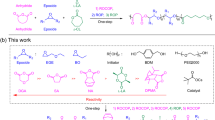
Abstract
Multiblock copolymers (MBCPs) comprising polyester and polyacrylate segments offer an efficient strategy for enhancing the performance of polyester and polyolefin blends but synthesis and structural modification of these MBCPs remains challenging. Here, we propose a method for synthesizing MBCPs via the switchable polymerization of epoxides, cyclic anhydrides, and acrylates using a dinuclear Co-complex, wherein the anhydride acts as a switcher. Detailed studies on the copolymerization process reveal that the successful synthesis of MBCPs is achieved by intramolecular bimetallic synergistic catalysis, producing MBCPs with controlled molecular weights and narrow dispersities. Owing to the high compatibility of the monomers, this method allows for producing MBCPs with diverse structures and block numbers. Moreover, the resulting MBCPs effectively enhance the performance of the polyester and polyacrylate blends, improving the toughness of polyesters. Studies on microphase separation show that MBCPs can effectively compatibilize immiscible blends, highlighting their potential as compatibilizers.
Similar content being viewed by others
Introduction
Polymeric materials have been widely applied in many aspects of manufacturing and life owing to their advantages such as lightweight, low cost, wide variety, and excellent performance1. In particular, the highly designable architectures of polymeric materials endow them with various functionalities for diverse applications2,3,4. Among these architectures, multiblock copolymers (MBCPs), in which at least three discrete linear chains comprising chemically identical repeating units are covalently coupled, have the merit of rich structures resulting from various monomer arrays5,6. Consequently, MBCPs provide a platform for fabricating a variety of advanced materials7, such as thermoplastic elastomers8,9,10, separation membranes11, lithography12, polysomes13,14,15, compatibilizers16, and organic optoelectronics17,18,19. Among these materials, compatibilizers can promote interfacial adhesion between immiscible polymers, resulting in a uniform distribution of the dispersed phase and a stable morphology20. These characteristics make compatibilizers effective for polymer blending modification and waste plastic upcycling21. MBCPs are promising compatibilizer candidates especially because of their enhanced ability to stitch domains together by repeatedly crossing the interface between two homopolymers22. For example, tetra-block copolymers comprising polyethylene and isotactic polypropylene enable morphological control, thereby transforming brittle materials into mechanically tough blends23. Therefore, developing an efficient methodology for the synthesis of MBCPs with readily tunable structures is important.
Polyacrylates and polyesters, synthesized via the polymerization of acrylates and alternative copolymerization of epoxides and cyclic anhydrides, respectively24,25, are two important kinds of polymeric materials that have been extensively applicated26,27,28. Particularly, owing to the rich variety of epoxides and cyclic anhydrides, the resulting polyesters have the merits of structural diversity and tunability29,30. Therefore, constructing MBCPs from polyacrylate- and epoxide-derived polyesters is expected to provide a viable platform for accessing diverse MBCPs with tunable properties. Although many MBCPs have been synthesized via the chemical coupling of distinct linear polymer chains or repeated addition procedures, the precise synthesis of MBCPs comprising polyester and polyacrylate segments in one pot is challenging31,32,33,34,35. This is because constructing different segments in one system is difficult owing to the differing polymerization manner and reaction conditions required for epoxides and acrylates36,37,38. In this context, developing an efficient synthetic methodology is the key to the production of this type of MBCPs, thus attaining extensive applications.
Recently, we reported an efficient bimetallic catalytic strategy for the synthesis of various polyacrylate-based di-block copolymers comprising polyester, polyether, and polycarbonate39. The polymerization of epoxides and acrylates proceeds in a sequence-controlled manner. In this process, the epoxide-involving copolymerization occurs first, followed by the polymerization of acrylate, which is initiated by the alkoxide species from the propagating polymer chains. We hypothesize that the in situ-generated carbanion attacks a cyclic anhydride, thus forming a carboxylate species that further triggers the copolymerization of epoxides and cyclic anhydrides (Fig. 1). In this case, the homopolymerization of acrylate can be switched to the copolymerization of epoxides and cyclic anhydrides, enabling the production of MBCPs through the periodic addition of cyclic anhydrides. Herein, we propose a one-pot strategy for synthesizing MBCPs comprising polyacrylate, polyester, segments via the switchable terpolymerization of epoxides, cyclic anhydrides, and acrylates catalyzed by a dinuclear cobalt (Co)-complex. A series of MBCPs with different block numbers (nbs) are synthesized via the periodic feeding of cyclic anhydrides and acrylates in the presence of excess epoxides. Moreover, MBCPs with hexa-blocks exhibit high strength when stitching immiscible polyesters and polyacrylates, resulting in a physical blend with enhanced mechanical performance. These results highlight the potential of MBCPs as compatibilizers.
