Abstract
Tissue morphogenesis shapes epithelial sheets via cell remodelling to form functional living organisms. While the mechanisms underlying single morphogenetic events are well studied, how one tissue undergoes multiple concomitant shape changes remains largely unexplored. To tackle this, we study the process of simultaneous mesoderm folding and extension in the gastrulating Drosophila embryo. This composite transformation relies on a sharply timed reorganization of the cortical actomyosin network into two distinct subcellular tiers to drive concomitant cell apical constriction and lateral intercalation for tissue folding and convergence-extension, respectively. Here we elucidate the spatio-temporal control of the two-tiered actomyosin network. We show that, within the geometric constraints imposed by the columnar shape of mesoderm epithelial cells, the nucleus acts as a barrier shielding the lateral cortex from interactions with the microtubule network, thereby regulating the distribution of the key signalling molecule RhoGEF2. The relocation of the nucleus, driven by the contraction of the first actomyosin tier and the resulting cytoplasmic flow, unshields the lateral cortex for RhoGEF2 delivery to direct the stereotypic formation of the second tier. Thus, the nucleus and its position function as a spatio-temporal cytoskeleton compartmentalizer establishing a modular scaffold powering multiple simultaneous cell remodeling for composite morphogenesis.
Similar content being viewed by others
Introduction
The morphogenesis of epithelia displays remarkable plasticity and robustness during embryo development: living tissues actively remodel their shape while maintaining their structural integrity to establish functional organisms. Tissue remodelling relies on the ability of epithelial cells to generate mechanical forces and to acquire new shape and position while maintaining cell-cell contacts1,2. To achieve this, cells remodel their cytoskeleton in an orchestrated fashion3,4,5 under the control of signalling cues established both at the local and organismal levels6.
An outstanding example of epithelial plasticity and robustness is composite morphogenesis, the process by which an epithelial tissue undergoes multiple and simultaneous shape transformations7. For instance, epithelia can simultaneously fold and extend to drive embryo gastrulation, neurulation or tubulogenesis8,9,10. While great advances have been made to unveil the mechanisms underlying single morphogenetic processes, how composite morphogenesis is directed and coordinated is largely unexplored. To tackle this question, we study the concomitant folding and extension of the prospective mesoderm during early Drosophila gastrulation. Recent work has shown that mesoderm epithelial cells establish two junctional tiers formed by actomyosin networks tethered to E-cadherin and tailored for distinct functions. The first tier is apically located and mediates cell apical constriction for tissue furrowing (Fig. 1a, b and b’, bottom)11. The second tier is positioned laterally ( ~ 10 µm from the apical side) and is planar cell polarized to initiate cell intercalation for tissue convergence-extension (Fig. 1a, b and b’, top)8. With this study, we aim to shine light on the mechanisms that spatio-temporally coordinate the formation of functionally distinct cytoskeletal networks for simultaneous cell shape and topology changes. While the two tiers share the same nature, their origin differs. The first tier is under the control of the cell apical-basal polarity12 and it is shaped by apical-medial actomyosin contractions13. The second tier is formed by the coalescence of the cell lateral actomyosin network, focusing E-cadherin to form a cluster8. Overall, the formation of the two-tier junctional system is under the control of the synergistic interaction between the embryo anterior-posterior (AP) and dorsal-ventral (DV) gene patterning systems8. How the lateral actomyosin network is spatio-temporally controlled is still unknown.
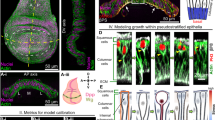
a The prospective mesoderm simultaneously folds and extends at the onset of Drosophila gastrulation. Cross-section (left), surface view (top-right) and sagittal section (bottom-right). White arrowhead and dashed line indicate the furrow position. Scale bar, 50 µm. b Sagittal view of mesoderm cells showing a two-tier organization of MyoII. Scale bar, 5 µm. b’ Time-lapse showing the lateral and apical pool of MyoII driving cell intercalation (top) and apical constriction (bottom), respectively. Scale bars, 5 µm. c Time-lapse of the mesoderm cross-section during ventral furrowing. Arrowheads indicate MyoII-LCs. Scale bar, 10 µm. d MyoII lateral intensity and nuclear position in function of time. Inset: temporal cross-correlation between the peak of nuclear migration speed and the peak of MyoII lateral recruitment. Apex-to-nucleus distance: distance between the apical side of the cell and the apical side of the nucleus. Data points represent the mean values with error bars indicating the 95% confidence interval (n = 3 embryos, N = 45 junctions, N = 30 nuclei). e Mesoderm front view (left) and cross-section (right) time-lapse showing apical constriction and the associated nuclear displacement. Scale bars, 10 µm. f Mean nuclear position in function of the mean apical cell surface at different phases of mesoderm invagination. Inset: raw individual values with an exponential fit (y = 69.212*e-0.096x). Data points represent the mean values with error bars indicating the 95% confidence intervals (n = 5 embryos, N = 925 cells, N = 925 nuclei). Source data are provided as a Source Data file.
In addition to the molecular signalling, the change in position and shape of cellular organelles play a role in cell remodelling14,15. During the phase of apical constriction of mesoderm epithelial cells, the cell nuclei migrate basally16,17. While a great body of work has highlighted the mechanisms controlling nuclear relocation and the role of nuclear mechanotransduction in cell polarity, migration, division, or fate determination18,19,20, how the position of the nucleus participates in tissue remodelling remains elusive. We now investigate this during mesoderm folding and extension. We provide evidence that the nucleus functions as a cellular compartmentalizer and that its relocation spatio-temporally organizes the cell cytoskeleton and the distribution of the Rho guanine exchange factor RhoGEF2 to establish a second actomyosin tier for cell intercalation.
Overall, by implementing advanced live imaging, laser manipulation, optogenetics, and molecular modelling, this study reveals the mechanisms underlying the spatiotemporal control of the cortical actomyosin cytoskeleton to form a modular scaffold for simultaneous morphogenetic events.
Results
MyoII upregulation along the cell lateral cortex follows nuclear migration
During the initial phase of gastrulation, MyoII accumulates both at the apical and lateral side of mesoderm cells to establish a two-tier actomyosin scaffold generating contractile forces for both apical constriction and cell intercalation (Fig. 1b, b’ bottom and top panel, respectively). To investigate the origin of the lateral tier, we monitored MyoII dynamics and the cellular processes occurring in the mesoderm tissue. Recent work has shown that increase of apical MyoII, mediating constriction of the cell apical surface (Fig. 1b, b’, bottom), precedes lateral MyoII upregulation8. Seminal studies have reported that, during apical constriction, mesoderm cell nuclei migrate toward the cell basal side16,17. To time the sequence of events, we performed a cross-correlation analysis between nuclear position and MyoII accumulation at the apical and lateral cortex over time. Our data shows that, while the migration of nuclei is synchronized with the increase of apical MyoII (Supplementary Fig. 1b, c), the peak of nuclear displacement rate precedes the peak of lateral MyoII accumulation rate by about 2 min (Fig. 1c, d, Supplementary Fig. 1a, c, Supplementary Movie 1). In addition, the apical-basal location of individual MyoII lateral clusters (MyoII-LCs) appears to correlate with the position of the corresponding nuclei (Supplementary Fig. 1d). Altogether, these evidences highlight a possible link between nuclear migration and the formation of the MyoII lateral tier.
In 2012, Gelbart and colleagues formulated the volume conservation principles by which apical constriction drives cytoplasm flow pushing the nucleus towards the cell basal side21. This model is based on the assumption that the cytoplasm is an incompressible fluid and that the nucleus works as an “impassable piston”. Tissue-scale cytoplasmic flow was then demonstrated by He et al. in 201422. While Gelbart and colleagues show a strong correlation between the apical constriction rate and the rate of nuclear displacement, we now directly tested the relationship between the apical cell surface area and the nuclear position over time. Our analysis shows that the position of the nucleus along the apical-basal axis of the cell is tightly coupled with the apical surface area of the cell (Fig. 1e, f).
Overall, our analyses further support the idea that MyoII-dependent apical constriction controls nuclear apical-basal position and show that the formation of the MyoII lateral tier follows nuclear migration.
