Abstract
Intrahepatic bile duct (IHBD) development produces a morphologically heterogeneous network of large “ducts” and small “ductules” by adulthood. IHBD formation is closely linked to developmental specification of biliary epithelial cells (BECs) starting as early as E13.5, but mechanisms regulating differential IHBD morphology remain poorly understood. Here, we show that duct and ductule development has distinct genetic requirements, with Sox9 required to form the developmental precursors to peripheral ductules in adult livers. By optimizing large-volume IHBD imaging, we find that IHBDs emerge as a homogeneous webbed structure by E15.5 and undergo morphological maturation through 2 weeks of age. Developmental knockout of Sox9 leads to decreased postnatal branching morphogenesis, resulting in adult IHBDs with normal ducts but significantly fewer ductules. In the absence of Sox9, BECs fail to mature and exhibit elevated TGF-β signaling and Activin A. Exogenous Activin A is sufficient to induce developmental gene expression and morphological defects in wild-type BEC organoids, while early postnatal inhibition of Activin A in vivo rescues IHBD morphogenesis in the absence of Sox9. Our data demonstrate that proper IHBD architecture relies on inhibition of Activin A by Sox9 to promote ductule morphogenesis, defining regulatory mechanisms underlying morphological heterogeneity.
Similar content being viewed by others
Introduction
Bipotential hepatoblasts give rise to hepatocytes and biliary epithelial cells (BECs) during liver development1. Developmental signaling pathways including TGF-β, Notch, and Yap/Hippo initiate BEC fate near the portal vein, while hepatocyte specification is believed to be a default fate of hepatoblasts in the absence of pro-BEC signaling2,3,4,5,6,7. Mature BECs form the intrahepatic bile ducts (IHBDs), which exhibit hierarchical morphology. Small, peripheral “ductules” drain into large “ducts” that join the extrahepatic biliary tree, which has a distinct developmental origin from IHBDs8.
IHBD morphogenesis progresses over time from the liver hilum towards the periphery, with BEC markers first detectable between E11.5 and E13.56,9. As BECs specify, they organize around the portal vein to form a ductal plate1. Conventional models propose that ductal plates, which are “stacked” on top of one another, give rise to discontinuous primitive ductal structures that interconnect and elongate through a type of branching morphogenesis termed tubulogenesis1,10. These structures rearrange and regress through late embryonic and early postnatal development to give rise to adult IHBDs10,11,12. Failure to form proper IHBD networks is associated with a wide range of human cholangiopathies with diverse and often poorly-defined genetic etiologies. However, the regulatory mechanisms underlying differential specification of duct versus ductule morphology and global IHBD architecture remain poorly understood.
SOX9 regulates branching morphogenesis in the lung, salivary gland, and thyroid13,14,15,16. In the liver, Sox9 is activated in the ductal plate at E11.5, and remains expressed in all BECs throughout adulthood9,17. Surprisingly, conditional deletion of Sox9 in hepatoblasts (Sox9cKO) has been reported to have minimal impact on IHBD development, resulting only in delayed BEC specification from E15.5-P69,18. However, subsequent studies of aged Sox9cKO mice identified defects in BEC polarity, loss of primary cilia, and development of liver cysts, raising questions about the role of Sox9 in establishing phenotypically normal IHBDs19. Here, we examined the role of Sox9 in regulating formation of morphologically heterogeneous IHBD architecture.
Using large-scale 3D imaging of IHBDs, we find that developmental loss of Sox9 disrupts branching morphogenesis, leading to reduced numbers of ductules in adult livers. The same approach reveals that early postnatal IHBDs develop as a continuous biliary “web” that is dependent on Sox9. Mechanistically, we show that Sox9 represses Activin A, which can be inhibited to partially rescue Sox9cKO-associated defects in IHBD development. Together, we identify differing genetic requirements for morphologically-distinct compartments of IHBDs: (1) Sox9-independent morphogenesis of large ducts and (2) Activin A-inhibited, Sox9-driven morphogenesis of small ductules.
Results
Ductal paucity in adult Sox9cKO mice
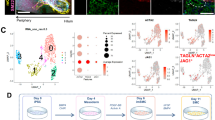
We crossed Sox9-floxed mice to Albumin-Cre mice to generate mice with conditional deletion of Sox9 in hepatoblasts (Sox9fl/fl:AlbCre; Sox9cKO). Sox9cKO mice undergo Cre recombination beginning at E10.5, resulting in loss of Sox9 in hepatocytes and BECs9,20. To avoid a previously reported liver cyst phenotype in aged Sox9cKO mice, we only studied adults between 6 and 12 weeks19. Because variable recombination efficiency and timing have been reported for the AlbCre allele, we first confirmed effective knockout by co-localizing SOX9 with the BEC-specific marker EpCAM by immunofluorescence (IF) in adult mice21. This revealed that only 3.2 ± 2.1% of BECs remained SOX9+ (Fig. 1a; Supplementary Fig. 1a). To determine if mosaicism was localized to certain anatomical compartments of the IHBDs, we measured duct diameter and colocalized EpCAM and SOX9 cells lining the IHBD lumen in adult mice (Supplementary Fig. 1a). We found that SOX9 recombination was efficient across IHBDs of different sizes (Supplementary Fig. 1b). Previous reports described loss of primary cilia in aged adult (6–12 months) and developing BECs following conditional ablation of Sox9 using both AlbCre and AlfpCre alleles9,19. We confirmed loss of primary cilia by IF for acetylated α-tubulin (Ac-tubulin) (Supplementary Fig. 2a; arrowheads)18,19. Aged Sox9cKO mice were also reported to have perturbed biliary polarity, including loss or mislocalization of Osteopontin (OPN)19. We observed the expected apical localization of OPN and F-actin in both ducts and ductules of Sox9cKO mice 6–12 weeks of age (Supplementary Fig. 2b; arrowheads). Therefore, 6–12 week Sox9cKO mice confirm previous reports of loss of primary cilia but demonstrate normal apical polarity, which may suggest that polarity defects emerge at later timepoints.
a IF of adult control and Sox9cKO mice confirms loss of Sox9 in Sox9cKO mice compared to control (scale bar represents 50 μm; white asterisks indicate portal vein). b Sox9cKO mice exhibit a loss of BECs and reduced IHBDs per portal field. c Whole tissue imaging of control liver lobes illustrates complex IHBD hierarchy, while d Sox9cKO mice exhibit significant loss of ductules, IHBD discontinuity, and reduced EpCAM expression (scale bars represent 0.5 mm; arrowheads mark representative ductules). e Sholl analysis demonstrates quantitative loss of branching complexity throughout Sox9cKO IHBDs (arbitrary units, a.u., represent distance normalized to lobe size). f AUC of proximal (0.00-0.50 a.u.) vs. distal (0.50–1.00 a.u.). Sholl intersections reveal significant loss of branching in distal IHBDs. g Sholl decay coefficient is unchanged between control and Sox9cKO IHBDs (p = 0.461) and h the mean value of total Sholl intersections (Nav) trends downward (p = 0.056). Total i volume (p = 0.433) and j length (p = 0.063) are not significantly different between control and Sox9cKO IHBDs. a-j n = 3 left lobes per group. b, f, g–j significance indicates a p < 0.5, an unpaired two-sided t-test, was used, and data are presented as mean values ± SEM. Source data are provided as a Source Data file.
In contrast with previous reports that loss of Sox9 only delays BEC specification, we observed reduced numbers of both EpCAM+ BECs and distinct ductal structures per portal field in Sox9cKO mice (Fig. 1b)9,18. To determine if ductal paucity results from decreased BEC proliferation, we colocalized EpCAM and Ki-67. Proliferation rates were unchanged between control and Sox9cKO BECs (Supplementary Fig. 2c). Since BECs are largely quiescent during homeostasis, we induced liver injury with thioacetamide (TAA) for 1 month, which resulted in extensive BEC proliferation22. Once again, we saw no change in BEC proliferation in Sox9cKO mice (Supplementary Fig. 2d). To determine if apoptosis contributes to Sox9cKO ductal paucity, we conducted IF for EpCAM and cleaved caspase-3. We did not detect apoptosis in either control or Sox9cKO BECs (Supplementary Fig. 2e). Therefore, adult Sox9cKO mice exhibit ductal paucity that is not a result of decreased proliferation or increased apoptosis.
Sox9 is required for proper IHBD morphology
To examine global IHBD morphology, we performed whole-tissue imaging using iDISCO+ and light sheet microscopy of adult control and Sox9cKO livers23. Controls demonstrated the expected IHBD hierarchy, with large ducts at the hilum decreasing in size towards the periphery and terminating in web-like ductules (Fig. 1c; Supplementary Movie 1,2). Sox9cKO ducts appeared slightly dilated but otherwise normal (Fig. 1d). There was no difference in the number of large ducts between Sox9cKO and control livers (Supplementary Fig. 3a). In contrast, Sox9cKO ductules were disorganized and discontinuous, with a general loss of peripheral webbing (Fig. 1d; Supplementary Movie 1,2). Ductules in Sox9cKO tissues also expressed lower EpCAM levels than controls (Fig. 1d; Supplementary Movie 2; arrowheads).
To better understand Sox9cKO ductal paucity, we performed quantitative analysis of 3D IHBD images. We reasoned that Sholl analysis, a common measure of neuron branch complexity, could be applied to quantify IHBDs24. With the hilum as the starting point, Sholl intersections were quantified at 1 µm spherical intervals, and plotted over distance normalized to lobe size. IHBD branching was reduced in Sox9cKO livers, supporting qualitative observations (Fig. 1e). To determine if ductal paucity was localized to distinct IHBD regions, we performed area under the curve (AUC) analysis on Sholl intersections. We found that proximal (0.00–0.50 arbitrary units; a.u.) branching complexity was minimally impacted, while distal AUC (0.50–1.00 a.u.) was significantly decreased in Sox9cKO livers, consistent with the loss of peripheral ductules (Fig. 1f). To further quantify Sox9 regulated changes in IHBDs, we conducted regression and curve-fitting analyses to characterize branching morphology25. Sholl decay coefficients, which describe the rate of change in branch density across a structure, were calculated from linear regression of semi-log plots of intersections vs. normalized distance (Supplementary Fig. 3b)26. Consistent with AUC, Sholl decay was increased in Sox9cKO samples, reflecting decreased branch density in the liver periphery (Fig. 1g; Supplementary Fig. 3b). Ductal paucity in adult mice was further supported by a downward trend in the average number of Sholl intersections (Nav) in Sox9cKO mice (Fig. 1h). We also compared the maximum number of Sholl intersections and the critical value (Nm), which is the maximum of the polynomial function and accounts for technical noise in biological samples25. We found a significant decrease in both values in Sox9cKO mice, demonstrating a decrease in maximum branch point intersections (Supplementary Fig. 3c). To determine the distance at which maximum branching occurs, we analyzed the critical radius (rc). Loss of peripheral branching in Sox9cKO mice is reflected by a slight shift in rc toward the hilum (Supplementary Fig. 3d). However, this difference does not reach statistical significance, which may suggest limitations in global Sholl measurements to identify localized phenotypes. To quantify overall IHBD size independent of branching, we measured total IHBD volume and length. We normalized these values to overall lobe volume. While the length of Sox9cKO IHBDs trended downward, overall volume was unchanged (Fig. 1i, j). This may be due to the relative contribution of large ducts, which are present and dilated in Sox9cKO mice, to total IHBD volume and length. Collectively, quantitative whole-tissue imaging reveals that ductal paucity in adult Sox9cKO mice is defined by reduced numbers of peripheral ductules.
