Abstract
Major depression is characterized by an array of negative experiences, including hopelessness and anhedonia. We hypothesize that inhibition of negative experiences or aversion may generate antidepressant action. To directly test this hypothesis, we perform multimodal behavioral screenings in male mice and identify somatostatin (SST)-expressing neurons in the region X (HBX) between the lateral and medial habenula as a specific type of antidepressant neuron. SST neuronal activity modulation dynamically regulates antidepressant induction and relief. We also explore the circuit basis for encoding these modulations using single-unit recordings. We find that SST neurons receive inhibitory synaptic inputs directly from cholecystokinin-expressing neurons in the bed nucleus of the stria terminalis and project excitatory axon terminals onto proenkephalin-expressing neurons in the interpeduncular nucleus. This study reveals a cell-type-specific circuit of SST neurons in the HBX that encodes antidepressant action, and the control of the circuit may contribute to improving well-being.
Similar content being viewed by others
Introduction
Depression is an emotional disease characterized by diverse depressive behaviors, including hopelessness and anhedonia. The varied responses to currently available treatments highlight its complex etiology and pathophysiology1,2,3,4. Although most medications require an extended period to manifest their effects, alternative therapies like deep-brain stimulation can promptly alleviate symptoms in certain individuals5,6,7, whereas they are ineffective in many others8,9,10. Several brain structures, including the medial prefrontal cortex and habenula, play a role in reward, emotion, and motivation11,12,13,14,15, and have thus been proposed to be relevant in therapeutic action. However, to date, testing this hypothesis directly has not been possible, as a cell-type-specific target for therapeutic use has yet to be identified.
Herein, we performed a series of comprehensive behavioral tests and identified somatostatin (SST)-expressing neurons in the region-X (HBX) between the lateral and medial habenula as positive valence neurons (hereafter referred to as SST neurons). We then developed multimodal genetic models to bidirectionally control SST neurons in mice and demonstrated that these controls bidirectionally modulate (induce or relieve) antidepressant action. Subsequently, we employed genetically engineered virus tracing using single-unit recordings and found that SST neurons received inhibitory synaptic inputs directly from cholecystokinin (CCK)-expressing neurons in the bed nucleus of the stria terminalis (BNST) and projected excitatory axon terminals exclusively onto proenkephalin (PENK)-expressing neurons in the interpeduncular nucleus (IPN). Finally, we provide compelling evidence that a cell-type-specific circuit of SST neurons encodes antidepressant action and that selectively targeting this circuit profoundly and rapidly relieves depressive-like behaviors.
Results
Activation of SST neurons in the HBX generated antidepressant action
To explore cell-type-specific neurons that causally influence both the induction and relief of multiple depression-related behaviors, including reduced motivation and anhedonia, we developed a large array of tools for controlling motivation and emotion in adult naïve mice. In the initial brief access test, water-deprived adult naïve mice were presented with water and 2, 12, or 20% sucrose solutions in successive 5-s trials. Consistent with prior research, we observed that increased sweetness led to a higher intake at lower concentrations, reaching a peak at an intermediate level, and then declining with further increases in concentration. Notably, mice significantly favored sucrose over water (Fig. 1a). In a subsequent two-bottle preference (TBP) test using nondeprived mice (n = 50), a heightened attraction to the 12% sucrose solution was observed.
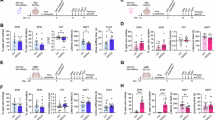
a The mice consume water, as well as 2%, 12%, and 20% sucrose solutions, data are presented as mean ± SEM (n = 8 mice per group, water vs. 2% sucrose ***p = 1.93 × 10−13, water vs. 12% sucrose ***p < 1.0 × 10−15, water vs. 20% sucrose ***p = 6.0 × 10−15, two-way ANOVA). b The scatter plot and the bar chart showing the sucrose preference in the TBP, the sucrose preference was estimated at 0.63 ± 0.006 (group 1: n = 13 mice); 0.74 ± 0.008 (group 2: n = 24 mice) and 0.86 ± 0.009 (group 3: n = 13 mice), data are presented as mean ± SEM (1 vs. 2 ***p = 8.76 × 10−11, 1 vs. 3 ***p < 1.0 × 10−15, 2 vs. 3 ***p = 2.53 × 10−10, one-way ANOVA). c The scatter plot and the bar chart showing the place preferences in CPP, which were estimated at 0.60 ± 0.004 in group 1 (n = 13); 0.69 ± 0.007 in group 2 (n = 24), and 0.81 ± 0.006 in group 3 (n = 13), data are presented as mean ± SEM (1 vs. 2 ***p = 1.43 × 10−8, 1 vs. 3 ***p < 1.0 × 10−15, 2 vs. 3 ***p = 8.0 × 10−15, one-way ANOVA). d Heatmaps of the locomotor activity of CPP and linear regression scatter plot showing the correlation between place preference and sucrose preference. Pearson’s r (2-tailed), p < 1.0 × 10−15. e A plot showing time spent in the central chamber in the CPP, data are presented as mean ± SEM (one-way ANOVA, ns, no significant difference). f Representative heatmaps and a plot showing time spent in the light box of high (n = 13) versus low (n = 13) sucrose preference mice in the LDT, data are presented as mean ± SEM (***p = 1.64 × 10−13, two-tailed t-test). “Low” for low-sucrose-preference groups and “High” for high-sucrose-preference groups. TBP: two-bottle preference; CPP: conditioned place preference; UCP: unconditioned place (water); CP: conditioned place (12% sucrose); LDT: light/dark test. Source data are provided as a Source Data file.
Sucrose preference, calculated as the number of licks for sucrose solution to total licks for both sucrose solution and water, varied among mice. To systematically categorize the mice, we employed arbitrary yet historically informed cutoffs based on individual sucrose preference scores. Specifically, we established thresholds at 70% and 80% sucrose preference to delineate the groups: low preference (group 1: n = 13), medium preference (group 2: n = 24), and high preference (group 3: n = 13) (Fig. 1b). We further subjected these 50 mice to a sucrose conditioned place preference (CPP) test and found that mice demonstrated a statistically significant increase in time spent within the chamber conditioned with 12% sucrose. The place preferences were estimated to be 0.60 ± 0.01 for group 1 (n = 13), 0.69 ± 0.01 for group 2 (n = 24), and 0.81 ± 0.01 for group 3 (n = 13) (Fig. 1c). By analyzing the sucrose and the place preferences, we categorized adult naïve mice into high- and low-sucrose-preference groups. High-sucrose-preference mice displayed a significantly larger sucrose preference and spent more time in the sucrose-conditioned place than low-sucrose-preference mice, whereas time spent in the central chamber did not differ between groups (Fig. 1d, e). Finally, in the light/dark test (LDT), high-sucrose-preference mice spent more time in the light box than low-sucrose-preference mice (Fig. 1f). Collectively, these results suggested that sucrose preference was indicative of antidepressant activity in mice.
Next, we generated reporter mice, FosCreERT2 (TRAP2), and labeled the temporally segregated active neuronal ensembles in these mice to map the neurons that encode antidepressant action (Figs. 2a–c and Supplementary Fig. 1a, b). Using RNA-scope staining, we confirmed the co-expression of SST mRNA in TRAP2-expressing neurons (Fig. 2d–f). We identified excitatory glutamatergic neurons that expressed SST in the HBX (hereafter referred to as SST neurons) and were highly activated by positive valence-related behaviors (Supplementary Figs. 2–4).
a Administration of 4-OHT following a sucrose-CPP test labeled neurons in several brain regions. b Representative images showing TRAP2ed neurons in the HBX, the NAc, and the BNST from mice with high- and low-sucrose-preference groups. c The numbers of TRAP2ed neurons in various brain regions from mice with high- and low-sucrose-preference groups, data are presented as mean ± SEM (n = 10 mice per group, BNST *p = 0.0098, MHb *p = 0.0097, MHb ***p = 1.23×10−7, ns, no significant difference, two-way repeated measures ANOVA). d Representative images showing that TRAP2ed neurons (mRNA) are expressed with SST mRNA. e A plot showing that 80.47% ± 0.61% TRAP2ed neurons co-localized with SST mRNA, data are presented as mean ± SEM (n = 3 mice). f A plot illustrating the co-localization analysis of TRAP2 and SST fluorescence. TRAP2: targeted recombination in active populations 2; 4-OHT: 4-hydroxytamoxifen; HBX: the region X of the habenula; NAc: the nucleus accumbens; BNST: bed nucleus of the stria terminalis; LHb: lateral habenula; MHb: medial habenula; SST: somatostatin. Source data are provided as a Source Data file.
Subsequently, we tested whether the bidirectional control (inhibition or excitation) of SST neurons could bidirectionally modulate (induce or relieve) multiple independent depression-related behaviors. Depression-related behaviors in mice are primarily assayed by employing established experimental protocols for systematic measurement of motivation, anhedonia, and depressive-like behaviors, demonstrating progressive deterioration under chronic stress exposure and measurable recovery following sustained antidepressant administration16,17,18,19. In investigations of depression-like behaviors, motivation is evaluated by exposing mice to constrained stressors, exemplified by procedures employed in the tail-suspension (TST), TBP, or social interaction (SIT) tests, and analyzing escape-related behaviors, including immobility, which is considered a sign of hopelessness and anhedonia20,21,22.
