Abstract
The delay between the origin of animals in the Neoproterozoic and their Cambrian diversification remains perplexing. Animal diversification mirrors an expansion in marine shelf area under a greenhouse climate, though the extent to which these environmental conditions directly influenced physiology and early organismal ecology remains unclear. Here, we use a biogeochemical model to quantify oxygen dynamics at the sunlit sediment-water interface over day-night (diel) cycles at warm and cold conditions. We find that warm temperatures dictated physiologically stressful diel benthic oxic-anoxic shifts over a nutrient-rich shelf. Under these conditions, a population-and-phenotype model further show that the benefits of efficient cellular oxygen sensing that can offer adaptations to stress outweigh its cost. Since diurnal benthic redox variability would have expanded as continents were flooded in the end-Neoproterozoic and early Palaeozoic, we propose that a combination of physiological stress and ample resources in the benthic environment may have impacted the adaptive radiation of animals tolerant to oxygen fluctuations.
Similar content being viewed by others
Introduction
Animals diversified dramatically during the first half of the Cambrian Period (540–490 million years ago; Ma)1 in the shallow benthic environment2 (Fig. 1A). Multiple studies have investigated how temperature, nutrients and oxygen gradients influenced animal diversity in the Cambrian Period3. However, the specific mechanisms by which these abiotic conditions influenced subsequent changes in animal diversity patterns at the Neoproterozic-Paleozoic boundary remains unresolved, and no single hypothesis fully explains the timing of the event and nor is it likely to4. For example, it is suggested that environmental oxygen concentrations increased above a threshold permissive for a diversity of animal species at some point in time, but we lack understanding of a line of events that would have spurred the diversification of animals after this threshold5. In contrast to how other biotic turnover events are linked to harsh conditions6, the Cambrian explosion is rarely explored as the result of physiological stress. Recent investigations, however, note that recurring marine anoxia and heterogenous water column oxygenation may have accelerated evolutionary innovations at the Neoproterozic-Paleozoic boundary7,8,9 and over the Phanerozoic Eon6,9. More generally, it is well established that cellular and physiological stress is a driver for change in evolutionary biology10. Rapid evolutionary change in organisms as diverse as Ordovician trilobites and modern fruit flies is associated with environmental fluctuations and resource availability above the needs of survival and maintenance11. For the Cambrian, however, it remains unknown, and we here aim to test, whether diel oxygen fluctuations would have been present to accelerate diversification10.
At this boundary, the temporal extent of potentially global glaciations of the Cryogenian (blue vertical fields) is depicted as well as this study’s region of interest (ROI). A The sandy shelf area in Laurentia and its increase in extent over the Neoproterozoic-Palaeozoic, from ref. 14 (black line, grey field represent bootstrap resampled error at ±1 std. dev.). B One modelled global average surface air temperature representation (at low latitude) modified from ref. 34, see also supplementary information and Supplementary Fig. 1 for comparison with clumped isotope data from ref. 27. Grey field represent ±1 std. dev and +25 °C marking a transition from cold (blue) and warm (red) climate states. A gradual shift from an icehouse to a greenhouse climate (blue-red colour). C The divergence of animal clades (one of several possible time-calibrated trees, based on ref. 96, with approximate divergence between lineages based on ref. 97 and ref. 71). Boxes represent early branching clades and Bilateria that today perform cellular oxygen sensing mechanisms with the Hypoxia-Inducible Factor (HIF) system (filled orange) or not (grey), based on refs. 43,53. The positions of Ctenophora and Placozoa are based on ref. 96.
The benthic Cambrian habitat in which animals primarily diversified2 expanded with large-scale global flooding of continental fragments from Rodinia and Pannotia12,13. In Laurentia, sandy sediment deposits in shallow settings increased in areal extent four-fold during the early Cambrian Period, as demonstrated by macrostratigraphic summaries of rock quantities in North America14 and consistent with strontium isotope data12,15,16 (Fig. 1A). This flooding resulted in a dramatic expansion in sunlit shallow water sediments where benthic microalgae photosynthesis likely regulated the diel oxygen dynamics and seafloor redox conditions, as in modern seas17,18. Given that microalgae contribute substantially to the benthic oxygen budget in the modern coastal ocean19,20 and became ecologically significant in the Cryogenian-Ediacaran21,22, their presence can be presumed to have contributed to pronounced diel benthic oxygen fluctuations in the end-Neoproterozoic and early Palaeozoic. Notably, the microalgae photosynthetic oxygen production and oxygen consumption through heterotrophic respiration at the sediment-water interface is fundamentally controlled by temperature23. Temperature also regulates diffusive transport mechanisms and oxygen solubility in seawater. With increasing temperature, diffusive transport mechanisms increase and oxygen solubility decrease that leads to faster transport rates but less availability of oxygen. During the Cambrian Period, global temperatures were generally defined by a greenhouse climate as indicated by modelling efforts24,25, oxygen isotope palaeothermometry26 and climatically sensitive lithologies27,28,29,30,31,32 (Fig. 1B, S1). Annual equatorial sea surface temperatures have been estimated as high as 30–38 °C in the Cambrian32. Although reconstructions suggest a variable climate over the post-Marinoan33, both shallow shelf area and global temperature increased between the Cryogenian and the Cambrian periods14,27,34. The higher temperatures would have increased photosynthesis-driven gross oxygen production, the molecular diffusion of oxygen, and the coupled oxygen consumption in benthic microalgae communities. Consequently, oxygen dynamics and variability in oxygen concentrations between day and night likely would have been amplified with higher temperatures, leading to temporally amplified daily redox fluctuations35 and more physiologically stressful conditions for early animals in the expanding shallow benthic habitats.
Physiological stress is a potential driver of the emergence of evolutionary innovations10. In addition to increasing genetic variation (e.g. mutations), environmental stress lets a population probe alternative phenotypic states36. Since beneficial stress-tolerant traits have high heritability under stressful conditions11, increased variance channelled through existing functional systems can both account for similarity and lead to adaptive radiation10. For a scenario where environmental stress leads to adaptive radiation, however, it is suggested that fluctuations are predictable and combined with a surplus of energy to drive organisms’ metabolism (beyond what is needed for survival and maintenance)11. In contrast, constant extreme physiological stress (e.g. saline pools) or low-energy environments (e.g. dark caves) would hinder phenotypic explorations and, therefore, adaptive radiation10,36.
A specific trait that allows extant animals to tolerate environmental fluctuations is cellular oxygen sensing37. Oxygen-sensing mechanisms regulated by Hypoxia-Inducible Factor alpha (HIF-α) allow animals to orchestrate tissue homoeostasis by sensing and responding to redox fluctuations37. Oxygen shortage can be physiologically stressful for multicellular organisms by affecting metabolic homoeostasis38, and extant animal cells coordinate a HIF-α response within minutes by, for example, shifting from aerobic to anaerobic cell metabolism39. This means that oxygen-sensing mechanisms accommodate stress-avoidance traits with high heritability under stressful conditions. Components in the current oxygen sensing mechanism have evolved from prokaryotes (e.g. the Per-ARNT-Sim or PAS domain)40, to choanoflagellates (e.g. the prolyl hydroxylation domain or PHD)41 and within animals37.
