Abstract
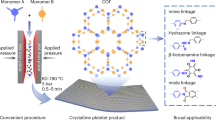
Covalent organic frameworks (COFs) are typically synthesized using solvothermal conditions with high temperature and long reaction time (≥120 °C, >72 h). Herein, we report a general and rapid microplasma electrochemistry strategy to synthesize COFs under ambient conditions. A series of flexible imine-bond COFs with high-crystallinity were prepared in minutes via this method, which showed 1000-fold higher space-time yield than solvothermal method. This approach also achieved the preparation of COFs with diverse linkages including rigid imine, hydrazone, β-ketoenamies and azine linkages. Moreover, four types of imine-based COFs were successfully synthesized in aqueous acetic acid, which avoided the use of harmful organic solvents, indicating that microplasma method is green and versatile for COF synthesis. The obtained COFs showed higher surface area and exhibited superior performance in volatile iodine uptake compared to those COFs prepared by solvothermal method. After screening more than ten types of COFs, the iodine adsorption capacity could be promoted from 2.81 to 6.52 g g−1. The efficiency, versatility, and simplicity of the microplasma method render it as a promising approach for the swift screening of COFs across a wide range of applications.
Similar content being viewed by others
Introduction
Covalent organic frameworks (COFs) are a novel class of crystalline organic porous materials extended of molecular building blocks linked by covalent bonds. Owing to their high surface area, high stability, structural designability, and multifunctional features1, COFs have received widespread attention in various fields, including separation2, catalysis3, sensing4, biomedicine5, energy storage6 and environmental remediation7. Solvothermal method is widely used to prepare COFs since it was first reported in 20058. While, the low energy efficiency makes it suffer from long reaction time (>72 h) and high temperature (≥120 °C) because most of the energy is consumed by reagents and solvents rather than the activation of chemical bonds. Moreover, the closed system and the addition of toxic organic solvents also highlight the challenges and promote the researchers to search for novel strategies to synthesize COFs rapidly at room temperature and atmospheric pressure.
Various room-temperature methods have been proposed as alternatives to the conventional solvothermal method. The solution-suspension approach could achieve COFs synthesis at room temperature with acetic acid as catalysts whereas it still required extended reaction time (3 days) and more specific building monomers9. The mechanochemical synthesis was conducted by directly grinding the reactants, leveraging the benefit of a solvent-free process. Banerjee’s group10 first prepared COFs after grinding for 40 min, however, the as-prepared COFs had lower surface area and poorer porosity compared with the solvothermal method. Then research concentrated on exploring innovative energy inputs to bolster universality and elevate crystallization rates. Building on this, microwave11,12 and light radiation13,14 were utilized to hasten the nucleation of crystallites, but these strategies typically required closed systems, elevated temperatures and bespoke equipment. Electron beam irradiation as a versatile approach was developed by Wang et al.15, and a variety of imine linkage COFs were rapidly synthesized under ambient conditions. Most recently, Chou et al.16 and Wang’s group17,18,19 reported the application of gamma irradiation method in synthesizing different types of COFs or functionalized COFs. While, the high cost and limited accessibility of electron beam equipment, along with the radiation safety hazards posed by gamma rays, limited the widespread adoption of these techniques, especially in laboratory-scale material screening. Additionally, the avoidance of toxic organic solvents is preferable. Cooper’s group20 developed a sonochemical method for preparing COFs in aqueous acetic acid, powered by high-energy ultrasound. However, this approach was limited to the synthesis of imine-based COFs, and the reaction time of 1 h remained much lengthy for the rapid production of diverse COFs. Therefore, it is still challenge to establish a more efficient, universal, and simpler strategy for the ultrafast synthesis of COFs at room temperature to fully harness the potential of COF chemistry.
Non-thermal plasmas, boasting the advantages of simple operation, environmental friendliness and a nonequilibrium reactive environment, have attracted much attention in the exploration for advanced materials synthesis at mild condition especially at room temperature and atmosphere pressure21,22,23,24,25,26. For COF synthesis, to the best of our knowledge, Hou’s group firstly proposed liquid dielectric barrier discharge (DBD) plasma-assisted method, which successfully fabricated various types of COFs27 and modified COF materials28,29,30 in less than 1 h, whereas the organic solvents were still necessary during the preparation process. In recent years, the Microplasma Electrochemistry (MIPEC) technique based on direct current glow discharge, has also been applied in materials synthesis by employing microplasma as a gas electrode, operating above the solution surface31. It merges the benefits of microplasma and electrochemistry by facilitating charge transfer to the solution and generating high-energy active species under mild conditions32,33. A notable advantage of MIPEC is its reliance on compact, user-friendly, and cost-effective equipment, making it particularly suitable for lab-scale material synthesis and screening. Consequently, the MIPEC method has achieved significant breakthroughs such as metal or metal oxide nanoparticles34,35, carbon dots36, polyphenol37,38,39, and metal-organic frameworks (MOFs)40, showing its potential in COF synthesis.
In this paper, MIPEC method was proposed as a simple and fast strategy to assemble COFs in minutes under room-temperature, the schematic of MIPEC method is shown in Fig. 1. Compared to solvothermal method, the obtained COFs has higher crystallinity, and MIPEC approach consumed five orders of magnitude less energy and had a three orders of magnitude higher space-time yield (STY). Besides, MIPEC is a versatile method and achieved the ultrafast preparation of eight flexible imine linkage COFs and five COFs with diverse linkages types, such as rigid imine, hydrazone, β-ketoenamies, and azine linkages, with high surface areas in less than 10 min at ambient conditions. Moreover, four types of imine-based COFs were also synthesized in aqueous acetic acid by MIPEC approach, which avoided the using of harmful organic solvents. The obtained COFs exhibited superior performance of volatile iodine uptake and MIPEC strategy provided a rapid synthesis method in screening COFs for radioactive iodine adsorption. The rapid, simple, green and versatility of MIPEC strategy with improved material performance makes it promising for the rapid discovery and screening of advanced COFs.
Results and discussion
MIPEC Synthesis of MP-COF-1
To prove the feasibility of MIPEC method, a kind of COF with imine linkage was prepared firstly. Due to the high rotational degree of freedom of flexible building blocks (FBB), it is a challenge to obtain highly ordered structure and crystallized COFs with FBB41. For the sake of clarity, COFs synthesized with MIPEC strategy are designated as MP-COF-x. Here, MP-COF-1, an imine-linkage COF derived from flexible units, was specifically prepared with the MIPEC strategy (Supplementary Fig. 1). As shown in Fig. 2a, the monomers of MP-COF-1, 2,4,6-tris-(4-formylphenoxy)-1,3,5-triazine (TPT-CHO) and 1,3,5-tris(4-aminophenyl)trianiline (TAPT) were dissolved in o-dichlorobenzene/n-butanol/6 M AcOH (2/2/1 by vol.), and then treated by microplasma cathode under ambient conditions with a discharge current of 10 mA for 3 min. A yellow solid was successfully prepared and isolated in 85% yield.
a Schematic diagram of MP-COF-1 synthesis process. b PXRD patterns and top view of MP-COF-1 in the simulated AA eclipsed stacking model. The experimental PXRD pattern (black), the Pawley refined pattern (red), the simulated pattern obtained from the modeled structure (blue), the difference pattern (green), the Bragg positions (pink). c High resolution XPS spectrum of N 1 s for MP-COF-1. d FT-IR spectra of MP-COF-1, TAPT and TPT-CHO. e N2 adsorption isotherm of MP-COF-1 measured at 77 K. The inset is BET plots of MP-COF-1. f PXRD patterns of MP-COF-1 synthesized with different methods.
