Abstract
Glycosylated RNAs (glycoRNAs), a recently discovered class of membrane-associated glyco-molecules, remain poorly understood in function and clinical value due to limited detection methods. Here, we show a dual recognition Förster resonance energy transfer (drFRET) strategy using nucleic acid probes to detect N-acetylneuraminic acid-modified RNAs, enabling sensitive, selective profiling of glycoRNAs on small extracellular vesicles (sEVs) from minimal biofluids (10 μl initial biofluid). Using drFRET, we identify 5 prevalent sEV glycoRNAs derived from 7 cancer cell lines. In a 100-patient cohort (6 cancer types and non-cancer controls), sEV glycoRNA profiles achieve 100% accuracy (95% confidence interval) in distinguishing cancers from non-cancer cases and 89% accuracy in classifying specific cancer types. Furthermore, drFRET reveal that sEV glycoRNAs specifically interact with Siglec proteins and P-selectin, which is critical for sEV cellular internalization. The drFRET strategy provides a versatile and sensitive platform for the imaging and functional analysis of sEV glycoRNAs, with promising implications for clinical applications.
Similar content being viewed by others
Introduction
The presence of modified RNAs on the surface of mammalian cells initially referred to as membrane-associated RNAs (maxRNAs), has been revealed by recent studies1. Currently, partial structural information and functional relevance of maxRNAs have been determined. For example, Flynn et al. confirmed the presence of N-glycosylation on specific small RNAs, termed glycosylated RNAs (glycoRNAs), utilizing biochemical methods2. Glycosylation of RNAs is important as various RNA modifications (e.g., pseudouridylation, methylation, and adenylation) play pivotal roles in diverse cellular processes (e.g., tRNA-mediated translation, RNA epigenetics, chromatin structure modulation, and RNA maturation)3,4,5,6. Zhang et al. discovered that glycoRNAs enhance the recruitment of neutrophils to inflammatory sites in vivo through their interaction with P-selectin on endothelial cells, and their expression and function are dependent on Sidt genes7. Despite recent progress, our understanding of glycoRNAs lags behind that of proteins, lipids, and glycans.
There is a significant need for the development of techniques to detect and analyze glycoRNAs. Several methods have been developed for the identification of glycoRNAs. Click chemistry and metabolic labeling-based blots or imaging techniques are effective in characterizing the RNA and glycan portions of glycoRNAs8,9, respectively. The integration of metabolic labeling with next-generation sequencing enables a precise profile of glycoRNA sequences10. In addition, the putative structures of glycoRNAs “glycan composition” can be precisely identified by mass spectrometry11. The ARPLA technique, developed by Ma et al., facilitates spatial imaging of cell surface glycoRNAs12. However, these methods often necessitate intricate experimental designs and rigorous conditions, and the simultaneous provision of sequence and spatial information remains challenging. Therefore, the development of simplified methods for sensitive and specific profiling of glycoRNAs is crucial to further investigate their molecular characteristics and biofunctions.
Numerous functional phospholipid bilayer structures undergoing dynamic transformation across the cell membrane carry a substantial amount of material information highly similar to that of the progenitor cells13. This raises intriguing questions regarding the role of glycoRNAs and their potential presence on other membranous structures. For instance, it is worth exploring whether glycoRNAs exist within membraned organelles or extracellular membrane structures, such as extracellular vesicles. Small extracellular vesicles (sEVs, typically 30–150 nm in diameter) are a subtype of phospholipid membrane-enclosed nanoscale vesicles that are naturally secreted by various cells14. Cells actively orchestrate intricate biological processes by secreting sEVs loaded with a diverse array of biomolecules (e.g., proteins, nucleic acids, lipids, and glycans), and the cargo-specific regulation and biogenesis of sEVs are indispensable cellular processes15,16. Therefore, it is highly likely that glycoRNAs are present on sEVs. Notably, the majority of biomolecules carried by sEVs are known functionally relevant in intercellular communication, immune responses and infection, metabolic and cardiovascular diseases, neurodegeneration, and cancer17,18,19,20,21. The anchoring of glycoRNAs on sEVs may reflect concealed biochemical information and exert significant regulatory effects on sEV function. Investigating the presence of glycoRNAs on sEVs and their impact on sEV function is a significant need that requires the development of suitable methods.
In this work, we present a dual recognition Förster resonance energy transfer (drFRET) strategy that combines dimensionality-reduction algorithms to enable the imaging of multiple glycoRNAs on sEVs with high sensitivity and selectivity. We utilize two distinct DNA probes: one as an N-acetylneuraminic acid (Neu5Ac) probe for glycan recognition (GRPs), and the other as an in situ hybridization probe (ISHPs) for detecting glycoRNAs. The drFRET method relies on the non-radiative energy transfer occurring through dipole-dipole coupling from an excited-state donor fluorophore to a ground-state acceptor when the proximity requirement is satisfied, which effectively prevents false-positive signals. Through drFRET, we successfully detect FRET signals of multiple glycoRNAs on cancer cell-secreted and serum-derived sEVs. The unweighted sum of the levels of five individual glycoRNAs on sEVs exhibits high accuracy in cancer diagnosis through the integration of dimensionality reduction analysis algorithms for the automated classification and visualization of six cancer types. Finally, we demonstrate that the ligands of Siglec proteins consist partially of sEV glycoRNAs, which play a pivotal role in the cellular internalization of sEVs. Overall, the drFRET approach advances the toolbox for glycoRNAs detection and functional analysis, offering promising biomarkers (sEV glycoRNAs) for early precise disease diagnosis.
Results
Presence of glycoRNAs in sEVs
Flynn et al. metabolically converted azide-modified sialic acid precursors (N-azidoacetylmannosamine-tetraacylated, Ac4ManNAz) into the corresponding azido sialic acid and incorporated them into the N-glycans of glycoRNA2. However, the incorporation of such metabolically labeled glycoRNAs into sEV biogenesis and subsequent integration into sEVs remains currently unknown (Fig. 1a). The metabolically labeled glycoRNAs, once integrated into sEVs, can be readily detected through click chemistry reactions (Fig. 1b). To investigate the presence of sialoglycans-modified RNAs (i.e., glycoRNAs) on sEVs, HeLa cells were metabolically labeled with 100 μM Ac4ManNAz, a metabolic chemical reporter (MCR), for 36 h. Subsequently, sEVs were extracted using classical differential ultracentrifugation while ensuring the inclusion of necessary RNase inhibitors. High-purity RNA was then obtained by employing rigorous chemical and enzymatic methods in conjunction with RNA extraction kits (refer to the Methods section for details). In brief, RNA was extracted using warm TRIpure (similar to TRIzol, containing acid phenol and guanidine salts), followed by ethanol precipitation, desalting through FastPure RNA columns, removal of protein contamination via high concentration proteinase K digestion, and subsequent repurification over columns. To visualize azide-labeled components, copper-free click chemistry was employed by treating RNA samples with dibenzocyclooctyne-polyethylene-glycol-4-biotin (DBCO-PEG4-biotin, 25 °C), followed by denaturation (95% formamide) at 65 °C. The resulting products were then separated using denaturing gel electrophoresis and analyzed by blotting (Fig. 1c).
a Incorporation of Ac4ManNAz, a glycan reporter, into sEV RNAs. Cartoon highlighting the glycoRNA anchoring onto the surface of sEVs, a process that has not been previously characterized. Ac4ManNAz, peracetylated N-azidoacetylmannosamine. b Schematic diagram illustrating strategy for metabolic labeling. c Schematic of sEV RNA extraction protocol. Prot. K, proteinase K; DBCO, dibenzocyclooctyne. d RNA blotting of RNA extracted from HeLa cell-derived sEVs and HeLa cells treated with 100 μM Ac4ManNAz for the indicated duration. After sEV isolation and RNA purification, Ac4ManNAz was conjugated to DBCO-PEG4-biotin, visualized via a chemiluminescent nucleic acid detection module (Strep, bottom), and scanned with an ultra-sensitive chemiluminescence imaging system. Before RNA transfer to the nitrocellulose membrane, total RNA was stained and imaged with YeaRed reagent (YeaRed, top) to interrogate quality and loading. The regions where glycoRNAs are present are highlighted in red text, while non-specific labeling (nsl) is also indicated. Molecular weight standard: DNA ladder (DNA Marker 2000, see Methods for more information). Notably, the use of a DNA ladder limited the precise determination of molecular weight. Representative images are shown. 3 s: exposure time. e The gray values of different lanes along the dotted line direction in (d) are shown in the histograms. f Dot blotting of RNA from HeLa cells-derived sEVs. Representative images from separate blots are shown. Data are representative of two independent experiments with similar results. g Expression of sEV glycoRNAs analyzed by chemiluminescence dot-blot. GlycoRNAs were metabolically labeled by 100 μM Ac4ManNAz and 100 μM Ac4GalNAz (N-azidoacetylgalactosamine-tetraacylated), respectively. An equal quantity of RNAs was spotted on the polyvinylidene difluoride membranes prior to staining. Data are representative of three independent experiments with similar results. Representative images from separate blots are shown. Data in (g) are shown as mean ± s.d. Normalized units (norm.); arbitrary units (arb.). Source data are provided as a Source Data file.
By employing denaturing electrophoresis and subsequent blotting, we detected biotin signals in RNA derived from HeLa cells as well as HeLa cell-derived sEVs, and we observed the presence of biotinylated species exclusively after metabolic labeling in both samples (Fig. 1d). Despite co-incubation of purified RNA with DBCO-PEG4-biotin, no biotin signal was observed in the absence of Ac4ManNAz labeling (lane 4 of Fig. 1d). Electrophoresis effectively separated major visible RNAs, including small RNAs/tRNA, 18S rRNA, and 28S rRNA (as inferred from distinct three-band patten in the case of using DNA ladder, Fig. 1d), and gray value (YeaRed) profiling of the lanes demonstrates consistent loading amounts of purified RNA (10 µg), and lanes with MCR labeling exhibit clear signals (2 and 3) compared to the controls without MCRs, indicating that the observed biotin signals originated from the incorporation of MCRs into RNA glycans (Fig. 1e). Previous studies have shown that distinct rRNA (18S and 28S) were diminished in extracellular vesicle samples, including sEVs, while small RNA species were enriched (e.g., in DKO-1 and GIi36 cell lines)22. The lack of reduction in rRNA levels in HeLa cell-derived sEVs may be influenced by multiple factors, such as systematic differences in biological samples, experimental conditions, and methodological variations; however, it is important to note that gel electrophoresis provides qualitative rather than precise quantitative data. In addition, the biotin signal migrates more slowly than rRNA, and any background signal detected at the main rRNA band location is negligible (Fig. 1d and e). These data provide evidence that the glycoRNA signal is not a result of non-specificity associated with the Ac4ManNAz-DBCO-PEG4-biotin labeling strategy. Furthermore, to further validate the linking between glycans and RNAs, we increased the loading amount of RNA (15 µg) and observed an increase in biotin signal; however, no signal was detected in the control group (lane 8), indicating a direct connection between MCRs and RNAs. Figure 1e illustrates the distribution of biotin signals within the lane, demonstrating minimal background labeling while highlighting prominent biotin signals for Ac4ManNAz-labeled sEV RNAs and cellular RNAs. Together, these data support the presence of glycoRNAs in sEVs.
