Abstract
Purifying C2H2 by removing trace CO2 is critically needed yet challenged by their analogous physical properties. Herein, we report a commercial resin adsorbent HP20 (Diaion® HP-20 Resin) loaded with polyethyleneimine (PEI@HP20) which selectively captures trace CO2 and excludes C2H2. PEI@HP20 possesses a high CO2 adsorption capacity (4.35 mmol/g) at 100 kPa and 298 K and a record CO2/C2H2 uptake ratio compared with all reported CO2-selective adsorbents. The ideal adsorbed solution theory selectivity reaches 1.33×107. The pilot-scale pressure-temperature swing adsorption on 2 kg PEI@HP20 further validated that it can obtain >99.99% purity C2H2 from CO2/C2H2(1/99, v/v) mixtures with a high yield of 344.7 g per cycle. The combination of multinuclear solid-state Nuclear Magnetic Resonance, Fourier Transform infrared spectroscopy and density functional theory calculations reveal that the performance of PEI@HP20 relies on a dual chemisorption/physisorption mechanism. This work highlights a promising method to develop green, low cost, high efficiency, and readily scalable CO2-selective adsorbent.
Similar content being viewed by others
Introduction
Acetylene (C2H2) serves as one of the most essential industrial feedstocks and fuel, which is widely used in the petrochemical industry and metalworking processes1. In recent years, the market value of C2H2 has steadily increased by more than 5% every year, and is expected to reach around $14 billion by 20272. C2H2 is mainly derived from the partial combustion of methane, steam cracking of hydrocarbons, and the calcium carbide process. However, trace CO2 (ca. <3%) is inevitably generated as a by-product during these procedures, which reduces the purity of C2H2 and may significantly hinder downstream applications3,4. For example, the purity of C2H2 used in atomic absorption spectroscopy usually needs to reach 99.6% and in the semiconductor industry, carbon containing components made from C2H2, such as photolithographic masks and dielectric films have more stringent requirements for the purity of C2H2, which is up to 99.99 %5,6,7. Therefore, it is essential to remove the CO2 impurity to obtain high grade C2H2 ( > 99.6%)8,9. Currently, the prevalent conventional separation technologies to get high-purity C2H2 include solvent extraction and chemical absorption, which both suffer from heavy energy penalties and high operating costs while also generating environmental pollution issues (Fig. 1)10,11. Thus, it is necessary to develop alternative separation technologies involving energy-efficient and environmentally benign processes to address these problems.
Adsorption separation based on porous materials has been envisaged for decades as a promising alternative separation technology with the possibility for high efficiency, low energy, and lower environmental impact compared to traditional separation methods (Fig. 1)12,13,14,15,16. However, similar physical properties such as molecular dimensions (C2H2: 3.3 × 3.3 × 5.7 Å3; CO2: 3.2 × 3.3 × 5.4 Å3), boiling points (C2H2: 189.3 K; CO2: 194.7 K), and the strict upper compression of C2H2 (2 bar) make the efficient separation of trace CO2 from CO2/C2H2 mixtures a challenging task17. Painstaking efforts have been dedicated to utilize porous adsorbents such as zeolites, carbon molecular sieves, Metal-Organic Frameworks (MOFs), and Hydrogen Organic Frameworks (HOFs) for separation of CO2/C2H2 mixtures18,19,20. Current adsorbents are generally classified into C2H2-selective adsorbents and CO2-selective adsorbents based on their different binding preferences21. Since CO2 is the contaminant, CO2-selective adsorbents are desirable for purification of C2H2, which can directly isolate C2H2 products to avoid the energy-intensive desorption process. About 40% of energy consumption can be saved compared to C2H2-selective adsorbents12,22. However, the majority of porous adsorbents typically exhibit preferential adsorption of the relatively acidic and polarizable C2H2 by hydrogen bonding and π-complexation23,24. CO2-selective adsorbents thus are more rare and a universal methodology of designing such adsorbent is as of yet, non-obvious. Multiple strategies, such as complementary electrostatic interactions within compact pore spaces25, blocking strong binding sites of C2H226, and the use of predesigned pore shapes27 have been used to prepare CO2-selective adsorbents. However, these reported CO2-selective adsorbents, typically fall short due to insufficient ability to capture trace CO2 and sensitivity to water inhibition. They are also unsuitable for industrial application at large-scale due to the high cost of synthesis and lack of mature molding technology. Therefore, it is urgent to develop a common strategy for constructing effective trace CO2-selective adsorbents with the potential of direct industrialization.
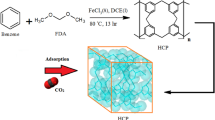
Here, we propose a facile synthetic strategy for CO2-selective adsorbents by immobilizing stable branched polyethyleneimine (PEI-800) into the commercially available porous resin HP20, which shows good preferential adsorption of trace CO2 over C2H2 through a dual chemisorption/physisorption mechanism (Fig. 1). Such an adsorbent combines the advantages from both chemical adsorption and physical adsorption, leading to a green, efficient, and energy saving separation process. Initial attempts involved modifying the pore volume and environment of the moderately C2H2-selective HP20 by gradually varying the loading of PEI-800 to achieve inverse CO2-selective adsorption, and the resultant adsorbent displayed booming CO2 sorption and suppressed C2H2 sorption. Among those samples, the HP20 loaded with 50 wt.% PEI-800 (referred to as PEI@HP20) displays the highest CO2 adsorption capacity (4.35 mmol/g at 100 kPa, 2.88 mmol/g at 1 kPa) among all the currently reported CO2-selective adsorbents and shows a new benchmark selectivity (up to 1.33 × 107) and a record-breaking uptake ratio of CO2/C2H2 (22.5), exhibiting unprecedented performance for CO2/C2H2 separation. Dynamic breakthrough experiments and pilot-scale pressure-temperature swing adsorption (PTSA) on 2 kg PEI@HP20 further validated the ability to obtain high purity C2H2 (99.99%) from the CO2/C2H2 (1/99, v/v) mixtures at both dry and 100% relative humidity. The in situ adsorption-desorption cyclic experiments over 100 cycles show that performance remained near-consistent, particularly after the first 13 cycles. The combination of gas sorption analysis, in situ multinuclear Solid State Nuclear Magnetic Resonance (SSNMR) and density functional theory (DFT) calculations, reveals the exceptional performance of PEI@HP20 in CO2/C2H2 separation lies in the formation of a reversible ammonium carbamate network inside HP20, followed by a simultaneous hydrogen-bond binding CO2 from the NH of ammonium carbamate. Additionally, the loading of amines greatly reduces HP20 porosity, suppressing the adsorption of C2H2. Thus, this work breaks the trade-off between adsorption capacity, selectivity, and cycling stability, and provides a general operable method for the construction of CO2-selective adsorbents. Moreover, the facile and inexpensive scale-up preparation strategy of the PEI@HP20 indicates it can be rapidly transferred into industrial production on demand.
