Abstract
Tendon injuries are frequently occurring disorders; it is clinically important to enhance tendon regeneration and prevent functional impairment post-injury. While tendon injuries in children heal quickly with minimal scarring, those in adults heal slowly and are accompanied by fibrotic scarring. Therefore, investigating the healing mechanisms after tendon injury, and identifying the factors that regulate the inherent regenerative capacity of tendons are promising approaches to promoting tendon regeneration. Here, we identify that the PI3K-Akt signalling pathway is preferentially upregulated in injured neonatal murine Achilles tendons. Inhibition of PI3K-Akt signalling in a neonatal murine Achilles tendon rupture model decreases cell proliferation and migration in both Scx-lineage intrinsic tenocytes and Tppp3-lineage extrinsic paratenon sheath cells. Moreover, the inhibition of PI3K-Akt signalling decreases stemness and promotes mature tenogenic differentiation in both Scx- and Tppp3-lineage cells. Collectively, these results suggest that PI3K-Akt signalling plays a pivotal role in neonatal tendon regeneration.
Similar content being viewed by others
Introduction
Tendon injuries are a frequent disorder that affects 16 million people per year in the United States1. It is clinically important to promote tendon regeneration to prevent functional impairment after injury. Tendons are dense connective tissues that connect muscles to bones and conduct muscle force to the bones2. Furthermore, tendons mainly comprise longitudinally aligned type 1 collagen, which can withstand strong mechanical loading3. Conversely, tendons have low cell density, poor vascularity, and slow cell division; therefore, injured tendons tend to heal more slowly than other tissues4. In addition, tendon injuries in adults often heal with fibrotic scarring, which is cumbersome due to its low physical strength that can lead to re-rupture and adhesions to surrounding tissues. In such cases, reconstructive surgery is required, which can lead to poor clinical outcomes such as pain and impaired mobility5. Conversely, tendon injuries in children heal quickly, with minimal scarring, and they are less likely to re-rupture or adhere6,7. Similar to humans, comparative studies on adult and neonatal mice have shown that neonatal mice acquire better tendon healing than adults; the former cured with intrinsic tenocytes, whereas the latter cured with scarring by extrinsic fibrotic cells8. Thus, the comparative analysis of the healing mechanisms following tendon injury, which undergo age-related changes, between adult and neonatal mice and the identification of factors governing the intrinsic regenerative potential in neonatal tendons, could offer a promising avenue for the development of innovative therapies aimed at enhancing tendon regeneration.
It is widely accepted that many cell types are involved in tissue regeneration after traumatic injuries, such as bone fractures and nerve injuries9,10. Similarly, intrinsic tendon cell populations, such as tenocytes, and extrinsic cell populations, such as tendon sheath-synovial cells, nontendinous mesenchymal stem cells (MSCs), and inflammatory cells, have been shown to play a major role in healing after tendon injury; however, their localisation and function remain controversial8,11,12,13. Tenocytes are usually a homogeneous population positive for Scleraxis (Scx)13. Scx, a basic helix-loop-helix transcription factor that regulates collagen and extracellular matrix gene expression, is essential for normal tendon development and differentiation14,15,16. Tendon regeneration was evaluated by visualising tendon cells in ScxGFP transgenic mice17,18 and Scx-EGFP knock-in mice19. However, it is challenging to distinguish between tenocyte-mediated intrinsic tendon regeneration and extrinsic regeneration due to the tenogenic differentiation of surrounding cells. Recently, lineage tracing using the Scx-CreERT2 mouse model showed that Scx-lineage cells proliferate and migrate to bridge the injured tendon gap, indicating that Scx-lineage intrinsic tenocytes contribute to tendon regeneration8,13,20,21. In contrast, Tppp3, a member of the tubulin polymerisation-promoting protein family, is specifically expressed in the tendon sheath, epitenon, and paratenon cells22; lineage tracing using the Tppp3-CreERT2 mouse model has shown that Tppp3-lineage cells differentiate into tenocytes, contributing to extrinsic tendon regeneration12. Furthermore, alpha-smooth muscle actin (αSMA) is expressed in myofibroblasts that appear early after tendon injury2 but is also expressed in resident tendon progenitor cells, including tendon fibroblasts within the uninjured tendon substance and paratenon/sheath/reticular cells23. These αSMA positive (+) progenitor cell populations reportedly proliferate and differentiate into Scx+ cells upon tendon injuries23. Therefore, both intrinsic and extrinsic cell populations are essential for tendon regeneration, and the mechanisms and factors that control these cell populations drive the physiological regenerative potential of neonatal tendons. Understanding the spatiotemporal role of these cell populations in physiological tendon regeneration is key to establishing novel tendon regeneration therapies.
To date, several signalling pathways regulating tendon regeneration have been identified in murine models of tendon injury. These include TGF-β20, IGF1-PI3K-Akt, and IGF1-ERK signalling24 in tenocytes, as well as Hedgehog25 and PDGF signalling in tendon sheath cells26. Tendons are force-transmitting tissues that contain abundant extracellular matrix, and extracellular stimuli are important for regulating their integrity. It has been shown that mechanical load induces the expression of tenogenic markers and collagen I through integrin β1-ILK-Akt-mTOR27 and PI3K-Akt signalling activation28. Furthermore, mechanical load induces tenogenic differentiation of MSCs through FAK signalling29. Since ILK and FAK are upstream of PI3K-Akt27,30, PI3K-Akt signalling could be implicated in tendon regeneration.
In this study, we investigate the mechanism underlying the physiological regulation of tendon regeneration in neonatal mice, aiming to determine how these mechanisms regulate intrinsic and extrinsic cell populations involved in tendon regeneration. Using RNA-sequencing analyses, we identify that PI3K-Akt signalling is specifically upregulated in injured neonatal murine Achilles tendons. In vitro treatment of tenocytes and paratenon sheath cells with a PI3K-Akt signalling inhibitor results in decreased cell proliferation, cell migration, and self-renewal while increasing tendon differentiation. Next, we induce PI3K-Akt signalling inhibition in a neonatal murine Achilles tendon rupture model using a PI3K inhibitor and lineage-specific overexpression of phosphatase and tensin homologue (Pten), which is a main negative regulator of PI3K-Akt signalling pathway31. This results in decreased cell proliferation and migration to the regenerating tendon in both Scx- and Tppp3-lineage cells. Interestingly, Tppp3-lineage cells are the primary cell source for regenerating tendons. Suppression of PI3K-Akt signalling in Tppp3-lineage cells specifically results in the thinning of regenerating tendons while an increase in thick fibres, which indicate mature collagen production, suggesting that suppression of PI3K-Akt signalling reduces the stemness and promotes the differentiation of tendon-regenerating cells. Collectively, PI3K-Akt signalling plays a pivotal role in physiological tendon regeneration in neonates by regulating the cell proliferative capacity, cell migration, and stemness of both Scx-lineage intrinsic tenocytes and Tppp3-lineage extrinsic paratenon sheath cells.
Results
PI3K-Akt signalling pathway is activated following tendon injury in neonatal mice
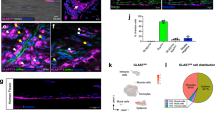
Given that neonatal mice can functionally recover and regenerate injured tendons via tendon differentiation to a greater extent than adult mice8, we attempted to identify the molecular regulatory mechanisms underlying tendon regeneration by comparing and evaluating gene expression in uninjured and injured tendons of neonatal (postnatal day 7, P7d) and adult (postnatal month 6, P6m) mice. P7d and P6m Scx-EGFP mice underwent Achilles tenotomy on the right hind limb (injured tendon) and sham operation on the left hind limb (uninjured tendon). Five days later, the Achilles tendons were harvested, and their gene expression was analysed by RNA-sequencing (Fig. 1a). In the injured tendons of neonatal mice, 134 and 297 genes were significantly upregulated and downregulated, respectively (Supplementary Fig. 1a, b). In the injured tendons of adult mice, 202 and 243 genes were significantly upregulated and downregulated, respectively (Supplementary Fig. 1a, b). KEGG pathway analysis revealed 29 and 77 pathways that were significantly elevated in neonatal and adult injured tendons, respectively (Fig. 1b). Among them, 24 pathways were shared between neonatal and adult mice. Among the five pathways specifically upregulated in neonatal injured tendons, we focused on the PI3K-Akt signalling pathway (Fig. 1b, c). Comparison of gene expression between injured and uninjured neonatal tendons revealed that 34 PI3K-Akt signalling pathway-related genes were elevated by more than 2-fold in injured neonatal tendons (Supplementary Fig. 1b). Although these 34 genes were also upregulated in the adult tenotomy group, the heat map showed that their expression patterns differed between the neonatal and adult tenotomy groups (Supplementary Fig. 1c). Consistent with the RNA-sequencing results, western blot analysis showed higher levels of phosphorylated Akt (pAkt) in tendon tissue collected 5 days after Achilles tenotomy in neonatal mice than in adult mice (Fig. 1d, e). Moreover, immunofluorescence showed strong pAkt expression in the tendon stub of neonatal mice 5 days after Achilles tenotomy (Fig. 1f). These results suggested that PI3K-Akt signalling is more remarkably activated in neonatal mice than in adult mice after tendon injury.
a Schematic of the sample collection protocol for RNA-sequence analysis. The 7-day-old (P7d) or 6-month-old (P6m) Scx-EGFP mice underwent Achilles tenotomy on the right hind limb (tenotomy group) and a sham operation (sham group) on the left hind limb. Injured and uninjured Achilles tendons were collected 5 days later. Five to eleven individual samples were pooled in each group and used in the RNA-sequencing analysis (7-day sham, n = 11; 7-day cut, n = 11; 6-month sham, n = 5; 6-month cut, n = 9). b KEGG pathway analyses for the neonatal and adult tenotomy groups; 29 and 77 upregulated pathways were identified in neonatal and adult tenotomy groups, respectively. A Venn diagram comparing the upregulated pathways between the neonatal and adult tenotomy groups shows five specifically upregulated pathways in neonatal mice. c The five specifically upregulated pathways in the neonatal tenotomy group. d Western blot analysis of injured neonatal (P7d) and adult (P6m) tendons on post-tenotomy day 5. Three independent samples were analysed in neonatal and adult mice. e Quantification of the pAkt/Akt ratio shown in (d). Akt phosphorylation (pAkt) was higher in neonates than in adults. Data are shown as the mean ± SEM of three independent samples in each group (two-tailed Student’s t test; P = 0.0344). f Haematoxylin-eosin staining (H&E) and fluorescent immunohistochemistry of pAkt in the injured neonatal (P7d) and adult (P6m) tendons on post-tenotomy day 5. The black square areas in the left panels are enlarged. Strong pAkt expression was observed at the distal stub of the neonatal injured tendon. Scale bars, 200 μm and 50 μm.