Currently, glycoRNA is predominantly believed to undergo N-glycan modification, with the glyco-terminus not always exhibiting sialylation; in certain instances, they may instead feature galactosylation. Subsequently, glycoRNAs were metabolically labeled using Ac4ManNAz (100 μM) and Ac4GalNAz (100 μM, N-azidoacetylgalactosamine-tetraacylated, an azide-labeled monosaccharide) and subsequently purified from HeLa cell-secreted sEVs. Equal amounts of sEV-derived RNAs were spotted onto polyvinylidene difluoride membranes for subsequent staining. We further confirmed the presence of glycoRNA on sEVs using the dot blotting method (a streamlined protocol). Equal amounts of sEV-derived RNAs were spotted onto polyvinylidene difluoride membranes for subsequent staining, and a clear biotin signal was only observed following MCRs treatment, consistent with previous results (Fig. 1f). In addition, dot-blot analysis revealed that HeLa cell-secreted sEVs were positive for MCRs, that is, Ac4ManNAz and Ac4GalNAz (Fig. 1g). The detection of MCR-related signals on sEV-extracted RNAs further confirms the presence of glycoRNA on sEVs. These results demonstrate the detectability of glycoRNAs on sEVs using conventional chemobiological assays, as well as their detection potential by altering MCRs to incorporate into diverse glycoRNA moieties. However, both RNA blotting and dot blotting are semi-quantitative techniques primarily used to qualitatively determine the presence of metabolically incorporated glycoRNAs on sEVs. These MCR-based metabolic labeling and click-based detection methods measure the MCRs incorporation rate and accessibility of the labeled glycans rather than the entire glycan pool on sEV glycoRNAs23,24. Moreover, current metabolic labeling-based methods such as RNA gel electrophoresis rely on MCRs and involve complex experimental procedures, while also lacking the capability for in situ detection of pre-existing glycoRNAs. The glycan structures of native glycoRNAs can be precisely characterized using mass spectrometry, which can be integrated with RNA sequencing to systematically compare the abundance, sialylation patterns, and sequences of glycoRNAs on sEVs and cells. Such comparisons would provide valuable insights into the differential roles and characteristics of glycoRNAs across various cellular contexts. Nonetheless, these techniques are intricate and complex, involving destructive processes and cumbersome isolation and purification steps, which currently preclude in situ imaging and functional analysis of sEV glycoRNAs. Therefore, we developed drFRET for the in situ detection of sEV glycoRNAs.
Development of drFRET for in situ imaging sEV glycoRNAs
Membrane-anchored hybrid molecules, such as glycoRNA on sEVs, occupy novel physical spaces, thereby creating new interfaces for biological functions25. More recently, our understanding of membrane-anchored glycoRNA has been expanding. A variety of flexible genetic and chemical tools, including diverse encoding and targeting biopolymers, have proven effective in elucidating the compositional and spatial features of glycoRNA. Given that proximity as a central concept in glycoRNA biology, it is imperative to develop robust proximity detection techniques for in situ imaging and functional analysis of sEV glycoRNA. Single-stranded nucleic acids (e.g., ssDNA) have wide applications in molecular imaging, biosensor design, and other fields as affinity reagents for various targets26,27. For in situ imaging glycoRNAs on sEVs, we devised drFRET utilizing two probes (Fig. 2a): GRPs containing ssDNAs with an aptamer sequence that specifically targets Neu5Ac12,27, which is covalently linked to Cyanine3 (Cy3); ISHPs with ssDNAs modified with Cyanine5 (Cy5) for in situ hybridization of RNA (Supplementary Table 1). ssDNA was engineered for Neu5Ac-specific binding, and the molecular dynamics simulation in Supplementary Fig. 1a illustrates the structural arrangement of a GRP and the molecular configuration of Neu5Ac, where appropriate spatial folding is an essential prerequisite for specific recognition. We initially employed total internal reflection fluorescence microscopy (TIRF) to visualize the potential spatial overlap between Neu5Ac and glycoRNAs on sEVs (Pearson’s r = 0.90), thereby elucidating the co-localization and heterogeneous distribution of both within sEV populations (Supplementary Fig. 1b). To validate the specificity of drFRET, we treated sEVs individually with RNase A and α2-3,6,8,9 Neuraminidase A prior to probe labeling, followed by capturing TIRF images. The α2-3,6,8,9 Neuraminidase A is a broad specificity sialidase, which cleaves linear and branched non-reducing terminal sialic acid residues from glyco-substrates (Supplementary Fig. 1c), while RNase A degrades RNA, theoretically attenuating the signal of ISHPs28,29. The GRPs signals significantly decreased when Neu5Ac was removed (P < 0.0001), while the ISHPs signals also diminished after glycoRNA hydrolysis (P < 0.01), demonstrating the specificity of both probes (Supplementary Fig. 1d).
a Schematic illustration of in situ FRET imaging of glycoRNAs. ISHPs were used for in situ hybridization of RNAs, whereas GRPs for specific targeting Neu5Ac. FRET occurs only when dual recognition of Neu5Ac and RNAs within a proximity of 10 nm. b Schematic of the confocal FRET imaging of sEV glycoRNAs. The bead-bound sEVs were uniformly deposited onto the focal plane of the microscope, and the FRET signal was automatically corrected using the sensitized emission method. The FRET efficiency and distance between GRPs (donor) and ISHPs (acceptor) were calculated. c Fluorophore photophysical properties, and our selection of wavelength for excitation (559 nm, green), optical filter (585 nm), and FRET collection band (650–700 nm, cyan). Excitation/readout optics and calculated inter-dye Förster radii (Rda). The ultraviolet-visible diffuse reflectance spectrum of the optically inert carrier fcPS is represented by the gray dashed line, which exhibits no absorption within the drFRET optical analysis bands.
Although TIRF imaging has revealed the co-localization (Pearson’s r = 0.90) of Neu5Ac with glycoRNA and the heterogeneous distribution of sEV populations (Supplementary Fig. 2), it is challenging to determine the connection between glycoRNAs and Neu5Ac using colocalization imaging. Hence, we developed drFRET for visualizing their spatial relationship. The inherent inverse-sixth-power distance dependence makes FRET a valuable tool for nanoscale proximity detection within a typical range of approximately 1–10 nm30. FRET signals between GRPs and ISHPs on sEVs were observed, indicating their close proximity. Treatment with DNase I (hydrolyzes phosphodiester bonds), RNase A, or their combination resulted in a significant reduction in the FRET signal by 91.5% (P = 0.0006), 88.6% (P = 0.0057), and 93.6% (P = 0.0021), respectively, suggesting that the colocalization-induced FRET signal predominantly occurred on the sEV surface rather than within its interior (Supplementary Fig. 3). Here, we settled the beads loaded with sEVs in a uniform focal plane. When glycoRNAs were present, the non-radiative energy transfer between GRPs and ISHPs was observed within the cross-section of the beads, demonstrating the formation of a FRET image highlighted by a circular outline in Fig. 2b. The calculated Förster radii (Rda = 53 Å, d and a are the donor and acceptor, respectively) underline their propensity for energy transfer (Fig. 2c). Considering the potential spectral crosstalk between the dyes on the probes, we employed the sensitized emission method to accurately correct FRET signals (referred to as corrected FRET or cdFRET, which is positively correlated with glycoRNA abundance). The cdFRET channels (see Methods for more information), depicted in cyan, are used for quantifying the glycoRNA content by recording its intensity in subsequently acquired images. In later experiments, we employed cdFRET intensities to compare the contents of multiple glycoRNAs.
In situ FRET imaging of glycoRNAs on cancer-derived sEVs
The high concentration (up to 1010 ml–1) and stability of tumor-derived sEVs in the bloodstream have been reported, enhancing their feasibility for acquisition31,32,33. In this study, sEVs were initially isolated and purified from cancer patient serum and cell culture supernatant for feasibility analyses (Supplementary Fig. 4). Morphological characterization of sEVs was conducted using transmission electron microscopy (TEM). sEVs exhibit typical spherical or cup shapes and form clusters, which is in accordance with previous findings (Fig. 3a)34. To efficiently enrich sEVs from biofluids and enable drFRET, we prepared HER2 protein-specific aptamer-functionalized polystyrene microbeads (~ 4 μm, fcPS, see Methods for more information) (Supplementary Fig. 5). It is worth noting that the HER2 protein possesses multiple large extracellular domains35, which facilitate DNA aptamers in contacting various regions of the extracellular domain and forming multi-point bindings36. This enhances both the stability and specificity of the binding interactions. The HER2 protein has been extensively utilized as a “catcher” for the specific capture of sEVs37,38,39. The sEVs captured by the HER2 aptamers were subsequently employed for drFRET assays. To prepare fcPS, we used commercially available aminated polystyrene beads and modified them using 3-maleimidopropionic acid N-hydroxysuccinimide ester (BMPS), an amine-to-sulfhydryl crosslinker. BMPS is a heterobifunctional crosslinker containing N-hydroxysuccinimide (NHS) ester and maleimide groups, enabling covalent conjugation between amine- and sulfhydryl-containing molecules. Consequently, BMPS-modified PS beads can readily react with thiol-modified sEV capture probes (sEVCP), such as HER2 protein-specific aptamers. The fcPS exhibits exceptional carrier properties without any surface plasmon resonance characteristics, and the ultraviolet-visible diffuse reflectance spectrum of the beads reveals minimal absorption in the emission bands of GRPs and ISHPs (also FRET collection bands), while not causing any quenching effect on the drFRET probes (Supplementary Fig. 6). sEV enrichment was confirmed by characterizing the microbeads with scanning electron microscopy (SEM) (Supplementary Fig. 7). Figure 3b shows that the fcPS-captured sEVs have typical spherical shapes and a smaller size distribution (calculated from SEM images) compared to sEVs (measured by nanoparticle tracking analysis) isolated by ultracentrifugation (Fig. 3c), which is consistent with previously reported findings40,41.
a Representative TEM images of sEVs derived from patient serum and cell culture supernatant, respectively. b SEM characterization of sEVs captured on beads. c Size distribution of sEVs characterized by NTA and SEM (n = 300 vesicles), respectively. d Confocal laser scanning microscopy (CLSM) images (cdFRET channel) of sEV glycoRNAs and various control groups in the absence of components for FRET imaging. sEVCP, sEV capture probe. m, cell culture medium. s, serum. Scale bars: 2 μm. e Quantification of average cdFRET intensity in (d). f Schematic illustration of specificity analysis for sEV glycoRNA FRET imaging and representative CLSM images of glycoRNAs on sEVs from cells treated with glycosylation inhibitors (left panel). Quantification of average cdFRET intensity (right panel). g Validation of the specificity for drFRET. sGRPs: glycan probe using DNA with scrambled sequence; us-ISHPs: unspecific RNA binding probes (see Supplementary Table 1 for detailed sequence information). n = 3 frames. All data are representative of three independent experiments. Data in (f and g) are shown as mean ± s.d. Statistical significance in (e, f, and g) was determined by a two-tailed Student’s t test. P-values are indicated in the charts.
To validate drFRET, we placed fcPS loaded with HeLa cell-derived sEVs in the optical focal plane to observe cdFRET channel (Fig. 3d). FRET signals of glycoRNAs were observed on both sEVs derived from cell culture medium (m) and serum sample (s) when all the essential drFRET components were present, as depicted in Fig. 3d and the respective channels of drFRET probes (Supplementary Fig. 8). However, the absence of sEVCP, sEVs, GRPs, or ISHPs in drFRET resulted in the significant reduction of all signals by over 20-fold (P < 0.01), demonstrating the necessity of all components (Fig. 3e). In addition, to determine the specificity of cdFRET signals for glycoRNAs, we manipulated glycoRNA expression using several glycosylation inhibitors2,12,42. After the inhibitor-treated (24 h) cells were cultured for 48 h (37 °C, 5% CO2), we isolated sEVs for drFRET to image glycoRNAs (Fig. 3f). cdFRET signals of sEV glycoRNAs were reduced by 71.1% (P = 0.0078), 69.3% (P = 0.0043), or 69.5% (P = 0.0106) compared to the control group after cells were treated respectively with castanospermine, NGI-1, or tunicamycin (Fig. 3f), whereas the signal of the signature protein CD63 in these sEVs samples showed only minimal changes, and the overall protein content of sEVs remained virtually unaltered (Supplementary Fig. 9), demonstrating drFRET’s specificity for the glycan moiety of sEV glycoRNA. In addition, replacing GRP with a scrambled DNA sequence (sGRP) reduced cdFRET signals by 92.8% (P = 0.0088), demonstrating the validity of drFRET for the glycan moiety of sEV glycoRNA. Moreover, replacing ISHPs with unspecific ISHP (us-ISHPs) resulted in a 92.6% (P = 0.0004) decrease in the cdFRET signal, demonstrating the specificity of drFRET for the RNA moiety of sEV glycoRNA. Furthermore, sEVs were treated with PNGase F, which are known to cleave the glycan moiety of glycoRNAs. This enzymatic treatment resulted in a significant > 99% (P = 0.0003) reduction in cdFRET intensity compared to untreated sEVs (Fig. 3g). Collectively, these important controls validate the specificity of drFRET in sEV glycoRNA assays.
