Abstract
Inositol pyrophosphates (PP-InsPs) are eukaryotic nutrient messengers. The N-terminal kinase domain of diphosphoinositol pentakisphosphate kinase (PPIP5K) generates the messenger 1,5-InsP8, the C-terminal phosphatase domain catalyzes PP-InsP breakdown. The balance between kinase and phosphatase activities regulates 1,5-InsP8 levels. Here, we present crystal structures of the apo and substrate-bound PPIP5K phosphatase domain from S. cerevisiae (ScVip1PD). ScVip1PD is a phytase-like inositol 1-pyrophosphate histidine phosphatase with two conserved catalytic motifs. The enzyme has a strong preference for 1,5-InsP8 and is inhibited by inorganic phosphate. It contains an α-helical insertion domain stabilized by a structural Zn2+ binding site, and a unique GAF domain that channels the substrate to the active site. Mutations that alter the active site, restrict the movement of the GAF domain, or change the substrate channel’s charge inhibit the enzyme activity in vitro, and Arabidopsis VIH2 in planta. Our work reveals the structure, enzymatic mechanism and regulation of eukaryotic PPIP5K phosphatases.
Similar content being viewed by others
Introduction
Inositol pyrophosphates (PP-InsPs) are eukaryotic nutrient messengers1. These intracellular signaling molecules consist of a densely phosphorylated six-carbon myo-inositol ring with pyrophosphate groups attached, for example, at position 1 (1-InsP7), position 5 (5-InsP7), or both positions (1,5-InsP8). PP-InsPs regulate cell signaling by a variety of mechanisms, including mediating protein-protein interactions, allosterically regulating enzyme or transporter activities, or post-translationally modifying proteins2,3.
A central role of PP-InsPs in cellular inorganic phosphate (Pi) homeostasis is conserved among fungi4,5,6, protozoa7, algae8, plants9,10,11,12,13,14,15, and animals16,17,18,19. Pi homeostasis is primarily controlled by 1,5-InsP8 binding to PP-InsP sensing SPX receptor proteins4,5,11,12,14,15,17. 1,5-InsP8 levels are low under Pi starvation conditions, whereas 1,5-InsP8 accumulates under Pi-sufficient growth conditions or upon Pi resupply5,13. As cellular Pi homeostasis and other essential cellular processes appear to be mechanistically linked to 1,5-InsP8 levels, understanding the regulation of 1,5-InsP8 biosynthesis and catabolism is of fundamental importance.
PP-InsPs are generated from InsP6 through a series of phosphorylation steps catalyzed by different small molecule kinases. In yeast, the inositol hexakisphosphate kinase Kcs120,21 and in plants, the inositol 1,3,4-trisphosphate 5/6-kinases ITPK1/222, preferentially transfer a second phosphate to position 5 of InsP6 or 1-InsP7 to generate 5-InsP7 and 1,5-InsP8, respectively. The diphosphoinositol pentakisphosphate kinases (PPIP5Ks), known as Vip1 in S. cerevisiae, Asp1 in Schizosaccharomyces pombe23,24,25, PPIP5K in amoeba and in animals16,26,27,28,29,30 or VIP/VIH in plants8,10,31,32,33,34, preferentially transfer a phosphate to position 1, to generate 1-InsP7 and 1,5-InsP8, respectively. Consistent with the function of 1,5-InsP8 as messenger in Pi homeostasis in Arabidopsis, vih1/vih2 loss-of-function mutants have severely reduced 1,5-InsP8 levels and display constitutive Pi starvation responses10,11,12.
In addition to the conserved Pi homeostasis-related phenotypes, loss-of-function of PPIP5K enzymes results in altered growth, cell and vacuolar morphology, chromosome segregation, host colonization and DNA damage-induced autophagy24,25,35,36. PPIP5K mutants are associated with hearing loss in humans and in mice37. In human cell lines, depletion of PPIP5K activity induced broad changes in cell metabolism38. In plants, PPIP5K activity has been linked to jasmonic acid and salicylic acid hormone signaling, and to cell wall architecture32,33,34,39.
There are several PP-InsP catabolic enzymes, including a family of histidine acid phosphatases located in C-terminal half of PPIP5Ks24. Thus, PPIP5Ks are dual-function enzymes that contain two distinct domains with opposing enzymatic activities: An N-terminal kinase domain that preferentially generates 1,5-InsP8 from 5-InsP725,26,40, and a C-terminal phosphatase domain with inositol 1-pyrophosphate phosphatase activity25,29,40,41,42. The relative PP-InsP kinase and phosphatase activities in PPIP5K are thought to modulate the cellular levels of 1,5-InsP82,10,16.
PPIP5K loss-of-function mutants show altered PP-InsP pools in various eukaryotes5,8,10,13,24,25,32,34,35,36,38,43. PPIP5K knockout mutants have reduced 1-InsP7 and 1,5-InsP8 levels, while the 5-InsP7 substrate of the PPIP5K kinase domain accumulates5,13,34,44. 1,5-InsP8 pools can be restored to wild-type like levels in genetic rescue experiments with phosphatase-dead but not with kinase-dead versions of the corresponding PPIP5K enzyme10,25,35,36,38.
How regulation of PPIP5Ks controls 1,5-InsP8 pools in response to changes in Pi nutrient availability is not well understood. Currently, it is known that the ATP/ADP ratio decreases in response to Pi starvation, which in turn reduces 1,5-InsP8 synthesis by the kinase domains of human PPIP5K1/2 or plant VIH1/210,16. Pi itself has been reported as an inhibitor of the phosphatase domain of human and fungal PPIP5Ks10,16,40.
The structure and enzymatic mechanism of the human and fungal PPIP5K kinase domains have been well established27,45,46. However, currently there is no structural information on the PPIP5K phosphatase domain and its enzymatic mechanism and regulation remain to be elucidated. Based on sequence comparisons, the PPIP5K phosphatase domain has been assigned to the histidine acid phosphatase superfamily24, specifically to the branch 2, which includes the well-characterized Escherichia coli and fungal phytases, as well as glucose-1-phosphate and acid phosphatases47. The PPIP5K phosphatase domain contains the catalytic RHxxR motif, which is conserved among histidine acid phosphatases24. Histidine acid phosphatases catalyze the hydrolysis of phosphomonoesters via a two-step mechanism. First, the conserved histidine acts as a nucleophile, forming a covalent phospho-histidine intermediate. In a second step, a water molecule hydrolyzes the intermediate, releasing free inorganic phosphate48. However, the lack of sequence conservation in the substrate-binding domains within the superfamily precludes predictions about substrate recognition and specificity. A “cryptic” pleckstrin homology (PH) domain located near the conserved RHxxR motif in PPIP5Ks has been hypothesized to play a critical role in substrate binding26. PH domains, typically found in signaling proteins, bind phospholipids and molecules derived from phospholipid headgroups, including inositol phosphates and PP-InsPs49.
Here, we report the structure of a PPIP5K phosphatase domain and characterize its substrate binding and enzymatic mechanisms, its regulation by nutrients and its structural organization within the full-length enzyme.
Results
Overall structure of the ScVip1 kinase and phosphatase domains
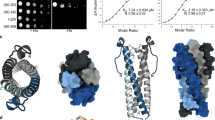
We first attempted to characterize the structure and catalytic mechanism of the histidine acid phosphatase domain (PD) from plant PPIP5Ks10,31,32. However, we were unable to express sufficient amounts of the isolated phosphatase domains from either Arabidopsis thaliana VIH1/VIH2 or from Marchantia polymorpha VIP134 (Supplementary Fig. 1). Therefore, we expressed and purified the phosphatase domains from S. cerevisiae Vip1 (ScVip1PD, residues 536–1107) as well as its N-terminal kinase domain (ScVip1KD, residues 186–522) in baculovirus-infected insect cells (Fig. 1a; see “Methods”).
The structure of the ScVip1KD was solved by molecular replacement to a resolution of ~1.2 Å (Supplementary Table 1). Apo crystals of the ScVip1PD initially diffracted to ~3.1 Å. The structure was solved by molecular replacement-single wavelength anomalous diffraction (MR-SAD) on a platinum derivative (Supplementary Table 1). In this structure, a large disordered loop (residues 851–918) was located and subsequently replaced by a short Gly-Ser-Ser-Gly linker. Crystallization of ScVip1PD Δ848-918 yielded a second crystal form diffracting at 3.4 Å resolution (Supplementary Table 1).
The structure of ScVip1KD in complex with adenosine diphosphate revealed the typical ATP-grasp fold and substrate binding sites previously seen in other PPIP5K kinase structures27,46 (Fig. 1a, b). ScVip1KD shares significant sequence and structural homology with HsPPIP5K2 and with the kinase domain of S. pombe Asp1 (Supplementary Figs. 2, 3). The C-terminal α11 helix of the ScVip1 kinase domain (residues 498–520) is well defined in our structure (Fig. 1b; Supplementary Fig. 2).
a Schematic diagram of the S. cerevisiae Vip1 kinase (orange, ScVip1KD) and phosphatase (blue, ScVip1PD) domains (https://www.uniprot.org/ Uniprot ID Q06685), highlighting structured regions as blocks (residues 186–520 and 536–1094) and putative linker regions as solid lines (residues 1–185, 521–535, and 1095–1146). b Ribbon diagram of the ScVip1KD structure bound to ADP (in bonds representation) and including an omit 2 (Fo–Fc) electron density map contoured at 1 σ (blue mesh). c Ribbon diagram of ScVip1PD Δ848-918, with the histidine acid phosphatase core and α-helical insertion domain shown in light blue, and the PPIP5K-specific GAF domain in dark blue, respectively. The position of the engineered GSSG linker is indicated with a dotted line. The inlet provides a view of the zinc binding site, with the Zn2+ ion shown as a sphere (in gray) and the coordinating histidine and cysteine residues depicted in bonds representation.
The two apo structures of ScVip1PD revealed a conserved histidine acid phosphatase core with two insertion domains (Fig. 1c). The N-terminus of ScVip1PD (residue 536) and the most C-terminal α9 helix (residues 1079–1094) are well defined by electron density (Fig. 1c). Based on these domain boundaries, we hypothesize that the ScVip1 kinase and phosphatase domains are surrounded by large, unstructured N- and C-terminal extensions, and are connected by a flexible ~15 amino acid linker (Fig. 1a; Supplementary Fig. 2, see below). Plant and human PPIP5Ks contain sequence-diverse linkers of similar length between their kinase and phosphatase domains (Supplementary Fig. 2). An unexpected metal binding site occupied by a Zn2+ ion is formed by His651 and His1064 from the histidine acid phosphatase core, and by Cys793 and His836 from the α-helical insertion domain (Fig. 1c; Supplementary Fig. 2; see below).