Sox9cKO BEC exhibits elevated TGF-β signaling and transcriptomic features of immaturity
We next performed bulk RNA-seq of FACS-isolated BECs from adult control and Sox9cKO mice (Supplementary Fig. 4a). Samples clustered by genotype on PCA (Supplementary Fig. 4b). We identified 766 upregulated and 422 downregulated genes in Sox9cKO BECs (q-value < 0.05; LFC > 1) (Fig. 2a; Supplementary Data 1). We performed gene set enrichment analysis (GSEA) on known biliary developmental signaling pathways. We found an enrichment of TGF-β and YAP signaling with no change in NOTCH and WNT signaling (Fig. 2b; Supplementary Fig. 4c). Since several of the top upregulated genes in Sox9cKO BECs were TGF-β related, we confirmed TGF-β signaling elevation by IF for pSMAD2 (Fig. 2a, c; Supplementary Fig. 4d). SMAD2 is phosphorylated and translocated to the nucleus to modify downstream gene expression when TGF-β superfamily ligands bind their heteromeric type I/type II receptor complexes27,28. Previous work has found an increase in TGFBRII following the loss of Sox9 and suggested TGFBRII inhibits biliary differentiation9. Of the known TGF-β superfamily receptors, we observed an increase in Tgfbr2 and a decrease in Amhr2 in Sox9cKO mice by RNA-seq, but no change in both type I and II receptor pairs (Fig. 2d). Of known TGF-β superfamily ligands, only Inhba was elevated in Sox9cKO mice by RNA-seq (Fig. 2e). Inhba is the gene precursor to Activin A. We confirmed Activin A elevation by IF, demonstrating substantially increased Activin A in Sox9cKO BECs (Fig. 2f; Supplementary Fig. 4e). In both control and Sox9cKO tissues, Activin A expression was predominantly observed in BECs and the surrounding peribiliary region, as well as in rare hepatocytes (Supplementary Fig. 4e). Activin A inhibits branching morphogenesis in salivary glands, kidney, and prostate epithelium in vitro29,30. Previous studies have suggested that Activin A promotes BEC specification, although its role remains unclear2. To examine Inhba coincident with BEC specification, we analyzed published transcriptomic data from developing hepatoblasts, hepatocytes, and BECs31. We confirmed that Inhba is expressed at low levels in hepatoblasts and increases over time in both embryonic hepatocytes and embryonic BECs, supporting a potential role for Activin A in hepatoblast differentiation (Supplementary Fig. 4g).
a Bulk RNA-seq identifies differentially expressed genes between Sox9cKO and control BECs. Differential expression (DE) analysis was performed, and genes with a log fold change ≥1 and a q < 0.05 were considered statistically significant. b GSEA demonstrates significant enrichment of TGF-β signaling in Sox9cKO BECs relative to control BECs. c Co-localization of pSMAD2 and EpCAM confirms elevated TGF-β signaling in Sox9cKO BECs (scale bar represents 5μm; white asterisks indicate portal vein). d Targeted DE analysis of TGF-β receptor expression in bulk RNA-seq reveals that Tgfbr2 is the only significantly upregulated receptor and Amhr2 is the only downregulated receptor (asterisk indicates significance, p < 0.05). e Targeted DE analysis of TGF-β ligand expression in bulk RNA-seq reveals that the only significantly upregulated ligand is Inhba (TGF-β ligands that are not detected are not shown: Bmp2, Bmp15, Nodal, Gdf3, and Gdf8; asterisk indicates significance, p < 0.05). f IF demonstrates elevated Activin A in EpCAM+ BECs in Sox9cKO livers (scale bar represents 50 μm; white asterisks indicate portal vein). g GSEA demonstrates that Sox9cKO BECs are de-enriched for adult BEC gene signature (NES: −2.70; p = 3.67E-10) and enriched for adult hepatocyte gene signature (NES: 2.31; p = 3.67E-10). h GSEA of liver development gene sets shows that the Sox9cKO BEC transcriptome resembles embryonic BECs (NES: 1.23; p = 6.06E-6) and embryonic hepatocytes (NES: 2.32; p = 3.00E-10), but not hepatoblasts (NES: 0.99; p = 0.55). i Co-localization of NCAM1 and EpCAM is significantly elevated in Sox9cKO compared to controls (scale bar represents 5 μm; white asterisks indicate portal vein; p < 0.5, unpaired two-sided t-test; data are representated as). a-i, n = 3 biological replicates per group. b, g, and h gene set enrichment analysis was performed, and a p < 0.5 was considered significant. Source data are provided as a Source Data file.
We next asked if Sox9cKO-associated ductal paucity corresponded with long-term defects in BEC specification or maturation. GSEA of published gene signatures from adult mice demonstrated that Sox9cKO BECs were enriched for hepatocyte-associated genes and de-enriched for BEC-associated genes, suggesting a loss of BEC identity (Fig. 2g)32. We reasoned that loss of Sox9 at E10.5 in the Sox9cKO model could result in an inability to suppress the “default” hepatocyte differentiation program associated with liver development6,7. To further determine if Sox9cKO BECs are more embryonic-like, we performed GSEA with published RNA-seq from: (1) E12.5-E14.5 hepatoblasts, (2) E15.5-E17.5 BECs, and (3) E15.5-E17.5 hepatocytes31. Embryonic BEC and hepatocyte gene signatures were significantly enriched in adult Sox9cKO BECs, but hepatoblast genes were not, suggesting that Sox9 is required for BEC transcriptomic maturation, but dispensable for loss of hepatoblast identity (Fig. 2h). To validate incomplete BEC maturation, we performed IF for NCAM1, which is reported to be expressed exclusively by developing or injured BECs and was highly elevated in Sox9cKO BECs by RNA-seq (Fig. 2a, i; Supplementary Figs. 4d, e)33. While 24.5 ± 3.5% of BECs in healthy, adult controls are NCAM1+, this number rose to 91.4 ± 5.2 % in Sox9cKO mice (Fig. 2g). Taken together, these data suggest that Sox9cKO BECs are transcriptomically distinct from hepatoblasts but similar to embryonic BECs, consistent with a failure to fully acquire mature BEC identity. Additionally, these defects are associated with upregulation of Activin A, a ligand that represses branching in other tissues that is thought to regulate early BEC specification.
Sox9 has subpopulation-specific impacts on BEC gene expression
To determine if the loss of ductules reflects subpopulation-specific changes, we performed scRNA-seq on FACS-isolated EpCAM+ BECs from control and Sox9cKO mice. We identified 11 clusters across 6,152 BECs (3,498 control; 2,654 Sox9cKO) (Fig. 3a; Supplementary Fig. 5a; Supplementary Data 2). Most control and Sox9cKO BECs clustered separately, with the exception of custers 3, 8, and 10, which contained a mixture of control and Sox9cKO BECs (Fig. 3a; Supplementary Fig. 5b). We next examined gene expression of reported duct and ductule biomarkers34,35,36,37,38. These markers were either: (i) non-specific and expressed broadly across all clusters or (ii) not detected in enough cells to infer duct/ductule identity (Supplementary Fig. 5c). Our lab recently characterized a Sox9EGFP transgene, finding that IHBDs contain subpopulations of Sox9EGFP-high BECs which are enriched in ductules17. Using our previously published bulk RNA-seq, we performed GSEA, which demonstrated enrichment of Sox9EGFP-high genes in clusters 1, 2, 5, 6, and 8 (Supplementary Fig. 3d)17. These clusters were grouped and classified as “ductules”, while clusters 0, 3, 4, 7, 9, and 10 were grouped and classified as “ducts” (Fig. 3b). To validate distinct identity, we determined differentially expressed genes (DEGs) between control duct and ductule clusters, which identified 516 upregulated and 634 downregulated genes in control ductules (Supplementary Fig. 6; Supplementary Data 3). Interestingly, gene expression analysis also identified Ncam1 and Inhba upregulation specifically in the Sox9cKO ductule cluster (Fig. 3c, d). To validate ductule-specific upregulation in Sox9cKO livers, we quantified IF relative to duct diameter and found an enrichment of Activin A/EpCAM cells in ducts with smaller diameters (Fig. 3d, e). Taken together, scRNA-seq suggests that loss of Sox9 has broad transcriptomic impacts on BECs, but that Inhba and Ncam1 upregulation is driven by a ductule-like subpopulation.
a Control and Sox9cKO BECs can be classified into 11 transcriptomically distinct clusters. b Based on gene expression, BEC clusters were grouped into control duct (0 and 7), control ductule (1 and 2), Sox9cKO duct (clusters: 4 and 9), Sox9cKO ductule (5 and 6), and mixed population (clusters: 3, 8, and 10). c scRNA-seq of FACS-isolated EpCAM+ BECs reveals subpopulation-specific transcriptomic changes in Sox9cKO. d Upregulation of Inhba and Ncam1 in Sox9cKO samples is specific to Sox9cKO ductule-like cells. e Co-localization of EpCAM and Activin A confirms enrichment in Sox9cKO BECs (n = 3 biological replicates per group; p < 0.5, unpaired two-sided t-test). Duct diameter was measured as basolateral-to-basolateral distance using EpCAM as a marker of BEC cell membrane. Activin A was enriched in Sox9cKO IHBDs with smaller diameter (p < 0.5, unpaired t-test). Source data are provided as Source Data file.
Sox9 is required for normal biliary organoid formation and cystic morphology
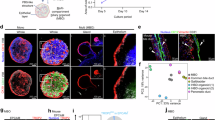
To determine if Sox9 is required for the establishment of biliary organoids, termed mouse intrahepatic cholangiocyte organoids (mICOs), FACS-isolated control and Sox9cKO BECs were plated as single cells and allowed to grow in mICO media conditions for 7 d39,40. Compared to controls, Sox9cKO BECs formed ~50% fewer mICOs (Fig. 4a, b). Strikingly, a majority of Sox9cKO mICOs failed to form the clearly defined lumens observed in control samples and characteristic of ductal epithelial organoids (Fig. 4a, c)40. We confirmed that these mICOs, which we termed “non-cystic”, continue to be viable by live cell imaging (Supplementary Movie 3). Despite non-cystic morphology, we observed no change in area or number of nuclei between control and Sox9cKO mICOs at 7 d of culture, consistent with our observation that proliferation is unaffected by loss of Sox9 in vivo (Fig. 4d; Supplementary Fig. 7a). Unlike control mICOs, which form a single lumen apically enriched in F-actin, non-cystic Sox9cKO mICOs either lacked a single, large lumen or had multiple, poorly-defined lumenal spaces (Supplementary Fig. 7a; Supplementary Movie 4). F-actin was apically polarized within both cystic and non-cystic mICOs (Supplementary Fig. 7a; Supplementary Movie 4). To assay proliferation, we quantified whole mount IF for Ki-67 and saw no change in the percent of proliferating nuclei between control and Sox9cKO mICOs (Supplementary Fig. 7b). To confirm our in vivo observations in vitro, we performed RT-qPCR and found loss of Sox9 and elevation of Ncam1 in Sox9cKO mICOs (Supplementary Fig. 7c). Inhba levels in Sox9cKO mICOs trended up compared to controls (Supplementary Fig. 7d). However, Inhba levels were significantly higher in Sox9cKO BECs in vivo when compared to mICOs, suggesting that culture conditions may suppress Inhba (Supplementary Fig. 7d). Together, these results establish that Sox9 is required for mICO formation and organization from single BECs in vitro.
a Single Sox9cKO BECs cultured for 7 d fail to form lumens typical of control mICOs (scale bars represent 50 μm). b Sox9cKO BECs form significantly fewer mICOs, a majority of which c exhibit non-cystic morphology. d mICO area is unchanged between control and Sox9cKO (p = 0.3630). e 50 ng/mL Activin A is sufficient to induce Sox9cKO morphology in WT BECs (scale bars represent 50 μm), but f unlike Sox9cKO (Sox9 Δ/Δ) did not impact mICO organoid formation (p = 0.0113). g Rates of non-cystic mICO morphology were similar between Sox9cKO and Activin A treated WT BECs (p < 0.0001). h Organoid area remained unchanged between all groups (p = 0.4680). i Ncam1 was significantly upregulated in Sox9cKO mICOs by RT-qPCR, and induced by Activin A in WT mICOs (p < 0.0001). j Activin A upregulates Sox9 expression in WT mICOs (p = 0.0004). a-d, i and j n = 3 biological replicates. f–h, n = 6 biological replicates. b–d and f–j, significance indicates a p < 0.5, and data are presented as mean values ± SEM. b–d, an unpaired two-sided t-test, was used. f–j Tukey’s test after one-way ANOVA was performed, and letters indicate grouping by significance. Source data are provided as a Source Data file.
