Abstract
γ-Aminobutyric acid (GABA) transporters (GATs) are sodium- and chloride-dependent transporters that mediate the reuptake of the inhibitory neurotransmitter GABA after its release from synaptic vesicles. GAT3 transports GABA from the synaptic cleft into astrocytes and modulates synaptic signaling. GAT3 has been implicated in various neurological disorders and neurodegenerative diseases, rendering it a therapeutically important drug target. To understand the mechanism of transport and inhibition, here we determine cryo-electron microscopy structures of human GAT3 in its apo form and in complex with the selective inhibitor SNAP-5114. Unexpectedly, we have discovered that SNAP-5114 acts as a noncompetitive inhibitor at GAT3. SNAP-5114 binds at the orthosteric substrate binding pocket of GAT3 in its inward-open conformation, in agreement with its noncompetitive inhibition of GABA transport. In the apo state, GAT3 also adopts an inward-open conformation with the orthosteric substrate binding pocket exposed to cytoplasm, while an extensive network of interactions closes the extracellular gate. The structures, complemented with mutagenesis and radioligand uptake assays, show that the increased orthosteric substrate binding pocket volume and bulky moieties of SNAP-5114, drive the selective inhibition of GAT3 over GAT1. Our structural and functional studies reveal the mechanism of selective inhibition of GAT3 and provide a framework for GAT3-targeted rational drug design.
Similar content being viewed by others
Introduction
γ-aminobutyric acid (GABA) is the principal inhibitory neurotransmitter and plays an essential role in the function and maturation of the central nervous system (CNS)1. Upon release from synaptic vesicles, GABA activates receptors on the postsynaptic membrane, lowering the probability of triggering an action potential2. Malfunctions in GABA signaling are involved in a range of neurological disorders such as epilepsy, autism, anxiety, schizophrenia, among others2. GABA transporters (GATs) remove GABA from the synaptic cleft and maintain the balance between excitatory and inhibitory neurotransmission. In mammals, four different sodium (Na+) and chloride (Cl−) dependent GABA transporters, GAT1-3 and betaine/GABA transporter (BGT1) have been identified and characterized for their physiological role3,4,5. GAT1 and GAT3 are exclusively expressed in the CNS, whereas GAT2 and BGT1 are expressed in various other tissues, including the kidney, liver, and blood-brain barrier6.
Human GAT3 and the homolog mouse GAT4 (mGAT4) mediate the clearance of GABA into glial cells and are involved in various physiological processes7,8. It plays an important role in CNS maturation, neuroinflammation, and modulation of phasic and tonic GABA receptor currents in postsynaptic GABAergic signaling9,10,11,12. The upregulation of GAT3 has been observed in schizophrenia, neuroinflammation, and Rhett syndrome, whereas GAT3 downregulation has been implicated in depression and alcohol use disorder10,13,14,15,16. Several studies have shown that inhibition of GAT3 can be a viable treatment for epilepsy, sleep disturbance in insomnia, pain, and depression, while GAT3 activity has been proposed to have beneficial effects in brain injury, alcoholism, and addiction recovery17,18,19,20,21.
GAT3 belongs to the neurotransmitter sodium symporter (NSS) family of transporters, that includes other GABA transporters (GAT1, GAT2, and BGT1), dopamine (DAT), norepinephrine (NET), serotonin (SERT), and glycine transporters (GlyT1 and GlyT2)22. Although no structure of GAT3 is available, several GAT1 structures have recently been reported and showed that GAT1 follows the canonical “LeuT” fold23, consisting of 12 transmembrane helices (TMs) and symports GABA together with 2 Na+ and 1 Cl− ions through a rocking-bundle mechanism24,25,26,27. TM1 and TM6 are unwound at the transporter core, forming the orthosteric substrate binding pocket. The relative position of the cytoplasmic end of TM1 modulates the intracellular gates formed among residues from TM1, TM6, and TM8, whereas the residues from the extracellular end of TM1, TM6, and TM10 form the extracellular gate24,28,29.
In the presence of a Na+/Cl− gradient across the plasma membrane, the transporter adopts an “outward-open” conformation, with a closed intracellular gate, while exposing the orthosteric binding pocket towards the extracellular lumen through an open extracellular gate. GABA binds to the orthosteric binding pocket, along with Na+ and Cl− ions. Once bound, GAT1 closes the extracellular gate, transitioning to the “occluded” conformation. Solvation of the Na+ binding site through the partial unwinding of the intracellular end of TM5 leads to Na+ release, which in turn facilitates the transition to an “inward-open” conformation and subsequent GABA release into the cytoplasm30.
Nipecotic acid is a GABA transporter substrate and can thus inhibit GABA transport competitively31,32. However, nipecotic acid is only moderately selective for GAT1 over GAT2 and GAT333. Nipecotic acid has been used as a scaffold for the development of a variety of GABA transporter inhibitors33,34,35,36,37,38. The FDA approved, nipecotic acid-derivative tiagabine is a potent, 10,000–20,000 fold selective and mixed-type inhibitor of GAT134,37,39. It competitively inhibits GABA transport, while also showing noncompetitive properties, suggesting an allosteric effect24,37,39. Based on these findings, we have previously proposed a two-step induced fit mechanism of inhibition24: First, tiagabine binds to the outward-open conformation of GAT1 in its orthosteric binding pocket, explaining competitive inhibition, followed by a transition to and subsequently stalling GAT1 in the inward-open conformation, thus, inhibiting the transporter noncompetitively24. Mutation and uptake studies identified Y60 as the key residue for tiagabine selectivity towards GAT124. Unlike GAT1, only moderately selective GAT3 inhibitors are available33,40. The high sequence similarity among GABA transporters makes it challenging to develop selective inhibitors. Although tiagabine is highly selective for GAT1, nipecotic-acid derivative SNAP-5114 shows 15–40 fold selectivity toward GAT3 over GAT1 whereas 5-(2-Thienyl)Isatin is ~167 fold selective for GAT333,40,41. 5-(2-Thienyl)Isatin is proposed to act as an allosteric modulator that might stabilize an outward-open conformation40. Subtype-selective inhibitors are important research tools to investigate the role of individual transporters in various physiological and pathological processes, as well as a therapeutic strategy for cell-type specific inhibition of GABA transport. Selective modulation of GAT3 transport activity will contribute to pharmacological evaluation of GAT3 as a potential target for the treatment of epilepsy, alcoholism, pain and neuroinflammation. Therefore, the identification of potent and selective inhibitors of GAT3 is of high importance. However, the mechanisms of GAT3-mediated GABA transport and inhibition are unknown. Despite its high importance as a potential therapeutic target, structural and functional studies of GAT3 are scarce.
To understand the molecular mechanism of GAT3 function and selective inhibition, in this study, we determine structures of human GAT3 in its apo form, and in complex with the selective inhibitor SNAP-5114 using cryo-electron microscopy (cryoEM). Our structures reveal the orthosteric substrate binding pocket and extensive network forming the extracellular gate. We observe a unique conformation of extracellular loop 4, which may have implications for extracellular gating and conformational transitions of GAT3. The SNAP-5114-bound structure and [3H]-GABA uptake assays, in combination with mutagenesis studies, provide insights into the mechanism of GAT3-selective inhibition. Our study will ultimately help to guide the rational structure-based design of GAT3-selective inhibitors as tool- and therapeutic compounds for the treatment of a spectrum of neurological and neurodegenerative disorders.