We further investigated the detection performance of drFRET. The cdFRET intensity shows a positive correlation with the amount of glycoRNAs on fcPS (Fig. 4a). As we increased the concentrations of sEVs, we observed a corresponding increase in cdFRET intensity, with drFRET showing a wide linear detection range from 105 to 109 ml−1 (R2 = 0.9988) and a low limit of detection (3 × standard deviation of the blank, LOD) of 7.7 × 104 ml−1 (Fig. 4b). To assess the quality of cdFRET signals from fcPS-captured sEVs for data acquisition, we utilized the distribution of cdFRET intensity across all sample points to assess whether the sample preparation and measurement process are in a controlled state, and illustrated the exceptional uniformity of cdFRET signals, thereby affirming the viability of drFRET for imaging and quantification purposes (Supplementary Fig. 10). Furthermore, probe-labeled sEVs were enriched onto fcPS within 30 min (Supplementary Fig. 11). These results show that drFRET can be applied to sensitively and specifically visualize glycoRNAs on sEVs within ~ 1.2 h (with potential additional time required for batch assays). In addition, we performed in situ FRET imaging of 5 glycoRNAs, including U1 small nuclear RNA, U3 small nuclear RNA, U35a small nuclear RNA, U8 small nuclear RNA, and Y5 small cytoplasmic RNA, on fcPS-captured sEVs derived from 7 cancer cell lines, and recorded the corresponding cdFRET signals (Fig. 4c and Supplementary Fig. 12). Specifically, we initially isolated sEVs from the culture supernatants of 7 cancer cell lines using differential ultracentrifugation and quantified their particle concentrations via nanoparticle tracking analysis (NTA) to ensure consistent sEV concentrations for the drFRET assay. We subsequently recorded cdFRET signals for 5 glycoRNAs associated with these sEVs. As demonstrated in Fig. 4c, cdFRET signals for sEV glycoRNAs derived from different cell lines exhibit variability (e.g., the cdFRET signal of U1 glycoRNA on MDA-MB-231 cell-derived sEVs was significantly lower than that on MCF-7 cell-derived sEVs, P = 0.0216). It is crucial to emphasize that comparisons of cdFRET measurements are only valid when performed between identical glycoRNA species, while direct comparisons between different glycoRNAs are not feasible owing to variations in the binding efficiencies of corresponding ISHPs to their respective target RNAs. Distinct cdFRET signals among these sEV glycoRNAs were observed, and the utilization of principal component analysis (PCA) for dimension reduction successfully segregated the samples based on variations in cdFRET signal distribution patterns (Fig. 4d).
a Representative cdFRET images of glycoRNA expression in low concentrations of sEVs (csEV: 5.0 × 103 − 5.0 × 109 ml−1) by FRET imaging. b Regression analysis of the average cdFRET intensity of glycoRNAs on bead-bound sEVs as a function of sEV concentration. n = 3 samples for each sEV count; data are presented as mean ± s.d. LOD, limit of detection. c In situ FRET imaging of glycoRNAs on fcPS-captured sEVs derived from different cell lines (upper panel), and quantification of average cdFRET intensity (lower panel). Scale bar, 4 μm. Different sEVs were isolated from different cell culture supernatants using differential ultracentrifugation, and their particle concentrations were quantified by NTA (for detailed methodology, see Methods). For drFRET assays, sEV samples with identical particle concentrations (5.0 × 109 ml−1) were employed, the original concentrations are provided in Supplementary Table 8. n = 3 samples for each cell line sEVs, data are shown as mean ± s.d. d PCA showing the discrimination of 7 cell line groups using the combination of the cdFRET signals of 5 sEV glycoRNAs. Sample groups are color-coded as indicated.
FRET imaging revealing multiple glycoRNA alterations in cancers
The difference in glycoRNA profiles on sEVs from cell lines of different tissue origins suggests that this could also occur in samples of clinical significance. Notably, several tumor-related biomarkers have been identified within sEVs in blood samples, and their types and expression levels strongly correlate with the carcinogenesis and progression of specific cancer categories such as pancreatic, colorectal, and ovarian cancer43,44,45. However, the current evidence is insufficient to establish a correlation between serum sEV glycoRNAs and the carcinogenesis and progression of diseases, and thus the diagnostic value of sEV glycoRNAs remains unknown. In this study, the diagnostic potential of 5 glycoRNAs on sEVs (fcPS-enriched from 10 μl initial serum sample) was assessed via drFRET in a clinical cohort including 88 cancer patients (breast, pancreas, liver, intestines, lung, and cervix cancer) and 12 non-cancer controls. The sample size is sufficiently large to evaluate diagnostic accuracy with the desired level of statistical precision (see Statistical analysis section for details). The drFRET response levels (cdFRET intensity) of multiple glycoRNAs on serum-derived sEVs were summarized in Fig. 5a. Elevated FRET signals originating from glycoRNAs were detected in each cancer group, exhibiting diverse intensities; conversely, weak cdFRET signals of glycoRNAs were detected in the non-cancer group (Supplementary Figs. 13–19). Next, we performed unsupervised hierarchical clustering analysis (ward.D2, euclidean) on the cdFRET signals of 5 specific glycoRNAs on sEVs within both the training cohort (n = 70) and an independent validation cohort (n = 30). The resulting heat map shows that the combination of these 5-glycoRNAs effectively segregated the patients and controls (the non-cancer samples are distinct from the cancer samples and form a separate subtree) into distinct clusters (Fig. 5a).
a A heat map constructed by non-supervised hierarchical clustering of the levels of five glycoRNAs in the training cohort (n = 70) and the validation cohort (n = 30) for differentiating the cancer and non-cancer control groups. Clustering analysis was performed with Ward linkage and Euclidean distance. Samples are marked and colored (bottom). Data are the mean of triplicate experiments. b ROC curves (left) for discriminating non-cancer (n = 8) and all cancer samples (n = 62) in the training cohort using a logistic regression model, and PRC curves (right). The gray diagonal line indicates the expected curve for random classification. Bottom: the AUC, GINI indexes, and overall model quality (OMQ) are shown for glycoRNAs (detailed data description and summary are presented in Supplementary Table 2–3). The OMQ values exceeding 0.5 indicate the effectiveness of good models, while below 0.5 suggests that the model is not merely a result of random prediction. c ROC curves (left) for discriminating non-cancer (n = 4) and all cancer samples (n = 26) in the validation cohort, and PRC curves (right). Bottom: the AUC, GINI indexes, and OMQ are shown for glycoRNAs (Supplementary Table 2–3). Source data are provided as a Source Data file.
The diagnostic performance was assessed by calculating receiver operating characteristic (ROC) curves. The unweighted sum of the levels of 5 individual sEV glycoRNAs (referred to as the SUM signature (sEVSUM (glycoRNA))) within both the training cohort and the validation cohort demonstrated a sensitivity of 100% (95% confidence interval (CI)), specificity of 100% (95% CI), accuracy of 100% (95% CI), and an area under the curve (AUC) of 1 for discriminating cancer from controls using ROC analysis. The single glycoRNA, in contrast, failed to achieve sufficiently high accuracy: in the training cohort, AUC values of glycoRNAs are 0.818, 0.823, 0.935, 0.874, and 0.968 for U1, U3, U35a, U8, and Y5, respectively (Fig. 5b). The GINI index of the precision-recall curve (PRC) shows that the drFRET-based model well predicts positive samples (Fig. 5b). GINI indexes of glycoRNAs are 1.000, 0.635, 0.645, 0.871, 0.748, and 0.935 for sEVSUM (glycoRNA), U1, U3, U35a, U8, and Y5, respectively (detailed data description and summary are presented in Supplementary Table 2–3). In the validation cohort, the AUC values of are 0.904, 0.808, 0.971, 0.894, and 0.923 for U1, U3, U35a, U8, and Y5, respectively (Fig. 5c); and the GINI indexes are 1.000, 0.808, 0.615, 0.942, 0.788, and 0.846 for sEVSUM (glycoRNA), U1, U3, U35a, U8, and Y5, respectively (Fig. 5c). The overall model quality (OMQ, a measure for the homogeneity across all clusters) reflects the performance of drFRET for precision cancer screening (Fig. 5b and c). OMQ values of glycoRNAs are 1.00, 0.70, 0.73, 0.88, 0.78, and 0.92 for sEVSUM (glycoRNA), U1, U3, U35a, U8, and Y5 in the training cohort; and 1.00, 0.79, 0.66, 0.91, 0.75, and 0.82 for sEVSUM (glycoRNA), U1, U3, U35a, U8, and Y5 in the validation cohort. Notably, the single drFRET assay based on Y5 also shows good performance in cancer screening (OMQ = 0.91) within the entire test set. The value of 0.91 indicates that, on average, a pair of records within the same cluster shares identical values in 91% of the active fields. Collectively, drFRET identified multiple glycoRNA alterations in cancers, and the sEVSUM (glycoRNA) signature demonstrated excellent performance (100% accuracy) for precision cancer screening.
Accurate cancer diagnostics enabled by sEV glycoRNA profiles
Next, our research endeavors to explore the clinical feasibility of employing sEV glycoRNA profiles for the precise diagnosis of multiple cancers. Previous studies have demonstrated the potential of tumor-derived sEVs as a non-invasive diagnostic target for multiple cancers46,47,48,49. After demonstrating the accurate discrimination between cancer and non-cancer samples using sEVSUM (glycoRNA), we next showcased the potential of sEV glycoRNAs for multiclass cancer classification. As depicted in Fig. 6a, although there are significant differences (P < 0.0001, two-tailed heteroscedastic t test) in the FRET signals of sEV glycoRNAs between cancer and non-cancer groups within the entire test set, it appears that a single sEV signature is insufficient to distinguish between different types of cancer due to substantial overlap in data distributions among these samples. Subsequently, we investigated the AUC values to assess the efficacy of sEV glycoRNAs in discriminating specific cancer types from five other distinct types within the entire test set (Supplementary Figs. 20, 21 and Supplementary Tables 4, 5). However, none of the subsets within the sEV glycoRNA panels demonstrated sufficient discriminatory power to achieve this objective (Fig. 6b). These results demonstrate the intricate interplay of multiple sEV glycoRNAs in cancer initiation and progression, underscoring the significance of sEVSUM (glycoRNA) in providing precise cancer-related information.
a The cdFRET intensity all five glycoRNAs and their unweighted sum (sEVSUM) of cancer-derived sEVs (n = 88; means ± s.e.m.) compared with sEVs from non-cancer controls (n = 12; means ± s.e.m.) within the entire test set. Statistical differences were determined by a two-tailed heteroscedastic t test. P-values are indicated in the charts. b AUC for evaluating the performance of sEV glycoRNAs in discriminating distinct cancer types (detailed data description and summary are presented in Supplementary Tables 4and 5). c Visualized classification performance in the entire test set using PCoA mapping, wherein cancer individuals were classified into different cancer types based on all five glycoRNAs (sEVSUM (glycoRNA)) as inputs. Sample groups are color-coded as indicated, and each dot represents a serum sample. Each ellipse represents the 95% CI for the barycentre of each group. d Confusion matrix providing a comprehensive summary of the multi-cancer classification results in the entire test set.
