Abstract
In the process of the unfolded protein response (UPR), the Hac1p protein is induced through a complex regulation of the HAC1 mRNA. This includes the mRNA localization on the endoplasmic reticulum (ER) membrane and stress-triggered splicing. In yeast, a specific ribosome ubiquitination process, the monoubiquitination of eS7A by the E3 ligase Not4, facilitates the translation of HAC1i, a spliced form of the HAC1 mRNA. Upon UPR, the mono-ubiquitination of eS7A increases due to the downregulation of Ubp3, a deubiquitinating enzyme of eS7A. However, the exact mechanisms behind these regulations have remained unknown. In this study, an E3 ligase, Grr1, an F-box protein component of the SCF ubiquitin ligase complex, which is responsible for Ubp3 degradation, has been identified. Grr1-mediated Ubp3 degradation is required to maintain the level of eS7A monoubiquitination that facilitates Hac1p translation depending on the ORF of HAC1i. Grr1 also facilitates the splicing of HAC1u mRNA independently of Ubp3 and eS7A ubiquitination. Finally, we propose distinct roles of Grr1 upon UPR, HAC1u splicing, and HAC1i mRNA translation. Grr1-mediated Ubp3 degradation is crucial for HAC1i mRNA translation, highlighting the crucial role of ribosome ubiquitination in translational during UPR.
Similar content being viewed by others
Introduction
Ribosome ubiquitination is a crucial modification of translation quality control pathways targeting the nascent polypeptide (RQC: Ribosome-associated Quality Control) and mRNA (NGD: No-Go Decay), as well as nonfunctional rRNA decay (18S NRD)1,2,3,4,5. In RQC, collision sensors Hel2 in yeast and ZNF598 in mammals form a K63-linked polyubiquitin chain on uS10 of the stalling ribosome in collided disomes or disomes6,7,8,9. The K63-linked polyubiquitin chain on uS10 is subsequently recognized by the RQC-trigger (RQT) complex and plays a crucial role in the dissociation of collided ribosomes into subunits to initiate the RQC pathway6,7,8,9,10,11,12. In yeast, the RQT complex is composed of the RNA helicase-family protein Slh1, ubiquitin-binding protein Cue3, and Rqt48. The ATPase activity of Slh1 is responsible for subunit dissociation of polyubiquitinated collided ribosomes8,10. The two subunits of the RQT complex, Cue3, and Rqt4 simultaneously recognize the K63-linked polyubiquitin chain formed on the stalling ribosome by Hel211. Similarly, ZNF598, the human homolog of Hel2, ubiquitinates ribosomal proteins uS10 and eS10 to initiate RQC in mammals. The human RQT (hRQT) complex is composed of the RNA helicase-family protein ASCC3, the ubiquitin-binding protein ASCC2, and TRIP46,12, which specifically recognizes the K63-linked polyubiquitin chain on uS10 in collided ribosomes, indicating that the triggering step of RQC is conserved from yeast to humans6.
The ubiquitination of the small subunit protein eS7A plays a crucial role in NGD9. Collided ribosomes are also subjected to the NGD pathway, which can be divided into two branches, depending on their coupling with RQC. NGD coupled with RQC is referred to as NGDRQC+, depending on the Hel2-mediated K63-linked polyubiquitination of uS10 and RQT-mediated subunit dissociation of the stalling ribosomes9. An endonuclease Cue2 is responsible for the cleavage at the vicinity of the collided ribosome13,14. In the absence of the subunit dissociation, the endonucleolytic mRNA cleavages upstream of the collision site are referred to as the NGDRQC-. The cleavages in NGDRQC- require K63-linked polyubiquitination of ribosomal protein eS7A via a two-step mechanism, the E3 ligase Not4 first monoubiquitinates eS7A which is then followed by Hel2-mediated polyubiquitination14. Polyubiquitination of eS7A is required for the recruitment of Cue2, which cleaves mRNA upstream of the collided ribosomes14.
Despite an increased understanding of the role of ribosome ubiquitination in quality control pathways, the physiological relevance of ribosome ubiquitination remains largely unknown. The Ccr4-Not complex initiates mRNA decay through the deadenylation and activation of decapping15,16. The Ccr4-Not complex monitors the translating ribosome for codon optimality via a specific interaction of the Not5 subunit with the ribosomal E-site17. This interaction only occurs when the ribosome lacks an accommodated A-site tRNA, indicative of low codon optimality. Ccr4-Not also interacts with the ribosome through mono-ubiquitination of eS7A by Not4 E3 ligase9,14,18,19,20. Deletion of Not4 and mutation of the four lysine residues targeted by Not4 (eS7A-4KR) strongly stabilized non-optimal and semi-optimal reporters17. This suggests that eS7A-ubiquitination is part of the same pathway as Not5-mediated sensing of translation rate, but occurs upstream of E-site probing, and the precise role of eS7A ubiquitination in codon optimality-dependent mRNA decay by Ccr4-Not remains elusive.
In recent studies, ribosomal ubiquitination has been linked to cellular responses to stress. K63 polyubiquitination may modulate oxidative stress responses, and ubiquitination of specific lysine residues in ribosomal proteins contributes to stress responses19,21,22,23,24,25,26,27,28. When cellular stresses lead to the accumulation of misfolded proteins within the endoplasmic reticulum (ER), it triggers a network of intracellular signaling pathways known as the unfolded protein response (UPR)29,30,31. In the UPR signaling pathway, HAC1 mRNA in budding yeast32,33,34 or XBP1 mRNA in metazoans32,33,34 is targeted to the ER-resident protein kinase and endonuclease Ire132,35,36. Subsequently, Ire1 cleaves an intervening sequence from HAC1/XBP1 mRNA, and the spliced form of HAC1/XBP1 mRNA encodes a transcription factor that stimulates the expression of numerous proteins, including ER-resident chaperones, to upregulate the protein-folding capacity of the cell. Induction of the UPR upregulates the ubiquitination of ribosomal proteins in mammals37 and yeast18. In yeast, the ubiquitination of eS7A, uS10, and uS3 was upregulated and Not4-mediated monoubiquitination of eS7A is required for resistance to tunicamycin (Tm), whereas E3 ligase Hel2-mediated polyubiquitination of uS10 and uS3 is not required18. Ribosome profiling revealed that monoubiquitination of eS7A is crucial for translational regulation, including upregulation of the spliced form of HAC1 (HAC1i) mRNA18. Downregulation of the deubiquitinating enzyme complex Ubp3-Bre5 increased the levels of ubiquitinated eS7A during UPR18. These results suggest that monoubiquitination of ribosomal protein eS7A plays a crucial role in translational control during the ER stress response in yeast. However, it remains unknown how Ubp3 is downregulated and how eS7A ubiquitination facilitates HAC1i mRNA translation upon UPR.
In this study, we identified an E3 ligase, Grr1, an F-box protein component of the SCF ubiquitin ligase complex responsible for Ubp3 downregulation during the UPR. Grr1 is required for proteasomal degradation of Ubp3, thereby increasing levels of ubiquitinated eS7A. eS7A ubiquitination facilitates HAC1i translation during UPR. Collectively, we propose that upon UPR, Grr1-mediated proteasomal degradation of Ubp3 upregulates eS7A mono-ubiquitination, thereby promoting HAC1i translation. We also found that Grr1 facilitates the splicing of HAC1u mRNA independently of Ubp3 and eS7A ubiquitination, indicating the unknown function of SCFGrr1 complex in the mRNA splicing on the ER membrane.
Results
Grr1 is responsible for the proteasomal degradation of Ubp3 and facilitates HAC1u splicing upon UPR
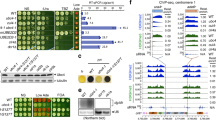
Ribosome ubiquitination increases significantly upon induction of the UPR in mammals and yeast18,37. In yeast, Ubp3 is the enzyme responsible for deubiquitylation of the ubiquitinated eS7A and is downregulated, which is consistent with the increased level of monoubiquitinated eS7A in response to UPR18. To understand how ribosome ubiquitination is regulated upon UPR in yeast, we performed genetic screening of E3 ubiquitin ligases that are required for Tm-resistance growth and Ubp3 degradation. Among 76 deletion mutants of the E3 ligases, we identified pep3Δ and grr1Δ mutants that conferred Tm-sensitive growth (Fig. 1A and Supplementary Fig. 1A). The level of Hac1 protein (Hac1p), an essential transcription factor induced by ER stress, was downregulated in the grr1Δ mutant, 4 h after Tm treatment (Fig. 1B, lanes 1-8). In contrast, the level of Hac1p was not decreased in the pep3Δ mutant cells (Fig. 1B, lanes 9-12). In the grr1Δ and pep3Δ mutant cells, induction of HAC1 mRNA by Tm treatment was intact (Fig. 1C). We quantified the splicing efficiency based on the levels of unspliced HAC1u and spliced HAC1i forms of HAC1 mRNA (Supplementary Fig. 1B–E), and the splicing efficiency in the grr1Δ mutant cells 2 h after Tm treatment was 52% of that in WT cells (Supplementary Fig. 1E), indicating that the splicing efficiency of HAC1 mRNA is moderately reduced. The level of HAC1i mRNA in grr1Δ mutant cells was 40% of that in GRR1 wildtype cells (Supplementary Fig. 1D), suggesting that Grr1 also contributes to Hac1p expression upon UPR at the posttranscriptional levels.