A PPIP5K unique GAF domain
Next, we performed structural homology searches with ScVip1PD as a search model using the DALI web server (http://ekhidna2.biocenter.helsinki.fi/dali/)50. We found that ScVip1PD shares the core phosphatase domain fold and the α-helical insertion domain with bacterial, plant and human histidine acid phosphatases (Fig. 2a; Supplementary Fig. 4). The relative orientation of the α-helical insertion domain is conserved among histidine acid phosphatases with very different substrate preferences (Fig. 2a), including phytases that hydrolyze InsP6 (Fig. 2b). We located a second insertion domain specific to PPIP5Ks in a position normally occupied by the much smaller β1-insert in other histidine acid phosphatases (Fig. 2a). Analysis with DALI revealed a weak structural homology of this second insertion domain with bacterial and human GAF51 or PAS52 domains involved in light, metabolite and cyclic nucleotide sensing53,54 (Fig. 2c; Supplementary Fig. 4). The GAF domain is conserved among fungal, plant and animal PPIP5Ks (Supplementary Fig. 2). The central β-sheet of the ScVip1PD GAF domain is reminiscent of the inositol phosphate/inositol polyphosphate binding surface previously identified in PH domains, but its topology is different (Fig. 2c)55,56.
a Structural comparison of ScVip1PD Δ848-918 (ribbon diagram, in blue) with the E. coli phytase AppA (https://doi.org/10.2210/pdb7z2s/pdb60, yellow to red), human prostatic acid phosphatase (https://doi.org/10.2210/pdb1nd6/pdb99, light- to dark-green), human liver fructose-2,6-bisphosphatase (https://doi.org/10.2210/pdb1k6m/pdb71, purple and dark gray) and the sensor histidine kinase phosphatase SixA from E. coli (https://doi.org/10.2210/pdb1ujc/pdb100, pink and light gray). b Structural superposition of ScVip1PD Δ848-918 and EcAppA (colors as in panel A, root mean square deviation r.m.s.d. is ~1.1 Å comparing 105 corresponding Cα atoms from the histidine acid phosphatase core). The Zn2+ binding site in ScVip1PD is not conserved among other histidine acid phosphatases. c Structural comparison of the GAF domain in ScVip1PD and structurally related GAF (https://doi.org/10.2210/pdb1f5m/pdb53, r.m.s.d. is ~0.9 Å comparing 26 corresponding Cα atoms in the β-sheet) and inositol polyphosphate-binding PH domains (https://doi.org/10.2210/pdb4y94/pdb56, https://doi.org/10.2210/pdb1unq/pdb55). Note that GAF and PH domains share similar sized β-sheets with different topologies.
ScVip1PD specifically hydrolyses 1,5-InsP8
1,5-InsP8 is the preferred substrate for the isolated ScVip1 phosphatase domain in quantitative nuclear magnetic resonance (NMR) phosphatase assays using 13C-labeled substrates (Fig. 3a; Supplementary Fig. 5). ScVip1PD is a specific inositol pyrophosphate phosphatase, with a high preference for the 1-position of PP-InsPs (Fig. 3a; Supplementary Fig. 5), as previously reported25. In malachite green-based phosphatase assays, we derived similar KM values for 1,5-InsP8 and 1-InsP7, but the catalytic efficiency of ScVip1PD for 1,5-InsP8 is ~18fold higher when compared to 1-InsP7 (Fig. 3b). Notably, 1,5-InsP8 is the reaction product of the N-terminal kinase domain of ScVip124.
a Table summary of the initial enzyme velocities (v0) for ScVip1PD versus 1,5-InsP8, 1-InsP7, or 5-InsP7 derived from an NMR-based phosphatase assay. Pseudo-2D NMR spectra of ScVip1PD incubated with different substrates (blue traces) are shown below, overlaid with the respective reaction products 5-InsP7, or InsP6 (red traces). b Initial enzyme velocities (v0) for ScVip1PD—catalyzed hydrolysis of 1,5-InsP8 and 5-InsP7 as a function of inositol pyrophosphate concentration and using a malachite green-based phosphatase assay. The Michaelis-Menten equation was fitted to the data using non-linear regression. Extracted kinetic parameters are summarized in the table as means (two biological replicates were performed, n = 2). c Structural superposition of the active sites of ScVip1PD Δ848-918 (in bond representation, in dark blue) and EcAppA (in orange, https://doi.org/10.2210/pdb1dkl/pdb, r.m.s.d. is ~1.0 Å comparing 67 corresponding Cα atoms from the histidine acid phosphatase core). In EcAppA, His17 is the catalytic residue, Asp304 is the proton-donor, and Arg16, Arg20, and Arg92 are involved in InsP6 substrate binding. Initial velocities of 1,5-InsP8 hydrolysis catalyzed by wild-type and mutant versions of ScVip1PD, measured by NMR-based (d) or malachite green-based (e) phosphatase assays (n = 2).
A sequence motif central to catalysis in histidine acid phosphatases is located in the active site cleft of ScVip1PD sandwiched between the phosphatase core and the α-helical insertion domain (Fig. 3c). The motif with the consensus sequence Arg-His-x-x-Arg (RHxxR, where x can be any amino acid) is structurally conserved between ScVip1PD and bacterial phytases (Fig. 3c). Mutation of either Arg547, His548 or Arg551 to alanine greatly reduces 1,5-InsP8 hydrolysis in NMR- and malachite green-based enzyme assays, respectively (Fig. 3d, e; Supplementary Fig. 5). This is consistent with the established function of the two arginine residues in InsP6 substrate binding in bacterial phytases57, and the essential role of the nucleophilic acceptor histidine in catalysis48. Asp304 in E. coli phytase is the proton donor involved in the degradation of the phosphohistidine intermediate58. In our ScVip1PD structures, a glutamate residue (Glu991) occupies the position of the phytase proton donor aspartate (Fig. 3c). Mutation of Glu911 to alanine activates the enzyme (Fig. 3d, e; Supplementary Fig. 5, see below). Similarly, deletion of the large disordered loop in the α-helical insertion domain (ScVip1PD Δ848-918) moderately activates the engineered enzyme in vitro (Fig. 3d).
In conclusion, the isolated phosphatase domain of ScVip1 is an inositol 1-pyrophosphate phosphatase with a substrate preference for 1,5-InsP8 and with an active site similar to InsP6 metabolizing phytases.
Inhibition of ScVip1PD by Pi
Gu et al. previously reported that low millimolar concentrations of Pi inhibited the inositol 1-pyrophosphate phosphatase activity of human PPIP5K1 and PPIP5K216. Addition of 10 mM Pi inhibited the phosphatase activity of ScVip110. We found that ScVip1PD is inhibited by Pi in a concentration-dependent manner, as inferred from NMR-based assays (Fig. 4a). As previously reported for SpAsp140, sulfate was less effective than Pi in inhibiting the ScVip1PD phosphatase activity (Fig. 4b). Nitrate had no detectable effect on the hydrolysis of 1,5-InsP8 (Fig. 4c). We conclude that Pi at concentrations found in the yeast cytosol59, directly inhibits the enzymatic activity of the isolated ScVip1 phosphatase domain.
NMR time course experiments for ScVip1PD-catalyzed hydrolysis of 100 µM 1,5-InsP8 to 5-InsP7 in the presence of increasing concentrations of (a) PO43-, (b) SO42-, or (c) at a constant concentration of 25 mM NaNO3. c InsP = 1,5-InsP8 (red line) or 5-InsP7 (green line). The measured initial velocity of 980 nmol min−1 mg−1 is similar to the initial velocity in absence of NaNO3 (1100 nmol min−1 mg−1, compare Fig. 3a).
GAF domain movements control enzyme activity
All four molecules in the asymmetric unit of our ScVip1PD crystals display the GAF domain in an “open” conformation (Fig. 5a; Supplementary Fig. 6; Supplementary Table 1). In ScVip1PD Δ848-918 crystals, two molecules in the asymmetric unit also adopt the “open” conformation (Fig. 5a; Supplementary Fig. 6; Supplementary Table 1). Crystal packing interactions between two adjacent GAF domains in our ScVip1PD Δ848-918 crystals are mediated in part by a second Zn2+ binding site. The Zn2+ ion is coordinated along a pseudo two-fold axis involving residues His573 and Glu575 from the GAF domain (Supplementary Fig. 6). Given that ScVip1PD behaves as a monomer in solution (Supplementary Fig. 7) and that the histidine and glutamate residues are not conserved among other PPIP5Ks (Supplementary Fig. 2), the second Zn2+ coordination site is likely a crystallization artifact (Supplementary Fig. 6).
a Structural superposition of apo ScVip1PD Δ848-918 reveals the GAF insertion domain in either its open (chain A, dark blue ribbon diagram) or closed (chain D, in light blue) conformation. The RHxxR and hinge regions are shown alongside (in bonds representation) b Details of the ScVip1PD Δ848-918 active site: Asp550 stabilizes the catalytic loop by engaging in a salt bridge (green dotted line) with the Zn2+ ion- coordinating His836. The active site and Zn2+ coordinating residues are depicted in bonds representation, the Zn2+ ion is shown as a gray sphere. Initial velocities of 1,5-InsP8 hydrolysis catalyzed by wild-type or mutant version of ScVip1PD, measured by NMR (c) or malachite green (d) phosphatase assays (n = 2). e Table summarizing the thermal melting profiles of wild-type or mutant ScVip1PD. The ΔTm (in °C) represent the difference between the melting temperature of a designated ScVip1PD mutant protein and the wildtype control.
Next, we replaced the catalytic loop residues Arg547, His548 and Arg551 in ScVip1PD Δ848-918 with alanine and obtained crystals in complex with the substrate 1,5-InsP8 diffracting at ~2.4 Å resolution (ScVip1PD Δ848-918 RHR-AAA, Supplementary Table 1). Notably, the GAF domain adopts a “closed” conformation in the substrate-bound structure (Fig. 5a). We hypothesized that the movements of the GAF domain are enabled by a hinge region in close proximity to the catalytic loop (RHxxR motif) of the enzyme (Fig. 5a, b). Consequently, mutation of the hinge residues Pro553, Gly646 and Gly647 (P553V-G646V-G647A) inactivated ScVip1PD in NMR- and malachite green-based phosphatase assays, respectively (Fig. 5b–d). This suggests that movement of the GAF domain is part of the enzymatic cycle of ScVip1PD. Notably, the catalytic loop (Arg547, His548, Arg551) does not appear to be involved in the domain movement when comparing the open and closed conformation structures (Fig. 5a). The conserved Zn2+ binding site in ScVip1PD positions His836 to form a salt bridge with Asp550, apparently stabilizing the catalytic loop (Fig. 5b). Mutation of the Zn2+ binding site residues His651 or Cys793 to alanine reduces the phosphatase activity in our enzyme assays, suggesting that the Zn2+ binding site is involved in the structural stabilization of the enzyme and, importantly, of its catalytic loop (Fig. 5b–e). Consistent with this, mutation of Zn2+ coordinating residues reduces the structural stability of ScVip1PD, whereas excess of ZnCl2 further stabilizes the enzyme in thermal shift assays in vitro (Fig. 5e, Supplementary Fig. 7).
The GAF domain is involved in substrate binding and channeling
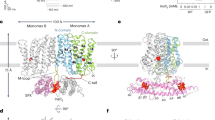
In our ScVip1PD Δ848-918 RHR-AAA—1,5-InsP8 complex structure, we located a crystallographic dimer stabilized by an intermolecular disulfide bridge involving a cysteine (Cys793) that, in apo structures, is part of the zinc-binding site (Supplementary Fig. 8). One molecule of 1,5-InsP8 is bound to each monomer, either on the surface of the enzyme involving the GAF domain or near the catalytic center (Fig. 6a; Supplementary Fig. 8). The GAF domain adopts a closed conformation in both monomers (Fig. 6a). In both binding sites, all phosphate groups of 1,5-InsP8 are well defined by electron density (Fig. 6b), with the 1-β-phosphate facing away from the active site (Fig. 6c). Conserved basic residues from the GAF domain and the α-insertion domain contribute to the formation of the outer substrate binding surface (Fig. 6c). A large network of lysine residues from the GAF domain and from the base of the α-insertion domain forms the second substrate binding surface, which is located in close proximity to the RHxxR catalytic loop (Fig. 6c). Most of the basic residues contributing to the outer and the inner 1,5-InsP8 binding surfaces map to the central β-sheet of the GAF domain (Fig. 6c, d). Comparison of the outer and inner substrate binding sites in ScVip1PD suggests that the 1,5-InsP8 substrate may undergo a ~90° rotation upon entering the active site (Fig. 6d).
a Ribbon diagrams of ScVip1PD Δ848-918 RHR-AAA bound to 1,5-InsP8 (in bonds representation). 1,5-InsP8 binds either at the surface of ScVip1PD Δ848-918 RHR-AAA (chain A, top) or close to the active site (chain B, bottom). b Detailed view of the 1,5-InsP8 molecules shown in two orientations and including a 2 (Fo–Fc) electron density map (blue mesh) contoured at 2.5 σ in chain A (top) or at 1 σ in chain B (bottom), respectively. “1-β-P” and “5-β-P” designate the positions of the β-phosphate of the pyrophosphate group attached to the position 1 or 5 of the myo-inositol ring. c Detailed view of the 1,5-InsP8 binding sites. The residues interacting with 1,5-InsP8 are shown in bonds representation, hydrogen bonds are indicted by dotted lines (in green). d Ribbon diagram of the isolated GAF domain from the ScVip1PD Δ848-918 RHR-AAA structure, depicting the relative positions of the outer (chain A) and inner (chain B, modeled) 1,5-InsP8 substrate (in bonds representation) binding sites.