The crystallinity of MP-COF-1 was determined by powder X-ray diffraction (PXRD) analysis. As shown in Fig. 2b, the main diffraction peak located at ~3.9° was attributed to the (100) planes, and other minor peaks appeared at ~6.8°, ~7.9°, ~10.4°, and ~25.8°, were assigned to the (110), (200), (210), and (001) facets, respectively. The crystalline structure of COF was simulated by Materials Studio (MS) software package. Pawley refinement pattern of experimental PXRD spectrum was obtained by using the reflex tool of MS software, giving the relevant information of the crystal structure of MP-COF-1. The space group was P6, a = b = 27.2161 Å, c = 3.7202 Å, α = β = 90°, γ = 120°, Rp = 1.86%, Rwp = 2.37%. No diffraction peaks of the raw materials were observed and the sharp diffraction peak confirmed the crystallinity of the COFs synthesized by MIPEC method. The PXRD pattern of MP-COF-1 was identical to the previously published work15. The XPS analysis exhibited that the COFs was consisted of C, N, O elements besides H element (Supplementary Fig. 2). And the N 1 s core level spectrum showed that the absorption peaks at 396.74 eV and 395. 95 eV for MP-COF-1 were ascribed to the nitrogen atoms within the triazine rings and the imine linkages, respectively (Fig. 2c). The two peaks in the spectra illustrated only two chemical states of N exist in the materials, implying MP-COF-1 had been prepared with high purity. As shown in Fig. 2d, in FT-IR spectra of the starting materials, the C = O stretching bands for TPT-CHO and N–H stretching bands for TAPT were appeared at 1701 cm-1 and 3377 cm-1. In the FT-IR spectra of MP-COF-1, with the intensity of C = O stretching bonds dramatically decreased and N–H bonds absolutely vanished, a new stretching vibration band at 1620 cm-1 appeared, which could be attributed to the C = N bond, indicating the completion of the aldehyde-amine condensation reaction. The solid-state 13C NMR spectrum (Supplementary Fig. 3) displayed the characteristic peak of imine carbons at ~154 ppm, which further confirmed the formation of imine bonds. The typical morphology of MP-COF-1 was observed via scanning electron microscopy (FE-SEM) images. SEM images showed that MP-COF-1 powder agglomerates were composed of nanoparticles of ~ 420 nm in diameter. (Supplementary Fig. 4).
N2 gas adsorption-desorption experiment at 77 K was measured to investigate the pore structure characteristics of MP-COF-1. MP-COF-1 displayed a type-I reversible adsorption isotherm with a sharp uptake below P/P0 = 0.01 (Fig. 2e), which demonstrated the presence of micropores in material. The Brunauer-Emmett-Teller (BET) surface area was calculated to be 1457 m2 g−1 (insert Fig. 2e), higher than the same type of COF prepared by electron beam irradiation15. The total pore volume was estimated to be 0.694 cm3 g-1 at P/P0 = 0.99. The pore size distribution was calculated by the nonlocal density functional theory (NLDFT) model. The pore width of MP-COF-1 was mainly located in the width of 0.9 ~ 1.8 nm, and the most probable aperture size was about 1.0 and 1.5 nm (Supplementary Fig. 5). The thermal stability of MP-COF-1 was assessed by thermogravimetric analysis (TGA) in Supplementary Fig. 6. MP-COF-1 displayed great thermal stability and could be stable up to 410 °C under nitrogen conditions.
We demonstrated the superiority of the MIPEC method via comparing different approaches for the synthesis of MP-COF-1. With the same precursors and solvents, PXRD pattens of the as-prepared COFs were shown in Fig. 2f. A brief exposure of the precursor solution to room temperature for 3 min resulted in few products formation with amorphous structures (3 min, without treatment). Extending the reaction time to 72 h, yielded some products with poor crystallinity (72 h, without treatment). Moreover, employing the conventional solvothermal method at 120 °C for 72 h led to a higher yield but with poorer crystallinity and a reduced surface area compared to the product synthesized via the MIPEC method (1251 vs 1457 m2 g−1, Supplementary Fig. 7). These contrast experiments underscored the crucial role of MIPEC method in the ultrafast synthesis of COFs.
The discharge time and current are the important parameters in the MIPEC synthesis, which could control the reaction process via adjusting electrochemistry input. As shown in Fig. 3a, d, with the increase of discharge time from 10 s to 3 min, the crystallinity and yields of the obtained products increased rapidly. It was found that 3 min was the optimal reaction time for COFs synthesis. When the discharge time was prolonged to 5 min, the yield showed almost no further increase and the crystallinity decreased due to the formation of defects in the synthesized COFs42,43 (Supplementary Fig. 8). Similarly, increasing the current from 5 mA to 20 mA improved the crystallinity and yields of COFs as shown in Fig. 3b, e. The reaction time for the COFs could be shortened as the discharge current increased, the products with high crystallinity and yield could be obtained with a short reaction time (30 s) when applied a relatively higher discharge current (20 mA). The concentration of precursor solution would also influence the crystallinity and the quality of COFs (Fig. 3c, f). An increased concentration of raw materials promoted more complete growth of nucleated particles during the diffusion process40, consequently leading to larger particle sizes, higher quality, and greater yield of the final products (Supplementary Fig. 9).
PXRD patterns of MP-COF-1 synthesized with (a) different time, (b) different discharge current, and (c) different concentration. The yield of MP-COF-1 synthesized with (d) different time, (e) discharge current, and (f) different concentration. The error bars in (d–f) are the standard deviations calculated from measurements of three independent samples across parallel experiments.
Besides, the energy consumption and efficiency of the MIPEC method were assessed. The energy consumption for making 100 mg of products via MIPEC method (the power of the discharge was 20 W and the discharge time was 3 min) was about five orders of magnitude lower than that of same amounts of products synthesized from the solvothermal method (the power of the oven for heating was 4 kW and the heating time was 72 h). The space-time yield (STY) of MIPEC method was about 3 orders of magnitude higher than the solvothermal method (5.07 × 103 kg m−3 d−1 vs 4.11 kg m−3 d−1, details in Supplementary Section 1.3). To the best of our knowledge, MIPEC achieved one of the highest STY for COFs synthesis in reported approaches44,45,46. The above results demonstrated that the MIPEC approach was successfully used to synthesize flexible COFs with imine linkage, and the reaction process of MIPEC synthesis was controllable. Compared to conventional solvothermal methods, the obtained COFs exhibited improved crystallinity and surface area, and the MIPEC strategy was much faster with lower energy consumption.