A grr1∆ and pep3∆ strains are sensitive to tunicamycin (Tm). Yeast cells lacking one of the E3 ligases and strains transformed with plasmids encoding each wild type were grown in SDC-Leu medium. 10-fold serial dilutions of OD600 = 0.3 cells were prepared in the 1.5 mL tubes and spotted onto Tm-added SDC-Leu and control plates. Plates were incubated at 30 °C for 2–3 days. B Hac1p expression is decreased in grr1∆ strains. Yeast cells were grown at 30 °C until OD600 = 0.2, then treated with 1 µg/mL of Tm for ~4 h and harvested. The samples were analyzed using western blotting with anti-Hac1 antibody. CBB staining was used as a loading control. C The splicing of HAC1 mRNA occurs in grr1∆ and pep3∆ cells. Yeast cells were grown and harvested as in (B). The samples were analyzed by northern blotting with DIG-labeled HAC1 probe. Methylene Blue staining was used as a loading control. D Ubp3 downregulation is suppressed in the grr1∆ cells. The indicated yeast cells expressing UBP3-3HA were grown and harvested as in (B). The samples were analyzed using western blotting with anti-HA and anti-α-Tubulin antibodies. E, F Grr1-dependent rapid decay of Ubp3 under ER stress conditions. The indicated yeast cells expressing UBP3-3HA were grown at 30 °C until OD600 = 0.2, then treated with 1 µg/mL of Tm for 1 h. Yeast cells were then treated with 0.25 mg/mL of cycloheximide (CHX) and harvested at the indicated times. The samples were analyzed with western blotting using anti-HA antibodies. CBB staining was used as a loading control. Dilutions are 100%, 75%, 50%, 25%, 12.5%, 6.25%. G The half-life of Ubp3-3HA in the wild-type and the grr1∆ mutant cells. The Ubp3-3HA/CBB levels shown in (E, F), Supplementary Fig. 2A, B were quantified and normalized relative to that at 0 min samples. Data represent n = 3 biologically independent experiments (mean ± SE), and P values were calculated by Two-sided Welch’s t-test. All experiments were repeated at least twice with biologically independent samples and showed similar results. Source data are provided as a Source Data file.
Grr1 is the F-box protein component of an SCF ubiquitin-ligase complex38,39. Therefore, we hypothesized that the upregulated Ubp3 decreases the monoubiquitinated eS7A in the grr1Δ mutant cells, thereby reducing the Hac1p level. The level of Hac1p 2 h after Tm treatment was reduced in the eS7A ubiquitination-defective eS7A-4KR cells, confirming that monoubiquitination of ribosomal protein eS7A plays a crucial role in translational control during the ER stress response in yeast (Supplementary Fig. 1F). Neither uS3 nor uS10 ubiquitination, which is essential for 18S NRD and RQC, affected the Hac1p level upon UPR (Supplementary Fig. 1G, H), indicating that eS7A ubiquitination plays a crucial role in ER stress response. The downregulation of Ubp3 was diminished in grr1Δ mutant cells (Fig. 1D, lanes 9-12), indicating that Grr1 is required for Ubp3 downregulation upon UPR. To examine whether Ubp3 is subjected to Grr1-dependent proteasomal degradation, we measured Ubp3-3HA stability using cycloheximide (CHX) chase followed by western blotting (Fig. 1E). Ubp3-3HA was more unstable 2 h after Tm treatment (Fig. 1E, lanes 6-10; half-life, t1/2 ~ 80 min, Fig. 1G and Supplementary Fig. 2A) than in the absence of ER stress (Fig. 1E, lanes 1-5; half-life, t1/2 ~ 120 min, Fig. 1G and Supplementary Fig. 2A). We also noticed that Ubp3-3HA was not efficiently degraded 4 h after Tm treatment in wildtype and the grr1Δ mutant cells (half-life, t1/2 > 120 min, Supplementary Fig. 2C, D). Although Ubp3 is proposed to be involved in ribophagy27, Ubp3-3HA was not stabilized in the atg1Δ mutant cells (Supplementary Fig. 3A, B) regardless of Tm treatment. However, Ubp3-3HA was stabilized after treatment with the proteasome inhibitor MG132 (Supplementary Fig. 3C, D, lanes 6-10) and in grr1Δ mutant cells regardless of Tm treatment (Fig. 1F, lanes 1-10; half-life, t1/2 > 120 min; Fig. 1G and Supplementary Fig. 2B). Moreover, RNA-seq and Ribo-seq demonstrated that levels of total and translated UBP3 mRNAs were not reduced 4 h after Tm treatment (Supplementary Fig. 3E, F). These results demonstrated that Grr1 is responsible for the efficient degradation of Ubp3, which may contribute to efficient HAC1i translation probably via an increase in eS7A ubiquitination.
Grr1 interacts with Ubp3
Grr1 associates with core SCF (Cdc53p, Skp1p, and Hrt1p/Rbx1p) to form the SCFGrr1 complex33,38,39,40. The F-box domain was responsible for binding to SCF, and the LRR domain interacted with the substrate for ubiquitination38,39 (Fig. 2A). The levels of Ubp3-3HA upon UPR were determined in the grr1Δ cells expressing Grr1 mutants with the deletion of the indicated domains (Fig. 2B, C). The downregulation of Ubp3-3HA was diminished in the grr1Δ mutants expressing Grr1 mutant proteins that lack the 311-361 residues F-box (Fbox) and the 413-740 residues LRR (LRR) domains (Fig. 2B, lanes 9-12). The quantification of Ubp3-3HA levels revealed that the reduction of Ubp3-3HA by 4 h Tm treatment was diminished in grr1Δ mutant cells and complemented by plasmids expressing wildtype Grr1 or Grr1-ΔN but not by Grr1-ΔFbox or Grr1-ΔLRR mutant proteins (Fig. 2B, C and Supplementary Fig. 4A). We also determined the stability of Ubp3-3HA in the grr1 mutant cells (Fig. 2D and Supplementary Fig. 4B). Ubp3-3HA destabilization by Tm treatment diminished in the grr1Δ mutant cells and complemented by plasmids expressing wildtype Grr1 or Grr1-ΔN but not by Grr1-ΔFbox or Grr1-ΔLRR mutant proteins (Fig. 2D and Supplementary Fig. 4B). Consistently, the grr1Δ mutants expressing Grr1-ΔFbox or Grr1-ΔLRR mutant conferred sensitivity to Tm (Fig. 2E) and the reduced Hac1p expression upon UPR (Fig. 2F). These indicated that the F-box and LRR domains of Grr1 are required for the downregulation of Ubp3-3HA upon UPR. The expression level of Grr1-ΔFbox or Grr1-ΔLRR mutant proteins was the same as the wild-type Grr1 (Fig. 2G). The expression level of Grr1-ΔN was significantly lower than that of wild-type Grr1 (Fig. 2G), but Grr1-ΔN complemented the grr1Δ mutant phenotype in Tm sensitivity (Fig. 2E) and the reduced Hac1p level upon UPR (Fig. 2F), indicating that the N-terminal region is dispensable for Grr1 function. To confirm the interaction of Ubp3-3HA with Grr1, we purified the wild-type and mutant Grr1-FLAG proteins that lack F-box (ΔFbox) or LRR (ΔLRR) domains and examined the co-immunoprecipitation of the Ubp3-3HA (Fig. 2H). Ubp3-3HA co-immunoprecipitated with all Grr1 proteins (WT, ΔFbox, ΔLRR)(Fig. 2H, lanes 11-16), indicating that Grr1 interacts with Ubp3-3HA independent of the F-box and LRR domains. Taken together, we conclude that Grr1 destabilizes Ubp3 dependent on the F-box and LRR domains.
A Schematic drawing of Grr1 domains. B Ubp3 downregulation is suppressed in the grr1∆ cells expressing GRR1(∆Fbox) and GRR1(∆LRR) mutant. The grr1∆ mutant cells expressing the indicated GRR1 mutants were grown and harvested as in Fig. 1B. The samples were analyzed using western blotting using anti-HA antibody. C The Ubp3-3HA/CBB levels shown in Supplementary Fig. 4A were quantified and normalized relative to that at 0 min samples. Data represent n = 3 biologically independent experiments (mean ± SE), and P values were calculated by Two-sided Welch’s t-test. D The half-lives of Ubp3-3HA in the indicated Grr1 mutant cells. The Ubp3-3HA/CBB levels shown in Supplementary Fig. 4B were quantified and normalized relative to that at 0 min samples. Data represent at least three biologically independent experiments (mean ± SE), and P values were calculated by Two-sided Welch’s t-test. E GRR1(∆Fbox) and GRR1(∆LRR) mutant cells are sensitive to tunicamycin (Tm). The grr1∆ cells expressing the indicated GRR1 mutants were grown in SDC-Ura medium. 10-fold serial dilutions of OD600 = 0.3 cells and spotted onto Tm-added SDC-Ura and control plates, then incubated at 30 °C for 2–3 days. F Hac1p is decreased in GRR1(∆Fbox) and GRR1(∆LRR) mutant cells. Yeast cells were grown and harvested as in Fig. 1B. The samples were analyzed using western blotting using anti-Hac1 antibody. G Expression levels of Grr1p mutants. The grr1∆ cells expressing 3HA-GRR1 mutants were grown and harvested as in Fig. 1B. The samples were analyzed as in (B). H Grr1 was coimmunoprecipitated with Ubp3 independently of ER stress, F-box, and LRR domains. The yeast cells expressing Ubp3-3HA and the indicated Grr1-FLAG mutants were grown as in Fig. 1B, treated with 1 µg/mL of Tm for 1.5 h, and then harvested. Grr1-FLAG proteins were affinity-purified using anti-FLAG beads and FLAG peptides. The elution samples were analyzed by western blotting using anti-HA, anti-FLAG antibodies. All experiments were repeated at least twice with biologically independent samples and showed similar results. Source data are provided as a Source Data file. CBB staining was used as a loading control on western blot analysis in (B, F, G).