Results
Initially, dinuclear Co-complex 1, in conjunction with PPNCl (PPN = bis(triphenylphosphine)iminium) as a cocatalyst, was employed for the copolymerization of cyclohexene oxide (CHO), phthalic anhydride (PA), and ethyl acrylate (EA). Co-complex 1 exhibited high efficiency in catalyzing this copolymerization as PA and EA were fully converted within 4.0 h, affording the copolymer with number-average molecular weight (Mn) and a narrow dispersity (Ð) of 41.6 kg/mol and 1.14, respectively (Supplementary Table 1, Entry 1). Structural characterization revealed that the copolymer possessed a block structure (Supplementary Figs. 1 and 2), in agreement with our previous report39. In addition, the use of the dinuclear Cr complex also led to the formation of block copolymers with a lower efficiency. Whereas, the use of Al complex resulted in the respective polymerization of CHO/PA and acrylate, which produced a mixture of polyester and poly(ethyl acrylate) (PEA) (Entries 2 and 3). Detailed exploration indicated that the ring-opening polymerization (ROP) of CHO could not proceed via the catalysis of 1/PPNCl (Entry 4). Similarly, the polymerization of EA was also inaccessible in the presence of excess or no PA in the presence of CHO (Entries 5 and 6). However, the addition of a small amount of PA led to efficient EA polymerization (Entry 7). These results indicated that the homopolymerization of EA could be initiated by the alkoxide species generated in situ from the copolymerization of CHO and PA. Therefore, we attempted to synthesize the corresponding MBCPs through a second feeding procedure, wherein copolymerization was conducted with a feed ratio of CHO:PA:EA:1:PPNCl = 2000:150:150:1:2 (in molar), followed by the addition of an additional 150 equiv. each of PA and EA after the initial EA was fully consumed. Correspondingly, the Mn of the resultant copolymer reached 25.8 kg/mol, with a narrow dispersity (Ð) of 1.13. The signals at δ 5.2 and 4.2 ppm, which represent poly(cyclohexene phthalate) (PCP) and PEA segments, respectively, are observed in the 1H NMR spectrum of the purified copolymer (Fig. 2a). Notably, no signals representing polyether generated from the ROP of CHO were observed, even when the reaction time was extended to 6 h. In addition, only one diffusion coefficient was observed in the diffusion-ordered spectroscopy (DOSY) NMR spectrum of the resultant copolymer (Fig. 2b), whereas two apparent diffusion coefficients were clearly observed in the 1H DOSY NMR spectrum of the PCP and PEA blends (Supplementary Fig. 3), suggesting the formation of a block structure.
To obtain more information on the structure of the resultant block copolymer, the second feeding process of the terpolymerization of CHO/PA/EA was monitored using in situ FTIR with a probe fitted to the reaction mixture and a feed ratio of CHO:PA:EA:1:PPNCl = 2000:150:150:1:2 with toluene as solvent (VCHO:Vtol = 1:1). The intensity of the peak at λ 903 cm–1, which is ascribed to the out-of-plane bending v(C–H) vibration of PA, decreased rapidly, accompanied with an increase in the intensity of the peak at 1283 cm–1, ascribed to the asymmetric v(C–O–C) stretching of the ester group in PCP (Fig. 3a, b). No obvious change in the intensity of the peak at 1193 cm–1, ascribed to the asymmetric v(C–O–C) stretching vibration of EA, was observed, indicating that only the copolymerization of CHO and PA occurred during this period. After PA was fully converted, the sharp decrease and increase in the intensities of the peaks at 1193 cm–1 and 1732 cm–1 (asymmetric v(C=O) stretching vibration of PEA), respectively, suggest the transformation of EA into PEA. Subsequently, the addition of another 150 equiv. each of PA and EA after full EA consumption retriggered the copolymerization of CHO and PA. Furthermore, the homopolymerization of residual EA was visible after PA was fully consumed. Monitoring this second feeding polymerization procedure using 1H NMR spectroscopy also confirmed that no EA conversion was observed before PA was fully converted in each feeding period. Besides, the addition of PA was capable of retriggering the sequence-controlled polymerization involving CHO/PA copolymerization and EA homopolymerization (Fig. 3c). Accordingly, the Mns of resultant copolymers gradually increased with the conversion of each monomer, while maintaining low dispersities (Ð < 1.2) (Fig. 3d). Combined with the single diffusion coefficient in the corresponding 1H DOSY NMR spectrum (Fig. 2b), these results confirmed that the resulting copolymer was a tetra-block copolymer (MBCP1) comprising PCP and PEA segments.
