Abstract
Decentralized water treatment technologies, designed to align with the specific characteristics of the water source and the requirements of the user, are gaining prominence due to their cost and energy-saving advantages over traditional centralized systems. The application of chemical water treatment via heterogeneous advanced oxidation processes using peroxide (O–O) represents a potentially attractive treatment option. These processes serve to initiate redox processes at the solid-water interface. Nevertheless, the oxidation mechanism exemplified by the typical Fenton-like persulfate-based heterogeneous oxidation, in which electron transfer dominates, is almost universally accepted. Here, we present experimental results that challenge this view. At the solid-liquid interface, it is demonstrated that protons are thermodynamically coupled to electrons. In situ quantitative titration provides direct experimental evidence that the coupling ratio of protons to transferred electrons is almost 1:1. Comprehensive thermodynamic analyses further demonstrate that a net proton-coupled electron transfer occurs, with both protons and electrons entering the redox cycle. These findings will inform future developments in O–O activation technologies, enabling more efficient redox activity via the tight coupling of protons and electrons.
Similar content being viewed by others
Introduction
The centralized water treatment model has been the dominant paradigm over the past century. However, this model is facing a number of challenges1,2, including high upfront costs associated with infrastructure development, the aging of existing infrastructure, limited resilience to population growth and climate change, and a lack of suitability for implementation in remote communities. It is therefore imperative to transition towards a more decentralized methodology, namely decentralized solutions that treat water at a smaller scale and in closer proximity to the point of utilization. Advanced oxidation processes (AOPs) represent an optimal solution for the treatment of water in decentralized systems3,4. In order to overcome the foundational impediments that have hitherto constrained the pragmatic deployment of conventional AOPs, including the reduction of their chemical and energy input requirements, efforts have been made to employ heterogeneous catalysts5,6. Transition metal oxides represent a common example of heterogeneous catalysts. In general, transition metal oxides (TMOs) are a plentiful class of materials with the potential to remain stable under oxidizing conditions. Moreover, the financial burden associated with the complete mineralization of organic pollutants (associated with production of CO2 and H2O) for AOPs is considerable. Accordingly, the optimal design objective of an AOP is to achieve partial oxidation of the organic pollutants, thereby producing inert, less concerning products in practice7,8. Indeed, the aforementioned process is consistent with the emerging paradigm of chemical oxidation, namely, the simultaneous control of pollution and resource recovery. One potential strategy for realizing this process is the conversion of organic pollutants into higher molecular weight polymers7,8,9,10, analogous to the synthesis of lignin by plants in nature through enzyme-mediated polymerization of phenolic compounds.
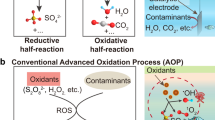
Persulfate-based heterogeneous oxidation represents a prototypical and extensively studied Fenton-like AOP, initiated by a solid-water interface11,12,13,14 (Supplementary Fig. 1). Benchmark TMOs-based catalysts activate the O–O bonds of persulfate precursors—peroxymonosulfate (PMS, HO–OSO3–) and peroxydisulfate (PDS, O3SO–OSO32–) (Supplementary Fig. 2)—enabling the linkage of TMOs metal redox transformation to the oxidation of organic pollutants. The process can be subdivided into high-valent, metal (–oxo)-induced, non-radical oxidation steps, as illustrated in Figs. 1A, B. This process has created a paradigm shift in the water treatment field; the historical focus on pollution control has shifted toward a more sustainable approach that incorporates resource recovery10,15. This shift changed the mode of organic elimination from mineralization to polymerization, enabling water purification to be associated with low-level carbon emissions7,8,9. Using this knowledge, significant advances have been made in the development of sustainable water treatments7,8,9. However, the high-valent, metal (–oxo)-induced, oxidation mechanism at solid-water interfaces is typically described using the electron transfer (ET)–centered view. Currently, three oxidation pathways are recognized16: an electron-transfer process (ETP), oxygen atom transfer (OAT), and hydrogen atom transfer (HAT) (Fig. 1C–E). All primarily involve redox-driven ET.
Activation of the O–O bond at a solid-water interface is actually initiated by two interlinked redox reactions. The first, termed (A), is the transition from M(m+n)+ [high-valent metal (–oxo) species] to Mm+ (low-valence metal), which serves as a key process in the oxidation of model pollutants. The second is (B), the transition from Mm+ to M(m+n)+, which involves cleavage of the O–O bond. The prevailing categorization of oxidation pathways as (C) ETP and (D) OAT has been found to be inadequate due to its predominant emphasis on electron transfer for pollutant oxidation, with a concomitant neglect of interface processes. The (E) HAT hypothesis is untenable because it assumes that the proton and electron originate and terminate in the same bond. In the case of transition metal-mediated O–O bond activation at a solid-water interface, the electron (e–) is typically found occupying the available d-state at the metal site, while the proton (H+) attaches to the interfacial O atom. P., model pollutants; Pox., oxidation products; P=Oox., oxygen atom transfer products; P•ox., hydrogen atom transfer products.
The electron transfer (ET)–centered view of redox reactions is increasingly challenged in the context of reaction thermochemistry17,18,19 due to the fact that chemical bonds are formed or broken via the temporary addition or removal of electrons. In such instances, an electron may be transferred in conjunction with a proton, through a mechanism termed proton-coupled electron transfer (PCET)20,21. This interdependent coupling occurs when the free energy perturbations associated with ET at the interface of a redox system cannot be disregarded17,22,23. The coupling implies that the initial and final states of ET significantly differ due to the presence of non-zero nuclear reorganization, which encompasses solvent reorganization and ion transfer. Here, we concentrate on charge-compensated cation coupling, in which the free energy of ET is reflective of the characteristics of the cations, rather than the just intrinsic properties of TMOs. This coupling ensures that thermodynamic equilibrium is reached, thereby implying that PCET is thermodynamically preferred. However, such joint participation of electrons and protons conflicts with the prevailing view of an interfacial Fenton-like heterogeneous persulfate-based oxidation reaction, in which the fundamental nature of the reaction is only regarded as the addition or removal of electrons (i.e., pure ET). There remains a lack of knowledge concerning the coupled proton transfer (PT) with ET of Fenton-like processes4,9; this lack of knowledge hinders improvement. The coupled protons have been largely overlooked in previous studies. This study explicitly demonstrates that proton coupling at a solid-water interface typically accompanies ET, stimulating further investigation of how redox water treatments could be improved.
The present study examines the proton-coupled nature of well-characterized interfacial redox reactions, with a particular focus on persulfate-based heterogeneous oxidation. The volcano relationship between the rate constant and the pH, exemplified by one benchmark TMOs-based catalyst (CuO), is confirmed. Mechanistic studies indicate that activation of the persulfate O–O bond is mediated by interfacial Cu sites via a concerted PCET (CPET) pathway, yielding high-valent metal (–oxo) species (CuIII) that serve as key intermediates during the oxidation of model pollutants including phenol (PhOH) and 2,6-dimethylphenol (2,6-M-PhOH) to organic radicals. Thus, both processes facilitate radical polymerization at a solid-water interface. The catalytic activity during oxidative polymerization is affected by the proton activity (pH); pH significantly alters the activity (up to 31.60-fold for CuIII). Quantitative acid–base titrations tracking the evolution of [H+] reveal that, during CPET, the H+/e– stoichiometry is almost 1:1. Changes in pH affect the CPET process of other benchmark TMOs (NiO and Co3O4) and thus the oxidation activities of NiIV = O and CoIV = O. Oxidative polymerization can be extended to another typical aromatic, aniline, yielding organic polymers. These results demonstrate the generalizability of CPET in terms of persulfate O–O activation at a solid-water interface.
