Abstract
Improvement of cold tolerance at the booting stage (CTB) in rice is a key strategy for cultivation in high-altitude and high-latitude regions. Here, we identify CTB3 gene, encoding a calmodulin-binding transcriptional activator that positively regulates cold tolerance at the booting stage in japonica rice. Two indels (57-bp and 284-bp) in the CTB3 promoter confer a differential transcriptional response to cold between the japonica and indica subspecies. OsTCP19 suppresses CTB3 expression by binding to these indels, negatively regulating cold tolerance. CTB3 activates the expression of TREHALOSE-6-PHOSPHATE PHOSPHATASE1 (OsTPP1), reducing trehalose 6-phosphate (Tre6P) levels, which increases sugar accumulation in panicles and improves cold tolerance. Additionally, favorable alleles of OsTCP19 and CTB3 are selected in japonica rice for cold adaptation. These findings highlight the important role of CTB3 in cold adaptation and its potential for improving cold tolerance in rice breeding.
Similar content being viewed by others
Introduction
Rice (Oryza sativa L.) is a major food crop with tropical origins and is sensitive to low temperatures. Temperate rice of the japonica subspecies is grown in a wider geographic range than indica and tropical japonica rice and can be grown in colder regions at higher altitudes and latitudes. In contrast, indica and tropical japonica rice are better suited for cultivation in regions with more abundant light and higher temperatures1,2. Considering the increased frequency of extreme weather events on a global scale, low-temperature stress is becoming a serious threat to rice production. In China, an estimated 3–5 million tons of rice production is lost annually due to low temperatures in the autumn3. From 1999 to 2012, rice production in China decreased by about 3.7% due to extreme cold4. Cold injury at the booting stage leads to spikelet sterility, a lower seed setting, and consequently a direct decrease in yield, resulting in irreparable losses5,6. Therefore, identifying new genes that confer cold tolerance at the booting stage would be beneficial for rice production.
Cold tolerance is a complex trait controlled by multiple genes in rice, with different genes playing roles at various developmental stages. At the early stage of germination, favorable alleles of genes such as qLTG3-1, OsLTPL159 and OsUBC12 promote rice germination and growth under low-temperature7,8,9. For instance, OsUBC12 encodes an E2 ubiquitin-conjugating enzyme, increase low temperature germinability in japonica rice by inhibiting ABA signaling9. At the seedling stage, genes such as LTG1, COLD1, qCTS9, bZIP73, HAN1, COLD11, COG1, COG2 and COG3 have been identified through linkage analysis or association studies10,11,12,13,14,15,16,17,18. For example, COLD1 encodes a G-protein signaling regulator that interacts with the G-protein α subunit RGA1 to perceive cold signals, activating Ca2+ channels to initiate cold-specific signaling10. COG1 encodes a receptor-like protein that, together with its co-receptor kinase OsSERL2, senses low temperatures and activates downstream defense responses through the MAPK cascade11. Other genes like LTG1, qCTS9, HAN1 and bZIP73 modulate cold tolerance through pathways involving auxin, brassinosteroid (BR) signaling, jasmonic acid (JA) and abscisic acid (ABA)12,13,14,15. COLD11, COG2 and COG3 are involved in rice responses to cold stress through distinct pathways, including DNA repair, cell wall remodeling and maintenance of photosynthesis, respectively16,17,18.
Although the studies on cold tolerance at the early stages have provided valuable insights, understanding the molecular mechanisms of cold tolerance at the booting stage is crucial due to its direct impact on grain yield. However, fewer genes for cold tolerance at the booting stage have been identified. bZIP73 involves in the bZIP73Jap–qLTG3-1Nip–sugar transport pathway, which promotes sugar transport to pollen at low temperatures19. CTB4a encodes a leucine-rich repeat receptor-like kinase that interacts with AtpB to increase energy supply, thereby improving the seed setting in rice grown at high altitudes20. CTB2 encodes a UDP-glucose sterol glucosyltransferase that mediates the accumulation of steryl glycoside and acyl steryl glycoside to maintain cell membrane permeability and reduce damage to pollen grains and pollen walls under cold stress conditions21. Other genes, such as Ctb1 (an F-box protein)22, qCTB7 (a PHD-finger domain protein)23 and OsLEA9 (a late embryogenesis abundant protein)24, have been implicated in cold tolerance, although their precise molecular roles remain to be clarified.
The calmodulin-binding transcriptional activator (CAMTA) family of transcription factors plays important roles in plant response to biotic and abiotic stresses. In Arabidopsis, AtSR1 (AtCAMTA3), AtCAMTA1 and AtCAMTA2 negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by suppressing the expression of AtSARD1 and AtCBP60g, which in turn suppresses plant immunity25. AtSR1, AtCAMTA1 and AtCAMTA2 along with AtCAMTA5 positively regulate freezing tolerance in Arabidopsis and can respond to low temperatures and activate the expression of AtDREB1 (CBF) genes and other cold-inducible genes26. AtSR1 activates several RNA interference (RNAi)-related genes for defense against viral invasion27. AtSR1 also functions as a transcriptional activator in drought stress and positively regulates drought tolerance28. Furthermore, CAMTA6 negatively regulate salt tolerance29. In rice, the CAMTA transcription factor OsCBT1 and OsCAMTA3 also negatively regulates plant immune responses to pathogens30,31, whereas the rice CAMTAs SCT1 and SCT2 synergistically repress wax synthesis regulatory 2 (OsWR2) expression and negatively regulate heat tolerance32. However, whether CAMTA family transcription factors play a role in rice cold acclimatization remains unknown.
In this work, we identify the CTB3 gene, which encodes a calmodulin-binding transcription factor controlling cold tolerance at the booting stage. The allele of CTB3 with the 57-bp and 284-bp deletions is derived from Chinese wild rice (O. rufipogon) and promotes the cold adaptation of japonica rice. OsTCP19 suppresses CTB3 expression by binding to the two indel regions and thereby negatively regulates cold tolerance at the booting stage. Furthermore, CTB3 activates the expression of OsTPP1, leading to reduced Tre6P content. The lower Tre6P content promotes the expression of sugar-transporter-related genes, which increase the accumulation of sugar in the panicles, thereby enhancing cold tolerance. Our results show that the two favorable alleles of OsTCP19 and CTB3 have been selected and retained in japonica rice to improve cold tolerance and promote the expansion of rice to high-latitude regions. These findings highlight the role of CTB3 in promoting cold adaptation and offer a potential target for improving cold tolerance in rice breeding.
Results
CTB3 positively regulates cold tolerance at the booting stage in rice
In our previous study, we discovered a locus related to cold tolerance at the booting stage, qCTB1t, by genome-wide association study and identified Os01g0923600 and Os01g0923800 as the major candidate genes2. To clarify the target gene, we obtained single knockout mutants of Os01g0923600 and Os01g0923800 in cold-tolerant Nipponbare (Nip) through the CRISPR/Cas9 approach (Supplementary Fig. 1a, b, 2a, b). We used three methods to assess the cold tolerance at the booting stage in rice, and the seed setting rates of the three main panicles were used to assess the cold tolerance of the plants (Supplementary Fig. 3). When grown under cold stress conditions in high-altitude area (CS-HAA), the knockout mutants of Os01g0923800 showed no difference from Nip (Supplementary Fig. 1c, d), while the knockout mutants of Os01g0923600 had lower seed setting rates than Nip (Supplementary Fig. 2c, d). We postulated that Os01g0923600 is the functional gene of the cold tolerance locus qCTB1t and named this gene as CTB3.
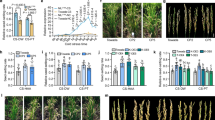
To identify the natural variations of CTB3, we performed haplotype analysis (Supplementary Data 1). Based on a phenotypic evaluation, CTB3-Hap1 displayed a higher seed setting rate than CTB3-Hap2 under CS-HAA conditions, indicating that CTB3-Hap1 is a cold-tolerant haplotype at the booting stage. The accessions containing CTB3-Hap1 mainly belong to the japonica subspecies, while those containing CTB3-Hap2 mainly belong to the indica subspecies (Fig. 1a, b). To confirm that CTB3 is involved in cold tolerance, we introduced a complementary construct containing a 2.5-kb promoter fragment fused with the coding region of CTB3 from Nip (CTB3-Hap1) into cold-sensitive Teqing (TQ, CTB3-Hap2) (Supplementary Fig. 2e, f). The two complementary lines (C1 and C2) showed higher seed setting rates than TQ under CS-HAA, under cold stress in a phytotron (CS-PT) and under cold stress in deep water (CS-DW) conditions (Fig. 1c, d). Under normal conditions, there was no significant difference in the seed setting rate of C1 and C2 and wild type TQ (Supplementary Fig. 2 h).
a Haplotypes analysis of CTB3. b Seed setting rates of the different haplotypes grown under CS-HAA conditions (n = 145/43/125 accessions). For box plots, boxes represent the interquartile range (25th to 75th percentiles), center lines indicate medians, and whiskers extend to the minima and maxima. c Panicles of the Teqing (TQ) and complementary lines under CS-PT conditions, Scale bar is 2 cm. d Statistical results for seed setting rates of TQ, C1, C2 under cold stress conditions. Data represent means ± SD (n = 15 plants). e, f Phenotype of plants and panicles of the CTB3-knockout and -overexpression lines grown under CS-HAA conditions. Scale bar is 2 cm. g Statistical results for seed setting rates of Nip, CTB3-cr1, CTB3-cr2 and CTB3-overexpression lines grown under cold stress conditions. Data represent means ± SD (n = 15 plants). h Pollen fertility evaluated by I2-KI dyeing. Scale bar is 100 μm. i Statistical results of pollen fertility. Data represent means ± SD (n = 10 plants). NC, Normal conditions. CS-DW, cold stress in deep water. CS-HAA, cold stress in high-altitude area. CS-PT, cold stress in a phytotron with 16–17 °C. Nip, Nipponbare. TQ, Teqing. In (b, d, g, i), Different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. Source data are provided as a Source Data file.
To further investigate the function of CTB3, we overexpressed CTB3 in Nip using the coding sequences (CDS) amplified from temperate japonica accession, Nip, and cold-sensitive indica accession, 9311 (Supplementary Fig. 1e, g). The CTB3-overexpression (OE) lines showed higher seed setting rates than Nip under all three cold stress conditions (Fig. 1e–g), and there was no significant difference in the seed setting rate between CTB3Hap1-OE and CTB3Hap2-OE lines. Under normal conditions, there was no significant difference in the seed setting rate of CTB3 knockout mutants, CTB3Hap1-OE, CTB3Hap2-OE lines and Nip (Supplementary Fig. 2i). These results indicate that CTB3 positively regulates cold tolerance at the booting stage. In addition, we evaluated the cold tolerance of CTB3 transgenic lines at the seedling stage. The results suggest that CTB3 also regulates cold tolerance at the seedling stage (Supplementary Fig. 4a, b).
