Abstract
Fibroblast growth factor 21 (FGF21), a metabolic hormone with pleiotropic effects, is beneficial for various cardiac disorders. However, FGF21’s role in heart failure with preserved ejection fraction (HFpEF) remains unclear. Here, we show that elevated circulating FGF21 levels are negatively associated with cardiac diastolic function in patients with HFpEF. Global or adipose FGF21 deficiency exacerbates cardiac diastolic dysfunction and damage in high-fat diet (HFD) plus N[w]-nitro-L-arginine methyl ester (L-NAME)-induced HFpEF mice, whereas these effects are notably reversed by FGF21 replenishment. Mechanistically, FGF21 enhances the production of adiponectin (APN), which in turn indirectly acts on cardiomyocytes, or FGF21 directly targets cardiomyocytes, to negatively regulate pyruvate dehydrogenase kinase 4 (PDK4) production by activating PI3K/AKT signals, then promoting mitochondrial bioenergetics. Additionally, APN deletion strikingly abrogates FGF21’s protective effects against HFpEF, while genetic PDK4 inactivation markedly mitigates HFpEF in mice. Thus, FGF21 protects against HFpEF via fine-tuning the multiorgan crosstalk among the adipose, liver, and heart.
Similar content being viewed by others
Introduction
Fibroblast growth factor 21 (FGF21), a member of the endocrine fibroblast growth factor (FGF) subfamily without mitogenic activities1, is produced by several organs and plays a crucial role in the regulation of fatty acid oxidation (FAO), energy expenditure, glucose homeostasis, and the function of the somatotropic axis and hypothalamic-pituitary-adrenal pathway2,3. Furthermore, pharmaceutical or genetic replenishment of FGF21 or its related analogs can significantly counteract obesity and its related metabolic disorders in rodents and primates4,5,6,7. Interestingly, growing evidence suggests that the glucose-lowering and insulin-sensitizing effects of peroxisome proliferator-activated receptor γ (PPARγ) agonists (such as thiazolidinedione) and the therapeutic benefits of peroxisome proliferator-activated receptor α (PPARα) agonists (like fenofibrate) on lipid profiles are, to some extent, due to the activation of FGF218,9.
FGF21 exerts its metabolic actions by binding to the receptor complex FGF receptor 1 (FGFR1) and β-klotho, a single transmembrane protein that is highly expressed in adipose tissue, liver, pancreas, and hypothalamus4,10,11. Adipocytes are the primary target of FGF21, where it increases glucose uptake1, modulates lipolysis12, enhances mitochondrial oxidative capacity13, augments PPARγ activity14, promotes the browning of white adipose tissue and the production of adiponectin (APN)15,16. In addition, FGF21 has also been shown to directly or indirectly exert its cardiovascular protective effects on blood vessels and heart17,18,19,20, acting as a mediator to coordinate the multi-organ crosstalk under various pathophysiological conditions.
Heart failure with preserved ejection fraction (HFpEF) is the most common type of heart failure (HF) worldwide, affecting about half of all HF patients. In the past ten years, the incidence and prevalence of HFpEF have steadily increased, affecting millions of individuals21. Despite its frequent and extensive investigation over several decades, the etiology of most cases of HFpEF remains undefined. Due to the heterogeneity of HFpEF and its diverse underlying etiologies, therapies used for heart failure with reduced ejection fraction (HFrEF) have shown limited efficacy in treating HFpEF, and there is currently a lack of pharmacological compounds specifically designed for it22. Recently, clinical studies indicated that circulating FGF21 concentrations were significantly increased and positively associated with echocardiographic parameters of diastolic function, LV end-diastolic pressure, and serum N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels in patients with HFpEF23,24, suggesting that elevated circulating FGF21 levels are related to the pathogenesis of HFpEF. Although the cardiac protective functions of FGF21 are widely characterized, little is known about its pathophysiological roles in HF, and the molecular mechanism whereby FGF21 protects against HF, especially HFpEF, remains poorly defined.
In this study, we identify FGF21 as a key molecule with a crucial regulatory role in HFpEF. Our results indicated that upregulated expression of FGF21 is a compensatory response to HFpEF and its related cardiac damage, and the mechanism whereby FGF21 protects against HFpEF is mediated by promoting mitochondrial bioenergetics through the activation of the APN-PI3K/AKT-PDK4 axis in mice.
Results
Circulating FGF21 levels are markedly elevated and negatively associated with cardiac function in subjects with HFpEF
FGF21 has been considered a novel metabolic factor associated with various metabolic and cardiovascular diseases (CVDs). To explore the clinical characteristics of FGF21 in patients with HFpEF, serum levels of FGF21 from 151 patients with HFpEF and 146 healthy controls were determined by immunoassay. Interestingly, serum levels of FGF21 were elevated in HFpEF patients compared with those in healthy controls [346.35 (180.03–732.09) pg/mL vs. 132.38 (78.61–237.98) pg/mL, p < 0.001] (Fig. 1A). To further explore whether FGF21 is related to cardiac function in patients with HFpEF, all clinical parameters of these 151 patients with HFpEF were divided into three groups based on FGF21 tertiles. Baseline characteristics according to FGF21 tertiles are shown in Table S1. Interestingly, the percentage of patients with New York Heart Association (NYHA) class IV cardiac status (2.0% vs. 4.0% vs. 16.0%) gradually increased, accompanied by elevated FGF21 levels among these subjects with HFpEF. Brain natriuretic peptide (BNP), a conventional biomarker of HF, also exhibited a similar pattern [164.0 (74.3–296.0) vs. 423.5 (254.0–600.5) vs. 554.0 (234.0–1093.0)] followed by the increased FGF21 levels. Moreover, further correlation analysis showed a significant association between increased serum FGF21 and BNP (r = 0.541, p < 0.001) and left ventricular ejection fraction (LVEF) (r = −0.204, p = 0.013) (Fig. 1B, C, Table S2). These data suggest that elevated serum FGF21 levels are related to the pathogenesis of HFpEF.
A Serum FGF21 levels of patients with HFpEF and healthy subjects were measured by immunoassay (HFpEF, n = 151; healthy, n = 146). B, C Correlation analysis between LogFGF21 and LogBNP, as well as left ventricular ejection fraction (LVEF) in subjects with HFpEF. (B, n = 151; C, n = 147). D Left ventricular systolic function of WT mice with HFpEF and controls. Top, M-mode echocardiographic tracings; Bottom, quantification of LVEF and left ventricular fractional shortening (LVFS). n = 6 mice per condition. E Left ventricular diastolic function. Top, pulsed-wave Doppler (top) and tissue Doppler (bottom) tracings of WT mice with or without HFpEF. Bottom, quantification of the ratio between the mitral E wave and A wave (E/A), and the mitral E wave and E′ wave (E/E′). n = 6 mice per condition. The lung weight to tibia length ratio (LW/TL, F) and the running distance during exercise exhaustion test (G) of WT mice with or without HFpEF. n = 6 mice per condition. H Serum FGF21 levels of WT mice with HFpEF and controls were measured by immunoassay. n = 6 mice per condition. FGF21 contents in epididymal white adipose tissue (eWAT, I), subcutaneous white adipose tissue (scWAT, J), liver (K), and heart (L) of mice with HFpEF and controls were tested by immunoblot, respectively. n = 6 mice per condition. Violin plots in (A) are presented as lines indicating the median and interquartile range. Bar graphs in (D–L) are shown as mean ± SEM. Statistical significance was determined by the two-sided Mann–Whitney U test (A), two-tailed unpaired Student’s t test (D–G, I–L), or two-tailed unpaired Student’s t test with Welch’s correction (H). The associations between cardiac function parameters and serum levels of FGF21 were estimated by Pearson correlation analysis (B, C).
Next, we further verified the expression profiles of FGF21 in the HFpEF mouse models generated by treatment with high-fat diet (HFD) plus N[w]-nitro-l-arginine methyl ester (L-NAME) as in previous reports25. Consistent with previous studies, there were no significant differences in the cardiac systolic function index, including LVEF and left ventricular fractional shortening (LVFS), tested by echocardiography, in these HFpEF mice compared with controls (Fig. 1D). However, a notable reduction in the E wave to A wave (E/A) ratio and a significant increase in the E wave to E’ wave (E/E′) ratio was observed in these HFpEF mouse models (Fig. 1E), followed by elevated lung weight to tibial length (LW/TL) ratio and decreased running distance (Fig. 1F, G), two critical markers of pulmonary congestion and exercise intolerance. Consistently, mice with HFpEF showed higher serum FGF21 levels compared to controls (Fig. 1H), and FGF21 protein contents in adipose and liver tissue of HFpEF mice were significantly higher than in the heart tissues (Fig. 1I–L), suggesting that FGF21 is associated with the pathogenesis of HFpEF.
FGF21 deficiency exacerbates cardiac diastolic dysfunction and cardiac damage in mice with HFpEF
To investigate the role of FGF21 in the pathogenesis of HFpEF, Fgf21 knockout (Fgf21 KO) mice were generated by replacing most of exon 1 and all of exons 2 and 3 of the Fgf21 gene with the IRES-LacZ-polyA/PGK-neo cassette and confirmed by polymerase chain reaction (PCR) (Fig. S1A). Interestingly, Fgf21 KO mice with HFpEF exhibited a significant increase in average systolic and diastolic pressures compared to wild-type (WT) controls, even though the body weights were comparable between Fgf21 KO mice and WT controls (Fig. 2A–C). Echocardiography analysis showed no significant differences in cardiac systolic function between the Fgf21 KO mice and WT littermates (Fig. 2D). However, Fgf21 KO mice with HFpEF showed a worsened exacerbation of diastolic dysfunction compared to WT controls, as evidenced by a notable reduction in the E/A ratio (1.08 ± 0.02 vs. 1.26 ± 0.02, p < 0.05) and a significant increase in the E/E’ ratio (28.06 ± 0.73 vs. 24.20 ± 0.55, p < 0.01, Fig. 2E). Furthermore, Fgf21 KO mice with HFpEF had a higher LW/TL ratio and a lower running distance than WT controls (Fig. 2F, G), suggesting that FGF21 deficiency worsens cardiac diastolic dysfunction, exercise intolerance and pulmonary congestion in mice with HFpEF.
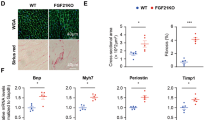
A Schematic diagram for evaluating cardiac function and cardiac remodeling in Fgf21 KO mice and WT controls with or without HFpEF. B Average systolic blood pressure (SBP, left) and diastolic blood pressure (DBP, right) of Fgf21 KO and WT mice with or without HFpEF. n = 6 mice per condition. C The body weight of mice mentioned above. n = 6 mice per condition. D Left ventricular systolic function. Top, M-mode echocardiographic tracings. Bottom, quantification of left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS). n = 6 mice per condition. E Left ventricular diastolic function. Top, representative pulsed-wave Doppler (top) and tissue Doppler (bottom) tracings. Bottom, quantification of the ratio between the mitral E wave and A wave (E/A), and the mitral E wave and E′ wave (E/E′). n = 6 mice per condition. The lung weight to tibia length ratio (LW/TL, F), running distance during exercise exhaustion test (G), and the heart weight to tibia length ratio (HW/TL, H) of mice mentioned above. n = 6 mice per condition. I Hematoxylin and eosin (H&E) and wheat germ agglutinin (WGA) staining of cardiac sections (left) and cardiomyocyte cross-sectional quantification (right) of Fgf21 KO and WT mice with or without HFpEF. Scale bars, 1 mm (H&E), and 25 μm (WGA). n = 50 cardiomyocytes per condition. J Cardiac mRNA expression levels of hypertrophic markers (Anp, Bnp, and Myh7). n = 6 mice per condition. K Dihydroethidium (DHE) staining of cardiac sections (left) and quantification of the intensity (right). Scale bars, 100 μm. n = 6 mice per condition. L Sirius red staining (left) and quantification of fibrotic areas (right). Scale bars, 50 μm. n = 6 mice per condition. M Cardiac contents of fibrotic factors (fibronectin, collagen I, and α-SMA) were tested by immunoblot. n = 6 mice per condition. Violin plots in (I) are presented as lines indicating the median and interquartile range; other bar graphs are presented as mean ± SEM. Statistical significance was determined by the two-way ANOVA, followed by Tukey’s multiple comparison test.