Nuclear migration controls lateral MyoII upregulation
Nuclear migration and lateral MyoII upregulation show spatial and temporal correlation. To test if the basal displacement of nuclei is necessary for MyoII-LC formation, we devised a set of experiments to interfere with nuclear migration. As nuclear positioning is strongly correlated with cell surface changes (Fig. 1f), we implemented infrared (IR) femtosecond (fs) laser ablation of the apical actomyosin network to abort apical constriction and eventually inhibit nuclear migration while preserving cell membrane integrity (Fig. 2a, b, Supplementary Fig. 2a and Supplementary Movie 2). It should be noted that IR fs laser ablation is a multi-photonic technique with high spatial specificity23 (i.e., depth resolution of about 1 µm) that is effective to selectively dissect the actomyosin network located at the apical side of cells (Supplementary Fig. 2d and e). Upon ablation, apical constriction is inhibited and nuclei fail to move basally (Fig. 2c, d). This evidence directly demonstrates a causal link between nuclear migration and apical constriction, corroborating the idea that nuclei behave as cellular “pistons”21. Remarkably, MyoII-LCs are inhibited in the zone where nuclei fail to displace basally (Fig. 2c, c’ and e and Supplementary Fig. 2c, c’). In the zone next to the ablated region (i.e., the control zone), cells rapidly shrink their apical surface area and display enhanced apical constriction as a consequence of released tension in neighbouring cells (Supplementary Fig. 2a, b, blue). As a result, nuclei in the control zone move significantly more basally than nuclei in non-ablated embryos (Fig. 2d), preserving or even enhancing the formation of MyoII-LCs (Fig. 2c, Supplementary Fig. 2f and Supplementary Movie 3). Intriguingly, enhanced formation of MyoII-LCs can similarly appear in non-ablated embryos that overexpress RhoGEF2 (Supplementary Fig. 2f’). Since laser dissection of the apical actomyosin network leads to significant recoil and cell area expansion, we sought to determine whether laser dissection per se might cause cortical dilution of the MyoII signal at the lateral cortex. To investigate this, we first measured changes in cell surface area at both the apical and lateral regions following laser ablation (see Supplementary Fig. 2g). Our analysis shows that cell surface expansion is significantly reduced at the lateral cortex compared to the apical site of ablation (Supplementary Fig. 2h). We then examined changes in junction length along both the AP and DV axes. Given the tension anisotropy in the mesoderm tissue (with higher tension along the AP axis)24,25, laser ablation along a line orthogonal to AP induces junctional lengthening along AP, whereas DV junction length remains unchanged (Supplementary Fig. 2i). These findings rule out the cortical dilution hypothesis.
a Mesoderm tissue upon laser dissection of the apical actomyosin network along two DV segments (dashed lines). Blue and red segments indicate control and cut cells, respectively. Scale bar, 10 µm. b Schematic representation of cell shape, nuclear position and MyoII distribution in the control (blue) and cut (red) region. Cross: dissection of the apical actomyosin network. Arrowheads: apical contraction forces. c Front view at 10 µm from the apical side and sagittal section of the mesoderm at the onset of and 7 min into gastrulation. Crosses indicate the sites of ablation (performed at the onset of gastrulation). Arrowheads indicate MyoII-LCs. Scale bar, 10 µm. c’ Magnified views of MyoII-LC distribution in the control (1) and cut (2) region. Scale bars, 5 µm. d Nuclear position in the control (blue) and cut (red) region and in control embryos (grey) devoid of laser dissection. Apex-to-nucleus distance corresponds to the distance between the apical side of the cell and the apical side of the nucleus. The boxplot shows the median (line), interquartile range (IQR, box), and data spread with whiskers extending to 1.5x the IQR.(n = 6 embryos, N = 90 nuclei). e MyoII intensity at 10 µm from the apical side at the onset of and 7 min into gastrulation in the region where nuclei fail to migrate as a result of apical laser ablation, in the control region and in control embryos. The barplot represents the mean values with error bars indicating the 95% confidence interval(n = 6 embryos, N = 88 junctions). f Cytoplasmic flow along the cell apical-basal axis in cut and control cells in function of changes in apical cell surface. Data points represent individual values (n = 5 embryos, N = 24 beads, N = 24 cells). P values in (d): 0.462, 0.0673, >0.9999 (onset of gastr.)/ <0.0001, 0.0003, <0.0001 (7 min into gastr.). P values in (e): 0.0736, 0.5272, >0.9999 (onset of gastr.)/>0.9999, <0.0001, <0.0001 (7 min into gastr.). Source data are provided as a Source Data file.
To further test the causal relationship between nuclear migration and lateral MyoII upregulation, we implemented optogenetics to prevent nuclear basal displacement. More specifically, we used two-photon optogenetics to ectopically activate MyoII at the cell basal side, resulting in cell basal constriction (Supplementary Fig. 3a, b and c)26,27. As a result, nuclei are kept close to the apical cell side (i.e., nuclear migration is impaired) and the formation of lateral MyoII-LCs is inhibited (Supplementary Fig. 3d, e and f). Altogether, this shows that nuclear migration is necessary for lateral MyoII upregulation.
To further dissect the relationship between the cell apical MyoII and the MyoII-LCs, we devised an experiment to decouple the apical actomyosin network from apical constriction and nuclear displacement. To that end, we laser dissected the apical actomyosin network along a line parallel to the AP axis during tissue furrowing soon after the establishment of MyoII-LCs (i.e., along a direction and during a phase of lower tissue tension - Supplementary Fig. 4a). This results in minimal apical surface dilation and consequent negligible change in nuclear position (Supplementary Fig. 4b, c). Under these experimental conditions, MyoII-LCs are preserved (Supplementary Fig. 4d, e) providing direct evidence that the apical actomyosin network is per se not necessary to maintain MyoII-LCs.
We then wondered if the formation of MyoII-LCs is a reversible process. To that end, we devised an experimental trial to reverse the cytoplasmic flow and move nuclei back to the apical surface. We injected fluorescent inert beads to monitor cytoplasmic flow (Supplementary Fig. 4f, f’) and performed IR fs laser ablation to dilate apically-constricted cells once MyoII-LCs were formed. Ablation results in apical cell surface dilation, leading to a reversal of the cytoplasmic flow (Supplementary Fig. 4g). The cytoplasmic flow scales with the changes in apical cell surface, and the flow directionality can be flipped from centripetal (i.e., from apical to basal) to centrifugal (i.e., from basal to apical) if cells shift from constriction to dilation (Fig. 2f). As a consequence, nuclear migration is also reversed in the ablated region (Supplementary Fig. 4g, h) and lateral MyoII is downregulated (Supplementary Fig. 4i). Overall, our data demonstrate that nuclear basal migration is necessary for the formation of MyoII-LCs, which is a reversible process.
Nuclear position defines the actomyosin coalescing zone and the location of MyoII lateral clusters
Our previous results show that nuclear migration is necessary for MyoII lateral upregulation and indicate that the location of MyoII-LCs is not stochastic but rather follows the position of nuclei (Supplemental Fig. 1d). To better decipher the relationship between nuclear migration and the upregulation of lateral MyoII, we considered four possible working models and performed a thorough quantitative analysis of the relative position between nuclei and MyoII-LCs. In general, a MyoII-LC is surrounded by four cells (i.e., four nuclei) and is located at the cortex between two adjacent cells (Fig. 3a). Therefore, we hypothesized that the position of the MyoII-LC could be controlled by the most apical (model 1) or the most basal (model 2) nucleus among the four, by the two adjacent nuclei (model 3 - Fig. 3a, A and P nuclei) or by the two non-adjacent nuclei (model 4 - Fig. 3a, D and V nuclei). To test the predictive capability of the four models, we measured the relative position between the four nuclei and the corresponding MyoII-LC. Our analysis shows that model 3, having the smallest standard deviation and highest R2, has the greatest predictive power (Fig. 3b and Supplementary Fig. 6f). Model 3 advances the idea that the MyoII-LC is located at half the distance between the apical side of the cell and the average position of the two adjacent nuclei (i.e., the halfway rule). This working principles is in line with a previous study that reports a coordinated coalescence of the actomyosin network on both sides of the interface between two neighboring cells8. This results in the formation of two MyoII clusters (one on each side of the interface) both located at the same apicobasal position and never at different locations one from the other. To challenge the predictive power of the halfway rule, we identified pairs of AP nuclei having high positional difference (defined as inter-nucleus depth > 5 µm, red-contoured data points in Supplementary Fig. 6g). Also in these extreme case the halfway rule is fulfilled (Supplementary Fig. 6g, inset).
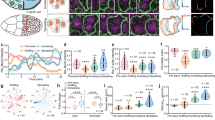
a Sagittal view of mesoderm cells showing MyoII-LC (white arrowhead) and the two corresponding nuclei. Dashed line highlights the cell outline. Scale bar, 5 µm. a’ Top: Schematic representation of two cell sharing a MyoII-LC. Bottom: schematic representation of the initiation of a cell intercalation event (A is anterior, P posterior, D dorsal and V ventral). b Quantification of MyoII-to-nuclei relative position (as described by the inset formula) in different conditions (refer to a’). Right axis: associated standard deviation for each condition. The boxplot shows the median (line), interquartile range (IQR, box), and data spread with whiskers extending to 1.5x the IQR. (n = 5 embryos, N = 50 clusters, N = 200 nuclei). c Top: time-lapse showing the ablation of the apical actomyosin network over an extended portion of the mesoderm. Bottom: magnified views showing MyoII distribution in the control (left) and cut (right) region. Nuclei, that fail to migrate, are visible in black at 10 µm depth in the cut region. Arrowheads indicate MyoII-LCs. Scale bars, 10 µm. d Schematic representation of the centrifugation protocol employed to drive enhanced nuclear basal migration (see Supplementary Methods). e Mesoderm front view at 15 µm depth and cross-section in centrifuged (top) and control (bottom) embryos. Arrowheads indicate MyoII-LCs. Cross-sections show nuclei in cyan and MyoII in purple. Scale bars, 10 µm. f Apico-basal distribution of MyoII-LCs in embryos undergoing reduced nuclear migration (blue), control embryos (grey) and centrifuged embryos (purple). Inset shows the cumulative density relative to the apico-basal position of MyoII-LCs (n = 12 embryos, N = 565 clusters). g MyoII-LC apico-basal position relative to the average AP nuclear position. Data points represent individual values (refer to a’). Apex-to-nucleus distance corresponds to the distance between the apical side of the cell and the apical side of the nucleus. Inset: quantification of MyoII-to-nuclei relative position for each individual cluster-nuclei pair. The boxplot shows the median (line), interquartile range (IQR, box), and data spread with whiskers extending to 1.5x the IQR. (n = 16 embryos, N = 140 clusters, N = 280 nuclei). Source data are provided as a Source Data file.
