Abstract
Synaptosomal-associated protein 25 kDa (SNAP25) is essential for vesicular trafficking and protein docking at presynaptic membranes in the nervous system, yet its role in the heart remains poorly understood. Here, we show an unrecognized function of SNAP25, which is selectively expressed in the atria, in regulating atrial electrical remodeling and the onset of atrial fibrillation (AF). SNAP25 protein is downregulated in the atria of AF patients. Cardiomyocyte-specific knockout of SNAP25 in male mice significantly shortens the atrial effective refractory period and action potential duration (APD), increasing susceptibility to AF, which is attributed to elevated Kv1.5 current and membrane expression. Blocking Kv1.5 channels effectively restores atrial APD and reduces AF incidence. Mechanistically, SNAP25 deficiency reduces the internalization of Kv1.5 from the cell surface membrane to early endosomes. In human iPSC-derived atrial cardiomyocytes, SNAP25 deficiency similarly elevates arrhythmic activity and accelerates repolarization. In conclusion, this study reveals that SNAP25 regulates AF susceptibility by controlling the trafficking of the atrial-specific Kv1.5 channel, highlighting SNAP25 as a promising therapeutic target for atrial arrhythmias.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in the clinic and can lead to various consequences, such as heart failure and stroke1,2,3,4,5. Despite its prevalence, our mechanistic understanding for the AF is still incomplete. Present antiarrhythmic drugs, primarily targeting ion channels, are often suboptimal and have the risk in promoting the fatal ventricular arrhythmias6,7,8,9. Increasing evidence suggests that regulating atrial-specific ion channels represents a significant hope for the intervention of AF10,11.
The onset of AF is multifaceted, often involving the remodeling of multiple ion channels, which leads to abnormal atrial action potential duration (APD) and atrial effective refractory period (ERP), ultimately contributing to the initiation and persistence of AF12,13,14,15. The dominant repolarizing K+ current in the atria, known as the ultrarapid delayed rectifier potassium current (IKur), is mediated by the potassium voltage-gated channel subfamily A member 5 (Kv1.5) channel, which is crucial for atrial repolarization and whose dysfunction is closely associated with AF16,17. Given the atrial-specific expression of Kv1.5 and its relationship with AF, targeting Kv1.5 has emerged as a promising therapeutic approach18,19,20,21. However, the regulatory mechanisms controlling the Kv1.5 channel remain largely unknown.
In excitable cells, the function and activity of ion channels rely on their proper localization and surface docking on the plasma membrane, a process that is dynamically regulated by endocytic and exocytic vesicle trafficking22,23,24. This process involves the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), and their interaction with ion channels can regulate the surface expression, localization and anchoring of these channels to the plasma membrane25,26. Synaptosomal associated protein of 25 kDa (SNAP25), a member of the SNARE complex, has been implicated in the regulation of vesicle trafficking in neuronal cells27,28. However, the presence and role of SNAP25 in the heart remain unexplored.
Here, we reveal an unexpected role of SNAP25 in regulating atrial electrical remodeling and susceptibility to AF through a specific mechanism involving the membrane trafficking of the atrial-selective Kv1.5 channel. This finding suggests that SNAP25 could be a potential target for AF intervention.
Results
SNAP25 is preferentially expressed in the atria
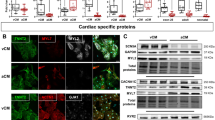
We first examined the expression of SNAP25 in adult mouse hearts using quantitative PCR (qPCR) and western blot analysis. The results showed that both SNAP25 mRNA and protein levels were significantly higher in the atria compared to the ventricles (Fig. 1a–c). Moreover, no significant difference in SNAP25 expression was observed between the right and left atria (Supplementary Fig. S1a, b). To determine the subcellular localization of SNAP25 in atrial cardiomyocytes, we performed immunofluorescence staining with anti-ANP (an atrial marker) and anti-SNAP25 antibodies. Confocal imaging revealed that SNAP25 was predominantly localized to the plasma membrane of atrial cardiomyocytes, with a weaker distribution observed in the cytoplasm (Fig. 1d). We also obtained atrial tissue samples from patients with or without atrial fibrillation (AF), and the detailed information of patients was provided in Supplementary materials (Supplementary Table 1). Western blot analysis showed a significant reduction in SNAP25 expression in atria from patients with AF compared to the sinus rhythm group (Fig. 1e, f). This finding was further supported by immunofluorescence analysis, which demonstrated a decreased expression of SNAP25 in atrial tissue from AF patients, as indicated by a weaker fluorescence signal (Fig. 1g, h). These results suggest that SNAP25 preferentially expresses in the atria and is downregulated in the atria of AF patients, implying a potential role of SNAP25 in pathological processes of AF.
a The mRNA expression of Snap25 in the atrium and ventricle of mice was detected by qPCR and its quantitative analysis, normalized to Gapdh levels (n = 4 samples per group) (P = 2.34e-5). The protein expression of SNAP25 in the atria and ventricles of mice was detected by western blot (b) and its quantitative analysis, normalized to GAPDH levels (c) (n = 4 samples per group) (P = 7.85e-5). d Immunofluorescence (IF) for detecting the subcellular localization of SNAP25 in mouse atrial cardiomyocytes (n = 3 samples per group, scale bar = 10 μm). SNAP25 (red), ANP (green), and DAPI (blue). The protein expression of SNAP25 in atria of the SR and AF patients was detected by western blot (e) and its quantitative analysis, normalized to GAPDH levels (f). The samples were taken from left atria (n = 5 samples per group). IF for detecting the localization and expression of SNAP25 in human atrial tissues (g) and quantitative analysis of mean fluorescence intensity (h) (n = 7 samples per group, scale bar = 50 μm). SNAP25 (red), ANP (green), and DAPI (blue). SR sinus rhythm, AF atrial fibrillation. Data are presented as mean ± SEM (a, c, f, and h). P values were calculated using two-tailed Student’s t-test. Source data are provided as a Source Data file.