On the other hand, Fgf21 KO mice with HFpEF showed a significant increase in the heart weight to tibial length (HW/TL) ratio compared to WT controls (Fig. 2H). Likewise, histopathological analysis showed that heart size and cardiomyocyte cross-sectional area, tested by hematoxylin and eosin (H&E) staining and wheat germ agglutinin (WGA) staining, respectively, were significantly higher in Fgf21 KO mice with HFpEF than that in WT controls (Fig. 2I). Consistently, the expression profiles of cardiac hypertrophic factors, including Anp, Bnp, and Myh7, were further strikingly enhanced in Fgf21 KO mice with HFpEF compared with WT controls (Fig. 2J), suggesting loss of FGF21 exacerbates HFpEF-induced cardiac hypertrophy.
Given that oxidative stress and fibrosis are two critical events during the pathogenesis of HFpEF, we next evaluated whether loss of FGF21 influences these critical events of HFpEF. As expected, HFD + L-NAME-induced HFpEF mice had higher cardiac reactive oxygen species (ROS) levels in WT mice compared to WT controls. Interestingly, HFpEF-induced elevated cardiac ROS levels were further worsened in Fgf21 KO mice with HFpEF compared with WT controls (Fig. 2K). Furthermore, cardiac fibrotic areas were significantly higher in Fgf21 KO mice with HFpEF than in WT controls (Fig. 2L). Consistently, the expression profiles of cardiac fibrosis markers, including fibronectin, collagen I, and α-smooth muscle actin (α-SMA), were also profoundly higher in Fgf21 KO mice with HFpEF than in WT controls (Fig. 2M). These data indicated that FGF21 deficiency accelerates HFpEF-induced cardiac damage.
Considering a significant increase of adipose FGF21 contents in HFpEF, we next explored the role of adipose-derived FGF21 in HFpEF. Adipoq-specific Cre (adipoq-Cre) mice were bred with Fgf21-floxed (Fgf21F/F) mice to generate adipocyte-specific Fgf21 KO (Fgf21Adipoq) mice (Fig. S1B). In line with the findings in Fgf21 KO mice, Fgf21Adipoq mice with HFpEF exhibited a notable elevation in average systolic and diastolic pressures but not in body weight, compared to Fgf21F/F controls (Fig. S2A–C). Accordingly, Fgf21Adipoq mice displayed more severe cardiac diastolic dysfunction, pulmonary congestion, and exercise intolerance than the controls (Fig. S2D–G). In addition, cardiac hypertrophy, cardiac oxidative stress, and cardiac fibrosis were notably exacerbated in Fgf21Adipoq mice with HFpEF compared to controls (Fig. S2H–M). Interestingly, cardiac damage in Fgf21Adipoq mice with HFpEF were comparable to those in Fgf21 KO mice (Figs. 2 and S2). These data indicated that adipose-derived FGF21 is indispensable in HFpEF-induced cardiac diastolic dysfunction and damage.
Replenishment of FGF21 profoundly improves HFpEF-induced cardiac diastolic dysfunction and cardiac damage in mice
To further elucidate the role of FGF21 in HFpEF, we next investigated whether replenishment of FGF21 could alleviate HFpEF-induced cardiac diastolic dysfunction and damage. As expected, administration of adeno-associated virus (AAV)-Fgf21 significantly elevated circulating FGF21 levels and its protein abundances in various organs such as white adipose tissue (WAT), liver, and heart of mice (Fig. S1C–I), and treatment with AAV-Fgf21 significantly lowered HFpEF-induced blood pressure elevation but did not influence the body weight of mice with HFpEF (Fig. S3A–C). Consistent with our above loss-of-function results, treatment with AAV-Fgf21 significantly increased the E/A ratio and decreased E/E′ ratio (E/A, 1.56 ± 0.04 vs. 1.31 ± 0.03, p < 0.05; E/E’, 21.85 ± 0.48 vs. 26.14 ± 0.59, p < 0.01, Fig. S3D), and markedly attenuated pulmonary congestion and improved exercise intolerance, compared with controls (Fig. S3E, F). Moreover, HFpEF-induced cardiac hypertrophy was dramatically alleviated by administration of AAV-Fgf21, as evidenced by decreased HW/TL ratio and cardiomyocyte area, and inhibited the mRNA expression of cardiac hypertrophic markers, including Anp, Bnp, and Myh7, compared with controls (Fig. S3G–I). Similarly, treatment with AAV-Fgf21 also reduced cardiac ROS content, cardiac fibrosis, and the expression levels of cardiac fibrotic factors, including fibronectin, collagen I, and α-SMA, compared with controls (Fig. S3J–L). Accordingly, overexpression of FGF21 also reversed FGF21 deficiency-induced cardiac diastolic dysfunction, cardiac hypertrophy, cardiac oxidative stress, and cardiac fibrosis in Fgf21 KO mice with HFpEF (Fig. S4). Therefore, these results demonstrate that replenishment of FGF21 ameliorates HFpEF-induced cardiac diastolic dysfunction and cardiac damage in mice.
FGF21 regulates glucose utilization and lipid homeostasis in HFpEF mice
Given that cardiac energy metabolism imbalance is the most critical event in the pathogenesis of HFpEF, we investigated whether FGF21 influences glucose and lipid metabolism in this context. Our data demonstrated that FGF21 deficiency exacerbated glucose and insulin intolerance in mice with HFpEF (Fig. S5A and B). Notably, Fgf21 KO HFpEF mice exhibited a significant decrease in cardiac mRNA levels of Glut1 but not Glut4, compared to WT controls, while other glycolysis-related enzymes, including Hk1, Hk2, and Pkm2 remained comparable (Fig. S5C). Furthermore, treatment with recombinant mouse FGF21 (rmFGF21) markedly restored the palmitic acid (PA)-induced decrease in glucose uptake in primary neonatal rat ventricular myocytes (NRVMs, Fig. S5D). In addition, cardiac phosphorylated pyruvate dehydrogenase (PDH) protein levels were significantly increased in Fgf21 KO mice with HFpEF compared to WT controls (Fig. S5E).
Regarding lipid metabolism, FGF21 deficiency significantly elevated plasma free fatty acid (FFA) and triglyceride (TG) levels and their contents in the hearts of mice with HFpEF compared with WT controls (Fig. S5F and G). Moreover, Oil Red O staining revealed a substantial increase in cardiac lipid accumulation in Fgf21 KO HFpEF mice compared with WT controls (Fig. S5H). In addition, treatment with rmFGF21 also significantly decreased PA-induced lipid uptake in primary NRVMs (Fig. S5I). Consistently, cardiac mRNA expression levels of fatty acid (FA) transporter Slc27a1 but not Cd36, Fabp4, and Lpl, as well as FAO-related enzymes, including Cpt1b, Acadm, Acadl, Acadvl, and Echs1, was increased in Fgf21 KO HFpEF mice compared to WT controls (Fig. S5J), and no significant difference in cardiac CPT1b, an essential enzyme of FAO, was seen between Fgf21 KO and WT controls with HFpEF (Fig. S5K). Collectively, these data suggest that the beneficial effects of FGF21 against HFpEF may be related to enhanced glucose uptake and oxidation, as well as enhanced lipid transport and reduced lipid accumulation in mice.
FGF21 promotes mitochondrial bioenergetics in mice with HFpEF
To clarify the mechanism whereby FGF21 protects against HFpEF-related cardiac damage, a comprehensive data-independent acquisition (DIA)-based proteomic analysis was performed to explore the differentially expressed proteins (DEPs) in the heart of AAV-Fgf21- or AAV-GFP-treated mice with HFpEF. Gene Set Enrichment Analysis (GSEA) - Gene Ontology (GO) analysis showed that the administration of AAV-Fgf21 significantly influenced mitochondrial function (Fig. 3A). Given that mitochondrial dysfunction is the critical event of HF, we next examined whether replenishment of FGF21 affects cardiac mitochondrial function in mice with HFpEF. Transmission electron microscopy analysis indicated that HFpEF caused significant changes in mitochondrial morphology, characterized by empty vacuoles, ruptured membranes, and sparse, disorganized cristae. Interestingly, these alterations were notably exacerbated in Fgf21 KO mice with HFpEF but significantly ameliorated after treatment with AAV-Fgf21 (Fig. 3B, C). Moreover, cardiac PDH activities and ATP levels were significantly decreased in mice with HFpEF, and these profiles were exacerbated in FGF21 null mice but significantly restored after AAV-Fgf21 treatment (Fig. 3D, E, G, J). Similarly, these patterns were also observed in cardiac pyruvate and acetyl-CoA contents (Fig. 3H, I, K, L). Moreover, there were no significant differences in cardiac expression levels of the five major electron transport chain (ETC) proteins among these HFpEF mice treated with AAV-Fgf21 or controls (Fig. 3F), suggesting that FGF21 deficiency worsens mitochondrial bioenergetics in mice with HFpEF.
A Gene Set Enrichment Analysis (GSEA) - Gene Ontology (GO) analysis of proteomics data from the heart tissue of AAV-Fgf21- or AAV-GFP-treated mice with HFpEF. B, C Top, transmission electron microscopy images of heart tissue in Fgf21 KO and WT mice with or without HFpEF, or AAV-Fgf21-treated mice with HFpEF. Scale bar, 1 μm. Bottom, quantifications of mitochondrial density (n = 5 mice per condition), total area (n = 5 mice per condition), and cristae number (n = 50 mitochondria per condition). D, E Cardiac ATP content in mice mentioned above. n = 5 mice per condition. F Cardiac mitochondrial respiratory electron transport chain (ETC) complex I–V subunits were tested by immunoblot. n = 3 independent experiments. The activity of pyruvate dehydrogenase in the heart tissues (G), cardiac pyruvate (H), and acetyl-CoA (I) levels in Fgf21 KO and WT mice with or without HFpEF. n = 5 mice per condition. The activity of pyruvate dehydrogenase in the heart tissues (J), cardiac pyruvate (K), and acetyl-CoA (L) levels in mice with HFpEF, treated with AAV-Fgf21 or AAV-GFP. n = 5 mice per condition. M–P Neonatal rat ventricular myocytes (NRVMs) were exposed to palmitic acid (PA) with a dosage of 500 μmol/L, then incubated with recombinant mouse FGF21 (rmFGF21) for 24 h. M Representative images of DCFH-DA staining and MitoSOX Green staining (top) and quantification of the DCFH-DA intensity and mitochondrial length (bottom). Scale bars, 50 μm for DCFH-DA staining, 10 μm (top) and 2 μm (bottom) for MitoSOX Green staining. n = 5 biological samples. N Representative images of JC-1 staining (left) and quantification of the red/green fluorescence intensity ratio (right). Scale bars, 50 μm. Aggregates, red; Monomers, green. n = 5 biological samples. O Real-time monitoring of the oxygen consumption rate (OCR) in NRVMs. n = 5 biological samples. P ATP contents tested by spectrophotometric methods. n = 5 biological samples. Violin plots in (B, C) are presented as lines indicating the median and interquartile range; other bar graphs are presented as mean ± SEM. The statistical significance of differences was assessed by two-way ANOVA with Tukey’s multiple comparison.
To further confirm whether FGF21 improves HFpEF-induced cardiac dysfunction and cardiac damage by enhancing mitochondrial bioenergetics, primary NRVMs were treated with PA or vehicle and then incubated with rmFGF21 for 24 h. As expected, treatment with rmFGF21 significantly attenuated PA-induced ROS accumulation, reduced mitochondrial fission, and normalized mitochondrial membrane potential (Fig. 3M, N). Furthermore, administration of rmFGF21 markedly restored PA-induced decreased mitochondrial oxygen consumption rate (OCR) and improved cellular ATP levels in NRVMs (Fig. 3O, P). These data showed that FGF21 can reverse PA-induced myocardial damage by enhancing mitochondrial bioenergetics.