PI3K-Akt signalling pathway affects tenocyte proliferation, migration, and stemness in vitro
The PI3K-Akt signalling pathway reportedly enhances cell proliferation and increases cell migration32,33,34,35,36. Tenocytes are a major cell source for tendon regeneration and play a crucial role in physiological tendon regeneration via intrinsic healing in neonatal mice8. To determine whether PI3K-Akt signalling is involved in physiological tendon regeneration in neonatal mice, we investigated the role of PI3K-Akt signalling in tenocytes in vitro, including proliferation, migration, stemness, and differentiation. We treated rat primary tenocytes with ZSTK474 (ZSTK), a PI3K inhibitor, to suppress PI3K-Akt signalling. ZSTK treatment strongly inhibited Akt phosphorylation in tenocytes (Supplementary Fig. 2). Immunocytochemical staining showed a decrease in the Ki67+ cell ratio in the ZSTK group, indicating a decreased cell proliferative capacity (Fig. 2a, b). The scratch and transwell migration assays revealed a decrease in cell migration potential in the ZSTK group (Fig. 2c–f). In addition, the CFU assay showed decreased colony numbers in the ZSTK group, indicating reduced self-renewal capacity (Fig. 2g, h). Notably, among the stem cell markers Nestin (Nes) and Cd44, which are expressed in tenocyte-derived tendon stem/progenitor cells (TSPCs)37,38,39,40,41,42,43,44, Nes expression was significantly decreased in the ZSTK group (Fig. 2i). Moreover, ZSTK treatment of tenocytes demonstrated that tenogenic markers, Scx, Mkx, Tnmd, Col1a1, and Col3a1, were significantly upregulated (Fig. 2i). In contrast, ZSTK treatment moderately reduced Sox9 and Acan expression, and had no significant impact on the expression of Col2a1 and Col10a1, showing no clear tendency for chondrogenic differentiation by PI3K-Akt signalling inhibition (Fig. 2i). Thus, PI3K-Akt signalling may be involved in the control of proliferation, migration, and stemness in the tenogenic differentiation of tenocytes.
a Immunocytochemical staining using a Ki67 antibody for tenocytes treated with vehicle (control; Ctrl) or the PI3K inhibitor ZSTK474 (ZSTK). b Ki67 positive cell ratio of tenocytes in (a) (n = 3 independent experiments). The ZSTK group showed significantly decreased cell proliferation compared with the control group (two-tailed Student’s t test; p = 0.0095). c The scratch test was used to assess the migration capacity of tenocytes treated with vehicle or ZSTK. d Quantification of the wound healing rate shown in (c) (n = 6 independent experiments). The ZSTK group showed significantly decreased cell migration (two-tailed Student’s t test; P < 0.0001 at 12 h, and P < 0.0001 at 24 h). e Transwell assay was used to assess the migration capacity of tenocytes after treatment with vehicle or ZSTK. Analyses were performed 24 h after seeding. f Quantification of migrated cells shown in (e) (n = 6 independent experiments). The ZSTK group showed significantly decreased cell migration (two-tailed Student’s t-test; p < 0.0001). g CFU-F assay was used to assess the self-renewal capacity of tenocytes treated with vehicle or ZSTK. Analyses were performed 7 days after seeding. h Quantification of colony formation unit numbers shown in (g) (n = 3 independent experiments). The ZSTK group showed significantly decreased colony formation compared to the control group (two-tailed Student’s t test; p = 0.0002). i RT-qPCR was used to compare RNA expression in tenocytes between the control and ZSTK groups. Nes expression was significantly decreased in the ZSTK group. Expression of tenocyte tendon differentiation markers (Scx, Mkx, Tnmd, Col1a1, and Col3a1) was significantly increased in the ZSTK group. Expression of chondrogenic markers (Sox9 and Acan) was significantly decreased in the ZSTK group (two-tailed Student’s t-test, n = 8 independent experiments). *Data are presented as mean ± SEM. Scale bars, 100 μm (a), 200 μm (c), 400 μm (e).
PI3K-Akt signalling is involved in tenocyte proliferation and migration in the early regenerative phase in vivo
Next, we generated tenocyte-specific reporter mice, Scx-CreERT2 (Scx-CE); Rosa26-tdTomato (R26-tdT), to label Scx-expressing cells as red (Supplementary Fig. 3a–h), and investigated the role of the PI3K-Akt signalling pathway in tenocytes [Scx-lineage (Scxlin) cells] in vivo during tendon regeneration after Achilles tenotomy. Tamoxifen was injected three times at P5–7 d, and Achilles tenotomy was performed 72 h later. ZSTK was administered and evaluated 2 h, 3 d, and 28 d after Achilles tenotomy (Fig. 3a). Scxlin cells were detected in the uninjured Achilles tendons (Supplementary Fig. 3g, h). A previous report indicated that Scxlin cell apoptosis at the tendon stub occurs 2 h after Achilles tenotomy45. PI3K has been reported to inhibit apoptosis by increasing the expression of anti-apoptotic genes, such as Bcl2 and XIAP46. However, analyses conducted 2 h after Achilles tenotomy showed that the area of tdTomato+ cells (Scxlin cell area) was reduced due to apoptosis in both the control and ZSTK groups. There was no significant difference in the number of TUNEL+ cells at the tendon stub between the two groups, indicating that PI3K-Akt signalling does not prevent apoptosis of Scxlin cells following neonatal tendon rupture (Fig. 3b, c). Three days after Achilles tenotomy, the number of Ki67+ cells in the tendon stub was significantly lower in the ZSTK group than in the control group (Fig. 3d, e). Additionally, in the control group, the tdTomato- cell area at the tendon stub caused by apoptosis 2 h after tenotomy was replenished with Scxlin cells (Fig. 3d). In contrast, in the ZSTK group, Scxlin cells did not populate to the tendon tips (Fig. 3d), and a significant decrease in the tdTomato+ cell area at the tendon stub was observed (Fig. 3f). These results indicate that the proliferation and migration capabilities of Scxlin cells were suppressed by the inhibition of PI3K-Akt signalling.
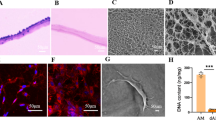
a Experimental scheme for evaluating tendon regeneration in vivo. Scx-CreERT2; Rosa26-tdTomato mice were injected with 0.075 mg of tamoxifen three times. Tamoxifen was washed out for 72 h, and tenotomy was performed (P 10-day). Vehicle (control; Ctrl) or ZSTK474 (ZSTK) was administered and evaluated 2 h, 3 d, and 28 d later. b Haematoxylin-eosin staining (H&E) and TUNEL staining of the Achilles tendon stub 2 h after tenotomy. Scx-lineage cells [tdTomato-positive (tdTomato+) cells] were stained with an RFP antibody. White dotted lines indicate the distal tendon stub. c Quantification of TUNEL-positive (TUNEL+) cells shown in (b). Data are shown as the mean ± SEM of three independent biological samples (three independent sections per mouse). A two-tailed Student’s t-test was used for statistical analyses. There was no significant difference in the number of apoptotic cells in tendon stubs between the control and ZSTK groups. d H&E and immunofluorescence staining using RFP and Ki67 antibodies for the Achilles tendon stub was performed 3 days post-tenotomy. The distal tendon stub is indicated by white dotted lines, while the tdTomato+ tendon area is indicated by yellow dotted lines. e Quantification of the percentage of Ki67 positive (Ki67+) cells shown in (d). Data are shown as the mean ± SEM of three independent biological samples (three independent sections per mouse). The percentage of Ki67+ cells significantly decreased in the ZSTK group (two-tailed Student’s t-test; p = 0.0439). f Quantification of the area of the tendon with tdTomato-positive (tdTomato+) cells shown in (d). Data are shown as the mean ± SEM of three independent biological samples (three independent sections per mouse). The ZSTK group showed a significantly smaller tdTomato+ area at the tendon stub than the control group (two-tailed Student’s t-test; p = 0.0003), indicating that the migration of tdTomato+ cells decreased. g Haematoxylin-eosin staining (H&E), Safranin O and fast green staining (SaO+FG), and Picrosirius Red (PSR) staining of Achilles tendons 28 days after tenotomy. The regenerated tendons and intact plantaris tendons are indicated by white dotted lines and black asterisks, respectively. h Magnified images of the black dotted squares in (g). i Quantification of neotendon thickness shown in (g). Data are shown as the mean ± SEM of four independent biological samples (two independent sections per mouse). The transverse diameter of the thickest part of the regenerated tendon, excluding distal and proximal tendon stubs, was measured as neotendon thickness. The ZSTK group showed a significant decrease in neotendon thickness compared with the control group (two-tailed Student’s t-test; p = 0.0232). j Percentage of thick fibres in the neotendon. The thick fibre area was measured in PSR staining sections. Data are shown as the mean ± SEM of four independent biological samples (two independent sections per mouse). The ZSTK group had a significantly higher percentage of thick fibres than the control group (two-tailed Student’s t-test; p = 0.0064). k Immunofluorescence staining was performed using an RFP antibody at the boundary between the regenerated tendon and the remaining distal tendon stub at 28 days after tenotomy. Lower migration of tdTomato+ cells into the regenerated tendons was observed in the ZSTK group than in the control group. CA; calcaneus. l Quantification of the distance of proximally migrating tdTomato-positive (tdTomato+) cells in neotendons shown in (k). The distance between the most proximal tdTomato+ cell and the calcaneus was measured. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The distance in the ZSTK group was significantly smaller than that in the control group (two-tailed Student’s t-test; p = 0.0311). m Quantification of the area with tdTomato+ cells shown in (k). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The area of tdTomato+ cells in the ZSTK group was significantly smaller than that in the control group (two-tailed Student’s t-test; p = 0.0309). n Immunofluorescence staining using the Col2 antibody. Yellow dotted circles indicate Col2 positive (Col2+) chondrometaplastic regions in regenerated tendons. o Quantification of the number of Col2+ cells shown in (n). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The number of Col2+ cells significantly decreased in the ZSTK group compared to the control groups (two-tailed Mann Whiteny-U test, p = 0.0286). p Immunofluorescence staining using the S100b antibody. Yellow dotted circles indicate S100b positive (S100b+) chondrometaplastic regions in regenerated tendons. q Quantification of the number of S100b+ cells shown in (p). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The number of S100+ cells significantly decreased in the ZSTK group compared to the control groups (two-tailed Student’s t-test, p = 0.0461). r Transmission electron microscope (TEM) pictures showing collagen fibrils in the neotendon at 28 days after tenotomy. s Distribution of the collagen fibril diameter in the neotendon. The diameter of 1000 collagen fibrils was measured in TEM pictures shown in (r) (three independent biological samples in each group). Collagen fibril diameter in the ZSTK group tends to be larger than that in the Ctrl group. * Scale bars, 100 nm (s), 50 μm (n, p), 100 μm (d, h), 200 μm (b, k), 400 μm (g).
