Abstract
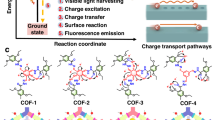
Ground-state charge transfer plays a vital role in improving the photocatalytic performance of D-A type covalent organic frameworks. However, limited studies have explored the modulation of photocatalytic performance in COFs-based photocatalysts through ground-state charge transfer. Here we show the formation of extremely intense ground-state charge transfer via a unique covalent bonding approach. We transform three-dimensional stacked COF-based S-scheme heterojunctions (FOOCOF-PDIU) into co-planar single-molecule junctions (FOOCOF-PDI). This co-planar single-molecule junction structure exhibits strong ground-state charge transfer compared to the traditional randomly stacked heterojunctions and individual COFs. Ground-state charge transfer induces charge redistribution and dipole moment formation, which enhances the built-in electric field intensity in single-molecule junctions. This enhanced built-in electric field promotes exciton dissociation and charge separation, resulting in improved photocatalytic efficiency. Therefore, a stable molecule-decorated COF with broad light absorption has been successfully obtained, whose hydrogen evolution rate can reach 265 mmol g−1 h−1. This work opens an avenue for exploiting photocatalytic mechanisms in COFs based on ground-state charge transfer effects.
Similar content being viewed by others
Introduction
Due to their tunable electronic properties and high surface areas, covalent organic frameworks (COFs)-based photocatalysts have recently gained increasing attention as promising candidates for solar photocatalysis1,2,3,4,5,6,7, which have emerged as potential photocatalysts for solar-driven water splitting to produce hydrogen8,9,10,11. These materials have shown great potential for photocatalytic applications but face challenges related to their poor charge carrier separation and high exciton binding energy, which limit their efficiency in the photocatalytic process12,13,14,15,16.
Constructing heterojunction is an effective approach to enhance the photogenerated charge carrier separation efficiency in COF-based semiconductors14,17. The heterojunction formed by stacking ideal superimposed layers facilitates the out-of-plane charge transfer, while the actual stacking deviations are unfavorable for photogenerated charge separation18. While optimizing the planarity of semiconductors can enhance the exciton dissociation and charge carrier separation in semiconductor photocatalysts19,20,21,22. Therefore, converting the stacked heterostructures into co-planar molecular junctions will greatly enhance the interfacial charge carrier transport efficiency between heterojunctions. However, the molecular junction-enhanced mechanism has been less studied so far, especially the ground-state charge transfer (GSCT) has rarely been reported.
GSCT enhances the separation of photogenerated electron-hole pairs through spatial and energetic mechanisms. Spatially, GSCT facilitates electron transfer from donor to acceptor molecules, increasing the distance between electrons and holes, and thus decreasing recombination. Energetically, the GSCT process results in a more favorable environment for hole transport on the donor due to the increased positive charge density. Moreover, the energy level alignment between the donor and acceptor after charge transfer reduces the Coulombic binding energy, further minimizing recombination23,24,25. Interestingly, such GSCT is posited to play a mediating role in charge separation within D-A organic heterojunctions, manifesting as a real electronic state. The GSCT is thought to modulate the kinetics of charge separation and recombination processes, thereby enhancing the performance of organic photovoltaic devices26,27. However, in most D-A type COFs, the formation of charge-separated states is typically observed under photoexcitation. The GSCT in D-A type COFs has not been extensively investigated in photocatalysis.
Here, the synthesis and study of two FOOCOF-based heterojunction photocatalysts, three-dimensional stacked COF-based S-scheme heterojunctions, and co-planar single-molecule junctions. Studies have revealed that single-molecule junctions FOOCOF-PDI, featuring covalent bonding, exhibit a stronger GSTC effect than FOOCOF-PDIU and FOOCOF. The crystal and chemical structure of the resulting FOOCOF-PDI were confirmed using powder X-ray diffraction (PXRD), Fourier-transform infrared (FT-IR) spectroscopy, and solid-state 13 C nuclear magnetic resonance (13 C NMR). Both experimental and theoretical calculations show that bridging PDI molecules to the end of COFs through GSTC results in COF-PDI possessing a stronger dipole moment and built-in electric field, which promotes exciton dissociation and carrier migration. Under visible light irradiation, with Pt as a cocatalyst, its photocatalytic hydrogen production activity reached 265 mmol g−1 h−1.
Results and Discussion
FOOCOF was synthesized the same as in our previous work28. Under the presence of acetic acid, FOOCOF-PDI was synthesized through the condensation of aldehyde groups at the end of FOOCOF with the amine groups of PDINH in a mixed solvent of o-dichlorobenzene/n-butanol (3/1, v/v) at 140 °C, as detailed in the additional information (Fig. 1). The FOOCOF/PDINH S-scheme heterojunction (FOOCOF-PDIU) is formed by ultrasound-assisted mixing of FOOCOF and PDINH.