To functionally test the halfway rule, we ectopically modulated the position of nuclei and monitored the location of the MyoII-LCs. First, we aimed to reduce nuclear migration. To that end, we downregulated apical constriction by ablating the apical actomyosin network across a portion of the mesoderm and used the non-ablated portion as an internal control. After ablation, the apical network is progressively restored, resulting in a delayed constriction and a reduced nuclear migration compared to the control region (Fig. 3c, Supplementary Fig. 5a, b and d, Supplementary Movie 4 and Supplementary Methods). Under these conditions, MyoII-LCs form more apically (Fig. 3c, f and Supplementary Fig. 5c, e). Next, we enhanced nuclear migration by implementing embryo centrifugation (Fig. 3d and Supplementary Methods), a technique previously used in cell culture28. In centrifuged embryos, nuclear migration is eventually enhanced and MyoII-LCs form more basally (Fig. 3e, f, Supplementary Fig. 6a, b, b’). Overall, in all conditions, the position of the vast majority of MyoII-LCs obeys the halfway rule (Fig. 3f, g). This data corroborates the notion that MyoII-LCs are tightly controlled by nuclear migration and shows that they stereotypically form halfway between the apex of the cell and the average position of the two adjacent AP nuclei.
What mechanism controls the halfway position of MyoII-LCs? Previous work has shown that MyoII-LCs result from the coalescence of the cell lateral actomyosin network under the control of the activating factor RhoGEF28. Considering that RhoGEF2 is enriched along the lateral cortex between the cell apical side and the nucleus (Supplementary Fig. 7a and a’ top), we can hypothesize that an isotropic contraction of the lateral actomyosin network would be sufficient to locate MyoII-LCs at the midpoint (Supplementary Fig. 7b). To test this, we used optogenetics to ectopically recruit RhoGEF2 along a cell lateral segment26. As a result, MyoII-LCs form in the middle of the activated segment (Supplementary Fig. 7c, d and Supplementary Movie 5), comforting the hypothesis that actomyosin isotropic contraction and coalescence determine MyoII-LC position. Previously, it was shown that MyoII coalescence drives focusing of E-cadherin and the formation of ad hoc spot adherens junctions at the lateral cortex8. Therefore, inhibition of basal nuclear movement should in principle disrupt both MyoII-LC formation and E-cadherin focusing. To test this, we used apical laser ablations (as previously described) to prevent nuclear displacement in a subset of mesoderm cells while monitoring E-cadherin signal at the lateral cortex. Our analysis validates the fact that preventing nuclear basal movement inhibits both MyoII-LC formation and E-cadherin focusing (Supplementary Fig. 7f, g).
A growing body of evidence has shown that nuclear deformation can lead to acute changes in genetic expression and cell behaviour driven by cytoskeletal remodelling18. To test if a mechanism involving nuclear deformation is at play during MyoII-LC formation, we imaged and measured nuclear changes in shape in mesoderm cells during this initial phase of gastrulation, starting from late cellularization. Our analyses show that both nuclear local curvature and global eccentricity do not vary during this time period (Supplementary Fig. 8a, a’, b, c).
Overall, our data supports a model based on nuclear position, rather than nuclear shape, controlling the size of a contractile zone that is established between the cell apical surface and the nucleus. The position of the MyoII-LCs results from the isotropic contraction and coalescence of the cortical actomyosin network within that space.
The nucleus works as a microtubule compartmentalizer regulating the cortical distribution of RhoGEF2
We have shown that the apical-basal position of nuclei determines the location of MyoII-LCs. Since ectopic accumulation of RhoGEF2 at the cell lateral cortex leads to local upregulation of MyoII (Supplementary Fig. 7a, b, c and d), we wondered if the nucleus per se controls the cortical recruitment of RhoGEF2. Since the cross-sectional area of the nucleus and of the cell are similar (Fig. 1e), we can hypothesize that the nucleus can physically limit cortical accessibility to cytosolic RhoGEF2. To test this, we implemented two-photon optogenetics to ectopically increase RhoGEF2 levels specifically along the cell cortex flanked by the nucleus. If the nucleus limits cortical accessibility to RhoGEF2, MyoII-LC formation is expected to be compromised upon activation. After activation, MyoII is upregulated and a cluster forms between nuclei similarly to control conditions (i.e., along the part of the cortex not flanked by nuclei - Supplementary Fig. 7e). This evidence argues against a direct regulation of RhoGEF2 by the nucleus, leaving open the possibility that the nucleus indirectly regulates RhoGEF2.
Previous studies have reported an association between RhoGEF2 and microtubule (MT) plus ends, via the end-binding protein EB129,30. EB1 live imaging in mesoderm cells during furrow formation shows MT plus ends radiating from the MT organizing centres (i.e., MTOCs, located at the apical side of the nucleus) towards the lateral cortex where MyoII eventually clusters (Supplementary Movie 6). Therefore, we hypothesized that nuclear position can regulate MT distribution. To test this, we measured EB1 density along the cell lateral cortex at the onset of gastrulation and 7 min later when nuclei migrate basally and RhoGEF2 is laterally enriched. EB1 density increases over time phenocopying RhoGEF2 dynamic distribution8 (Fig. 4a, b and Supplementary Movie 7). To directly test if the nuclear position controls MT plus-end distribution, we inhibited nuclear displacement (using the same experimental design presented in Fig. 2b) and measured EB1 cortical distribution. In the region where nuclear migration is impaired, the cortical levels of EB1 significantly drop (Fig. 4c, d). This demonstrates that nuclear migration controls the density of MT plus ends along the cell lateral cortex. To explore how the nucleus can dictate MT plus-end distribution, we developed an in silico model using the Cytosim simulator15 by implementing in vivo measurements of MT properties and cell geometry as boundary conditions (Supplementary Fig. 9a–e and Supplementary Movie 8). We measured the density of MT plus ends contacting the lateral cortex at different apical-basal positions and in multiple conditions (e.g., with the nucleus at a more apical or more basal location, see Supplementary Methods). Our analyses show that MT plus ends contact the lateral cortex mostly in the zone between the apical side of the cell and the nucleus and that the plus-end density drops beyond the nucleus position (Fig. 4e–h). We then systematically varied both subcellular geometric features and MT properties to assess their contribution to this process. While the distance between centrosomes plays a mild effect, the only two parameters playing a major role are MT rigidity and nuclear size: more rigid MTs and a larger nucleus more effectively limit the number of plus end-to-cortex contacts beyond the nucleus position (Fig. 4i and Supplementary Fig. 9f–l). These evidences outline a model in which the nucleus works as an MT compartmentalizer physically shielding the cortex from MT plus ends.
a Sagittal view of mesoderm cells showing EB1 distribution along the cell lateral cortex at the onset of (top) and 7 min into gastrulation (bottom). Scale bar, 10 µm. b Cortical over cytoplasmic ratio of EB1 at the lateral cortex of mesoderm cells (5 to 15 µm) at the onset of and 7 min into gastrulation. The boxplot shows the median (line), interquartile range (IQR, box), and data spread with whiskers extending to 1.5x the IQR. (n = 3 embryos, N = 29 cortices). c Left: EB1 distribution at 10 µm depth before (top) and after (bottom) apical laser dissection (dashed line) and nuclear displacement. Right: magnified views of the control region (blue) and the region where nuclei relocate apically after ablation (red). Scale bars, 10 µm, 5 µm. d EB1 intensity along the cell lateral cortex at 10 µm depth in the control (blue) and in the apical nuclear relocation (red) region. The barplot represents the mean values with error bars indicating the 95% confidence interval (n = 5 embryos, N = 146 cells). e 3D representation of the MT network by mathematical simulation with the nucleus located apically, basally and with MTOCs separated from the nucleus. f Percentage (and associated confidence interval) of MT plus ends along the cell lateral cortex between the apical side and the nucleus (blue) and between the nucleus and the basal side (red) in different conditions (refer to e). g, h, i, MT plus-end distribution along the cell lateral cortex in the three different conditions (refer to e). = 100 simulations per condition. The blue and red areas indicate locations between the apical side of the cell and the nucleus and between the nucleus and the basal side of the cell, respectively (see inset). j RhoGEF2 distribution in mesoderm cells at 10 µm from the apical side upon live-injection of water (top) or Colcemid (bottom). Scale bar, 5 µm. k Cortical over cytoplasmic ratios of RhoGEF2 in control embryos (water live-injected or water photo-activated) or upon MT disruption (Colcemid live-injection or SbTub4AP photo-activation with a 405 nm laser). The boxplot shows the median (line), interquartile range (IQR, box), and data spread with whiskers extending to 1.5x the IQR. (n = 12 embryos, N = 145 cells). l Mesoderm front view time-lapse at 10 µm from the apical side showing MyoII distribution in WT (left) or T48 mutant (right) embryos. Scale bar, 10 µm. m Normalized density of MyoII-LCs in WT or T48 mutant embryos. The barplot represents the mean values with error bars indicating the 95% confidence interval (n = 12 embryos, N = 2664 cells). P value in (b): <0.0001. P values in (d): >0.9999 (before abl.), <0.0001 (after abl.). P values in (k): 0.0766 (Water live), <0.0001 (Colcemid live), >0.9999 (Water photo-activated), <0.0001 (SbTub4AP photo-activated). P value in (m): <0.0001. Source data are provided as a Source Data file.