Results
Preparation and characterization of PEI@HP20
HP20, used herein, forms as a ball-shaped porous copolymer generated from polymerization of styrene and divinylbenzene28. The porosity of HP20 was determined by N2 gas isotherms at 77 K, from which a Brunauer-Emmett-Teller (BET) surface area of 772 m2/g and an average pore size of 8.5 nm was derived (Supplementary fig. 1). HP20 was found by TGA to be thermally stable up to 400 °C (Supplementary fig. 2a). PEI-800 is a commercially available macromolecular polyethyleneimine thermally stable up to 280 °C (Supplementary fig. 2a), with average molecular mass of 800 g/mol and molecular dynamics diameter of 16.2 Å freely allowed into HP20 (Supplementary fig. 2b). PEI@HP20 was obtained by wet impregnation loading a specified amount of PEI-800 into commercially available HP20 particles in pure water (Fig. 2a and Supplementary fig. 3). The optimized PEI@HP20 with the highest CO2 uptake contained 50 wt.% of PEI-800 (Supplementary fig. 4). Thus, subsequent PEI@HP20 mentioned herein refers to the sample obtained under this condition. The chemical composition and structure of HP20 before and after PEI-800 impregnation were first characterized by Fourier Transform infrared (FT-IR) spectroscopy (Fig. 2b). For pristine HP20, the broad band centered at 3422 cm−1 is attributed to the O-H stretching of adsorbed water29. The three bands at 3020 cm−1, 2926 cm−1, and 2876 cm−1 are assigned to the aliphatic and aromatic C-H stretching vibrations in the polystyrene resin, and the peaks at 1603 cm−1, 1487 cm−1, and 1447 cm−1 are also due to the aromatic frame30,31. Compared with HP20, some different bands in FT-IR spectra were exhibited in the sorbent after PEI-800 modification. The center of the broad peak was shifted from 3422 cm−1 to 3366 cm−1, corresponding to the N-H stretching vibrations of PEI-800 molecules32. The two broad peaks at 2926 cm−1 and 2851 cm−1 were attributed to -CH2- stretching vibrations in the PEI-800 molecules33. Additionally, the peaks appearing at 1315 cm−1 and 1416 cm−1 were associated with the N-COO− stretching vibration of the carbamate species produced between amine groups and CO2 from air34,35.
a A schematic illustration of the structure of PEI@HP20. b FT-IR spectra of pristine HP20 and PEI@HP20. c The local structure of PEI@HP20 along with C and H atom labels. Only non-equivalent sites are labelled and atoms with very close chemical shifts are regarded as one site. d 1H MAS and e 1H → 13C CP/MAS SSNMR spectra of HP20 and PEI@HP20, as obtained at a magnetic field of 14.1 T. f 2D 1H − 1H SQ − SQ spectrum of PEI@HP20 at a mixing time of 25 ms, along with text denoting specific atomic correlations.
The local structure of PEI@HP20 (Fig. 2c) was further investigated by multinuclear SSNMR experiments. As shown in Fig. 2d, the 1H MAS SSNMR spectrum of HP20 features three resonances located at 7.0, 2.4, and 1.1 ppm, which are assigned to phenyl protons (Hphenyl), -CH (Ha) and -CH2/CH3 (Hb/c) of HP20, respectively. For the PEI@HP20 sample, the 1H spectrum exhibits two extra strong signals at 3.9 and 2.0 ppm, which originate from the -CH2 (Hd) and amine groups (He/f) of PEI-800 (Figs. 2c and 2d)36. The 1H → 13C CP/MAS SSNMR spectrum of HP20 contains six broad peaks (Fig. 2e) which are assigned to C1-C6 of HP20 (Fig. 2c). Similar to the 1H spectrum of PEI@HP20, the 13C spectrum of PEI@HP20 also shows multiple extra carbon signals located in the range of 40–60 ppm (Fig. 2e), which belong to the C7-C10 of PEI-800 (Fig. 2c)37. Unlike the broad HP20 proton signals (Hphenyl, Ha, Hb/c) and carbon resonances (C1-C6), the proton and carbon signals of PEI-800 in PEI@HP20 sample are all relatively sharp, suggesting the loaded PEI-800 are in quasi-liquid state. The encapsulation of PEI-800 inside HP20 is further confirmed by 2D 1H–1H Single Quantum-Single Quantum (SQ–SQ) experiment. The 1H–1H SQ–SQ SSNMR spectrum (Fig. 2f) presents multiple correlation peaks, which are assigned to the coupling between hydrogen atoms of PEI-800 (Hd and He/f) and hydrogen atoms of HP20 (Hphenyl, Ha and Hb/c), suggesting there is close spatial proximity between them and precluding the possibility that PEI@HP20 is a simple mixture of PEI-800 and HP20. In contrast, the 1H–1H SQ–SQ SSNMR spectrum of the physical mixture of PEI + HP20 shows only one correlation peak between He/f of PEI-800 and Hphenyl of HP20 (Supplementary fig. 5a), confirming that PEI-800 in PEI@HP20 is encapsulated in the pore of HP20 and it has been weakly adsorbed on the surface of HP20 in PEI + HP20. Additionally, the N2 gas isotherms show that the pore volume of PEI@HP20 gradually decreases with increasing PEI-800 loading (Supplementary fig. 5b), indicating the loaded PEI-800 hinders the adsorption of N2 and further validating that PEI-800 resides into the pores of HP20. Moreover, PEI@HP20 is directly synthesized from commercially available HP20 and PEI-800 in water without any other harmful solvents, making the synthesis method simple, green, readily scalable.