We then expressed an enhanced version of halorhodopsin-3.0 (NpHR3) or green fluorescent protein (GFP) in SST neurons via viral delivery of the NpHR3-GFP or GFP virus into the HBX of SST-CRE/Ai14 mice (Fig. 3a), producing the generation of SSTNpHR3GFP or SSTGFP mice (Fig. 3b and Supplementary Figs. 5a, b). We performed a comparative analysis between SSTNpHR3GFP mice and SSTGFP controls. Consequently, we observed a notable decrease in escape-related behavior that was transient during the illumination of SST neurons in the TST (Fig. 3c). To investigate whether the observed behavioral reduction reflected a generalized locomotor impairment versus selective deficits in escape motivation, we employed optogenetic inhibition of SST neurons in the identical cohort through an open-field-test (OFT). Motor activity levels showed consistent measurements between the groups (Supplementary Fig. 5c).
a Injection of the NpHR3-GFP virus into the HBX of SST-CRE/Ai14 mice, in which Ail4 was expressed in SST neurons by crossing SST-CRE mice with CRE-dependent Ail4 reporters. b Representative images showing bilateral expression of NpHR3-GFP and fiber location in Ail4-labeled SST neurons (n = 8 mice). c–e Optogenetic inhibition (yellow: light on) of SSTNpHR3 neurons induces depression-like behaviors, based on the TST (c), the TBP (d), and the SIT (e). Data are presented as mean ± SEM (n = 8 mice per group, ON: ***p = 1.03 × 10−5 in (c), ON: **p = 1.57 × 10−4 in (d), ON: ***p = 3.87 × 10−7 in (e) two-way repeated measures ANOVA). f Illustration showing the injection of the ChR2-GFP virus into the HBX of SST-CRE mice and representative images showing the expression of ChR2-GFP in SST neurons (n = 8 mice). g, h Experimental design (g) and optogenetic activation of SSTChR2GFP neurons validated by recording single-unit spikes in freely behaving mice (h), data are presented as mean ± SEM in (h). i–l Optogenetic activation (blue: light on) of SSTChR2GFP neurons generates antidepressant action, based on the LDT (i), the EPM (j), the SIT (k), and the OFT (l) before (baseline), during (on), and after (off) light illumination. In this study, depression-like behaviors were induced by CMS. The non-CMS mice were used as controls. Data are presented as mean ± SEM (n = 8 mice per group, ON: CMSGFP vs. CMSChR2 ***p = 1.48 × 10−8 in (i), ON: CMSGFP vs. CMSChR2 ***p = 1.29 × 10−7 in (j), ON: CMSGFP vs. CMSChR2 ***p = 6.53 × 10−9 in (k), ON: CMSGFP vs. CMSChR2 ***p = 1.64 × 10−8 in (l), ns, no significant difference, two-way repeated measures ANOVA). TST: tail suspension test; SIT: social interaction test; CMS: chronic mild stress; EPM: elevated plus maze; OFT: open field test. Source data are provided as a Source Data file.
Next, we employed a time-sensitive assay with high temporal precision to evaluate behavioral despair states, the TBP test23,24. Behavioral quantification was performed by analyzing lick events directed toward spouts dispensing either water or 12% sucrose solution throughout a 90-minute testing period. This protocol comprised three sequential phases: initial 30-minute baseline recording (light-off), subsequent 30-minute optogenetic intervention (light-on), and final 30-minute post-stimulation observation (light-off). Strikingly, SSTNpHR3GFP mice exhibited a marked decrease in sucrose preference specifically during the optogenetic intervention, whereas SSTGFP controls remained unaffected (Fig. 3d). Critically, no epoch-dependent variations in total licks were detected between groups (Supplementary Fig. 5d). We detected a substantial decline in the social index during the optogenetic intervention, specifically in the SSTNpHR3GFP mice, indicating a decrease in social interaction, which is a key aspect of depression-like behavior (Fig. 3e). Therefore, optogenetic inhibition of SST neurons reliably induced depression-like behavior, corresponding to the traditionally defined behavioral states of anhedonia18,19.
To test the potential causal link with depression-related phenotypes, we probed whether SST neuron activation could counteract their optogenetic inhibition. We activated SST neurons and achieved temporally precise excitation with light by expressing channelrhodopsin-2-E123A, a genetically engineered light-gated ChR2 ion channel fused with GFP in SST neurons. By injecting the ChR2-GFP or GFP virus into the HBX region of SST-CRE mice, we ultimately generated SSTChR2GFP or SSTGFP mice (Fig. 3f).
Given the established association between clinical depression and prolonged stress exposure over extended periods25,26, we employed the extended-duration chronic mild stress (CMS) protocol3. This experimental design involved administering variable mild stressors through a twice-daily regimen maintained consistently across a 21-day experimental timeline (Figs. 3g, Supplementary Table 1 and Methods). CMS was validated to reliably decrease motivation3, demonstrated through diminished escape-related responses to stressors and decreased sucrose preference reflecting anhedonia. The experimental setup consisted of four groups. SSTChR2GFP and SSTGFP control mice, which were either exposed to CMS or housed in a standard environment. We performed multiple behavioral assays, examining the animals during the baseline (light-off), optogenetic intervention (light-on), and post-stimulation observation (light-off) epochs. To produce the phasic activation of SST neurons, we employed a physiological, sparse bursting light stimulation protocol3,27,28, (eight pulses at 20 Hz, 5-ms pulse width, every 5 s; Fig. 3h). Behavioral motivation was assessed in each of the four groups using the LDT. During baseline, CMS significantly decreased the duration of time spent in the light box by over 70% relative to that in the controls. Upon illumination, CMS-SSTChR2GFP mice showed a marked elevation in the time spent within the light box relative to that of the CMS-SSTGFP group (Fig. 3i). In the TST, CMS also markedly inhibited escape-related behaviors by ~50% compared to control mice. Upon illumination, CMS-SSTChR2GFP mice displayed a notable enhancement in escape-related behaviors when compared to those of control CMS-SSTGFP mice (Supplementary Fig. 6a).
Optogenetic intervention of SST neurons rapidly alleviated depression-like effects in CMS-SSTChR2GFP but not CMS-SSTGFP mice in the TBP test, with no observed differences in total licks between experimental groups (Supplementary Fig. 6b). In addition, in the SIT, CMS-SSTChR2GFP mice exhibited a notable enhancement in social interaction behavior upon illumination compared with that of the CMS-SSTGFP group (Fig. 3k). Thus, phasic illumination of SST neurons in CMS-SSTChR2GFP but not CMS-SSTGFP mice reversed depression-like phenotypes to control levels. We subsequently examined anxiety-like behaviors using an elevated plus maze, in which the time spent in the open arms was quantified. In line with prior research29,30, our findings revealed that CMS substantially increased anxiety-like behaviors relative to those in non-stressed controls. Notably, the duration spent in the open arm showed a significant increase during illumination was observed in CMS-SSTChR2GFP but not CMS-SSTGFP control mice (Fig. 3j and Supplementary Fig. 6c). We also investigated whether the optogenetic activation of SST neurons affected locomotion by evaluating the activation of SST neurons in the same animals during exploration in the OFT. The distance traveled showed no statistically significant variation between the groups (Fig. 3l and Supplementary Fig. 6d). Together, these results demonstrated that the bidirectional control of SST neurons can rapidly modulate (induce or ameliorate) multiple depression-related behaviors.
SST neurons receive inputs from CCK neurons and project onto PENK neurons
Downstream synaptic targets of SST neurons in the HBX have yet to be studied. To systematically identify a cell-type-specific circuit that may underlie the control mechanisms of SST neurons in bidirectionally modulating (inducing or inhibiting) depression-related behaviors, we delivered a GFP virus into the HBX region of SST-CRE mice (Fig. 4a, b). The density of SST projections was assessed in the interpeduncular nucleus/ventral tegmental area (IPN/VTA) (Figs. 4c and Supplementary Fig. 7a–d). We conducted a comprehensive mapping of activated neurons throughout the brain following optogenetic activation of SSTChR2GFP neurons (Fig. 4d). Our findings indicated that neurons activated by photo-stimulation of SSTChR2GFP neurons were predominantly localized in the IPN, with additional activation observed in the region between the VTA and IPN (Figs. 4e, f and Supplementary Fig. 8a, b).
a–c Schematic diagram (a), representative images showing the expression of SSTGFP neurons (b) and the axon projection targets of HBXSST neurons (c) (n = 3 mice). d–f Schematic diagram (d), representative images (e, n = 3 mice), and a plot (f) showing the axon projection targets of HBXSST neurons and c-fos anti-body staining is utilized to identify the activation of neurons within the IPN. Data are presented as mean ± SEM (n = 3 mice per group, **p = 3.78 × 10−4, two-tailed t-test). g–k SST neurons project excitatory synapses onto PENK neurons. Schematic diagram (g) and representative images showing the expression of ChR2-GFP in SST neuronal axon terminals (h, n = 3 mice) and tdT in PENK neurons. Whole-cell patch-clamp recordings from PENK neurons were validated by biocytin labeling through recording electrodes (i, n = 4 mice). The evoked EPSCs by optogenetic stimulation of axon terminals of SSTChR2GFP neurons were completely blocked by 20 μM CNQX (j) or 1 μM TTX and reversed by 4-AP (k). Data are presented as mean ± SEM (n = 10 neurons from 7 mice, ***p = 3.96 × 10−11 in (j), ACSF vs. TTX ***p = 2.68 × 10-9 in (k), TTX vs. TTX/4-AP ***p = 1.75 × 10-6 in (k), paired sample two-tailed t-test for (j), one-way repeated measures ANOVA for (k), ns, no significant difference). l–o SST neurons receive inhibitory synaptic inputs from CCK neurons. Schematic diagram (l) and representative images showing the expression of TVA/G and GFP in SST neurons (m, n = 3 mice) and GFP in CCK neurons (n, n = 3 mice). The evoked IPSCs (o) recorded from SST neurons were completely blocked by 20 μM BIC or 1 μM TTX (also see Supplementary Fig. 11). Data are presented as mean ± SEM (n = 10 neurons from 6 mice, ACSF vs. TTX ***p = 5.07 × 10−12, ACSF vs. BIC ***p = 5.01 × 10−10, paired sample two-tailed t-test). VTA: ventral tegmental area; IPN: interpeduncular nucleus; PENK: proenkephalin; ACSF: artificial cerebrospinal fluid; CNQX: 6-cyano-7-nitroquinoxaline-2,3-dione; TTX: tetrodotoxin; 4-AP: 4-amino phenylacetic acid; BNST: bed nucleus of the stria terminalis; BIC: bicuculline. Source data are provided as a Source Data file.
To determine the downstream neuronal targets of SST neurons, we stereotaxically co-administered a recombinant herpes simplex virus type 1 (H129ΔTK-GFP) and a TK-tdTomato (TK-tdT) virus into the HBX of SST-CRE mice (Supplementary Figs. 9a, b). This injection led to the expression of GFP in SST neuronal targets in the IPN/VTA, in which PENK-expressing neurons comprise a major neuronal cell type, hereafter referred to as PENK neurons (Supplementary Fisg. 9c–e). To confirm that PENK neurons are downstream targets of SST neurons, we generated PENK-DRE mice in which DRE recombinase was expressed under the control of the PENK promoter. We then injected the rAAV2/9-hSyn-RSR-TVA/G-GFP and ΔG-RV-DsRed viruses into the IPN of PENK-DRE mice (Supplementary Fig. 9f). This injection induced simultaneous expression of GFP with DsRed in PENK neurons and DsRed in SST neurons (Supplementary Figs. 9g–j), demonstrating the direct synaptic input from SST to PENK neurons.