Cardiomyocyte-specific knockout of SNAP25 increases susceptibility to AF
To explore the role of SNAP25 in the heart, we generated cardiomyocyte-specific SNAP25 conditional knockout (SNAP25 cKO) mice by deleting exon 4 using CRISPR-Cas9 (Fig. 2a). The knockout efficiency was confirmed through qPCR and western blot, showing a significant reduction in SNAP25 mRNA and protein levels in the atria of SNAP25 cKO mice compared to wild-type (WT) mice (Fig. 2b–d). Immunofluorescence analysis further confirmed the successful ablation of the SNAP25 in cKO mice, as shown in Fig. 2e, f. Echocardiographic analysis revealed normal cardiac structure and function (Supplementary Fig. S1c-e). Additionally, wheat germ agglutinin staining showed no differences in cardiac morphology between WT and SNAP25 cKO mice (Supplementary Fig. S1f, g), indicating no signs of cardiac dilation or hypertrophy. Baseline heart rate and electrocardiogram (ECG) parameters (PR, RR, QRS, and QTc intervals) also showed no significant differences between WT and SNAP25 cKO mice (Supplementary Fig. S2a, b). These findings suggest that SNAP25 deficiency does not affect baseline ventricular structure, function, or electrophysiology in mice.
a Schematic diagram of SNAP25 cKO transgene mice construct. b The mRNA level of atrial Snap25 in WT and SNAP25 cKO mice were detected by qPCR, and its quantitative analysis, normalized to Gapdh levels (n = 4 samples per group). The protein level of atrial SNAP25 in WT and SNAP25 cKO mice were detected by western blot (c), and the quantification of SNAP25 protein level (d) (n = 5 samples per group) (P = 5.82e-5). Immunofluorescent localization of SNAP25 in ANP-expressing atrial cardiomyocytes from WT and cKO mice (e), and pooled data for the mean fluorescence intensity across the cells (f) (n = 5 samples per group). SNAP25 (red), ANP (green), and DAPI (blue), scale bar = 10 μm. g Quantification of atrial ERP in WT and SNAP25 cKO groups (n = 5 samples per group). Representative simultaneous recordings of lead-2 surface ECGs and intracardiac ECGs in WT and SNAP25 cKO mice (h), and the bar graph shows the increased incidence of AF inducibility in SNAP25 cKO versus WT mice (i) (n = 11 samples per group). j The quantification of AF duration in WT and cKO mice (n = 11 samples per group). WT wild-type, cKO SNAP25 conditional knockout, ECGs electrocardiograms, AF atrial fibrillation, ERP effective refractory period. Data are presented as median with interquartile ranges (IQR) (b, j) or mean ± SEM (d-g, i). P values were calculated using Mann-Whitney test (b, j), two-tailed Student’s t-test (d-g), or Chi-square test (i). Source data are provided as a Source Data file.
To investigate whether SNAP25 deficiency promotes atrial arrhythmias, we conducted in vivo programmed electrical stimulation studies as previously described29. A Millar catheter was inserted into the right atrium, and intracardiac electrical stimulation was used to induce AF in anesthetized WT and SNAP25 cKO mice, in the presence of acetylcholine (1 mg/kg). Notably, surface ECG and intracardiac electrograms demonstrated that SNAP25 cKO mice were significantly more susceptible to acetylcholine- and pacing-induced AF compared to WT mice. Specifically, 72.7% (8 out of 11) of SNAP25 cKO mice developed AF, compared to 18.2% (2 out of 11) of WT mice (Fig. 2h, i). Meanwhile, the averaged AF duration was significantly longer in the cKO mice compared to the WT mice (Fig. 2j). These findings indicate that SNAP25 cKO mice have an increased susceptibility to AF.
Shortening of the atrial ERP contributes to increased susceptibility to AF in SNAP25 cKO mice
Since atrial effective refractory period (ERP) and conduction velocity (CV) are critical electrophysiological factors in the initiation and maintenance of AF12, we examined whether these parameters were altered in SNAP25 cKO mice. The results revealed a significant reduction in atrial ERP in SNAP25 cKO mice compared to WT mice using an S1-S2 pacing protocol, where a series of baseline stimuli (S1) at a cycle length of 100 ms were followed by a progressively premature extra stimulus (S2) decremented in 2-ms steps until loss of capture (Fig. 2g). To assess CV, we conducted optical mapping on Langendorff-perfused hearts from both groups. Activation maps showed no significant difference in atrial CV between SNAP25 cKO and WT mice (Supplementary Fig. S2c, d), indicating that atrial conduction was unaffected by SNAP25 deficiency. These findings suggest that SNAP25 deficiency promotes atrial electrical remodeling and AF primarily through the shortening of atrial ERP rather than alterations in atrial CV.
The shortening of atrial ERP corresponds to a reduction in atrial APD in SNAP25 cKO mice
The atrial ERP is closely related to the atrial APD29. As expected, membrane potential recordings from optical mapping revealed a shortened APD at 50% repolarization (APD50) in the SNAP25 cKO group (Fig. 3a, b). To further examine the impact of SNAP25 deficiency on atrial APD, we performed patch-clamp experiments on isolated atrial cardiomyocytes. In line with the optical mapping data, atrial cardiomyocytes from SNAP25 cKO mice showed significant shortening of both APD50 and APD at 90% repolarization (APD90) under 1 Hz pacing, compared to WT mice (Fig. 3c, d). These findings indicate that SNAP25 deficiency contributes to atrial electrical remodeling, characterized by a shortened atrial ERP and APD, which increases susceptibility to AF.
a Typical optical APD50 maps and AP signal records in perfused hearts. b Statistical and quantitative analysis of APD50 in WT and SNAP25 cKO mice (n = 5 samples per group). c Representative AP traces of single atrial cardiomyocyte from WT and SNAP25 cKO mice. d Statistical and quantitative analysis of APD50 (P = 2.72e-7) and APD90 (P = 1.41e-6) in atrial cardiomyocytes from WT and SNAP25 cKO mice (n = 6 samples per group). APD, action potential duration; APD50, APD at 50% repolarization; APD90, APD at 90% repolarization. Data are presented as mean ± SEM. P values were calculated using two-tailed Student’s t-test. Source data are provided as a Source Data file.
The shortening of atrial APD in SNAP25 cKO mice is attributed to the upregulation of Kv1.5
Ion channel remodeling is widely regarded as the primary electrical substrate responsible for shortening atrial APD during AF13,30. To further investigate the ionic mechanisms underlying the shortened atrial ERP and APD observed in SNAP25-deficient atria, we used whole-cell patch-clamp techniques to measure key ion channel currents that shape action potential (AP) morphology, including transient outward potassium current (Ito), ultrarapid delayed rectifier potassium current (IKur), sodium current (INa), and L-type calcium current (ICa, L). The results showed no significant differences in Ito densities between WT and SNAP25 cKO mice. In contrast, we observed an enhancement of IKur in SNAP25-deficient atrial cardiomyocytes compared to the WT group (Fig. 4a–c). Additionally, evaluations of INa and ICa, L revealed no significant differences in their densities between SNAP25-deficient and WT atrial cardiomyocytes (Fig. 4d–g). These findings suggest that SNAP25 deficiency leads to the enhancement of the Kv1.5 current.
a Peak Ito and IKur current traces of atrial cardiomyocytes from WT and SNAP25 cKO groups. Cells were depolarized from −40 mV to +50 mV in 10 mV steps. Inset shows voltage protocol. b, c Peak current amplitudes normalized to cell capacitance, and current-voltage curves for peak Ito (b) and IKur (c) (n = 3 samples per group). Representative traces of voltage-dependent changes of late INa in single atrial cardiomyocyte from WT and SNAP25 cKO mice (d), and summarized current-voltage relationship for late INa (e). Cells were depolarized from −90 mV to +30 mV in 5 mV steps. Insert shows schematic voltage-clamp protocol (n = 3 samples per group). Representative ICa, L traces from WT and SNAP25 cKO mice (f), and summarized current-voltage relationship for ICa, L densities (g). Cells were depolarized from −60 mV to +60 mV in 5 mV steps. Insert shows schematic voltage-clamp protocol (n = 3 samples per group). IKur, ultrarapid delayed rectifier potassium current; Ito, transient-outward K+ current; INa, Na+ current; ICa, L, L-type Ca2+ current. Data are presented as mean ± SEM (b, c, e, and g). P values were calculated using two-way ANOVA. Source data are provided as a Source Data file.