Results
Structure determination of GAT3
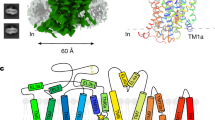
To gain insights into the transport and inhibition mechanisms of GAT3, we expressed and purified full-length wild-type human GAT3 (GAT3WT) from HEK293S GnTi− cells in the presence of SNAP-5114, however, structure determination efforts using cryoEM were unsuccessful (Supplementary Fig. 1). We adopted a recently described fiducial marker-based strategy to facilitate structure determination of GAT3, by an epitope graft, which enables antibody fragment (Fab) binding25. The epitope for Drosophila melanogaster DAT (dDAT) Fab 9D5 was grafted onto human GAT3 by incorporating mutations N327H, Y497S, D498E, N499D, E501R, Y506F, and R507P (Fig. 1a). The epitope-grafted GAT3 transports GABA and binds to the 9D5 (Supplementary Fig. 2). In [3H]-GABA uptake assays, epitope-grafted GAT3 displays inhibition by SNAP-5114 with a half-maximal inhibitory concentration (IC50) of 0.31 µM (95% confidence interval (CI) 0.24–0.41 µM), close to GAT3WT, with an IC50 of 0.45 µM (95% CI 0.36–0.58 µM) (Fig. 1b). The full-length GAT3 construct with the epitope graft, referred to as GAT3EM, was used for all subsequent structural studies using cryoEM.
a Sequence alignment between the epitope region of dDAT and hGAT3WT results in epitope-graft mutant GAT3EM. Mutations are highlighted in colored boxes. b [3H]-GABA uptake assay to measure inhibition potency of SNAP-5114 comparing GAT3WT and GAT3EM. The dot represents a single normalized data point with fit calculated for the average of all data points (three independent measurements (n = 3), each in triplicates). Source data are provided as a Source Data file. c CryoEM map of SNAP-5114-bound GAT3EM (forest green) with Fab 9D5 (gray) at 3.42 Å. SNAP-5114 in yellow. d CryoEM map of apo GAT3EM (light blue) with 9D5 (gray) at 3.51 Å.
We determined the cryoEM structure of the GAT3EM-9D5 complex in the presence of SNAP-5114 and 5-(2-thienyl)Isatin (“isatin”) at 3.42 Å resolution (Fig. 1c and Supplementary Fig. 3). Local resolution of the transmembrane region and orthosteric pocket of GAT3EM ranged between 2.6–3.2 Å, thus enabling side chain modeling. The density at the orthosteric pocket of GAT3 clearly resembled SNAP-5114 and allowed for unambiguous modeling of SNAP-5114. We could not detect any obvious density for isatin in the map, although it was proposed to bind in the “S2” site40. Therefore, the structure is referred to as GAT3SNAP. We speculated that the two compounds stabilize opposing conformational states, resulting in mutually exclusive binding. To obtain a 5-(2-thienyl)Isatin-bound structure, we determined a GAT3EM-9D5 structure in the presence of 100 µM isatin at 3.51 Å overall resolution with the local resolution of the transporter ranging between 2.6–3.2 Å (Fig. 1d and Supplementary Fig. 4). However, again no density for isatin was observed and the transporter was again locked in the inward-open conformation. Due to the lack of any discernible ligand density, the second structure is termed GAT3Apo. We observed structural heterogeneity at the cytoplasmic face of the orthosteric pocket among reconstructions, as discussed later in detail.
GAT3 architecture
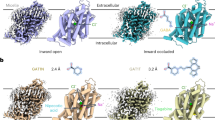
The overall structure of GAT3 is composed of 12 transmembrane helices (TM) arranged in two structurally related inverted repeats, connected through intracellular (ICL) and extracellular (ECL) loops, adopting the canonical LeuT fold (Fig. 2a). Superposition of GAT3 with inward-open GAT1 (PDB Id: 7Y7V)26, SERT (PDB ID: 7LI8)42 and GlyT1 (PDB ID: 6ZBV)43 displays similar structural arrangements, with root mean square deviation (RMSD) values of 0.88 Å, 1.38 Å, and 0.98 Å, respectively with major differences in the cytoplasmic end of TM1 and select ECLs (Fig. 2b). The N- and C-terminus of GAT3 are located at the cytoplasmic face with terminal residues (1-56 and 595-632) disordered. The C-terminus of GAT3 folds underneath the transporter and interacts with the helical segment in ICL1, potentially contributing to structural maintenance of the transporter (Fig. 2a). The ICLs and ECLs of GAT3 vary in length and contain short helical segments. The largest ECL connecting TM3 and TM4 (ECL2) includes the conserved disulfide bond between residues C171 and C180 potentially constraining the movement of ECL (Fig. 2b, top inset). The ECL2 and ECL4 fold on top of the transporter and plug the translocation path of GAT3. In NSSs, ECL4 contains two helical segments, EL4a and EL4b, that fold back onto the translocation path and participate in extracellular gate closing (Fig. 2b). In GAT3, the EL4a unwinds due to a potential steric clash with Y81 of TM1b. The unwound residues obtain a unique conformation among all the NSS structures (Fig. 2b, top inset). Consequently, residue E372 from ECL4 forms hydrogen bonds with W207 of helical segment in ECL2 and Y540 of ECL6, restricting access to the translocation path (Fig. 2b, top inset). Mutation of GAT1 EL4a residue S359 shows a modest effect on GABA interaction while weakening nipecotic acid-mediated GAT1 inhibition27. Therefore, the helical segments of ECLs likely plays an important role in transport, through modulation of required conformational states44,45. Analogous to other LeuT transporters, the core of TM1 and TM6 are unwound and are divided into TM1a, TM1b, and TM6a, TM6b, respectively. The TM5 is partially unwound at the GlyX9Pro motif leading to the potential solvation of the Na2 site30 (Fig. 2a, left). In addition, TM10 contains a one-turn π-helix that could be important for the transport activity of GAT346. Furthermore, TM1a at the GAT3 N-terminus sways away from the transporter core, exposing the orthosteric pocket to the cytoplasm (Fig. 2a, c). Consequently, the transporter adopts an inward-open conformation in the apo state. The conformation of TM1a is divergent from the inward-open conformations of GAT1 and SERT and most closely resembles GlyT1 (Fig. 2b, bottom inset).
a Model of GAT3Apo (light blue). TM helices are labeled with the corresponding number, along with an orthosteric substrate binding pocket (red circle) and extracellular gates (black rectangle). The Cl− ion is depicted as a green sphere. b Structural superposition of GAT3Apo with inward-open SERT (PDB Id: 7LI8), GlyT1 (PDB Id: 6ZBV), and GAT1 (PDB Id: 7Y7V). The differences in TM1a and ECLs are highlighted with a zoom-in view in the bottom and top insets, respectively. The partial unwinding of the EL4a helical segment in ECL4 leads to a unique conformation of ECL4 in GAT3 compared to other NSS transporters. ECL4 residue E372 interacts with W207 (ECL2) and Y540 (ECL6) and may participate in transport gating. The position of the orthosteric pocket is depicted as a red hexagon. The disulfide bond in GAT3 is depicted as sticks. c The orthosteric pocket of GAT3Apo. Cyan and green rectangles highlight the ceiling of the orthosteric pocket and Cl− coordination (green sphere). d Zoomed-in view of the extracellular gate (left inset), orthosteric pocket ceiling (right, top inset), and Cl− coordination (right, bottom inset). The color of the inset corresponds to positions in (a, c).
Structural studies of GAT1 in complex with GABA and nipecotic acid derivative inhibitors provided insights into the orthosteric binding pocket of GABA transporters, including residue responsible for the selectivity of tiagabine towards GAT1 over other GATs24,26,27. The structure of GAT3Apo provided insights into the orthosteric pocket of GAT3. In the transporter core, TM1, TM3, TM6, TM7, and TM8 with distal contributions from TM2, form the orthosteric pocket (Fig. 2c). The majority of orthosteric pocket residues are highly conserved throughout the NSS family, and particularly among GABA transporters (Supplementary Fig. 5a). The pocket shows a negative electrostatic potential; therefore, the presence of positive Na+ ions is important for the recognition and binding of substrate GABA (Supplementary Fig. 5b). The pocket is capped by the side chains of F308 of TM6a and Y147 of TM3 from the extracellular side (Fig. 2d, top right inset). Mutations in either of the equivalent residues, F294 and Y140, significantly reduce the transport activity of GAT147,48.