PI3K-Akt signalling is involved in regenerating tendon thickening, and functional and mechanical properties in the late regenerative phase after tendon injury in vivo
At 28 days post-tenotomy, fluorescence stereomicroscopic analysis showed an enhanced tdTomato fluorescence signal in the Achilles tendon stub of the control group, whereas the signal was weak in the Achilles tendon stub of the ZSTK group (Supplementary Fig. 4a). Histological images showed that the neotendons were significantly thinner in the ZSTK group, whereas picrosirius red staining showed larger thick fibre area in the ZSTK group, indicating mature collagen production (Fig. 3g–j). In addition, compared to the control group, the distance of proximally migrating tdTomato+ cells and the area of tdTomato+ cells in neotendons were significantly reduced in the ZSTK group, indicating decreased proliferation and migration of Scxlin cells (Fig. 3k–m). Furthermore, the Col2+ and S100b+ cell number was significantly decreased in the ZSTK group, suggesting that the inhibition of PI3K-Akt signalling impaired chondrometaplasia formation (Fig. 3n–q). Intriguingly, consistent with the results of picrosirius red staining, transmission electron microscope (TEM) analyses showed that the fibril diameter of neotendons was larger in the ZSTK group than in the control group (Fig. 3r, s).
Functionally, ZSTK group showed significantly shorter running distance than control group (Fig. 4a, Supplementary Fig. 4b). Computer gait analyses using the ALMA (Automated Limb Motion Analysis) toolbox47 which is based on “DeepLabCut48” showed that the ankle joint amplitude of injured hindlimbs was restored to levels comparable to the intact hindlimbs in the control group, whereas ankle joint amplitude of injured hindlimbs significantly decreased in comparison with those of intact hindlimbs in the ZSTK group (Fig. 4b, c). Furthermore, although stride length and gait cycle duration were restored in both groups, the stance phase of the injured hindlimbs in the ZSTK group remained significantly prolonged compared to that in the control group (Fig. 4d). Biomechanical testing showed that the tensile strength and stiffness of neotendons in the ZSTK group were significantly lower than those in the control group (Fig. 4e). These results indicate that prolonged inhibition of PI3K-Akt signalling results in suboptimal functional and mechanical regeneration of injured tendons while reducing chondrometaplasia and increasing the collagen fibril diameter in neotendons.
a Running distance of the mice at 28 days after tenotomy. The mice in the ZSTK group showed significantly shorter running distances than those in the Ctrl group (Ctrl, n = 6; ZSTK, n = 5) (two-tailed Student’s t-test, p = 0.0087). b Treadmill videos were recorded using an iPhone positioned parallel to the treadmill belt. According to the Automated Limb Motion Analysis (ALMA) method47, the coordinates of six hindlimb joints (iliac crest, hip, knee, ankle, metatarsophalangeal (MTP) joint, and toe) were tracked in the treadmill kinematic paradigm, and the coordinates were processed using the ALMA toolbox to generate hindlimb trajectories. c Ankle joint amplitude (range of motion angles) during running at 28 days after tenotomy. In the ZSTK group, the angle on the injured side was significantly decreased compared to that on the intact side (p = 0.0081). In contrast, in the Ctrl group, the angle on the injured side remained unchanged compared to that on the intact side (p = 0.3479). Moreover, the angle on the injured side in the ZSTK group was significantly smaller than that on the injured side the Ctrl group (p = 0.0005) (Ctrl, n = 16; ZSTK, n = 12, two-way ANOVA test). d Gait analyses at 28 days after tenotomy. Stride length and cycle duration were comparable between the two groups. Compared to the Ctrl group, the stance phase of the injured side was significantly prolonged, and the swing phase was significantly shortened in the ZSTK group (Ctrl, n = 16; ZSTK, n = 12) (two-way ANOVA test, p = 0.0102 and 0.0102, respectively). e Biomechanical test of the regenerated tendons at 28 days after tenotomy. Tensile strength (N) and stiffness (N/mm) in the ZSTK group were significantly lower compared to the Ctrl group (Ctrl, n = 8; ZSTK, n = 7) (two-tailed Student’s t-test, p = 0.0335 and 0.0198, respectively).
PI3K-Akt signalling pathway is involved in Scx-lineage cell migration, proliferation, and chondrometaplasia in vivo
Pharmacological inhibition of PI3K-Akt signalling by ZSTK administration may affect cell types other than Scxlin cells in tendon regeneration. Therefore, we investigated the role of the PI3K-Akt signalling pathway in the intrinsic healing of Scxlin cells by lineage-specific inhibition of the PI3K-Akt signalling pathway. Conditional Pten-overexpressing mice, Rosa26-loxP-stop-loxP-Pten (R26-LSL-Pten), were generated (Supplementary Fig. 5a–e). Mice were crossed to obtain Scx-CE; R26-tdT; Scx-EGFP (Ctrl) and Scx-CE; R26-LSL-Pten; R26-tdT; Scx-EGFP (PtenOE) mice. Ctrl and PtenOE mice were injected with tamoxifen three times at P5–7 d, followed by Achilles tenotomy after 72 h. Tendon regeneration was evaluated 3 and 28 days after Achilles tenotomy (Fig. 5a, Supplementary Fig. 6a). Similar to ZSTK treatment, the number of tdTomato+ Ki67+ cells and tdTomato+ area in the tendon stub were significantly lower in the PtenOE group at day 3 (Supplementary Fig. 6b–e). Similarly, the tdTomato+ cell migrating area and the distance of proximally migrating tdTomato+ cells in neotendons in the PtenOE group were significantly reduced at day 28 (Fig. 5b–d). However, in contrast to the significant reduction in neotendon thickness in the ZSTK-administered mice (Fig. 3g–i), the PtenOE group did not exhibit a significant reduction in neotendon thickness (Fig. 5b, e). Notably, although the neotendons of the PtenOE group exhibited decreased numbers of tdTomato+ cells, EGFP and Tnmd expression was detected in the neotendon, suggesting compensatory healing by non-Scxlin cells (Fig. 5b, f–i).
a Schematic representation of the experiment. To investigate Scx-lineage (Scxlin) cell-specific suppression of the PI3K-Akt signalling pathway, Scx-CreERT2; Rosa26-tdTomato; Scx-EGFP (Ctrl), Scx-CreERT2; Rosa26-loxP-stop-loxP-Pten (Rosa26-LSL-Pten); Rosa26-tdTomato; Scx-EGFP (PtenOE) mice were used. After tamoxifen injection, Scx-lineage (Scxlin) cell-specific overexpression of Pten is induced. Yellow and red fluorescence indicates Scxlin tenocyte and non-tenocyte, respectively, whereas green and non-fluorescence indicate non-Scxlin tenocyte and non-tenocyte. Mice were injected with tamoxifen (0.075 mg) three times. Tamoxifen was washed out for 72 h, and subsequently, a tenotomy was performed (P 10-day) and evaluated 28 days later. b Haematoxylin-eosin (H&E), picrosirius red (PSR), and immunofluorescent staining of regenerated tendons 28 days after tenotomy. RFP and GFP antibodies were used for immunofluorescence analysis. Regenerated tendons and tendon stubs are indicated by white dotted lines. Intact plantaris tendons are indicated by asterisks. CA; calcaneus. c Quantification of the area with tdTomato-positive cells (Scxlin cells) shown in (b). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The area of tdTomato+ cells significantly decreased in the PtenOE group (two-tailed Student’s t-test; p = 0.0011). d Quantification of the distance of proximally migrating Scxlin cells in the neotendons shown in (b). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The distance in the PtenOE group was significantly smaller than that in the control group (two-tailed Student’s t-test; p = 0.0159). e Quantification of neotendon thickness shown in (b). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). No significant differences were observed between the two groups (two-tailed Student’s t-test). f Magnified images of the yellow dotted squares (b). g The quantification of the EGFP-positive (EGFP+) cell ratio in the neotendons shown in (f). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The percentage of EGFP+ cells was significantly increased in the PtenOE group (two-tailed Student’s t-test; p = 0.0215). h Immunofluorescent staining at regenerated tendons using RFP and Tnmd antibodies. i Quantification of the Tnmd-positive (Tnmd+) cell ratio in the neotendons shown (h). Data are shown as the mean ± SEM of four independent biological samples (two independent sections per mouse). No significant difference in the percentage of Tnmd+ cells was observed between the Ctrl and PtenOE groups (two-tailed Student’s t-test; p = 0.3963). j Immunofluorescent staining using RFP and Col2 antibodies in the chondrometaplasia region is indicated by yellow dotted lines. k Magnified images of Fig. 5j. l Quantification of the number of Col2+ cells in regenerative tendons shown in (j). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). No significant differences were observed between the two groups (two-tailed Student’s t-test, p = 0.4649). m Quantification of the number of Col2+ Scxlin (tdTomato+) cells in the regenerative tendons shown in (j). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Chondrometaplasia derived from Scxlin cells (tdTomato+ cells) significantly decreased in the PtenOE group (two-tailed Student’s t-test, p = 0.0286). n Immunofluorescent staining using RFP and S100b antibodies. Yellow dotted circles indicate chondrometaplasia. o Magnified images of Fig. 5n. p Quantification of the number of S100b+ cells in regenerative tendons shown in (n). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). No significant differences were observed between the two groups (two-tailed Student’s t-test). q Quantification of the number of the S100b+ Scxlin (tdTomato+) cells in the regenerative tendons shown in (n). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Chondrometaplasia derived from Scxlin cells (tdTomato+ cells) significantly decreased in the PtenOE group (two-tailed Student’s t-test, p = 0.0427). * Scale bars, 50 μm (f, h, k, o), 200 μm (b, j, n).