a Representative 3D stack plot of the FTIR spectra collected every 45 s during the CHO/PA/EA copolymerization. b Peak intensity variation of each component in the reaction mixture over time monitored using in situ FTIR. c Time-elapsed 1H NMR spectra in CDCl3 of the reaction process. d Gel permeation chromatography traces of the resultant copolymers at various conversions; Mn values are given in kg/mol.
With an established strategy for producing MBCPs in hand, we attempted to enrich the diversity of MBCPs derived from epoxides and acrylates, thus providing a platform for accessing tailor-made functionalities. Leveraging the rich diversity of epoxides and anhydrides, polyacrylate-based tetra-block copolymers comprising different polyester segments can be synthesized (Fig. 4 and Supplementary Fig. 4). In addition, the use of other acrylates contributes to the synthesis of MBCPs with various structures, including poly(methyl acrylate) (PMA), PEA, and poly(butyl acrylate) (Supplementary Fig. 5). Furthermore, MBCPs with higher nbs, such as hexa- (MBCP2) and octa-block copolymers (MBCP3), can be synthesized via two- and three-time PA/EA feeding procedures, respectively (Supplementary Fig. 6).
Subsequently, a detailed investigation was conducted to determine the formation of the MBCP and the role of PA during the copolymerization process via complementary experiments and kinetic studies using in situ FTIR spectroscopy. The copolymerization of CHO, PA, and EA was initially carried out with a feed ratio of CHO:PA:EA:1:PPNCl = 2000:150:150:1:2, followed by the addition of PA (150 equiv.) to the reaction mixture at an EA conversion of ~62%. At this point, the reaction of CHO and PA restarted, and the transformation of EA was interrupted (as shown in Supplementary Fig. 7). Once the newly added PA was fully consumed, EA transformation resumed, leading to the formation of a MBCP with a molecular weight (Mn) of 21.8 kg/mol and a dispersity (Ð) of 1.16. These results indicate that PA serves as a switcher that can toggle the polymerization of EA to the copolymerization of CHO and PA, thus producing MBCPs.
A kinetic study of the terpolymerization of CHO, PA, and EA was conducted as a function of catalyst loading with respect to the conversion rate of the copolymerization of CHO/PA and homopolymerization of EA. The initial rate (kobs) of PA conversion, determined as the slope of the fitted absorbance versus time curve, was directly proportional to the catalyst concentration (Supplementary Fig. 8 and Supplementary Table 3). For the copolymerization of CHO and PA, a linear fit to ln(kobs) as a function of ln([1]) was obtained with a slope of 1.07, indicating a first-order dependence on the concentration of catalyst 1. For the subsequent EA transformation process, a first-order dependence on the concentration of catalyst 1 was also obtained (Supplementary Fig. 9 and Supplementary Table 4). These results suggest that the terpolymerization of CHO, PA, and EA proceeds in an intramolecular catalytic manner, that is, the terpolymerization of CHO, PA, and EA proceeds inside the cleft of the dinuclear Co-complex in an intramolecular bimetallic synergistic catalytic manner. A mechanism for the 1/PPNCl-catalyzed terpolymerization of CHO, PA, and EA is proposed as follows. In bimetallic synergistic catalysis, the copolymerization of CHO with PA proceeds first in the presence of excess CHO, followed by the homopolymerization of EA, thus yielding a PCP-b-PEA di-block copolymer. With the addition of more PA and EA, the reaction between the in situ-generated carbanion and PA, wherein carboxylate ions are produced and are capable of retriggering the copolymerization of CHO and PA, switches the homopolymerization of EA to copolymerization. Then, periodic terpolymerization of CHO, PA, and EA proceeds to produce MBCPs (Fig. 5). Notably although the corresponding mononulcear Co-complex was capable of catalyzing this terpolymerization, no further conversion of PA or EA was detected after adding another portion of PA/EA (Supplementary Table 2), suggesting the switchable polymerization of CHO/PA/EA is unable to proceed in a monometallic catalysis manner.