FGF21 decreases cardiac PDK4 expression in mice with HFpEF
To further clarify the mechanism of FGF21 against HFpEF, we investigated whether there were some potential downstream targets to mediate the protective effect of FGF21 against HFpEF. A total of 165 DEPs, including 134 DEPs downregulated and 31 DEPs upregulated, were screened by proteomic analysis between HFpEF mice treated with AAV-Fgf21 and AAV-GFP, which was defined by fold change ≤ 0.83 or fold change ≥ 1.2 and p < 0.05 (Fig. 4A). Interestingly, pyruvate dehydrogenase kinase 4 (PDK4), a member of the PDH kinase family that negatively regulates the activity of the PDH complex by phosphorylating its subunits, was found among 134 downregulated DEPs (Fig. 4B). Furthermore, the proteomic analysis showed a significant decrease in cardiac PDK4 contents in AAV-Fgf21-treated HFpEF mice (Fig. 4C) compared with controls. Consistently, cardiac PDK4 protein levels were significantly increased in mice with HFpEF compared with WT controls, and these profiles on cardiac PDK4 levels were further elevated in Fgf21 KO mice with HFpEF but significantly alleviated when treated with AAV-Fgf21 (Fig. 4D, E). These results suggest that PDK4 might be one of the downstream target molecules that may negatively modulate the protective effect of FGF21 against HFpEF in mice.
A Volcano plot of proteomic analysis in AAV-Fgf21- or AAV-GFP-treated mice with HFpEF. B Heatmap of the differentially expression proteins (DEPs) in the heart tissues of AAV-Fgf21- or AAV-GFP-treated mice with HFpEF. C Relative expression levels of cardiac PDK4 in AAV-Fgf21- or AAV-GFP-treated mice with HFpEF from the proteomic analysis data. n = 4 mice per condition. Cardiac PDK4 levels in Fgf21 KO and WT mice with or without HFpEF (D) or AAV-Fgf21- or AAV-GFP-treated HFpEF mice (E), tested by immunoblot, respectively. n = 6 mice per condition. The expressional difference of cardiac PDK4 in the mitochondria (F) and non-mitochondria (G) of cardiomyocytes in HFpEF mice and controls was examined by immunoblot, respectively. n = 6 mice per condition. Data are presented as mean ± SEM. Statistical significance was determined by the two-tailed unpaired Student’s t test (A, F, G), two-sided Brown-Forsythe and Welch ANOVA with Dunnett’s multiple comparison test (C), or two-way ANOVA with Tukey’s multiple comparison test (D, E).
To confirm the expression site of PDK4 in the pathological process of HFpEF, mitochondrial and non-mitochondrial fractions of cardiac tissues were used to test the expression status of PDK4 in mice with HFpEF and controls. Consistent with the elevated cardiac PDK4 contents in mice with HFpEF, mitochondrial PDK4, but not non-mitochondrial PDK4 of the heart tissue, was increased remarkably in HFpEF mice compared to controls (Fig. 4F, G), suggesting that PDK4 is expressed in the mitochondria of cardiomyocytes.
Cardiac-specific silencing of PDK4 reverses HFpEF-induced cardiac diastolic dysfunction and cardiac damage in FGF21 null mice
To further determine the role of PDK4 in the protective effect of FGF21 against HFpEF, AAV-mediated specific short hairpin RNA of PDK4 (Pdk4-shRNA) with a murine cTNT core promoter was delivered into WT and Fgf21 KO mice with HFpEF by tail-vein injection. As expected, a noticeable reduction in PDK4 expression was observed after two weeks of Pdk4-shRNA treatment (Fig. S6A). Moreover, genetic inhibition of PDK4 did not affect the body weight and blood pressure of HFpEF mice but significantly reversed HFpEF-induced cardiac diastolic dysfunction and notably improved pulmonary congestion and exercise intolerance in both Fgf21 KO and WT mice (Fig. 5A–E). Consistently, genetic knockdown of PDK4 led to a decrease in the HW/TL ratio, heart size, cardiomyocyte areas, and the mRNA expression levels of hypertrophic markers, including Anp, Bnp, and Myh7, in both Fgf21 KO and WT mice with HFpEF compared to controls (Fig. 5F–H). Similarly, treatment with Pdk4-shRNA markedly attenuated HFpEF-induced cardiac ROS production and cardiac fibrosis in both FGF21 null and WT mice compared to controls (Fig. 5I–K). Notably, cardiac knockdown PDK4-induced beneficial effects on HFpEF-related cardiac diastolic dysfunction and cardiac damage were comparable between FGF21 null mice and WT controls (Fig. 5). These findings suggest that suppression of PDK4 reverses FGF21 deficiency-induced HFpEF-related cardiac diastolic dysfunction and damage in Fgf21 KO mice with HFpEF.
A Average systolic blood pressure (SBP, left) and diastolic blood pressure (DBP, right) of Fgf21 KO and WT mice with HFpEF, after treatment with or without Pdk4 shRNA. n = 6 mice per condition. B The body weight of mice mentioned above. n = 6 mice per condition. C Left ventricular diastolic function of mice mentioned above. Top, pulsed-wave Doppler (top) and tissue Doppler (bottom) tracings. Bottom, quantification of the ratio between the mitral E wave and A wave (E/A), and the mitral E wave and E′ wave (E/E′). n = 6 mice per condition. D The lung weight to tibia length ratio (LW/TL) of mice mentioned above. n = 6 mice per condition. Running distance during exercise exhaustion test (E) and the heart weight to tibia length ratio (HW/TL, F) of mice mentioned above. n = 6 mice per condition. G Hematoxylin and eosin (H&E) and wheat germ agglutinin (WGA) staining of cardiac sections (left) and cardiomyocyte cross-sectional area quantification (right) in mice mentioned above. Scale bars, 1 mm (H&E), and 25 μm (WGA). n = 50 cardiomyocytes per condition. H The mRNA expression levels of cardiac hypertrophic marker genes (Anp, Bnp, and Myh7) in mice mentioned above. n = 6 mice per condition. I Dihydroethidium (DHE) staining (left) of heart tissues and quantification (right) of the intensity, Scale bars, 100 μm. n = 6 mice per condition. J Sirius red staining of heart tissues (left) and quantification of the fibrotic areas (right). Scale bars, 50 μm. n = 6 mice per condition. K Cardiac contents of fibrotic factors, including fibronectin, collagen I, and α-SMA tested by immunoblot. n = 6 mice per condition. L Cardiac p-PDH, PDH and PDK4 contents of mice tested by immunoblot. n = 6 mice per condition. M Cardiac pyruvate dehydrogenase activity in mice mentioned above. n = 6 mice per condition. Cardiac pyruvate (N), acetyl-CoA (O), and ATP (P) contents in mice mentioned above. n = 6 mice per condition. Violin plots in (G) are presented as lines indicating the median and interquartile range; other bar graphs are presented as mean ± SEM. Statistical significance was determined by the two-way ANOVA, followed by Tukey’s multiple comparison test.
Since PDK4 is a critical regulator in PDH phosphorylation, which negatively regulates pyruvate oxidation and attenuates mitochondrial bioenergetics, we next explored whether blockage of PDK4 influences cardiac mitochondrial bioenergetics in mice. As expected, genetic suppression of PDK4 reversed HFpEF-induced elevated phosphorylated PDH levels and increased PDH activities in both FGF21 null and WT mice, followed by reduced pyruvate accumulation and enhanced acetyl-CoA and ATP production (Fig. 5L–P). These findings suggest that PDK4 is a downstream negative regulator that modulates the beneficial effects of FGF21 against HFpEF-induced cardiac diastolic dysfunction and cardiac damage.
Overexpression of PDK4 exacerbates HFpEF-induced cardiac diastolic dysfunction and damage in both FGF21 null mice and WT controls
To further explore the role of PDK4 in the pathogenesis of HFpEF, both Fgf21 KO and WT mice with HFpEF were treated with AAV vectors carrying PDK4 (AAV-Pdk4) or AAV-GFP with cTNT core promoter by tail-vein injection. As expected, a significant increase in cardiac PDK4 expression was observed in these mice after two weeks of AAV-Pdk4 treatment (Fig. S6B). Interestingly, administration of AAV-Pdk4 markedly worsened HFpEF-induced cardiac diastolic dysfunction, pulmonary congestion, and exercise intolerance in Fgf21 KO and WT mice compared to AAV-GFP-treated controls. However, it did not affect the body weight and blood pressure of Fgf21 KO and WT mice (Fig. S7A–E). In addition, treatment with AAV-Pdk4 notably increased HW/TL ratio, enlarged heart size and cardiomyocyte area, and elevated cardiac mRNA levels of hypertrophic factors, including Anp, Bnp, and Myh7, in both Fgf21 KO and WT mice with HFpEF (Fig. S7F–H), compared to AAV-GFP controls.
On the other hand, the pathological staining analysis also showed that administration of AAV-Pdk4 significantly exacerbated HFpEF-induced ROS production and cardiac fibrosis in Fgf21 KO and WT mice compared to AAV-GFP controls (Fig. S7I–K). Consistently, treatment with AAV-Pdk4 significantly aggravated HFpEF-induced increased phosphorylated PDH levels and lowered PDH activities, accompanied by elevated pyruvate accumulation and decreased acetyl-CoA and ATP production in Fgf21 KO and WT mice, compared to controls (Fig. S7L–P). Importantly, PDK4-induced adverse effects on HFpEF-related cardiac diastolic dysfunction and cardiac damage were comparable between FGF21 null mice and WT controls (Fig. S7). Therefore, these results demonstrated that FGF21 deficiency does not influence the adverse effect of PDK4 on the pathological process of HFpEF.
FGF21 suppresses PDK4 expression by activation of PI3K/AKT signals in cardiomyocytes
Previous research indicated that the PI3K/AKT signaling pathway is involved in the various biological processes of FGF21, including glucose and lipid metabolism and insulin sensitivity26,27. Therefore, we further explored whether FGF21 modulates cardiac PDK4 expression by activating the PI3K/AKT pathway. Our results showed that cardiac p-PI3K and p-AKT contents significantly decreased in mice with HFpEF compared to controls, and these profiles were further intensified in FGF21 null mice (Fig. 6A), accompanied by elevated cardiac PDK4 expression (Fig. 4D). In contrast, HFpEF-induced decreased cardiac p-PI3K and p-AKT contents were notably reversed after treatment with AAV-Fgf21 (Fig. 6B), followed by lowered cardiac PDK4 production (Fig. 4E), suggesting that FGF21-regulated cardiac PDK4 expression may be related to activating the PI3K/AKT signals in mice.
A Cardiac p-PI3K, PI3K, p-AKT, AKT levels in Fgf21 KO and WT mice with or without HFpEF. n = 6 mice per condition. B Cardiac p-PI3K, PI3K, p-AKT, AKT contents in mice with HFpEF, treated with AAV-Fgf21 or AAV-GFP. n = 6 mice per condition. C–I Neonatal rat ventricular myocytes (NRVMs) were incubated with palmitic acid (PA) before administration of recombinant mouse FGF21 (rmFGF21), and then treated with LY294002 or vehicle for 24 h. For Flag-Pdk4 transfection, NRVMs were infected with Flag-Pdk4 for 48 h, then incubated with PA and rmFGF21, followed by treatment with LY294002 or vehicle. C PDK4, p-PDH, and PDH levels in NRVMs were tested by immunoblot. n = 5 biological samples. The activity of pyruvate dehydrogenase (D), pyruvate (E), acetyl-CoA (F), and ATP (G) contents in NRVMs. n = 5 biological samples. H Representative images of DCFH-DA staining and MitoSOX Green staining (top) and quantification of the DCFH-DA intensity and mitochondrial length (bottom). Scale bars, 50 μm for DCFH-DA staining, 10 μm (top) and 2 μm (bottom) for MitoSOX Green staining. n = 5 biological samples. I Representative images of JC-1 staining (left) and quantification of the red/green fluorescence intensity ratio (right). Scale bars, 50 μm. Aggregates, red; Monomers, green. n = 5 biological samples. Data are presented as mean ± SEM. Statistical significance was determined by two-way ANOVA, followed by Tukey’s multiple comparison test (A, B). Other assays were assessed by one-way ANOVA, followed by Tukey’s multiple comparison test.