We next compared the open conformation of apo ScVip1PD with the substrate-bound structures (Fig. 7a). We found that the conformational changes strongly alter the surface charge distribution of the enzyme, with the GAF domain opening and closing on a large and well conserved basic surface area surrounding the substrate binding sites and formed by the GAF and α-insertion domains (Fig. 7a, b). By mapping the positions of the 1,5-InsP8 molecules bound to the outer and inner substrate binding sites, we uncovered a potential substrate binding channel (Fig. 7c). We hypothesize that the 1,5-InsP8 substrate first targets the outer substrate binding site of ScVip1PD in its open conformation. Substrate binding to this outer site may trigger closure of the GAF domain, resulting in rotation of the substrate and its translocation to the active site (Fig. 7c). In its closed conformation, the PPIP5K GAF domain contributes to the formation of a substrate channel that may facilitate transport of 1,5-InsP8 to the catalytic center as well as the exit of the 5-InsP7 product from the enzyme (Fig. 7c).
a Electrostatic surface charge distribution of the apo ScVip1PD Δ848-918 (top) and 1,5-InsP8-bound ScVip1PD Δ848-918 RHR-AAA (bottom) structures (in surface representation). b Evolutionary conservation (from cyan, variable to pink, invariant) of surface residues mapped onto the apo ScVip1PD Δ848-918 (top) and 1,5-InsP8-bound ScVip1PD Δ848-918 RHR-AAA (bottom) structures. Views as in (a). Residue conservation was derived from the multiple sequence alignment shown in Supplementary Fig. 2 and analyzed with Consurf101. c Cutaway front view of the apo ScVip1PD Δ848-918 (shown as molecular surface), with the outer and inner 1,5-InsP8 molecules depicted in bonds representation (top). Same view for the 1,5-InsP8-bound ScVip1PD Δ848-918 RHR-AAA structure (bottom). The closed GAF domain forms the substrate channel. d Groups of mutated residues involved in 1,5-InsP8 channeling (in bonds representation) mapped onto the ScVip1PD Δ848-918 RHR-AAA structure (shown as molecular surface). The active site of ScVip1PD is indicated with a red asterisk. Initial velocities of 1,5-InsP8 hydrolysis for wild-type or mutant versions of ScVip1PD, measured by NMR-based (e) or malachite green-based (f) phosphatase assays (n = 2).
Mutation of highly conserved residues from the outer substrate binding site (Lys558, Lys605, Lys732, Lys817 to alanine) (Fig. 7d) inactivated the enzyme (Fig. 7e, f), suggesting that 1,5-InsP8 is indeed initially bound to the surface of the enzyme before entering the active site. We next mutated residues that contribute to the PPIP5K substrate channel. Mutation of Lys817, Asp819, and Ser821 originating from the α-helical insertion domain (Fig. 7d) moderately reduced the 1,5-InsP8 phosphohydrolase activity of ScVip1PD in both NMR- and malachite green-based assays (Fig. 7e, f). In contrast, mutation of 1,5-InsP8 binding residues from the GAF domain (Glu576, Lys623, Gln625, Lys627) inactivated the enzyme (Fig. 7d–f). Mutations close to the active site of the enzyme (Lys554, Lys557, Lys644) likewise reduced the activity of ScVip1PD (Fig. 7d–f), but in addition structurally destabilized the enzyme (Supplementary Fig. 7). We therefore generated an additional single Lys554 to alanine mutant, which is structurally intact (Supplementary Fig. 7) but which also showed very low enzyme activity in our assays (Fig. 7d–f). Taken together, our structure-function analyses suggest that PPIP5K phosphatase domains contain two 1,5-InsP8 binding sites that contribute to substrate channeling into the active site.
An active site glutamate mediates substrate specificity of ScVip1PD
As described above, the canonical phytase proton donor aspartate is replaced by Glu991 in ScVip1, which adopts different conformations in our apo and substrate-bound crystal structures (Figs. 3c; 8a, b). Replacement of Glu991 with alanine activates ScVip1PD (Fig. 3d, e). In addition, we observed that the ScVip1PD Glu991 to Ala mutant readily accepts 1-InsP7 as a substrate in both NMR and malachite green-based phosphatase assays, but remains unable to hydrolyze InsP6 (Fig. 8c, d). It has been previously reported that mutation of Asp304 in AppA strongly alters the stereospecificity of the E. coli enzyme60. Notably, multiple inositol polyphosphate phosphatases that lack strong stereospecificity for InsP6 are characterized by an alanine residue at this position (Fig. 8a, b)61. We conclude that Glu991, which is conserved among PPIP5Ks from different species (Supplementary Fig. 2) is involved in the selection of the 1,5-InsP8 substrate. The inositol ring occupies a different position in our ScVip1PD—1,5-InsP8 complex structure when compared to known phytases (Fig. 8b). We speculate that this is an adaptation to the hydrolysis of a pyrophosphate substrate. The long and uniquely shaped substrate channel may play an additional role in the selection of 1,5-InsP8 (Fig. 7c).
a Structure-based sequence alignment of the presumed catalytic motifs in ScVip1PD, E. coli AppA, and the multiple inositol polyphosphate phosphatase MINPP from Bacteroides thetaiotaomicron. The RHxxR motif is conserved among all enzymes, while the proton donor aspartate in EcAppA is replaced by Glu991 in ScVip1PD and by an alanine residue in MINPP. b Structural comparison of the ScVip1PD, EcAppA (https://doi.org/10.2210/pdb7z2s/pdb60) and MINPP (https://doi.org/10.2210/pdb4fdu/pdb61) active sites. Glu991 (in bonds representation, colored from cyan to blue) adopts different conformation in our structures. Asp304 controlling substrate stereospecificity in EcAppA60, and Ala324 in the highly promiscuous MINPP enzyme are highlighted in blue. Initial velocities of 1,5-InsP8, 1-InsP7, and 5-InsP7 hydrolysis for wild-type or Glu991Ala versions of ScVip1PD, measured by NMR-based (c) or malachite green-based (d) phosphatase assays (n = 2).
Mutation of key motifs inhibits the function of the VIH2 phosphatase domain in planta
We next sought to functionally characterize the substrate binding and catalytic mechanisms of ScVip1PD in yeast. Previous studies in fission yeast revealed a requirement of the PPIP5K Asp1 in ensuring fidelity of chromosome segregation by increasing microtubule stability35. Like the fission yeast ortholog, ScVip1 is encoded by a non-essential gene in budding yeast24. We analyzed the Vip1 genetic interaction network in numerous Synthetic Genetic Array (SGA) screens to identify mutant backgrounds in which Vip1 function becomes critical62,63. These studies revealed synthetic sick/lethal interactions between Vip1 and components required to maintain chromosome stability62. One such component is Bim164, a conserved +end microtubule binding protein, which in complex with Kar9 forms the cortical microtubule capture site and delays the exit from mitosis when the mitotic spindle is oriented abnormally65. We generated a vip1∆bim1∆ mutant strain that is synthetic sick (Supplementary Fig. 9a) and used it for complementation assays. We found that both expression of wild-type full-length ScVip1 and of a mutant targeting the RHxxR motif (ScVip1R547A-H548A-551A), which impairs the catalytic function of the phosphatase (Fig. 3d, e), fully restored growth to wild-type like levels (Supplementary Fig. 9a). The ScVip1E991A activating mutant (Fig. 3d, e) also behaved similar to wild type, as did a mutant targeting the outer substrate binding site in the phosphatase domain (ScVip1K558A-K605A-K732A) (Supplementary Fig. 9a).
In an alternative approach, we found that overexpression of the isolated kinase domain is lethal for yeast cells grown on low phosphate (Pi) containing media (ScVip1KD in Supplementary Fig. 9b). This effect was not observed when overexpressing either full-length or kinase-dead (ScVip1KD K414A-D487A) versions of the enzyme (Supplementary Fig. 9b). In contrast, overexpression of either wild-type or catalytically inactive versions of ScVip1PD was not lethal when yeast were grown on low Pi-containing media (Supplementary Fig. 9b).
Returning to our original plan to characterize the catalytic mechanism of plant PPIP5K phosphatases (see above), we next mapped all residues and sequence motifs characterized in ScVip1PD to AtVIH2 from Arabidopsis thaliana, which shares 25% sequence identity with ScVip1PD (Fig. 9a; Supplementary Fig. 2). We have previously reported that constitutive expression of a phosphatase-dead variant of full-length AtVIH2 (AtVIH2R372A-H373A) in Col-0 wild-type background reduced rosette size and intracellular Pi levels10. We used these phenotypes to perform structure-function studies with the AtVIH2 phosphatase domain. Constitutive expression of wild-type AtVIH2 had no detectable effect on either rosette area or cellular Pi levels (Fig. 9b–e). Expression of a kinase-dead version of the enzyme (AtVIH2K219A-D292A) resulted in hyperaccumulation of cellular Pi, as previously reported (Fig. 9b–e)10. Three different mutant combinations targeting the catalytic core of the phosphatase domain in Arabidopsis VIH2 (compare Fig. 3c–e) were all associated with significantly reduced rosette areas and cellular Pi levels (highlighted in blue in Fig. 9a–e). Mutations targeting the conserved structural Zn2+ binding site (compare Fig. 5b–e) were also associated with reduced rosette areas and cellular Pi levels (highlighted in purple in Fig. 9a–e). A mutant in the phosphatase hinge region showed less severe growth phenotypes and only slightly reduced Pi levels (shown in red in Fig. 9a–e), whereas the corresponding mutations in ScVip1PD strongly affected the activity and structural stability of the enzyme in vitro (Fig. 5a, c–e). Consistent with the moderate effect of mutations in the α-helical insert contributing to the outer 1,5-InsP8 binding site (Fig. 7d–f), the corresponding AtVIH2R722S-D724A-T726A mutant looks similar to the AtVIH2 wild-type expressing control (Fig. 9a–e). Importantly, mutations targeting either the outer or inner GAF domain binding surfaces (compare Fig. 7d–f) both resulted in a severe reduction in rosette area and in low Pi levels (shown in yellow in Fig. 9a–e). In conclusion, our mutational analysis of the AtVIH2 phosphatase domain confirms the importance of the catalytic center of the enzyme and highlights the critical contributions of the outer and inner 1,5-InsP8 binding sites in the PPIP5K-specific GAF domain (Fig. 9; Supplementary Fig. 10).
a Ribbon diagram of the ScVip1PD Δ848-918 structure, with the histidine phosphatase core in white, the α-helical insert in light gray, and the GAF domain in dark gray. Residues involved in phosphatase catalysis, Zn2+ ion coordination, GAF domain movement, and 1,5-InsP8 binding are shown in bond representation. Corresponding residues in AtVIH2 are also indicated, illustrating the conserved functional regions. b Rosette phenotype of 3 weeks old Col-0 wild-type plants and lines expressing full-length (FL) Flag-tagged versions of AtVIH2 under the control of the ubiquitin-10 (UBI10) promoter. c Quantification of the rosette area. Box plots show 16 plants per genotype (center black line, median; box, interquartile range (IQR); whiskers, lowest/highest data point within 1.5 IQR of the lower/upper quartile; raw data are denoted by a colored dot). Multiple comparisons of the genotypes vs. Col-0 were performed according to a Dunnett test102,103 as implemented in GraphPad prism v10.3.0 (***p < 0.001, **p < 0.005, *p < 0.01) (n = 16). d Quantification of cellular Pi levels. For each genotype, 8 individual plants were measured using 3 technical replicates (n = 8). The estimated Pi concentration was normalized by tissue fresh weight. e Western blot of Flag-tagged AtVIH2. The theoretical molecular mass of AtVIH2 is ~118 kDa (indicated by a black arrow; 1:Col-0, 2:AtVIH2WT,3:AtVIH2K219A-D292A, 4:AtVIH2R372A-H373A, 5:AtVIH2R372A-H373A-R376A, 6:AtVIH2R372A-H373A-R376A-E882A, 7:AtVIH2H741A, 8:AtVIH2C696A, 9:AtVIH2H505A-H741A-H975A, 10:AtVIH2P378V-G500V-G501A, 11:AtVIH2R722S-D724A-T726A, 12:AtVIH2K383A-K447A-K598A-R722A, 13:AtVIH2K379A-K381A-K498A. These experiments were performed twice, compare Fig. S10.
