Abstract
The effectiveness of the hepatitis E vaccine in high-risk groups, such as chronic hepatitis B (CHB) patients, remains understudied. A key clinical manifestation of CHB is the persistent positivity of hepatitis B surface antigen (HBsAg). We conducted a test-negative design study involving 2,926 HBsAg-positive individuals (born 1941–1991; median age 49.0; male-to-female ratio of 1.4), identified through a hepatitis surveillance system, as part of the phase 3 trial (NCT01014845) of the recombinant hepatitis E vaccine HEV 239 (Hecolin). This system monitored suspected hepatitis cases and performed diagnoses across 11 townships in Dongtai, Jiangsu, China, from 2007 to 2017. Vaccine effectiveness of HEV 239 was assessed by comparing vaccination status between confirmed 96 hepatitis E cases and 2830 test-negative controls, using logistic regression adjusted for sex and age. We found that HEV 239 vaccination was associated with a reduced risk of hepatitis E among HBsAg-positive individuals, with an estimated effectiveness of 72.1% [95% confidence interval (CI) 11.2–91.2], and 81.5% (95% CI 35.9–94.6) among phase 3 trial participants. Our findings show that HEV 239 is highly effective in HBsAg-positive adults, supporting its future recommended use in this population.
Similar content being viewed by others
Introduction
Hepatitis E virus (HEV) stands as a prominent cause of viral hepatitis, constituting a global health concern1. Despite being largely self-limiting, HEV infection can lead to severe complications, particularly among individuals with pre-existing chronic liver diseases (CLD), immunocompromised patients, and pregnant women2,3. Meta-analysis results have revealed significantly elevated risks of liver failure (OR: 5.5, 95% CI: 1.5-20.1) and mortality (OR: 5.0, 95% CI: 1.9-13.3) in CLD patients with HEV superinfection compared to those without4. This issue is further exacerbated by the considerable overlap of hepatitis B virus (HBV) and HEV endemicity in many Asian countries, where HEV infection is an important trigger of acute-on-chronic liver failure5,6. Additionally, the reactivation of chronic hepatitis B (CHB) led by acute HEV superinfection7,8, and adverse outcomes among organ transplant recipients or HIV-infected patients caused by HBV and HEV dual-infection9,10,11, have been reported in high-income countries.
The recombinant hepatitis E vaccine, HEV239 (Hecolin; Xiamen Innovax Biotech, Xiamen, China), has been the sole vaccine available for hepatitis E since 201212,13. Hecolin has demonstrated excellent long-term efficacy, safety, and immunogenicity in adults13,14,15, and shown to be safe and immunogenic in hepatitis B carriers or CHB as well16,17. However, the lack of additional efficacy or effectiveness data in CLD or CHB patients hinders the prioritization of this vaccine for these populations, leaving a critical gap in hepatitis E prevention strategies targeting the vulnerable groups5,18,19.
In areas of high endemicity, such in China, HBV is transmitted mostly perinatally from infected mothers to neonates, while the risk of progression from acute to chronic HBV infection is 90% when infection occurs in infants20. It is estimated that ~93 million individuals in China are living with HBV infection, with an HBsAg prevalence of about 8% among those aged 15–59 years21,22. And the vast majority (about 90%) of hepatitis B reported in China are clinically diagnosed as CHB23,24. Given that hepatitis B surface antigen (HBsAg) positivity is one of the clinical manifestations of CHB and considering the large population of adult CHB in China, here we conducted a test-negative design study to assess the vaccine effectiveness (VE) of Hecolin against symptomatic hepatitis E among adults who tested positive for HBsAg, representing individuals at high risk of CHB.