In light of the aforementioned animal results, we further clarify whether FGF21 modulates PDK4 expression by triggering PI3K/AKT signals in primary NRVMs. As expected, administration of NRVMs with rmFGF21 significantly suppressed PDK4 and p-PDH expression but upregulated p-PI3K and p-AKT levels in a dose-dependent manner (Fig. S8A). Moreover, treatment of NRVMs with the PI3K inhibitor LY294002 significantly increased PDK4 production in a dosage-dependent manner (Fig. S8B), suggesting that FGF21-suppressed PDK4 production may be mediated by activating the PI3K/AKT signal pathway in cardiomyocytes.
Then, we further investigated the role of PI3K/AKT signals in FGF21-suppressed PDK4 production when exposed to PA. Interestingly, PA-induced upregulated PDK4 production and elevated phosphorylated PDH contents were markedly alleviated by administration of rmFGF21 in NRVMs. However, these rmFGF21-triggered effects were strikingly abolished by treatment with the PI3K inhibitor LY294002 or transfection with a Flag-Pdk4 plasmid, respectively (Fig. 6C), which was accompanied by decreased PDH activities, increased pyruvate accumulation, and lowered acetyl-CoA and ATP production (Fig. 6D–G). Similarly, the beneficial effects of FGF21 against PA-induced elevated myocardial ROS production, mitochondrial fission, and abnormal mitochondrial membrane potentials were also abolished after administration of PI3K inhibitor LY294002 or transfection with Flag-Pdk4 plasmid, respectively (Fig. 6H, I). Therefore, these results suggest that FGF21-triggered negative regulation of PDK4 is mediated by triggering the PI3K/AKT signals in cardiomyocytes.
APN partly mediates the protective effects of FGF21 on HFpEF-induced cardiac diastolic dysfunction and cardiac damage in mice
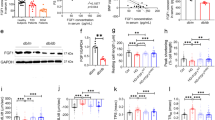
APN, a hormone mainly expressed in adipocytes, is crucial in various biological processes, including metabolic regulation, anti-inflammatory, and anti-apoptotic activities28. Our previous reports have demonstrated that APN is indispensable in mediating FGF21 actions on insulin sensitization and anti-atherosclerosis16,18. We next investigated whether FGF21 exerts its anti-HFpEF effects by inducing APN. Notably, circulating APN levels were significantly reduced in mice with HFpEF and further lowered in FGF21 null mice with HFpEF (Fig. S9A) but profoundly reversed after treatment with AAV-Fgf21 (Fig. S9B), suggesting that the protective effects of FGF21 against HFpEF may be related to regulating APN production.
To further clarify the role of APN in FGF21 against HFpEF-induced cardiac diastolic dysfunction and cardiac damage, Apn knockout (Apn KO) mice were generated and confirmed by PCR analysis (Fig. S9C). Interestingly, APN deficiency did not affect mice’s body weight and blood pressure with HFpEF (Fig. 7A, B). Consistent with previous studies29, APN deficiency considerably accelerated HFpEF-induced cardiac diastolic dysfunction and cardiac damage compared to WT controls (Fig. 7C–K). Intriguingly, the protective effects of AAV-Fgf21 against HFpEF-induced cardiac diastolic dysfunction were significantly alleviated in Apn KO mice compared to WT controls, accompanied by a reduction in the improvement of pulmonary edema and exercise intolerance (Fig. 7C–E). Moreover, the beneficial effects of FGF21 against HFpEF-induced cardiac hypertrophy were largely attenuated in Apn KO mice compared to WT controls (Fig. 7F–H). In addition, the protective effects of AAV-Fgf21 on attenuating HFpEF-induced cardiac oxidative stress, cardiac fibrosis, and its related cardiac fibrotic factors (fibronectin, collagen-I, and α-SMA) expressions were manifestly attenuated in Apn KO mice as compared with WT controls (Fig. 7I–K). Finally, AAV-Fgf21-induced lowered cardiac p-PDH expression, elevated cardiac PDH activities, decreased pyruvate accumulation, and promoted acetyl-CoA and ATP production were also largely lowered in APN null mice with HFpEF, compared with WT control (Fig. 7L–P). Therefore, these data suggest that APN plays an indispensable role in the protective effects of FGF21 against HFpEF-induced cardiac diastolic dysfunction and cardiac damage in mice.
A Average systolic blood pressure (SBP, left) and diastolic blood pressure (DBP, right) of Apn KO and WT mice with HFpEF after treatment with AAV-Fgf21 or AAV-GFP, respectively. n = 6 mice per condition. B The body weight of mice mentioned above. n = 6 mice per condition. C Left ventricular diastolic function of Apn KO and WT mice with HFpEF, treated with AAV-Fgf21 or AAV-GFP, respectively. Top, representative pulsed-wave Doppler (top) and tissue Doppler (bottom) tracings of mice. Bottom, quantification of the ratio between the mitral E wave and A wave (E/A), and the mitral E wave and E′ wave (E/E′). n = 6 mice per condition. D The lung weight to tibia length ratio (LW/TL) of mice mentioned above. n = 6 mice per condition. Running distance during exercise exhaustion test (E) and the heart weight to tibia length ratio (HW/TL, F) of mice mentioned above. n = 6 mice per condition. G Hematoxylin and eosin (H&E) and wheat germ agglutinin (WGA) staining of cardiac sections (left) and cardiomyocyte cross-sectional area quantification (right) in mice mentioned above. Scale bars, 1 mm (H&E), and 50 μm (WGA). n = 50 cardiomyocytes per condition. H The mRNA expression levels of cardiac hypertrophic marker genes (Anp, Bnp, and Myh7) in mice mentioned above. n = 6 mice per condition. I Dihydroethidium (DHE) staining (left) of heart tissues and quantification (right) of the intensity, Scale bars, 100 μm. n = 6 mice per condition. J Sirius red staining of heart tissues (left) and quantification of the fibrotic areas (right). Scale bars, 50 μm. n = 6 mice per condition. K Cardiac contents of fibrotic factors, including fibronectin, collagen I, and α-SMA tested by immunoblot. n = 6 mice per condition. L Cardiac PDK4, p-PDH and PDH contents of mice tested by immunoblot. n = 6 mice per condition. M Cardiac pyruvate dehydrogenase activity in mice mentioned above. n = 6 mice per condition. Cardiac pyruvate (N), acetyl-CoA (O), and ATP (P) contents in mice mentioned above. n = 6 mice per condition. Violin plots in (G) are presented as lines indicating the median and interquartile range; other bar graphs are presented as mean ± SEM. Statistical significance was assessed by two-way ANOVA, followed by Tukey’s multiple comparison test.
The APN-PI3K/AKT-PDK4 axis mediates the beneficial effects of FGF21 against mitochondrial dysfunction in cardiomyocytes
Because the therapeutic effects of APN against HFpEF are found to be associated with improving myocardial oxidative stress and mitochondrial function in mice30, and our above data indicated that PDK4 is a negative regulator in mitochondrial bioenergetics during the pathogenesis of HFpEF (Figs. 5 and S7), we next explored whether APN mediates the beneficial effects of FGF21 against HFpEF by regulating PDK4-related mitochondrial bioenergetics. Consistently, cardiac PDK4 contents were increased in WT mice with HFpEF and further elevated in APN null mice with HFpEF (Figs. 4B–E and 7L), accompanied by exacerbated cardiac diastolic dysfunction and cardiac damage (Fig. 7C–K). Interestingly, administration of AAV-Fgf21 significantly blocked HFpEF-induced elevated cardiac PDK4 contents in WT mice but not in APN null mice (Fig. 7L), suggesting that PDK4 may be a downstream target to regulate the protective effect of APN against HFpEF negatively.
Considering that the PI3K/AKT signaling pathway is involved in various biological processes of APN31,32,33, we further explored whether APN modulates PDK4 expression by activating PI3K/AKT signals in primary NRVMs. Consistently, administration of PA markedly increased PDK4 expression and upregulated p-PDH levels but significantly attenuated PI3K and AKT phosphorylation and lowered PDH activities (Fig. 8A, B). However, PA-induced adverse effects were notably reversed after incubation with subcutaneous adipose tissues of WT (wt-SAT) but not Apn KO (ko-SAT) mice for 24 h (Fig. 8A, B), as accompanied by elevated APN secretion in culture media of NRVMs (Fig. S9D), suggesting that incubation with wt-SAT suppressed PDK4 expression may be related to activating PI3K/AKT signals by triggering APN production. Interestingly, these effects caused by co-culture with wt-SAT and incubation with PA were notably attenuated by PI3K inhibitor LY294002 (Fig. 8A, B), as accompanied by increased pyruvate accumulation and myocardial ROS levels, decreased acetyl-CoA and ATP production, promoted mitochondrial fission, abnormal mitochondrial membrane potentials, and decreased mitochondrial OCR (Fig. 8C–H), suggesting that adipose-derived APN negatively regulates PDK4 expression and promotes mitochondrial bioenergetics by activation of PI3K/AKT signals in NRVMs.
Neonatal rat ventricular myocytes (NRVMs) were co-cultured with subcutaneous adipose tissues isolated from WT (wt-SAT) or Apn KO (ko-SAT) mice for 24 h after 48 h of Flag-Pdk4 transfection, then treated with palmitic acid (PA), followed by incubation with recombinant mouse FGF21 (rmFGF21) or rmFGF21 plus LY294002 for 24 h. A p-PI3K, PI3K, p-AKT, AKT, PDK4, p-PDH, and PDH levels in NRVMs were tested by immunoblot. n = 4 biological samples. The activity of pyruvate dehydrogenase (B), pyruvate (C), acetyl-CoA (D), and ATP (E) contents in NRVMs. n = 5 biological samples. F Representative images of DCFH-DA staining and MitoSOX Green staining (top) and quantification of the DCFH-DA intensity and mitochondrial length (bottom). Scale bars, 50 μm for DCFH-DA staining, 10 μm (top) and 2 μm (bottom) for MitoSOX Green staining. n = 5 biological samples. G Representative images of JC-1 staining (left) and quantification of the red/green fluorescence intensity ratio (right). Scale bars, 50 μm. Aggregates, red; Monomers, green. n = 5 biological samples. H Real-time monitoring of the oxygen consumption rate (OCR) in NRVMs. n = 5 biological samples. Data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA, followed by Tukey’s multiple comparison test.
On the other hand, our data also showed that administration of rmFGF21 and co-culture with wt-SAT caused a synergistic effect on adipose-derived APN-triggered negative regulation of PDK4 expression and positive promotion of mitochondrial function, as evidenced by further decreased PDK4 expression, upregulated PDH activities, decreased pyruvate accumulation and myocardial ROS levels, promoted acetyl-CoA and ATP production, reduced mitochondrial fission, and improved abnormal mitochondrial membrane potentials and mitochondrial OCR in NRVMs (Fig. 8).
In line with our results mentioned above, PDK4 is a negative regulator of mitochondrial metabolism, so we investigated the role of PDK4 in APN-triggered improved mitochondrial dysfunction. Surprisingly, co-culture with wt-SAT and administration of rmFGF21-triggered beneficial effects on PDH-mediated pyruvate oxidation and mitochondrial bioenergetics were largely blocked when transfected with Flag-Pdk4 plasmids in NRVMs (Fig. 8). Together, these data indicated that the beneficial effects of FGF21 against PA-induced mitochondrial dysfunction are mediated by triggering the APN-PI3K/AKT-PDK4 axis.
Discussion
Despite extensive research on the metabolic function of FGF21, its contribution to the pathogenesis of HFpEF has never been investigated. This study provides novel evidence that FGF21 deficiency exacerbates HFD + L-NAME-induced cardiac diastolic dysfunction and cardiac damage in mice, and replenishment of FGF21 effectively reverses these adverse effects, suggesting that FGF21 is an endogenous protector against HFpEF. In line with these findings, elevated circulating FGF21 levels in patients with HFpEF may serve as a compensatory response of the body to prevent cardiac diastolic dysfunction and cardiac damage. To support this notion, increased circulating FGF21 levels have been shown to act as a compensatory mechanism to counteract atherosclerosis18, angiotensin II-induced hypertension20, carbon tetrachloride-induced acute liver injury34, and ventilator-induced lung injury35.