The thermal properties of the PCP- and PEA-based MBCPs with different nbs were investigated using differential scanning calorimetry (DSC). The glass transition temperatures (Tgs) of PCP and PEA are 142.7 and −14.2 °C, respectively (Supplementary Fig. 10). Two glass transitions at −13.9 and 124.6 °C are clearly observed in the second heating for the di-block copolymer. In contrast, only one glass transition at 123.3 °C is observed for the corresponding tri-block copolymer. The increase in nbs of MBCPs leads to a decrease in Tgs, with a Tg of 83.2 °C detected for MBCP3. The reduction in the Tgs of the PCP segments within the MBCPs as the nbs increased suggests their improved microphase compatibility, indicating their potential as compatibilizers (Fig. 6)40,41,42.
In fact, previous works have revealed that MBCPs are effective compatibilizers in stitching two immiscible polymers, thus offering enhanced mechanical performances19,21. In pursuit of the compatibilization performance of obtained MBCPs, an investigation was conducted to explore the toughening performance of the MBCPs by incorporating them into PCP/PMA blends (50:50 wt%) (B1), wherein PCP is a typical brittle material with a maximal strength (σb) of 67.5 MPa and elongation at break (εb) of only 6.0% and PMA is soft material with a σb of 8.17 MPa and εb of 811%, respectively (Supplementary Figs. 11 and 12). Tensile test revealed that this blend is brittle, with an εb of only 137% and a σb of 5.1 MPa (Fig. 7a). In contrast, the εb and σb of the blends containing 3 wt% MBCP2 (B2) were 633% and 5.4 MPa, respectively. The viscoelasticity of the two blends was further investigated using a dynamic mechanical analyzer (DMA). The storage modulus (E’) of B2 was 3150 MPa at 0 °C, which is approximately nine times that of B1 (Fig. 7b). Two glass transitions were detected for B2, whereas only one was observed for B1. This is because B1 was too brittle to stretch with the increasing test temperature. To elucidate the reason for the enhanced toughness of the PMA and PCP blend, the microphase behavior of this blend was investigated. Thermal characterization revealed that the difference between the Tgs of the PCP and PMA blends was reduced by the incorporation of MBCP2. Initially, two distinct glass transitions were identified at 16.8 °C for PMA and 138.8 °C for PCP within blends; upon the addition of 3 wt% MBCP2, these Tgs shifted to 18.9 and 130.0 °C, respectively (Fig. 7c). The narrow gap between the two Tgs values shows a tendency similar to that of MBCPs, suggesting improved fusion of these two separated microphases with the addition of MBCP2. This is further supported by the morphology characterization of this blend using scanning electron microscopy (SEM), which revealed a sea-island structure for this blend, indicating an immiscible nature of these two polymers (Fig. 7d). The morphology becomes smoother when MBCP2 was added, suggesting that the blend transformed into a uniform microphase. Similar results were obtained from the characterization of this blend using atomic force microscopy (AFM). These results suggested that the addition of MBCP2 to the blend made the PCP and PMA mixture more uniform, thus enhancing the toughness of the physical blends (Fig. 7e)43,44,45,46.