To visualize the distribution of samples (n = 100) across the most significant and predictive parameters, we conducted principal coordinates analysis (PCoA, an unconstrained or indirect gradient analysis ordination method) using sEVSUM (glycoRNA) as the input. The cancer classification results were visually represented on a two-dimensional plane, revealing distinct spatial segregation among the different groups (Fig. 6c). The spatial overlap observed among the different groups may potentially indicate the pathological metastasis of specific cancers. The quantitatively summarized results from PCoA are presented in Fig. 6d, depicted as a confusion matrix. The cancer diagnosis achieved an overall accuracy of 89% (95% CI) across 6 different cancer types, which could be increased using the top two prediction-of-cancer types. Interestingly, the remarkable accuracy (89%) achieved by the drFRET-based clinical sEV glycoRNAs assay for cancer diagnosis is comparable to that of sEV protein/glycan assay-based disease diagnostics previously reported50,51. This result is encouraging and highlights the potential of drFRET as a highly sensitive and specific technique for monitoring glycoRNAs. Additionally, it demonstrates the potential of multiple glycoRNAs on clinical sEVs as promising biomarkers for precise multi-disease diagnosis.
drFRET revealing the interaction between sEV glycoRNAs and Siglec proteins
sEV membrane biomolecules are typically involved in molecular interactions with binding partners in cis- or in trans- at sEV-cell junctions52. Considering the presence of glycoRNAs on sEVs, it is highly plausible that they engage in interactions with conventional glycan receptors. Previous studies have shown that glycoRNAs can influence cell function by specifically binding to Siglecs and P-selectin through their glycan terminal sialic acids (Neu5Ac)2,7. Here, we sought to visualize the putative interactions between sEV glycoRNAs and sialic-acid-binding immunoglobulin-like lectins (Siglecs) by drFRET and to further elucidate the regulatory role of glycoRNAs on sEV function. To investigate the spatial proximity between glycoRNAs and glycan receptors, we substituted the GRPs with Cy3-modified proteins (human Siglec-Fc 10, human Siglec-Fc 11, and P-selectin) for drFRET (Fig. 7a), and the spatial structures and sizes were determined, demonstrating drFRET can effectively accommodate this spatial scale (Fig. 7b). We initially assessed the binding of sEV glycoRNAs to Siglec-10 using FCM analysis. Approximately 98.9% of a population of sEV-bound fcPS microspheres showed positivity with Siglec-10 staining, contrasting with a mere 0.75% observed in the unstained control group. This binding was stably attenuated (78.1%) by pre-treatment of fcPS-bound sEVs with RNase A (P = 0.0033), and restored (98.3%, P = 0.0035) by the addition of specific inhibitors (1% v/v) to block the activity of RNase A (Fig. 7c and d). These results demonstrate the specific binding of sEV glycoRNAs to Siglec-10 and indicate that only ~ 23% of the signals from Siglec-10 bound to sEVs is from glycoRNAs.
a Schematic diagram of the in situ FRET imaging of sEV glycoRNA binding to Siglec proteins. Predicted binding locations of the ISHPs and Siglec-Fc proteins (e.g., Siglec-10) are highlighted. b The AlphaFold predicted structures of Siglec-10, Siglec-11, and P-selectin. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. AlphaFold produces a per-residue model confidence score (pLDDT) between 0 and 100. Some regions below 50 pLDDT may be unstructured in isolation. AlphaFold Protein Structure Database, https://alphafold.ebi.ac.uk/. c FCM analysis of bead-bound sEVs pre-treated with the indicated enzymes or inhibitors, followed by staining with the Cy3-conjugated Siglec-10. The gated region indicates that the population shifted toward high Siglec-10 binding. d Quantitative analysis of the positivity rate (Cy3 channel) depicted in (c). e Validation of the specific binding of glycoRNAs to Siglec-10. sEVs were pre-treated with RNase A and analyzed by FRET imaging (top). Scale bars: 10 μm (raw); 2 μm (enlarged). Quantification of average cdFRET intensity (bottom). f Representative cdFRET images of glycoRNA captured by using Cy3-conjugated Siglec-11 and Cy3-conjugated P-selectin for FRET assay. Scale bars: 10 μm (raw); 2 μm (enlarged). The red numbers represent the average intensities of the cdFRET signals. Data in (d and e) are shown as mean ± s.d. Statistical significance in (d and e) was determined by a two-tailed Student’s t test. P-values are indicated in the charts. All data are representative of three independent experiments.
The recent findings by Flynn et al. (2021) indicate a high level of sialylation in N-glycans associated glycoRNAs2. Building upon this knowledge, we employed drFRET to visualize the interaction between the Neu5Ac portion of multiple glycoRNAs and Siglec-10. The cdFRET signal quantitatively reflects the extent of Siglec-10 binding to each specific glycoRNA (Fig. 7e). The observed variation in Siglec-10 binding among these five glycoRNAs may be attributed to the differences in Neu5Ac capping rates on glycoRNA N-glycans. The cdFRET signals generated between Siglec-10 and ISHPs were effectively reduced (83.8% for U1, P = 0.0034; 78.3% for U3, P = 0.0088; 79.8% for U35a, P = 0.0003; 90.5% for U8, P = 0.0015; and 72.2% for Y5, P = 0.0016) upon pre-treatment with RNase A, confirming the direct interaction between Siglec-10 and Neu5Ac of sEV glycoRNAs (Fig. 7e). Moreover, we have investigated the interaction between Siglec-11 and 5 sEV glycoRNAs, as well as the interaction between sEV glycoRNAs and P-selectin via FRET imaging (Fig. 7f). These data demonstrate that sEV glycoRNAs have served as direct Siglec and selectin receptor ligands.
Reducing cellular uptake by ablating surface RNAs on sEVs
Considering that tumor-derived sEVs establish pre-metastatic niches (PMNs) and reprogram cellular functions in multiple non-metastatic organs53, we further investigated the effect of glycoRNAs on the cellular internalization of cancer-derived sEVs in multiple cell lines. Glycan receptors, such as Siglec and selectin, are widely expressed on the cell surface and play crucial roles in intercellular communication by facilitating cell-cell adhesion and cell-pathogen interactions54. In this study, we hypothesize that glycoRNAs enhance sEVs’ adhesion to the cell surface, promoting their uptake and facilitating extensive PMNs establishment (Fig. 8a). GlycoRNA enhances the internalization by promoting sEV adhesion to the cell surface, while ablating surface RNAs reduces the glycan scaffold on sEVs, thereby diminishing sEV-cell interaction and ultimately decreasing the cellular uptake of sEVs (Fig. 8b). We quantified the internalization of HepG2 (an in vitro model of human hepatoma), LOVO (an in vitro model of human colorectal cancer), and HeLa cell-derived sEVs in LX2, NCM−460, and endothelial cells respectively using CLSM. Specifically, following the uptake of DiI-labeled sEVs (pre-treated, and with excess enzymes and dyes removed) by cells (37 °C, 5% CO2), Tubulin TrackerTM Green was employed to fluorescently stain polymerized microtubule proteins in viable cells (37 °C, 5% CO2) for determining cellular spatial contour and distinguishing between surface-bound vesicles and internalized vesicles. Simultaneously, the nucleus was stained using Hoechst 33258 (37 °C, 5% CO2). Subsequently, the cells were fixed using 4% paraformaldehyde for confocal imaging and three-dimensional (3D) scanning (for more information, refer to Methods).
a Cartoon model of glycoRNA-mediated cellular uptake of tumor-derived sEVs. b Ablating surface RNAs reduces sEV-endothelial cell interaction. c Confocal micrographs showing different internalization of sEVs pre-treated with PNGase F, α2-3,6,8,9 Neuraminidase A, and RNase A, respectively. Arrowheads indicate internalized sEVs. Micrographs are representative of three independent experiments. The intensities of different fluorochromes along the white lane in the magnified images are shown in the histograms on the right. Scale bars: 20 μm (raw); 10 μm (magnification). The pseudo-color bar in the inset represents fluorescence intensity. d Analysis of sEV internalization in cells. Data are shown as mean ± s.d. Statistical significance was determined by a two-tailed Student’s t test. P-values are indicated in the charts. e Representative orthogonal slice images of DiI-labeled sEVs in a Hoechst 33258-labeled HUVEC cell. Left: fixed cells were subjected to Z-scanning, resulting in the acquisition of 150 images that were subsequently utilized for 3D reconstruction. Right: the orthogonal view reveals a predominant intracellular distribution of sEVs. All data are representative of three independent experiments.
Figure 8c demonstrates that these sEVs are internalized into the cell interior rather than being bound to its surface. The internalized vesicles exist in a dispersed or aggregated state and consistently tend to accumulate near the nucleus. The right panel illustrates the spatial distribution of the three channels within the cell (alongside the line depicted in a magnified view on the left panel). This clearly indicates that sEVs’ distribution always falls within Tubulin’s range of distribution, without any spatial overlap between sEVs and the nucleus but exhibiting a close relationship instead (Fig. 8c). Notably, previous studies have underscored the crucial role of glycans in facilitating the cellular internalization of sEVs. Here, we employed PNGase F to eliminate almost all N-linked oligosaccharides from glyco-substrates from the surface of sEVs and α2-3,6,8,9 Neuraminidase A to remove Neu5Ac from the surface of sEVs. We observed reduced accumulation of sEVs upon removal of these sugar moieties (82.7% for PNGase F, P = 0.0096; 67.7% for α2-3,6,8,9 Neuraminidase A, P = 0.0156) within endothelial cells (Fig. 8d). Following the protocol previously reported2,7,12, we employed exogenous RNase A to ablate glycoRNA from sEVs (with untreated sEVs serving as the control group and RNase A-treated sEVs as the experimental group). The internalization level of sEVs in cells was significantly reduced upon RNase A treatment (69.8%, P = 0.0347), as demonstrated in Fig. 8d. This can be attributed to the ablation of RNAs from the surface of sEVs, leading to a reduction of glyco-molecules associated with glycoRNAs and consequently diminishes sEV internalization. Subsequently, we performed 3D imaging of these cells (Fig. 8e), acquiring confocal images at various depths (n = 150 frames) along the z-axis while maintaining a fixed xy plane position. The right panel demonstrates the orthogonal view at this coordinate (marked by the intersection of the yellow dotted line) using pseudo-color to visualize sEVs. Our results reveal that these internalized vesicles exhibit a tendency to aggregate in close proximity to the nucleus while maintaining spatial separation from it. Furthermore, observation of internalized sEVs across different sections perpendicular to the z-axis strongly supports their intracellular presence rather than surface binding, as no sEV signal would be detected in this location if they were surface-bound. We also observed reduced accumulation of sEVs (derived from HepG2 and LOVO cells) pre-treated with PNGase F, α2-3,6,8,9 Neuraminidase A, and RNase A within LX2 and NCM-460 cell lines (Supplementary Fig. 22). Collectively, the data above provide compelling evidence supporting the important role of surface RNAs in mediating the cellular internalization of sEVs (as inferred from the observed changes in internalization levels rather than from direct adhesion measurements), with this functionality being primarily attributed to the glycans on glycoRNAs.
Discussion
In this study, we present an imaging method called drFRET for the in situ visualization of various representative glycoRNAs on sEVs derived from multiple cancer cell lines and clinical serum samples. We used GRPs and ISHPs for dual recognition of the glycan and RNA components of sEV glycoRNAs, respectively, followed by employing a sensitized emission method to correct the FRET signal arising from non-radiative energy transfer between GRPs and ISHPs to effectively prevent false-positive signals. drFRET is a scalable universal tool for highly sensitive and specific sEV glycoRNA imaging. We observed that cdFRET signals from sEV glycoRNAs originating from different cell lines (n = 7) display variability. Importantly, cdFRET measurements are only reliable when conducted between identical glycoRNA species. Direct comparisons between different glycoRNAs are not feasible due to variations in the binding efficiencies of corresponding ISHPs to their respective target RNAs. Furthermore, the imaging of sEV glycoRNAs was accomplished using drFRET with a minimal volume of initial biofluids (10 μl) in just 70 min (Supplementary Table 6). In addition, drFRET shows a wide linear detection range from 105 to 109 ml−1 (R2 = 0.9988) with a LOD of 7.7 × 104 ml−1, allowing for the profiling of sEVs in diverse biofluids.
We established drFRET as a clinically feasible liquid biopsy approach, enabling the precise profiling of multiple glycoRNAs on serum-derived sEVs. The drFRET-based sEV glycoRNA assay demonstrates high specificity and sensitivity in distinguishing between cancer patients and non-cancer individuals (100% (95% CI), AUC = 1 for ROC curve, GINI index = 1 for PRC curve, and OMQ = 1). This remarkable performance was consistently observed in both the training cohort (n = 70) and an independent validation cohort (n = 30). In the entire cohort (n = 100), we recorded the positive rates (i.e., cdFRET signals of sEV glycoRNAs) for each individual. Herein, we used PCoA (an unconstrained or indirect gradient analysis ordination method) algorithm to automatically classify six different cancer types using sEVSUM (glycoRNA) (unweighted sum of the positive rates of sEVs) as the input, and an overall accuracy of 89% (95% CI) were obtained for the classification of cancer types. Notably, the single drFRET assay based on Y5 also shows good performance in cancer screening (OMQ = 0.91); however, it shows a limited ability to discriminate different cancer types (Supplementary Fig. 23). Although a reduced number of sEV glycoRNA combinations may exhibit favorable performance in cancer screening, meticulous selection to align with diverse clinical application scenarios becomes imperative (Supplementary Fig. 23).