We then functionally tested the role of MT in driving MyoII-LCs. To that end, we used Colcemid to depolymerize MTs while preserving MTOC integrity (Supplementary Fig. 10a, b). Colcemid injection during late cellularization leads to a strong downregulation of MyoII-LCs (Supplementary Fig. 10f). Previous work has shown that MT disruption does not affect the initial phase of apical constriction that is characterized by cell stochastic ratcheted pulsatile contractions11,31. Nevertheless, while the first phase is indeed preserved, our data show that the following phase, characterized by concerted apical constriction, is strongly compromised (Supplementary Fig. 12a, a’). More specifically, a great number of cells fail to apically constrict, phenocopying mesoderm cells from embryos injected with a 5 mM ROCK-inhibitor Y-27632, ultimately resulting in furrow formation failure. (Supplementary Fig. 12b, Supplementary Movie 9 and 10). In Colcemid-injected embryos, cells that still apically constrict show a basally located nucleus while un-constricted cells maintain their nucleus to their apical side (Supplementary Fig. 12c). These evidences rule out MTs as active contributors to nuclear migration and corroborate the notion that the displacement of nuclei is driven by constriction-dependent cytoplasmic flow. The fact that cells deprived of MTs and with a displaced nucleus show no distinctive MyoII-LC formation (Supplementary Fig. 10f), demonstrates that both MTs and nuclear migration are necessary but not sufficient per se to form MyoII-LCs.
A previous study has shown that, during early gastrulation, MTs are nucleated from both centrosomal and acentrosomal pools, the latter being regulated by the minus-end binding protein Patronin31. To disentangle the contribution of the two MT networks in MyoII-LC formation, we used RNAi-mediated downregulation of Patronin to selectively disrupt the MT acentrosomal pool (Supplementary Fig. 11a). Consistent with previous reports, Patronin downregulation leads to a partially compromised cell apical constriction resulting from heterogeneous constriction patterns, yet without disrupting overall mesoderm invagination31. In a Patronin downregulated background, MyoII-LCs still form (Supplementary Fig. 11b) suggesting that the acentrosomal MT network is unnecessary for MyoII-LC formation. Nevertheless, the formation of heterogeneous constriction patterns results in an enhanced variability in nuclear and MyoII-LC apicobasal position across the tissue with a relative distance yet fulfilling the halfway rule (Supplementary Fig. 11c, d). These experiments do not exclude the possibility that the acentrosomal microtubule network still contributes to MyoII-LC formation. A plausible hypothesis is that centrosomal microtubules are much more numerous than acentrosomal microtubules and therefore are sufficient to drive the formation of MyoII-LCs.
Mesoderm cell intercalation is initiated by MyoII-LCs that are distributed in a planar cell polarized (PCP) fashion with higher levels along DV junctions8. RhoGEF2, alpha-tubulin and EB1 also show the same PCP distribution (Supplementary Fig. 12d–g). In addition, RhoGEF2 show high levels at the two MTOCs (Supplementary Fig. 7a’, bottom) where EB1 is also highly concentrated (Supplementary Fig. 9d). We can thus raise the hypothesis that MT plus ends are predominantly targeting DV junctions for local RhoGEF2 delivery and consequent PCP actomyosin activation. To test this hypothesis, we monitored changes in cortical RhoGEF2 density upon MT disruption. To that end, we performed time-controlled injection of Colcemid during lateral MyoII upregulation (i.e., after apical constriction and nuclear migration – Supplementary Fig. 10c and Supplementary Methods). After injection, RhoGEF2 is downregulated within 20 s (Fig. 4j, k). A similar result is obtained when implementing photo-controlled depolymerization of MTs using SbTub4AP17 (Fig. 4k, Supplementary Fig. 10d, e and g and Supplementary Movie 11). Overall, this demonstrates that MT plus ends spatio-temporally control RhoGEF2 cortical recruitment.
The transmembrane protein Transcript 48 (T48) has been shown to be expressed in mesoderm cells and to act as a cortical anchor to capture RhoGEF214,18. Live imaging of T48 overexpression shows that T48 can indeed locate at the cell cortex (Supplementary Fig. 12h, h’). In T48 mutant embryos, while apical constriction and nuclear migration is overall preserved (showing in most cases only a mild phenotype – Supplementary Movies 12 and 13), MyoII-LCs are inhibited (Fig. 4l, m). Moreover, in this mutated background, mesoderm cells exchange neighbours in an aberrant fashion (previously described as bowtie intercalation1 - Supplementary Fig. 12i). This shows that T48 is pivotal for the establishment of MyoII-LCs.
Overall, our data show that the nucleus functions as an MT compartmentalizer controlling the cell cortex accessibility. Therefore, nuclear migration enables MT to redistribute along the lateral cortex and to deliver RhoGEF2 with spatio-temporal precision, with the support of T48, to form MyoII-LCs.
Discussion
Composite morphogenesis, characterized by multiple and simultaneous tissue shape transformations, is a vital process during embryo development7. We investigate the mechanisms underlying the concomitant folding and extension of the prospective mesoderm during Drosophila embryo early development. During gastrulation, mesoderm epithelial cells establish a junctional modular system supported by a two-tier actomyosin scaffold at the cell apical and lateral side, mediating apical constriction and lateral cell intercalation for concomitant folding and extension, respectively8. This study dissects the spatio-temporal coordination of the two-tiered scaffold, shedding light on the subcellular control of cytoskeletal networks.
We show that the relocation of the nucleus within mesoderm epithelial cells is the coordinating and triggering mechanism leading to the stereotypic formation of the lateral actomyosin tier. At the onset of gastrulation, mesoderm cells apically constrict in a concerted fashion driving a basally directed cytoplasmic flow that pushes nuclei towards the cell basal side. This nuclear movement triggers a sudden change in the architecture of the MT network that delivers RhoGEF2 to the lateral cortex. As a result, the cortical actomyosin, activated by RhoGEF2 signalling, coalesces isotropically to form the lateral tier. Remarkably, cortical RhoGEF2 levels drop within tens of seconds after MT depolymerization and lateral MyoII accumulation can be enhanced when ectopically increasing the rate of nuclear relocation. Overall, this supports a model in which RhoGEF2 cortical recruitment is tightly controlled by the time-integrated MT-to-cortex interaction.
Nuclear repositioning is pivotal in several processes including topological remodeling of epithelia32, body axis determination33, nervous system development34, cell proliferation35 and cell death36. We now show that the relocation of this large organelle also plays a paramount role in tissue morphogenesis by regulating cytoskeletal dynamics. Often, nuclear relocation relies on MTs37,38,39,40. Nevertheless, the actomyosin cytoskeleton can also power the displacement of the nucleus. For instance, actomyosin contraction can drive cytoplasmic advection, dragging the nucleus into the centre of the mouse oocyte41 and spreading nuclei in the Drosophila syncytium42, or it can generate directed traction forces, pulling the nucleus during cell apoptosis in pseudo-stratified epithelia36. We demonstrate that, in epithelial mesoderm cells, the nucleus acts as a piston (more likely not completely “impassable”43) pushed basally by the cell cytoplasm that behaves as an incompressible fluid, as previously theorized21. We further show that basally directed cytoplasmic flow results from actomyosin contraction forces driving the constriction of the apical side22.
Columnar cells, characterized by an elongated prism shape, are common in epithelia and play roles in absorption, secretion, protection, and structural support. This study highlights a function for column-shaped cells in tissue morphogenesis and embryo development. The elongated geometry of mesoderm cells provides a cellular platform that is functional to establish subcellular niches, with different biochemical and mechanical properties, engaged in distinct morphogenetic transformations. In such a long and narrow cell, the nucleus acts as a cytoskeleton compartmentalizer and its relocation modulates the activity of the actomyosin cortex by controlling the MT-to-cortex interaction. Thus, the nucleus functions as an apico-basal organizer structuring the actomyosin cortex in a multi-tiered scaffold, possibly complementing or overcoming the cell apico-basal signalling that is downregulated specifically in the mesoderm during this early gastrulation phase44.
In the prospective mesoderm, RhoGEF2 is involved in the regulation of the actomyosin cytoskeleton both apically (i.e., the first tier45,46,47,48,49,50) for apical constriction and laterally (i.e., the second tier8) for cell intercalation. Intriguingly, in the neighbouring ectoderm, cell intercalation is under the control of both RhoGEF2 and DpRhoGEF11430. What is the basis for such difference? The dual control in the ectoderm could rely on a moderate expression of RhoGEF2 to avoid strong apical contractile forces and to maintain a more fluid-like medial-apical meshwork, facilitating junction remodelling51 while preserving the cell apical surface area. Therefore, a second RhoGEF (i.e., DpRhoGEF114), excluded from the cell apical-medial region and specifically recruited at junctions, is necessary to provide the sufficient signaling control for cell intercalation30. Conversely, in the mesoderm, RhoGEF2 may be expressed at higher levels to provide alone a sufficient activating signal for both cell shape and topological changes.