Extension of MIPEC method to other COFs
In addition, we extended the synthesis of flexible imine-linked COFs from MP-COF-1 to seven other flexible imine-linked COFs, and these COFs were constructed by MIPEC strategy under similar conditions with various monomers and solvents (details in Supplementary Section 1.2). MP-COF-2 was composed of 2,4,6-tris(4-formylphenoxy)-1,3,5-triazine and p-phenylenediamine. MP-COF-3 to MP-COF-8 were synthesized with the condensation of 2,4,6-tris(4-aminophenoxy)-1,3,5-triazine and different aldehyde monomers, which were terephthalic aldehyde, 2,5-dihydroxyterephthalaldehyde, 2,5-dimethoxyterephthalaldehyde, 2,2′-bipyridine-4,4′-dicarboxaldehyde, 1,3,5-tris(p-formylphenyl)benzene, 2,4,6-tris(4-formylphenyl)-1,3,5-triazine respectively. The crystallinity and formation of MP-COF-2 to MP-COF-8 were confirmed by PXRD and FT-IR spectra. All the MP-COFs exhibited good crystallinity and showed diffraction peaks that were consistent with the simulated structures (Figs. 4 and 5). The FTIR spectra confirmed the consumption of the starting monomer and the formation of imine linkages (Supplementary Fig. 10). The permanent porosity of MP-COF-2 to MP-COF-8 was measured by nitrogen adsorption–desorption isotherms at 77 K (Supplementary Figs. 13–14). For MP-COF-2 to MP-COF-6, the adsorption isotherms showed characteristic type IV features of mesoporous materials and the adsorption isotherms of MP-COF-7 and MP-COF-8 presented characteristic type I features of microporous material. The BET surface areas of MP-COF-2 to MP-COF-8 were calculated to be 1240, 1508, 451, 1011, 313, 519, and 1329 m2 g−1, respectively. The pore size distribution of MP-COF-2−MP-COF-8 were centered at around 3.204, 3.204, 3.204, 3.204, 3.553, 1.478, and 1.478 nm, respectively. A summary of the pore structure data was shown in Supplementary Table 2.
To further demonstrate the universality of MIPEC method in the synthesis of COFs crystalline materials, a series of COFs with various linkages types, including the rigid imine, hydrazone, β-ketoenamies and azine linkages COFs, were successfully synthesized with a similar procedure as for MP-COF-1 (details in Supplementary Section 1.2). MP-COF-9 with rigid imine bond was consisted of 1,3,5-tris(4-aminophenyl)benzene and 2,5-dimethoxyterephthalaldehyde. MP-COF-10 with rigid imine bond was consisted of 1,3,5-triformylbenzene and 3,3’-dimethylbiphenyl-4,4’-diamine. MP-COF-11 with hydrazone bond was consisted of 1,3,5-triformylbenzene and 2,5-diethoxybenzene-1,4-dicarbohydrazide. MP-COF-12 with β-ketoenamies bond was consisted of 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde and 3,3’-dimethylbiphenyl-4,4’-diamine. MP-COF-13 with azine bond was consisted of 2,4,6-tris(4-formylphenoxy)-1,3,5-triazine and hydrazine hydrate. The crystallinity and formation of MP-COF-9 to MP-COF-13 were confirmed by the PXRD and FT-IR spectra. The PXRD pattern confirmed the high crystallinity and they were consistent with the simulated structures by Materials Studio software (Fig. 6). The Pawley refinement pattern was fitted well with the experimental PXRD pattern. The FTIR spectra verified the consumption of the starting monomers and the formation of the imine, hydrazone, β-ketoenamies and azine linkages (Supplementary Fig. 11). The permanent porosity of MP-COF-9 to MP-COF-13 was measured by nitrogen adsorption–desorption isotherms at 77 K (Supplementary Fig. 15). For MP-COF-9, MP-COF-11 and MP-COF-13, the adsorption isotherms showed characteristic type IV features of mesoporous materials and the adsorption isotherms of MP-COF-12 showed typical type I features of microporous material. The BET surface areas of MP-COF-9 to MP-COF-13 were calculated to be 828, 225, 1076, 463, and 297 m2 g-1, respectively. The pore size distribution of MP-COF-9 to MP-COF-13 was centered at around 3.409, 3.055, 2.433, 1.725, and 2.607 nm, respectively. A summary of the pore structure data was shown in Supplementary Table 2. The SEM images of MP-COF-2 to MP-COF-13 showed COFs powder agglomerates were composed of nanoparticles (Supplementary Figs. 17–28). TGA data of MP-COF-2 to MP-COF-13 showed that they were stable up to 400 °C (Supplementary Figs. 33–34).
Research for Synthesis Mechanism of MIPEC
In solvothermal method, acetic acid often serves as catalyst to accelerate the Schiff-base reaction between amines and aldehydes monomers. To obtain the products with high crystallinity, elevated temperature and long reaction time were required to the self-correcting of defects by the dynamic reversible conversion of covalent bond47,48 (Fig. 2f). While MIPEC method synthesized COFs with high crystallinity in just 3 min at room temperature, the reaction mechanisms of MIPEC and solvothermal method exhibited substantial differences. Consequently, we further explored the synthesis reaction mechanism of MP-COF-1 by MIPEC strategy.
The MIPEC method was a combination of electrochemistry and plasma chemistry. There were some researches on the electrochemical synthesis of COFs with solid electrodes, primarily focusing on the synthesis of COF films49,50,51,52. In our previous work on MOFs synthesis with MIPEC, the electrochemistry effect attributed to the deprotonation of ligands by the free electrons in the microplasma, promoting the MOFs nucleation along with the plasma chemistry40. Initially, we ascertained the impact of electrochemistry on the synthesis process of COFs. As shown in Supplementary Fig. 36. there was almost no product formed in 3 min by only using solid electrodes, which demonstrated the electrochemistry had negligible influence on the formation of COFs in MIPEC method (details in Supplementary Section 1.3).
The effect of plasma in MIPEC synthesis of COFs was studied. As acetic acid was used as the catalyst in solvothermal method, to eliminate the effect of acetic acid, the synthesis of MP-COF-1 were carried out without acetic acid catalyst. 0.5 mL ionic liquid was added into the precursor solution instead of acetic acid to enhance its conductivity for discharging. The addition of ionic liquid could not trigger or promote the synthesis reaction without microplasma (details in Supplementary Section 1.4, Supplementary Fig. 37). As demonstrated in Fig. 7a, the MP-COF-1 (0.5 mL BMImNTf2 + 0 mL water, MIPEC) could be successfully synthesized by the MIPEC method in the absence of acetic acid. Furtherly, the reaction was conducted out in the non-contact discharge reactor (details in Supplementary Section 1.4, Supplementary Fig. 38), in which microplasma was generated above the precursor solution. Thus, the liquid phase only contained the monomers, o-dichlorobenzene and n-butanol solvents, excluding the influence of acids, ionic liquids, and electrochemical effects. Microplasma could be generated above the precursor solution. The MP-COF-1 (No addition, Non-contact Microplasma) also could be synthesized in 3 min. These results demonstrated that the microplasma could initiate and promote the fast formation of COFs. However, the crystallinity of obtained COFs (0.5 mL BMImNTf2 + 0 mL water, MIPEC; No addition, Non-contact Microplasma) were much poorer than MP-COF-1 (2.5 mL 6 M AcOH, MIPEC) synthesized by MIPEC with addition of acetic acid. The crystallization of the synthesized COFs by MIPEC might be affected by other factors.
a PXRD pattern of MP-COF-1 synthesized by MIPEC with addition of acetic acid (2.5 mL AcOH, MIPEC), without acetic acid (0.5 mL BMImNTf2 + different water addition, MIPEC; 5 mL water, Non-contact microplasma; No addition, Non-contact microplasma). EPR spectra of the precursor solution (b) with addition of acetic acid (2.5 mL AcOH, MIPEC), (c–d) without acetic acid (0.5 mL ionic liquids + different water addition, MIPEC) before and after treated by MIPEC, DMPO = 100 mM. The EPR signals of DMPO-•OH, DMPO-n-BuO•, DMPO-•CH2CH2OH are marked as yellow circle, red diamond and orange star, respectively. e–f without acetic acid (No addition, Non-contact microplasma; 5 mL water, Non-contact microplasma) before and after treated by Non-contact microplasma. g The proposed formation mechanism of MP-COF-1 with ROS generated by MIPEC.