To ascertain the functional status of synaptic transmission between SST and PENK neurons, we crossed PENK-DRE with SST-CRE mice, generating PENKDRE/SSTCRE mice (Fig. 4g). Injection of the ChR2-GFP virus into the HBX and the tdT virus into the IPN of PENKDRE/SSTCRE mice led to the expression of ChR2-GFP in SST neurons (SSTChR2GFP) and tdT in PENK neurons (Fig. 4h, i). The whole-cell patch-clamp recordings of PENK neurons were performed in slices. At a holding potential of -70 mV, excitatory postsynaptic currents (EPSCs) were detected and elicited through blue laser stimulation onto SSTChR2GFP efferent axon projections. Evoked EPSCs were sensitive to TTX and the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionicacid receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (Fig. 4j, k). These data demonstrated that SST neurons directly and functionally project excitatory synapses onto PENK neurons.
To unambiguously identify afferent inputs to SST neurons, we generated mutant mouse lines with SST neuron-specific expression of the avian viral receptor TVA and rabies G proteins (SSTTVA/G mice) by injecting TVA/G virus particles into the HBX of SST-CRE mice. Synaptic retrograde GFP-encoding ΔG-rabies viruses were administered via microinjection into the same region (Fig. 4l). This resulted in the GFP labeling of SSTTVAtdT neurons (Fig. 4m) and their respective presynaptic neurons in several brain regions, with large clusters of labeled neurons observed in the BNST and nucleus of the diagonal band of Broca (Supplementary Figs. 10a, b). We focused on synaptic inputs from the BNST because it plays a role in valence surveillance and participates in modulating emotional/arousal states and regulating reward/aversion31,32,33. The BNST, a structurally diverse brain region, comprises 11 transcriptionally distinct neuronal populations34. To determine which of these neuronal populations project to SST neurons, we performed histochemical labeling and found that almost all labeled neurons in the BNST expressed VGAT and CCK, hereafter referred to as CCK neurons, but not protein kinase C-delta or VgluT2 (Figs. 4n and Supplementary Figs. 10a–c). This suggested that CCK neurons project to SST neurons and are GABAergic inhibitory neurons.
To determine the functional synaptic transmission between CCK and SST neurons, we generated three enzymatic recombination mutant mice (TERM mice), in which FLP recombinase was expressed in CCK neurons and CRE and DRE were expressed in SST (SST-CRE) and PENK (PENK-DRE) neurons, respectively (Supplementary Fig. 11a). We expressed ChR2-GFP in CCK neurons (CCKChR2GFP neurons) and tdT in SST neurons (SSTtdT neurons) by injecting ChR2-GFP and tdT viruses into the BNST and HBX regions of TERM mice, respectively (Supplementary Fig. 11b). Whole-cell patch-clamp recordings were conducted on SST neurons to evoke inhibitory postsynaptic currents (IPSCs) by optogenetic stimulation of CCKChR2GFP neuronal axon terminals. Evoked IPSCs were sensitive to TTX and 20 μM of the GABAA receptor antagonist, bicuculline (Fig. 4o). Taken together, these findings demonstrated that SST neurons located in the HBX receive inhibitory GABAergic inputs from CCK neurons in the BNST and directly and functionally project inhibitory synapses onto PENK neurons in the IPN.
Bidirectional control of CCK neurons modulated antidepressant action
Subsequently, we determined whether inhibitory synaptic inputs from CCK neurons affect depression-related states of SST neurons. We expressed NpHR3 in CCK neurons by injecting the NpHR3-GFP or GFP viruses into the BNST of CCK-FLP mice (Fig. 5a), leading to the generation of CCKNpHR3GFP or CCKGFP mice, respectively (Figs. 5b, c and Supplementary Fig. 12a). We performed multiple behavioral assessments, examining animal behavior across the three distinct epochs (light-off; light-on; light-off) (Fig. 5d). Optogenetic suppression of CCK neurons revealed distinct behavioral characteristics that were consistent with antidepressant action and could be reliably induced and reversed based on multiple behavioral assessments, including the CPP (Fig. 5e), TST (Fig. 5f), TBP (Fig. 5g), and SIT tests (Supplementary Fig. 12b), without affecting locomotion (Supplementary Fig. 12c). Given that CCK neurons functionally project inhibitory synaptic inputs onto SST neurons, the antidepressant action induced by the optogenetic inhibition of CCK neurons may be mediated by activating SST neurons. We then labeled activated SST neurons with c-fos and observed that the quantity of activated SST neurons in the HBX was highly correlated with the positive valence states in mice following the inhibition of CCK neurons (Fig. 5h). NpHR3 has been extensively used to suppress neuronal activity; however, this method affects all downstream target regions. We used eOPN3 (Gi/o mosquito rhodopsin), which is known to selectively inhibit synaptic release while maintaining the fidelity of action potentials in axons, thus providing a more specific approach to synaptic manipulation35,36,37. We expressed eOPN3 in CCK neurons by injecting the eOPN3 or tdT virus into the BNST of CCK-FLP mice (Supplementary Fig. 13a). This led to the generation of CCKeOPN3 or CCKtdT mice. We then implanted optical fibers bilaterally into the HBX of mice (Supplementary Fig. 13b). This strategy aimed to inhibit the terminals of CCK neurons in the HBX. Compared with CMS-CCKtdT mice, optogenetic inhibition of the terminals of CCK neurons in the HBX of CMS-CCKeOPN3 mice significantly attenuated depression-like phenotypes, as evidenced by the CPP, TST, TBP, SIT, and OFT tests (Supplementary Fig. 13c–g).
a–d Schematic diagram (a) and the expression of NpHR3 in CCK neurons (b, n = 10 mice) and their exon terminals (c, n = 10 mice) and single unit recordings of CCK neuronal inhibition from freely behaving mice (d) with fiber locations, error bar represents mean ± SEM in (d). e–g Optogenetic inhibition (yellow: light on) of CCK neurons generates antidepressant action, based on the sucrose-CPP (e), the TST (f), and the TBP assays (g). Data are presented as mean ± SEM (n = 10 mice per group, ON: CMSGFP vs. CMSNpHR3 **p = 2.92 × 10−4 in (e), ON: **p = 4.03 × 10−4 in (f), ON: ***p = 3.43 × 10−5 in (g), two-way repeated measures ANOVA, ns, no significant difference). Representative heatmaps in Fig. 5e show the CPP of CMS CCKNpHR3GFP mice before and during light illumination. In this study, depression-like behaviors in CCKGFP and CCKNpHR3GFP mice were induced by CMS, and the non-CMS CCKGFP and CCKNpHR3GFP mice were used as controls. h Plots showing the percentage of activated SST neurons following optogenetic inhibition of CCK neurons highly correlate with antidepressant action based on the CPP and the TBP assays. Pearson’s r (2-tailed). Data are presented as mean ± SEM (n = 10 mice per group, p = 4.2 × 10−3 for CPP, p = 1.28 × 10-4 for TBP). i–k Schematic diagram (i) and the expression of ChR2-GFP in CCK neurons (j, n = 10 mice), and single-unit recordings of CCK neuronal excitation from freely behaving mice (k) with fiber locations, error bar represents mean ± SEM in (k). l–n Optogenetic activation (blue: light on) of CCK neurons induces depression-related phenotypes, based on the assays in the TST (l), the sucrose-CPP (m), and the TBP (n). Data are presented as mean ± SEM (n = 10 mice per group, ON: GFP vs. ChR2 ***p = 3.83 × 10−5 in (l), ON: **p = 1.46 × 10-4 in (m), ON: ***p = 7.50 × 10−7 in (n), ns, no significant difference, two-way repeated measures ANOVA). CCK: cholecystokinin; UCP: unconditioned place (water); CP: conditioned place (12% sucrose). Source data are provided as a Source Data file.
To test the causal link between the antidepressant action and optogenetic inhibition of CCK neurons, we also assessed CCK neuron activation and examined whether it could induce depression-like phenotypes similar to those induced by CMS or through optogenetic inhibition of SST neurons (Fig. 5i). To achieve temporally precise CCK neuron activation with light illuminations, we expressed GFP-fused ChR2 in CCK neurons by injecting the ChR2-GFP virus into the BNST region of CCK-FLP mice, resulting in the generation of CCKChR2GFP or CCKGFP mice, respectively (Fig. 5j). We performed multiple behavioral assessments, examining the animals during the three epochs (light-off; light-on; light-off) (Fig. 5k). Following light illumination, the CCKChR2GFP mice exhibited a significant reduction in escape-associated behavior (Fig. 5l), a decrease in social interaction in the SIT (Supplementary Fig. 14a) relative to that of the CCKGFP group, and significantly reduced place and sucrose preference in both the CPP (Fig. 5m) and TBP assessments (Fig. 5n), without affecting locomotion (Supplementary Fig. 14b). Therefore, optogenetic modulation of CCK neurons in CCKChR2GFP but not CCKGFP mice induced a depression-like phenotype similar to that caused by CMS or through optogenetic suppression of SST neurons. Together, these findings revealed that optogenetic activation of CCK neurons produces profound depression-like behavior in aspects of both motivation and the ability to experience pleasure and that selective inhibition of CCK neurons alleviates the depression-like phenotype through the activation of SST neurons in the HBX.