Activin A signaling promotes non-cystic mICO phenotype
Next, we sought to determine if elevated Activin A is sufficient to promote the non-cystic phenotype observed in Sox9cKO mICOs. We isolated wild-type (WT) BECs from C57Bl/6 mice and plated them in mICO conditions with or without Activin A for 7 d. Sox9cKO BECs were isolated in parallel as a positive control for non-cystic morphology. Importantly, Y-27632 has been shown to reduce TGF-β2 signaling in human trabecular organoids but has no established connection to Activin A, and no other mICO media components are known to impact Activin A signaling41. Treating WT BECs with Activin A did not recapitulate loss of organoid formation seen in Sox9cKO mICOs, but drove non-cystic morphology at approximately the same rate observed in Sox9cKO mICOs (Fig. 4e–g). Activin A did not impact mICO size (Fig. 4h). RT-qPCR revealed that Ncam1 was significantly upregulated by Activin A in WT mICOs, similar to levels observed in Sox9cKO (Fig. 4i). We also found that Sox9 was upregulated in Activin A treated mICOs (Fig. 4j). These data support a link between Activin A and functional and gene regulatory defects observed in Sox9cKO BECs.
To confirm that downstream Activin A signaling is responsible for non-cystic mICO morphology, we isolated and cultured single control and Sox9cKO BECs for 7 d in standard mICO conditions with or without SB-431542, an activin receptor-like kinases 4/5/7 (ALK4/5/7) inhibitor that prevents SMAD2/3 phosphorylation. While SB-431542 did not impact the number of mICOs formed, it completely rescued non-cystic mICO morphology in Sox9cKO samples and increased organoid size in both control and Sox9cKO mICOs (Fig. 5a–d). Ncam1, which was elevated in untreated Sox9cKO BEC organoids relative to controls, was significantly downregulated in both control and Sox9cKO mICOs by SB-431542 (Fig. 5e). Additionally, Sox9 was significantly downregulated by SB-431542 in controls (Fig. 5f). These results demonstrate that ALK4/5/7-mediated signaling is responsible for the non-cystic morphology observed in Sox9cKO mICOs and positively regulates Sox9 and Ncam1 expression. With in vivo data demonstrating increased Activin A in Sox9cKO BECs, our mICO data suggest that Activin A signaling drives a negative feedback loop by upregulating Sox9.
a Culturing single BECs with 10 μM SB-431542 for 7 d rescues non-cystic morphology in Sox9cKO mICOs (scale bar represents 50 μm). b Decreased mICO formation by Sox9cKO BECs is not impacted by SB-431542 (p < 0.0001), but c non-cystic morphology is completely rescued (p < 0.0001). Notably, rare non-cystic mICOs in the control group are also significantly reduced (letters indicate grouping by significance, p < 0.5, one-way ANOVA). d SB-431542 treatment increases mICO size independent of Sox9 (p = 0.0062). e RT-qPCR demonstrates that SB-431542 downregulates Ncam1 in control and Sox9cKO mICOs (p = 0.0020) and f Sox9 in control mICOs (p < 0.0001). a–f, n = 3 biological replicates. b–f significance indicates a p < 0.5, data are presented as mean values ± SEM, Tukey’s test after one-way ANOVA was performed, and letters indicate grouping by significance. Source data are provided as a Source Data file.
Late IHBD development proceeds via proliferative expansion of a continuous ductal “web”
To determine timepoints when Sox9 might promote ductule morphogenesis, we applied iDISCO+ to IHBD development. We imaged EpCAM+ IHBDs in WT livers at E15.5, E17.5, P1, P5, and P14. Consistent with previous reports, IHBDs formed at the hilum at early developmental timepoints and advanced toward the periphery through P5 (Fig. 6a)10. We noted very little discontinuity between BECs, which was previously reported through E1710. While rare peripheral ductal structures were disconnected from the main mass of EpCAM+ cells at E15.5, no disconnected structures were observed at E17.5 or later (Fig. 6a, b). Ductal structures were present as a tight “web” lacking a clear hierarchy of ducts and ductules from E15.5 through P1 (Fig. 6a, b). While clear duct-ductule hierarchy was still lacking at P1, rare segments began to exhibit slightly enlarged size (Fig. 6c). By P5, distinct large ducts webbed with peripheral ductules were observed (Fig. 6a). Even by P14, IHBDs continue to be webbed and lack the distinct, interlobar large ducts that are characteristic of adult IHBDs, though minimally webbed large ducts appear to be emergent at the hilum (Fig. 6d). These data reinforce that IHBD morphogenesis is a dynamic postnatal process, with IHBDs starting to demonstrate a clear hierarchy by P5, but not resembling a fully mature morphology by 2 weeks of age.
a IHBDs develop primarily as an interconnected web of EpCAM+ BECs from E15.5 through P14, originating at the hilum and extending to the periphery by P1, with rare disconnected BECs visible closer to the periphery at E15.5 (yellow arrowhead marks non-continuous BECs; scale bar represents 0.5 mm). b High-magnification images demonstrate discontinuous BECs at E15.5, which are absent by E17.5 (scale bars represent 150 μm; dashed blue lines denote portal veins). c At P1, early duct-ductule hierarchy begins to emerge as some IHBDs become enlarged but remain intertwined with web-like peripheral IHBDs (scale bars represent 150 μm; yellow arrowhead denotes enlarged IHBD; dashed blue lines denote portal veins). d Duct-ductule hierarchy is clearly established at P14, but large hilar ducts continue to be surrounded by web-like peripheral IHBDs (scale bars represent 150 μm). e Co-localization of Ki-67 and EpCAM reveals proliferative BECs throughout IHBD development (scale bar represents 50 μm; white asterisks indicate portal vein). f Quantification of BEC proliferation reveals abundant embryonic proliferation and decreasing, but high proliferation rates relative to adult tissue during postnatal IHBD development (p < 0.0001). g EpCAM+ BECs per portal field trend upwards E15.5-P5 until decreasing P14 and into adulthood (p = 0.0847). f, g significance indicates a p < 0.5, data are presented as mean values ± SEM, Tukey’s test after one-way ANOVA was performed, and letters indicate grouping by significance. Source data are provided as a Source Data file.
Models for IHBD development focus on BEC specification from hepatoblasts, but proliferation of BECs post-specification is less well-characterized. To determine the contribution of BECs to IHBD development, we quantified Ki-67 at the same timepoints assayed by iDISCO+. 37.7 ± 4.6% of BECs expressed Ki-67 at E15.5 and 41.0 ± 3.4% at E17.5 (Fig. 6e, f). Postnatally, BEC proliferation dropped to ~20% at P1 through P14 (Fig. 6e, f). A final decrease in BEC proliferation occurred between P14 and adulthood with 3.30 ± 1.1% of BECs proliferating at 8wks of age, consistent with proliferation rates in adult control and Sox9cKO livers (Fig. 6e, f; Supplementary Fig. 2c). To determine if there is a relationship between BEC proliferation and IHBD expansion, we quantified BECs per portal field using conventional IF. BEC numbers followed a similar trend to proliferation, initially increasing in number and then trending downwards postnatally (Fig. 6e, g). This downward trend is coincident with postnatal IHBD remodeling and the emergence of duct-ductule hierarchy. These data suggest that BECs are key contributors to IHBD expansion during development following hepatoblast differentiation, and may have substantial impacts on adult IHBD morphology.
Loss of Sox9 results in early postnatal ductal paucity
Conventional histology and iDISCO+ revealed that P5 IHBDs are highly proliferative and demonstrate early signs of duct-ductule hierarchy. To study the impact of Sox9 on IHBD morphogenesis, we collected livers from P5 control and Sox9cKO mice. We measured duct diameter and colocalized EpCAM and SOX9 cells lining the IHBD lumen in P5 mice. Overall, Sox9 recombination was highly efficient, with only 6.7 ± 1.0% of BECs still expressing SOX9 (Supplementary Fig. 1C). BECs that escaped recombination increased as IHBD diameter increased, but remained at or under 41% in all ducts examined (Supplementary Fig. 1d). We found that P5 Sox9cKO mice exhibit ductal paucity compared to control livers, consistent with adult samples (Fig. 7a, b). The percent of Ki-67+/EpCAM+ BECs was unchanged between P5 Sox9cKO and control tissues, supporting that ductal paucity in Sox9cKO livers is not caused by reduced proliferation, even in developing IHBDs (Fig. 7a, c). To examine global IHBD morphogenesis, we subjected P5 livers to iDISCO+. Similar to large ducts in adult mice, P5 Sox9cKO mice exhibited ducts extending from the hilum to the periphery with no morphological defects (Fig. 7d, e; Supplementary Movie 5). However, these main branches were largely devoid of the peripheral ductules observed at P5 in WT and control livers (Fig. 7d, e; Supplementary Movie 5). To determine changes to IHBD branch complexity in control and Sox9cKO livers, we performed Sholl analysis on the whole liver specifying the liver hilum as the starting point. Unlike adult Sox9cKO livers, which showed changes localized to the periphery, Sholl analysis demonstrated a loss of IHBD complexity across entire P5 Sox9cKO livers (Fig. 7f; Supplementary Fig. 8a). This result was confirmed by large decreases in both proximal (0.0–0.5) and distal (0.5–1.0) AUC in Sox9cKO livers (Supplementary Fig. 8b). Sholl decay was unchanged between P5 control and Sox9cKO livers, reflecting uniform branch density within each group and reinforcing a global loss of branching in Sox9cKO (Supplementary Fig. 8c). This universal loss of branching in P5 Sox9cKO tissue was further apparent in reduced average branch density, as well as actual and calculated maximum number of branch point intersections (Supplementary Figs. 8d-f). P5 Sox9cKO IHBDs also decreased in overall length and volume (Supplementary Fig. 8g, h). The distance at which maximum branching occurs (rc) was unchanged between control and Sox9cKO, further reinforcing global changes in IHBD morphology in developing Sox9cKO livers (Supplementary Fig. 8i). Together, these data demonstrate that ductal paucity in adult Sox9cKO mice originates during development and suggest that Sox9 is critical for morphogenesis of web-like precursors to ductules.
a Co-localization of Ki-67 and EpCAM at P5 (scale bar represents 50 μm; white asterisks indicate portal vein) shows that Sox9cKO mice exhibit b significant loss of BECs per portal field, but (c) no change in proliferation. d Whole tissue imaging of P5 livers reveals substantial loss of web-like peripheral IHBDs in Sox9cKO (scale bar represents 0.5 mm). e To visualize ducts and ductules, we masked positive signal and colored the mask based on volume between branch points. Ducts were defined as having a volume ≥2.0e5 μm3 (illustrated in yellow; scale bar represents 0.5 mm). Ductules were defined as having a volume <2.0e5 μm3 (illustrated in red). Periportal regions were isolated and enlarged (right panels). Sox9cKO IHBDs exhibit broad ductule loss. f P5 Sholl analysis demonstrates a loss of branching throughout the liver. g E17.5 whole tissue image of IHBDs labeled with EpCAM exhibit unchanged branching between control and Sox9cKO (scale bars represent 0.5 mm). h E17.5 Sholl analysis confirmed no changed in branching between control and Sox9cKO. a–c n = 3 biological replicates per group. d–f, n = 2 whole livers per group. g, h n = 3 whole livers per group. b and c significance indicates a p < 0.5, an unpaired two-sided t-test was used, and data are presented as mean values ± SEM. Source data are provided as a Source Data file.
