Abstract
Functional nanowire ink formulations require elaborate control over their composition, rheological properties, and fluidic properties to optimize their printing processes. They also require harsh post-fabrication treatments to maximize the performance of the resulting printed flexible devices, making it challenging to uniformly deposit nanowire-based architectures and ensure device reproducibility and scalability. Here, we propose a strategy for developing silver nanowire (AgNW) ink formulations, where hyperbranched molecules (HPMs) are employed as both dispersant and stabilizer for nanowires. The three-dimensional architecture with functional groups on the periphery of HPMs enables the preparation of thixotropic HPMs-AgNW inks with solid contents of up to 20 wt.% in both aqueous and organic solvents using a low amount of HPMs (AgNW and HPMs weight ratio = 1:0.001). The HPMs-AgNW inks can be printed into patterns with a resolution of 20 μm on various flexible substrates without needing harsh post-treatments. We obtain bar-coated transparent electrodes (sheet resistance of 17.1 Ω sq−1 at 94.7% transmittance), slot-die-coated flexible conductive patterns, screen-printed conductive lines (conductivity exceeding 6.2 × 104 S cm−1), and 3D printed stretchable wires. Importantly, this HPMs-stabilized formulation strategy is general for various functional nanowires, enabling the integration of a diverse set of nanowire-based wearable electronic systems.
Similar content being viewed by others
Introduction
In the past few decades, printed electronics have attracted widespread interest in both the scientific and industrial communities1,2,3. Printing technologies allow for low-cost, high-volume, and versatile manufacturing with minimal environmental impact and also allow flexible or wearable electronics to enter broader markets4,5. To create printed wearable electronics, a long-standing material science challenge is to develop printable and flexible electrodes. Metal nanowires, such as AgNWs, are attractive for assembling compliant electrodes for flexible electronics due to their high electrical conductivity and mechanical flexibility6,7. However, as with all nanowire materials with large aspect ratios, it is difficult to obtain printable suspensions of AgNWs compatible with flexible substrates and various printing processes. AgNWs have a nanometer diameter but a micrometer length and tend to aggregate in their suspensions8. Large amounts of organic polymer-based dispersants, such as poly(vinylpyrrolidone) (PVP), are usually required to formulate with AgNWs to form stable suspensions. These polymeric additives tightly adsorb and wrap the nanowires to prevent their aggregation via the formation of steric effects or electric repulsion9. Although they enable AgNW suspension to assemble printed electronics, adhesive polymer binders inevitably impede inter-nanowire connections and block the formation of tight nanowire junctions10,11. This significantly deteriorates the electrical conductivity of printed AgNW patterns10,11. Harsh post-processing methods such as solvent washing and high-temperature annealing are inevitably used to remove such polymer additives, but this increases the process’s complexity and damages the printed patterns and/or flexible substrates12,13. Other challenges remain in printed AgNW electrodes/conductors, such as constructing high-resolution, complex structures and assembling large-scale patterns with high uniformity14,15,16.
To tackle these long-standing issues, here we propose a general design principle for formulating AgNW suspensions by exploiting HPMs as general dispersants. This approach enabled the scalable manufacturing of electrodes on various flexible substrates via various printing technologies. In contrast to conventional linear polymer dispersants or surfactants, HPMs have a three-dimensional (3D) architecture with linear or branched units inside and functional groups on their periphery17,18,19. These distinct structural features endow HPMs with many features, such as a large number of reactive end-groups and few or non-chain entanglements, allowing them to serve as effective dispersants for nanowire suspensions20,21. HPMs provide abundant terminal groups that serve as anchoring moieties to bond with nanowires. The remaining terminal groups on the globular surface of HPMs can interact with solvent molecules to sterically stabilize nanowires and prevent their aggregation22,23. Moreover, few or non-entangled state of HPM can significantly reduce their amount used to stabilize the nanowire dispersions22,23.
Based on this, we formulated stable AgNW inks in both aqueous and organic phases using low contents of HPMs as dispersants and stabilizers (AgNW and HPMs weight ratio = 1:0.001). Various thixotropic AgNW inks with solid contents ranging from 0.1 to 20 wt.% were obtained using this strategy. Bar coating, screen printing, slot-die coating, and extrusion-based 3D printing, were employed to print dilute or gel-like AgNW inks onto flexible substrates, involving paper, plastic, fabric, and elastomer, to construct flexible or even stretchable patterns with good resolution and uniformity. The as-printed AgNW patterns showed a high electrical conductivity without needing harsh post-treatment processes such as solvent washing or high-temperature annealing to remove residual dispersants. The bar-coated AgNW transparent electrode showed a transmittance of 94.7% with a sheet resistance of 17.1 Ω sq−1, the slot-die-coated AgNW electrodes withstood more than 10,000 bending cycles, the screen-printed AgNW conductors reached a high conductivity of 6.2 × 104 S cm−1 and printing resolution of 20 μm, and the 3D-printed AgNW patterns could be stretched up to 60% strain. Importantly, this HPMs formulation strategy was generally applicable to various functional nanowire suspensions. These versatile conductive and semiconducting nanowire inks enabled the efficient fabrication of printed wearable integrated systems for energy harvesting, wireless communication, sensing, and photodetection.
Results
Formulation and characterization of AgNW-HPMs inks
We selected and investigated two HPMs as dispersants for the formulation of AgNW inks: an amino-terminated hyperbranched polyamide (NHPM) and a thiol-terminated hyperbranched polymer (SHPM) (Fig. 1a and Supplementary Fig. 1). NHPM and SHPM were synthesized according to refs. 24,25. AgNWs with an average length of 40 μm and a diameter of 40 nm were synthesized via the polyol method (Fig. 1c)26,27. The as-prepared AgNWs were purified using our previously reported dynamic agitation-induced centrifugal filtration method to remove the PVP binder and impurities such as silver nanorods and silver nanoparticles28. SHPM and NHPM dispersants were respectively added and mixed with purified AgNWs at a weight ratio of 0.001:1 to prepare SHPM-AgNW and NHPM-AgNW inks using different solvents with nanowire concentrations in the range of about 0.1–20 wt.%. The terminal groups of NHPM and SHPM formed strong coordination bonds with AgNW: amino-silver (N-Ag) and thiol-silver (S-Ag) with binding energies of −1.37 and −1.20 eV (Supplementary Fig. 2), respectively. The highly branched topologies of NHPM and SHPM provided many peripheral end-groups that could bind with AgNWs. The globular structures offered sufficient steric hindrance to suppress AgNW aggregation, and the remaining unbonded terminal groups interacted with solvent molecules to promote AgNW to disperse (Fig. 1b). Thus, the dispersibility and stability of HPMs-grafted AgNWs can be greatly improved compared with the linear polymeric analog.
a Molecular structure of NHPM and SHPM. b Illustration showing AgNWs being stabilized by SHPM (S-Ag bond: orange, solvation: blue, Ag: gray, S: red). c SEM image of as-prepared AgNWs. TEM images of (d) NHPM-AgNW and (f) SHPM-AgNW. EDS element maps of (e) Ag (green), C (red), N (wathet), and O (magenta) for NHPM-AgNW and (g) Ag (green), C (red), S (yellow), and O (magenta) for SHPM-AgNW. h XPS spectra of AgNW (black), NHPM-AgNW (peach), and SHPM-AgNW (blue) in the Ag 3d region. i Viscosity as a function of shear rate for NHPM-AgNW IPA inks with solid contents of 7.1 wt.% (gray), 13.2 wt.% (peach), and 20.3 wt.% (blue). j Rheological properties of the NHPM-AgNW IPA inks during shear thinning and shear recovery (7.1 wt.%: gray, 13.2 wt.%: peach, and 20.3 wt.% blue). k G”/G’ as a function of shear stress for NHPM-AgNW IPA inks (7.1 wt.%: gray, 13.2 wt.%: peach, and 20.3 wt.% blue). Source data are provided as a Source Data file.