Today, all animals with true tissues (excludes Porifera) and a benthic component to their life cycle (generally excludes Ctenophora) respond transcriptionally to O2 fluctuations via HIF-α39,42 (Fig. 1C). These animals share a joint core to this sensing by an oxygen dependent degradation domain (ODDD) of the bHLH-PAS protein where a proline can be targeted by a PHD protein (if O2 is present) and hinder gene transcription (for details on Molecular dynamics within Hypoxia Inducible Factor (HIF), see supplementary discussion). However, the components of the pathway vary in their presence within animal groups and in terms of how they sense and respond to oxygen fluctuations. For example, an additional C-terminal transactivation domain (CTAD) can enable the protein to bind with transcriptional coactivators of and a factor inhibiting HIF (FIH) can limit the regulation of genes43. Differences in oxygen sensitivity and regulatory roles are also described for the two different prolines (P564 and P402) targeted by PHD, and an N-terminal transactivation domain (NTAD)43. These variations are likely to contribute to fine-tuning of both O2 sensitivity and range of regulated genes44,45, such that rudimentary oxygen sensing mechanisms could have been a functional but evolving system that channelled stress-induced adaptations.
A downside of oxygen sensing functionality via HIF-α is its energy cost for the cell. For example, the pathway involves a continuous production of a protein that is either degraded (in the presence of oxygen) or stabilised to induce a response (at oxygen shortage). Therefore, we here tested trade-offs for oxygen sensing as benefits and costs vary, against the suggestion that fluctuating benthic O2 concentrations led to physiological stress in which improved HIF-α functionality optimised fitness.
In this work, we hypothesise that the combination of physiological stress and the substantial expansion of nutrient-rich sunlit benthic habitats from the late Neoproterozoic to the early Palaeozoic promoted phenotypic probing and, subsequently, the adaptive radiation of animals (Fig. 2A, B). We further hypothesise that stress-induced adaptations could have been channelled through the regulatory system of oxygen-sensing mechanisms. To explore this, we first investigate benthic O2 fluctuations over day- and night cycles (Fig. 2C) at varying temperatures and carbon loads using a 1-dimensional (1D) biogeochemical model (Fig. 2D)46. We then assess trade-offs between the benefits of an efficient oxygen-sensing mechanism and its energetic costs for early animal fitness by modelling ecological (population) and evolutionary (strategy) dynamics of organisms with effective or poor oxygen-sensing mechanisms (eOSM or pOSM) existing in the same environment.
A schematic visualisation of the shallow sandy shelf and habitat expansion, the corresponding amplified extent of benthic microalgae habitats in the photic zone (green line), and the hypothetical diel O2 dynamics at (A) the icehouse (blue) and (B) the greenhouse (beige) climate end-member scenarios, with cold versus warm shallow shelf settings. C A schematic understanding of benthic microalgae communities (green line) at the sediment-water-interface where it regulates sediment oxygen concentration dynamics by photosynthetic gross O2 production during daylight and by heterotrophic O2 consumption during nighttime. D The applied 1D biogeochemical model illustrating the diffusion boundary layer (DBL), the sediment-water (SW) interface and two modelled steady state O2 concentration profiles as they appear during daylight (red line, photosynthesis-driven net oxygen productive) and during night (blue line; net O2 consuming), respectively. Model parameters are atmospheric oxygen (ppO2), temperature (T), salinity (S), production Q10 (Q10p), respiration Q10 (Q10r) and TOC adjustment factor (coc).
Results
Shifts in daily benthic O2 fluctuations on an expanding shelf
Biogeochemical model: We applied a 1D biogeochemical model to constrain O2 concentration dynamics and predict the concurrent physiological stress imposed by rapidly fluctuating (redox) conditions over a 21-h day and night cycle (early Palaeozoic)47. The partial differential equation model includes oxygen gross production, consumption and diffusive transport equations (Fig. 2C, Supplementary Table 1 for parameter values and supplementary methods for model description). The model results were validated against measured data from modern marine microphytobenthic studies at sunlit shallow-water sandy sediments (Supplementary Fig. 2). As the model is qualitatively generic and reflects key biophysical processes, identical dynamics and shape of profiles would be expected if validated against ocean shelf or even deep-sea sediments48. The modelled dynamics are universal for muddy, mixed, carbonate and sandy sediments and include oxygen scavenging by, e.g. iron and sulphide reduction35. The model was used to derive day and night O2 concentration profiles and to quantify the impact on these of changing temperature and total organic carbon (TOC) availability (see ‘Method’ section for modelled scenarios). Since rapid changes in O2 concentrations can be physiologically stressful to benthic animals when adjustments to their ATP-turnover are less swift49, we also quantified the transition time between day and night conditions.
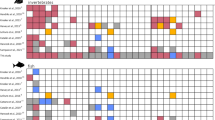
Biogeochemical model results: These first-order experiments found that in a cold scenario (+5 °C), daily oxygen fluctuations and diel amplitude would have been modest with weak oxic conditions during the day and with hypoxic conditions during night lasting for ~11 h (Fig. 3A, C). In contrast, a warm model scenario (+25 °C)demonstrated profoundly amplified daily fluctuations with abrupt changes from fully oxygenated conditions during the day to true anoxia during the night in less than 0.3 h (Fig. 3B, D). Regardless of changes in TOC (0.1 to 1 relative to Phanerozoic levels3), a hot climate (at +25° C and +40° C, compared to a cold at +5° C) led to O2 concentrations that were high during the day and low during night hours (Fig. 3E), shorter transition times with hypoxic conditions (Fig. 3F) and longer exposure to anoxia at the sediment water interface (Fig. 3G). A visual comparison of the results for 20 %PAL versus 50 %PAL at varying TOC adjustment factors (Supplementary Tables 3–4) depicts that a delicate balance of sub-modern ppO2, sub-modern TOC and temperature could lead to severe diel redox fluctuations at the sediment-water interface (Supplementary Figs. 4–5). The robustness of the model results with respect to parameter values was tested by running a Monte-Carlo simulation (50.000 times over, see Methods for details).
Diel O2 fluctuations were modelled across the sediment boundary layer (0.05 cm) and 1.25 cm into the sediment at diel scales at (A) the cold scenario (+5 °C) and (B) the warm scenario (+25 °C). The illustrations include 2.5 days of 21 h each. Diel oxygen amplitudes during day (yellow) and night hours (green), and the transition time (ttrans; graded coloured) in hypoxic conditions that benthic animals would encounter become considerably more abrupt, going from (C) the cold to (D) the warm conditions. Also, TOC availability increased from the Cryogenian to the Cambrian with a factor of 0.1–0.8 relative to the Phanerozoic mean, respectively3. (E-G) Modelling results at +5 °C, +25 °C, and +40 °C and TOC settings of 0.1 (white circles), 0.25 (light grey diamonds) and 1 (dark grey triangles) relative to Phanerozoic mean are illustrated for (E) maximum benthic O2 concentrations in the night and day, schematic field with colour codes for oxygen concentrations as in A-B; (F) transition times (h day−1) through hypoxic conditions (green field); (G) extent of (h per day) benthic nocturnal anoxia (grey field). In (E), modelling results for maximum benthic O2 concentrations at night (scale to the left) at the different TOC settings are marked with full red outline and emphasised with a blue field. Modelling results for maximum benthic O2 concentrations in the day (scale to the right) at the different TOC settings are marked with dashed green outline and emphasised with a yellow field.