The positioning of glycoRNAs on sEVs enables their specific binding directly to cell surface receptors. We revealed the interaction between five representative sEV glycoRNAs and Siglec-10, Siglec-11, and P-selectin using drFRET, validating the binding specificity. We further investigated the impact of glycoRNAs on the internalization of cancer-derived sEVs. Our findings demonstrate a significant reduction in cellular uptake when sEVs lack N-linked oligosaccharides, Neu5Ac, or glycoRNAs, suggesting that glycoRNAs may interact with specific glycan receptors on the cell surface to regulate sEV internalization. However, the field of surface RNA and glycoRNA, particularly those anchored on the surface of sEVs, is still in its infancy. Many intriguing questions remain to be explored in future studies. For instance, it remains unclear whether the previously reported maxRNAs are glycosylated, meaning it is uncertain if glycoRNA constitutes part or all of the surface RNA. In addition, our data do not rule out the possibility that non-glycosylated sEV surface RNAs may also play functional roles in sEVs. Future research endeavors could focus on developing more sophisticated tools to comprehensively investigate the effects of glycoRNAs, beyond merely surface RNAs, on the functionality of sEVs. Clinically, the expression of cellular glycan receptors is correlated with disease severity in patients. Here, the Siglec-specific binding of sEV glycoRNAs was successfully confirmed using drFRET, elucidating the potential intersection between Siglec biology and sEV glycoRNA as a significant focus for future research endeavors.
drFRET, as an optical measurement method for investigating spatial topological relationships, offers several advantages. (1) It exhibits a high spatial resolution and effectively mitigates the occurrence of false positive signals, which other methods cannot provide. (2) drFRET does not necessitate pre-treatment (e.g., metabolic labeling) and enables imaging and detection of naturally unlabeled glycoRNA in diverse sample types. (3) drFRET is versatile and customizable, facilitating the detection or imaging of almost any glycoRNAs. Furthermore, glycoRNA demonstrates unique merits in diagnostics. Firstly, glycoRNAs represent a promising class of biomolecules with unique chemical, structural, and biophysical properties that distinguish them from other biomolecules, such as non-glycosylated RNAs, glycoproteins, glycolipids, etc. The integration of glycans substantially confers the spatial conformational diversity of native RNA, modifies the hydrophilicity and charge distribution of RNA scaffolds, and introduces additional functional dimensions (such as flexible membrane anchoring and involvement in immune activation and regulation) while preserving the fundamental functions of RNA. Aberrant RNA glycosylation may complement the functions of glycoproteins and glycolipids to adapt to complex nutritional conditions and disease states, albeit with potential differences in response specificity and sensitivity. For instance, during the initial stages of certain diseases, specific RNAs may demonstrate abnormal glycosylation patterns, leading to notable divergence in the chemical composition of glycoRNAs compared to their normal state, while other glycosylation scaffolds may remain relatively stable. Therefore, incorporating glycoRNA into diagnostic frameworks could significantly enhance the information dimension for disease diagnosis, and glycoRNA-related differences may serve as a critical basis for disease diagnosis and monitoring. Secondly, RNA glycosylation and its membrane anchoring may enhance the stability and accessibility of glycoRNA in the circulatory system: glycan introduces steric hindrance that regulates RNA spatial structure, thereby increasing glycoRNA’s resistance to ribonucleases; glycoRNA-induced solubility changes can improve water solubility; the complex nano-environment on the surface of sEVs may stabilize glycoRNA by promoting extensive interactions with proteins, lipids, small molecules, and ions, this not only anchor glycoRNA more firmly but also minimize its free movement on the vesicle, reducing the risk of structural damage from collisions; during circulation, the nano-environment (e.g., secondary protein corona, charged surface, etc.) formed on sEVs can physically shield glycoRNA, decreasing its susceptibility to degradation and clearance; the anchoring of glycoRNAs to sEVs enhances tissue penetration and circulatory capabilities, which are otherwise limited when glycoRNAs exist independently. These compiled factors attribute to the enhanced availability of sEV glycoRNAs in various biofluids, thereby significantly improving the sensitivity of non-invasive cancer detection methods. Notably, while these hypotheses are mechanistically plausible and supported by analogous systems, a multidisciplinary approach integrating enzymology, structural biology, and synthetic biochemistry could render the stability of glycoRNAs testable. The clinical implications of sEV glycoRNAs, although nascent, are promising, especially for diagnostics. Further experimentation to confirm their stability-driven roles could enhance this potential. Thirdly, ISHPs can be easily designed without the need for intricate screening procedures, such as those required for protein aptamers. Moreover, ISHPs possess the capability to perform multiple detection procedures effortlessly, including continuous monitoring and conformational monitoring of glcoRNAs.
The present version of drFRET has several limitations that remain to be addressed. For example, the cdFRET signal of glycoRNA is significantly higher than that from the background and negative controls; however, the overall intensity remains relatively weak, potentially due to the low expression level of the target glycoRNA. Consequently, the signals detected for various sEV glycoRNAs and their differences are modest and may not exhibit effectiveness in other systems (especially true when performing dimensionality reduction, as the small differences resulting from methodological constraints can be exaggerated and lead to erroneous conclusions). To enhance sensitivity, additional amplification or optical enhancement can be incorporated into drFRET. Furthermore, drFRET enables quantitative analysis of the relative abundance of glycoRNA in sEV populations isolated from biofluids, which mitigates heterogeneity among sEV particles. To achieve more precise quantitative analysis, such as determining the copy numbers of glycoRNAs per vesicle, techniques like super-resolution imaging or nano-flow cytometry can be employed to acquire single-vesicle glycoRNA information. Additionally, given that glycoRNA resides within the intricate nano-environment on the surface of sEVs, it is likely to exhibit extensive interactions or physical proximity with surrounding proteins, lipids, and small molecules, etc. The drFRET measurements may have captured signals from RNA-binding glycoproteins, RNA-glycoprotein crosslinks, or RNA and glycans in close proximity but not covalently linked. Consequently, employing multiple cross-validation methods or developing more advanced proximity labeling techniques would substantially enhance the discovery of sEV glycoRNA biology. It is important to note that fcPS only captures sEVs using single aptamers, which may primarily target HER2-positive sEVs, thus somewhat limits the specificity of capture and could potentially affect the drFRET assay of sEV glycoRNA, despite their relative cdFRET intensities might remain unchanged. Multiplexed sEV capture (e.g., combining with classical protein markers and phospholipid insertions, etc.) may mitigate variability arising from differential binding affinities between sEVs and capture reagents. Standardizing the captured sEVs, whether in terms of quantity, content, or specific marker levels such as CD63, could facilitate absolute quantification of sEV glycoRNA content or molecular copy numbers. This would significantly enhance the utility of sEV glycoRNA in cancer diagnosis. Finally, drFRET necessitates an RNA hybridization step for the identification of specific glycoRNAs, which in turn requires accurate sequence information pertaining to glycoRNA. Consequently, drFRET is not applicable to glycoRNAs with unknown sequences, particularly those that are vesicle-specific. Henceforth, the development of a drFRET based on general RNA recognition reagents may facilitate unbiased analysis of glycoRNAs. Ultimately, the clinical implementation of drFRET would necessitate a comprehensive system that offers streamlined sample workflow, integrated instrumentation, robust data processing pipelines, and interpretation algorithms.
Methods
Ethical statement
All clinical studies in this work were approved by the Biological and Medical Ethics Committee of Northeastern University (approval: EC-2023B022). All relevant ethical regulations were complied with. The non-cancer serum samples were collected from 12 healthy volunteers in the laboratory (all relevant ethical regulations were complied with). Serum samples were collected from patients with breast cancer (15 cases), pancreas cancer (21 cases), liver cancer (19 cases), intestines cancer (16 cases), lung cancer (11 cases), and cervix cancer (6 cases) at the First Affiliated Hospital of China Medical University (all relevant ethical regulations were complied with). All participants provided written informed consent. A total of 100 serum samples were evaluated in this study (Supplementary Table 7), and all serum samples were analyzed fresh or flash-frozen and stored at − 80 °C for future analysis. As this study served as a pilot study aimed at developing a methodology for the potential identification of multiple cancers, no sample size calculation was conducted to explore its clinical utility.
Cell culture
The human breast cancer cell lines MCF-7 (Cat. #: TCHu 74) and MDA-MB-231 (Cat. #: TCHu227), the human pancreatic cancer cell line PANC (Cat. #: TCHu 98), the human cervical cancer cell line HeLa (Cat. #: TCHu187), the human hepatoma cell line HepG2 (Cat. #: TCHu 72), the human colorectal cancer cell line LOVO (Cat. #: TCHu 82), and the human lung cancer cell line A549 (Cat. #: TCHu150) were supplied by Stem Cell Bank, Chinese Academy of Sciences (China). The normal human colon mucosal epithelial cell line NCM-460 (Cat. #: CP-H040), the human hepatic stellate cell line LX2 (Cat. #: CL-0560), and the human umbilical vein endothelial cells (HUVEC, Cat. #: CL-0675) cells were provided by Procell (Wuhan, China). MCF-7, MDA-MB-231, HeLa, HepG2, LOVO, LX2, and HUVEC cells were cultured in Dulbecco’s modified Eagle’s medium (Seven Biotechnology Co., Ltd., Beijing, China) supplemented with 10% (v/v) fetal bovine serum (FBS, Biological Industries, Kibbutz Beit Hamek, Israel) and 1% (v/v) penicillin-streptomycin (Seven Biotechnology Co., Ltd., Beijing, China) in a humidified 37 °C incubator with 5% CO2, and maintained at densities between 5 × 105 and 2 × 106 cells ml−1. PANC, A549, and NCM-460 cells were cultured in RPMI-1640 medium (Seven Biotechnology Co., Ltd., Beijing, China) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) penicillin-streptomycin in a humidified 37 °C incubator with 5% CO2, and maintained at densities between 5 × 105 and 2 × 106 cells ml−1. All the cell lines were authenticated by suppliers and tested to be free of mycoplasma contamination.
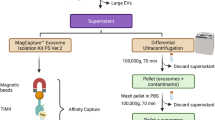
sEV isolation by differential ultracentrifugation
The sEVs were isolated and purified using the differential ultracentrifugation method as described in previous studies and adhering to the guidelines outlined in the Minimal Information for Studies of Extracellular Vesicles55. Cells were cultured in a vesicle-depleted medium for 48 h (~ 80% cell confluence) prior to the collection and characterization of sEVs. The collected media containing sEVs were initially centrifuged at 300 × g for 10 min using a Hitachi Refrigerated Centrifuge (Hitachi, Tokyo, Japan), followed by an additional centrifugation at 2000 × g and 4 °C for 10 min to remove cells. Subsequently, the supernatant underwent further centrifugation at 10,000 × g and 4 °C for 90 min. The collected supernatant containing sEVs was filtered using a 0.22 μm membrane filter (regenerated cellulose, Millipore), followed by ultracentrifugation at 100,000 × g and 4 °C for 180 min. All the collected sEV samples were directly utilized for the feasibility analysis of drFRET. The same protocol was employed for the isolation of sEVs from serum samples (for morphological characterization). The enriched sEVs were stored at − 80 °C prior to further use.
Blotting analysis of Ac4ManNAz-labeled RNA
The presence of glycoRNAs in cells and cell-derived sEVs was confirmed through metabolic labeling with Ac4ManNAz, followed by RNA blotting, as previously described in a published report2,7,42. After metabolic labeling, the total RNA of sEVs was extracted and subjected to proteinase K digestion, when necessary, for efficient removal of protein contamination. Subsequently, a purification column was employed for further cleaning of the purified RNA. The purified RNA was then labeled via bioorthogonal click chemistry, followed by denaturing gel electrophoresis and subsequent analysis via RNA blotting. The presence of biotinylated species in RNA extracted from metabolically labeled cell-derived sEVs was confirmed, as depicted in Fig. 1d. Despite undergoing the same incubation, electrophoresis, and RNA blotting experiments, no detectable signal was observed from cell-derived sEVs RNA without Ac4ManNAz treatment. This suggests that Ac4ManNAz was metabolically integrated into the glycan portion of sEV glycoRNAs, thereby indicating the presence of glycoRNAs in these vesicles.