The adhesion of HPMs on AgNWs was characterized by transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). The TEM images show a molecular layer with a thickness of about 1 nm on both NHPM-AgNW and SHPM-AgNW surfaces (Fig. 1d, f). EDS confirmed the existence and uniform distribution of N and S atoms over the NHPM-AgNW and SHPM-AgNW surfaces, respectively (Fig. 1e, g). Such a thin dispersant layer is not expected to affect the formation of intimately connected junctions between AgNWs after printing. This should facilitate electron transport throughout the entire nanowire network without needing to remove residual HPMs. XPS confirmed the formation of Ag-S and Ag-N coordination bonds in SHPM-AgNW and NHPM-AgNW, respectively. The Ag 3d5/2 and Ag 3d3/2 peaks shifted to lower binding energies in the spectra of both SHPM-AgNW and NHPM-AgNW compared with that of pure AgNW (Fig. 1h).
The rheological behavior of the AgNW inks stabilized by HPMs was investigated using a cone-plate rheometer. As an example, we prepared three NHPM-AgNW inks in isopropanol (IPA) with nanowire concentrations of 7.1, 13.2, and 20.3 wt.%. Figure 1i shows that the viscosity of NHPM-AgNW IPA inks increased as the solid content increased. The viscosities at a shear rate of 0.1 s−1 for the AgNW inks with solid contents of 7.1, 13.2, and 20.3 wt.% were ≈ 90, 160, and 517 Pa, respectively, which were much lower than that of commercially available and previously reported AgNW inks and gels stabilized by conventional linear polymer dispersants29,30,31. This low viscosity was attributed to the low HPM content and their unique topological structures32,33. The non-entangled state and abundant end-groups allowed SHPM and NHPM to exhibit a high solubility but low viscosity in solvents20,21,22,23. Figure 1i shows that all NHPM-AgNW IPA inks with different solid contents displayed shear-thinning thixotropic behavior, indicating the formation of a dynamic nanowire network29. The decrease in the viscosity under a high shear rate rapidly recovered after reducing the shear rate (Fig. 1j), which should allow for the continuous extrusion/printing and subsequent quick solidification of the AgNW inks34,35.
The viscoelastic properties of the NHPM-AgNW IPA inks were further characterized in a stress sweep step test. The curves of the variation in the storage modulus G’ (elastic component) and loss modulus G” (viscous component) for AgNW inks with various solid contents as a function of shear stress are displayed in Supplementary Fig. 3 and are divided into two regions. Figure 1k illustrates the variation of tan δ (G”/G’) as a function of shear stress. In region I, G’ and G” decreased as the shear stress increased, while G’ remained higher than G”. This indicates that the AgNW network gradually broke down as the shear stress increased, but the inks still exhibited elastic-dominated behavior (G’ > G”)35,36. Region II began at the shear stress at which G’ = G” (G’/G” = 1 in Supplementary Fig. 3), and extended to higher shear stresses when G” > G’29,36. This rheological behavior indicates that the AgNW inks crossed over from solid-like to liquid-like behavior in region II29,35,36. The high aspect ratio of AgNWs (similar to linear polymer chains), uniform dispersion of AgNWs (stabilized by NHPM), and dynamic network (assembled from AgNWs) were crucial to the rheological behavior of the AgNW inks29.
The dispersibility and stability of AgNWs in solvents were strongly correlated to the solubility of HPMs. Accordingly, a series of NHPM-AgNW inks in deionized water, IPA, and ethanol, and SHPM-AgNW inks in N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and N-methylpyrrolidone (NMP) were formulated with various solid contents. The photographs of these AgNW inks are shown in Fig. 2 and Supplementary Fig. 4. Stable NHPM-AgNW IPA inks were obtained with AgNW contents in the range of about 10–200 mg mL−1. The NHPM-AgNW IPA inks formed a gel-like state when the solid content exceeded 150 mg mL−1 due to the formation of a dense NHPM-AgNW network (Fig. 2a)29. Dilute AgNW inks with low solid contents were compatible with printing methods that require low ink viscosity such as spray coating, bar coating, and slot-die printing. In contrast, viscous concentrated AgNW inks can be used with printing methods that require high ink viscosity, such as screen printing and extrusion-based 3D printing. Thus, these HPMs-stabilized AgNW inks with different solid contents can be used for various printing methods. This HPMs formulation method is general for different functional nanowires including metallic copper nanowires (CuNWs, Supplementary Fig. 5), semiconducting tellurium nanowires (TeNWs, Supplementary Fig. 6), and zinc oxide nanowires (ZnONWs, Supplementary Fig. 7) (Fig. 2d–f). Thixotropic and stable NHPM-CuNW inks, NHPM-TeNW inks, and NHPM-ZnONW inks (Supplementary Figs. 8–10) were also prepared using low amount of NHPM (weight ratio of 0.001:1 to nanowires) as dispersants with nanowire contents of up to about 20 wt.% (Supplementary Figs. 11–13). These HPMs-stabilized nanowire inks can be stored for over one month without obvious precipitation.
Printability and performance of AgNW-HPMs inks
To investigate the general printability of the nanowire inks, different printing methods were used to print AgNW inks and other nanowire inks with the same solid content to form different patterns on flexible or wearable substrates. First, we fabricated a transparent electrode via bar coating 1 wt.% NHPM-AgNW IPA ink on a polyethylene terephthalate (PET) substrate with a length of 50 cm and a width of 30 cm (Fig. 3a, b), followed by trying at 80 °C for 5 min. The SEM image (Fig. 3c) shows that the AgNWs contacted each other and formed tight junctions to facilitate electron transport throughout the entire AgNW network. The sheet resistance was measured to be 17.1 and 7.1 Ω sq−1 with a transmittance (550 nm) of 94.7% and 90.9%, respectively. We have found that annealing (at temperature higher than 120 °C) or washing the as-prepared AgNW transparent electrodes would not further improve their conductivity. The transparent electrodes fabricated from our NHPM-AgNW ink without harsh posttreatment exhibited superior optoelectronic performance to ITO/glass and previously reported AgNW transparent electrodes that required additional posttreatment processes such as high-temperature annealing or solvent washing to remove residual additives (Fig. 3d)37,38,39. Moreover, the performance of transparent electrodes is typically quantified in terms of a figure-of-merit (FoM) defined as the ratio of the direct current conductivity to the optical conductivity at a given wavelength40. Our AgNW transparent electrode had a FoM value up to 543.4 at a transmittance (550 nm) of 90.9%, which is higher than that for ITO/glass and most reported AgNW-based transparent electrodes (Supplementary Table 1)37,38,39,41,42,43,44,45,46,47. Scalable slot-die coating of 7.1 wt.% NHPM-AgNW IPA ink formed a high-quality and uniform large-area AgNW film on a flexible PET substrate (Fig. 3e, f). We used laser scribing to construct AgNW patterns on the conductive film with a resolution of 30 μm for integrated electronic applications (Fig. 3g and Supplementary Fig. 14). There was no decrease in conductivity for the AgNW film after 10,000 bending cycles with a bending angle of 180° (Fig. 3h). This verifies the high flexibility and stability of the printed AgNW networks.