CO2 and C2H2 sorption and selectivity
Single-component C2H2 and CO2 gas adsorption isotherms of HP20 and PEI@HP20 were first collected at 273, 298, and 308 K (Fig. 3a). The results show that the CO2 adsorption capacity of HP20 at 100 kPa and 298 K is 0.47 mmol/g, and so is C2H2-selective with a C2H2 capacity of 0.78 mmol/g. HP20 exhibits typical type III adsorption isotherms for both CO2 and C2H2 which display weak interactions with HP20. In contrast, PEI@HP20 displays a typical type I adsorption isotherm for CO2 with rapid saturation up to 4.35 mmol/g at 100 kPa (298 K), which implies the strong binding interaction between PEI@HP20 and CO2. In contrast, the adsorption of C2H2 in PEI@HP20 is greatly reduced to 0.19 mmol/g (Fig. 3a). The dramatically increased CO2 adsorption (4.35 mmol/g vs 0.47 mmol /g) and the largely suppressed C2H2 capacity (0.19 mmol/g vs 0.78 mmol/g) in PEI@HP20 show it to achieve the inverse preferential adsorption of CO2, therefore potentially enabling the ideal separation of CO2/C2H2 mixtures. It is apparent that the impregnation of PEI-800 into HP20 largely enhances CO2 adsorption and concurrently suppresses C2H2. In fact, the CO2 adsorption capacity of PEI@HP20 at 100 kPa (4.35 mmol/g) and 1 kPa (2.88 mmol/g) surpasses all the reported state-of-the-art CO2-selective adsorbents for separation of CO2/C2H2 mixtures (Fig. 3b/c, and Supplementary Table 1) including ALF (3.85 mmol/g, 0.31 mmol/g, Fig. 3b/c and Supplementary fig. 6)38, Zn-ox-mtz (3.27 mmol/g, 2.02 mmol/g, Fig. 3b/c and Supplementary fig. 7)39, Cd-NP (2.59 mmol/g, 0.10 mmol/g, Fig. 3b/c and Supplementary fig. 8)40 and P/D SD (3.40 mmol/g, 2.60 mmol/g, Fig. 3b/c and Supplementary fig. 9)41. The uptake ratio of CO2/C2H2 in PEI@HP20 also creates a new benchmark of 22.5 which is superior to all other reported CO2-selective adsorbents (Fig. 3d), such as typical P/D SD (18.9)41, ALF (12.4), MUF-16 (12.0)42, Zn-ox-mtz (7.6), Cu-F-pymo (11.6)43, Cd-NP (4.4), and Tm-OH-bdc-1a (2.8)44. To quantitatively evaluate the separation potential of PEI@HP20 for the mixture of CO2/C2H2, the ideal adsorbed solution theory (IAST) selectivity was calculated for CO2/C2H2 (1/99, v/v) at 298 K (Supplementary Figs. 10-11). The results display an IAST selectivity of PEI@HP20 as 1.33 × 107 (Fig. 3e), which is four orders of magnitude higher than that of the currently best CO2-selective physical adsorbent Zn-ox-mtz (2290)39, and is also far beyond the chemical adsorbents P/D-SD (7.98 × 105)41.
a CO2 and C2H2 sorption isotherms of HP20 (left) and PEI@HP20 (right) at 273, 298, and 308 K. b, c Comparison of CO2 and C2H2 adsorption between PEI@HP20 and typical CO2-selective adsorbents at 298 K. d Comparison of the CO2/C2H2 uptake ratio at 1 kPa and 100 kPa with best-performing CO2-selective adsorbents at 298 K. e Comparison of the CO2 uptake capacity and IAST selectivity for CO2/C2H2 (1/99, v/v) mixture with advanced CO2-selective adsorbents at 298 K. f A comprehensive properties comparison in a pentagonal radar chart for PEI@HP20 and other reported adsorbents.
In order to fully compare PEI@HP20 with reported CO2-selective adsorbents, an index, termed as ∆q, which combines adsorption capacity and IAST selectivity, was introduced to further assess the CO2/C2H2 separation performance45,46,47. As depicted in Fig. 3f and Supplementary Fig. 12, PEI@HP20 exhibited the highest ∆q (284.7 mmol/g) for CO2/C2H2 (1/99, v/v), which is higher than the benchmark porous adsorbents ALF (12.2 mmol/g)38, Zn-ox-mtz (192.12 mmol/g)39, and P/D-SD (222.5 mmol/g)41. To meet the requirements for industrial applications, CO2-selective adsorbents must satisfy various standards such as high selectivity, economic feasibility, stability, and facile scale up (Supplementary Table 2-3). This work comprehensively compared these criteria between PEI@HP20 and other previously reported materials (Fig. 3f). These results solidly underline the good preferential CO2 capture performance of PEI@HP20 from C2H2, especially for trace CO2 removal.
Laboratory-scale breakthrough experiments and Pilot-scale PTSA
To directly evaluate the actual dynamic separation performance of PEI@HP20 for CO2/C2H2 mixtures, fixed-bed breakthrough experiments were carried out at 298 K, in which the CO2/C2H2 (1/99, v/v) mixture was used to simulate a realistic industrial scenario. In the breakthrough experiment, a mixture gas with a flow rate of 30 mL/min was passed through a PEI@HP20-packed column (Fig. 4a and Supplementary Fig. 13). As expected, the impurity CO2 is immediately adsorbed by PEI@HP20, and high-purity C2H2 is directly obtained at the outlet, indicating the minimal C2H2 adsorption (Fig. 4a). The CO2 adsorption capacity of PEI@HP20 was calculated to be 132 g/kg by the breakthrough experiment, which was consistent with the IAST theoretical calculation. Furthermore, a dynamic breakthrough experiment under humid condition (relative humidity (RH) = 100 %) was also conducted (Fig. 4a). The results show that the separation performance of PEI@HP20 exhibits almost no change under both dry and humid environments, suggesting it is suitable to work under humid conditions. (Fig. 4a). By comparing the best CO2-selective adsorbent Zn-ox-mtz, the breakthrough time of PEI@HP20 is 2512 min/g, which is much longer than that of Zn-ox-mtz (1525 min/g) at the same gas flow rate (Supplementary Fig. 14).
a PEI@HP20 breakthrough curves for a CO2/C2H2 (1/99, v/v) mixture at dry and humid environments; the experiment was carried out at 298 K and the inset shows the dimensions of the PEI@HP20 packed column. b 5 cycles of column breakthrough experiments at 298 K. c In situ CO2 adsorption-desorption test for PEI@HP20 over 100 cycles. d Regeneration performance of PEI@HP20 at different temperatures under a purge gas of N2. e Schematic model for a 2-bed PTSA process. f Dynamic sorption-desorption cycling process on PEI@HP20 for CO2/C2H2 (1/99, v/v) mixture (Process I: Adsorption at 298 K for 30 min; process II: Vacuum heating desorption at 75 °C for 15 min; process III: N2 purge assisted cooling for 15 min).
For practical industrial applications, the stability and regeneration ability of materials must be considered. As depicted in Supplementary Fig. 15, the CO2 adsorption capacity of PEI@HP20 at 298 K remained unchanged after it was exposed to air for at least one year, which shows the high stability. In addition, three batches of the material were made with the same synthetic method, and their CO2 adsorption isotherms at 298 K were found to be consistent (Supplementary Fig. 16), confirming the high reproducibility. More importantly, the separation performance of PEI@HP20 does not decay after at least five consecutive breakthrough cycles, showing the full retention of separation performance (Fig. 4b). 100 in situ cycles of CO2 adsorption (25 °C)-desorption (100 °C) were also conducted for PEI@HP20 (Fig. 4c). The result shows that the CO2 capacity has a slight 5.5% attenuation in the first 13 cycles, then it remains almost unchanged in the following 87 cycles. CO2 desorption performance is the main factor to determine the stability and energy consumption of the adsorbent, so it is vital in actual separation processes. Therefore, the CO2 desorption kinetics experiments of PEI@HP20 were explored. After CO2 adsorption equilibration at 25 °C, the desorption rates under different temperatures were examined. Only less 15 min were required to completely remove CO2 at 80 °C, which can be shortened to 8 min when the temperature is increased to 100 °C. In contrast, when the PEI-800 is used directly for CO2 capture which shows a significantly low CO2 adsorption capacity, which may lack enough adsorption sites due to its own agglomeration, and at least 200 min is required to release CO2 at 100 °C (Supplementary fig. 17). The favorable desorption kinetics of PEI@HP20 has obvious advantages compared with other amine-functional porous materials. For example, COF-99948 requires 60 min to completely remove CO2 at 80 °C, een-MOF49 requires 1 h at 140 °C, while HPD450/PEI-5050 require 30 min at 120 °C, respectively.