Evolutionary shifts to the sensing and responses to daily O2 fluctuations
Population and phenotype model: After finding that daily benthic redox fluctuations on an expanding Cambrian shelf would have presented animals with severe daily O2 fluctuations, we explored the ecological (population) and evolutionary (strategy) dynamics of species with effective and poor oxygen-sensing mechanisms (eOSM or pOSM), respectively, existing in these environments. To do this, we used a mathematical modelling framework named G functions50,51 that simultaneously captures ecological (population) and evolutionary (oxygen-sensing strategy) dynamics over time. In other words, the same set of equations can qualitatively capture the interplay between changes in oxygen levels, ecological dynamics and species competition, and adaptation via oxygen sensing mechanisms. The capacity to effectively sense and respond to changing oxygen levels (rate of phenotypic switching) presumably incurs a cost, but it would be an adaptive advantage for an organism to swiftly change from aerobic to anaerobic cell metabolism in this environment. For example, for anaerobic metabolism to produce ATP that matches that of aerobic metabolism (per glucose molecule), eighteen times more glucose is required. An increased metabolic rate is feasible, and the organism can remain highly functional, if also rate-limiting enzymes keep up. Expression of phosphofructokinase, the rate limiting and irreversible step in this pathway, is rapidly upregulated by HIF-α49. Organisms with eOSM would have the benefit of responding quickly to changing oxygen conditions, but at some cost. The costs of rapid cellular phenotypic plasticity could include cells refining oxygen sensing or maintaining the intra-cellular machinery to rapidly upregulate anaerobic metabolism (or their rate-limiting enzymes). Our model extends the classic Lotka-Volterra competition equations and imposes a cost for efficient oxygen-sensing. Namely, the population dynamics are given by Eq. (1):
where the per capita growth rates of a species are given by Eq. (2):
and the carrying capacity is a function of how well the focal individual’s strategy matches the environment, Eq. (3):
In these equations, v represents a species’ cellular metabolism phenotype (strategy), u = (u1, u2) and x = (x1, x2) are the strategies and population sizes of each species in the population, respectively. The evolutionary dynamics are then derived by Fisher’s fundamental theorem52 as Eq. (4):
As a simplified L-V competition model, we assume that competition among species is independent of their strategies. All individuals have the same adverse impacts on each other and compete equally in a density-dependent fashion. This leads to growth in a logistic manner, wherein the carrying capacity depends on how well adapted the species is to its (continually changing) environment. Finally, we included a cost of oxygen sensing through the last term in the G-function. We assume this cost scales linearly with the capacity for oxygen sensing. The eOSM and pOSM species are differentiated solely by the eOSM species having a higher capacity for oxygen sensing, s, than the pOSM species. Thus, the eOSM species can switch its cellular metabolism more rapidly than the pOSM species. For further details on model derivation, please consult the ‘Methods’ section.
To capture the ecological (population) and phenotype (strategy) dynamics of each species (eOSM and pOSM), we performed three simulations, in which we let the optimal phenotypic strategy (γ) vary in a sinusoidal fashion with added stochastic noise. We simulated this over 500 time-steps (pseudo time; i.e. an arbitrary time unit) to capture the dynamics before the continental flooding, as in the early Palaeozoic. For simplicity and visualisation purposes, the flooding that led to the expanded shallow shelf area was implemented as a switch. To simulate the flooding, we increased the periodic O2 fluctuations (driven by microalgal production and consumption), stochastic O2 fluctuations (driven by nutrient cycling), or both.
Population and phenotype model results: The results of the simulations can be seen in Fig. 4A–C. When oxygen fluctuated stochastically, the benefit of tracking environmental oxygen levels with an efficient oxygen sensing was greatly outweighed by its cost and pOSM species dominated (Fig. 4A). When oxygen fluctuated periodically (as if daily), eOSM species dominated the population within ~500 (pseudo) time-steps after the switch, suggesting the benefit of perceiving and responding to redox fluctuations in a spatiotemporal manner (Fig. 4B). In the case of both periodic and stochastic fluctuations, eOSM species were greatly favoured, which drove the pOSM species to extinction within 250 (pseudo) time-steps (Fig. 4C). Although a qualitative estimate in pseudo-time, the biological event rate exceeds that of fluctuations, and both are instant in comparison to atmospheric changes of oxygen concentrations. The trends observed in the plots of eco-evolutionary dynamics generally hold for a wide range of parameter values (for details on Model of population dynamics and speed of phenotypic plasticity, see Supplementary discussion and Supplementary Figs. 7–8). In all cases, the pOSM and eOSM species attempt to track the environmental oxygen levels by adjusting their strategy. The eOSM species, which can more effectively track the oxygen levels due to their improved oxygen-sensing capability, displayed greater changes in strategy. Note that the cost of the oxygen sensing mechanism has a large influence on the outcome of the competition experiments (Supplementary Fig. 8). The competition experiments demonstrated that eOSM species are favoured across the range of costs of the mechanism tested in environments with stochastic and periodic fluctuations, and favoured for most tested costs (d < 0.09) in environments with periodic fluctuations. In contrast, pOSM species are favoured for most tested costs (d > 0.01) in environments with only stochastic fluctuations. The shape and magnitude of the cost function was chosen arbitrarily for the purposes of this qualitative modelling study, but for a given environment, there will be a threshold value for \(d\), below which eOSM species will outcompete pOSM species and above which pOSM species will outcompete eOSM species. Furthermore, it is possible that extreme costs can overshadow the main trends observed in this in silico study.
Competitive eco-evolutionary dynamics between organisms with poor oxygen sensing (pOSM) and efficient oxygen sensing mechanisms (eOSM) demonstrate that an increase (dashed line) in the frequency of (A) stochastic oxygen fluctuations (driven by nutrient cycling) is not enough to favour the eOSM species, (B) periodic oxygen fluctuations (driven by microalgal production and consumption) eventually favour eOSM, and (C) of both stochastic and periodic O2 fluctuations directly favours eOSM. D All sediment types deposited at the North American craton at the PC/C boundary as extracted from Macrostrat demonstrate an ~8-fold increase in areal extent of particularly shallow (sand and carbonate) settings (modified from14). E Schematic of our suggested cascade of events with stress (diel benthic redox fluctuations) developing with the greenhouse climate in the end-Neoproterozoic (striped black-yellow) and increase of metabolic energy (green) as a driver for diversification of animals (genus level) with a presumed distribution of species with pOSM (grey) and eOSM (orange). The schematic representation of animal diversity (number of genera, singletons omitted) and disparity (number of classes, grey box) is modified from ref. 74. F Molecular differences in the HIF-like structures between the Porifera, Placozoa, Cnidaria, Bilateria and within Bilateria43,53, with phylogeny of animal lineages based on ref. 96, with approximate divergence between lineages based97 and ref. 71). A maximum diversity of components to the OSM is depicted per lineage in a simplified phylogeny97 (to Lophotrochozoa (L), Ecdysozoa (E), Ambulacraria (A) and Chordata (C) for Bilateria), based on sequences in ref. 53 and ref. 43 (see SI text and Supplementary Table 4 for details). Proteins were not drawn in scale and the position of the components are approximate. The HIF-like bHLH (grey) and PAS (blue) is common to all, while the P564 (green triangle) appear from Cnidaria and Placozoa and CTAD (in Cnidaria (pale red when uncertain, see Supplementary Table 4) and Bilateria (red). Within Bilateria, multiple combinations of P564, CTAD plus P402 (purple triangle), NTAD (yellow, pale yellow when uncertain) and FIH (black triangle and grey when uncertain) are present. *asparagine 803 which is the FIH interaction site.** depicts human reference with also HIF-2α.
