Abstract
In diverse Phytophthora-plant pathosystems, Phytophthora secretes XLP1 (PsXEG1-Like Protein), a non-enzymatic paralog that functions as a decoy to protect XEG1 (Xyloglucan-specific Endoglucanase) from host inhibitors. Here, we show that the genus-specific selection pressures on the XEG1/XLP1 gene pair are crucial for host adaptation and are closely linked to Phytophthora host range. Our findings reveal that the XEG1/XLP1 gene pair originated within Phytophthora and subsequently evolved into genus-specific genes, undergoing functional divergence driven by preferential selection. Positive selection sites within the XEG1/XLP1 gene pair in Phytophthora contribute to this functional divergence and are associated with the host range variability of Phytophthora as evidenced by multivariate statistical analyses. Furthermore, mutations at key selection sites in Phytophthora sojae and Phytophthora capsici significantly impair their pathogenicity, with P. capsici exhibiting almost no colonization expansion on tobacco and pea. Notably, natural Phytophthora populations harbor mutations at the positive selection sites, indicating ongoing evolutionary pressures on the XEG1/XLP1 gene pair.
Similar content being viewed by others
Introduction
Phytophthora, which diverged about 70 million years ago, belongs to oomycetes, a group that looks similar to filamentous fungi but is classified as Stramenopiles, which includes diatoms and brown algae1,2,3,4. The most notorious species is Phytophthora infestans, which caused the potato famine in the 1840s5. The Phytophthora genus consists of 180 known species that infect a wide variety of plants in both managed and natural ecosystems, including crops, trees, and ornamentals6,7. The host ranges of Phytophthora species vary from 1 to 327 host species8.
Apoplastic and cytoplasmic effectors have evolved to facilitate parasitism by suppressing plant immunity and altering plant physiology to promote the growth and spread of parasites9,10. The apoplastic effectors in Phytophthora are intricately linked to their host range, and there is a notable correlation between apoplastic effector abundance and the breadth of host species8. Apoplastic effectors in Phytophthora encompass various proteins, such as glycoside hydrolases, protease inhibitors, necrosis-inducing proteins (NLPs), proteases, lipases, and lipid-binding proteins. Among them, glycoside hydrolases are predominant within the suite of apoplastic effectors8.
Glycoside hydrolase 12 (GH12) family proteins, such as XEG1, are present in Phytophthora species and are thought to have originated through horizontal gene transfer from fungi11. P. sojae XEG1 (PsXEG1) exhibits xyloglucan-hydrolyzing activity and plays a crucial role in virulence by degrading plant cell wall components12. However, soybean counters this attack by producing an apoplastic glucanase inhibitor protein, GmGIP1, which binds to PsXEG1 and blocks its contribution to virulence. To evade this defense, P. sojae secretes a paralogous protein, PsXLP1, which lacks enzymatic activity but binds GmGIP1 with higher affinity than PsXEG1. This competitive binding effectively liberates PsXEG1, allowing it to facilitate infection13. In addition to GmGIP1, soybean employs a second layer of defense by secreting an aspartic protease, GmAP5, into the apoplast. GmAP5 binds to and degrades PsXEG1, thereby diminishing its virulence14. Remarkably, N-glycosylation of PsXEG1 provides two advantages: it shields the protein from degradation by GmAP5 and reduces its inhibition by GmGIP114, enhancing PsXEG1’s effectiveness in supporting P. sojae infection. Moreover, PsXEG1 functions as a pathogen-associated molecular pattern (PAMP) that is recognized by the host, triggering PAMP-triggered immunity (PTI) and inducing cell death in soybean12. However, during natural infections, PTI responses elicited by PsXEG1 are actively suppressed by pathogen-derived effectors, such as RXLR effectors15. The pivotal apoplastic effector XEG1 and its paralog XLP1 constitute a focal point of coevolutionary struggle between P. sojae and soybean. Additionally, the gene pair encoding PsXEG1 and PsXLP1 is conserved in many Phytophthora species, highlighting the crucial role of the XEG1/XLP1 gene pair in the biology and adaptation of Phytophthora species.
Here, evolutionary analyses reveal that the XEG1/XLP1 gene pair originated within the Phytophthora genus and evolved into a genus-specific gene pair. The functions of XEG1/XLP1 have diverged significantly among Phytophthora species, particularly in inducing cell death, triggering defense gene expression, and producing reducing sugars in Nicotiana benthamiana. Ancestral genes reconstruction and replacement experiments in P. sojae provide strong evidence of functional divergence, highlighting evolutionary shifts from ancestral to modern sequences. Fourteen positive selection sites were identified in XEG1/XLP1, with no correlation to general genotypic relationships in P. capsici. Bidirectional mutational experiments between ancestral and modern sequences confirmed the role of positive selection pressure in driving functional divergence. Multivariate analyses further revealed a strong correlation between XEG1/XLP1 divergence and host range diversity, with three key positive selection sites identified. In P. capsici, mutations at a key positive selection site were validated through inoculation experiments on fourteen plant species, revealing that the positive selection site impacts infection capabilities on five plant species. Notably, P. capsici exhibited almost no colonization expansion on tobacco and pea. Selection pressure sites were also detected in natural Phytophthora populations, emphasizing their evolutionary significance. In P. sojae, mutations reverting XEG1/XLP1 to ancestral forms at positive selection sites indicated that they enhance host adaptability. Additionally, two natural mutations at positive selection pressure site 62 were validated as critical for P. sojae host adaptation. Together, these findings highlight that genus-specific selection pressures on XEG1/XLP1 are essential for host adaptation and closely linked to host range diversity in Phytophthora.
Results
Origin and functional divergence of the XEG1/XLP1 gene pair in Phytophthora
To study the distribution of GH12 family proteins, we analyzed 66 oomycete species spanning multiple genera, including Albugo, Aphanomyces, Bremia, Hyaloperonospora, Nothophytophthora, Peronospora, Peronosclerospora, Phytophthora, Phytopythium, Pilasporangium, Plasmopara, Pythium, and Saprolegnia. Most of these species are recognized as plant pathogens, while Saprolegnia, Aphanomyces, and some species of Pythium, such as Pythium insidiosum, are known to infect animals or humans (Supplementary Data 1 and 2). The majority of GH12 protein sequences were only identified in plant pathogens, including Phytophthora, Bremia, Peronospora, Peronosclerospora, and Hyaloperonospora, with Phytophthora exhibiting the highest abundance (Fig. 1a). To investigate the evolution of these GH12 family proteins, we constructed a phylogenetic tree utilizing all the GH12 family proteins found in oomycetes and those previously identified in fungi and bacteria12. The result revealed that GH12 proteins from fungi and bacteria form distinct branches, separate from GH12 proteins of oomycetes. Within oomycetes, the GH12 proteins grouped into four distinct clades (Supplementary Fig. 1), exhibiting both quantitative expansion and evolutionary diversity. Notably, the orthologs of PsXEG1 and PsXLP1 formed independent subclades and exhibited later evolutionary divergence compared with other GH12 proteins of oomycetes (Fig. 1b and Supplementary Fig. 1). Both subclades contain 41 Phytophthora species, and only P. pinifolia and P. hibernalis are missing due to low-quality sequencing assemblies.