The ScVip1 kinase and phosphatase domains are independent enzymatic modules
We expressed and purified full-length SpAsp1 from E. coli (Fig. 10a), as previously described40, applied the apo enzyme to carbon-coated grids and performed negative-stain electron microscopy (Fig. 10b, see “Methods”). We calculated a low-resolution envelope from two-dimensional projections of single particles and docked the experimental structures of the sequence-related ScVip1 kinase and phosphatase domains (Fig. 10c, Supplementary Fig. 2). Full-length SpAsp1 adopts a L-shaped conformation (Fig. 10c). The N-terminal kinase and the C-terminal phosphatase domains are perpendicular to each other (Fig. 10c). The exact orientation of the kinase domain is somewhat ambiguous but is reasonably constrained by the size of the 16 amino acid linker (Fig. 10c). In our model, the catalytic centers of the kinase and phosphatase modules are far apart, at least in the conformation adopted by the apo enzyme on the electron microscope grid (Fig. 10c). Consistent with this more open conformation of PPIP5K, the inositol 1-pyrophosphate phosphatase activity of ScVip1KD-PD (residues 186–1107) is comparable to that of ScVip1PD (Fig. 10d). Next, we performed hydrogen/deuterium exchange mass spectrometry experiments on ScVip1KD-PD. Addition of 1,5-InsP8 to the apo enzyme protects regions from the α-helical insertion domain and the GAF domain (Fig. 10e; Supplementary Fig. 11), further supporting our substrate binding model for ScVip1PD (Fig. 6c), and the proposed dynamics of the GAF domain (Figs. 5a, 7a–c). Addition of an ATP substrate analog reveals additional protected regions in the vicinity of the active site of the kinase domain (Fig. 10e; Supplementary Fig. 11). No changes consistent with the formation of new kinase – phosphatase domain interfaces are observed upon addition of either the phosphatase or kinase substrate (Fig. 10e; Supplementary Fig. 11). Taken together, these experiments suggest that the PPIP5K kinase and phosphatase modules are enzymatically and structurally independent entities.
a Coomassie blue-stained SDS-PAGE of purified SpAsp1. b A representative micrograph of the negatively stained SpAsp1 used for image processing. 1565 micrographs were collected in total. The scale bar corresponds to 50 nm. c Low resolution envelope of SpAsp1 determined by negative stain electron microscopy (left). Individual crystal structures of ScVip1KD (orange ribbon diagram) and ScVip1PD Δ848-918 (blue ribbon diagram) were docked into the L-shaped negative stain map of SpAsp1 (right). The linkers truncated in the crystal structures of ScVip1 are potentially visible in the SpAsp1 envelope (black dashed lines). d Initial velocities of 1,5-InsP8 hydrolysis catalyzed by ScVip1PD or ScVip1KD-PD, measured by NMR-based phosphatase assays. e Hydrogen-deuterium exchange data mapped on the ScVip1KD-PD model. The protected regions in ScVip1 after incubation with non-hydrolysable 1,5-InsP8 (PCP-IP8) are shown in light green, the protected regions after incubation with non-hydrolysable 1,5-InsP8 (PCP-IP8) and ATP (AMPPNP) analogs, respectively are shown in dark green.
Discussion
PPIP5Ks play a central role in inositol pyrophosphate metabolism and signaling2. The enzyme activity, substrate specificity, 3-dimensional structure and catalytic mechanism of the N-terminal kinase domain of PPIP5K are well established26,27,28,40,46,66, but the C-terminal phosphatase domain has only been partially characterized biochemically25,29,40,41,42, and has resisted structural analysis2. We were able to overcome this problem by focusing on the phosphatase domain of ScVip1, by using a eukaryotic expression system, by fine-mapping the phosphatase domain boundaries and by engineering of a large unstructured loop (see “Methods”, Supplementary Table 1). The structure of ScVip1PD confirms its evolutionary relationship with bacterial and animal histidine acid phosphatases24. We found that the active site of ScVip1PD is very similar to that of InsP6 hydrolyzing phytases, with both enzymes sharing the structurally conserved RHxxR catalytic motif24,47 (Fig. 3c–e). It is noteworthy that ScVip1PD specifically catalyzes the hydrolysis of a phosphoanhydride bond, not of a phosphomonoester as seen with other histdine acid phosphatases (Fig. 3).
A second catalytic histidine (His993 in ScVip1, His807 in SpAsp1) was originally defined by sequence comparison with E. coli AppA23,24, where the corresponding His303 is involved in InsP6 substrate binding and in catalysis48,57. Structural comparisons of EcAppA and ScVip1PD reveal that this motif has been incorrectly assigned. His303 in the bacterial phytase corresponds to a poorly conserved lysine residue (Lys990) in ScVip1 (Supplementary Figs. 11, 2). His993 in ScVip1 or His807 in SpAsp1 are not part of the active site but rather map to an α-helical segment buried in the phosphatase domain (Supplementary Fig. 12). Thus, substitution of SpAsp1 H807 with alanine may disrupt the structural integrity of the enzyme rather than affect its catalytic activity, providing an alternative explanation for the reduced enzyme activities and associated phenotypes reported for this mutant variant35,41,42. Our in vitro (Fig. 3) and in planta (Fig. 9) analyses of the substrate binding and catalytic mechanism of the enzyme now provide a reliable set of mutant combinations for engineering phosphatase-dead PPIP5Ks for genetic rescue experiments in various eukaryotes. Our complementation assays in yeast (Supplementary Fig. 9) and in Arabidopsis10 both suggest that the catalytic activity of the PPIP5K phosphatase domain is dispensable for growth. This is likely due to apparent partial redundancy (biochemical and genetic) of PPIP5K phosphatases with other inositol pyrophosphate phosphatases in yeast67,68 and in plants34.
A conserved aspartate (Asp304) has been previously reported as the proton donor in E. coli phytase58 and mutation of Asp304 strongly alters the substrate stereospecificity of this enzyme60. In ScVip1PD this aspartate is replaced by glutamate (Glu991), which is conserved among PPIP5Ks from different species, and which adopts different conformations in our apo and substrate-bound structures (Fig. 8a, b). The ScVip1E991A mutant exhibits an overall increase in enzyme activity and reduced stereospecificity (Fig. 8c, d). We hypothesize that both Asp304 in E. coli phytase and Glu991 in ScVip1PD influence the size and charge distribution of the substrate binding site, with mutations increasing the processivity of the enzyme at the expense of substrate selectivity.
Mapping previously identified SpAsp1 missense mutations from a genetic suppressor screen onto our ScVip1PD structure revealed that the His686 (His836 in ScVip1PD) to Tyr and Cys643 (Cys793 in ScVip1PD) to Tyr mutants target the structural Zn2+ binding site in PPIP5K (Fig. 1c). This further highlights the contribution of the Zn2+ binding site to the structural integrity of the enzyme69 (Supplementary Fig. 7).
In the case of ScVip1PD, a KM of ~5 μM has been reported for 1-InsP725. Using saturating substrate concentrations, we have derived KM values of ~70 μM and 45 μM for 1-InsP7 and 1,5-InsP8, respectively, and 1,5-InsP8 is the preferred substrate of ScVip1PD in vitro (Fig. 3a, b). For comparison, the PP-InsP phosphatase Siw14 from yeast has a KM for 5-InsP7 of ~10 μM70. The cytosolic concentration of 1,5-InsP8 in S. cerevisiae has been recently estimated at ~0.3 μM5. However, the local concentration in proximity to the N-terminal Vip1 kinase domain may be higher.
Our structures reveal that the GAF domain is a distinctive structural feature of PPIP5K phosphatases (Fig. 2a). In phytases, the β1-insert maps to the position of the GAF domain. It has been previously demonstrated that this β1-insert undergoes conformational changes upon InsP6 binding57 (Fig. 2). Our structures reveal that the PPIP5K GAF domain undergoes much larger conformational changes and plays a pivotal role in substrate binding and in channeling of the substrate to the active site (Figs. 5–7). This is further highlighted by the growth phenotypes of mutant versions of AtVIH2 targeting the GAF domain (Fig. 9). Despite the low degree of sequence conservation, substrate binding, catalysis and regulation appear to be well conserved among PPIP5Ks from different organisms (Fig. 9).
In our 1,5-InsP8 complex structure, one substrate molecule is bound to the outer GAF domain, the second molecule maps close to the active site (Fig. 6a–c). These structures and our mutational analysis define inositol polyphosphates/pyrophosphates as additional ligand candidates for GAF domains53. It is noteworthy that a PP-InsP “capture site” outside the active site has also been identified in the PPIP5K kinase domain45. Orthophosphate is a known inhibitor of human and yeast PPIP5K phosphatase domains, thereby regulating 1,5-InsP8 levels and contributing to phosphate homeostasis10,16,40. We speculate that the inhibitory effect of orthophosphate in ScVip1PD (Fig. 4a) may be caused by the binding of this metabolite to the highly basic substrate channel, where it may block access of the 1,5-InsP8 substrate to the outer substrate binding surface (Fig. 7a, c). We attempted to further characterize this intriguing regulatory mechanism, but ScVip1PD crystals grown in the presence of Pi, sulfate or tungstate did not diffract.
The low-resolution envelope of full-length SpAsp1 and our hydrogen/deuterium exchange mass spectrometry experiments collectively indicate that the PPIP5K kinase and phosphatase domains share only a small interface. The active sites of the kinase and phosphatase domains are situated at a considerable distance from one another, which is incompatible with the hypothesis of direct substrate channeling (Fig. 10c, e). A similar L-shaped arrangement of kinase and phosphatase domains has been previously observed in the crystal structure of fructose-2,6-bisphosphate kinase phosphatase71. Consistent with this very open architecture of the enzyme, the phosphatase activity of full-length ScVip1 is not drastically different from that of isolated ScVip1PD (Fig. 10d). However, this appears not to be the case for the kinase activity in human PPIP5K2, where the isolated kinase domain appeared much more active compared to the full-length enzyme37. The domain boundaries of the PPIP5K kinase and phosphatase domains suggest the presence of large unstructured N- and C-terminal loops, as well as the occurrence of a large disordered linker region within the α-helical insertion domain the phosphatase module (Fig. 1a, c; Supplementary Fig. 2). These sequence-diverse linker regions may represent protein interaction sites37,72 that could alter the architecture and regulation of the full-length enzyme. Moreover, the linkers are found phosphorylated in yeast (Supplementary Fig. 2), suggesting that additional regulatory mechanisms of PPIP5K remain to be elucidated.