Due to significant loss of branching by P5 Sox9cKO IHBDs, we looked to E17.5 to determine if ductal paucity arises at the earliest stages of IHBD morphogenesis. While more SOX9+ “escaper” BECs were observed at E17.5 than at P5, a majority were SOX9- (23.5 ± 12.2% SOX9+/EpCAM+) (Supplementary Fig. 1e). At E17.5 and P5, several SOX9+ EpCAM- cells were observed surrounding control portal fields which were absent in Sox9cKO, and may represent uncommitted, intermediate hepatobiliary progenitors (Supplementary Figs. 1c, e; arrowheads). Intriguingly, whole tissue imaging at E17.5 revealed no change in proximal or distal IHBD density between control and Sox9cKO (Fig. 7g). We confirmed this using conventional histology, which also showed no change in EpCAM+ cells per portal field (Fig. 7g; Supplementary Fig. 9a). Sholl and AUC analyses further supported this finding, with no change in branch complexity proximally or distally between E17.5 control and Sox9cKO (Fig. 7h; Supplementary Figs. 9b–e). While there was no change in IHBD length between control and Sox9cKO, IHBD volume trended down in E17.5 Sox9cKO IHBDs, but did not reach significance (Supplementary Figs. 9f, g). Taken together, these results reveal that Sox9 is required after E17.5 for IHBD patterning and maturation.
Activin A inhibition partially rescues branching morphogenesis in Sox9cKO livers
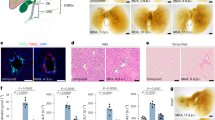
We reasoned that Activin A inhibition might rescue Sox9cKO IHBD morphology during development, similar to the phenotypic rescue observed in Sox9cKO mICOs. We treated Sox9cKO pups at P1 and P3 with an anti-Activin A (α-ActA) monoclonal antibody, previously shown to neutralize Activin A in vivo, or IgG control (Fig. 8a)42. Conventional IF demonstrated that α-ActA treatment increased BEC numbers per portal field compared to IgG-injected Sox9cKO mice (Fig. 8b, c). Surprisingly, neutralization of Activin A decreased the number of Ki-67+ BECs, as well as overall liver volume as measured by light-sheet imaging (Fig. 8b, d; Supplementary Fig. 10a). These data suggest that Activin A promotes BEC proliferation and liver size while inhibiting BEC specification during postnatal development.
a Workflow demonstrating 2 µg/g Activin A neutralizing antibody (α-ActA) or IgG antibody injection occurs on P1 and P3 with tissue collection on P5. b Co-localization of Ki-67 and EpCAM in IgG and α-ActA treated livers demonstrates that Activin A neutralization in vivo (scale bar represents 50 μm; white asterisks indicate portal vein) c increases BEC numbers per portal field while d inhibiting BEC proliferation. e Whole tissue imaging of EpCAM+ IHBDs reveals that Activin A neutralization partially rescues Sox9cKO ductal paucity, resulting in increased branching near the hilum (scale bar represents 0.5 mm). f Sholl analysis supports increased proximal branching and decreased distal branching in α-ActA treated livers, which is corroborated by (g) AUC demonstrating increased hilar branch complexity (0.00–0.25 a.u.), no change at 0.25–0.50 a.u., and decreased branching distally (0.50–1.00a.u.). h Sholl decay coefficient is increased following Activin A neutralization reflecting increased localized branching near the hilum. b–d, n = 3 biological replicates per group. c and d significance indicates a p < 0.5, an unpaired two-sided t-test was used. c, d, and g data are presented as mean values ± SEM. e–h n = 2 whole livers per group. Source data are provided as a Source Data file.
To determine if Activin A impacts duct vs. ductule morphogenesis, we performed iDISCO+ on α-ActA and IgG P5 Sox9cKO livers. Light sheet imaging revealed a clear web-like ductule network surrounding the main ducts near the hilum of α-ActA Sox9cKO mice, absent in IgG samples (Fig. 8e; Supplementary Movie 6). Sholl analysis confirmed increased IHBD branch intersections near the liver hilum in α-ActA Sox9cKO (Fig. 8f; Supplementary Fig. 10b). Because the rescue was observed near the hilum, we carried out AUC analysis on quartiles of normalized Sholl intersections. AUC was increased in α-ActA Sox9cKO livers in the most proximal quartile (0.00–0.25a.u.) and decreased approaching the periphery (0.50–0.75 a.u. and 0.75–1.00 a.u.) (Fig. 8g). Decreased peripheral branching may be related to decreased proliferation in α-ActA. Increased hilar branching in α-ActA Sox9cKO livers was further supported by increased Sholl decay coefficient (Fig. 8h). No differences were noted between IgG and α-ActA livers for Nav, Max, rc, or total IHBD length and volume, which may be attributed to the shift in branching from the periphery to the hilum in α-ActA (Supplementary Figs. 10c–g). A slight decrease in Nm was observed, consistent with differences in peripheral branching (Fig. 8f; Supplementary Fig. 10d). These data support a critical role for Activin A in postnatal IHBD branching morphogenesis, where it promotes BEC proliferation but limits web-like branching from main ducts.
Discussion
Sox9 is one of the earliest markers of BECs in the ductal plate and is associated with BEC identity. Previous studies have shown that Sox9 regulates the timing of early BEC specification and that its absence can worsen ductal paucity in a mouse model of Alagille Syndrome (ALGS), but it is thought to be dispensable for IHBD formation9,43. In ALGS, SOX9 levels are linked to the severity of the disease, showing reduced levels in severe cases43. By applying an in toto imaging approach to IHBD morphology, we show here that loss of Sox9 in hepatoblasts results in normal IHBD morphology through E17.5 but a failure to form web-like ductal structures in postnatal IHBD development. Importantly, this manifests as lifelong disorganization of IHBD architecture in adult mice, characterized by reduced numbers of peripheral ductules. The lack of ductal paucity at E17.5 suggests Sox9 does not play a critical role in initial biliary specification but is required for postnatal branching morphogenesis. Comparing developmental and adult whole-tissue imaging of IHBDs suggests that adult ductules are derived directly from web-like structures surrounding the portal vein during development. These are notably absent in Sox9cKO livers at P5, while ducts running parallel to the portal vein persist, identifying distinct genetic requirements for developmental morphogenesis of ducts and ductules. Ductules may also arise, in part, from hepatocytes in late development, which is a potential explanation for why we observe a loss of ductules, but no change in BEC proliferation in Sox9cKO livers at P5. This has been suggested as a compensatory mechanism in diseases associated with ductal paucity and shown in a mouse model of Alagille Syndrome32,44,45. It would also be consistent with observations of SOX9+, EpCAM- cells with hepatocyte morphology at E17.5 and P5, observed here in control mice. Because our Sox9cKO mice lack Sox9 in both BECs and hepatocytes, our data support an important role for Sox9 in ductule development, regardless of underlying precursor populations or cellular mechanisms resulting in ductule morphogenesis.
Mechanistically, we show that Sox9 represses Inhba/Activin A, which is a well-established inhibitor of branching morphogenesis in multiple tissues29,30. BEC specification is known to involve a gradient of TGF-β and Activin A that is highest near the portal vein2. Our analyses show that BECs produce Inhba/Activin A, which may contribute to an autocrine or paracrine “niche” that promotes TGF-β signaling and regulates local IHBD architecture. As with morphological defects, elevated TGF-β signaling, and Ncam1 expression are present in Sox9cKO ductule-like cells, suggesting that Sox9 plays a key role in BEC maturation by repressing Activin A signaling in a subpopulation-specific manner.
In postnatal livers, Activin A inhibition rescues hilar branching, providing functional evidence for a Sox9-Inhba relationship and demonstrating that downregulation of Activin A is sufficient to rescue ductule morphogenesis even in the absence of Sox9. This aligns with work in other tissues showing that extended treatment of tissue explants with Activin A can result in a complete loss of branched epithelial networks30. It is possible that Sox9cKO IHBD branching rescue is highest at the hilum because Activin A neutralizing antibodies may not penetrate throughout the entire tissue. Circulating molecules introduced by i.p. injection primarily travel through the portal vein, so antibodies would likely be at the highest concentration in the liver near the hilum46. While mICO culture conditions do not produce branching structures, epithelial organoids form through 3D self-organization, which may parallel morphogenic processes in vivo47,48. It is notable that Sox9cKO mICOs also exhibit morphological defects that can be modulated by Activin A signaling. Increasing or inhibiting Activin A signaling in vitro is sufficient to: (i) induce morphological defects and Sox9 and Ncam1 expression in WT mICOs, and (ii) rescue lumenal phenotypes in Sox9cKO mICOs, respectively. These data point to a negative feedback loop, where Activin A induces Sox9, which in turn represses Inhba/Activin A. Our IF data suggest that BECs are an important source of Activin A but that other cells in the peribiliary niche may contribute as well. Collectively, our data establish the repression of Activin A by Sox9 as an important event in BEC maturation and IHBD branching morphogenesis. Interestingly, high Activin A was recently shown to be a negative predictor of jaundice clearance following hepatoportoenterostomy in biliary atresia, suggesting an inverse relationship between Activin A and IHBD function in humans49. We consider it likely that other signaling pathways also play a role in fine-tuning IHBD branching morphogenesis, including established regulators like Yap and Notch, and may provide alternative explanations for partial rescue of Sox9cKO branching by Activin A inhibition in vivo7.
Technical limitations in whole-tissue imaging of adult and developing livers, including tissue size, density, and opacity, have been long-standing barriers to studying IHBD biology. Models for IHBD development have relied on computational reconstruction of tissue sections or retrograde ink injection of ductal lumens10,50. The latter is particularly advantageous in avoiding technical artifacts associated with tissue sectioning and image reconstruction. However, ink injections only label continuous structures, may not perfuse into finer ductules, and are not amenable to quantitative analysis. In contrast, iDISCO+ and light sheet microscopy facilitate direct, sensitive visualization of BECs by IF without physical manipulation of IHBD lumens. Notably, we observe that pronounced, large-diameter ducts are not clearly established until P5, in conflict with earlier ink injection studies describing emergence of duct-ductule hierarchy at E17.510. It is possible that introducing ink into developing IHBDs may increase intraluminal forces and, subsequently, the diameter of main branches in the developing ductal web. Surprisingly, we find that IHBDs continue to exhibit highly ramified ductule branches as late as P14, and do not have reduced numbers of ductules surrounding large hilar ducts, as observed in 6-12wk old mice. Together, these data establish iDISCO+ and light-sheet microscopy as valuable tools for understanding IHBD biology and underscore the need to expand understanding of postnatal IHDB remodeling.