The extracellular gate, formed by TM1b, TM6a, and TM10 residues, is occluded. The sidechain guanidinium group of R75 forms a hydrogen bond network with Q305 of TM6a, D467 of TM10 α-helix, and S472 of TM10 π-helix (Fig. 2d, left). Additionally, the amine group of the indole moiety of W74 forms a hydrogen bond with the backbone carbonyl group of P375 of ECL4. The residues participating in extracellular gate closure are conserved and show comparable interactions as GAT1 (Supplementary Fig. 5a). The mutations in corresponding GAT1 residues W68, R69, and D451 significantly reduce the transport highlighting the importance of extracellular gating residues in transporter function28,29,49.
The TM10 π-helix is a conserved feature of the GABA transporter subfamily, compared to monoamine transporters of the SLC6 family (Supplementary Fig. 5a, c). As mentioned earlier, S472 participates in the extracellular gating of GAT3. In addition to R75, the hydroxyl group of S472 interacts with T304 of TM6a, further reinforcing the closure of the extracellular gate (Fig. 2d). In GAT1, the π-helix residues S456 and G457, equivalent to S472 and G473 in GAT3, play important roles in transport activity29. Substitutions of GAT1 G457 eliminated the transport activity, whereas S456 substitutions retained transport activity, although at a significantly lower level46. A comparison of inward-open GAT3 with an AlphaFold-predicted outward-open conformation showed that G473 acts as a hinge residue to facilitate the dilation of TM10 away from the translocation path and participates in extracellular gate opening. These comparisons suggest that the TM10 π-helix plays an important role in GABA transport and conformational dynamics of the transporter. However, further studies are required for a detailed examination of the role of this π-helix in GAT3 function.
GAT3 requires Na+ and Cl− gradients for GABA transport. We identified the putative Na+ and Cl− -binding sites of GAT3 by sequence and structural alignment with GAT1 (Supplementary Fig. 5a). The GAT3Apo cryoEM map shows higher resolution for the GAT3 transmembrane region (Supplementary Fig. 4c). We examined the cryoEM map for putative ion densities at these sites. We did not observe any density at either of the Na+ binding sites, however, a discernible density at the Cl− site was observed. The Cl− ion is coordinated by conserved residues Y92, Q305, S309, and S345 (Fig. 2d, bottom right inset) and the coordination is identical to GAT1 (Supplementary Fig. 5a). The S340E mutant mGAT4, equivalent to S345 of hGAT3, transports GABA in a Cl−-independent manner50. The glutamate substitution supposedly mimics the negative charge of chloride ions and enables Cl−-independent transport. Interestingly, GAT3 Q305 also interacts with R75 of TM1b and participates in the extracellular gate formation. GAT1 Q291, equivalent to Q305 of hGAT3, is critical for Cl− binding, and mutations in Q291 render GAT1 transport deficient and at loss of transport-coupled currents51,52. Therefore, the Cl− occupancy at the Cl−-binding site may participate in the translocation path gating. However, further studies are required to probe the link between transporter conformational dynamics and Cl− occupancy.
SNAP-5114 binding site
The GAT3 selective inhibitor SNAP-5114 is a nipecotic acid derivative that contains three 4-methoxyphenyl moieties, connected to nipecotic acid through a methoxyethyl bridge (Fig. 3a). To elucidate the structural basis of GABA transport inhibition, we determined a cryoEM structure of GAT3 in complex with SNAP-5114, and observed that GAT3, unexpectedly, adopts an inward-open conformation (Fig. 1c).
a Models of GAT3 inhibitor SNAP-5114, and substrate nipecotic acid and GABA. The chemical structures are drawn using ChemDraw (PerkinElmer). b Model of GAT3 (forest green cartoon) in inward-open conformation bound to SNAP-5114 (yellow sticks). Cl− is depicted as a green sphere. The location of TM1a is labeled. c Polar interaction network of SNAP-5114 at the GAT3 orthosteric pocket. d GAT3 residues participating in hydrophobic interactions with SNAP-5114’s piperidine ring (top) and lipophilic domain (bottom) are depicted as sticks.
SNAP-5114 binds at the orthosteric pocket and forms polar and hydrophobic contacts with the transporter (Figs. 1c and 3b–d). The nipecotic acid moiety of SNAP-5114 occupies an identical site as nipecotic acid, GABA, and tiagabine in GAT1 (Supplementary Fig. 6). The carboxylic acid group interacts with the backbone atoms of L70, G71, and S309 and the side-chain hydroxyl groups of Y147, like the interactions of substrate GABA and inhibitors tiagabine and nipecotic acid in GAT1 (Fig. 3c). The residue Y140 in GAT1, which corresponds to Y147 in GAT3, forms a hydrogen bond with the carboxyl group of GABA and is crucial for substrate binding48. The conserved residues interacting with the carboxylic group might be responsible for the limited selectivity of nipecotic acid for GABA transporters. The piperidine ring of nipecotic acid is stabilized through hydrophobic interactions with L143, Y147 of TM3, F308, A311, L314 of TM6, and C414 of TM8 (Fig. 3d, top). The 4-methoxyphenyl moieties form polar and hydrophobic interactions with residues from TM1a, TM2, TM6b, TM7, and TM8 (Figs. 3c, d, bottom). The 4-methoxy groups form hydrogen bonds with the backbone of L320 of TM6 and S410 of TM8, in addition to the sidechain of S410 (Fig. 3c). SNAP-5114 analogs that lack one, two, or all three 4-methoxy moieties show lower inhibition potencies at GAT3, whereas these analogs display higher potency for GAT1 than SNAP-511433. Furthermore, replacing two of the 4-methoxy moieties with the 4-methyl groups showed a modest decrease in inhibition33. The phenyl groups are stabilized in the pocket with hydrophobic contacts with E66, F104, C316, L317, L337, and V413 (Fig. 3d, bottom). SNAP-5114-derived analogs, in which one of the 4-methoxyphenyl moiety was replaced with smaller functional groups, showed a significant decrease in inhibitory potency highlighting the importance of three aromatic moieties in mGAT4 (hGAT3) inhibition35. The E66 residue caps the orthosteric pocket at the bottom towards the cytoplasm, limits solvent access, and likely prevents the release of SNAP-5114 from GAT3.
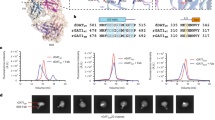
All GABA transporters (GATs) mediate the uptake of GABA and contain highly conserved residues in the orthosteric pocket for Na+, Cl−, and GABA interaction (Supplementary Fig. 5a). Structural comparisons of tiagabine-bound inward-open GAT1 and inward-open GAT3 in complex with SNAP-5114 showed that the nipecotic acid moieties of both inhibitors occupy a similar position and interact with conserved residues, however, the position and interactions of bis(3-methyl-2-thienyl) moiety of tiagabine and tris(4-methoxyphenyl) moiety of SNAP-5114 are divergent (Fig. 4a and Supplementary Fig. 6a, b). Although the orthosteric pocket is highly conserved among GAT1 and GAT3, four residues E66, C316, V413, and C414 differ from corresponding GAT1 residues Y60, S302, C399, and T400, respectively (Fig. 4b). Residues E66, C316, V413 and C414 show hydrophobic interactions with SNAP-5114 (Fig. 3d). SNAP-5114 inhibits GAT3 with 15–40 times more potency than GAT133,40,41,53. In our [3H]-GABA uptake assays, SNAP-5114 inhibits GABA transport by GAT1 with an IC50 of >100 µM, almost 100-fold higher compared to GAT3 (Supplementary Fig. 7f, h). In GAT1, the selectivity for tiagabine is mainly driven by residue Y6024. The equivalent residue of GAT1 Y60 in GAT3 is E66. Therefore, we hypothesized that residue E66 in GAT3 might have an influence on the selectivity of SNAP-5114 towards GAT3. The E66Y mutation decreased GAT3 expression and showed ~8-10% transport of wildtype (Supplementary Fig. 7a, b and g, h). Surprisingly, the E66Y mutation did not affect the IC50 of SNAP-5114, as measured in our [3H]-GABA uptake inhibition assay (Supplementary Fig. 7g-h). This finding suggested a different mechanism of SNAP-5114 selectivity in GAT3, compared to the selectivity of tiagabine towards GAT1. Accordingly, we mutated the remaining non-conserved residues in the GAT3 orthosteric pocket to equivalent GAT1 amino acids. We created three GAT3 mutants containing a single mutation at C316S, a double mutant V413C/C414T, and a triple mutant C316S/V413C/C414T. All mutants showed expression levels similar to GAT3WT, however, the transport level was decreased (Supplementary Fig. 7). The GAT3 triple mutant C316S/V413C/C414T increased the SNAP-5114 IC50 20–30-fold, displaying an additive effect concerning the single (C316S) and double (V413C/C414T) mutant (Fig. 4c). From a structural perspective, it is conceivable that the increased separation between TM1a and ICL3 in GAT3, compared to GAT1, increases the pocket volume enabling accommodation of a bulkier ligand moiety (tris(4-methoxyphenyl)) of SNAP-5114 (Fig. 4b). The introduction of a methyl group in one of the 4-methoxyphenyl moieties led to a modest improvement in subtype selectivity without affecting GAT3 inhibition potency54. In addition, nipecotic acid derivatives with either two or three phenyl groups in the lipophilic domain show distinct preferences toward GABA transporters36. The structure-activity relationship (SAR) studies suggest the importance of the bulkier tris-aryl moiety and GAT3-inhibitor hydrophobic interactions, driving subtype selectivity. Therefore, the differences in the inhibitor size, transporter orthosteric pocket residues, and volume might drive selectivity of SNAP-5114 towards GAT3, over GAT1. Our [3H]-GABA uptake assays support the binding of SNAP-5114 at the orthosteric pocket and provide insights into transporter-inhibitor interactions.