A cell-type-specific circuit of SST neurons encodes antidepressant action
Next, we investigated the neural representations of antidepressant action in the downstream targets of CCK neurons under conditions of baseline and phasic CCK neuron inhibition. We recorded neural activity from SST neurons in freely behaving mice while performing optogenetic inhibition of CCK neurons (Fig. 6a, b). Thirty minutes of data were recorded during behavioral performance in a TBP task, in which transitions between licks for water versus sucrose were clearly demarcated, providing a prospect to explore the SST neural dynamics that underlie antidepressant action. We validated that the optogenetic inhibition of CCKNpHR3 neurons reliably activated SST neurons (Fig. 6c). We observed a substantial population of SST neurons (n = 98) that exhibited phasic changes in response to light pulses or licks in response to sucrose. The majority of SST neurons (93 out of 98) were activated by light illumination of CCKNpHR3 neurons. Of these neurons, only five SST neurons showed different phasic responses to light pulses compared with those in lick events (Fig. 6e), whereas 88 of the 93 SST neurons showed phasic excitation associated with licks for sucrose (Figs. 6d and Supplementary Movie 1).
a Schematic diagram. b Experimental schedule and representative images showing the expression of BNSTCCKNpHR3 and HBXSSTChR2 (n = 7 mice). c Representative recordings of single units from CCKNpHR3 and SSTChR2 neurons, showing that optogenetic inhibition of CCK neurons activates SST neurons in the TBP test (also see Supplementary Movie 1). d, e 93 SST neurons were recorded from 7 mice in the TBP test. Peri-event raster histograms for representative neurons (d), showing phasic excitation associated with both light inhibition and lick events (88 out of 93 cells), and representative neurons (e), showing different phasic responses with light pulses and lick events (5 out of 93 cells). f Raster plots of single units in response to lick events in an example neuron from control and CMS mice (84 SST neurons were recorded from 7 control mice, ranging from 8 to 17; 71 SST neurons were recorded from 7 CMS mice, ranging from 5 to 12). g Bar plot of firing frequency of the SST neurons, with and without optogenetic inhibition (yellow: light on) of CCK neurons, data are presented as mean ± SEM (n = 7 mice per group, Baseline *p = 8.42 × 10−3, Lick *p = 5.48 × 10−3, two-way repeated measures ANOVA). h, i Peri-event raster histograms for representative neurons from control mice (h), showing phasic excitation of SST neurons associated with lick events during light off and on and representative neurons from CMS mice (i), showing different phasic responses of SST neurons with lick events. j Single-unit firings from SST neurons correlate to antidepressant action. Plots showing the percentage of SST neurons with action potential firings in response to the lick events during light off and on epochs in individual control (blue) and CMS (pink) mice. Pearson’s r (2-tailed), CMS p = 6.82 × 10−3 and Control p = 3.13 × 10−3 for Light off, CMS p = 3.69 × 10−4 and Control p = 2.42 × 10−3 for Light on. Data are presented as mean ± SEM (n = 7 mice per group, two-way repeated measures ANOVA). TERM: triple enzymatic recombination mutant; CMS: chronic mild stress. Data are provided as a Source Data file.
We observed a significant increase in action potential firing from CCK neurons in mice subjected to CMS compared with that in control mice (Supplementary Fig. 15a, b). Furthermore, our analysis revealed that CMS did not alter the intrinsic properties of these neurons, including resting membrane potential (Supplementary Fig. 15c), input resistance (Supplementary Fig. 15d), and action potential threshold (Supplementary Fig. 15e).
In addition to phasic responses, we also observed that SST neurons were associated with decreased action potential firings and sucrose preference in CMS mice compared with those in controls (Figs. 6f, g and Supplementary Fig. 15f). Seventy-eight out of the 91 SST neurons from control mice (86%; Fig. 6h) encoded active behaviors, as observed by phasic responses associated with licks for sucrose both during the baseline and optogenetic intervention period. Of the 91 SST neurons from CMS mice, 75 showed different phasic responses to sucrose licks during the baseline and optogenetic intervention epochs. (Fig. 6i). Optogenetic inhibition of CCK neurons significantly increased sucrose licks during light-on periods compared to light-off periods. Furthermore, the proportion of SST neurons exhibiting phasic activation patterns during CCK neuron inhibition positively correlated with the degree of behavioral state activation (Fig. 6j). These data indicated that the ensemble assemble activity of SST neurons under the control of CCK neurons encodes antidepressant action.
Activation of PENK neurons generated antidepressant action
Having determined the robust phasic electrophysiological responses of SST neurons to antidepressant action, we observed that SST neuron excitation was causally involved in antidepressant action and that inhibitory synaptic inputs from CCK neurons were required for coding these actions. We examined whether the bidirectional control of PENK neurons (downstream synaptic targets of SST neurons) could bidirectionally modulate neural representations of behaviorally despair-like states. We expressed ChR2-GFP in SST neurons (SSTChR2GFP neurons) by injecting the ChR2-GFP virus into the HBX of TERM mice, concurrently, we injected NpHR3-tdT into the IPN to enable the expression of NpHR3-tdT in PENK neurons (Fig. 7a, b). We used these genetic settings to simultaneously assess and control the active responses of both SST and PENK neurons to behavioral challenges by conducting synchronized single-unit electrophysiology with optogenetics in freely moving mice. We observed well-isolated single units from PENK neurons after the optogenetic excitation of SST neurons (Fig. 7c). Of the 127 recorded PENK neurons, 83 neurons (65%) exhibited phasic activation patterns during lick events under both baseline and SST neuron optogenetic stimulation conditions. Notably, 81 neurons (97.6% of responsive cells) maintained consistent lick-related firing patterns across these experimental paradigms (Fig. 7d), whereas only two of these 83 neurons differentially represented licks in the baseline and SST neuron optogenetic stimulation conditions (Fig. 7e). We also found that action potential firing from PENK neurons was robustly decreased in CMS mice when contrasted with control mice (Figs. 7f, g and Supplementary Fig. 16). Optogenetic stimulation of SST neurons significantly modified the representation of antidepressant action in PENK neurons; of 117 PENK neurons, 82 (70.1%) were found to selectively code antidepressant action during both baseline and optogenetic intervention epochs (Fig. 7h–j). To validate the electrophysiological recordings from CCK neurons, we engineered PENK neurons expressing GCaMP6m (PENKGCaMP6m) and performed Ca2+ imaging of these neurons in response to strobe light illumination (Figs. 8a, b and Supplementary Fig. 17a, b). As supported by single-unit recordings, we observed a substantial boost in Ca2+ activity in PENK neurons in response to the optogenetic inhibition of CCK neurons and detected time-locked Ca2+ dynamics in a positive valence state (Fig. 8c, d). Finally, we bidirectionally manipulated PENK neurons using optogenetics in PENKChR2GFP and PENKNpHR3GFP mice (Fig. 8e, f) and demonstrated that the optogenetic stimulation of PENK neurons attenuated depressive-like behaviors (Figs. 8g and Supplementary Fig. 18b). However, the selective inhibition of these same neurons induced depression-like behaviors similar to those induced by CMS (Fig. 8h and Supplementary Fig. 18d). Notably, the optogenetic control of PENK neurons had no effect on locomotion (Supplementary Fig. 18a, c).
a, b Schematic diagram (a) and representative images (b, n = 9 mice) showing the expression of ChR2-GFP in SST neurons in the HBX, the projections of SSTChR2GFP and the expression of NpHR3-tdT in PENK neurons (PENKNpHR3tdT) in the IPN. c Action potential firings isolated from SST and PENK neurons are modulated by optogenetic controls of SSTChR2GFP. d, e Peri-event raster histograms for representative neurons showing phasic excitation associated with both light stimulation of SST neurons and lick events (d, example cell 1, 81 out of 83 cells) or different phasic responses with light pulses versus lick events (e, example cell 2, 2 out of 83 cells). f, g Raster plots (f) of single units in response to licks (pink) in an example neuron from control and CMS mice. Bar plots (g) of firing frequency of the PENK neurons during 30 min of the recording epoch, with and without optogenetic activation (blue: light on) of SST neurons, data are presented as mean ± SEM (n = 9 mice per group, 91 PENK neurons were recorded from 9 control mice, ranging from 7 to 15, 99 PENK neurons were recorded from 9 CMS mice, ranging from 8 to 13, ns, no significant difference, ***p = 4.86 × 10−5, two-way repeated measures ANOVA). h, i Peri-event raster histograms for representative PENK neurons from CMS mice (h) and control mice (i), showing different phasic responses with lick events during light off and on epochs. j Single-unit firings from PENK neurons correlate to antidepressant action. Plots showing the percentage of the numbers of neurons with action potential firings in response to lick events during light-off and light-on epochs, in individual control (blue) and CMS (pink) mice. Pearson’s r (2-tailed), CMS p = 5.91 × 10−4 and Control p = 1.05 × 10−3 for Light off, CMS p = 1.44 × 10−4 and Control p = 1.37 × 10−2 for Light on. Data are presented as mean ± SEM (n = 9 mice per group). TERM: triple enzymatic recombination mutant. Source data are provided as a Source Data file.
a, b Schematic diagram (a) and representative images (b, n = 3 mice) showing the expression of NpHR3-GFP in CCK neurons within the BNST and the expression of GCaMP6m in PENK neurons within the IPN, along with the depiction of optrode and optical fiber implantation sites in TERM mice. c, d Single unit recordings in CCK neurons (c) and peri-event plots of Ca2+ dynamics in PENK neurons (d) in response to optogenetic inhibition of CCK neurons in freely behaving mice, error bar represents mean ± SEM in (d). e, f Peri-event raster histograms for single-unit firings from PENKChR2GFP (e) and PENKNpHR3GFP (f) neurons are bidirectionally modulated by light illuminations, error bar represents mean ± SEM in (e) and (f). g Plots showing that optogenetic activation (blue: light on) of PENKChR2GFP neurons generates antidepressant action. Data are presented as mean ± SEM (n = 8 mice per group, ON: CMSGFP vs. CMSChR2 ***p = 5.97 × 10−6 for TST, ON: CMSGFP vs. CMSChR2 **p = 1.75 × 10−4 for TBP, ns, no significant difference, two-way repeated measures ANOVA). h Plots showing that selective inhibition (yellow: light on) of PENKNpHR3GFP neurons generates behavioral despair-like states. Data are presented as mean ± SEM (n = 8 mice per group, ON: GFP vs. NpHR3 ***p = 1.91 × 10−7 for TST, ON: GFP vs. NpHR3 ***p = 1.14 × 10−9 for TBP, ns, no significant difference, two-way repeated measures ANOVA). Source data are provided as a Source Data file.
Discussion
This study examined the key question of whether the activation of SST neurons in the HBX generates antidepressant action. We identified a type of antidepressant SST neuron in the habenula that encodes a positive valence. We demonstrated that multiple independent antidepressant actions could be induced by the activation of the SST neurons. We also searched for a specific type of SST neuron circuit in the HBX and found that these neurons received inhibitory synaptic inputs directly from CCK neurons in the BNST and projected excitatory synaptic inputs onto PENK neurons in the IPN. Bidirectional control of CCK neurons modulates neural representations of antidepressant action generated by the activation of SST or PENK neurons. These findings provide circuit-level insights into neural representations of antidepressant action in a cell-type-specific circuit of SST neurons.