The population and phenotype model is agnostic to which organisms would represent pOSM or eOSM species. To relate the phenotype model, the flooding event and early animal diversification in the Cambrian Period (Fig. 4D, E) to the early evolution of animals, we mapped differences in the composition of HIF-driven oxygen sensing across the animal clade based on sequences in ref. 53 and ref. 43 (Fig. 4F, supplementary discussion and Supplementary Table 4, for details). While HIFs that today compose the OSM of modern animals contain components (e.g. genes, domains, or proteins) that can be traced back to the origins of yeast and even to prokaryotes37, its building blocks expand and differ in functions within and between early branching animals. For example, oxygen sensing in animals with e.g. only P564 would be simpler than in animals e.g. with P564 and P402 or with P564 and CTAD. Components to the OSM like the P402, CTAD, NTAD and Factor Inhibiting HIF (FIH) regulate a wide scope of target genes at a wide range of conditions, some of them with clear importance for development37. From what we know of the importance of these components in Bilateria54,55,56, the different configurations amongst animals43,53 reflect varying efficiency and costs to sense and respond to redox fluctuations.
Discussion
By applying two lines of first-order reconstructions, we demonstrate that daily O2 fluctuations in a photic benthic marine setting could be severe enough to induce physiological stress and promote specific adaptations among early animals at flooding events in the end-Neoproterozoic to early Palaeozoic. Biogeochemical modelling shows that the benthic biota on, for example the Cambrian shelf that expanded 4-8 times14, could have been exposed to fully oxic conditions during the day to, after an abrupt shift, nocturnal anoxia (Fig. 3). In contrast, the modelling indicates that a cold setting would offer only modest daily O2 fluctuations. More specifically, in a +5 °C setting (where atmospheric O2 is set at <6% and TOC lower than on average in the Phanerozoic), the setting is never completely devoid of oxygen (weakly oxic in the day and hypoxic at night). Furthermore, our qualitative modelling of population and phenotypic dynamics demonstrated that investment in an energetically costly strategy (e.g. efficient oxygen-sensing or eOSM) pays off in fitness when species are exposed to periodic chemical fluctuations (Fig. 4B). The evolutionary success of animals with eOSM is even clearer when compounded with stochastic effects (Fig. 4C), such as those manifesting during algal blooms that could induce water column anoxia in the already poorly oxygenated Cambrian oceans7,8. Our results therefore call for a re-evaluation of factors driving the Cambrian explosion, highlighting how shifts in abiotic parameters and associated physiological stress over both spatial and short temporal scales could spur the adaptations observed in populations seen today.
Stress-driven needs and adaptations
The significance of physiological stress across brief time spans in driving evolutionary innovation is frequently discussed, particularly in pivotal transitions throughout life’s history6. For example, metabolic stress through both warm climate and oxygen variability is suggested for biotic turnover at the Permian/Triassic57,58. The Cambrian Explosion, however, has been treated differently, with studies focusing primarily on long-term changes in the atmosphere and the water-column (of e.g. O2 or TOC) creating permissive conditions3. Our results pinpoint that seemingly inhospitable conditions—the fast and daily transitions to nocturnal anoxia—could act on the expanding Cambrian shelf setting. Since this respiration-driven nocturnal anoxia is primarily dictated by the temperature, it is worth noting that the paleotemperature record for this period is scarce. Evidence of global ice ages, however, anchors the coarse assumption that benthic O2 dynamics changed from a colder mid-Neoproterozoic with limited shelf area to a warmer early Palaeozoic scenario with broadly flooded continental shelves. This means that even if flooding eroded evidence of larger Cryogenian shelves, benthic O2 fluctuations in cold settings would have remained modest. In the Cambrian Period, flooded continents and a new benthic niche (produced by the new weathering regime of sands, muds and carbonate) at least to mid latitudes would have experienced the severe O2 fluctuations between day and night. To inhabit the new fertile grounds, animals must have also tolerated associated physiological stress, paced by daily and severe oxygen fluctuations. To tolerate stress, animals manage metabolism.
Stress associated with recurring anoxia creates a need for animals to manage metabolism, like balancing the enzymes needed for glycolysis. Recurring anoxia also creates the need to manage other necessary functions. For example, anoxic nights associate with sulphide oozing from shelf sediments. Dissolved sulphides can diffuse across cell membranes without transporters (gasotransmitters) and are toxic to mitochondrial respiration59. Modern bivalves anchored in sulfidic sediments handle such exposure by an endosymbiotic relationship with sulphur-metabolising bacteria that convert the sulphide to less toxic forms60. Under these conditions, iron homoeostasis is also affected in multicellular eukaryotic organisms. To compare with oxic conditions and circumneutral pH, ferrous iron is oxidised and precipitates stepwise from nanoparticular Fe(III) crystallites to a crystalline ferric Fe(III) (oxyhydr) oxides61. Eukaryotic cells (like those of animals) transport this insoluble but bio-essential ferric iron across its membrane with mechanism like ferric-chelate reductase62. However, under anoxic marine conditions, reduced iron [ferrous or Fe(II)] dominates, meaning that animal cells risk exposure to iron deficiency63. Today, animals rely on the HIF system to mitigate these implications of exposure to anoxia. HIF-α participates in the upregulation of rate-limiting enzymes during glycolysis49, sulphide tolerance64,65 and fine-tuning of the cell’s iron budget62,63 and thus help secure a stable energy supply to cells. To compare, yeast regulate iron uptake, storage and use via three independently regulated routes and cannot mitigate iron fluctuations via a single mechanism66. Therefore, the adaptation of refined oxygen-sensing via HIF-α may have allowed animals to endure daily and stochastic benthic redox fluctuations.
Adaptations that early animals evolved to handle redox fluctuations remain unknown and hard to test. That the core of the HIF-α mechanism evolved once is clear43, but also that several of its components to sense and respond to redox fluctuations appear and vary within the animal clade (for details on these components, see also supplementary discussion and Fig. 4F). For example, the ancestral components (e.g. PAS and bHLH domain proteins)40,41,67 with the addition of a proline targeted for hydroxylation (P564) are described as present in Placozoa and Cnidaria, whereas the CTAD is undoubtedly present first in Bilateria42,43 (see Fig. 4F and Supplementary Table 4 for comparisons).
In bilaterian animals, however, the variation of combinations increase with e.g. a second proline (P402) that can be hydroxylated and genes transactivated via the N-terminal or NTAD45,68,69. Within the NTAD, the interaction site for the Factor Inhibiting HIF (FIH) is also noted within Bilateria. FIH can hydroxylate an asparagine residue at O2 tensions that are higher than when the proline (P564) is hydroxylated70. This means that animals with FIH can demonstrate HIF-α that is stabilised or hydroxylated (and therefore degraded) differently than in animals without FIH. Based on how FIH, CTAD, NTAD and the prolines are known to differentially register and respond to O2 fluctuations54,55,56, cellular oxygen-sensing mechanisms appears be become refined between Placozoa and Cnidaria, between Cnidaria and Bilateria and especially within Bilateria (compare e.g. variants within Protostomia in Fig. 4F). The known differences and functions of these components could have been separating Placozoa (pOSM) from Cnidaria (eOSM), or species with pOSM from eOSM within cnidarians or bilaterians; whether living in the Ediacaran71 or Cambrian. If so, the environmental stress in the Ediacaran and early Palaeozoic can be connected to a physiological impact and to adaptations within and between animal groups that led to diversification through novel niche filling. As discussed above, to achieve rapid cellular phenotypic plasticity would come with a cost and eOSM species can in specifically periodic conditions bear that cost. This leads us to see that the evolution of traits like oxygen sensing and oxygen responses may have important functional roles that could have contributed to animal diversification. Therefore, our population and phenotype model results combined with previous work on the differences within the molecular differences in animal OSM are consistent with the view that adaptations of rudimentary oxygen-sensing capacities gave certain animals a competitive advantage over other early metazoans to cope (by phenotypic switching) within the opened shallow sunlit niches with diurnal benthic oxygen fluctuations.