Results
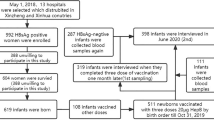
In the phase 3 trial of Hecolin and its extended follow-up study (NCT01014845)13, a cumulative total of 313,536 suspected hepatitis events were noted over a 10 year period (from August, 2007 to October, 2017) in the study area, of which 12,787 were found to have alanine aminotransferase (ALT) ≥ 2.5 upper limit of normal (ULN), and 12,707 completed the laboratory testing for HEV-related markers. Through the preliminary hepatitis typing testing, a total of 3282 events were found to be positive for HBsAg, and finally 2926 individuals born between 1941 and 1991 were identified and included in the analysis of this study, with a median age of 49.0 years [interquartile range (IQR) 40.0–58.0] and a male-to-female ratio of 1.4. 96 out of 2926 (3.3%) individuals with positive HBsAg were confirmed as hepatitis E cases and categorized as test-positive cases, while the remaining 2830 were categorized as test-negative controls (Fig. 1). The distribution of acute infection detection markers among the tested population is detailed in Supplementary Table 1. No non-trial participants were found to have received any dose of Hecolin during the observation period. To assess the VE of Hecolin in HBsAg-positive adults, we compared vaccination status between confirmed hepatitis E cases and controls, using logistic regression adjusted for sex and age.
ALT alanine aminotransferase, ULN upper limit of normal, HEV hepatitis E virus, HBsAg hepatitis B surface antigen. *Hepatitis E-related laboratory testing was not completed for 80 events because of insufficient sample availability. Individuals exhibiting hepatitis-like symptoms (fatigue or loss of appetite) lasting for three or more consecutive days underwent ALT testing. Suspected hepatitis cases were defined as those with ALT levels at least 2.5 times the ULN, indicating acute hepatitis. Preliminary hepatitis typing tests, including assays for HBsAg, hepatitis B core antibody (HBcAb), hepatitis A virus IgM antibody, hepatitis C virus IgG antibody and anti-HEV IgM, was performed on the first serum sample from each suspected case. A second serum sample was required to be collected 2–6 weeks later and also tested for anti-HEV IgM. Any positive result for anti-HEV IgM in either of the two samples prompted regular follow-up sampling until ALT levels normalized or symptoms resolved. All serial serum samples were sent to a central laboratory for hepatitis E diagnosis. A positive diagnosis for hepatitis E was made if at least two acute HEV markers were present (i.e., HEV RNA, IgM anti-HEV, and a fourfold or greater increase in IgG anti-HEV).
In the analysis of clinical characteristics comparing test-positive cases with test-negative controls (Table 1), we observed that the median age at onset of hepatitis-like symptoms in cases was 53.5 years (IQR 44.0-63.0), which was significantly older (P = 0.0028) compared to controls (49.0 years, IQR 40.0–58.0); this difference primarily arose from the unscreened non-trial participants. Although there was no significant difference in sex distribution between the two groups overall, among trial participants the proportion of male test-positive cases was significantly higher (75% vs 51%, P = 0.0350). Furthermore, ALT levels were significantly higher in test-positive cases (15.2 ULN vs 5.6 ULN, P < 0.0001), irrespective of whether they were trial or non-trial participants. Taking the test-negative controls as reference, we found a significant association between Hecolin vaccination status and a reduced proportion of hepatitis E [Crude odds ratio (OR): 0.30, 95% confidence interval (CI) 0.09-0.94]. After controlling for sex and age, the corresponding estimated VE for at least one dose was 72.1% (95% CI 11.2–91.2). Moreover, when restricted to the 600 trial participants, the estimated VE appeared higher. Specifically, the VE of at least one dose of Hecolin among trial participants was 81.5% (95%CI 35.9–94.6) (Table 2). The specific age distributions of test-positive cases and test-negative controls are detailed in Supplementary Table 2.