Dopaminergic neurons in the dorsal raphe nucleus and VTA send out signals that are received by excitatory glutamatergic neurons in the BNST38,39, which are known to play crucial roles in the onset and manifestation of depression-like behaviors, and following antidepressant treatments40,41. Given that SST neurons in the HBX are inhibited by CCK neurons in the BNST and project excitatory synapses onto PENK neurons in the IPN/VTA, we proposed a circuit-based working model of antidepressant action by SST neurons (Supplementary Fig. 20). In this model, excitatory glutamatergic neurons in the BNST innervated by dopaminergic neurons in the IPN/VTA42 may regulate local inhibitory CCK neurons in the BNST and, in turn, modulate the antidepressant action of SST neurons in the HBX. Overactivity within the lateral habenula (LHb), one of the three components of the habenula, is associated with depression-like symptoms in both animal models and humans43,44. Specifically, an increase in burst firing in the LHb neurons has been shown to induce depressive-like behaviors in mice45,46. Although neurons in the LHb do not project to SST neurons in the HBX, they directly project to the VTA via excitatory synaptic connections47,48. Thus, stress signals from the LHb may be transmitted to the VTA and, in turn, activate CCK neurons in the BNST and inhibit SST neurons in the HBX, leading to the development of depression-like behaviors. Consistent with these results, a recent study reported that bright light treatment effectively reduced burst firing occurring in the LHb and attenuated depression-like behaviors in rodents49,50. Thus, modulating the IPN/VTA→ BNST→ HBX/LHb subnetworks, such as through deep brain stimulation (DBS), may represent a promising strategy for the rapid relief of depression-like symptoms in humans.
In the context of existing trials targeting the habenula for DBS51,52,53, our proposed circuit-based model offers a complementary perspective on the neurobiological mechanisms underlying depression. Several studies conducted on patients have investigated the efficacy of habenula DBS in alleviating symptoms of treatment-resistant depression (TRD). For instance, a pilot study demonstrated that habenula DBS could reduce depressive symptoms in patients resistant to conventional therapies, underscoring the habenula’s role as a critical node in mood regulation and its potential as a target for neuromodulation54. Building on these findings, our study identifies key subnetworks that represent a pathway modulating depressive behaviors, involving the regulation of local inhibitory CCK neurons in the BNST and the excitatory influence on PENK neurons in the IPN/VTA. This pathway provides a more nuanced understanding of how the habenula and its connected circuits contribute to depression, potentially explaining the mixed outcomes observed in habenula DBS trials. Future research should explore the therapeutic potential of targeting this specific circuit, either in conjunction with or as an alternative to direct habenula stimulation. Such an approach may offer a more comprehensive and precise strategy for treating depression, particularly in cases where habenula DBS alone has shown limited efficacy.
Most psychopharmacological treatments for depression take weeks or months to achieve effects in clinical settings5,6. Alternative therapeutic approaches, including DBS55,56 and ketamine57,58, demonstrate swift efficacy but remain effective for only a limited proportion of patients. Thus, the acute behavioral changes observed in this study reflect the degree to which direct control of a cell-type-specific circuit of SST neurons might bypass traditional therapeutic uses, hence, temporarily generating antidepressant action. Consistent with this notion, we observed that the optogenetic inhibition of CCK neurons in the BNST or the activation of SST neurons in the HBX stimulated excitatory glutamatergic projections to PENK neurons. Thus, our data confirm the role of the habenula in relaying positive valences with motivation to the IPN, a brain region known to be associated with reward and motivation59, and translating such states into behaviorally relevant outputs for coding antidepressant action. Our chronic stimulation data revealed that the repetitive activation of SST neurons in the HBX generated a positive valence and elicited a long-lasting reversal of depression-like behaviors (Supplementary Fig. 19). Additional investigations will be necessary to elucidate the underlying molecular mechanisms between the chronic activation of a cell-type-specific antidepressant circuit and the corresponding rescue of these behaviors.
Together, our data provide an experimental bridge between a cell-type-specific circuit of SST neurons that encode positive valence in animal models with antidepressant action. Thus, direct control of this cell-type-specific circuit will offer a promising strategy for the therapeutic intervention of a subset of major depression-like behaviors and, in general, could serve as an effective means for correcting maladaptive behaviors.
Methods
Ethical compliance
Our study complies with all applicable ethical regulations. The study received full approval from the Committee on the Ethics of Animal Experiments at Huazhong University of Science and Technology, ensuring that animal welfare was prioritized throughout the research process.
Mice
Male mice were used to avoid the potential differences between sexes. Mice were bred and reared under the same conditions in accordance with institutional guidelines and the Animal Care and Use Committee of the animal core facility at Huazhong University of Science and Technology, Wuhan, China and housed in groups of three to five mice/cage under a 12-h light-dark cycle, with lights on at 8:00 am, at a consistent ambient temperature (21 ± 1 °C) and humidity (50 ± 5%)60,61,62. Behavioral assessments were conducted throughout the light phase of the cycle (otherwise indicated in the specific experiments). Fos2A-iCreER/+ (TRAP2, Stock No: 030323) and ROSA26-CAG-stop-flox-tdTomato (Ai14, Stock No: 007914) mice were purchased from the Jackson Laboratory and crossed these transgenic lines to obtain the double heterozygous (TRAP2:Ai14) mice.
To selectively target CCK neurons in the BNST, SST neurons in the HBX, and PENK neurons in the IPN, we generated TERM mice, in which CRE and FLP recombinases were expressed in SST (SST-CRE) and CCK (CCK-FLP) neurons, respectively, whereas DRE recombinase was expressed in PENK (PENK-DRE) neurons. In order to generate TERM mice with C57BL/6 genetic background, we generated double knock-in mice through the crossing of SST-CRE and CCK-FLP mice. After that, these double knock-in mutant mice were bred with the PENK-DRE mice to acquire triple knock-in mutants, namely TERM mice.
SST-CRE (SST-IRES-CRE C57BL/6 J) was generated in our previous studies63. CCK-FLP (CCK-IRES-FLP) mice were generated by Cyagen Biosciences, Inc., Suzhou, China. PENK-DRE mutation was generated by Cyagen Biosciences, Inc., Suzhou, and had an IRES2 sequence and a DRE recombinase gene inserted immediately downstream of the PENK translational STOP codon. The targeting vector contained, from 5’ to 3’, a partial PENK sequence spanning exon 1 to intron 1 (with an frt3 site in intron 1), a partial PENK exon 2 sequence up to and including the endogenous stop codon, an internal ribosome entry site 2 (IRES2) sequence (allows translation initiation in the middle of an mRNA sequence), a DRE recombinase gene, a bovine growth hormone poly-A sequence, an AttB site, a PGK promoter-Neomycin resistance gene-PGK poly-A cassette, an frt5 site, an mRNA splice acceptor, the 3’ portion of the hygromycin gene (Hygro2) with SV40 late poly-A signal, and an AttP site. This construct was electroporated into C57BL/6 F1-derived G4 embryonic stem (ES) cells. Correctly targeted ES cells were injected into recipient blastocysts. PENK-DRE mice were bred with C57BL/6 J wild-type mice for 5 generations.
Brief access test
We performed a 30-minute brief access test in mice64. In brief, each mouse was allowed to freely access only one sipper, starting each trial voluntarily. During initial testing, a sipper delivered one aliquot (2 mL) for each detected lick response for 5 sec before being blocked for 8 s via a sliding door. Mice could then initiate a new trial. A computer-controlled valve system (Lafayette Instrument Company, US) delivered water and sucrose solutions (2%, 12%, or 20%) across three trial blocks, with one aliquot per trial. Cumulative licks were recorded for all trials. Mice were acclimated to the apparatus and trained with water after 20 h of deprivation. Upon achieving stable lick behavior, testing commenced under 10-hour deprivation conditions. To avoid the variability, we averaged lick ratios from 2-consecutive tests for individual mice.
Two-bottle preference test (TBP)
We examined the sucrose preference of mice by using a two-bottle preference test23. Each test lasts for 10 min. Each mouse was acclimated to the behavioral apparatus and granted access to two bottles, one of which was filled with water and the other with a 12% sucrose solution. We recorded the licks per sipper and calculated the preference ratios, which are the licks for sucrose divided by the total licks for sucrose and water. To avoid side biases, we examined mice under different conditions over 4 consecutive days, switching the positions of the sucrose and water bottles every day. For each mouse, we calculated the average sucrose preference across 4-consecutive testing days.
Conditioned place preference test (CPP)
The CPP test was adopted from a previous study65,66, with minor modifications. The CPP setup was constructed with two visually distinct chambers partitioned by an intermediary neutral zone (Noldus, Holland): a left compartment, measuring 25 cm × 20 cm, featured darkened wall surfaces and flooring composed of metallic mesh panels; a center chamber measuring 15 cm × 20 cm with gray walls and gray plastic floor; and a right chamber measuring 25 cm × 20 cm with white walls and a mesh metal floor. Mouse location within the apparatus during each preference test was monitored using a computerized photobeam system (Noldus, Holland). Briefly, on day 1, individual mice were placed in the center chamber and allowed to freely explore the entire apparatus for 15 min (habituation). On day 2, drops of water were paired with the mice that were confined to one of the side chambers for 20 min (20 μL per minute), and in the second session, mice were confined to the other side chamber and paired with drops of 12% sucrose solution for 20 min (20 μL per minute). The order of sucrose conditioning sessions was counterbalanced. On day 3, mice were permitted to freely explore the entire experimental device for 20 min to assess their place preference.
In the optogenetic CPP experiment, the initial habituation phase (Day 1) involved transferring individual mice to the central neutral zone for a 20-minute acclimatization period with full apparatus access. Subsequent conditioning procedures (Day 2) comprised 30-minute sessions where mice were assigned to a compartment under baseline optical conditions (non-activated fiber connection). After 6 h, during photostimulation conditioning, mice were allocated to the stimulus-associated chamber (blue/yellow wavelength exposure) for 30-minute exposure intervals. A balanced randomization protocol ensured equitable spatial conditioning distribution across the cohort. Post-conditioning evaluation (Day 3) involved a 20-minute unrestricted exploration trial with full chamber access, during which spatial preference was determined through continuous quantification of chamber occupancy durations using automated tracking systems. hChR2 excitation was achieved using precisely timed photonic delivery systems generating 20-Hz pulsed blue light (pulse duration: 20 ms) (473 nm, 5 mW, DPSS laser, Inper Co., Ltd., Hangzhou, China). Continuous yellow light (595 nm for NpHR3.0, 532 nm for eOPN3, 10 mW) was used to activate NpHR3.0 or eOPN3.
The place preference score was calculated by dividing the time spent in the conditioned place by the total time spent in the conditioned and unconditioned place during the test.