The PXRD pattern of FOOCOF is shown in Fig. 2, featuring prominent peaks at 4.88°, 9.69° and 11.63° (Fig. 2A and Figure S1), corresponding to the 001, 211, and 102 planes of FOOCOF (a = b = 29.80 Å and c = 15.00 Å with the residual factors of Rp = 5.06% and Rwp = 5.02%). The structure of FOOCOF remains unchanged after PDI modification, indicating the covalent connection of PDI molecules shows a low effect on FOOCOF (Fig. 2B). XRD pattern of FOOCOF-PDIU shows distinct PDINH peaks (Figure S2). In the FTIR spectrum, the peak at 1267 cm−1 corresponding to the C-N bond strengthened29,30, indicating the successful covalent connection of PDINH at the end of FOOCOF (Fig. 2C). Meanwhile, the peak at 1591 and 3423 cm−1 were attributed to C=O and -NH2, respectively. The NMR spectrum revealed a peak at 184.5 ppm, which is attributed to the carbonyl group of the fluorenone moiety. Peaks at 137 and ≈146 ppm corresponded to C-N and C-NH bonds, confirming the successful synthesis of FOOCOF. In the NMR spectrum of FOOCOF-PDI, the peak attributed to C-N exhibits a enhanced intensity, confirming the successful covalent connection of PDINH at the end of FOOCOF (Fig. 2D). XPS analysis further confirmed the structural characteristics of FOOCOF-PDI. The C 1s spectrum was resolved into three peaks at binding energies of 284.8, 286.1, and 288.9 eV, corresponding to C=C, C=O, and C=N, respectively. The N 1s spectrum displayed peaks at 399.6 and 398.8 eV, attributed to the nitrogen atoms of the C-N and C=N bonds. In the O 1s spectrum, the peak at 531.1 eV corresponded to the C=O bonds of FOOCOF and PDI; the peak at 532.9 eV was attributed to oxygen species adsorbed (Figures S3–5). The prepared samples’ UV-vis diffuse reflectance spectra (UV-DRS) display an extended absorption tail reaching up to 750 nm (Fig. 2E and Figure S6), indicating a broad harvesting range. Therefore, the bandgaps for FOOCOF, FOOCOF-PDI and PDINH are determined to be 1.86, 1.79 and 1.89 eV, respectively (Figure S7). According to VB-XPS, the valence bands of FOOCOF, FOOCOF-PDI and PDINH are determined to be 0.94, 1.02 and 2.37 V vs RHE, respectively. E(RHE) = E(NHE) + 0.059 pH. Therefore, the valence bands of FOOCOF, FOOCOF-PDI and PDINH are determined to be 0.53, 0.61 and 1.96 V vs NHE, respectively (Figure S8–10). Accordingly, the conduction bands of FOOCOF, FOOCOF-PDI and PDINH were −1.33, −1.18 and 0.07 V, respectively (Fig. 2F and Figure S11). The prepared samples thermodynamically meet the requirements for photocatalytic water splitting. Meanwhile, the thermodynamic conditions are satisfied for the formation of a S-scheme heterojunction between FOOCOF and PDINH.
A Experimental PXRD patterns of FOOCOF with corresponding Pawley refinement, simulated eclipsed (AA) stacking patterns, differences between refinement and experimental, and Bragg positions, (B) PXRD patterns, (C) FTIR spectrum, (D) Solid-state CP/MAS 13C NMR spectra, (E) UV–vis differential reflectance spectral pattern and (F) band level of FOOCOF and FOOCOF-PDI. G TEM image of FOOCOF, (H) High-resolution TEM (HRTEM) images of FOOCOF; (I) TEM images of FOOCOF-PDI.
SEM and TEM were employed to investigate the morphological impact of PDINH covalently connecting COFs. The SEM images (Figures S12–14) reveal the smooth, layered stacking morphology of FOOCOF, and the covalent attachment of PDINH does not induce any discernible changes in the sample’s morphology. The TEM images (Fig. 2G and Figure S15) further divulge the nanosheet structure of FOOCOF. Additionally, the obvious lattice stripes can be seen in the figure, indicating the high crystallinity of the prepared sample (Fig. 2H). After the formation of single-molecule junctions, no obvious change could be found in the TEM images (Fig. 2I and Figure S16). Meanwhile, no PDINH molecule could be found in the FOOCOF-PDI (Fig. 2I and Figure S17). The reversible nitrogen adsorption isotherm of FOOCOF exhibits the typical type IV isotherm characteristic and displays mesoporous features (Figure S18). The BET surface area of FOOCOF is calculated to be 95.52 m2 g−1. The pore size distribution is 1.3 nm, which is in agreement with the theoretical model of 1.3 nm. Covalently connecting PDI results in a slight decrease in the BET surface area to 76.81 m2 g−1, yet the pore size remains unchanged. Thermogravimetric analysis (TGA) was performed to investigate the thermal stability of the COFs. As shown in Figure S19, FOOCOF decomposes at 250 °C, while the thermal stability is improved after covalently connecting PDI.
In-situ photodeposition of Pt as a cocatalyst and 0.1 M ascorbic acid as a sacrificial agent were utilized in the photocatalytic water splitting under visible light. The photocatalytic hydrogen evolution activity of FOOCOF and FOOCOF-PDI was evaluated with 2 wt% Pt as the cocatalyst. FOOCOF exhibited an average hydrogen evolution rate of 103 mmol g−1 h−1 under visible light irradiation for three hours. After formatting S-scheme heterojunctions, the average hydrogen evolution rate of FOOCOF-PDIU increased to 164 mmol g−1 h−1. Upon the formation of the single molecular junction, FOOCOF-PDI showed a notable enhancement in the average hydrogen evolution rate, reaching 265 mmol g−1 h−1 under visible light irradiation for three hours, indicating a improvement in the photocatalytic activity of COFs upon the formation of single molecular junctions (Fig. 3A, B). Meanwhile, the performance is comparable to that of the other COF-based photocatalysts (Table S1)31,32,33,34,35. When the Pt content is less than 2%, the hydrogen production activity of the sample is lower than 265 mmol h−1 g−1 due to the lack of active sites. Due to the light shielding effect, the photocatalytic activity of the samples gradually decreased with the increase of Pt content and catalysts dose (Figures S20 and S21). Considering the weighing error when using 1 mg of catalyst, we still take the hydrogen production activity at 2 mg as the standard. The hydrogen evolution rate of FOOCOF-PDI is greatly dependent on the pH of the reaction solution (Figure S22). The hydrogen production activity is higher under acidic conditions in ascorbic acid solution than under alkaline conditions in TEOA solution. In addition, no H2 is produced when ethanol is used as a sacrificial agent. FOOCOF-PDI maintained 90 percent stability in the 24-hour cycle test (Fig. 3C). As evidenced by the experimental results, the sample maintained stability.The black plots on the surface of COF could attributed to the cocatalysts Pt (Figures S23 and S24). To analyze the wavelength-dependent photocatalytic performance of FOOCOF-PDIs, the apparent quantum efficiency (AQE) as a function of wavelength was monitored (Figure S25). The AQE value of FOOCOF is up to 36.3% at 420 nm.