We report that RhoGEF2 is planar cell polarized in the mesoderm tissue. How is RhoGEF2 PCP achieved? We show that microtubule plus ends, that tightly regulate the cortical distribution of RhoGEF2, contact the cell lateral cortex more often along cell sides parallel to the DV axis. This supports a model where MTs deliver RhoGEF2 via EB129,30,52 preferentially along DV junctions. How is the MT plus-end PCP established? Plus-end PCP is not the result of mesoderm cell anisotropic shape during tissue furrowing since reducing shape anisotropy by laser ablation does not downregulate but rather enhances MyoII PCP. MT PCP could instead result from a polarized stabilization of the EB1-RhoGEF2-T48 complex at the cortex29. Since the mesoderm has a similar genetic background as the bordering ectoderm, further work is necessary to test if the factors controlling MyoII PCP in the ectoderm53,54 are also acting in the mesoderm.
A recent study has reported that T48 contributes to regulate cellularization55. We now show that T48 also plays a specific function in the formation of the two-tier actomyosin scaffold at the onset of gastrulation. While T48 is overall unnecessary for the formation of the apical actomyosin network44, it is key for the establishment of the lateral tier. Our data corroborates the notion that T48 is a cortical protein, yet future work is necessary to determine how T48 is specifically distributed along the different cortical sides of mesoderm cells. In T48 knockout embryos, mesoderm cells undergo bowtie intercalations. This aberrant T1 process, also reported in the bicoid, nanos and torso triple mutant8, is characterized by a stochastic distribution of cell cross-sectional areas probably driven by an unsynchronized nuclear migration. Concertina (the alpha-subunit of the GPCR protein Mist) is another known signalling factor participating in RhoGEF2 localization29 during early mesoderm morphogenesis50. A renewed effort is necessary to dissect the role of Concertina in the establishment of the two-tier actomyosin scaffold.
Mesoderm morphogenesis, during the early phases of gastrulation, provides a powerful paradigm to study simultaneous tissue folding and extension. Cell columnarisation, apical constriction, nuclear basal migration and microtubule architecture remodelling have also been reported during other vital processes (e.g., during neurulation56) in which tissues concomitantly fold and extend. Now, this study opens a path to unravel the cellular mechanisms underlying such complex tissue transformation.
Methods
Fly stocks and genetics
Drosophila stocks used for live imaging were: Membrane-GFP (Resille::GFP, Spider::GFP), myosin-mCherry (Sqh::mCherry), membrane-mCherry (GAP43::mCherry), centrosome-GFP (cnn::GFP), nuclei-GFP (Ubi:Gfp::nls). UASp EB1::GFP, UAsp tubulin::mCherry, UASp T48::GFP were driven maternally by one copy of (67 C) matαTub-Gal4VP16 driver. T48 mutant embryos were collected from homozygous T48-, Spider::GFP/T48-, Spider::GFP parents. For optogenetic modulation of basal cell surface, embryos were collected from ubi: Gap43::mCherry/+; UASp Cry2::RhoGEF2/+; UASp CIBN::pmGFP/osk Gal4 mothers. For photoactivation of MyoII localization, embryos were collected from Sqh::mCherry/ UASp Cry2::RhoGEF2; UASp CIBN::pmGFP/osk Gal4 mothers. The complete list of stocks and genetics is available in Supplementary Data 1.
Drosophila embryo preparation
Cages containing male and virgin Drosophilae of selected genotypes were established one day prior to imaging and were maintained at 18 °C. Subsequently, the cages were shifted to 25 °C for egg laying. Cages were changed every 3 h to precisely time embryo development with the onset of Drosophila gastrulation. For optogenetic experiments, cages were kept at a temperature of 20 °C. Freshly laid embryos were collected on 1% agar plates placed within Petri dishes. To remove the chorion, embryos were briefly exposed to a 50% bleach solution for 30 sec. Following this treatment, embryos were thoroughly rinsed with distilled water and transferred on agar for mounting.
Sample mounting
For confocal and spinning-disk microscopy, Drosophila embryos were collected using a custom-made needle and carefully oriented in water on MatTek dishes with a 0.17 mm coverslip bottom. For light-sheet imaging, embryos were mounted within a glass capillary filled with a 0.5% Gelrite solution, ensuring their long axis was parallel to the capillary.
Confocal microscopy
Embryos were imaged using two distinct confocal microscopes. The Zeiss LSM 880 AiryScan was employed to achieve high-resolution imaging for subcellular structures, utilizing a 40X objective with a numerical aperture (NA) of 1.1. For laser dissection, optogenetic experiments, and live injection experiments, the Zeiss LSM 780 was utilized, equipped with a 40X objective (1.2 NA). Appropriate filter sets for fluorescent channels were used for each experiment. When Z-stacks were performed, they were acquired using a Piezo stage with a step size of either 1, 2 or 5 μm, depending on the need for different temporal resolutions. For rapid Z-stack imaging, a Nikon TI2-Eclipse spinning disk microscope with a Nikon 60X objective (1.1 NA) was used.
Light-sheet microscopy (In Toto 4D Imaging)
In toto 4D imaging of Drosophila embryos was conducted using the Luxendo MuviSPIM selective-plane illumination light-sheet microscope. This instrument was equipped with a 20X objective featuring a numerical aperture of 1.0. Additionally, a 1.5X lens was incorporated into the optical path. Z-stacks were acquired with a step size of 1 μm. During each acquisition, embryos were imaged from two opposing orthogonal views, capturing both a 0° dorsal-ventral view and a 90° lateral view. This approach resulted in the acquisition of four 3D stacks for every individual time point. The fusion of these four stacks was performed using Matlab 4, resulting in a final isotropic pixel resolution of 0.29 μm.
Actomyosin meshwork laser dissection
A femtosecond-pulsed infrared laser (IR fs, MaiTai) integrated with a Zeiss LSM 780 NLO confocal microscope, tuned to a wavelength of 950 nm, was employed for ablating the apical actomyosin meshwork. Experiments were conducted using a 40X objective with 1.2 NA. The Zeiss Zen software was configured with bleach mode (110 mW laser power at the focal plane, single iteration, 2.15 μs pixel dwell time and 1 s frame rate). To investigate the modulation of apical cell surface constriction and nuclear positioning, single ablations were exclusively conducted in the dorso-ventral axis of the mesoderm tissue. In cases where ablations were performed prematurely, they were repeated at the appropriate developmental timing to observe the intended effects. For experiments aimed at delaying furrow invagination, a grid pattern of iterative ablations targeting the apical actomyosin network was employed to reduce anisotropic tearing and moderate tear amplitude. Ablations were performed each time the network showed signs of recovery to hinder the restoration of the actomyosin network and further delay constriction in the cut region.
Optogenetic recruitment of RhoGEF2
Cry2 optogenetic experiments were carried out on Zeiss 780 LSM confocal microscope with a 40 × 1.2 NA objective, using bleach mode on Zeiss Zen software, with 2-P MaiTai tunable laser. Photo-recruitment of RhoGEF2 catalytic domain was achieved by line or rectangular activation on cortex using 950 nm, 18 mW laser power at the focal point, 40 iterations, 3.5 μs pixel dwell.
Drosophila microinjections
Dechorionated embryos were dried using silica beads for 7–10 min, then mounted on heptane glue-coated coverslips in halocarbon oil before microinjection. Microinjection was conducted using micro-needles prepared by a Narishige vertical puller, using borosilicate glass capillaries with filament. Microinjections were executed using an Eppendorf FemtoJet micro-injector. Embryos were injected until they had re-expanded to their original volume from the desiccated state. For the inhibition of MyoII activity, embryos were microinjected laterally with Y-27632, prepared from stock solutions in water with concentrations of 10, 25, or 50 mM. Microtubule depolymerization was achieved by injecting embryos with 2.5 mM Colcemid diluted in water. Control experiments were conducted by injecting water into the embryos. To photo-induce microtubule depolymerization, embryos were injected with 5 mM SBTub-4AP, while being maintained in darkness, then illuminated with a 405 nm laser (3.15 μs pixel dwell time, 10 iterations) at specific time points of interest. For assessment of cytoplasmic flow, fluorescent beads with a diameter of 1 μm (FluoSphere) were diluted in water at a 1:4 ratio and ventro-laterally injected into embryos. All injections were performed at the late cellularization stage, which was confirmed by monitoring the depth of cellularization front within a range of 25–30 μm.
Drosophila embryo centrifugation
Drosophila embryos were subjected to centrifugation using an Eppendorf Centrifuge 5430 R equipped with a swing-bucket rotor to achieve fully-orthogonal force application. Embryos at late cellularization stage were collected, mounted ventral side up on coverslips and loaded onto custom 3D-printed Eppendorf insert. Centrifugation conditions, such as speed and duration were optimized to achieve nuclear apicobasal displacement while preserving embryo early gastrulation. Final settings used in the study were: 6000 x g/rcf for a duration of 2 min. After centrifugation, embryos were retrieved, mounted in water on MatTek dishes as previously described for microscopy live-imaging. In centrifuged embryos, the number of rows of apically constricting cells remains comparable to that of control embryos (Supplementary Fig. 6d). Furthermore, analysis of apical cell surfaces revealed that cells in centrifuged embryos, with more basally positioned nuclei, exhibited significantly reduced apical cell surfaces (Supplementary Fig. 6e). This observation underscores the established relationship governed by the principle of volume conservation.