Methods
Expression construct cloning
The kinase (residues 186–522, ScVip1KD), phosphatase (residues 536–1107, ScVip1PD), loop-truncated phosphatase (residues 536–1107, Δ848-918, ScVip1PDΔ848-918), and kinase-phosphatase tandem domains (residues 186–1107, ScVip1KD-PD) from Saccharomyces cerevisiae Vip1 were cloned from synthetic genes (Twist Bioscience) codon-optimized for expression in Trichoplusia ni (BTI-Tnao38) cells in vector pBB3, providing tobacco etch virus protease (TEV) cleavable N-terminal 10xHis and twin StrepII affinity tags (Source Data). Point mutations in the ScVip1 phosphatase domain were generated by site-directed mutagenesis and subcloned in pBB3 (Source Data). For crystallization optimization, the ScVip1PD Δ848-918 construct was engineered by replacing the flexible loop with a short Gly-Ser-Ser-Gly linker. The ScVip1PD Δ848-918 construct was further optimized for co-crystallization with 1,5-InsP8 by replacing Arg547, His548, and Arg551 in the catalytic center with alanines. Full-length Asp1 from Schizosaccharomyces pombe was cloned from a synthetic gene codon-optimized for expression in Escherichia coli into the pMH-HSSumo vector, providing N-terminal small ubiquitin-like modifier fusion protein (Sumo) containing 7xHis and StrepII affinity tags, as described40,73.
Protein expression and purification
ScVip1KD, ScVip1PD, ScVip1PD Δ848-918, ScVip1PD Δ848-918 RHR-AAA, ScVip1KD-PD, and their point-mutated versions were expressed in Trichoplusia ni BTI-Tnao38 cells (Boyce Thompson Institute, #160776, Ximbio) using recombinant baculoviruses74. Typically, 10 mL of recombinant P3 baculovirus was used to infect 200 mL of BTI-Tnao38 insect cells growing in SF900 media (Gibco) at a cell density of ~2.0 × 106 cells/mL. The cells were incubated for 72 h at 28 °C with shaking at 110 rpm. Cells were harvested by centrifugation at 1000 × g for 20 min at 4 °C when cell viability was 80–85% and GFP fluorescence indicated >50% infection rate. The cell pellets were flash-frozen in liquid N2 and stored at −80 °C in lysis buffer containing 50 mM Bis-Tris HCl (pH 7.5), 500 mM NaCl, protease inhibitor cocktail tablets (cOmplete EDTA-free; Roche), and DNase I (Roche). For protein purification, cells were thawed and lysed by sonication (Branson Sonifier DS450). The lysate was clarified by ultracentrifugation at 55,000 × g for 60 min at 4 °C and filtered through 0.45 µm (Durapore Merck) filters. The supernatant was loaded onto a Ni2+ affinity column (HisTrap HP 5 mL, Cytiva), washed with 50 mM Bis-Tris HCl (pH 7.5), 500 mM NaCl, 20 mM imidazole, 0.5 mM EDTA, and eluted with wash buffer supplemented with 300 mM imidazole (pH 8.0) directly onto a Strep-Tactin XT 4Flow 5 mL column (IBA). The final elution from the Strep-Tactin column was performed using BXT buffer (IBA) supplemented with NaCl to reach a final concentration of 500 mM. The eluted fractions were incubated with TEV protease for 16 h at 4 °C to remove the 10xHis and twin StrepII affinity tags. The cleaved protein was separated from the tags by a second Ni2+ affinity chromatography step. Further purification was achieved by size-exclusion chromatrography on a HiLoad Superdex 200 16/600 pg column (Cytiva) equilibrated in 20 mM HEPES (pH 7.5) and 150 mM NaCl. The peak fractions were concentrated to 5–15 mg/mL and immediately used for crystallization experiments or flash-frozen in liquid nitrogen and stored at −80 °C for enzymatic assays.
The expression and purification protocol for full-length SpAsp1 was derived from the work of Benjamin et al.40. Protein expression was done in E. coli BL21 (DE3) RIL cells grown in Terrific Broth containing 50 μg/mL kanamycin, 34 μg/mL chloramphenicol, and 1% (v/v) ethanol. When the culture reached an OD600nm of 0.8, protein expression was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 18 °C for 16 h. Cells were harvested by centrifugation and resuspended in a buffer containing 50 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 10% glycerol, supplemented with a complete protease inhibitor cocktail tablet, DNase I, and 0.5 mg/mL lysozyme (Roth). Cell lysis was achieved by sonication. The lysate was then clarified by centrifugation at 46,500 × g for 1 h, and the supernatant was loaded onto a Ni2+ affinity column (HisTrap HP 5 mL, Cytiva) equilibrated in 50 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 10% (v/v) glycerol. After a washing step with the same buffer, SpAsp1 was eluted with buffer supplemented with 250 mM imidazole (pH 8.0). The 7xHis-StrepII-SUMO tag was cleaved by treatment with Ulp1 protease during an overnight incubation at 4 °C75. The tag-free SpAsp1 protein was separated from the fusion tag by a second Ni2+ affinity chromatography step. SpAsp1 was then diluted into 50 mM Tris-HCl (pH 8.0) and 10% (v/v) glycerol to reach a final NaCl concentration of 50 mM and loaded onto a HiTrap Heparin HP 5 mL column (Cytiva) equilibrated with 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, and 10% (v/v) glycerol. SpAsp1 was eluted against a gradient of 50 mM Tris-HCl (pH 8.0), 1 M NaCl, and 10% (v/v) glycerol. Finally, SpAsp1 was applied to a HiLoad Superdex 200 16/600 pg column (Cytiva) equilibrated in 20 mM Hepes (pH 7.5) and 150 mM NaCl. The peak fractions containing SpAsp1 were concentrated and immediately used for negative stain electron microscopy.
Crystallization and data collection
ScVip1KD (8.0 mg/mL in 20 mM Hepes pH 7.5, 150 mM NaCl) was incubated with 5 mM ADP and 20 mM MgCl2 for 1 h on ice prior to crystallization. Crystals developed in hanging drops composed of 1.0 μL of protein solution and 1.0 μL of crystallization buffer (23 % [v/v] PEG 3,350, 0.1 M citric acid/BIS-Tris propane pH 6.4) suspended over 1.0 mL of the latter as reservoir solution. A complete dataset to 1.18 Å resolution was collected at beam line X06DA of the Swiss Light Source, Villigen, Switzerland (Supplementary Table 1). Triclinic crystals of apo ScVip1PD developed in hanging drops composed of 1.5 μL of protein solution (15 mg/mL in 20 mM Hepes pH 7.4, 150 mM NaCl) and 1.5 μL of crystallization buffer (20 % [v/v] PEG 3,350, 0.2 M NH4NO3). Crystals were derivatized for 18 h in crystallization buffer supplemented with 0.1 mM K2PtCl4 and subsequently snap-frozen in liquid N2 after serial transfer in crystallization buffer supplemented with 20 % (v/v) glycerol as cryoprotectant. A single anomalous diffraction (SAD) dataset at the ‘white line’ Pt L3 edge was collected at beam line X06DA (Supplementary Table 1). Native crystals of ScVip1PD developed in 20% v/v (+/-)-2-Methyl-2,4-pentanediol, 0.1 M Tris pH 8.0. Crystals were cryoprotected by addition of glycerol to a final concentration of 20% (v/v) and a complete dataset with apparent orthorhombic symmetry was collected at beam line X06DA to 3.05 Å resolution (Supplementary Table 1). Crystals of the engineered ScVip1PD Δ848-918 construct (10 mg/mL in 20 mM Hepes pH7.5, 150 mM NaCl, 0.5 mM (CH3CO2)2Zn) developed in 2 μL drops containing 10 % (w/v) polyvinylpyrrolidone K15, 5 mM CoCl2, 0.1 M Tris pH 7.0 as crystallization buffer and 0.1 μL additive solution (5 mM 13:0 Lyso PC aka 1-tridecanoyl-2-hydroxy-sn-glycero-3-phosphocholine) using microseeding protocols. Crystals were cryoprotected by serial transfer into crystallization buffer containing glyerol to a final concentration of 30% (v/v) and snap frozen in liquid N2. Data collection at beam line X06DA yielded a complete dataset at 3.4 Å resolution (Supplementary Table 1). The ScVip1PD Δ848-918 RHR-AAA—1,5-InsP8 complex crystallized in sitting drops containing 0.25 μL protein solution (12 mg/mL in 20 mM Hepes 7.5, 150 mM NaCl, 2.5 mM 1,5-InsP8) and 0.25 μL of crystallization buffer (Morpheus II screen condition H2, 32.5% v/v Precipitant Mix 6: 25% w/v PEG 4000, 40% w/v 1,2,6-Hexanetriol; 0.04 M Polyamines: 0.01 M Spermine tetrahydrochloride, 0.01 M Spermidine trihydrochloride, 0.01 M 1,4-Diaminobutane dihydrochloride, 0.01 M DL-Ornithine monohydrochloride; 0.1 M Buffer System 4: pH6.5 MOPSO, Bis-Tris)76. Crystals were cryoprotected by serial transfer in crystallization buffer supplemented with ethylene glycol to a final concentration of 25 % (v/v) and diffracted up to 2.36 Å resolution (Supplementary Table 1). Data processing was done with XDS77 (version June 30, 2023).
Crystallographic structure solution and refinement
The structure of ScVip1KD was solved by molecular replacement method using the program PHASER78 and the isolated kinase domain of HsPPIP5K2 as search model (https://doi.org/10.2210/pdb3t9a/pdb). The solution comprises a monomer in the asymmetric unit. The structure was completed in alternating cycles of manual model building in COOT79 and restrained refinement with anisotropic atomic displacement parameters as implemented in PHENIX.REFINE80. The structure of ScVip1PD was solved by MR-SAD using a low redundancy SAD dataset collected from a K2PtCl2-derivatized crystal and a fragment of the Francisella tularensis histidine acid phosphatase core domain (https://doi.org/10.2210/pdb3it3/pdb) in the program PHASER-EP81. The resulting solution comprised four molecules in the asymmetric unit, four Pt2+ and four Zn2+ ions (Supplementary Table 1). The starting phases and model were used for non-crystallographic symmetry (NCS) density modification in PHENIX.RESOLVE82 and the resulting 3.5 Å electron density map was sufficient to build a nearly complete poly-alanine model that was used to determine the structure of ScVip1PD apo by molecular replacement. ScVip1PD apo crystals had an apparent C 2 2 2 symmetry, however no packing solution could be identified with PHASER78 and analysis with PHENIX.XTRIAGE suggested an abnormal distribution of intensities. The structure was subsequently solved in space group P 1 and input into the program ZANUDA83 for space group validation. ZANUDA returned a solution in space group P 21, with four molecules in the asymmetric unit (Supplementary Table 1). The a and c axes are almost identical, with a twin fraction of ~0.5, similar to previous reports84,85. The structure was completed in alternating cycles of manual model building and restrained NCS refinement with twin operator l, -k, h in PHENIX.REFINE80. A large, apparently disordered loop (residues 851–918) was identified in this structure and subsequently replaced by a short Gly-Ser-Ser-Gly linker. The structures of ScVip1PD Δ848-918 and ScVip1PD Δ848-918 RHR-AAA were solved by molecular replacement using the ScVip1 apo structure as search model and refined with PHENIX.REFINE80 (Supplementary Table 1). The stereochemistry of the refined models was assessed with PHENIX.MOLPROBITY86. Structural diagrams were generated with CHIMERA87.