In addition, the effects of the weight content and nbs of the MBCPs on the compatibilization performance were explored in detail by examining the mechanical properties of the PCP/PMA blends (50:50 wt%). With increasing MBCP2 content from 1 (B3) to 3 (B2) and 5 wt% (B4), the Tgs of PMA within the blends increased from 17.7 to 18.9 and 20.4 °C, respectively, together with a decrease in the Tgs of PCP from 135.7 to 130.0 and 125.1 °C (Fig. 8a). Morphological characterization using SEM and AFM revealed that the microphase compatibility was improved by increasing the weight content of the MBCPs, resulting in uniform blends (Supplementary Figs. 13 and 14). Correspondingly, tensile tests showed that improved microphase compatibility resulted in higher toughness. For example, increasing the MBCP2 weight content from 1 (B3) to 3 wt% (B2) resulted in dramatic improvements in strength and elongation, as σb and εb increased from 5.1 to 5.4 MPa and from 172% to 633%, respectively (Fig. 8b). Further increasing the MBCP2 content to 5 wt% leads to a further enhanced εb of 5.6 MPa, while σb decreases to 388%. A similar pattern was observed for the viscoelasticity of these blends, wherein E’ increased with the addition of MBCP2 (Fig. 8c). The highest E’ was achieved in the blend containing 3 wt% MBCP2, accompanied by a comparable loss factor (tan δ) and higher glass transition temperatures. Consequently, the addition of 3 wt% MBCP2 improved the microphase compatibility of the two immiscible polymers. Based on these results, the influence of MBCPs on the compatibilization performance of these MBCPs was examined in the presence of MBCPs with varying nbs (Supplementary Figs. 15 and 16). MBCPs with higher nbs could slightly narrow the gap between two Tgs of these two segments (Fig. 8d). With the addition of 3 wt% MBCP1 (B5), the εb and σb increased to 366% and 5.4 MPa, respectively (Fig. 8e). With the addition of 3 wt% MBCP3 (B6), εb increased to ~650% with a comparable σb of 5.5 MPa. Similarly, the higher the nb of the MBCP, the higher the E’ of the resultant PCP/PMA blend, wherein the highest E’ reached was 3320 MPa for B6 (Fig. 8f). Notably, MBCP2 and MBCP3 exhibited comparable compatibility enhancement effects in terms of both tensile properties and storage modulus. Consequently, the MBCP derived from PCP and PMA with hexa-blocks is a robust compatibilizer that enhances the toughness of PCP/PMA blends. To test this hypothesis, the compatibilization performance of MBCP2 was examined in a PCP/PMA blend with a weight ratio of 70:30. The σb and εb of this blend are 31 MPa and 4.7%, respectively (Supplementary Fig. 17), indicating their brittle nature. The addition of different amounts of MBCP2 is devoted to the enhanced tensile performances, wherein the highest σb and εb reach 43 MPa and 6.9%, respectively for the blend with added 3 wt% of MBCP2.
Discussion
In this study, we developed an effective strategy for synthesizing MBCPs, comprising polyester and polyacrylate segments, through the switchable copolymerization of epoxides, anhydrides, and acrylates in the presence of a dinuclear Co-complex. A detailed investigation of the copolymerization process revealed that bimetallic synergistic catalysis is key to achieving this switchable polymerization, wherein polyacrylate-based MBCPs, containing polyesters and polycarbonate segments, with different nbs can be synthesized via periodic feeding of cyclic anhydrides and acrylates in the presence of excess epoxides. The resultant MBCPs exhibited increased phase compatibility with an increase in nb, which renders them suitable for use as compatibilizers. The blends resulting from the addition of a PCP-b-PEA hexa-block copolymer to the PCP/PMA blend (50:50 wt%) exhibited enhanced tensile strength and elongation at break. Systematic characterization of these blends using SEM and AFM revealed that the addition of MBCPs improved the microphase separation of these mixtures, thus enhancing the mechanical performance of the blends. This results in the enhanced mechanical performance of these blends, highlighting the potential of MBCPs as a compatibilizer. Further studies focusing on the development of efficient strategies for producing α-olefins-derived MBCPs with diverse structures are currently underway.
Methods
Materials
Propylene oxide and CHO were purchased from Leyan and dried over calcium hydride (CaH2) overnight. Methyl acrylate, EA, and n-butyl acrylate were purchased from Energy Chemical and dried over CaH2 overnight, followed by vacuum distillation. Succinic anhydride and PA were purified by sublimation before being used. PPNCl (bis(triphenylphosphine)iminium chloride, Energy Chemical) was recrystallized by layering a saturated dichloromethane solution with diethyl ether. All other chemicals and reagents were purchased from commercial sources and used as received.
Characterization methods
NMR
1H and 13C NMR spectra were recorded on a Varian INOVA-400 MHz type (1H, 400 MHz; 13C, 100 MHz) spectrometer. Their peak frequencies were referenced versus an internal standard (TMS) shifts at 0 ppm for 1H NMR and against the solvent, CDCl3 at 77.4 ppm for 13C NMR, respectively.