To clarify whether CTB3 affects the development of anthers or pistils, we observed the pistils and anthers of Nip, CTB3-cr1 and CTB3Hap1-OE1 plants under normal and CS-PT conditions. The pistils showed no obvious morphological changes under the CS-PT condition, but the anthers of the knockout mutant were deformed and crumpled, and the number of mature pollens was decreased (Supplementary Fig. 5a). In contrast, the damage to anthers in CTB3Hap1-OE1 was less than that in Nip. Meanwhile, the number of normal anther chambers was significantly lower in the mutant than in Nip, while the number of normal anther chambers was significantly higher in the overexpression line than in Nip (Supplementary Fig. 5b, c). Furthermore, the knockout mutant showed lower pollen fertility, and the overexpression line showed higher pollen fertility, compared to Nip, while there were no significant differences in pollen fertility under normal conditions (Fig. 1h, i). Based on these observations, we hypothesized that CTB3 promotes cold tolerance at the booting stage by affecting the development of anthers and pollen.
Expression pattern and subcellular localization of CTB3
Tissue expression analysis showed that CTB3 is expressed in all tissues, including node, stem, leaf, sheath, root and panicle at different stages (Supplementary Fig. 6a). CTB3 expression was rapidly induced after cold treatment, reaching a peak at 1 day (Supplementary Fig. 6b). A subcellular localization assay showed that CTB3 is localized in the nucleus (Supplementary Fig. 6c). Yeast two-hybrid experiments and luciferase assays showed that CTB3 has self-activating activity (Supplementary Fig. 7a, b). These results are consistent with CTB3 function as a transcriptional activator. Transcriptional activation experiments in yeast cells suggested that the transcriptional activation domain of CTB3 is located within 136–306 aa at the N terminus, downstream of the CG-1 DNA-binding domain (Supplementary Fig. 7c).
Two indels in the CTB3 promoter confer cold tolerance
To analyze the variation of CTB3, we selected Nip and 9311 for sequencing and identified several single nucleotide polymorphisms (SNPs) and insertions/deletions (indels) in the CTB3 sequence (Supplementary Data 2). We characterized the indel variations in 525 rice germplasm resources and found that 99.45% of the indica rice accessions contain 57-bp and 284-bp sequences in the CTB3 promoter region that are missing from 87.1% of the japonica rice accessions. Notably, 130 out of 138 temperate japonica rice accessions (94.2%) do not contain the 57-bp and 284-bp sequences in the CTB3 promoter (Supplementary Fig. 8).
To clarify the functional significance of these variants, we performed candidate gene-based association analysis and found that the 57-bp and 284-bp indels were strongly associated with cold tolerance at the booting stage (Fig. 2a). To elucidate the function of the two indel variants, we mutated these two regions in CTB3-Hap2, which normally contains the 57-bp and 284-bp sequences, to delete one or both sequences. The transient expression assays demonstrated that deleting either the 57-bp or the 284-bp sequences alone increased the promoter activity. Simultaneously removing both sequences from the CTB3-Hap2 promoter, the activity of CTB3-Hap2 (-∆57/-∆284 bp) significantly rose to match the level observed in the promoter of CTB3-Hap1 (Fig. 2b). We also compared the expression levels of germplasm resources containing different haplotypes. The cold-induced expression of CTB3 in the CTB3-Hap1 accessions was significantly higher than that in the CTB3-Hap2 accessions (Fig. 2c), which is consistent with their differences in cold tolerance at the booting stage (Fig. 2d). We further constructed a near-isogenic line of CTB3 (NILCTB3-Hap1) in the background of a cold-sensitive indica variety, Deyou17 (DY17, CTB3-Hap2). The NILCTB3-Hap1 lines carried the CTB3-Hap1 allele from the temperate japonica variety, Lijiangxiaoheigu (LJXHG). Compared with DY17, the cold-induced expression of CTB3 was higher in the NILCTB3-Hap1 plants (Fig. 2e).
a Candidate gene based association analysis of CTB3 using 178 rice accessions. b Transient dual-luciferase expression assay in rice protoplasts. CTB3 promoter fragments were cloned from Nip (Hap1) or 9311 (Hap2). Data are mean ± SD (n = 3 biological replicates). c Relative expression levels of ten CTB3-Hap1 accessions and ten CTB3-Hap2 accessions under CS-PT at 1 day. The expression levels of CTB3 in different accessions at 0 h were set as 1. Data are mean ± SD (n = 3 biological replicates). d Seed setting rates of the different haplotypes grown under CS-HAA conditions (n = 5 plants). For box plots, boxes represent the interquartile range (25th to 75th percentiles), center lines indicate medians, and whiskers extend to the minima and maxima. e Cold induced expression analysis of CTB3 at the booting stage in panicles of NILCBT3-Hap1 and DY17 under CS-PT conditions at different times. Data are means ± SD (n = 3 biological replicates). CS-HAA, cold stress in high-altitude area. CS-PT, cold stress in a phytotron with 16–17 °C. Nip, Nipponbare. In (a), P values were determined under the mixed linear model (MLM) using a two-sided Fisher’s exact test, implemented in TASSEL 5.0. In (b), different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. In (c-e), significant difference was determined by two-sided Student’s t-test (indicated by asterisks, *P < 0.05, **P < 0.01, ***P < 0.001). Source data are provided as a Source Data file.
Using gene editing technology, we generated a transgenic line D395, with the 57-bp and 284-bp sequences in the promoter region of CTB3 deleted in the background of a high-quality and conventional indica variety, Huanghuazhan (HHZ, CTB3-Hap2) (Supplementary Fig. 4c, d). After chilling treatment at the seedling stage, the survival rate of D395 was significantly higher than that of HHZ (Supplementary Fig. 4e). The cold-induced expression of CTB3 was higher in D395 compared to HHZ (Supplementary Fig. 4f, g). In addition, two variants in CTB3 coding region showed the same protein subcellular localization and a similar effect in enhancing cold tolerance (Fig. 1e–g and Supplementary Fig. 6), which suggests that they do not affect the protein function.
Overall, these results demonstrate that these two indels in the CTB3 promoter are the key functional variants leading to the differences in cold tolerance at the booting stage in rice accessions.
OsTCP19 acts upstream of CTB3 and negatively regulates cold tolerance at the booting stage
Sequence comparison revealed that the 57-bp insertion in 9311 is a repeat sequence, with Nip containing only one copy of this sequence, while 9311 contains two tandem copies. Additionally, the 284-bp insertion includes a 46-bp sequence that is identical to 46-bp of the 57-bp sequence (Supplementary Fig. 9a). We used the PlantRegMap (http://plantregmap.gao-lab.org/) website to analyze the 57-bp sequence33. The results showed that TCP family transcription factors may be able to bind to this 57-bp sequence (Supplementary Data 3). Subsequent analysis uncovered potential binding motifs for OsTCP19 on both the 57-bp and 284-bp sequences (Supplementary Fig. 9b), suggesting that the number of OsTCP19 binding motifs could be crucial for the regulation of CTB3 expression. We performed yeast one-hybrid (Y1H) assays for some cloned TCP family transcription factors and found that OsTCP19 had the strongest ability to bind to the CTB3 promoter (Supplementary Fig. 9c). We proposed that OsTCP19 could be an upstream regulator of CTB3. OsTCP19 was expressed in all tissues, and its expression was downregulated by cold (Supplementary Fig. 9d, e).
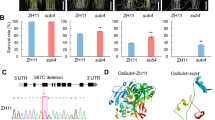
To test our prediction, we obtained two independent OsTCP19-knockout mutants and two OsTCP19-overexpression lines in the Nip background and detected the transcript levels of CTB3 (Supplementary Fig. 10a, b). The cold-induced expression level of CTB3 in the OsTCP19-overexpression lines were significantly lower than that in Nip, whereas the cold-induced expression levels in the OsTCP19-knockout mutants were higher than that in Nip (Fig. 3a). In transient expression assays, after co-transforming 35S:OsTCP19 with the ProCTB3-Hap1:LUC or ProCTB3-Hap2:LUC constructs in rice protoplasts, the activity of these promoters was suppressed, as compared with that of the vector control (Fig. 3b), indicating that OsTCP19 represses the activity of the CTB3 promoter. Chromatin immunoprecipitation with quantitative PCR (ChIP-qPCR) assays using the panicles of OsTCP19-GFP-overexpression lines showed that DNA fragment P1 of the CTB3-Hap1 promoter, which contains the predicted binding motif T1, was significantly enriched (Fig. 3c). Y1H assays showed that OsTCP19 could directly bind to the CTB3-Hap1 and CTB3-Hap2 promoters (Fig. 3d). Gel electrophoresis mobility shift assay (EMSA) showed that OsTCP19 significantly reduced the migration of the T1 and T2 probes, which contain the predicted OsTCP19 binding motifs (Fig. 3e). These results indicate that OsTCP19 directly binds to its binding sites on the CTB3 promoter and suppresses the expression of CTB3. Importantly, CTB3-Hap1 possesses only one OsTCP19 binding motif on its promoter, whereas CTB3-Hap2 contains three OsTCP19 binding motifs (Fig. 3e), resulting in differences in CTB3 expression and consequently divergent cold responses.
a Relative expression levels of the CTB3 gene in OsTCP19-knockout mutants and OsTCP19-overexpression lines. Data are mean ± SD (n = 3 biological replicates). b OsTCP19 represses the activity of CTB3 promoter, as shown by firefly luciferase/Renilla luciferase activity ratio (LUC/REN). Data are mean ± SD (n = 4 biological replicates). 62SK-Empty represents the pGreenII 62-SK-Empty vector, while 62SK-OsTCP19 represents the 35S construct used as the effector. ProCTB3-Hap1 represents ProCTB3-Hap1: LUC and ProCTB3-Hap2 represents ProCTB3-Hap2: LUC, used as reporters. c ChIP-qPCR assay to assess the binding of OsTCP19 to the CTB3 promoter in vivo. Mock, control samples without antibody. Data are mean ± SD (n = 3 biological replicates). d Yeast one-hybrid assays testing the binding of OsTCP19 to the promoter of CTB3. e Electrophoretic mobility shift assay (EMSA) of OsTCP19 binding to T1 and T2 on the promoter of CTB3. A representative experiment from at least two independent experiments is shown. f Panicles of OsTCP19-knockout mutants and -overexpression lines grown under CS-HAA. Scale bar is 2 cm. g Statistical results for seed setting rates of OsTCP19-knockout mutants and OsTCP19-overexpression lines grown under NC, CS-HAA and CS-PT conditions. Data are mean ± SD (n = 15 plants). h Panicles of Nip, CTB3-cr1, OsTCP19-cr1, CTB3-cr1/osctp19-1 and CTB3-cr1/osctp19-2 grown under CS-HAA conditions. Scale bar is 2 cm. i Statistical results for seed setting rates of Nip, CTB3-cr1, OsTCP19-cr1, CTB3-cr1/osctp19-1 and CTB3-cr1/osctp19-2 under NC, CS-HAA and CS-PT conditions. Data are mean ± SD (n = 15 plants). NC, Normal conditions. CS-HAA, cold stress in high-altitude area. CS-PT, cold stress in a phytotron with 16–17 °C. Nip, Nipponbare. In (c), significant difference was determined by two-sided Student’s t test (indicated by asterisks, *P < 0.05, **P < 0.01, ***P < 0.001). In (a, b, g, i), different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. Source data are provided as a Source Data file.