Results
ET-Only mechanisms fail to explain non-monotonic kinetic/pH dependencies
Our study began with pH activity profiling with representative reactants, namely benchmark TMOs (CuO, NiO, and Co3O4)24, a persulfate oxidant, and model pollutants in a buffer-free batch reactor. The studied pH range is detailed in Supplementary Note 1. Under buffer-free conditions, the catalytic activity of the model CuO-activated PMS process (Supplementary Fig. 3) expressed as an apparent rate constant (kapp in L min–1 g–1) (Supplementary Fig. 4) is not pH-dependent (PCET predicted either a volcanic or caldera-shaped kinetic dependence on pH)19,25,26,27; a similar result has been previously reported28 (Supplementary Note 2). We hypothesized that the discrepancy could be resolved by considering the instantaneous change in interfacial proton concentration29 triggered by a reaction-induced local pH shift. One potential solution might utilize borate and glycine buffers, which attenuate the nearly interfacial local pH gradient within acceptable tolerances (Supplementary Note 3). A novel finding emerged under well-buffered conditions: a large kinetic pH effect manifested as a kinetic gap of an order of magnitude (31.60-fold) (Fig. 2A, Supplementary Fig. 5 and Supplementary Table 1). Critically, a failure to consider the interfacial and bulk solution pH gradients can mask identification of this effect, leading to the commonly observed weak rate-pH scaling effect (Supplementary Note 4). Another potentially counterintuitive result was the non-monotonic nature of the pH-dependent kinetics; the maximum kapp occurred at a unique inflection point of pH 6.2 (Fig. 2A, Supplementary Fig. 5 and Supplementary Table 1). The volcano-shaped activity versus pH profiles of other benchmark catalysts, including NiO and Co3O4 (Figs. 2B, C, Supplementary Figs. 6, 7 and Supplementary Table 1), provide further evidence to support this conclusion. These profiles show that the pH-dependent activity variations described above are common. Such pH-determined behavior indicates proton activity at the solid-water interface. This proton activity has not yet been considered by researchers focused on typical interfacial redox reactions, such as persulfate-based oxidations. The non-monotonic kinetic/pH dependencies cannot be fully explained by the conventional ET-focused view; it is essential to consider PCET.
The apparent rate constant (kapp in L min–1 g–1) versus pH and the molar ratio of consumed PMS to removed PhOH for (A) CuO, (B) NiO, and (C) Co3O4 is shown here for mean values and error bars. Error bars indicate the standard deviation of duplicate measurements (n = 2). Shaded regions represent confidence intervals (95%). Experimental conditions: [PMS] = 0.3 mM, [PhOH] = 0.15 mM, catalyst = 0.2 g L–1, T = 25 ± 2 °C, 0.2 M borate or glycine buffer.
Unnoticed PT at a solid-water interface
To identify previously unrecognized protons, we used electron equivalents that could resolve the reaction path. Electron conservation is not consistent with the conventional radical-oxidation/pollutant-mineralization mechanism (Fig. 2A, Supplementary Table 2 and Supplementary Note 5)4,30; non-radical oxidation is also involved, as indicated by the near-unity molar ratio of consumed PMS to removed phenol as the pH increases from 6.0 to 9.0 when CuO is utilized (Fig. 2A). This phenomenon was expected10. However, the relative importance of the radical and non-radical paths remains unclear. Chemical oxygen demand (COD) measurements and thermogravimetric analysis (TGA) revealed that non-radical oxidation explained > 81.51% of PhOH removal (see Supplementary Methods 1–3, and Supplementary Fig. 8). Semi-quantitative electron paramagnetic resonance (EPR) studies and radical-probing experiments [using benzoic acid31,32 and iopromide33 to probe for ·OH/SO4•− and SO4•−, respectively] (Supplementary Method 4, Supplementary Figs. 9 and 10) showed that ·OH, SO4•−, O2•−, and 1O2—in either the bulk solution or surface-bound form—were not engaged in oxidation. Radicals explained less than 19.12% of PhOH oxidation (Supplementary Note 6); the non-radical pathway is the primary mechanism, rather than a supporting mechanism.
Next, we explored the intermediates and products of the non-radical pathway. The surface-accumulated PhOH oxidation product was a crosslinked polymer (Supplementary Fig. 8) that did not peel from the surface into the solvent (Supplementary Note 7). We inferred that a reactive phenoxy radical (PhO·) intermediate (not yet directly detected) explains formation of the crosslinked polymer (Supplementary Note 8), considering that PhOH exhibits three active H-sites (at the ortho-/para-positions of phenolic–OH). If this inference is correct, concealment of two-thirds of the H-sites (at the two ortho-positions) might hinder crosslinking. We thus implemented a hypothesis-driven approach; we modified PhOH to 2,6-M-PhOH (with only one active H-site in the para-position). As expected, 91.06% of 2,6-M-PhOH was converted into non-crosslinked products (Supplementary Methods 5–7 and Supplementary Fig. 11). Specifically, 33.82% of products were chain-like polyphenyl ethers (formed via C–O polymerization) (Fig. 3A and Supplementary Fig. 12), whereas 57.24% of products were 3,3’,5,5’-tetramethyldiphenoquinone (created via C–C coupling) (Fig. 3B and Supplementary Fig. 13); all products formed via oxidation of 2,6-M-PhOH. The polymerization and coupling products were identified using COD measurements, TGA, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), gel permeation chromatography (GPC), and liquid chromatography-mass spectrometry (LC-MS) (Figs. 3A, B, Supplementary Figs. 11–14 and Supplementary Note 9). The predominant PhO· intermediate ( > 91.06%) is similar to those previously reported9,10. Importantly, the oxidation of PhOH to yield PhO· involves a PT34,35; this aspect cannot be disregarded.
A The MALDI-TOF-MS spectrum of the oxidation products of 2,6-M-PhOH washed with toluene. The inset in (A) is a partially magnified view of the MALDI-TOF-MS spectrum. It can be seen that the mean mass interval of 120.1 corresponds to the polymeric unit of poly-(2,6-dimethyl-1,4-phenylene oxide) (PPO), as well as a schematic of the polymerization reaction pathway. B The high-resolution mass spectrum of the oxidation products of 2,6-M-PhOH washed with ethanol. The exact theoretical mass of 3,3′,5,5′-tetramethyldiphenoquinone (positive ion acquisition mode, +H) is 241.1228, which agrees with the experimental value in (B) (i.e., 241.1308). The inset in (B) shows a schematic of the surface coupling reaction pathway. The results from (A and B) indicate that the predominant formation is of the PhO· intermediate during PhOH oxidation. C EPR spectra of the CuO/PMS system at pH 6.8, 7.4, and 9.0 with 0.1-M DMPO as a spin-trapping agent. The positive correlation between the EPR peak height and the kinetic activity indicates that the pH-dependent production of CuIII occurs. Experimental conditions: [PMS] = 0.3 mM, [CuO] = 0.2 g L–1, T = 25 ± 2 °C, 0.2 M borate buffer. D The ultraviolet-visible spectrum of the CuIII-periodate complex showed a distinct light absorption at 420 nm, indicating the formation of CuIII. Experimental conditions: [NaIO4] = 0.5 mM, [PhOH] = 0.15 mM, [PMS] = 0.3 mM, [CuO] = 0.2 g L–1, T = 25 ± 2 °C, pH = 7.4, 0.2 M borate buffer.