a Distribution and quantity of GH12 family proteins in oomycetes infecting plants and animals. Distinct genera are represented by distinct colors. The outer circle indicates the quantity of the presence and absence of signal peptides in GH12 family proteins across various oomycetes, while the inner circle represents the host range diversity of Phytophthora pathogens, with four colors denoting distinct host ranges. The number of homologous proteins without signal peptides of Peronospora effusa is indicated by numbers. Icons depict representative host plants or animals infected by these pathogens. b Phylogenetic tree of GH12 family proteins across oomycetes, fungi, and bacteria. The phylogeny was constructed using sequences from selected species within these groups. Subclades containing PsXEG1 and PsXLP1 are highlighted, showing that these proteins form a well-supported clade within the Phytophthora genus. Detailed protein information and phylogenetic tree data are available in Supplementary Data 1, 2 and Source Data. c Evolutionary analysis of the XEG1/XLP1 subclades. The purifying selection rates (ω) for each clade were estimated based on the nonsynonymous to synonymous ratio (ω < 1), indicating purifying selection. d Phenotypic characterization of Phytophthora XEG1 and XLP1 in Nicotiana benthamiana. Multiple phenotype assays were conducted to evaluate the functional effects of XEG1 and XLP1 in different Phytophthora species in Nicotiana benthamiana. Electrolyte leakage, indicative of cell death, was quantified 48 h post-infiltration. Each point represents the average of nine biological replicates (n = 9) from three experiments for one gene. Reducing sugar production levels in the apoplastic fluid were measured for Phytophthora XEG1 and XLP1 about 20 h in N. benthamiana. Each point represents the average of nine biological replicates (n = 9) from three experiments for one gene. Relative expression levels of total defense marker genes were assessed 24 h after infiltration using qRT-PCR. Each point represents the average of seven marker genes from three biological replicates (n = 3) of three experiments for one gene. In the box plots, dots represent min-to-max value of individual data points, the line indicates the median, and the box boundaries indicate the 25th and 75th percentiles. Statistical significance was determined using a two-sided t-test. Source data are provided as a Source Data file.
To further understand the evolutionary dynamics of these subclades, we conducted purifying selection analysis using two datasets. The first focused on the GH12 proteins from Phytophthora (Fig. 1c), and the second incorporated GH12 proteins from bacteria, fungi, and oomycetes (Supplementary Fig. 2a). Both PsXEG1 and PsXLP1 subclades showed evidence of purifying selection, as indicated by nonsynonymous-to-synonymous substitution rate ratios (ω) below 1 in both datasets (Fig. 1c and Supplementary Fig. 2a). In addition, both subclades also exhibited a distinct conservative pattern (Supplementary Fig. 2b).
To determine whether the genes within the PsXEG1 and PsXLP1 subclades are XEG1 and XLP1 genes in the corresponding Phytophthora species, we performed a detailed scan of the Phytophthora genomes to identify adjacent upstream and downstream genes, followed by collinearity analysis (Supplementary Data 3). The analysis confirmed that the genes within the PsXEG1 and PsXLP1 subclades are indeed orthologous to XEG1 or XLP1 in the respective Phytophthora species, suggesting that the XEG1/XLP1 gene pair originated within Phytophthora. Given that PsXEG1 and PsXLP1 in P. sojae are arranged in a head-to-head configuration within the genome, we focused on orthologs exhibiting this arrangement for further analysis, encompassing 26 Phytophthora species (Supplementary Fig. 2c, d).
To investigate the functional differences of XEG1 and XLP1 across various Phytophthora species, we evaluated their abilities to induce cell death, trigger defense gene expression, and produce reducing sugars in N. benthamiana using Agrobacterium-mediated transient expression. For the assessment of cell death induction, we measured electrolyte leakage as an indicator of cell death intensity induced by XEG1 and XLP1 orthologs. P. palmivora was excluded due to unsuccessful gene synthesis. Except for P. idaei XLP1, all tested PsXEG1 and PsXLP1 orthologs elicited varying degrees of cell death compared to the empty vector control with GFP (Supplementary Fig. 3a). To evaluate their ability to trigger defense responses, we examined the expression of defense genes related to PTI, salicylic acid (SA), ethylene (ET), and jasmonic acid (JA) at 12-, 24-, and 36-h post-infiltration. Most defense genes were induced by XEG1 or XLP1 at 24 h, with Acre31, CYP71D20, PAL10, AOS, and Pti5 showing stronger induction than WRKY8 (Supplementary Fig. 4). For the capacity to generate reducing sugars, we measured the apoplastic reducing sugar levels in N. benthamiana expressing the corresponding XEG1 or XLP1 (Supplementary Fig. 5a). In 18 species, XEG1 orthologs exhibited higher reducing sugar production compared to their respective XLP1. In contrast, XLP1 orthologs exhibited higher activity in four species (Phytophthora europaea, Phytophthora chlamydospore, Phytophthora parvispora, and Phytophthora pseudosyringae) and similar activity to XEG1 in two species (Phytophthora aleatoria and Phytophthora idaei) (Supplementary Fig. 5b, c). These proteins were effectively expressed at the expected size in N. benthamiana, as confirmed using western blot analysis (Supplementary Fig. 3b). A comprehensive analysis of both XEG1 and XLP1 revealed that XEG1 led to higher levels of defense response genes and reducing sugars compared to XLP1, although there were no significant differences in cell death (Fig. 1d). Furthermore, variations in the abilities of XEG1 and XLP1 from different Phytophthora species to induce cell death, trigger defense genes, and produce reducing sugars highlight the functional diversification of these genes (Supplementary Fig. 6).
Functional divergence in the ancestral nodes of XEG1 and XLP1
To elucidate the functional divergence of XEG1 and XLP1 during Phytophthora evolution, we reconstruct the ancestral genes of XEG1 (XEGa) and XLP1 (XLPa), as well as their common ancestor gene (XELa) using PamlX16 (Supplementary Fig. 7). We initially assessed the ability of XEGa, XLPa, and XELa to cause cell death and analyzed structural differences. Even though the cell death-inducing capacity of XEGa and XLPa was only approximately two-fifths of that of PsXEG1, both XELa, XEGa and XLPa effectively induced cell death and were expressed correctly in N. benthamiana (Supplementary Fig. 8b–e). Structural comparison analyses showed that common ancestor XELa shares similar overall conformations with the corresponding XEG1 and XLP1 from P. sojae and Phytophthora parasitica, respectively, with only minor differences in specific regions (Supplementary Fig. 8a, f).