Ac4ManNAz treatment
For metabolic labeling, HeLa cells were seeded at a density of 5 × 105 cells per flask (T25 flask, Corning, USA) and incubated overnight at 37 °C with 5% CO2. Once the cells completely adhered to the bottom of the flask (~ 80% cell confluence), cells were subjected to 100 μM (in 6 ml vesicle-depleted medium) Ac4ManNAz (T303546, Aladdin Reagent Co., Ltd., China) treatment for an additional 36 h, followed by subsequent isolation of sEVs for gel electrophoresis and blotting according to the protocols described in “sEV isolation by differential ultracentrifugation”.
sEV RNA extraction and purification
The extraction and purification of sEV RNA were conducted according to a previously reported methodology with minor modifications2,7,42. For RNA extractions, the manufacturer’s protocol was followed with the following details. Specifically, HeLa cells (washed twice with PBS, 1200 × g, 4 °C, 5 min) or 200 μl HeLa cell-derived sEVs (concentrating more sample can improve the amount of sEV RNA obtained) were treated with 1 ml of TRIpure reagent (RN0102, Aidlab Biotechnologies Co., Ltd., China) and incubated at 25 °C (5 min) to denature non-covalent interactions (e.g., fully dissociate the nucleic acid-protein complex). Chloroform extraction and isopropanol precipitation procedures were carried out in accordance with the manufacturer’s protocol. Phase separation was performed by adding 0.2 ml of chloroform, vortexing to mix (15 s) incubating for 5 min on ice, and finally spinning at 12,000 × g for 15 min at 4 °C. The RNA in the aqueous phase was carefully removed (< 80%), transferred to a fresh tube, and mixed with 0.5 × volumes of 100% isopropanol. This solution was transferred into FastPure RNA Columns III, then centrifuged at 10,142 × g for 30 s, with the fluid always discarded. Subsequently, the column was reinserted into the collection tube, followed by the addition of 500 μl Buffer RW1 and discarding of the waste liquid through centrifugation at 10,142 × g for 30 s. After placing the column back in the collection tube, 500 μl Buffer RW2 was added and centrifuged at 10,142 × g for 30 s (this step was repeated twice). Place the column into a sterile tube (DNase/RNase-free) and add 50 μl of Buffer TB (incubate at room temperature for 2 min), followed by centrifugation at 10,142 × g for 1 min to obtain the RNA solution. The obtained RNA was subjected to protein digestion by adding Proteinase K (1 μg of Pro K, Sangon Biotech Co., Ltd., A600451) and incubating for 30 min at 37 °C, followed by purification using the aforementioned method (stored at – 80 °C and avoid repeated freezing and thawing). All RNA samples generated in this study underwent initial purification using these steps prior to subsequent analysis, and RNA content was determined by OneDrop™ series ultramicro-spectrophotometer (onedrop, OD1000 +, http://www.onedrop.cn/). Reagents and instruments utilized in the RNA purification process were supplied by the manufacturer, while the specific procedures were conducted in accordance with the instructions provided by FastPure Cell/Tissue Total RNA Isolation Kit V2 (RC112, Vazyme).
Copper-free click conjugation to sEV RNA
The experiments were conducted under copper-free conditions to prevent the presence of copper in the solution during the conjugation of biotin to the azido sugars. Dibenzocyclooctyne-polyethylene-glycol-4-biotin (DBCO-PEG4-biotin, D338359, Aladdin Reagent Co., Ltd., China) was used for labeling azide-containing molecules or compounds. Ac4ManNAz-labeled RNA (9 µl) extracted from cells or cell-derived sEVs was mixed with 10 µl 1 × volumes of “dye-free” Gel Loading Buffer II (df-GLBII, 95% deionized formamide, 18 mM EDTA, and 0.025% SDS) (8546 G, Thermo Scientific) and 1 µl 10 mM DBCO- PEG4-biotin (final concentration: 500 µM). Conjugation was performed at 25 °C for 10 min. Biotin-labeled RNA was then purified over the column as mentioned above and analyzed by gel electrophoresis as described below.
RNA gel electrophoresis, blotting, and imaging
Blotting analysis of Ac4ManNAz-labeled RNA was conducted conceptually similar to a previously reported methodology with the following modifications2,7,42. Biotin-labeled RNA was resuspended in 20 µl df -GLBII, and incubated at 65 °C for 10 min, and chilled on ice for 3 min to denature RNA. Thereafter, samples (10 µg or 15 µg RNA) were then loaded to a 1% agarose formaldehyde-denaturing gel and electrophoresed in 1 × MOPS buffer (M8150, Solarbio Science & Technology Co., Ltd., China) at 110 V for 40 min (room temperature). For band orientation, the DNA Marker 2000 (ZD20001, Zoonbio Biotechnology Co., Ltd., China) was utilized as a ladder (supplied in the storage and loading buffer and can be directly applied onto a gel). Notably, the use of a DNA ladder limited the precise determination of molecular weight, while RNA ladders would improve molecular weight accuracy. The total RNA in the gel was visualized using a gel imager (2500, Tanon Science & Technology Co., Ltd., China) after YeaRed staining (YeaRed Nucleic Acid Gel Stain, 10202ES76, Yeasen Biotechnology Co., Ltd.). The RNA was then transferred to a nitrocellulose membrane (NC membrane, 0.45 µm) (RPN303B, Amersham) following the northern blot procedure for 3.5 h at room temperature using 1 × SSC buffer (pH = 7.0). Afterward, the NC membrane was washed with 2 × SSC (0.1% SDS), and then RNA was cross-linked to the NC membrane using UV-C light (10 min). The NC membrane was subsequently placed in a hybridization oven (LF-III, Ningbo Scientz Biotechnology Co., Ltd., China) and washed twice with 2 × SSC (0.1% SDS, 5 min, 30 °C, 414 × g) and twice more with 0.5 × SSC (0.1% SDS, 15 min, 30 °C, 414 × g). Instead of previously reported methods, we used the Chemiluminescent Nucleic Acid Detection Module (89880, Thermo Scientific) here to detect glycoRNA on the NC membrane. Specifically, to block the NC membrane, 20 ml Blocking Buffer was added and incubated for 15 min with gentle shaking. After blocking, the Stabilized Streptavidin-Horseradish Peroxidase Conjugate was diluted to 1:300 in Blocking Buffer and incubated with the membrane for 15 min with gentle shaking. Transfer the membrane to a new container and rinse briefly (four times, 5 min) with 20 ml of 1 × wash solution (by adding 40 ml of 4 × Wash Buffer to 120 ml ultrapure water). Then, transfer the membrane to a new container and add 30 ml of Substrate Equilibration Buffer for 5 min with gentle shaking. After removing the membrane from the Substrate Equilibration Buffer, an edge of the membrane on a paper towel was carefully blotted to remove excess buffer, and pour the Substrate Working Solution (by adding 6 ml Luminol/Enhancer Solution to 6 ml Stable Peroxide Solution) onto the membrane so that it completely covers the surface for 5 min without shaking. Next, remove the membrane from the Working Solution and blot an edge of the membrane on a paper towel to remove excess buffer. The NC membrane was then scanned with an ultra-sensitive chemiluminescence imaging system (4600, Tanon Science & Technology Co., Ltd., China). Shorter or longer exposures may be used to obtain the desired signal. The dot blotting analysis of glycoRNA follows a similar procedure as described above, with the exception that gel electrophoresis and membrane transfer steps are omitted. Instead, 8 µl of the RNA sample was directly dropped onto polyvinylidene difluoride membranes, followed by subsequent steps, including washing, cross-linking, blocking, coupling, and exposure as described above. For detailed instructions, please refer to the manufacturer’s protocol.
Nanoparticle tracking analysis of sEVs
To determine the concentration and size distribution of sEVs, nanoparticle tracking analysis (NanoSight NS500, Malvern Instruments) was conducted (20 ± 3 °C). sEV samples were diluted in 1 × PBS buffer (pH = 7.4) to achieve an estimated concentration of ~ 109 ml−1 to match the NTA’s counting compatibility. The NTA detection experiments were conducted three times for all sEV samples. The videos collected in each particle counting experiment were directly utilized for particle enumeration and statistical analysis of size distribution (Supplementary Table 8). The size distribution data were acquired and analyzed utilizing the ZetaView software (version 8.05.14 SP7).
Characterization of sEVs using electron microscopies
SEM was performed to characterize the fcPS-bound sEVs derived from cells. The fcPS was utilized to capture sEVs, and the dehydrated fcPS-bound sEVs were coated with a thin gold film using a high-resolution ion beam coater, followed by SEM characterization using a field emission scanning electron microscope (SU8010, Hitachi, Tokyo, Japan). SEM images were subsequently used to calculate the size of the sEVs. The morphology of sEVs (derived from cells and serum samples, respectively) was confirmed by means of transmission electron microscopy (TEM). Specifically, the sEV samples were dropped onto a carbon-coated copper grid (Ted Pella) and left for 20 min. After blotted with filter paper, the grid containing sEVs was immersed in 0.5% aqueous phosphotungstic acid for 30 s. The grid was subsequently extracted, air-dried, and examined under a field emission transmission electron microscope (Tecnai G2 F20, FEI, USA).
Preparation of fcPS
We prepared fcPS as a solid phase carrier for sEVs, 3-maleimidopropionic acid N-hydroxysuccinimide ester (BMPS, M122239), a heterobifunctional crosslinker containing N-hydroxysuccinimide (NHS) ester (react with primary amines to form amide bonds) and maleimide groups (react with sulfhydryl groups to form stable thioether bonds), allowing covalent conjugation of amine- and sulfhydryl-containing molecules. sEVCP containing sulfhydryl groups (SH-aptamerHER2) can be conjugated to microspheres through BMPS. Specifically, we initially modified the coating of aminated polystyrene microspheres (PS, 4 μm, P107792) with BMPS, and subsequently coupled SH-aptamerHER2 for the immune-enrichment of sEVs. The experimental methodology involved the following steps: (i) 4 μl of 5% w/v PS was dispersed in 100 μl of 10 mM BMPS in 1 × PBS (pH 7.4, filtered through a 0.22 μm regenerated cellulose membrane filter from Millipore). The mixture was incubated at 25 °C for 3 h, followed by centrifugation (13,914 × g) for 3 min to isolate the PS-BMPS, and then washed three times with PBS. (ii) The PS-BMPS was dispersed in 50 μl of 10 μM SH-(T)9-AptHER2 (pre-incubated at 25 °C for 2 h with 1 mM TCEP to prevent disulfide bond formation by the terminal sulfhydryl). The mixture was incubated for 2 h at 25 °C in darkness. (iii) The samples were treated with 50 μl of 4 mM 6-Mercapto-1-hexanol (MCH, M123042) in PBS for 1 h at 25 °C and then blocked with 50 μl BSA (2% w/v in PBS) for another hour at 25 °C to prevent potential nonspecific adsorption. Finally, fcPS were washed three times with PBS, dispersed in 10 μl PBS (2% w/v), and stored at 4 °C for later use.
Flow cytometry of stained sEVs
For sEV analysis, the vesicles were captured on fcPS. The fluorescence signals of fluorophores (e.g., Cy3) were measured utilizing a BD AccuriTM C6 Plus Flow Cytometer. The fluorescence intensities of fcPS (excluding debris) were determined by quantifying 20,000 events (Fig. 7c) using FloJo software (version FlowJo_V10).