A Time course of photocatalytic H2 evolution of as-prepared samples. B Average hydrogen evolution rate and (C) cycling stability for as prepared samples. D UV-vis absorption, (E) ESR spectra in the dark and (F) surface potential difference of FOOCOF, FOOCOF-PDIU and FOOCOF-PDI. Surface potentials via KPFM for (G) FOOCOF-PDIU and (H) FOOCOF-PDI. I Normalized built-in electric field strength of FOOCOF, FOOCOF-PDIU and FOOCOF-PDI.
When ultraviolet light irradiates the charge transfer complex, electrons can undergo a transition from the ground state to the charge-transfer level, resulting in the formation of a new absorption band. This absorption band is referred to as the charge transfer absorption band. The enhanced GSCT was first confirmed by UV-vis absorption (Fig. 3D). Notably, after the formation of FOOCOF-PDI, a new absorption peak arises at a longer wavelength (∼650 nm), which indicates the formation of a new charge-transfer complex36,37. Then, we found that FOOCOF-PDI exhibited a distinct dark-state signal in the dark state through ESR, evidencing GSCT (Fig. 3E and Figure S26). Furthermore, the GSCT impact on the carrier extraction capability were investigated using scanning Kelvin probe microscopy (SKPM). As shown in Fig. 3F and Figure S27–28, the potential of FOOCOF-PDI is approximately 0.32 V higher than that of pure FOOCOF and FOOCOF-PDIU, indicating interface vacuum level shift due to electron transfer23,36,38. These results corroborate the GSCT in FOOCOF-PDI. We measured the fluorescence lifetime of the prepared samples, and the lifetime of FOOCOF-PDI is longer than that of FOOCOF. The average lifetimes for both FOOCOF and FOOCOF-PDI were fitted by using bi-exponential decay kinetics. The calculated average lifetime of FOOCOF is 21.3 ns. However, upon formation single-molecule junctions, the average lifetime of FOOCOF-PDI increases more than 4 times (Figure S29). The enhancement in the lifetime upon complex formation between FOOCOF and PDI suggests the formation of long-lived free carriers.
GSCT can result in the generation of dipole moments39. Generally, the dipole moment of a single molecule can effectively drive charge transfer within the molecule. Once the molecular units undergo highly ordered stacking, an intrinsic electric field is generated; otherwise, the dipoles would cancel out due to the disordered assembly40,41. We analyzed the built-in electric field of the prepared samples. Due to the existence of dipoles, the built-in electric field is enhanced. In general, the strength of the built-in electric field can serve as a kinetic factor driving charge separation, thus necessitating a quantitative evaluation42. According to the theory of Zhang et al., the determination of the material’s embedded electric field is monotonically positively correlated with its surface charge density and zeta potential43. Utilizing KPFM (Kelvin probe force microscopy) to detect the surface charge density of the sample, it is shown that the highest potential of FOOCOF-PDI reaches up to 103.5 mV (Fig. 3G, H). Combined with the zeta potential results shown in Figure S30, it confirms that the built-in electric field of FOOCOF-PDI are 2.1 and 1.5 times stronger than that of FOOCOF and FOOCOF-PDIU (Fig. 3I).
The interface dipoles caused by charge transfer can promote the dissociation of excitons into free carriers, as demonstrated in numerous prior studies44. The early detection of excitons (within 0.5 ps) in the prepared COFs was carried out using femtosecond transient absorption spectroscopy (fs-TAS). The prepared sample exhibited a negative peak at 450–600 nm and a positive peak at 850–1100 nm, which are attributed to the ground-state bleach (GSB) and excited-state absorption (ESA), respectively. These signals correspond to the excited-state relaxation. Therefore, the photogenerated electron and hole pairs in the sample contribute to both the GSB and the broad ESA signals (Fig. 4A, E, Figure S31 and S32 A). It has been confirmed that the photogenerated electrons mainly participate in the interfacial transfer45. Figure 4A–H and Figure S32 A–C show that the prepared sample exhibited a broad negative peak (F1) near 500 nm. Combined with steady-state fluorescence, it can be inferred that F1 is the stimulated emission band of photogenerated excitons. Notably, the broadening of the fs-TAS absorption peak corresponds to the relaxation of bound excitons and the increase in carrier density46,47. Hence, compared to FOOCOF, the broadening of F1 in FOOCOF-PDI indicates a weakening exciton effect and a more efficient exciton dissociation. For further quantitative analysis, a double-exponential function was used to fit the decay of F1, with the results shown in Fig. 4D, H. τ1 is related to rapid exciton dissociation at high exciton densities, whereas τ2 is associated with rapid charge recombination and capture. The smaller τ1 value for FOOCOF-PDI (0.52 ps) compared to FOOCOF (0.62 ps) confirms the more effective exciton dissociation of FOOCOF-PDI, consistent with the binding energy of the excitons. After 0.5 ps, rapid separation of photoinduced electron-hole pairs occurs, coexisting with some fluorescence emission, and the remaining photoinduced electrons undergo an electron capture process. When the laser excites the photo-excited electrons from the CB of the material to higher energy levels, it leads to the capture of the reduced photoinduced absorption or the reduction in transmittance48. Therefore, the intensity and decay of the photoinduced absorption represent the capture and reduction of charges. The increase in τ2 indicates the beneficial effect of capturing long-lived shallow electrons on the transfer of active electrons, achieving high-efficiency photocatalytic hydrogen production performance. Through comparison between single molecule junctions and heterojunctions, it was further found that the formation of heterojunctions can capture long-lived electrons, although the electron lifetime is relatively shorter compared to single-molecule junctions (Figure S32). We measured the temperature-dependent photoluminescence spectrum to test the exciton binding energy (Fig. 4I-G). The intensity of PL for both FOOCOF and FOOCOF-PDI decreased with increasing the temperature from 80 to 290 K, corresponding to the thermally activated nonradiative recombination process. The exciton binding of FOOCOF-PDI (18.35 meV) was lower than that of FOOCOF (25.36 meV). Reducing the exciton binding energy enhances the quantum efficiency of semiconductor materials, which refers to the efficiency of generating an electron upon absorbing a photon. This higher quantum efficiency translates to greater conversion of light energy into chemical energy, leading to improved hydrogen production efficiency. Decreasing the exciton binding energy also extends the lifetime of electrons and holes. The prolonged lifetime allows electrons more time to migrate to the catalyst surface and participate in reduction reactions, ultimately enhancing photocatalytic efficiency. The vigorous photocurrent intensity and lower slope mean the enhanced charge concentration in FOOCOF-PDI from the efficient exciton dissociation (Fig. 4K, L). We have measured electrochemical impedance spectroscopy (EIS) to detect the role of single-molecule junction during the photocatalytic reaction. In the EIS spectrum, the as prepared samples typically exhibits two distinct regions, each reflecting different electrochemical processes. In the high frequency region, the impedance spectrum displays a smaller semicircle with a slope close to 0. This region primarily reflects charge transfer processes at the electrode surface, including electron transfer and ion migration between the electrode material and the electrolyte. The diameter of the semicircle represents the charge transfer resistance, with smaller values indicating easier charge transfer. In the low frequency region, the impedance spectrum exhibits a larger semicircle with a slope approaching −1. This region primarily reflects the diffusion of ions within the electrode material, i.e., the diffusion rate of ions within the electrode structure. A slope close to −1 indicates Warburg impedance, signifying that ionic diffusion is the primary factor controlling the reaction rate. FOOCOF and FOOCOF-PDIU exhibit a larger radius compared to FOOCOF-PDI, indicating that the single-molecule junction can reduce electron-transfer impedance in FOOCOF-PDI49,50 (Figure S33).