The OsTCP19-knockout mutants had higher seed setting rates than Nip under CS-HAA and CS-PT conditions, whereas the OsTCP19-overexpression lines had significantly lower seed setting rates than Nip (Fig. 3f, g). This suggests that OsTCP19 negatively regulates cold tolerance at the booting stage. To analyze the genetic relationship between OsTCP19 and CTB3, we developed CTB3-cr1/osctp19 double knockout mutants (Supplementary Fig. 10c). The seed setting rates of CTB3-cr1/osctp19-1 and CTB3-cr1/osctp19-2 were significantly lower than that of Nip, but not significantly different from that of CTB3-cr1 (Fig. 3h, i). These results suggest that CTB3 possibly acts downstream of OsTCP19 and could be in the same genetic pathway involved in regulating cold tolerance at the booting stage.
OsTPP1 is directly targeted by CTB3 and positively regulates cold tolerance at the booting stage
To identify the downstream genes regulated by CTB3, we collected panicles from the CTB3-cr1 and Nip plants after 1 day of CS-PT treatment and performed RNA sequencing (RNA-seq). We identified 1020 differentially expressed genes (DEGs), including 810 downregulated genes and 210 upregulated genes (Supplementary Fig. 11a, Supplementary Data 4). GO enrichment analysis of DEGs identified several highly significant terms in biological processes and in molecular functions (Supplementary Fig. 11b, c). We focused on GO terms related to energy supply and carbohydrate (sugar) metabolism due to previous studies demonstrating that cold tolerance at the booting stage involving mechanisms like energy supply, sugar transport, pollen development and gibberellin (GA) signaling19,20,21,34. Within the ‘carbohydrate metabolic process’ term, we identified OsTPP1, a cold tolerance gene that plays a crucial role in trehalose metabolism and sugar signaling35,36,37. Additionally, several sugar-transport-related (STG) genes, including Os05g0169700, OsSweet15, OsSweet11b, OsMST6 and OsSweet7d, were identified within the ‘sugar transmembrane transporter activity’ term, further supporting the role of CTB3 in maintaining sugar metabolism and transport under cold stress. Transcriptome data showed that the transcript levels of all these genes were significantly lower in the panicles of CTB3-cr1 than in Nip panicles (Supplementary Fig. 11d). Reverse transcription quantitative PCR (RT-qPCR) analysis showed that the expression of these genes was reduced in the panicles of CTB3-knockout mutants and increased in the panicles of CTB3-overexpression lines as compared with that in Nip under the CS-PT conditions (Supplementary Fig. 12a). Y1H assays revealed that CTB3 bound to the OsTPP1 promoter, but not to the promoters of the other genes (Supplementary Fig. 12b). Therefore, we identified OsTPP1 as the major downstream candidate gene regulated by CTB3 for further analysis.
CAMTA family transcription factors specifically bind to the CG-box motifs (G/A/C)CGCG(C/G/T) to regulate the expression of downstream genes25,26. By using the PlantRegMap website to predict the promoter of OsTPP133, we found four potential CAMTA family protein binding motifs, which we named as CG1, CG2, CG3 and CG4 (Supplementary Data 5). Y1H assays showed that CTB3 binds to the F3 region of the OsTPP1 promoter, which contains four potential CG-box motifs (Fig. 4a). Subsequently, we performed ChIP-qPCR using the panicles of CTBHap1-OE1 plants at the booting stage, which showed that DNA fragments containing the CG3 binding motif were enriched more than 6-fold, and the degree of enrichment was higher than that in CG1, CG2 and CG4 (Fig. 4b). To further verify that CTB3 directly binds to the CG3 binding motif in the OsTPP1 promoter in vitro, we performed EMSA and revealed that MBP-CTB3-NT (N-terminal of CTB3) bound to the labeled probe. The addition of unlabeled probes significantly attenuated these binding, and mutated unlabeled probes could not compete for the binding ability (Fig. 4c). Furthermore, luciferase activity was enhanced when co-expressed with 35S:CTB3 with proOsTPP1: LUC vectors, as compared to the empty vector control, under normal and cold conditions (Fig. 4d). In addition, we also examined the cold-induced expression level of OsTPP1 in CTB3-Hap1 and CTB3-Hap2 accessions. OsTPP1 expression was significantly higher in CTB3-Hap1 accessions than in CTB3-Hap2 accessions under the CS-PT conditions (Supplementary Fig. 13). Taken together, these results suggest that CTB3 positively regulates OsTPP1 expression by directly binding to its promoter.
a Y1H assays testing the binding of CTB3 to the promoter of OsTPP1. The OsTPP1 promoter was divided into three segments F1, F2 and F3 and ligated into the pLacZi2μ vector. b ChIP-qPCR assay showing CTB3 binding to the OsTPP1 promoter in vivo. Data represent means ± SD (n = 3 biological replicates). c Electrophoretic mobility shift assay of CTB3 binding to the CG3 motif on promoter of OsTPP1. A representative experiment from at least two independent experiments is shown. d Transient dual luciferase expression assay of CTB3 in the transcriptional regulation of OsTPP1 in protoplasts. Protoplasts were incubated at 4 °C for 30 min. Data are mean ± SD (n = 3 biological replicates). 62SK-Empty represents pGreenII 62-SK-Empty vector, 62SK-CTB3 represents 35S: CTB3, as effector. ProOsTPP1 represents ProOsTPP1: LUC, as reporters. e Panicles of DJ, tpp1-1, and tpp1-2 grown under CS-DW conditions. Scale bar is 2 cm. f Statistical results for relative seed setting rates of DJ, tpp1-1, tpp1-2 under NC, CS-DW and CS-PT conditions. Data represent means ± SD (n = 15 plants). g Pollen fertility evaluated by I2-KI dyeing. Scale bar is 100 μm. h Statistical results of pollen fertility. Data represent means ± SD (n = 10 plants). i Panicles of Nip, CTB3-cr1, CTB3-cr1/OsTPP1-OE1, CTB3-cr1/OsTPP1-OE2 grown under CS-HAA. Scale bar is 2 cm. j Statistical results for seed setting rates of Nip, CTB3-cr1, CTB3-cr1/OsTPP1-OE1, CTB3-cr1/OsTPP1-OE2 under NC, CS-HAA and CS-PT conditions. Data represent means ± SD (n = 15 plants). NC, Normal conditions. CS-DW, cold stress in deep water. CS-HAA, cold stress in high-altitude area. CS-PT, cold stress in a phytotron with 16–17 °C. Nip, Nipponbare. DJ, Dongjing. In (b), significant difference was determined by two-sided Student’s t-test (indicated by asterisks, *P < 0.05, **P < 0.01, ***P < 0.001). In (d, f, h, j), different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. Source data are provided as a Source Data file.
OsTPP1 was expressed at the highest level in leaves (Supplementary Fig. 12c), and its expression was upregulated in panicles by cold (Supplementary Fig. 12d). To investigate the function of OsTPP1 at the booting stage, we characterized the phenotype of OsTPP1-knockout mutants (tpp1-1 and tpp1-2) and temperate japonica rice, Dongjing (DJ) under CS-DW and CS-PT conditions. The seed setting rates of the knockout mutants were significantly lower than those of DJ after cold treatments, indicating that OsTPP1 positively regulates cold tolerance at the booting stage (Fig. 4e, f). In addition, the ratio of normal anther chambers was significantly lower in tpp1-1 under the CS-PT conditions compared with DJ, and anther damage was more severe in tpp1-1 (Supplementary Fig. 14b, c). We observed pollen viability using I2-KI staining and found that the pollen fertility of tpp1-1 and tpp1-2 was lower than that of DJ under the CS-PT conditions (Fig. 4g, h). However, there was no significant difference in pistil morphology between these knockout mutants and DJ (Supplementary Fig. 14a). These observations suggest that OsTPP1 promotes cold tolerance at the booting stage by affecting pollen development.
To confirm the genetic relationship between CTB3 and OsTPP1, we overexpressed OsTPP1 in the background of the CTB-cr1 knockout mutant (Supplementary Fig. 12e). The overexpression lines had higher seed setting rates than CTB3-cr1 and Nip, indicating that OsTPP1 can rescue the CTB3-cr1 phenotype (Fig. 4i, j), indicating that CTB3 and OsTPP1 could act in the same genetic pathway in regulating cold tolerance at the booting stage.