Selective inhibition of PI3K-Akt signalling in Scxlin cells did not affect the total cell number of Col2 and S100b-labelled chondrometaplastic regions (Fig. 5j–l, n–p). However, the number of the Scxlin cell-derived Col2- and S100b-expressing cells was significantly decreased in the PtenOE group (Fig. 5m, q), indicating that the inhibition of the PI3K-Akt signalling pathway suppressed chondrometaplasia in Scxlin cells. In addition, in the Ctrl group, small populations of Col2+ tdTomato+ and S100b+ tdTomato+ cells were detected in chondrometaplastic regions (25.0% and 14.5% of chondrometaplatic regions, respectively; Fig. 5j–q). This observation suggests that non-Scxlin cells are the major cell source of chondrometaplasia.
PI3K-Akt signalling pathway affects paratenon sheath cell proliferation, migration, and stemness in vitro
Based on these findings, we found that both Scxlin cell-induced intrinsic healing and extrinsic healing mediated by non-Scxlin cells are important for neotendon formation. To date, Tppp3lin paratenon sheath cells12 and αSMAlin resident tendon progenitor cells23 have been proposed as non-Scxlin cell sources for tendon regeneration. To determine the contribution of these lineage cells to neotendon formation, we generated additional lineage-specific reporter mice, namely Tppp3-CreERT2 (Tppp3-CE); R26-tdT and αSMA-CreERT2 (SMA-CE); R26-tdT mice to respectively label Tppp3- and αSMA-expressing cells red (Supplementary Fig. 7a–h). The Achilles tendons of each mouse were excised 72 h after tamoxifen administration and evaluated 14 days later (Supplementary Fig. 8a). Interestingly, αSMAlin cells were rarely found in neotendons, whereas most cells were Tppp3lin cells (Supplementary Fig. 8b, c). Therefore, in subsequent experiments, we focused on Tppp3+ paratenon sheath cells. Tppp3-expressing primary paratenon sheath cells obtained from rat Achilles tendons were treated with ZSTK and assessed in terms of proliferation, migration, stemness, and gene expression (Supplementary Fig. 9a, b). Immunocytochemical staining revealed a significant decrease in the Ki67+ cell ratio in the ZSTK group (Fig. 6a, b). The scratch, transwell migration, and CFU assays revealed significant decreases in migration capacity and colony number in the ZSTK group (Fig. 6c–h). In addition, stem cell markers, Nes and Cd44, were downregulated, and tendon markers, Scx, Mkx, Tnmd, Col1a1, and Col3a1, were upregulated significantly (Fig. 6i). In the analysis of cartilage markers, Sox9, Col2a1, and Col10a1 exhibited upregulation, although the increase in Col2a1 expression was not statistically significant. These findings suggest a positive trend towards chondrogenic differentiation upon inhibition of PI3K-Akt signalling (Fig. 6i). These results revealed that the PI3K-Akt signalling pathway regulates proliferation, migration, stemness, and tendon differentiation in paratenon sheath cells.
a Immunocytochemical staining using Ki67 antibody for paratenon sheath cells treated with vehicle (control; Ctrl) or ZSTK474 (ZSTK). b Ki67 positive cell ratio of paratenon sheath cells in (a) (n = 4 independent experiments). The ZSTK group showed significantly decreased cell proliferation relative to the control group (two-tailed Student’s t-test; p = 0.0006). c The scratch test was used to assess the migration capacity of the paratenon sheath cells treated with vehicle or ZSTK. d Quantification of the wound healing rate shown in (c) (n = 6 independent experiments). The ZSTK group showed significantly decreased cell migration (two-tailed Student’s t-test; p < 0.0001 at 12 h, and p < 0.0001 at 24 h). e Transwell assay was used to assess the migration capacity of paratenon sheath cells treated with vehicle or ZSTK. Analyses were performed at 24 h after seeding. f Quantification of migrated cells shown in (e) (n = 6 independent experiments). The ZSTK group showed significantly decreased cell migration (two-tailed Student’s t-test; p < 0.0001). g CFU-F assay was used to assess the self-renewal capacity of paratenon sheath cells treated with Vehicle or ZSTK. Analyses were performed seven days after seeding. h Quantification of colony formation units shown in (g) (n = 3 independent experiments). The ZSTK group showed significantly decreased colony formation compared to the control group (two-tailed Student’s t-test; p = 0.0009). i Real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to evaluate RNA expression in paratenon sheath cells in the control and ZSTK groups. Expression of tendon stem cell markers, Nes and Cd44, was significantly decreased in the ZSTK group. Expression of tendon differentiation markers (Scx, Mkx, Tnmd, Col1a1, and Col3a1) was significantly increased in the ZSTK group. Expression of chondrogenic markers (Sox9 and Col10a1) was significantly increased in the ZSTK group (two-tailed Student’s t-test, n = 8 independent experiments). * Data are presented as mean ± SEM. Scale bars, 100 μm (a), 200 μm (c), 400 μm (e).
PI3K-Akt signalling pathway is involved in Tppp3-lineage cell migration, proliferation, tendon differentiation, chondrometaplasia, and regenerating tendon thickening in vivo
Subsequently, we confirmed the role of the PI3K-Akt signalling pathway in extrinsic healing via Tppp3lin cells in vivo. To specifically inhibit the PI3K-Akt signalling pathway in Tppp3lin cells, Tppp3-CE; R26-tdT; Scx-EGFP (Ctrl) and Tppp3-CE; R26-LSL-Pten; R26-tdT; Scx-EGFP (PtenOE) mice were generated; tendon regeneration was evaluated 3 and 28 days after Achilles tenotomy (Fig. 7a, Supplementary Fig. 10a). Similar to Scxlin cells, the Ki67+ tdTomato+ cell ratio in the neotendons were significantly lower in the PtenOE group at day 3 (Supplementary Fig. 10b, c). At day 28, histological images showed neotendon thinning in the PtenOE group (Fig. 7b, c). Picrosirius red staining showed a significantly increased area of thick fibres in the PtenOE group (Fig. 7b, d). These results suggest that inhibition of the PI3K-Akt signalling pathway resulted in the thinning of neotendons and the production of more mature collagen fibres. To assess the migration capacity, the percentage of tdTomato+ cells in the neotendons was calculated and found to be significantly lower in the PtenOE group (Fig. 7b, e). Furthermore, to evaluate whether Tppp3lin cells differentiated into tendons, the percentage of EGFP+ tdTomato+ and Tnmd+ tdTomato+ cells was calculated. The percentage of EGFP+ tdTomato+ cells was comparable between the Ctrl and PtenOE groups; however, the percentage of Tnmd+ tdTomato+ cells was significantly higher in the PtenOE group (Fig. 7b, f–i, Supplementary Fig. 10d). Additionally, regenerated tendons in the PtenOE group showed significantly higher expression of Scx, Mkx, Tnmd, and Col1a1 than those in the Ctrl group (Supplementary Fig. 10e), suggesting that Tppp3lin cells with inhibited PI3K-Akt signalling pathway were more likely to differentiate into mature tendons.
a Schematic representation of the experiment. To investigate Tppp3-lineage (Tppp3lin) cell-specific suppression of the PI3K-Akt signalling pathway, Tppp3-CreERT2; Rosa26-tdTomato (control; Ctrl), Tppp3-CreERT2; Rosa26-loxP-stop-loxP-Pten (Rosa26-LSL-Pten); Rosa26-tdTomato (PtenOE) mice were used. After tamoxifen injection, Tppp3-lineage (Tppp3lin) cell-specific overexpression of Pten is induced. Yellow and red fluorescence indicate Tppp3lin tenocyte and non-tenocyte, respectively, whereas green and non-fluorescence indicate non-Tppp3lin tenocyte and non-tenocyte. Mice were injected with tamoxifen (0.075 mg) three times. Tamoxifen was washed out for 72 h, and subsequently, a tenotomy was performed (P 10-day) and evaluated 28 days later. b Haematoxylin-eosin (H&E), picrosirius red (PSR), and immunofluorescent staining of regenerated tendons 28 days after tenotomy. RFP and Tnmd antibodies were used for immunofluorescence analysis. Regenerated tendons are indicated by white dotted lines, and intact plantaris tendons are indicated by a black asterisk. The region marked by yellow dotted squares was enlarged in (h). c Quantification of neotendon thickness. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Regenerated tendons were significantly thinner in the PtenOE group than in the control group (two-tailed Student’s t-test, p = 0.0460). d Percentage of thick fibres in the neotendon. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The PtenOE group had a significantly higher percentage of thick fibres than the control group (two-tailed Student’s t-test; p = 0.0336). e Quantification of the percentage of tdTomato-positive (+) cells (Tppp3lin cells) in regenerated tendons shown in (b). Data are shown as the mean ± SEM of five independent biological samples (three independent sections per mouse). The area of tdTomato+ cells significantly decreased in the PtenOE group (two-tailed Student’s t-test; p = 0.0052). f Magnified images of the yellow dotted squares in Supplementary Fig. 10d. g The percentage of EGFP+ Tppp3lin (tdTomato+) cells among tdTomato+ cells in the neotendons. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). EGFP+ tdTomato+ cells ratio was similar between the Ctrl and PtenOE groups (two-tailed Student’s t-test, p = 0.9696). h Magnified images of the yellow dotted squares in (b). Immunofluorescent staining of regenerated tendons 28 days after tenotomy using RFP and Tnmd antibodies. i The percentage of Tnmd+ Tppp3lin (tdTomato+) cells among tdTomato+ cells in the neotendons. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Tnmd+ tdTomato+ cells ratio was higher in the PtenOE group than in the control group (two-tailed Student’s t-test, p = 0.0010). j Immunofluorescent staining using RFP and Col2 antibodies. Yellow dotted circles indicate chondrometaplasia. k Magnified images of (j). l Quantification of the number of Col2+ cells in the regenerative tendons shown in (j). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The number of Col2+ cells in the PtenOE group decreased, although it was not significant (two-tailed Student’s t-test; P = 0.2158). m Quantification of the number of Col2+ Tppp3lin (tdTomato+) cells in the regenerative tendons. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Chondrometaplasia derived from Tppp3lin cells (tdTomato+ cells) decreased in the PtenOE group although not significant (two-tailed Student’s t-test, p = 0.1301). n Immunofluorescent staining using RFP and S100b antibodies. Yellow dotted circles indicate chondrometaplasia. o Magnified images of (n). p Quantification of the number of S100b+ cells in the regenerative tendons shown in (n). Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). The number of S100b+ cells in the PtenOE group significantly decreased (two-tailed Student’s t-test; p = 0.0329). q Quantification of the number of S100b+ Tppp3lin (tdTomato+) cells in the regenerative tendons. Data are shown as the mean ± SEM of four independent biological samples (three independent sections per mouse). Chondrometaplasia derived from Tppp3lin cells (tdTomato+ cells) significantly decreased in the PtenOE group (two-tailed Student’s t-test, p = 0.0125). * Scale bars, 50 μm (f, h, k), 100 μm (o), 200 μm (b, j, n).