Significantly, the CO2-selective separation amine-functional adsorbent P/D-SD also requires just 15 min to completely remove CO2 at 100 °C, however, the mass of P/D-SD continuously decreases after the CO2 is removed, indicating the decomposition of the amine active group and makes it very vulnerable (Supplementary Fig. 18). The instability during the CO2 desorption intrinsically hampers the lifetime and regeneration of the P/D-SD adsorbent. These results confirm that PEI@HP20 not only has excellent CO2 adsorption performance but also has rapid and stable regeneration ability.
It is a tremendous challenge to take technology from laboratory to industrial applications. The large-scale production and testing on a pilot-scale is critical to promote a new material for real-world applications. Considering the good separation performance of PEI@HP20 in laboratory-scale column breakthrough experiments, the pilot-scale PTSA on 2 kg of PEI@HP20 was set up for industrial implementation in removing trace CO2 from C2H2 under 100% RH conditions (Fig. 4e and Supplementary Fig. 19). After optimization, the flow rate of feed mixture gas was determined to be 20 L/min, the adsorption stage was controlled for 30 min at room temperature, and then the CO2 was completely desorbed under vacuum at 75 °C for 15 min. Finally, N2 was used to purge PEI@HP20 for 15 min to assist cooling and the next cycle experiment was repeated at 298 K (Fig. 4f). The continuous cycle operation of the dual-bed PTSA device is achieved by controlling the adsorption and desorption times to be consistent. The result showed that the purity of C2H2 harvested from the CO2/C2H2 (1/99, v/v) mixture can reach above 99.99% (Fig. 4f). With a flow rate of 20 L/min, the average C2H2 productivity of the dual-bed PTSA device is calculated to be 689.4 g per cycle (Fig. 4f). The pilot-scale PTSA experiment successfully simulated the actual capability of PEI@HP20 toward CO2/C2H2 separation and fully demonstrates the significant potential in large-scale industrial application.
CO2/C2H2 separation mechanism studies
As mentioned above, the excellent performance of PEI@HP20 in separation of CO2/C2H2 mixtures lies in its strong affinity towards CO2 and negligible adsorption of C2H2. A combination of SSNMR spectroscopy, FT-IR, and DFT calculations were performed to investigate the mechanism of CO2/C2H2 separation in PEI@HP20. In order to study C2H2 and CO2 adsorption and dynamics within PEI@HP20, isotopically labeled gases, including C2D2 and 13CO2, were loaded inside PEI@HP20 for in situ static variable temperature (VT) SSNMR experiments. The natural abundances for deuterium and 13C are ca. 0.016% and 0.96%, respectively. C2D2 and 13CO2 used in the experiments are all 99% isotopically labeled with 2H and 13C, so the observed 2H and 13C NMR signals in the static SSNMR experiments should arise from C2D2 and 13CO2 guests. The static 2H VT SSNMR spectra of C2D2-loaded PEI@HP20 (termed as PEI@HP20-C2D2) are given in Fig. 5a. The 2H SSNMR spectrum at 298 K features a narrow sharp resonance arising from free C2D251. This indicates that PEI@HP20 has a very weak binding interaction towards C2D2, which is consistent with the neglectable adsorption capacity of C2H2 (Fig. 3a)52. When the temperature was decreased to 213 K, a very weak broad resonance can be observed beneath the dominant narrow central resonance, suggesting that PEI@HP20 can only adsorb a small amount of C2D2 at very low temperature.
In situ static VT 2H and 13C SSNMR spectra of PEI@HP20 loaded with saturated C2D2 (a) and 13CO2 (b) along with simulations (see the corresponding top legend). c 13C CP/MAS SSNMR spectrum of PEI@HP20 − 13CO2. The * symbols denote spinning sidebands. d 1H MAS SSNMR spectra of PEI@HP20 and PEI@HP20 − 13CO2. The different colored resonances beneath the experimental spectra are deconvolutions of individual signal contributions to the overall 1H SSNMR spectra. e 1H → 13C HETCOR spectra and correlation assignments. The static VT 2H SSNMR spectra of PEI@HP20-C2D2 were collected at a magnetic field strength of 9.4 T, with the other measurements obtained at a magnetic field strength of 14.1 T.
In contrast, the static 13C SSNMR spectra of 13CO2-loaded PEI@HP20 (termed as PEI@HP20-13CO2) at 298 K contains two broad signals and one weak sharp resonance that belongs to free 13CO2 (Fig. 5b). The breadth of a 13CO2 resonance is strongly associated with its dynamics which a narrower resonance suggesting a larger degree of CO2 motion and a broader resonance arising from CO2 with limited mobility53. The downfield broader resonance with a width of ca. 270 ppm (purple signal) has an isotropic chemical shift (δiso) of 164 ppm, suggesting it corresponds to chemisorbed CO254,55. The relatively narrower resonance (green signal) on more upfield has a width of ca. 170 pm and a δiso of 132 ppm which is close to the δiso of free CO2 (125 ppm). This signal belongs to physically adsorbed CO2 within PEI@HP2056. The observation of both physically and chemically adsorbed CO2 indicate the adsorption of CO2 in PEI@HP20 is a dual chemisorption/physisorption process57. At elevated temperature, the two adsorbed 13CO2 resonances both become narrower and the free CO2 signal increases steadily, suggesting some of the adsorbed CO2 are released from PEI@HP20 at higher temperature. At 373 K, the absence of the upfield resonance suggests that all the physisorbed CO2 desorbs into free CO2. The signal of the chemisorbed CO2 almost vanishes at 391 K, suggesting the adsorbed CO2 in PEI@HP20 can be quickly desorbed at a moderate regeneration temperature.