IKur, encoded by the Kv1.5 channel, is a key component of the human atrial repolarizing K+ current17. Consistent with the increased IKur observed, SNAP25 deletion led to enhanced Kv1.5 expression in atrial membrane fractions and reduced its expression in cytoplasmic components (Supplementary Fig. S3a, b). These findings indicate that SNAP25 deficiency results in the upregulation of atrial-specific Kv1.5 membrane expression, along with increased IKur currents, which may provide a molecular mechanism for the shortened atrial ERP and APD.
The Kv1.5 blocker alleviates the shortening of atrial APD and reduces the occurrence of AF in SNAP25 cKO mice
To further confirm that the upregulation of Kv1.5 membrane expression in atrial cardiomyocytes is responsible for the shortening of atrial APD in SNAP25-deficient cells, we used the selective Kv1.5 blocker diphenyl phosphine oxide-1 (DPO-1, 1 µM) to pharmacologically inhibit Kv1.5 and assess its role in APD shortening. Both optical mapping and patch-clamp experiments demonstrated that Kv1.5 inhibition with DPO-1 restored the APD in SNAP25-deficient atria to levels comparable to those of WT mice (Fig. 5a–d), indicating that the upregulated Kv1.5 membrane expression is indeed responsible for the APD shortening in SNAP25 cKO mice.
a Typical optical APD50 maps and AP signal records in perfused hearts from WT and SNAP25 cKO mice treated with DPO-1 (1 µM). b Statistical and quantitative analysis of APD50 (n = 5 samples per group). c Representative AP traces of single atrial cardiomyocyte from WT and SNAP25 cKO mice treated with DPO-1. d Statistical and quantitative analysis of APD50 and APD90 (n = 5 samples per group). Representative simultaneous recordings of lead-2 surface ECGs and intracardiac ECGs in WT and SNAP25 cKO mice treated with DPO-1 (e), and the bar graph shows the incidence of AF in the WT and SNAP25 cKO mice treated with DPO-1 (f) (n = 5–8 samples per group). g The quantification of AF duration in WT and SNAP25 cKO mice treated with DPO-1 (n = 5 samples for WT group, n = 8 samples for cKO group). Data are presented as mean ± SEM (b, d, and f) or median with interquartile ranges (IQR) (g). P values were calculated using two-tailed Student’s t-test (b, d), Chi-square test (f), or Mann-Whitney test (g). DPO-1, diphenyl phosphine oxide-1; ns, not significant. Source data are provided as a Source Data file.
Since SNAP25 deficiency enhances Kv1.5 membrane expression, contributing to atrial electrical remodeling and AF susceptibility, we further investigated whether Kv1.5 inhibition with DPO-1 could prevent atrial arrhythmogenesis in SNAP25 cKO mice. Two weeks after tamoxifen injections, both cKO and WT mice were treated with DPO-1 (0.5 mg/kg). The results showed that DPO-1 effectively prevented both the induction and maintenance of AF in SNAP25 cKO mice (Fig. 5e–g). These findings collectively suggest that Kv1.5 blockade mitigates atrial electrical remodeling and reduces AF occurrence in SNAP25 cKO mice.
SNAP25 accelerates the internalization of Kv1.5 channel from the surface membrane to early endosomes
To elucidate the mechanisms behind the increased surface expression of Kv1.5 in atrial cardiomyocytes following SNAP25 deletion, we performed a co-immunoprecipitation assay using mouse atrial tissue lysates. This assay revealed that a significant portion of SNAP25 co-immunoprecipitated with Kv1.5, confirming their interaction (Fig. 6a). Immunofluorescence staining further demonstrated that SNAP25 and Kv1.5 co-localized on the plasma membrane of atrial cardiomyocytes, with minimal overlap in the cytoplasm (Fig. 6b). Moreover, super-resolution structured illumination microscopy (SIM) revealed that SNAP25 and Kv1.5 are spatially co-localized and modulate the trafficking of Kv1.5 to the plasma membrane of atrial cardiomyocytes (Supplementary Fig. S3c). These findings suggest that SNAP25 may regulate the translocation of Kv1.5 between the cell surface and intracellular compartments.
a SNAP25 co-immunoprecipitated with Kv1.5 in mouse atrial tissues. The immunoprecipitating antibody is indicated above each lane (n = 3–5 samples per group). b IF imaging of Kv1.5 (red) and SNAP25 (green) in isolated mouse atrial cardiomyocytes using confocal microscopy, with yellow indicating colocalization. Zoom-in demonstrating the local association of SNAP25 with Kv1.5. White boxes indicate the orientation of the signal intensity profiling (n = 3–5 samples per group, scale bar = 10 μm). Colocalization analysis revealed a high percentage of overlapping SNAP25 and Kv1.5. c IF imaging of Kv1.5 (red) and Cav-3 (green) or EEA1 (green) in isolated mouse atrial cardiomyocytes using super-resolution structured illumination microscopy (SIM). Pearson’s correlation coefficients were performed for assessing the colocalization levels of Kv1.5 with Cav-3 or EEA1 (P = 9.05e-9) in WT and SNAP25 cKO group. Colocalization analysis displayed the colocalization of Kv1.5 and Cav-3 in plasma membrane of atrial cardiomyocyte from SNAP25 cKO mice, and the colocalization of Kv1.5 and EEA1 in cytoplasm of atrial cardiomyocyte from WT mice. Scale bar = 10 μm (n = 14 cells per group). Western blot detected the expression of surface proteins of SNAP25 and Kv1.5 in atrial tissues from WT, SNAP25 cKO, and SNAP25 cKO+Dyn group (d), and quantitative analysis (e) (n = 3 samples per group). IF Immunofluorescence, EEA1 early endosome antigen 1, Dyn Dynasore, Cav-3 Caveolin-3, ns not significant. Data are presented as median with interquartile ranges (IQR) (c, up) or mean ± SEM (c, down; e). P values were calculated using Mann-Whitney test (c, up), two-tailed Student’s t-test (c, down) or one-way ANOVA (e). Source data are provided as a Source Data file.