a Structural superposition of GAT1-tiagabine complex (PDB Id: 7SK2, orange) and SNAP-5114-bound GAT3 (forest green). The differences in TM1a and TM6b are highlighted with arrows, and the corresponding distances are labeled. b Differences in GAT1 and GAT3 orthosteric pocket with bound tiagabine and SNAP-5114, respectively. The non-conserved residues of GAT1 and GAT3 are shown as sticks. c Measurement of SNAP-5114 inhibition potency for wild-type (WT) and mutant GAT3 using [3H]-GABA uptake assay. The experiment was repeated in three biologically independent experiments (n = 3), each data point was measured in triplicates. The dots represent a single normalized data point with fit calculated for the average of all data points. d The output of [3H]-GABA uptake kinetic assays using GAT3-expressing HEK293F cells. The assay was performed with increasing concentrations of SNAP-5114, preincubated with cells, before the addition of [3H]-GABA. The experiment was repeated three times (n = 3). The dots represent a single normalized data point with fit calculated for the average of all data points. Source data are provided as a Source Data file.
Noncompetitive GAT3 inhibition by SNAP5114
Nipecotic acid-derived inhibitors show varying degrees of selectivity and inhibition mechanisms for GABA transporters. They have been described as substrate inhibitors, noncompetitive, or mixed-type inhibitors27,32,37. Tiagabine, a mixed-type inhibitor, is proposed to bind GAT1 in an outward-open conformation followed by its transition to inward-open conformation trapping the transporter upon Na+ release, through a two-step induced-fit mechanism of inhibition24. In the GAT3SNAP structure, SNAP-5114 also occupies the orthosteric pocket in the inward-open conformation (Fig. 3b–d). Therefore, we investigated the mechanism of SNAP-5114-mediated GAT3 inhibition by carrying out [3H]-GABA kinetic uptake experiments, in response to changes in SNAP-5114 concentration. We preincubated GAT3WT-expressing HEK293F cells with varying concentrations of SNAP-5114 and subsequently measured [3H]-GABA uptake levels to determine Michaelis constant (Km) and maximum reaction velocity at saturating concentration of substrate (Vmax). The Michaelis-Menten plots of SNAP-5114 showed a significant decrease in Vmax values with increasing SNAP-5114 concentration, however, Km values remained relatively constant, suggesting a noncompetitive mode of inhibition (Fig. 4d and Supplementary Fig. 8).
Noncompetitive inhibitors of SLC6 transporters act allosterically, and either directly bind at the allosteric S2 site in the extracellular vestibule, or lock the transporter in inward-open conformation55,56,57. Ibogaine, a noncompetitive inhibitor of SERT, stabilizes its inward-open conformation56. The conformationally selective Fab enabled the determination of the SERT-ibogaine structure in an outward-open conformation58. However, when a conformationally selective Fab was removed, SERT-ibogaine attained either an occluded or inward-open conformation depending on the presence of cations Na+ or K+58. Other examples of noncompetitive inhibitors stabilizing the inward-open conformation include the inhibition of GlyT1 by ALX-5407 and Cmpd143,59.
In the GAT3SNAP structure, SNAP-5114 binds to the transporter in its inward-open conformation. We speculate three possibilities for SNAP-5114-mediated inward-open conformation stabilization. (A) A two-step mechanism of noncompetitive inhibition in which SNAP-5114 binds at the outward-open conformation and rapidly transitions the transporter to the inward-open conformation, analogous to our model for tiagabine inhibition of GAT124. (B) SNAP-5114 diffuses across the plasma membrane and binds at the orthosteric pocket intracellularly. (C) SNAP-5114 utilizes a combination of possibilities A and B.
The introduction of polar groups might affect the plasma membrane diffusion, therefore, the potency of GAT3 inhibition should correlate with the degree of polarity. However, in SAR studies focused on the lipophilic domain of SNAP-5114, no correlation was observed between the polarity and inhibitory potency of the derivatives suggesting the mechanism based on diffusion across the plasma membrane might be less likely35. These observations suggest the two-step induced-fit mechanism of noncompetitive inhibition of GAT3 by SNAP-5114 (Fig. 5). In this mechanism, we propose that SNAP-5114 binds to the outward-open GAT3 conformation, along with Na+ and Cl− ions. Subsequently, GAT3 transitions into its inward-open conformation, through an intermediate occluded state. In the inward-open state, Na+ ions exit into cytoplasm, whereas SNAP-5114 and Cl− remain bound to GAT3. Stalling of GAT3 in the inward-open state disables GABA transport in a non-competitive manner (Fig. 5).
SNAP-5114 (red circle), along with Na+ and Cl− ions, bind to GAT3 in outward-open conformation. Upon binding, GAT3 transitions to inward-open via an intermediatory occluded conformation. Na+ ions are released into the cytoplasm, whereas the SNAP-5114 and Cl− remain bound. The cryoEM structure of GAT3 in the complex with SNAP-5114 corresponds to this state in which GAT3 obtains inward-open conformation with SNAP-5114 and Cl− bound to the transporter (dashed rectangle).
Discussion
GAT3-mediated GABA uptake participates in CNS maturation, GABA homeostasis and regulates GABAergic signaling7. Both enhancement and inhibition of GAT3 represent potential therapeutic avenues for treating neurodegenerative diseases and neurological disorders. In this study, we determined structures of apo, and inhibitor (SNAP-5114) bound GAT3 in the inward-open conformation, gaining structural and mechanistic insights into GAT3 transport and inhibition. Like the apo structure of GAT1, we did not observe any Na+ density in either structure, whereas a Cl− density was present suggesting GAT3Apo is captured in the post-Na+ and substrate-release state. Unexpectedly, SNAP-5114 inhibits GAT3 noncompetitively and traps the transporter in an inward-open conformation, post Na+ release (Figs. 1c, 3b, 4d). Our [3H]-GABA uptake assays of GAT3 mutants and the structural comparison with GAT1 suggested that subtype-selectivity is driven by the overall larger mass of SNAP-5114 and differences in orthosteric binding pocket residues and volume (Fig. 4 and Supplementary Fig. 7). The comparison of the GAT3 inward-open structure with other inward-open NSSs, two features of GAT3 were standing out: (A) the relative conformation of TM1a (Fig. 2b, bottom right inset) and (B) a unique conformation of EL4a (Fig. 2b, top right inset).