Single vesicle imaging
The following protocol was employed for the single vesicle imaging of sEVs. First, to investigate the spatial overlap of GRPs and ISHPs on sEVs, 10 µl of HeLa cell-derived sEVs (2.0 × 1011 particles ml−1) were mixed with 10 µl of drFRET probes (containing 2 µM GRPs and 2 µM ISHPs) in 1 × coupling buffer (c-buffer: 50 mM Tris-HCl buffer and 10 mM Mg2+, pH 7.4). The mixture was incubated at 37 °C for 30 min. Then the excess drFRET probes were removed using Amicon ultra 100 K centrifugal filters (Merck Millipore Inc., USA) at a speed of 16,066 × g for 10 min at 4 °C. Finally, the fluorescence of ISHPs (λex Cy5: 635 nm) and GRPs (λex Cy3: 559 nm) on sEVs was visualized using total internal reflection fluorescence microscopy (TIRF, Olympus), followed by colocalization analysis.
TIRF was used to investigate the recognition specificity of GRPs and RNBs, respectively. Here, is a brief description of the steps: (i) For investigating the recognition specificity of GRPs, 10 µl of HeLa cell-derived sEVs (2.0 × 1011 particles ml−1) was mixed with 10 µl of α2-3,6,8,9 Neuraminidase A (2 U µl−1 in c-buffer, NEB Bioke, P0722), and incubated at 37 °C for 1 h. Then 10 µl of GRPs (2 µM in c-buffer) was added and incubated at 37 °C for 30 min, followed by removal of excess GRPs by ultrafiltration (MWCO: 100 kDa). (ii) For investigating the recognition specificity of ISHPs, 10 µl of sEV samples (HeLa cell line) was mixed with 10 µl of RNase A (5 µg µl−1 in c-buffer, Sangon Biotech Co., Ltd., B600473) and incubated at 37 °C for 1 h, Then 10 µl of ISHPs (2 µM in c-buffer) was added and incubated at 37 °C for 30 min, followed by removal of excess GRPs by ultrafiltration (100 kDa). Finally, the fluorescence signals of GRPs and ISHPs on single sEVs were observed using TIRF.
In situ imaging of sEV glycoRNAs with drFRET
The following protocol was implemented for the in situ imaging of sEV glycoRNAs. Specifically, the fcPS were equilibrated at room temperature for 20 min prior to use. A total of 10 μl sample containing sEVs (cell culture supernatant “m” or patient serum “s”) was mixed with 10 µl of drFRET probes (containing 2 µM GRPs and 2 µM ISHPs in c-buffer). The mixture was then incubated at 37 °C for 30 min (denoted as mixture A). Simultaneously, 10 µl of fcPS (2% w/v) was blocked with a 50 µl mixture of BSA (2% w/v, Sangon Biotech Co., Ltd., A600332) in c-buffer and 5 µM poly T oligonucleotides at 25 °C for 30 min. The blocked fcPS were separated through centrifugation (13,914 × g, 3 min). Thereafter, fcPS were dispersed in mixture A and incubated at 25 °C for 30 min to capture sEVs, followed by three subsequent washes (13,914 × g, 3 min) with a c-buffer. The cdFRET channels of fcPS containing sEVs were ultimately captured through confocal laser scanning microscopy (CLSM, Olympus) for subsequent analysis. All incubation steps were conducted at 170 rpm in a thermostatic culture oscillator to ensure a continuous oscillatory flow on fcPS. Three independent measurements of each imaging group (n = 3 samples for each cell line sEVs) were performed and validated for further statistical analysis, showing similar trends. For each measurement, signals from ≥ 10 randomly selected fcPS were analyzed quantitatively using the collected images.
To acquire cdFRET images, we applied the sensitized emission method to correct the FRET signals56. In brief, seven images were acquired from the following three samples (Fluorophore, drFRET probes): a reference sample with only the donor (D, GRPs) dyed to acquire images of below-mentioned a and b, a reference sample with only the acceptor (A, ISHPs) dyed to acquire images of below-mentioned c and d, and a sample for FRET observation with both D and A dyed to acquire images of below-mentioned e, f, and g. To run sensitized emission, the following conditions are required: (i) all images should have intensity data; (ii) each image of reference samples (a, b, c, and d) should have undergone background correction. The formula of drFRET was used:
where DSBT is GRPs spectral bleed-through, ASBT is ISHPs spectral bleed-through, f is channel A (cA) with D excited (De) (with D and A), a is cD with De (D only), b is cA with De (D only), c is cA with De (A only), d is cA with Ae (A only), e is cD with De (with D and A), and g is cA with Ae (with D and A), QGRPs is GRPs quantum yield, QISHPs is ISHPs quantum yield, and R0 is Förster distance between GRPs and ISHPs. For drFRET imaging, fcPS were imaged using an FV−1200 CLSM (Japan) with a 100 × objective lens at λex/ λem = 559/ (650–700) nm for cdFRET. The average cdFRET intensity of all fcPS samples (three frames) was calculated using Image J software (version 1.8.0.112).
Validation of the specificity of drFRET
To validate the specificity of drFRET via pharmacological interventions, HeLa cells were treated with various glycosylation inhibitors to manipulate the expression of glycoRNAs. Cell-secreted sEVs were isolated for drFRET imaging, and cdFRET intensity was recorded for significant difference analysis. Briefly, HeLa cells were seeded at a density of 5 × 105 cells per flask (T25 flask, Corning, USA) and incubated overnight at 37 °C with 5% CO2. Once the cells completely adhered to the bottom of the flask, they were subjected to drug treatment for an additional 24 h using 4 ml culture medium (10% v/v FBS) respectively containing the following inhibitors: PBS for control, 10 μM castanospermine (MCE, HY-N2022), 1 μM tunicamycin (T101151, Aladdin Reagent Co., Ltd., China), and 10 μM NGI-1 (MCE, HY-117383). After removing the medium containing the inhibitor, cells were further cultured in a vesicle-depleted medium for 48 h, followed by subsequent isolation of sEVs for drFRET imaging according to the protocols described in “sEV isolation by differential ultracentrifugation”. To further validate the specificity of drFRET through probe control and enzymatic treatments, sGRPs and us-ISHPs were employed following the drFRET procedure, as previously described in Section “In situ imaging of sEV glycoRNAs with drFRET”. For PNGase F treatment, 10 µl of 50 U µl−1 PNGase F (NEB Bioke, P0704) in GlycoBuffer (NEB) were added to incubate with sEVs along with the drFRET probe, while other groups received corresponding volumes of buffer solution. The subsequent drFRET incubation and test procedures were conducted accordingly (refer to Fig. 3g for results).
In situ imaging of the specific interaction between sEV glycoRNAs and glycan receptors
To in situ visualize the interaction between sEV glycoRNAs and Siglec-10, Siglec-11, and P-selectin using drFRET, we initially prepared Cy3-modified derivatives of Siglec-10, Siglec-11, and P-selectin. All reagents required for the modification were included in the Cy3 conjugation kit (Sangon Biotech Co., Ltd., D601061). Specifically, 2 μl of Cy3 reagent (1 mg ml−1) was mixed with 300 μl of 10 μg human Siglec-10 recombinant protein (6 × His tag C-Terminus, > 95%, Sangon Biotech Co., Ltd., D145080), 300 μl of 10 μg human Siglec-11 recombinant protein (E84A, K145Q, CHO, hFc; MCE; HY-P79127), or 300 μl of 10 μg P-selectin (HEK293, His; MCE; HY-P70803), and incubated for 30 min at 37 °C in the dark. Subsequently, the excess Cy3 was eliminated by employing a desalting centrifuge column at 1346 × g (6 min, 4 °C) and subjected to three washes with a purification buffer. The resulting Cy3-modified protein samples were dispersed in 500 μl of coupling buffer and stored at − 20 °C for later use.
For drFRET imaging, a total of 10 μl HeLa cell-derived sEVs (1.2 × 1011 particles ml−1) were mixed with mixture A and mixture B to investigate the specific binding between sEV glycoRNAs and Siglec-10. Mixture A consists of 10 μl of 4 µM ISHPs in c-buffer and 10 μl of aforementioned Cy3-modified Siglec-10, while mixture B was 10 μl of c-buffer as a control or 10 μl of 10 µg µl−1 RNase A in c-buffer. These mixtures were incubated at 37 °C for 30 min. Simultaneously, 10 µl of fcPS (2% w/v) were blocked in accordance with the method outlined in “In situ imaging of sEV glycoRNAs with drFRET”, and then dispersed in the above mixtures to yield mixture C. Mixture C were incubated at 25 °C for 30 min to capture sEVs, followed by three washes with c-buffer. The cdFRET channels were captured using CLSM (Olympus) for subsequent analysis. All incubation steps were conducted at 170 rpm in darkness. It is worth noting that the concentration of RNase A used was much lower than that of the blocking agent BSA, and its impact on sEV capture was negligible (Supplementary Fig. 24).
Validation of the binding specificity of sEV glycoRNAs to Siglec-10
To elucidate the binding specificity of the sEV glycoRNA towards Siglec-10, we treated sEVs with RNase A (in the presence or absence of RNase A inhibitor) prior to drFRET. Experimental steps are as follows: (i) 10 μl of sEVs (derived from HeLa cells, 1.2 × 1011 particles ml−1) were mixed with 10 μl of Cy3-modified Siglec-10, mixture D (10 μl of c-buffer for control, or 10 μl of 10 µg µl−1 RNase A), and mixture E (10 μl of c-buffer for control; or 10 μl of 1% v/v RNase A inhibitor, Sangon Biotech Co., Ltd., B540004), and incubated at 37 °C for 30 min. (ii) sEVs were then enriched on fcPS, followed by FCM analysis. The data were analyzed and visualized with FloJo software.
Confocal imaging of the cellular internalization of sEVs
To image the in vitro cellular uptake of sEVs, endothelial cells (HUVEC), LX2 cells, and NCM-460 cells were cultured in four-well confocal dishes (NEST Biotechnology Co., Ltd.), respectively. The culture medium was removed once the cells reached 80% confluence. The sEVs derived from HeLa cells (2.0 × 1011 particles ml−1), HepG2 cells (1.5 × 1011 particles ml−1), and LOVO cells (4.1 × 1010 particles ml−1) underwent enzymatic treatment, followed by fluorescence labeling. Briefly, four groups of sEV samples were pre-prepared: untreated sEVs, PNGase F-treated sEVs, α2-3,6,8,9 Neuraminidase A-treated sEVs, and RNase A-treated sEVs. Notably, each group of sEV samples was independently prepared. For the evaluation of cellular internalization in each sEV sample, a distinct enzyme was used for each sample, and confocal imaging was performed independently. Specifically, 10 µl of sEVs were mixed with 10 µl of α2-3,6,8,9 Neuraminidase A (2 U µl−1 in c-buffer), 10 µl of RNase A (5 µg µl−1 in c-buffer), 10 µl of 50 U µl−1 PNGase F (NEB Bioke, P0704) in GlycoBuffer (NEB), 10 µl of c-buffer (as a control), respectively. The mixtures were incubated at 37 °C for 1 h (170 rpm), followed by the addition of 10 μl of 10 µM lipophilic membrane dye (DiI, Beyotime, C1991S). After the treated sEV samples were stained for 10 min at 37 °C, excess dye was removed by ultracentrifugation (100,000 × g, 4 °C, 20 min). The labeled sEV samples were dispersed into 500 μl of vesicle-depleted medium and incubated with the cells (80% confluence) for 6 h at 37 °C with 5% CO2. Next, the cells were rinsed (3 × 5 min) in filtered PBS, followed by fixation using pre-cooled methanol at − 20 °C for 4 min, and washed three times with PBS. The internalization of sEVs was observed and quantified using CLSM (100 × lens, λex 559 nm).
To distinguish between surface-bound and internalized vesicles, HaLa cell-derived sEVs (2.0 × 1011 particles ml−1) were co-incubated with endothelial cells (following the previously established protocol), and the cells were washed three times with filtered PBS. Subsequently, 500 μl pre-heated 1 × Tubulin TrackerTM Green (Beyotime, C2213S) (containing solvent-promoting and stain enhancer, detailed information can be obtained from https://www.beyotime.com/index.htm), was utilized for fluorescently staining polymerized microtubule proteins in viable cells to determine the cellular cytoskeleton profile (37 °C for 1 h). Afterward, the stained cells were washed three times with 1 × stain enhancer (Beyotime). Subsequently, to stain the nucleus, 500 μl of 1 × Hoechst 33258 (Beyotime, C1017) was added and incubated for 30 min at 37 °C in a dark environment, followed by three washes with PBS. Finally, the cells were fixed with 4% paraformaldehyde at 25 °C for 10 min and subsequently washed three times with PBS. The internalization of sEVs was observed, and the 3D scanning of cells was performed using CLSM (100 × lens, λex 559 nm for DiI, λex 488 nm for Tubulin Tracker, and λex 405 nm for Hoechst 33258).