Caveolin-3, a structural protein associated with sarcolemma invaginations in the T-tubules, is predominantly localized in the plasma membrane of cardiomyocytes31. To investigate the distribution of Kv1.5 and caveolin-3, we performed immunostaining on atrial cardiomyocytes from WT and SNAP25-deficient mice. In WT cells, Kv1.5 was widely distributed throughout the cytoplasm, however, in SNAP25-deficient cells, Kv1.5 showed enhanced co-localization with caveolin-3 on the plasma membrane (Fig. 6c), indicating increased membrane localization of Kv1.5 in the absence of SNAP25.
To determine if SNAP25 regulates Kv1.5 internalization via the endosomal pathway, we examined Kv1.5 co-localization with early endosome antigen 1 (EEA1), an early endosome marker, using super-resolution SIM. The analysis revealed Kv1.5 co-localizing with EEA1 in early endosomes (Fig. 6c). We then used Dynasore, a dynamin inhibitor that disrupts endocytosis by preventing the scission of vesicles, to explore the role of endocytosis. Atrial cardiomyocytes from WT and SNAP25 cKO mice were treated with 1 µM Dynasore, followed by western blot to assess Kv1.5 and SNAP25 membrane expression. Dynasore treatment effectively blocked the increase in Kv1.5 membrane expression seen in SNAP25-deficient cells (Fig. 6d, e), suggesting that the constitutive exocytosis of Kv1.5 is disrupted. Overall, our results indicate that SNAP25 deletion promotes Kv1.5 membrane trafficking through a constitutive exocytosis process.
SNAP25 deficiency increases arrhythmogenicity in human induced pluripotent stem cell-derived atrial cardiomyocyte monolayers
To further establish the connection between SNAP25 deficiency and increased AF susceptibility, we employed human induced pluripotent stem cell-derived atrial cardiomyocytes (iPSC-aCMs) as a preclinical model for arrhythmia induction32,33,34. Small interfering RNA targeting SNAP25 (si-SNAP25) was designed and transfected into cultured human iPSC-aCMs. The knockdown efficiency was confirmed via qPCR and western blot, both of which revealed a significant reduction in SNAP25 mRNA and protein levels in the si-SNAP25 group compared to the si-NC group (Fig. 7a–c). Following transfection, the human iPSC-aCMs were exposed to 1 µM CoCl2 to induce arrhythmias. Multi-electrode array (MEA) recordings showed that 70% of cells in the SNAP25 knockdown group exhibited arrhythmogenic activity with beat-to-beat irregularity, mimicking an AF-like phenotype, whereas only 12.5% of cells in the si-NC group showed abnormal rhythms while treatment with DPO-1 (1 µM) significantly reduced AF inducibility, with only 20% of cells showing an AF phenotype compared to 70% in the vehicle-treated group (Fig. 7d, e).
a Relative mRNA expression of SNAP25 in si-NC and si-SNAP25 group; GAPDH is used for the loading control (n = 3 independent experiments in each group) (P = 8.8e-4). Representative western blot bands (b) and normalized expression of SNAP25 protein expression in si-NC and si-SNAP25 group (c) (n = 3 independent experiments in each group). Representative MEA traces from human iPSC-aCMs monolayers (d), and a summary of the incidence of human iPSC-aCMs arrhythmia in si-NC, si-SNAP25, and si-SNAP25 + DPO-1 group (e) (n = 8-10 independent experiments in each group). f Representative AP traces of human iPSC-aCMs from si-NC, si-SNAP25, and si-SNAP25 + DPO-1 group. g Statistical and quantitative analysis of APD50 and APD90 in si-NC, si-SNAP25, and si-SNAP25 + DPO-1 group (n = 5 independent experiments in each group). Data are presented as mean ± SEM (a, c, e, and g). P values were calculated using two-tailed Student’s t-test (a, c), Chi-square test (e), or one-way ANOVA (g). Source data are provided as a Source Data file.
We also assessed the effect of SNAP25 knockdown on APD in the human iPSC-aCMs using the patch-clamp technique. The results demonstrated that SNAP25 knockdown led to a significant shortening of both APD50 and APD90 compared to the si-NC group, and treatment with DPO-1 reversed the APD shortening in the si-SNAP25 group to levels comparable to those in the control group (Fig. 7f, g). These findings, consistent with results from SNAP25 cKO mice, reinforce the conclusion that SNAP25 deficiency contributes to AF onset through APD shortening, driven by the upregulation of Kv1.5 membrane expression.
Discussion
Although SNAP25 has been widely studied in neurons27,35, its role in the heart is largely unexplored. Our study provides insights into the previously unrecognized function of SNAP25, a SNARE complex protein36,37 selectively expressed in the atria, in regulating the membrane trafficking of atrial Kv1.5, thereby influencing atrial electrical remodeling and contributing to AF onset.
In this study, cardiomyocyte-specific deletion of SNAP25 resulted in the redistribution of Kv1.5 to the plasma membrane, increasing the density of IKur. This change contributed to the shortening of atrial ERP and APD, thereby enhancing susceptibility to AF in mice. Compared to sinus rhythm, SNAP25 protein levels were downregulated in the atria of both in mice and patients with AF. And immunofluorescence studies further revealed co-localization of SNAP25 and Kv1.5 in mouse atrial cardiomyocytes, with SNAP25 mediating the internalization of Kv1.5 from the cell surface into early endosomes, thus negatively regulating Kv1.5 membrane trafficking. Electrophysiological analysis of human iPSC-aCMs further demonstrated that SNAP25 knockdown increased the induction of AF-like arrhythmias. These findings highlight the role of SNAP25 in modulating atrial electrical remodeling and AF through its regulation of Kv1.5 membrane trafficking.
The biological regulation of Kv1.5 is complex, with its modulation via intracellular trafficking being a key aspect of normal and abnormal cell electrophysiology22,23,24,29,38. In this study, we uncovered a mechanism regulating Kv1.5 membrane expression and current based on vesicular transport. The processes governing the metabolism of Kv1.5 in atrial cardiomyocytes are not yet fully understood. Increased surface expression of ion channels might result from enhanced protein synthesis, metabolic dysfunction, or reduced internalization for degradation39,40. Our findings suggest that SNAP25 deficiency influences Kv1.5 surface expression by modulating its endocytosis. This function parallels the roles of the dynein motor complex23, myosin-V motor proteins24, and redox-sensitive sulfenic acid modification38 in Kv1.5 membrane trafficking. Collectively, our study identifies a previously unappreciated pathway critical for the regulation of Kv1.5 trafficking during atrial electrical remodeling.
Traditional anti-arrhythmic drugs often produce non-specific ventricular side effects due to their lack of selectivity for specific ion channels6,7. Kv1.5, an ion channel predominantly expressed in the atria, has emerged as a crucial therapeutic target for AF. Notably, a marked translational gap persists between the therapeutic efficacy demonstrated in large animal models and clinical outcomes in AF patients41. This disparity may arise from two principal factors: the inadequate target specificity of the existing inhibitors and species-specific variations in Kv1.5 channels42. Therefore, the development of next-generation Kv1.5-selective modulators with improved pharmacodynamic properties, coupled with novel drug delivery technologies, may overcome these challenges and enhance clinical outcomes in human populations.