We next sought to identify the active site for conversion of PhOH to PhO·. This site engages in selective oxidation of primary organic pollutants; the removal efficiencies differ between electron-rich (with electron-donating groups, -OH and/or -NH2) and electron-deficient (with electron-withdrawing groups, -COOH and/or -NO2) organics (Supplementary Fig. 15). Moreover, the photoluminescence spectra resemble volcanoes (Supplementary Fig. 16), suggesting that CuIII is the critical intermediate initiating PhO· generation. This suggestion is supported by previous results14 and our EPR spectra (Fig. 3C) that revealed the typical seven-line EPR signal of 5,5-dimethyl-1-pyrrolidone-N-oxyl (DMPOX) was produced via oxidation of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) by CuIII. The formation of DMPOX entails the exclusion of 1O2 and abrupt generation of a substantial quantity of ·OH, as described in Supplementary Fig. 9 and Supplementary Note 10. The pronounced pH-dependent correlation between kinetic activity (Supplementary Fig. 5) and EPR peak height (Fig. 3C) indicates that low-valent CuII undergoes oxidation to high-valent CuIII in a pH-dependent manner. A similar conclusion can be drawn regarding the final oxidation products of methyl phenyl sulfoxide (PMSO); these are biphenyl compounds or hydroxylated products, as revealed by spectrally matched LC-MS (Supplementary Fig. 17), and they were previously suspected to result from direct CuIII oxidation14. Ultraviolet–visible spectral analysis revealed a transient CuIII–periodate complex36,37 (Fig. 3D and Supplementary Note 11), providing additional evidence to support our conclusion. Next, we conducted open-circuit potential versus time (OCPT) tests (Supplementary Method 8 and Supplementary Fig. 18) to track the efficiency of the CuIII-to-CuII transition with and without PhOH. Prior to PhOH addition, the dominant valence state was that of electrochemically generated CuIII. However, the accelerated quenching of CuIII upon the addition of PhOH indicated that CuIII oxidizes PhOH as the critical active site. Thus, PhO· production reflects oxidation by CuIII.
Evaluations of other benchmark TMOs, thus NiO and Co3O4, revealed that high-valence NiIV = O and CoIV = O were kinetically competent oxidants, associated with 97.35% (NiIV=O) and 91.83% (CoIV=O) PhOH oxidation to PhO·. The details are presented in Figs. 2B, C, Supplementary Figs. 6, 7 and 19. These results are comparable to the ~100% PhOH-to-PhO· reactions catalyzed by transition metal (Cu, Ni, Co, and Fe) single-atom catalysts9. Our experiments show that high-valent metal (–oxo) species (CuIII, NiIV = O, and CoIV = O) are key intermediates of PhOH coupling/polymerization; they extract an H-atom (H•) from PhOH to generate PhO· (Eqs. 1 and 2). Furthermore, our research platform enabled investigation of how high-valent metal (–oxo) species engage in interfacial PCET (I-PCET), facilitating a deeper understanding of the I-PCET mechanism.
Evidence for I-PCET
Two general mechanisms, hydrogen atom transfer (HAT) and CPET9, involve extraction of H• from PhOH by electrophilic high-valent metal (–oxo) species (CuIII, NiIV = O, and CoIV = O). The kinetic isotope effect (KIE), defined as KIE = kPhOH(H)/kPDOD(D), represents a valuable method for distinguishing between the HAT and CPET mechanisms. The dissimilarities in proton versus deuteron wavefunction overlap are reflected in the KIE value, which is frequently close to unity for CPET. This has been corroborated through both computational and experimental means21,38,39. The low kinetic isotope effect of k (Supplementary Method 9 and Supplementary Fig. 20) and its high temperature dependence [i.e., KIE inversion at higher temperatures26] (Supplementary Fig. 21), show that CPET is involved. Thus, the proton (H+) and electron (e–) respectively originate and terminate the distinct bonds. In contrast, HAT requires H+ and e– to both originate and terminate the same bonds40. In the context of benchmark transition metal oxides (CuO, NiO, and Co3O4), CPET is plausible considering that e– populates the available d states of the metal and H+ attaches to the interfacial O atom (Eq. 3). These observations indicate that oxidation of PhOH to PhO· is a form of coupled H+/e– transfer in a manner of H• loss (1:1 H+:e– stoichiometry), followed by CPET-mediated H+/e– addition to distinct interfacial trapping sites (Fig. 4A). Thus, the high-energy intermediates typically observed during sequential ET and PT steps are absent. The negligible variation in solution pH after high-valent metal (–oxo) species react with PhOH reinforces this conclusion (Supplementary Fig. 22). Notably, the I-PCET mechanism initiated by a solid-water interface involves the formation of a high-valent metal (–oxo) species via heterolytic O–O bond activation17 (Fig. 1B). Nevertheless, an understanding of the I-PCET process is compromised by the limited data regarding the coupled proton that accompanies the generation of high-valent metal (–oxo) species via O–O bond cleavage. More research focused on this proton is needed.
A Oxidation of PhOH to PhO·, which involves the abstraction of H• from PhOH by the electrophilic CuIII, accompanied by the reduction of CuIII to CuII. B Formation of CuIII from CuII through heterolytic O–O bond activation, where the molar ratio of transferred electrons to coupled protons is close to 1:1, as shown in this study. This indicates that the interfacial O–H bond is directly involved in the oxidation of CuII to CuIII, and the O–H bond is simultaneously broken in response to the temporary removal of an electron from CuII to CuIII.
Previous studies concerning the formation of high-valent metal (–oxo) species at solid-water interfaces did not identify PT9,14,41. A possible explanation is that reactions mediated by coupled protons tend to be unnoticed because they are ubiquitous. In contrast, the thermochemistry [i.e., energy difference between bonds broken and formed that affects reaction equilibria, typically described using a “square scheme” (Supplementary Fig. 23)17,19] implies that ET and PT are thermodynamically coupled to ensure charge equilibrium17,23,40. Accordingly, the coupled movement of proton charges compensates for the transferred electrons. The mechanism may be either a concerted PCET (CPET) [where the H+ and e– are transferred in the same kinetic step42], a separate but coupled pathway [PT precedes ET (PTET)], or ET followed by PT (ETPT)26. We propose that the formation of a high-valent metal (–oxo) species involves a net PCET, thus comprising an inextricably coupled transfer of e– and H+. Qualitative experimental support for this hypothesis is provided by the model CuO-activated PMS reaction, which is associated with a pH reduction indicating a loss of H+ to solution (Supplementary Fig. 24). However, quantitative verification has been lacking, partly due to the difficulty associated with determining the H+/e– stoichiometry m/n [an integer ratio according to Dalton’s law (Eq. 4)]. Because the bond dissociation free energy (BDFE) of X–H (Eq. 5) is the “gold standard” thermochemical descriptor of I-PCET17,23,40, it is reasonable to speculate that tight H+/e– coupling involves cleavage of the surface-H bond, increasing pKa (pKa = –logKa, where Ka is the acid dissociation constant) at the surfaces of metal oxides and yielding high-valent metal (–oxo) species upon electron removal (Figs. 1B and 4B). This speculation assumes that H+ generation upon dissociation of the surface-H bond will be stoichiometric, accompanied by ET. Consequently, trapping and quantification of coupled protons (dissociated H+) could directly yield to the critical m/n stoichiometry governing formation of high-valent metal (–oxo) species, enabling a comprehensive understanding of I-PCET. Thus, we present stoichiometric-H+ experimental measurements obtained via appropriate, liquid-phase acid–base titrations (Supplementary Note 12). We quantify dissociated-H+ levels by determining changes in total surface-hydroxyl density43,44.