Schematic illustration of (a) bar coating, e slot-die coating, i screen printing, and (o) extrusion-based 3D printing HPM-AgNW inks with different concentrations (HPM-AgNW inks: gray). b Photograph of flexible AgNW transparent electrode made by bar-coating 1 wt.% NHPM-AgNW IPA ink on a PET substrate. c SEM image of the tight junction formed between AgNWs. d Comparison of the optoelectrical properties of our AgNW transparent electrode with other published results of AgNW transparent electrodes (this work: peach, ref. 37: gray, ref. 38: blue, ref. 39: purple, ITO/glass: yellow)37,38,39. f Photograph of flexible AgNW conductive film made by slot-die coating 7.1 wt.% NHPM-AgNW IPA ink on PET substrate. g Optical micrograph of AgNW interdigitated electrodes with an electrode width of 60 μm and a gap of 30 μm, made by laser etching a slot-die printed AgNW film. h Relative resistance changes of the slot-die coated AgNW electrodes for 10,000 bending cycles with a bending angle of 180°. j Optical microscopy image of AgNW patterns with a spacing of 20 μm made by screen printing 13.2 wt.% NHPM-AgNW IPA ink on a cellulose paper substrate. k Optical image of screen-printed AgNW lines with widths of 30, 50, 100, 150, 200, and 250 μm. SEM images of (l) screen-printed AgNW lines with widths of 30 μm and m smooth edge of the screen-printed AgNW line. n Calculated sheet resistance of the screen-printed AgNW lines with different line widths (blue points and red line). Error bars (blue) represent the standard deviations from the sheet resistance results of five samples. p Optical image of a coiled electrode made by 3D printing a 20.3 wt.% NHPM-AgNW IPA ink on a PDMS substrate. q Optical image of a 3D-printed AgNW serpentine electrode stretched to 60% strain. r Relative resistance changes of an AgNW serpentine electrode upon increasing the strain (blue points and red line). Source data are provided as a Source Data file.
We also directly printed AgNW electrodes on a cellulose paper substrate via screen printing using viscous NHPM-AgNW IPA ink with a solid content of 13.2 wt.% (Fig. 3i). The printability of the NHPM-AgNW IPA ink ensured that diverse patterns could be printed with a high resolution and uniformity. Uniform AgNW patterns with a line spacing of 20 μm (Fig. 3j) and AgNW lines with different widths (30, 50, 100, 150, 200, and 250 μm, Fig. 3k, l and Supplementary Fig. 15) could be printed with smooth edges (Fig. 3m). The as-printed AgNW lines with a width of 30 μm achieved a low sheet resistance of 0.64 Ω sq−1, corresponding to conductivity of 6.2 × 104 S cm−1 (Fig. 3n). This conductivity is comparable to or even higher than that of most electrodes printed from previously reported or commercial silver-based inks that require high post-annealing/sintering temperatures (Supplementary Table 2)48,49,50,51,52,53. The gel-like state and thixotropic behavior of 20.3 wt.% NHPM-AgNW IPA ink allowed it to be adapted for extrusion-based 3D printing. A benchtop robot (FiSNAR F7304N) with a preprogrammed procedure was used to construct complex patterns on planar and curved surfaces with irregular geometries (Fig. 3o, p). A serpentine-patterned electrode was printed on a stretchable polydimethylsiloxane (PDMS) substrate (Fig. 3q), which reduced mechanical stresses and retained stable conductivity under a strain of 60% (Fig. 3r). The serpentine-patterned electrode exhibited better stretchability than that of the straight-patterned electrode (Supplementary Fig. 16). In addition to AgNW inks, CuNW, TeNW, and ZnONW could also be printed into uniform, high-resolution patterns using printing methods (Supplementary Figs. 17–19). The rheological, electrical, and mechanical properties and the versatility of nanowire inks (Supplementary Table 3) stabilized by HMPs allowed for the construction of wearable integrated electronics using only printing methods.
Application demonstration
Printed circuits and electronics are in demand for various emerging wearable devices. As a demonstration, we printed different wearable electronics, including a near-field communication (NFC) communication system, a temperature-sensing textile, and a photodetector array (10 × 10 pixels) using AgNW and other semiconducting nanowire inks with different concentrations. We first screen-printed electrodes and an antenna using the 13.2 wt.% NHPM-AgNW IPA ink on a paper substrate with 6 cm in length and 5 cm in width for NFC applications (Fig. 4a). The printed antenna was composed of 10 turns of coils with a line width of 250 μm and a coil spacing of 250 μm. This AgNW antenna illuminated 4 LEDs via wireless energy harvesting from a smartphone (Fig. 4b). A flexible NFC tag comprised of a AgNW antenna and a chip with data storage and exchange functions was suitable for wireless sensing, such as an access card for a standard electronic door lock, an identification label for personal information, and to control a smartphone app (Fig. 4c, d, Supplementary Fig. 20, and Supplementary Movies 1–3).
a Photograph of printed AgNW NFC tag on a paper substrate with a length of 6 cm and width of 5 cm. b Photograph of electricity being transmitted to a AgNW NFC antenna from a smartphone to illuminate 4 LEDs. Application examples of the AgNW NFC tag being used as (c) an access card for standard electronic door locks and (d) an identification label for personal information and control by a smartphone app. e Photograph of a sensory e-textile composed of a printed AgNW circuit, FPCB, battery, and printed TeNW temperature sensor on the backside. A sensory e-textile worn on a human wrist to monitor skin temperature changes, where the temperature is transmitted to a smartphone with a custom app. f Relative resistance change of the sensory e-textile over time by varying the temperature from 0.1 to 20 °C (blue). g Relative resistance changes of the sensory e-textile as a function of temperature (blue points and red line). h Time trace of skin temperature changes monitored by the sensory e-textile compared with a commercial CTPR (CTPR: black, sensory e-textile: blue). i Time trace of skin temperature changes of a volunteer’s wrist monitored by the sensory e-textile during rest (blue) and exercise (red). j Time trace of skin temperature changes from different parts of a volunteer’s body monitored by the sensory e-textile (A: black, B: red, C: blue, D: yellowish green, E: purple). Source data are provided as a Source Data file.
We also fabricated a wearable electronic textile (e-textile) for the real-time monitoring of human body temperature changes. This wearable sensory e-textile was composed of screen-printed AgNW circuits, a TeNW-based printed temperature sensor, a flexible printed circuit board (FPCB, with a built-in Bluetooth module), and a textile substrate with fabric cover factor of 0.98 (Fig. 4e and Supplementary Fig. 21). The temperature sensor was fabricated by directly screen printing an NHPM-TeNW H2O ink (solid content of 16.7 wt.%) onto the backside of a textile substrate to ensure reliable contact with human skin. While TeNW exhibited moderate biocompatibility54, a thin surface coating (EcoFlex) has been coated onto the printed TeNWs to prevent direct contact with human skin and further improve the adhesion between TeNWs and textile substrate. The sensory e-textile closely and stably adhered to the human body and measured the body temperature, which was transmitted to a smartphone in real-time and processed by a custom program (Fig. 4e and Supplementary Fig. 22). The conductivity of TeNW exhibited a temperature-dependent behavior near room temperature that aligned with its intrinsic semiconductor properties55. This property made TeNW suitable for temperature sensing material. As shown in Fig. 4f, g, the as-printed TeNW sensor exhibited a high temperature coefficient of resistance (TCR) of −1.5% °C−1 with a sensing resolution of 0.1 °C. A commercial paperless temperature recorder (CPTR) was used as a reference to assess the thermal sensing accuracy and reliability of our sensory e-textile. The continuous temperature values measured by our sensory e-textile under various conditions were consistent with the reference values obtained by the CPTR (Fig. 4h). When worn on a human wrist, our sensory e-textile monitored skin temperature changes during rest or exercise (Fig. 4i), and it could also be attached to different parts of the body to continuously and stably detect temperature changes (Fig. 4j).