CTB3 mediates sugar supply in rice to confer cold tolerance
OsTPP1 encodes TREHALOSE-6-PHOSPHATE PHOSPHATASE 1, which dephosphorylates trehalose 6-phosphate (Tre6P) to trehalose (Tre)38. Heterologous expression of OsTPP1 in maize (Zea mays) promoted the expression of STG genes by inhibiting Tre6P accumulation in florets to prevent maize seed loss and improve yield under drought stress36,37. Therefore, we first examined the Tre6P content in panicles of the CTB3 transgenic lines and Nip plants. The Tre6P content was lower in CTB3Hap1-OE1 and higher in CTB3-cr1 compared with Nip under the CS-PT conditions (Fig. 5a). We also examined the Tre6P content in tpp1-1 and DJ and the Tre6P content was significantly higher in tpp1-1 than in DJ under normal and CS-PT conditions (Fig. 5b). Subsequently, we determined the expression of STG genes, including Os05g0169700, OsSweet15, OsSweet11b, OsMST6 and OsSweet7d, in the panicles of DJ, tpp1-1 and tpp1-2. The expression levels of these STG genes were significantly lower in the tpp1-1 and tpp1-2 panicles than in DJ panicles under the CS-PT conditions (Fig. 5c). Then, we detected the sugar contents in the panicles of the CTB3 transgenic lines and OsTPP1-knockout mutants under normal and CS-PT conditions. After the CS-PT treatment, the contents of sucrose, glucose and fructose were significantly lower in the CTB3-cr1 and CTB3-cr2 panicles, but significantly higher in the panicles of CTB3-overexpression lines, compared to those in Nip (Fig. 5d–f). The contents of these sugars were significantly lower in the tpp1-1 and tpp1-2 panicles than in DJ panicles after the CS-PT treatment (Fig. 5g–i). Furthermore, we identified that exogenous application of Tre6P reduced the cold tolerance of two CTB3-overexpression lines (Supplementary Fig. 15). These results imply that CTB3 activates the expression of OsTPP1 under cold stress, and OsTPP1 protects pollen development and fertility under cold stress by reducing the Tre6P content and affecting sugar accumulation.
a, b Trehalose-6-phosphate contents in the panicles of Nip, CTB3-cr1, CTB3Hap1-OE1, DJ, tpp1-1 at the booting stage under CS-PT treatment at 0 h and 1 day. Data are mean ± SD (n = 3 biological replicates). c RT-qPCR assays of transcript levels of five sugar transport-related genes in the panicles of DJ, tpp1-1, and tpp1-2 grown under CS-PT conditions at 1 day. Data were averaged over three biological replicates and visualized in a heat map using log2-fold changes in expression ratios relative to Nip at 0 h. d–f Sucrose, glucose and fructose content in the panicles of Nip, CTB3-cr1, CTB3-cr2, CTB3Hap1-OE1, CTB3Hap1-OE2 before and after CS-PT treatment for 7 days. Data are mean ± SD (n = 3 biological replicates). g–i Sucrose, glucose, and fructose content in the panicles of DJ, tpp1-1 and tpp1-2 before and after CS-PT treatment for 7 days. Data are mean ± SD (n = 3 biological replicates). Nip, Nipponbare. DJ, Dongjing. In (a, b, d–i), different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. Source data are provided as a Source Data file.
Finally, we examined the transcript levels of OsTPP1 and STG genes in OsTCP19 transgenic lines. Under CS-PT conditions, the transcript levels of OsTPP1 and STG genes were significantly higher in OsTCP19-cr1 and OsTCP19-cr2 panicles, but lower in OsTCP19-OE1 and OsTCP19-OE2 panicles, compared with Nip panicles (Supplementary Fig. 16). This indicated that OsTCP19 negatively regulates the expression of OsTPP1 and STG genes, thereby reducing cold tolerance at the booting stage.
CTB3 is under selection during domestication of temperate japonica rice
The geographic distribution of CTB3 alleles showed that CTB3-Hap1 accessions are mainly found in Northern China and Japan, whereas CTB3-Hap2 accessions are mainly distributed in Southern China, Africa, India, Thailand, Vietnam and other low-latitude regions (Fig. 6a, b). The geographic distribution of CTB3-Hap1 accessions showed a tendency to expand northward, suggesting that CTB3-Hap1 may facilitate the adaptation of rice to cold environments.
Geographical distribution of the different haplotype materials of CTB3 in the world (a) and in China (b). The x- and y-axes indicate longitude and latitude, respectively. c Haplotype network of CTB3. d Phylogenetic tree of CTB3 generated from 477 diverse rice accessions, including the Tej, Trj, Ind, Aro, Aus and O. rufipogon, showing divergence between the CTB3-Hap1 and CTB3-Hap2. e Nucleotide diversity of CTB3. The x-axis denotes the position of CTB3, and the y-axis indicates average π values, π value ×100 for convenience of highlighting differences.
To analyze the origin and spread of CTB3, we downloaded and compared the sequences of the CTB3 gene region in 14 wild rice genomes39,40,41,42. The information on SNPs and indels of wild rice was combined with that of 525 common rice cultivars (Supplementary Data 1). To identify the domestication process of the 57-bp and 284-bp alleles in wild and cultivated rice, we performed a minimum spanning tree analysis (Fig. 6c). The result showed that CTB3 diverged in wild rice: both 57-bp and 284-bp insertion or deletion already existed in wild rice, and different variants were retained in the japonica and indica subpopulations (Fig. 6c). Phylogenetic tree analysis of CTB3 revealed separate clustering for indica and japonica rice. The CTB3-Hap1 accessions were mainly temperate japonica rice, clustered with Chinese wild rice. The CTB3-Hap2 accessions were mainly indica rice, clustered with wild rice from the Indian and Thai regions (Fig. 6d).
To identify whether CTB3 was under selection during domestication, we performed nucleotide diversity analysis. The nucleotide diversity of CTB3 in the temperate japonica accessions (π = 0.000239) was significantly lower than that in wild rice (π = 0.001507, average of Or-I, Or-II and Or-III), but the diversity was higher in the flanking regions of the gene was than in the gene region itself (Fig. 6e and Supplementary Table 1). This implied that CTB3 was subjected to positive selection in temperate japonica. The neutrality test showed that the Tajima’ D value of CTB3 was –2.38646 (P < 0.01) in temperate japonica cultivars (Supplementary Table 1), which could indicate that CTB3 was subject to positive selection in the temperate japonica. Together these results suggested that CTB3-Hap1 may have been under positive selection during domestication of temperate japonica rice and that this facilitated japonica adaptation to cold climates.
OsTCP19 is under selection during domestication of japonica rice
To investigate whether OsTCP19 plays a role in rice cold acclimation, we performed haplotype analysis. There were two haplotypes of OsTCP19 in japonica rice and five haplotypes in indica rice (Supplementary Fig. 17a). T-Hap1 exhibited higher cold tolerance than other OsTCP19 haplotypes at the booting stage, defining it as a cold-tolerant haplotype (Supplementary Fig. 17b). Phylogenetic tree analysis revealed that T-Hap1 is found mainly in japonica, as well as in a few indica rice cultivars; T-Hap5 (OsTCP19-H) is mainly present in indica, Aro, Aus, and the other haplotypes are mainly found in indica (Supplementary Fig. 17c). T-Hap1 mainly clustered with some O. rufipogon III varieties, suggesting that the cold-tolerant haplotype T-hap1 may have originated directly from O. rufipogon III (Supplementary Fig. 17c).
The minimum spanning tree showed that OsTCP19 has diverged in wild rice, and OsTCP19-H and OsTCP19-L may have directly originated from different wild rice ancestors. T-Hap1 directly originated from wild rice and has been selected to be retained in japonica and a few indica (Supplementary Fig. 17d). Geographic distribution analysis revealed that T-Hap1 accessions were mainly distributed in northeastern and northern China as well as in Japan and North Korea; and OsTCP19-H accessions were mainly distributed in India and Bangladesh (Supplementary Fig. 18). These results indicated that T-Hap1 may have been retained in the japonica subpopulation due to selection by cold environments.
To assess whether OsTCP19 is under selection, we first calculated the average nucleotide diversity of OsTCP19. The nucleotide diversity in temperate japonica (π = 0.00034), tropical japonica (π = 0.00025) and japonica (π = 0.00077) was significantly lower than that in wild rice (π = 0.00295, average of Or-I, Or-II and Or-III) or indica subpopulations (π = 0.00237) (Supplementary Table 2). In addition, the Tajima’ D values were –2.56474 (P < 0.001) in temperate japonica, –1.83346 (P < 0.05) in tropical japonica and –2.33494 (P < 0.01) in japonica, suggesting that OsTCP19 was positively selected in the japonica subpopulation (Supplementary Table 3). The Tajima’ D value was 2.41223 in indica (P < 0.05), suggesting that OsTCP19 may have been subjected to balancing selection in the indica subpopulation (Supplementary Table 3). These results indicated that T-hap1 may have been selected in japonica rice to improve cold tolerance.
Taking these results together, we suggested that T-Hap1 may be directly derived from a few O. rufipogon III and has been retained in the japonica subpopulation by positive selection, which promotes the adaptation of japonica rice to cold habitats at the booting stage.
OsTCP19 and CTB3 jointly contribute to the improvement of cold tolerance
To investigate the contribution of different allele combinations of OsTCP19 and CTB3 to cold tolerance, we performed a combined haplotype analysis and obtained six haplotype combinations, designated as groups I–VI. Among them, group I, containing the two favorable alleles of OsTCP19 and CTB3, exhibited the highest seed setting rate and was present only in japonica rice. Group II, containing the OsTCP19 favorable allele, showed higher seed setting rate than other groups in indica rice. This result implies that T-Hap1 may have initially promoted the improvement of cold tolerance in japonica rice and some indica rice, while CTB3-Hap1 further promoted cold tolerance improvement in intra-japonica subspecies (Fig. 7a, b).
a Combined haplotypes of OsTCP19 and CTB3. b Comparison of haplotypes of different combinations in the japonica and indica rice (n = 128/30, 11/9/36/32/11 accessions). For box plots, boxes represent the interquartile range (25th to 75th percentiles), center lines indicate medians, and whiskers extend to the minima and maxima. c Evolutionary relationships of OsTCP19 and CTB3 revealed by a combined haplotype network. d Allelic changes of OsTCP19 and CTB3 during rice breeding. Improved varieties (IMP) and landraces (LAN). e Panicles of LJXHG, NILCTB3-Hap1 and DY17 grown under CS-HAA conditions. Statistical results for seed setting rates of LJXHG, NILCTB3-Hap1 and DY17 under CS-HAA (f), normal (g), and CS-PT (h) conditions. Data represent means ± SD (n = 15 plants). i Model of the roles of CTB3 in cold tolerance at the booting stage. Solid lines represent direct regulation and dashed lines represent indirect regulation. The thickness of the line represents the strength of the regulation. Arrows indicate up-regulation, and lines ending with a horizontal bar indicate down-regulation. NC, Normal conditions. CS-HAA, cold stress in high-altitude area. CS-PT, cold stress in a phytotron with 16–17 °C. LJXHG, Lijiangxiaoheigu. DY17, Deyou17. In (b) of Jap group, significant difference was determined by two-sided Student’s t-test (indicated by asterisks, *P < 0.05, **P < 0.01, ***P < 0.001). In (b) of Ind group and (f–h), different lowercase letters above the bars indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple range test. Source data are provided as a Source Data file.
To clarify the evolutionary relationship between OsTCP19 and CTB3, we constructed a joint minimal spanning tree of the two genes. The accessions containing the OsTCP19 favorable haplotype is mainly present in O. rufipogon III (Fig. 7c). The CTB3 favorable haplotype may be a new natural variation aroused in a few O. rufipogon III containing OsTCP19 favorable haplotype. The two favorable haplotype OsTCP19(+) and CTB3(+) may have been selected and retained in japonica rice as it adapted to colder climate (Fig. 7c).