Grr1 facilitates eS7A ubiquitination
Not4-mediated monoubiquitination of eS7A is required to produce Hac1p during the UPR18; therefore, we assessed whether the upregulation of eS7A ubiquitination is dependent on Grr1. We found that the upregulation of eS7A ubiquitination diminished in grr1Δ mutant cells after the addition of Tm (Fig. 3A), indicating that Grr1 is required for the upregulation of eS7A ubiquitination upon UPR. In addition, the upregulation of eS7A ubiquitination partially attenuated in pep3Δ mutant cells after the addition of Tm (Fig. 3A). We also confirmed that defects in Ubp3 downregulation in the grr1Δ mutants expressing Grr1-ΔFbox or Grr1-ΔLRR proteins results in the reduction of eS7A mono-ubiquitination. The levels of mono- and diubiquitinated eS7A were reduced in grr1Δ cells (Fig. 3B, lanes 3-4) and grr1Δ cells expressing Grr1-ΔFbox or Grr1-ΔLRR mutants (Fig. 3B, lanes 9-12). The levels of mono and di-ubiquitinated eS7A did not significantly change in grr1Δ cells expressing wild-type Grr1 and Grr1-ΔN mutant protein (Fig. 3B, lanes 5-8), in which Ubp3-3HA downregulation was partly restored (Fig. 2B). The quantification of the non-, mono-ubiquitinated eS7A proteins revealed that the ratio of monoubiquitinated eS7A was reduced in the grr1Δ mutant cells (Fig. 3C), and the reduction in the grr1Δ mutant cells was complemented by plasmids expressing wildtype Grr1 or Grr1-ΔN but not by Grr1-ΔFbox or Grr1-ΔLRR mutant proteins (Fig. 3C, Supplementary Fig. 5A, C). These are consistent with the stability of Ubp3-3HA in the grr1 mutant cells (Fig. 2D and Supplementary Fig. 4B). Not4 monoubiquitinates eS7A at the four lysine residues (Fig. 3D)41, with K83 ubiquitination primarily responsible for mRNA quality control9. We examined cell growth in the presence of Tm, eS7A ubiquitination, and Hac1p production upon UPR in four eS7A mutants containing a single lysine residue, susceptible to Not4-mediated monoubiquitination, eS7A-K72only, eS7A-K76only, eS7A-K83only and eS7A-K84only. K83 but no other lysine residues were responsible for resistance to Tm (Fig. 3E) and Hac1p expression upon UPR (Fig. 3F, middle panels). Consistently, K83 was found to be the major ubiquitination site of eS7A (Fig. 3F, top panels), confirming that K83 ubiquitination is primarily responsible for eS7A ubiquitination thereby Hac1p production upon UPR. We quantified the non-, mono-ubiquitinated eS7A proteins and demonstrated that the ratio of monoubiquitinated eS7A was reduced in the eS7A-K83only grr1Δ mutant cells (Fig. 3G, H, Supplementary Fig. 5B, D), indicating a crucial role of Grr1 in maintaining the level of eS7A ubiquitination at K83 upon UPR.
A eS7A ubiquitination is decreased in the grr1∆ cells. The indicated yeast cells expressing eS7A-HA were grown and harvested as in Fig. 1B. The samples were analyzed using western blotting using anti-HA, anti-Hac1 and anti-α-Tubulin antibodies. B eS7A ubiquitination is decreased in GRR1(∆Fbox) and GRR1(∆LRR) mutants. The grr1∆ cells expressing the GRR1 mutants and eS7A-HA were grown and harvested. The samples were analyzed as in (A). C The eS7A-HA levels shown in Supplementary Fig. 5A were quantified and normalized relative to that at 0 min samples. eS7A-Ub levels were normalized to total eS7A levels (eS7A-Ub and free eS7A). Data represent n = 4 biologically independent experiments (mean ± SE), and P values were calculated by Two-sided Welch’s t-test. D The schematic view of eS7A ubiquitination sites. This image was prepared by UCSF ChimeraX software using PDB file 8CBJ20. E K83 of eS7A is essential for tunicamycin resistance. eS7a∆eS7b∆ cells expressing the indicated eS7A-HA mutants were grown in YPD medium. 10-fold serial dilutions of OD600 = 0.3 cells were prepared in the 1.5 mL tubes and spotted onto Tm added YPD and control plates. Plates were incubated at 30 °C for 2–3 days. F K83 of eS7A is a major ubiquitination site and essential for Hac1p expression. eS7a∆eS7b∆ cells expressing eS7A-HA mutants were grown and harvested as in (A). The samples were analyzed using western blotting with anti-HA, anti-Hac1 and anti-EF3 antibodies. G eS7A ubiquitination at K83 is decreased in grr1∆ strains. Yeast cells harboring plasmids encoding eS7A(K83 only)-HA were grown and harvested as in (A). The samples were analyzed using western blotting with anti-HA antibody. CBB staining was used as a loading control. H The eS7A-HA levels shown in Supplementary Fig. 5B were quantified and normalized relative to that at 0 min samples. eS7A-Ub levels were normalized to total eS7A levels (eS7A-Ub and free eS7A). Data represent n = 4 biologically independent experiments (mean ± SE), and P values were calculated by Two-sided Welch’s t-test. All experiments were repeated at least twice with biologically independent samples and showed similar results. Source data are provided as a Source Data file.
Grr1 is required for HAC1i translation during the UPR in yeast
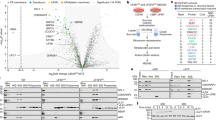
These results suggest that Grr1 plays a role in HAC1 translation by downregulating Ubp3 during the UPR in yeast. To test whether Grr1 is involved in translational control of the UPR, we performed RNA-seq and ribosome profiling. To investigate the regulation of translation in response to ER stress, we estimated translation efficiency (TE) by assessing both mRNA abundance and ribosome occupancy. eS7A ubiquitination-dependent translational regulation was monitored at 4 h. Translational responses were observed in grr1Δ mutant cells, with statistically significant changes in the translation of HAC1 mRNA (Fig. 4A, B; Q < 0.05). To examine the Grr1-mediated ubiquitination dependency of the involved mRNAs, TE fold-change by Tm treatment revealed 191 and 154 mRNAs categorized as up- and downregulated, respectively (Fig. 4B, C). These subsets were identified using the formula “log2 TE fold change ( + Tm) - log2 TE fold change (-Tm)”, with mRNAs scored as > 2 and < −2 defined being up- and down-regulated, respectively.
A, B Fold change of translation efficiency (TE) in WT and grr1∆ cells. Red and blue dots represent genes with upregulated and downregulated TE in WT strains, respectively (Q < 0.05, calculated by Two-sided Likelihood ratio test). The ribosome profiling and RNA-seq results represent two independent biological replicates. C The genes with upregulated and downregulated TE in WT (Q < 0.05, calculated by Two-sided Likelihood ratio test). Genes with the log2 fold changes greater than 2 or less than −2 are shown. D Map of the P-site position of footprints at HAC1i CDS in WT and the grr1∆ cells.
We identified HAC1 as the most significantly translationally upregulated gene during the UPR as previously reported42,43 (Fig. 4A–D). In comparing TE in cells 4 h after the addition of Tm based on reads of HAC1, we found that the TE of HAC1 mRNAs was approximately 1.96-fold lower in grr1Δ than in wild-type (WT) cells (Fig. 4C). The regulation of some of the HAC1 target mRNAs44,45,46 by Tm treatment was also affected in the grr1Δ mutant cells (Supplementary Fig. 6A). Ribo-seq demonstrated that the footprint on HAC1u mRNA (Supplementary Fig. 6B, -Tm) was significantly lower than HAC1i mRNA (Supplementary Fig. 6B, +Tm), indicating that translation of HAC1u mRNA is inefficient. Given that the footprint on HAC1u mRNA was low, the splicing efficiency of HAC1u mRNA should affect TE. In the grr1Δ mutant cells, the splicing efficiency 4 h Tm treatment was 1.93-fold lower than WT (Supplementary Fig. 1E, 82.3% in WT, and 42.5% in grr1Δ), which could account for the 1.96-fold lower TE of HAC1 mRNAs in grr1Δ than in wild-type (WT) cells. The footprints at the initiation codon were not changed in the grr1Δ mutant cells (Supplementary Fig. 6B), and the peaks in the 5’UTR were low and not changed in the mutant cells (Supplementary Fig. 6B), suggesting that translation initiation may not be affected. Mapping of the footprints throughout HAC1i mRNA in WT and grr1Δ mutant cells HAC1i mRNA contains potential translation pause sites (Fig. 4D), suggesting that Grr1 may be involved in translation elongation of HAC1i mRNA.
Translation of HAC1i mRNA requires eS7A ubiquitination regardless of ER stress
UPR induces the transcription and splicing of HAC1u mRNA ensuring Hac1p expression upon UPR. We constructed a 5H3 clone containing the HAC1 ORF, 5′ and 3′ untranslated regions (5′ and 3′ UTRs) but lacking an intron (Fig. 5A) and examined Hac1p levels in hac1Δ mutant cells harboring 5H3 clones under the control of various promoters (Fig. 5B). Hac1p is induced upon Tm treatment in hac1Δ mutant cells harboring 5H3 under the control of the HAC1 promoter, as reported (Fig. 5B, lanes 5-6). Hac1p was expressed from 5H3 under the control of the GPD, TEF1 and ADH1 promoters, regardless of Tm treatment (Fig. 5B, lanes 7-12). Hac1p was induced upon UPR in hac1Δ mutant cells expressing 5H3 under the CYC1 promoter (Fig. 5B, lanes 13-14), suggesting that CYC1 transcription is induced upon UPR. We examined HAC1 mRNA levels in hac1Δ mutant cells harboring 5H3 clones under the control of various promoters (Fig. 5C, D and Supplementary Fig. 7A–C). The transcriptional induction of HAC1 and CYC1 promoter by Tm treatment was confirmed. We also confirmed the expression levels of Hac1p in hac1Δ mutant cells harboring 5H3 clones under the control of various promoters were sufficient for Tm resistance (Fig. 5E).
A Schematic drawing of HAC1u mRNA and 5H3(HAC1-intronless) mRNA. B Hac1p expression from 5H3 reporters with different promoters. Cells were grown and harvested and the samples were analyzed as in Fig. 1B. C Induction of 5H3 from various promoters by Tm treatment. Yeast cells were grown at 30 °C until OD600 = 0.2, then treated with 1 µg/mL of Tm for ~ 2 h and harvested. The samples were analyzed by northern blotting with DIG-labeled HAC1 probe. Methylene Blue staining was used as a loading control. Dilutions are 100%, 75%, 50%, 25%. D Fold change of 5H3 mRNA/25S rRNA levels shown in Fig. 5C and Supplementary Fig. 7A, B were quantified and normalized relative to that at 0 min samples. E The correlation between Hac1p levels and tunicamycin resistance. 10-fold serial dilutions of OD600 = 0.3 cells grown in SDC-Ura medium were spotted onto SDC-Ura plates. Plates were incubated at 30 °C for 2–3 days. F eS7A ubiquitination is required for efficient Hac1p expression from 5H3 clones under control of HAC1 or TEF1 promoters. The indicated cells were harvested as in (C). The samples were analyzed as in Fig. 1B. G HAC1i and 5H3 mRNA levels in eS7A(WT) and eS7A(4KR). Yeast cells were harvested, and the total RNA samples were analyzed using northern blotting as in (C). H 5H3 mRNA/25S rRNA levels shown in (G) and Supplementary Fig. 7C were quantified and normalized relative to 2 h in hac1∆ encoding HAC1p-5H3. I Grr1 is required for efficient Hac1p expression from 5H3 under control of HAC1 or TEF1 promoters. Cells were harvested as in (C), and samples were analyzed as in (F). J HAC1i and 5H3 mRNA levels in WT and grr1∆. Yeast cells were harvested, and the samples were analyzed as in (C). K 5H3 mRNA/25S rRNA levels shown in (J) and Supplementary Fig. 7D were quantified and normalized relative to 2 h in WT. All experiments were repeated at least twice with biologically independent samples and showed similar results. Data represent n = 3 biologically independent experiments (mean ± SE). Source data are provided as a Source Data file.
