Abstract
Due to the analogous physicochemical properties and weak coordination ability of alkali and alkaline earth metals, accurate separation of radioactive 90Sr from groundwater or seawater still presents a big challenge in environmental radioactivity remediation. Here we mimic the complexation behavior of molecular crown-ether carboxylic acids to construct an elegant negatively charged supramolecular trap in an anionic crown ether-based metal-organic framework (ZJU-X99) for precisely catching Sr2+. Owing to the synergistic effects of electrostatic interactions arising from the In(COO)4- nodes and supramolecular host-guest recognition from the 18-crown-6 rings, ZJU-X99 exhibits rapid adsorption kinetics (1 min), high adsorption capacity (263 mg/g), and exceptional selectivity for Sr2+ even when 1000-fold of Na⁺, K⁺ and Cs⁺ coexist. Relative to alkali metals, Sr2+ ions are intricately ensconced within the supramolecular trap, resulting in lowest binding energy and minimal structural alterations. Dynamic column experiments and radioactive 90Sr decontamination trials further validate its practical application prospects. Our findings offer valuable insights into the design of supramolecular frameworks featuring tailored binding sites for targeted ions.
Similar content being viewed by others
Introduction
The separation of alkali and alkaline earth metals is of utmost importance in various fields such as energy storage1, water purification2, spent fuel reprocessing3, and medical isotope preparation4. For instance, 90Sr generated from the nuclear waste serves as a β-radiation emitter releasing high-energy β particles. The release of 90Sr into the environment from the nuclear accidents has caused certain contamination of groundwater, rivers, and seawater, attracting widespread concern5. Upon entering the human body, it readily associates with bones, potential causing bone cancer6. Conversely, 90Y, primarily derived from the β decay of 90Sr, holds vast prospects in nuclear medicine7. Given the adjacency of alkali and alkaline earth metals in the periodic table and their closely resembling physicochemical properties, coupled with their weak coordination ability and high hydration energy, the attainment of selective separation poses a significant challenge8.
Over the past few decades, various techniques have been emerged for the efficient separation of Sr2+ ions, including ion exchange9, liquid-liquid extraction10, precipitation11, and membrane separation12, but these methods suffer from several drawbacks such as secondary waste generation, high energy consumption, complex operations, and poor selectivity13. In contrast, adsorption stands out due to its energy efficiency, low cost, simplicity, and recyclability, being regarded as a green technology14. A multitude of adsorbent materials have been explored for the separation of alkali and alkaline earth metals, encompassing metal sulfides15, zeolites16, zirconium phosphate17, metal-organic frameworks (MOFs)18, crystalline silicotitanates (CST)19, and hexacyanoferrates20. Nevertheless, most of them lacked specific binding sites tailored for Sr2+, resulting in diminished adsorption performance particularly in the presence of high concentrations of competing ions. Addressing these challenges necessitates the development of selective recognition sites and the enhancement of strong binding affinity for Sr2+.
The strategic construction of selective recognition sites via host-guest chemistry holds significant promise for enhancing ion separation. Crown ethers, as macrocyclic molecules with selective complexation properties, have found widespread application in ion recognition21, providing opportunities for nuclear waste treatment and medical isotopes separation22. To augment ion selectivity, the integration of crown ethers into solid-state materials has emerged as a promising strategy. Traditional methods of incorporating these crown ethers, such as direct physical adsorption or chemical grafting, often resulted in molecular aggregation and pore blockage within the adsorbents23. This, in turn, could impede adsorption kinetics and reduce overall capacity. An approach involving embedding crown ethers within the structured frameworks of MOFs provides an effective solution to this issue24. Different crown ether units could be modularly introduced as host-guest functional groups into MOFs and single-crystal analysis has revealed that the cavity of 18-crown-6 and Sr2+ was well matched25. Despite this, the competitive ion environment and the inherently weak binding affinity of crown ethers necessitate further optimization to improve both selectivity and capacity. Recent studies suggested that macrocyclic chelating agents with negatively charged carboxylic groups could significantly enhance the binding efficiency for heavier alkaline earth metals26. Given that the atomic radius of Sr2+ closely resembles those of Na+ and K+, but with a notably higher charge density, endowing supramolecular crown ether functional groups together with a negatively charged skeleton within a solid matrix is hypothesized to strengthen the interactions between Sr2+ ions and the adsorbent’s framework (Fig. 1a).
a Structure design of ideal Sr2+ adsorbent. b Anionic In(COO)4− node and ligand used in ZJU-X99 (TBADB-18Cr6 = 4,4’,5,5’-terabenzoic acid dibenzo-18-crown-6). c View of the three-dimensional structures of ZJU-X99. d Negatively-charged supramolecular trap of ZJU-X99. e Supramolecular trap presented by electrostatic potential surface region which less than −0.17 eV (blue). f The size of the pore 1 and pore 2 in ZJU-X99. g The solvent-accessible void volume of ZJU-X99. Atom colors: In, orange; O, red; C, light gray; H, white.
Inspired by this, we proposed a synthesis strategy to construct a negatively charged supramolecular trap in an anionic indium-based MOF containing crown ether units (ZJU-X99), which was assembled by negatively charged In(COO)4− nodes and 4,4’,5,5’-terabenzoic acid dibenzo-18-crown-6 (18Cr6) linkers. The unique combination of suitably sized crown ether units and the anionic framework rendered ZJU-X99 exceptionally proficient at selectively adsorbing Sr2+ ions, achieving remarkably high adsorption capacities. This study represents the synergistic effects of electrostatic interactions and host-guest recognition mechanisms within crown ether-based anionic frameworks targeting alkali and alkaline earth metals.