To evaluate chondrometaplasia, we measured the Col2+ and S100b+ cell numbers and found that the total number of Col2+ and S100b+ cells decreased in the PtenOE group (Fig. 7j–l, n–p). Notably, a large number of tdTomato+ cells were present in the Col2- and S100b-positive chondrometaplastic region of the control group (57.1% and 25.6% of chondrometaplatic regions, respectively; Fig. 7j–q). However, the number of Col2+ tdTomato+ and S100b+ tdTomato+ cells was decreased in the PtenOE group relative to the control group, although not significant in the Col2+ cell region (Fig. 7m, q). These results suggested that Tppp3lin cells are a major source of chondrometaplasia and that the PI3K-Akt signalling pathway regulates chondrometaplasia in Tppp3lin cells.
Discussion
To date, several types of tendon-regenerating cells have been identified, including Scx+ cells8,13,20,49, Tppp3+ cells12, Osteocalcin+ cells25, and αSMA+ cells23. However, the roles and functions of these cells in injured tendon regeneration are not completely understood. In this study, we found that the PI3K-Akt signalling drives physiological regeneration in injured neonatal tendons. In addition, by analysing how this signalling regulates Scxlin and Tppp3lin cells, the major tendon-regenerating cells, we identified a cell type-specific role of PI3K-Akt signalling in tendon regeneration.
Because PI3K-Akt signalling positively regulates cell proliferation and migration in other cell types32,33,34,35,36, we expected that PI3K-Akt signalling would positively affect tendon regeneration. Regarding Scxlin cell regeneration, pharmacological and genetic inhibition of PI3K-Akt signalling via Pten overexpression decreased cell proliferation, migration, and self-renewal. Interestingly, analyses using Scx-CE; R26-LSL-Pten; R26-tdT; Scx-EGFP mice showed that Scxlin cell-specific PI3K-Akt signalling inhibition decreased Scxlin cell migration into neotendons; however, EGFP-expressing cells derived from non-Scxlin cells were observed in neotendons and some of them differentiated into Tnmd-expressing tenocytes. The results showed that Scxlin cell-specific PI3K-Akt signalling inhibition did not alter tendon thickness. Best et al.50 reported that deletion of Scxlin -cells via the inducible diphtheria toxin receptor (Scx-Cre; Rosa26-LSL-DTR mice) increased the abundance of αSMA+ cells in regenerative tendons, leading to increased stiffness and maximal load of failure of regenerative tendons. These findings suggest that decreased intrinsic healing via Scxlin cells was compensated for by extrinsic healing via non-Scxlin cells. Kaji et al.20 demonstrated that TGF-β signalling regulated tenocyte migration upon tendon injuries. TGF-β and PI3K-Akt signalling reportedly interact both positively and negatively51,52. However, we did not observe any obvious interactions between them in tenocytes, suggesting that PI3K-Akt signalling may be a distinct signalling pathway associated with tendon healing (Supplementary Fig. 11a, b). Similarly, in Tppp3lin cell regeneration, pharmacological and genetic inhibition of PI3K-Akt signalling decreased cell proliferation, migration, and self-renewal. Previous studies have shown that PI3K-Akt signalling regulates MSC differentiation and that its inhibition suppresses tenogenic differentiation53,54. In contrast to these studies, our study showed that inhibition of PI3K-Akt signalling in paratenon sheath cells increased the expression of tendon markers, including Scx, Mkx, Tnmd, Col1a1, and Col3a1 in vitro, and that its inhibition in Tppp3lin cells increased the expression of Tnmd, a mature tendon marker, in neotendons in vivo. Thus, these findings suggest that PI3K-Akt signalling plays multiple roles in Scxlin and Tppp3lin cells during tendon regeneration, promoting cell proliferation, migration, and self-renewal, while suppressing tenogenic differentiation toward the mature state.
To understand the regulation of tenogenic differentiation by the PI3K-Akt signalling pathway, we focused on the stemness of tendon-regenerating cells. TSPCs are tissue-resident stem cells contributing to tendon regeneration55. They exist in the tendons and paratenon and are characterised by the expression of TSPC markers, including Nes and CD4438,55,56. TSPCs are derived from tendon-resident Scx+ tenocytes and Tppp3+ paratenon sheath cells38,55,56. Recently, a subpopulation of Tppp3lin cells, which co-express Pdgfra, was proposed as a tendon stem cell population12. Consistent with these results, we found Nes- and CD44-expressing populations in both Scxlin and Tppp3lin cells in uninjured Achilles tendons and their paratenons (Supplementary Fig. 12). PI3K-Akt signalling plays a fundamental role in the maintenance of stemness in several cell types57,58,59,60,61. Our in vitro study revealed that inhibition of PI3K-Akt signalling decreased self-renewal in the CFU assay of both tenocytes and paratenon sheath cells. Furthermore, inhibition of PI3K-Akt signalling decreased Nes expression in tenocytes and Nes and Cd44 expression in paratenon sheath cells while increasing the expression of tendon differentiation markers in both cell types. These results indicate that PI3K-Akt signalling regulates the stemness of Scxlin and Tppp3lin cells and that its inhibition promotes tenogenic differentiation through the loss of stemness.
Several studies have identified the sources of chondrometaplasia and heterotopic ossification, including Scxlin, Tppp3lin, Pdgfralin, and Hoxa11lin cells8,62,63. Our in vitro analyses revealed that inhibition of PI3K-Akt signalling caused slight and distinct changes in the expression of cartilage markers, resulting in a decrease in tenocytes and an increase in paratenon sheath cells. Interestingly, in contrast, pharmacological and Scxlin and Tppp3lin cell-specific inhibition of PI3K-Akt signalling decreased chondrometaplasia in vivo. Furthermore, our findings indicated that Scxlin cells made a minor contribution to chondrometaplasia, while Tppp3lin cells were a major contributor. Consistent with our results, recent studies showed that approximately 20% of cells in the cartilage region of adult injured Achilles tendons are derived from Tppp3lin cells62. Additionally, inhibition of PI3K-Akt signalling using a PI3K inhibitor (LY294002) prevented the formation of ectopic cartilage and bone in the injured tendons in vivo64. Although we were unable to explain the discrepancy between our in vitro and in vivo results on chondrogenic differentiation of tenocytes and paratenon sheath cells, these findings suggest that PI3K-Akt signalling plays a positive role in ectopic cartilage and bone formation in injured Achilles tendons.
Given the findings in this study, the dynamic control of the PI3K-Akt signalling activity could be a potential target for physiological tendon regeneration. During the early period (inflammatory and proliferating phases) after tendon injury, cell proliferation, migration, maintenance of stemness, and self-renewal of Scxlin and Tppp3lin cells could be promoted by PI3K-Akt signalling activation. In contrast, during the late period (remodelling phase), mature tenogenic differentiation of Scxlin cells and Tppp3lin-derived regenerated cells could be promoted by PI3K-Akt signalling suppression, leading to regeneration of thicker and more mature tendons with less chondrometaplasia.
However, our study has several limitations and challenges that need to be overcome. Firstly, it should be noted that the constitutive activation of the PI3K-Akt signalling pathway presents a potential risk for the development of cancer65,66,67. Previous experiments have shown that cancer development, including leukaemia, necessitates prolonged Akt activation through Pten deletion for a minimum of 8–10 weeks68,69,70,71. Therefore, short-term activation of the PI3K-Akt signalling pathway only in the early stages following tendon injury may mitigate the risk of cancer development. Furthermore, to enhance tendon regeneration, we administered the Akt activator SC79 to a mouse model of Achilles tendon injury, specifically targeting 6-month-old mice. However, a 4-week systemic administration of SC79 resulted in toxicity, such as severe weakening of the mice, and consequently led to poor functional recovery (Supplementary Fig. 13a–k). Hence, the development of a drug delivery system, such as controlled-release materials, for spatial signalling activation is essential to achieve localised drug effects while minimising systemic side effects72. Further studies are warranted to comprehend the regenerative potential of the PI3K-Akt signalling pathway in adult tendon injuries.
Secondly, we did not observe any remarkable impact of SMAlin cells on neonatal tendon regeneration, which is inconsistent with previous studies20,23. This discrepancy could be attributed to variations in the transgenic constructs used. Specifically, their SMA-CreERT2 mice have a shorter regulatory element (Acta2 promoter spanning 1.1 kb to the 1st intron 2.7 kb) compared to ours73.
Thirdly, we utilised a combination of different species in our research, specifically mice for in vivo experiments and rats for in vitro experiments. While using the same species throughout would be ideal, we attempted to culture tenocytes and paratenon sheath cells from transgenic mice in vitro. However, the collection of paratenon sheath cells from P7d transgenic mice proved technically challenging (Supplementary Fig. 9a). A recent study revealed that murine and rat tenocytes exhibit a similarly low proliferative capacity, which closely resembles that of human tenocytes. Additionally, although the global gene expression profiles of mice and rats differed, they displayed similar changes in the expression of tendon markers (Scx, Tnmd, Tnc, Col1, Col3, and Col5) in response to inflammation74.