Light/dark test
The experimental setup for behavioral assessment utilized an acrylic enclosure (40 cm × 20 cm × 20 cm) partitioned into two equal-volume compartments (20 cm3 each) through central vertical division. The dark section received full-spectrum attenuation via black aluminum cladding, while the light counterpart maintained ambient light transmission. Intercompartmental mobility was enabled through a 52 cm ground-level access port centered on the partition wall, supplemented by a parallel 0.5 cm-wide vertical conduit for uninterrupted optogenetic tether routing. Mice were initially positioned in the light compartment under continuous 5-minute video graphic monitoring via ceiling-mounted digital tracking apparatus, with spatial navigation patterns systematically quantified throughout the observation. The time spent on the sides was analyzed and monitored using a computerized photo-beam system (Noldus, Holland).
Chronic mild stress (CMS)
Stressed mice were housed separately from non-stressed control mice in a quiet room with a reverse 12-hour light-dark cycle, supplied with food and water ad libitum, and subjected to a standard CMS protocol3. In brief, two stressors per day for 21 consecutive days before behavioral testing. Mice were exposed to one stressor during the day and a different stressor during the night; white noise for 3 h; strobe light illumination for 3 h; damp bedding (200 mL water poured into sawdust bedding) for 6 h, forced swim in a forced swim test tank with water for 6 min or tail suspension in a tail suspension test apparatus for 6 min. Experimental protocols were approved by the Animal Care and Use Committee of the animal core facility at Huazhong University of Science and Technology, Wuhan, China.
TRAP2 strategy
Young adult male TRAP2-Ai14 mice, aged 90 ± 2 days, were utilized for TRAP2 investigations and classified into High and Low sucrose preference groups based on the Conditioned Place Preference (CPP) test. On Day 1, each mouse was placed in the central chamber and permitted to freely explore the entire apparatus for 15 min to acclimate. On Day 2, the mice were confined to one of the side chambers for 20 min, during which they were paired with water drops for 20 min at a rate of 20 μl per minute. Subsequently, in a separate session, the mice were confined to the opposite side chamber for an additional 20 min, where they were paired with 12% sucrose solution drops at the same rate. The sucrose conditioning sessions were counterbalanced to control for potential side biases. On Day 3, behavioral testing was conducted, and the test mouse was introduced into the CPP apparatus and allowed to explore freely for 20 min. Sixty minutes following the behavioral test, the mouse received an intraperitoneal injection of 50 mg/kg 4-OHT. Three hours post-administration, the mouse was returned to its home cage. Histological examinations were performed five days after the 4-OHT administration.
For the pleasant touch experiment, one week prior to the experiment, mice were singly housed and gently stroked in their cages to acclimate them to the environment. A handheld soft brush (6 cm wide, soft fan-shaped powder brush) was used to gently stroke the furry back skin of the mouse at a constant speed (18 ~ 22 cm/second) and force (maximum 23 ~ 25 mN) from the cervical spine to the lumbar expansion area (T11 ~ L2)67. Mice were placed in a dimly lit room and individually habituated for 120 min. Eight sessions were performed, with each session including three trials of 100-second pleasant touch interspersed with 5-minute rests. Sixty minutes following the behavioral test, the mouse received an intraperitoneal injection of 50 mg/kg 4-OHT. Three hours post-administration, the mouse was returned to its home cage. Histological examinations were performed five days after the 4-OHT administration.
For the foot shock experiment, mice were placed in a dimly lit room and individually habituated for 120 min. A total of 15 randomly arranged foot shocks (1 mA, 2 sec) were administered to the mice within a 10-minute period in a fear conditioning chamber (Coulbourn Instruments, USA)68,69. Similarly, 60 min following the conclusion of the experiment, the mouse received an intraperitoneal injection of 50 mg/kg 4-OHT. Three hours post-administration, the mouse was returned to its home cage. Histological examinations were conducted five days subsequent to the 4-OHT administration.
Virus particles and injections
The rAAV2/9-hSyn-DIO-NpHR3GFP (titer: 1.59 × 1013 VG/mL), rAAV2/9-hSyn-DIO-GFP (titer: 2.06 × 1013 VG/mL), rAAV2/9-hSyn-DIO-ChR2GFP (titer: 2.17 × 1013 VG/mL), rAAV2/9-hSyn-DIO-ChR2-tdTomato (titer: 2.2 × 1013 VG/mL), rAAV2/9-hSyn-RSR-TVA/G-GFP (titer: 1.78 × 1013 VG/mL), rAAV2/9-hSyn-RSR-tdTomato (titer: 1.97 × 1013 VG/mL), rAAV2/9-hSyn-DIO-TVA/G-tdTomato (titer: 5.35 × 1012 VG/mL), rAAV2/9-VGAT-fDIO-ChR2GFP (titer: 1.09 × 1013 VG/mL), rAAV2/9-hSyn-DIO-tdTomato (titer: 1.40 × 1013 VG/mL), rAAV2/9-VGAT-fDIO-NpHR3-GFP (titer: 1.67 × 1013 VG/mL), rAAV2/9-hSyn-RSR-GCaMP6m (titer: 1.88 × 1013 VG/mL), rAAV2/9-hSyn-RSR-NpHR3- tdTomato (titer: 1.45 × 1013 VG/mL) and rAAV2/9-hSyn-DIO-hM3DqGFP (titer: 2.28 × 1013 VG/mL) were purchased from Taitool Bioscience (Shanghai, China). The ΔG-RV-GFP (titer: 8.51 × 108 VG/mL) and ΔG-RV-DsRed (titer: 7.01 × 108 VG/mL) were purchased from BrainVTA (Wuhan, China). The rAAV2/9-EF1ɑ-fDIO-eOPN3-mScarlet (titer: 5.03 × 1012 VG/mL) was purchased from BrainCase (Shenzhen, China). The rAAV2/9-hSyn-DIO-TKtdTomato (titer: 1.75 × 1013 VG/mL) and H129ΔTK-GFP (titer: 1.00 × 109 VG/mL) were described63.
The surgical and viral injection procedures have been previously described63. In brief, mice were anesthetized with 4% isoflurane and maintained with 1% isoflurane using an animal anesthesia system (Shenzhen RWD Life Science Co., Ltd.) and secured in a stereotaxic frame (Shenzhen RWD Life Science Co., Ltd.). The scalp was disinfected with 75% alcohol, followed by a midline scalp incision and cranial drilling with a 0.6 mm drill bit. AAV viruses were injected into the habenula (HBX; AP: −1.50 mm, DV: −2.53 mm, ML: ±0.25 mm), bed nucleus of the stria terminalis (BNST; AP: +0.12 mm, DV: −4.25 mm, ML: ± 0.8 mm), or interpeduncular nucleus (IPN; AP: −3.61 mm, DV: −4.58 mm, ML: ±0 mm). The virus solution was administered at a total volume of 300 nL bilaterally or unilaterally at an infusion rate of 80 nL/min via a 10 μL syringe connected to a micro-injection pump (KD Scientific, Cat# 78-8130). Post-infusion, the needle remained positioned for 40 min to ensure proper diffusion. Mice were subsequently transferred to a warming pad until full recovery. All behavioral tests were conducted 4 to 8 weeks post-viral injection. The expression pattern of the virus was confirmed by immunofluorescence staining and imaging using a confocal microscope (Zeiss, LSM 800).
Open field test (OFT)
The experimental configuration employed a behavioral arena with standardized dimensions (50 cm × 50 cm × 40 cm). The mouse was attached to a cable that was joined to the outer portion of the chronically implanted optical fiber. Mice were placed in the center of the chamber and permitted to freely explore for 12 min. In the open field test, the central area was defined as a 625 cm² region, constituting 25% of the total open field area. The corner areas were each delineated as 235 cm² sections at the four corners. We measured the velocity of a mouse in the field using TopScan tracking software (Noldus, Holland).
Social interaction test (SIT)
The three-chamber apparatus, with dimensions of 50 cm in length and 42 cm in width, had dividing walls with doorways (13 cm wide) to facilitate mouse access to each chamber22,70. During the initial phase, two empty cylindrical acrylic cages (diameter 10 cm, height 15 cm) were placed in the lateral chambers. A test mouse was introduced into the central chamber and allowed to investigate the cylinders for 10 min. In the next phase, one cylinder housed an unfamiliar adult male mouse, while the other contained a similarly sized and colored object. The test mouse was then returned to the central chamber and permitted to explore the setup freely for an additional 10 min. Both sessions were video-recorded, and interaction times with the novel mouse (T-mouse) and the object (T-object) were measured. The sociability index was calculated using the formula: (T-mouse − T-object) / (T-mouse + T-object). The equipment was disinfected with 75% ethanol between trials.
Tail suspension test (TST)
The mice were subjected to the tail suspension test by attaching them 1 cm from the tip of the tail with adhesive tape, suspended ~50 cm above a contact-free surface. To prevent climbing or hanging, plastic tubes were secured around the tails. In cases where optogenetic manipulation was employed, it was initiated immediately after placing the mice in the testing chamber and lasted for 6 min. The behavior of the mice was recorded with a digital camera, and the period of immobility was assessed via TopScan tracking software (Noldus, Holland).
Elevated plus maze (EPM)
For the EPM test, we used a standard mouse EPM-sized maze (50 cm height of the maze from the floor, 63 cm full length of each arm type, 6 cm arm width, 15 cm tall closed arms, with 0.5 cm tall/wide ledges on the open arms). At the beginning of the assay, a mouse was positioned in the center region of the maze. Its behavior was tracked for 10 min and later analyzed using TopScan tracking software (Noldus, Holland). Parameters such as the number of entries and time spent in the open arm were analyzed.