Results
Synthesis and crystal structure
Colorless transparent hexagonal flake-shaped (Fig. 2a) crystals of ZJU-X99 ([NH2Me2][In(TBADB-18Cr6)]·H2O, TBADB-18Cr6 = 4,4’,5,5’-terabenzoic acid dibenzo-18-crown-6) were obtained by heating a mixture of 0.071 mmol (21.4 mg) In(NO3)3·xH2O and 0.071 mmol (60 mg) TBADB-18Cr6 in a 6 mL solution of N,N-dimethylformamide (DMF), acetonitrile, and acetic acid (volume ratio = 2 mL/2 mL/2 mL) at 120 °C for 72 h (85.4% yield based on In). Single-crystal X-ray diffraction analysis revealed that ZJU-X99 crystallized in the monoclinic space group C2/c, exhibiting an open 2-fold interpenetrated 3D anionic framework structure. Its asymmetric unit consists of one In3+ ion, one TBADB-18Cr6 molecule, one water molecule, and one [NH2Me2]+ (Supplementary Fig. 1). Each In3+ ion is coordinated by eight oxygen atoms from four different TBADB-18Cr6 ligands in a bidentate manner, forming In(COO)4− polyhedral clusters (Fig. 1b). Every TBADB-18Cr6 ligands serve as 4-connected nodes and coordinate with four In3+ to construct a single-layer network with a pore size of 7.8 Å × 11.3 Å (Supplementary Fig. 2). Two single-layer network duplexes 2-fold interpenetrated architecture, resulting in a 3D anionic framework (Fig. 1c). [NH2Me2]+ cations are located within the channel as charge balancing species (Supplementary Fig. 3). Remarkably, the close proximity (3.0 Å and 3.3 Å) between the O2 and O14 atoms on the In(COO)4- clusters and the crown ether plane suggests that the negatively charged supramolecular trap formed by the In(COO)4- clusters and 18Cr6 ring can be utilized to selectively capture cations with specific sizes (Supplementary Fig. 4 and Fig. 1d). Electrostatic potential surface (ESP) mappings further highlighted the presence of a negatively charged supramolecular trap wrapped between In(COO)4− and TBADB-18Cr6, exhibiting the most negative electrostatic potential distribution (< -0.17 eV) (Fig. 1e). Regarding to previous supramolecular crown-ether MOFs, the neutral clusters and crown ether units are too distant to cooperatively construct such unique supramolecular trap. In addition, there are two types of channels along the b-axis, with dimensions of 6.4 Å × 8.3 Å and 4.6 Å × 7.3 Å, facilitating rapid ion exchange (Fig. 1f). Topological analysis indicated that In atom and TBADB-18Cr6 unit act as 4-connected nodes respectively, resulting in a binodal 4,4-connected framework with a topology of pts (Supplementary Fig. 5). The solvent accessible volume is 16.8% calculated by PLATON (Fig. 1g).
a Optical image of ZJU-X99. b Powder X-ray diffraction patterns of ZJU-X99 after soaked in the pH range from 0 to 11 and β radiation. c CO2 adsorption/desorption isotherms and pore-size distribution profiles (Inset) for ZJU-X99. d Adsorption kinetics curves of ZJU-X99 toward Sr2+ (C0 = 10 mg/L). e Adsorption isotherm curves of Sr2+ by ZJU-X99 compared with ZJU-X100. f Effect of Na+, K+, and Cs+ on the adsorption of Sr2+ by ZJU-X99 (C0 = 10 mg/L). g A comparison of Sr2+ uptake capacity with reported adsorbents. h Dynamic adsorption and elution curves of ZJU-X99 (C0 = 8–12 mg/L).
PXRD patterns of the synthesized material matched the simulated pattern well (Fig. 2b), confirming the phase purity of the sample. The permanent porosity of ZJU-X99 was confirmed through CO2 adsorption/desorption isotherms, with a surface area of 293 m2/g (Fig. 2c). TG and variable-temperature PXRD analysis revealed that ZJU-X99 remained stable below 300 °C, indicating its high thermal stability (Supplementary Fig. 7a, b). To assess the stability of the material under practical application conditions, ZJU-X99 was soaked in solutions with pH values ranging from 0 to 11 for 12 h or exposed to 200 kGy β and γ radiation. The PXRD patterns, In3⁺ leaching rate, weight retention percentage, FTIR spectra, and CO2 adsorption/desorption isotherms of sample were tested. The PXRD patterns and FT-IR spectra of ZJU-X99 remained nearly unchanged under pH conditions of 1 to 11 and radiation exposure (Fig. 2b and Supplementary Fig. 8). Under these conditions, the low In3⁺ leaching rate, high weight retention percentage and the minimal decline in specific surface area (Supplementary Figs. 9–12) confirm the excellent stability of ZJU-X99 across a broad pH range and under irradiation conditions.
Efficient capture of Sr2+ ions
Adsorption kinetics experiments demonstrated that ZJU-X99 reached adsorption equilibrium within 1 min, exhibiting a remarkable removal efficiency of 99% for Sr2+ (Fig. 2d). As illustrated in Supplementary Table 3, the adsorption rate of ZJU-X99 was significantly faster compared to the majority of other adsorbents. Such rapid adsorption kinetics and high removal efficiency demonstrated the excellent suitability of ZJU-X99 for nuclear emergency accident remediation. This is attributed to the electrostatic interaction and the host-guest recognition of the negatively charged supramolecular trap constructed by In(COO)4- cluster and the 18Cr6 unit, which synergistically enhanced the adsorption of Sr2+. Simultaneously, the 18Cr6 unit served as a one-dimensional channel to facilitate the entry of Sr2+ into the framework. The Langmuir model fitted the adsorption isotherm data of ZJU-X99 for Sr2+ well, yielding a maximum adsorption capacity of 263 mg/g (Fig. 2e and Supplementary Fig. 13), surpassing those of most previously reported crown ether composite materials (DtBuCH18C6/Sipolymer27, DCH18C6 functionalized resin28, Fe3O4@SiO2@DtBuCH18C629, TNTs@DCH18C630), anionic MOFs (SZ-731, SZ-49, SZ-632, FJSM-InMOF18), metal sulfides (ZnSnS-133, NaTS34, KNbS35, KZrTS36), and zeolites37 (Fig. 2g). It is worth noting that the maximum adsorption capacity of ZJU-X99 for Sr2+ exceeded those of crown ether-based neutral MOFs (SNU-20038, MOF-18Cr624, and ZJU-X10025), attributable to the substitution of neutral metal nodes with anionic In(COO)4−, thereby increasing negative charge density of the framework. ZJU-X99 exhibited excellent Sr2+ removal performance within the pH range of 3–11, with a removal efficiency exceeding 99% (Supplementary Fig. 14). After irradiation with β and γ radiation at 200 kGy, the removal efficiency of Sr2+ could still maintain at above 96% (Supplementary Fig. 15). Even after 5 cycles of adsorption-desorption, the material could still remove over 97% of Sr2+ (Supplementary Fig. 16).