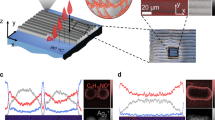
We also constructed a photodetector array (10 × 10 pixels) on a flexible paper substrate (5 × 5 cm2) by using AgNW as interdigitated electrodes and ZnONW as active layers (Fig. 5a and Supplementary Fig. 23). The interdigitated electrodes with a width of 60 μm and interspaces of 60 μm (Fig. 5b) were fabricated by slot-die coating NHPM-AgNW IPA ink (solid content of 7.1 wt.%) on paper, followed by laser etching. A 9.2 wt.% NHPM-ZnONW IPA ink was then screen-printed onto the interdigitated electrodes to construct a UV photodetector array. Figure 5c displays a representative SEM image of the ZnONW uniformly covering the interdigitated electrodes and channel. Figure 5d presents the I–V curves of one photodetector from the photodetector array in the dark and under illumination by a 365 nm UV light at different light intensities. The linear I–V characteristics indicate good Ohmic contact between the semiconducting ZnONW and AgNW electrodes. Supplementary Fig. 24 shows the current curves for one ZnONW photodetector in the dark and under 365 nm UV illumination upon increasing the light intensity from 0.01 to 1.0 mW cm−2 at a 1 V bias. The rise and decay times during the on/off cycles under 0.1 mW cm−2 illumination were 49.5 and 38.6 s, respectively. These are in line with the expected values for pure ZnO-based photodetectors56. Figure 5e illustrates the photo-dark current ratio and responsivity of one device illuminated by different light intensities under a 1 V bias. Under a light intensity of 0.1 mW cm−2, the device achieved a responsivity of 1.35 A W−1 and a photo-dark current ratio of over 9400. These values are superior to most reported ZnO-based photodetectors (Supplementary Table 4)56,57,58,59,60,61,62,63,64,65. An assessment of all 100 pixels of the photodetector array showed that the output current was uniform. The photocurrent statistics revealed that about 90% of values fell within the interval of 3.93 ± 0.24 μA (Fig. 5f). Three designed masks with “N”, “K”, and “U” opening patterns were used to cover the photodetector array under 0.1 mW cm−2 illumination. As displayed in Fig. 5g–i, the letters “N”, “K”, and “U” could be clearly identified from the mapping result of photocurrent on the photodetector array.
a Printed 10 × 10 photodetector array on paper. b Optical micrograph of a single photodetector pixel. Scale bar, 500 μm. c SEM image of the channel of a printed ZnONW electrode. d I–V curves of one device from a photodetector array illuminated by a 365 nm light with different intensities (0.01 mW cm−2: purple, 0.03 mW cm−2: burgundy, 0.1 mW cm−2: blue, 0.3 mW cm−2: red, 1.0 mW cm−2: gray) and in the dark (yellow). e Photo-dark current ratio (blue) and responsivity (red) of one device from the photodetector array illuminated by a 365 nm light with different intensities under a 1 V bias. f The photocurrent statistics of all 100 pixels under a 365 nm light with 0.1 mW cm−2 illumination at 1 V bias voltage (peach). g–i Mapping of photocurrent distribution of the photodetector array covered by masks with “N”, “K”, and “U” opening patterns under 0.1 mW cm−2 illumination at a 1 V bias voltage. Source data are provided as a Source Data file.
Discussion
By employing HPMs as high-efficiency and general dispersants, we have developed stable AgNW inks in both aqueous and organic solvents with a wide range of solid contents. The 3D architecture of HPMs with functional groups on their periphery enabled their many terminal groups to strongly bond with AgNWs, while the remaining ones interacted with solvent molecules. As a result, the HPMs-modified AgNWs were stably dispersed in solvents with concentrations of up to 20 wt.% by using only a low amount of HPMs. The resultant thixotropic AgNW inks were printed onto different flexible substrates using various printing methods to construct various high-resolution conductive patterns without needing any harsh post-treatment step. This HPMs formulation strategy was generally applicable to various conductive and semiconducting nanowire inks. The reliable printing of such a wide range of functional nanowires enabled the fabrication of wearable integrated electronic systems, including NFC tags, sensory e-textiles, and photodetector arrays with high performance and resolution. Our HPMs formulation strategy for functional nanowire inks lays the foundation for manufacturing all-printed and wearable electronics with large-scale and uniform integration.
Methods
Materials
Hydroxyl-ended hyperbranched polyester (HBP-2) was provided by Wuhan Hyperbranched Polymers Science & Technology Co., Ltd., China. 3-mercaptopropionic acid (MPA, ≥ 98%), p-toluenesulfonic acid (TsOH, ≥ 98.5%), anhydrous sodium sulfate (≥ 99%), sodium hydroxide (NaOH, ≥ 96%), sodium chloride (NaCl, ≥ 99.5%), PVP (K88-96), toluene (TL), acetone (ACE, ≥ 99.5%), ethanol (EtOH, ≥ 99.5%), isopropanol (IPA, ≥ 99.7%), DMF (≥ 99.5%), DMSO (PharmPure™), and NMP ( ≥ 99%) were purchased from Aladdin Chemical Reagent Co., Ltd., China. Diethylenetriamine (DETA, > 99%), methyl acrylate (MA, ≥ 99%), and ethylenediamine (EDA, ≥ 99%) were supplied by Sinopharm Chemical Reagent Co., Ltd. Ethylene glycol (EG, > 99%) was purchased from Concord Co., Ltd., China. Silver nitrate (AgNO3, ≥ 99.8%) was obtained from Beijing InnoChem Science & Technology Co., Ltd., China. Sodium tellurite (Na2TeO3, 99%) and L-ascorbic acid (≥ 99%) were purchased from Sigma-Aldrich. The filtration membrane (8 µm average pore size) was purchased from Zhongli Filter Equipment Factory Co., Ltd., China. PET film (thickness 0.125 mm) was purchased from Shenzhen Tenglong Packaging Materials Co., Ltd., China. Copper nanowires (CuNWs, length ≈5 μm and diameter ≈200 nm) were purchased from Nangong Bole Metal Material Co., Ltd., China. Zinc oxide nanowires (ZnONWs, length ≈5 μm and diameter ≈50 nm) were purchased from Xuzhou Jiechuang Material Technology Co., Ltd., China. The PDMS mixture (SYLGARD 184) was purchased from Dow Corning Co., Ltd., China. All reagents were of analytical grade and used as received.