To gain insights into conformational transitions during the transport of GABA, we superimposed the inward-open GAT3SNAP structure with inward-occluded, GABA-bound GAT1 (PDB ID: 7Y7W, RMSD: 0.85 Å over 458 Ca atoms)26. The structural alignment showed that the intracellular half of the transporter undergoes noticeable conformational changes (Supplementary Fig. 9). As expected, TM1a sways outwards from TM6b, in the inward-open conformation (Supplementary Fig. 9, bottom inset). We observe an unwound TM5 in the inward-open GAT3 conformation, whereas TM5 is intact in the occluded conformation of GAT1 (Supplementary Fig. 9, top inset). Interestingly, the cytoplasmic half of GAT3 TM8 resembles inward-open GAT1, whereas GAT3 TM9 obtains a conformation comparable to inward-occluded GAT1. Conformational changes in TM8, an intermediate between inward-occluded and inward-open, might be responsible for starting the cascade for Na+, and subsequently, substrate release (Fig. 5). GAT1 residue D395, located at TM8, equivalent to GAT3 D409, coordinates Na+ at the Na2 site (Supplementary Fig. 5a). The conformational change in TM8 will disrupt Na+ coordination and lead to solvation and release of Na+. The release of Na+ from the Na2 site would disrupt the remaining Na+ at Na1 and substrate interactions with TM1a. The unwinding of the last helical turn of TM1a can lead to the opening of the intracellular gates to release substrate and Na+ (Na1) (Fig. 5).
Overall, our study provides insights into GAT3 architecture and the mechanism of noncompetitive and selective inhibition by SNAP-5114. Even though SNAP-5114 shows modest selectivity and µM potency, the GAT3 structure in complex with SNAP-5114 will provide the groundwork for future studies to identify potent and selective activators and inhibitors against this therapeutically important drug target. The discovery of novel and selective GAT3 ligands will enable identification of molecular mechanisms of GAT3 mediated GABA transport, neuroinflammation, and GABAergic signaling in alcohol use disorders, schizophrenia and epilepsy.
Limitations of the study
Detergent-solubilized GAT3 adopted an inward open conformation in its apo state and in complex with SNAP-5114. Our repeated trials to obtain an Isatin-bound GAT3 structure were unsuccessful. We hypothesize that Isatin-bound GAT3 obtains an outward-open conformation, however the low affinity of Isatin for GAT3 might be the limiting factor for determining a GAT3 structure in complex with Isatin. Alternatively, it is conceivable that a detergent environment or fiducial might influence the transporter conformation. Future structural studies of GAT3 with lipids in nanodiscs or liposomes are required to ascertain the effect of membrane lipids on the transporter conformation. Provided the fiducial 9D5 is added after incubation with ligand and initial purification, we hypothesize that it does not affect the transporter conformation. However, we cannot rule out the possibility of 9D5 fab biasing GAT3 towards inward-open conformation, and warrants further studies to examine 9D5 fab’s effect on transport activity and conformation of GAT3. We acknowledge that although our study proposes a mechanism of selective inhibition of GAT3, we do not report any structure-activity relationship (SAR) studies of GAT3 inhibitors. Nevertheless, we note that our study will aid in rational structure-based ligand discovery for GAT3.
Methods
Constructs and cell culture
Codon-optimized genes encoding full-length wildtype human gat3 (gat3WT) and epitope-grafted gat3 (gat3EM) were synthesized by Twist Biosciences. The gat3WT and gat3EM genes were subcloned into pBacMam60 and pcDNA3.1(-) (Invitrogen) vectors for expression and functional studies, respectively. The final constructs contained genes fused with a C-terminal human rhinovirus 3C protease (HRV-3C) cleavage site (LEVLFQ|GP) followed by a flexible linker (GSGGGGSGGGG), enhanced green fluorescent protein (eGFP), TwinStrep tag, and a His10-tag. The codon-optimized genes encoding 9D5 Fab heavy and light chains were synthesized and subcloned in the pFastBac-Dual expression vector (Genscript). The light and heavy chains contained N-terminal GP64 signal sequences under p10 and polyhedrin promoter control, respectively. The heavy chain C-terminal was fused with an HRV-3C protease site, a “GS” linker, and a His10-tag to facilitate affinity purification.
Proteins were expressed using a bac-to-bac expression system (Gibco) using baculovirus infection of either Spodoptera frugiperda Sf9 insect cells (Expression Systems) or human embryonic kidney (HEK) 293S GnTI− cells (American Type Culture Collection (ATCC) CRL-3022). Bacmids were prepared by transforming the expression vector into Escherichia coli DH10Bac competent cells. P1/P2 viruses were produced in Sf9 cells according to the manufacturer’s protocol and used for large-scale protein expression. Functional studies were performed in HEK293F (ATCC CRL-1573) cells transfected with wild-type or mutant GAT3-pcDNA3.1(-) and GAT1-pcDNA3.1(-). Sf9 cells were cultured in ESF921 protein-free insect cell culture media (Expression Systems). HEK293S GnTi− and HEK293F cells were maintained in FreeStyle 293 Expression Medium media (Gibco) supplemented with and without 1% fetal bovine serum (FBS) (Cytiva), respectively.
Expression and purification of 9D5
The Fab fragment 9D5 was expressed in Sf9 cells and secreted into media. For expression, Sf9 cells at 2 × 106 cells per ml density were infected with the P1 baculovirus at a multiplicity of infection (MOI) of 10. The media was supplemented with 1% protein boost additive (PBA) to increase the expression, and the cells were grown at 27 °C for 72 h. Following expression, cells were harvested by centrifugation at 2000 rpm (931 × g) for 20 min at 4 °C. The supernatant was collected, and its pH was adjusted to 7.5 by titrating with 1 M Tris-HCl pH 8.0. The supernatant was supplemented with MgCl2 and CaCl2 at a final concentration of 5 mM and ultracentrifuged at 200,000 × g for 25 min at 4 °C. The supernatant was incubated with Ni-NTA resin (Roche) for 3 h at 4 °C in the presence of an in-house produced protease inhibitor (PI) cocktail (2 mM AEBSF, 14 µM E-64, 1 µM leupeptin and 0.3 µM aprotinin) and 10 mM imidazole. Following incubation, the resin was collected in a gravity column and washed with 5 column volume (CV) wash buffer 1 (20 mM HEPES pH 7.5, 200 mM NaCl, 10 mM imidazole) and 10 CV wash buffer 2 (20 mM HEPES pH 7.5, 200 mM NaCl, 30 mM imidazole). The Fab was eluted with 6 CV of elution buffer (20 mM HEPES pH 7.5, 200 mM NaCl, 300 mM imidazole). Elution fractions were pooled, concentrated, and injected onto size-exclusion chromatography (SEC) column Superdex200 10/300 GL Increase (Cytiva) with buffer (20 mM pH 7.5 HEPES, 100 mM NaCl). SEC fractions corresponding to Fab were pooled, concentrated using 10-kDa cutoff centrifugal filter (Amicon, Merck Millipore), flash-frozen in liquid nitrogen in 100 µg aliquots at 1.14 mg/ml, and stored at −80 °C for future use.
Expression and purification of GAT3EM-9D5
The GAT3EM was expressed in HEK293S GnTI− cells. The baculoviruses were used to infect HEK293S GnTi− cells at a cell density of 2 × 106 cells per ml at an MOI of 5. The culture was supplemented with 10 mM sodium butyrate at the time of infection to boost protein production. Cells were grown at 37 °C and 5% CO2 for 72 h post-infection. Cells were harvested by centrifugation at 2000 rpm (931 × g) for 20 min at 4 °C and stored at −80 °C until further use.
The frozen cell pellets were thawed and resuspended in a low salt buffer (10 mM HEPES, 10 mM MgCl2, and 20 mM KCl) and PI cocktail. The cells were homogenized with a Dounce homogenizer, and then 1 M NaCl was added followed by more homogenization. The lysed cells were centrifuged at 186,000 × g for 35 min at 4 °C. This process was repeated one more time with the centrifuge pellet. Finally, the pellet/membranes were resuspended in storage buffer (10 mM HEPES, 10 mM MgCl2, 20 mM KCl, and 30% glycerol), flash-frozen using liquid nitrogen, and stored at −80 °C for future use.