In silico 3D modelling of microtubule architecture
CytoSIM computational software was employed to simulate microtubule dynamics. Detailed three-dimensional representations of a cell and microtubules took into account parameters described in configuration files (Supplementary Data 2). Parameters were estimated using experimental measurements when possible and configured to replicate specific biological contexts. Unknown parameters were varied to assess their weight in the proposed model. A custom-made python script was used to extract and sort cortical MT distribution. You can find more info on CytoSIM official repository https://gitlab.com/f-nedelec/cytosim.
2D and 3D segmentation of cells and nuclei
2D segmentations of apical cell surfaces in drug injection and ablation contexts were carried out using respectively ASTEC and IMARIS software by analysing individual optical sections of the acquired membrane images with watershed segmentation. 3D segmentation of nuclei was achieved through volume rendering and initial automated thresholding using nuclear fluorescent signal. Segmentation parameters and thresholds were then optimized to capture cellular and nuclear details. All segmented data were sorted and analysed using a custom-made Python script and R software.
Statistics and reproducibility
Statistical analyses were conducted using GraphPad Prism 7 and R software. Descriptive statistics, which include means and standard deviations, were calculated for all relevant data. The normality of each dataset was tested using the Shapiro-Wilk test. Appropriate statistical tests were selected and described for each experiment in Supplementary Data 3. A threshold p-value of 0.05 was employed to determine statistical significance. The 95% confidence intervals were visually represented on data plots. All legends marked as ‘au’ denote arbitrary units. Representative micrographs shown in this study correspond to experiments that were performed independently at least three times. This applies to the data presented in Fig. 1a, b; 2a, c; 3a, e; 4c. The experiment showcased in Fig. 4j (Water live injection) was performed twice independently.
Image analyses and quantifications
Cell area and nuclear positioning measurements
Changes in cell surface and nuclear position were monitored throughout the internalization of the mesoderm by recording time-lapse movies at different intervals, with a depth of 35 µm (Z step = 1 µm). Temporal realignment of embryos was achieved by using the onset of apical constrictions as the reference point, designated as t = 0 min. The apex-to-nucleus distance was computed by measuring the Z position of the apical side of the nucleus within each cell and then subtracting the Z position of the apical surface. Manual monitoring was employed to establish the association between each cell surface and its corresponding nucleus.
Nuclear migration and MyoII intensity measurements
To quantify nuclear migration and MyoII lateral intensity, time points were re-stacked to maintain consistency in the invaginating furrow position across all frames. To do so, progression of furrow invagination was monitored over time and black planes were added to the front and back of the image stacks to match the depth of the furrow tip. Apex-to-nucleus distance and lateral MyoII intensity were evaluated in the middle portion of the mesoderm, which exhibited sufficient flatness for analysis. Embryos were temporally aligned by using initial appearance of apical MyoII clusters as the reference point for the onset of gastrulation (t = 0 min). Randomly chosen nuclei were tracked throughout gastrulation, spanning 30 time points (ΔT = 22 s), to establish the migration curve. MyoII lateral intensity was determined by Z-projecting image slices between 8 and 12 µm from the apical side after re-stacking, capturing lateral MyoII signal during mesoderm internalization. Randomly selected junctions were then monitored throughout mesoderm internalization. Apical MyoII intensity was measured using large regions of interest (ROIs) encompassing the mesoderm tissue, from the end of cellularization (identified by the position of the cellularization front) until the furrow reached a depth where tracking became impractical. MyoII accumulation and the rate of nuclear migration were obtained by computing the first derivative of the average MyoII intensity and average nuclear migration curves, respectively. Further analysis involved cross-correlation between the peak rate of nuclear migration and the peak of lateral MyoII accumulation to estimate the lag time between nuclear migration and the appearance of lateral MyoII clusters.
Nuclear positioning and lateral MyoII intensity measurements in embryos with compromised nuclear migration
Time-lapse movies with a depth of 30 µm (z-step = 2 µm) were examined to track nuclear positioning and MyoII distribution following apical laser dissection and opto-induced inhibition of nuclear migration. Lateral MyoII intensity was assessed in both control and cut/opto regions using the line measurement tool in ImageJ, positioned 10 µm from the apical side. Special care was taken to exclude low nuclear signal to prevent intensity bias in the cut region. The apex-to-nucleus distance was determined by measuring the distance between the apical side of the nucleus and the apical side of the cell. To facilitate comparison between control embryos and ablated embryos (comprising both control and cut regions), gastrulation was timed and Z-stacks in control and ablated embryos were temporally synchronized for analysis specifically 7 min after the onset of gastrulation.
E-cad intensity measurements in embryos with compromised nuclear migration
Time-lapse movies with a depth of 30 µm (z-step = 2 µm) were examined to track E-cad distribution following apical laser dissection. Lateral E-cad intensity was assessed in both control and cut regions using the line measurement tool in ImageJ, positioned 10 µm from the apical side. Special care was taken to exclude low nuclear signal to prevent intensity bias in the cut region.
Apicobasal flow velocity and apical cell surface ratio measurements
Time-lapse movies with a depth of 35 µm (z-step = 2 µm) were analysed to follow apical cell surface changes and fluorescent bead apicobasal displacement. Prior to and post apical laser dissection, the Z-position of fluorescent beads was measured in both control and ablated regions. Given the tissue invagination during this period, adjustments were made to each bead’s Z-position by subtracting the apical surface Z-position at each time point. Bead apicobasal displacement was determined by calculating the difference in Z-position between beads before and after laser dissection. Flow velocity was subsequently obtained by dividing this displacement by the time delay between the two measured time points. Similarly, the apical surfaces of cells containing beads were subjected to 2D segmentation before and after apical laser dissection at corresponding time points. To quantify changes, the apical cell surface ratio was computed by dividing the tracked cell surface after laser dissection by the respective surface before laser dissection.
Apical laser dissection effect on MyoII fluorescence intensity
To mitigate the possibility of signal loss attributed to the application of femtosecond IR laser pulses at the apical region, MyoII fluorescence intensity measurements were taken before and after apical laser dissection in both control and cut regions at different positions: the apical side and at a distance of 10 µm from the apical side. Intensity ratios were then calculated by dividing the fluorescence intensity after laser dissection by the intensity measured before laser dissection.
MyoII apicobasal position measurements
Time points were restacked to account for progressive invagination of the mesoderm tissue. Furrow invagination was monitored over time and black planes were added to the front and back of the stacks accordingly, corresponding to the distance invaginated by the furrow tip. By navigating time-lapse movies of mesoderm invagination, MyoII apicobasal position was determined using time points at which MyoII appeared most coalesced on reconstructed sagittal sections, corresponding to final cluster establishment on the apicobasal axis. Measurements were performed in control, centrifuged and grid-pattern ablated embryos when the furrow reached a depth of 5 µm.
MyoII-to-nuclei relative position measurements
Myosin apicobasal position was determined as it is most focused and initiates cell intercalation processes (four-way junction formation). In control and centrifuged embryos, cell intercalations were identified by screening time-lapse movies during the initial 15 min of gastrulation. Reference points for intercalation apicobasal position were established using newly formed four-way junctions enriched in MyoII, which coalesced on the apicobasal axis. In centrifuged embryos, lateral MyoII position analysis focused on nuclei sets with enhanced displacement to account for heterogeneity of nuclear repositioning. In delayed (grid-patterned ablation) embryos, iterative apical laser dissections did not allow for continuous junction monitoring. To standardize measurements, lateral MyoII Z-positions in control, centrifuged, and delayed embryos were realigned relative to the apical Z-position of mesoderm cells. Nuclear positioning was determined by measuring the apex-to-nucleus distance for adjacent nuclei along the anterior-posterior (AP) axis that shared a MyoII-enriched lateral junction. Ratios were calculated by dividing the apicobasal position of lateral MyoII clusters by the average apex-to-nucleus distance of the two adjacent AP nuclei.
Nucleus morphology changes
Nucleus curvature was measured on mesoderm nuclei at different time points of gastrulation using Z-projected mid-sections of nuclear contours, obtained from computed edges from nuclear signal (Ubi-nls::GFP signal). Semi-automated curvature analysis was performed using ImageJ Kappa plugin and B-spline curve fitting. Nucleus prolate eccentricity was measured by 3D segmentation of mesoderm nuclei using IMARIS software at different time points of gastrulation.
EB1 intensity measurements
EB1 intensity dynamics were tracked throughout gastrulation via sagittal sections of the mesoderm tissue. Embryos were temporally synchronized, and measurements were performed both at the onset (marked by the initial recruitment of apical MyoII) and 7 min into gastrulation. Using the line measurement tool in ImageJ, EB1 cortical intensity along the lateral cortex (2 to 15 µm) was quantified. Concurrently, associated cytoplasmic signal within the same region was measured, excluding nuclear signal from the region of interest (ROI) to prevent intensity bias, particularly in the first time point. Ratios were computed by dividing cortical measurements by corresponding cytoplasmic measurements. In laser-dissected embryos, lateral EB1 intensity was assessed in en face sections of the mesoderm tissue at 10 µm from the apical surface. EB1 signal was not normalized to cytoplasmic intensity due to the presence of nuclei occupying that space in the cut regions, which could bias ratios towards higher values. Background subtraction was conducted by utilizing three regions of interest (ROIs) per embryo to determine the average background signal. It was observed that both EB1 density and intensity decreased in the cut region, indicating that the loss of EB1 signal did not arise from a more diffuse signal as cells expanded their perimeter.