The interference resistance is a crucial consideration in screening adsorbent materials. The selectivity capability of ZJU-X99 in removing Sr2+ was tested in the presence of Na+, K+, Rb+, Cs+, and Mg2+. In the presence of a 10-fold concentration of monovalent cations, the removal efficiency of Sr2+ remained above 99%. When Mg2+ was present at a 10-fold concentration, the removal efficiency of Sr2+ was maintained at over 93% (Supplementary Fig. 17). Considering the excess competing ions such as Na+ and K+ present in real environments, the removal efficiency of Sr2+ was investigated as a function of the concentration of competing ions. Surprisingly, when the concentrations of Na+, K+, and Cs+ were increased to 1000-fold, ZJU-X99 was still able to remove 99% of Sr2+ (Fig. 2f). Even in the presence of complex competing ions including 1000-fold Na+, 1000-fold K+, 1000-fold Cs+, 1-fold Mg2+ and 1-fold Ca2+, more than 99% Sr2+ was captured by the adsorbent (Supplementary Fig. 18). Compared to the reported layered metal sulfides39, anionic metal-organic frameworks9,18,40, and anionic inorganic adsorbents41,42, ZJU-X99 maintains excellent adsorption percentage for Sr2+ in the presence of higher concentrations of competing ions (Supplementary Table 6). It is reported that macrocyclic chelating agents with large negative charges are advantageous for binding heavy alkaline earth metals26. Consistent with this finding, the synergism of the electrostatic interactions from the negatively charged In(COO)4- nodes and supramolecular host-guest interaction from 18Cr6 groups effectively enhanced the high selectivity of Sr2+.
To assess the material’s practical application potential, ZJU-X99 was utilized as the stationary phase and packed into an adsorption column with an inner diameter of 5.7 mm for dynamic adsorption experiments (Supplementary Fig. 20). Sr2+ solution was passed through the adsorption column via a peristaltic pump at a flow rate of 0.3 mL/min. Effluent was collected by an automatic collection for analysis of residual Sr2+ concentration. After the adsorbent was fully saturated, Sr2⁺ was desorbed using a 0.1 M HCl solution, followed by rinsing the column with deionized water for the next adsorption process. As shown in Fig. 2h, upon reaching the breakthrough point (Ct/Co = 5%), the treatment volume was approximately 285 mL (2850 bed volume), indicating that only 0.1 mL (0.1 g) of ZJU-X99 could process 285 mL of Sr2+ solution. This substantial reduction in waste volume underscores its high removal efficiency, primarily attributed to the rapid adsorption kinetics and high adsorption capacity of ZJU-X99 towards Sr2+. The treatment volume of ZJU-X99 remained above 2490 bed volumes throughout the three cycles, demonstrating the material’s stability and effectiveness in removing Sr2⁺. In the dynamic breakthrough experiment with 10-fold excess Na+, K+, and Cs+, the treatment volume of ZJU-X99 for Sr2+ remained at 2580 mL (Supplementary Fig. 21), indicating the material’s selectivity for Sr2⁺. In addition, various solid-to-liquid ratios of ZJU-X99 were employed to examine the application potential for the removal of real radioactive 90Sr, under the condition of a solid-to-liquid ratio of 10:1, ZJU-X99 demonstrated the capability to remove over 95% of 90Sr (Supplementary Fig. 22), indicating its promising practical application prospects for radioactive waste management.
Revealing the adsorption process
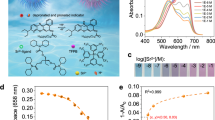
To investigate the adsorption mechanism of Sr2+ in ZJU-X99, scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDS), powder X-ray diffraction (PXRD), and X-ray photoelectron spectroscopy (XPS) were used to analyze ZJU-X99 before and after adsorption of Sr2+. As shown in Supplementary Fig. 23, the SEM-EDS results confirmed the uniform distribution of Sr within the crystal. The PXRD peak of ZJU-99 retained after the adsorption of Sr2+ (Supplementary Fig. 24), indicating excellent chemical stability of ZJU-X99 attributed to the high chemical bond energy of In-O and the interpenetrated 3D structure. Thermogravimetric (TG) test results showed that Sr2+⊂ZJU-X99 maintained a high decomposition temperature (300 °C), which meant that the adsorption of Sr2+ did not affect the thermal stability of the material (Supplementary Fig. 25). In the XPS spectrum of ZJU-X99 after Sr2+ adsorption, characteristic peaks of Sr 3p and Sr 3d (135.5 eV and 133.8 eV correspond to Sr 3d3/2 and Sr 3d5/2 peaks, respectively, Supplementary Fig. 26 and Fig. 3a) newly appeared, demonstrating the successful incorporation of Sr2+ into the ZJU-X99 structure24. The significant reduction of the N 1s peak at 399.2 eV (Supplementary Fig. 26) in Sr2+⊂ZJU-X99 confirmed the ion exchange reaction between [NH2Me2]+ and Sr2+ ions40. In the high-resolution O 1s XPS spectrum after Sr2+ adsorption, a 0.3 eV shift of the C-O peak was observed and a new O-Sr peak appeared at 532.1 eV for ZJU-X99 (Fig. 3b). This suggests the existence of interactions between Sr2+ and the 18Cr6 moiety24. In comparison to ZJU-X99, the high-resolution C 1s XPS spectrum of Sr2+⊂ZJU-X99 showed a 0.1 eV shift of the C-O peak (Supplementary Fig. 27), further confirming the binding of Sr2+ with the 18Cr6 unit.
a High-resolution Sr 3d spectrum of Sr2+⊂ZJU-X99. b High-resolution O 1s spectra of ZJU-X99 before and after Sr2+ adsorption. c Solid-state 1H NMR spectra of ZJU-X99 before and after Sr2+ adsorption. d Solid-state 13C NMR spectra of ZJU-X99 before and after Sr2+ adsorption. e Sr K-edge XANES spectra for Sr2+⊂ZJU-X99 and SrO. f EXAFS analysis of the k-space spectrum of Sr2+⊂ZJU-X99. g Fourier transforms of K-edge EXAFS spectra of Sr2+⊂ZJU-X99. h WT contour plots for Sr2+⊂ZJU-X99. i WT contour plots for SrO.