From physical harshness to diversification
Animal diversity today follows latitudinal gradients, largely attributed to the tropics as being more productive72. The higher productivity offers extensive habitats and biotic feedbacks where species themselves create environmental heterogeneity that, in turn, promotes the evolution and coexistence of even more species72. When an ecosystem is highly productive and incurs high biotic stress through competition, predation and competition between different mutualistic associations, a kind of bio-diversity pump ensues. Therefore, a setting with high productivity like the tropics today promote and can tolerate tighter niche packing that involves rapid and efficient use of available nutrients and resources from the lower to higher trophic levels. However, tight niche packing of an ecosystem does not necessarily promote diversification. When an ecosystems instead is governed by abiotic and physical stress, adaptations strive to mitigate physical harshness at the expense of traits aimed at suppressing competitors and avoiding predation11. These new needs and adaptations are independent of population density. Indeed, the density-independent needs of species under physical harshness generate less intense frequency-dependent interactions that can promote species diversification73,74, especially when combined with a surplus of metabolic energy11.
The shelf habitat of the say Cryogenian Period would have offered a fairly constant area and metabolic energy for as long as there was an icehouse climate27,34 (Supplementary Fig. 1), little need for oxygen sensing adaptations (modest fluctuations). Metabolic rates, metabolic scope, and the need to rapidly pre-empt others for resources would have been low. The overall ecological environment would have been harsh for early animal life even if the chemical environment was not. In fact, the modest fluctuations in oxygen would have been the result of low primary productivity and turnover of organic matter. With the warm climate and enlarged continental shelves of the Ediacaran (as a shift from possibly one75,76 or several77,78 extensive glaciations) and Cambrian periods, primary productivity would have increased dramatically both in terms of per unit area as well as more area exhibiting high productivity. However, a consequence of this primary productivity would have been biotically driven stress from severe daily O2 fluctuations – an additional physical stressor for animal life and a drag on diversification. Hence, we see the evolution of efficient handling of oxygen fluctuations (as via HIF-α) as constrain-breaking adaptations that mitigated the stress of benthic oxygen fluctuations, permitted fuller utilisation of the primary productivity, shifted the system from one of primarily physical harshness to one of extreme biotic harshness, and unleashed the biodiversity pump of the Cambrian Explosion.
Diversification with and without HIF-α
Animals without HIF-α (e.g. sponges79) and with HIF-α (Placozoa, Cnidaria and Bilateria) diversify in the Cambrian43. Cnidaria are represented as early as in the Ediacaran biota80, thus implying that animals with a core to the HIF-α pathway (e.g. P564 in the ODDD targeted by PHD) were present from before continental flooding in the Cambrian Period. If the HIF-α pathway indeed mitigated stress for early animals as it does in extant Bilateria (like upregulating rate-limiting enzymes for glycolysis)49, it is fair to assume that heritability to modification of the HIF-pathway would be high. HIF target genes, furthermore, regulate cell differentiation in Bilateria, which is key to tissue architecture and homoeostasis81,82. Indeed, cell differentiation is one out of four key characteristics to underpin most animal phenotypes that have been realised over the Phanerozic83. This would indicate that HIF, at least in Bilateria, allowed for metabolic versatility and adaptations that also pertain to body plans (i.e. disparity). In contrast, sponges diversified without a HIF-α pathway and constitute a negative control to the hypothesis that HIF-α was key to the Cambrian explosion. While being diverse, sponges appear to lack the ability to form true tissues, as their cells generally do not connect in basement membranes84,85. Instead, the limited number of sponge cell types86 demonstrate high plasticity through trans-differentiation84,85 and mobility from outer to inner (mesohyl) layers87. Sponges also appear to rhythmically open and close towards the environment, which regulates internal O2 from oxic to anoxic88,89. The interval of opening and closing the osculum differs between species (e.g. of ~40 min or 80–170 min intervals), which leads to canals being re-modelled each time the sponge contracts and relaxes again88,89. Although it remains unclear if the rhythmic changes to internal O2 also alter the fate of sponge cells, it is clear that cell differentiation is an oxygen-driven process90. Therefore, one can speculate that pulses of internal anoxia (for stemness) and oxic conditions (for cell differentiation) represent a unique adaptation for regulating cell fate and maintenance in sponges. With an adaptation to control fluctuations internally, sponges would have bypassed the need for cellular OSM and diversified in their own right as density-independent needs (from abiotic stress) and access to metabolic energy changed with the Neoproterozoic to early Palaeozoic flooding events.
The interaction of abiotic, ecological and functional factors for the Cambrian explosion
Based on our results, we offer the general principle that global and long-term geophysical changes converged with local and short-term biotic periodicity in a habitat with increased metabolic energy to drive the Cambrian explosion of primarily phyla possessing HIF-α (Fig. 5). When eustatic sea-level was high under the greenhouse climate, shallow sands and new carbonate platforms offered animals a rich marine ‘tableland’ but with severe abiotic harshness. At points, these conditions could have become too harsh, possibly explaining Cambrian intervals that are barren of animal fossils91. That physiological stress was involved in the Cambrian diversification may also be indicated by how extinctions were proportionally higher92 than later in the Phanerozoic and generic longevity much expanded over the earliest Palaeozoic (from 0.8 myr in the Cambrian to 6.3 myr in the Ordovician)93. This data may be taken to reflect how a stressful environment was intertwined with both death and new adaptations. However, the overall success of animals with efficient handling of the redox fluctuations would have reached beyond simply enduring this setting, allowing animals with specific adaptations, like sponges or Hifozoa43, to diversify.
In the mid-Neoproterozoic, a narrow and unchanged shelf habitat offered icehouse-dictated (blue for cold temperatures in air and water) modest diel benthic fluctuations (left panels). Towards the end-Neoproterozoic, warmer climate (pink for warm temperatures in air and water) increased amplified diel redox fluctuations and physiological stress while the unchanged habitat and low productivity maintained harsh ecological conditions (middle panels). In the early Palaeozoic, global flooding of shallow shelf area with the severe diel benthic redox periodicity expands dramatically, thus lifting the biological stress by surplus of metabolic energy in the short term (right panels). The combination of stress-induced needs for oxygen-sensing and increased metabolic energy led to new needs, adaptations and radiation of animals with adequate oxygen sensing mechanism, e.g. sponges (without HIF-α) and Bilateria (with HIF-α).
Previous explorations into the animal diversification in the Neoproterozoic-early Palaeozoic have largely overlooked the combined role of daily benthic redox fluctuations and physiological stress with changed metabolic energy as drivers. Recent observations, such as how early animals diversified despite globally low or oscillating O25,33, inspire a shifted perspective on stress-induced evolutionary change.