To explain the utilization of OsTCP19 and CTB3 in breeding, we investigated the allele frequencies of OsTCP19 and CTB3 in 505 cultivated rice accessions (Supplementary Data 6). OsTCP19 has a relatively high allele frequency in landraces and improved japonica rice; thus, T-hap1 may have been used to improve cold tolerance in japonica rice during early breeding. The favorable allele frequency of CTB3 in improved varieties was increased compared to landrace varieties in japonica rice, which means CTB3 has been better utilized. In addition, the frequency of the OsTCP19/CTB3 alleles also increased in the improved varieties, suggesting that this combination of haplotype is favorable in breeding for cold tolerance. In indica rice, the frequencies of the favorable alleles of OsTCP19 and CTB3 were very low in the landrace varieties and reduced in the improved varieties, and indica rice not contained OsTCP19/CTB3 alleles (Fig. 7d). These results suggest that OsTCP19 and CTB3 have been widely used to improve cold tolerance in japonica rice and promote the expansion of rice to high-latitude regions. Furthermore, gene complementation assays showed that CTB3-Hap1 improved the cold tolerance of TQ (CTB3-Hap2) at the booting stage (Fig. 1c, d). Meanwhile, NILCTB3-Hap1 lines showed higher seed setting rates than DY17 (CTB3-Hap2) under both CS-HAA and CS-PT conditions (Fig. 7e-h). These results indicate that CTB3-Hap1 can be used for improving rice cold tolerance at the booting stage.
Discussion
Cold damage at the booting stage refers to low-temperature stress occurring just before flowering, which directly affects the development of reproductive organs, including spikelet differentiation and pollen viability, ultimately leading to a reduction in yield5,6. At present, only a limited number of CTB genes have been cloned from natural populations20,21,22,23,24. Among them, CTB4a enhances seed setting rates by maintaining energy supply20, and CTB2 protects pollen development by sterol glycosides under cold stress conditions21. However, the functional mechanisms of other key loci, such as Ctb1, qCTB7 and OsLEA9 remain largely unexplored22,23,24. Therefore, investigating cold-tolerance genes at the booting stage is essential for addressing the unique challenges posed by low temperatures in rice.
We previously obtained a cold tolerance loci, qCTB1t, at the booting stage by genome-wide association study2. In this study, we showed that CTB3 encodes a CAMTA family transcription factor that positively regulates rice cold tolerance at the booting stage and was under selection during domestication of temperate japonica rice. CTB3 exhibited a rapid response to cold stress at the transcriptional level and induces the expression of the downstream gene OsTPP1. We speculated that OsTPP1 influences the expression of genes related to sugar transport may through Tre6P, thereby modulating sugar accumulation under cold stress conditions (Fig. 7g).
Two natural indel variants exist in the CTB3 promoter, association analysis of candidate genes, along with transient expression assays, demonstrated that these two indels are the key functional variants of CTB3 (Fig. 2a, b). OsTCP19 negatively regulates cold tolerance by binding to the CTB3 promoter and suppressing CTB3 expression (Fig. 3). We found that CTB3-Hap1 possesses a single OsTCP19 binding site, while CTB3-Hap2 contains three OsTCP19 binding sites, which differentially affects CTB3 promoter activity and thus CTB3 expression levels (Fig. 3e). Inhibition of the activity of the CTB3-Hap2 promoter by these two indels resulted in reduced cold tolerance of the CTB3-Hap2 accessions (Fig. 1a,b). We transferred CTB3-Hap1 into TQ (CTB3-Hap2) and backcrossed it into DY17 (CTB3-Hap2) to create inbred lines. The cold tolerance of the complemented lines C1 and C2 was enhanced, as compared to TQ (Fig. 1c, d). The cold tolerance of NILCTB3-Hap1 was also significantly improved, as compared to DY17 (Fig. 7e-h). Meanwhile, the transgenic line D395, which exhibited the deletion of both the 57-bp and 284-bp indels in the promoter region of CTB3, showed enhanced cold tolerance as compared to HHZ (Supplementary Fig. 4e). Thus, this CTB3 haplotype can be employed to enhance cold tolerance in indica rice and some japonica rice. The two indel variants in the CTB3 promoter can serve as molecular markers for enhancing cold tolerance in breeding programs and as target sites for gene editing.
In the CTB3-cr1 background, we obtained OsTCP19-knockout and OsTPP1-overexpression transgenic plants. Cold tolerance of CTB3-cr1/ostcp19-1 and CTB3-cr1/ostcp19-2 did not increase but was similar to that of CTB3-cr1, which suggests that OsTCP19 could be an upstream regulator of CTB3 that negatively regulates cold tolerance at the booting stage by inhibiting CTB3 expression (Fig. 3h, i). CTB3-cr1/OsTPP1-OE1 and CTB3-cr1/OsTPP1-OE2 had higher cold tolerance than CTB3-cr1 and Nip (Fig. 4i,j), suggesting that OsTPP1 is a target gene of CTB3, and that CTB3 regulates cold tolerance by activating OsTPP1 expression. In addition, we found that the T-hap1 allele of OsTCP19 initially enhanced cold tolerance at the booting stage in rice (Supplementary Fig. 17a, b). Taken together, these results clarify the importance of CTB3 and its possibly upstream and downstream interactors for cold tolerance at the booting stage (Fig. 7g).
Tre6P is a critical signaling molecule in plant carbon metabolism, serving not only as an intermediate in trehalose synthesis but also playing a key role in regulating sucrose levels, energy use and overall plant growth and development36,37,38,43,44,45,46,47,48,49,50. As shown in recent studies, Tre6P has been demonstrated to inhibit SnRK1 activity in vitro by binding to the catalytic subunit KIN1046. High levels of Tre6P are thought to inhibit SnRK1 kinase activity, which is postulated to promote biosynthetic processes, such as amino acid and organic acid synthesis, thereby supporting growth; low levels of Tre6P might release SnRK1 activity, thus enhancing catabolic processes under stress conditions43,44,45,46. However, this model has not yet been validated in planta. Tre6P plays a crucial role in many plant developmental processes, including embryo development, flowering, shoot branching and root branching45,47,48. Reducing Tre6P levels by overexpressing OsTPP1 in developing maize ears promotes sucrose partitioning to seeds, prevents kernel abortion and increases yield under drought conditions36,37. Additionally, class II Tre6P synthase-like proteins suppress SnRK1 by affecting its nuclear localization49.
In rice, OsTPP1 plays a pivotal role in enhancing rice yield and defending against damage caused by low temperatures. Under normal conditions, overexpression of OsNAC23 represses OsTPP1, leading to increased Tre6P levels in leaves, which promotes sucrose partitioning to sink organs such as grains, increasing rice yield50. While, in response to cold stress at the seedling stage, OsbHLH002 activates OsTPP1, driving trehalose accumulation to improve cold tolerance35. Although trehalose is an important osmotic stressor, the function of OsTPP1 in determining fertility in cold tolerance at the booting stage may also be mediated through Tre6P and sucrose. Heterologous expression of OsTPP1 in maize ears using the OsMADS6 promoter resulted in reduced Tre6P and increased sucrose levels in spikelets, boosting yield under normal and drought conditions36. The increase in sucrose concentration is linked to the activation of SWEET genes, release of SnRK1 activity and enhanced photosynthesis37. SnRK1 coordinates carbon partitioning and energy depletion by interacting with transcription factors and regulating their transcriptional activity49. Our results indicate that CTB3 activates OsTPP1 expression, while OsTPP1 promotes the conversion of Tre6P to trehalose, and that low levels of Tre6P may promote the expression of STG genes (Fig. 5a–c). We speculate that the STG genes may be regulated by low levels of Tre6P through the SnRK1 kinase36,37,49. In rice, STG genes, including OsSweet1b, OsSweet4, OsSweet7c, OsSweet14, OsSweet11a, OsSweet11b, OsSweet15 and OsMST8, have been demonstrated to play crucial roles in pollen development and seed filling51,52,53,54. Transcriptome data and RT-qPCR assays showed that the transcript levels of OsTPP1 and five STG genes were lower in CTB3-cr1 panicles than in Nip panicles (Supplementary Fig. 12a), and the transcript levels of STG genes were also lower in tpp1-1 and tpp1-2 panicles than in DJ panicles (Fig. 5c). Therefore, CTB3 and OsTPP1 may affect STG expression through Tre6P, which in turn improves cold tolerance at the booting stage by modulating sugar translocation and accumulation due to the eventual accumulation of sugars can reduce damage to anthers and pollen at low temperatures.
Our study identifies CTB3, a member of the Calmodulin-binding Transcription Activator (CAMTA) family, as a positive regulator of cold tolerance at the booting stage in rice. In rice, other CAMTA family members also participate in various stress responses. For instance, OsCBT1 and OsCAMTA3 negatively regulate plant immune30,31, while SCT1 and SCT2 synergistically suppress heat tolerance32. In Arabidopsis, AtSR1 coordinates freezing tolerance and pathogen immunity along with other AtCAMTAs25,26. AtSR1 may receive pathogen-triggered calcium signals through the IQ and CaMBD regions55, suggesting a role in integrating calcium signaling. AtSR1 and AtCAMTA5 act as key transcriptional activators in sensing rapid cooling, rapidly inducing cold-responsive genes like DREB1B and DREB1C26. These findings suggest that CAMTA proteins are multifunctional regulators involved in integrating various environmental signals.
In transient expression assays, we found the full-length of CTB3 significantly activated the OsTPP1 promoter activity, in contrast, the truncated CTB3ΔIQ/CaMBD did not enhance the promoter activity of OsTPP1 (Supplementary Fig. 19). This result suggests that the IQ motif and CaMBD of CTB3 might be important for its transcriptional activity, possibly by mediating responses to Ca²+/calmodulin signaling under cold stress. Considering the GO enrichment analysis indicating the involvement of CTB3 in processes like aminosugar and chitin metabolism, polysaccharide metabolism and cell wall organization, CTB3 may also play roles in disease resistance in rice (Supplementary Fig. 11b, c). Future studies focusing on how CTB3 receives and transduces adaptive stress signals and disease resistance will contribute to a deeper understanding of the multifunctionality of CAMTA proteins in rice.