Gel permeation chromatography (GPC)
Molecular weight and molecular weight distribution of the polymers with low solubility at room temperature were determined by gel permeation chromatography (GPC) with the PL-GPC220 equipped with a triple detection array, including a differential refractive index detector, a two-angle light scattering detector, and a fourbridge capillary viscometer at 150 °C using 1,2,4-trichlorobenzene as the eluent.
Differential scanning calorimetry (DSC)
The analysis of DSC was carried out with a NETZSCH DSC 206 thermal analyzer. Conditions: under the N2 atmosphere, the sample was annealed at 80 °C for 5 min and cooled to 0 °C at a rate of −10 K/min, then heated to 180 °C at a rate of 10 K/min. The DSC curve shows the first heating section of the thermodynamic process.
Thermo-gravimetric analyzer (TGA)
Thermo-gravimetric analysis of the resulting polymers was measured on a Mettler-Toledo TGA/SDTA851e. Nitrogen with the flow of 50 mL/min was used as a protective gas and the heating rate during the whole test was 10 K/min. All samples are heated to 600 °C.
In situ IR experiment
In situ, IR experiments of tracing recycling of the polymer in solvent were carried out with a Mettler-Toledo React IR 15.
Scanning electron microscopy (SEM)
Ultrahigh Resolution Field Emission SEM (JSM-7900F) equipped with an RBED detector was employed to observe the morphology of samples under 20000 times magnification.
Atomic force microscopy (AFM)
The surface morphology of the samples was observed using an AFM model JPK Nanowizard 4XP. The test was performed in QI mode, with a scan area of 5 × 5 µm, utilizing probes model SNL-A (tip radius less than 10 nm) to obtain high-quality images.
Dynamic mechanical analysis (DMA)
A rectangular spline for the Dynamic Mechanical Properties Test with a scale of 18 × 4 × 2 mm was obtained by cutting the samples. The test was performed at small tension film mode, 0.1% strain, 1 Hz, 3.0 °C min−1 on a Mettler-Toledo DMA/SDTA 1+.
Tensile test
The dog-bone samples (75-mm gauge length, 4-mm width, and thickness of 2 mm) from injection molding after twin-screw blending were employed. Stress/strain experiments were performed at a strain rate of 5 mm/min on an INSTRON 6800.
Blend preparation
The use of these multiblock copolymers for the compatibilization of a PCP/PMA (50/50 wt%) blends was investigated. The samples were prepared using an internal mixer at a set temperature and at a rotor speed of 100 rpm for 10 min. After that, the mixed samples were extruded from the twin-screw machine into the injection mold, in which the dog-bone injection mold was selected. The samples were cooled to room temperature after injection molding and subjected to a tensile test.
Data availability
The authors declare that the data supporting this study are available within the paper and the Supplementary Information File. All other data are available from the authors upon request.
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
EL-Ghoul, Y., Alminderej, F. M., Alsubaie, F. M., Alrasheed, R. & Almousa, N. H. Recent advances in functional polymer materials for energy, water, and biomedical applications: a review. Polymers 13, 4327 (2021).
Fiandra, E., Shaw, L., Starck, M., McGurk, C. J. & Mahon, C. S. Designing biodegradable alternatives to commodity polymers. Chem. Soc. Rev. 52, 8085 (2023).
Sun, H., Kabb, C. P., Sims, M. B. & Sumerlin, B. S. Architecture-transformable polymers: reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 89, 61–75 (2019).
Beyer, V. P., Kim, J. & Becer, C. R. Synthetic approaches for multiblock copolymers. Polym. Chem. 11, 1271–1291 (2020).
Clothier, G. K. K. et al. Multiblock copolymer synthesis via RAFT emulsion polymerization. Chem. Soc. Rev. 52, 3438 (2023).
Feng, H.-B., Lu, X.-Y., Wang, W.-Y., Kang, N.-G. & Mays, J.-W. Block copolymers: synthesis, self-assembly, and applications. Polymers 9, 494 (2017).
Bolton, J.-M., Hillmyer, M.-A. & Hoye, T.-R. Sustainable thermoplastic elastomers from terpene-derived monomers. ACS Macro Lett. 3, 717–720 (2014).