The accurate structure and location of adsorbed CO2 in PEI@HP20 is further investigated by magic-angle spinning (MAS) NMR experiments. The 13C CP/MAS spectrum of PEI@HP20-13CO2 (Fig. 5c) features two sharp resonances at ca. 165.4 and 132.2 ppm and weak broad resonances located between 40-60 ppm. The signal at 165.4 ppm is assigned to the chemisorbed CO2 and the resonance at 132.2 ppm originates from physisorbed CO2, which matches well with the observed chemically and physically adsorbed CO2 in various amine functional silica54,55 and metal-organic frameworks materials58,59. The weak broad resonances originate from the carbon atoms of PEI@HP20. The 1H MAS spectrum of PEI@HP20-13CO2 was also collected and compared to that of PEI@HP20 (Fig. 5d). The largely reduced signal of NH2/NH (i.e He/f) in the 1H MAS SSNMR spectrum of PEI@HP20-13CO2 (Fig. 5d) suggests chemical bonding occurs between CO2 and PEI@HP20 on the amine groups, forming ammonium carbamate and/or carbamic acid. In addition, a new signal located at ca. 5 ppm can be observed from the deconvolution of the spectrum, which corresponds to the proton of -NHRCO2−. This assignment is further supported by the two-dimensional (2D) 1H → 13C heteronuclear correlation (HETCOR) spectrum. The 2D 1H → 13C HETCOR experiment can probe interatomic proximities and reveal the spatial relationship between atoms in PEI@HP20-13CO2 (Fig. 5e). The spectrum features two correlation groups of 13C resonances at 165.4 and 132.2 ppm, which belong to chemically and physically adsorbed CO2, respectively. The 13C resonance at 165.4 ppm has two strong 1H correlations and two minor 1H correlations. The correlations at (1.1, 165.4 ppm) and (8.8, 165.4 ppm) are assigned to Hb/c (i.e. CH2/CH3 hydrogen of HP20) and Hphenyl (i.e. phenyl hydrogen of HP20), suggesting the produced ammonium carbamate and/or carbamic acid are proximal to HP20. The correlations at (4.7, 165.4 ppm) and (12.3, 165.4 ppm) are assigned to the proton atoms of -NHRCO2− and NHR3+ species, respectively, indicating the formation of ammonium carbamate chains in PEI@HP20-13CO2 and the positive ammonium groups are hydrogen-bonded with carbamate (see the insets in Fig. 5e)60,61. The existence of -NHRCO2− suggests the reaction of CO2 with amine groups occurs in the primary amine (i.e. -NH2) instead of the secondary amine -NH- of PEI-800 since the reaction of CO2 with the -NH- would form a tertiary nitrogen without attached hydrogens. The formation of ammonium carbamate chains has been also widely observed in amine functional MOFs and the absence of strong correlation at ca. 13 ppm precludes the existence of carbamic acid60. For the physisorbed CO2, the 1H correlations at (1.1, 132.2 ppm) and (4.7, 132.2 ppm) are assigned to Hb/c and hydrogen of -NHRCO2−, suggesting the location of physisorbed CO2 is near -NHRCO2− and CH2/CH3 of HP20. The formation of -NHRCO2− and NHR3+ species are also supported by FT-IR spectra. As showed in Supplementary fig. 20, the peaks at 1315 cm−1 and 1416 cm−1 were attributed to the formation of carbamates which display strong chemical interaction between PEI@HP20 and CO262,63,64. Peaks found at 2078 cm−1, 2333 cm−1, and 2358 cm−1 were attributed to hydrogen bonding between primary/secondary ammonium ions, carbamates, and CO2, validating that PEI@HP20 possesses physical adsorption effects on CO265,66.
The formation of ammonium carbamate and the adsorption of physisorbed CO2 in PEI@HP20 were further corroborated by DFT calculations. Several possible structures between CO2 and PEI-800 were modeled (Supplementary Fig. 21) and the corresponding 1H and 13C NMR chemical shifts were then calculated to compare with the experimental values in Fig. 4c-e to ascertain the most suitable structure. For the chemisorbed CO2, the ammonium carbamate chain formed from primary amine with strong bonding to adjacent NHR3+ has the best match with the experimental results: the calculated carbamate 13C NMR chemical shift is 165.9 ppm (Fig. 6a) which is close to the observed 165.4 ppm (Fig. 5c). Meanwhile, the calculated 1H NMR shifts of -NHRCO2− and NHR3+ are 4.6 and 13.7 ppm (Fig. 6a), respectively, which are also in good agreement with the experimental data (4.7 and 12.3 ppm, Fig. 5e). In the ammonium carbamate chain structure, the distances between the carbon and oxygen atoms of carbamate with the proton of ammonium are measured as 1.92 and 1.02 Å, respectively (Fig. 6a). As for the physisorbed CO2, the calculation suggests the NH of -NHRCO2− is the primary adsorption site of CO2 via forming a H-bond with the oxygen of CO2 (Fig. 6b). The calculated chemical shift of CO2 is 132.4 ppm which matches well with the experimental data (132.2 ppm). The distance between oxygen of CO2 and hydrogen of -NHRCO2− is measured as 3.11 Å. NH groups in many MOFs have been reported to exhibit strong binding interaction towards CO267,68,69. Based on the combined multinuclear SSNMR experiments and computational data in this study, the structure of PEI@HP20-CO2 can now be proposed, as illustrated in Fig. 6c. The pores of HP20 contains abundant PEI-800 and the primary amine NH2 reacts with CO2 to form ammonium carbamate. Due to the strong hydrogen bonding between carbamate and the proton of ammonium in adjacent PEI-800, the PEI-800 derived ammonium carbamate chains formed into network-like structure which are located in the inhomogeneous pores of HP20. The formation of ammonium carbamate is responsible for the steep adsorption of CO2 at low pressure (<1 kPa, Fig. 1c). Meanwhile, the NH group of ammonium carbamate acts as strong binding site for the physisorbed CO2 which contributes to the continual adsorption of CO2 above 1 kPa.
The DFT calculated local structures of ammonium carbamate (a) and physisorbed CO2 (b) in PEI@HP20 along with the calculated NMR parameters and atom distances. c the proposed structures of chemically and physically adsorbed CO2 in PEI@HP20. Color code: carbon: grey, hydrogen: white, nitrogen: blue, oxygen: red.
Discussion
In summary, we have successfully obtained the new PEI-800 loaded adsorbent, PEI@HP20, by a simple, green, and scalable wet impregnation method. This adsorbent sets a new benchmark for the preferential capture of trace CO2 from C2H2 with ultra-high CO2/C2H2 selectivity, high CO2 adsorption capacity, low regeneration temperature, and excellent cycle stability. The pilot-scale PTSA test demonstrates the great potential of PEI@HP20 for large-scale industrial application. The excellent performance is enabled through a dual physisorption/ chemisorption mechanism. Multinuclear and in situ SSNMR experiments reveal that the chemisorption is associated with the formation of an ammonium carbamate network and the NH group of ammonium carbamate is responsible for the subsequent physisorption. The physicochemical co-adsorption mechanism of CO2 in PEI@HP20 is further confirmed by DFT calculations in which a detailed structure model is successfully obtained. Thus, this work provides a common and efficient method for the development of CO2 selective adsorbents, and greatly promotes the industrialization of adsorption separation technology in the field of C2H2 purification.