When the MIPEC method was operated in acetic acid system, besides the acetic acid, water also present in the reaction container. In order to investigate the influence of water on COFs crystallization, different amount of water was added into the reaction system (details in Supplementary Section 1.4). A markedly increase of COF crystallinity was observed with the addition of water (Fig. 7a, 0.5 mL BMImNTf2 + 1/2.5/5 mL water, MIPEC; 5 mL water, Non-contact Microplasma). Compared to the COFs formed without water, the larger BET surface area (1195 vs 868 m2 g−1) and crystalline grain size of MP-COF-1 were observed in the presence of water (Supplementary Figs. 39–40). These results indicated that water had an obvious influence on the crystal growth process during the MIPEC synthesis of COFs, which was in line with other reports53,54. The direct dehydration of aldehyde and amine monomers could easily lead to the formation of poor crystalline or even disordered network structures55, as shown in our experiment. Water played a crucial role in the crystallization process, potentially due to its ability to regulate dehydration reactions and enhance the reversibility of aldehyde amine condensation reactions56, thereby facilitating defect repair and promoting the formation of highly ordered crystal structures57,58.
The electron paramagnetic resonance (EPR) analysis was used to further analyze the synthesis mechanism in this reaction system. 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as the common spin-trap agents was added into the precursor solution, and the EPR signals of different reaction system was obtained after treating by microplasma cathode for 3 min. For the precursor solution only containing ionic liquid (0.5 mL BMImNTf2 + 0 mL water, MIPEC) or no addition (No addition, Non-contact microplasma), as shown in Fig. 7b–f, we observed a signal with hyperfine couplings AN = 1.361 mT, AHβ = 0.683 mT of DMPO-n-BuO•. For the precursor solution added ionic liquid and water, a typical spectrum of 1:2:2:1 quartet signal with hyperfine couplings AN = AHβ = 1.48 mT and a signal with hyperfine couplings AN = 1.598 mT and AHβ = 2.283 mT were presented (shown in Fig. 7b and Supplementary Fig. 41, 0.5 mL BMImNTf2 + 1/2.5/5 mL water, MIPEC), which were assigned to DMPO-•OH and DMPO-•CH2CH2OH59,60. In the initial reaction system included acetic acid (2.5 mL 6 M AcOH, MIPEC), the formation of •OH and •CH2CH2OH were also observed as depicted in Fig. 7c. No signal was detected in all the precursor solution without microplasma treatment (Fig. 7b–f, Supplementary Fig. 41). These results suggested that the inducing and accelerating of COFs nucleation could be ascribed to the plasma chemistry effects of microplasma, as demonstrated in Fig. 7g. A large number of high-energy active species from the microplasma could generate reactive oxygen species (ROS, such as O2-, HO2• and OH•) in reaction solution38,61. In our work, ROS species included BuO•, •CH2CH2OH, and •OH, could come from n-butanol solvents and water (H2O) with the attack of high-energy active species in microplasma. These ROS species could activate monomer molecules to produce monomer radical, such as radical–produced imino radical (TAPT•), effectively reduced the energy barrier of the initial step for the formation of a C─N bond, promoting the rapid nucleation of COFs15,16,62. Furthermore, through generating high-energy active reactive species from microplasma electrodes, the reaction system could be highly activated to quickly fix the most stable conformation of the flexible building block, improving the crystallinity of the COFs. It did not need a long time and high temperature to self-correction of defects to obtain crystalline products like convention solvothermal method15. Therefore, MIPEC method could synthesized high crystallinity COFs in just 3 min at ambient temperature.
Our mechanistic hypothesis could well explain the experimental results. With longer discharge time, more ROS species could be generated and particles had longer growth time, which was attributed to both the nucleation and growth processes of COFs to obtain the higher yield and the bigger size of the crystal grain (Fig. 3a, d Supplementary Fig. 42). Increasing the discharging current could also bring more ROS species, which helped to promote the nucleation process of COFs to increased yield of products, but the crystal grain size of the COFs was not significantly changed (Fig. 3b, e, Supplementary Fig. 43). The concentration of the solution, the addition and content of water mainly affected the growth of COFs. The higher concentration led to the larger products size (Figs. 3c, f, Supplementary Fig. 9). The presence of water played a critical role in the crystal growth of COFs, which helped to regulate the crystal structure and generate products with larger grain size (Fig. 6a, Supplementary Fig. 40).
Green synthesis with MIPEC
Aqueous synthesis of COFs is attractive because it eliminates the necessity of using organic solvents and offering a more environmentally friendly approach. So far, there is only a few reports of COFs preparation in aqueous solution system20,63,64,65, and it still demands high temperatures (≥120 °C) and/or long reaction times (1 h-5 days). In this work, we tried to synthesize four type COFs with imine linkages without organic solvent (details in Supplementary Section 1.2). MP-COF-14 and MP-COF-15 were composed of 2,5-dimethoxyterephthalaldehyde with 1,3,5-tris(4-aminophenyl)benzene and 1,3,5-tris(4-aminophenyl)trianiline. MP-COF-16 and MP-COF-17 were composed of 1,3,5-triformylbenzene with 1,3,5-tris(4-aminophenyl)trianiline and 1,3,5-tris(4-aminophenyl)benzene. These imine-based COFs were synthesized in a mixture of 6 M AcOH solution by MIPEC method in 3 min with a discharge current of 10 mA. As shown in Fig. 8, all the MP-COFs exhibited good crystallinity and the diffraction peaks were consistent with the simulated structures. The Pawley refinement pattern fitted well with the experimental PXRD pattern. The FTIR spectra confirmed the consumption of the starting monomer and the formation of the imine, hydrazone, β-ketoenamies and azine linkages (Supplementary Fig. 12). The permanent porosity of MP-COF-14 to MP-COF-17 was measured by nitrogen adsorption–desorption isotherms at 77 K (Supplementary Fig. 16). For MP-COF-14 and MP-COF-15, the adsorption isotherms showed characteristic type IV features of mesoporous materials, the BET surface areas were calculated to be 1782 and 1802 m2 g−1, respectively. The MP-COF-16 and MP-COF-17 were typical type I adsorption isotherms, which indicated the formation of micropores. The BET surface areas of MP-COF-16 to MP-COF-17 were calculated to be 1035 and 1138 m2 g−1, respectively. The pore size distribution of MP-COF-14 to MP-COF-17 were centered at around 3.204, 2.890, 0.926, 0.926 nm, respectively. The SEM images of MP-COF-14 to MP-COF-17 showed COFs powder agglomerates composed of nanoparticles or winding nanowire (Supplementary Figs. 29–32). These COFs exhibited remarkable thermal stability, with no noticeable weight loss observed until decomposition occurred above 400 °C (Supplementary Fig. 35).
As a result, we synthesized four imine-based COFs with high crystallinity in aqueous acetic acid using MIPEC strategy, which avoided the use of harmful organic solvents. MIPEC emerges as a green and versatile technique for COF synthesis, capable of not only preparing diversity of COFs but also being adaptable to various solvent systems.
Iodine adsorption screening by MIPEC
Rapid preparation and screening of high-performance COFs for specific applications under real conditions is practically significant, offering valuable guidance for COF design and synthesis. Capturing volatile iodine isotopes like 129I or 131I is crucial due to their long half-lives (∼1.57 × 107 years for 129I) and potential impact on human health and the environment66,67. In the recent years, COFs have been regarded as an ideal candidate for capturing iodine, and developing COFs with high I2 adsorption capacities attracted lots of interest. In general, two key features of COFs directly affect the iodine capture performance: physical structure (e.g., surface area, pore size, and pore volume) and chemical interaction (e.g., introducing functional groups, electron-rich heteroatoms, and aromatic networks) between COFs adsorption sites and iodine molecule. In 2018, Jiang’s group68 proposed concept of the 2D covalent organic porous materials for iodine capture, suggesting that this capability was dependent solely on their physical structure rather than the binding sites of the COFs. While Liu’s group69 reported that the binding energy between COFs and iodine molecules would remarkably affect the iodine capture. However, it is still not clear the dominant role and combined effect of these factors in COFs predesign for the efficient iodine capture, and most COFs required strict synthesis conditions and long time. Here, MIPEC preparation was used as a fast-screening platform to discover the potential COFs candidates for iodine capture applications.