NMR-based phosphatase assay
ScVip1 phosphatase assays were performed as previously described10. Reactions contained 100 µM of the respective [13C6]-labeled PP-InsP in 20 mM Hepes pH 7.0, 150 mM NaCl, 0.2 mg/mL BSA, and D2O to a total volume of 600 µL. Reactions were supplemented with sodium phosphate, sodium sulfate, or sodium nitrate, as indicated. Reaction mixtures were pre-incubated at 37 °C and the reaction was started by adding the respective amount of enzyme (ScVip1PD (WT): 10 nM for 1-InsP7 and 1,5-InsP8, 700 nM for 5-InsP7; 1.5 µM R547A-H548A-R551A; 1.25 µM R547A; 1.5 µM H548A; 1.5 µM R551A; 10 nM Δ848-918; 1.5 µM K554A; E991A: 2 µM for InsP6, 20 nM for 1-InsP7, 10 nM for 1,5-InsP8; 1 µM P553V-G646V-G647A; 10 nM C793A; 10 nM H651A; 350 nM K558A-K605A-K732A-K817A; 1 µM K554A-K556A-K644A; 10 nM K817S-D819A-S821A; 50 nM E576A-K623A-Q625S-K627A). Enzyme concentrations were varied in order to monitor the biochemical reactions within the detection range and the time frame of our NMR method. Substrate concentrations were kept at 100 µM, which was required to ensure robust detection by NMR. Turnover of PP-InsPs was monitored continuously at 310 K using a nuclear magnetic resonance spectroscopy (NMR) pseudo-2D spin-echo difference experiment88. Individual NMR spectra were recorded on a Bruker AV-III spectrometer (Bruker Biospin) equipped equipped with a cryo-QCI probe operating at 600 MHz for1H and 151 MHz for13C nuclei. Measurements and NMR data analysis were performed using TopSpin 3.5. Individual NMR spectra were recorded every 86 s, with a number of scans per spectrum of 128. Spectral width amounted to 16.663 ppm (13.03 ppm to −3.62 ppm). The relative intensity changes of the C2 peaks of the respective PP-InsPs as a function of reaction time were used for quantification10,88. Data analysis was carried out with GraphPad PRISM 5. Depending on the degree of reaction progress, kinetic parameters were derived from the respective progress curves using two types of equations. When the reactions were at low conversion (<25%), the reactions were considered to be in the initial range, where the progress curves had a linear shape. In these cases, the reaction rates were derived by linear regression in GraphPad PRISM. The slope of the graph representing the reaction product (typically 5-InsP7) corresponded to the non-normalized reaction velocity (unit: µM min-1). This reaction velocity was normalized by dividing the first derivative of the equation by the mass concentration of the enzyme, yielding v0 with the unit nmol mg-1 min-1. If the enzymatic activity and NMR measurement time were sufficient to monitor higher conversions, kinetic parameters were calculated using non-linear regression in GraphPad PRISM. Here, the “one-phase decay “ equation (Y = (Y0 − Plateau)*exp(-K*X) + Plateau) was used to fit the hyperbolically shaped progress curve. The non-normalized reaction velocity was calculated from the first derivative of the equation which corresponds to -K(Y0-Plateau). As for linear procress curves, the parameters corresponding to the hydrolysis product (typically 5-InsP7) were applied to the equation. Again, the non-normalized reaction velocity was subsequently normalized by dividing by the mass concentration of the enyme, yielding v0 with the unit nmol mg−1 min−1. To validate the kinetic parameters calculated with the “one-phase decay “ equation, reaction velocities of hyperbolically shaped progress curves were also subjected to linear regression of the initial linear range, fitted using linear regression, and then normalized by dividing by the respective enzyme mass concentration.
In order to determine the substrate preference of ScVip1PD, reaction mixtures containing 20 mM HEPES pH 7.0, 150 mM NaCl, 0.2 mg/mL BSA and 100 µM of the respective [13C6]-labeled (PP-)InsP were prepared and pre-incubated at 37 °C. ScVip1PD was added subsequently (100 nM for 1-InsP7 and 1,5-InsP8, 1 µM for InsP6 and 5-InsP7) and the reactions were incubated at 37 °C for 3 h. As a control, H2O was added instead of the enzyme. Reactions were quenched by heating to 95 °C for 5 min. The samples were centrifuged at 15,000 × g for 5 min and the supernatant was analyzed by a1H-13C-BIRD-HMQC 2D NMR as described88.
Malachite green-based phosphatase assay
The phosphatase activity of ScVip1PD was determined using the Malachite Green Phosphate Assay Kit (Sigma-Aldrich). This assay detects the release of inorganic phosphate catalyzed by ScVip1PD from substrates such as 1,5-InsP8, 1-InsP7, 5-InsP7, or InsP6 during the phosphatase reaction89,90. For the assay, 100 nM of ScVip1PD was incubated at 37 °C for 2,4, and 8 min in 100 µL reaction mixtures containing 20 mM Hepes (pH 7.5), 150 mM NaCl and varying concentrations of substrates (1,5-InsP8 ranging from 8 µM to 700 µM; 1-InsP7 ranging from 1 µM to 2000 µM, unless indicated otherwise). The reaction was quenched by mixing 40 µL of the reaction mixture with 10 µL of malachite green reagent. The color formation was measured after 25 min on a plate reader (Tecan Spark) at 620 nm. When precipitation of the reagent occurred at high phosphate concentrations (>100 µM), the reaction mixture was 2–8 times diluted in buffer prior quenching. All experiments were performed twice (n = 2). The quantity of released inorganic phosphatase was estimated using the standard curve of OD620 nm versus phosphate standard concentrations. Blanks OD620 nm were measured without enzyme at each time point to discard effect of non-enzymatic hydrolysis of PP-InsPs or the eventual presence of inorganic phosphate in the buffer. The kinetic parameters (v0, Vmax, and KM) were calculated using the linear regression analysis and the Michaelis-Menten model implemented in GraphPad PRISM (version 10.3.0.507).
Thermal shift assay
Thermal shift assays were conducted with 25 to 100 µM of wild-type or mutant ScVip1PD in 20 mM Hepes (pH 7.5), 150 mM NaCl, and 8fold dilution of SYPRO Orange dye (Thermo Fisher Scientific). Protein samples were heated with an increasing gradient of 0.05 °C/s from 25 to 99 °C and melting curves were recorded using a QuantStudio 5 Real-Time PCR System system (Thermo Fisher Scientific).
Analytical size-exclusion chromatography
Gel filtration experiments were performed using a Superdex 200 Increase 10/300 GL column (GE Healthcare) pre-equilibrated in 20 mM Hepes pH 7.5, 150 mM NaCl. 200 μl of the respective protein was loaded sequentially onto the column, and elution at 0.75 ml/min was monitored by ultraviolet absorbance at 280 nm. Peak fractions were analyzed by SDS–PAGE gel electrophoresis.
Yeast strains and plasmids
The Saccharomyces cerevisiae strains used in this study are listed in Source Data. Genomic disruptions at genomic loci were performed as previously reported91. Preparation of media, yeast transformations, and genetic manipulations were performed according to established protocols. The plasmids and primers used for site-directed mutagenesis used in this study are listed in Source Data. All recombinant DNA techniques were performed according to established procedures using Escherichia coli TOP10 cells for cloning and plasmid propagation. Gene mutations were generated with QuickChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). All cloned DNA fragments and mutagenized plasmids were verified by Sanger-sequencing (Microsynth, AG).
Molecular cloning and generation of stable transgenic A. thaliana lines
The Golden Gate system was used to generate plasmids for Arabidopsis transformation92. Mutant versions of the AtVIH2 coding sequence were synthesized (Twist bio-science, San Francisco, CA) (Source Data). The different mutants were then cloned into the level one (L1) vector (Source Data). To construct the binary vector L1 vectors containing the AtUBI10 promoter (proUBI10), the corresponding AtVIH2 coding sequence, a FLAG tag, the Nos terminator, and Fast Red as a fluorescent marker for the seed coat. The plasmids were transformed into A. tumefaciens strain GV3101. The floral dip method was then used to transform 5-week-old plants (Col-0)93. T1 plants were selected by red fluorescence using a Nikon SMZ18 stereomicroscope equipped with an RFP-B filter. Lines with single T-DNA insertions were selected by the segregation analysis. All experiments were performed using stable T3 generation lines (Source Data).
Plant material, seed sterilization, and plant growth conditions
Seeds were sterilized using the chloride gas protocol. Seeds were then sown on ½MS media containing 1% (w/v) sucrose, MES (0.5 g/L), pH 5.7 and agar (8 g/L). 120 mm square Petri dishes were incubated for 48 h at 4 °C in the dark and transferred to a growth chamber at 22 °C with a 16 h/8 h light/dark cycle under a fluorescent light source. 7 d old seedlings were transferred to soil in 8 cm pots and grown as indicated.
Image acquisition and rosette area quantification
To evaluate the rosette phenotypes of the different T3 lines, 16 plants (in 4 pots) were analyzed. After 2 weeks in soil, top images were recorded and the rosette area was manually segmented in Fiji94. Specifically, images were converted to 8 bit followed by manual selection of individual plants. Contrast was adjusted, and the rosette was selected using the threshold. The scale (240 px to 1.5 cm) was set before quantification. Finally, the area of the segmented image was determined using the Particle Quantify function as implemented in Fiji.
Determination of cellular Pi concentration
For Pi quantification 3-weeks-old plants were analyzed (8 independent plants per sample). One cotyledonous leaf per plant was harvested, the fresh weight was recorded and the leaf was transferred to an Eppendorf tube (1.5 ml) containing 500 µl miliQ H2O. The tubes were then frozen in liquid nitrogen and boiled at 80 °C for 5 min. This step was repeated two times. Measurement was performed using a colorimetric molybdate assay89. The master solution contains contained 72 µL of ammonium molybdate solution (0.0044 % [w/v] of ammonium molybdate tetrahydrate, 0.23 % [v/v] of 18 M H2SO4), 16 µL of 10 % (w/v) acetic acid and 12 µL of miliQ H2O. For each reaction, 20 µl of sample were added in triplicate to a 96-well plate and incubated with 100 µl of working solution for 1 h at 37 °C. Absorbance was measured at 820 nm in a Spark plate reader (Tecan). The concentration was determined according to the standard curve with a concentration range of 1, 0.5, 0.2, 0.1, and 0 mM Na2HPO4.
Western blotting
The recombinant expression of 10xHis-tagged AtVIH1PD, AtVIH2PD and MpVIP1PD phosphatase domains in baculovirus-infected BTI-Tnao38 insect cells was assayed by western blotting. Typically, 40 mL of recombinant P2 baculovirus was used to infect 200 mL of BTI-Tnao38 insect cells growing in SF900 media (Gibco) at a cell density of ~1.5 × 106 cells/mL. The cells were incubated for 72 h at 28 °C while shaking at 110 rpm. Cells were harvested by centrifugation at 1000 × g for 20 min at 4 °C when cell viability was 80–85% and GFP fluorescence indicated >50% infection rate. Proteins were extracted from ~4 g of cell pellet, resuspended in 20 mM Hepes pH 7.5, 500 mM NaCl, 3 mM β-mercaptoethanol, supplemented with a cOmplete protease inhibitor cocktail tablet and DNase I. Cells were lysed by sonication and the lysate was clarified by ultracentrifugation at 55,000 × g for 60 min at 4 °C. The pellet, solubilized in 8 M urea, and the supernatant, were transferred in 30 µl of SDS loading buffer and incubated at 95 °C for 5 min. A 10% BIS-Tris acrylamide SDS-PAGE was run with the MES running buffer. Proteins were transferred to a 0.2 µm PVDF membrane (IB34002, Thermo Scientific™) using a semi-dry protocol (iBlot3, Thermo Scientific). Membranes were blotted with TBS-Tween (0.1%) - milk (5%) for 1 h at room temperature. For 10xHis tag detection, membranes were incubated with a Anti-His6-Peroxidase (11 965 085 001 Merck/Sigma-Aldrich), mouse monoclonal antibody (clone BMG-His-1, LOT 16830100) using a dilution of 1:3000. Detection was performed using SuperSignal West Femto Maximum Sensitivity Substrate (34095, Thermo Scientific).