Discussion
In this study, we demonstrate that Hecolin confers long-lasting (2007–2017) and substantial protection against hepatitis E in populations positive for HBsAg. To our knowledge, prior studies have not evaluated the effectiveness of the hepatitis E vaccine in HBsAg-positive populations, who are considered at high risk for CHB. While the question of whether HBsAg-positive individuals or those with CLD exhibit a higher prevalence of HEV infection remains debatable25,26,27,28, it is widely recognized that HEV superinfection in CLD patients is associated with more severe clinical outcomes29,30,31. The United States and several European countries have advocated for hepatitis A vaccination in CLD patients to reduce the disease burden of dual-infection32,33. However, in some countries especially in Asia where the incidence of hepatitis E is higher than that of hepatitis A, there have been no plans to promote hepatitis E vaccination among CLD patients, primarily due to a lack of data on the safety and efficacy or effectiveness of Hecolin in this population. Given that CLD patients represent a high-risk group that could benefit significantly from the hepatitis E vaccine, clinical data supporting the use of Hecolin in these patients is urgently needed18. The findings regarding the high effectiveness of Hecolin among HBsAg-positive individuals here provide valuable insights, which implied promising prospects for hepatitis E vaccine deployment in such population, especially within regions grappling with high incidence of CHB or CLD.
Intriguingly, we observed that the VE was 9.4% lower when non-trial participants were included compared to when they were excluded, suggesting potential confounding factors. Trial-participants were required to have no history of hepatitis B or E at enrollment; and subsequent antibody assessments revealed no differences in baseline anti-HEV IgG positivity rates between the vaccine and placebo groups (Supplementary Fig. 1), supporting the point that both groups had similar HEV exposure risks after screening and randomization. In contrast, the non-trial participants were not pre-screened, and their unknown infection history and disease status likely contributed to the observed discrepancy in the VE results. For instance, studies of SARS-CoV-2 vaccines using test-negative designs have shown that failing to account for prior infection history can underestimate VE34. Notably, compared to traditional case-control or cohort designs, the test-negative design used in this study is less susceptible to bias arising from differences in healthcare-seeking behavior between cases and controls35,36. However, the potential influence of other unmeasured or unknown confounders cannot be ruled out.
Previous studies have linked acute HEV superinfection with accelerated hepatocytes damage and liver failure in patients with CHB, as evidenced by elevated transaminases and deteriorating liver function, respectively37. Nevertheless, the impact of HEV superinfection in HBV DNA replication and its underlying mechanisms remain unclear. A concept that hepatocytes injury is predominantly triggered by HEV and only a trivial effect from HBV in hepatocytes injury has been proposed by several researchers37,38,39. Cheng et al. reported that HBV infection was dormant during acute HEV infection, and the profound clinical effect during coinfection was significantly triggered by HEV38. In our study, we also observed elevated ALT levels among individuals co-infected with HEV and HBV compared to those with HBV monoinfection, supporting this intriguing point. Conversely, a few studies argue that HBV exerts a dominant effect in coinfection or that HEV has no impact on HBV DNA replication25,40. Thus, further research is warranted to elucidate the underlying mechanisms of the immune-mediated responses initiated by both HBV and HEV viruses, which may enhance our understanding of the effects of dual infection and lead to improved strategies of prevention and treatment.
Some limitations must be considered in this study. First, the hepatitis surveillance system was initially designed to identify hepatitis E, which resulted in notable shortcomings in diagnosing other types of hepatitis. CHB is often defined by HBsAg positivity for >6 months but we did not continuously monitor this in our study. Consequently, it was almost impossible to accurately differentiate between acute and chronic infections of hepatitis B based on the available laboratory data. Second, the limited number of observed hepatitis E cases was insufficient to support further stratified analyses, including stratification by time period, age, or sex groups. Additionally, since the vast majority of vaccine recipients included in the analysis completed the three-dose regimen, it is currently not possible to compare the VE for individuals among different dosage groups. Third, beyond the limitation of unknown infection history and disease status in the non-trial population, this study did not explore the impact of environmental exposures, socioeconomic factors, baseline liver function before infection, or missing data on the outcomes. It should be noted that 10 years of case surveillance in the study region revealed that over 90% of hepatitis E were caused by HEV genotype-4 (with no genotype-1 cases observed since 2012)13. This zoonotic genotype is primarily associated with individual dietary hygiene practices and occupational exposure. Last, it is generally recognized that case-control studies achieve optimal power when vaccination coverage ranges between 20% and 80%41. In this study region, however, the Hecolin coverage rate was ~19%, potentially reducing the statistical power of the analysis.