Whole-tissue imaging also builds on conventional models of early IHBD expansion. Newly specified BECs are thought to form at and expand away from the hilum as discontinuous luminal cysts, interconnecting to form IHBDs1,50. Consistent with this model, we observed rare peripheral EpCAM+ cells at the edges of IHBD webs at E15.5. However, by E17.5 and beyond, all EpCAM+ cells appear integrated into a continuous IHBD network. Because we do not examine earlier timepoints, it is possible that joining of discontinuous cysts is the predominant mechanism for formation of the hilar IHBD web that is apparent by E15.5. Additionally, our studies rely on the expression of EpCAM+ as it is amendable to iDISCO+ protocols. However, it remains possible that very early BECs or BEC precursors that do not yet express EpCAM may contribute to IHBD expansion in a discontinuous manner, even at timepoints later than E15.5.
BEC proliferation during IHBD development has also remained ambiguous. Prior studies speculated that BECs have minimal contributions to IHBD growth after specification from hepatocytes, implying that IHBD development is driven primarily by continued differentiation of a progenitor population51. However, recent scRNA-seq and chromatin assays have established that the liver is devoid of cells with genetic characteristics of hepatoblasts after E14.5, calling into question how IHBDs continue to expand after this time31. Our embryonic biliary proliferation results demonstrate more proliferation than prior qualitative assessment of Ki-67 by IF, but are consistent with recent quantification by flow cytometry6,52. We also find that postnatal biliary proliferation remains robust through at least P14, further emphasizing contributions of specified BECs to developing IHBDs. Interestingly, inhibition of Activin A with neutralizing antibodies in vivo suggests that Activin A promotes BEC proliferation, while prior in vitro studies show that Activin A inhibits hepatocyte proliferation53. These results, along with upregulation of Inhba expression in early BECs and hepatocytes, point to cell type specific roles for Activin A that may reflect intrinsic regulatory differences in early epithelial lineage divergence from hepatoblasts. Further work will be required to understand the regulation of regional BEC proliferation, as well as how embryonic expansion and postnatal remodeling of IHBDs contribute to adult morphology.
SOX9 is known to promote branching morphogenesis in other tissues, where it is often localized to actively advancing or branching tips. One key difference in the liver is that SOX9 is expressed by all intrahepatic BECs9. Our data affirm that IHBDs undergo complex branching morphogenesis to form a Sox9-dependent webbed structure of IHBDs that has been described as a biliary plexus1. It is possible that variable local levels of Sox9 in IHBDs could drive branch formation. Heterogeneous expression of Sox9 has been reported in the pancreatic ducts, which exhibit similarly “stochastic” branching, with highest Sox9 expression in small terminal ducts54. Similarly, our lab has previously shown that Sox9 levels are heterogeneous in IHBDs, with highest Sox9 levels in ductules17. Therefore, it is possible that high Sox9 levels drive spatially localized branching programs in parallel with epithelial maturation, in part through suppression of Activin A. Collectively, our studies refine models for IHBD development by identifying distinct genetic requirements for Sox9-independent morphogenesis of large ducts and Sox9-dependent morphogenesis of small ductules. Defining the molecular regulation of IHBD morphological heterogeneity is important for understanding human developmental cholangiopathies and may yield new opportunities for therapeutic intervention.
Methods
Animal studies
Sox9fl/fl mice (JAX #013106) were crossed to Albumin-Cre (JAX #003574) mice to establish Sox9cKO (Sox9fl/fl:AlbCre) AlbCre was maintained at heterozygosity. Mice were of mixed background and adults were 6–12 wks of age at tissue collection. Mice were housed in an environment maintained at ~22 °C with 40–50% relative humidity and subjected to a 12-h light-dark cycle. Animals were supplied with water and chow ad libitum. Timed pregnancies were performed by breeding 1–2 female mice with 1 male mouse and checking for the presence of a vaginal plug between 6:00 and 8:00 A.M. the following morning. Upon detection of a vaginal plug, females were solo housed. The detection of a vaginal plug was denoted as gestational day 0.5. Males and females were used throughout the study. Mice were PCR genotyped using published primers20,55. For liver injury, mice were administered 0.3% (wt/vol) thioacetamide (TAA) ad libitum in drinking water for 1 month.
To neutralize Activin A, control and Sox9cKO neonates were administered 2 µg/g anti-Activin A (Biotechne; MAB3381) or mouse IgG1 (Biotechne; MAB002) in sterile PBS at P1 and P3 by intraperitoneal injection. Injection volumes were 30 µL per mouse. Neonates were anesthetized for injection by indirect immersion to the neck in wet ice for 2–4 min as previously described56. Liver tissue was collected at P5. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University.
Perfusion and tissue processing
Adult and 2wk animals were sacrificed and tissues fixed by intracardiac perfusion with cold PBS followed by cold 4% paraformaldehyde (PFA; Thermo Fisher; 41678-5000) using a peristaltic pump (Kamoer DIpump550). Embryos and neonatal mice under 10 days of age were not perfused. Instead, livers were dissected, swirled in Dulbecco’s Phosphate-Buffered Saline (DPBS; Thermo Fisher; 14190250) to wash off excess blood, and drop fixed in cold 4% PFA. For all samples, dissected livers were fixed overnight in 4% PFA at 4 °C. The next day, tissues for whole-mount imaging were moved to 0.05% sodium azide (Ricca Chemical; RC7144.8) in PBS at 4 °C until further processing. Tissues for conventional histology were moved to 30% sucrose overnight, then embedded in Tissue-Tek Optimal Cutting Temperature media (Sakura Finetek USA, Torrance, CA) and frozen on dry ice. Conventional histology tissues and sections were stored at –80 °C until use.
Intrahepatic bile duct dissociation and FACS
BECs were dissociated as previously described, with minor modifications17,40. Liver lobes were dissected avoiding extrahepatic duct and gallbladder to prevent contamination of intrahepatic biliary preps with extrahepatic biliary tissue. Tissue was rinsed in DPBS and then minced into small pieces (<0.5 cm2) using a razor blade. The sample was transferred to a 50 mL conical and suspended in prewarmed 37 °C Liver Wash Buffer [LWB; DMEM-H (Fisher Scientific; 11965118), 1% fetal bovine serum (Genesee Scientific; 25-525H), 1% GlutaMAX (Fisher Scientific; 35050061), 1% Pen/Strip (Fisher Scientific; 15140122)]. Tissue was allowed to sediment and then supernatant discarded. The tissue was resuspended in LWB and then discarded after sedimentation two more times. The tissue was pelleted in a swinging bucket centrifuge at 4 °C and 600 g for 5 min. Supernatant was discarded and the sample was resuspended in room temperature (RT) Liver Digest Buffer [0.15 mg/mL collagenase type XI (Sigma Aldrich; C9407), 0.3 U/mL dispase (Corning; 354235), 200 µg/mL DNase (Sigma Aldrich; DN25)] and incubated at 37 °C on a rotator for 90 min. Every 30 min the sample was retrieved and resuspended by pipetting vigorously with a P1000 micropipette set to 1 mL to further promote liver dissociation. After 90 min, an equivalent volume of LWB was added to the dissociation and the tissue was pelleted at 4 °C and 200 g for 5 min. The supernatant was carefully decanted to ensure the pellet remained intact and then the sample was washed again with LWB, pelleted, and supernatant decanted. Samples were resuspended in 5 mL RBC lysis buffer (BioLegend; 420301) diluted in molecular grade water and incubated on ice for 5 min with periodic agitation. RBC Lysis was quenched using 15 mL DPBS and the sample was pelleted at 4 °C and 600 g for 5 min and supernatant decanted. For whole duct organoid experiments, dissociation was stopped at this point and duct fragments were used for organoid culture.
To further dissociate liver tissue to single cells for FACS, samples were resuspended with 3 mL TrypLE (Fisher Scientific; 12605010) + 10 µM Y-27632 (Selleck Chemicals; S1049) and incubated at 37 °C for 12 min. Every 2 min, the sample was pipetted vigorously for 1 min with a P1000 micropipette set to 1 mL, to mechanically dissociate IHBDs. After 12 min, the TrypLE was quenched using 10 mL DPBS, and the sample was pelleted at 4 °C and 600 g for 5 min. The supernatant was decanted, and pellet resuspended in Sort Media [Advanced DMEM/F12 (Thermo Fisher; 12634028) with 2% B27 supplement without vitamin A (Thermo Fisher; 12587010), 1% N2 supplement (Thermo Fisher; 17502048), 2 mM Glutamax (Thermo Fisher; 35050061), 10 mM HEPES (Thermo Fisher; 15630080), 100 U/mL Pen/Strip (Fisher Scientific; 15140122), and 0.1% DNase (Sigma-Aldrich; DN25)]. After thoroughly resuspending, sample was filtered through a 40 µm cell strainer (Falcon; 352340).
Single cells were stained with anti-CD31-APC (1:100) (BioLegend; 102510), anti-CD45-APC (1:100) (BioLegend; 103112), and anti-CD326-APC/Cy7 (1:100) (BioLegend; 118218) for 1 h on ice. Cells were rinsed in Sort Media, pelleted at 4 °C and 600 g for 5 min, and supernatant decanted. Cells were resuspended in sort media. 5 uL of 7-AAD (BioLegend; 420404) and 5 μL Annexin V-APC (BioLegend; 640941) were added to resuspended cells to distinguish dead and dying cells, respectively. Cells were immediately analyzed and collected on a Sony SH800S fluorescence-activated cell sorter or BD FACSAria II (BD Biosciences). Gating schemes are shown in Supplementary Fig. 2a.
Mouse intrahepatic biliary organoid culture
8000 cells per sample for organoid growth experiments or 20,000 cells for organoid staining experiments were isolated using FACS and collected into 500 μL of Sort Media in 1.5 mL polypropylene tubes for biliary organoid culture. Cells were centrifuged at 6000 g for 5 min. Pellet was visualized by eye, supernatant was discarded, and cells were resuspended in 1 part Advanced DMEM/F12 and 2 parts Cultrex Reduced Growth Factor Basement Membrane Extract (R&D Systems; 3533-010-02) for a final solution of 66% Cultrex. 96 well plates were prewarmed to 37 °C and cell suspensions were plated as 10 μL droplets and allowed to polymerize at 37 °C for 30 min. After polymerization, Biliary Single Cell Media [50% Advanced DMEM/F12, 40% WNT3A-conditioned media, 10% RSPO1-conditioned media, 2% B27 supplement without vitamin A, 1% N2 supplement, 2 mM Glutamax, 10 mM HEPES, 100 U/mL Pen/Strip, 50 ng/mL recombinant murine EGF (Thermo Fisher; PMG8041), 100 ng/mL recombinant human Noggin (VWR International; 120-10 C), 100 ng/mL recombinant human FGF10 (Peprotech; 100-26), 10 nM recombinant human gastrin (Sigma; G9145), 5 ng/mL recombinant human HGF (PeproTech; 100-39H), 10 mM nicotinamide (Sigma Aldrich; N0636-100G), 10 µM forskolin (Selleck Chemicals; S2449), and 10 µM Y-27632]. Media was changed every other day. For treatments with 50 ng/mL Activin A (BioLegend; 592002) or 10 μM SB431542 (Selleck Chem; S1067), the reagents were added to the Biliary Single Cell Media for the entire culture period. At the end of 7 days, organoids were analyzed for organoid formation, area, and morphology. Organoid area was measured using bright field images taken using Olympus IX-83 inverted epifluorescent microscope and cellSens Imaging software. Non-cystic morphology was defined as biliary organoids that lack a well-defined lumen. For gene expression analysis, organoids were resuspended in 500 μL RNA Lysis Buffer (Invitrogen RNAqueous Micro Kit; AM1931) and lysates were stored at −80 °C.