Fourthly, the choice of tendon injury model (for example, complete transection with or without repair, partial injury, and different types of tendons) may lead to variations in the types of cells involved in tendon regeneration. In the case of the patellar tendon defect model involving punch injury12 and the complete transection model of the Achilles tendon of the gastrocnemius62, the contribution of Tppp3lin cells to regenerative tendons was found to be 20–30% at 3–4 weeks post-injury. Similarly, our complete transection model of the Achilles tendons of the gastrocnemius and soleus revealed that Tppp3lin cells were the primary contributors to regenerative tendons, while Scxlin cells were present in smaller numbers. In contrast, the suture repair model of flexor digitorum longus tendons showed a contribution of 60–80% by Scxlin cells to regenerative tendons at 3–4 weeks post-injury. Therefore, it should be noted that our results cannot be generalised to all types of tendon injuries.
Finally, we used P10d mice for in vivo experiment because we could not optimise tamoxifen injections for P0-2d newborn mice. Importantly, regenerative window in neonatal tissues is shown to narrow after P7d75, therefore P5d neonatal tendon injury models have been used8,20. However, no studies have clearly determined when the regenerative window of tendons closes. Notably, previous studies show that the proliferative capacity of tendon cells at P7–14 d remains higher than that in adult76, and P7–14 d represents a critical stage for tendon maturation77. These findings suggest that regenerative window of tendons may close by P14d. We confirmed that the neotendons in P5d and P10d Scx-EGFP mice contained a large number of EGFP+ cells (61.2% and 42.1%, respectively) whereas those in P1m and P6m mice contained few EGFP+ cells (1.7% and 2.2%, respectively) (Supplementary Fig. 14a–e). These results suggest that the regenerative window is still open in P10 mice. Although not ideal, the use of P10d mice may be acceptable.
In conclusion, the regeneration of injured neonatal tendons primarily involves Scxlin tenocytes and Tppp3lin paratenon sheath cells. The activation of PI3K-Akt signalling is crucial for the physiological regeneration of tendons in neonatal mice, as it promotes cell proliferation and migration, and regulates the stemness of both Scxlin and Tppp3lin cells (Fig. 8). Based on these findings, we suggest that precise control of the PI3K-Akt signalling could serve as a promising therapeutic target for the regeneration of damaged tendons.
When tendons are injured, regeneration is primarily mediated by tenocytes (Scx+ cells) and paratenon sheath cells (Tppp3+ cells). PI3K-Akt signalling pathway stimulates the proliferation and migration of these cell types. However, inactivation of the PI3K-Akt signalling pathway impairs cell proliferation and migration. In contrast, PI3K-Akt signalling regulates the stemness of both Scx+ and Tppp3+ cells. Inactivation of PI3K-Akt signalling leads to a loss of stemness and induces terminal differentiation into mature tenocytes. As a result, the neotendon fails to thicken, impairing functional and mechanical regeneration. Overall, the PI3K-Akt signalling pathway is necessary for physiological tendon regeneration in neonatal mice, and its inactivation alters the tendon regenerative potential.
Methods
Animal experiments
All animal experiments were approved by the Gifu University Animal Experiment Committee (28-19, 30-077, 2019-183, 2021-094, 2021-238) and complied with Animal Research: Reporting in Vivo Experiments guidelines. Scx-EGFP19 and Tppp3-CreERT278,79 mice have been established previously. Rosa26-LSL-tdTomato (Ai9)80 mice were purchased from the Jackson Laboratory (https://www.jax.org/). Both male and female mice were randomly used without bias. All mice were kept ad libitum in a temperature-controlled environment (23 ± 2 °C) under a 12-hour light/dark cycle.
Vector construction, gene targeting, and generation of knock-in and transgenic mice
To generate Rosa26-stop-HA-Pten mice, the HA-Pten sequence was cloned into pENTR_mCherry (Plasmid #139554; Addgene) and inserted into a destination vector; pRosa26-DEST (Plasmid #21189; Addgene) by LR recombination (Gateway™ LR Clonase™ II Enzyme mix, Thermo Scientific). pRosa26-stop-HA-Pten targeting vector was electroporated into V6.5 murine embryonic stem cells (ESCs) (C57BL/6 × 129/SV background) and correctly targeted ESC lines were confirmed using genomic PCR81.
For the Scx-CreERT2, the Red/ET bacterial artificial chromosome (BAC) recombination system was used to introduce an ires-CreERT2-pA-rox-pPGK-EM7-BsdR-pA-rox cassette into 3’-UTR of Scx gene (BAC clone; RP23-415D19) to generate a targeting vector. The obtained vector was electroporated into V6.5 ESCs, and correct targeting of the ESC lines was confirmed by genomic PCR and Southern blotting. Similar to Scx-EGFP mice19, the drug-selection cassette of Scx-CreERT2 mice was removed by crossing with Tg(CAG-dre)1Afst mice82. The ESC-derived chimeric mice were crossed with C57BL/6 mice at least five times.
For αSMA-CreERT2, using the Red/ET BAC recombination system CreERT2-pA-FRT-pPGK-EM7-NeoR-pA-FRT cassette was introduced into the start codon of Acta2 (BAC clone RP23-370F21). The correctly targeted αSMA-CreERT2 BAC, that contained about 92 kb of genomic DNA upstream of the CreERT2 translational start codon, was microinjected into fertilised eggs (C57BL/6 background) to generate transgenic mice.
Tamoxifen administration
Cre-mediated recombination was achieved by delivering tamoxifen (TMX; Sigma-Aldrich). TMX was prepared as 2.5 mg ml-1 stock in corn oil (Wako) and administered by intraperitoneal (i.p.) injection at 10 μl per g body weight for P5–7 (0.075 mg/day × 3 times), and mice were injured after 2 days.
Tendon injury model
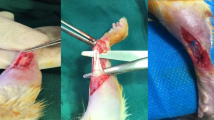
Three types of mixed anaesthetics (Vetorphale, Meiji Seika Phalmarma, Japan; Dorbene, Kyoritsu, Japan; and Dormicum, Maruishi, Japan) were used83. The right Achilles tendon of 10-day-old (P10d) transgenic mice and 6-month-old (P6m) wild type C57BL/6 mice was exposed through a central incision and transected 1.5 mm above the bottom of the foot, with the plantaris tendon left intact. The skin was closed using 7–0 nylon sutures (Natsume, Japan). In order to mimic a surgical procedure, incisions were made over the Achilles tendons and subsequently closed using sutures as a sham operation. After Achilles tenotomy, 2% hydroxypropyl cellulose (Tokyo Chemical Industry) (control) and 100 mg/kg of PI3K inhibitor ZSTK474 (Chemscene) were orally administered to P10d mice once a day for 1, 3, and 28 days. Dimethyl sulfoxide (DMSO) (Wako) (control) and 40 mg/kg of Akt activator SC79 (Selleck Biotechnology) were intraperitoneally administered to P6m mice once a day for 28 days.
RNA isolation, RT-PCR, and real-time quantitative RT-PCR
RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany) for tendon tissues and the RNeasy Plus Mini Kit (Qiagen) for cultured cells. Complementary DNA (cDNA) was synthesised using PrimeScript™ RT reagent Kit (Takara, Japan). RT-polymerase chain reaction (RT-PCR) and qRT-PCR were performed using KOD-Fx-Neo (Toyobo) and TB Green Premix Ex Taq II (Takara), respectively. Transcript levels were normalised by β-actin. The expression level in the control group was set to 1. PCR primers used are listed in Supplementary Table 1.
Primary culture of tenocytes and paratenon sheath cells
Primary tenocytes and paratenon sheath cells were isolated from 7–14-day-old CD (SD) rats using a well-established procedure25,55,84,85. In brief, the paratenon tissue was harvested by applying pressure to the Achilles tendons with micro forceps under a surgical microscope (Supplementary Fig. 9). The remaining Achilles tendons were used as the tendon tissue. The harvested tendon and paratenon tissues were minced and digested with 2 mg/ml type I collagenase (Worthington) at 37 °C for 30 min. Digested tendons were centrifuged, and the pellet was suspended in Minimum Essential Medium α (MEM α, Thermo Fisher Scientific), supplemented with 20% foetal bovine serum (FBS, BioWest), 55 μM 2-mercaptoethanol (Thermo Fisher Scientific) and 100 U/ml penicillin/streptomycin (Wako) at 37 °C under 5% CO2 and the medium was changed every 2–3 days. At 80–90% confluence, cells were trypsinised with 0.25% Tripsin-EDTA (Thermo Fisher Scientific), centrifuged, and resuspended in growth medium as passage 1 cells. After 80–90% confluence, aliquots of 3 × 106 cells/ml were cryopreserved in CELLBANKER 1 plus (Nippon Zenyaku Kogyo, Japan). All experiments were performed using cells at passage 3 cells.
Scratch migration assay and transwell migration assay
Primary rat tenocytes and paratenon sheath cells were treated with 5 μg/ml Mitomycin C (Wako) for 2 h to halt cell division. They were then treated with 1 μM PI3K inhibitor ZSTK474 or DMSO for 2 h. For the scratch migration assay, the cells were seeded in 12-well plates at a density of 1 × 105 cells and supplemented with 1 μM ZSTK474 or DMSO. After 24 h, the plates were scratched with a 200 μl pipette tip. Phase contrast imaging was performed at 12 h and 24 h using an Olympus IX83 microscope. For the transwell migration assay, the cells were seeded in Costar Transwell chambers on 24-well plates (Corning Incorporated, NY, USA) at a density of 0.6 × 105 cells with serum-free medium supplemented with 1 μM ZSTK474 or DMSO. The bottom plates were filled with medium containing 20% FBS and supplemented with 1 μM ZSTK474 or DMSO. After 24 h, the transwell chambers were washed with PBS, and the cells were fixed with 4% paraformaldehyde (PFA) for 15 min and stained with 0.1% crystal violet for 10 min at room temperature. Non-migrating cells on the upper side of the chambers were removed by scraping. The membranes were then attached to glass slides, and the stained cells were analysed using a microscope (BX51, Olympus, Tokyo, Japan).