Methods
Synthesis of PEI@HP20
A series of branched polyethyleneimine (PEI-800, Mw = 800) functionalization materials containing 30, 40, 50, and 60 wt.% PEI were prepared by the impregnation method. HP20 resin was first dried at 100 °C by heating under low vacuum for 12 h using a BSD-PM2 pretreatment station. Then, a stoichiometric amount of PEI was dissolved in 50 mL of water, and 500 mg of dried HP20 was added to the solution followed by stirring for 12 h. The water was removed by rotary evaporation, and white PEI@HP20 particles were then dried overnight under vacuum. The loading of PEI in the HP20 was determined using the following equation:
PEI + HP20: the sample was prepared by directly mixing 50 mg of PEI-800 and 50 mg of HP20. Before the NMR test, the sample was dried at 100 °C under vacuum for three hours.
Gas sorption experiments
Gas sorption isotherms were collected on the BSD-PM2 instrument, in which the sample was activated by heating at 373 K under vacuum for 10 h prior to analysis. The experimental temperatures were controlled by liquid N2 bath (77 K) and water-ethylene glycol bath (273, 298, and 308 K), respectively.
FT-IR experiments
FT-IR experiments were carried out on Nicolet IS10 FT-IR spectrometer produced by Shimadzu. Potassium bromide, HP20, and PEI@HP20 were pre-dried overnight in a vacuum oven at 80 °C. In a typical procedure, 1 ( ± 0.1) mg sample and 100 ( ± 0.2) mg potassium bromide were ground in an agate mortar. They were then kept in a vacuum oven at 80 °C for 3 h. After cooling to room temperature and refilling with nitrogen, the pellet was immediately transferred to a Nicolet IS10 FT-IR spectrometer for testing. The FT-IR spectra of PEI@HP20-CO2 were obtained after the tablets were placed in CO2 atmosphere for 30 min.
Sample preparation for SSNMR experiments
For magic angle spinning (MAS) SSNMR experiments, the HP20 and PEI@HP20 samples were directly packed into rotors. For PEI@HP20-13CO2, ca. 50 mg PEI@HP20 sample was first packed into a 7 mm diameter glass tube and activated under vacuum while heating at 120 °C for 12 h. Then the activated PEI@HP20 was exposed to 1 bar of 13C labelled CO2 (13CO2: Sigma-Aldrich, 99% 13C enriched) in a in-house vacuum line for 1 h to enable PEI@HP20 to adsorb enough 13CO2. Then, the glass tube was flame-sealed off from the vacuum line. For MAS SSNMR test, the sealed glass tube with PEI@HP20-13CO2 was first broken and then, the PEI@HP20-13CO2 powder was quickly packed into rotor for the subsequent test.
For in situ VT SSNMR experiments, the sample preparation is the same as the above PEI@HP20-13CO2 except 5 mm glass tubes were used and the flame-sealed glass tubes were directly inserted into NMR probe. For PEI@HP20-C2D2, the used gas was 99% 2H enriched C2D2 purchased from CDN Isotopes.
SSNMR experiments
1H,13C, and in situ VT 13C SSNMR experiments at 14.1 T were performed on a Bruker WB Avance Neo 600 MHz spectrometer. 1H MAS spectra were performed on a 2.5 mm HFXY four-resonance MAS probe with a spinning frequency of 25.0 kHz, π/2 pulse width of 2.5 μs and a recycle delay of 3 s. The 2D 1H − 1H SQ − SQ spectra were collected on a 2.5 mm HFXY four-resonance MAS probe using a mixing time of 25 ms, 512 t1 increments of 30 μs with 128 scans, and a recycle delay of 2 s. 13C cross polarization magic angle spinning (13C CP/MAS NMR) spectra were acquired with a 4 mm double-resonance MAS probe with a spinning rate of 10.0 kHz, a contact time of 3 ms, a recycle delay of 3 s and 5000 number of scans. The 1H-13C heteronuclear correlation (HETCOR) experiment was performed by a 4 mm double-resonance MAS probe with a spinning rate of 10.0 kHz and a contact time of 2 ms. A total of 64 t1 increments of 40 μs with 1000 scans and 1.5 s recycle delay were used. For the homonuclear decoupling in the indirect dimension, the frequency-switched Lee-Goldburg (FSLG) pulse was used at the 1H nutation frequency of 100 kHz5.
In situ VT 2H SSNMR experiments at 9.4 T were acquired using a Varian InfinityPlus wide-bore NMR spectrometer equipped with a 5 mm HX static Varian/Chemagnetics probe. The experiments were referenced using D2O(l) at 0.0 ppm as a secondary reference and performed using a 90°–90° solid echo pulse sequence, with a 90° pulse width of 3.75 µs and an interpulse delay of 45.0 µs. The calibrated 2H recycle delay was 2.0 s and the number of scans required for static 2H VT SSNMR experiments are 256 (298 K), 256 (273 K), 512 (253 K), 512 (233 K), 1024 (213 K).
In situ VT 13C SSNMR experiments at 14.1 T were acquired using a 5 mm SOL HX static probe. The experimental chemical shifts were referenced to TMS at 0.0 ppm and performed using single pulse sequence with a π/2 pulse of 6 μs and a recycle delay of 3 s. The number of scans used for static 13C VT SSNMR experiment were 2500 (298 K), 1800 (323 K), 1300 (353 K), 1300 (373 K), 1300 (391 K).
Breakthrough experiments
The breakthrough experiments were carried out in in-house built dynamic gas breakthrough equipment (Supplementary fig. 13). In the breakthrough system, the flow rates of all gases are regulated by mass flow controllers (Sevenstar Co., Beijing, China, D07-19B). An activated sample of PEI@HP20 or Zn-ox-mtz was packed into a high borosilicate glass column (1.6 cm inner diameter × 70 cm length) and the remaining volume in the column was filled by glass wool. Helium gas (with a flow rate of 20 mL·min−1 for 600 min at 373 K) was initially purged into the packed column to ensure that no other gases were detected in the effluent. Then, the desired gas mixtures (CO2/C2H2: 1/99) with a flow rate of 30 mL·min−1 at 298 K was dosed into the column. Effluent from the bed was monitored by gas chromatography (Nexis GC-2030). Between breakthrough experiments, the adsorbent was regenerated by a helium flow of 20 mL·min−1 for 600 min at 373 K to guarantee the complete removal of the adsorbed gas. Based on the mass balance, the gas sorption capacities can be determined as follows:
where\({\Delta }q\) is the equilibrium adsorption capacity of gas i (mmol·g−1), Ci is the feed gas concentration, V is the volumetric feed flow rate (mL·min−1), t is the adsorption time (min), F0 and F are the inlet and outlet gas molar flow rates, respectively, and m is the mass of the adsorbent (g). The 100% humidity is controlled as follows: after the raw mixture gas is fully humidified by the humidity-generating device equipped with saturated potassium sulfate solution, the humidity of the discharged wet raw gas is about 100%, which was used as the raw gas in the breakthrough experiment under humid conditions for investigating the moisture resistance of the adsorbent.
Data availability
The data generated in this study are provided in the Supplementary Information. Additional data are available from the corresponding author upon request. Source data are provided with this paper.
References
Pässler, P. et al. Acetylene. vol. 1 (Wiley-VCH, 2011).