The class I anti-arrhythmic drug quinidine has been shown to induce dose-dependent internalization of Kv1.5 in atrial cardiomyocytes18. Additionally, microtubules and the actin cytoskeleton are involved in regulating Kv1.5-mediated currents, with disruptions in Kv1.5 trafficking linked to AF and sudden death in mice23,24,43. However, upstream regulatory molecules targeting Kv1.5 are not atria-specific, limiting their clinical applicability. Our findings suggest that SNAP25, a molecule preferentially expressed in the atria, selectively modulates the surface density of Kv1.5, providing a promising and potentially safer approach for AF therapy.
The human iPSC-aCMs serve as a preclinical model for studying the mechanisms underlying arrhythmia induction32,33. In our study, knockdown of SNAP25 in the human iPSC-aCMs s increased the incidence of abnormal arrhythmias with beat-to-beat irregularity, mimicking an AF-like phenotype. Although human iPSC-aCMs offer valuable insights into AF electrophysiology, they remain developmentally immature and do not fully replicate the characteristics of adult atrial myocardium44,45. Given the significant anatomical, histological, and physiological differences between animals and humans, outcomes from animal models may not be simply transferred to human studies46. However, we validated our findings in the human iPSC-aCMs, suggesting that the role of SNAP25 in regulating atrial electrophysiology may also apply to humans. These results enhance the potential of SNAP25 as a therapeutic target for human AF, highlighting its clinical translational significance.
Other SNARE family members may also influence Kv1.5 membrane trafficking, but we have not examined their roles individually. Additionally, while we utilized cardiomyocyte-specific SNAP25 knockout mice, we did not assess SNAP25 expression and function in other cell types, such as fibroblasts, immune cells, and endothelial cells, leaving open the possibility of their indirect involvement in the pathogenesis of AF. Currently, there are no available activators for either SNAP25 or the SNARE complex, complicating effective rescue trials with tool compounds. This limitation could potentially be addressed by atrial-specific overexpression of SNAP25 using an ANP promoter-driven adeno-associated virus as a gene transfer vector47,48, which should be validated in future studies.
In summary, our study identifies SNAP25 as a novel atrial-specific molecule that regulates atrial electrical remodeling and susceptibility to AF by modulating the membrane expression and function of the atria-specific Kv1.5 channel, highlighting it as a potential new target for AF intervention.
Methods
Animals
This study followed the guidelines outlined in the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the Animal Care and Use Committee of Tongji University School of Medicine. The researchers were blinded to the mice genotypes during the experiments and data analysis.
Ethics and acquirement of human atrial samples
The acquisition of human atrial samples was approved by the ethics committee of Union Hospital, Wuhan, China, and was in compliance with the principles outlined in the Declaration of Helsinki. Atrial tissue samples were collected from the patients with sinus rhythm (SR) or atrial fibrillation (AF) who underwent surgical surgery. Written informed consent was obtained from all the participants.
Generation of SNAP25 cKO mice
Cardiomyocyte-specific SNAP25 conditional knockout (SNAP25 cKO) mice were generated using the Cre/LoxP system. Briefly, Snap25flox/flox; α-MHC Cre mice were generated by crossing the floxed SNAP25 (Snap25flox/flox) and the inducible cardiac-specific Cre (α-MHC Cre) transgenic mice. SNAP25 cKO mice were established by intraperitoneal injection of 10 mg/mL tamoxifen (T5684, Sigma) continuously for 5 consecutive days in Snap25flox/flox; α‐MHC Cre mice at the age of 6-8 weeks. Littermate male Snap25flox/flox and α-MHC Cre mice were considered as the controls. Snap25-floxed mice were maintained on a genetic background of C57BL/6 J. All mice were housed in temperature-controlled cages (18-25 °C and 40-60% humidity) with a 12 h light–dark cycle, and given free access to water and food. Echocardiographic parameters were assessed 2 weeks after injection, and the hearts were harvested for electrophysiological and molecular studies. All experiments using mice were performed in a blinded manner.
Echocardiography analysis
To evaluate left ventricular function and dimension, transthoracic two-dimensional echocardiography was performed on mice under general anesthesia (inhalation of 2% isoflurane, 0.8-1.2 L/min) using a Visual Sonics Vevo 770 ultrasound system (Visual Sonics) equipped with a 30 MHz linear array transducer. M-mode tracings in the parasternal short axis view were used to measure left ventricular anterior and posterior wall thicknesses and left ventricular internal diameters at end-systole and end-diastole, which were used to calculate left ventricular fractional shortening and the ejection fraction according to standard formulas.
ECG recording and programmed intracardiac stimulation
The mice were anesthetized with isoflurane (2.0%) (VME, Matrx, USA) and fixed on the console. A surface echocardiogram (ECG) was recorded (FE132-0641, AD instruments Co Ltd., New South Wales, Australia) and analyzed with LabChart 8 (AD instruments Co Ltd., New South Wales, Australia).
Programmed intracardiac stimulation was performed to assess atrial fibrillation (AF)-inducibility as previously described49. Briefly, mice were anesthetized with 2.0% isoflurane in 100% oxygen (1.0 L per minute) and a 1.1 F octapolar electrophysiology catheter (EPR-800, Millar Instruments, Houston, TX) was inserted via the jugular vein into the right atrium and right ventricle. Data was acquired using a computer-based system (Emka Technologies, Falls Church, VA) and data was recorded via a surface ECG and intracardiac bipolar electrograms. Then, the mice received intraperitoneal injection of acetylcholine (1 mg/kg, 0.1 mL) within 5 seconds. Next, programmed intracardiac stimulation was performed in the right atria through 2 ms current pulses with an external stimulator (STG-3008, MultiChannel Systems, Reutlingen, Germany) after acetylcholine administration. The atrial effective refractory period (ERP) was obtained by standard clinical pacing protocols and defined as the longest S1-S2 interval failing to generate a propagated beat in atria with S2 based on. AF was defined as the occurrence of rapid, fragmented atrial electrograms with irregular R-R intervals lasting at least 1 second. The incidence of inducible AF was calculated as the percentage of the AF-positive mice divided by the total number of mice studied.
Optical mapping
Optical mapping was performed as described previously50. Briefly, hearts were dissected and cannulated via the aorta and retrograde perfused and superfused with Tyrode’s solution (2–5 mL/min). To load hearts with the voltage-sensitive dye, Di-4-ANEPPS (10 μM, AAT bioquest, USA) was slowly injected into a drug-port over 10 min period. Afterwards, blebbistatin (10 μM, MCE, USA) was delivered to heart via the drug-port to eliminate motional artifacts. The emitted fluorescence Vm-signal was long-passed ( > 700 nm) and acquired via MiCAM optical mapping system (SciMedia, USA). The results were analyzed by the BrainVision software (SciMedia, USA).