The need for equivalent protons can be represented by ascertaining dissociative H+ behavior in a model of TMOs-mediated O–O bond activation during I-PCET reaction (Figs. 1B and 4B). In the presence of 0.1 mM HO–OSO3– and excess benchmark TMOs (2.0 g L–1 CuO) (Supplementary Note 13), the overall dissociated-H+ concentration was 0.093 mM, implying a H+/e– molar ratio of 1:2 attributable to O–O bond cleavage along with 2-e– transfer (Fig. 5A, Supplementary Fig. 25 and Supplementary Table 3). This result conflicts with the predicted 1:1 H+/e– stoichiometry of CuIII formation17,23,40. Furthermore, an identical but O2-free reaction yielded a similar result (Supplementary Fig. 26 and Supplementary Table 3). The contradictory outcomes are attributable to the presence of H+ acceptor in HO–OSO3– (Eqs. 2 and 6); H+ transferred to the product-H2O cannot be determined via titration. If this hypothesis is correct, replacement of HO–OSO3– with O3SO–OSO32– via H+-acceptor site-directed mutagenesis might yield a stoichiometrically compatible H+ level (Eqs. 2 and 7). As anticipated, the ratio of transferred e– to detected H+ was 1:1 (Fig. 5B, Supplementary Figs. 27, 28 and Supplementary Table 3), confirming the existence of a rigorous solid-water interface PCET in which the O–H bond is directly involved (Fig. 4B). To confirm that this I-PCET was not unusual, we extended the range of high-valent metal (–oxo) species from a form that is typically unstable, thus CuIII45 (as previously described), to stable complexes (NiIV=O and CoIV = O)45 and measured the H+/e– ratios of transition-metal-oxide (NiO and Co3O4)-mediated O–O bond activations. As expected, 2-coupled H+ was involved in NiIV = O and CoIV = O formation, linked to 2-e– transfer (Supplementary Figs. 29–36 and Supplementary Table 3). The H+/e– stoichiometry is 1:1, evincing the generality of this I-PCET mechanism.
The capture and quantification of the proton was achieved by the implementation of an appropriately performed liquid-phase acid–base titration for (A) CuO/PMS (conditions: [PMS] = 0.1 mM, [CuO] = 2.0 g L–1) and (B) CuO/PDS (conditions: [PDS] = 0.1 mM, [CuO] = 2.0 g L–1). The molar ratio of detected proton (H+) to transferred electron (e–), as determined by titration, is 1:2 for (A), while the ratio is 1:1 for (B). These results indicate that the formation of high-valent metal (–oxo) species is accompanied by the dissociation of the surface-H bond.
Thermokinetic analysis
A comprehensive understanding of I-PCET requires kinetic and thermodynamic details. Our initial objective was to gain kinetic insights into the pH-dependent solid-water interface–hosted CPET mechanism. This involves the almost stoichiometric formation of PhO· and high-valent metal (–oxo) species (Supplementary Note 14), which is relevant in the model CuO-activated PMS context. The duplicated kapp values are plotted as a function of pH in Fig. 2A, which reveals the dependence on proton activity. This dependence is confirmed by the fact that log(kapp) (approximately) scales with the Brønsted slope or the Brønsted α with the pH. The α predicted by Marcus theory is ~1/2 under the low driving force of a free energy barrier23,26 (Fig. 6A and Supplementary Note 15). It is imperative to emphasize that the Brønsted α, which establishes a linear correlation between the logarithm of the CPET rate constant [log(kCPET)] and the logarithm of the equilibrium constant [log(keq)] or the scaled driving force (|ΔG°CPET | )46, would provide an invaluable insight into the sensitivities of reaction barriers (rates) to changes in free energy (i.e., the driving force) during rate-limiting I-PCET. Figure 6A shows that as the pH increases from 6.0, kapp rises in a log-linear manner up to pH 6.2, with a slope of 0.45 ± 0.01 log(L min–1 g–1) pH−1; after pH 6.2, kapp decreases with a slope of –0.52 ± 0.01 log(L min–1 g–1) pH−1 up to pH 9.0. Indeed, the initial fractional slope of log(kapp) pH−1 (0.45 ± 0.01) is consistent with an approximately α-1/2 scaling, as predicted by Marcus theory23,26. This slope suggests that the relationship between log(kapp) and pH is analogous to the plot of log(kCPET) versus log(keq) or the plot of the barrier force versus the CPET driving force, ΔG‡ versus |ΔG°CPET | . These relationships highlight the pivotal role of PT, which agrees with the proposed I-CPET mechanism19,23,26,47. Moreover, log(kapp) pH−1 scaling, largely independent of buffer concentration (Supplementary Fig. 37), provides further support for the I-PCET mechanism because it is not perturbed by solvent. However, the electron-only or proton-only pathway is medium-dependent, and charges move48. Note that the Brønsted slope exhibits an apparently negative α-scaling based on pH [–0.52 ± 0.01 log(kapp) pH−1] (Fig. 6A) across the extensive pH range of 6.2 to 9.0. The reason for this negative α-scaling is unclear, and further investigations are required.
Linear correlations were observed between the logarithm of the kapp value and pH, with different slopes for the following systems: (A) CuO/PMS, (B) NiO/PMS, (C) Co3O4/PMS, and (D) NiO-air/PMS. Error bars indicate the standard deviation of duplicate measurements (n = 2). Experimental conditions: [PMS] = 0.3 mM, [PhOH] = 0.15 mM, catalyst = 0.2 g L–1, T = 25 ± 2 °C, 0.2 M borate or glycine buffer. BDFE is the primary energetic parameter.