Solid-state 1H and 13C NMR were employed to investigate the adsorption of Sr2+ onto ZJU-X99 before and after adsorption. In the solid-state 1H NMR spectra (Fig. 3c), the peak at 2.7 ppm was attributed to the hydrogen atoms on the crown ether43. Upon Sr2+ adsorption, the 1H NMR peak of 18Cr6 moiety shifted and broadened, indicative of a coordination interaction between Sr2+ and the 18Cr6 unit, which restricted the motion of the crown ether unit43,44. The chemical shift at 7.0 ppm corresponded to the hydrogen atoms on the phenyl group43. After Sr2+ adsorption, the signal of hydrogen atoms on the phenyl ring shifted, possibly due to the exchange out of [NH2Me2]+ that originally interacted with the benzene groups in the pore. In the solid-state 13C NMR spectra (Fig. 3d), the signal at 65–70 ppm was attributed to the carbon atoms of the crown ether unit45. Distinct signals in the range of 150–110 ppm were assigned to the carbon atoms on the phenyl group46. The signal at 35.8 ppm was attributed to the [NH2Me2]+47, which decreased after Sr2+ adsorption, indicating the successful exchange of [NH2Me2]+ by Sr2+. Moreover, compared to ZJU-X99, the carbonyl peak at 177.0 ppm in Sr2+⊂ZJU-X99 changed from a single peak to a double peak, indicating interactions between carbonyl oxygen and Sr2+, further confirming that Sr2+ entered the cavity of 18Cr6 groups and coordinated with the oxygen atoms of In(COO)4− after exchanging with [NH2Me2]+.
XAFS spectroscopy measurements were conducted on Sr2+⊂ZJU-X99 to investigate the coordination numbers and distances of Sr with surrounding atoms. The XANES spectrum showed an absorption edge energy of 16119 eV for Sr2+⊂ZJU-X99 (Fig. 3e), indicating no change in the oxidation state of Sr48. Figure 3f, g showed that the Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) spectra of Sr2+⊂ZJU-X99 in k-space and R-space. The fitted parameters are presented in the Supplementary Table 7. Analysis of the spectra indicated the presence of eight O atoms surrounding the Sr atom. Specifically, six Sr-O bonds exhibited an average distance of 2.8 Å, while the remaining two O exhibited an average distance of 3.1 Å from the central Sr atom. Additionally, there were twelve C atoms around the Sr atom. Among them, eight C atoms had an average distance of 3.6 Å from the central Sr atom, while the remaining four C atoms had an average distance of 3.9 Å from the central Sr atom. These findings were consistent with the data on Sr-O and Sr-C bond lengths obtained from the following single-crystal structure analysis. The Wavelet Transform (WT)-EXAFS of Sr K-edge provided molecular structural information in k-space and R-space, yielding relevant information about the coordination environment of Sr2+. As depicted in the Fig. 3h, i, the maximum signal in the WT spectrum of Sr2+⊂ZJU-X99 was similar to the Sr-O bond in SrO, suggesting the formation of Sr-O bonds in ZJU-X99 upon Sr2+ adsorption.
Single-crystal-to-single-crystal transformation
Some crown ether units exhibit flexibility and render the resulting MOFs unsuitable for single-crystal XRD characterization, leading to a lack of in-depth exploration of atomic-level adsorption mechanisms. ZJU-X99 exhibited outstanding chemical stability and a strong affinity for Sr2+ ions. The single-crystal structure of ZJU-X99 after Sr2+ adsorption could be readily obtained through immersion in saturated Sr2+ solution. Single-crystal-to-single-crystal (SC-SC) transformation occurred in ZJU-X99 after Sr2+ adsorption (Fig. 4a), which revealed the exchange of [NH2Me2]+ in the channels with Sr2+ (Supplementary Figs. 3 and 4a). The asymmetric unit consists of Sr2+⊂ZJU-X99 comprises one In atom, one TBADB-18Cr6 molecule, and one Sr2+ (Supplementary Fig. 28a). It belongs to C2/c space group and after adsorption the crystal lattice parameters were changed from 21.784 Å and 10.078 Å to 21.9165 Å and 9.7073 Å along the a and b-axis, respectively. Sr2+ forms a SrO8 polyhedral structure coordinated with six oxygen atoms from the 18Cr6 ring and two oxygen atoms from the In(COO)4− node, with Sr-O distances ranging from 2.8 Å to 3.1 Å (Fig. 4e, i), which is exactly consistent with the EXAFS analysis. Sr2+ is located 1.2 Å above the plane of oxygen atoms in the 18Cr6 (Supplementary Fig. 28b). The crown ether maintained the shallow boat conformation, with bending angles slightly changing from 24.7° to 28.4° (Supplementary Fig. 29), indicating a highly tailored fit of this negatively charged supramolecular trap in ZJU-X99 for Sr2+.
View of the three-dimensional structures of Sr2+⊂ZJU-X99 (a), Na+⊂ZJU-X99 (b), K+⊂ZJU-X99 (c), Cs+⊂ZJU-X99 (d). Representation of Sr2+ (e), Na+ (f), K+ (g), Cs+ (h) in the negatively charged supramolecular trap. Conformation of 18Cr6 unit in ZJU-X99 after complexation with Sr2+ (i), Na+ (j), K+ (k), Cs+ (l). Atom colors: In, orange; O, red; C, light gray; H, white; Sr, navy blue; Na, sky blue; K, light blue; Cs, powder blue.
To elucidate the high selectivity of ZJU-X99 towards Sr2+, we also obtained single-crystal structures after adsorption of Na+, K+, and Cs+ ions (Fig. 4b–d). Minimal variations in unit cell parameters of ZJU-X99 upon adsorption of metal ions with different sizes (Supplementary Table 8) suggest negligible changes in the overall framework of the crystal. The lengths of M-O bonds (M = Na, K, Cs), as presented in the Fig. 4j, k, l, exhibit close numerical values across different ions. Na+ within ZJU-X99 exhibits a positional disorder and was split into two positions during structure optimization, with the occupancy rates of 0.85 and 0.15 (Fig. 4j and Supplementary Fig. 31). The 18Cr6 unit undergoes distortion for the portion with an occupancy rate of 0.15, transitioning from a shallow boat conformation to a planar structure. A certain distortion of the crown ether rings is also observed for K+⊂ZJU-X99, with one O-C-O bond distorted by 90° (Fig. 4k and Supplementary Fig. 32). Cs+ within ZJU-X99 exhibits a positional disorder, with occupancy rates of 0.25 for two sites (Fig. 4l and Supplementary Fig. 33). The 18Cr6 unit undergoes some distortions, transitioning to a planar structure. The lower occupancy rate and distortion suggest that the specific ion recognition sites of ZJU-X99 are not well-suited for the larger Cs+ ion (167 pm)49. Compared to Sr2+⊂ZJU-X99, all other three ions entering the trap need to overcome corresponding energy barriers for structural distortions and conformational transitions, indicating that the negatively charged supramolecular trap in ZJU-X99 is better suited for Sr2+. This is consistent with the previous results that macrocyclic chelating agents with large negative charges favored binding to heavy alkaline earth metals26.