Statistics & reproducibility
To estimate the required sample size for ROC analysis of diagnostic accuracy, we compared the area under the ROC curve (AUC) with a null hypothesis value of 0.5. Statistical calculation was performed at various levels of type I error (α) and type II error (β) using IBM SPSS Statistics (version 27.0.1), specifically through the “Power Analysis of Independent-Samples T Test” module (detailed procedures are available at https://www.ibm.com/docs/en/spss-statistics/30.0.0?topic=means-power-analysis-independent-samples-t-test). The computed sample sizes, which are detailed in Supplementary Table 9, indicate that a total sample size of 100 (consisting of 12 non-cancer controls and 88 cancer patients) is sufficient for statistical evaluation of diagnostic accuracy, assuming α = 0.05 and a power of 95%. This calculation ensures that drFRET has adequate statistical power to detect a significant difference in diagnostic accuracy, with a low risk of both α and β.
For sample binary classification, PCoA algorithms (the key parameters of the operational panel are presented in Supplementary Table 10) were established on the features that show significant differences between non-cancer individuals and individuals with cancers. The predicted cancer types or health status were projected onto a two-dimensional space using PCoA57. ROC and PRC curves were constructed for five individual markers (glycoRNAs) and the sEVSUM (glycoRNA) signature to evaluate the accuracy of drFRET for cancer detection. ROC and PRC curve construction, AUC and GINI index calculation, and quality evaluation was performed using IBM SPSS Statistics (version 27.0.1). The accuracy represents the overall probability of correctly classifying an individual. The 95% confidence intervals were calculated using a binomial distribution. Statistical analysis was conducted using the Student’s t test. The differences were considered to be significant at P < 0.05. (**P < 0.01, ***P < 0.001, and ****P < 0.0001), and otherwise were indicated as “ns” (not significant). All the measurements were performed in triplicate unless otherwise specified, and the results were presented as the mean ± standard deviation. The fluorescence intensity of all images was quantified using Image J software (version 1.8.0.112). The fluorescence intensity was determined after adjusting all images to a uniform threshold. Graphs presenting the data were generated using Origin software. The original images were arranged using Adobe Illustrator 2022. The parameters and variables derived from the drFRET dataset were found to be reproducible. Detailed descriptions of the statistical analysis were provided in the respective figure legends. No statistical method was used to pre-determine sample size. No data were excluded from the analyses. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated in this study are provided in the paper, Supplementary Information, and Source Data file. The raw data used in this study are available in the Figshare database under accession DOI code https://doi.org/10.6084/m9.figshare.2789686858. Source data are provided in this paper.
Code availability
Principal Coordinates Analysis (PCoA) and Uniform Manifold Approximation and Projection (UMAP) were performed by using pcoa-ellipse and umap-ellipse tools in Hiplot, respectively. Code for performing PCoA is available at https://github.com/openbiox/hiplot-org-plugins/tree/6e2685e1f4bd4e2b842b4d92abbb2751381f3bb4/advance/pcoa-ellipse. Code for performing UMAP is available at https://github.com/openbiox/hiplot-org-plugins/tree/6e2685e1f4bd4e2b842b4d92abbb2751381f3bb4/advance/umap-ellipse.
References
Huang, N. et al. Natural display of nuclear-encoded RNA on the cell surface and its impact on cell interaction. Genome Biol. 21, 225 (2020).
Flynn, R. A. et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 184, 3109–3124 (2021).
Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 22, 375–392 (2021).
Helm, M. & Motorin, Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat. Rev. Genet. 18, 275–291 (2017).
Li, Y. et al. N6-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat. Genet. 52, 870–877 (2020).
Yu, S. & Kim, V. N. A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing. Nat. Rev. Mol. Cell Biol. 21, 542–556 (2020).
Zhang, N. N. et al. Cell surface RNAs control neutrophil recruitment. Cell 187, 846–860 (2024).
Guo, X. et al. Capture of the newly transcribed RNA interactome using click chemistry. Nat. Protoc. 16, 5193–5219 (2021).
Meng, L. et al. Metabolic RNA labeling for probing and imaging RNA in bacteria. Nucleic Acids Res. 48, 12566–12576 (2020).
David, B. et al. Arrow pushing in RNA modification sequencing. Chem. Soc. Rev. 50, 9482–9502 (2021).
Kimura, S., Dedon, P. C. & Waldor, M. K. Comparative tRNA sequencing and RNA mass spectrometry for surveying tRNA modifications. Nat. Chem. Biol. 16, 964–972 (2020).
Ma, Y. et al. Spatial imaging of glycoRNA in single cells with ARPLA. Nat. Biotechnol. 42, 608–616 (2024).
Michell, R. Cell membranes. Nature 261, 619 (1976).
Hendrix, A. et al. Extracellular vesicle analysis. Nat. Rev. Methods Primers 3, 56 (2023).
Raghu, K. & Kathleen, M. M. The role of extracellular vesicles in cancer. Cell 186, 1610–1626 (2023).
Dixson, A. C. et al. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476 (2023).
Becker, A. et al. Extracellular vesicles in cancer: Cell-to-Cell mediators of metastasis. Cancer Cell 30, 836–848 (2016).
Robbins, P. D. & Morelli, A. E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14, 195–208 (2014).
Carlos, C. et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc. Natl. Acad. Sci. USA 115, 12158–12163 (2018).
Budnik, V., Ruiz-Cañada, C. & Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 (2016).
Zhang, C. et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Mol. Cancer 23, 18 (2024).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445 (2019).
Dayoung, P. D. et al. Membrane glycomics reveal heterogeneity and quantitative distribution of cell surface sialylation. Chem. Sci. 9, 6271 (2018).
Batt, A. R. et al. Metabolic chemical reporters of glycans exhibit cell‐type‐selective metabolism and glycoprotein labeling. Chembiochem 18, 1177–1182 (2017).
Kageler, L., Perr, J. & Flynn, R. A. Tools to investigate the cell surface: Proximity as a central concept in glycoRNA biology. Cell Chem. Biol. 32, 1132–1144 (2024).
Dunn, M. R., Jimenez, R. M. & Chaput, J. C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 1, 0076 (2017).
Yue, H. et al. Systematic screening and optimization of single-stranded DNA aptamer specific for N-acetylneuraminic acid: a comparative study. Sens. Actuators B Chem. 344, 130270 (2021).
Albrecht, S. et al. Comprehensive profiling of glycosphingolipid glycans using a novel broad specificity endoglycoceramidase in a high-throughput workflow. Anal. Chem. 88, 4795–4802 (2016).
Raines, R. T. Ribonuclease A. Chem. Rev. 98, 1045–1065 (1998).
Algar, W. R. et al. FRET as a biomolecular research tool—understanding its potential while avoiding pitfalls. Nat. Methods 16, 815–829 (2019).
Chatterjee, M. et al. Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS. Nat. Med. 30, 1771–1783 (2024).
Nieuwland, R. & Siljander, P. R.-M. A beginner’s guide to study extracellular vesicles in human blood plasma and serum. J. Extracell. Vesicles 13, e12400 (2024).
Zhang, Y. et al. ICP-MS and Photothermal dual-readout assay for ultrasensitive and point-of-care detection of pancreatic cancer exosomes. Anal. Chem. 93, 11540–11546 (2021).
Shao, H. et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 6, 6999 (2015).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Zhu, G. et al. Combinatorial screening of DNA aptamers for molecular imaging of HER2 in cancer. Bioconjug. Chem. 28, 1068–1075 (2017).
Hu, R. et al. Exploring aptamer-based metasurfaces for label-free plasmonic biosensing of breast tumor‐derived exosomes. Adv. Opt. Mater. 12, 2401180 (2024).
Su, X. et al. Integrated SERS-vertical flow biosensor enabling multiplexed quantitative profiling of serological exosomal proteins in patients for accurate breast cancer subtyping. ACS Nano 17, 4077–4088 (2023).
Mun, B. et al. 3D-Nanostructured microfluidic device arranged in a herringbone pattern for the highly effective capture of HER2-Positive cancer-derived exosomes in urine. Chem. Eng. J. 482, 148851 (2024).
Kanwar, S. S. et al. Microfuidic device (ExoChip) for on-chip isolation, quantifcation and characterization of circulating exosomes. Lab Chip 14, 1891–1900 (2014).
Zhang, P. et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat. Biomed. Eng. 3, 438–451 (2019).
Xie, Y. et al. The modified RNA base acp3U is an attachment site for N-glycans in glycoRNA. Cell 187, 1–10 (2024).
Katherine, S. Y. et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med. 9, eaal3226 (2017).
Yoshioka, Y. et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 5, 3591 (2014).
Li, J. et al. An aptamer-based nanoflow cytometry method for the molecular detection and classification of ovarian cancers through profiling of tumor markers on small extracellular vesicles. Angew. Chem. Int. Ed. 63, e2023142 (2024).
Zhao, H. et al. A hydrogel-based mechanical metamaterial for the interferometric profiling of extracellular vesicles in patient samples. Nat. Biomed. Eng. 7, 135–148 (2023).
Wood, H. Extracellular vesicle α-synuclein marks PD risk. Nat. Rev. Neurol. 20, 63 (2024).
Liang, K. et al. Nanoplasmonic quantification of tumour-derived extracellular vesicles in plasma microsamples for diagnosis and treatment monitoring. Nat. Biomed. Eng. 1, 0021 (2017).
Lee, Y. et al. Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol. Cancer 22, 33 (2023).
Zhang, Y. et al. Plasmonic colorimetric biosensor for sensitive exosome detection via enzyme-induced etching of gold nanobipyramid@MnO2 nanosheet nanostructures. Anal. Chem. 92, 15244–15252 (2020).
Li, Y. et al. Thermophoretic glycan profiling of extracellular vesicles for triple-negative breast cancer management. Nat. Commun. 15, 2292 (2024).
Zhu, L. et al. Coupling aptamer-based protein tagging with metabolic glycan labeling for in situ visualization and biological function study of exosomal protein-specific glycosylation. Angew. Chem. Int. Ed. 60, 18111–18115 (2021).
Wang, G. et al. Tumour extracellular vesicles and particles induce liver metabolic dysfunction. Nature 618, 374–382 (2023).
Smith, B. A. H. & Bertozzi, C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 20, 217–243 (2021).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 13, e12404 (2024).
Elangovan, M. et al. Characterization of one- and two-photon excitation fluorescence resonance energy transfer microscopy. Methods 29, 58–73 (2003).
Li, J. et al. Hiplot: a comprehensive and easy-to-use web service boosting publication-ready biomedical data visualization. Brief. Bioinformatics 23, bbac261 (2022).
Ren, T. J. et al. FRET imaging of glycoRNA on small extracellular vesicles enabling sensitive cancer diagnostics. Data sets. figshare, https://doi.org/10.6084/m9.figshare.27896868 (2025).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 22374016, Z.X., No. 21874015, Z.X.). In addition, we thank Professor Jianhua Wang for providing access to the total internal reflection fluorescence microscopy equipment, and Professor Yongliang Yu for the flow cytometer.
Author information
Authors and Affiliations
Contributions
Z.X. directed the research. T.R. and Z.X. designed the project and experiments. T.R. performed the experiments and analyzed the data. Y.T. assisted in the experiments, Q.Z. assisted with data analysis. Y.Z. collected the serum samples of individuals with cancers. T.W. contributed to fcPS preparation. Y.W. and C.Y. assisted with data interpretation. T.R. and Z.X. wrote the manuscript. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ren, T., Zhang, Y., Tong, Y. et al. FRET imaging of glycoRNA on small extracellular vesicles enabling sensitive cancer diagnostics. Nat Commun 16, 3391 (2025). https://doi.org/10.1038/s41467-025-58490-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58490-2
This article is cited by
-
GlycoRNA, a novel RNA modification
Discover Oncology (2025)