Protein extraction and western blotting
Tendons were frozen in liquid nitrogen, homogenised, and resuspended in 150 μl T-PER™ Tissue Protein Extraction Reagent (Thermo Fisher Scientific) containing Halt™ Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific). The protein concentration was measured using the Protein Assay BCK Kit (Wako). SDS-PAGE was performed on NuPAGE™ 10%, Bis-Tris, 1.0 mm, Mini Protein Gels (Thermo Scientific). Following protein transfer, the membranes were treated with a blocking reagent containing 5% bovine serum albumin (BSA, Sigma-Aldrich) for 1 h at room temperature. Primary antibodies were applied in Can Get Signal Solution 1 (Toyobo) overnight at 4 °C and secondary HRP-linked antibodies in Can Get Signal Solution 2 (Toyobo) for 1 h at room temperature. Blots were scanned with ImageQuant™ LAS4000 mini4000 (Cytiva) and quantified using ImageJ. Quantified band intensities were normalised by β-actin and plotted on the graph. The primary antibodies used for western blotting were anti-HA-Tag [C29F4] (#3724) (Cell Signaling Technology; 1:1000), anti-Phospho-Akt (Ser473) [D9E] (#4060) (Cell Signaling Technology 1:1000), anti-Akt (#9272) (Cell Signaling Technology; 1:1000), anti-SMAD2/3 [D7G7] (#8685) (Cell Signaling Technology; 1:1000), anti-Phospho-SMAD2 (Ser465/467)/SMAD3 (Ser423/425) [D27F4] Rabbit mAb #8828 (Cell Signaling Technology; 1:1000), and anti-β-Actin [13E5] Rabbit mAb #4970 (Cell Signaling Technology; 1:2000). Full-size images of the blots are shown in the Source Data file and Supplementary Information (Supplementary Fig. 15).
Whole-mount fluorescence imaging
The hind limbs were skinned to expose the Achilles tendons, and whole-mount fluorescence images were captured using an M165 FC (Leica).
Histology and immunostaining
All tissue samples were fixed with 4% PFA overnight, decalcified with pH 7.2 EDTA buffer (G-Chelate Mild, GenoStuff, Japan) for 10 d at 4 °C, and embedded in paraffin. The samples were cut into 5-μm-thick sections. Haematoxylin and eosin (H&E), safranin O/fast Green, Masson’s trichrome, and picrosirius red staining were performed using standard protocols. The thickest part was measured in the sagittal section to determine the neotendon thickness. In picrosirius staining, red, orange, and yellow fibres indicated mature thick fibres, whereas green fibres indicated immature thin fibres86. For immunohistochemistry, the antibodies used were anti-GFP (NB600-308) (Novus; dilution 1:100), anti-RFP (ARG55744) (arigo Biolaboratories; dilution 1:200), anti-Ki67 [SP6] (ab16667) (Abcam; dilution 1:200), anti-Phospho-Akt (Ser473) [D9E] (#4060) (Cell Signaling Technology; dilution 1:100), anti-Phospho-Akt (Ser473) [736E11] (#3787) (Cell Signaling Technology; dilution 1:100), anti-tenomodulin (ab203676) (Abcam; dilution 1:200), anti-S100 beta [EP1576Y] (ab52642) (Abcam; dilution 1:200), anti-Collagen II (ab34712) (Abcam; dilution 1:200), anti-Nestin (N5413) (Sigma-Aldrich; dilution 1:100), and anti-CD44 [IM7] (14-0441-82) (Thermo Fisher Scientific; dilution 1:100). The stained cells were analysed by microscopy (BX51; Olympus). For immunofluorescence, secondary antibodies were conjugated with Alexa Fluor 488 and Alexa Fluor 594 (Thermo Fisher Scientific; dilution 1:200), and the nuclei were counterstained with DAPI (Cell Signalling; dilution 1:200). The stained cells were analysed using a fluorescence microscope (IX83, Olympus). TUNEL imaging was performed using In Situ Cell Death Detection Kit, Fluorescein (Roche).
Immunocytochemistry
Cultured cells were washed with PBS and fixed with 2% paraformaldehyde (PFA, Wako) for 15 min at room temperature. The primary antibody used was anti-Ki67 [SP6] (ab16667) (Abcam; dilution 1:200) and anti-HA-Tag [C29F4] (#3724) (Cell Signaling Technology; dilution 1:200), and the cells were incubated overnight at 4 °C. Alexa Fluor 488 and Alexa Fluor 594 (Thermo Fisher Scientific; dilution 1:200) secondary antibody was subsequently added to the cells, which were then analysed under a fluorescence microscope (IX83, Olympus).
Transmission electron microscope (TEM) analyses
TEM analyses of the Achilles tendons were performed at Tokai Electron Microscopy Inc. (Aichi, Japan). The preparation of specimens for TEM observations was previously described87. Briefly, specimens were immersed in a mixture containing 2% PFA and 2% glutaraldehyde diluted in 0.1 M cacodylate buffer (pH 7.4) for 16 h at 4 °C. Subsequently, they were post-fixed with 2 % osmium tetraoxide in 0.1 M cacodylate buffer for 3 h at 4 °C, dehydrated in ethanol, and embedded in Quetol-812 (Nisshin EM Co., Tokyo, Japan). The specimens were ultra-thin-sectioned at 70 nm with a diamond knife using an ultramicrotome (Ultracut UCT; Leica, Vienna, Austria) and were stained with uranyl acetate and lead stain solution (Sigma-Aldrich) before TEM observations. The sections were observed under a TEM (JEM-1400Plus; JEOL Ltd., Tokyo, Japan) at 100 kV.
Functional gait analysis
The mice were placed on the TMS-6N treadmill machine (MELQUEST, Toyama, Japan). The gait protocol was based on a previous study, as shown in the Supplementary Table 288 and their total gait distance was measured. In addition, all mice were recorded on video during gait. We used an open-source computational toolbox, “Automated Limb Motion Analysis (ALMA)” which is based on pose estimation from “DeepLabCut”47,48. The ankle joint amplitude (range of motion angles), stride length (cm), cycle duration (seconds), % swing and % stance strides were automatically calculated using the ALMA toolbox.
Stretch test
The mechanical properties of the Achilles tendons were measured using a creep metre (RE-3305S, Yamaden, Tokyo, Japan) as described previously89. The samples were fixed with custom-made two grips, which were pulled at a constant speed of 0.05 mm/s until failure, and the tensile strength (max force) (N) and failure strain (mm) were measured. The stiffness was manually determined from the slope in the linear region of the failure-stress curve. Stiffness was calculated as the following formula: Stiffness = Stress (N)/Strain (mm)89. As the accurate cross-sectional area could not be measured due to the small size of the samples, the tensile strength (MPa) and Young’s modulus were not evaluated.
CFU-F assays
For the colony-forming unit fibroblast (CFU-F) assay, single-cell suspensions of tenocytes (1000 cells/well) were seeded in 6-well plates. After 24 h, 1 μM ZSTK474 (Selleck Chemicals) or DMSO was added; the growth medium and reagents were changed every 2 days. Seven days after seeding, the colonies were fixed with 2% PFA for 15 min at room temperature, stained with 0.1% crystal violet (Muto Pure Chemicals, Japan) for 10 min, and rinsed twice with water. Colonies containing 30–50 or more cells were defined as single colony units, and the number of colonies was counted using the ImageJ software.
RNA-sequencing
Achilles tendons were harvested from 7-day- and 6-month-old male Scx-EGFP mice treated with tenotomy or sham surgery using a surgical microscope. In the tenotomy group, the distal stumps of the Achilles tendon without the surrounding connective tissue were selectively collected. RNA was isolated using the RNeasy Plus Mini Kit (Qiagen).
The integrity of the isolated RNA was verified using the Bioanalyzer RNA6000 pico kit (Agilent Technologies). Because the RNA integrity values of the samples were less than 8, RNA-Seq libraries were prepared from 10 ng of RNA using the NEBNext Ultra II Directional RNA Library Prep Kit for the Illumina kit, NEBNext rRNA Depletion Kit (Human/Mouse/Rat), and NEBNext Multiplex Oligos for Illumina. Each library was sequenced on a MiSeq (Illumina) using the MiSeq Reagent Kit V3 150 cycle (Illumina) with 75 base pair-end reads. Differentially expressed genes were identified using an exact test after normalisation. The RNA-sequencing data were analysed using DAVID.
Quantification and statistics
Statistical analyses were performed using GraphPad Prism 10.4.1. All quantitative data are shown as the mean ± SEM. The sample sizes and counting procedures are reported in each figure legend. Statistical P values were calculated using a parametric two-tailed unpaired Student’s t-test, nonparametric two-tailed Mann–Whitney U test, and two-way ANOVA test. P values < 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available upon request and provided in Supplementary Information and Source Data file. Source data are provided with this paper. The RNA-sequencing data have been deposited in the Gene Expression Omnibus (GEO) database under the accession code GSE236106. Source data are provided with this paper.
References
Wu, F., Nerlich, M. & Docheva, D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2, 332–342 (2017).
Voleti, P. B., Buckley, M. R. & Soslowsky, L. J. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 14, 47–71 (2012).
Buckley, M. R. et al. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect Tissue Res. 54, 374–379 (2013).
Hart, D., Kydd, A. & Reno, C. Gender and pregnancy affect neuropeptide responses of the rabbit Achilles tendon. Clin. Orthop. Relat. Res. 365, 237–246 (1999).
Shaw, A. V. et al. Outcome measurement in adult flexor tendon injury: a systematic review. J. Plast. Reconstr. Aesthet. Surg. 75, 1455–1466 (2022).
Elhassan, B., Moran, S. L., Bravo, C. & Amadio, P. Factors that influence the outcome of zone I and zone II flexor tendon repairs in children. J. Hand Surg. Am. 31, 1661–1666 (2006).
Cooper, L., Khor, W., Burr, N. & Sivakumar, B. Flexor tendon repairs in children: outcomes from a specialist tertiary centre. J. Plast. Reconstr. Aesthet. Surg. 68, 717–723 (2015).
Howell, K. et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci. Rep. 7, 45238 (2017).
Bahney, C. S. et al. Cellular biology of fracture healing. J. Orthop. Res. 37, 35–50 (2019).
Zhang, R. C. et al. Mesenchymal stem cell treatment for peripheral nerve injury: a narrative review. Neural Regen. Res. 16, 2170–2176 (2021).
Sunwoo, J. Y., Eliasberg, C. D., Carballo, C. B. & Rodeo, S. A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 38, 1666–1675 (2020).