To validate the functional divergence between XEG1/XLP1 and XELa during Phytophthora evolution, we used Cas9-mediated homologous gene replacement in P. sojae (P6497) to generate homozygous lines wherein PsXEG1 or PsXLP1 were replaced with XELa (T9XELa-PsXEG1, T41XELa-PsXEG1, T20XELa-PsXLP1, and T38XELa-PsXLP1) (Supplementary Fig. 9a, b). All transformants showed normal mycelium growth compared to the control (Supplementary Fig. 9c, d). Replacing PsXEG1 or PsXLP1 with XELa reduced the virulence of P. sojae on soybeans (Fig. 2a–c). But compared to the XEG1 or XLP1 knockout mutants, the reduced impact on the pathogenicity of replacement transformants resulted in relatively minor changes, which may be attributed to the retained hydrolytic activity of the ancestral genes (Supplementary Fig. 9e). These results confirmed the functional divergence of XELa after its initial generation.
a Virulence of P. sojae transformants on soybean hypocotyls. Representative symptoms at 48 h post-inoculation (hpi). The transformants include XELa replacing PsXEG1 (T9, T41, and control, CK_G) and XELa replacing PsXLP1 (T20, T38, and control, CK_P). Experiments were repeated three independent times with similar results. b, c Impact of PsXEG1 or PsXLP1 replacement on virulence in etiolated soybean hypocotyls. b Lesion lengths on hypocotyls produced by P. sojae (n = 15). c Relative biomass of the wild-type and transgenic P. sojae mutants infecting etiolated soybean hypocotyls, measured by gDNA qPCR (n = 9). b, c In the box plots, dots represent min-to-max value of individual data points, the line indicates the median, and the box boundaries indicate the 25th and 75th percentiles. Three independent experiments obtained similar results. Pairwise significance was determined using Duncan’s multiple range test (p < 0.01), with different letters representing significant differences. d Comparison of different ancestral nodes of Phytophthora XEG1 and XLP1 regarding their ability to induce cell death, produce reducing sugar, and their relative expression of defense genes. The age of the common ancestor proteins in millions of years is indicated at the corresponding node. The background shows a maximum likelihood evolutionary tree used to reconstruct ancestral genes, with the outgroup and partial terminal genes combined and nodes labeled accordingly. The pie chart illustrates the variations in activity efficiency, with green indicating an increase and blue indicating a decrease in the evolutionary pathways. At each ancestral node, the pie represents the average fold change in phenotypic measures normalized to XEGa or XLPa, respectively: reducing sugar (A), electrolyte leakage (B), and the relative expression of defense genes (C), including Pti5, Acre31, CYP71D20, and PAL10. The bottom pie chart corresponds to electrolyte leakage (n = 9 biological replicates), the right pie to the relative expression of defense genes (n = 3 biological replicates), and the left pie to reducing sugar production levels (n = 9 biological replicates). Source data are provided as a Source Data file.
To delineate the divergence of XEG1 and XLP1 in Phytophthora, we reconstructed the ancestral genes of XEG1 and XLP1 at each evolutionary node of Phytophthora using PamlX16. These ancestral genes were subsequently synthesized and efficiently expressed in N. benthamiana, as confirmed using western blot (Supplementary Fig. 10a, d). We then examined the capacity of them to trigger cell death, induce defense gene expression, and produce reducing sugars via Agrobacterium-mediated transient expression. Consistent with the XLP1 decoy model, the XLP1 subclade showed a considerable decline in the capacity of ancestral nodes to induce defense marker genes Pti5, Acre31, CYP71D20, or PAL10 expression and production of reducing sugar. While the XEG1 subclade showed upregulation in immunity, the process of producing reducing sugar did not show a clear pattern (Fig. 2d).
To investigate these differing patterns, we compared the protein sequences and analyzed17 the correlation between amino acid site variation and their associated phenotypes. Each clade exhibited a preference for mutations at specific amino acid sites. The XEG1 subclade showed mutations at sites 20, 31, 32, and 58, while the XLP1 subclade displayed mutations at sites 27, 28, 33, 88, 91, and 120 (Supplementary Fig. 10a). A correlation heatmap of these variant sites and phenotypic changes revealed that certain sites were closely related to specific phenotypes. Specifically, sites 31, 91, and 120 were linked to enhanced phenotype traits in the XEG1 subclade, while sites 20, 27, 28, 33, and 58 were associated with reduced phenotype traits in the XLP1 subclade (Supplementary Fig. 10b). Furthermore, synergistic effects between different amino acid sites were observed (Supplementary Fig. 10c). These findings suggest that the critical role of amino acid site variations in driving the functional differentiation of Phytophthora XEG1 and XLP1.
Roles of selection pressure sites of XEG1/XLP1 in Phytophthora host ranges
To evaluate the selection pressure sites in XEG1 and XLP1 following the formation of Phytophthora, we employed the MEME (Mixed Effects Model of Evolution)18,19 to analyze the nonsynonymous and synonymous substitution rate ratio (ω) and conducted significance testing for the ω values across all analyzed XLP1 and XEG1. The results identified 14 positive selection sites in the XEG1/XLP1 gene pair, visualized through an amino acid logo (Supplementary Fig. 11a). Considering previous studies demonstrating that XEG1 is subjected to three layers of host defense, including inhibitors (GmGIP1), proteases (GmAP5), and receptors (RXEG1)12,13,14,20, we analyzed the overlap between the binding sites of these host defense molecules and the positive selection sites within the XEG1/XLP1 gene pair. Apart from amino acid residue W at position 28, nearly all positive selection sites do not overlap with the binding sites of GmGIP1, GmAP5, or RXEG1. This suggests that the divergence in positive selection sites may be driven by other evolutionary factors (Supplementary Fig. 11a).
To determine whether positive selection sites simply reflect the general genotypic relationship between species, we conducted a hypothesis-driven analysis. We hypothesized that if positive selection sites were indicative of general genotypic relationships, species-specific characteristics would manifest as shared differentiating sites in the XEG1/XLP1 sequences of each Phytophthora species when compared to other Phytophthora XEG1/XLP1 sequences. To test this hypothesis, we selected P. capsici and performed pairwise comparisons of their XEG1/XLP1 sequences with those from other Phytophthora species (Supplementary Fig. 12a). The results revealed that, compared with the XEG1/XLP1 sequences of 13 other Phytophthora species, the shared differentiating sites among different P. capsici isolates showed no overlap with the positive selection sites in XEG1/XLP1 (Supplementary Fig. 12b). These findings suggest that the positive selection sites in XEG1/XLP1 are not indicative of general genotypic relationships between species but instead reflect species-specific evolutionary pressures.
To investigate the impact of these sites on the functional differentiation of XEG1/XLP1, we constructed the bidirectional mutants of the ancestral gene (XELa) and PsXEG1/PsXLP1 (Supplementary Fig. 11b). We determined their capacities to trigger cell death and produce reducing sugars in N. benthamiana using Agrobacterium-mediated transient expression. During the bidirectional mutation process of PsXEG1 or PsXLP1 and XELa, most of the sites were implicated in the reduction of sugar production capacity, although the PsXLP1 mutant did not exhibit any typical phenotypic changes (Fig. 3a). Following site identification and considering the differences between Phytophthora XEG1/XLP1 and XELa sequences, six sites were selected for further mutation analysis in other Phytophthora species (Supplementary Fig. 11b). These six sites significantly altered their ability to produce reducing sugar (Fig. 3b). Additionally, electrolyte leakage data showed that mutations at positive selection pressure sites variably affected their abilities to induce cell death (Fig. 3a, b). These proteins were efficiently expressed in N. benthamiana (Supplementary Fig. 13). These results indicate that positive selection pressure sites are involved in the functional capacities of Phytophthora XEG1/XLP1.