A–C Transient absorption spectra of FOOCOF. D Normalized transient absorption kinetics for FOOCOF. E–G Transient absorption spectra of FOOCOF-PDI. H Normalized transient absorption kinetics for FOOCOF-PDI. Temperature-dependent photoluminescence spectrum of integrated PL intensity as a function of the temperature of (I) FOOCOF and (J) FOOCOF-PDI (inset: temperature-dependent PL spectra from 80 to 290 K). K Photocurrent response and (L) Mott–Schottky curve of FOOCOF, FOOCOF-PDIU and FOOCOF-PDI.
To investigate the influence of GSCT on exciton dissociation, the electrostatic surface potential (ESP) of the optimized structures of FOOCOF and FOOCOF-PDI molecules was calculated, as depicted in Fig. 5A, B. The calculation of the multipole moment expansion of the molecular ESP revealed the broadest distribution of multipole moments for FOOCOF-PDI DFT, indicating a clear charge separation center. Hence, the single-molecule junction ensures the stable existence of a strong intrinsic electric field38,51, which is crucial for exciton dissociation, carrier separation and migration (Figure S34). Without a single-molecule junction, the electrons involved in the reaction tend to accumulate at the edges of the COF material. They are unable to interact with the reaction solvent immediately. However, after forming a single-molecule junction, it can be observed that the electrons tend to accumulate in the middle of the PDI, as the entire PDI molecule is in direct contact with the solvent, serving as the reaction site (Fig. 5C). Therefore, the formation of the molecular junction promotes the dissociation of excitons and guides free electrons to the interface edge sites, thereby enhancing the separation of charge carriers, reducing the recombination of charge carriers, and improving the efficiency of interfacial reactions. The structures were fully optimized, followed by time-dependent density functional theory (TD-DFT) calculations to gain insights into the exciton generation properties of the COF photocatalyst. The electron-hole distribution in the first excited singlet state (S2) of the two COFs indicates a difference in exciton binding energy (Eb) due to localized excitation. The exciton binding energy of FOOCOF-PDI is 1.887 eV, lower than the 2.274 eV of FOOCOF, indicating a weaker exciton effect in FOOCOF, consistent with its photocatalytic activity (Fig. 5D, E). Considering that calculating only fragments would be affected by edge and conjugation effects, we expanded the model and performed calculations on FOOCOF and FOOCOF-PDI (Figure S35). It was found that, under photoexcitation, the calculated results with continuous fragment structures are consistent with the fragment results. This suggests that the exciton effect may be one of the controlling factors in this process. FOOCOF and FOOCOF-PDI mainly absorb peaks composed of transitions from S0 to S2. The S2 state of FOOCOF can be described as HOMO-1 → LUMO + 1. The transition of S2 exhibits a molecular intra-charge transfer characteristic: electrons transfer from TP to the fluorenone site. Therefore, the effective separation of electron-hole pairs enhances the lifetime of carriers52. After the formation of the single-molecule junction, the S2 state of FOOCOF can be described as HOMO → LUMO + 2 (Fig. 5F, G). Electrons transfer from FOOCOF to the PDI site. This indicates that electrons tend to reside in the middle of the PDI, and the entire PDI molecule is the reaction site directly in contact with the solvent. Furthermore, we calculated the Gibbs free energy change (ΔG) for hydrogen adsorption at O atoms in both fluorenone sites and PDI sites accordingly from FOOCOF and FOOCOFs-PDI. It is noticeable that the fluorenone sites are energetically more favorable than the PDI sites. Moreover, the hydrogen adsorption free energy tends to decrease upon the formation of single-molecue junctions (Figure S36).
Electrostatic surface potential (ESP) distributions of (A) FOOCOF and (B) FOOCOF-PDI, blue and red represented electron accumulation and depletion, respectively. C Electric potential along X axile for (100) plane. D Exciton binding energies and electron-hole distributions of (D) FOOCOF and (E) FOOCOF-PDI, blue and green represent positive and negative phases. TD-DFT calculated electronic transition of (F) FOOCOF and (G) FOOCOF-PDI, blue and green represent positive and negative phases.