Characterization
The morphology of nanowires was imaged using a JSM-7800F field-emission scanning electron microscope (JEOL, Japan). TEM analysis was performed on a JEM-2800 transmission electron microscope (JEOL, Japan). XPS was conducted on a Thermo Scientific Kα X-ray photoelectron spectrometer (ThermoFisher, USA). The crystallinity of the nanowires was characterized by powder X-ray diffraction (Rigaku D/Max-2500, Japan). Optical microscopy images were obtained using an upright metallurgical microscope (Leica DM750 M, Germany). Digital photos of the samples were acquired using a Canon 5D Mark III camera. Optical transmission spectra of NHPM-AgNWs films were obtained using a UV-2600 spectrometer (Shimadzu, Japan). The sheet resistance of NHPM-AgNWs films was measured on an ST-2258C four-probe instrument (Suzhou Jingge Electronic Co., Ltd., China). The rheological properties of the nanowire inks were measured using a DHR-2 rheometer (TA Instruments, USA) at room temperature (25.0 ± 0.3 °C). The steady-state flow step (SSFS) test was used to measure the shear viscosity of the inks at shear rates in the range of 0.1–1000 s−1. The peak hold step (PHS) test was performed with constant shear rates in three intervals (0.1 s−1 shear rate for 30 s, 200 s−1 for 30 s, and 0.1 s−1 for 60 s). The stress sweep step (SSS) test was performed with an oscillation stress range of 1–1000 Pa at a frequency of 1 Hz. The temperature and temperature distribution of sensors were measured using K-type thermocouples and infrared thermal imagers (Testo 869, Germany), respectively. Laser etching was performed on an LPKF ProtoLaser R4 (LPKF, Germany) with a pulsed laser (λ = 515 nm) at a repetition rate of 100 Hz. All measurements were carried out at room temperature.
FoM calculation
The FoM is a representative quantity used to evaluate the performance of transparent electrodes, and could be expressed as:
where σdc is the direct current conductivity of the film, σop (λ) is the optical conductivity at wavelength of λ (550 nm), Rs is the sheet resistance, and T is the transmittance at λ (550 nm)40.
Electrical conductivity measurements
The electrical conductivity (ρ) of the as-prepared patterns was calculated using Eq. (2).
where L is the length of the as-prepared patterns, R is the electrical resistance, and S is the cross-section area of each pattern measured from the SEM images.
Resistance-change measurements
The electrical response of the slot-die coated AgNW electrode was measured by subjecting it to cyclic bending with a bending angle of 180° for 10,000 bending-unbending cycles. A motorized linear stage with a built-in controller (Zaber Technologies) was used to bend the samples by varying the distance of two stepper motors. Changes in the resistance of the samples were recorded using a digital multisource meter (Keithley 2400, Tektronix).
Stretching tests of 3D-printed flexible serpentine-patterned electrodes were performed using a motorized linear stage with a built-in controller (Zaber Technologies) by varying the distance between the two stepper motors. Changes in the resistance of the samples were recorded using a digital multisource meter (Keithley 2400, Tektronix).
Temperature sensing measurements
To conduct temperature-variable sensing measurements, a TeNW-based temperature sensor was first placed on a temperature-control module calibrated using a Peltier module to precisely control the temperature. Throughout this procedure, a Keithley 2400 digital multisource meter recorded changes in the resistance of the TeNW-based temperature sensors at different temperatures. The performance of the TeNW-based temperature sensing device was evaluated by calculating the TCR using Eq. (3).
where T and ΔR/R0 refers to the temperature and relative resistance change, respectively66.
Optoelectronic sensing measurements
The optoelectronic properties of the photodetectors were measured using a digital multisource meter (Keithley 2400). A 365 nm UV lamp was used as the UV source, and the optical power intensity was varied using a combination of neutral density filters. The photocurrent was measured at 1 V under UV illumination. The photocurrent on-off ratio was calculated by Eqs. (4) and (5):
where Ilight and Idark are the current when the UV illumination was switched on and off, respectively.
The responsivity was calculated using Eq. (6).
where R, P, and A are the responsivity, incident light power density, and effective area of an individual pixel, respectively67. All measurements were performed at room temperature under ambient conditions.
Computational studies
Vienna Ab-inito Simulation Package (VASP) was used to conduct all DFT calculations68,69. The Perdew-Burke-Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) was used to describe the exchange-correlation effects70,71. The core-valence interactions were accounted for by using the projected augmented wave (PAW) method72. The energy cutoff for plane wave expansions was set to 400 eV, and 3 × 3 × 1 Monkhorst-Pack grid k-points were selected to sample the Brillouin zone integration. Structural optimization was completed for an energy and force convergence set at 1.0 × 10−4 eV and 0.01 eV Å−1, respectively.
The binding energy (Eb) was defined as:
where the slab and cluster subscripts refer to the metal surface and adsorbate molecule, respectively.
NHPM synthesis
DETA (0.30 mol) and MA (0.30 mol) were added to a 250 mL three-necked flask equipped with a mechanical stirrer, a nitrogen inlet, and a water trap attached with a condenser24. The mixture was mechanically stirred at 0–5 °C for about 12 h, and then 0.05 mol EDA was dripped into the flask. The mixture was slowly heated to 70 °C and allowed to react for 1 h and then heated to 130 °C and reacted for an additional 7 h under a nitrogen atmosphere. The yield was about 96%, resulting in a yellowish liquid product named NHPM. Its theoretical molecular weight was calculated to be 1002 g mol−1 according to its molar ratio.
SHPM synthesis
SHPM was synthesized via the esterification of MPA and HBP-225. HBP-2 (0.03 mol), 0.43 mol MPA, 1.62 g p-toluenesulfonic acid, and 75 mL toluene were added consecutively to a three-necked flask equipped with a mechanical stirrer, a nitrogen inlet, and a water trap attached with a condenser. The reaction was carried out for 12 h at 80 °C. After the reaction mixture was cooled to room temperature, the obtained solution was washed with 5 wt.% NaOH (3 × 7.5 mL). The organic layer was dried with anhydrous sodium sulfate, filtered, and then distilled under reduced pressure. The yellow liquid product was obtained with a yield of about 93%. The product was named SHPM, and its theoretical molecular weight was calculated to be 2260 g mol−1 according to its molar ratio.
AgNW synthesis
AgNWs with an average length of 40 μm and diameter of 40 nm were synthesized via the polyol method reported in ref. 28. PVP and ethylene glycol (160 mL, 12.5 mg mL−1) were added to a brown conical flask, heated at 150 °C, and then stirred until PVP was completely dissolved. The solution was cooled to room temperature, and then sodium chloride in ethylene glycol (16 mL, 0.42 mg mL−1) and silver nitrate in ethylene glycol (40 mL, 50 mg mL−1) were successively added to the solution. After vigorous magnetic stirring for 5 min, the brown conical flask was transferred to an oven preheated to 110 °C for 12 h. The as-prepared AgNWs mixture was diluted with ethanol to 0.5 mg mL−1. The 6 L diluted mixture was poured into a filtration membrane-based cylindrical chamber with a pore size of 8 µm and then mechanically stirred at 200 rpm28. Ethanol slowly flowed continuously into the filtration setup, and the ethanol purification process was stopped after 80 min. While stirring and washing, the solution was concentrated to 1 mg mL−1, and the purified AgNWs solution was collected.
TeNW synthesis
NHPM (0.549 mmol) was ultrasonically dissolved in 160 mL deionized water, and then 22.7 mmol L-ascorbic acid was added and magnetically stirred until a clear solution was formed. Na2TeO3 (0.939 mmol) was added to the homogeneous solution, and the obtained suspension was continuously heated and stirred at 90 °C for 24 h. The crude reaction mixture was cooled to room temperature and centrifuged successively with deionized water and ethanol until the supernatant was transparent. The obtained precipitates were ultrasonically dispersed in 15 mL of distilled water and then freeze-dried to obtain tellurium nanowires (NHPM-TeNWs) with an average length of 1.5 μm and diameter of 50 nm.