Previous studies have clearly demonstrated that OsTCP19 negatively regulates rice tillering56. The OsTCP19-H allele, associated with increased tillering, enhances nitrogen-use efficiency at low or medium nitrogen levels56. Our haplotype analysis showed that T-Hap5 belonged to haplotype OsTCP19-H with high nitrogen-use efficiency (Supplementary Fig. 17a). Haplotype analysis of OsTCP19 showed that T-hap1 is the cold-tolerant haplotype, which belongs to the OsTCP19-L allele with lower nitrogen-use efficiency (Supplementary Fig. 17a, b). Geographic distribution analysis revealed that the T-hap5 (OsTCP19-H) accessions were primarily distributed in India and Bangladesh (Supplementary Fig. 18). Previous reports have shown that nitrogen-poor regions are mainly distributed in the northeastern India and Bangladesh along the basin of the Ganges River, where light may be abundant and temperatures are high. OsTCP19-H improves nitrogen-use efficiency and promotes rice tillering and growth in this ecological environment. In contrast, T-hap1 (OsTCP19-L) may be more favorable for regions with higher latitudes and had been selected for adaptation to cold environments (Supplementary Fig. 18). Combined haplotype analysis revealed that OsTCP19 first diverged in wild rice (Fig. 7c). A few O. rufipogon III containing favorable alleles of OsTCP19 and CTB3 may have conferred this combination of cold-tolerant haplotypes to japonica rice, where they have been subjected to selection and fixed in japonica rice to improve cold acclimatization.
The indica accessions exhibited the lowest nucleotide diversity (π = 0.000132) among all subpopulations and a significantly negative Tajima’s D value (-2.55351, P < 0.001) (Supplementary Table 1), suggesting that CTB3 may have evolved under strong selection in indica varieties. This indicates that different alleles of CTB3 were selected in temperate japonica and indica rice, with CTB3-Hap1 prevalent in temperate japonica and CTB3-Hap2 common in indica varieties. Similar patterns have been reported for other cold tolerance genes. For instance, different alleles of bZIP73 are fixed in japonica and indica rice, respectively15. Distinct alleles of COLD11—specifically, the 3GCG and (TCG + 3GCG) × 2 alleles—may have been subjected to strong selection16. It is worth noting that SCT1 and SCT2 are two orthologous genes of CTB3, which negatively regulate thermotolerance in rice32. The above reminds us that CTB3-Hap2 may contribute to high-temperature adaptation in indica varieties. Therefore, we speculate that the selection of different alleles of CTB3 may be related to environmental temperature differences in the growing regions of japonica and indica rice. The role of CTB3 in adaptation to different environmental conditions, particularly in relation to temperature variations between japonica and indica rice growing regions, needs to be further investigated.
Methods
Plant materials
To obtain CRSPR/Cas9 vector, we designed two 20-bp targets in the coding sequences (CDSs) of CTB3 and OsTCP19, and ligated them to the vector pHUE41157. To construct the overexpression vector for CTB3, we obtained the full-length CDS of CTB3 from the cDNA of Nipponbare (Nip) and 9311 by PCR and ligated it into the pCM1307 vector. To construct the overexpression vector for OsTCP19, we obtained the full-length CDS of OsTCP19 from the cDNA of Nip and ligated it to the vector pSuper1300-GFP. To obtain the overexpression vector for OsTPP1, we obtained the full-length CDS of OsTPP1 from the cDNA of Nip and ligated it to the vector pSuper1300-MYC. To obtain the complementary vector for CTB3, we obtained the promoter of CTB3 from Nip by PCR and ligated the 2.5-kb promoter with the full-length CDS into the vector pCM163. The plasmid was transferred into Agrobacterium tumefaciens strain EHA105 and transformed using the Agrobacterium transformation method58.
We obtained knockout and overexpression transgenic materials of CTB3 and OsTCP19 in the Nip background; OsTCP19 knockout and OsTPP1-MYC overexpression transgenic materials in the CTB3-cr1 background; and CTB3 complementary lines in the Teqing background. OsTPP1-knockout mutants, tpp1-1, tpp1-2 and wild-type Dongjing (DJ) were kindly provided by Prof. Jian Zhang of State Key Lab of Rice Biology, China National Rice Research Institute50.
Lines containing CTB3-Hap1 fragments were screened from recombinant inbred lines (F10) that were constructed from the cold-tolerant japonica rice Lijiangxiaoheigu and the cold-sensitive indica rice Deyou 17 (DY17) and were subsequently backcrossed three times with DY17 to produce NILs (BC3F3) for CTB3.
Phenotype evaluation
Three methods were adopted to evaluate the cold tolerance at the booting stage. For cold stress at high altitude area (CS-HAA), plants were grown in Baiyi, Kunming, Yunnan Province, China (altitude 1974 m), where minimum temperatures during the reproductive stage fall below 20 °C, naturally inducing cold stress suitable for evaluating cold tolerance (Supplementary Fig. 3a–c). At maturity, three primary panicles from each of at least 15 plants per genotype were harvested to assess seed setting rates.
For cold stress in deep water (CS-DW), plants were grown under normal conditions at the Shangzhuang Experimental Station in Beijing. Panicles at the booting stage were tagged (Supplementary Fig. 3d), and labeled plants were placed in boxes, which were subsequently transferred into deep water maintained at 16–18 °C, ensuring that the tagged panicles were completely submerged (Supplementary Fig. 3b). The cold treatment lasted for seven days. After treatment, plants were returned to the field to continue growing under normal conditions. Seed setting rates of the treated panicles were assessed at maturity. Relative seed setting rates were calculated by dividing the seed setting rate after the cold treatment by that under normal conditions. Cold tolerance of each genotype was evaluated using at least 15 plants, with three panicles treated per plant.
For cold stress in phytotron (CS-PT, 16–17 °C), similar to CS-DW, plants were grown under normal conditions until the booting stage and panicles were tagged. The tagged plants were moved to a controlled environment chamber (phytotron) set at 16–17 °C for seven days to impose cold stress (Supplementary Fig. 3b). After the treatment, plants were returned to the field to resume normal growth. Relative seed setting rates were assessed as described above.
For chilling treatments at the seedling stage, seeds were surface sterilized with 1% sodium hypochlorite solution and then germinated. Seedlings were grown in an incubator at 28°C day/25°C night under a short-day photoperiod (10 h light/14 h dark) and a light intensity of 120μmol·m-²·s-¹. After two weeks, they were transferred to an incubator maintained at 4°C with the same light conditions. After the cold treatment, the seedlings were returned to the incubator with normal temperature conditions for a further two weeks to recover. Survival rates were then measured.
Exogenous application of trehalose-6-phosphate
Trehalose-6-phosphate treatment was performed as previously described with minor modifications48. Briefly, the CTB3-overexpression lines and the wild-type Nip were transferred to an artificial climate chamber, and 1 mL of 1 mM Tre6P solution was injected into the labeled panicles after 0 h, 1 d, 2 d, 3 d, 4 d, 5 d and 6 d of CS-PT treatment. At the same time 1 mL of sterilized water (0 mM Tre6P) was injected into the labeled panicles of the control group to serve as a control for the Tre6P treatment. After 7 d of CS-PT treatment and transplanting to the field, the labeled panicles were harvested at maturity for seed setting test statistics and the relative seed setting rate was counted for cold tolerance at the booting stage.
Microscopy
Spikes were collected into formalin/glacial acetic acid/alcohol (FAA) solution under normal conditions and after cold treatment. Normal and abortive pollen were counted after staining using 1% I2-KI solution. For pistils, the morphology was observed directly without treatment. For anthers, after dehydration embedded in paraffin, paraffin sections were stained utilizing toluidine blue. All samples were observed and photographed using the microscope (Olympus CX23).
RNA isolation and RT–qPCR
Panicles of NIL-CTB3, DY17 and Nip were sampled under CS-PT conditions at different times. Different tissues (roots, stems, leaves, leaf sheaths, and different lengths of spikes) of Nip were sampled under normal conditions. The transgenic lines of CTB3, OsTCP19, OsTPP1 and their wild-types were subjected to low temperature treatment under CS-PT conditions. The panicles of these transgenic lines and their wild types were sampled before and after cold treatment. Total RNA was extracted using RNAiso Plus, and 2ug RNA was reverse transcribed using M-MLV reverse transcriptase (Takara, Kyoto, Japan). RT-qPCR was performed using an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA). Each experiment consisted of three biological and technical replicates. OsActin1 was used as an internal control.
Subcellular localization
To clarify the subcellular localization of CTB3, we ligated two types of CTB3 full-length CDSs from Nip and 9311, respectively, into the vector pSuper1300-GFP with the 35S promoter. The GFP plasmid was co-transformed with the plasmid Pro35S:RGN1-mCherry59, encoding a nuclear-tagged protein fused to the fluorescent protein mCherry, into the rice protoplasts. Fluorescence of GFP and red fluorescent protein was detected and photographed using the LSM 880 confocal laser scanning microscope (Zeiss, Oberkochen, Germany).
Transcriptional activity analysis
The full-length and truncated CDS of CTB3 were amplified and ligated into the pGBKT7 plasmid. All vectors were transferred into the yeast strain AH109 along with the pGADT7 empty vector. The transcriptional activation of various CTB3 proteins in yeast was assessed by their capacity to grow on media lacking leucine, tryptophan, histidine and adenine.
The full-length CDSs of two haplotypes of CTB3 were amplified and ligated into the 35S-GAL4BD vector. GAL4-VP16 was used as a positive control, and the GAL4 empty vector was used as a negative control. In addition, the 5-copy-driven LUC gene of GAL4/UAS serves as a reporter gene, and the 35S promoter-driven REN gene serves as an internal transformation control. The GAL4 plasmids, LUC plasmids and REN plasmids were transformed together into rice protoplasts. A Promega luminometer (GloMax® Explorer System) was used to measure the activity of LUC and REN using the dual LUC reporter assay kit60. The LUC/REN ratio was considered as the transcriptional activity of the CTB3 protein.
The full-length CDSs of OsTCP19 and CTB3 were amplified and cloned into the vector pGreenII 62-SK to obtain 35S:OsCTB3 and 35S:OsTCP19 as effectors. The CTB3 promoter of about 2.5-kb was amplified from Nip and 9311 and cloned into the vector pGreenII 0800LUC to obtain proCTB3-Hap1: LUC and proCTB3-Hap2: LUC. The two indel fragments of the CTB3-Hap2 promoter were progressively deleted to obtain the type of Hap2 (-∆57-bp), Hap2 (-∆284-bp), Hap2 (-∆57/-∆284-bp) by PCR amplification and recombination. Similarly, the OsTPP1 promoter of about 2.0-kb was amplified from Nip and inserted into pGreenII 0800LUC to obtain proOsTPP1: LUC as the reporter gene vector. Plasmids with different corresponding combinations were transformed into rice protoplasts, and the LUC activity was detected after incubation at 28 °C for 12 h. The LUC/REN values represent the transcriptional regulation ability of CTB3 and OsTCP19. Based on normal incubation, the transcriptional activity of CTB3 protein on OsTPP1 promoter was assayed after cold treatment by incubation at 4 °C for 30 min.