For purification, membranes were thawed and incubated with 100 µM ligand, 2 mg per ml iodoacetamide, and PI cocktail for 30 min at 4 °C. The salt concentration was increased by adding HEPES pH 7.5 and NaCl to a final concentration of 20 mM and 200 mM, respectively. Furthermore, the membrane mixture was supplemented with DNaseI and solubilized with 1% lauryl maltose neopentyl glycerol (LMNG) (Anatrace) and 0.1% cholesteryl hemisuccinate (CHS) (Anatrace) at 4 °C while stirred for 3 h. The insoluble membranes were removed by centrifugation at 235,400 × g for 45 min at 4 °C. The supernatant was filtered through a 0.22 µm filter and incubated with Strep-Tactin®XT 4Flow® resin (IBA Lifesciences) for 90 min at 4 °C. The resin was collected in a gravity column and washed with 15 CV buffer (20 mM HEPES, 200 mM NaCl, 0.001% LMNG, 0.0001% CHS, ligand). The resin was resuspended in 1 CV buffer and collected in a 5 mL tube. The HRV-3C protease was added to the resin-buffer mixture and incubated for 1 h at 4 °C. For GAT3EM, 200 µg 9D5 Fab was added to the resin-buffer-protease mixture and incubated overnight at 4 °C. The next day, the mixture was poured into a gravity column and flow-through was collected. The resin was washed with 1 CV buffer and wash was collected and mixed with the flow-through. The protein was concentrated and injected on SEC column Superdex200 10/300 GL Increase (Cytiva) for further cleanup. SEC fractions corresponding to the GAT3EM-9D5 complex were pooled and concentrated for grid freezing.
The GAT3EM-9D5 complex purified with 25 µM 5-(2-thienyl)Isatin (AstaTech) supplemented in the buffer will be referred to as GAT3Apo from here onwards. Similarly, the GAT3EM-9D5 complex purified with SNAP-5114 (Sigma-Aldrich) and 5-(2-thienyl)Isatin ligands at 5 µM concentrations in the buffer will be called GAT3SNAP. The GAT3WT was also purified using the same procedure with the omission of the 9D5 Fab addition step during purification.
Thermostability measurement
Thermostability was measured using 7-diethylamino-3-(4’-maleimidylphenyl)-4-methyl coumarin (CPM) dye61,62. The reaction was initiated by incubation of 2.5 µg purified transporter with 4 µg/ml CPM in 100 µL total reaction volume made up of purification buffer for 15 min at 4 °C. The reaction mixture was subjected to temperature ramp-up in the range of 25–95 °C at 2 °C per minute rate using a RotorGene (Qiagen) real-time PCR instrument. The fluorescent signal was measured with a 350 nm excitation and a 440 nm emission filter. The melting temperature (Tm) was determined by fitting raw data by non-linear regression with the equation: Y = Bottom + (Top-Bottom)/(1 + (IC50/X)^HillSlope) in GraphPad Prism.
CryoEM sample preparation and data collection
Freshly purified GAT3EM-9D5 samples were concentrated to 3.3–3.6 mg/ml for vitrification. Quantifoil R1.2/1.3 Au 200-mesh grids were glow-discharged at 20 mA for 1 min using a PELCO easiGlo glow discharge system (Ted Pella). For vitrification, 2.5 µL of sample was applied and blotted for 4.0–4.5 s at 100% humidity, 4 °C and plunged into liquid ethane using a Mark IV Vitrobot (Thermo Fisher Scientific).
Single particle cryoEM data were collected on an FEI Titan Krios microscope G3i (Thermo Fisher Scientific) operated at 300 keV, equipped with a BioQuantum GIF energy filter (Gatan) with 20 eV slit width using EPU data acquisition software (ThermoFisher Scientific). Movies containing 50 frames were collected at a nominal magnification of 130,000× with 1.8 s exposure time and a total dose of 50–52 e−/Å2 using a K3 direct electron detector (Gatan, Amtrak) operating in counted super-resolution mode with 2× hardware binning, resulting in a nominal pixel size of 0.647 Å. The defocus range for the data collection was set from −0.8 µm to −3.4 µm.
CryoEM data processing
Multi-frame movies were aligned and motion-corrected using MotionCor263. All subsequent data processing was performed in cryoSPARC (v4.5.3; Structura Biotechnology) unless stated otherwise64. Motion-corrected micrographs were imported into cryoSPARC, and contrast transfer function (CTF) parameters were estimated using patch CTF estimation, with default parameters. Micrographs with CTF resolution estimates worse than 4 Å were discarded. Reference-free Blob Picker was used for particle picking on a subset of curated micrographs. These particles were subjected to two-dimensional (2D) classification and were used to obtain low-resolution (4-6 Å) reconstructions. These volumes were used to create templates for template picking. Particles for the full dataset were picked using Template Picker, inspected manually, and extracted with 512 pixels box size, Fourier cropped to 128 (bin = 4, pixel size = 2.588 Å). Two rounds of 2D classification were performed to remove junk particles, and subsequently, a clean particle stack was re-extracted (bin = 2, pixel size = 1.294 Å). Particles were subjected to iterative rounds of ab-initio reconstruction and heterogenous refinement. Finally, a three-dimensional (3D) volume with distinguishable features was refined using non-uniform refinement followed by local refinement using masks focused on either the entire or the transporter-only volume65. The global resolution was calculated using a gold-standard Fourier Shell Correlation (GSFSC) criterion of 0.143. The “Local resolution estimation” tool in cryoSPARC was used for local resolution calculation.
For the GAT3SNAP sample, after initial 2D classification-based filtering, a clean stack of 443,799 particles was extracted from 10,105 curated micrographs with 512 pixels box size (bin = 2, pixel size = 1.294 Å). The particles were subjected to iterative cycles of 2D classification, multi-class ab-initio reconstructions, and heterogeneous refinement. The particles from heterogeneous refinement output were reextracted (box size=512 pixels, pixel size = 0.647 Å) and classified using reference-free 3D classification. One class with 216,211 particles was further refined using non-uniform and local refinement and yielded a reconstruction at 3.42 Å resolution.
For the GAT3Apo sample, after initial processing, 817,311 particles were extracted from 8,242 curated micrographs with 512 pixels box size (bin = 2, pixel size = 1.294 Å). Another round of 2D classification yielded a clean stack of 559,079 particles. The particles were classified using multi-class heterogeneous refinement with one good class and three junk classes. The good class was obtained by refining a subset of particles using non-uniform refinement. The junk classes were created by terminating ab-initio jobs at iteration 100–120 and marking them complete. After two rounds of heterogeneous refinement, 259,404 particles were subjected to global CTF refinement and classified using reference-free 3D classification66. Subsequently, one 3D class with 158,075 particles was refined using non-uniform, and local refinement and reconstruction at 3.51 Å was obtained.
3D variability analysis
The conformational heterogeneity in the cryoEM maps was inspected using the 3D variability analysis (3DVA) module in cryoSPARC67. The particles and mask from the final reconstructions were used as input. A filter resolution of 6 Å was applied and “optimal” per-particle scale factors were used. The output was visualized in ChimeraX (UCSF) as a volume series68.
Model building and refinement
The AlphaFold predicted structure of human GAT3 (AlphaFold protein structure database entry AF-P48066-F1) and crystal structure of 9D5 Fab (PDB id: 4XP5) were used as initial models for rigid body fitting in UCSF ChimeraX68,69,70,71. Models were manually adjusted using Coot to fit the cryoEM map and refined in phenix.real_space_refine72,73. The ligand-restrained file was generated using eLBOW (Phenix)74. Molprobity was used to calculate the model geometry and validation statistics75. Model refinement statistics were generated in Phenix and are listed in Supplementary Table 1. Figures were prepared using ChimeraX, PyMOL (Schrodinger), and Adobe Illustrator 2020.