Density functional theory calculations analysis
The exceptional selectivity of ZJU-X99 for Sr2+ was further identified by periodic density functional theory (DFT) calculations compared to other alkaline earth metal ions (Mg2+, Ca2+), alkali metal ions (Na+, K+, Cs+) and water molecule. The optimized negatively charged supramolecular complex closely resembles their crystal structures, with small root mean square deviations (RMSDs) and bond lengths varying within 0.06 angstroms. The reaction energy ΔE for the hydrate metal ions M(H2O)6+/2+ adsorbed by ZJU-X99 and generate M+/2+⊂ZJU-X99 and water molecules were calculated since the solvent seriously affects the cation recognition of crown ether9,50. As shown in Fig. 5a, the ΔE adheres to a recognition selectivity of M2+ > M+ > H2O. Moreover, Sr2+ cation shows stronger ΔE than Mg2+ and Ca2+ with different ionic radii suggesting its higher selectivity to ZJU-X99. The reaction Gibbs free energy ΔG were calculated to further evaluate the thermodynamic feasibility (Fig. 5b). Sr2+ cation gives the lowest ΔG value of −155.98 kcal/mol which indicates the remarkable thermodynamic stability of Sr2+⊂ZJU-X99 complex and the exceptional selectivity of ZJU-X99 for Sr2+ compared to Mg2+ and Ca2+. For the alkali metal ions, the ΔG adheres to a recognition selectivity of K+ > Cs+ > Na+ which is consistent with the results in literature50 confirming the reliability of theoretical calculations. The calculated charge density difference of M+/2+⊂ZJU-X99 (Supplementary Figs. 34–38) show the charge accumulation from ZJU-X99 to Sr2+ (Fig. 5c) markedly stronger than that of Cs+⊂ZJU-X99 (Fig. 5d). The M2+⊂ZJU-X99 show similar charge density difference (Fig. 5e) while the corresponding ΔG of ZJU-X99 to Sr2+, Ca2+ and Mg2+ are −155.98, −151.55 and −148.75 eV which indicates the higher adsorption ability of ZJU-X99 to Sr2+ than Ca2+ and Mg2+. In the meanwhile, the ZJU-X99 show relatively weaker adsorption to M+ ions (Supplementary Fig. 39) and water molecule according to the less charge transfer between them. Besides, the average Mayer bond orders of Sr-O bonds shorter than 0.20 (Supplementary Table 13) and the overlap between Sr 4d and O 2p orbitals are relatively weak (Supplementary Fig. 40), which is in accordance with the highly ionized characteristics of Sr2+, and further demonstrates the dominance of charge-driven ionic bonds.
a, b The reaction energy ΔE and Gibbs free energy ΔG of ZJU-X99 to metal ions and water molecule. The calculated charge density difference of Sr2+⊂ZJU-X99 (c) and Cs+⊂ZJU-X99 (d). e The extracted charge density difference of M+/2+⊂ZJU-X99 and H2O⊂ZJU-X99 and the corresponding ΔG (isovalue = 0.02, 0.01 and 0.0005 a.u. for M2+, M+ and H2O, respectively).
Discussion
In summary, we discovered a highly efficient and selective adsorbent material, ZJU-X99, with a unique negatively charged supramolecular trap for precisely catching Sr2+ from complex mixtures. ZJU-X99 exhibited exceptional stability even under high doses of irradiation and across a wide pH range. Moreover, it offered several notable advantages in the realm of Sr2+ ions separation, including a maximum adsorption capacity of 263 mg/g, rapid attainment of adsorption equilibrium within 1 min and 99% removal efficiency of Sr2+ from solutions containing 1000 times the concentration of Na+, K+, or Cs+. These advantages were stemmed from the synergism of electrostatic interactions arising from the negatively charged In(COO)4- nodes and supramolecular host-guest recognition from 18Cr6 groups. In addition, ZJU-X99 exhibited satisfactory performance in treating radioactive 90Sr solutions and could significantly reduce the volume of contaminated water when used as a column packing material. In comparison to monovalent alkali metal ions, the entrapment of Sr2+ within ZJU-X99 induced minimal structural distortion and conformational alterations as evidenced by single-crystal structure analysis. Further theoretical calculations corroborated these observations, elucidating the exceptional selectivity of ZJU-X99 towards Sr2+ ions over other alkaline and alkaline earth ions originated from thermodynamically favorable complexation reaction and strong charge-driven electrostatic attractions. This work established a design strategy for supramolecular macrocycle-based MOFs featuring anionic frameworks, providing a comprehensive understanding the factors governing ion affinity and selectivity. These insights offer valuable guidance for the design and optimization of advanced materials tailored for efficient ion separation applications.
Methods
Materials and characterizations
All starting materials were purchased from commercial companies and used without further purification. Powder X-ray diffraction (PXRD) patterns were recorded on a Rigaku Ultima IV diffractometer equipped with Cu Kα radiation from 3° to 50° with a step of 0.02°. Various-temperature PXRD was obtained on a Rigaku SmartLab X-ray diffraction instrument at 30 – 300 °C from 3° to 50° with a step of 0.02°. The porous properties of the samples were measured by CO2 physisorption at 273 K on an automated specific surface area and micropore analyzer (TriStar II Plus). TG analysis was performed in a nitrogen atmosphere with a rate of 10 °C min−1 using a TA Instruments SDT Q600. X-ray photoelectron spectroscopy (XPS) spectra were acquired on a Thermo Scientific ESCALAB 250Xi. The β irradiation was carried out using electron beams generated from an electron accelerator. The γ irradiation was carried out using 60Co γ-ray source apparatus. EDS images were acquired on a Scanning Electron Microscopy with an Energy Dispersive X-ray Spectrometer (SEM–EDS, Hitachi SU8010). The concentrations of Sr2+ in solutions were analyzed by an ICP–MS (Agilent Technologies 7800). The solid-state 1H and 13C NMR spectra were collected with a Bruker AVANCE NEO 600WB spectrometer. EXAFS analyses were performed at the Shanghai Synchrotron Radiation Facility. The single-crystal X-ray diffraction data collection of ZJU-X99, Sr2+⊂ZJU-X99, Na+⊂ZJU-X99, K+⊂ZJU-X99 and Cs+⊂ZJU-X99 were recorded on a Bruker D8-Venture diffractometer with a Turbo X-ray Source (Cu–Kα radiation). The data frames were collected using the program APEX 3 and processed using the program SAINT routine in APEX 3. The crystal structures were refined by the full-matrix least-squares on F2 using the SHELXTL-2018 program51.