We then examined whether eS7A ubiquitination is essential for the translation of HAC1i mRNA without ER stress. Hac1p was expressed regardless of UPR in eS7A-WThac1Δ cells expressing 5H3 under the control of HAC1 and TEF1 promoters (Fig. 5F, lanes 5-6 and 9-10) but not in eS7A-4KRhac1Δ mutant cells that lack eS7A ubiquitination (Fig. 5F, lanes 7-8 and 11-12), indicating that eS7A ubiquitination is essential for translation of HAC1i mRNA without ER stress. Upon UPR, the HAC1 mRNAs expressed by TEF1 promoter were increased 1.3-fold in eS7A-WThac1Δ but not increased in the eS7A-4KRhac1Δ cells (Fig. 5G, lanes 13-16, Fig. 5H and Supplementary Fig. 7C). In ER stress conditions, the levels of HAC1 mRNAs in the eS7A-WThac1Δ cells were decreased 0.69-fold in eS7A-4KRhac1Δ cells (Fig. 5G, lanes 10 and 12, Fig. 5H). We then assessed the role of Grr1 in HAC1i translation by using 5H3 constructs. Hac1p was expressed regardless of UPR in grr1Δhac1Δ mutant cells expressing 5H3 under the control of HAC1 and TEF1 promoters (Fig. 5I, lanes 5-6 and 9-10). Importantly, Hac1p levels in grr1Δhac1Δ cells (Fig. 5I, lanes 7-8 and 11-12) were lower than those in hac1Δ cells (Fig. 5I, lanes 5-6 and 9-10). We also determined HAC1 mRNA levels in hac1Δ mutant cells harboring 5H3 clones under the control of HAC1 and TEF1 promoters (Fig. 5J). The HAC1 mRNAs expressed by the TEF1 promoter were moderately increased by Tm treatment in hac1Δ and not increased in grr1Δhac1Δ cells (Fig. 5J, lanes 13-16, Fig. 5K and Supplementary Fig. 7D). In normal conditions, the levels of HAC1 mRNAs in hac1Δ and grr1Δhac1Δ cells were almost the same (Fig. 5J, lanes 13 and 15, Fig. 5K and Supplementary Fig. 7D), although the Hac1 protein was decreased in the grr1Δhac1Δ cells than in hac1Δ cells (Fig. 5I, lanes 9 and 11), indicating that Grr1 facilitates translation of HAC1i mRNA independent of ER stress.
ORF of HAC1i mRNA is required for eS7A ubiquitination-mediated translation stimulation
To understand how eS7A ubiquitination facilitates HAC1i mRNA translation under ER stress, we examined the putative cis elements required for the regulation. The HAC1i mRNA 3′ UTR contains a cis-acting 3′-bipartite element (BE) at nucleotides C1134 to A1192 (adenine of the AUG codon is +1) that promotes co-localization of the translationally repressed HAC1i mRNA under ER stress47,48. The protein kinases Kin1 and Kin2 contribute to HAC1u mRNA processing by phosphorylation of Pal1 and Pal2 which bind to the 3′-BE of HAC1 mRNA. HAC1u mRNA is stored in the cytoplasm in the absence of ER stress, and its translation is tightly suppressed by a base-pairing interaction between the intron and the 5′ UTR. Excision of the intron by Ire1-dependent splicing in response to ER stress led to robust translation of HAC1i mRNA, with the resulting Hac1p upregulating UPR target gene expression. We constructed HAC1 with the deletion of 3′-BE or 5′-BP, a region responsible for base-pairing with 3′ UTR or both (Supplementary Fig. 8A, ΔBE, ΔBP, ΔBP + ΔBE). The Hac1p was induced 2 hrafter Tm addition in the hac1Δ mutant cells harboring the 5H3 clones under the control of the HAC1 promoter (Supplementary Fig. 8B, lanes 5-12), indicating that these cis elements are not essential for the induction of Hac1p upon UPR. Consistently, hac1Δ mutant cells expressing HAC1 conferred resistance to Tm similar to wild-type cells (Supplementary Fig. 8C).
To identify the cis-elements involved in eS7A monoubiquitination-dependent translation activation of HAC1i mRNA, we constructed plasmids that contain the HAC1i ORF with or without 5′ and 3′ UTRs of HAC1 (Fig. 6A). The transcription from the TEF1 promoter is terminated by the CYC1 terminator in clones that contain the HAC1i ORF without 3′ UTR of HAC1, and we named them 5HCt and HCt due to CYC1 terminator-mediated transcription termination. The hac1Δ mutant cells expressing the 5H3, 5HCt, H3, and HCt mRNAs by the TEF1 promoter (TEF1p-5H3, 5HCt, H3, HCt) conferred resistance to Tm similar to wild-type cells (Fig. 6B), indicating that UTRs are dispensable for the expression of Hac1p by the TEF1 promoter at a sufficient level for Tm resistance. The Hac1p was expressed in cells harboring TEF1p-5H3, 5HCt, H3, and HCt clones (Fig. 6C, lanes 7-14). We extensively quantified and found that the levels of 3xFLAG-Hac1p were increased by 5′ and 3′ UTRs (Fig. 6C, lanes 7, 9, 11, 13; Fig. 6D). We examined the role of eS7A ubiquitination in Hac1p derived from these clones. The levels of 3xFLAG-Hac1p expressed in all HAC1i clones were significantly diminished in eS7A-4KR cells (Fig. 6C, lanes 7-14; Fig. 6D and Supplementary Fig. 8D). We also quantified the 3xFLAG-HAC1 mRNAs expressed from TEF1p-5H3, 5HCt, H3, and HCt clones in the eS7A and eS7A-4KR cells 2 h after Tm treatment (Fig. 6E, F). The HAC1 mRNAs expressed from TEF1p-5H3, 5HCt, H3 were moderately decreased in eS7A-4KR cells than in eS7A cells (Fig. 6E, lanes 5-10, Fig. 6F and Supplementary Fig. 8E). The 3xFLAG-HAC1 mRNAs expressed from TEF1p-HCt were moderately increased in the eS7A-4KR cells than in the eS7A cells (Fig. 6E, lanes 11-12, Fig. 6F). Given that 3xFLAG-Hac1p level was reduced in the eS7A-4KR cells (20% of WT, Fig. 6D), we suspected that translation efficiency of HCt was strongly reduced in eS7A-4KR cells. We constructed a 3xFLAG-GFP clone that contains the GFP open reading frame (ORF) under the control of the TEF1 promoter and the CYC1 terminator, and we compared it with a similar HCt clone. The levels of 3xFLAG-GFP were not affected in the eS7A-4KR cells (Fig. 6G, H). In contrast, the 3xFLAG-Hac1 protein derived from the HCt clone was only 20% of the control level, although the mRNA level was increased in the eS7A-4KR cells (Fig. 6C–F). These findings support the model that eS7A ubiquitination facilitates the translation of HAC1i mRNA, depending on potential cis-elements within the ORF. Inhibition of proteasome activity by MG132 treatment did not rescue the reduction of Hac1p in eS7A-4KR cells (Fig. 6I, J), suggesting that the downregulation of Hac1p in the eS7A-4KR mutant cells may not be due to the facilitation of proteasomal degradation of the Hac1p. Finally, we propose that eS7A ubiquitination facilitates the translation of HAC1i mRNA depending on putative cis element(s) in the ORF.
A Schematic drawing of 3xFLAG-HAC1i and 3xFLAG-GFP mRNAs. B Tunicamycin sensitivity of hac1∆ cells expressing 3xFLAG-HAC1i reporters. 10-fold serial dilutions of the indicated cells were spotted onto the plates. Plates were incubated at 30 °C for 2–3 days. C 3xFLAG-Hac1p expression is increased by eS7A ubiquitination independent of UTRs of HAC1. Yeast cells harboring plasmids encoding 3xFLAG-HAC1i shown in (A) under the control of TEF1 promoters were harvested as in Fig. 1B. The samples were analyzed using western blotting with anti-FLAG antibody. CBB staining was used as a loading control. Dilutions are 100%, 75%, 50%, 25%, 12.5%, 6.25%. D The 3xFLAG-Hac1p/CBB levels shown in (C) and Supplementary Fig. 8D were quantified and normalized relative to that from eS7A(WT) expressing 3xFLAG-5H3. The percentages indicate the protein levels ratio in eS7A(4KR) to that in eS7A(WT). E 3xFLAG-HAC1i mRNA levels in eS7A(WT) and eS7A(4KR). Yeast cells were harvested, and the samples were analyzed as in Fig. 5G, using DIG-labeled 3xFLAG probe. F 3xFLAG-HAC1i mRNA/25S rRNA levels shown in (E) and Supplementary Fig. 8E were quantified and normalized as in (D). G 3xFLAG-GFP expressions are almost the same between eS7A(WT) and eS7A(4KR). Yeast cells expressing 3xFLAG-GFPCt by TEF1 promoters were harvested, and the samples were analyzed as in (C). H The 3xFLAG-GFP/CBB levels shown in (G) were quantified and normalized relative to that from eS7A(WT). I Proteasome-dependent degradation of 3xFLAG-Hac1p in eS7A(WT) and eS7A(4KR). Yeast cells harboring plasmids encoding 3xFLAG-HCt under control of TEF1 promoter were grown at 30 °C until OD600 = 0.2, then treated with 1 µg/mL of Tm and 50 µM of MG132 for 2 h and harvested. The samples were analyzed as in (C). J The 3xFLAG-Hac1p/CBB levels shown in (I) were quantified and normalized relative to that in eS7A(WT) with 50 µM MG132 treatment. All experiments were repeated at least twice with biologically independent samples and showed similar results. Data represent n = 3 biologically independent experiments (mean ± SE), and P-values were calculated by Two-sided Welch’s t-test. Source data are provided as a Source Data file.