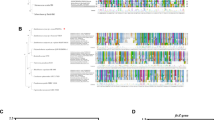
a, b Mutational analysis of putative functional sites in XELa and Phytophthora XEG1/XLP1. Single amino acid point mutation names are indicated on the left (a) and at the top (b). Details of the mutants are in Supplementary Fig. 13. “-” means no test. “EL” means electrolyte leakage. “PRS” means production of reducing sugar. Each measurement of a mutant protein was based on the mean value, with data normalized to the wild-type proteins (n = 9, biological replicates). Experiments were repeated three independent times with similar results. Statistical significance was determined by a two-sided t-test (*p (T ≤ t) <0.05, **p (T ≤ t) <0.001). The green (a) and red (b) colors represent enhanced phenotypes of the mutants, blue indicates weakened phenotypes, and gray represents little to no change. Cartoons were free and downloaded from the website (https://www.flaticon.com or https://icons8.com/icons). c Summary of the occurrence of variable importance projection (VIP) sites at each amino acid position within the five components related to Supplementary Fig. 14. Red stars represent sites under positive selection pressure. d Disease symptoms of wild-type and P. capcisi transformants. “M45” refers to the substitution of the positive selection site at position 45 in P. capsici XEG1 and XLP1 (PcaXEG1 and PcaXLP1, respectively) with the ancestral amino acid. Infection of P. capsici transformants and wild-type on the leaves of N. benthamiana, pepper, Nicotiana tabacum (tobacco), pea, potato, and snow pea plants inoculated with mycelial plugs, respectively. The leaves were photographed under UV light. Each experiment was repeated three times with similar results. e Positive selection site 45 influences the expansion capabilities of P. capsici across different host plants. The dotted line indicates the diameter of the hyphae plugs. In the box plots, dots represent min-to-max value of individual data points (n = 9 and n = 12 for N. benthamiana), the line indicates the median, and the box boundaries indicate the 25th and 75th percentiles. Red dots represent almost no infection of P. capcisi. All mutants were compared with the wild-type strains, and statistical significance was determined using a two-sided t-test (*p (T ≤ t) <0.05, **p (T ≤ t) <0.001). Each experiment was repeated three times. Source data are provided as a Source Data file.
Considering the findings by Kronmiller et al., which suggest that hydrolases may influence the host range dynamics of Phytophthora, we further analyzed the relationship between sequence variations in Phytophthora XEG1 and XLP1 and host range diversity. Following the classification system established by Kronmiller et al., we categorized Phytophthora species into four host range groups: narrow (confined to one plant host genus), multiple (within two to nine host genera), wide (spanning 16 to 55 host genera), and huge (spanning 107 to 327 host genera)8. Multivariate statistical analysis revealed that 31 amino acid sites, identified as variable importance projection (VIP) sites, were associated with host range variation among Phytophthora species (Supplementary Fig. 14). Based on the frequency of these sites across five components, five amino acid sites were identified as being closely related to changes in host range. Among these, three sites (positions 45, 88, and 103) were found to be under positive selection pressure (Fig. 3c), indicating that the positive selection acting on XEG1/XLP1 in Phytophthora is closely correlated with their host ranges diversity (Fig. 3c and Supplementary Fig. 14).
To determine the relationship between association sites and the host range of Phytophthora species, we selected Phytophthora capsici, a species with a wide host range (infecting over 16 plant genera), for further investigation. Among the three positive selection sites previously identified as being associated with host range variation, site 45 ranked highest based on the variable importance projection (VIP) contribution (Fig. 3c and Supplementary Fig. 14). Thus, site 45 of P. capsici XEG1 (PcaXEG1) and P. capsici XLP1 (PcaXLP1) was chosen for mutation analysis. Utilizing Cas9-mediated homologous gene replacement in P. capsici (LT1534), we generated homozygous lines in which the alanine (A) and threonine (T) at site 45 in PcaXEG1 and PcaXLP1 were replaced with serine (S) from the ancestral gene XELa, respectively (T2, T12: PcaXEG1A45S; T8, T12: PcaXLP1T45S) (Supplementary Fig. 15a, b). All transformants exhibited normal mycelium growth (Supplementary Fig. 15c, d). We further conducted infection assays on N. benthamiana, pepper, Nicotiana tabacum (tobacco), pea, potato, snow pea, green bean, edamame, tomato, carrot, yellow potato, cowpea, purple lentil, and red peanut. The results showed that while the mutants exhibited no significant differences in infection capacity on green bean, edamame, tomato, carrot, yellow potato, cowpea, purple lentil, red peanut, and potato, and their pathogenicity was significantly reduced on N. benthamiana, pepper, tobacco, pea, and snow pea. Notably, the mutants displayed almost no expansion on tobacco and pea when comparing the inoculation plug diameter to the pathogen growth diameter (Fig. 3d, e and Supplementary Fig. 15e). These findings demonstrate that the positive selection sites in XEG1/XLP1 differentially affect P. capsici’s ability to infect various hosts, further suggesting that these sites contribute to the pathogen’s host range.
To investigate that whether the selection pressure sites of XEG1 and XLP1 are present in natural populations of Phytophthora species, we analyzed the variations in the XEG1 and XLP1 across more than 128 previously documented Phytophthora isolates21,22,23. Positive selection sites, including positions 20, 22, 28, 62, 88, 91, 103, and 120, were identified within these populations. However, the frequency of mutations and the specific positions of these sites varied among different Phytophthora species (Fig. 4a, d and Supplementary Fig. 16). Given the currently limited number of sequenced Phytophthora populations, some positive selection sites, such as position 45, were not detected in the existing datasets. However, these findings strongly suggest the presence of selection pressures within natural Phytophthora populations and indicate ongoing evolutionary forces acting on the XEG1/XLP1 gene pair.
a Schematic comparison of various XEG1 and XLP1 mutants across multiple Phytophthora genomes. Each row represents a different genome, and mutation sites are highlighted in green, positive selection sites are indicated with dark gray, and missing sequences are displayed in light gray. Detailed mutation information is available in Supplementary Data 4. b, c Positive selection pressure sites enable the pathogenicity of P. sojae. d Natural mutation sites of P. sojae isolates. A map depicting the geographic distribution of P. sojae single nucleotide polymorphisms (SNPs). Variations in PsXEG1 and PsXLP1 across homozygous and heterozygous mutant SNPs are presented for each isolate. Detailed mutation information is provided in Supplementary Data 5. e, f Positive selection pressure site 62 in P. sojae population isolates enables the pathogenicity of P. sojae. b, e Representative images of disease symptoms were photographed at 48 hpi. c, f Relative biomass of mutant strains and wild-type at 48 hpi was quantified using genomic DNA qPCR. In the box plots, dots represent min-to-max value of individual data points (n = 9 biological replicates), the line indicates the median, and the box boundaries indicate the 25th and 75th percentiles. Statistical differences were assessed using Duncan’s multiple range test, with different letters indicating significant differences (p < 0.01). Source data are provided as a Source Data file. g Model: XEG1 and XLP1 originated within the Phytophthora genus. Over time, evolutionary differentiation within the genus led to the development of XEG1 and XLP1 from ancestral genes across various Phytophthora species. Genus-specific selection pressures acting on the XEG1/XLP1 gene pair have been pivotal for host adaptation and are strongly associated with the variability of Phytophthora host ranges.