In summary, our implementation of a biogeochemical model, paired with geological data, led to predictions that are consistent with the fossil record. Our results suggest that severe daily redox variations in an expanding sunlit benthic shallow shelf created niches in the end-Neoproterozoic and early Palaeozoic that could have promoted an adaptive radiation of organisms with efficient handling of oxygen fluctuations, such as those regulated by HIF-α. Our G function model that captures environmental oxygen fluctuations, population dynamics and species competition, and adaptation via oxygen sensing mechanisms demonstrated how periodic fluctuations could favour species with efficient oxygen sensing mechanisms, despite its metabolic costs. Further studies may detail low-oxygen environments that prevailed in the shallow shelf and their adjoining biotic adaptations, emphasising the role of daily benthic redox stress in shaping the animal evolution in the benthic environment.
Methods
Biogeochemical model: Model parameters were tuned for sandy sediments and to mimic cold (5 °C) and warm (25 °C, and 40 °C) shelf conditions with 20–50% of present atmospheric levels (%PAL) of O2 and 0.1–1 times the Phanerozoic load of organic carbon. While the term hypoxia was originally used to describe internal stress on an animal, it has also come to describe the external ocean medium94. The specific definitions of anoxic to oxic conditions used here are: anoxic <0.02 µM, severely hypoxic between 0.02 and 22 µM, hypoxic between 22 and 65 µM and oxic >65 µM95.
To test whether the model results were robust with respect to parameter values, we ran a Monte-Carlo simulation of the model 50,000 times over a large range of ppO2, TOC and temperature responses for production and respiration rates (Q10Prod and Q10Resp), accommodating for most possible seafloor conditions (Supplementary Table 1). These simulations supported the generality of the model by demonstrating that a short transition time (<1 h) from daytime oxic to nighttime anoxia/severe hypoxic conditions in the diffusive boundary layer (DBL) was met in 67% of the cases and that the model output is primarily sensitive to TOC adjustment factors and ppO2 (various scenarios shown in Supplementary Fig. S6). A compilation of TOC contents in more than 13,650 shale samples from the end-Neoproterozoic and Phanerozoic eons indicates that TOC in the Cryogenian were lower than in the Cambrian Period and the Phanerozoic overall3. Quantitative differences arise if parameters are set for muddy, mixed, or carbonate sediments, but the same fluctuating patterns and qualitative trends remain alike48.
Population and phenotype model: The G function framework we use allows us to track both how the population sizes of the species change over time (ecology) as well as how their strategies evolve in response to changing environments (evolution). We modelled the population dynamics of two species, one with high phenotypic plasticity for cellular metabolism in response to dissolved oxygen conditions and one with low (i = 1,2), Eq. (1):
where the fitness-generating function, \(G(v,{{{\boldsymbol{u}}}},{{{\boldsymbol{x}}}})\), describes the expected per capita growth of a species as influenced by its cellular metabolism phenotype (strategy), v, the strategies of each species in the population, u = (u1, u2), and the population sizes of each species, x = (x1, x2). In this case, the relevant strategy describes an abstract trait that allows cells of the multicellular organism to switch along a continuum between anaerobic and aerobic metabolism. Thus, this equation captures the change in the population size of a given species as a product of its current population size and its per capita growth rate. We let the rate of phenotypic switching of cells of the organism depend on (a) the slope of the fitness gradient and (b) how fast the species can change phenotype in response to the fitness gradient. The slope of the fitness gradient is given by \(\frac{{dG}}{{dv}}\). How fast the species can change in response to this fitness gradient depends on the oxygen sensing mechanism; the more developed this is, the more quickly a species can switch. These components together allow for the derivation of the equation for phenotype dynamics52, Eq. 2:
where \({s}_{i}\) is a measure of the capacity for cellular oxygen sensing that allows the organisms to closely track and respond to their environment. To define our fitness-generating function, G, we used simplified Lotka-Volterra (L-V) competition equations, Eq. 3:
We let the carrying capacity be a function of the focal individual’s strategy. We assume that there is some optimal strategy value γ. The closer the individual’s strategy is to γ, the higher its carrying capacity, Eq. 4:
We assume that deviations of strategy values from γ decrease carrying capacity in a Gaussian fashion. We allow γ to change over time as a result of fluctuating oxygen levels in the environment. A particular dissolved oxygen level corresponds monotonically to a particular value for γ. In the model, the ecological impact of a species’ phenotype to oxygen levels comes through its carrying capacity. As a simplified L-V competition model, we assume that competition among species is independent of their strategies. All individuals have the same adverse impacts on each other and compete equally in a density-dependent fashion. This leads to growth in a logistic manner, wherein the carrying capacity depends on how well adapted the species is to its (continually changing) environment. Finally, we included a cost of oxygen sensing through the last term in the G-function. We assume this cost scales linearly with the capacity for oxygen sensing. The eOSM and pOSM species are differentiated solely by the eOSM species having a higher capacity for oxygen sensing, s, than the pOSM species. Thus, the eOSM species can switch its cellular metabolism more rapidly than the pOSM species.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Code availability
The biogeochemical model is implemented using the Mobius framework, and the source code can be found in Supplementary Code 1 and at: https://github.com/NIVANorge/Mobius/tree/master/Modules/SedimentOxygen. Files used for running the model can be found at: https://github.com/NIVANorge/Mobius/tree/master/Applications/SedimentOxygen. The source code to the eco-evolutionary model can be found at in the Supplementary Code 2.
References
Erwin, D. H. et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
Vermeij, G. J. The origin of skeletons. Palaios 4, 585–589 (1989).
Sperling, E. A. & Stockey, R. G. The temporal and environmental context of early animal evolution: considering all the ingredients of an “explosion”. Integr. Comp. Biol. 58, 605–622 (2018).
Mills, D. B. & Canfield, D. E. Oxygen and animal evolution: did a rise of atmospheric oxygen “trigger” the origin of animals? Bioessays 36, 1145–1155 (2014).
Sperling, E. A. et al. Breathless through time: oxygen and animals across earth’s history. Biol. Bull. 243, 184–206 (2022).
Wood, R. & Erwin, D. H. Innovation not recovery: dynamic redox promotes metazoan radiations. Biol. Rev. 93, 863–873 (2018).
Hammarlund, E. U. et al. Early Cambrian oxygen minimum zone-like conditions at Chengjiang. Earth Planet. Sci. Lett. 475, 160–168 (2017).
Reinhard, C. T., Planavsky, N. J., Olson, S. L., Lyons, T. W. & Erwin, D. H. Earth’s oxygen cycle and the evolution of animal life. Proc. Natl Acad. Sci. USA 113, 8933–8938 (2016).
Wood, R. et al. Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nat. Ecol. Evol. 3, 528–538 (2019).
Badyaev, A. V. in Variation (eds Hallgrímsson, B. & Hall, B. K.) 277–302 (Academic Press, 2005).
Parsons, P. A. The importance and consequences of stress in living and fossil populations: from life-history variation to evolutionary change. Am. Nat. 142, S5–S20 (1993).
Peters, S. E. & Gaines, R. R. Formation of the ‘Great Unconformity’ as a trigger for the Cambrian explosion. Nature 484, 363–366 (2012).
Tasistro-Hart, A. R. & Macdonald, F. A. Phanerozoic flooding of North America and the Great Unconformity. Proc. Natl Acad. Sci. 120, e2309084120 (2023).
Segessenman, D. C. et al. in Laurentia: Turning Points in the Evolution of a Continent 220 0 (Geological Society of America, 2023).
van der Meer, D. G. et al. Reconstructing first-order changes in sea level during the Phanerozoic and Neoproterozoic using strontium isotopes. Gondwana Res. 44, 22–34 (2017).