In the widely accepted CPET mechanism, BDFE constitutes the central energetic parameter17,23,26. We thus hypothesized that a change in ΔG°PT (the free energy for PT) according to pH would exhibit a strong correlation with the abovementioned, complex pH dependence. The negative α-scaling of log(kapp) pH−1 reflects the fact that ΔG°PT determines PT-keq17,26; moreover, ΔG°ET (the free energy for ET) is in close balance with ΔG°PT to maintain a remarkably constant BDFE23. This constant BDFE indicates that the dependence of ΔG°PT on pH49 enables ΔG°PT to be tuned via regulation of the acidity and basicity of proton donors and acceptors, respectively. The free energy relationship lends further support to the notion that the rate would exhibit distinct responses to alterations in both ΔG°PT and ΔG°ET. This notion can be adequately described using Marcus-type formulations50,51. Specifically, if ΔG°PT increases when ΔG°ET is already large, ΔG°CPET may approach the intrinsic barrier (λ) of the CPET, resulting in a rate that is near the peak of the Marcus parabola26,52. Note also that the irregular dependence of kapp on pH, combined with the pH-varying ΔG°PT, indicates changes in the base and oxidant strengths (ΔG°PT and ΔG°ET). Considering the structural diversity of the Cu-site, and the redox evolution of the site during the catalytic cycle (Figs. 1B and 4B), it is unsurprising that ΔG°PT and ΔG°ET vary, consistent with the fact that the valence state of the Cu-site switches between CuII and CuIII, thereby explaining the variations in ΔG°CPET caused by changes in both ΔG°PT and ΔG°ET. These variations are attributable to differences in base and oxidant strengths of the Cu-site. In particular, CuII and CuIII, which are Lewis acids, vary based on protonation and deprotonation interactions; ΔG°PT disproportionally influences kapp. Because pKa serves as an index of proton-donating ability, substantial changes in the Cu-site pKa during oxidation of CuII to CuIII would modify the relative contributions of ΔG°PT and ΔG°ET to ΔG°CPET. The linear ΔG°CPET could become non-monotonic with respect to pH, based on the co-existence of CuII and CuIII. Indeed, simultaneous deprotonation of CuII and CuIII is promoted at high pH ( ≥ 6.2). The titrated pKa of CuII is 6.45 ± 0.31 (Supplementary Fig. 38); the pKa of CuIII is presumably less than 7.0 (Supplementary Note 16). These disparate pKa values result in two very distinct effects on the ΔG°PT, influencing the CuII-to-CuIII PT responsible for formation of high-valent metal (–oxo) species (ΔG°PT1) (Eq. 8, Figs. 1B and 4B), and on the CuIII-to-CuII PT, triggering PhOH-to-PhO· oxidation (ΔG°PT2) (Eq. 3, Figs. 1A and 4A). Thus, in contrast to ΔG°PT1, which chemically favors a pH increase (from 6.0 to 7.0) (Supplementary Fig. 38 and Supplementary Note 17), ΔG°PT2 is chemically unfavorable within the same pH region (Supplementary Fig. 39). The contributions of ΔG°PT1 and ΔG°PT2 to PT-keq are combined when determining ΔG°PT; these combined contributions explain the negative α-scaling log(kapp) pH−1 correlation. Thus, ΔG°PT, rather than pH, controls the kinetics; ΔG°PT declines as the pH increases from 6.2 to 9.0. The linear correlation between kapp and ΔG°PT is a defining characteristic of an I-PCET reaction. Next, we considered the BDFE, which is conceptually analogous to the free energy sums of pKa and E° (the equilibrium potential at the standard state of proton activity, pH 0) plus the ΔG° for H+ + e– → H• (CG)17. We thus explored kapp behavior very close to the CuO pKa because if the surface Brønsted-basic site (i.e., surface hydroxides near CuII) served as a proton donor, PT would be exquisitely sensitive to pKa and the inflection point of the volcano-shaped activity profile would (approximately) equal the pKa17. As anticipated, the experimental pKa was 6.45 ± 0.31 (Supplementary Fig. 38), indicating that the surface Brønsted-basic hydroxide site (also termed the Lewis-acid site redox–linked PT component) participated in PCET in a manner analogous to that of enzyme-catalyzed reactions53. This finding confirmed that PT plays a pivotal role in the kinetics. The CuIII-to-CuII PT is the reverse of the above steps (Figs. 1A, 4A and Eq. 3). At higher pH values (≥ 6.2), unfavorable protonation of CuIII inhibits PT from PhOH (corresponding to PhOH-to-PhO·) (Supplementary Fig. 39). Thus, re-reduction of CuIII is regulated by PT. Note that calculation of ΔG°PT is not yet feasible; the structure and energetics of the transition-state CuIII remain unknown. Consequently, it is not possible to build a rigorous mechanistic model that accurately predicts kinetic trends. However, the primary I-PCET thermochemical parameter (i.e., the BDFE that dictates PCET reactivity) can be investigated via slow scan–rate cyclic voltammetry (CV)54 (Supplementary Fig. 40). The quasi-reversible Eeq plotted as a function of the applied pH yielded an intercept E° value of 0.405 ± 0.04 V versus a standard hydrogen electrode (SHE) at pH 0; the E° was then converted to a BDFE of 62.1 ± 1.4 kcal mol–1 using a more general form of thermochemical cycling23 (see Supplementary Note 18 for details) (Fig. 6A). Together, these outcomes substantiate the fact that PT is coupled with ET via redox Cu-sites, emphasizing the mechanistic attributes of I-PCET.
The thermodynamic analyses were further extended to NiIV = O and CoIV = O to emphasize the generality of I-PCET. The volcano-shaped kapp profiles versus pH (Figs. 2B and 2C) were fitted to extract the log-linear dependence of kapp on pH and log(kapp) pH−1 slopes. The values for NiIV = O are 0.33 ± 0.01 (pH 5.0 to 8.0) and –0.36 ± 0.02 (pH 8.0 to 8.8); for CoIV = O, these values are 0.31 ± 0.03 (pH 5.8 to 6.3) and –0.34 ± 0.02 (pH 6.3 to 9.0) (Fig. 6B, C). Notably, the Brønsted α values were considerably smaller than the anticipated 1/223,26, indicating that ΔG°CPET substantially increases and then gradually approaches the CPET λ predicted by Marcus theory23. However, the interpretation of such shallow slopes is unclear, although several recent rate/driving force studies of PCET also showed very small α-values55,56,57. The Bernasconi principle of non-perfect synchronization (NPS)58 assumes that fundamental reactions involving multiple concurrent processes (e.g., electron localization/delocalization and bonding/cleavage) may proceed via “unbalanced” or “asynchronous” transition states58,59,60. We thus suggest that the transition state of I-PCET involves concerted transfer of H+ and e–, but in an asynchronous manner. Then, a larger α leads to a more pronounced PT “character” of the transition state and a greater sensitivity to changes in PT-driving forces61,62. Accordingly, the PT characteristics of the rate-determining transition states may differ among NiIV = O, CoIV = O, and CuIII, reflecting variations in CPET synchronicity. The observed variations in α may indicate one- and/or two-electron valence interconversions at Lewis-acid metal sites. These include CuIII ⇄ CuII (1-e–), CoIV = O ⇄ CoIII/CoII (1- and 2-e–), and NiIV = O ⇄ NiII (2-e–) (switchable electronic states, or “redox isomers”), as well as Lewis-acid metal site–dependent valence tautomerism63 (with lesser ETs and relatively greater PTs leading to increased α values; for example, CuIII ⇄ CuII associated with more complete PT during CPET compared with CoIV = O ⇄ CoIII/CoII and NiIV = O ⇄ NiII). In contrast to CuIII, the volcano-shaped activity inflection point for NiIV = O occurs at pH 8.0 (Fig. 6B), which considerably differs from the pKa values of NiO (pKa1 = 4.29 ± 0.32, pKa2 = 9.10 ± 0.22) (Supplementary Fig. 41). Notably, the NiIV = O inflection point is very close to pHpzc (pHpzc = 8.15 ± 0.05 according to quantitative titration) (Supplementary Fig. 41); this similarity has not been previously reported. To illustrate the importance of the inflection point at pHpzc, the surface Brønsted acid–base characteristics of the model catalyst NiO were manipulated to obtain NiO-air (Supplementary Figs. 42, 43 and Supplementary Note 19), and a strikingly analogous outcome was observed (Fig. 6D). The pHpzc (7.55 ± 0.03 of NiO-air according to quantitative titration) affected the activity-inflection point pH (7.6) (Fig. 6D, Supplementary Figs. 42 and 43). The pHpzc also predicted the activity-inflection point pH of another model catalyst, Co3O4 (Supplementary Fig. 44). One possible explanation is that high-valent metal species require electronically stable oxygenic ligands (NiIV=O and CoIV = O), rather than less stable metal–oxo species (CuIII)45. Alternatively, the electrostatic effects of a double-layer charge structure may be involved. A deviation from the pHpzc pH triggered double-layer reorganization and thus shifts in ΔG°CPET64. Moreover, the BDFE values of NiO and Co3O4 were calculated to be 83.2 ± 1.2 and 83.8 ± 1.4 kcal mol–1, respectively (Figs. 6B, C, Supplementary Figs. 45 and 46), and a thermochemical framework is presented (Fig. 7), thereby emphasizing the explicit dependence on PT.