Radiolabeled [3H]-GABA uptake assay
Whole-cell radiolabeled GABA uptake assays under initial rate kinetics were performed with [3H]-GABA (specific activity 75.3 Ci/mmol; Revvity) to determine the half-maximal inhibitory concentration (IC50) of SNAP-5114 for wild-type and mutant GAT3. HEK293F cells were transfected with 2 µg DNA per million cells and polyethyleneimine (PEI) (DNA to PEI ratio of 1–2) at 1 × 106 cells per ml cell density. Forty-eight hours post-transfection, cells were diluted to 1 × 106 and seeded at 100,000 cells per well in poly-D-lysine coated 96-well plates (Corning) and incubated overnight. Cells were washed with assay buffer (10 mM HEPES, 150 mM NaCl, 1 mM MgSO4, 5 mM KCl, and 10 mM D-Glucose) twice and incubated with SNAP-5114 for 15 min at room temperature followed by an incubation with 30 nM [3H]-GABA and 25 µM unlabeled GABA for 45 min at room temperature. Next, cells were washed with assay buffer twice and lysed with 1% SDS in the assay buffer. Subsequently, MicroScint scintillation liquid (PerkinElmer) was mixed with the lysed cells. The mixture was transferred to Isoplate-96 plates (PerkinElmer/Revvity). Finally, the luminescence level was measured by counting for 1 min using a MicroBeta2 platereader (PerkinElmer). Data were analyzed using non-linear regression curve fitting (model: [Inhibitor] vs response—variable slope (four parameters); equation: Y = 1/(1 + (IC50/X)^HillSlope)) in GraphPad Prism. Assays were performed in duplicate or triplicates and repeated three times on separate days to account for transfection efficiency variation.
Radiolabeled [3H]-GABA kinetic uptake assay
Whole-cell radiolabeled GABA kinetic uptake assays were performed with [3H]-GABA (Revvity) to determine the mode of SNAP-5114 inhibition of GAT3. Transiently GAT3WT expressing HEK293F cells were plated into poly-D-lysine coated 96-well plates as mentioned above. The wells were washed with assay buffer twice and incubated with buffer/SNAP-5114 for 15 min at room temperature. The 1:1000 ratio of [3H]-GABA and unlabeled GABA dilution series (50 µM, 25 µM, 12.5 µM, 6.25 µM, 3.125 µM, 1.56 µM, 0.78 µM “cold” GABA) was added to the wells and incubated for 15 min at room temperature. Next, cells were washed with assay buffer twice and lysed with 1% SDS in the assay buffer followed by MicroScint scintillation liquid addition. The radioactivity level was measured by counting for 1 min using MicroBeta 2 Plate Counter (PerkinElmer). Data were analyzed using Michaelis-Menten kinetic fit in GraphPad Prism to calculate the Vmax and Km. Assays were repeated three times on separate days to account for transfection efficiency variation.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support this study are available from the corresponding authors upon request. The final cryoEM maps have been deposited in the Electron Microscopy Data Bank (EMDB) with the accession codes EMD-45799 (GAT3SNAP) and EMD-45800 (GAT3Apo). The atomic coordinates have been deposited in the Protein Data Bank (PDB) with the accession codes 9CP4 (GAT3SNAP) and 9CP5 (GAT3Apo). Source data are provided with this paper.
References
Watanabe, M., Maemura, K., Kanbara, K., Tamayama, T. & Hayasaki, H. GABA and GABA Receptors in the Central Nervous System and Other Organs. in International Review of Cytology Vol. 213 (ed Jeon, K. W.) 1–47 (Academic Press, 2002).
Tang, X., Jaenisch, R. & Sur, M. The role of GABAergic signalling in neurodevelopmental disorders. Nat. Rev. Neurosci. 22, 290–307 (2021).
Guastella, J. et al. Cloning and expression of a rat brain GABA transporter. Science 249, 1303–1306 (1990).
Borden, L. A., Smith, K. E., Hartig, P. R., Branchek, T. A. & Weinshank, R. L. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J. Biol. Chem. 267, 21098–21104 (1992).
Yamauchi, A. et al. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J. Biol. Chem. 267, 649–652 (1992).
Liu, Q. R., López-Corcuera, B., Mandiyan, S., Nelson, H. & Nelson, N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. J. Biol. Chem. 268, 2106–2112 (1993).
Minelli, A., DeBiasi, S., Brecha, N. C., Zuccarello, L. V. & Conti, F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 16, 6255–6264 (1996).
Ying, Y. et al. GABA transporter mGat4 is involved in multiple neural functions in mice. Biochim. Biophys. Acta Mol. Cell Res. 1871, 119740 (2024).
Kawamoto, M., Ohno, K., Kuriyama, K., Kubo, T. & Sato, K. Developmental changes in GABA transporter (GAT1 and GAT3) mRNA expressions in the rat olfactory bulb. Brain Res. Dev. Brain Res. 126, 137–145 (2001).
Hernandez-Rabaza, V. et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflammation 13, 83 (2016).
Keros, S. & Hablitz, J. J. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J. Neurophysiol. 94, 2073–2085 (2005).
Beenhakker, M. P. & Huguenard, J. R. Astrocytes as gatekeepers of GABAB receptor function. J. Neurosci. 30, 15262–15276 (2010).
Schleimer, S. B., Hinton, T., Dixon, G. & Johnston, G. A. R. GABA transporters GAT-1 and GAT-3 in the human dorsolateral prefrontal cortex in schizophrenia. Neuropsychobiology 50, 226–230 (2004).
Dong, Q., Kim, J., Nguyen, L., Bu, Q. & Chang, Q. An astrocytic influence on impaired tonic inhibition in hippocampal CA1 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 40, 6250–6261 (2020).
Zink, M., Vollmayr, B., Gebicke-Haerter, P. J. & Henn, F. A. Reduced expression of GABA transporter GAT3 in helpless rats, an animal model of depression. Neurochem. Res. 34, 1584–1593 (2009).
Augier, E. et al. A molecular mechanism for choosing alcohol over an alternative reward. Science 360, 1321–1326 (2018).
Dalby, N. O. et al. Anticonvulsant properties of two GABA uptake inhibitors NNC 05-2045 and NNC 05-2090, not acting preferentially on GAT-1. Epilepsy Res. 28, 51–61 (1997).
Narita, M. et al. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain 152, 1358–1372 (2011).
Kataoka, K., Hara, K., Haranishi, Y., Terada, T. & Sata, T. The antinociceptive effect of SNAP5114, a gamma-aminobutyric acid transporter-3 inhibitor, in rat experimental pain models. Anesth. Analg. 116, 1162–1169 (2013).
Cho, F. S. et al. Enhancing GAT-3 in thalamic astrocytes promotes resilience to brain injury in rodents. Sci. Transl. Med. 14, eabj4310 (2022).
Kruyer, A., Dixon, D., Angelis, A., Amato, D. & Kalivas, P. W. Astrocytes in the ventral pallidum extinguish heroin seeking through GAT-3 upregulation and morphological plasticity at D1-MSN terminals. Mol. Psychiatry 27, 855–864 (2022).
Navratna, V. & Gouaux, E. Insights into the mechanism and pharmacology of neurotransmitter sodium symporters. Curr. Opin. Struct. Biol. 54, 161–170 (2019).
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437, 215–223 (2005).
Motiwala, Z. et al. Structural basis of GABA reuptake inhibition. Nature 606, 820–826 (2022).
Nayak, S. R. et al. Cryo-EM structure of GABA transporter 1 reveals substrate recognition and transport mechanism. Nat. Struct. Mol. Biol. 30, 1023–1032 (2023).
Zhu, A. et al. Molecular basis for substrate recognition and transport of human GABA transporter GAT1. Nat. Struct. Mol. Biol. 30, 1012–1022 (2023).
Joseph, D., Nayak, S. R. & Penmatsa, A. Structural insights into GABA transport inhibition using an engineered neurotransmitter transporter. EMBO J 41, e110735 (2022).