EB1 comets density upon photo-induced MT depolymerization
The density of EB1 comets was assessed upon photo-induced MT depolymerization. For every embryo, three random regions of interest were chosen, and comet counts were measured for each region across time points subsequent to MT depolymerization. To establish EB1 comet density, comet counts were standardized based on the area of the analyzed region of interest. Comets that remained partially attached to the centrosomes/MTOC were excluded from the comet count.
EB1 dynamic distribution relative to junction orientation
Mesoderm tissue dynamics during gastrulation were captured from front views with high temporal resolution (ΔT = 0.73 s). Imaging was conducted on a single stack positioned 12 µm from the apical side, enabling prolonged tracking of the lateral cortex of mesoderm cells as the tissue underwent invagination. Membrane signal served as a reference for identifying intercalation events and guiding the blind measurement of EB1 signal. EB1 fluorescence intensity was quantified at junctions aligned along the anterior-posterior axis, dorsal-ventral (DV) axis, and DV axis during intercalation. Signal integration over time was normalized by the total measurement duration. To address embryo-to-embryo variation, background normalization was executed using the average background signal derived from three distinct regions for each embryo.
RhoGEF2 intensity measurements
RhoGEF2 signal dynamics were examined following microtubule disruption through either the live-injection of the depolymerizing agent Colcemid or timed photo-induced depolymerization using the prodrug SbTub4AP. Front view single optical sections of mesoderm cells were captured at a depth of 12 µm from the apical side and tracked over time (ΔT = 3.5 s). Junctional and cytoplasmic levels of RhoGEF2 were quantified using both line and segmented area tools in ImageJ, at the last time point before activation and several frames thereafter (ΔT = 14 s). Cortical to cytoplasmic ratios were computed by dividing the measurements obtained from these two regions. Cells exhibiting pronounced expansion of cell area and nuclear movement towards the apical side induced by MT depolymerization were excluded from analysis. Upon significant loss of RhoGEF2 junctional signal or an increase in cytoplasmic signal, certain cell boundaries became indistinguishable and analysis relied on the restoration of the region of interest (ROI) selection between the first and second frames analyzed. Control analysis was conducted using the same protocol and similar time points in embryos either live-injected with water or injected with water during late cellularization then subjected to UV photo-activation (settings equivalent to those applied in SbTub4AP embryos).
RhoGEF2 PCP distribution
RhoGEF2 distribution was monitored along front view optical sections of the mesoderm tissue, positioned 12 µm from the apical side, during gastrulation. Upon detecting an elevation in RhoGEF2 cortical signal within mesoderm cells, the orientation of RhoGEF2-enriched junctions was assessed relative to the anterior-posterior (AP) axis, using the membrane reporter as a reference to precisely determine junction orientation.
Apical cell surface modulation upon pharmacological MT depolymerization or ROCK inhibition
To assess apical cell surface modulation following pharmacological treatments, the segmentation of mesoderm cell apical surfaces was performed throughout gastrulation, following previously described segmentation methods. For each embryo, a region of interest encompassing the mesoderm tissue was defined in the frame corresponding to 7 min after the initiation of gastrulation. Analysis included only cells whose centroids fell within this defined region and could be consistently tracked in each frame throughout gastrulation. The datasets presented here depict the distribution of apical cell surfaces at two specific time points: at the onset of gastrulation and 7 min into gastrulation.
Apical cell surface and associated nuclear positioning upon MT depolymerization
To allow efficient tracking of changes in apical cell surfaces with adequate temporal resolution, only the most apical Z positions of the tissue were captured (with a total depth of 12 µm from the apical surface). Consequently, nuclei that were highly displaced could not be effectively monitored. To address this limitation, three categories of nuclear displacement were established: apical nuclei (0–5 µm), intermediate nuclei (5–10 µm), and displaced nuclei (>10 µm). Nuclear position was quantified as the apex-to-nucleus distance (restacked accordingly to the position of the apical cell surface). For each time point in both water and Colcemid-injected embryos, randomly selected nuclei were paired with their respective apical cell surfaces. These surfaces were segmented using the polygon area tool in ImageJ.
Lateral MyoII density in control and T48 mutant backgrounds
MyoII signal dynamics were tracked over front view Z-stacks of the mesoderm tissue (total depth = 35 µm) throughout gastrulation. To maintain consistency with the invaginating furrow, each frame was spatially restacked relative to the apical position of the furrow tip, incorporating black planes either above or below the stack. This ensured the tracking of the same plane relative to the apical surface over time. Following spatial restacking, a region of interest (ROI) was defined within the mesoderm tissue, spanning the cells closest to the ventral midline. Z positions ranging from 8 to 12 µm were subjected to maximum Z-projection, resulting in a movie tracking this portion of the lateral cortex over time. MyoII lateral clusters were then enumerated during the first 10 min of gastrulation (t = 0 min corresponding to the initial accumulation of apical MyoII). A cluster was characterized as a coalesced high-intensity MyoII signal. Clusters were counted only once, even if the same junction was subsequently enriched again in MyoII at a later time point. The total number of cells within the defined ROI was then quantified for each embryo, and the count of MyoII clusters was normalized by the number of cells to derive the average density of lateral MyoII per embryo.
Cortical MT plus-ends apicobasal distribution from 3D in silico CytoSIM model
Boundary conditions, including cell length, cell radius, nucleus radius, nucleus-to-membrane spacing, centrosome radius, and inter-centrosomal distance, were measured in vivo to establish a template for the in silico model. Simulation of microtubule (MT) dynamics was conducted under various conditions, simulating 10 min of developmental time with 10,000 frames and a time step ΔT = 0.06 s. A custom Python script was utilized to extract the positions of cortical MT plus-ends, defined as MT plus-ends within a 0.5 µm range from the cortex. The apicobasal position of each MT plus-end at the cortex was then saved for each frame of the simulation. Subsequently, all apicobasal positions of cortical MT plus-ends were plotted as a function of their respective boundary conditions, such as the position of the nucleus and the distance between the nucleus and centrosomes. 100 simulations were conducted per condition. Each simulation involved randomizing the location of MT nucleation on the centrosomes or the occurrence of catastrophe/rescue events. This approach aimed to capture variability and ensure robustness in the analysis, ultimately obtaining an average trend in cortical MT plus-ends distribution per condition. To evaluate the individual contributions of various parameters in the simulation — such as MT dynamic properties (growth rate, shrinking rates, absolute number of polymers) and cell geometry (nucleus radius, centrosome position) — simulations were conducted 25 times for each parameter, with variations spanning several orders of magnitude. Employing the same extraction protocol for MT plus ends, subsequent plots of MT distribution were generated.
In silico cortical MT plus-end distribution relative to the nucleus
The mid-position of the nucleus was extracted from the template file used for the in silico model in each condition. A custom Python script counted the number of cortical MT plus ends located in two compartments: either above the nucleus (defined as the range from the nucleus mid-position to the apical surface) or below” the nucleus (from the nucleus mid-position to the basal side of the cells). Percentages of MT distribution relative to the nucleus were calculated by dividing the count in each compartment by the total count of cortical MT plus ends in the simulation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study and material are available on request from the corresponding author (M.R.). Source data are provided with this paper.
Code availability
The codes used for image analysis are available upon request from the corresponding author (M.R.). CytoSim software is available at https://gitlab.com/f-nedelec/cytosim. The custom Python script used for the analysis of CytoSIM simulations is provided as Supplementary Data 2 and is available at https://github.com/RepositoryNR/Custom_Template_CytoSIM.git.
Change history
19 February 2026
In the original version of this article the following information was incorrect in the Acknowledgement section: This work has been supported by the French government, through the France 2030 investment plan managed by the Agence Nationale de la Recherche, as part of the Université Côte d'Azur's Initiative of Excellence, reference ANR-15-IDEX-01. The original article has been updated.
References
Heisenberg, C. P. & Bellaiche, Y. Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013).
Lecuit, T. & Yap, A. S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539 (2015).
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22, 536–545 (2012).
Roper, K. Microtubules enter centre stage for morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190557 (2020).
Pradeau-Phelut, L. & Etienne-Manneville, S. Cytoskeletal crosstalk: A focus on intermediate filaments. Curr. Opin. Cell Biol. 87, 102325 (2024).
Gilmour, D., Rembold, M. & Leptin, M. From morphogen to morphogenesis and back. Nature 541, 311–320 (2017).
John, A. & Rauzi, M. Composite morphogenesis during embryo development. Semin Cell Dev. Biol. 120, 119–132 (2021).
John, A. & Rauzi, M. A two-tier junctional mechanism drives simultaneous tissue folding and extension. Dev. Cell 56, 1469–1483.e1465 (2021).
Nishimura, T., Honda, H. & Takeichi, M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097 (2012).