Different COFs were exposed to excess iodine vapor in a closed system at 75 °C for 48 h under ambient pressure (details in Supplementary Section 3.1). The adsorption capacities of the materials under different adsorption times were detected by gravimetric measurement. During the process of adsorption, the color of COFs changed gradually from light yellow to dark black (Supplementary Fig. 44). As shown in Fig. 9a, the adsorption capacity of MP-COF-1 was 3.46 g g−1, which was higher than that produced via the conventional solvothermal method (ST-COF-1, 2.81 g g−1). The average adsorption rate determined at 80% of the adsorption capacity (defined as K80%, the unit is g h−1) is often used to evaluate the adsorption performance. The K80% value of MP-COF-1 was 0.22 g h−1, which was larger than that of ST-COF-1 (0.17 g h−1).
a Gravimetric iodine uptake of MP-COF-1 synthesized by MIPEC and solvothermal method as a function of time at 75 °C and ambient pressure. b Gravimetric iodine uptake of MP-COF-1 to MP-COF-13 synthesized by MIPEC method as a function of time at 75 °C. c Comparison of the static I2 adsorption capacities of reported high-performance COF materials. The specific I2 uptake values and the corresponding references are listed in Supplementary Table 4. d Density functional theory (DFT) calculations of the binding energies and ESP analysis (left) (C, gray; H, white; O, red; N, blue; I, purple) of TPT-CHO-TAPT and TFB-BD(Me)2 with I2. The molecular structure of TPT-CHO-TAPT and TFB-BD(Me)2 (right). TPT-CHO-TAPT and TFB-BD(Me)2 are model molecules for calculations that represent MP-COF-1 and MP-COF-10, respectively. The error bars in (a, b) are the standard deviations calculated from measurements of three independent samples across parallel experiments.
This phenomenon was analyzed through the study of iodine adsorption mechanism. Firstly, adsorption kinetics of iodine on MP-COF-1 were applied to investigate the adsorption mechanism. The fitting results (details in Supplementary Section 3.2, Supplementary Fig. 45, Supplementary Table 3) showed that the adsorption data better fitted the pseudo-second-order kinetic model with a higher linear correlation coefficient R2, suggesting that the adsorption process of iodine on MP-COF-1 were mainly controlled by chemisorption. The adsorption of iodine on MP-COF-1 was supposed to involve the sharing or exchange electrons between adsorbent and adsorbate70. According to the results of PXRD patterns, XPS, and Raman spectra (Supplementary Section 3.3, Supplementary Figs. 46–51), the iodine existed as polyiodide anions (I3− and I5−) in MP-COF-1, and indicated that the n → σ* charge transfer (CT) reaction occurred between the guest iodine molecules and the electron-rich skeleton of COFs material71. The ordered arrangement of the skeleton facilitated the entry of iodine molecules into COFs72, occurring CT reaction, resulting in a higher iodine adsorption rate and capacity for MP-COF-1 compared to ST-COF-1 (Fig. 2f)
Typically, large surface areas and pore volume are considered to facilitate the performance of iodine adsorption66. MP-COF-1 had a larger surface area than ST-COF-1, which was attributed to the diffusion and transport of I273 and provided a high concentration of nucleophilic N sites to adsorb electron-deficient I267. In order to verify the hypnosis, the iodine adsorption performance of MP-COF-5 was further evaluated. The iodine capture uptake (5.30 g g−1 vs 4.27 g g−1, Supplementary Fig. 52) and the adsorption rate parameter of K80% of MP-COF-5 (0.48 g h−1 vs 0.33 g h−1) were also higher than that synthesized by solvothermal method. This result further proved the COFs synthesized by MIPEC method have better adsorption performance.
In addition, other COFs with various monomers and linkages were applied to capture iodine to screened out COFs rapidly with high iodine adsorption performance (Fig. 9b). And the adsorption capacity of MP-COF-1 to MP-COF-13 were 3.46, 3.72, 4.12, 3.07, 5.30, 4.39, 3.89, 2.88, 5.77, 6.52, 1.43, 1.66, and 3.02 g g−1 in 48 h, respectively. All the products exhibited high adsorption capacities of iodine. More importantly, MP-COF-10 was screened out with the highest iodine uptake (6.52 g g−1). To further evaluate the adsorption performance of MP-COF-10 synthesized by MIPEC, we compared MP-COF-10 with the published high-performance COFs (>4.00 g g−1) under similar experimental conditions (Fig. 9c, Supplementary Table 4). MP-COF-10 exhibited an above-average iodine capacity among the reported COFs (Supplementary Table 4), with the added advantage of a more facile and rapid MIPEC synthesis process, which notably requires only room-temperature conditions.
In general, high surface area and pore volume are thought to be favorable for iodine adsorption. MP-COF-10 exhibited the highest adsorption capacity but a relatively low BET surface areas (225 m2 g−1). We inferred that the ultrahigh adsorption performance of MP-COF-10 was mainly ascribed to the characteristics of the binding sites, which complied with the theory of Liu’s group69. In order to prove that, the density functional theory (DFT) calculations were performed based on B3LYP method74,75. As shown in Fig. 9d, TPT-CHO-TAPT and TFB-BD(Me)2 were used as the model molecules to represent MP-COF-1 and MP-COF-10. The calculated binding energies of TFB-BD(Me)2 with I2 was −11.25 kcal mol−1, which was lower than TPT-CHO-TAPT (−7.05 kcal mol−1), indicating that TFB-BD(Me)2 had a stronger affinity toward iodine. What’s more, the electrostatic potential (ESP) on the van der Waals (vdW) surface were analyzed and mapped onto the surface of the model molecules76. As shown in Fig. 9d, the vdW surface of I2 was mainly shown the positive ESP (red area) distribution, while the negative ESP (blue area) distribution of TFB-BD(Me)2 covered much greater scope than TPT-CHO-TAPT, which was conducive to the electron-deficient I2 to occur the CT reaction and form polyiodide anions67,77. The theoretical calculations results were consistent with our inferring and experimental conclusions.
These results proved the significant role of MIPEC method in rapidly synthesizing and screening COFs for iodine adsorption applications, because neither the physical property nor the adsorption sites were solely deterministic for iodine adsorption performance. In the context of various structures and multiple influencing factors associated with COFs, rapid preparation for screening proved to be the simplest and most efficient method. MIPEC method facilitates the quick and straightforward synthesis of diverse COFs, thereby providing a substantial array of options for selection. MP-COF-10 was screened out with the higher iodine capacity. Moreover, the COFs synthesized via MIPEC method exhibit high crystallinity and superior physical structures, both of which enhance their iodine adsorption performance. Furthermore, the results in our work indicated that, for MP-COFs with the distinct monomers or structures, the chemical adsorption effects overweighed the influence of physical structures, their adsorption capabilities of iodine capture were more likely to depend on the chemical binding energy rather than their textural properties such as specific surface areas and pore volumes. For MP-COFs with the same monomers (such as MP-COF-1 synthesized by different method), the better crystallinity and larger surface areas of those COFs were profit to the diffusion of iodine molecules and provides more binding sites, leading to the enhanced adsorption performance. The rapid preparation and screening with the MIPEC method could provide some reference data and valuable insights for the design and research of COFs with high iodine capture. Furthermore, leveraging MIPEC strategy, this rapid and versatile method is also well-suited for screening COFs in various specific application scenarios beyond iodine adsorption.