The PTET and ETPT pathways are situated in the upper and lower sections, respectively. The pathway bisecting the diagram is CPET, in which electrons and protons are transferred without the formation of intermediates. Note that schematic diagrams of the transition states of the interfacial reaction have been positioned at the corresponding angles on the circles (i.e., at 0°, 90°, 180°, and 270°).
To enhance the overall understanding of I-PCET via transition metal-mediated O–O bond activation, we now summarize the principal mechanistic features that distinguish I-PCET from well-studied reactions such as electrocatalytic PCET (E-PCET) (Supplementary Note 20), with a primary focus on proton activity (pH), particularly at the solid-water interface. One difference is the noncovalent, inner-sphere hydrogen-bonding interaction (Supplementary Fig. 47); the hydrogen bonding strength between a protic PMS-oxidant and a metal site limits the proton tunneling rate, thus affecting the generation of high-valent metal (–oxo) species. Furthermore, the I-PCET mechanism is based on the kinetic dependencies of the surface Brønsted acid–base parameters, particularly pKa and pHpzc. This relationship is primarily attributable to changes in free energy, which greatly affect keq. Additionally, in contrast to the electrified interface of E-PCET, where an aqueous electrochemical double layer controls the kinetics and thermodynamics of both PT and ET65, the solvation interface hosts the chemical potentials of protons and electrons; the potentials are tuned according to the redox potentials of the metal centers (CuIII, NiIV = O, and CoIV = O), thus favoring I-PCET. Finally, I-PCET is defined by concerted but asynchronous PT and ET. The difference in synchronicity of CPET is attributable to the valence tautomerism of the metal center.
To further extend polymerizations mediated by persulfate-based oxidation, we examined another typical aromatic, aniline. As expected, PT created organic radicals, followed by crosslinked and polymerization products (Supplementary Fig. 48). These data illustrate a broader range of organic contaminants that can be polymerized.
Discussion
This study highlights the pivotal roles of coupled protons in redox reaction activities at the solid-water interfaces of various persulfate-based, oxidative polymerization model systems. Careful acid–base titration analyses revealed a 1:1 proton:electron stoichiometry regarding the key fundamental components (PT and ET) of interfacial redox reactions. TMOs-mediated O–O bond activation proceeds via interfacial ET coupled to proton movement within a solvent, a complete PCET process. These findings greatly improve the overall understanding of interfacial redox activity. Proton and electron coupling determine the reaction rate. Such coupling removes the need for high-energy chemical intermediates23 and is thermodynamically favorable. Our findings suggest that reaction rates can be increased by modifying the force that drives interfacial PT through pH alteration during the overall PCET reaction. The data also indicate that the principal parameters of benchmark TMOs-based catalysts, namely the Lewis acid/base (electron withdrawing/donating) potentials and Brønsted acid/base (proton donor/acceptor) characteristics, govern I-PCET. This enables rational catalyst design. Further research is required to derive a thermochemical model that fully describes the relationship between the reaction rate and the driving force, particularly with respect to how coupled electrons and protons (i.e., net PCET) proceed across a solid-water interface. We suggest that analogous reactions are also significantly affected by the hitherto underappreciated reaction parameter of proton activity.
Methods
Chemicals
2,6-dimethylphenol (C8H10O, 99.0%), 2,6-dimethylaniline (C8H11N, 99.0%), methyl phenyl sulfone (C7H8O2S, 98.0%), methyl phenyl sulfoxide (C7H8OS, 98.0%), nitrobenzene (C6H5NO2, 99.0%), aniline (C6H7N, ≥ 99.5%), methanol (CH4O, 99.9%), acetonitrile (C2H3N, 99.9%), ferulic acid (C10H10O4, 99.0%), potassium ferricyanide (K3FeC6N6, ≥ 99.5%), 4-aminoantipyrine (C11H13N3O, 98.0%), sodium sulfate anhydrous (Na2SO4, 99.0%), boric acid (H3BO3, ≥ 99.5%), sodium tetraborate decahydrate (Na2B4O7·10H2O, 99.3%), glycine (C2H5NO2, ≥ 99.5%), amylose ((C6H10O5)n), potassium phosphate dibasic anhydrous (K2HPO4, 98.0%), benzoic acid (C7H6O2, ≥ 99.0%), p-phthalic acid (C8H6O4, 99.0%), tetrahydrofuran (C4H8O, ≥ 99.9%), potassium iodide (KI, 99.5%), carbon (C, 10–20 nm), Co3O4 (30 nm, 99.5%), sodium hydroxide (NaOH, 97.0%), potassium hydroxide (KOH, 95.0%), tetracycline (C22H24N2O8), sulfamethoxazole (C10H11N3O3S, 98.0%), and coumarin (C9H6O2, 98.0%) were procured from Shanghai Macklin Biochemical Co., Ltd, China. Peroxymonosulfate (2KHSO5·KHSO4·K2SO4, ≥ 42.0% KHSO5 basis), cupric chloride dihydrate (CuCl2·2H2O), and peroxydisulfate (K2S2O8, ≥ 99.0%) were procured from Shanghai Aladdin Biochemical Technology Co., Ltd, China. Ammonia solution (NH3·H2O, 25.0–28.0%), toluene (C6H5CH3, ≥ 99.5%), terephthalic acid (C8H6O4, 99.0%), sulfuric acid (H2SO4, 95.0–98.0%), and hydrochloric acid (HCl, 36.0–38.0%) were purchased from Chongqing Chuandong Chemical Co., Ltd, China, while phenol (C6H5OH, ≥ 99.0%) was procured from Chengdu Jingshan Chemical Test Co., Ltd, China. Barium chloride dihydrate (BaCl2·2H2O, ≥ 99.5%), sodium nitrate (NaNO3, ≥ 99%), tert-butyl alcohol (C4H10O, ≥ 99.0%), ammonium chloride (NH4Cl, ≥ 99.5%), and potassium dichromate (K2Cr2O7, ≥ 99.8%) were purchased from Chengdu Chron Chemical Co., Ltd, China. Tween-20 was procured from Tianjin Berens Biotechnology Co., Ltd, China. All chemicals were used as received without further purification, and deionized (DI) water (R = 18.25 MΩ) was employed in all the experiments.