Our previous results have indicated that elevated circulating FGF21 levels are seen in several cardiovascular disorders, including atherosclerosis18, diabetic cardiomyopathy19, myocardial infarction36, and hypertension20, suggesting that elevated FGF21 expression is related to the pathogenesis of cardiovascular disorders. Among patients with HFrEF and HFpEF, serum FGF21 levels are also significantly increased and positively associated with cardiac diastolic function, LV end-diastolic pressure, NT-pro-BNP and IL-6 levels, and lower skeletal muscle mass23,24,37. Consistent with these reports, our results demonstrated that elevated circulating levels of FGF21 are markedly increased in patients with HFpEF and HFD + L-NAME-induced HFpEF mice, accompanied by exacerbated cardiac diastolic dysfunction and cardiac damage (Fig. 1A, D–H). FGF21 is initially found to be expressed in liver cells and acts on adipocytes in an endocrinal manner to exert its biological function. It is also highly expressed in adipocytes, cardiomyocytes, and muscle cells in response to physiological and pathological stress38. In this study, our results showed that elevated FGF21 contents are significantly elevated in the liver and adipose tissue but not the heart of HFD + L-NAME-induced HFpEF mice (Fig. 1I–L), suggesting that liver and adipose tissues may be served as the primary sites for FGF21 production in response to HFpEF, and FGF21 presents its beneficial effects against HFpEF via fine-tuning the multiorgan crosstalk. In support of this notion, our previous studies indicated that FGF21 protects against atherosclerosis and angiotensin II-induced hypertension via the crosstalk of multiorgan, including liver, adipose, and vessels18,20.
Cardiac energy metabolism disorder is considered a pivotal event in the pathological process of HFpEF39,40. Cardiac energy metabolism is a finely-tuned process that can be disrupted by abnormal mitochondrial ultrastructure and function, loss of metabolic flexibility in substrate utilization, and incomplete ETC. During HF, cardiomyocytes lose their metabolic flexibility, leading to a shift in substrate utilization away from FAO towards glycolytic metabolism, which is uncoupled from pyruvate oxidation41,42,43,44. The decrease in pyruvate oxidation, caused by the overall deregulation of mitochondrial oxidative capacity and alterations in PDH activity, further impairs cardiomyocyte energy metabolism45,46. Moreover, a decline in cardiac pyruvate oxidation rate has been observed in several myocardial injury models, concomitant with impaired mitochondrial and cardiac function46,47,48. In the present study, HFpEF-triggered reduced PDH activities are notably counteracted by administration of AAV-Fgf21, followed by elevated cardiac acetyl-CoA and ATP contents and improved HFpEF-induced cardiac diastolic dysfunction and cardiac damage (Figs. 3 and S3), suggesting that FGF21 protecting against HFpEF-induced cardiac damage may be mediated at least in part by improvement of mitochondrial energy metabolism disorder through promotion of pyruvate oxidation. Considering that FGF21 exhibits a potent hypotensive effect in angiotensin II-treated mice49, these findings indicate that the protective effects of FGF21 on HFpEF may rely on its capacity to lower blood pressure and ameliorate cardiac metabolic disorders and mitochondrial dysfunction.
Mitochondrial damage, a critical event in the pathological process of HFpEF, has been confirmed in several rodent models of HFpEF and unequivocally contributed to cardiac diastolic dysfunction and cardiac damage. Clinical and animal-based studies indicate that mitochondrial dysfunction exacerbates excessive ROS production, substrate utilization obstacles, and an imbalance between ATP production and requirement, finally promoting cardiac damage. Our in vivo data also showed that FGF21 deficiency accelerates mitochondrial dysfunction in mice with HFpEF, as evidenced by intensified mitochondrial ultrastructure abnormalities, elevated cardiac ROS production, and lowered cardiac acetyl-CoA and ATP production (Figs. 2 and 3). In contrast, these adverse effects are primarily reversed by administration of AAV-Fgf21 (Figs. 3 and S3), suggesting that FGF21 protects against HFpEF-induced cardiac damage by improving mitochondrial dysfunction. However, the positive impact of FGF21, which is not linked to the modulation of mitochondrial ETC but rather to the promotion of substrate utilization, highlights its promising therapeutic potential. To support this notion, our in vivo data also showed that no significant differences in the vital ETC protein expression are seen between mice with HFpEF after treatment with AAV-Fgf21 or AAV-GFP (Fig. 3F).
Pyruvate dehydrogenase kinases (PDKs), the key enzymes of glucose oxidation in mitochondria, negatively regulate the activity of the PDH complex. As a member of PDKs, PDK4 is significantly and rapidly induced in the heart and other tissues in response to various metabolic stimuli, including fasting and a high-fat diet50. PDK4 deletion also improves left ventricular function and reduces remodeling after myocardial infarction51. In addition, silencing of PDK4 alleviates cardiac dysfunction and cardiomyocyte apoptosis in immature rat models of ischemic reperfusion52. In contrast, overexpression of PDK4 heightened calcineurin-induced cardiac hypertrophy and fibrosis53. These data collectively indicate that PDK4 plays a pivotal role in the pathogenesis of cardiovascular disorders. Consistently, our data showed that cardiac PDK4 contents are notably increased in WT mice with HFpEF (Fig. 4B–E), accompanied by exacerbated HFpEF-induced cardiac diastolic dysfunction and cardiac damage (Figs. 2 and S3), and overexpression of PDK4 significantly intensified these adverse effects, followed by lowered PDH activities and attenuated mitochondrial bioenergetics (Fig. S7), suggesting that PDK4 is a crucial regulator related to regulating cardiac energy metabolism during the pathological progression of HFpEF. To support this notion, our in vivo and in vitro data showed that genetic suppression of PDK4 expression notably attenuates HFpEF-induced cardiac mitochondrial dysfunction as evidenced by decreased pyruvate accumulation and promoted acetyl-CoA and ATP production (Figs. 5 and 6).
Our and other previous studies have demonstrated the effects of FGF21 on the elevation of circulating APN in rodents and humans16,54. In adipocytes, FGF21 can stimulate the gene expression and the protein secretion of APN in a PPARγ-dependent manner16. APN, an adipose-derived hormone with pleiotropic biofunctions, exerts anti-inflammatory and anti-hypertrophic effects, maintains body energy homeostasis, and is implicated in the development of hypertension, systolic and diastolic HF55,56. In humans, hypoadiponectinemia is an independent risk factor for vascular inflammation and atherosclerosis57. Moreover, animal-based studies also show that hypoadiponectinemia, which is frequently observed in individuals with obesity, may influence alterations in the extracellular matrix (ECM) and cardiomyocyte hypertrophy, and facilitate cardiac fibrosis, ultimately leading to the progression of HF58,59,60. In line with these reports, our results demonstrated that loss of APN exacerbates HFpEF-induced cardiac diastolic dysfunction and cardiac damage in mice, concomitant with intensified mitochondrial dysfunction as evidenced by decreased PDH activities and lowered cardiac acetyl-CoA and ATP production (Fig. 7). Moreover, the beneficial effects of FGF21 against HFpEF and its related cardiac damage are vastly alleviated in APN null mice. On the other hand, our in vitro results also indicated that adipose-derived APN negatively regulates PDK4 expression and promotes mitochondrial bioenergetics by activation of PI3K/AKT signals in NRVMs, and a synergistic effect on improving mitochondrial function is observed when treated with rmFGF21 and wt-SAT in NRVMs (Fig. 8). Taken together, these results demonstrated that the beneficial effect of FGF21 against HFpEF is mediated at least in part by induction of APN. To support this notion, APN is found to act as an obligatory downstream mediator of FGF21 on energy metabolism and insulin sensitivity16.
In conclusion, this study reveals and highlights the critical role of FGF21 in the pathogenesis of HFpEF. FGF21 exerts its beneficial effect against HFpEF by triggering PI3K/AKT signals through both APN-dependent and -independent manners, negatively regulating PDK4 expression, and promoting mitochondrial bioenergetics in mice (Fig. S10). Considering the potential benefits of FGF21 in cardiometabolic disease, our findings not only identify the FGF21-PDK4 axis as a novel therapeutic target with potential applications for treating this challenging condition but also open up an intriguing avenue for the development of targeted therapies for HFpEF management.
Methods
Study population
In this study, we investigated the serum levels of FGF21 and analyzed its association with biochemical parameters in patients with HFpEF and healthy controls. Blood samples of 151 subjects with HFpEF from a prospective study between 2014 and 2020 were kindly provided by Dr. Yulin Li, Beijing Anzhen Hospital, Capital Medical University. Adult patients with typical HF symptoms (breathlessness, coughing up pink frothy sputum, lower limb swelling, or jugular venous distension) with a LVEF over 50% or relevant structural heart changes assessed by echocardiography were included61. Subjects with acute HF, acute coronary syndrome, congenital heart disease, pericarditis, pulmonary heart disease, valvular heart disease, abnormal liver function, severe renal dysfunction, severe endocrine disorders, tumors, and acute infection shortly were excluded. Blood samples of 146 healthy control subjects (blood donors) were prospectively collected. All participants gave written informed consent before taking part in this study. All patients participated in the study process without compensation. This study was approved by the Human Ethics Committees of Beijing Anzhen Hospital in concordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Animal experiments
Fgf21 KO mice were provided by Beijing Viewsolid Biotech Ltd. Genotyping primers for Fgf21 KO mice were as follows: P1, 5’-GACTGTTCAGTCAGGGATTG-3’; P2, 5’-CCCGTGATATTGCTGAAGAG-3’; and P3, 5’-ACAGGGTCTCAGGTTCAAAG-3’. Fgf21F/F mice (Cyagen Biosciences, Suzhou, China) were bred with Adipoq-Cre mice (Cyagen Biosciences, Suzhou, China) to create Fgf21Adipoq mice. Genotyping primers for Fgf21F/F mice were described as follows: F1, 5’-TGTACCTGACCTAACCTCTCTCAG-3’; R1, 5’-CAGTGTCTTCCCTCAACTTTTCTC-3’. Genotyping primers for Adipoq-Cre mice were described as follows: F1, 5’-GGATGTGCCATGTGAGTCTG-3’; R1, 5’-ACGGACAGAAGCATTTTCCA-3’. Apn KO mice were generated by the Shanghai Model Organisms Center. Genotyping primers for Apn KO mice were as follows: F1, 5’-TGGATGCTGCCATGTTCCCAT-3’; R1, 5’-CTTGTGTCTGTGTCTAGGCCTT-3’; and R2, 5’-CTCCAGACTGCCTTGGGA-3’. All mice were on a C57BL/6 background. All the mice were housed in a specific-pathogen-free (SPF) space with consistent temperature (23 ± 1 °C) and a 12-h light/dark cycle and had unrestricted access to irradiated food and water. The Sprague Dawley (SD) rats were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. The HFpEF mouse model was established following the previously described procedure25. Eight to ten-week-old male mice were fed a HFD (D12492, Research Diets, USA) and concurrently administered L-NAME (51298-62-5, Sigma, USA) dissolved in drinking water (0.5 g/L) for 12 weeks. Meanwhile, age-matched control mice had a chow standard diet and regular drinking water. AAV vectors were introduced into mice by tail vein injection as described by previous reports62. The animal experiments conducted in this study were approved by the Animal Research Ethics Committee of Wenzhou Medical University.
Construction of AAV vectors for overexpression of FGF21 and PDK4
Recombinant adeno-associated virus 9 (AAV9) expressing FGF21 was constructed and produced by Wuhan Viral-therapy Biotechnologies Co. Ltd. The amplification process involved the use of specific primers: 5’-ATTGAATTCGCCACCATGGAATGGATGAGATCTAGAGTTG-3’ and 5’-AGAGGATCCTCAGGACGCATAGCTGGGGCTTCGG-3’. The DNA segment was inserted into the pAAV-IRES-ZsGreen plasmid to construct the pAAV-mFGF21-IRES-ZsGreen vector. AAV9 expressing PDK4 was constructed and produced by Shanghai OBiO Technology Co., Ltd. The amplification process involved the use of specific primers: 5’-GGCACCTATTGGTCTTACTGAC-3’ and 5’-CCTTGAAGGGGTAGCCGAT-3’. The DNA segment was inserted into the pAAV-cTNT-GdGreen-WPRE plasmid to construct the pAAV-cTNT-Pdk4-3xFLAG-P2A-GdGreen-WPRE vector. AAV vectors were prepared using the 3-plasmid transfection adenovirus-free protocol as previously described63. Briefly, the plasmid carrying the target gene cassette was cotransfected into HEK293T cells with a packaging plasmid and an adenovirus helper plasmid using PEI. The crude viral lysate was purified by two rounds of cesium chloride 2-tier centrifugation. After quantifying the viral stock titer, the stock was dissolved in HN buffer (50 mM HEPES, pH 7.4, 0.15 M NaCl) before injection.