Wei, G.-Y. et al. Long-term evolution of terrestrial inputs from the Ediacaran to early Cambrian: clues from Nd isotopes in shallow-marine carbonates, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 535, 109367 (2019).
Hancke, K. et al. Effects of temperature and irradiance on a benthic microalgal community: a combined two-dimensional oxygen and fluorescence imaging approach. Limnol. Oceanogr. 59, 1599–1611 (2014).
Attard, K. et al. Seafloor primary production in a changing Arctic Ocean. Proc. Natl Acad. Sci. USA 121, e2303366121 (2024).
Gattuso, J. P. et al. Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production. Biogeosciences 3, 489–513 (2006).
Falkowski, P. G. & Raven, J. A. in Aquatic Photosynthesis 2nd edn, 364–410 (Princeton University Press, 2007).
Morris, J. L. et al. The timescale of early land plant evolution. Proc. Natl Acad. Sci. USA 115, E2274 (2018).
Brocks, J. J. et al. Lost world of complex life and the late rise of the eukaryotic crown. Nature 618, 767–773 (2023).
Hancke, K., Hancke, T. B., Olsen, L. M., Johnsen, G. & Glud, R. N. Temperature effects on microalgal photosynthesis-light responses measured by O2 production, pulse-amplitude-modulated fluorescence, and 14C assimilaton. J. Phycol. 44, 501–514 (2008).
Nardin, E. et al. Modeling the early Paleozoic long-term climatic trend. Geol. Soc. Am. Bull. 123, 1181–1192 (2011).
Valdes, P. J., Scotese, C. R. & Lunt, D. J. Deep ocean temperatures through time. Clim 17, 1483–1506 (2021).
Wotte, T., Skovsted, C. B., Whitehouse, M. J. & Kouchinsky, A. Isotopic evidence for temperate oceans during the Cambrian Explosion. Sci. Rep. 9, 6330 (2019).
Bergmann, K. et al. A billion years of temperature variability: a key driver of Earth’s long-term habitability. ESS Open Archive. https://doi.org/10.1002/essoar.10511918.1 (2022).
Scotese, C. R., Song, H., Mills, B. J. W. & van der Meer, D. G. Phanerozoic paleotemperatures: the earth’s changing climate during the last 540 million years. Earth Sci. Rev. 215, 103503 (2021).
Vérard, C. & Veizer, J. On plate tectonics and ocean temperatures. Geology 47, 881–885 (2019).
Hearing, T. W. et al. An early Cambrian greenhouse climate. Sci. Adv. 4, eaar5690 (2018).
Boucot, A. J., Xu, C., Scotese, C. R. & Morley, R. J. in Phanerozoic Paleoclimate: An Atlas of Lithologic Indicators of Climate (SEPM Society for Sedimentary Geology, 2013).
Wong Hearing, T. W. et al. Quantitative comparison of geological data and model simulations constrains early Cambrian geography and climate. Nat. Commun. 12, 3868 (2021).
Krause, A. J., Mills, B. J. W., Merdith, A. S., Lenton, T. M. & Poulton, S. W. Extreme variability in atmospheric oxygen levels in the late Precambrian. Sci. Adv. 8, eabm8191 (2022).
Mills, B. J. W. et al. Modelling the long-term carbon cycle, atmospheric CO2, and Earth surface temperature from late Neoproterozoic to present day. Gondwana Res. 67, 172–186 (2019).
Hancke, K. & Glud, R. N. Temperature effects on respiration and photosynthesis in three diatom-dominated benthic communities. Aquat. Microb. Ecol. 37, 265–281 (2004).
Potticary, A. L., Morrison, E. S. & Badyaev, A. V. Turning induced plasticity into refined adaptations during range expansion. Nat. Commun. 11, 3254 (2020).
Hammarlund, E. U., Flashman, E., Mohlin, S. & Licausi, F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 370, eaba3512 (2020).
Sokolova, I. M., Frederich, M., Bagwe, R., Lannig, G. & Sukhotin, A. A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1–15 (2012).
Semenza, G. L. Life with oxygen. Science 318, 62–64 (2007).
Taylor, B. L. & Zhulin, I. B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63, 479–506 (1999).
Rytkönen, K. T., Williams, T. A., Renshaw, G. M., Primmer, C. R. & Nikinmaa, M. Molecular evolution of the metazoan PHD–HIF oxygen-sensing system. Mol. Biol. Evol. 28, 1913–1926 (2011).
Loenarz, C. et al. The hypoxia‐inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 12, 63–70 (2011).
Mills, D. B. et al. The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments. eLife 7, e31176 (2018).
Bouthelier, A., Meléndez-Rodríguez, F., Urrutia, A. A. & Aragonés, J. Differential contribution of N- and C-terminal regions of HIF1α and HIF2α to their target gene selectivity. Int J. Mol. Sci. 21, 9401 (2020).
Yan, Q., Bartz, S., Mao, M., Li, L. & Kaelin, W. G. Jr The hypoxia-inducible factor 2alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol. Cell Biol. 27, 2092–2102 (2007).
Norling, M. D., Jackson-Blake, L. A., Calidonio, J. L. G. & Sample, J. E. Rapid development of fast and flexible environmental models: the Mobius framework v1.0. Geosci. Model Dev. 14, 1885–1897 (2021).
Bartlett, B. C. & Stevenson, D. J. Analysis of a Precambrian resonance-stabilized day length. Geophys. Res. Lett. 43, 5716–5724 (2016).
Glud, R. N. Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289 (2008).
Soñanez-Organis, J. G., Peregrino-Uriarte, A. B., Sotelo-Mundo, R. R., Forman, H. J. & Yepiz-Plascencia, G. Hexokinase from the white shrimp Litopenaeus vannamei: cDNA sequence, structural protein model and regulation via HIF-1 in response to hypoxia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 158, 242–249 (2011).
Bukkuri, A. & Brown, J. S. Evolutionary Game Theory: Darwinian Dynamics and the G Function Approach. Games 12, 72 (2021).
Vincent, T. L. & Brown, J. S. Evolutionary Game Theory, Natural Selection, and Darwinian Dynamics (Cambridge University Press, 2005).
Fisher, S. A. & Burggren, W. W. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid. Redox Signal. 9, 1339–1352 (2007).
Graham, A. M. & Presnell, J. S. Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PloS ONE 12, e0179545 (2017).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Iwai, K. et al. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl Acad. Sci. USA 96, 12436–12441 (1999).
Lando, D. et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 (2002).
Song, H. et al. Anoxia/high temperature double whammy during the Permian-Triassic marine crisis and its aftermath. Sci. Rep. 4, 4132 (2014).
Penn, J. L., Deutsch, C., Payne, J. L. & Sperling, E. A. Temperature-dependent hypoxia explains biogeography and severity of end-Permian marine mass extinction. Science 362, eaat1327 (2018).
Hansen, M. H., Ingvorsen, K. & Jorgensen, B. B. Meohanisms of hydrogen sulfide release from coastal marine sediments to the atmosphere. Limnol. Oceanogr. 23, 68–76 (1978).
Stewart, F. J., Newton, I. L. G. & Cavanaugh, C. M. Chemosynthetic endosymbioses: adaptations to oxic–anoxic interfaces. Trends Microbiol 13, 439–448 (2005).