Proteins were extracted from 3-weeks-old plants. About 100 mg of sample were collected in 2 ml Eppendorf tubes and frozen in liquid nitrogen. The tissue was homogenized in a tissue lyzer (MM400, Retsch). Then, 300 µl of 1x PBS buffer supplemented with the plant-specific protease inhibitor cocktail (PE0230, Merck) was added to each tube and incubated for 30 min at 4 °C in an orbital shaker (Intelli-Mixer RM-2M, ELMI). The samples were then centrifuged at 10,000 × g for 20 min at 4 °C. Then, 150 µl of the supernatant was transferred in a new tube containing 30 µl of SDS loading buffer and incubated at 95 °C for 5 min. A 10% BIS-Tris acrylamide SDS-PAGE was run with the MES running buffer. Proteins were transferred to a 0.2 µm PVDF membrane (IB34002, Thermo Scientific) using a semi-dry protocol (iBlot3, Thermo Scientific). Membranes were blotted with TBS-Tween (0.1%) - milk (5%) for 1 h at room temperature. For FLAG detection, membranes were incubated with a monoclonal Anti-FLAG-M2-Peroxidase (HRP) antibody (A8592-5X1MG Merck/Sigma-Aldrich, clone M2, LOT # SLBH1183V) using a dilution of 1:5000. Detection was performed using SuperSignal West Femto Maximum Sensitivity Substrate (34095, Thermo Scientific).
Electron microscopy and image processing
The full-length SpAsp1 envelope was analyzed using negative-stain electron microscopy. A total of 5 μl of monomeric peak fractions from a gel filtration run (concentration 0.01 mg/mL) were applied to glow-discharged, carbon-coated copper grids (400 mesh, Electron Microscopy Sciences). After incubation for 1 min, excess liquid was blotted off. The grids were then sequentially passed through one drop of buffer (20 mM Hepes, pH 7.5, and 150 mM NaCl) and two drops of 2% (w/v) uranyl acetate solution, with the sample incubated in the last drop for 1 min before blotting and air drying. A total of 1,565 micrographs were recorded at the DCI-Geneva (cryoGEnic) electron microscopy platform using a Talos L120C microscope operating at 120 kV and equipped with a Falcon II direct electron detector. Data acquisition was automated using EPU software (Thermo Fisher Scientific) with a pixel size of 1.531 Å and a total electron dose of 52 e-/Ų. Data processing was performed using CryoSPARC (version 4.1)95. Initially, contrast transfer function (CTF) parameters were estimated using patch-based CTF estimation. Micrographs with a CTF fit better than 10 Å resolution were selected, resulting in 1497 micrographs. Manual picking of 177 SpAsp1 particles, which were extracted in boxes of 250 × 250 pixels, were used to train the blob picker tuner. After this process, 239 exposures were rejected due to containing either too few or excessively high numbers of picked particles, leaving a particle stack of 270,221 particles (averaging 220 particles per micrograph) with defocus values between 0.3 and 1.3 µm. Two rounds of reference-free 2D class averaging were employed to further refine the dataset and remove the stain artifacts, resulting in 107,485 particles. An ab initio reconstruction with six classes was followed by 2D classification and non-uniform refinement for each class. The best 3D reconstruction, representing full-length SpAsp1, comprised 11,837 particles.
Hydrogen/deuterium exchange mass spectrometry
HDX-MS experiments were performed at the UniGe Protein Platform (University of Geneva, Switzerland) following an established protocol with minimal modifications96. Details of reaction conditions and all data are presented in Supplementary Fig. 11. HDX reactions were performed in 50 μl volumes with a final protein concentration of 2.1 μM of ScVip1 and a 100-fold molar excess of AMP-PNP and/or PCP-IP897. Briefly, 107 picomoles of protein were pre-incubated with ligands for 1 h on ice in a final volume of 7 μl. The deuterium on-exchange reaction was initiated by adding 43 µl of D2O exchange buffer (10 mM Tris pH 8/150 mM NaCl in D2O) to the protein-ligand mixture. Reactions were carried-out at room temperature for 2 incubation times (30 s, 300 s) and terminated by the sequential addition of 20 μl of ice-cold quench buffer (4 M Gdn-HCl/1 M NaCl/0.1 M NaH2PO4 pH 2.5/1 % (v/v) formic acid). Samples were immediately frozen in liquid nitrogen and stored at −80 °C for up to 2 weeks. All experiments were repeated in triplicate (n = 3). To quantify deuterium uptake into the protein, samples were thawed and injected in a UPLC system immersed in ice with 0.1 % FA as liquid phase. The protein was digested via two immobilized pepsin columns (Thermo Scientific #23131), and peptides were collected onto a VanGuard precolumn trap (Waters). The trap was then eluted, and peptides were separated with a C18, 300 Å, 1.7 μm particle size Fortis Bio 100 × 2.1 mm column over a gradient of 8–30 % buffer C over 20 min at 150 μl/min (buffer B: 0.1 % [v/v] formic acid; buffer C: 100 % acetonitrile). Mass spectra were acquired on an Orbitrap Velos Pro (Thermo), for ions from 400 to 2200 m/z using an electrospray ionization source operated at 300 °C, 5 kV of ion spray voltage. Peptides were identified by data-dependent acquisition of a non-deuterated sample after MS/MS and data were analyzed by Mascot 2.6 using a database composed of purified proteins and known contaminants. Precursor mass tolerance was set to 10 ppm and fragment mass tolerance to 0.6 Da. Protein digestion was set as nonspecific. All peptides analyzed are shown in Supplementary Fig. 11. Deuterium incorporation levels were quantified using HD examiner software version 3.3 (Sierra Analytics), and quality of every peptide was checked manually. Results are presented as percentage of maximal deuteration compared to theoretical maximal deuteration level. Changes in deuteration level between two states were considered significant if >7% and >0.5 Da and p < 0.02 (unpaired t-test) for a single deuteration time.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support this study are available from the corresponding authors upon request. Crystallographic coordinates and associated structure factors have been deposited with the Protein Data Bank (PDB): 9GR8 (ScVip1KD—AD); 9GRH (ScVip1PD—apo); 9GRN (ScVip1PD Δ848-918—apo) and 9GRO (ScVip1PD Δ848-918 RHR-AAA—1,5-InsP8). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository98 with dataset identifier PXD056020. The source data underlying Figs. 3a, b, d, e, 4a–c, 5c–e, 7e, f, 8c, d, 9c, d, 10d, e, and Supplementary Figs. S7c, S10b, c are provided as a Source Data file. Source data are provided with this paper.
References
Shears, S. B. Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling. J. Cell. Physiol. 233, 1897–1912 (2018).
Gu, C., Li, X., Zong, G., Wang, H. & Shears, S. B. IP8: a quantitatively minor inositol pyrophosphate signaling molecule that punches above its weight. Adv. Biol. Regul. 91, 101002 (2024).
Nguyen Trung, M., Furkert, D. & Fiedler, D. Versatile signaling mechanisms of inositol pyrophosphates. Curr. Opin. Chem. Biol. 70, 102177 (2022).
Wild, R. et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352, 986–990 (2016).
Chabert, V. et al. Inositol pyrophosphate dynamics reveals control of the yeast phosphate starvation program through 1,5-IP8 and the SPX domain of Pho81. Elife 12, RP87956 (2023).
Guan, Z. et al. The cytoplasmic synthesis and coupled membrane translocation of eukaryotic polyphosphate by signal-activated VTC complex. Nat. Commun. 14, 718 (2023).
Cordeiro, C. D., Saiardi, A. & Docampo, R. The inositol pyrophosphate synthesis pathway in Trypanosoma brucei is linked to polyphosphate synthesis in acidocalcisomes. Mol. Microbiol. 106, 319–333 (2017).
Couso, I. et al. Synergism between inositol polyphosphates and TOR kinase signaling in nutrient sensing, growth control, and lipid metabolism in Chlamydomonas. Plant Cell 28, 2026–2042 (2016).
Stevenson-Paulik, J., Bastidas, R. J., Chiou, S.-T., Frye, R. A. & York, J. D. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc. Natl Acad. Sci. USA 102, 12612–12617 (2005).
Zhu, J. et al. Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife 8, e43582 (2019).
Dong, J. et al. Inositol pyrophosphate InsP8 acts as an intracellular phosphate signal in Arabidopsis. Mol. Plant https://doi.org/10.1016/j.molp.2019.08.002 (2019).
Ried, M. K. et al. Inositol pyrophosphates promote the interaction of SPX domains with the coiled-coil motif of PHR transcription factors to regulate plant phosphate homeostasis. Nat. Commun. 12, 384 (2021).
Riemer, E. et al. ITPK1 is an InsP6/ADP phosphotransferase that controls phosphate signaling in Arabidopsis. Mol. Plant 14, 1864–1880 (2021).
Zhou, J. et al. Mechanism of phosphate sensing and signaling revealed by rice SPX1-PHR2 complex structure. Nat. Commun. 12, 7040 (2021).
Guan, Z. et al. Mechanistic insights into the regulation of plant phosphate homeostasis by the rice SPX2—PHR2 complex. Nat. Commun. 13, 1581 (2022).
Gu, C. et al. The significance of the bifunctional kinase/phosphatase activities of diphosphoinositol pentakisphosphate kinases (PPIP5Ks) for coupling inositol pyrophosphate cell signaling to cellular phosphate homeostasis. J. Biol. Chem. 292, 4544–4555 (2017).
Li, X. et al. Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling. PNAS 117, 3568–3574 (2020).
Wang, Z. et al. Rapid stimulation of cellular Pi uptake by the inositol pyrophosphate InsP 8 induced by its photothermal release from lipid nanocarriers using a near infra-red light-emitting diode. Chem. Sci. 11, 10265–10278 (2020).
Li, X. et al. Homeostatic coordination of cellular phosphate uptake and efflux requires an organelle-based receptor for the inositol pyrophosphate IP8. Cell Rep. 43, 114316 (2024).
Saiardi, A., Erdjument-Bromage, H., Snowman, A. M., Tempst, P. & Snyder, S. H. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr. Biol. 9, 1323–1326 (1999).
Dubois, E. et al. In Saccharomyces cerevisiae, the inositol polyphosphate kinase activity of Kcs1p is required for resistance to salt stress, cell wall integrity, and vacuolar morphogenesis. J. Biol. Chem. 277, 23755–23763 (2002).
Laha, D. et al. Arabidopsis ITPK1 and ITPK2 have an evolutionarily conserved phytic acid kinase activity. ACS Chem. Biol. 14, 2127–2133 (2019).
Fridy, P. C., Otto, J. C., Dollins, D. E. & York, J. D. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J. Biol. Chem. 282, 30754–30762 (2007).
Mulugu, S. et al. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316, 106–109 (2007).
Dollins, D. E. et al. Vip1 is a kinase and pyrophosphatase switch that regulates inositol diphosphate signaling. Proc. Natl Acad. Sci. USA 117, 9356–9364 (2020).
Gokhale, N. A., Zaremba, A. & Shears, S. B. Receptor-dependent compartmentalization of PPIP5K1, a kinase with a cryptic polyphosphoinositide binding domain. Biochem. J. 434, 415–426 (2011).
Wang, H., Falck, J. R., Hall, T. M. T. & Shears, S. B. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nat. Chem. Biol. 8, 111–116 (2012).
Weaver, J. D., Wang, H. & Shears, S. B. The kinetic properties of a human PPIP5K reveal that its kinase activities are protected against the consequences of a deteriorating cellular bioenergetic environment. Biosci. Rep. 33, e00022 (2013).
Nair, V. S. et al. Inositol pyrophosphate synthesis by diphosphoinositol pentakisphosphate kinase-1 is regulated by phosphatidylinositol(4,5)bisphosphate. Biosci. Rep. 38, BSR20171549 (2018).
Desfougères, Y. et al. The inositol pyrophosphate metabolism of Dictyostelium discoideum does not regulate inorganic polyphosphate (polyP) synthesis. Adv. Biol. Regul. 83, 100835 (2022).
Desai, M. et al. Two inositol hexakisphosphate kinases drive inositol pyrophosphate synthesis in plants. Plant J. 80, 642–653 (2014).
Laha, D. et al. VIH2 regulates the synthesis of inositol pyrophosphate InsP8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell 27, 1082–1097 (2015).