Statista Research Department. Market value of acetylene worldwide in 2022 and 2027. Statista https://www.statista.com/statistics/933160/global-market-value-of-acetylene/ (2023).
Lin, R.-B. et al. Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material. J. Am. Chem. Soc. 139, 8022–8028 (2017).
Li, J.-R., Kuppler, R. J. & Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Plessis, P. & Marmet, P. Electroionization study of acetylene and fragment ions. Int. J. Mass Spectrom. Ion-. Process. 70, 23–44 (1986).
Lokachari, N. et al. New experimental insights into acetylene oxidation through novel ignition delay times, laminar burning velocities and chemical kinetic modelling. Proc. Combust. Inst. 37, 583–591 (2019).
Jha, P. & Vininski, J. V. Acetylene process gas purification methods and systems. Worldwide patent WO2010065880A2 (2010).
Fan, W. et al. Optimizing multivariate metal–organic frameworks for efficient C2H2/CO2 separation. J. Am. Chem. Soc. 142, 8728–8737 (2020).
Peng, X. et al. One-step ethylene purification from a seven-component cracking gas mixture with sorbent-sorbate induced-fit. CCS Chemistry N/A, 1–13 (2024).
Fang, M., Yi, N., Di, W., Wang, T. & Wang, Q. Emission and control of flue gas pollutants in CO2 chemical absorption system – A review. Int. J. Greenh. Gas. Control 93, 102904 (2020).
Thompson, J. G. et al. Determining the Henry’s volatility coefficient of nitrosamines in CO2 capture solvents. Int. J. Greenh. Gas. Control 73, 104–110 (2018).
Yang, R. T. Gas Separation by Adsorption Processes. vol. 1 (World Scientific, 1997).
Wu, X.-C. et al. A large-scale synthesizable superhydrophobic C2H6-selective MOF for C2H6/C2H4 separation. Microporous Mesoporous Mater. 378, 113257 (2024).
Jiang, Y.-P. et al. Ideal molecular splitter with object adaptive for benchmark n-butylene/iso-butylene separation. ACS Mater. Lett. 6, 4972–4979 (2024).
Zhao, L. et al. Exhaled anesthetic xenon regeneration by gas separation using a metal–organic framework with sorbent-sorbate induced-fit. Angew. Chem. Int. Ed. 63, e202407840 (2024).
Zhao, L. et al. Robust ultra-microporous metal-organic frameworks for highly efficient natural gas purification. Nano Res. 16, 12338–12344 (2023).
Lide, D. R. CRC Handbook of Chemistry and Physics. vol. 85 (CRC press, 2004).
Ma, B. et al. A zeolitic octahedral metal oxide with ultra-microporosity for inverse CO2/C2H2 separation at high temperature and humidity. Angew. Chem. Int. Ed. 61, e202209121 (2022).
Li, Y. et al. A microporous hydrogen bonded organic framework for highly selective separation of carbon dioxide over acetylene. Angew. Chem. Int. Ed. 62, e202311419 (2023).
Wang, X. et al. Metal-organic frameworks for C2H2/CO2 separation: Recent development. Coord. Chem. Rev. 482, 215093 (2023).
Wang, W. et al. Discriminatory gate-opening effect in a flexible metal–organic framework for inverse CO2/C2H2 separation. Small 19, 2302975 (2023).
Mersmann, A., Fill, B., Hartmann, R. & Maurer, S. The potential of energy saving by gas‐phase adsorption processes. Chem. Eng. Technol. 23, 937–944 (2000).
Wang, L. et al. Efficient capture of C2H2 from CO2 and CnH4 by a novel fluorinated anion pillared MOF with flexible molecular sieving effect. Nano Res. 16, 3536–3541 (2023).
Berkbigler, G. et al. C2H2/CO2 separation with a chain-type Zn pyrazolate MOF. Eur. J. Inorg. Chem. 27, e202300548 (2024).
Zhang, L. et al. Isoreticular contraction of cage-like metal–organic frameworks with optimized pore space for enhanced C2H2/CO2 and C2H2/C2H4 separations. J. Am. Chem. Soc. 146, 7341–7351 (2024).
Ji, Z. et al. Synergistic C2H2 binding sites in hydrogen-bonded supramolecular framework for one-step C2H4 purification from ternary C2 mixture. Angew. Chem. Int. Ed. 63, e202411175 (2024).
Jiang, M. et al. Controlling pore shape and size of interpenetrated Anion-Pillared ultramicroporous materials enables molecular sieving of CO2 combined with ultrahigh uptake capacity. ACS Appl. Mater. Interfaces 10, 16628–16635 (2018).
Chen, Z. et al. Polyethylenimine-impregnated resin for high CO2 adsorption: an efficient adsorbent for CO2 capture from simulated flue gas and ambient air. ACS Appl. Mater. Interfaces 5, 6937–6945 (2013).
Liu, F., Wang, S., Lin, G. & Chen, S. Development and characterization of amine-functionalized hyper-cross-linked resin for CO2 capture. N. J. Chem. 42, 420–428 (2018).
Shi, X. et al. Highly enhancing the characteristics of immobilized thermostable β-glucosidase by Zn2+. Process Biochem. 66, 89–96 (2018).
Balgis, R., Sago, S., Anilkumar, G. M., Ogi, T. & Okuyama, K. Self-organized macroporous carbon structure derived from phenolic resin via spray pyrolysis for high-performance electrocatalyst. ACS Appl. Mater. Interfaces 5, 11944–11950 (2013).
He, H., Zhuang, L., Chen, S., Liu, H. & Li, Q. Structure design of a hyperbranched polyamine adsorbent for CO2 adsorption. Green. Chem. 18, 5859–5869 (2016).
Witoon, T. Polyethyleneimine-loaded bimodal porous silica as low-cost and high-capacity sorbent for CO2 capture. Mater. Chem. Phys. 137, 235–245 (2012).
Cheng, D., Liu, Y., Wang, H., Weng, X. & Wu, Z. Enhanced CO2 adsorptive performance of PEI/SBA-15 adsorbent using phosphate ester based surfactants as additives. J. Environ. Sci. 38, 1–7 (2015).
Yu, B. et al. Characterisation and kinetic study of carbon dioxide absorption by an aqueous diamine solution. Appl. Energy 208, 1308–1317 (2017).
Holycross, D. R. & Chai, M. Comprehensive NMR studies of the structures and properties of PEI polymers. Macromolecules 46, 6891–6897 (2013).
Dillon, E. P., Crouse, C. A. & Barron, A. R. Synthesis, characterization, and carbon dioxide adsorption of covalently attached polyethyleneimine-functionalized single-wall carbon nanotubes. ACS Nano 2, 156–164 (2008).
Zhang, Z. et al. Exclusive recognition of CO2 from hydrocarbons by aluminum formate with hydrogen-confined pore cavities. J. Am. Chem. Soc. 145, 11643–11649 (2023).