Catalyst preparation
The metal oxide catalysts were synthesized through controlled precipitation and calcination methods (see Supplementary Synthesis for details of catalyst preparation). In summary, CuO was obtained by calcining Cu(OH)2 precursors, which were synthesized by reacting CuCl2 with NaOH at pH 9.0 (adjusted with ammonia solution) under stirring for 2.0 h. The resulting Cu(OH)2 precipitate underwent centrifugation, washing, freeze-drying, and calcination at 180 °C for 2.0 h in air. NiO was prepared from β-Ni(OH)2 precursors, synthesized by reacting NiCl2 with NaOH at pH 9.0 (adjusted with ammonia solution) and 90 °C for 2.0 h. The β-Ni(OH)2 was processed in a similar manner to the Cu(OH)2 and calcined at 450 °C for 5.0 h (with a heating rate of 3.0 °C min–1) in either an N2 or air atmosphere to produce NiO and NiO-air, respectively. Co3O4 (30 nm, 99.5%) was sourced from Shanghai Macklin Biochemical Co., Ltd, China.
Catalytic oxidation experiments
The catalytic oxidation experiments were investigated using a temperature-controlled system (25 ± 2 °C) with agitation in 150 mL beakers, with each experiment performed in duplicate. The reaction mixture comprised predetermined quantities of catalyst, target pollutant, and oxidants (PMS/PDS) in aqueous solution, maintaining a 2:1 molar ratio of oxidant to pollutant. The initial pH was modulated using borate or glycine buffers. During the reaction, 1.0 mL samples were extracted, immediately quenched with Na2S2O3, and filtered through membranes prior to analysis. Further details can be found in Supplementary Analysis.
Analytical methods
The Supplementary Methods detail a comprehensive suite of analytical methods, encompassing both qualitative and quantitative approaches: HPLC, TOC and COD measurements, TGA, EPR, LC-MS, MALDI-TOF-MS, GPC, UV-vis spectroscopy, iodometric method, BaCl2 turbidimetric method, electrochemical testing, KIE experiments, liquid-phase acid-base titration, and probing experiments.
Data availability
The data underpinning this study’s findings are presented in the main text and supplementary files, with source data provided alongside the paper. Raw data can be accessed by contacting the corresponding author. Source data are provided with this paper.
References
Larsen, T. A., Hoffmann, S., Luthi, C., Truffer, B. & Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 352, 928–933 (2016).
Pooi, C. K. & Ng, H. Y. Review of low-cost point-of-use water treatment systems for developing communities. Npj Clean. Water 1, 11 (2018).
Yu, Z. W. et al. Decoupled oxidation process enabled by atomically dispersed copper electrodes for in-situ chemical water treatment. Nat. Commun. 15, 1186 (2024).
Hodges, B. C., Cates, E. L. & Kim, J. H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Zhang, Y. J. et al. Metal oxyhalide-based heterogeneous catalytic water purification with ultralow H2O2 consumption. Nat. Water 2, 770–781 (2024).
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 1, 166–175 (2018).
Zhang, X. et al. Nanoconfinement-triggered oligomerization pathway for efficient removal of phenolic pollutants via a Fenton-like reaction. Nat. Commun. 15, 917 (2014).
Gao, X., Yang, Z. C., Zhang, W. & Pan, B. C. Carbon redirection via tunable Fenton-like reactions under nanoconfinement toward sustainable water treatment. Nat. Commun. 15, 2808 (2024).
Liu, H. Z. et al. Tailoring d-band center of high-valent metal-oxo species for pollutant removal via complete polymerization. Nat. Commun. 15, 2327 (2024).
Zhang, Y. J. et al. Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination. Nat. Commun. 13, 3005 (2022).
Zhang, D. P. et al. Dynamic active-site induced by host-guest interactions boost the Fenton-like reaction for organic wastewater treatment. Nat. Commun. 14, 3538 (2023).
Guo, Z. Y. et al. Electron delocalization triggers nonradical Fenton-like catalysis over spinel oxides. Proc. Natl Acad. Sci. 119, e2201607119 (2022).
Guo, Z. Y. et al. Crystallinity engineering for overcoming the activity-stability tradeoff of spinel oxide in Fenton-like catalysis. Proc. Natl Acad. Sci. 120, e2220608120 (2023).
Huang, M. et al. Facilely tuning the intrinsic catalytic sites of the spinel oxide for peroxymonosulfate activation: From fundamental investigation to pilot-scale demonstration. Proc. Natl Acad. Sci. 119, e2202682119 (2022).
Liu, L. D. et al. Nonradical activation of peroxydisulfate promoted by oxygen vacancy-laden NiO for catalytic phenol oxidative polymerization. Appl. Catal., B 2019 254, 166–173 (2019).
Yan, Y. Q. et al. Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes. Environ. Sci. Technol. 57, 12153–12179 (2023).
Mayer, J. M. Bonds over electrons: Proton coupled electron transfer at solid–solution interfaces. J. Am. Chem. Soc. 145, 7050–7064 (2023).
Schrauben, J. N. et al. Titanium and zinc oxide nanoparticles are proton-coupled electron transfer agents. Science 336, 1298–1301 (2012).
Warburton, R. E., Soudackov, A. V. & Hammes-Schiffer, S. Theoretical modeling of electrochemical proton-coupled electron transfer. Chem. Rev. 122, 10599–10650 (2022).
Rimgard, B. P. et al. Proton-coupled energy transfer in molecular triads. Science 377, 742–747 (2022).
Parada, G. A. et al. Concerted proton-electron transfer reactions in the Marcus inverted region. Science 364, 471–475 (2019).
Westendorff, K. S., Hülsey, M. J., Wesley, T. S., Román-Leshkov, Y. & Surendranath, Y. Electrically driven proton transfer promotes Brønsted acid catalysis by orders of magnitude. Science 383, 757–763 (2024).
Agarwal, R. G. et al. Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 122, 1–49 (2022).
Ren, W. et al. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes. Environ. Sci. Technol. 56, 78–97 (2021).
Huynh, M. H. V. & Meyer, T. J. Proton-coupled electron transfer. Chem. Rev. 107, 5004–5064 (2007).
Tyburski, R., Liu, T., Glover, S. D. & Hammarström, L. Proton-coupled electron transfer guidelines, fair and square. J. Am. Chem. Soc. 143, 560–576 (2021).
Lewis, N. B., Bisbey, R. P., Westendorff, K. S., Soudackov, A. V. & Surendranath, Y. A molecular-level mechanistic framework for interfacial proton-coupled electron transfer kinetics. Nat. Chem. 16, 343–352 (2024).
Wei, Y. et al. Ultrahigh peroxymonosulfate utilization efficiency over CuO nanosheets via heterogeneous Cu(III) formation and preferential electron transfer during degradation of phenols. Environ. Sci. Technol. 56, 8984–8992 (2022).
Zhang, M. K. et al. How buffers resist electrochemical reaction-induced pH shift under a rotating disk electrode configuration. Anal. Chem. 93, 1976–1983 (2021).
Parvulescu, V. I., Epron, F., Garcia, H. & Granger, P. Recent progress and prospects in catalytic water treatment. Chem. Rev. 122, 2981–3121 (2021).
Ren, W. et al. Electro-induced carbon nanotube discrete electrodes for sustainable persulfate activation. Environ. Sci. Technol. 56, 14019–14029 (2022).
Jing, Y. & Chaplin, B. P. Mechanistic study of the validity of using hydroxyl radical probes to characterize electrochemical advanced oxidation processes. Environ. Sci. Technol. 51, 2355–2365 (2017).
Zhang, T. et al. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 48, 5868–5875 (2014).
Chen, T. S. et al. Neutral phenolic contaminants are not necessarily more resistant to permanganate oxidation than their dissociated counterparts: Importance of proton-coupled electron transfer. Environ. Sci. Technol. 57, 17620–17628 (2023).