Roles of selection pressure sites of XEG1/XLP1 in Phytophthora host adaptability
To investigate the impact of positive selection sites on Phytophthora host adaptability, in addition to the amino acid at position 45, we also selected position 31, a positive selection site that significantly impacts PsXEG1 hydrolysis activity (Fig. 3a). At position 31, serine (S) in PsXEG1 was mutated to alanine (A), and at position 45 in PsXLP1, alanine (A) was mutated to serine (S) from the ancestral gene XELa. Homozygous lines were generated using the CRISPR/Cas9 system (T10, T14: PsXEG1S31A; T11, T49: PsXLP1A45S) (Supplementary Fig. 17a). Compared with wild-type (WT) and control (CK) lines, the mutants PsXEG1S31A, and PsXLP1A45S exhibited normal growth (Supplementary Fig. 17b, c). However, the pathogenicity of the mutants was reduced, as evidenced by a decline in biomass and lesion length measurements (Fig. 4b, c). These results indicate that common driving pressure sites in Phytophthora contribute to host adaptability and suggest that the evolutionary changes in sequences from ancestral forms to current Phytophthora species have enhanced their host adaptability.
Considering the presence of natural mutations at position 62, identified as a positive selection site in the natural population of P. sojae, we focused on a threonine (T) to isoleucine (I) mutation in XLP1 at this site (Fig. 4d). This mutation involves a shift from a polar to a nonpolar amino acid, altering the direction of the branching chain. Additionally, in Phytophthora kernoviae, another mutation at position 62 introduced an arginine (R), which has a long branch chain and alters the structural charge (Supplementary Fig. 18a). To explore the functional role of position 62, we used the CRISPR/Cas9 system to mutate this position to either isoleucine (I) or arginine (R), generating transformants (T5, T7: PsXLP1T62I; T14, T45: PsXLP1T62R) (Supplementary Fig. 18b). Compared with wild-type (WT) and control (CK) lines, mutants PsXLP1T62I, PsXLP1T62R exhibited normal growth (Supplementary Fig. 18c). The pathogenicity of PsXLP1T62I mutants increased significantly, whereas it declined in PsXLP1T62R mutants. Biomass measurements of P. sojae were consistent with lesion length (Fig. 4e, f). These results indicate that Phytophthora’s common evolutionary pressure is present in natural populations and influence Phytophthora’s ability to adapt to host.
Discussion
Our results propose a model in which Phytophthora-specific selection pressures on XEG1 and its paralog XLP1 are closely linked to the host range diversity of Phytophthora beyond their roles host adaptability (Fig. 4g). Originating from horizontal gene transfer events from fungi, XEG1 underwent gene duplication, giving rise to a paralog positioned in head-to-head configuration with its counterpart11,13. This gene pair has evolved into a genus-specific feature of Phytophthora, serving as a focal point of coevolutionary struggle in diverse Phytophthora-plant pathosystems. Previous research has underscored the role of XEG1 in virulence through hydrolytic activities, countered by host defenses via inhibitors, proteases, and recognition receptors12,13,14,20. In response, Phytophthora utilizes strategies such as decoys, glycosylation, and other effectors to evade host defenses13,14,15, underscoring its significance beyond its virulence-related functions. Our findings demonstrate that positive selection sites in XEG1/XLP1 are integral to the host adaptability of Phytophthora. The ancestral genes of XEG1/XLP1 exhibit significant sequence divergence compared to modern XEG1/XLP1, with several positive selection sites identified in these differences. Mutating these sites in Phytophthora XEG1/XLP1 to their ancestral counterparts resulted in reduced pathogenicity, suggesting a positive correlation between these evolutionary changes and host adaptability. Additionally, the involvement of XEG1/XLP1 in the host range diversity of Phytophthora further emphasizes their importance as genus-specific genes in Phytophthora. Beyond this, considering that some XLP1 orthologs exhibit hydrolytic activity, our results imply that XLP1 may have additional roles beyond its decoy function in P. sojae, potentially contributing to other essential functions in different Phytophthora species.
Natural selection, a fundamental mechanism of evolutionary change, drives the evolution of adaptive traits. Effectors exhibiting sequence and structural polymorphisms can elude the counter-defenses of hosts24,25. Previous investigations have indicated that the XEG1/XLP1 pair experiences diverse selection pressures from host inhibitors, proteases, and receptors. However, our observations indicate that the binding sites of XEG1 or XLP1 with these three classes of host proteins do not coincide with sites subjected to selection pressure. This suggests that the XEG1/XLP1 gene pair in Phytophthora is under a different type of selection pressure, possibly related to the hydrolytic activity of XEG1. Despite previous in vitro enzyme activity studies showing that XEG1/XLP1 can hydrolyze xyloglucan, its degradation substrates in host plants require further study. Additionally, this widespread selection pressure in natural Phytophthora populations implies the persistence of this driving force.
Dynamic changes in the host range of Phytophthora are likely influenced by multiple factors beyond the genetic evolution of specific genes. Human activities play a significant role in the long-distance dispersal of plant pathogens, facilitating the introduction of Phytophthora species into new regions and affecting host adaptability. For instance, the global nursery trade has contributed to the emergence of new hosts for P. ramorum26. In addition, P. ramorum and P. kernoviae have been found on hikers’ boots in infested areas, underscoring the role of human-mediated dispersal in spreading these pathogens27,28,29. Understanding the geographic origins of invading Phytophthora species, their evolutionary trajectories, and potential sources of migration will require more extensive genomic data, making this an important direction for future research.
The host range dynamics of Phytophthora are further influenced by the functional diversity of apoplastic effectors. Apoplastic effectors of Phytophthora encompass various proteins such as glycoside hydrolases, protease inhibitors, necrosis-inducing proteins (NLPs), proteases, lipases, and lipid-binding proteins8. The protease inhibitor EPIC1 of Phytophthora mirabilis is involved in the transition from the four o’clock flower to potato hosts. This study further suggests the role of apoplastic effectors in the host range dynamics of Phytophthora30. Host availability alone does not solely dictate oomycete diversification4. The evolutionary trajectory of pathogens may have been influenced by environmental changes or facilitated through horizontal gene transfer events from fungi9,31, bacteria32, and animals33. A comparative genomic analysis involving 31 Phytophthora genomes identified 44 horizontal candidates, who are also associated with enzymes involved in plant cell wall deconstruction, evasion, and protection from host defenses8, including XEG111. Although this study suggests that XEG1/XLP1 may contribute to alterations in the host range of Phytophthora, XEG1/XLP1 does not represent the sole determinant, warranting further investigation into additional genes and factors.
Methods
Plant and microbe growth conditions
Nicotianna benthamiana, Nicotiana tabacum, and other plants were grown in vermiculite in a greenhouse at 25 °C under a 16-h light/8-h dark cycle. The etiolated soybean cultivar Hefeng 47, susceptible to Phytophthora sojae strain P6497, was grown in the dark for 4 days at 25 °C in vermiculite.