Grr1 is required for eS7A ubiquitination-mediated translation stimulation
We then examined whether Grr1 facilitates the translation of HAC1 mRNA dependent on HAC1 ORF. To examine whether ORF of HAC1 is responsible for the Grr1 mediated regulation, we determined 3xFLAG-Hac1p derived from the HCt clone and 3xFLAG-GFP derived from the GFPCt clone in grr1Δ cells (Fig. 7A and Supplementary Fig. 9A). In the grr1Δ mutant cells, Hac1 derived from HCt was at 43% of the control level, while 3xFLAG-GFP levels in the grr1Δ mutant cells were at 75% of those in the control cells (see Fig. 7A and Supplementary Fig. 9A), indicating that HAC1 ORF is responsible for the Grr1-mediated regulation. We then examined the roles of eS7A ubiquitination and Grr1 in HAC1 translation. we determined 3xFLAG-Hac1p derived from 5H3 clone in eS7A-K83only, grr1ΔeS7A-K83only, eS7A-K83R, grr1ΔeS7A-K83R cells (Fig. 7B, C and Supplementary Fig. 9B). The levels of 3xFLAG-Hac1p were decreased by the deletion of Grr1 in the eS7A-K83only cells, but not in eS7A-K83R cells, indicating that Grr1 facilitates HAC1 translation in an eS7 ubiquitination-dependent manner. We also determined 3xFLAG-Hac1p derived from HCt clone in ubp3Δ, ubp3Δgrr1Δ cells (Fig. 7D, E and Supplementary Fig. 9C). The levels of 3xFLAG-Hac1p were not decreased by the deletion of Grr1 in the ubp3Δ cells, indicating that Grr1 facilitates HAC1 translation in an UBP3-dependent manner. To examine whether the low Hac1 protein levels in the grr1Δ cells could result from protein degradation by proteosomes, we determined the stability of Hac1 protein in wild-type and grr1Δ cells 2 h after Tm treatment (Supplementary Fig. 9D, E). The half-life of 3xFLAG-Hac1p was not changed in grr1Δ cells, indicating that Hac1 degradation does not contribute to the Grr1-mediated downregulation upon UPR (Supplementary Fig. 9D, E). Finally, we performed in vitro translation using lysates prepared from ski2Δ and ski2Δgrr1Δ mutant cells (Fig. 7F–H). Translation of 2xPA-HAC1i mRNA was significantly reduced in the reaction using grr1Δ mutant lysates but translation of 2xPA-GFP mRNA was not significantly reduced with in vitro translation using ski2Δ and ski2Δgrr1Δ mutant cell lysates (Fig. 7F–H). These strongly suggest Grr1 is required for translation of HAC1i mRNA.
A 3xFLAG-Hac1p/CBB and 3xFLAG-GFP/CBB levels shown in Supplementary Fig. 9A were quantified and normalized protein levels relative to that in WT. B 3xFLAG-Hac1p expressions are decreased in grr1∆eS7A(K83 only), eS7A(K83R) and grr1∆eS7A(K83R). Yeast cells harboring plasmids encoding 3xFLAG-HCt under control of TEF1 promoters were grown at 30 °C and harvested at log phase. The samples were analyzed using western blotting with anti-FLAG antibody. CBB staining was used as a loading control. Dilutions are 100%, 75%, 50%, 25%, 12.5%, 6.25%. C 3xFLAG-Hac1p/CBB levels shown in (B) and Supplementary Fig. 9B were quantified and normalized protein levels relative to that in eS7A(K83 only). Data represent n = 3 biologically independent experiments (mean ± SE). D 3xFLAG-Hac1p expressions are decreased in grr1∆, but not in grr1∆ubp3∆. Yeast cells harboring plasmids encoding 3xFLAG-HCt under control of TEF1 promoters were grown at 30°C and harvested at log phase. The samples were analyzed as in (B). E 3xFLAG- Hac1p/CBB levels shown in (D) and Supplementary Fig. 9C were quantified and normalized protein levels relative to that in WT. Data represent n = 4 (WT, grr1∆) or 8 (ubp3∆, grr1∆ubp3∆) biologically independent experiments (mean ± SE). F Schematic drawings of T7 promoter−2xPA-(HAC1i or GFP)-polyA70 mRNAs. G 2xPA-Hac1 translation is decreased in grr1∆ski2∆ lysate. In vitro translation was performed using 2xPA-HAC1i-polyA70 and 2xPA-GFP-polyA70 mRNAs at 17 °C for 30 min. The samples were analyzed using western blotting with anti-PA antibody. Dilutions are 100%, 75%, 50%, 25%, 12.5%, 6.25%. H 2xPA-Hac1/2xPA-GFP levels shown in (G) were quantified and normalized protein levels relative to that in ski2∆ lysate. I Model for the crucial role of Grr1-mediated degradation of Ubp3 in the translation of HAC1i mRNA. Not4-mediated monoubiquitination of eS7A is required for efficient translation of HAC1i mRNA. The SCFGrr1 complex is responsible for the downregulation of Ubp3, which facilitates eS7A ubiquitination and HAC1i translation. All experiments were repeated at least twice with biologically independent samples and showed similar results. Data in (A, H) represent n = 4 biologically independent experiments (mean ± SE). P values were calculated by Two-sided Welch’s t-test. Source data are provided as a Source Data file.
Discussion
Hac1p is expressed specifically in response to ER stress and its expression is ensured by the multi-step regulation of HAC1 mRNA30,49. This includes localization to the ER membrane, tight repression of translation, and Ire1p-mediated splicing. During ER stress responses in yeast, ubiquitination of ribosomal protein eS7A is necessary for translational control of HAC1i mRNA. The deubiquitinating enzyme complex Ubp3-Bre5 regulates eS7A ubiquitination18, and UPR leads to an increase in the level of monoubiquitinated eS7A by the downregulation of Ubp3. In this study, we identified Grr1, a subunit of the SCFGrr1 complex, as the essential E3 ligase responsible for the downregulation of Ubp3 (Figs. 1, 2), which facilitates eS7A ubiquitination (Fig. 3) and the translation of HAC1i (Figs. 4–7). Grr1 is co-immunoprecipitated with Ubp3 and is required for Ubp3 degradation, regardless of ER stress (Figs. 1, 2). The F-box and LRR domains of Grr1 are necessary for Ubp3 degradation but dispensable for the substrate binding (Fig. 2H). Grr1 is essential for the downregulation of Ubp3 during the UPR (Fig. 2B–D) and is critical for Hac1p expression (Fig. 2F) and Tm resistance (Fig. 2E). Notably, Grr1 mutants that are defective in Ubp3 downregulation (e.g., the Grr1-ΔFbox or Grr1-ΔLRR mutants) exhibited defects in Hac1p expression (Fig. 2F). Importantly, Grr1 is necessary for maintaining eS7A ubiquitination levels, regardless of Tm treatment (Fig. 3). Finally, we confirmed that in ubp3Δ and eS7A-4KR background, GRR1 deletion could not reduce the Hac1p level (Fig. 7A–E and Supplementary Fig. 9A–C), indicating that Grr1 facilitates Hac1p expression in the eS7A ubiquitination and Ubp3-dependent manner. Based on our findings, we propose that Grr1-mediated Ubp3 degradation is crucial for maintaining eS7A ubiquitination during UPR. We observed the increase of eS7A mono-ubiquitination in wild-type cells expressing eS7A-HA (Fig. 3B, C) or eS7AK83 only-HA (Fig. 3G, H). Importantly, the levels of eS7A mono-ubiquitination were increased upon UPR in the grr1Δ mutant cells expressing wildtype Grr1 or Grr1-ΔN but not by Grr1-ΔF or Grr1-ΔLRR mutant proteins (Fig. 3B, C). Given that wildtype Grr1 or Grr1-ΔN could complement the grr1Δ mutant defects in Ubp3 downregulation and Hac1p expression and Tm sensitivity, we propose that Grr1 is crucial to maintain the level of eS7A mono-ubiquitination upon UPR that is required for Hac1p expression.
The SCFGrr1 complex plays a crucial role in nutrient uptake and cell proliferation by interacting with various targets33,50. The F-box Grr1 selects and discriminates substrates, in part, via its leucine-rich repeat (LRR) domain33,38,39,40,50,51. In the Grr1-mediated downregulation of Ubp3, the LRR domain is required for regulation but not interaction. Grr1 interacts with Ubp3 independent of Tm treatment (Fig. 2); however, the downregulation of Ubp3 by Grr1 only under Tm treatment conditions (Fig. 1) indicates that Grr1-mediated degradation of Ubp3 under stress conditions requires unknown regulation to induce Ubp3 degradation after interaction with E3 ligase. Ubp3 roles in quality controls, indicating that the deubiquitination is a key reaction to ensure the accuracy of gene express21,23,24,25,27,28. The results of this study revealed the crucial role of ubiquitin-proteasomal degradation of the deubiquitinating enzyme under stress conditions in maintaining translation of transcription factor Hac1 in yeast. We have observed that F-box and LRR domains are required for Ubp3 destabilization by Tm treatment (Fig. 2D and Supplementary Fig. 4) and normal growth (Fig. 2E). Grr1 associates with core SCF to form the SCFGrr1 complex. The CLN1/2 and GIC2 are well-characterized substrate of the SCFGrr1 complex and required for the G1/S cell cycle transition. Therefore, we suspect that F-box and LRR domains are required for CLN1/2 and GIC2 and the G1/S cell cycle transition.