In summary, we have successfully designed and synthesized the single-molecule junction of FOOCOF-PDI. Following the formation of this molecular junction, the photocatalytic activity of FOOCOF has improved. In the presence of Pt as a cocatalyst, the photocatalytic hydrogen evolution activity of FOOCOF-PDI reaches 265 mmolg−1 h−1, which is 2.57 times higher than that of FOOCOF. The experimental results indicate that the formation of the single-molecule junctions induces a GSCT to enhance the intrinsic electric field. The dipole effect effectively enhances exciton dissociation. This work has theoretical and application value for deeply studying the GSCT-inducing exciton dissociation and charge separation in COF-based photocatalysts.
Method
Materials
All chemicals and reagents were of analytical grade materials and used as received without further purification. The o-1,2-Dichlorobenzene (1,2-Dichlorobenzene, 99%), anhydrous n-BuOH (n-Butanol, 99.4%), Pyrrolidine (Py, 98%), Ascorbic acid (>99.0%), 2,7-Dinitro-9-fluorenone (≥98%) and, 4,9,10-Perylenetetracarboxylic dianhydride were purchased from Macklin Chemicals 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde was supplied by Jilin Chinese Academy of Sciences-Yanshen Technology Co. Ltd.
Synthesis of PDINH
The PDINH monomer was prepared through a nucleophilic substitution reaction between 3,4,9,10-Perylenetetracarboxylic dianhydride and hydrazine monohydrate. In a typical procedure, 7.84 g (0.02 mol) of the dianhydride was dissolved in 100 mL of ethanol within a 250 mL round-bottom flask under a nitrogen environment. The mixture was cooled to 0 °C using an ice bath, and 10 mL (0.2 mol) of hydrazine monohydrate was then introduced slowly over a 10-minute period. Following 30 min of agitation in the ice bath, the reaction was warmed to ambient temperature before being heated under reflux at 70 °C for a duration of 1 h.
Synthesis of 2,7-diamino-9 H-fluoren-9-one
In a typical procedure, 9.45 g (35 mmol) of 2,7-dinitro-9-fluorenone was dissolved in 375 mL of ethanol at room temperature. To this solution was added a separately prepared aqueous solution (650 mL) of sodium sulfide nonahydrate (37.85 g, 157.5 mmol) and sodium hydroxide (15 g, 375 mmol). The resulting mixture was refluxed for 5 h and then cooled to 0–5 °C. The precipitate was collected by filtration and washed extensively with water, 5% (w/v) sodium hydroxide solution, water, cold ethylene glycol, ether, and hexane. Vacuum drying, followed by recrystallization from acetone-ethanol (1:1 v/v), afforded 6.10 g (83%) of 2,7-diamino-9H-fluoren-9-one as purple needle-shaped crystals.
Synthesis of FOOCOF
In a Pyrex tube, 16.8 mg (0.08 mmol) of 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde and 25.0 mg (0.12 mmol) of 2,7-diamino-9H-fluoren-9-one were combined with o-1,2-Dichlorobenzene (0.75 mL), n-BuOH (0.25 mL), and pyridine (Py, 0.1 mL). The mixture was subjected to ultrasonic mixing and then degassed by three freeze-pump-thaw cycles to a vacuum of 100 mTorr before being sealed. The sealed tube was heated to 120 °C and maintained at this temperature for 72 h. Following the reaction, the brown solid was separated via centrifugation and purified by washing with tetrahydrofuran (100 mL) and anhydrous acetone (200 mL).
Synthesis of FOOCOF-PDI
In a Pyrex tube, PDINH (1 mg) and FOOCOF (10 mg) were combined with o-1,2-Dichlorobenzene (0.75 mL), n-BuOH (0.25 mL), and acetic acid (0.05 mL). The mixture underwent three freeze-pump-thaw cycles to remove dissolved gases, achieving a vacuum pressure of 100 mTorr, before being sealed. The sealed tube was heated to 140 °C for 36 h. After the reaction, the resulting brown solid was filtered, with DMSO and ethanol aid the filtration, and purified by sequential Soxhlet extraction with methanol, acetone, and hexane (each for 24 h).
Photocatalytic hydrogen evolution method and parameters
We carried out photocatalytic reactions in a 100 mL three-necked flask, using a 300 W xenon lamp (PLS-SXE300, λ > 420 nm) to simulate sunlight at an intensity of 160 mV cm−2. We used a 420 nm cutoff filter to remove UV light. Specifically, we dispersed 2 mg of photocatalyst in 40 mL of 0.1 M ascorbic acid solution. We used ultrasonication to ensure uniform dispersion and then purged with N2 to create an anaerobic environment. We allowed the reaction to proceed for 1 h under continuous illumination. Finally, we extracted 0.4 mL of the generated gas intermittently and analyzed it using a gas chromatograph (GC-9560, TCD). We calculated the apparent quantum efficiency (AQE) of the samples as follows.
where np is the mole number of product obtained (mol), NA is the Avogadro constant (6.022 × 1023 mol−1), P is the optical density (W cm-2), S is the light irradiation area (cm2), t is the light irradiation time (s), λ is the monochromatic light wavelength (m), h is Planck’s constant (6.626 × 10−34 J s), and c is the speed of light (3 × 108 m s−1).
Powder X-ray diffraction (PXRD) analysis
PXRD analysis was performed on a Rigaku Smartlab diffractometer utilizing Cu Kα radiation (λ = 1.54178 Å). The diffractometer was operated in reflection geometry at 40 kV and 40 mA.
FTIR analyses
A Nicolet Avatar 6700 FT-IR spectrometer (Thermo Fisher, USA) was utilized to record FTIR spectra.
Solid-state diffuse reflectance ultraviolet-visible spectroscopy analysis
A Shimadzu UV-2600 UV-vis-NIR spectrophotometer was employed to analyze the UV-Vis diffuse reflection spectra of the photocatalysts.
N2 Physisorption measurements
The porous properties of the COF materials were characterized by nitrogen adsorption at 77 K using an Autosorb-iQ-MP analyzer. Before measurement, samples were outgassed under vacuum (P < 10−3 Torr) at room temperature and subsequently heated at 120 °C for 12 h to ensure complete removal of adsorbed species. The specific surface area was determined by the BET method applied to the nitrogen adsorption isotherm data in the relative pressure range of 0.01–0.9.