Wan, Y., He, J., Zhang, Y. & Chen, E. Y.-X. One-step synthesis of lignin-based triblock copolymers as high temperature and UV-blocking thermoplastic elastomers. Angew. Chem. Int. Ed. 61, e202114946 (2022).
Steube, M., Johann, T., Barent, R. D., Müller, A. H. E. & Frey, H. Rational design of tapered multiblock copolymers for thermoplastic elastomers. Prog. Polym. Sci. 124, 101488 (2022).
Solimando, X. et al. Controlled grafting of multi-block copolymers for improving membrane properties for CO2 separation. Polymer 255, 125164 (2022).
Wen, Z.-X. et al. Progress in polyhedral oligomeric silsesquioxane (POSS) photoresists: a comprehensive review across lithographic systems. Polymers 16, 846 (2024).
Wang, Y., Fan, J. & Darensbourg, D. J. Construction of versatile and functional nanostructures derived from CO2-based polycarbonates. Angew. Chem. Int. Ed. 54, 10206–10210 (2015).
Ren, F.-Y. et al. Amphiphilic polycarbonate micellar rhenium catalysts for efficient photocatalytic CO2 reduction in aqueous media. Angew. Chem. Int. Ed. 61, e202200751 (2022).
Kerwar, S. S., Kohn, L. D., Lapiere, C. M. & Weissbach, H. In vitro synthesis of procollagen on polysomes. Proc. Natl. Acad. Sci. USA 69, 2727–2731 (1972).
Lin, T.-W. et al. Advances in nonreactive polymer compatibilizers for commodity polyolefin blends. Chem. Rev. 124, 9609–9632 (2024).
Segalman, R. A., McCulloch, B., Kirmayer, S. & Urban, J. J. Block copolymers for organic optoelectronics. Macromolecules 42, 9205–9216 (2009).
Vo, N. T. P. et al. Autonomous self-healing supramolecular polymer transistors for skin electronics. Nat. Commun. 15, 3433 (2024).
Cooper, C. B. et al. Autonomous alignment and healing in multilayer soft electronics using immiscible dynamic polymers. Science 380, 935–941 (2023).
Ragaert, K., Delva, L. & Geem, K. V. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017).
Si, G.-F., Li, C., Chen, M. & Chen, C.-L. Polymer multi-block and multi-block+ strategies for the upcycling of mixed polyolefins and other plastics. Angew. Chem. Int. Ed. 62, e202311733 (2023).
Self, J. L. et al. Linear, graft, and beyond: multiblock copolymers as next-generation compatibilizers. JACS Au 2, 310–321 (2022).
Eagan, J. M. et al. Combining polyethylene and polypropylene: enhanced performance with PE/iPP multiblock polymers. Science 355, 814–816 (2017).
Longo, J. M., Sanford, M. J. & Coates, G. W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure−property relationships. Chem. Rev. 116, 15167–15197 (2016).
Wang, Y. et al. Switchable polymerization triggered by fast and quantitative insertion of carbon monoxide into cobalt–oxygen bonds. Angew. Chem. Int. Ed. 59, 5988–5994 (2020).
DiCiccio, A. M., Longo, J. M., Rodríguez-Calero, G. G. & Coates, G. W. Development of highly active and regioselective catalysts for the copolymerization of epoxides with cyclic anhydrides: an unanticipated effect of electronic variation. J. Am. Chem. Soc. 138, 7107–7113 (2016).
Korpusik, A. B. et al. Degradation of Polyacrylates by One-pot sequential dehydrodecarboxylation and ozonolysis. J. Am. Chem. Soc. 145, 10480–10485 (2023).
Ho, G.-M., Zulueta, M. M. L. & Hung, S.-C. Stereoselective one-pot synthesis of polypropionates. Nat. Commun. 8, 679 (2017).
Li, J. et al. Enantioselective resolution copolymerization of racemic epoxides and anhydrides: efficient approach for stereoregular polyesters and chiral epoxides. J. Am. Chem. Soc. 141, 8937–8942 (2019).
Yu, X. et al. Unraveling substituent effects on the glass transition temperatures of biorenewable polyesters. Nat. Commun. 9, 2880 (2018).
Bai, Y., Wang, H., He, J. & Zhang, Y. Rapid and scalable access to sequence-controlled DHDM multiblock copolymers by FLP polymerization. Angew. Chem. Int. Ed. 59, 11613–11619 (2020).