Agrobacterium tumefaciens and Escherichia coli strains were cultured on Luria-Bertani (LB) agar at 28 °C and 37 °C, respectively. P. sojae (P6497) and Phytophthora capsici (LT1534) were cultured on 10% (v/v) V8 medium at 25 °C in the dark.
Plasmid construction
Plasmids are often constructed according to standard molecular engineering procedures. Part of the Phytophthora XEG1 and XLP1 genes, as well as the ancestral genes constructed in the Pbin-3 × HA vector, were synthesized depending on predicted introns, as listed in Supplementary Data 2 and 6 (Generalbiol, Chuzhou, China). Single guide RNAs (sgRNAs) targeting PsXEG1, PsXLP1, PcaXEG1, and PcaXLP1 genes were constructed in the plasmid pYF515. Homologous recombination plasmids for ancestral replacement or single amino acid mutations, each flanked by 1 kb of homology arms, were constructed in the PVX-3 × HA vector.
Proteome database search and sequence analysis
Proteome data from Supplementary Data 1 were used for Hidden Markov Model (HMM) searches against the conserved Glycohydro 12 domain (PFAM ID: PF01670). Non-redundant protein sequences were retrieved and analyzed for protein domains and signal peptides using SMART (http://smart.embl-heidelberg.de) and SignalP 6.0 (http://www.cbs.dtu.dk/services/SignalP-6.0). Enzyme sites within GH12 proteins were predicted using Conserved Domains (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Genomic DNA (gDNA) used for collinearity analysis, as well as cDNA sequences for experimental analysis, were validated against current RNA-sequencing data and protein annotation files (GFF). Any regions with potential annotation errors or lacking RNA-seq reads from the Sequence Read Archive were manually reannotated based on genomic data and listed in Supplementary Data 2.
Collinearity analyses were performed using the One-step MCScanX module in TBtools (v2.067), which generated the “. collinearity”, “. gff”, and “dualSynteny.ctl” files. Due to low-quality genome assemblies, the orthologs of PsXEG1 and PsXLP1 were located on separate contigs in nine Phytophthora species, complicating the identification of these as genuine genes or pseudogenes. In six species, only a single XEG1 or XLP1 was found, and paired gene relationships could not be determined. Upstream and downstream regions of XEG1 orthologs were highlighted, and dual synteny plots were created for visualization.
For natural mutation analysis in Phytophthora isolates (Fig. 4a, d), reference protein sequences from Supplementary Data 2 were used to query each isolate’s translated nucleotide database via NCBI’s tblastn. More details are listed in Supplementary Data 4 and 5.
Phylogenetic analysis
The phylogenetic tree of oomycete species: OrthoFinder v2.3.12 (https://github.com/davidemms/OrthoFinder/releases) was used to find orthogroups and orthologs, use the multiple sequence alignment species tree method (-M msa), infer a rooted species tree for the species being analyzed, and map the gene duplication events from the gene trees to branches in the species tree. Hosts of pathogens cited by Wikipedia and references listed in Supplementary Data 1. Cartoons were free and downloaded from the website (https://www.flaticon.com or https://icons8.com/icons). The phylogenetic tree used to estimate the time when ancestral genes began to diverge also used the same method in Orthofinder.
Phylogenetic analysis of all GH12 proteins and used to calculate selection pressure for XEG1 and XLP1 subclades were performed using an approximate maximum likelihood method implemented in IQ-Tree v1.6.12. All the trees were assessed with 1000 bootstraps, displayed using iTOL v6 (http://itol.embl.de).
TimeTree (Fig. 2d) analysis was conducted using the Reltime method and the JTT matrix-based model in MEGA7, with the input of 28 concatenated amino acid sequences generated by OrthoFinder v2.3.12. OrthoFinder v2.3.12 was used to identify orthogroups and orthologs, with parameters “-M msa” for gene tree inference and the STAG algorithm for rooted species tree inference.The divergence node time between Phytophthora cinnamomi and P. sojae was fixed, setting the maximum divergence time at 10.2 and the minimum divergence time at 9.9.
Conservation analysis
Evolutionary conservation of amino acid positions in XEG1/XLP1 orthologs was assessed using the ConSurf server (https://consurf.tau.ac.il). Based on ConSurf conservation scores, the conservation patterns of XEG1 and XLP1 clades were visualized on 3D structures modeled from PDB: 7w3v (chain A) using PyMOL (https://pymol.org/edu/). The 3D structures of PsXLP1, PpXEG1, PpXLP1, and ancestral proteins were predicted using AlphaFold2 via ColabFold v1.5.5 (https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb#scrollTo=G4yBrceuFbf3). Structural alignment for comparison was performed using the pair-fit method in PyMOL (version 2.5.2). Weblogo 3 (https://weblogo.threeplusone.com/create.cgi) was used to create logo plots showing conserved sites in proteins with intact domain organizations.
Selection pressure analysis of XEG1/XLP1 clades
Selection pressure was analyzed using the CODEML module in PAML v4.9j, which employs two branching models to calculate the dN/dS ratio34. Phylogenetic trees were constructed based on protein alignments using the maximum likelihood method in IQ-Tree v1.6.12, and tree files were generated (.tree). The cDNA sequences of coding regions (codons 1 to 723) were aligned using ClustalW in MEGA7, followed by manual gap removal, and the resulting files were converted to the “.nuc” format. Selection pressure was assessed by editing the.ctl file and analyzing in PAML v4.9j. Branches corresponding to the XEG1 and XLP1 clades were labeled as “$1” and “$2,” respectively. One outgroup used by all fungi, oomycetes, and bacteria, and another outgroup used by the Phytophthora GH12 family proteins, where XEG1 and XLP1 were paired. For model comparison, we obtained lnL1 = −107,588.8137 and lnL0 = −107,601.6545, with a pchisq value of 2.65E−06 (5% significance level, Supplementary Fig. 2a). Similarly, we obtained lnL1 = −55,764.8344 and lnL0 = −55,774.1569, with a pchisq value of 8.94E−05 (5% significance level, Fig. 1c).
Reconstruction of ancestral XEG1/XLP1 proteins sequences
Protein sequences corresponding to the hypothetical ancestors of the Phytophthora XEG1/XLP1 pairs were inferred using the CODEML program in package pamlX. A total of 52 sequences of XEG1/XLP1 pairs and 3 nearest outgroup proteins were used in the prediction. Amino acid sequences of coding regions were aligned using ClustalW, with gaps removed based on the PsXEG1 sequence (codons 1-241), resulting in a new alignment file (.pml). Phylogenetic trees were constructed using the maximum likelihood method in MEGA7, and the resulting tree file (.tree) was generated. Ancestral sequences were predicted using the CODEML program with the alignment, amino acid rate file (“wag.dat”), and model 3 (Empirical+F).
Selection pressure sites analysis of Phytophthora XEG1/XLP1
Selection sites were analyzed using the MEME method (Maximum Likelihood Estimation of Evolutionary Models) within the HyPhy 2.5.48 software package. This method identifies amino acid positions evolving faster than expected under a neutral model, indicating positive selection. A total of 52 XEG1/XLP1 coding region sequences were aligned using ClustalW in MEGA7 and generated new alignment file. Phylogenetic trees were constructed using the maximum likelihood method in MEGA7, and the ‘foreground’ branches were marked on the PhyloTree website (https://phylotree.hyphy.org/) to update the phylogenetic tree. To assess the statistical significance of ω, likelihood ratio tests (LRTs) were performed, comparing a neutral model (no selection) and a selection model. Sites with ω > 1 and a significant p value of 0.05 were identified as under positive selection pressure.