X-ray photoelectron spectroscopy
Surface elemental analysis was performed by XPS on a VG ESCALAB 250 instrument, utilizing Al Kα radiation (hv = 1486.6 eV) as the excitation source. The analysis parameters were as follows: spot size 400 μm, base pressure < 5.0 × 10−7 mbar, operating voltage 12 kV, and filament current 6 mA. Survey spectra were collected with a pass energy of 100 eV and a step size of 1 eV, while high-resolution spectra were obtained with a pass energy of 50 eV and a step size of 0.1 eV. Charge correction was applied to all spectra by referencing the C 1 s peak to 284.80 eV. The number of cycles for high-resolution scans was optimized for each element to ensure adequate signal-to-noise ratio, with a minimum of five cycles.
High-resolution transmission electron microscopy (HRTEM) and scanning electron microscopy (SEM)
Microstructural characterization was carried out using a Thermo Fisher Talos F200S TEM. The morphology of the samples was examined using a Zeiss EVO MA 15 field emission SEM.
Electron spin resonance spectroscopy (ESR)
A Bruker EMX CW micro spectrometer, operating at X-band (≈9.87 GHz) and equipped with a high-sensitivity ER 4119HS-WI cavity, was used to perform electron spin resonance (ESR) measurements at 293 K. Samples were photo-excited using a 300 W xenon lamp, with wavelengths below 420 nm removed by a cut-off filter (LOT Oriel).
Electrochemical analysis
The working electrodes were fabricated by first creating a photocatalyst slurry consisting of 5 mg of the photocatalyst powder and 4 mL of ethanol, followed by ultrasonic dispersion. A total of 500 μL of the resulting slurry was applied to a 2 cm × 3.5 cm fluorine-doped tin oxide (FTO) glass substrate via drop-casting, repeated ten times to ensure uniform coverage. The electrodes were then dried in an oven and annealed at 150 °C for 1 h under a N2 gas flow. The resulting electrodes were planar in nature. Transient photocurrent responses were measured using a CHI660E electrochemical workstation (CHI Shanghai, Inc.) in a standard three-electrode configuration. The fabricated electrode served as the working electrode, while a Pt wire and Ag/AgCl (saturated KCl) electrode were used as the counter and reference electrodes, respectively. A quartz cell with dimensions of 7 cm × 7 cm × 7 cm was used for the measurements. Visible light irradiation (λ ≥ 420 nm) was provided by a 300 W Xe arc lamp equipped with a sharp cut-off filter. The initial applied voltage was 0 V, and the data acquisition interval was set to 0.1 s. Electrochemical impedance spectroscopy (EIS) measurements were conducted using an IM6e impedance analyzer over a frequency range from 0.01 Hz to 105 Hz under dark conditions. A 300 W Xe arc lamp, in conjunction with a cut-off filter (λ > 420 nm), was used as the light source. The electrolyte consisted of a 0.1 M Na2SO4 aqueous solution. Mott-Schottky plots were generated in the same 0.1 M Na2SO4 electrolyte, with an AC potential frequency of 1000 Hz. The electrolyte solution was prepared by dissolving 1.48 g of Na2SO4 in deionized water and adjusting the final volume to 100 mL, resulting in a solution with a pH value of 6.68 ± 0.04. The electrolyte was freshly prepared prior to each set of measurements.
Fluorescence spectrum Steady-state PL
An Edinburgh Instruments FLS980 spectrometer was used to measure photoluminescence (PL) spectra at varying temperatures, as well as the phosphorescence spectrum. The exciton binding energy was subsequently determined according to the equation::
AFM-SKPM and KFPM analysis
Kelvin probe force microscopy (AFM-KPFM and AFM-SKPM) was employed on a Bruker Dimension Icon atomic force microscope to measure the surface potential distribution of the samples.
Zeta potential analysis
Zeta potential measurements were conducted using a Malvern Zetasizer Nano ZS ZEN2600.
Transient absorption spectroscopy analysis
A Helios pump-probe system (Ultrafast Systems LLC), driven by a femtosecond laser (Coherent, 35 fs, 1 kHz, 800 nm), was used for transient absorption spectroscopy. White-light continuum probe pulses (430–603 nm) were generated via focusing a fraction (~10 μJ) of the 800 nm fundamental beam onto a 1 mm CaF2 crystal. The 365 nm pump pulses were derived from an optical parametric amplifier (TOPAS-800-fs).
Calculations details and discussions
The B3LYP functional with the 6-311G* basis set, as implemented in Gaussian 09, was used to optimize molecular geometries and characterize electronic structure. Excitation energies and oscillator strengths of the transient species were calculated using TD-DFT at the TD-B3LYP/6-311G* level. Implicit solvation models and dispersion corrections were incorporated into the calculations. Multiwfn (version 3.8) was used for electronic orbital analysis, electrostatic potential calculations, and hole-electron analysis, while VMD (version 1.9.3) was used for visualizing the orbitals. The initial COF structural model was constructed within Materials Studio (Materials Visualizer) using the P222 space group (sql topology, FOOCOF) obtained from the Reticular Chemistry Structure Resource (RCSR). Subsequently, the structure was energy-minimized using the universal force field within the Forcite module. Experimental PXRD patterns were subjected to Pawley refinement using the Reflex module. Integrated intensities were obtained using pseudo-Voigt profile fitting. The refined parameters included unit cell parameters (a, b, c), FWHM parameters (U, V, W), profile parameters (NA, NB), and the zero-point error. A 20th-order polynomial function was used to model the background. Finally, PXRD patterns were simulated from the optimized structure using the Reflex module.