Synthesis of ZJU-X99
The ligand of TBADB-18Cr6 (60 mg, 0.071 mmol) and In(NO3)3·xH2O (21.4 mg, 0.071 mmol) were added in 6 mL of DMF/acetonitrile/acetic acid (v/v/v, 2 mL/2 mL/2 mL) solution. The mixture was placed into a Teflon-lined stainless-steel autoclave and heated at 120 °C for 72 h. After cooling to room temperature, the crystals were isolated by filtration, then washed with DMF, EtOH and dried at 50 °C for 12 h (Yield: 85.4% based on In atoms). The crystal data of ZJU-X99 is presented in Supplementary Table 1.
Adsorption experiments
Adsorption Kinetics Experiments. The adsorption kinetics experiments were carried by dispersing ZJU-X99 into Sr2+ solution with a solid-liquid ratio of 1:1, respectively. The mixture was stirred at room temperature. The supernatant solution was collected by syringe in different time intervals from 1 to 120 min, then filtered by 0.22 µm nylon membrane filters. The filtrates of residual Sr2+ were diluted with 5% HNO3 and the concentrations of Sr2+ were determined by ICP–MS. The removal percentages were calculated by the equation as follows:
where C0 (mg/L) is the initial concentration, Ct (mg/L) is the concentration of Sr2+ after stirring at different times.
Adsorption Isotherm Experiments. The sorption isotherm experiments were measured by exposing ZJU-X99 into different concentrations of Sr2+ solution with a solid-liquid ratio of 1:1. The samples were stirred for 12 h at room temperature and filtered by 0.22 µm nylon membrane filters. The concentrations of residual Sr2+ in aqueous solution were determined by ICP–MS. The sorption capacity of ZJU-X99 toward Sr2+ was calculated by the following equation:
Where C0 (mg/L) is the initial concentration, Ce (mg/L) is the equilibrium concentration, m (g) is the mass of sorbent, and V (L) is the volume of the solution.
Langmuir and Freundlich sorption models were used to fit the sorption isotherms. The Langmuir isotherm model is expressed as given below:
where qe (mg/g) is the sorption capacity at equilibrium, qm (mg/g) is the maximum sorption capacity and KL is the Langmuir constant, Ce (mg/L) is the equilibrium concentration of Sr2+. The Freundlich isotherm model is shown as follows:
where KF is a constant about sorption capacity and n is related to the intensity of sorption.
Adsorption Selectivity Experiments. The adsorption selectivity experiments of ZJU-X99 toward Sr2+ were performed by adding ZJU-X99 into 10 ppm of Sr2+ solution containing corresponding proportions of competing anions with a solid-liquid ratio of 1:1, respectively. The solution was stirred for 12 h at room temperature. The samples were filtered and the concentrations of residual Sr2+ were analyzed by ICP–MS, respectively.
Reusability test
ZJU-X99 was firstly immersed in Sr2+ (10 ppm) solution with a solid-liquid ratio of 1:1 for 3 h at room temperature. Then the samples were filtered and the solid was desorbed by 0.1 M HCl solution for the regeneration of ZJU-X99. The regenerated adsorbents were used in subsequent adsorption experiments.
Column experiments
0.1 g of ZJU-X99 was placed in a polyethylene column with an inner diameter of 5.7 mm. The loading height is approximately 4 mm, resulting in a bed volume of 0.1 mL. To prevent solid sample loss, two sieve plate with a pore size of 20 μm was placed at the top and bottom of the column. A solution containing 8–12 mg/L Sr2+ was then introduced into the column at a flow rate of 0.3 mL/min. Solution flow rate and sample collection were regulated by a peristaltic pump and automatic collector. After the material reached full saturation, Sr2⁺ was desorbed using a 0.1 M HCl solution, followed by rinsing the column with deionized water to prepare it for the subsequent adsorption cycle.
90Sr decontamination test
Caution! 90Sr is a high-energy β radiation emitter that poses significant health risks and all experiments involving 90Sr are conducted exclusively in authorized laboratories designed for radiological research. Strict adherence to established safety precautions and protocols for handling radioactive substances is mandatory. The 90Sr decontamination test was conducted by adding ZJU-X99 to 90Sr solutions (3704 cpm) with different solid-liquid ratios of 1:1, 10:1, 25:1, 50:1 and 100:1. After shaking for 10 min, the supernatant was filtered and the activity of 90Sr was measured by a liquid scintillation counting (LSC, PerkinElmer Tri-carb 4910TR) technique.
Density functional theory calculations
The periodic density functional theory (DFT) calculations were performed with PBE52 exchange-correlation functional within the generalized gradient approximation (GGA) as implemented in the CP2K 9.153,54 software package. The norm-conserving55 GTH pseudopotentials were performed for core electrons while the polarizable double-zeta quality basis sets were performed for valence electrons with 4, 5, 6, 9, 10, 13 valence electrons for C, N, O, alkali metal ions, alkaline earth metal and In, respectively. Considered the existence of Na+, the long-range electrostatics terms were calculated with a plane-wave basis set with a 1000 Ry cutoff and the Grimme’s DFT-D3 dispersion corrections were used with Becke-Johnson damping function56,57. Because of the large unit cell size, only the Gamma point was considered. The global geometry optimization was performed while for the frequency calculations, the vibration of metal ions and [In(COO)4-TBADB-18Cr6] were calculated and other atoms were constrained. For the cluster model, the structures of ZJU-X99 containing TBADB-18Cr6, In(COO)4- polyhedral clusters, and coordinated metal were extracted from their respective crystal structures. The geometry optimization was performed at the B3LYP level58,59,60 using the Gaussian16 package61. The heavy atoms of the aromatic ring were positionally constrained. The C, H, and O atoms were described using the 6–31 g(d, p) basis set62, while metal ions were described using the Stuttgart small-core effective core potential and their corresponding SEG basis set63,64.