Shukla, A. et al. Wheat inositol pyrophosphate kinase TaVIH2-3B modulates cell-wall composition and drought tolerance in Arabidopsis. BMC Biol. 19, 261 (2021).
Laurent, F. et al. Inositol pyrophosphate catabolism by three families of phosphatases regulates plant growth and development. PLoS Genet 20, e1011468 (2024).
Pascual-Ortiz, M. et al. Asp1 bifunctional activity modulates spindle function via controlling cellular inositol pyrophosphate levels in Schizosaccharomyces pombe. Mol. Cell. Biol. 38, e00047-18 (2018).
Gu, C. et al. KO of 5-InsP7 kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype. Proc. Natl Acad. Sci. USA 114, 11968–11973 (2017).
Yousaf, R. et al. Mutations in Diphosphoinositol-Pentakisphosphate Kinase PPIP5K2 are associated with hearing loss in human and mouse. PLoS Genet. 14, e1007297 (2018).
Gu, C. et al. Metabolic supervision by PPIP5K, an inositol pyrophosphate kinase/phosphatase, controls proliferation of the HCT116 tumor cell line. Proc. Natl Acad. Sci. USA 118, e2020187118 (2021).
Gulabani, H. et al. Arabidopsis inositol polyphosphate kinases IPK1 and ITPK1 modulate crosstalk between SA-dependent immunity and phosphate-starvation responses. Plant Cell Rep. 41, 347–363 (2022).
Benjamin, B. et al. Activities and structure-function analysis of fission yeast inositol pyrophosphate (IPP) kinase-pyrophosphatase Asp1 and its impact on regulation of pho1 gene expression. mBio 13, e01034-22 (2022).
Pöhlmann, J. et al. The Vip1 inositol polyphosphate kinase family regulates polarized growth and modulates the microtubule cytoskeleton in fungi. PLoS Genet. 10, e1004586 (2014).
Wang, H. et al. Asp1 from Schizosaccharomyces pombe binds a [2Fe-2S]2+ cluster which inhibits inositol pyrophosphate 1-phosphatase activity. Biochemistry 54, 6462–6474 (2015).
Gu, C., Wilson, M. S. C., Jessen, H. J., Saiardi, A. & Shears, S. B. Inositol pyrophosphate profiling of two HCT116 cell lines uncovers variation in InsP8 levels. PLoS ONE 11, e0165286 (2016).
Qiu, D. et al. Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry. Nat. Commun. 11, 6035 (2020).
Wang, H. et al. Synthetic inositol phosphate analogs reveal that PPIP5K2 has a surface-mounted substrate capture site that is a target for drug discovery. Chem. Biol. 21, 689–699 (2014).
Benjamin, B. et al. Structures of fission yeast inositol pyrophosphate kinase Asp1 in ligand-free, substrate-bound, and product-bound states. mBio 13, e03087–22 (2022).
Rigden, D. J. The histidine phosphatase superfamily: structure and function. Biochem. J. 409, 333–348 (2008).
Ostanin, K. et al. Overexpression, site-directed mutagenesis, and mechanism of Escherichia coli acid phosphatase. J. Biol. Chem. 267, 22830–22836 (1992).
Scheffzek, K. & Welti, S. Pleckstrin homology (PH) like domains—versatile modules in protein–protein interaction platforms. FEBS Lett. 586, 2662–2673 (2012).
Holm, L. & Rosenström, P. Dali server: conservation mapping in 3D. Nucl. Acids Res. 38, W545–W549 (2010).
Aravind, L. & Ponting, C. P. The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 22, 458–459 (1997).
Ponting, C. P. & Aravind, L. PAS: a multifunctional domain family comes to light. Curr. Biol. 7, R674–R677 (1997).
Ho, Y. J., Burden, L. M. & Hurley, J. H. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J. 19, 5288–5299 (2000).
Henry, J. T. & Crosson, S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 65, 261–286 (2011).
Milburn, C. C. et al. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 375, 531–538 (2003).
Wang, Q. et al. Autoinhibition of Bruton’s tyrosine kinase (Btk) and activation by soluble inositol hexakisphosphate. Elife 4, e06074 (2015).
Lim, D., Golovan, S., Forsberg, C. W. & Jia, Z. Crystal structures of Escherichia coli phytase and its complex with phytate. Nat. Struct. Biol. 7, 108–113 (2000).
Ostanin, K. & Van Etten, R. L. Asp304 of Escherichia coli acid phosphatase is involved in leaving group protonation. J. Biol. Chem. 268, 20778–20784 (1993).
Auesukaree, C. et al. Intracellular phosphate serves as a signal for the regulation of the PHO pathway in Saccharomyces cerevisiae. J. Biol. Chem. 279, 17289–17294 (2004).
Acquistapace, I. M. et al. Insights to the structural basis for the stereospecificity of the Escherichia coli Phytase, AppA. IJMS 23, 6346 (2022).
Stentz, R. et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 6, 646–656 (2014).
Pan, X. et al. A robust toolkit for functional profiling of the yeast genome. Mol. Cell 16, 487–496 (2004).
Masinas, M. P. D., Usaj, M. M., Usaj, M., Boone, C. & Andrews, B. J. TheCellVision.org: a database for visualizing and mining high-content cell imaging projects. G3 10, 3969–3976 (2020).
Schwartz, K., Richards, K. & Botstein, D. BIM1 encodes a microtubule-binding protein in yeast. Mol. Biol. Cell 8, 2677–2691 (1997).
Manatschal, C. et al. Molecular basis of Kar9-Bim1 complex function during mating and spindle positioning. Mol. Biol. Cell 27, 3729–3745 (2016).
An, Y., Jessen, H. J., Wang, H., Shears, S. B. & Kireev, D. Dynamics of substrate processing by PPIP5K2, a versatile catalytic machine. Structure 27, 1022–1028.e2 (2019).
Sanchez, A. M., Schwer, B., Jork, N., Jessen, H. J. & Shuman, S. Activities, substrate specificity, and genetic interactions of fission yeast Siw14, a cysteinyl-phosphatase-type inositol pyrophosphatase. mBio 14, e02056–23 (2023).
Ghosh, S. et al. Activities and genetic interactions of fission yeast Aps1, a Nudix-type inositol pyrophosphatase and inorganic polyphosphatase. mBio 15, e0108424 (2024).
Garg, A., Shuman, S. & Schwer, B. A genetic screen for suppressors of hyper-repression of the fission yeast PHO regulon by Pol2 CTD mutation T4A implicates inositol 1-pyrophosphates as agonists of precocious lncRNA transcription termination. Nucleic Acids Res. 48, 10739–10752 (2020).
Wang, H., Gu, C., Rolfes, R. J., Jessen, H. J. & Shears, S. B. Structural and biochemical characterization of Siw14: a protein-tyrosine phosphatase fold that metabolizes inositol pyrophosphates. J. Biol. Chem. 293, 6905–6914 (2018).
Lee, Y.-H., Li, Y., Uyeda, K. & Hasemann, C. A. Tissue-specific structure/function differentiation of the liver isoform of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. J. Biol. Chem. 278, 523–530 (2003).
Machkalyan, G., Trieu, P., Pétrin, D., Hébert, T. E. & Miller, G. J. PPIP5K1 interacts with the exocyst complex through a C-terminal intrinsically disordered domain and regulates cell motility. Cell. Signal. 28, 401–411 (2016).
Hothorn, M., Dabi, T. & Chory, J. Structural basis for cytokinin recognition by Arabidopsis thaliana histidine kinase 4. Nat. Chem. Biol. 7, 766–768 (2011).
Hashimoto, Y., Zhang, S. & Blissard, G. W. Ao38, a new cell line from eggs of the black witch moth, Ascalapha odorata (Lepidoptera: Noctuidae), is permissive for AcMNPV infection and produces high levels of recombinant proteins. BMC Biotechnol. 10, 50 (2010).
Malakhov, M. P. et al. SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J. Struct. Funct. Genom. 5, 75–86 (2004).
Gorrec, F. The MORPHEUS protein crystallization screen. J. Appl Crystallogr 42, 1035–1042 (2009).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry land cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Cryst. D. 60, 2126–2132 (2004).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Cryst. D. 68, 352–367 (2012).
Read, R. J. & McCoy, A. J. Using SAD data in Phaser. Acta Crystallogr D. Biol. Crystallogr 67, 338–344 (2011).
Terwilliger, T. C. SOLVE and RESOLVE: automated structure solution and density modification. Meth. Enzymol. 374, 22–37 (2003).
Lebedev, A. A. & Isupov, M. N. Space-group and origin ambiguity in macromolecular structures with pseudo-symmetry and its treatment with the program Zanuda. Acta Cryst. D. 70, 2430–2443 (2014).
Ban, N. et al. Placement of protein and RNA structures into a 5 A-resolution map of the 50S ribosomal subunit. Nature 400, 841–847 (1999).
Wittmann, J. G. & Rudolph, M. G. Pseudo-merohedral twinning in monoclinic crystals of human orotidine-5’-monophosphate decarboxylase. Acta Crystallogr D. Biol. Crystallogr 63, 744–749 (2007).
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Harmel, R. K. et al. Harnessing 13C-labeled myo-inositol to interrogate inositol phosphate messengers by NMR. Chem. Sci. 10, 5267–5274 (2019).
Ames, B. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8, 115–118 (1966).
Baykov, A. A., Evtushenko, O. A. & Avaeva, S. M. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171, 266–270 (1988).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Binder, A. et al. A modular plasmid assembly kit for multigene expression, gene silencing and silencing rescue in plants. PLoS ONE 9, e88218 (2014).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Wang, H. et al. Autoregulation of class II alpha PI3K activity by its lipid-binding PX-C2 domain module. Mol. Cell 71, 343–351.e4 (2018).
Hostachy, S. et al. Dissecting the activation of insulin degrading enzyme by inositol pyrophosphates and their bisphosphonate analogs. Chem. Sci. 12, 10696–10702 (2021).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Ortlund, E., LaCount, M. W. & Lebioda, L. Crystal structures of human prostatic acid phosphatase in complex with a phosphate ion and α-benzylaminobenzylphosphonic acid update the mechanistic picture and offer new insights into inhibitor design. Biochemistry 42, 383–389 (2003).
Hamada, K. et al. Crystal structure of the protein histidine phosphatase SixA in the multistep His‐Asp phosphorelay. Genes Cells 10, 1–11 (2005).
Landau, M. et al. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 33, W299–W302 (2005).
Dunnett, C. W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50, 1096–1121 (1955).
Varadi, M. et al. AlphaFold Protein Structure Database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 52, D368–D375 (2024).
Acknowledgements
We thank P. Rieu and A. Caregnato for critical reading of the manuscript. This work was supported by Sinergia grant CRSII5_209412 from the Swiss National Science Foundation (to D.F., V.G.P., and M.H.) and by a Howard Hughes Medical Institute International Research Scholar Award 55008733 (to M.H.).
Author information
Authors and Affiliations
Contributions
P.R. and K.L. expressed and purified proteins, K.L. and P.R. crystallized proteins and solved and refined structures with the help of M.H. P.R. and S.M.B. performed and analyzed enzyme assays with the help of D.F. D.F. contributed PP-InsP reagents. P.R. performed thermal shift assays and size-exclusion chromatography experiments. D.P-C. and V.G.P. designed and performed yeast experiments. F.R. performed plant genetic experiments, Pi measurements and western blotting. P.R. performed and analyzed negative stain electron microscopy experiments. K.L. and O.V. performed and analyzed HDX-MS experiments. P.R. and M.H. wrote the manuscript. All authors edited the manuscript and approved the final document.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raia, P., Lee, K., Bartsch, S.M. et al. A small signaling domain controls PPIP5K phosphatase activity in phosphate homeostasis. Nat Commun 16, 1753 (2025). https://doi.org/10.1038/s41467-025-56937-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56937-0