Construction of AAV vectors for knockdown of PDK4
AAV vectors for knockdown of PDK4 were constructed and produced by Shanghai OBiO Technology Co., Ltd. Briefly, AAV9 driving the expression of short hairpin RNA (shRNA) targeting Pdk4 or control shRNA were used in this study: AAV-cTNT-EGFP-P2A-3xFLAG-shRNA(Pdk4)-WPRE and AAV-cTNT-EGFP-P2A-3xFLAG-shRNA(NC)-WPRE. The shRNA against Pdk4 was cloned into the shRNA AAV vector, and the recombinant plasmid was co-transfected into HEK293T cells using PEI. At 72 h after transfection, cells were collected by centrifugation at 800 g, 4 °C for 10 min. The cell pellet was resuspended in lysis buffer (150 mM NaCl and 20 mM Tris, pH 8.0) by vortexing and then incubated with concentration reagent overnight at 4 °C. After quantifying the concentration of the AAV vector, the viral stock was dissolved in HN buffer (50 mM HEPES, pH 7.4, 0.15 M NaCl) before injection. The shRNA sequences used in this study are as follows: shRNA (Pdk4): 5’-AGACGCTATCATCTACTTAAA-3’; shRNA (NC): 5’-TTCTCCGAACGTGTCACG-3’.
Exercise exhaustion test
After three days of acclimatization to treadmill exercise, an exhaustion test was conducted on the mice, as reported previously25. Mice ran uphill on a treadmill (Harvard Instruments) at an incline of 20°. The treadmill exercise started with a warm-up speed of 5 m/min for 4 min, then increased to 14 m/min for 2 min. Subsequently, the speed was raised by 2 m/min every 2 min until the mice reached a point of exhaustion. Exhaustion was defined by the mice’s inability to resume running within 10 s of direct contact with an electric shock grid. The running distance was recorded.
Tissue and blood collection
Mice were anesthetized and sacrificed at the specified time points, and serum was collected. The heart and other related tissues were collected after being transcardially perfused with 20 mL of PBS. The heart and lung tissues were cleaned with PBS and then weighed. Portions of the tissues were rapidly frozen using liquid nitrogen for long-term storage at −80 °C for subsequent protein and RNA extraction. Furthermore, a portion of these tissues was fixed with 4% paraformaldehyde for 24 h and subsequently embedded in paraffin for pathological staining analysis. Likewise, a part of the heart tissue was embedded in the optimal cutting temperature (OCT) compound (4583, SAKURA, USA) for subsequent frozen sectioning.
Echocardiography
Echocardiographic analysis was performed to assess the cardiac function of HFpEF model mice and control mice using a Vevo 3100 Ultrasound System (VisualSonics, Toronto, Canada) equipped with an MS400 transducer. In brief, mice were maintained in an anesthetized state using 1.5% isoflurane in pure oxygen. Ventricular M mode ultrasound was utilized at the papillary muscle level to evaluate LVEF, LVFS, and other parameters of cardiac systolic function. Apical four-chamber views were utilized to assess the peak flow velocities during early diastole (E wave) and late diastole (A wave). In addition, this view also measured the early-diastolic peak velocity (E′ wave) of the mitral annulus. Data analysis was performed on VevoLAB software (v5.6.1, Fujifilm VisualSonics) according to the analysis guidelines.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
The glucose tolerance test was performed in overnight-fasted (19:00-10:00) mice by an intraperitoneal injection of D-glucose (2 g/kg body weight, G5767, Sigma, USA). Insulin tolerance test was conducted with an intraperitoneal injection of insulin (0.5 U/kg body weight, Actrapid HM, Denmark) in mice after 6 h of fasting (09:00-15:00). Blood was collected from the tail vein at various time points as specified in each experiment for measurement of glucose levels with a glucose meter (Accu-Chek, Roche, Switzerland).
Measurement of blood pressure
Blood pressure was measured using tail-cuff systems (BP2000, Visitech Systems, USA). Mice were individually placed in holders on a temperature-controlled platform (37 °C), and recordings were conducted under steady-state conditions. After four days of prior training, measurements were performed during the daytime (8:00 to 11:00). Blood pressure was recorded for at least four consecutive days, with readings averaged from a minimum of eight measurements per session.
Immunoblot analysis
Tissues or cultured cells were lysed and centrifuged. The supernatant was used to determine total protein concentration with the BCA assay (23227, Thermo Scientific, USA). Equal amounts (20 μg) of complete protein were subjected to SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (IPVH00010, Millipore, Germany). Membranes were blocked with 5% milk and then incubated with primary antibodies against GAPDH (HRP-60004, Proteintech, China), VDAC1 (A19707, ABclonal, China), FGF21 (ab171941, Abcam, USA), Fibronectin (ab45688, Abcam, USA), Collagen I (ab260043, Abcam, USA), α-SMA (ab124964, Abcam, USA), PDK4 (12949-1-AP, Proteintech, China), p-PDH (ab177461, Abcam, USA), PDH (18068-1-AP, Proteintech, China), PI3K (T40064, Abmart, China), p-PI3K (T40065, Abmart, China), AKT (9272S, CST, USA), p-AKT (12694S, CST, USA), total OXPHOS Rodent (ab110413, Abcam, USA) and CPT1b (22170-1-AP, Proteintech, China) at 4 °C overnight. Membranes were washed and incubated with secondary antibodies at room temperature for 1 h. Specific protein bands were visualized by exposure machine (Amersham ImageQuant 800, GE, USA) using enhanced-chemiluminescent (ECL) substrate (K-12045-D50, Advansta, USA) and quantitatively processed with ImageJ software.
ELISA detection
Serum samples collected from humans and mice were stored at −80 °C before analysis. The serum FGF21 levels were measured using a commercial ELISA kit (31180, 32180, Immunoassay Services, HK), according to the manufacturer’s protocol. Similarly, according to the manufacturer’s protocol, the APN levels in serum and cell culture medium were measured using a standard ELISA kit (32010, Immunoassay Services, HK).
RNA extraction and quantitative real-time PCR
Total RNA was isolated from frozen left ventricle tissue by the Trizol Reagent (15596018, Invitrogen, USA). Complementary DNA was then synthesized from 500 ng total RNA by reverse transcription with an Improm-II reverse transcription kit (A5001, Promega, USA) with random hexamer primers. Real-time quantitative polymerase chain reaction (qPCR) was performed with a real-time PCR system (CFX96, Bio-Rad, USA) with specific primers described in Table S3. The level of specific gene expression was normalized to the GAPDH gene.
Histological analysis
Heart tissues were dissected, fixed with 4% paraformaldehyde, embedded in paraffin, and then cut into 5-μm-thick sections. The tissue sections underwent deparaffinization and rehydration through immersion in xylene and alcohol. To assess heart morphology, the sections were stained with H&E. H&E staining was performed according to the manufacturer’s protocol using a commercial kit (G1120, Solarbio, China). Cardiomyocyte cross-sectional size was assessed by WGA (L4895, Sigma, USA) staining. Briefly, the sections were stained with WGA working buffer (1:100) for 1 h at 37 °C in the dark. Afterward, the sections were washed and counterstained with 4’,6-diamidino-2-phenylindole (DAPI, 9542, Sigma, USA). To compare the cardiomyocyte cross-sectional area, the cross-sectional areas of 50 cardiomyocytes from 5 to 6 samples were analyzed using ImageJ. The ventricular fibrosis area was assessed by Sirius red (S1020, Biohao, China) staining. In brief, dewaxed and rehydrated heart sections were incubated with Sirius red dye for 40 min at room temperature. To determine the extent of fibrosis, we calculated the average percentage of the fibrotic area from four randomly selected fields per heart using ImageJ software. For Oil Red O staining, 5-μm-thick frozen heart sections were air-dried for 10 min at room temperature. After washing with PBS, the sections were dipped into 60% isopropyl alcohol three times and then placed into Oil Red O working solution [0.5% (w/v) Oil Red O (O1391, Sigma, USA) dissolved in isopropyl alcohol] for 15 min. The sections were washed three times with 60% isopropyl alcohol and once with water for 30 min. The percentage of Oil Red O positive staining (to the cardiac area) was calculated and quantified using ImageJ software. All images were captured using an optical microscope (BX53F2O, Olympus, Japan).
ROS detection
Briefly, 5 μm thick heart-frozen sections were fixed in methyl alcohol for 15 min at room temperature and then washed thrice with PBS. Afterward, the sections were exposed to dihydroethidium (DHE) dye (1 μM, D11347, Thermo Scientific, USA) at 37 °C for 1 h without light. After three washes in PBS, the slides were sealed with DAPI. Images were captured using an optical microscope (BX53F2O, Olympus, Japan). Intracellular ROS levels were assessed using DCFH-DA (S0033S, Beyotime, China). After aspirating the cell culture medium, diluted DCFH-DA was introduced into the cell culture dish to achieve a final concentration of 10 μM and incubated at 37 °C for 30 min. Cells underwent three washes with serum-free cell culture medium to eliminate any residual DCFH-DA that had not penetrated the cells. Subsequently, images were captured using an inverted microscope (TI-S, Nikon, Japan). The fluorescence intensity was analyzed by ImageJ.
2-NBDG staining
Intracellular glucose uptake was assessed using 2-NBDG (ST2078, Beyotime, China) staining. After aspirating the cell culture medium, diluted 2-NBDG was introduced into the cell culture dish to achieve a final concentration of 100 μM and incubated at 37 °C for 30 min. Cells underwent three washes with PBS to eliminate any residual 2-NBDG that had not penetrated the cells. Subsequently, images were captured using an inverted microscope (TI-S, Nikon, Japan). The 2-NBDG fluorescence intensity was analyzed by ImageJ.
BODIPY staining
Intracellular FA uptake was assessed using BODIPY (D3821, Thermo Fisher Scientific, USA) staining. In brief, the cell culture medium was aspirated, and cells were incubated with 4 μM BODIPY dye for 30 min in the dark. After washing, the cells were stained with DAPI (9542, Sigma, USA) at 37 °C for 30 min. Subsequently, images were captured using an inverted microscope (TI-S, Nikon, Japan). The fluorescence intensity was analyzed by ImageJ.
Transmission electron microscopy
After euthanasia, mice were immediately transcardially perfused with PBS. After perfusion, the hearts were excised and dissected into small pieces of 1 mm3 and fixed in 2.5% glutaraldehyde and 1% osmium tetroxide for 2 h at room temperature. Subsequently, the tissues were embedded, sliced into 90-nm thin sections using ultramicrotomy, and captured using transmission electron microscopy (JEM-F200, JEOL, Japan). The mitochondrial number, size and cristae were assessed with ImageJ.
Proteomics
Proteomics analysis was conducted by Applied Protein Technology Co., Ltd. using 12 samples. Four WT mice were fed a standard diet and injected with AAV-GFP, four WT mice were subjected to the HFpEF mouse model and treated with AAV-GFP, and the remaining four WT mice were subjected to the HFpEF mouse model and treated with AAV-Fgf21. After 12 weeks, the hearts were collected and immediately frozen in liquid nitrogen. Approximately 100 mg of heart tissue from each mouse was dissolved in SDT buffer (4% SDS, 100 mM DTT, 150 mM Tris-HCl, pH 8.0) and quantified using the BCA Protein Assay Kit (Bio-Rad). Peptide fragments were prepared through protein precipitation, filtration, washing, and enzymatic digestion. The peptides were then desalted, concentrated via vacuum centrifugation, and reconstituted in solution.