For whole-mount organoid experiments, organoids were grown from ductal fragments. Like single cells, ductal fragments were resuspended in 2 parts Cultrex and 1 part Advanced DMEM/F12. Single-cell media was used for the first 2 days in culture following biliary isolation or passaging. After 2 days, the media was changed to Biliary Expansion Media [90% Advanced DMEM/F12, 10% RSPO1-conditioned media, 2% B27 supplement without vitamin A, 1% N2 supplement, 2 mM Glutamax, 10 mM HEPES, 100 U/mL Pen/Strip, 50 ng/mL recombinant murine EGF, 100 ng/mL recombinant human FGF10, 10 nM recombinant human gastrin, 5 ng/mL recombinant human HGF, 10 mM nicotinamide, and 10 µM forskolin]. Organoids were passaged every 5–7 days.
For statistical comparison, one-way ANOVA was used. A value of p < 0.05 was considered significant. Statistical analyses were carried out in Prism 10.1.0. All values are depicted as mean ± SEM.
Immunofluorescence on tissue sections
10 μm tissue sections were cut on a cryostat (Leica; CM1860) and allowed to dry for 4–6 h at RT. Sections were washed 3 × 5 min with PBS, permeabilized for 20 min with 0.3% Triton X-100 (Sigma; T8787-250ML) in PBS, and then blocked in 5% normal donkey serum (NDS; Jackson Immuno; 017-000-121) for 45 min. If using mouse primary antibody, tissues were then blocked with 1x mouse on mouse (MOM) blocking reagent (Vector Laboratories; MKB-2213) in PBS for 1 h, washed 2 × 5min with PBS, and rinsed briefly with PBS twice before proceeding with protocol. As a control for non-specific MOM staining, we stained tissue with secondary antibody only. For all slides, primary antibody was applied overnight at 4 °C in PBS. The next day, primary antibody solution was discarded, and slides were rinsed 3 × 5 min with PBS. Secondary antibody raised against the host species of the primary antibody was applied in PBS for 1 hr at RT. IF staining for F-actin, phalloidin-647 (Abcam; ab176759) was applied 1:1000 with secondary. After staining, antibody solution was discarded and bisbenzimide (Sigma; 14530) was applied at 1:1000 in PBS for 10 min to label nuclei. Slides were then washed 3 × 5 min with PBS, mounted with ProLong Glass Antifade (Thermo Fisher; P36980), and allowed to cure overnight at RT in the dark. Images were acquired on a laser scanning Nikon A1R HD25 Confocal Microscope (40× and 60× oil objectives with 0.14 mm working distance) or the Nikon CSU-W1 SoRa Spinning Disk (60× oil with 0.12 mm working distance) in the Emory Integrated Cellular Imaging. To image polarity markers OPN and F-actin, a 10 μm z-stack was acquired with a step size of 0.2 μm. When IHBD diameter was measured on tissue sections, EpCAM was used as a cell border marker. FIJI was used to measure the diameter across the bile duct from the basolateral side of each BEC. Acquisition settings were consistent between control and Sox9cKO samples. Statistical analyses were carried out in Prism 10.1.0 (GraphPad Software). For statistical comparison, unpaired two-sided t-test was used. A value of p < 0.05 was considered significant. Statistical analyses were carried out in Prism 10.1.0. All values are depicted as mean ± SEM.
Antibody usage for immunostaining
Primary antibodies were used at the following concentrations for immunostaining: 2.5 μg/mL anti-Activin A (R&D Systems; MAB3381), 0.75 μg/mL anti-pSMAD2 (Ser465/467) (Cell Signaling; 3108 T), 2.5 μg/mL anti-EpCAM/CD326 (BioLegend; 118201), 1 μg/mL anti-Ki-67 (Invitrogen; PA5-19462), 7 μg/mL anti-Ac-alpha-Tubulin (K40)(Cell Signaling; 5335), 0.16 μg/mL anti-Cleaved Caspase 3 (D175) (Cell Signaling; 9661), 0.2 μg/mL anti-NCAM1 (Cell Signaling; 99746 T), 5 μg/mL anti-OPN/SPP1 (R&D; AF808), 1 μg/mL anti-SOX9 (Millipore; AB5535), 2.5 μg/mL anti-EpCAM/CD326-647 (BioLegend; 118212). Secondary antibodies used for immunostaining: 4 μg/mL donkey anti-rabbit Alexa Fluor 555 (ThermoFisher; A31572), 4 μg/mL donkey anti-rat Alexa Fluor 488 (ThermoFisher; A21208), 4 μg/mL donkey anti-mouse Alexa Fluor 555 (ThermoFisher; A31570), and 5 μg/mL goat anti-rabbit HRP (ThermoFisher; G21234).
Tyramide signal amplification (TSA)
TSA was used for the secondary detection of pSMAD2. Slides were washed 3x5min with PBS. During the final wash, peroxidase block was made by combining 1 part H2O2 (30% H2O2 stock; Sigma; 216763) and 9 parts methanol (Fisher Scientific; A4134). Slides were submerged in a slide mailer with peroxidase block for 10 min at room temperature. After treatment, slides were washed 3 × 5 min with PBS, and primary antibody was applied overnight in PBS at 4 °C. The next day, primary antibody solution was discarded, and slides were washed 3 × 5min with PBS. Secondary staining was applied including 5 μg/mL goat anti-rabbit HRP (ThermoFisher; G21234) for 1 h. Slides were washed 3 × 5min in 100 mM borate, pH 8.5 (J.T. Baker; 0084-01) supplemented with 0.1% Tween-20 (Thermo Fisher; 85113). During the final wash, the TSA reaction buffer was assembled by adding TSA vivid fluorophore (1:500; Tocris Biosciences; 7526 to 100 mM borate, pH 8.5, 0.1% Tween-20, and 0.003 % H2O2. Solution was applied to tissue and incubated in the dark for 10 min. Slides were then washed 3 × 5 min with 0.1% Tween-20 in PBS to reduce background. Finally, bisbenzimide was applied 1:1000 for 10 min. Slides were washed 3x5min with PBS and mounted for imaging as described above.
Whole mount organoid staining
Whole mount organoids were stained using two methods. (i) Organoids grown from whole duct fragments were larger in size and amenable to retrieval from Cultrex and staining as previously described (Supplementary Fig. 7b)57. All steps from organoid retrieval through fixation were conducted with tubes and pipette tips pre-coated in 1% (wt/vol) PBS–BSA (Sigma Aldrich; A9647), to prevent organoid loss. After fixation, BSA-coated plastics were not used. Organoids were retrieved from Cultrex by removing culture media and adding 500uL Cultrex Organoid Harvesting Solution (R&D Systems; 3700-100-01) to the wells of a 48-well plate. The plate was incubated on a nutating platform for 45–60 min at 4 °C. After incubation, organoids were retrieved by resuspending well contents 5–10 times, then transferred to a 15 mL tube using a 1 mL pipette tip. The tube was filled to 10 mL with cold PBS and pelleted at 70 g for 3 min at 4 °C. Organoids were fixed by resuspension in 1 mL of 4% PFA for 45 min at 4 °C and gently resuspended halfway through the incubation. Next, organoids were permeabilized with 10 mL of cold 0.1% (vol/vol) PBS-Tween for 10 min at 4 °C. Organoids were pelleted at 70 g for 5 min at 4 °C and supernatant discarded. Organoids were transferred to a 24-well plate in 200 μL of cold (4 °C) Organoid Wash Buffer (OWB; 0.1% Triton X-100 and 2 mg/mL BSA in PBS) and incubated at 4 °C to block the organoids. 200 μL of OWB was left in each well for buffer changes to reduce organoid loss. Rabbit anti-Ki-67 primary antibody was added 1:500 in 200 μL OWB and added to the pre-existing 200 μL in the well for a final antibody concentration of 1:1000 and incubated overnight at 4 °C on a nutating platform. The next day, primary antibody was diluted using 1 mL of OWB. Organoids were washed 3 × 2 h on a nutating platform at 4 °C with 1 mL OWB buffer changes. Once organoids had settled to the bottom of the plate, 1 mL of OWB was removed and donkey anti-rabbit Alexa Fluor 555 was applied at 1:250 in 200μL OWB and added to the pre-existing 200 μL OWB for a final concentration of 1:500 and incubated overnight at 4 °C on a nutating platform. The next day, bisbenzimide diluted 1:500 in 200 μL OWB and added to the 200 μL volume of OWB remaining in wells for a final concentration of 1:1000 for 30 min. Organoids were washed in 1 mL OWB 3 × 2 h on a nutating platform at 4 °C. Next, as much OWB was removed as possible, and organoids were cleared by resuspension in 60% (vol/vol) glycerol and 2.5 M fructose in water. For mounting, a barrier pen (Vector Labs; H-4000) and double-sided sticky tape were used to make a thin well to contain the organoids and organoids were mounted with a cover slip. Whole mount organoids were quantified and imaged using a Nikon A1R HD25 Confocal Microscope in the Emory Integrated Cellular Imaging Core and Olympus IX-83 inverted fluorescence microscope. Analyses were performed using 1μm step size, z-stacks through the entire organoid, and max-intensity projections in FIJI. Statistical analyses were carried out in Prism 10.1.0. For statistical comparison, unpaired t test was used. A value of p < 0.05 was considered significant. Statistical analyses were carried out in Prism 10.1.0. All values are depicted as mean ± SEM.
(ii) Organoids grown from single cells were much smaller, and most were lost when retrieved from Cultrex. Therefore, we performed an organoid whole mount staining protocol that does not retrieve organoids from extracellular matrix. We performed this protocol as previously described with some modifications58. We removed culture media and overlayed prewarmed 37 °C freshly made 2% PFA for 20 min at RT. Wells were washed twice with 1xPBS at RT for 5 min. mICOs were permeabilized with 0.5% Triton X-100 in PBS for 20 min at RT. Wells were washed twice with 100 mM glycine in PBS. To label F-actin, phalloidin was applied in IF Wash (0.1% BSA, 0.2% Triton-X, 0.2% Tween-20 in PBS) for 45 min. Phalloidin stain was removed and DAPI (Millipore Sigma; D9542) was applied 1:1000 for 10 min. mICOs were washed 3 × 20 min in IF Wash then stored in 1XPBS at 4 °C until imaging the same week. Images were acquired using the Olympus IX-83 inverted epifluorescent microscope LUCPLFLN 40X/0.6 objective (working distance is 2.7–4.0 mm) capturing the entire organoid through the z-plane with a 2μm step size.
Whole tissue staining and iDISCO+ clearing
Whole liver IHBD labeling/iDISCO+ was performed as previously described23. Perfusion-fixed livers were washed 30 min in PBS, then dehydrated in methanol (Fisher Scientific; A4134) diluted in DI water: 20%, 40%, 60%, 80%, 100%, and 100% for 1 h each. Samples were delipidated overnight in 66% dichloromethane (DCM; Sigma Aldrich; 270997-1L)/33% methanol at RT on a rocker. The samples were then washed 2 × 1 h in 100% methanol and chilled at 4 °C before bleaching overnight at 4 °C using 5% H2O2 in methanol. The next day, samples were rehydrated with methanol diluted in DI water: 80%, 60%, 40%, 20%, and PBS for 1 h each at RT. Samples were washed twice with PTx.2 (0.2% TritonX-100 in PBS) for 1 h.