Agrobacterium tumefaciens infiltration
We transformed verified plasmids with the strain GV3101 and cultured them on liquid medium (LB) with antibiotics at 28 °C for 36 h. We resuspended the Agrobacterium in infiltration buffer (10 mM MgCl2, 200 mM acetosyringone, 10 mM MES, pH 5.7) after identifying the correct clone. The phenotypes of cell death induced by XELa, XEGa, XLPa, PpXEG1, PpXLP1, and PsXEG1 were early and strong, in order to compare the differences, the final concentration of OD600 = 0.2 was chosen. For the other electrolyte leakage measurement experiments, the defense marker gene induction experiments, the reducing sugar production experiments, and the protein extraction triggered by various Phytophthora XEG1 and XLP1, different ancestral node proteins, and mutational sites in XELa and Phytophthora XEG1/XLP1, the final concentration for each strain in the infiltration medium was diluted to OD600 = 0.4. Each gene or mutant was injected on the leaves, avoiding the veins.
Electrolyte leakage assay
To identify the ability of different Phytophthora XEG1 and XLP1 to induce cell death, we performed measurements at 48 h. In order to distinguish the influence of site mutation differences, we measured experiments at about 30 h. XELa, XEGa, XLPa, PpXEG1, PpXLP1, PsXEG1 inducing cell death were measured more earlier at about 28 h, when the leaves were not completely necrotic. For cell death, we performed a point injection into an area of approximately more than 1 cm in diameter, and marked.
Five 1 cm leaf discs were cut after infiltration and floated in 5-mL Ep tubes containing deionized water for three hours at room temperature. Measurements were made of the first electrolyte leakage levels. After boiling for 10 min and cooling to room temperature, the final electrolyte leakage values were determined. A conductivity meter was used to measure the electrolyte leakage values (Con 700; Consort, Turnhout, Belgium). Electrolyte leakage was measured by dividing the second value by the first value and multiplying by 100.
Production of reducing sugar assays
The apoplastic fluid (AF) in N. benthamiana leaves was isolated 20 h after infiltration in N. benthamiana. Inject approximately 3–4 circles per leaf. Five 2.5-cm leaf discs were cut and floated in 25-mL Ep tubes containing 1× PBS. After pumping for 2 min for each timer with the vacuum pump, slowly open the air valve. Use absorbent paper to carefully dry the buffer on the surface of the leaf discs and place them in the centrifuge device. Centrifuge at 1500 × g for 10 min. Ten μL of AF was transferred into PCR tubes, including 100 μL of DNS solution (DNS, D7800; Solarbio) and 90 μL of distilled water. Incubate the tubes at 37 °C for 30 min, then heat them at 99.9 °C for 10 min. Measure them with a SpectraMax® M5 microplate reader at 540 nm. The standard curve was measured with 0, 0.2, 0.4, 0.6, 0.8, and 1 mg/L standard of D-glucose solution (Sangon) in Na-acetate buffer (0.05 M, pH 5.5), which had been Milli-Q filtered.
Immunoprecipitation and western blotting
Protein extractions and immunoblots were performed as previously described13. Antibodies used: Anti-HA (Mouse Antibody, 1:5000 dilution, Abmart, lot#324184),RDye 800CW Goat anti-Mouse IgG(H + L) antibody(926-32210, LI-COR, 1:10,000).
Whole genome resequencing data analysis
The adapter low-quality reads were filtered using Trim Galore. The Burrows-Wheeler Transform Alignment (BWA) v0.7.5a was then used to mapped clean reads to the P. sojae reference genome v3.0. The Samtools software package v1.8 was used to sort and convert mapped bam files. We used IGV v2.16.1 to view the “.bam” files and count the PsXEG1 and PsXLP1 mutations. More details are listed in Supplementary Data 5.
Statistics and reproducibility
Samples were allocated randomly into experimental groups. Samples sizes were determined based on the generation of convincing and compelling results. Investigators were blinded to the experiments and assays. At least three biological replicates were performed in all experiments. The number of replicates is indicated in the figure legend. Data were analyzed using boxplots created with Graphpad Prism version 8.0 software (https://www.graphpad.com/). Whiskers mean “min to max, and show all points”. In the box plots, dots represent the min-to-max value of all individual data points, the line indicates the median, and the box boundaries indicate the upper (25th percentile) and lower (75th percentile) quartiles. For data exclusions, only analysis of mutations in natural population isolates of P. sojae. The sequence reads with low quality are more likely derived from sequencing errors, and were thus excluded. To reduce the effect of sequencing error on assembly, we performed the quality control of raw data using Trimmomatic (v. 0.38).
The normal heatmaps were constructed and visualized using TBtools (v2.067). The heatmaps in Supplementary Fig. 10 were created with MetaboAnalyst 6.0. For converting the ancestral sequence changes of the XEG1 clade compared to XEGa and the XLP1 clade compared to XLPa into binary formats. Taking the change of amino acid at site 20 as an example, the “G-G” code is 0000001, and the “A-A” code is 0000010. Taking the change in the site 90 amino acid as an example, in the XEG1 clade, the unchanged “T-T” code is 0001111, and the changed “T-S” code is 0010000. In the XLP1 clade, the unchanged “S-S” code is 0001101, and the changed “S-T” code is 0100000. Encode the dynamically changing amino acids of all ancestor nodes into binary format. At the same time, the phenotype is divided into five levels, enhanced is set to 5, weakly enhanced is set to 4, unchanged is set to 3, weakly weakened is set to 2, and weakened is set to 1. Then use MetaboAnalyst 6.0 for analysis; no sample normalization was set.
Partial least squares discriminant analysis (PLS-DA) analysis was used the ‘AlignIO’ module in Biopython v1.75 to handle multiple sequence alignment (MSA) convert into binary format files. We labeled the host ranges set for different Phytophthora pathogens and analyzed with MetaboAnalyst 6.0. The PLS regression is performed using the plsr function provided by R pls package. The classification and cross-validation are performed using the corresponding wrapper function offered by the caret package. Sample normalization was normalized by median, data transformation and data scaling were used the default value. Select maximum components to search is 5, cross-validation (CV) method is 5-fold CV, and performance measure is Q2. Permutation test statistic was selected separation distance (B/W), and set permutation numbers 1000, empirical p value was 0.003.
P. sojae and P. capsici transformation
The CRISPR-mediated gene replacement technique was used to produce homologous gene replacement mutants13,35.
P. sojae growth and virulence assays
The steps are similar to those reported in the previous article13.
P. capsici growth and virulence assays
WT and all transformants of P. capsici were cultured on 10% V8 medium at 25 °C in the dark. After 4–5 days, we examined the colony diameters on the V8 medium. Mycelial plugs were infected on half of the plant leaves, and the inoculated leaves were placed in a plastic box with high humidity, the size of the lesions on the inoculated leaves was measured under UV light.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Supplementary Information/Source Data file. The PDB code for the plant receptor like protein RXEG1 in complex with xyloglucanase XEG1 structure: 7W3V. The detailed SRA project of NCBI data cited in the article also can be found at Supplementary Data. Source data are provided with this paper.