Periodic DFT calculations were carried out using the CP2K software package. The exchange-correlation energy was described using the PBE functional, with the inclusion of Grimme’s DFT-D3 dispersion correction incorporating the BJ-damping scheme. A MOLOPT basis set, supplemented with appropriate diffuse functions, was employed within the framework of the Gaussian and plane waves method. The DZVP-MOLOPT-GGA-GTH basis set was used for geometry optimization, energy determination, vibrational mode analysis, and time-dependent density functional theory calculations. The energy cutoff for the plane-wave basis was set to 600 eV, and convergence was ensured to within 10-6 Hartree. The optimized geometry, corresponding to the lowest energy structure, was then used for subsequent calculations. Thermochemical parameters were corrected using the rigid-rotor harmonic oscillator (RRHO) approximation within the Shermo program at standard conditions (1.01325 bar and 298.15 K). The chemical potential of the proton-electron pair (H+/e−) was equated to one-half the gas-phase H2 chemical potential at standard hydrogen electrode (SHE) conditions, following the computational hydrogen electrode (CHE) approach. The free energy change for each elementary step was calculated using the following equation:
For HER, the reaction path is as follows:
Data availability
All data are available in the main text or the supplementary information. Source data are provided with this paper.
References
Wang, H. et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 49, 4135–4165 (2020).
Guan, Q. et al. Metalated covalent organic frameworks: from synthetic strategies to diverse applications. Chem. Soc. Rev. 51, 6307 (2022).
Liang, Z. et al. Covalent organic frameworks: fundamentals, mechanisms, modification, and applications in photocatalysis. Chem. Cata. 2, 2157–2228 (2022).
Lu, M. et al.Rational design of crystalline covalent organic frameworks for efficient CO2 photoreduction with H2O. Angew. Chem. Int. Edit. 58, 12392–12397 (2019).
Huang, N. et al. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Diercks, C. S. et al. The atom, the molecule, and the covalent organic framework. Science 355, 1585 (2017).
Chen, Z. et al. Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance. Nat. Commun. 14, 1106 (2023).
Zhao, C. et al. Recent advances in conjugated polymers for visible-light-driven water splitting. Adv. Mater. 32, 1907746 (2020).
Yang, J. et al. Protonated imine-linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 60, 19797–19803 (2021).
Ma, S. et al. Photocatalytic hydrogen production on a sp2-carbon-linked covalent organic framework. Angew. Chem. Int. Edit. 61, e202208919 (2022).
Wang, X. et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 10, 1180–1189 (2018).
Shen, R. C. et al. Efficient photocatalytic hydrogen evolution by modulating excitonic effects in ni-intercalated covalent organic frameworks. Adv. Energy Mater. 13, 2203695 (2023).
Lu, M. et al. Confining and highly dispersing single polyoxometalate clusters in covalent organic frameworks by covalent linkages for CO2 photoreduction. J. Am. Chem. Soc. 144, 1861–1871 (2022).
Zhang, M. et al. Semiconductor/covalent-organic-framework Z-scheme heterojunctions for artificial photosynthesis. Angew. Chem. Int. Edit. 59, 6500–6506 (2020).
Li, Y. et al. In situ photodeposition of platinum clusters on a covalent organic framework for photocatalytic hydrogen production. Nat. Commun. 13, 1355 (2022).
Wang, S. et al. Cobaloxime-integrated covalent organic frameworks for photocatalytic hydrogen evolution coupled with alcohol oxidation. Angew. Chem. Int. Edit. 62, e202311082 (2023).
Wang, Y. et al. Unraveling the photo-induced dynamic behavior of COF-based Z-scheme heterostructure monolithic aerogels. Matter 7, 3145–3162 (2024).
Weng, W. et al. Chiral covalent organic framework films with enhanced photoelectrical performances. J. Am. Chem. Soc. 146, 13201–13209 (2024).
Mukherjee, S. et al. Scalable integration of coplanar heterojunction monolithic devices on two-dimensional In2Se3. ACS Nano 14, 17543–17553 (2020).
Zhong, H. et al. Fused dithienogermolodithiophene low band gap polymers for high-performance organic solar cells without processing additives. J. Am. Chem. Soc. 135, 2040–2043 (2013).
Lan, Z. A. et al. Reducing the exciton binding energy of donor-acceptor-based conjugated polymers to promote charge-induced reactions. Angew. Chem. Int. Edit. 58, 10236–10240 (2019).
Lan, Z.-A. et al. A fully coplanar donor-acceptor polymeric semiconductor with promoted charge separation kinetics for photochemistry. Angew. Chem. Int. Edit. 60, 16355–16359 (2021).
Xu, K. et al. Ground-state electron transfer in all-polymer donor-acceptor heterojunctions. Nat. Mater. 19, 738 (2020).
Park, S. et al. Temperature-dependent electronic ground-state charge transfer in van der waals heterostructures. Adv. Mater. 33, 2008677 (2021).
Sekita, M. et al. Intense ground-state charge-transfer interactions in low-bandgap, panchromatic phthalocyanine-tetracyanobuta-1,3-diene conjugates. Angew. Chem. Int. Edit. 55, 5560–5564 (2016).
Deibel, C. et al. Role of the charge transfer state in organic donor-acceptor solar cells. Adv. Mater. 22, 4097–4111 (2010).
Akaike, K. et al. Impact of ground-state charge transfer and polarization energy change on energy band offsets at donor/acceptor interface in organic photovoltaics. Adv. Funct. Mater. 20, 715–721 (2010).
Hao, L. et al. Fluorenone-based covalent organic frameworks with efficient exciton dissociation and well-defined active center for remarkable photocatalytic hydrogen evolution. Appl. Catal. B Environ. Energy. 330, 122581 (2023).
Yang, Y. et al. Engineering β-ketoamine covalent organic frameworks for photocatalytic overall water splitting. Nat. Commun. 14, 593 (2023).
Weng, W. et al. The effect of enantioselective chiral covalent organic frameworks and cysteine sacrificial donors on photocatalytic hydrogen evolution. Nat. Commun. 13, 5768 (2022).
Bai, J. X. et al. Topology-induced local electric polarization in 2D thiophene-based covalent organic frameworks for boosting photocatalytic H2 evolution. Chinese J. Catal. 59, 225–236 (2024).