Isolation of atrial cardiomyocytes
Atrial cardiomyocytes were isolated from the Langendorff-perfused hearts of adult WT and SNAP25 cKO mice. Briefly, the heart was removed, mounted on a Langendorff apparatus and perfused with Tyrode’s solution to wash out the blood. Then, the heart was perfused with Ca2+-free Tyrode’s solution buffer containing 0.5 mg/mL collagenase type II (Worthington, USA) and 1 mg/mL BSA for digestion at 37 °C for 15–20 min. When the heart became flaccid, the atria were dissected out from the heart, cut into small pieces, and resuspended in modified Kraftbruhe (KB) solution containing (in mM) 100 potassium glutamate, 10 potassium aspartate, KCl 25, KH2PO4 10, MgSO4 2, taurine 20, creatine 5, EGTA 0.5, glucose 20, and HEPES 5, and 1 mg/mL BSA (pH adjusted to 7.2 with KOH). Then, the cell-containing solution was filtered with a 100 μm filter. For electrophysiological experiments, gradient recalcification of the acutely isolated cells was performed by administering 1 M Ca2+ solution every 30 min until a final concentration of 1.8 mM was reached.
Patch-clamp recording
Whole-cell patch clamping for action potential (AP) and current recoding was performed as previously described24,51. Borosilicate glass microelectrodes had tip with 2–5 MΩ resistances filled with the intracellular solution. Once gigaseal resistances were achieved, series resistance and cell-capacitance were compensated. The data were collected in the current clamp mode or the voltage clamp mode through an EPC-10 amplifier (HEKA, Germany) with standard patch-clamp techniques at room temperature (RT). For AP recording, after achieving a whole-cell patch-clamp configuration, the AP was elicited by elicited by 3 ms supra-threshold current steps at 1 Hz.
For K+ current recordings, extracellular solutions containing (in mM): NaCl 110, KCl 4, MgCl2 1, CaCl2 1.8, HEPES 10, and glucose 1.8; and was adjusted to pH 7.35 with NaOH. The pipette solution containing (in mM): K-aspartate 120, MgCl2 0.5, NaCl 6, HEPES 10, glucose 10, KCl 25, K2ATP 4, EGTA 0.06, pH = 7.2. For recording ultrarapid delayed rectifier potassium current (IKur) and transient-outward K+ current (Ito), the membrane was depolarized from −40 mV to +50 mV for 500 ms in 10 mV steps.
The Na+ current (INa) was recorded with an external solution containing (in mM): Tris-Cl 120, NaCl 20, CsCl 5.4, MgCl2 1.2, CaCl2 1.8, HEPES 5, glucose 10, pH 7.4 adjusted with NaOH, whereas the pipette solution containing (in mM) CsCl 130, NaCl 10, MgCl2 1, EGTA 5, HEPES 10, MgATP 4, Na2GTP 0.03, pH 7.2 adjusted with KOH. For recording INa, the membrane potential was depolarized from −90 mV to +30 mV for 400 ms in 5 mV steps.
For L-type Ca2+ current (ICa, L) recordings, the extracellular solutions containing (in mM): NaCl 150, CsCl 5.4, MgCl2 1.2, CaCl2 1.8, HEPES 5, glucose 10, pH 7.4 adjusted with NaOH. The pipette solution containing (in mM): CsCl 130, MgCl2 1, EGTA 5, HEPES 10, TEA-Cl 10, MgATP 4, Na2GTP 0.03, pH 7.2 adjusted with KOH. For recording ICa, L, the membrane potential was depolarized from −60 mV to +60 mV for 400 ms in 5 mV steps. Waveform protocols can also be found in the figure legends.
Currents were recorded 2 min after achieving a whole-cell configuration. The series resistance and capacitance were compensated, and leak currents were subtracted. All the individual currents were normalized to membrane capacitance, expressed as the current density (pA/pF). And all the data were analyzed with Patchmaster 2.42 (HEKA, Germany).
Quantitative PCR (qPCR)
Total RNA was extracted from fresh mouse atrial tissues and cardiomyocytes using RNAiso plus reagent (Takara, 9109) according to the manufacturer’s protocols, and quantified by a Nanodrop; 1000 ng of total RNA was used to generate cDNA with the PrimeScript RT reagent Kit (Takara, RR037A). For quantitative assessment of gene expression, quantitative real‐time polymerase chain reaction analysis was performed. Specific mRNAs were quantified by SYBR green real‐time master mix (Toyobo, QPK‐201) on ABI QuantStudio 6 real‐time polymerase chain reaction system (Applied Biosystems) under standard manufacturer’s protocol. The gene primer sequences were as follows: mouse Snap25: forward, 5′-CAAATTTAACCACTTCCCAGCA-3′; reverse, 5′-CAGAATCGCCAGATCGACAG-3′; and mouse Gapdh: forward, 5′-AGGTCGGTGTGAACGGATTTG-3′; reverse, 5′-GCCCAATACGACCAAATCC-3′.
Biotin-labeled surface cellular protein isolation
For biotin-labeled surface cellular protein isolation, cells were incubated with 1 mg/mL sulfo-NHS-LC-Biotin (Pierce, USA) for 30 min at 4 °C. Unreacted biotin was quenched by a quenching solution containing 100 mM glycine in PBS. The cells were subsequently lysed in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1% sodium deoxycholate, 1% NP-40, 1 mM PMSF and 1 mM EDTA). The lysates containing biotinylated proteins were incubated with prewashed streptavidin-agarose beads (Pierce, USA) at 4 °C overnight. After being washed three times, the biotin-bound surface proteins were eluted with loading buffer for western blot.
Western blot and coimmunoprecipitation analysis
Total proteins were extracted through a conventional method using RIPA lysis buffer (Beyotime Biotechnology, China) with protease inhibitor cocktail (Beyotime Biotechnology, China) at 4 °C. The membrane and cytoplasmic proteins were extracted according to the MinuteTM kit (SM-005, Invent). Protein concentrations were determined by BCA protein assay (Beyotime Biotechnology, China), and equal amounts of total proteins were separated by 10% SDS-PAGE (Thermo Fisher Scientific, USA), and then transferred onto PVDF membranes (Millipore, USA). Next, the membranes were blocked with 5% nonfat milk in 0.1% Tween 20 washing buffer for 1 h at RT, and then incubated with primary antibodies including anti-SNAP25 (ab109105, Abcam), anti-Kv1.5 (APC-004, Alomone), anti-Caveolin-3 (ab2912, Abcam), and anti-GAPDH (NB100-56875, Novus) overnight at 4 °C. On the next day, the membranes were incubated with the conjugated secondary antibody (Invitrogen, USA) for 1 h at RT and the bands were visualized using ChemiDoc Touch Gel Imaging System (Bio-Rad, USA). The blots were quantified by densitometry using ImageJ software (NIH).