In summary, MIPEC method has been developed to enable the rapid synthesis of flexible COFs with imine bonds, as well as rigid COFs incorporating various linkages, including rigid imine, hydrazone, β-ketoenamies, and azine. As a general and environmental-friendly method, MIPEC not only achieved synthesizing diverse COFs with different monomer and linkages, but also could be applied in different solution system such as organic and non-toxic aqueous solvent. Compared to the solvothermal route, MIPEC approach was approximately 3 orders of magnitude faster and consumed 5 orders of magnitude less energy, showing one of the highest STY (5.07 × 103 kg m−3 d−1) for COFs synthesis in reported approaches without optimizing experimental conditions. What’s more, the obtained COFs demonstrated great performance in volatile iodine uptake, especially the iodine adsorption capacity of MP-COF-10 reached 6.52 g g−1. MIPEC as a rapid screening strategy provided valuable insights and references for the design and relevant research of COFs with high iodine adsorption performance. We believe the MIPEC method, owing to its efficiency, generality, and environmental friendliness, is not only attractive for producing advanced crystalline materials under mild conditions but also presents a significant opportunity for high-throughput screening and discovering new materials for various specific applications.
Methods
Materials
2,4,6-Tris-(4-formylphenoxy)-1,3,5-triazine (98%), 2,4,6-tris(4-aminophenyl)-1,3,5-triazine (98%), 2,4,6-tris-(4-formylphenoxy)-1,3,5-triazine (98%), 2,4,6-tris(4-aminophenoxy)-1,3,5-triazine (98%), 2,5-dihydroxyterephthalaldehyde (98%), 2,5-dimethoxyterephthalaldehyde (98%), 2,2′-bipyridine-4,4′-dicarboxaldehyde, 1,3,5-tris(p-formylphenyl)benzene (96%), 1,3,5-tris(4-aminophenyl)benzene (98%), 1,3,5-triformylbenzene (98%), 1,3,5-triformylbenzene (98%), 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (98%) were purchased from Jilin Chinese Academy of Sciences-Yanshen Technology Co., Ltd. p-Phenylenediamine (98%), 3,3’-dimethylbiphenyl-4,4’-diamine (98%), mesitylene (98%), 3,3’-dimethylbiphenyl-4,4’-diamine (98%), acetic acid (AR) was purchased from Meryer (Shanghai) Chemical Technology Co., Ltd. n-Butanol (AR), tetrahydrofuran (AR), 1,4-dioxane (99.5%, Extra Dry)were purchased from shanghai tian scientific Co., Ltd. Terephthalic aldehyde (98%), o-dichlorobenzene (AR) was purchased from Anhui Zesheng Technology Co., Ltd. Ethanol absolute (AR) was purchased from Modern Oriental (Beijing) Technology Development Co., Ltd. 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (98%) were purchased from Shanghai Macklin Biochemical Co., Ltd. 2,5-diethoxybenzene-1,4-dicarbohydrazide (97%) was purchased from Beijing HWRK Chem Co., Ltd. was purchased from shanghai tian scientific Co., Ltd. Hydrazine hydrate (98%) was purchased from Sigma-Aldrich. 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide (98%) was purchased from Qingdao Aolike New Material Technology Co., Ltd. All reagents and solvents are commercially available and used without further purification.
Experimental device of MIPEC
The microplasma electrochemistry (MIPEC) system consisted of a sealed glass H-cell (Tianjin Aida Hengsheng Technology Co., Ltd) and two electrodes (Supplementary Fig. 1). The precursor solution was added into cathodic side and the anolyte solutions that consisted of solvents without monomers were filled in the anodic side. The solution in the anodic and cathodic sides was prevented from mixing by a fritted glass plug. The argon purged the sealed system for the air replacement. The Pt wire (1 mm o.d., Tianjin Aida Hengsheng Technology Co., Ltd) electrode was partially immersed in solution to serve as the anode. On the cathode side, a stainless-steel capillary tube (250 μm i.d., Dikma Technologies Inc.) was fed with argon gas flow and vertically positioned 2 mm above the surface of the precursor solution. The microplasma was generated through electrical breakdown of the argon gas flow with the rate of 40 sccm (standard cubic centimeters per minute) under a high voltage (~2000 V), which was provided by a DC power supply.
Synthesis of MP-COF-1
Typically, 2,4,6-tris-(4-formylphenoxy)-1,3,5-triazine (TPT-CHO, 88.3 mg, 0.2 mmol) and 1,3,5-tris(4-aminophenyl)trianiline (TAPT, 70.9 mg, 0.2 mmol) were dissolved in 12.5 mL solvent consisted of 5 mL o-dichlorobenzene (o-ODC), 5 mL n-Butanol (n-BuOH) and 2.5 mL acetic acid (6 M). The mixture was sonicated to get a homogeneous dispersion and transferred to the cathode cell. After the discharge of 10 mA for 3 min, the yellow precipitate was collected by centrifugation, washed with THF/ethanol absolute for two times, and dried in an oven at 60 °C for 12 h to afford the yellow powder in 85% isolated yield. Other MP-COFs were synthesized by MIPEC with similar process of MP-COF-1, see Supplementary Section 1.2 and Supplementary Table 1 for further details.
Characterization method
The Powder X-ray diffraction (PXRD) patterns of the COFs were collected on a diffractometer (Bruker, D8 Advance) and measured with Cu Kα radiation (λ = 1.54056 Å) with a step size of 0.01°. Fourier transform infrared absorption spectrums (FT-IR) were characterize by the spectrophotometer (Thermo-Nicolet Nexus 470 FT-IR) in the wavenumber range of 500–4000 cm−1. X-ray Photoelectron Spectroscopy (XPS) was recorded on the photoelectron spectrometer (ULVAC-PHI, PHI Quantro SXM) with a mono X-ray source Al Kα excitation (1361 eV). N2 adsorption-desorption analysis was conducted on a specific surface and aperture analyzer (Quantachrome Instruments, Autosorb iQ). Solid-state NMR experiments were performed on JEOL JNM-ECZ600R spectrometer at a resonance frequency of 150 MHz. 13C CP/MAS NMR spectra were recorded using a 4 mm MAS probe and a spinning rate of 12 kHz. The specific surface area was determined by the Brunauer-Emmett-Teller (BET) adsorption model, and the pore size distribution was calculated by the nonlocal density functional theory (NLDFT) model. Thermogravimetric Analysis (TGA) curves was carried out on a TGA/DSC simultaneous thermal gravimetric analyzer (TA Instruments, SDT Q600) under N2 atmosphere with a heating rate of 10 °C/min. Scanning electron microscopy (SEM) images of the COFs were observed by a field-emission scan electron microscope (Zeiss, GeminiSEM 300) at 8.0 kV acceleration voltage. Raman spectra was measured with the 785 nm excitation wavelength on the Raman spectrometer (Horiba France SAS, Xplora Plus) in 100-300 cm−1. The electron paramagnetic resonance (EPR) spectra were record using an X-band EPR spectrometer (JEOL, JES FA-200). The operating parameters were as following: microwave power = 1.0 mW; central magnetic field = 336 mT; sweep width = ±5 mT; sweep time 100 s; modulation frequency = 100.00 kHz; and time constant = 0.03 s.