NHPM-AgNW inks formulation
AgNW dispersed in ethanol (1 mg mL−1) were coordinated with the NHPM dispersant at an optimal weight ratio of 1:0.001 and then centrifuged. Less amount of HPMs would affect the dispersibility of AgNWs in the inks. More HPMs would decrease the conductivity of the printed patterns. The precipitates were dispersed in different solvents with different nanowire concentrations to prepare NHPM-AgNW inks.
SHPM-AgNW inks formulation
AgNW dispersed in ethanol (1 mg mL−1) were coordinated with the SHPM dispersant at a weight ratio of 1:0.001 and then centrifuged. The precipitates were dispersed in different solvents with various nanowire concentrations to prepare SHPM-AgNW inks.
NHPM-TeNW inks formulation
TeNW dispersed in IPA (0.5 mg mL−1) were mixed with the NHPM dispersant at a weight ratio of 1:0.001 and then centrifuged. The precipitates were dispersed in different solvents with various nanowire concentrations to prepare NHPM-TeNW inks.
NHPM-ZnONW inks formulation
ZnONW dispersed in deionized water (0.5 mg mL−1) were mixed with the NHPM dispersant at a weight ratio of 1:0.001 and then centrifuged. The precipitates were dispersed in different solvents with various nanowire concentrations to prepare NHPM-ZnONW inks.
NHPM-CuNW inks formulation
CuNW dispersed in ethanol (1 mg mL−1) were coordinated with the NHPM dispersant at a weight ratio of 1:0.001 and then centrifuged. The precipitates were dispersed in different solvents with various nanowire concentrations to prepare NHPM-CuNW inks.
Fabrication of AgNW transparent electrode
PET films were secured to an automatic coater equipped with a Meyer rod (RDS 6). Then, NHPM-AgNW ink was pipetted at the top of the film, and the Meyer rod was quickly drawn down over the NHPM-AgNW ink to spread it across the PET into a thin, uniform film. Different densities of NHPM-AgNW were formed on the substrate surface by controlling the nanowire concentration. The films coated with NHPM-AgNWs were dried in the air for 5 min at 80 °C without any additional harsh posttreatment process to optimize their conductivity. Washing and annealing (at temperature higher than 120 °C) the as-printed AgNWs would not further improve their conductivity.
Slot-die coating nanowire inks and laser etching
NHPM-AgNW IPA ink (solid content of 7.1 wt.%) was printed onto a paper substrate by slot-die coating and dried in the air for 5 min at 80 °C to form a conducting layer. The optoelectronic sensing device was produced using an LPKF ProtoLaser R4 (LPKF Laser & Electronics AG, power = 8 W) with a pulsed laser (λ = 515 nm) at a repetition rate of 100 Hz. The etching width and depth were varied by modulating the laser spot size and pulse intensity. Ten pairs of interdigitated electrodes were prepared, with 19 interspaces between adjacent electrodes and a fixed interspace between interdigitated AgNWs lines of 30 μm. The width and length of the interdigitated AgNWs lines were 60 μm and 1400 μm, respectively. The as-prepared NHPM-ZnONW IPA ink (solid content of 80 mg mL−1) was screen-printed onto the interdigitated electrodes to finish the construction of ultraviolet photodetectors.
Screen printing nanowire inks
Screen-printing tests were performed on a screen printer (TC-4060k, Dongguan Ta Chen Screen Printing Machine & Materials Co., Ltd.) using a precision stainless-steel screen mesh (400 mesh count, Dongguan XiangPeng Screen Printing Equipment Co., Ltd.). A squeegee with an ergonomic holder formed an angle of ≈45° with the screen mesh. The printing speed was ≈100 mm s−1, and the printing force was ≈32.2 N. NHPM-AgNW IPA ink (solid content of 13.2 wt.%) was used to print patterns. The printed patterns were dried in the air for 5 min at 80 °C.
Extrusion-based 3D printing nanowire inks
Serpentine patterns and coils were prepared by extrusion-based 3D printing using a pre-programmed benchtop robot. NHPM-AgNW IPA ink (solid content of 20.3 wt.%) was extruded using an air-powered fluid dispenser (FiSNAR, DC 100) with a needle diameter of 250 μm, a pressure of 15.0 bar, and a movement speed of 3 mm s−1. The liquid PDMS mixture (SYLGARD 184, Dow Corning, with a weight ratio of silicone elastomer to curing agent of 10 to 1) was coated on a glass slide and cured at 80 °C for 60 min to obtain a layer that was 300 μm thick. Then, a serpentine pattern was printed on a PDMS film, and the electrode was encapsulated by PDMS using a drop-casting method and then dried at 70 °C for 1 h.
NFC tag fabrication
An NFC antenna was screen printed on a paper substrate (length ≈6 cm and width ≈5 cm) using the NHPM-AgNW IPA ink (solid content of 13.2 wt.%), and then a jumper resistor, LEDs, and chip (IC-CUID, ISO 1443) were welded to obtain AgNW NFC tags. The printed antenna was composed of 10-turn coils with a line width of 250 μm and a coil spacing of 250 μm. Using the NFC function of a mobile phone, the access card program, personal information, and application package were written into the AgNW NFC tags, respectively. The AgNW NFC tags were used as an access card for standard electronic door locks, an identification label for personal information, and to control a smartphone app.
Fabrication of sensory e-textile
A TeNW-based temperature sensor was screen printed, and the printing speed, force, and angle between the stencil and squeegee were optimized for the NHPM-TeNW H2O ink (solid content of 16.7 wt.%). The conductive electrodes with a trace width and spacing of 1.0 mm and 1.8 cm were screen printed using the NHPM-AgNW IPA ink (solid content of 13.2 wt.%) on the surface of polyester fabric. The pattern (length ≈2.5 cm and width ≈1 cm) of the screen-printing plate was installed in the screen printer, and then the NHPM-TeNW H2O ink was applied onto the screen-printing plate and printed onto the polyester fabric by sliding the squeegee over the stencil. The printed device was dried at 60 °C for 5 min to evaporate the water before subsequent tests.
The wearable e-textile was composed of screen-printed AgNW circuits, a TeNW-based temperature sensing device, a flexible printed circuit board (FPCB, with a built-in Bluetooth module), and a polyester fabric substrate. The wearable e-textile integrated a microcontroller unit (MCU) and battery management system. The MCU possessed a built-in Bluetooth module and an analog-to-digital converter (ADC). The battery supplied power to the wearable e-textile using a battery management system. To collect the resistance signal, the wearable e-textile provided an excitation signal to the TeNW-based temperature sensor. The ADC collected the voltage signal from the TeNW-based temperature sensor and then converted it into a resistance signal through an internal program of the chip (ESP32-PICO-D4, Espressif Systems (Shanghai) Pte., Ltd., China). This was performed according to the principle of resistance voltage division, and then data analysis and calculations were performed. Subsequently, the temperature data were wirelessly transmitted to the smartphone application via the Bluetooth module.