A pooled sample, prepared by combining equal aliquots from all experimental samples, was used for DDA library generation and quality control. All fractions for the DDA library were analyzed using a Thermo Scientific Q Exactive HF-X mass spectrometer coupled with an Easy nLC 1200 chromatography system. Peptides were first loaded onto an EASY-Spray™ C18 Trap column (Thermo Scientific, P/N 164946, 3 μm, 75 μm × 2 cm) and then separated on an EASY-Spray™ C18 LC Analytical Column (Thermo Scientific, ES802, 2 μm, 75 μm × 25 cm) using a linear gradient of buffer B (80% acetonitrile and 0.1% formic acid) at a flow rate of 250 nl/min over 90 min. Mass spectrometry (MS) detection was performed in positive ion mode with a scan range of 300–1800 m/z. The MS1 scan resolution was set to 60,000 (at 200 m/z), with an AGC target of 3 × 106 and a maximum injection time (IT) of 25 ms. Dynamic exclusion was set to 30 s. Each full MS-SIM scan was followed by 20 ddMS2 scans, with an MS2 scan resolution of 15,000, an AGC target of 5 × 104, a maximum IT of 25 ms, and a normalized collision energy of 30 eV.
Peptides from each sample were also analyzed in DIA mode. Each DIA cycle included one full MS-SIM scan and 30 DIA scans, covering a mass range of 350–1800 m/z. The MS1 scan resolution was set to 120,000 (at 200 m/z), with an AGC target of 3 × 106 and a maximum IT of 50 ms. DIA scans were performed at a resolution of 15,000, with an AGC target of 3 × 106, auto maximum IT, and a normalized collision energy of 30 eV. The runtime was 90 min, using a linear gradient of buffer B (80% acetonitrile and 0.1% formic acid) at a flow rate of 250 nl/min. QC samples, prepared by pooling equal aliquots from all experimental samples, were injected at the start of the MS study and after every six injections to monitor instrument performance.
For DDA library data, the FASTA sequence database was searched using Spectronaut™ 14.4.200727.47784 (Biognosys) software. The parameters were set as follows: trypsin was used as the protease, allowing two missed cleavages. Carbamidomethylation of cysteine (C) was set as a fixed modification, while oxidation of methionine (M) and N-terminal acetylation were set as variable modifications. DIA data analysis was performed using Spectronaut™ 14.4.200727.47784 software, searching against the previously constructed spectral library. The main parameters for the analysis were as follows: (1) Retention time prediction type: dynamic iRT; (2) MS2-level interference correction: enabled; (3) Cross-run normalization: enabled. All results were filtered based on a Q value cutoff of 0.01, corresponding to a false discovery rate (FDR) of less than 1%. GSEA-GO analysis was performed by using gseGO functions, through the clusterProfiler R package. Proteins with an absolute fold change ≤0.83 or fold change ≥1.2 and p < 0.05 were considered as DEPs. Volcano plot and hierarchical clustering heatmaps were created with the ggplot library.
Evaluation of mitochondrial membrane potential
The JC-1 mitochondrial membrane potential detection kit (G1515, Servicebio, China) was used to evaluate the changes in mitochondrial membrane potential following the manufacturer’s instructions. Briefly, cells were washed with PBS, followed by immersion in a JC-1 dye solution at 37 °C for 30 min. After the incubation, cells underwent two additional washes with a dilution buffer, and images were recorded using an inverted microscope (TI-S, Nikon, Japan). The green or red fluorescence intensity was analyzed by ImageJ.
Evaluation of mitochondrial morphology
Mitochondrial morphology was assessed using the MitoTracker Green probe. Briefly, NRVMs were seeded on glass-bottom confocal dishes. Cells were incubated with Hoechst 33342 (C1022, Beyotime, China, 1:100) at 37 °C for 10 min. Subsequently, the cells were washed twice with PBS and then incubated with the MitoTracker Green probe (40742ES50, Yeasen, China, 1:5000) at 37 °C for 30 min. Afterward, the cells were washed with PBS and imaged using a super-resolution microscope (AIR-SIM-STORM, Nikon, Japan). The mitochondrial sizes were assessed with ImageJ.
Measurement of acetyl-CoA contents
For acetyl-CoA level measurement, the heart tissues and cells were collected in PBS buffer and then subjected to homogenization on ice. The mixture was centrifuged at 12,000 g, 4 °C for 5 min to obtain the supernatant. Subsequently, the concentration of acetyl-CoA in the collected supernatant was measured following the instructions provided by the manufacturer of the acetyl-CoA assay kit (E-EL-0125, Elabscience, China). Absorbance was measured at 450 nm using a microplate reader (51119080, Thermo Scientific, USA). The content of acetyl-CoA was then calculated based on the standard curve.
Measurement of pyruvate contents
Pyruvate measurement was performed according to the manufacturer’s protocol using the pyruvate assay kit (BC2205, Solarbio, China). Briefly, the tissues and cells were added to the extraction solution and homogenized on ice. After a 30 min incubation, the mixture was then centrifuged at 8000 g for 10 min at 25 °C (room temperature). 75 μL of supernatant and 150 μL of the assay reagent were added to a 96-well plate, and the absorbance at 570 nm was measured using a multimode microplate reader (51119080, Thermo Scientific, USA). The content of pyruvate was then calculated based on the standard curve.
ATP measurement
ATP measurement was performed according to the manufacturer’s protocol using the ATP assay kit (S0027, Beyotime, China). The heart tissues and cells were lysed on ice and then centrifuged at 12,000 g, 4 °C for 5 min to obtain the supernatant. The supernatant and 20 μL of standard ATP solution (concentrations ranged from 0.01 to 10 μM) were added to 96-well black opaque plates, then 100 μL of detection buffer was added to each well. ATP concentrations were measured using a multimode microplate reader (Synergy Neo2, Biotek, USA) and calculated using a standard curve.
Measurement of PDH activity
The PDH activity microplate assay kit (BC0385, Solarbio, China) was used to measure the PDH activity of heart tissues and cells. Briefly, the tissues and cells were added to PBS and homogenized on ice. Subsequently, the mixture was centrifuged at 11,000 g for 10 min at 4 °C. Then 10 μL of supernatant was added to a 96-well plate, followed by 180 μL of detection buffer to each well. The absorbance at 605 nm was measured using a microplate reader (51119080, Thermo Scientific, USA). The absorbance values were recorded at both 10 s and 1 min 10 s and then these values were substituted into the calculation formula.
Plasma and tissue lipid content
The FFA content assay kit (BC0595, Solarbio, China) was used to measure the FFA content of heart tissues and serum. Briefly, blood samples were left at room temperature for 1 h, then centrifuged at 3000 g for 15 min at 4 °C. The upper serum layer was collected and stored on ice. Tissues were washed with physiological saline, and surface moisture was absorbed with filter paper. The tissues were then homogenized on ice in an extraction buffer at a ratio of 1:5. The homogenate was centrifuged at 8000 g for 10 min at 4 °C, and the supernatant was collected and stored on ice. 30 μL of supernatant was added to a 96-well plate, followed by a detection buffer to each well. The absorbance at 550 nm was measured using a microplate reader (51119080, Thermo Scientific, USA). The FFA content was then calculated based on the standard curve.
The TG content assay kit (E1013-50, Applygen, China) was used to measure the TG content of heart tissue. Tissues were washed with physiological saline, and surface moisture was absorbed with filter paper. Lysis buffer was added according to the manufacturer’s instructions. The tissue was homogenized using an electric high-speed homogenizer. The homogenate was then centrifuged at 12,000 g for 10 min. The supernatant was heated at 70 °C for 10 min, followed by centrifugation at 2000 g for 5 min at room temperature. The upper clear liquid was collected for measurement. Ten μL of supernatant was added to a 96-well plate, followed by a detection buffer to each well. The absorbance at 550 nm was measured using a microplate reader (51119080, Thermo Scientific, USA). The TG content was then calculated based on the standard curve.
The TG content assay kit (A110-1-10, Nanjing Institute of Bioengineering, China) was used to measure the TG content of serum. Briefly, blood samples were left at room temperature for 1 h, then centrifuged at 3000 g for 15 min at 4 °C. The upper serum layer was collected and stored on ice. Then, 2.5 μL of supernatant was added to a 96-well plate, followed by the addition of detection buffer to each well. The plate was shaken to mix thoroughly and incubated at 37 °C for 10 min. The absorbance at 500 nm was measured using a microplate reader (51119080, Thermo Scientific, USA). The TG content was then calculated based on the formula provided in the manufacturer’s instructions.
Cell bioenergetic profiling
The OCR of NRVMs were measured with a Seahorse XFe96 Extracellular Flux Analyzer (Seahorse XFe96, Agilent Technologies, USA). In brief, the cells were seeded on the assay microplate at 10000 cells/well density with 10% DMEM. Following specific stimulation, the culture medium was replaced with an assay medium, which included 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate. The plate was then incubated at 37 °C in a CO2-free incubator for 1 h. The assay microplate was then sequentially injected with the following compounds: 1.5 mM oligomycin (ATP synthase inhibitor); 2 mM FCCP (mitochondrial uncoupler); 0.5 mM Rot/AA (complex III and complex I inhibitors). The mitochondrial oxidation respiratory functions were determined by measuring the changes in OCR after adding FCCP, oligomycin, or Rot/AA. The data were analyzed using the Wave package.
NRVMs isolation and culture
NRVMs were isolated from the hearts of neonatal SD rats64,65,66. After disinfection with 75% alcohol, the chests of newborn rats were dissected, and their hearts were removed. The excised hearts were washed twice with cold PBS and sliced into approximately 1 mm³ pieces with scissors. The heart pieces were digested with 0.08% collagenase II (17101015, Gibco, USA) at 37 °C in a water bath for 30 min. The supernatant was collected, and digestion was stopped by adding 5 mL stop solution (10% DMEM). The digestion was repeated 4 to 6 times, and each repeated digestion cycle was 10 min. The obtained supernatant was centrifuged at 800 g for 10 min. Subsequently, the cell pellets were re-suspended in 10% DMEM. The cell suspension was seeded into culture dishes and cultured at 37 °C in a 5% CO2-humidified incubator for 1.5 h. Following differential attachment, the supernatant containing unattached myocytes was seeded in cell containers till confluence for the experiment. For PA treatment, the cells were supplemented with 500 μM PA (P0500, Sigma, USA) for 24 h. 10% fatty acid-free bovine serum albumin (BSA) was used as a control. NRVMs were exposed to 500 μM PA or 10% BSA, followed by incubation with rmFGF21 (ab63277, Abcam, USA) and LY294002 (S1105, Selleck, USA) for 24 h. Following a 24 h stimulation with PA, the NRVMs were collected and used to perform various experiments. For NRVMs and adipose tissue co-culture experiments, NRVMs were cultured with mouse subcutaneous adipose tissue isolated from WT or Apn KO mice in 10% DMEM.
Plasmids and cell transfection
The PDK4 plasmid was constructed and procured from Shanghai OBiO Technology Co. Ltd. The amplification process involved the use of specific primers: 5’-CGCAAATGGGCGGTAGGCGTG-3’ and 5’-AGAGACAGCAACCAGGAT-3’. The DNA segment was cloned into the GL123-basic vector and produced the pSLenti-EF1-mCherry-P2A-Puro-CMV-Pdk4-3xFLAG-WPRE plasmid. Before transfection, the cells were seeded in cell containers. Transfections were performed according to the manufacturer’s protocol using the Lipofectamine 3000 Kit (L3000150, Invitrogen, USA). In brief, 1 μg plasmid (plus 2 μL P3000) and 2.5 μL Lipofectamine 3000 were each mixed separately in 100 μL Opti-MEM (31985070, Gibco, USA) and then mixed well and allowed to stand for 5 min before being added to the cells. Cells were incubated with the liposome-DNA complexes for 48–72 h before being harvested for experiments.
Statistical analysis
Statistical analyses for human data were performed using SPSS 26.0 (IBM) software. Results for continuous data with normal distribution were reported as average ± standard deviation (SD) assessed using analysis of independent sample t-test. In contrast, data with non-normal distribution were reported as median (interquartile range) assessed using analysis of the Mann–Whitney U test. Categorical variables were expressed as absolute numbers and percentages evaluated using the χ2 test or Fisher’s exact test. ANOVA with the multiple-comparisons test with Bonferroni correction and the Kruskal-Wallis test were performed, when appropriate. Pearson correlation coefficient for a continuous variable and Kendall tau-b correlation coefficient for a categorical variable were used to analyze the associations between cardiac function parameters and serum levels of FGF21 (log-transformed as required). Two-sided p < 0.05 was considered as indicating statistical significance.