Posth, N. R., Canfield, D. E. & Kappler, A. Biogenic Fe(III) minerals: from formation to diagenesis and preservation in the rock record. Earth Sci. Rev. 135, 103–121 (2014).
Anderson, G. J. & Vulpe, C. D. Mammalian iron transport. Cell Mol. Life Sci. 66, 3241–3261 (2009).
Anderson, C. P. & Leibold, E. A. Mechanisms of iron metabolism in Caenorhabditis elegans. Front Pharm. 5, 113 (2014).
Budde, M. W. & Roth, M. B. The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide. Genetics 189, 521–532 (2011).
Shen, C., Nettleton, D., Jiang, M., Kim, S. K. & Powell-Coffman, J. A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans*. J. Biol. Chem. 280, 20580–20588 (2005).
Philpott, C. C. & Protchenko, O. Response to Iron deprivation in Saccharomyces cerevisiae. Eukaryot. Cell 7, 20–27 (2008).
Ledent, V. & Vervoort, M. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 11, 754–770 (2001).
Chan, D. A., Sutphin, P. D., Yen, S. E. & Giaccia, A. J. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol. Cell. Biol. 25, 6415–6426 (2005).
Zoccola, D. et al. Structural and functional analysis of coral Hypoxia Inducible Factor. PLoS ONE 12, e0186262 (2017).
Ivan, M. & Kaelin, W. G. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol. Cell 66, 772–779 (2017).
Dunn, F. S. et al. The developmental biology of Charnia and the eumetazoan affinity of the Ediacaran rangeomorphs. Sci. Adv. 7, eabe0291 (2021).
Rohde, K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65, 514–527 (1992).
Niklas, K. J. Morphological evolution through complex domains of fitness. Proc. Natl Acad. Sci. USA 91, 6772–6779 (1994).
Marshall, C. R. Explaining the Cambrian “Explosion” of animals. Annu. Rev. Earth Planet. Sci. 34, 355–384 (2006).
Wang, R. et al. A Great late Ediacaran ice age. Natl. Sci. Rev. 10, nwad117 (2023).
Wang, R., Yin, Z. & Shen, B. A late Ediacaran ice age: the key node in the Earth system evolution. Earth Sci. Rev. 247, 104610 (2023).
Linnemann, U. et al. An Upper Ediacaran Glacial Period in Cadomia: the Granville tillite (Armorican Massif)—sedimentology, geochronology and provenance. Geol. Mag. 159, 999–1013 (2022).
Niu, Y.-z. et al. Ediacaran Cordilleran-type mountain ice sheets and their erosion effects. Earth Sci. Rev. 249, 104671 (2024).
Zhuravlev, A. Y. & Wood, R. A. The two phases of the Cambrian explosion. Sci. Rep. 8, 16656 (2018).
Dunn, F. S. et al. A crown-group cnidarian from the Ediacaran of Charnwood Forest, UK. Nat. Ecol. Evol. 6, 1095–1104 (2022).
Vaapil, M. et al. Hypoxic conditions induce a cancer-like phenotype in human breast epithelial cells. PLoS ONE 7, e46543 (2012).
Buravkova, L. B., Andreeva, E. R., Gogvadze, V. & Zhivotovsky, B. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion 19, 105–112 (2014).
Deline, B. et al. Evolution of metazoan morphological disparity. Proc. Natl Acad. Sci. 115, E8909–E8918 (2018).
Fernàndez-Busquets, X. et al. Cell adhesion-related proteins as specific markers of sponge cell types involved in allogeneic recognition. Dev. Comp. Immunol. 26, 313–323 (2002).
Sogabe, S. et al. Pluripotency and the origin of animal multicellularity. Nature 570, 519–522 (2019).
Valentine, J. W., Collins, A. G. & Meyer, C. P. Morphological complexity increase in metazoans. Paleobiology 20, 131–142 (1994).
Ruppert, E. E., Fox, R. S. & Barnes, R. D. Invertebrate zoology: a functional evolutionary approach. (Belmont, Calif. Thomson, 2003).
Kumala, L. & Canfield, D. E. Contraction dynamics and respiration of small single-osculum explants of the demosponge halichondria panicea. Front. Mar. Sci. 5. https://doi.org/10.3389/fmars.2018.00410 (2018).
Nickel, M. Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae). J. Exp. Biol. 207, 4515–4524 (2004).
Ivanovic, Z. & Vlaski-Lafarge, M. Anaerobiosis and Stemness: An Evolutionary Paradigm for Therapeutic Applications (Academic Press, 2015).
Peters, S. E. The problem with the Paleozoic. Paleobiology 33, 165–181 (2007).
Bambach, R. K., Knoll, A. H. & Wang, S. C. Origination, extinction, and mass depletions of marine diversity. Paleobiology 30, 522–542 (2004).
Bowring, S. A. et al. Calibrating rates of early Cambrian evolution. Science 261, 1293–1298 (1993).
Haxen, E. R. et al. Hypoxic” Silurian oceans suggest early animals thrived in a low-O2 world. Earth Planet. Sci. Lett. 622, 118416 (2023).
Sperling, E. A., Knoll, A. H. & Girguis, P. R. The ecological physiology of earth’s second oxygen revolution. Annu. Rev. Ecol. Evol. Syst. 46, 215–235 (2015).
Philippe, H. et al. Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol. 9, e1000602 (2011).
Telford, M. J., Budd, G. E. & Philippe, H. Phylogenomic insights into animal evolution. Curr. Biol. 25, R876–R887 (2015).
Acknowledgements
We thank Robert R. Gaines, Christopher Scotese, James Sample and Robert Austin for continuously helpful input and criticism. We are also grateful to members at the Tissue Development and Evolution (TiDE) group, the Department of Geology at Lund University and the Nordic Center for Earth Evolution (NordCEE) at the University of Southern Denmark for help with both conceptual and practical experiments. We thank the Norwegian Institute for Water Research (NIVA) for the access to the data platform and the Mobius framework. The authors are grateful for funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme, grant agreement No 949538 (E.U.H., M.I., E.B.); Norwegian Institute for Water Research (K.H.); VILLUM FONDEN grant 15397 (N.R.P.); Crafoord Foundation Grant 20220633 (A.B., C.C., E.U.H); The Royal Swedish Academy of Sciences MG2022-0019 (A.B.); The National Science Foundation Graduate Research Fellowship Pr. 1746051 (A.B.).
Funding
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
Design of the study: E.U.H., K.H.; Building and calibration of the 1D diffusion model: E.U.H., K.H., M.D.N.; Building the G-function framework: A.B. J.S.B.; Performing experiments/modelling: E.U.H., K.H., M.D.N.; A.B. M.I.; Composing the HIF component map: M.I.; Analyses of results: E.U.H., K.H., M.D.N.; M.I., S.E.P., N.R.P., A.B., K.J.P., S.R.A., C.C., M.I., R.A.G., E.B. J.S.B.; Writing the manuscript: E.U.H., A.B., K.H., M.D.N.; N.R.P., S.E.P., C.C., E.B. K.J.P., S.R.A., R.A.G., J.S.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Thomas Wong Hearing and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammarlund, E.U., Bukkuri, A., Norling, M.D. et al. Benthic diel oxygen variability and stress as potential drivers for animal diversification in the Neoproterozoic-Palaeozoic. Nat Commun 16, 2223 (2025). https://doi.org/10.1038/s41467-025-57345-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57345-0