Harvey, T., Flamenco, S. & Fan, C. M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 21, 1490–1503 (2019).
Best, K. T. & Loiselle, A. E. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J. 33, 8578–8587 (2019).
Lejard, V. et al. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J. Biol. Chem. 282, 17665–17675 (2007).
Espira, L. et al. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J. Mol. Cell Cardiol. 47, 188–195 (2009).
Murchison, N. D. et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708 (2007).
Pryce, B. A., Brent, A. E., Murchison, N. D., Tabin, C. J. & Schweitzer, R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev. Dyn. 236, 1677–1682 (2007).
Sugimoto, Y., Takimoto, A., Hiraki, Y. & Shukunami, C. Generation and characterization of ScxCre transgenic mice. Genesis 51, 275–283 (2013).
Komura, S. et al. Induced pluripotent stem cell-derived tenocyte-like cells promote the regeneration of injured tendons in mice. Sci. Rep. 10, 3992 (2020).
Kaji, D. A., Howell, K. L., Balic, Z., Hubmacher, D. & Huang, A. H. Tgfbeta signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. Elife 9, e51779 (2020).
Best, K. T. et al. NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Sci. Signal. 13, eabb7209 (2020).
Staverosky, J. A., Pryce, B. A., Watson, S. S. & Schweitzer, R. Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev. Dyn. 238, 685–692 (2009).
Dyment, N. A. et al. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One 9, e96113 (2014).
Disser, N. P. et al. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J. 33, 12680–12695 (2019).
Wang, Y. et al. Osteocalcin expressing cells from tendon sheaths in mice contribute to tendon repair by activating Hedgehog signaling. Elife 6, e30474 (2017).
Harvey, T., Flamenco, S. & Fan, C. M. A Tppp3+Pdgfra+ tendon stem cell populationcontributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 21, 1490–1503 (2019).
Mousavizadeh, R. et al. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci. Rep. 10, 12644 (2020).
Wang, T. et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 13, eabe5738 (2021).
Xu, B. et al. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J. Cell Physiol. 227, 2722–2729 (2012).
Xia, H., Nho, R. S., Kahm, J., Kleidon, J. & Henke, C. A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem. 279, 33024–33034 (2004).
Garcia-Cao, I. et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149, 49–62 (2012).
Franke, T. F., Kaplan, D. R. & Cantley, L. C. PI3K: Downstream AKTion blocks apoptosis. Cell 88, 435–437 (1997).
Zhou, H., Li, X.-M., Meinkoth, J. & Pittman, R. N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 151, 483–494 (2000).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Han, P. et al. Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation. J. Cell Physiol. 234, 17382–17391 (2019).
Hemmings, B. A. Akt signaling–linking membrane events to life and death decisions. Science 275, 628–630 (1997).
Tempfer, H. et al. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem. Cell Biol. 131, 733–741 (2009).
Yin, Z. et al. Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality. Sci. Adv. 2, e1600874 (2016).
Mienaltowski, M. J., Adams, M. S. & David, E. B. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res. Ther. 5, 86 (2014).
Feng, H. et al. Tendon-derived cathepsin K-expressing progenitor cells activate Hedgehog signaling to drive heterotopic ossification. J. Clin. Invest. 130, 6354–6365 (2020).
Alberton, P. et al. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 24, 597–609 (2015).
Rui, Y. F. et al. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 16, 1549–58 (2010).
Zhou, Z. et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell 9, 911–915 (2010).
Durgam, S. S., Altmann, N. N., Coughlin, H. E., Rollins, A. & Hostnik, L. D. Insulin enhances the in vitro osteogenic capacity of flexor tendon-derived progenitor cells. Stem Cells Int. 2019, 1602751 (2019).
Maeda, T. et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr. Biol. 21, 933–941 (2011).
Liu, R. et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 11, 797 (2020).
Aljovic, A. et al. A deep learning-based toolbox for Automated Limb Motion Analysis (ALMA) in murine models of neurological disorders. Commun. Biol. 5, 131 (2022).
Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019).
Sakabe, T. et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J. Biol. Chem. 293, 5766–5780 (2018).
Best, K. T. et al. Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. Elife 10, e62203 (2021).
Zhang, L., Zhou, F. & ten Dijke, P. Signaling interplay between transforming growth factor-β receptor and PI3K/AKT pathways in cancer. Trends Biochem. Sci. 38, 612–620 (2013).
Conery, A. R. et al. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat. Cell Biol. 6, 366–372 (2004).
Theodossiou, S. K. et al. Akt signaling is activated by TGFbeta2 and impacts tenogenic induction of mesenchymal stem cells. Stem Cell Res. Ther. 12, 88 (2021).
Cong, X. X. et al. Activation of AKT-mTOR signaling directs tenogenesis of mesenchymal stem cells. Stem Cells 36, 527–539 (2018).
Bi, Y. et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219–1227 (2007).
Walia, B. & Huang, A. H. Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J. Orthop. Res. 37, 1270–1280 (2019).
Paling, N. R., Wheadon, H., Bone, H. K. & Welham, M. J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 279, 48063–48070 (2004).
Kingham, E. & Welham, M. Distinct roles for isoforms of the catalytic subunit of class-IA PI3K in the regulation of behaviour of murine embryonic stem cells. J. Cell Sci. 122, 2311–2321 (2009).
Yu, J. S. & Cui, W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143, 3050–3060 (2016).
Park, S. Y. et al. Inactivation of PI3K/Akt promotes the odontoblastic differentiation and suppresses the stemness with autophagic flux in dental pulp cells. J. Dent. Sci. 17, 145–154 (2022).
Zhao, Q. et al. Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int. J. Mol. Med. 36, 857–864 (2015).
Yea, J. H. et al. Tppp3+ synovial/tendon sheath progenitor cells contribute to heterotopic bone after trauma. Bone Res. 11, 39 (2023).
Pagani, C. A. et al. Novel lineage-tracing system to identify site-specific ectopic bone precursor cells. Stem Cell Rep. 16, 626–640 (2021).
Chen X. et al. Inhibition of PI3K/AKT signaling pathway prevents blood-induced heterotopic ossification of the injured tendon. J. Orthop. Transl. 44, 139–154 (2024).
Testa, J. R. & Bellacosa, A. AKT plays a central role in tumorigenesis. Proc. Natl Acad. Sci. USA 98, 10983–10985 (2001).
Altomare, D. A. & Testa, J. R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24, 7455–7464 (2005).
Wang, R. C. et al. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 338, 956–959 (2012).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Liu, S. et al. A novel controlled PTEN-knockout mouse model for prostate cancer study. Front. Mol. Biosci. 8, 696537 (2021).
Horie, Y. et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J. Clin. Invest. 113, 1774–1783 (2004).
Wang, S. et al. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl Acad. Sci. USA 103, 1480–1485 (2006).
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5, 951–967 (2021).
Grcevic, D. et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 30, 187–196 (2012).
Oreff, G. L., Fenu, M., Vogl, C., Ribitsch, I. & Jenner, F. Species variations in tenocytes’ response to inflammation require careful selection of animal models for tendon research. Sci. Rep. 11, 12451 (2021).
Montero, A. M. & Huang, A. H. The regenerative capacity of neonatal tissues. Development 149, dev199819149 (2022).
Grinstein, M. et al. A distinct transition from cell growth to physiological homeostasis in the tendon. Elife 8, e48689 (2019).
Fan, C. et al. A Cd9+ Cd271+ stem/progenitor population and the SHP2 pathway contribute to neonatal-to-adult switching that regulates tendon maturation. Cell Rep. 39, 110762 (2022).
Komura, S. et al. Cell-type dependent enhancer binding of the EWS/ATF1 fusion gene in clear cell sarcomas. Nat. Commun. 10, 3999 (2019).
Goto, A. et al. C-X-C domain ligand 14-mediated stromal cell-macrophage interaction as a therapeutic target for hand dermal fibrosis. Commun. Biol. 6, 1173 (2023).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Hohenstein, P. et al. High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics 1, 3 (2008).
Anastassiadis, K. et al. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis. Models Mech. 2, 508–515 (2009).
Kawai, S., Takagi, Y., Kaneko, S. & Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60, 481–487 (2011).
Zhang, J. & Wang, J. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 11, 1471–2474 (2010).
Cho, Y. et al. CTRP3 exacerbates tendinopathy by dysregulating tendon stem cell differentiation and altering extracellular matrix composition. Sci. Adv. 7, eabg6069 (2021).
Cai, J. et al. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int. J. Nanomed. 13, 3481–3492 (2018).
Yoshioka, H. et al. Deletion of Tfam in Prx1-Cre expressing limb mesenchyme results in spontaneous bone fractures. J. Bone Miner. Metab. 40, 839–852 (2022).
Dougherty, J. P., Springer, D. A. & Gershengorn, M. C. The treadmill fatigue test: a simple, high-throughput assay of fatigue-like behavior for the mouse. J. Vis. Exp. 111, 54052 (2016).
Tsutsumi, H. et al. Generation of a tendon-like tissue from human iPS cells. J. Tissue Eng. 13, 20417314221074018 (2022).
Acknowledgements
We are grateful to Dr. Konstantinos Anastassiadis and Dr. A. Francis Stewart for their gift of Tg(CAG-dre)1Afst mice82. We thank Tomoyo Ukai, Miki Hirosawa, and Kyosuke Kondo for their technical support. This study was supported by the Takeda Science Foundation and JSPS KAKENHI (grant numbers: 16H06845, 18K09101, 18K16617, 21H03052, and 23K21465). This work was supported by JSPS KAKENHI (grant numbers: JP18K09101 and JP18K16617; AdAMS).
Author information
Authors and Affiliations
Contributions
A.G., S.K., and H. Akiyama proposed the study concept, designed and performed the experiments, and wrote the manuscript. K.K., R.M., T.M., A.H., Y.I., and J.T. performed the experiments. H.T., H. Aoki, M.O., Y.I., A.K., T.K., H. Asahara, and Y.Y. provided technical instructions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Makoto Ikeya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goto, A., Komura, S., Kato, K. et al. PI3K-Akt signalling regulates Scx-lineage tenocytes and Tppp3-lineage paratenon sheath cells in neonatal tendon regeneration. Nat Commun 16, 3734 (2025). https://doi.org/10.1038/s41467-025-59010-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59010-y