Electrophysiology and optogenetics ex vivo
To investigate synaptic transmission from CCK neurons to SST neurons or from SST neurons to PENK neurons, we expressed ChR2 in CCK neurons or SST neurons. The HBX or the IPN slices (300 μm) from mice, in which ChR2GFP was expressed in CCK or SST neurons, and GFP was expressed in SST or PENK neurons, were prepared as previously described60,62,63. Brain tissue sections were initially placed in an incubation chamber filled with oxygenated artificial cerebrospinal fluid (ACSF, in mM: 124 NaCl, 3 KCl, 26 NaHCO3, 1.2 MgCl2, 1.25 NaH2PO4, 10 C6H12O6, and 2 CaCl2 at pH 7.4, 305 mOsm) maintained at 32°C for 30 min, followed by a 60-minute equilibration period at 22°C. Individual slices were subsequently moved to a submerged recording chamber perfused with oxygenated ACSF (2 mL/min flow rate) at ambient temperature (22°C). Whole-cell patch-clamp recordings were conducted on SST/PENK neurons under visual guidance using an Axioskop 2FS upright microscope with infrared differential interference contrast (IR-DIC) optics and a Hamamatsu C2400-07E camera system. Synaptic responses were induced through 3 ms light pulses of 473 nm (~5 mW power output) generated by a DPSS laser (Inper Co., Ltd., Hangzhou, China) targeting CCK or SST axon terminals in the HBX or the IPN. The patch pipette solution contained the following components at specified concentrations: 140 mM potassium gluconate, 10 mM HEPES buffer, 0.2 mM EGTA, 2 mM sodium chloride, 2 mM magnesium-ATP, and 0.3 mM sodium-GTP, with all reagents dissolved in the intracellular medium. For recording CCK→SST synaptic transmission, GABAA receptor-mediated IPSCs at a 0 mV holding potential under NBQX application. These currents were suppressed through 1 μM tetrodotoxin (TTX, Abcam, 146038), subsequently restored following 100 μM 4-aminopyridine (4-AP, TOCRIS, 0940) administration, and exhibited sensitivity to 20 μM bicuculline (BIC, TOCRIS, 0130). For recording SST→PENK synaptic transmission, the external ACSF solution contained GABAA-receptor antagonists including 20 μM bicuculline (TOCRIS, 0130). Recordings of AMPA-mediated EPSCs were conducted at a − 70 mV holding potential under pharmacological isolation with 20 μM BIC. These currents were suppressed by 1 μM TTX, restored following 100 μM 4-AP application, and demonstrated sensitivity to 20 μM CNQX (TOCRIS, 0190). Continuous signal acquisition was performed at a sampling rate of 10 kHz with 2 kHz low-pass filtering. Data analysis utilized ClampFit 10.2 (Molecular Devices) employing template-based event detection algorithms set at a 5 pA amplitude threshold for precise quantification of synaptic events.
In vivo optogenetics
To investigate the modulation of depression-related behaviors by optogenetic excitation or inhibition of target neurons (CCK, SST, PENK), we expressed ChR2 or NpHR3 in these neurons. Following virus injection, two-channel unjacketed optical fibers (200-μm diameter, INPER Co., Ltd) were implanted into the BNST, HBX, or IPN and secured to the skull via screws and dental cement. Experiments were conducted for experiments 15 days post-implantation.
Optrode recordings
To confirm the efficiency of ChR2 and NpHR3, we utilized optrodes to detect single-unit activity in SST-positive neurons of the HBX and CCK-positive neurons of the BNST. Specifically, rAAV2/9-VGAT-fDIO-NpHR3-GFP or rAAV2/9-VGAT-fDIO-ChR2-GFP was injected into the BNST of CCK-FLP mice, while rAAV2/9-hSyn-DIO-ChR2-GFP was injected into the HBX of SST-CRE mice. Three weeks post-viral injection, single-unit recordings were conducted using an optrode in the BNST of awake, freely moving mice.
For recording the activity of CCK-positive neurons in the BNST and SST-positive neurons in the HBX, rAAV2/9-VGAT-fDIO-NpHR3-GFP, and rAAV2/9-hSyn-DIO-ChR2-tdTomato were injected into the BNST or HBX of TERM mice, respectively. Three weeks post-viral injection, single-unit recordings were performed using an optrode in the BNST and HBX of awake, freely moving mice. The optrode was vertically advanced into the BNST and HBX using a stereotaxic instrument, ensuring the electrode tip was in the BNST/HBX while the optic fiber was positioned above, thus minimizing damage to the BNST/HBX.
To record the activity of SST-positive neurons in the HBX and PENK-positive neurons in the IPN, rAAV2/9-hSyn-DIO-ChR2-GFP, and rAAV2/9-hSyn-RSR-NpHR3-tdTomato were injected into the HBX or IPN of TERM mice, respectively.
Custom-fabricated optrodes were utilized in this study, consisting of a 200 µm diameter optical fiber attached to eight bipolar electrode wires. These wires were threaded through silicon tubes that were glued parallel to the fiber, serving as guides during the implantation process. These optrodes were mounted on a driver that facilitated vertical adjustment of the optrode assembly and were incrementally lowered in 30 µm steps for each recording session. The optrode was gradually descended to detect light-responsive neurons, with ~150 µm separating the two tips. At the conclusion of the experiment, the accuracy of the optrode positioning was evaluated through immunofluorescence imaging.
In the recordings, we identified neurons as CCK, SST, or PENK based on their response to light stimulation. In the BNST region, neurons that were inhibited by yellow light were classified as CCK-positive neurons. In the HBX region, neurons that showed a significant increase in firing rate when stimulated with blue light were classified as SST-positive neurons. In the IPN region, neurons that exhibited a significant decrease in firing rate when stimulated with yellow light were classified as PENK-positive neurons. Only these classified neurons were included in the subsequent analysis. To identify ChR2-tagged neurons, laser pulse trains (20 Hz) were delivered intermittently every minute. A unit was identified as ChR2-expressing whenever laser pulses triggered spikes with short first-spike latency (under 6 ms for all units in our sample). To identify NpHR3-tagged neurons, a protocol of 5 s of yellow light using a 589 nm laser was sufficient to decrease the spontaneous activity of NpHR3-expressing BNSTCCK or IPNPENK neurons.
After identifying the target neurons, the mice were permitted to rest for 30 min before undergoing behavioral recordings.
In vivo fiber photometry experiments
To image in vivo calcium transients of the PENK neurons, we unilaterally injected rAAV2/9-hSyn-RSR-GCaMP6m into the IPN region of TERM mice. Two weeks later, a fiber with a diameter of 200-μm and a length of 5 mm (NA:0.37, Inper Co., Ltd, China) was slowly implanted into the PENK region. Through the use of screws and dental cement, the fiber was affixed to the skull. The placement of optical fibers was verified in all mice by generating 30-μm-thick coronal sections at the implantation sites, and calcium transients were recorded via commercial fiber photometry systems (Inper Co., Ltd, China).
Chemogenetic manipulation
For chemogenetic experiments, we bilaterally injected rAAV2/9-hSyn-DIO-hM3Dq-GFP into the HBX region of SST-CRE mice. Two weeks post-injection, following the induction of the chronic mild stress (CMS) model, behavioral assessments were conducted. Mice received intraperitoneal (i.p.) injections of clozapine-N-oxide (CNO; 2 mg/kg, BrainVTA) dissolved in 0.9% saline or an equivalent volume of saline, 30 min prior to the commencement of the experiments.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (Sigma, P-010) and underwent intracardiac perfusion with 100 mL of saline (0.9% w/v NaCl) followed by 4% paraformaldehyde (PFA, dissolved in double-distilled water). After perfusion, the mice were decapitated, and their brains were fixed in 4% PFA for 24 h. The fixed brains were then dehydrated in a sucrose gradient (10%, 20%, and 30% sucrose, dissolved in 4% PFA, stored at 4 °C) with each sucrose gradient step lasting for 24 h. Coronal or sagittal brain sections were sliced via a cryostat (Leica Microsystems, CM1850). Free-floating brain sections underwent immunohistochemical analysis following previously established protocols60,62,63. Briefly, 30 μm free-floating coronal sections were stained and blocked in 3% normal bovine serum for 1 h at room temperature. Subsequently, the sections were incubated in 50 mM Tris-HCl buffer containing 3% bovine serum and 0.3% Triton X-100 with the primary antibody rabbit anti-c-Fos (1:400, SYSY, 226008), rabbit anti-TH (1:500, Abcam, ab112), mouse anti-NeuN (1:500, Millipore, MAB377), rabbit anti-SST (1:500, ABclonal, A9274) and rabbit anti- PKCδ (1:500, Abcam, ab182126) for 24 h at 4°C. Following a 1-hour incubation at room temperature with adsorbent-bound Alexa Fluor secondary antibodies (Invitrogen, diluted 1:250 in 3% bovine serum albumin, BSA), the sections were rinsed. They were then briefly co-stained with DAPI (4’,6-diamidino-2-phenylindole) for five minutes, rinsed again, dried, and coverslips were applied with fluorescence mounting medium.
Imaging of single or dual labeling was conducted with a confocal laser scanning microscope. Confocal image series were acquired at 0.5 μm intervals within the target area, generating optical stacks consisting of 6∼12 images per series. Absolute counts of single, dual, or triple cells were quantified by preparing sections (image stack) using established methods60,62,63. Slides were coded prior to quantitative analysis by an experimenter blinded to the study conditions. Quantification was performed by another experimenter who was also blinded to experimental conditions and treatments60,62,63.
RNAscope fluorescent in situ hybridization (FISH)
Mice were anesthetized using pentobarbital sodium (Sigma, P-010) and subsequently decapitated. The brains were rapidly dissected, and frozen in Optimal Cutting Temperature (OCT) compound (Sakura Tissue-Tek, 4583) using a dry ice bath. Serial sections of the brain samples were cut to a thickness of 16μm using a cryostat (Leica, CM1850), affixed to SuperFrost Plus microscope slides (Epredia,4951PLUS-001E), and stored at −80 °C until subsequent processing, after which they were sectioned, immediately fixed in 4% paraformaldehyde for 30 min at 4 °C and stained using the Advanced Cell Diagnostics RNAscope Multiplex Fluorescent V2 Assay protocol (ACDBio, 323100) protocol. Subsequently, sections were then stained with antisense probes for SST (ACDBio, 404631-C1), vGluT2 (ACDBio, 319171-C2), CCK (ACDBio, 402271-C1), PENK (ACDBio, 318761-C1), TH (ACDBio, 317621-C4), GFP (ACDBio, 409071-C2), EGFP (ACDBio, 400281-C3), tdTomato (ACDBio, 317041-C2, 317041-C3), and VGAT (ACDBio, 319191-C3). Finally, the coverslip was applied to the sections via Vectashield hard-set antifade mounting medium, which included DAPI (ThermoFisher, P36935). Images were captured through a laser confocal microscope (Zeiss LSM 800, Zeiss), and all brain sections were imaged under consistent camera settings.
Statistical analyses
Data were collected using various tools for specific applications: Inper was utilized for fiber photometry recording and optogenetic manipulation; Ethovision XT 14.0 (Noldus) was employed for behavioral tests; the MultiClamp 700B amplifier (Molecular Devices) was used for in vitro electrophysiological recordings; ZEN v2.3 (ZEISS) facilitated confocal imaging; and Plexon was utilized for in vivo electrophysiological recordings.