In conclusion, this test-negative design study observed a notable effectiveness of Hecolin against hepatitis E in HBsAg-positive populations, shedding light on the potential expansion of vaccine applicability to broader populations. However, further studies are warranted to enhance the evidence regarding the efficacy or effectiveness and safety of Hecolin in individuals with pre-existing CLD.
Methods
Study design and setting
This test-negative design study was done as part of the long-term follow-up for a single-centre, double-blind, randomized, placebo-controlled, phase 3 trial of Hecolin in adults in Dongtai County, Jiangsu Province, China, spanning from August, 2007 to October, 2017 (ClinicalTrial.gov, NCT01014845)13,14,15. As previously described, between 22 August and 6 November, 2007, a total of 112,604 adults aged 16–65 years from 11 townships were enrolled and randomly assigned (stratified by age and sex) in a 1:1 ratio to received three doses of Hecolin or placebo (hepatitis B vaccine)14. Among these participants, all individuals from the Anfeng and Qindong townships were selected as the immunogenicity subgroup for the phase 3 clinical trial, based on considerations such as sample size and feasibility, to evaluate the immunogenicity and immune persistence13,14,15.
At the beginning of the Hecolin phase 3 trial, a comprehensive active hepatitis surveillance system, including 205 clinical sentinel sites (village clinics, private clinics, town hospitals, municipal hospitals, etc.), was established to cover the entire study region and maintained for 10 years post-vaccination. Details of the surveillance methods have been described previously13,14,15. Briefly, individuals exhibiting hepatitis-like symptoms (fatigue or loss of appetite) lasting three or more consecutive days underwent alanine aminotransferase (ALT) testing. Suspected hepatitis cases were defined as those with ALT levels at least 2.5 times higher than the upper limit of normal (ULN), indicating acute hepatitis. A preliminary hepatitis typing testing, including tests for HBsAg, HBcAb, anti-HAV IgM, anti-HCV IgG and anti-HEV IgM, was performed on the first serum sample from each suspected hepatitis case. A second serum sample was required to be collected 2–6 weeks later and also tested for anti-HEV IgM. Any occurrence of anti-HEV IgM positivity in either of the two samples prompted regular follow-up sampling until ALT levels normalized or symptoms resolved. All serial serum samples were sent to a central laboratory for testing HEV-related markers (HEV RNA, IgM and IgG anti-HEV). Suspected cases with detectable anti-HEV IgM antibody or anti-HEV IgG antibody levels at least twice as high as any previous sample during the same illness episode underwent HEV RNA testing.
To evaluate the effectiveness of Hecolin in HBsAg-positive individuals, this test-negative design study focused on the HBsAg-positive population identified through hepatitis typing within the surveillance system. Confirmed hepatitis E cases were classified as test-positive, while non-confirmed cases were classified as test-negative.
Participants
Through the hepatitis surveillance system, Dongtai County conducted hepatitis case monitoring for over 10 years, covering ~290,000 permanent residents born between 1941 and 1991 (the target population of the phase 3 trial of Hecolin). Following the large-scale phase 3 trial, the Hecolin vaccination coverage rate in the target population (born between 1941 and 1991) was ~19%. Participants with health-seeking behaviors entered the test-negative design study through the surveillance system, including the following two groups: a. Individuals who participated in the Hecolin phase 3 trial and met the inclusion criteria, including being aged 16–65 years in 2007, having no history of hepatitis B or E, no immunodeficiency, no severe chronic diseases, and meeting other trial-specific eligibility requirements; b. Local residents of study region who did not participate in the Hecolin clinical trial, received no interventions, and were within the same age range as trial participants.
Sex information was determined based on identity cards (trial participants) or medical records (non-trial participants). Site investigators employed personal identity information, such as fingerprints and photographs, obtained from phase 3 trial to identify whether suspected cases observed in the surveillance system were indeed trial-participants. For trial-participants, Hecolin vaccination status was obtained from the electronic records of the phase 3 clinical trial, while for non-trial-participants, the vaccination status would be check by the local Immunization Information System. It is important to note that although Hecolin became commercially available in China in 2012, its initial uptake (self-paid vaccination) was low due to insufficient public awareness and education. Furthermore, the timing of vaccine introduction varied across urban and rural areas with different economic levels. Prior to performing this study, investigators reviewed local vaccination records and consulted regional sales, revealing that there was no self-paid vaccination of Hecolin in Dongtai from 2012 to 2017.