References
Gunderson, J. H., Elwood, H., Ingold, A., Kindle, K. & Sogin, M. L. Phylogenetic relationships between chlorophytes, chrysophytes, and oomycetes. Proc. Natl Acad. Sci. USA 84, 5823–5827 (1987).
Jiang, R. H. & Tyler, B. M. Mechanisms and evolution of virulence in oomycetes. Annu. Rev. Phytopathol. 50, 295–318 (2012).
Thines, M. Phylogeny and evolution of plant pathogenic oomycetes—a global overview. Eur. J. Plant Pathol. 138, 431–447 (2014).
Matari, N. H. & Blair, J. E. A multilocus timescale for oomycete evolution estimated under three distinct molecular clock models. BMC Evol. Biol. 14, 1–11 (2014).
Haas, B. J. et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461, 393–398 (2009).
Yang, X., Tyler, B. M. & Hong, C. An expanded phylogeny for the genus Phytophthora. IMA Fungus 8, 355–384 (2017).
Lo Presti, L. et al. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 66, 513–545 (2015).
Kronmiller, B. A. et al. Comparative genomic analysis of 31 Phytophthora genomes reveals genome plasticity and horizontal gene transfer. Mol. Plant Microbe Interact. 36, 26–46 (2023).
Thomma, B. P., Nürnberger, T. & Joosten, M. H. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23, 4–15 (2011).
Jones, J. D. G., Staskawicz, B. J. & Dangl, J. L. The plant immune system: from discovery to deployment. Cell 187, 2095–2116 (2024).
Richards, T. A. et al. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc. Natl Acad. Sci. USA 108, 15258–15263 (2011).
Ma, M. Z. et al. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27, 2057–2072 (2015).
Ma, Z. et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science 355, 710–714 (2017).
Xia, Y. et al. N-glycosylation shields Phytophthora sojae apoplastic effector PsXEG1 from a specific host aspartic protease. Proc. Natl Acad. Sci. USA 117, 27685–27693 (2020).
Wang, Q. et al. Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 23, 2064–2086 (2011).
Xu, B. & Yang, Z. PAMLX: a graphical user interface for PAML. Mol. Biol. Evol. 30, 2723–2724 (2013).
Pang, Z. et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52, gkae253 (2024).
Kosakovsky Pond, S. L. & Frost, S. D. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 22, 1208–1222 (2005).
Pond, S. L. K., Frost, S. D. & Muse, S. V. HyPhy: hypothesis testing using phylogenies. Bioinformatics 21, 676–679 (2005).
Sun, Y. et al. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 610, 335–342 (2022).
Mandal, K., Dutta, S., Upadhyay, A., Panda, A. & Tripathy, S. Comparative genome analysis across 128 Phytophthora isolates reveal species-specific microsatellite distribution and localized evolution of compartmentalized genomes. Front. Microbiol. 13, 806398 (2022).
Zhang, X. et al. Whole genome re-sequencing reveals natural variation and adaptive evolution of Phytophthora sojae. Front. Microbiol. 10, 2792 (2019).
Arsenault-Labrecque, G. et al. Stable predictive markers for Phytophthora sojae avirulence genes that impair infection of soybean uncovered by whole genome sequencing of 31 isolates. BMC Biol. 16, 1–16 (2018).
Attah, V. et al. Duplication and neofunctionalization of a horizontally transferred xyloglucanase as a facet of the Red Queen coevolutionary dynamic. Proc. Natl Acad. Sci. USA 121, e2218927121 (2024).
Cao, Y. et al. Structural polymorphisms within a common powdery mildew effector scaffold as a driver of coevolution with cereal immune receptors. Proc. Natl Acad. Sci. USA 120, e2307604120 (2023).
Grünwald, N. J., LeBoldus, J. M. & Hamelin, R. C. Ecology and evolution of the sudden oak death pathogen Phytophthora ramorum. Annu. Rev. Phytopathol. 57, 301–321 (2019).
Webber, J. F. & Rose, J. Dissemination of aerial and root infecting Phytophthoras by human vectors. Proc. Sudden Oak Death Third Sci. Symp. 195–198, https://research.fs.usda.gov/treesearch/29882 (2008).
Frankel, S. J., Kliejunas, J. T. & Palmieri, K. M. Proceedings of the Sudden Oak Death Third Science Symposium. General Technical Report PSW-GTR-214 491, 214 (US Department of Agriculture, Forest Service, 2008).
Mullett, M. S. et al. Phylogeography and population structure of the global, wide host-range hybrid pathogen Phytophthora × cambivora. IMA Fungus 14, 4 (2023).
Dong, S. et al. Effector specialization in a lineage of the Irish potato famine pathogen. Science 343, 552–555 (2014).
Misner, I., Blouin, N., Leonard, G., Richards, T. A. & Lane, C. E. The secreted proteins of Achlya hypogyna and Thraustotheca clavata identify the ancestral oomycete secretome and reveal gene acquisitions by horizontal gene transfer. Genome Biol. Evol. 7, 120–135 (2015).
Belbahri, L., Calmin, G., Mauch, F. & Andersson, J. O. Evolution of the cutinase gene family: evidence for lateral gene transfer of a candidate Phytophthora virulence factor. Gene 408, 1–8 (2008).
Lévesque, C. A. et al. Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol. 11, 1–22 (2010).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Fang, Y. & Tyler, B. M. Efficient disruption and replacement of an effector gene in the oomycete Phytophthora sojae using CRISPR/Cas9. Mol. Plant Pathol. 17, 127–139 (2016).
Acknowledgements
We thank Prof. M. Wang (Nanjing Agricultural University), Prof. K. Duan (Nanjing Agricultural University) for insightful discussions and constructive suggestions. This research was supported by grants from the National Key Research and Development Program of China (2022YFF1001500, Y.W.), National Natural Science Foundation of China Outstanding Youth Fund (Overseas, Z.M.), Jiangsu Province Distinguished Professor (Special Support, Z.M.) and National Natural Science Foundation of China (32102172, Y.X.). Bioinformatic workstation was supported from the Bioinformatics Center, Nanjing Agricultural University.
Author information
Authors and Affiliations
Contributions
W.-Y.C. and M.-Z.C. administrate the project. W.-Y.C., M.-Z.C. and Z.Q. conceived the idea, guided the project, and wrote the manuscript with feedbacks from other authors. Z.Q., C.X., C.B., J.-L.Y., Y.-H.X., M.J., Z.-X.Y., W.-X.Y., L.-H.S., L.-S.Z., W.-K.X., J.-H.B., X.-J.H., S.-H.D., Z.-Z.C., Q.M., C.H. and X.-Y.Q. performed experiments and analyzed the results. D.-S.M., Y.-W.W. and W.Y. provided advice and reagents and discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Stefan Kusch and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Chen, X., You, H. et al. Specific selection on XEG1 and XLP1 genes correlates with host range and adaptability in Phytophthora. Nat Commun 16, 3638 (2025). https://doi.org/10.1038/s41467-025-58770-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58770-x