Fabrication of photodetector array
A photodetector array (10 × 10 pixels) on a paper substrate (5 × 5 cm2) was fabricated using AgNWs as interdigitated electrodes and ZnONWs as the active layer. The interdigitated electrodes were fabricated by slot-die coating the NHPM-AgNW IPA ink (solid content of 7.1 wt.%) on paper, followed by laser etching. Five pairs of interdigitated electrodes were prepared, with nine interspaces between adjacent electrodes. The interspace between interdigitated AgNWs lines was controlled at 60 μm, and the width and length of the interdigitated AgNWs lines were 60 μm and 800 μm, respectively. Then, the as-prepared NHPM-ZnONW IPA ink (solid content of 9.2 wt.%) was screen-printed onto the interdigitated electrodes to finalize the construction of the ultraviolet photodetector array.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request. Source data are provided with this paper.
References
Lee, B. et al. Omnidirectional printing of elastic conductors for three-dimensional stretchable electronics. Nat. Electron. 6, 307–318 (2023).
Lv, J. et al. Printed sustainable elastomeric conductor for soft electronics. Nat. Commun. 14, 7132 (2023).
Kam, D., Rulf, O., Reisinger, A., Lieberman, R. & Magdassi, S. 3D printing by stereolithography using thermal initiators. Nat. Commun. 15, 2285 (2024).
Song, Y. et al. 3D-printed epifluidic electronic skin for machine learning–powered multimodal health surveillance. Sci. Adv. 9, eadi6492 (2023).
Zhuang, Q. et al. Permeable, three-dimensional integrated electronic skins with stretchable hybrid liquid metal solders. Nat. Electron. 7, 598–609 (2024).
Xu, Y. et al. Phase-separated porous nanocomposite with ultralow percolation threshold for wireless bioelectronics. Nat. Nanotechnol. 19, 1158–1167 (2024).
Liu, Y. et al. Ag–thiolate interactions to enable an ultrasensitive and stretchable mxene strain sensor with high temporospatial resolution. Nat. Commun. 15, 5354 (2024).
Nguyen, V. H. et al. Advances in flexible metallic transparent electrodes. Small 18, 2106006 (2022).
Ge, Y. et al. Direct room temperature welding and chemical protection of silver nanowire thin films for high performance transparent conductors. J. Am. Chem. Soc. 140, 193–199 (2018).
Sim, H. et al. Organic-stabilizer-free polyol synthesis of silver nanowires for electrode applications. Angew. Chem. Int. Ed. 55, 11814–11818 (2016).
Wan, J. et al. Enhanced charge collection of AgNWs-based top electrode to realize high-performance, all-solution processed organic solar cells. Sci. China Chem. 67, 3347–3356 (2024).
Azani, M.-R., Hassanpour, A. & Torres, T. Benefits, problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 10, 2002536 (2020).
Yang, Y., Duan, S. & Zhao, H. Advances in constructing silver nanowire-based conductive pathways for flexible and stretchable electronics. Nanoscale 14, 11484–11511 (2022).
Um, D.-S. et al. High-resolution filtration patterning of silver nanowire electrodes for flexible and transparent optoelectronic devices. ACS Appl. Mater. Interfaces 12, 32154–32162 (2020).
Wan, T. et al. Facile patterning of silver nanowires with controlled polarities via inkjet-assisted manipulation of interface adhesion. ACS Appl. Mater. Interfaces 12, 34086–34094 (2020).
Kim, Y. U. et al. Patterned sandwich-type silver nanowire-based flexible electrode by photolithography. ACS Appl. Mater. Interfaces 13, 61463–61472 (2021).
Jiang, Y. et al. Strong and ultra-tough ionic hydrogel based on hyperbranched macro-cross-linker: Influence of topological structure on properties. Angew. Chem. Int. Ed. 62, e202310832 (2023).
Mi, X. et al. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 130, 100977 (2022).
Xu, Z. et al. Recyclable thermoset hyperbranched polymers containing reversible hexahydro-s-triazine. Nat. Sustain. 3, 29–34 (2020).
Chen, S. et al. Preparation of nanocomposites with epoxy resins and thiol-functionalized carbon nanotubes by thiol-ene click reaction. Polym. Test 77, 105912 (2019).
Zhao, B. et al. Piezoelectric nanogenerators based on electrospun PVDF-coated mats composed of multilayer polymer-coated BaTiO3 nanowires. ACS Appl. Nano Mater. 5, 8417–8428 (2022).
Un, M. & Temel, G. Preparation of water dispersible carbon nanotubes using photoinduced hyperbranched copolymerization and noncovalent interactions. Eur. Polym. J. 105, 398–404 (2018).
Chen, S. et al. Load transfer of thiol-ended hyperbranched polymers to improve simultaneously strength and longation of CNTs/epoxy nanocomposites. Eur. Polym. J. 120, 109254 (2019).
Zhang, D. et al. Preparation of mesoporous silica from electrolytic manganese slags by using amino-ended hyperbranched polyamide as template. ACS Sustain. Chem. Eng. 5, 10258–10265 (2017).
Chen, S., Zhang, J., Zhou, J., Zhang, D. & Zhang, A. Dramatic toughness enhancement of benzoxazine/epoxy thermosets with a novel hyperbranched polymeric ionic liquid. Chem. Eng. J. 334, 1371–1382 (2018).
Sun, Y., Gates, B., Mayers, B. & Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2, 165–168 (2002).
Sun, Y., Mayers, B., Herricks, T. & Xia, Y. Polyol synthesis of uniform silver nanowires: a plausible growth mechanism and the supporting evidence. Nano Lett. 3, 955–960 (2003).
Wang, H., Tang, H., Liang, J. & Chen, Y. Dynamic agitation-induced centrifugal purification of nanowires enabling transparent electrodes with 99.2% transmittance. Adv. Funct. Mater. 28, 1804479 (2018).
Liang, J., Tong, K. & Pei, Q. A water-based silver-nanowire screen-print ink for the fabrication of stretchable conductors and wearable thin-film transistors. Adv. Mater. 28, 5986–5996 (2016).
Shukla, D., Liu, Y. & Zhu, Y. Eco-friendly screen printing of silver nanowires for flexible and stretchable electronics. Nanoscale 15, 2767–2778 (2023).
Hemmati, S., Barkey, D. P. & Gupta, N. Rheological behavior of silver nanowire conductive inks during screen printing. J. Nanopart. Res. 18, 249 (2016).
Yu, C. et al. Hyperbranched polymers containing epoxy and imide structure. Prog. Org. Coat. 151, 106031 (2021).
Wang, Y., Chen, S., Guo, W., Miao, M. & Zhang, D. The precise effect of degree of branching of epoxy-ended hyperbranched polymers on intrinsic property and performance. Prog. Org. Coat. 127, 157–167 (2019).
Rudež, R., Pavlič, J. & Bernik, S. Preparation and influence of highly concentrated screen-printing inks on the development and characteristics of thick-film varistors. J. Eur. Ceram. Soc. 35, 3013–3023 (2015).
Faddoul, R., Reverdy-Bruas, N. & Blayo, A. Formulation and screen printing of water based conductive flake silver pastes onto green ceramic tapes for electronic applications. Mater. Sci. Eng. B 177, 1053–1066 (2012).
Wang, Q., Ji, X., Liu, X., Liu, Y. & Liang, J. Viscoelastic metal-in-water emulsion gel via host–guest bridging for printed and strain-activated stretchable electrodes. ACS Nano 16, 12677–12685 (2022).