In addition, for the trial-participants in the immunogenicity subgroup, serum samples were collected at baseline and at multiple time points post-vaccination. The details of the serological follow-up have also been previously reported13,14,15.
Definitions
We defined confirmed hepatitis E cases (i.e., the test-positive group) as individuals who sought medical care and met the following criteria: symptoms persisting for at least 3 days, ALT level ≥ 2.5 ULN, and at least two of the following: positive HEV RNA, positive anti-HEV IgM antibody, and a fourfold or greater increase in anti-HEV IgG antibody concentrations13. Suspected hepatitis cases that were tested but did not fully meet these criteria were defined as the test-negative group.
Laboratory measurements
ALT concentrations were quantified by the Dongtai Centre for Disease Control and Prevention (CDC) or the municipal hospitals using commercial assay kits (BioSino, Beijing, China) in international units per litre of blood (units/L), and reported as multiples of the ULN. According to the kit instructions, the ULN values stand at 40 units/L for men and 31 units/L for women13,15. Hepatitis typing and anti-HEV IgG antibody detection were conducted using commercial ELISA kits (Beijing Wantai Biological Pharmacy, Beijing, China; see Supplementary Table 3 for detailed performance data of the kits) following standardized procedures provided by the manufacturer. Preliminary hepatitis typing, involving serum samples from all suspected hepatitis cases, was performed by Dongtai CDC, while HEV-related markers testing for the final diagnosis of hepatitis E, as well as immunogenicity measurements for the subgroup, were done by the central laboratory. HBsAg and anti-HEV IgM levels were expressed as a ratio of signal to cutoff (s/co), with a positive result defined as ≥ 1.0 s/co13,42. Concentrations of anti-HEV IgG were determined in parallel with WHO reference reagent (NIBSC code 95/584) and expressed in WHO unit (WU) per mL13.
Statistical analysis
All suspected hepatitis patients with HBsAg positivity were included in this study and categorized into two groups according to their diagnosis results for hepatitis E (i.e., test-positive cases and test-negative controls). The characteristics of cases and controls were compared using χ2 tests, t-tests with logarithmic transformation or Wilcoxon rank-sum tests. In the test-negative design analyses, unconditional logistic regression models were utilized to compute the odds ratio (OR) and its corresponding 95% confidence intervals (CIs) to assess the association between vaccination and the status of being a case or control. The potential confounders such as age (at suspected symptom onset) and sex were included as covariates in the models to evaluate adjusted OR (aOR). Vaccine effectiveness (VE) estimates were then derived as (1-aOR) × 100%.
All analyses were performed using SAS software (version 9.4), and missing data were not imputed. Reported P-values were two-sided with a significance level (α) of 0.05.
Ethics declaration
This work was approved by the ethics committee of the Jiangsu Provincial Center for Disease Control and Prevention and Xiamen University. Written informed consent was obtained from each participant of the Hecolin clinical trial, while the requirement for non-participants included in the analysis to provide informed consent was waived due to the study’s observational nature without any deviation from the local medical practice, and all data for the analysis were anonymized.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The individual-level raw data in this study were collected as part of a clinical trial and subject to confidentiality restrictions. The processed data are available under restricted access. Researchers may submit a detailed study proposal (non-commercial purpose) to access processed data from 6 months to 36 months after publication, directed to zhangj@xmu.edu.cn or yingyingsu@xmu.edu.cn. The corresponding authors will promptly review the request and determine whether the requested data can be shared, and will respond within 8 weeks of receiving the request. Any data that can be shared will be released via a material transfer agreement. The data will be available for 1 year once access has been granted.
References
Kamar, N. et al. Hepatitis E virus infection. Nat. Rev. Dis. Primers 3, 17086 (2017).