Leresche, F., Ludvíková, L., Heger, D., Gunten, U. & Canonica, S. Quenching of an aniline radical cation by dissolved organic matter and phenols: a laser flash photolysis study. Environ. Sci. Technol. 54, 15057–15065 (2020).
Wang, L. H. et al. Trace cupric species triggered decomposition of peroxymonosulfate and degradation of organic pollutants: Cu(III) being the primary and selective intermediate oxidant. Environ. Sci. Technol. 54, 4686–4694 (2020).
Wang, Y. et al. Natural polyphenols enhanced the Cu(II)/peroxymonosulfate (PMS) oxidation: the contribution of Cu(III) and OH·. Water Res. 186, 116326 (2020).
Edwards, S. J., Soudackov, A. V. & Hammes-Schiffer, S. Analysis of kinetic isotope effects for proton-coupled electron transfer reactions. J. Phys. Chem. A. 113, 2117–2126 (2009).
Glover, S. D., Parada, G. A., Markle, T. F., Ott, S. & Hammarström, L. Isolating the effects of the proton tunneling distance on proton-coupled electron transfer in a series of homologous tyrosine-base model compounds. J. Am. Chem. Soc. 139, 2090–2101 (2017).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Wu, Q. Y., Yang, Z. W., Wang, Z. W. & Wang, W. L. Oxygen doping of cobalt-single-atom coordination enhances peroxymonosulfate activation and high-valent cobalt–oxo species formation. Proc. Natl Acad. Sci. 120, e2219923120 (2023).
Darcy, J. W., Koronkiewicz, B., Parada, G. A. & Mayer, J. M. A continuum of proton-coupled electron transfer reactivity. Acc. Chem. Res. 51, 2391–2399 (2018).
Mahdavi-Shakib, A. et al. The role of surface hydroxyls in the entropy-driven adsorption and spillover of H2 on Au/TiO2 catalysts. Nat. Catal. 6, 710–719 (2023).
Szekeres, M. & Tombácz, E. Surface charge characterization of metal oxides by potentiometric acid–base titration, revisited theory and experiment. Colloids Surf. A Physicochem. Eng. Asp. 414, 302–313 (2012).
Larson, V. A., Battistella, B., Ray, K., Lehnert, N. & Nam, W. Iron and manganese oxo complexes, oxo wall and beyond. Nat. Rev. Chem. 4, 404–419 (2020).
Sayfutyarova, E. R., Lam, Y. C. & Hammes-Schiffer, S. Strategies for enhancing the rate constant of C–H bond cleavage by concerted proton-coupled electron transfer. J. Am. Chem. Soc. 141, 15183–15189 (2019).
Hammes-Schiffer, S. Proton-coupled electron transfer: Moving together and charging forward. J. Am. Chem. Soc. 137, 8860–8871 (2015).
Noh, H. & Mayer, J. M. Medium-independent hydrogen atom binding isotherms of nickel oxide electrodes. Chem. 8, 3324–3345 (2022).
Chen, C. L., Chen, Y. T., Demchenko, A. P. & Chou, P. T. Amino proton donors in excited-state intramolecular proton-transfer reactions. Nat. Rev. Chem. 2, 131–143 (2018).
Liu, T. F. et al. elucidating proton-coupled electron transfer mechanisms of metal hydrides with free rnergy-and pressure-dependent kinetics. J. Am. Chem. Soc. 141, 17245–17259 (2019).
Bourrez, M., Steinmetz, R., Ott, S., Gloaguen, F. & Hammarström, L. Concerted proton-coupled electron transfer from a metal-hydride complex. Nat. Chem. 7, 140–145 (2015).
Marcus, R. A. & Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 811, 265–322 (1985).
Chen, K. S. et al. Atomically defined mechanism for proton transfer to a buried redox centre in a protein. Nature 405, 265–322 (2000).
Samantaray, Y., Martin, D. J., Agarwal, R. G., Gibson, N. J. & Mayer, J. M. Proton-coupled electron transfer of cerium oxide nanoparticle thin-film electrodes. J. Phys. Chem. C. 127, 4015–4020 (2023).
Qiu, G. Q. & Knowles, R. R. Rate–driving force relationships in the multisite proton-coupled electron transfer activation of ketones. J. Am. Chem. Soc. 141, 2721–2730 (2019).
Markle, T. F., Marcy, J. W. & Mayer, J. M. A new strategy to efficiently cleave and form C–H bonds using proton-coupled electron transfer. Sci. Adv. 4, eaat5776 (2018).
Darcy, J. W., Kolmar, S. S. & Mayer, J. M. Transition state asymmetry in C–H bond cleavage by proton-coupled electron transfer. J. Am. Chem. Soc. 141, 10777–10787 (2019).
Bernasconi, C. F. The principle of imperfect synchronization: I. Ionization of carbon acids. Tetrahedron 41, 3219–3234 (1985).
Bernasconi, C. F. The principle of nonperfect synchronization: more than a qualitative concept? Acc. Chem. Res. 25, 9–16 (1992).
Bím, D., Maldonado-Domínguez, M., Rulíšek, L. & Srnec, M. Beyond the classical thermodynamic contributions to hydrogen atom abstraction reactivity. Proc. Natl Acad. Sci. 115, E10287–E10294 (2018).
Jencks, W. P. A primer for the Bema Hapothle. an empirical approach to the characterization of changing transition-state structures. Chem. Rev. 85, 511–527 (1985).
Teindl, K., Patrick, B. O. & Nichols, E. M. Linear free energy relationships and transition state analysis of CO2 reduction catalysts bearing second coordination spheres with tunable acidity. J. Am. Chem. Soc. 145, 17176–17186 (2023).
Raczyńska, E. D., Kosińska, W., Ośmiałowski, B. & Gawinecki, R. Tautomeric equilibria in relation to pi-electron delocalization. Chem. Rev. 105, 3561–3612 (2005).
Li, P. et al. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 5, 900–911 (2022).
Swift, M. W., Swift, J. W. & Qi, Y. Modeling the electrical double layer at solid-state electrochemical interfaces. Nat. Comput. sci. 1, 212–220 (2021).
Acknowledgements
This research was funded by the Natural Science Foundation of Chongqing (grant CSTB2022NSCQ-MSX0448 to L.D.L.), the Sichuan Science and Technology Program (grant 2023NSFSC0801 to L.D.L.), and the Chongqing Municipal Education Commission (grants KJQN202200202 and KJQN202100214, both to L.D.L.).
Author information
Authors and Affiliations
Contributions
This study was conceptualized, designed, and supervised by L.D.L., with J.H.C., W.T.L., and K.Y.C. managing data curation and J.H.C., W.T.L., and L.D.L. conducting formal analysis. The investigation was carried out by J.H.C., W.T.L., K.Y.C., H.J.T., Z.T.L., and S.A., while L.D.L., W.T.L., and J.H.C. developed the methodology. Validation was performed collaboratively by J.H.C., W.T.L., K.Y.C., H.J.T., Z.T.L., S.A., and L.D.L. The original manuscript was co-written by L.D.L., J.H.C., and W.T.L., with L.D.L. conducting the review and editing. All authors participated in the results discussion and manuscript development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shujuan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, JH., Li, WT., Cai, KY. et al. Proton-coupled electron transfer controls peroxide activation initiated by a solid-water interface. Nat Commun 16, 3789 (2025). https://doi.org/10.1038/s41467-025-58917-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58917-w