RNA-seq
Total RNA was extracted from rice panicles following cold treatment using the CS-PT method, and three biological replicates were prepared for both the knockout mutant CTB3-cr1 and the wild type Nip. RNA-seq libraries were constructed using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina (Illumina, San Diego, CA, USA) and sequenced on the Illumina NovaSeq 6000 platform by Beijing Novogene Co., Ltd. The raw reads were first subjected to quality control using fastp (version 0.19.7)61 to remove low-quality sequences and adapters, resulting in high-quality clean reads. These reads were then aligned to the rice reference genome using HISAT2 and only uniquely mapped reads were retained for further analysis62. Gene expression levels were quantified by normalizing the raw read counts to account for sequencing depth differences across samples. Differential expression analysis was performed using DESeq263, where differentially expressed genes (DEGs) were identified based on an absolute log2(fold change) > 1 and an FDR (padj) <0.01. For functional annotation, Gene Ontology (GO) term enrichment analysis was performed using an online GO tool (https://www.omicshare.com). The RNA-seq data were deposited in the NCBI Sequence Read Archive (SRA) under accession number PRJNA1106408. All differential gene data are available in Supplementary Data 4.
Yeast-one-hybrid
The full-length CDSs of CTB3 and OsTCP19 were inserted into pB42AD (pJG4-5) vector, named pB42AD-CTB3 and pB42AD-OsTCP19. The promoter fragment of CTB3, OsTPP1, Os05g0169700, OsSweet15, OsSweet11b, OsMST6 and OsSweet7D were obtained from Nip and cloned into the pLACZi2μ vector. The promoter of OsTPP1 was divided into three segments and cloned into the pLACZi2μ vector. Various combinations of pB42AD and pLACZi2μ plasmids were co-transformed into yeast EGY48. The interaction between transcription factors and promoters was assessed by observing changes in color on medium containing β-D-galactopyranoside but lacking uracil (Ura) and tryptophan (Trp) in strains with different plasmid combinations.
Chromatin immunoprecipitation-qPCR assay
Panicles of 35S: FLAG-CTB3 transgenic plants and 35S: OsTCP19-GFP transgenic plants were collected and cross-linked in 1% formaldehyde64. Briefly, after cross-linking, the reaction was terminated by the addition of glycine solution. After being ground into powder in liquid nitrogen, the samples underwent chromatin isolation followed by ultrasonic fragmentation. After removal of sample contaminants, anti-FALG (Sigma F1804) or anti-GFP (TransGen HT801) antibodies were added and immunopbrecipitation was performed. DNA fragments were obtained after precipitation, decrosslinking and DNA purification. Primer design was performed based on the predicted binding sites of OsTCP19 and the predicted binding sites of the CAMTA family, and the ChIP-enriched DNA fragments were analyzed by RT-qPCR.
Electrophoretic mobility shift assay
The full-length CDS of OsTCP19 was ligated to the vector pGEX-4T-1 to obtain the GST-OsTCP19 construct. The truncated sequence of CTB3 containing the DNA-binding structural domain was ligated to the vector pMAL-c5x to obtain the MBP-CTB3-NT construct. The fusion protein was expressed and purified before EMSA experiments. DNA probes containing predicted binding motifs were synthesized and biotin-labeled at the 5’ end; unlabeled probes were used as competition probes, and probes with mutations in the binding site were used as mutant competition probes. EMSA experiments were performed according to the instructions of the chemiluminescent EMSA kit (Beyotime, GS009), and the biotin-labeled probes were detected using a Tanon 5200 chemiluminescent imaging system (Tanon, Shanghai, China).
Measurement of endogenous substances contents
Rice panicles were collected before and after 1 day of cold treatment using the CS-PT method. The Tre6P content was determined by Wuhan Greensword Creation Technology Co. Ltd., following LC-MS/MS analysis (Thermo Scientific Ultimate 3000 UHPLC coupled with TSQ Quantiva)65. Tre6P was extracted using a chloroform-acetonitrile (3:7, v/v) mixture65. In brief, samples were ground to fine powder in liquid nitrogen, and 5 μL of [13C6]G6P (5 μg/mL) was added to each sample as the internal standard. Tre6P was extracted with an ice-cold chloroform-acetonitrile solution at –20 °C for 2 h. Ice-cold water was then added, and the mixture was vortexed and centrifuged at 4 °C. The upper aqueous acetonitrile phase was separated and re-extracted with ice-cold water. The aqueous acetonitrile phases were combined, evaporated to dryness, and purified using a C18 SPE cartridge. Finally, the extracted compounds were derivatized with an 8-DMQ solution. Tre6P derivatives (T6P-DMQ) were separated on a Waters Acquity UPLC BEH HILIC (100 mm × 2.1 mm i.d., 1.7 m) column under a 22 min gradient elution at a flow rate of 1.0 mL/min using 0.3% formic acid (FA) and 40 mM ammonium formate (HCOONH4) aqueous solution (A) and acetonitrile (ACN) (B). Quantification was conducted in selective reaction monitoring (SRM) mode with the following ion source parameters: positive ion mode, 3500 V spray voltage, ion transfer tube temperature at 350 °C, and auxiliary, sheath, sweep gases set at 10, 20, 4 Arb, respectively. T6P-DMQ was monitored using the transition m/z 564.1 > 142.1 with a collision energy of 38 eV. Tre6P concentrations were calculated based on the peak area ratio (analyte/internal standard) and normalized to plant tissue weight.
Panicles were collected before and after CS-PT treatment for sugar content measurement. For sucrose measurements, the samples were co-heated with alkali to destroy the reducing sugars in them, which were hydrolyzed to glucose and fructose under acidic conditions. Fructose content was measured using the resorcinol method66. Glucose content was measured using the glucose oxidase method67.
Haplotype analysis
Resequencing data for the CTB3 and OsTCP19 genes were obtained from the 3000 Rice Genomes Project68, and indel information on the OsTCP19 promoter was obtained from laboratory-held resequencing data. The CTB3 promoter was compared with the gene region by segmented PCR amplification followed by sequencing to analyze the sequence differences between Nip and 9311 (Supplementary Data 2). Primers were designed and PCR amplification was used to identify insertion deletions in both indels (Supplementary Fig. 8). We used the phenotypes of the materials under CS-HAA conditions for the haplotype analysis2. In this study, SNPs with significant association signals at the promoter and the two insertion/deletion (indel) variants, as well as SNPs in the coding region causing amino acid changes, were used to classify CTB3 haplotypes in the germplasms (Supplementary Data 1). To search for potential functional SNPs or indels of CTB3, the variant information of the two indels was integrated into the CTB3 gene resequencing data. Gene-based association analysis was performed in Tassel 5.0 using mixed linear model69. Haplotype classification of OsTCP19 was performed using all SNP and indel variants from the database. The geographical distribution of CTB3 and OsTCP19 haplotypes was annotated using the Maps R package (R v4.3.3) under a GPL-2 license.
Selection and evolutionary analysis
For the selection analysis, sequence datasets for the CTB3 and OsTCP19 gene regions and upstream and downstream 20-kb regions were obtained from deep sequencing data of our lab, the 3000 Rice Genome Project, and publicly available wild rice data1. Custom PERL scripts were used to calculate the π values (Nucleotide diversity) for the different subpopulations and the Tajima’ D values were calculated using the DNASP5.10 software70.
To construct phylogenetic tree and haplotype network for CTB3, 14 wild rice sequences in the CTB3 gene region were obtained from publicly available wild rice (O. rufipogon) genomes and compared and organized39,40,41,42, and the SNPs and Indels variation information of wild rice and cultivated rice were integrated together for analysis. To construct phylogenetic tree and haplotype network of OsTCP19, the SNPs information of wild rice and cultivated rice were integrated together for analysis69. The phylogenetic tree was constructed using the maximum-likelihood method with 1000 bootstrap replicates in MEGA7 software71. The tree was then visualized and annotated using EvolView (https://www.evolgenius.info/EvolView). For the construction of haplotype network, the sequence data was analyzed using DnaSP 5.1070 to generate NEX, HAP and ARP files. The NEX files were subsequently imported into PopART 1.7 software to construct the haplotype network using the minimum spanning method72.
Primers
All primers used in this study are shown in the Supplementary Data 7.
Statistical analyses
Data are presented as mean ± standard deviation with error bars. For pairwise group comparisons, inter-group significance was assessed through two-tailed t tests implemented in Microsoft Excel 2016. When analyzing datasets containing three or more experimental groups, we employed one-way ANOVA combined with Duncan’s multiple range test using IBM SPSS software (version 21.0).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within this paper and its Supplementary Information files. The genetic materials used in this study are available from the corresponding authors upon request. The RNA-seq data generated in this study have been deposited in the NCBI Sequence Read Archive database under BioProject accession PRJNA1106408. The processed transcriptomic data are available in the Gene Expression Omnibus (GEO) database under accession number GSE288722. Sequence data of the genes in this study is available from the RAPDB data libraries following accession: CTB3 (Os01g0923600) [https://rapdb.dna.affrc.go.jp/locus/?name=Os01g0923600], OsTCP19 (Os06g0226700) [https://rapdb.dna.affrc.go.jp/locus/?name=Os06g0226700], OsTPP1 (Os02g0661100) [https://rapdb.dna.affrc.go.jp/locus/?name=Os02g0661100], OsSweet15 (Os02g0513100) [https://rapdb.dna.affrc.go.jp/locus/?name=Os02g0513100], OsSweet11b (Os09g0508250) [https://rapdb.dna.affrc.go.jp/locus/?name=Os09g0508250], OsMST6 (Os07g0559700) [https://rapdb.dna.affrc.go.jp/locus/?name=Os07g0559700], UBQ5 (Os01g0328400) [https://rapdb.dna.affrc.go.jp/locus/?name=Os01g0328400], UBQ10 (Os02g0161900) [https://rapdb.dna.affrc.go.jp/locus/?name=Os02g0161900], and OsActin1 (Os03g0718100) [https://rapdb.dna.affrc.go.jp/locus/?name=Os03g0718100]. Source data are provided with this paper.
Code availability
The Perl scripts that are used to calculate the nucleotide diversity can be obtained from Zendo [https://doi.org/10.5281/zenodo.14587313].