Larrue, H., Abravanel, F. & Péron, J. M. Hepatitis E, what’s the real issue? Liver Int. 40, 43–47 (2020).
Ma, Z., de Man, R. A., Kamar, N. & Pan, Q. Chronic hepatitis E: advancing research and patient care. J. Hepatol. 77, 1109–1123 (2022).
Qiu, L. X. et al. Prognosis of hepatitis E infection in patients with chronic liver disease: a meta-analysis. J. Viral Hepat. 30, 101–107 (2023).
Nasir, M. & Wu, G. Y. HEV and HBV dual infection: a review. J. Clin. Transl. Hepatol. 8, 313–321 (2020).
Mezzano, G. et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 71, 148–155 (2022).
Aslam, A., Susheela, A., Iriana, S., Chan, S. S. & Lau, D. Acute hepatitis E superinfection leading to chronic hepatitis B reactivation. BMJ Case Rep. 2018, bcr2017223616 (2018).
Obeidat, A. E., Monti, G., Sae-Ow, W., Shinoda, H. & Lim, H. Hepatitis E virus superinfection: an underrecognized trigger of acute hepatitis B virus flare. Cureus 13, e13809 (2021).
Zhou, X. et al. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev. Med. Virol. 23, 295–304 (2013).
Kamar, N. et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 140, 1481–1489 (2011).
Piroth, L. et al. Epidemiology, diagnosis and treatment of chronic hepatitis B in HIV-infected patients (EPIB 2005 STUDY). Aids 21, 1323–1331 (2007).
Zhang, J., Zheng, Z. & Xia, N. Prophylactic hepatitis E vaccine. Adv. Exp. Med. Biol. 1417, 227–245 (2023).
Huang, S. et al. Long-term efficacy of a recombinant hepatitis E vaccine in adults: 10 year results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 403, 813–823 (2024).
Zhu, F. C. et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 376, 895–902 (2010).
Zhang, J. et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 372, 914–922 (2015).
Wu, T. et al. Immunogenicity and safety of hepatitis E vaccine in healthy hepatitis B surface antigen positive adults. Hum. Vaccin. Immunother. 9, 2474–2479 (2013).
HEPATOLOGY. Posters (Abstracts 301–2389). Hepatology 68, 184–1353 (2018).
WHO. Hepatitis E vaccine: who position paper, May 2015. Vaccine 34, 304–305 (2016).
Peron, J. M., Larrue, H., Izopet, J. & Buti, M. The pressing need for a global HEV vaccine. J. Hepatol. 79, 876–880 (2023).
Trépo, C., Chan, H. L. & Lok, A. Hepatitis B virus infection. Lancet 384, 2053–2063 (2014).
Fang, K. et al. Trends in disease burden of hepatitis B infection in Jiangsu province, China, 1990-2021. Infect. Dis. Model 8, 832–841 (2023).
Liang, X. et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine 27, 6550–6557 (2009).
Meng, T. T. et al. [Analysis on hepatitis B cases reported from surveillance points in China, 2019]. Zhonghua. Liu. Xing. Bing. Xue. Za. Zhi. 42, 1532–1536 (2021).
Miao, N., Wang, F. Z., Zheng, H., Zhang, G. M. & Yin, Z. D. Estimation of incidence of viral hepatitis B and analysis on case characteristics in China,2013-2020. Zhonghua. Liu. Xing. Bing. Xue. Za. Zhi. 42, 1527–1531 (2021).
Hoan, N. X. et al. Hepatitis E virus superinfection and clinical progression in hepatitis B patients. EBioMedicine 2, 2080–2086 (2015).
Zhang, L. et al. Comparison of hepatitis E virus seroprevalence between HBsAg-positive population and healthy controls in Shandong province, China. BMC Infect Dis. 18, 75 (2018).
Bayram, A., Eksi, F., Mehli, M. & Sözen, E. Prevalence of hepatitis E virus antibodies in patients with chronic hepatitis B and chronic hepatitis C. Intervirology 50, 281–286 (2007).