Pantanowitz, S., Bendahan, A. & Kanner, B. I. Only one of the charged amino acids located in the transmembrane alpha-helices of the gamma-aminobutyric acid transporter (subtype A) is essential for its activity. J. Biol. Chem. 268, 3222–3225 (1993).
Ben-Yona, A. & Kanner, B. I. An acidic amino acid transmembrane helix 10 residue conserved in the neurotransmitter:sodium:symporters is essential for the formation of the extracellular gate of the γ-aminobutyric acid (GABA) transporter GAT-1. J. Biol. Chem. 287, 7159–7168 (2012).
Malinauskaite, L. et al. A mechanism for intracellular release of Na+ by neurotransmitter/sodium symporters. Nat. Struct. Mol. Biol. 21, 1006–1012 (2014).
Krogsgaard-Larsen, P. & Johnston, G. A. Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J. Neurochem. 25, 797–802 (1975).
Johnston, G. A., Stephanson, A. L. & Twitchin, B. Uptake and release of nipecotic acid by rat brain slices. J. Neurochem. 26, 83–87 (1976).
Dhar, T. G. et al. Design, synthesis and evaluation of substituted triarylnipecotic acid derivatives as GABA uptake inhibitors: identification of a ligand with moderate affinity and selectivity for the cloned human GABA transporter GAT-3. J. Med. Chem. 37, 2334–2342 (1994).
Borden, L. A. et al. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur. J. Pharmacol. 269, 219–224 (1994).
Böck, M. C., Höfner, G. & Wanner, K. T. N-Substituted Nipecotic Acids as (S)-SNAP-5114 Analogues with Modified Lipophilic Domains. ChemMedChem 15, 756–771 (2020).
Tóth, K., Höfner, G. & Wanner, K. T. Synthesis and biological evaluation of novel N-substituted nipecotic acid derivatives with an alkyne spacer as GABA uptake inhibitors. Bioorg. Med. Chem. 26, 3668–3687 (2018).
Braestrup, C. et al. (R)‐N‐[4, 4‐bis (3‐methyl‐2‐thienyl) but‐3‐en‐1‐yl] nipecotic acid binds with high affinity to the brain γ‐aminobutyric acid uptake carrier.J. Neurochem. 54, 639–647 (1990).
Knutsen, L. J. et al. Synthesis of novel GABA uptake inhibitors. 3. Diaryloxime and diarylvinyl ether derivatives of nipecotic acid and guvacine as anticonvulsant agents. J. Med. Chem. 42, 3447–3462 (1999).
Kragholm, B. et al. Discovery of a subtype selective inhibitor of the human betaine/GABA transporter 1 (BGT-1) with a non-competitive pharmacological profile. Biochem. Pharmacol. 86, 521–528 (2013).
Damgaard, M. et al. Identification of the first highly subtype-selective inhibitor of human GABA transporter GAT3. ACS Chem. Neurosci. 6, 1591–1599 (2015).
Höfner, G. & Wanner, K. T. Evaluation of GABA uptake in subcellular fractions of bovine frontal cortex and brainstem. Neurosci. Lett. 364, 53–57 (2004).
Yang, D. & Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 7, eabl3857 (2021).
Shahsavar, A. et al. Structural insights into the inhibition of glycine reuptake. Nature 591, 677–681 (2021).
Rannversson, H. et al. Importance of the extracellular loop 4 in the human serotonin transporter for inhibitor binding and substrate translocation. J. Biol. Chem. 290, 14582–14594 (2015).
Esendir, E. et al. Extracellular loops of the serotonin transporter act as a selectivity filter for drug binding. J. Biol. Chem. 297, 100863 (2021).
Dayan, O. et al. An extra amino acid residue in transmembrane domain 10 of the γ-aminobutyric acid (GABA) transporter GAT-1 Is required for efficient ion-coupled transport. J. Biol. Chem. 292, 5418–5428 (2017).
Dayan, O., Ben-Yona, A. & Kanner, B. I. The aromatic and charge pairs of the thin extracellular gate of the γ-aminobutyric acid transporter GAT-1 are differently impacted by mutation. J. Biol. Chem. 289, 28172–28178 (2014).
Bismuth, Y., Kavanaugh, M. P. & Kanner, B. I. Tyrosine 140 of the gamma-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 272, 16096–16102 (1997).
Kleinberger-Doron, N. & Kanner, B. I. Identification of tryptophan residues critical for the function and targeting of the gamma-aminobutyric acid transporter (subtype A). J. Biol. Chem. 269, 3063–3067 (1994).
Zomot, E. et al. Mechanism of chloride interaction with neurotransmitter: sodium symporters. Nature 449, 726–730 (2007).
Mari, S. A. et al. Role of the conserved glutamine 291 in the rat gamma-aminobutyric acid transporter rGAT-1. Cell. Mol. Life Sci. 63, 100–111 (2006).
Ben-Yona, A., Bendahan, A. & Kanner, B. I. A glutamine residue conserved in the neurotransmitter:sodium:symporters is essential for the interaction of chloride with the GABA transporter GAT-1. J. Biol. Chem. 286, 2826–2833 (2011).
Borden, L. A. et al. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Recept. Channels 2, 207–213 (1994).
Pabel, J. et al. Development of an (S)-1-{2-[tris(4-methoxyphenyl)methoxy]ethyl}piperidine-3-carboxylic acid [(S)-SNAP-5114] carba analogue inhibitor for murine γ-aminobutyric acid transporter type 4.ChemMedChem 7, 1245–1255 (2012).
Salomon, K. et al. Dynamic extracellular vestibule of human SERT: unveiling druggable potential with high-affinity allosteric inhibitors. Proc. Natl. Acad. Sci. USA. 120, e2304089120 (2023).
Jacobs, M. T., Zhang, Y.-W., Campbell, S. D. & Rudnick, G. Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J. Biol. Chem. 282, 29441–29447 (2007).
Plenge, P. et al. The antidepressant drug vilazodone is an allosteric inhibitor of the serotonin transporter. Nat. Commun. 12, 5063 (2021).
Coleman, J. A. et al. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 569, 141–145 (2019).
Wei, Y. et al. Transport mechanism and pharmacology of the human GlyT1. Cell 187, 1719–1732.e14 (2024).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Alexandrov, A. I., Mileni, M., Chien, E. Y. T., Hanson, M. A. & Stevens, R. C. Microscale fluorescent thermal stability assay for membrane proteins. Structure 16, 351–359 (2008).
Ayers, F. C., Warner, G. L., Smith, K. L. & Lawrence, D. A. Fluorometric quantitation of cellular and nonprotein thiols. Anal. Biochem. 154, 186–193 (1986).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci 32, e4792 (2023).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Varadi, M. et al. AlphaFold Protein Structure Database in 2024: providing structure coverage for over 214 million protein sequences. Nucleic Acids Res 52, D368–D375 (2024).
Wang, K. H., Penmatsa, A. & Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 521, 322–327 (2015).
Casañal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci 29, 1069–1078 (2020).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Acknowledgements
We thank K. Villers and D. Landaker for technical support for recombinant protein expression in mammalian and Sf9 cells; the late R. Oania for general laboratory support; T. Osinski at the USC Center for Advanced Research Computing (CARC) for support with computing resources and H. Khant for maintenance and help with operation of the cryoEM instrumentation. This research was supported by a US National Institutes of Health grant R01 GM144965-02 (to C.G.). We acknowledge the Center of Excellence for Nano Imaging (CNI) at the University of Southern California for microscope time.
Author information
Authors and Affiliations
Contributions
C.G. conceived, designed, and supervised the study. R.Y. performed sample preparation for the cryoEM and biochemistry experiments. R.Y. performed cryoEM data collection, data analysis, and data processing. R.Y. and G.W.H. performed model building and refinement. R.Y. and C.G. prepared figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Elena Bossi, Claus Loland, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yadav, R., Han, G.W. & Gati, C. Molecular basis of human GABA transporter 3 inhibition. Nat Commun 16, 3830 (2025). https://doi.org/10.1038/s41467-025-59066-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59066-w