Data availability
Data supporting the findings of this study are available from the manuscript and its Supplementary Information. Source Data are provided with this manuscript. All data are available from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2299624 (ZJU-X99), 2314304 (Sr2+⊂ZJU-X99), 2344894 (Na+⊂ZJU-X99), 2344895 (K+⊂ZJU-X99) and 2344896 (Cs+⊂ZJU-X99). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Chen, X. et al. Spatially separated crystallization for selective lithium extraction from saline water. Nat. Water 1, 808–817 (2023).
Yao, Y. et al. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat. Sustain. 4, 138–146 (2021).
Tang, J. H. et al. Highly selective cesium(I) capture under acidic conditions by a layered sulfide. Nat. Commun. 13, 658 (2022).
Zhang, S. et al. Efficient separation of strontium in different environments with novel acid-resistant silica-based ion exchanger. Sep. Purif. Technol. 322, 124347 (2023).
Burns, P. C., Ewing, R. C. & Navrotsky, A. Nuclear fuel in a reactor accident. Science 335, 1184–1188 (2012).
Feng, L. et al. Ultrasensitive and highly selective detection of strontium ions. Nat. Sustain. 6, 789–796 (2023).
Kawamura, T., Ito, T. & Kim, S. Y. Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J. Radioanal. Nucl. Chem. 320, 9–14 (2019).
Wang, Y., Zhang, W., Zeng, X., Deng, T. & Wang, J. Membranes for separation of alkali/alkaline earth metal ions: A review. Sep. Purif. Technol. 278, 119640 (2021).
Zhang, J. et al. Distinctive two-step intercalation of Sr2+ into a coordination polymer with record high 90Sr uptake capabilities. Chem 5, 977–994 (2019).
Haupt, S. et al. Extraction properties of 25, 27-bis(carbonylmethoxy) calix[4]arenes towards Sr2+: competitive extraction and extraction in a synthetic groundwater. J. Radioanal. Nucl. Chem. 300, 779–786 (2014).
Tokunaga, K., Kozai, N. & Takahashi, Y. A new technique for removing strontium from seawater by coprecipitation with barite. J. Hazard. Mater. 359, 307–315 (2018).
Wei, Y. et al. Radioactive strontium ions sieving through reduced graphene oxide membrane. J. Membr. Sci. 689, 122181 (2024).
Zhang, X., Gu, P. & Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 215, 543–553 (2019).
Asim, U. et al. Morphology controlled facile synthesis of MnO2 adsorbents for rapid strontium removal. J. Ind. Eng. Chem. 98, 375–382 (2021).
Li, W., Peng, Y., Ma, W., Huang, X. & Feng, M. Rapid and selective removal of Cs+ and Sr2+ ions by two zeolite-type sulfides via ion exchange method. Chem. Eng. J. 442, 136377 (2022).
Faghihian, H., Moayed, M., Firooz, A. & Iravani, M. Synthesis of a novel magnetic zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Colloid Interface Sci. 393, 445–451 (2013).
Liu, R. et al. Calcium-intercalated zirconium phosphate by granulation: a strategy for enhancing adsorption selectivity of strontium and cesium from liquid radioactive waste. Inorg. Chem. 62, 5799–5809 (2023).
Gao, Y. J. et al. An easily synthesized microporous framework material for the selective capture of radioactive Cs+ and Sr2+ ions. J. Mater. Chem. A 6, 3967–3976 (2018).
Milcent, T., Hertz, A., Barré, Y. & Grandjean, A. Influence of the Nb content and microstructure of sitinakite-type crystalline silicotitanates (CSTs) on their Sr2+ and Cs+ sorption properties. Chem. Eng. J. 426, 131425 (2021).
Yao, C. & Dai, Y. Layer-by-layer assembled Prussian blue compounds deposited on polyurethane foams for adsorption of strontium. J. Radioanal. Nucl. Chem. 332, 4113–4124 (2023).
Wei, P. et al. New wine in old bottles: Prolonging room-temperature phosphorescence of crown ethers by supramolecular interactions. Angew. Chem. Int. Ed. 59, 9293–9298 (2020).
Ripon, R. I., Begum, Z. A. & Rahman, I. M. Selective separation of radionuclides from aqueous matrices using crown Ether: A review. Microchem. J., 199, 110161 (2024).
Zhang, A. et al. Preparation of macroporous silica-based crown ether materials for strontium separation. J. Porous Mater. 17, 153–161 (2010).
Feng, L. et al. Decorating Channel Walls in Metal–Organic Frameworks with Crown Ethers for Efficient and Selective Separation of Radioactive Strontium (II). Angew. Chem. Int. Ed., 62, e202312894 (2023).
Li, L. et al Incorporating Two Crown Ether Struts into the Backbone of Robust Zirconium-Based Metal-Organic Frameworks as Custom-Designed Efficient Collectors for Radioactive Metal Ions. Adv. Sci., 11, 2308663 (2024).
Gilhula, J. C. et al. Advances in heavy alkaline earth chemistry provide insight into complexation of weakly polarizing Ra2+, Ba2+, and Sr2+ cations. Sci. Adv. 10, eadj8765 (2024).
Zhang, A., Wei, Y. & Kumagai, M. Synthesis of a novel macroporous silica-based polymeric material containing 4, 4’,(5’)-di(tert-butylcyclohexano)-18-crown-6 functional group and its adsorption mechanism for strontium. React. Funct. Polym. 61, 191–202 (2004).
Ye, G., Bai, F., Wei, J., Wang, J. & Chen, J. Novel polysiloxane resin functionalized with dicyclohexano-18-crown-6 (DCH18C6): Synthesis, characterization and extraction of Sr(II) in high acidity HNO3 medium. J. Hazard. Mater. 225, 8–14 (2012).
Yi, R., Ye, G., Wu, F., Lv, D. & Chen, J. Magnetic solid-phase extraction of strontium using core–shell structured magnetic microspheres impregnated with crown ether receptors: a response surface optimization. J. Radioanal. Nucl. Chem. 308, 599–608 (2016).