The mechanisms underlying the role of eS7A ubiquitination in the translational regulation of ER stress remains unknown. Ribosome profiling revealed that HAC1i was the most significantly upregulated gene during the UPR (Fig. 4). Translation of HAC1i mRNA was significantly lower in eS7-4KR cells18 and grr1Δ cells than in wild-type cells (Fig. 4). One possibility is that eS7A ubiquitination affects the interaction between the 40S subunit and translation initiation factors in the pre-initiation complex, thereby modulating the translation initiation of HAC1i mRNA. The cycle of ubiquitination and deubiquitination of the 40S ribosomal subunit, eS7A, is important for efficient translation19,20. The two deubiquitinating enzymes, Otu2 and Ubp3, for eS7A cause defects in protein synthesis19,20. Otu2 specifically binds to free 40S ribosomes and promotes the dissociation of mRNAs from 40S ribosomes during recycling. Despite the crucial role of Otu2 in translation initiation, the otu2Δ mutant was resistant to Tm20. Ubp3 inhibited the polyubiquitination of eS7A in polysomes to maintain eS7A in a mono-ubiquitinated form, however, otu2Δ suppressed moderate sensitivity of the ubp3Δ mutant to Tm (Supplementary Fig. 10). The otu2 mutation that disrupts the deubiquitinating activity of Otu2 could not rescue the Tm resistance of ubp3Δotu2Δ cells, suggesting that this suppression depends on the lack of the deubiquitinating activity of Otu2 (Supplementary Fig. 10). These suggest that translation initiation may be involved but not crucial for HAC1i translation.
We observed that eS7A ubiquitination is essential for the translation of HAC1i mRNA, which lacks 5’ UTR, 3’ UTR, and introns, even under normal conditions (Figs. 5–7). This suggests that the ORF of HAC1i mRNA contains elements required for eS7A ubiquitination-mediated regulation, and eS7A ubiquitination facilitates the translation elongation of HAC1i mRNA. Together with the previous footprints of eS7A-WT and eS7A-4KR also revealing putative translation pausing sites, we propose that eS7A ubiquitination facilitates the translation elongation of HAC1i mRNA depending on the cis-element in ORF (Fig. 7I). Given that Ubp3 deubiquitinates the ribosomal protein eS7A in the 80S and polysome fractions, the maintenance of eS7A ubiquitination in translating ribosomes could contribute to the efficient translation elongation of HAC1i mRNA.
The Ccr4-NOT complex monitors translating ribosomes for codon optimality via E-site binding of the N-terminal domain of the Not5 subunit17. eS7A ubiquitination contributes to the interaction of the Ccr4-Not complex with the ribosome, and thereby codon-optimality-mediated mRNA degradation. The phenotypes of Not4 deletion or the eS7A-4KR mutation were hardly aggravated by additional deletion of Not5-NTD but reduced Not5 association with polyribosomes17, suggesting that eS7A-ubiquitination by Not4 is involved in the same pathway as Not5 but occurs upstream of E-site probing, and Not5 binding to ribosomes is dependent on prior eS7A-ubiquitination. Our results suggest that Not4-mediated eS7A ubiquitination is required for the efficient translation elongation of HAC1i mRNA. Although the Ccr4-Not complex association with ribosomes is required for codon optimality-mediated mRNA decay, the stability of the 50% optimal HIS3 reporter mRNA was not changed in the eS7A-4KR or not5Δ mutant cells17. The codon optimality of HAC1i mRNA is 50%, and its mRNA level did not decrease in the eS7A-4KR mutant cells18. More importantly, GFP, with the same codon optimality as HAC1i mRNA in yeast, can suppress eS7A-mediated regulation. Taken together, we suspect that codon optimality is not solely responsible for the regulation of HAC1i translation. In mammalian cells, the secondary structure of mRNA contributes to translation and possibly mRNA stability, although its mechanism remains unknown. A more precise analysis of the cis-elements in HAC1i mRNA and the involvement of trans factors, including Ccr4-NOT, is needed to elucidate the mechanisms underlying the eS7A ubiquitination-mediated regulation of HAC1i mRNA translation elongation. Further experiments will shed light on how eS7A ubiquitination stimulates the translation of HAC1i mRNA, providing insight into the crucial role of ribosome ubiquitination in stress responses in addition to well-characterized roles in translation quality controls.
In this study, we also analyzed the transcription and splicing of HAC1 mRNA. The statistical analysis of the results indicates that no statistically significant difference in HAC1 mRNA levels between wild-type (WT) and grr1Δ mutant cells (Fig. 5K and Supplementary Fig. 1C, D). In contrast, Grr1 facilitates the splicing of HAC1u mRNA (Supplementary Fig. 1B–E). The splicing of HAC1u mRNA does not change in the eS7A-4KR and the ubp3Δ mutant cells18. We propose that Grr1 regulates HAC1u mRNA splicing independently of Ubp3 and eS7A ubiquitination. Further analysis will uncover the Grr1 function in the co-localization and HAC1 mRNA and Ire1 protein on the ER membrane required for HAC1u mRNA splicing. Given that Grr1-mediated Ubp3 degradation is crucial for maintaining eS7A ubiquitination during UPR, we propose that Grr1 facilitates the splicing of HAC1u mRNA independently of Ubp3 and eS7A ubiquitination, as well as the translation of HAC1i mRNA in a Ubp3- and ubiquitination of eS7A-dependent manner (Fig. 7I). Further investigation into how Grr1 facilitates HAC1u mRNA splicing and translation of HAC1i mRNA depending on the cis-element in ORF.
Methods
Yeast strains and genetic methods
The Saccharomyces cerevisiae strains used in this study are listed in Supplementary Data 1. Yeast Knock Out (YKO) library strains (BY4741) (Open Biosystems) used in E3 ligase screening are indicated in Supplementary Fig. 1A. Gene disruption, C-terminal tagging and N-terminal tagging were performed following previously reported methods52,53,54. Yeast Saccharomyces cerevisiae W303-1a based strains were obtained by established recombination techniques using PCR-amplified cassette sequences (kanMX4, hphMX4, hphNT1, natMX4, or HISMX6). To construct strains of essential ribosomal protein genes, the shuffle strains transformed with plasmids expressing mutant ribosomal protein products were grown on SDC plate containing 0.1%(w/v) of 5-fluoroorotic acid (5-FOA, #F9001-5, Zymo Research) and isolated URA3 absent strains.
Plasmid constructs
DNA cloning was performed with PCR amplification by using gene specific primers and KOD FX Neo (#KFX-201, TOYOBO), and by using T4 DNA ligase (#M0202S, NEB). All cloned DNAs amplified by PCR were verified by sequencing. Plasmids used in this study are listed in Supplementary Data 2. Sequences used in Figs. 6 and 7 are listed in Supplementary Data 3. These plasmids contain part of an ARS, part of a CEN and CYC1 terminator.
Yeast culture and media
All yeast cells were cultured with YPD or synthetic complete (SC) medium with 2% glucose at 30 °C and harvested by centrifugation and discarding medium. To induce ER stress, yeast cells were grown at 30 °C until OD600 = 0.2, then treated with 1 µg/mL of tunicamycin (Tm, #208-08243, Wako) for ~4 h and harvested. A proteasome inhibitor MG132 (#HY-13259, MCH) was added with tunicamycin to a final concentration of 50 µM. All cell pellets were frozen in liquid nitrogen immediately after harvest and stored at −80 °C until used.
RNA isolation, RNA electrophoresis and northern blotting
Yeast cells were harvested at indicated time points ( ~ 4 h) after Tm added, as described above. Total RNA solutions were prepared by using one-step hot formamide RNA extraction method and RNA electrophoresis was performed as described55,56. Yeast cell pellet in 1.5 mL tube (stand on ice) was resuspended with 50 µL of FAE solution and heated at 70 °C for 10 min. After centrifugation at 17,800 x g for 1 min at room temperature, 40 µL of the supernatant containing total RNA was collected. RNA samples containing 1.2 µg of total RNA were incubated at 70 °C for 5 min and left to stand on ice for 10 min. Each sample was electrophoresed at 200 V for 50 min on a 1.2% HT-FA agarose gel in HT buffer, followed by transfer of RNA to Hybond-N+ membrane (GE healthcare) with 20x SSC for over 18 h using capillary system. RNA was cross-linked on the membrane by CL-1000 ultraviolet crosslinker (UVP) at 120 mJ/cm2. Membrane was incubated with DIG Easy Hyb Granules (#11796895001, Roche) for 1 h at 50 °C in a hybridization oven and DIG-labelled probe prepared using PCR DIG Probe Synthesis Kit (#11636090910, Roche) was added and incubated for over 18 h. The membrane was washed with wash buffer I for 15 min in a hybridization oven, followed by washing twice with wash buffer II for 15 min in a hybridization oven. The membrane was then washed with 1x maleic acid buffer for 10 min at room temperature and incubated with Blocking Reagent (#11096176001, Roche) for 30 min. Anti-Digoxigenin-AP, Fab fragments (#11093274910, Roche) was added to the Blocking Reagent and the membrane was further incubated for 1 h. After that, the membrane was washed three times with wash buffer III for 10 min and equilibrated by equilibration buffer. The membrane was reacted with CDP-star (#11759051001, Roche) for 10 min, and chemiluminescence was detected by LAS-4000 (GE healthcare). Quantification of band intensities were performed using ImageQuant TL software (Cytiva).
-
FAE solution (98% deionized Formamide, 10 mM EDTA)
-
RNA sample (50% deionized Formamide, 30 mM HEPES, 30 mM Triethanolamine, 0.4 M Formaldehyde (#061-00416, Wako), 0.5 mM EDTA, 0.02% bromophenol blue)
-
HT buffer (30 mM HEPES, 30 mM Triethanolamine)
-
1.2% HT-FA agarose gel (Agarose LE Analytical Grade (#V3125, Promega), 30 mM HEPES, 30 mM Triethanolamine, 0.4 M Formaldehyde)
-
20x SSC (3 M NaCl, 300 mM Trisodium citrate dihydrate)
-
Wash buffer I (2x SSC, 0.1% SDS)
-
Wash buffer II (0.1x SSC, 0.1% SDS)
-
1x maleic acid buffer (100 mM maleic acid, 150 mM NaCl, pH 7.0, adjusted by NaOH)
-
Wash buffer III (1x maleic acid buffer, 0.3% tween 20)
-
Equilibration buffer (100 mM Tris-HCl pH 9.4, 100 mM NaCl)
Trichloroacetic acid (TCA) precipitation for protein preparation
Yeast cell pellet in 1.5 mL tube (stand on ice) was resuspended with 1 mL of ice-cold double-distilled water containing 1 mM phenylmethylsulfonyl fluoride (PMSF, #164-12181, Wako). 100 µL of yeast suspension was diluted with 900 µL of double-distilled water followed by OD600 measurement. To the rest of yeast suspension (900 µL), 135 µL of Lysis buffer was added and incubated on ice for 15 min. After that, 135 µL of 50% TCA was added to samples and incubated on ice for 10 min. After centrifugation of lysates (17,800 x g, 10 min, 4 °C), the supernatant was discarded completely, and the pellet was dissolved in HU buffer (250 µL/OD600) and heated at 65 °C for 10 min. After centrifugation at 17,800 x g for 5 min at room temperature, the supernatant was used as a sample for SDS-PAGE.