To permeabilize, samples were incubated overnight at 37 °C with 80% PTx.2, 23 mg/mL glycine (Millipore Sigma; G7126), and 20% DMSO (Sigma Aldrich; D2650). The next day samples were moved to 0.1% Tween-20, 0.1% TritonX-100, 0.1% deoxycholate (Sigma Aldrich; D2510), and 20% DMSO in PBS overnight at 37 °C. Samples were blocked for 2 d at 37 °C in Blocking Solution [84% PTx.2, 6% NDS (Jackson Immuno; 017-000-121), 10% DMSO]. Samples were stained with unconjugated 2.5 µg/mL anti-CD326 (BioLegend; 118201) or anti-CD326-647 conjugated antibody (BioLegend; 118212) diluted in PTwH [0.002% Tween-20 and 4 µg/mL heparin (Sigma; H3393 in PBS]/ 5%DMSO/3% NDS and for 7 d. Samples were washed in PTwH 4–5× until the next day. For samples labeled with unconjugated primary antibody, secondary antibody was applied for 5 d at 37 °C in PTwH/ 5%DMSO/3% NDS, then washed in PTwH 4–5× until the next day.
E15.5-P5 livers were embedded in 1% agarose (Genesee Scientific; 20-102GP) prior to methanol dehydration, to facilitate sample mounting for imaging. Samples were dehydrated in methanol diluted in DI water: 20%, 40%, 60%, 80%, 100%, and 100% for 1 hr each at RT, then delipidated by shaking for 3 hr on a rocker in 66% DCM/33% Methanol at RT. To remove methanol, samples were washed 2 × 15 min with 100% DCM, shaking on a gyrating rocker at RT. To clear tissue, samples were incubated in dibenzyl ether (DBE; Millipore Sigma; 108014) overnight, taking care to prevent air from oxidizing the sample. Samples were either imaged in DBE or ethyl cinnamate (ECi; Sigma; 112372). For samples imaged in ECi, they were cleared in DBE for at least 24 h then moved to ECi at least 24 h before imaging. See Supplementary Data 4 for detailed descriptions of staining, clearing, and imaging conditions for each sample.
Light sheet imaging
Cleared tissue samples were imaged using Miltenyi UltraMicroscope Blaze Lightsheet (Blaze) in the Emory Integrated Cellular Imaging Core (Blaze; ex 640, em 680/30), LaVision BioTec UltraMicroscope II (UMII) in the Biomedical Microscopy Core at the University of Georgia, or UMII in the UNC Microscopy Services Laboratory (ex 640, em 680/30). Whole tissue images using the Miltenyi UltraMicroscope Blaze Lightsheet were acquired using a 1.1× MI PLAN objective (WD ≤ 17 mm) or 12× MI PLAN objective (WD ≤ 10.9 mm) in ECi. LaVision BioTec UltraMicroscope II whole tissue images were acquired using Olympus MVPLAPO 2×/0.5 objective with a custom LaVision BioTec dipping cap (WD ≤ 10 mm) in DBE (detailed information in Supplementary Data 4). Laser power was adjusted based on intensity of the fluorescent signal. Z-step size for whole tissue images was 5μm. High magnification images of the adult tissue were captured on the UMII using a 6.3× zoom, and the Z-step size was 1 μm. High magnification of developmental tissue was captured using the Blaze with a 12× objective, 1× zoom, and 1 μm step size.
Whole tissue image processing and data analysis
3D rendering and video generation for the 3D images was done on high-powered workstations in the Emory Integrated Cellular Imaging Core with 512 GB RAM, NVIDIA graphics card, and 8–64 core processors. We used Imaris (v.10.0 and v.10.1, Bitplane) with the Imaris for Neuroscientists package for 3D visualization and image analyses. Image processing and analyses were conducted by using the surfaces function in Imaris and the Labkit extension or Imaris Machine Learning Training for pixel classification to segment positive staining59. In Labkit, GPU-accelerated segmentation was enabled. Following segmentation, images were masked to set non-segmented regions to 0 pixel intensity and segmented regions to 1000 pixel intensity to create binary images. Binary images were used with filament tracer in Imaris. In filament tracer, the “autopath (loops) with Soma and no Spine” was selected. One starting point was defined at the liver hilum. Multiscale points were used for seed points. Once the filament was defined, 3D Sholl analysis of IHBDs was carried out by concentric 3D spheres being implemented every 1 µm around the starting point. The number of intersections at each sphere was measured at 1 µm step sizes. To quantify large ducts in adult tissue, large ducts were defined as having a volume in between branch points ≥ 2e6 μm3. Large ducts were visualized in 3D in Imaris and counted. For statistical comparison, unpaired t test was used. A value of p < 0.05 was considered significant. Statistical analyses were carried out in Prism 10.1.0. All values are depicted as mean ± SEM.
Sholl analysis
Descriptive statistical analyses of Sholl data acquired in Imaris were performed in R, following a previously described framework (Supplemental Data 4)60,61. Sholl distance was normalized to a range of 0 to 1 by dividing each Sholl step size by maximum distance assigned a positive intersectional value in Imaris. To compute the polynomial model of Sholl intersections, we designed an algorithm utilizing the caret package to select the best-fit polynomial degree (up to a maximum of 7) using Monte-Carlo Cross-Validation (MCCV) to decrease overfitting62. MCCV was performed for 20 iterations with 80% of the data used as a training set and 20% as a testing set, and the polynomial degree with the lowest average Root Mean Square Error was used to fit a final polynomial model on the entire dataset. The mean value (Nav), critical value (Nm), and critical radius (rc) metrics were computed from this final polynomial model, as previously described 6. From the polynomial model, the mean value indicates the average number of Sholl intersections across the sample, the critical value (maximum of the polynomial model) indicates the maximum number of Sholl intersections, and the critical radius indicates the distance from the hilum where Nm occurs60. To calculate Sholl decay, Sholl intersections were log-transformed to fit linear models of the Sholl dataset. R-squared values and Sholl decay coefficients (slope of the linear model multiplied by −1) were calculated for each model. To represent trends between biological replicates in the same experimental group, trendlines for Sholl intersections were calculated with locally estimated scatterplot smoothing regression, shown in Figs. 1, 7, and 863.
Bulk RNA-sequencing
Sample collection, library construction, and sequencing
25,000 BECs were FACS isolated from 3 control and 3 Sox9cKO adult mice. Cells were sorted directly into 500 μL RNA Lysis Buffer. After isolation, lysates were immediately placed on ice and stored at −70 °C until RNA purification. RNA purification was carried out using the RNAqueous Micro Kit (Invitrogen; AM1931) following manufacturer instructions. RNA concentrations were assessed using Qubit fluorometer (Q33238; Thermo Fisher Scientific) following the protocol for the Quant-iT Qubit RNA HS Assay Kit (Invitrogen; Q32852). Library construction started from 4.9 ng of RNA per sample made using the SMARTer Stranded Total RNA-seq Kit v3-Pico Input Mammalian (Takara; 634486). The quality of libraries was checked with the with the High Sensitivity DNA Reagents Kit (Agilent; 5067-4626) on a 2100 Bioanalyzer and cDNA concentrations were obtained using Qubit BR dsDNA Assay Kit (Fisher Scientific; Q32853). All samples were sequenced by the Illumina Next-seq 550 instrument with paired-end 2x75bp read length and sequenced to a depth of at least 25 M reads per sample in the Emory Integrated Genomics Core.
Data analysis
Reads were demultiplexed, mapped to the reference genome (GRCm39), and deduplicated based on UMIs using Cogent NGS Analysis Pipeline (CogentAP v2.0). The CogentAP pipeline uses Cutadapt v3.4, STAR v2.7.8a, SAMtools v 0.1.19, bedtools v2.29.2, and Subread v2.0.064,65,66,67,68. The gene matrix file was output by the CogentAP pipeline. Differential expression (DE) analysis was done using DESeq2 package v1.36.069. The apeglm package v1.18.0 16 was used for effect size estimation and lfcShrink log2 fold-change values70. Genes were considered differentially expressed if LFC ≥ 1 and q value < 0.05. The clusterProfiler package v4.7.1 was used to perform Gene Set Enrichment Analysis (GSEA). WNT signaling gene set (MM8992) is from the Molecular Signatures Database71,72. All other gene sets for GSEA analyses have previously been published6,32,73,74,75.
Single cell RNA-sequencing
Sample collection, library construction, and sequencing
125,000–135,000 BECs per sample were isolated using FACS, processed, and fixed following the manufacturer’s protocol for whole cells (Parse Biosciences; SB1001). scRNA-seq libraries were prepared using split-pool barcoding as described in the Evercode WT V2 kit (Parse Biosciences; SB2001) according to the manufacturer’s instructions. Libraries were sequenced on one lane of an Illumina NovaSeq S4 flow cell (R1: 74 cycles, i7 index: 6 cycles, R2: 86 cycles, i5 index: 0 cycles) in the Emory Nonhuman Primate Genomics Core.
Data processing and quality control
For scRNA-seq data, fastq files corresponding to Sox9 control and Sox9cKO samples across all Parse sublibraries were demultiplexed using Parse Biosciences pipeline v0.9.6. Reads were mapped to mouse reference genome GRCm39 and transcripts counted in STAR v2.710a within the Parse pipeline 11. Gene expression matrices were loaded into Seurat (v4.1.0) for all data visualization and downstream analysis76. For quality control, we removed cells fulfilling any of these criteria from downstream analyses: less than 500 unique molecular identifiers (UMIs) or less than 200 features. We also removed contaminating endothelial cells based on expression of CD31.
Cell clustering and data analysis
Seurat objects of two samples were merged with merge() function. Data was normalized with the default Seurat’s LogNormalize method using NormalizeData() function. After normalization, variable features were found using FindVariableFeatures() and data was scaled with ScaleData() function. Statistically reliable clusters were determined using Clustree v0.4.4 to identify 0.6 as the earliest resolution where clusters stabilize77. Clustering was completed by using the RunPCA(), FindNeighbors(),and FindClusters() functions with the first 26 principal components and a clustering resolution of 0.6. Nonlinear dimensionality reduction was performed using the uniform manifold approximation and projection (UMAP) technique with the RunUMAP() function implemented in Seurat package. Cluster-specific markers were identified by applying the FindAllMarkers() function with the implemented in Seurat MAST test78. Gene sets enrichment scores were calculated using UCell package v2.1.179. The scCustomize package v1.1.1 was used to adjust colors on the FeaturePlots80.
To determine the percent of control and Sox9cKO cells in each cluster, cell counts were normalized between control and Sox9cKO, and the percent of control and Sox9cKO cells in each cluster was calculated (Supplementary Fig. 5b). Clusters with <80% of one sample type are considered mixed clusters. Sox9EGFP-high cells have been previously reported to be enriched in small ductules (GEO accession number GSE151387).17. Therefore, to assess differentially expressed genes in ducts and ductules and between control and Sox9cKO tissues, we regrouped samples as control or Sox9cKO ductules if the cluster’s mean enrichment score of Sox9EGFP-high gene signature is > 0.163 (Supplementary Figs. 2f-g).
Reanalysis of developing hepatoblast, cholangiocyte, and hepatocyte data
To determine the gene signature associated with developing epithelial cell types in the liver, we reanalyzed published RNA-seq31. The count matrix was downloaded from GEO (accession number GSE142089). Differential expression analysis was done using DESeq2. For DE, samples collected at different time points (E12.5-E17.5) were merged by cell type to form three groups: hepatoblasts, cholangiocytes, and hepatocytes. Samples from each cell type “group” were compared against all other samples to identify genes significantly upregulated in a single cell type (control duct, control ductule, Sox9cKO duct, Sox9cKO ductule, and mixed population). We also compared control duct and control ductule groups to identify genes significantly upregulated. Following the differential expression analysis, genes with adjusted p-value ≤ 0.01 and log2FoldChange > 0.5 were chosen for calculating enrichment scores.