Yang, S.-Q. et al. Immobilization of the polar group into an ultramicroporous metal–organic framework enabling benchmark inverse selective CO2/C2H2 separation with record C2H2 production. J. Am. Chem. Soc. 145, 13901–13911 (2023).
Xie, Y. et al. Electrostatically driven selective adsorption of carbon dioxide over acetylene in an ultramicroporous material. Angew. Chem. Int. Ed. 133, 9690–9695 (2021).
Huang, J. et al. Enhanced adsorption of trace carbon dioxide from acetylene using polyethyleneimine-diethanolamine blends. Chem. Eng. J. 493, 152859 (2024).
Qazvini, O. T., Babarao, R. & Telfer, S. G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 12, 197 (2021).
Shi, Y. et al. Highly selective adsorption of carbon dioxide over acetylene in an ultramicroporous metal–organic framework. Adv. Mater. 33, 2105880 (2021).
Ma, D. et al. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 8, 11933–11937 (2020).
Zhang, Z. et al. Optimal pore chemistry in an ultramicroporous metal–organic framework for benchmark inverse CO2/C2H2 separation. Angew. Chem. Int. Ed. 60, 17198–17204 (2021).
Krishna, R. Metrics for Evaluation and Screening of Metal–Organic Frameworks for Applications in Mixture Separations. ACS Omega 5, 16987–17004 (2020).
Wang, L. et al. Interpenetration symmetry control within ultramicroporous robust boron cluster hybrid MOFs for benchmark purification of acetylene from carbon dioxide. Angew. Chem. Int. Ed. 60, 22865–22870 (2021).
Zhou, Z. et al. Carbon dioxide capture from open air using covalent organic frameworks. Nature 635, 96–101 (2024).
Choi, D. S. et al. Highly selective CO2 separation from a CO2/C2H2 mixture using a diamine-appended metal–organic framework. J. Mater. Chem. A 9, 21424–21428 (2021).
Bai, G. et al. Polyethylenimine (PEI)-impregnated resin adsorbent with high efficiency and capacity for CO2 capture from flue gas. N. J. Chem. 43, 18345–18354 (2019).
Chen, M. et al. Analyzing gas adsorption in an amide-functionalized metal organic framework: Are the carbonyl or amine groups responsible? Chem. Mater. 30, 3613–3617 (2018).
Chen, S. et al. Cleaving carboxyls: Understanding thermally triggered hierarchical pores in the metal–organic framework MIL-121. J. Am. Chem. Soc. 141, 14257–14271 (2019).
Kong, X. et al. CO2 dynamics in a metal–organic framework with open oetal sites. J. Am. Chem. Soc. 134, 14341–14344 (2012).
Mafra, L. et al. Structure of chemisorbed CO2 species in amine-functionalized mesoporous silicas studied by solid-state NMR and computer modeling. J. Am. Chem. Soc. 139, 389–408 (2017).
Pinto, M. L., Mafra, L., Guil, J. M., Pires, J. & Rocha, J. Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem. Mater. 23, 1387–1395 (2011).
Chen, S., Lucier, B. E. G., Boyle, P. D. & Huang, Y. Understanding the fascinating origins of CO2 adsorption and dynamics in MOFs. Chem. Mater. 28, 5829–5846 (2016).
Zhu, Z. et al. High-capacity, cooperative CO2 capture in a diamine-appended metal–organic framework through a combined chemisorptive and physisorptive mechanism. J. Am. Chem. Soc. 146, 6072–6083 (2024).
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 519, 303–308 (2015).
Mao, V. Y. et al. Cooperative carbon dioxide adsorption in alcoholamine- and alkoxyalkylamine-functionalized metal–organic frameworks. Angew. Chem. Int. Ed. 59, 19468–19477 (2020).
Forse, A. C. et al. Elucidating CO2 chemisorption in diamine-appended metal–organic frameworks. J. Am. Chem. Soc. 140, 18016–18031 (2018).
Kim, E. J. et al. Cooperative carbon capture and steam regeneration with tetraamine-appended metal–organic frameworks. Science 369, 392–396 (2020).
Zhai, Y. & Chuang, S. S. C. The nature of adsorbed carbon dioxide on immobilized amines during carbon dioxide capture from air and simulated flue gas. Energy Technol. 5, 510–519 (2017).
Bacsik, Z., Atluri, R., Garcia-Bennett, A. E. & Hedin, N. Temperature-induced uptake of CO2 and formation of carbamates in mesocaged silica modified with n-propylamines. Langmuir 26, 10013–10024 (2010).
Jung, H., Jeon, S., Jo, D. H., Huh, J. & Kim, S. H. Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents. Chem. Eng. J. 307, 836–844 (2017).
Gómez Castaño, J. A., Fantoni, A. & Romano, R. M. Matrix-isolation FTIR study of carbon dioxide: Reinvestigation of the CO2 dimer and CO2 ⋯N2 complex. J. Mol. Struct. 881, 68–75 (2008).
Fredin, L., Nelander, B. & Ribbegård, G. On the dimerization of carbon dioxide in nitrogen and argon matrices. J. Mol. Spectrosc. 53, 410–416 (1974).
Moreau, F. et al. Unravelling exceptional acetylene and carbon dioxide adsorption within a tetra-amide functionalized metal-organic framework. Nat. Commun. 8, 14085 (2017).
Xiang, Z., Leng, S. & Cao, D. Functional group modification of metal–organic frameworks for CO2 capture. J. Phys. Chem. C. 116, 10573–10579 (2012).
Zhu, Y. et al. Three N–H functionalized metal–organic frameworks with selective CO2 uptake, dye capture, and catalysis. Inorg. Chem. 53, 7692–7699 (2014).
Acknowledgements
Y.P. and S.C. acknowledge financial support from the National Natural Science Foundation of China (no. 22201304 and no. 22105091), the Science Foundation of China University of Petroleum, Beijing (2462021QNXZ011, 2462022YXZZ007). and Fundamental Research Funds for the Central Universities (lzujbky-2024-ey06). Y.H. thanks the Natural Sciences and Engineering Research Council of Canada for a Discovery Grant.
Author information
Authors and Affiliations
Contributions
Y-L.P., S.C., designed the project and experiments. Y-L.P. and J-S.Z. prepared the samples and collected FTIR and gas sorption data. S.C., Z.W., J.X., W.Z., B.E., G.L. and Y.H. collected and processed NMR data, Z.W. conducted DFT calculations. Z.Z., Y.J., X-C.W. and J.L. collected TG data. Y-L.P., J-S.Z. and S.C. wrote the first draft of manuscript and Y-L.P., S.C., Y.H.A., G.C. and M.J.Z. performed manuscript edits and revisions. All authors contribute to the final manuscript and the interpretation of the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks, the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zou, JS., Wang, ZP., Andaloussi, Y.H. et al. Benchmarking selective capture of trace CO2 from C2H2 using an amine-functionalized adsorbent. Nat Commun 16, 2598 (2025). https://doi.org/10.1038/s41467-025-57972-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-57972-7