For coimmunoprecipitation analysis, proteins were extracted from atrial tissue in mice. After quantification, protein lysates (same amounts in each group) were incubated for 2 hours at 4 °C with primary antibodies against the SNAP25 (Abcam), the Kv1.5 (Alomone), or isotype control immunoglobulin G (Sigma, USA) at a concentration of 1:50. Then, 50 μL Dynabeads (Invitrogen, USA) was added to each tube, and the mixtures were incubated for another 2 hours at 4 °C. Afterward, the bead-antibody complexes were collected after being washed three times, and the samples were eluted with loading buffer and analyzed by western blot.
Immunofluorescence
Isolated mouse atrial cardiomyocytes were placed on glass slides pre-coated with 20 μg/mL of laminin (37 °C, 30 minutes). After the cells adhered properly, a gentle wash with PBS was applied to remove unattached cells, and cells were fixed in 4% paraformaldehyde for 20 minutes, permeabilized in PBS with 0.5% Triton X‐100 (Sigma, T9284) for 15 minutes and then blocked with 5% goat serum (Gibco, 16210072) in PBS with 0.1% Tween 20 for 60 minutes. After being incubated with primary antibodies against anti-SNAP25 (Abcam, USA), anti-Kv1.5 (Alomone), ant-EEA1 (Abcam, USA), anti-Caveolin-3 (Santa) and anti-ANP (Proteintech) at 4°C overnight, cells were washed with PBS with 0.1% Tween 20 and incubated with Alexa Fluor conjugated secondary antibodies at RT for 1 h in the dark. Next, cells were stained with DAPI (Sigma, D9542) for 15 minutes. Finally, images were captured by the Lecia SP8 laser confocal microscopy with DAPI, CY5, and FITC fluorescence excitation filters or Zeiss super-resolution microscopy (Elyra 7 Lattice SIM).
For atrial tissue preparation, the paraffin-embedded sections underwent sequential deparaffinization in xylene followed by graded ethanol rehydration. Antigen retrieval was achieved through microwave-mediated heating in citrate buffer (10 min, 95 °C). Immunofluorescence protocols proceeded as follows: sections were blocked with 10% normal goat serum (NGS) in PBS for 1 hour at RT, then incubated with primary antibodies against SNAP25 (1:200, Abcam) and ANP (1:500, Proteintech) in blocking buffer at 4 °C overnight. After three 5-min PBST (0.1% Tween-20/PBS) washes, sections were exposed to species-matched Alexa Fluor-conjugated secondary antibodies (1:1000) in 2% NGS/PBST for 1 hr at RT. Nuclear counterstaining was performed with DAPI (1 µg/mL) for 5 min. For membrane visualization, sections were treated with Alexa Fluor 594-conjugated WGA (50 µg/mL, Thermo Fisher) in PBS for 1 hour at RT, followed by three PBS rinses. All fluorescence imaging was conducted using a Leica TCS SP8 confocal microscope.
Neonatal rat atrial cardiomyocyte isolation and transfection
Primary atrial cardiomyocyte isolation was performed using P1-P3 neonatal Sprague-Dawley rats. The experimental procedure was conducted as follows: atrial tissues were enzymatically dissociated in calcium-free HBSS (Gibco) containing 0.125 mg/mL trypsin (Gibco), 0.1 mg/mL collagenase IV (Sigma), and 10 mg/mL DNase II (Sigma) under constant agitation at 37 °C. Sequential digestion was achieved through 4-6 cycles of 5-min interval supernatant collection, with each fraction immediately neutralized in HBSS supplemented with 10% FBS. The pooled cell suspension was subsequently centrifuged at approximately 100 x g for 10 min and resuspended in complete DMEM (Gibco) containing 10% FBS and 100 mM 5-bromo-2’-deoxyuridine (Sigma). After filtration through a 100 μm cell strainer (BD Falcon), differential adhesion was employed by pre-plating cells on 10 cm culture dishes for 2 hours at 37 °C to deplete fibroblasts. The enriched cardiomyocyte population was then transferred to 1% gelatin-coated culture vessels. Dual plasmid transfection was performed using Lipofectamine 3000 (Invitrogen) according to manufacturer specifications, introducing SNAP25-GFP and Kv1.5-mCherry fusion constructs (GenScript Biotech Corp.). And the images were ultimately obtained by Zeiss super-resolution microscopy (Elyra 7 Lattice SIM).
Human iPSC-derived atrial cardiomyocytes culture and transfection
Human induced pluripotent stem cell-derived atrial cardiomyocytes (iPSC-aCMs) and related reagents were purchased from the Institute of Biophysics of the Chinese Academy of Sciences (Beijing, China) and Nanjing cosmos Biotechnology Co., Ltd. These cardiomyocytes are terminally differentiated cells with limited regenerative capacity. Their use is not subject to the “Biosecurity Law of the People’s Republic of China” or the “Human Genetic Resources Regulation of the People’s Republic of China.” Therefore, no ethical approval is required. The human iPSC-aCMs were cultured according to a standard procedure. Gene silencing was performed using siRNA against SNAP25, which was transfected into the human iPSC-aCMs at a final concentration of 50 nmol/L with Lipofectamine RNAiMAX (13778-150, Invitrogen). For quantitative assessment of gene expression, qPCR analysis was performed. The gene primer sequences were as follows: human: SNAP25: forward, 5′-TCCGCAGGGTAACAAATGAT-3′; reverse, 5′-TGGCCTCATCAATTCTGGTT-3′; and GAPDH: forward, 5′-ACATCGCTCAGACACCATG-3′; reverse, 5′-TGTAGTTGAGGTCAATGAAGGG-3′.
Multielectrode array (MEA) recording
The human iPSC-aCMs were cultured in a T25 flask precoated with vitronectin (0.01 μg/μL, Cauliscell, China) in cardiomyocyte maintenance medium (Cauliscell, China) at 37 °C and 5% CO2. The medium was refreshed every 2 days. At day 12, the cells were dissociated and resuspended in cardiac recovery medium at a density of 3 × 106 cells/mL. Aliquots of the cell suspensions (10 μL) were plated on CytoView MEA 24-well plates (Axion BioSystems, USA). The MEA device automatically adjusted and controlled the environment (37 °C and 5% CO2) to maintain the temperature and pH of the medium. The data were acquired using Maestro MEA System (Axion Biosystems, USA). The induction of arrhythmic events on MEA recording was carried out as previously reported with some modification52, the human iPSC-aCMs were treated with 1 μM CoCl2.
Statistical analysis
Normality was assessed using the Shapiro-Wilk test and Kolmogorov–Smirnov test. For comparisons between two groups, either a Student’s t-test or a nonparametric test was used. For comparisons involving multiple groups, one-way or two-way ANOVA was applied. Statistics on percentages were performed using Chi-square test. Statistical significance was defined as a P < 0.05. Normally distributed data are presented as mean ± standard error of the mean (SEM), while non-normally distributed data are presented as median with interquartile range (IQR). All statistical analyses were conducted using GraphPad Prism 9.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the manuscript and its Supplementary Information. All other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Brundel, B. et al. Atrial fibrillation. Nat. Rev. Dis. Prim. 8, 21 (2022).