Ma, J. et al. A facile preparation of dicyclohexano-18-crown-6 ether impregnated titanate nanotubes for strontium removal from acidic solution. Solid State Sci 90, 49–55 (2019).
Zhang, J. et al. Efficient Sr-90 removal from highly alkaline solution by an ultrastable crystalline zirconium phosphonate. Chem. Commun. 57, 8452–8455 (2021).
Li, G. et al. A hydrolytically stable anionic layered indium–organic framework for the efficient removal of 90Sr from seawater. Dalton Trans 48, 17858–17863 (2019).
Wang, K. Y., Ding, D., Sun, M., Cheng, L. & Wang, C. Effective and rapid adsorption of Sr2+ ions by a hydrated pentasodium cluster templated zinc thiostannate. Inorg. Chem. 58, 10184–10193 (2019).
Zhang, Z. et al. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem. Eng. J. 372, 1205–1215 (2019).
Liang, C. et al. Preparation of potassium niobium sulfide and its selective adsorption properties for Sr2+ and Co2+. J. Radioanal. Nucl. Chem. 322, 377–387 (2019).
Ma, C. et al. Novel Zr-Doped Thiostannate Spinning Fiber (Fiber-KZrTS) for Highly Efficient and Renewable Recovery of Cesium and Strontium from Geothermal Water. ACS Appl. Mater. Interfaces 15, 13589–13599 (2023).
Smičiklas, I., Dimović, S. & Plećaš, I. Removal of Cs+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl. Clay Sci. 35, 139–144 (2007).
Guo, C. et al. Efficient capture of Sr2+ from acidic aqueous solution by an 18-crown-6-ether-based metal organic framework. CrystEngComm 23, 3349–3355 (2021).
Li, J. et al. Rapid and selective uptake of Cs+ and Sr2+ ions by a layered thiostannate with acid–base and irradiation resistances. ACS EST Water 1, 2440–2449 (2021).
Kim, Y. et al. Selective Sr2+ capture in an In3+-based anionic metal-organic framework. Chem. Eng. J. 484, 149321 (2024).
Lv, T. T. et al. Rapid and highly selective Sr2+ uptake by 3D microporous rare earth oxalates with the facile synthesis, high water stability and radiation resistance. Chem. Eng. J. 435, 134906 (2022).
Guo, Y. L. et al. Efficient removal of Sr2+ ions by a one-dimensional potassium phosphatoantimonate. Chem. Eng. J. 460, 141697 (2023).
Kim, M. S. & Tsukahara, T. Studies on coordination and fluorescence behaviors of a novel uranyl ion-selective chemosensor bearing diaza 18-crown-6 ether and naphthalimide moieties. ACS Earth Space Chem 4, 2270–2280 (2020).
Shin, S. H. R. et al. Porous Liquids as Electrolyte: A Case Study of Li+ and Mg2+ Ion Transport in Crown Ether-Based Type-II Porous Liquids. ACS Mater. Lett. 5, 330–335 (2022).
Li, J. et al. Water-Mediated Hydrogen Bond Network Drives Highly Crystalline Structure Formation of Crown Ether-Based Covalent Organic Framework for Sr Adsorption. ACS Appl. Mater. Interfaces 15, 59544–59551 (2023).
Cao, X. et al. Pyrazine and crown ethers: functional covalent organic polymers for (solar-assisted) high capacity and rate performance lithium-organic battery. Mater. Today Chem. 26, 101082 (2022).
Franssen, W. M., van Heumen, C. M. & Kentgens, A. P. Structural Investigations of MA1-xDMAxPbI3 Mixed-Cation Perovskites. Inorg. Chem. 59, 3730–3739 (2020).
Jensen, M. P., Dzielawa, J. A., Rickert, P. & Dietz, M. L. EXAFS investigations of the mechanism of facilitated ion transfer into a room-temperature ionic liquid. J. Am. Chem. Soc. 124, 10664–10665 (2002).
Li, X. et al. Strain Regulation of Mixed-Halide Perovskites Enables High-performance Wide-Bandgap Photovoltaics. Adv. Mater., 36, 2401103 (2024).
Jing, Z. et al. Alkali Metal Ion Recognition by 18-Crown-6 in Aqueous Solutions: Evidence from Local Structures. J. Phys. Chem. B 127, 4858–4869 (2023).
Sheldrick, G. & Shelxtl, P. Siemens Analytical X-Ray Instruments, 1995 (Madison, WI, 1990).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. cp2k: atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 15–25 (2014).
VandeVondele, J. et al. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Miehlich, B., Savin, A., Stoll, H. & Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 157, 200–206 (1989).
Frisch, M. gaussian 09, Revision d. 01, 201, (Gaussian. Inc, Wallingford CT, 2009).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973).
Lim, I. S., Schwerdtfeger, P., Metz, B., Stoll, H. All-electron and relativistic pseudopotential studies for the group 1 element polarizabilities from K to element 119. J. Chem. Phys. 122, 104103 (2005).
Lim, I. S., Stoll, H. & Schwerdtfeger, P. Relativistic small-core energy-consistent pseudopotentials for the alkaline-earth elements from Ca to Ra. J. Chem. Phys. 124, 034107 (2006).
Acknowledgements
We acknowledge the support from the National Natural Science Foundation of China (No. 22276167) and Zhejiang Provincial Natural Science Foundation (LRG25B060002). We thank X.Y. Xiao (the State Key Laboratory of Silicon Materials, School of Materials Science and Engineering, Zhejiang University) for Single-crystal X-ray diffraction data collection and analysis. We thank Dr S.F. Zhang (Institute of Nuclear-Agricultural Science, Zhejiang University) for the quantitative analysis of 90Sr. We thank J. Q. Yuan and X. Y. Zheng (Analysis Center of Agrobiology and Environmental Sciences of Zhejiang University) for measurement the concentration of Sr2+.
Author information
Authors and Affiliations
Contributions
C.L. Xiao conceived and directed the project. L. Li., Z.L. Li and H.X. Guan performed the experiments and characterizations. Z.Y. Liu and X.C. Xu designed and performed the computational studies. L. Xu and X.F. Yang performed the 90Sr decontamination experiments. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sahel Fajal and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Liu, Z., Xu, X. et al. A negatively-charged supramolecular trap for precisely catching strontium ion. Nat Commun 16, 2606 (2025). https://doi.org/10.1038/s41467-025-57844-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57844-0