Zhang, Y., Guo, J., Xu, D., Sun, Y. & Yan, F. One-pot synthesis and purification of ultralong silver nanowires for flexible transparent conductive electrodes. ACS Appl. Mater. Interfaces 9, 25465–25473 (2017).
Li, B., Ye, S., Stewart, I. E., Alvarez, S. & Wiley, B. J. Synthesis and purification of silver nanowires to make conducting films with a transmittance of 99%. Nano Lett. 15, 6722–6726 (2015).
Lee, J. et al. Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 4, 6408–6414 (2012).
Bellchambers, P. et al. High performance transparent silver grid electrodes for organic photovoltaics fabricated by selective metal condensation. Adv. Mater. 35, 2300166 (2023).
Chou, C.-Y., Liu, H.-S. & Liou, G.-S. Highly transparent silver nanowire–polyimide electrode as a snow-cleaning device. RSC Adv. 6, 61386–61392 (2016).
Chen, W.-H., Li, F.-W. & Liou, G.-S. Novel stretchable ambipolar electrochromic devices based on highly transparent AgNW/PDMS hybrid electrodes. Adv. Opt. Mater. 7, 1900632 (2019).
Zhao, W. et al. Centrifugal inertia-induced directional alignment of AgNW network for preparing transparent electromagnetic interference shielding films with joule heating ability. Adv. Sci. 11, 2406758 (2024).
Dcosta, J. V., Ochoa, D. & Sanaur, S. Extensive properties investigation of AgNWs-PEDOT:PSS based transparent conductive electrode for ultraflexible organic photodetector applications. Adv. Electron. Mater. 10, 2300559 (2024).
Xia, Y., Yalagala, B. P., Karimullah, A. S., Heidari, H. & Ghannam, R. Beyond flexibility: transparent silver nanowire electrodes on patterned surfaces for reconfigurable devices. Adv. Eng. Mater. 26, 2301165 (2024).
Li, W., Yang, S. & Shamim, A. Screen printing of silver nanowires: balancing conductivity with transparency while maintaining flexibility and stretchability. npj Flex. Electron. 3, 13 (2019).
Chen, X. et al. Ultra-flexible and transparent AgNWs-based electrodes for joule heating. Mater. Sci. Semicond. Process 174, 108237 (2024).
Lin, Y. et al. Highly conductive and compliant silver nanowire nanocomposites by direct spray deposition. ACS Appl. Mater. Interfaces 14, 57290–57298 (2022).
Lin, Y. et al. High-resolution and large-size stretchable electrodes based on patterned silver nanowires composites. Nano Res. 15, 4590–4598 (2022).
Huang, Q. & Zhu, Y. Gravure printing of water-based silver nanowire ink on plastic substrate for flexible electronics. Sci. Rep. 8, 15167 (2018).
Park, J. Y., Lee, W. J., Kwon, B.-S., Nam, S.-Y. & Choa, S.-H. Highly stretchable and conductive conductors based on Ag flakes and polyester composites. Microelectron. Eng. 199, 16–23 (2018).
Imparato, A., Mauro, A. D. G. D., Ricciardi, M. R. & Antonucci, V. Thermoforming of printed conductive silver on flexible substrates. Macromol. Symp. 413, 2400109 (2024).
Tybrandt, K. & Vörös, J. Fast and efficient fabrication of intrinsically stretchable multilayer circuit boards by wax pattern assisted filtration. Small 12, 180–184 (2016).
Li, C. et al. Advances of bioactive tellurium nanomaterials in anti-cancer phototherapy. Mater. Adv. 3, 6397–6414 (2022).
Bordoloi, U., Ghosh, S., Kumar Kedia, S. & Srivastava, P. Correlation between structural, morphological, and electrical transport properties of nano-dimensional tellurium thin film by low energy ion beam irradiation. Mater. Sci. Eng. B 305, 117399 (2024).
Nasiri, N., Bo, R., Wang, F., Fu, L. & Tricoli, A. Ultraporous electron-depleted ZnO nanoparticle networks for highly sensitive portable visible-blind UV photodetectors. Adv. Mater. 27, 4336–4343 (2015).
Gedamu, D. et al. Rapid fabrication technique for interpenetrated ZnO nanotetrapod networks for fast UV sensors. Adv. Mater. 26, 1541–1550 (2014).
Hu, L. et al. An optimized ultraviolet-a light photodetector with wide-range photoresponse based on ZnS/ZnO biaxial nanobelt. Adv. Mater. 24, 2305–2309 (2012).
Tian, W. et al. Low-cost fully transparent ultraviolet photodetectors based on electrospun ZnO-SnO2 heterojunction nanofibers. Adv. Mater. 25, 4625–4630 (2013).
Ahn, S.-E. et al. Origin of the slow photoresponse in an individual sol-gel synthesized ZnO nanowire. Appl. Phys. Lett. 90, 153106 (2007).
Ick Son, D., Yeon Yang, H., Whan Kim, T. & Il Park, W. Photoresponse mechanisms of ultraviolet photodetectors based on colloidal ZnO quantum dot-graphene nanocomposites. Appl. Phys. Lett. 102, 021105 (2013).
Cheng, J., Zhang, Y. & Guo, R. ZnO microtube ultraviolet detectors. J. Cryst. Growth 310, 57–61 (2008).
Lupan, O. et al. Ultraviolet photoconductive sensor based on single ZnO nanowire. Phys. Status Solidi A-Appl. Mat. 207, 1735–1740 (2010).
Manekkathodi, A., Lu, M.-Y., Wang, C. W. & Chen, L.-J. Direct growth of aligned zinc oxide nanorods on paper substrates for low-cost flexible electronics. Adv. Mater. 22, 4059–4063 (2010).
Liu, K., Sakurai, M., Liao, M. & Aono, M. Giant improvement of the performance of ZnO nanowire photodetectors by Au nanoparticles. J. Phys. Chem. C 114, 19835–19839 (2010).
Liu, H. et al. Approaching intrinsic dynamics of mxenes hybrid hydrogel for 3D printed multimodal intelligent devices with ultrahigh superelasticity and temperature sensitivity. Nat. Commun. 13, 3420 (2022).
Wu, W. et al. Ultrathin and conformable lead halide perovskite photodetector arrays for potential application in retina-like vision sensing. Adv. Mater. 33, 2006006 (2021).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFA1203304) and the National Natural Science Foundation of China (52173238 and 52473214).
Author information
Authors and Affiliations
Contributions
J.L. supervised the project. J.L. conceived and designed the research. X.M., L.L., S.Y., and X.J. participated in materials preparation, device fabrication, device test or interpretation of results. P.W. and W.Z. contributed SEM characterizations. J.L. and X.M. analyzed the data and co-wrote the manuscript. All authors analyzed and discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experiments were performed according to the university guidelines (The Ethics Guidelines for Research Involving Human Subjects or Human Tissue from Nankai University, Nankai University Institutional Review Board, 201990). The evaluation of the wearable e-textile for human participants followed all the ethical regulations according to the protocol above. The participants (age range 20–30 years) were recruited from Nankai university through advertisement by posted notices. All participants gave written informed consent before participation in the study. The authors affirm that human research participants provided informed consent for publication of the images in Fig. 4.
Peer review
Peer review information
Nature Communications thanks Naoji Matsuhisa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mi, X., Liu, L., Yang, S. et al. Ink formulation of functional nanowires with hyperbranched stabilizers for versatile printing of flexible electronics. Nat Commun 16, 2590 (2025). https://doi.org/10.1038/s41467-025-57959-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57959-4