Sanchez-Corrales, Y. E., Blanchard, G. B. & Roper, K. Radially patterned cell behaviours during tube budding from an epithelium. Elife 7, https://doi.org/10.7554/eLife.35717 (2018).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 (2009).
Harris, T. J. & Peifer, M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167, 135–147 (2004).
Weng, M. & Wieschaus, E. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J. Cell Biol. 212, 219–229 (2016).
Salle, J. et al. Asymmetric division through a reduction of microtubule centering forces. J. Cell Biol. 218, 771–782 (2019).
Petrie, R. J., Koo, H. & Yamada, K. M. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014).
Kam, Z., Minden, J. S., Agard, D. A., Sedat, J. W. & Leptin, M. Drosophila gastrulation: analysis of cell shape changes in living embryos by three-dimensional fluorescence microscopy. Development 112, 365–370 (1991).
Leptin, M. & Grunewald, B. Cell shape changes during gastrulation in Drosophila. Development 110, 73–84 (1990).
Kalukula, Y., Stephens, A. D., Lammerding, J. & Gabriele, S. Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 23, 583–602 (2022).
Gundersen, G. G. & Worman, H. J. Nuclear positioning. Cell 152, 1376–1389 (2013).
Almonacid, M., Terret, M. E. & Verlhac, M. H. Nuclear positioning as an integrator of cell fate. Curr. Opin. Cell Biol. 56, 122–129 (2019).
Gelbart, M. A. et al. Volume conservation principle involved in cell lengthening and nucleus movement during tissue morphogenesis. Proc. Natl Acad. Sci. USA 109, 19298–19303 (2012).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Rauzi, M. & Lenne, P. F. Probing cell mechanics with subcellular laser dissection of actomyosin networks in the early developing Drosophila embryo. Methods Mol. Biol. 1189, 209–218 (2015).
Martin, A. C., Gelbart, M., Fernandez-Gonzalez, R., Kaschube, M. & Wieschaus, E. F. Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010).
Fierling, J. et al. Embryo-scale epithelial buckling forms a propagating furrow that initiates gastrulation. Nat. Commun. 13, 3348 (2022).
Izquierdo, E., Quinkler, T. & De Renzis, S. Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nat. Commun. 9, 2366 (2018).
Krueger, D., Tardivo, P., Nguyen, C. & De Renzis, S. Downregulation of basal myosin-II is required for cell shape changes and tissue invagination. EMBO J 37, https://doi.org/10.15252/embj.2018100170 (2018).
Zhu, R., Antoku, S. & Gundersen, G. G. Centrifugal Displacement of Nuclei Reveals Multiple LINC Complex Mechanisms for Homeostatic Nuclear Positioning. Curr. Biol. 27, 3097–3110.e3095 (2017).
Rogers, S. L., Wiedemann, U., Hacker, U., Turck, C. & Vale, R. D. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14, 1827–1833 (2004).
De Las Bayonas, A. G., Philippe, J. M., Lellouch, A. C. & Lecuit, T. Distinct RhoGEFs Activate Apical and Junctional Contractility under Control of G Proteins during Epithelial Morphogenesis. Curr. Biol. 29, 3370–337 (2019).
Ko, C. S., Tserunyan, V. & Martin, A. C. Microtubules promote intercellular contractile force transmission during tissue folding. J. Cell Biol. 218, 2726–2742 (2019).
de Leeuw, N. F., Budhathoki, R., Russell, L. J., Loerke, D. & Blankenship, J. T. Nuclei as mechanical bumpers during epithelial remodeling. J. Cell Biol. 223, ARTN https://doi.org/10.1083/jcb.202405078 (2024).
Almonacid, M., Terret, M. E. & Verlhac, M. H. Control of nucleus positioning in mouse oocytes. Semin Cell Dev. Biol. 82, 34–40 (2018).
Bertipaglia, C., Goncalves, J. C. & Vallee, R. B. Nuclear migration in mammalian brain development. Semin Cell Dev. Biol. 82, 57–66 (2018).
Meyer, E. J., Ikmi, A. & Gibson, M. C. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr. Biol. 21, 485–491 (2011).
Ambrosini, A., Rayer, M., Monier, B. & Suzanne, M. Mechanical Function of the Nucleus in Force Generation during Epithelial Morphogenesis. Dev. Cell 50, 197–211.e195 (2019).
Miyazaki, A., Kato, K. H. & Nemoto, S. Role of microtubules and centrosomes in the eccentric relocation of the germinal vesicle upon meiosis reinitiation in sea-cucumber oocytes. Dev. Biol. 280, 237–247 (2005).
Tissot, N. et al. Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte. Nat. Commun. 8, 15168 (2017).
McNally, K. L., Martin, J. L., Ellefson, M. & McNally, F. J. Kinesin-dependent transport results in polarized migration of the nucleus in oocytes and inward movement of yolk granules in meiotic embryos. Dev. Biol. 339, 126–140 (2010).
Tsai, J. W., Chen, Y., Kriegstein, A. R. & Vallee, R. B. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J. Cell Biol. 170, 935–945 (2005).
Almonacid, M. et al. Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 17, 470–479 (2015).
Deneke, V. E. et al. Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos. Cell 177, 925–941.e917 (2019).
Gross, S. P., Guo, Y., Martinez, J. E. & Welte, M. A. A determinant for directionality of organelle transport in Drosophila embryos. Curr. Biol. 13, 1660–1668 (2003).
Weng, M. & Wieschaus, E. Polarity protein Par3/Bazooka follows myosin-dependent junction repositioning. Dev. Biol. 422, 125–134 (2017).
Leptin, M. Twist and Snail as Positive and Negative Regulators during Drosophila Mesoderm Development. Gene Dev. 5, 1568–1576 (1991).
Costa, M., Wilson, E. T. & Wieschaus, E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, 1075–1089 (1994).
Fox, D. T. & Peifer, M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 134, 567–578 (2007).
Kolsch, V., Seher, T., Fernandez-Ballester, G. J., Serrano, L. & Leptin, M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386 (2007).
Manning, A. J., Peters, K. A., Peifer, M. & Rogers, S. L. Regulation of Epithelial Morphogenesis by the G Protein-Coupled Receptor Mist and Its Ligand Fog. Sci. Signaling 6, ARTN https://doi.org/10.1126/scisignal.2004427 (2013).
Kerridge, S. et al. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat. Cell Biol. 18, 261–26 (2016).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Lin, B., Luo, J. & Lehmann, R. An AMPK phosphoregulated RhoGEF feedback loop tunes cortical flow-driven amoeboid migration in vivo. Sci. Adv. 8, eabo0323 (2022).
Paré, A. C. et al. A positional Toll receptor code directs convergent extension in (vol 515, pg 523, 2014). Nature 527, https://doi.org/10.1038/nature15719 (2015).
Karki, S. et al. Serotonin signaling regulates actomyosin contractility during morphogenesis in evolutionarily divergent lineages. Nat. Commun. 14, 5547 (2023).
Horo, U., Clarke, D. N. & Martin, A. C. Drosophila Fog/Cta and T48 pathways have overlapping and distinct contributions to mesoderm invagination. Mol. Biol. Cell 35, ar69 (2024).
Vijayraghavan, D. S. & Davidson, L. A. Mechanics of Neurulation: From Classical to Current Perspectives on the Physical Mechanics that Shape, Fold, and Form the Neural Tube. Birth Defects Res. 109, 153–168 (2017).
Acknowledgements
We thank S. De Renzis, E. Wieschaus, S. Streichen, T. Lecuit, T. Klein and Y. Bellaiche for providing fly stocks; members of the Rauzi lab for critical reading of the manuscript; A. John for initial support, A. B. Tanari for live injection support; B. Delorme for developing ImageJ tools and Python support; S. Dmitrieff and F. Nédelec for support with the Cytosim simulator; O. Thorn-Seshold for sharing the photoactivable drug for microtubule depolymerization and support; G.J.L. Wuite, G. Bergamaschi and Laserlab Europe for support on nuclei optical-tweezing trials; S. Noselli, Y. Bellaiche, M. Théry and all members of the Rauzi lab for the fruitful discussions; the PRISM imaging facility for technical support. MR thanks the company Bruker Luxendo for fruitful collaborations and support. NR was supported by the scholarship MENRT and is at present supported by the FRM. This work has been supported by the French government, through the France 2030 investment plan managed by the Agence Nationale de la Recherche, as part of the Université Côte d’Azur’s Initiative of Excellence, reference ANR-15-IDEX-01, the ATIP-Avenir program of the CNRS, the Human Frontier Science Program (CDA00027/2017-C), the Region SUD PACA Research and ERC Booster programs, the ANR PRC program (ANR-22-CE13-0024), the ANR PRCI program (ANR-23-CE13-0042); support from the CNRS to the MITI 80 PRIME program and support from Inserm to the Booster Program Mecacell3D.
Author information
Authors and Affiliations
Contributions
N.R. and M.R. planned the experiments. N.R. performed the experiments. M.R. performed initial preliminary experiments. N.R. performed data processing and quantifications. N.R. and M.R. analyzed the data. MR and NR wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roby, N., Rauzi, M. Nuclear position controls the activity of cortical actomyosin networks powering simultaneous morphogenetic events. Nat Commun 16, 1587 (2025). https://doi.org/10.1038/s41467-025-56880-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56880-0