutated melanoma to MAPK inhibitors. Clin. Cancer Res. 25, 6852–6867 (2019).
Luo, J. et al. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the Warburg effect by cetuximab. Cancer Lett. 384, 39–49 (2017).
Li, X. et al. AMPK-mediated energy homeostasis and associated metabolic effects on cancer cell response and resistance to cetuximab. Oncotarget 6, 11507–11518 (2015).
McDonnell, E. et al. Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Rep. 17, 1463–1472 (2016).
Li, Y. J. et al. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 39, 111044 (2022).
Ali, A. et al. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 10, https://doi.org/10.15252/emmm.201708313 (2018).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017).
Morrissy, A. S. et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 529, 351–357 (2016).
Roh, V. et al. Cellular barcoding identifies clonal substitution as a hallmark of local recurrence in a surgical model of head and neck squamous cell carcinoma. Cell Rep. 25, 2208–2222.e2207 (2018).
Peng, K. et al. Identification and validation of cetuximab resistance associated long noncoding RNA biomarkers in metastatic colorectal cancer. Biomed. Pharmacother. 97, 1138–1146 (2018).
Yarchoan, M. et al. A phase 1 study of TPST-1120 as a single agent and in combination with nivolumab in subjects with advanced solid tumors. J. Clin. Oncol. 40, 3005 (2022).
Dierge, E. & Feron, O. Dealing with saturated and unsaturated fatty acid metabolism for anticancer therapy. Curr. Opin. Clin. Nutr. Metab. Care 22, 427–433 (2019).
Dierge, E. et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 33, 1701–1715 e1705 (2021).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Orjuela, S., Huang, R., Hembach, K. M., Robinson, M. D. & Soneson, C. ARMOR: an Automated Reproducible MOdular Workflow for preprocessing and differential analysis of RNA-seq data. G3 9, 2089–2096 (2019).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Machiels, J. P. et al. Activity and safety of afatinib in a window preoperative EORTC study in patients with squamous cell carcinoma of the head and neck (SCCHN). Ann. Oncol. 29, 985–991 (2018).
Schmitz, S., Bindea, G., Albu, R. I., Mlecnik, B. & Machiels, J. P. Cetuximab promotes epithelial to mesenchymal transition and cancer associated fibroblasts in patients with head and neck cancer. Oncotarget 6, 34288–34299 (2015).
Young, H. et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 35, 1773–1782 (1999).
Lorenz, M. A., Burant, C. F. & Kennedy, R. T. Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal. Chem. 83, 3406–3414 (2011).
Dewulf, J. P. et al. ECHDC1 knockout mice accumulate ethyl-branched lipids and excrete abnormal intermediates of branched-chain fatty acid metabolism. J. Biol. Chem. 297, 101083 (2021).
Yuan, M. et al. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat. Protoc. 14, 313–330 (2019).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Acknowledgements
This work was supported by grants from the Fonds de la Recherche Scientifique (F.R.S.-FNRS, CDR J001023), WELBIO (Walloon Excellence in Life Sciences and Biotechnology, X250624), the Belgian Foundation against Cancer (2022-168), and the Joseph Maisin Foundation, This was carried out in the frame of the COST Action STRATAGEM CA17104, supported by COST (European Cooperation in Science and Technology). C.C. is a F.R.S.-FNRS Research Associate and a WELBIO junior fellow. V.V.D.B. and J.V. are FNRS-Aspirant PhD fellows. E.V. is a Télévie PhD fellow. O.F. is a senior WELBIO investigator. S.S. is a FNRS postdoctoral fellow. The authors are grateful to the 2IP imaging (RRID:SCR_023378; Dr Caroline Bouzin) and flow cytometry (Dr Davide Brusa) platforms (IREC-UCLouvain) for technical support. Figure panels 1c, 8a, k and S8j were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Conceptualization: V.V.D.B., J.P.M., O.F., S.S. and C.C.; Experimental work: V.V.D.B., J.V., E.V., I.R., H.Z., S.B., L.M., E.Y.L., C.G., O.L., H.D., A.M., M.D., P.V. and A.Wa.; Data analysis and interpretation: V.V.D.B., A.W., S.S. and C.C.; Methodology: J.A., S.V.L., R.H., C.v.M., B.F.J., L.A., I.G., G.T.B., R.B. and Y.L.; Writing—review & editing: V.V.D.B., A.W., J.P.M., O.F., S.S. and C.C.; Funding and resources: J.P.M., O.F., A.W., S.S. and C.C.; Supervision: S.S. and C.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Andrew Hoy, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Van den bossche, V., Vignau, J., Vigneron, E. et al. PPARα-mediated lipid metabolism reprogramming supports anti-EGFR therapy resistance in head and neck squamous cell carcinoma. Nat Commun 16, 1237 (2025). https://doi.org/10.1038/s41467-025-56675-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56675-3
This article is cited by
-
Multi-omics integration in deciphering non-small cell lung cancer drug resistance: current status, challenges, and future prospects
Hereditas (2026)
-
Metabolomic and transcriptomic profiling of HNSCC identifies AMIGO2 as a therapeutic target modulating tumor microenvironment
npj Precision Oncology (2025)
-
Anti-tumor efficacy and Vδ2 T-cell activation via EGFR antibody-drug conjugates featuring novel aminobisphosphonates
Scientific Reports (2025)