RT-qPCR
RNA lysate from sorted single cells or organoids was thawed on ice and purified using the RNAqueous Micro Kit (Invitrogen; AM1931) following manufacturer instructions. RNA concentrations were assessed using Qubit fluorometer (Q33238; Thermo Fisher Scientific) following the protocol for the Quant-iT Qubit RNA HS Assay Kit (Invitrogen; Q32852). cDNA was generated using the iScript cDNA Synthesis Kit (BioRad; 1708891) according to manufacturer guidelines. cDNA was diluted 1:3 in molecular-grade water. RT-qPCR was carried out using Taqman probes and SsoAdvanced Universal Probes Supermix (BioRad; 1725284). Relative fold change of expression was carried out using the delta-delta CT method with 18S as the internal reference gene81. Taqman assays in this study are: 18S (Hs99999901_s1), Ncam1 (Mm01149710), Inhba (Mm00434339_m1), and Sox9 (Mm00448840_m1). For statistical comparison, one-way ANOVA was used. A value of p < 0.05 was considered significant. Statistical analyses were carried out in Prism 10.1.0. All values are depicted as mean ± SEM.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All high-throughput sequencing data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under the following accession numbers: bulk RNA-seq GSE249385; scRNA-seq GSE249558. Previously published sequencing data used in this study include embryonic liver scRNA-sequencing data GSE142089 and Sox9EGFP liver bulk RNA-sequencing data GSE151387. Source data are provided with this paper.
References
Lemaigre, F. P. Development of the intrahepatic and extrahepatic biliary tract: a framework for understanding congenital diseases. Annu. Rev. Pathol. Mech. Dis. 15, 1–22 (2020).
Clotman, F. et al. Control of liver cell fate decision by a gradient of TGFβ signaling modulated by Onecut transcription factors. Genes Dev. 19, 1849–1854 (2005).
Geisler, F. et al. Liver‐specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology 48, 607–616 (2008).
Tchorz, J. S. et al. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 50, 871–879 (2009).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010).
Yang, L. et al. A single-cell transcriptomic analysis reveals precise pathways and regulatory mechanisms underlying hepatoblast differentiation. Hepatology 66, 1387–1401 (2017).
Gérard, C., Tys, J. & Lemaigre, F. P. Gene regulatory networks in differentiation and direct reprogramming of hepatic cells. Semin. Cell Dev. Biol. 66, 43–50 (2017).
Roskams, T. A. et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39, 1739–1745 (2004).
Antoniou, A. et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology 136, 2325–2333 (2009).
Tanimizu, N. et al. Intrahepatic bile ducts are developed through formation of homogeneous continuous luminal network and its dynamic rearrangement in mice. Hepatology 64, 175–188 (2016).
Roskams, T. & Desmet, V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat. Rec. 291, 628–635 (2008).
Terada, T. & Nakanuma, Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am. J. Pathol. 146, 67–74 (1995).
Rockich, B. E. et al. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl Acad. Sci. 110, E4456–E4464 (2013).
Gonçalves, A. N., Correia-Pinto, J. & Nogueira-Silva, C. ROBO2 signaling in lung development regulates SOX2/SOX9 balance, branching morphogenesis and is dysregulated in nitrofen-induced congenital diaphragmatic hernia. Respir. Res. 21, 302 (2020).
Chatzeli, L., Gaete, M. & Tucker, A. S. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144, 2294–2305 (2017).
Liang, S. et al. A branching morphogenesis program governs embryonic growth of the thyroid gland. Development 145 https://doi.org/10.1242/dev.146829 (2018).
Tulasi, D. Y. et al. Sox9(EGFP) defines biliary epithelial heterogeneity downstream of yap activity. Cell Mol. Gastroenterol. Hepatol. 11, 1437–1462 (2021).
Poncy, A. et al. Transcription factors SOX4 and SOX9 cooperatively control development of bile ducts. Dev. Biol. 404, 136–148 (2015).
Xu, W. P. et al. Deletion of Sox9 in the liver leads to hepatic cystogenesis in mice by transcriptionally downregulating Sox63 J. Pathol. https://doi.org/10.1002/path.5636 (2021)
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Postic, C. & Magnuson, M. A. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 (2000).
Kamimoto, K. et al. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. eLife 5, e15034 (2016).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Sholl, D. A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 87, 387–406 (1953).
Ristanović, D., Milošević, N. T., & Štulić, V. Application of modified Sholl analysis to neuronal dendritic arborization of the cat spinal cord. J. Neurosci. Methods 158, 212–218 (2006).
Stanko, J. P., Easterling, M. R. & Fenton, S. E. Application of Sholl analysis to quantify changes in growth and development in rat mammary gland whole mounts. Reprod. Toxicol. 54, 129–135 (2015).
Martinez-Hackert, E., Sundan, A. & Holien, T. Receptor binding competition: a paradigm for regulating TGF-β family action. Cytokine Growth Factor Rev. 57, 39–54 (2021).
Morikawa, M., Derynck, R. & Miyazono, K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol. 8 https://doi.org/10.1101/cshperspect.a021873 (2016).
Cancilla, B. et al. Regulation of prostate branching morphogenesis by Activin A and Follistatin. Dev. Biol. 237, 145–158 (2001).
Ritvos, O. et al. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech. Dev. 50, 229–245 (1995).
Yang, L. et al. The default and directed pathways of hepatoblast differentiation involve distinct epigenomic mechanisms. Dev. Cell 58, 1688–1700.e1686 (2023).
Schaub, J. R. et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018).
Fabris, L. et al. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am. J. Pathol. 156, 1599–1612 (2000).
Luo, Z. L. et al. Bile acid transporters are expressed and heterogeneously distributed in rat bile ducts. Gut Liver 13, 569–575 (2019).
Glaser, S. S. et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab. Investig. 89, 456–469 (2009).
Francis, H. et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am. J. Physiol. Cell Physiol. 295, C499–C513 (2008).
Alpini, G. et al. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology 53, 628–639 (2011).
Renzi, A. et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G634–G643 (2011).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Ota, C., Ida, Y., Ohguro, H. & Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 10, 20292 (2020).
Spottiswoode, N. et al. Role of activins in hepcidin regulation during malaria. Infect. Immunity 85, 10.1128/iai.00191-00117 (2017)
Adams, J. M. et al. Sox9 is a modifier of the liver disease severity in a mouse model of alagille syndrome. Hepatology 71, 1331–1349 (2020).
Ernst, L. M., Spinner, N. B., Piccoli, D. A., Mauger, J. & Russo, P. Interlobular bile duct loss in pediatric cholestatic disease is associated with aberrant cytokeratin 7 expression by hepatocytes. Pediatr. Dev. Pathol. 10, 383–390 (2007).
Fabris, L. et al. Analysis of liver repair mechanisms in alagille syndrome and biliary atresia reveals a role for notch signaling. Am. J. Pathol. 171, 641–653 (2007).
Al Shoyaib, A., Archie, S. R. & Karamyan, V. T. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm. Res 37, 12 (2019).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Aloia, L. et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 21, 1321–1333 (2019).
Džepina, P. et al. Expression of activin A in liver tissue and the outcome of patients with biliary atresia. Front Pediatr. 12, 1457837 (2024).
Takashima, Y., Terada, M., Kawabata, M. & Suzuki, A. Dynamic three-dimensional morphogenesis of intrahepatic bile ducts in mouse liver development. Hepatology 61, 1003–1011 (2015).
Tanimizu, N., Miyajima, A. & Mostov, K. E. Liver progenitor cells fold up a cell monolayer into a double-layered structure during tubular morphogenesis. Mol. Biol. Cell 20, 2486–2494 (2009).
Carpentier, R. et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 141, 1432–1438 (2011).
Haridoss, S. et al. Activin A is a prominent autocrine regulator of hepatocyte growth arrest. Hepatol. Commun. 1, 852–870 (2017).
Rezanejad, H. et al. Heterogeneity of SOX9 and HNF1β in pancreatic ducts is dynamic. Stem Cell Rep. 10, 725–738 (2018).
Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002).
Navarro, K. L. et al. Mouse anesthesia: the art and science. ILAR J. 62, 238–273 (2021).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Beşikcioğlu, H. E. et al. Protocol for whole-mount immunofluorescence staining of ECM gel-embedded innervated pancreatic organoids. STAR Protoc. 5, 103132 (2024).
Arzt, M. et al. LABKIT: Labeling and segmentation toolkit for Big Image data. Front. Comput. Sci. 4 https://doi.org/10.3389/fcomp.2022.777728 (2022).
Ferreira, T. A. et al. Neuronal morphometry directly from bitmap images. Nat. Methods 11, 982–984 (2014).
R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Kuhn, M. Building predictive models in R using the Caret Package. J. Stat. Softw. 28, 1–26 (2008).
Cleveland, W. S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74, 829–836 (1979).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019).
Castanza, A. S. et al. Extending support for mouse data in the Molecular Signatures Database (MSigDB). Nat. Methods 20, 1619–1620 (2023).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Coulouarn, C., Factor, V. M. & Thorgeirsson, S. S. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology 47, 2059–2067 (2008).
Villanueva, A. et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 143, 1660–1669.e1667 (2012).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Zappia, L. & Oshlack, A. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. GigaScience 7 https://doi.org/10.1093/gigascience/giy083 (2018).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Andreatta, M. & Carmona, S. J. UCell: robust and scalable single-cell gene signature scoring. Comput Struct. Biotechnol. J. 19, 3796–3798 (2021).
scCustomize: Custom Visualizations & Functions for Streamlined Analyses of Single Cell Sequencing (2021).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45–e45 (2001).
Acknowledgements
We thank Dr. Pablo Ariel (UNC) for providing training and technical support in light sheet imaging. We thank Dr. April Reedy (Emory) for training on the Ultramicroscope Blaze and Stoyan Ivanov (Emory) for training in 3D image analysis in Imaris. We thank Dr. Saul Karpen (Emory) for providing Alb-Cre mice and Dr. Scott Magness (UNC) for providing Sox9fl/fl mice. We thank Drs. Saul Karpen, Paul Dawson, Ken Moberg, Jitendra Thakur, and members of the Gracz Lab for constructive discussions and critical reading of the manuscript. This study was funded by the NIH/NIDDK under award numbers R01DK132653 (Gracz), R35GM142503 (Gracz), F31DK134199 (Hrncir), and T32GM135060 (Hrncir). Research reported in this publication was supported in part by the Emory Integrated Genomics Core (EIGC) shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292, by the Emory University Emory Integrated Cellular Imaging Core Facility (RRID:SCR_023534) and NIH under Award Number S10 OD032320-01, the Emory NPRC Genomics Core is supported in part by NIH P51 OD011132, by the North Carolina Biotech Center Institutional Support Grant 2016-IDG-1016, the Biomedical Microscopy Core at the University of Georgia, and the Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Author information
Authors and Affiliations
Contributions
H.R.H. and A.D.G. designed experiments and analyzed data. H.R.H., B.G., and C.B.H. conducted experiments. O.J. carried out Sholl analyses. S.B. carried out computational RNA-seq analyses. H.R.H. and A.D.G. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hrncir, H.R., Goodloe, B., Bombin, S. et al. Sox9 inhibits Activin A to promote biliary maturation and branching morphogenesis. Nat Commun 16, 1667 (2025). https://doi.org/10.1038/s41467-025-56813-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56813-x