-
Lysis buffer (2 M NaOH, 1 M 2-Mercaptoethanol)
-
HU buffer (8 M Urea, 200 mM Tris-HCl pH 6.8, 5% SDS, 1 mM EDTA, 0.01% BPB, 100 mM DTT)
Protein electrophoresis and western blotting
Protein samples were separated by SDS-PAGE, and were transferred onto PVDF membrane (Immobilon-P, Millipore). After blocking with 5% skim milk in PBS-T, the membrane was incubated with primary antibodies overnight at 4 °C. The membrane was washed with PBS-T for three times followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. In the case of HA-tagged protein detection, the membrane was incubated with HRP-conjugated anti-HA antibodies. After washing with PBS-T for three times, chemiluminescence was detected by LAS4000 (GE Healthcare) or Amersham ImageQuant 800 (Cytiva). Primary antibodies for western blotting in this study were listed in Supplementary Data 4. Quantification of band intensities were performed using ImageQuant TL software (Cytiva) and Multi Gauge software (Fuji film).
-
PBS-T (10 mM Na2HPO4/NaH2PO4 pH 7.5, 0.9% NaCl, 0.1% Tween-20)
Spot assay
Yeast cells were cultured with 3 mL YPD or SC medium with 2% glucose at 30 °C overnight and adjusted to OD600 = 0.3. 10-fold serial dilutions were prepared in the 1.5 mL tubes and spotted onto 1 µg/mL Tm added plates and control plates. Plates were incubated at 30 °C for several days.
Ribosome Profiling (Ribo-seq) and RNA-seq
Library preparation was performed as described18 with following modifications. After 4 h of tunicamycin treatment, yeast cells were harvested by filtration and frozen by liquid nitrogen. They were lysed by lysis buffer for Ribo-seq (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 1% Triton X-100, 0.1 mg/mL cycloheximide, 25 U/mL Turbo DNase (Invitrogen, AM2238)). Clear lysates were obtained by centrifugation at 20,000 x g for 10 min at 4 °C. Clear lysate containing 20 μg total RNA was treated with 1.25 unit of RNase I (#E0067-10D1, LGC Biosearch Technologies) per µg RNA for 45 min at 25 °C. RNase I treatment was quenched by the addition of 200 units of SUPERase・In RNase Inhibitor (Invitrogen, AM2694). RNA-digested lysate was layered on a sucrose cushion (1 M sucrose, 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.1 mg/mL cycloheximide, 20 U/mL SUPERase・In RNase Inhibitor), followed by centrifugation in a TLA-110 rotor (Beckman) at 542,715 x g for 1 h at 4 °C. The ribosomal pellet was resuspended in 120 µL of splitting buffer (20 mM Tris-HCl pH 7.5, 300 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM DTT, 20 U/mL of SUPERase・In RNase Inhibitor) to dissociate ribosomes from the mRNA fragments, and then passed through the 100 kDa MWCO Amicon Ultra-0.5 device (Millipore, UFC5100). The non-ribosomal flow-through (approximately 100 µL) was collected and mixed with 300 µL TRIzol reagent (Invitrogen, 15596018). RNA fragments were extracted by Direct-zol RNA MicroPrep (Zymo Research). For the ribosome profiling analysis, the whole cell lysate containing 20 μg of total RNA was treated with 1.25 units/1 µg RNA of RNase I (#E0067-10D1, LGC Biosearch Technologies) at 25 °C for 45 min. As linker DNA, 5’-(Phos)NNNNNIIIIITGATCGGAAGAGCACACGTCTGAA(ddC)-3’ where (Phos) indicated 5’ phosphorylation and (ddC) indicates a terminal 2’, 3’-dideoxycytidine, was used. The Ns and Is indicate random barcodes for eliminating PCR duplication and multiplexing barcodes, respectively. The linkers were pre-adenylated with 5’ DNA Adenylation kit (NEB), and then used for the ligation reaction. The rRNAs were removed using a mixture of riboPOOL (siTOOLs Biotech) and homemade customized biotinylated oligos. An oligo 5’-(Phos)NNAGATCGGAAGAGCGTCGTGTAGGGAAAGAG(iSp18)GTGACTGGA GTTCAGACGTGTGCTC-3’, where (Phos) indicated 5’ phosphorylation and Ns indicate random barcode, was used for reverse transcription. PCR was performed with oligos, 5’-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTC-3’ and 5’- CAAGCAGAAGACGGCATACGAGATJJJJJJGTGACTGGAGTTCAGACGTGTG-3’, where Js indicate reverse complement of the index sequence discovered during Illumina sequencing. The libraries were sequenced on a HiSeq X Ten (Illumina) at Macrogen Japan corp.
For the RNA-seq analysis, total RNA was extracted from lysate using Direct-zol RNA Microprep w/ Zymo-Spin IC Columns (#R2062, funakoshi). The libraries were sequenced on a DNBSEQ-G400RS High-throughput Sequencing Kit (MGI Tech Co., Ltd.) at Bioengineering Lab, Japan. The reads were mapped to yeast transcriptome, removing duplicated reads based on random barcode sequences. The analyzes for ribosome profiling were restricted to 27-29 nt reads for WT and grr1∆ data sets. The DESeq2 package was used to calculate the fold change of mRNA expression and translation efficiency (TE).
Cycloheximide chase assay
To analyze the Ubp3-3HA stability, yeast cells were treated with 0.25 mg/mL cycloheximide after 1 h of tunicamycin treatment. At the indicated time points, yeast cells were treated with 30 mM sodium azide and harvested by centrifugation and discarding medium. A proteasome inhibitor MG132 (#HY-13259, MCH) was added simultaneously with tunicamycin to a final concentration of 50 µM. To analyze the 3xFLAG-Hac1p stability, yeast cells were treated with tunicamycin for 2 h, then harvested by centrifugation and discarding medium, and resuspended in 6 mL of SDC medium with 1 µg/mL tunicamycin and incubated at 30 °C for 5 min. As 0 min samples, 1.4 mL of yeast cell cultures were treated with 30 mM sodium azide and harvested by quick centrifugation. Then yeast cells were treated with 0.25 mg/mL cycloheximide and 1 mL of yeast cell cultures were treated with 30 mM sodium azide and harvested by quick centrifugation at indicated time points. Protein stability was determined by western blotting.
Immunoprecipitation using FLAG beads
After 1.5 h of tunicamycin treatment, then yeast cells were harvested by centrifugation (8983 x g, 5 min, room temperature) and discarding medium. All cell pellets were frozen in liquid nitrogen immediately after harvest and stored at −80 °C until used. After grinding the cell pellets in liquid nitrogen with a mortar and a pestle, the cell pellets were lysed with Lysis buffer for IP to prepare crude lysate. Anti DYKDDDDK tag Antibody Beads (# 016-22784, Wako) were added to the crude lysate and rotated at 4 °C for 1 h. The beads were washed 7 times with Wash buffer for IP. FLAG-tagged proteins were eluted twice by Elution buffer for IP with FLAG peptide at 4 °C for 1 h. After TCA precipitation, samples were subjected to SDS-PAGE and western blotting.
-
Lysis buffer for IP (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 10 mM MgCl2, 2 mM 2-Mercaptoethanol, 0.01% NP-40, 1 tablet/10 mL cOmplete mini EDTA-free (# 11836170001, Roche))
-
Wash buffer for IP (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 10 mM MgCl2, 2 mM 2-Mercaptoethanol, 0.01% NP-40)
-
Elution buffer for IP with FLAG peptide (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 10 mM MgCl2, 2 mM 2-Mercaptoethanol, 0.01% NP-40, 100 µg/mL FLAG® Peptide (# F3290, Sigma aldrich))
In vitro translation
In vitro translation was performed as describe7 with following modifications. Reporter mRNAs was produced using the mMessage mMachine Kit (Thermo Fischer) and the sequences were listed in Supplementary Data 3. To prepare yeast cell-free translation extract, yeast cells were grown in YPD medium to an OD600 of 1.5–2.0, then washed with water and 100 mM KCl, and finally incubated with 10 mM DTT in 100 mM Tris-HCl pH 8.0 for 15 min at room temperature. After centrifugation, cells were resuspended in YPD/1 M sorbitol (3.3 mL per 1 g of cell pellet). To generate spheroplasts, 2.69 mg zymolyase per 1 g of cell pellet was added to cells and incubated for 90 min at 30 °C. Spheroplasts were then washed three times with YPD/1 M sorbitol and once with 1 M sorbitol, and lysed with a douncer in lysis buffer for IVT. From the lysate, an S100 fraction was obtained by low-speed centrifugation followed by ultracentrifugation of the supernatant. The S100 was passed through a PD10 column (Cytiva). In vitro translation was performed at 17 °C for 30 min using a large excess of template mRNA (2 µg per 20 µL of extract), and stopped by mixing with a quarter volume of 4xSB (final 1xSB) and flash frozen in liquid nitrogen. Samples were thawed and heated at 95 °C for 5 min. After centrifugation at 17,800 x g for 5 min at room temperature, the supernatant was used as a sample for SDS-PAGE.
-
Lysis buffer for IVT (20 mM HEPES-KOH pH 7.5, 100 mM KOAc, 2 mM Mg(OAc)2, 10% Glycerol, 1 mM DTT, 0.5 mM PMSF, 1 tablet/10 mL cOmplete mini EDTA-free)
-
4xSB (200 mM Tris-HCl pH 6.8, 8% SDS, 40% Glycerol, 0.04% BPB, 100 mM DTT)
Statistics and reproducibility
In Fig. 4, Q values were calculated by Two-sided Likelihood ratio test. For other statistical analyses, P values were calculated by Two-sided Welch’s t-test. All experiments were repeated at least twice with biologically independent samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequencing data of RNA-seq and ribosome profiling have been deposited in the NCBI Gene Expression Omnibus and are accessible through GEO series accession number GSE260735 (RNA-seq) and GSE260736 (Ribo-seq), respectively. Source data are provided with this paper.