For the analysis of mouse experiments, GraphPad Prism 8 was used. All the mice used in the animal study were randomly allocated into control and experimental groups. A double-blind experimental analysis mode was performed. Data were tested for normality before parametric statistics were applied using the Shapiro-Wilk normality test. For normally distributed data, a two-tailed unpaired Student’s t test was used for comparisons of two groups with equal variances, and a two-tailed unpaired Student’s t test with Welch’s correction was used for unequal variances. In contrast, for non-normally distributed data, the Mann–Whitney U test (2 groups) was utilized. For comparisons of more than two groups, a one-way ANOVA followed by Tukey’s multiple comparison test was used for equal variances, and Brown-Forsythe and Welch ANOVA with Dunnett’s multiple comparison test were used for unequal variances. Two-way ANOVA with Tukey’s multiple comparison test was used to test the difference among groups with various factors (e.g., genotype or treatment). The specific statistical analyses conducted for each experiment are detailed in the corresponding figure legends. Two-sided p < 0.05 was considered as indicating statistical significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data have been deposited to the Integrated Proteome Resources repository with the dataset identifier PXD051242. All data generated or analyzed in this study, including anonymized patient-derived data, are available within the article and its supplementary information files. Source data are provided with this paper.
References
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635 (2005).
Fisher, F. M. & Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 78, 223–241 (2016).
Salminen, A., Kaarniranta, K. & Kauppinen, A. Regulation of longevity by FGF21: Interaction between energy metabolism and stress responses. Ageing Res. Rev. 37, 79–93 (2017).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008).
Kharitonenkov, A. et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781 (2007).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Flippo, K. H. & Potthoff, M. J. Metabolic Messengers: FGF21. Nat. Metab. 3, 309–317 (2021).
Li, H. et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 61, 797–806 (2012).
Kim, K. H. & Lee, M. S. FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs. J. Endocrinol. 226, R1–R16 (2015).
Ito, S. et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mechanisms Dev. 98, 115–119 (2000).
Adams, A. C., Cheng, C. C., Coskun, T. & Kharitonenkov, A. FGF21 requires betaklotho to act in vivo. PloS ONE 7, e49977 (2012).
Chen, W. et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J. Biol. Chem. 286, 34559–34566 (2011).
Chau, M. D., Gao, J., Yang, Q., Wu, Z. & Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl Acad. Sci. USA 107, 12553–12558 (2010).
Murata, Y., Konishi, M. & Itoh, N. FGF21 as an endocrine regulator in lipid metabolism: from molecular evolution to physiology and pathophysiology. J. Nutr. Metab. 2011, 981315 (2011).
Fisher, F. M. et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013).
Joki, Y. et al. FGF21 attenuates pathological myocardial remodeling following myocardial infarction through the adiponectin-dependent mechanism. Biochem. Biophys. Res. Commun. 459, 124–130 (2015).
Lin, Z. et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 131, 1861–1871 (2015).
Wu, F. et al. FGF21 ameliorates diabetic cardiomyopathy by activating the AMPK-paraoxonase 1 signaling axis in mice. Clin. Sci. 131, 1877–1893 (2017).
Pan, X. et al. FGF21 prevents Angiotensin II-Induced hypertension and vascular dysfunction by activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 27, 1323–1337.e1325 (2018).
Redfield, M. M. & Borlaug, B. A. Heart failure with preserved ejection fraction: a review. Jama 329, 827–838 (2023).
Sharma, K. & Kass, D. A. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ. Res. 115, 79–96 (2014).
Ianoș, R. D. et al. Diagnostic performance of serum biomarkers fibroblast growth factor 21, Galectin-3 and Copeptin for heart failure with preserved ejection fraction in a sample of patients with type 2 Diabetes Mellitus. Diagnostics 11, 1577 (2021).
Chou, R. H. et al. Circulating fibroblast growth factor 21 is associated with diastolic dysfunction in heart failure patients with preserved ejection fraction. Sci. Rep. 6, 33953 (2016).
Schiattarella, G. G. et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356 (2019).
Pan, Y. et al. Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. J. Cell Mol. Med. 23, 1059–1071 (2019).
Rosales-Soto G., Diaz-Vegas A., Casas M., Contreras-Ferrat A. & Jaimovich E. Fibroblast growth factor-21 potentiates glucose transport in skeletal muscle fibers. J. Mol. Endocrinol. 65, 85–95 (2020).
Hui, X., Lam, K. S., Vanhoutte, P. M. & Xu, A. Adiponectin and cardiovascular health: an update. Br. J. Pharm. 165, 574–590 (2012).
Tanaka, K. et al. Effects of adiponectin on calcium-handling proteins in heart failure with preserved ejection fraction. Circ. Heart Fail 7, 976–985 (2014).
Francisco, C., Neves, J. S., Falcão-Pires, I. & Leite-Moreira, A. Can Adiponectin help us to target diastolic dysfunction? Cardiovasc. Drugs Ther. 30, 635–644 (2016).
Liu, L. et al. Adiponectin attenuates lipopolysaccharide-induced apoptosis by regulating the Cx43/PI3K/AKT pathway. Front. Pharm. 12, 644225 (2021).
Xu, Z. P. et al. Adiponectin Attenuates Streptozotocin-Induced Tau Hyperphosphorylation and Cognitive Deficits by Rescuing PI3K/Akt/GSK-3β Pathway. Neurochem. Res. 43, 316–323 (2018).
Zhou, Y., Wei, L. L., Zhang, R. P., Han, C. W. & Cao, Y. Globular adiponectin inhibits osteoblastic differentiation of vascular smooth muscle cells through the PI3K/AKT and Wnt/β-catenin pathway. J. Mol. Histol. 52, 1067–1080 (2021).
Yang, X. et al. FGF21 alleviates acute liver injury by inducing the SIRT1-autophagy signalling pathway. J. Cell Mol. Med. 26, 868–879 (2022).
Ding, P. et al. Fibroblast growth factor 21 attenuates ventilator-induced lung injury by inhibiting the NLRP3/caspase-1/GSDMD pyroptotic pathway. Crit. Care 27, 196 (2023).
Xie, W. et al. Serum FGF21 levels predict the MACE in patients with myocardial infarction after coronary artery bypass graft surgery. Front. Cardiovasc. Med. 9, 850517 (2022).
Refsgaard Holm, M. et al. Fibroblast growth factor 21 in patients with cardiac cachexia: a possible role of chronic inflammation. ESC Heart Fail 6, 983–991 (2019).
Geng, L., Lam, K. S. L. & Xu, A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667 (2020).
Bottomley, P. A. et al. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci. Transl. Med. 5, 215re213 (2013).
Capone, F. et al. Cardiac metabolism in HFpEF: from fuel to signalling. Cardiovasc Res. 118, 3556–3575 (2023).
Bertero, E. & Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 15, 457–470 (2018).
Karwi, Q. G., Uddin, G. M., Ho, K. L. & Lopaschuk, G. D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5, 68 (2018).
Tran, D. H. & Wang, Z. V. Glucose metabolism in cardiac hypertrophy and heart failure. J. Am. Heart Assoc. 8, e012673 (2019).
Mori, J. et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ. Heart Fail 5, 493–503 (2012).
Cluntun, A. A. et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 33, 629–648.e610 (2021).
Gopal, K. et al. FoxO1 inhibition alleviates type 2 diabetes-related diastolic dysfunction by increasing myocardial pyruvate dehydrogenase activity. Cell Rep. 35, 108935 (2021).
Tong, D. et al. NAD(+) repletion reverses heart failure with preserved ejection fraction. Circ. Res. 128, 1629–1641 (2021).
Ni, J. et al. Rg3 regulates myocardial pyruvate metabolism via P300-mediated dihydrolipoamide dehydrogenase 2-hydroxyisobutyrylation in TAC-induced cardiac hypertrophy. Cell Death Dis. 13, 1073 (2022).
Pan, X. et al. FGF21 prevents angiotensin ii-induced hypertension and vascular dysfunction by activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 27, 1323–1337.e1325 (2018).
Pilegaard, H. & Neufer, P. D. Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proc. Nutr. Soc. 63, 221–226 (2004).
Cardoso, A. C. et al. Mitochondrial substrate utilization regulates cardiomyocyte cell cycle progression. Nat. Metab. 2, 167–178 (2020).
Yin, Q., Wang, P. & Wu, X. MicroRNA -148 alleviates cardiac dysfunction, immune disorders and myocardial apoptosis in myocardial ischemia-reperfusion (MI/R) injury by targeting pyruvate dehydrogenase kinase (PDK4). Bioengineered 12, 5552–5565 (2021).
Zhao, G. et al. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 294, H936–H943 (2008).
Holland, W. L. et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013).
Fang, H. & Judd, R. L. Adiponectin regulation and function. Compr. Physiol. 8, 1031–1063 (2018).
Hopkins, T. A., Ouchi, N., Shibata, R. & Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 74, 11–18 (2007).
Shioji, K. et al. [Hypoadiponectinemia implies the development of atherosclerosis in carotid and coronary arteries]. J. Cardiol. 46, 105–112 (2005).
Ouchi, N., Shibata, R. & Walsh, K. Cardioprotection by adiponectin. Trends Cardiovasc. Med. 16, 141–146 (2006).
Kruszewska J., Cudnoch-Jedrzejewska A. & Czarzasta K. Remodeling and fibrosis of the cardiac muscle in the course of obesity-pathogenesis and involvement of the extracellular matrix. Int. J. Mol. Sci. 23, 4195 (2022).
Wang, H. T. et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J. Transl. Med. 10, 145 (2012).
Ke, B. et al. Aldosterone dysregulation predicts the risk of mortality and rehospitalization in heart failure with a preserved ejection fraction. Sci. China Life Sci. 65, 631–642 (2022).
Xie, W. et al. Myocardial infarction accelerates the progression of MASH by triggering immunoinflammatory response and induction of periosti. Cell Metab. 36, 1269–1286.e1269 (2024).
Ito, T. et al. Adenoassociated virus-mediated prostacyclin synthase expression prevents pulmonary arterial hypertension in rats. Hypertension 50, 531–536 (2007).
Gao, W. et al. Inhibiting receptor of advanced glycation end products attenuates pressure overload-induced cardiac dysfunction by preventing excessive autophagy. Front. Physiol. 9, 1333 (2018).
Lei, I. et al. Acetyl-CoA production by specific metabolites promotes cardiac repair after myocardial infarction via histone acetylation. Elife 10, e60311 (2021).
Liu, Y. et al. Spexin protects cardiomyocytes from hypoxia-induced metabolic and mitochondrial dysfunction. Naunyn Schmiedebergs Arch. Pharm. 393, 25–33 (2020).
Acknowledgements
This research was supported by China National Funds for Distinguished Young Scientists (No. 81925004 to Z.L.) and the National Key Research and Development Program of China (No. 2020YFA0803801 to Z.L.).
Author information
Authors and Affiliations
Contributions
Z.L., F.W., Y.L. and X.Liu. conceptualized the study; K.Z., B.W., D.Z. and N.W. performed investigative studies; J.G., B.W., J.Y., C.W., N.W. and X.G. performed data analysis; Z.L., K.Z., J.G. and D.Z. interpreted the results and wrote the manuscript. X.Li., W.L., A.X., Y.L. and F.W. provided essential mouse lines for this study and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kaiwen Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, K., Gan, J., Wang, B. et al. FGF21 protects against HFpEF by improving cardiac mitochondrial bioenergetics in mice. Nat Commun 16, 1661 (2025). https://doi.org/10.1038/s41467-025-56885-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56885-9
This article is cited by
-
Metabolic rewiring and inter-organ crosstalk in diabetic HFpEF
Cardiovascular Diabetology (2025)
-
Sodium–Glucose Cotransporter 2 Inhibitor Ameliorate Angiotensin II-Induced Hypertension and Vascular Injury by Upregulating FGF21
Inflammation (2025)
-
Systemic Interactions in HFpEF: A Multiorgan Perspective on Pathways and Therapeutic Targets
Journal of Cardiovascular Translational Research (2025)