Engelis, N. G. et al. Sequence-controlled methacrylic multiblock copolymers via sulfur-free RAFT emulsion polymerization. Nat. Chem. 9, 171–178 (2017).
Matsuo, Y., Konno, R., Ishizone, T., Goseki, R. & Hirao, A. Precise synthesis of block polymers composed of three or more blocks by specially designed linking methodologies in conjunction with living anionic polymerization system. Polymers 5, 1012–1040 (2013).
Steube, M. et al. Isoprene/styrene tapered multiblock copolymers with up to ten blocks: synthesis, phase behavior, order, and mechanical properties. Macromolecules 51, 10246–10258 (2018).
Gody, G., Maschmeyer, T., Zetterlund, P. B. & Perrier, S. Rapid and quantitative one-pot synthesis of sequence-controlled polymers by radical polymerization. Nat. Commun. 4, 2505 (2013).
Lu, X.-B. & Ren, B.-H. Partners in Epoxide Copolymerization Catalysis: approach to high activity and selectivity. Chin. J. Polym. Sci. 40, 1331–1348 (2022).
Ren, W.-M., Wang, R.-J., Ren, B.-H., Gu, G.-G. & Yue, T.-J. Mechanism-inspired design of heterodinuclear catalysts for copolymerization of epoxide and lactone. Chin. J. Polym. Sci. 38, 950–957 (2020).
Xia, Y., Scheutz, G. M., Easterling, C. P., Zhao, J. & Sumerlin, B. S. Hybrid block copolymer synthesis by merging photoiniferter and organocatalytic ring-opening polymerizations. Angew. Chem. Int. Ed. 60, 18537–18541 (2021).
Fu, X.-Y. et al. A powerful strategy for synthesizing block copolymers via bimetallic synergistic catalysis. Angew. Chem. Int. Ed. 63, e202401926 (2024).
Patterson, D. & Robard, A. Thermodynamics of polymer compatibility. Macromolecules 11, 690–695 (1978).
Retsos, H. & Anastasiadis, S. H. Interfacial tension in binary polymer blends in the presence of block copolymers. 2. Effects of additive architecture and composition. Macromolecules 37, 524–537 (2004).
Zhang, C. et al. Melt crystallization behavior and crystalline morphology of polylactide/poly(ε-caprolactone) blends compatibilized by lactide-caprolactone copolymer. Polymers 10, 1181 (2018).
Grover, T. L. & Guymon, A. Effect of block copolymer self-assembly on phase separation in photopolymerizable epoxy blends. Macromolecules 57, 4717–4728 (2024).
Ge, Q.-Y. & Dou, Q. Preparation of supertough polylactide/polybutylene succinate/epoxidized soybean oil bio-blends by chain extension. ACS Sustain. Chem. Eng. 11, 9620–9629 (2023).
Li, G. et al. Stable-dispersed organic/inorganic hybrids for enhanced comprehensive performances of vinyl ester resin. Polymer 294, 126700 (2024).
Reinaldo, J. S. et al. Thermal, mechanical, and morphological properties of multicomponent blends based on acrylic and styrenic polymers. Polym. Test. 82, 106265 (2020).
Acknowledgements
T.-J.Y. and W.-M.R. acknowledge support from the National Natural Science Foundation of China (NSFC, 22101040 and 22171037). T.-J.Y. acknowledges the support from the China Postdoctoral Science Foundation (BX2021050 and 2021M690517). X.-B.L. acknowledges the support from the National Key Research and Development Program of China (2021YFA1501704). Gratitude is expressed to the Fundamental Research Funds for the Central Universities (DUT22LAB609).
Author information
Authors and Affiliations
Contributions
W.-M.R. conceived the project. W.-M.R. and T.-J.Y. directed the research. F.-X.Y. and G.-X.H. carried out experiments and collected the overall data. T.-J.Y. wrote the original manuscript draft, X.-B.L. and W.-M.R. revised and finalized the manuscript. All co-authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alex Plajer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, XY., Yue, TJ., Guo, XH. et al. Synthesis of highly effective polyester/polyacrylate compatibilizers using switchable polymerization. Nat Commun 16, 2154 (2025). https://doi.org/10.1038/s41467-025-57449-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57449-7