Zhu, F. C. et al. Epidemiology of zoonotic hepatitis E: a community-based surveillance study in a rural population in China. PLoS ONE 9, e87154 (2014).
Tseng, T. C. et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 72, 1105–1111 (2020).
Zhao, H. et al. Hepatitis E virus superinfection impairs long-term outcome in hospitalized patients with hepatitis B virus-related decompensated liver cirrhosis. Ann. Hepatol. 28, 100878 (2023).
Choi, J. W. et al. Acute hepatitis E virus superinfection increases mortality in patients with cirrhosis. BMC Infect. Dis. 22, 62 (2022).
CDC. Hepatitis A Vaccine. https://www.cdc.gov/hepatitis-a/vaccination/index.html (2024).
NHS. Hepatitis A. https://www.nhs.uk/conditions/hepatitis-a/ (2022).
Wiegand, R. E. et al. Bias and negative values of COVID-19 vaccine effectiveness estimates from a test-negative design without controlling for prior SARS-CoV-2 infection. Nat. Commun. 15, 10062 (2024).
Haber, M. et al. A probability model for evaluating the bias and precision of influenza vaccine effectiveness estimates from case-control studies. Epidemiol. Infect. 143, 1417–1426 (2015).
Ozasa, K. & Fukushima, W. Commentary: test-negative design reduces confounding by healthcare-seeking attitude in case-control studies. J. Epidemiol. 29, 279–281 (2019).
Kilonzo, S. B. et al. Superinfective hepatitis E virus infection aggravates hepatocytes injury in chronic hepatitis B. Curr. Med. Sci. 39, 719–726 (2019).
Cheng, S. H. et al. Influence of chronic HBV infection on superimposed acute hepatitis E. World J. Gastroenterol. 19, 5904–5909 (2013).
Yeh, C. T., Yeh, C. S., Chu, Y. D. & Chiang, Y. J. Seroclearance of hepatitis B surface antigen following hepatitis E exacerbation on chronic hepatitis E and B dual infection in a renal transplant recipient: a case report. J. Med. Case Rep. 12, 50 (2018).
Zhang, X. et al. Comparison of effects of hepatitis E or A viral superinfection in patients with chronic hepatitis B. Hepatol. Int. 4, 615–620 (2010).
Verani, J. R. et al. Case-control vaccine effectiveness studies: preparation, design, and enrollment of cases and controls. Vaccine 35, 3295–3302 (2017).
Wen, G. P. et al. Prevalence of hepatitis E virus and its associated outcomes among pregnant women in China. Pathogens 12, 1072 (2023).
Acknowledgements
This study was supported by National Key Research and Development Plan (2023YFC2307602) (to J.Z.), the National Natural Science Foundation of China (81991490) (to N.X.), Xiang An Innovation Laboratory (2023XAKJ0101006) (to Y.H.), Postdoctoral Fund (2023M732935) (to Y.H.), the Open Research Fund of State Key Laboratory of Vaccines for Infectious Diseases (Grant No. 2024SKLVDkf08) (to C.Z.), and the Fundamental Research Funds for the Central Universities (20720220006) (to N.X.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.Z. and N.X. conceived of the study. H.J., X.Z., Y.W., C.Y., D.L. and X-F.Z. were investigators. Z.Z., X.H., J.L., K.Z., M.L. and L.C. were responsible for sample testing or management. S.H. and Y.S. managed the data. C.Z. performed the statistical analysis. C.Z. and X.L. drafted the manuscript, and J.Z., Y.S., T.W. and Y.H. critically revised it.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Francisco Diaz-Mitoma and Wen-Qiang He for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhuang, C., Liu, X., Huang, X. et al. Effectiveness of a hepatitis E vaccine against medically-attended symptomatic infection in HBsAg-positive adults from a test-negative design study. Nat Commun 16, 1699 (2025). https://doi.org/10.1038/s41467-025-57021-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57021-3
This article is cited by
-
Long-term protection from naturally acquired immunity against hepatitis E virus reinfection
Nature Communications (2025)
-
Substantial spillover burden of rat hepatitis E virus in humans
Nature Communications (2025)