References
Joazeiro, C. A. P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 20, 368–383 (2019).
Monaghan, L., Longman, D. & Cáceres, J. F. Translation-coupled mRNA quality control mechanisms. Embo J. 42, e114378 (2023).
Brandman, O. & Hegde, R. S. Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 23, 7–15 (2016).
Shao, S. & Hegde, R. S. Target Selection during Protein Quality Control. Trends Biochem Sci. 41, 124–137 (2016).
Inada, T. Quality controls induced by aberrant translation. Nucleic Acids Res 48, 1084–1096 (2020).
Narita, M. et al. A distinct mammalian disome collision interface harbors K63-linked polyubiquitination of uS10 to trigger hRQT-mediated subunit dissociation. Nat. Commun. 13, 6411 (2022).
Matsuo, Y., Uchihashi, T. & Inada, T. Decoding of the ubiquitin code for clearance of colliding ribosomes by the RQT complex. Nat. Commun. 14, 79 (2023).
Matsuo, Y. et al. Ubiquitination of stalled ribosome triggers ribosome-associated quality control. Nat. Commun. 8, 159 (2017).
Ikeuchi, K. et al. Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. Embo J. 38 https://doi.org/10.15252/embj.2018100276 (2019).
Best, K. et al. Structural basis for clearing of ribosome collisions by the RQT complex. Nat. Commun. 14, 921 (2023).
Matsuo, Y. et al. RQT complex dissociates ribosomes collided on endogenous RQC substrate SDD1. Nat. Struct. Mol. Biol. 27, 323–332 (2020).
Hashimoto, S., Sugiyama, T., Yamazaki, R., Nobuta, R. & Inada, T. Identification of a novel trigger complex that facilitates ribosome-associated quality control in mammalian cells. Sci. Rep. 10, 3422 (2020).
D’Orazio, K. N. et al. The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during No Go Decay. Elife 8 https://doi.org/10.7554/eLife.49117 (2019).
Tomomatsu, S. et al. Two modes of Cue2-mediated mRNA cleavage with distinct substrate recognition initiate no-go decay. Nucleic Acids Res 51, 253–270 (2023).
Collart, M. A. Global control of gene expression in yeast by the Ccr4-Not complex. Gene 313, 1–16 (2003).
Collart, M. A. The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip Rev. RNA https://doi.org/10.1002/wrna.1332 (2016).
Buschauer, R. et al. The Ccr4-Not complex monitors the translating ribosome for codon optimality. Science 368 https://doi.org/10.1126/science.aay6912 (2020).
Matsuki, Y. et al. Ribosomal protein S7 ubiquitination during ER stress in yeast is associated with selective mRNA translation and stress outcome. Sci. Rep. 10, 19669 (2020).
Takehara, Y. et al. The ubiquitination-deubiquitination cycle on the ribosomal protein eS7A is crucial for efficient translation. iScience 24, 102145 (2021).
Ikeuchi, K. et al. Molecular basis for recognition and deubiquitination of 40S ribosomes by Otu2. Nat. Commun. 14, 2730 (2023).
Fang, N. N., Zhu, M., Rose, A., Wu, K. P. & Mayor, T. Deubiquitinase activity is required for the proteasomal degradation of misfolded cytosolic proteins upon heat-stress. Nat. Commun. 7, 12907 (2016).
Jung, Y. et al. Modulating cellular balance of Rps3 mono-ubiquitination by both Hel2 E3 ligase and Ubp3 deubiquitinase regulates protein quality control. Exp. Mol. Med 49, e390 (2017).
Kraft, C., Deplazes, A., Sohrmann, M. & Peter, M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell Biol. 10, 602–610 (2008).
Kraft, C. & Peter, M. Is the Rsp5 ubiquitin ligase involved in the regulation of ribophagy? Autophagy 4, 838–840 (2008).
Kvint, K. et al. Reversal of RNA polymerase II ubiquitylation by the ubiquitin protease Ubp3. Mol. Cell 30, 498–506 (2008).
Oling, D., Masoom, R. & Kvint, K. Loss of Ubp3 increases silencing, decreases unequal recombination in rDNA, and shortens the replicative life span in Saccharomyces cerevisiae. Mol. Biol. Cell 25, 1916–1924 (2014).
Ossareh-Nazari, B. et al. Cdc48 and Ufd3, new partners of the ubiquitin protease Ubp3, are required for ribophagy. EMBO Rep. 11, 548–554 (2010).
Ossareh-Nazari, B. et al. Ubiquitylation by the Ltn1 E3 ligase protects 60S ribosomes from starvation-induced selective autophagy. J. Cell Biol. 204, 909–917 (2014).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Xu, C., Bailly-Maitre, B. & Reed, J. C. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest 115, 2656–2664 (2005).
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Willems, A. R. et al. SCF ubiquitin protein ligases and phosphorylation-dependent proteolysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 1533–1550 (1999).
Rüegsegger, U., Leber, J. H. & Walter, P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell 107, 103–114 (2001).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Tirasophon, W., Welihinda, A. A. & Kaufman, R. J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 (1998).
Higgins, R. et al. The Unfolded Protein Response Triggers Site-Specific Regulatory Ubiquitylation of 40S Ribosomal Proteins. Mol. Cell 59, 35–49 (2015).
Flick, J. S. & Johnston, M. GRR1 of Saccharomyces cerevisiae is required for glucose repression and encodes a protein with leucine-rich repeats. Mol. Cell Biol. 11, 5101–5112 (1991).
Skowyra, D. et al. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and Rbx1. Science 284, 662–665 (1999).
Kusama, K. et al. Dot6/Tod6 degradation fine-tunes the repression of ribosome biogenesis under nutrient-limited conditions. iScience 25, 103986 (2022).
Panasenko, O. O. & Collart, M. A. Presence of Not5 and ubiquitinated Rps7A in polysome fractions depends upon the Not4 E3 ligase. Mol. Microbiol 83, 640–653 (2012).
Mori, K., Kawahara, T., Yoshida, H., Yanagi, H. & Yura, T. Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells 1, 803–817 (1996).
Kawahara, T., Yanagi, H., Yura, T. & Mori, K. Endoplasmic reticulum stress-induced mRNA splicing permits synthesis of transcription factor Hac1p/Ern4p that activates the unfolded protein response. Mol. Biol. Cell 8, 1845–1862 (1997).
Hu, Z., Killion, P. J. & Iyer, V. R. Genetic reconstruction of a functional transcriptional regulatory network. Nat. Genet. 39, 683–687 (2007).
MacIsaac, K. D. et al. An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinforma. 7, 113 (2006).
Defenouillère, Q. et al. The induction of HAD-like phosphatases by multiple signaling pathways confers resistance to the metabolic inhibitor 2-deoxyglucose. Sci Signal 12 https://doi.org/10.1126/scisignal.aaw8000 (2019).
Anshu, A., Mannan, M. A., Chakraborty, A., Chakrabarti, S. & Dey, M. A novel role for protein kinase Kin2 in regulating HAC1 mRNA translocation, splicing, and translation. Mol. Cell Biol. 35, 199–210 (2015).
Ghosh, C. et al. Phosphorylation of Pal2 by the protein kinases Kin1 and Kin2 modulates HAC1 mRNA splicing in the unfolded protein response in yeast. Sci. Signal 14 https://doi.org/10.1126/scisignal.aaz4401 (2021).
Karagöz, G. E., Acosta-Alvear, D. & Walter, P. The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum. Cold Spring Harb. Perspect Biol. 11 https://doi.org/10.1101/cshperspect.a033886 (2019).
Benanti, J. A., Cheung, S. K., Brady, M. C. & Toczyski, D. P. A proteomic screen reveals SCFGrr1 targets that regulate the glycolytic-gluconeogenic switch. Nat. Cell Biol. 9, 1184–1191 (2007).
Berset, C. et al. Transferable domain in the G(1) cyclin Cln2 sufficient to switch degradation of Sic1 from the E3 ubiquitin ligase SCF(Cdc4) to SCF(Grr1). Mol. Cell Biol. 22, 4463–4476 (2002).
Mathur, R. & Kaiser, P. PCR-mediated epitope tagging of genes in yeast. Methods Mol. Biol. 1205, 37–44 (2014).
Janke, C. et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Shedlovskiy, D., Shcherbik, N. & Pestov, D. G. One-step hot formamide extraction of RNA from Saccharomyces cerevisiae. RNA Biol. 14, 1722–1726 (2017).
Inada, T. & Aiba, H. Translation of aberrant mRNAs lacking a termination codon or with a shortened 3’-UTR is repressed after initiation in yeast. EMBO J. 24, 1584–1595 (2005).
Acknowledgements
The authors thank Dr. Tohru Yoshihisa, University of Hyogo, for providing antibody against the Hac1 protein and for critically reading the manuscript. The authors thank the members of their laboratories for discussions and critical comments on the manuscript. This work was supported by AMED (JP23gm1110010, JP223fa627001 to T.I.), MEXT/JSPS KAKENHI (Grant Numbers JP19H05281, 21H05277, 22H00401 to T.I., 21H00267, 23H04249 and 23K23869,18H05498 to Y.S.), Research grants from Takeda Science Foundation (T.I.), and JST PREST Grant Number JPMJPR21EE (Y.M.).
Author information
Authors and Affiliations
Contributions
Genetic and biochemical experiments were performed by N.S., N.Y., Y.Mastuki, and S.T. under the supervision of T.I. N.S. performed the ribosome profiling experiments under the supervision of S.L, Y.Matsuo, and T.I. N.S. and T.I. wrote the manuscript. T.I. primarily conceived the idea and designed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Madhusudan Dey, Adrien Rousseau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sato, N., Nakano, Y., Matsuki, Y. et al. Crucial roles of Grr1 in splicing and translation of HAC1 mRNA upon unfolded stress response. Nat Commun 16, 2172 (2025). https://doi.org/10.1038/s41467-025-57360-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57360-1