Dai, L. et al. Enhancement of visible-light-driven hydrogen evolution activity of 2D π-conjugated bipyridine-based covalent organic frameworks via post-protonation. Angew. Chem. Int. Edit. 62, e202300224 (2023).
Dong, W. B. et al. Isomeric oligo(phenylenevinylene)-based covalent organic frameworks with different orientation of imine bonds and distinct photocatalytic activities. Angew. Chem. Int. Edit. 62, e202216073 (2023).
Li, Z. P. et al. Three-component donor-π-acceptor covalent-organic frameworks for boosting photocatalytic hydrogen evolution. J. Am. Chem. Soc. 145, 8364–8374 (2023).
Lin, Z. et al. Boosting photocatalytic hydrogen evolution enabled by SiO2-supporting chiral covalent organic frameworks with parallel stacking sequence. Chinese. J. Catal. 64, 87–97 (2024).
Liu, T. et al. Ground-state electron transfer in all-polymer donor:acceptor blends enables aqueous processing of water-insoluble conjugated polymers. Nat. Commun. 14, 8454 (2023).
Kar, S. et al. Unveiling the role of a ground state charge transfer complex in carbon nanoparticles for highly efficient metal-free solar hydrogen production. J. Mater. Chem. A 12, 4712–4726 (2024).
Liao, Q. et al. Tailoring and modifying an organic electron acceptor toward the cathode interlayer for highly efficient organic solar cells. Adv. Mater. 32, 1906557 (2020).
Bao, Q. Y. et al. Trap-assisted recombination via integer charge transfer states in organic bulk heterojunction photovoltaics. Adv. Funct. Mater. 24, 6309–6316 (2014).
Guo, Y. et al. Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution. Nat. Commun. 13, 2067 (2022).
Yang, J. et al. A full-spectrum porphyrin-fullerene D-A supramolecular photocatalyst with giant built-in electric field for efficient hydrogen production. Adv. Mater. 33, 2101026 (2021).
Zhang, Z. J. et al. A highly crystalline perylene imide polymer with the robust built-in electric field for efficient photocatalytic water oxidation. Adv. Mater. 32, 1907746 (2020).
Li, J. et al. Giant enhancement of internal electric field boosting bulk charge separation for photocatalysis. Adv. Mater. 28, 4059–4064 (2016).
Aarnio, H. et al. Spontaneous charge transfer and dipole formation at the interface between P3HT and PCBM. Adv. Energy. Mater. 1, 792–797 (2011).
Bie, C. et al. A bifunctional CdS/MoO2/MoS2 catalyst enhances photocatalytic H2 evolution and pyruvic acid synthesis. Angew. Chem. Int. Edit. 61, e202212045 (2022).
Yu, J. et al. Excited-state electronic properties in zr-based metal-organic frameworks as a function of a topological network. J. Am. Chem. Soc. 140, 10488–10496 (2018).
Qan, Y. et al. Photocatalytic molecular oxygen activation by regulating excitonic effects in covalent organic frameworks. J. Am. Chem. Soc. 142, 20763–20771 (2020).
Wei, Z. et al. Steering electron-hole migration pathways using oxygen vacancies in tungsten oxides to enhance their photocatalytic oxygen evolution performance. Angew. Chem. Int. Edit. 60, 8236–8242 (2021).
Barman, S. et al. Metal-free catalysis: a redox-active donor-acceptor conjugated microporous polymer for selective visible-light-driven CO2 reduction to CH4. J. Am. Chem. Soc. 143, 16284–16292 (2021).
Haldar, R. et al. Charge transfer in metal-organic frameworks. Chem. Commun. 59, 1569–1588 (2023).
Liu, D. et al. Rational design of PDI-based linear conjugated polymers for highly effective and long-term photocatalytic oxygen evolution. Adv. Mater. 35, 2300655 (2023).
Shen, R. C. et al. Realizing photocatalytic overall water splitting by modulating the thickness-induced reaction energy barrier of fluorenone-based covalent organic frameworks. Adv. Mater. 35, 2305397 (2023).
Acknowledgements
The authors thank National Natural Science Foundation of China (22378148, 52472110, 22308113) and Natural Science Foundation of Guangdong Province (2024A1515012433) for their support.
Author information
Authors and Affiliations
Contributions
R.C.S., C.H., and L.H. contributed equally to this work. R.C.S. and C.H., conceived and designed the experiments. R.C.S., L.H., and X.L. designed, synthesized, and characterized the materials. C.H. performed the theoretical calculations. G.J.L. performed the TAS analysis. R.C.S. and L.H. performed the TEM measurements. P.Z. helped with the manuscript discussion. Q.Y. revised the manuscript Data. were interpreted by all authors and the manuscript was prepared by R.C.S. and X.L. All authors contributed to this work, read the manuscript, and all data are reported in the main text and supplemental materials.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yongfa Zhu and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, R., Huang, C., Hao, L. et al. Ground-state charge transfer in single-molecule junctions covalent organic frameworks for boosting photocatalytic hydrogen evolution. Nat Commun 16, 2457 (2025). https://doi.org/10.1038/s41467-025-57662-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57662-4
This article is cited by
-
Enhanced Photocatalytic Antibiotic Degradation Through BiOBr/TiO2 Heterojunction Engineering: Synergistic Charge Separation and Band Alignment Effects
Chemical Research in Chinese Universities (2025)
-
Topological covalent organic frameworks for sustainable photocatalysis
Science China Materials (2025)
-
2D sp2 carbon-conjugated covalent organic frameworks: photocatalytic platforms for solar energy conversion
Rare Metals (2025)
-
Bi and Ag Nanoclusters Dual Plasmonic-cocatalysts Decorating Bi4Ti3O12 Perovskite for Efficient Photocatalysis
Chemical Research in Chinese Universities (2025)
-
Epitaxial Vertical Growth of Carbon Nitride-based Homojunction Composites for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride
Chemical Research in Chinese Universities (2025)