Theoretical calculations
The studied molecules were full optimized at the DFT level with B3LYP functional75,78 implemented in Gaussian 16, in which the corrections of Grimme’s D3BJ dispersion79 were combined to account for the weak interactions between the model molecule and iodine. Valence double-zeta basis sets with ‘new’ polarization functions of the Karlsruhe group80 were used for all elements, where the Stuttgart-Dresden effective core potentials were used for I81. We abbreviated the basis sets used in this study as def2-SVP for convenience. The vibrational frequency calculations were also carried out for each of the molecules to confirm the optimized geometries to be the minima. The binding energy Eb (kcal mol-1) between the fragments is computed as:
where E(complex), E(M) and E(I2) are calculated energies of the model complex formed between model molecules and iodine, model molecules, and iodine. The ESP analysis was completed with Multiwfn software76,82 and then plot ESP colored van der Waals surface in VMD83 software.
Data availability
The experimental data generated in this study are provided in the Supplementary Information and the Source Data File. The crystal structure information with unit cell parameters of MP-COF-1 to MP-COF-17 are listed in Source Data File. Source data are provided with this paper.
References
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Knebel, A. & Caro, J. Metal-organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 17, 911–923 (2022).
López-Magano, A. et al. Recent advances in the use of covalent organic frameworks as heterogenous photocatalysts in organic synthesis. Adv. Mater. 35, 2209475 (2023).
Cao, Y., Wu, R., Gao, Y. Y., Zhou, Y. & Zhu, J. J. Advances of electrochemical and electrochemiluminescent sensors based on covalent organic frameworks. Nano Micro. Lett. 16, 37 (2024).
Shi, Y. Q., Gao, F., Zhang, Q. C. & Yang, J. L. Covalent organic frameworks: recent progress in biomedical applications. Acs. Nano. 17, 1879–1905 (2023).
Liu, W. B. et al. Highly connected three-dimensional covalent organic framework with flu topology for high-performance Li-S batteries. J. Am. Chem. Soc. 145, 8141–8149 (2023).
Xia, C. L. et al. Latest advances in layered covalent organic frameworks for water and wastewater treatment. Chemosphere 329, 138580 (2023).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks-supporting. Science 310, 1166–1170 (2005).
Bagheri, A. R. & Aramesh, N. Towards the room-temperature synthesis of covalent organic frameworks: a mini-review. J. Mater. Sci. 56, 1116–1132 (2021).
Biswal, B. P. et al. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 135, 5328–5331 (2013).
Campbell, N. L., Clowes, R., Ritchie, L. K. & Cooper, A. I. Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem. Mater. 21, 204–206 (2009).
Wei, H. et al. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 51, 12178–12181 (2015).
Kim, S. et al. Rapid photochemical synthesis of sea-urchin-shaped hierarchical porous COF-5 and its lithography-free patterned growth. Adv. Funct. Mater. 27, 1700925 (2017).
Kim, S. & Choi, H. C. Light-promoted synthesis of highly-conjugated crystalline covalent organic framework. Commun. Chem. 2, 60 (2019).
Zhang, M. et al. Electron beam irradiation as a general approach for the rapid synthesis of covalent organic frameworks under ambient conditions. J. Am. Chem. Soc. 142, 9169–9174 (2020).
Elewa, A. M. et al. Room-temperature synthesis of covalent organic frameworks using gamma-irradiation in open-air conditions. Small 20, 2311472 (2024).
Zhang, M. X. et al. Radiation-induced one-pot synthesis of grafted covalent organic frameworks. Sci. China. Chem. 66, 1781–1787 (2023).
Zhang, M. X. et al. Radiation-assisted assembly of a highly dispersed nanomolybdenum-functionalized covalent organic framework. Acs. Appl. Mater. Interfaces. 16, 22504–22511 (2024).
Zhao, X. et al. One-pot synthesis of a mixed-valent copper(I/II)-coordinated covalent organic framework induced by gamma-ray radiation. Inorg. Chem. 63, 12333–12341 (2024).
Zhao, W. et al. Using sound to synthesize covalent organic frameworks in water. Nat. Synth. 1, 87–95 (2022).
Heng, Y. P., Yu, L., Chen, Y. R., Chen, X. W. & Wang, W. L. Plasma-assisted material preparation strategies and property optimization. Phys. Status. Solidi. A. 2400702 https://doi.org/10.1002/pssa.202400702 (2024).
He, J. et al. Dielectric barrier discharge plasma for nanomaterials: fabrication, modification and analytical applications. Trac Trends. Anal. Chem. 156, 116715 (2022).
Jiang, X. et al. Plasma-catalysed reaction Mn+ + L-H → MOFs: facile and tunable construction of metal-organic frameworks in dielectric barrier discharge. Chem. Commun. 55, 12192–12195 (2019).
Hou, X., Wen, X. H., He, J. & Hou, X. D. Fast preparation of Eu(BTB) MOFs in dielectric barrier discharge liquid plasma for luminescent sensing of trace iron. Luminescence 37, 2050–2058 (2022).
Wen, X. H. et al. Synthesis and real-time monitoring of the morphological evolution of luminescent Eu(TCPP) MOFs. Nanoscale 15, 11582–11591 (2023).
He, J. et al. Dielectric barrier discharge induced Oxid-Ti3C2Tx/UIO-66-NH2 composites for efficient phosphopeptides enrichment. Mater. Design. 233, 112193 (2023).
He, J. et al. Low Power, Low temperature and atmospheric pressure plasma-induced polymerization: facile synthesis and crystal regulation of covalent organic frameworks. Angew. Chem. Int. Ed. 60, 9984–9989 (2021).
Yuan, C. et al. Cyclodextrin incorporation into covalent organic frameworks enables extensive liquid and gas chromatographic enantioseparations. J. Am. Chem. Soc. 34, 18956–18967 (2023).
Peng, Q. Q. et al. Regulating the local electron density and adsorption energy of COF-based single copper sites for highly efficient Fenton-like photo-oxidation. J. Mater. Chem. A 12, 29033–29043 (2024).
Wen, G. J., Peng, Q. Q., Yuan, C., He, J. & Hou, X. D. Nickel single atoms anchored on a bipyridine-based covalent organic framework: boosting active sites for photodegradation of acetaminophen. Nanoscale 17, 322–332 (2024).
Chiang, W. H., Mariotti, D., Sankaran, R. M., Eden, J. G. & Ostrikov, K. K. Microplasmas for advanced materials and devices. Adv. Mater. 32, e1905508 (2020).
Richmonds, C. et al. Electron-transfer reactions at the plasma-liquid interface. J. Am. Chem. Soc. 133, 17582–17585 (2011).
Rumbach, P., Witzke, M., Sankaran, R. M. & Go, D. B. Decoupling interfacial reactions between plasmas and liquids: charge transfer vs plasma neutral reactions. J. Am. Chem. Soc. 135, 16264–16267 (2013).
Luo, J. H. et al. Electrodeposition of UO2 nanoparticles in molten salt with microplasma gaseous cathode. Sep. Purif. Technol. 332, 125829 (2024).
Luo, J. H. et al. Non-contact gaseous microplasma electrode as anode for electrodeposition of metal and metal alloy in molten salt. Chin. Chem. Lett. 35, 108483 (2024).
Wang, Z. et al. Microplasma-assisted rapid synthesis of luminescent nitrogen-doped carbon dots and their application in pH sensing and uranium detection. Nanoscale 7, 20743–20748 (2015).
Wang, Z. et al. Microplasma electrochemistry controlled rapid preparation of fluorescent polydopamine nanoparticles and their application in uranium detection. Chem. Eng. J. 344, 480–486 (2018).