ImageJ (1.52a) (Fiji) was used for c-Fos counting. ClampFit 10.2 (Molecular Devices) was employed to analyze data, while Ethovision XT 14.0 (Noldus) assisted in video analysis. Inper Plot V1.7.9 was utilized for Ca2+ imaging data analysis, and Plexon was used for spike sorting.
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., CA, USA) and SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± SD/SEM. Power analysis was not conducted prior to the experiment to determine the sample size. Instead, sample sizes were based on those reported in previously published studies. All samples were included in the analysis, except those where virus expression or the positioning of the fiber/optrode deviated from the target. The normality of the data was assessed using the Shapiro-Wilk test to determine whether parametric or non-parametric statistical methods were appropriate. Electrophysiological data were analyzed using paired Student’s t-test (Fig. 4j, o) or one-way repeated measures ANOVA (Fig. 4k) with Bonferroni post hoc comparisons. Behavioral data from optogenetic experiments were analyzed using two-way repeated measures ANOVA with Bonferroni post hoc comparisons, while behavioral data from chemogenetic experiments were analyzed using two-way ANOVA with Bonferroni post hoc comparisons. Statistical significance was considered at * p < 0.01, ** p < 0.001 and *** p < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data for figures and Supplementary Figs. of this paper are available in the source data file. Source data are provided with this paper.
References
Malgaroli, M., Calderon, A. & Bonanno, G. A. Networks of major depressive disorder: a systematic review. Clin. Psychol. Rev. 85, 102000 (2021).
Miranda, R., Valderrama, J., Tsypes, A., Gadol, E. & Gallagher, M. Cognitive inflexibility and suicidal ideation: mediating role of brooding and hopelessness. Psychiatry Res. 210, 174–181 (2013).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Gonda, X. et al. Genetic variants in major depressive disorder: from pathophysiology to therapy. Pharmacol. Therapeutics 194, 22–43 (2019).
Scangos, K. W., Makhoul, G. S., Sugrue, L. P., Chang, E. F. & Krystal, A. D. State-dependent responses to intracranial brain stimulation in a patient with depression. Nat. Med. 27, 229–231 (2021).
Smith-Apeldoorn, S. Y., Veraart, J. K., Spijker, J., Kamphuis, J. & Schoevers, R. A. Maintenance ketamine treatment for depression: a systematic review of efficacy, safety, and tolerability. lancet Psychiatry 9, 907–921 (2022).
Ramirez, S. et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA psychiatry 73, 456–464 (2016).
Marwaha, S. et al. Novel and emerging treatments for major depression. Lancet (Lond., Engl.) 401, 141–153 (2023).
Mayberg, H. S. et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005).
Ables, J. L. & Park, K. Ibañez-Tallon I. Understanding the habenula: a major node in circuits regulating emotion and motivation. Pharmacol. Res. 190, 106734 (2023).
Hu, H., Cui, Y. & Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 21, 277–295 (2020).
Matsumoto, M. & Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115 (2007).
Proulx, C. D., Hikosaka, O. & Malinow, R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 17, 1146–1152 (2014).
Yang, Y. et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018).
Cerniauskas, I. et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 104, 899–915 (2019).
Dong, Y. et al. Stress relief as a natural resilience mechanism against depression-like behaviors. Neuron 111, 3789–3801.e3786 (2023).
Lee, E. H., Park, J. Y., Kwon, H. J. & Han, P. L. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat. Commun. 12, 6682 (2021).
Singh, I. et al. Structure-based discovery of conformationally selective inhibitors of the serotonin transporter. Cell 186, 2160–2175.e2117 (2023).
Lim, B. K., Huang, K. W., Grueter, B. A., Rothwell, P. E. & Malenka, R. C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Nestler, E. J. & Hyman, S. E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Sacai, H. et al. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat. Commun. 11, 5140 (2020).
Florsheim, E. B. et al. Immune sensing of food allergens promotes avoidance behaviour. Nature 620, 643–650 (2023).
Liu, M. Y. et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 13, 1686–1698 (2018).
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Sci. (N. Y., NY) 301, 386–389 (2003).
Liu, R. T. Stress generation: future directions and clinical implications. Clin. Psychol. Rev. 33, 406–416 (2013).
Berrios, J. et al. Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nat. Commun. 7, 10702 (2016).
Hoopfer E. D., Jung Y., Inagaki H. K., Rubin G. M., Anderson D. J.P1 Interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015).
Huang, J. et al. Mesenchymal stromal cells alleviate depressive and anxiety-like behaviors via a lung vagal-to-brain axis in male mice. Nat. Commun. 14, 7406 (2023).
Yan, L. et al. Stress increases hepatic release of lipocalin 2 which contributes to anxiety-like behavior in mice. Nat. Commun. 15, 3034 (2024).
Giardino, W. J. et al. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21, 1084–1095 (2018).
Jennings, J. H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013).
Rodriguez-Romaguera, J. et al. Prepronociceptin-expressing neurons in the extended amygdala encode and promote rapid arousal responses to motivationally salient stimuli. Cell Rep. 33, 108362 (2020).
Welch, J. D. et al. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell 177, 1873–1887 (2019).
He, X. J. & Banghart, M. R. It’s lights out for presynaptic terminals. Neuron 109, 1755–1757 (2021).
Labouesse, M. A. et al. A non-canonical striatopallidal go pathway that supports motor control. Nat. Commun. 14, 6712 (2023).
Mahn, M. et al. Efficient optogenetic silencing of neurotransmitter release with a mosquito rhodopsin. Neuron 109, 1621–1635 (2021).
Paquelet, G. E. et al. Single-cell activity and network properties of dorsal raphe nucleus serotonin neurons during emotionally salient behaviors. Neuron 110, 2664–2679 (2022).
Marcinkiewcz, C. A. et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101 (2016).
Qi, G. et al. NAc-VTA circuit underlies emotional stress-induced anxiety-like behavior in the three-chamber vicarious social defeat stress mouse model. Nat. Commun. 13, 577 (2022).
Zhou, W. et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat. Neurosci. 22, 1649–1658 (2019).
Fellinger, L. et al. A midbrain dynorphin circuit promotes threat generalization. Curr. Biol.: CB 31, 4388–4396 (2021).
Ma, S. et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature 622, 802–809 (2023).
Zheng, Z. et al. Hypothalamus-habenula potentiation encodes chronic stress experience and drives depression onset. Neuron 110, 1400–1415 (2022).
Cui, Y. et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 (2018).
Fan, Z. et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell 186, 560–576 (2023).
Rossi, M. A. et al. Transcriptional and functional divergence in lateral hypothalamic glutamate neurons projecting to the lateral habenula and ventral tegmental area. Neuron 109, 3823–3837.e3826 (2021).
Takahashi, A. et al. Lateral habenula glutamatergic neurons projecting to the dorsal raphe nucleus promote aggressive arousal in mice. Nat. Commun. 13, 4039 (2022).
Huang, L. et al. A visual circuit related to habenula underlies the antidepressive effects of light therapy. Neuron 102, 128–142 (2019).
Liu, X. et al. Burst firing in output-defined parallel habenula circuit underlies the antidepressant effects of bright light treatment. Adv. Sci. (Weinh., Baden. -Wurtt., Ger.) 11, e2401059 (2024).
Abraham, M. E. et al. Investigating deep brain stimulation of the habenula: a review of clinical studies. Neuromodulation: J. Int. Neuromodulation Soc. 26, 292–301 (2023).
Germann, J. et al. Deep brain stimulation of the habenula: systematic review of the literature and clinical trial registries. Front. psychiatry 12, 730931 (2021).
Zhang, C. et al. Bilateral Habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features. Transl. psychiatry 12, 52 (2022).
Sartorius, A. et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. psychiatry 67, e9–e11 (2010).
Alagapan, S. et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature 622, 130–138 (2023).
Sheth, S. A. & Mayberg, H. S. Deep brain stimulation for obsessive-compulsive disorder and depression. Annu. Rev. Neurosci. 46, 341–358 (2023).
Dean, R. L. et al. Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. Cochrane database Syst. Rev. 9, Cd011612 (2021).
McIntyre, R. S. et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am. J. psychiatry 178, 383–399 (2021).
Klenowski, P. M. et al. A neuronal coping mechanism linking stress-induced anxiety to motivation for reward. Sci. Adv. 9, eadh9620 (2023).
Jing, W. et al. A circuit of COCH neurons encodes social-stress-induced anxiety via MTF1 activation of Cacna1h. Cell Rep. 37, 110177 (2021).
Li, X. et al. Synaptic dysfunction of Aldh1a1 neurons in the ventral tegmental area causes impulsive behaviors. Mol. neurodegeneration 16, 73 (2021).
Li, X. et al. Molecularly defined and functionally distinct cholinergic subnetworks. Neuron 110, 3774–3788 (2022).
Li, X. et al. A circuit of mossy cells controls the efficacy of memory retrieval by Gria2I inhibition of Gria2. Cell Rep. 34, 108741 (2021).
de Araujo, I. E. et al. Food reward in the absence of taste receptor signaling. Neuron 57, 930–941 (2008).
Coimbra, B. et al. Role of laterodorsal tegmentum projections to nucleus accumbens in reward-related behaviors. Nat. Commun. 10, 4138 (2019).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Liu, B. et al. Molecular and neural basis of pleasant touch sensation. Sci. (N. Y., NY) 376, 483–491 (2022).
He, A. et al. VGLUT3 neurons in median raphe control the efficacy of spatial memory retrieval via ETV4 regulation of VGLUT3 transcription. Sci. China Life Sci. 65, 1590–1607 (2022).
Xiu, J. et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat. Neurosci. 17, 1552–1559 (2014).
Wang, J. et al. The basal forebrain to lateral habenula circuitry mediates social behavioral maladaptation. Nat. Commun. 15, 4013 (2024).
Acknowledgements
This study is supported by the National Key Research and Development Program of China (Grant No. 2019YFE0121200), and the National Natural Science Foundation of China (Grants: 31721002 and 81920208014 to YL).
Author information
Authors and Affiliations
Contributions
Y.L., A.H., and D.L. initiated, designed, and supervised the study; L.L. and W.J. performed behavioral studies and immunohistochemistry studies, and virus and mutant mouse generation. Y.G. performed electrophysiological studies and western blot studies. All authors analyzed the data. L.L., Y.L., and W.J. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jessica Ables, Steven Shabel and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, L., Jing, W., Guo, Y. et al. A cell-type-specific circuit of somatostatin neurons in the habenula encodes antidepressant action in male mice. Nat Commun 16, 3417 (2025). https://doi.org/10.1038/s41467-025-58591-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58591-y