References
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
Guo, H. F. et al. Differentiation, evolution and utilization of natural alleles for cold adaptability at the reproductive stage in rice. Plant Biotechnol. J. 18, 2491–2503 (2020).
Zhu, Y. J. et al. Identification and fine mapping of a stably expressed QTL for cold tolerance at the booting stage using an interconnected breeding population in rice. PLoS One 10, e0145704 (2015).
Fu, J. et al. Extreme rainfall reduces one-twelfth of China’s rice yield over the last two decades. Nat. Food 4, 416–426 (2023).
Li, J. H., Zhang, Z. Y., Chong, K. & Xu, Y. Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 268, 153576 (2022).
Han, J. J. et al. MORN motif-containing protein OsMORN1 and OsMORN2 are crucial for rice pollen viability and cold tolerance. Plant J. 119, 998–1013 (2024).
Fujino, K. et al. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl Acad. Sci. USA 105, 12623–12628 (2008).
Zhao, J., Wang, S. S., Qin, J. J., Sun, C. Q. & Liu, F. The lipid transfer protein OsLTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 18, 756–769 (2020).
Zhang, C. Z. et al. A transposon insertion in the promoter of OsUBC12 enhances cold tolerance during japonica rice germination. Nat. Commun. 15, 2211 (2024).
Ma, Y. et al. COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221 (2015).
Xia, C., Liang, G., Chong, K. & Xu, Y. Y. The COG1-OsSERL2 complex senses cold to trigger signaling network for chilling tolerance in japonica rice. Nat. Commun. 14, 3104 (2023).
Lu, G. et al. Rice LTG1 is involved in adaptive growth and fitness under low ambient temperature. Plant J. 78, 468–480 (2014).
Zhao, J. et al. A novel functional gene associated with cold tolerance at the seedling stage in rice. Plant Biotechnol. J. 15, 1141–1148 (2017).
Mao, D. H. et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc. Natl Acad. Sci. USA 116, 3494–3501 (2019).
Liu, C. T. et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 9, 3302 (2018).
Li, Z. T. et al. Natural variation of codon repeats in COLD11 endows rice with chilling resilience. Sci. Adv. 9, eabq5506 (2023).
Feng, J. L. et al. COG2 negatively regulates chilling tolerance through cell wall components altered in rice. Theor. Appl. Genet. 136, 19 (2023).
Liu, D. F. et al. COG3 confers the chilling tolerance to mediate OsFtsH2-D1 module in rice. N. Phytol. 241, 2143–2157 (2024).
Liu, C. T. et al. The bZIP73 transcription factor controls rice cold tolerance at the reproductive stage. Plant Biotechnol. J. 17, 1834–1849 (2019).
Zhang, Z. Y. et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 8, 14788 (2017).
Li, J. L. et al. Stepwise selection of natural variations at CTB2 and CTB4a improves cold adaptation during domestication of japonica rice. N. Phytol. 231, 1056–1072 (2021).
Saito, K., Hayano-Saito, Y., Kuroki, M. & Sato, Y. Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci. 179, 97–102 (2010).
Yang, L. M. et al. qCTB7 positively regulates cold tolerance at booting stage in rice. Theor. Appl. Genet. 136, 135 (2023).
Lou, Q. J. et al. Cold-adaptive evolution at the reproductive stage in Geng/japonica subspecies reveals the role of OsMAPK3 and OsLEA9. Plant J. 111, 1032–1051 (2022).
Sun, T. J. et al. Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol. Plant 13, 144–156 (2020).
Kidokoro, S. et al. Different cold-signaling pathways function in the responses to rapid and gradual decreases in temperature. Plant Cell 29, 760–774 (2017).
Wang, Y. et al. A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe 29, 1393–1406.e7 (2021).
Zeng, H. Q. et al. Arabidopsis CAMTA3/SR1 is involved in drought stress tolerance and ABA signaling. Plant Sci. 319, 111250 (2022).
Shkolnik, D., Finkler, A., Pasmanik-Chor, M. & Fromm, H. CALMODULIN-BINDING TRANSCRIPTION ACTIVATOR 6: A key regulator of Na (+) homeostasis during germination. Plant Physiol. 180, 1101–1118 (2019).
Koo, S. C. et al. The calmodulin-binding transcription factor OsCBT suppresses defense responses to pathogens in rice. Mol. Cells 27, 563–570 (2009).
Yu, S. B., Li, S. P., Wang, W. & Tang, D. Z. OsCAMTA3 negatively regulates disease resistance to magnaporthe oryzae by associating with OsCAMTAPL in rice. Int. J. Mol. Sci. 25, 5049 (2024).
Kan, Y. et al. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 8, 53–67 (2022).
Tian, F., Yang, D. C., Meng, Y. Q., Jin, J. P. & Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 48, D1104–D1113 (2020).
Tang, J. Q. et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell 34, 4495–4515 (2022).
Zhang, Z. Y. et al. OsMAPK3 Phosphorylates OsbHLH002/OsICE1 and Inhibits Its Ubiquitination to Activate OsTPP1 and Enhances Rice Chilling Tolerance. Dev. Cell 43, 731–743.e735 (2017).
Nuccio, M. L. et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat. Biotechnol. 33, 862–869 (2015).
Oszvald, M. et al. Trehalose 6-phosphate regulates photosynthesis and assimilate partitioning in reproductive tissue. Plant Physiol. 176, 2623–2638 (2018).
Fichtner, F. & Lunn, J. E. The role of trehalose 6-phosphate (Tre6P) in plant metabolism and development. Annu. Rev. Plant Biol. 72, 737–760 (2021).
Zhou, Y. et al. Pan-genome inversion index reveals evolutionary insights into the subpopulation structure of Asian rice. Nat. Commun. 14, 1567 (2023).
Brozynska, M. et al. Sequencing of Australian wild rice genomes reveals ancestral relationships with domesticated rice. Plant Biotechnol. J. 15, 765–774 (2017).
Zhang, F. et al. Long-read sequencing of 111 rice genomes reveals significantly larger pan-genomes. Genome Res. 32, 853–863 (2022).
Zhao, Q. et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 50, 278–284 (2018).
Yadav, U. P. et al. The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 65, 1051–1068 (2014).
Figueroa, C. M. et al. Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J. 85, 410–423 (2016).
Fichtner, F. et al. Functional Features of TREHALOSE-6-PHOSPHATE SYNTHASE1, an Essential Enzyme in Arabidopsis. Plant Cell 32, 1949–1972 (2020).
Blanford, J. et al. Molecular mechanism of trehalose 6-phosphate inhibition of the plant metabolic sensor kinase SnRK1. Sci. Adv. 10, eadn0895 (2024).
Fichtner, F. et al. Regulation of shoot branching in arabidopsis by trehalose 6-phosphate. N. Phytol. 229, 2135–2151 (2021).
Morales-Herrera, S. et al. Trehalose-6-phosphate signaling regulates lateral root formation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 120, e2302996120 (2023).
Van Leene, J. et al. Mapping of the plant SnRK1 kinase signalling network reveals a key regulatory role for the class II T6P synthase-like proteins. Nat. Plants 8, 1245–1261 (2022).
Li, Z. Y. et al. The OsNAC23-Tre6P-SnRK1a feed-forward loop regulates sugar homeostasis and grain yield in rice. Mol. Plant 15, 706–722 (2022).
Yang, J., Luo, D., Yang, B., Frommer, W. B. & Eom, J. S. SWEET11 and 15 as key players in seed filling in rice. N. Phytol. 218, 604–615 (2018).
Wu, L. B. et al. OsSWEET11b, a potential sixth leaf blight susceptibility gene involved in sugar transport-dependent male fertility. N. Phytol. 234, 975–989 (2022).
Li, P., Wang, L. H., Liu, H. B. & Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 10, 98–108 (2022).
Zhang, H. et al. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22, 672–689 (2010).
Yuan, P. G., Tanaka, K. & Poovaiah, B. W. Calmodulin-binding transcription activator AtSR1/CAMTA3 fine-tunes plant immune response by transcriptional regulation of the salicylate receptor NPR1. Plant Cell Environ. 44, 3140–3154 (2021).
Liu, Y. Q. et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 590, 600–605 (2021).
Xing, H. L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 271–282 (1994).
Li, G. L. et al. RGN1 controls grain number and shapes panicle architecture in rice. Plant Biotechnol. J. 20, 158–167 (2022).
Hellens, R. P. et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13 (2005).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Shao, Y. L. et al. OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell 31, 1257–1275 (2019).
Luo, X. T. et al. Sensitive analysis of trehalose-6-phosphate and related sugar phosphates in plant tissues by chemical derivatization combined with hydrophilic interaction liquid chromatography–tandem mass spectrometry. J. Chromatogr. 1592, 82–90 (2019).
Varandas, S. et al. Glucose and fructose levels on grape skin: Interference in Lobesia botrana behaviour. Anal. Chim. Acta 513, 351–355 (2004).
Kabasakalian, P., Kalliney, S. & Westcott, A. Enzymatic blood glucose determination by colorimetry of N, N -diethylaniline-4-aminoantipyrine. Clin. Chem. 20, 606–607 (1974).
Wang, W. S. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49 (2018).
Bradbury, P. J. et al. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Leigh, J. W. & Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecology Evolut. 6, 1110–1116 (2015).
Acknowledgements
We thank Prof. Jian Zhang from State Key Lab of Rice Biology, China National Rice Research Institute for the gift of transgenic materials of OsTPP1, tpp1-1, tpp1-2 and the wild-type DJ. This work was supported by the grants from the National Key Research and Development Program of China (2023YFF1000400 to Jinjie Li), National Natural Science Foundation of China (32372080 to Jinjie Li) and China Postdoctora Science Foundation (2024M763581 to Y.L.).
Author information
Authors and Affiliations
Contributions
Jinjie Li designed the research. J.L., H.G. together with Q.L., Z.G., Y.G., S.G., B.X. and S.H. performed most of experiments and analyzed the data. P.X. generated OsTCP19-related transgenic plants. Y.Z. was responsible for cold tolerance evaluation under CS-HAA. R.S., A.D., W.Y., M.Z. and Y.L. performed part of the experiments. X.S., Z.Z, H.Z, D.M., C.C and Z.L. conceived and supervised the research. J.L., H.G. and Jinjie Li wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Guo, H., Lou, Q. et al. Natural variation of indels in the CTB3 promoter confers cold tolerance in japonica rice. Nat Commun 16, 1613 (2025). https://doi.org/10.1038/s41467-025-56992-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56992-7
This article is cited by
-
Suppressing an auxin efflux transporter enhances rice adaptation to temperate habitats
Nature Communications (2025)