Wang, Z. et al. Microplasma-assisted rapid, chemical oxidant-free and controllable polymerization of dopamine for surface modification. Polym. Chem. 8, 4388–4392 (2017).
Qing, Q. et al. Microplasma-induced rapid polymerization of tannic acid for surface modification. New J. Chem. 48, 3286–3293 (2024).
Wei, G. et al. Microplasma electrochemistry (MIPEC) strategy for accelerating the synthesis of metal organic frameworks at room temperature. Chin. Chem. Lett. 32, 497–500 (2021).
Guo, X. et al. Mechanistic insight into hydrogen-bond-controlled crystallinity and adsorption property of covalent organic frameworks from flexible building blocks. Chem. Mater. 30, 2299–2308 (2018).
Bora, H. J. et al. Enabling ultrahigh surface area of covalently-linked organic framework for boosted CO2 capture: an air liquid interfacial plasma as post-furnishing protocol. Chem. Eur. J. 29, e202300756 (2023).
Bora, H. J. et al. Unveiling the potential of covalent organic framework electrocatalyst for enhanced oxygen evolution. Langmuir 40, 9751–9760 (2024).
Wang, X. L. et al. Dynamic imine exchange reactions for facile synthesis of imine-linked covalent organic frameworks. Chem. Mater. 35, 10070–10077 (2023).
Liu, X. L. et al. A general large-scale synthesis approach for crystalline porous materials. Nat. Commun. 14, 7022 (2023).
Khalil, S. Transforming Covalent Organic Framework Synthesis for Advanced Applications: from Solution Processing to High-throughput Production in Flow Reactors. Doctoral Dissertation, Rice Univ. (2023).
Rowan, S. J., Cantrill, S. J., Cousins, G. R., Sanders, J. K. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Rotter, J. M. et al. Covalent organic framework films through electrophoretic deposition-creating efficient morphologies for catalysis. Chem. Mater. 31, 10008–10016 (2019).
Tavakoli, E. et al. In situ bottom-up synthesis of porphyrin-based covalent organic frameworks. J. Am. Chem. Soc. 141, 19560–19564 (2019).
Wang, X. Y. et al. Electrosynthesis of ionic covalent organic frameworks for charge-selective separation of molecules. Small 18, 2107108 (2022).
Wang, L. L. et al. Electrocleavage synthesis of solution-processed, imine-linked, and crystalline covalent organic framework thin films. J. Am. Chem. Soc. 144, 8961–8968 (2022).
Li, H. Y. et al. Nucleation and growth of covalent organic frameworks from solution: the example of COF-5. J. Am. Chem. Soc. 139, 16310–16318 (2017).
Smith, B. J. & Dichtel, W. R. Mechanistic studies of two-dimensional covalent organic frameworks rapidly polymerized from initially homogenous conditions. J. Am. Chem. Soc. 136, 8783–8789 (2014).
Smith, B. J., Overholts, A. C., Hwang, N. & Dichtel, W. R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 52, 3690–3693 (2016).
Guan, C. Z., Wang, D. & Wan, L. J. Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation. Chem. Commun. 48, 2943–2945 (2012).
Liu, X. H. et al. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 135, 10470–10474 (2013).
Stewart, D. et al. Stable and ordered amide frameworks synthesised under reversible conditions which facilitate error checking. Nat. Commun. 8, 1102 (2017).
DuBose, C. M., Rehorek, D., Oehler, U. M. & Janzen, E. G. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 4, 259–303 (1987).
Su, J. & Du, J. Electron Paramagnetic Resonance Spectroscopy Principles and Applications (Science publishing Company, 2022).
Chen, Q., Li, J. S. & Li, Y. F. A review of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 48, 424005 (2015).
Zhang, Y. Q. et al. Molecularly soldered covalent organic frameworks for ultrafast precision sieving. Sci. Adv. 7, eabe8706 (2021).
Thote, J. et al. Constructing covalent organic frameworks in water via dynamic covalent bonding. Iucrj 3, 402–407 (2016).
Lu, J. et al. Large-scale synthesis of azine-linked covalent organic frameworks in water and promoted by water. New J. Chem. 43, 6116–6120 (2019).
Martín-Illán, J. A. et al. Green synthesis of imine-based covalent organic frameworks in water. Chem. Commun. 56, 6704–6707 (2020).
Pei, C., Ben, T., Xu, S. & Qiu, S. Ultrahigh iodine adsorption in porous organic frameworks. J. Mater. Chem. A 2, 7179–7187 (2014).
Xie, Y. Q. et al. Efficient and simultaneous capture of iodine and methyl iodide achieved by a covalent organic framework. Nat. Commun. 13, 2878 (2022).
Wang, P. et al. Exceptional iodine capture in 2D covalent organic frameworks. Adv. Mater. 30, 1801991 (2018).
Song, S. N., Shi, Y., Liu, N. & Liu, F. Q. Theoretical screening and experimental synthesis of ultrahigh-iodine capture covalent organic frameworks. ACS. Appl. Mater. Interfaces. 13, 10513–10523 (2021).
Pooresmaeil, M. & Namazi, H. Hydrogels Based on Natural Polymers. Ch. 14 (Elsevier, Amsterdam, 2020).
Xiong, S. et al. Uniform poly (phosphazene–triazine) porous microspheres for highly efficient iodine removal. Chem. Commun. 54, 8450–8453 (2018).
Wang, C. et al. A 3D covalent organic framework with exceptionally high iodine capture capability. Chem. Eur. J. 24, 585–589 (2018).
Zhang, Z. Y. et al. Chemically stable guanidinium covalent organic framework for the efficient capture of low-concentration iodine at high temperatures. J. Am. Chem. Soc. 144, 6821–6829 (2022).
Kim, K. & Jordan, K. D. Comparison of density functional and MP2 calculations on the water monomer and dimer. J. Phys. Chem. 98, 10089–10094 (1994).
Stephens, P. J., Devlin, F. J., Chabalowski, C. F. & Frisch, M. J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994).
Zhang, J. & Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys.Chem. Chem. Phys. 23, 20323–20328 (2021).
Jiang, B. et al. Efficient gaseous iodine capture enhanced by charge-induced effect of covalent organic frameworks with dense tertiary-amine nodes. Chin. Chem. Lett. 33, 3556–3560 (2022).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Weigend, F., Ahlrichs, R., Florian, W. & Reinhart, A. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Kirk, A. P., Detlev, F., Erich, G., Hermann, S. & Michael, D. Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16–18 elements. J. Chem. Phys. 119, 11113–11123 (2003).
Lu, T. & Chen, F. W. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. Model. 14, 33–38 (1996).
Acknowledgements
Financial support from National Natural Science Foundation of China (Grant No. 22422606 to Y.L., 22376059 to Z.W., 22273121 to X.-G. X., U2341289 to Y.L.) is acknowledged.
Author information
Authors and Affiliations
Contributions
Z.W. and Y.L. conceived the experiments; Q.Q. performed and analyzed with experiments and wrote the manuscript draft; J.L. and J.W. assisted in the microplasma experiment; S.L. assisted in the adsorption of iodine experiment; J.C. participated in the results disscussion; X.-G.X. performed the computational studies; Z.W., X.-G.X. and Y.L. surpervised the studies and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qing, Q., Luo, J., Liu, S. et al. General synthesis of covalent organic frameworks under ambient condition within minutes via microplasma electrochemistry approach. Nat Commun 16, 2571 (2025). https://doi.org/10.1038/s41467-025-57892-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57892-6