Baman, J. R. & Passman, R. S. Atrial Fibrillation. Jama 325, 2218 (2021).
Michaud, G. F. & Stevenson, W. G. Atrial Fibrillation. N. Engl. J. Med. 384, 353–361 (2021).
Seiffge, D. J. et al. Secondary stroke prevention in people with atrial fibrillation: treatments and trials. Lancet Neurol. 23, 404–417 (2024).
Spatz, E. S. & Herrin, J. Screening for atrial fibrillation to prevent stroke: increasing enthusiasm but outcomes still lag. Eur. Heart J. 44, 205–207 (2023).
Saljic, A., Heijman, J. & Dobrev, D. Recent Advances in Antiarrhythmic Drug Therapy. Drugs 83, 1147–1160 (2023).
Calvo, D., Filgueiras-Rama, D. & Jalife, J. Mechanisms and Drug Development in Atrial Fibrillation. Pharmacol. Rev. 70, 505–525 (2018).
Van Gelder, I. C., Rienstra, M., Crijns, H. J. & Olshansky, B. Rate control in atrial fibrillation. Lancet (Lond., Engl.) 388, 818–828 (2016).
Ang, Y. S., Rajamani, S., Haldar, S. M. & Hüser, J. A New Therapeutic Framework for Atrial Fibrillation Drug Development. Circulation Res. 127, 184–201 (2020).
Dobrev, D. & Nattel, S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet (Lond., Engl.) 375, 1212–1223 (2010).
Wiedmann, F. et al. Treatment of atrial fibrillation with doxapram: TASK-1 potassium channel inhibition as a novel pharmacological strategy. Cardiovascular Res. 118, 1728–1741 (2022).
Nattel, S., Heijman, J., Zhou, L. & Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circulation Res. 127, 51–72 (2020).
Heijman, J., Voigt, N., Nattel, S. & Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circulation Res. 114, 1483–1499 (2014).
Schmitt, N., Grunnet, M. & Olesen, S. P. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiological Rev. 94, 609–653 (2014).
Burg, S. & Attali, B. Targeting of Potassium Channels in Cardiac Arrhythmias. Trends Pharmacol. Sci. 42, 491–506 (2021).
Fedida, D. et al. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circulation Res. 93, 744–751 (2003).
Christophersen, I. E. et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur. Heart J. 34, 1517–1525 (2013).
Schumacher, S. M. et al. Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5. Circulation Res. 104, 1390–1398 (2009).
Friederich, P. & Pfizenmayer, H. The novel Kv1.5 channel blocker vernakalant for successful treatment of new-onset atrial fibrillation in a critically ill abdominal surgical patient. Br. J. Anaesth. 107, 644–645 (2011).
Ravens, U. & Odening, K. E. Atrial fibrillation: Therapeutic potential of atrial K(+) channel blockers. Pharmacol. Therapeutics 176, 13–21 (2017).
Wirth, K. J. et al. Atrial effects of the novel K(+)-channel-blocker AVE0118 in anesthetized pigs. Cardiovascular Res. 60, 298–306 (2003).
Balse, E. et al. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc. Natl Acad. Sci. USA 106, 14681–14686 (2009).
Choi, W. S. et al. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circulation Res. 97, 363–371 (2005).
Schumacher-Bass, S. M. et al. Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes. Circulation Res. 114, 982–992 (2014).
Leung, Y. M., Kwan, E. P., Ng, B., Kang, Y. & Gaisano, H. Y. SNAREing voltage-gated K+ and ATP-sensitive K+ channels: tuning beta-cell excitability with syntaxin-1A and other exocytotic proteins. Endocr. Rev. 28, 653–663 (2007).
Ji, J. et al. SNAP-25, a SNARE protein, inhibits two types of K channels in esophageal smooth muscle. Gastroenterology 122, 994–1006 (2002).
Selak, S. et al. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 63, 357–371 (2009).
Lau, C. G. et al. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J. Neurosci.: Off. J. Soc. Neurosci. 30, 242–254 (2010).
Geng, L. et al. SNX17 (Sorting Nexin 17) Mediates Atrial Fibrillation Onset Through Endocytic Trafficking of the Kv1.5 (Potassium Voltage-Gated Channel Subfamily A Member 5) Channel. Circulation Arrhythmia Electrophysiol. 12, e007097 (2019).
Brundel, B. J. et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 103, 684–690 (2001).
Sanchez-Alonso, J. L. et al. Functional LTCC-β(2)AR Complex Needs Caveolin-3 and Is Disrupted in Heart Failure. Circulation Res. 133, 120–137 (2023).
Brown, G. E. et al. Engineered cocultures of iPSC-derived atrial cardiomyocytes and atrial fibroblasts for modeling atrial fibrillation. Sci. Adv. 10, eadg1222 (2024).
Guo, F. et al. Patient-Specific and Gene-Corrected Induced Pluripotent Stem Cell-Derived Cardiomyocytes Elucidate Single-Cell Phenotype of Short QT Syndrome. Circulation Res. 124, 66–78 (2019).
Devalla, H. D. et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 7, 394–410 (2015).
Alten, B. et al. Role of Aberrant Spontaneous Neurotransmission in SNAP25-Associated Encephalopathies. Neuron 109, 59–72.e55 (2021).
Zhou, Q. et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 548, 420–425 (2017).
Jahn, R., Cafiso, D. C. & Tamm, L. K. Mechanisms of SNARE proteins in membrane fusion. Nat. Rev. Mol. Cell Biol. 25, 101–118 (2024).
Svoboda, L. K. et al. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. Circulation Res. 111, 842–853 (2012).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Reyat, J. S. et al. PITX2 deficiency leads to atrial mitochondrial dysfunction. Cardiovascular Res. 120, 1907–1923 (2024).
Schüttler, D. et al. Animal Models of Atrial Fibrillation. Circulation Res. 127, 91–110 (2020).
Pavri, B. B. et al. MK-0448, a specific Kv1.5 inhibitor: safety, pharmacokinetics, and pharmacodynamic electrophysiology in experimental animal models and humans. Circulation Arrhythmia Electrophysiol. 5, 1193–1201 (2012).
Loewen, M. E. et al. Shared requirement for dynein function and intact microtubule cytoskeleton for normal surface expression of cardiac potassium channels. Am. J. Physiol. Heart Circ. Physiol. 296, H71–H83 (2009).
Goversen, B., van der Heyden, M. A. G., van Veen, T. A. B. & de Boer, T. P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on I(K1). Pharmacol. Therapeutics 183, 127–136 (2018).
Feyen, D. A. M. et al. Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes. Cell Rep. 32, 107925 (2020).
Robinson, N. B. et al. The current state of animal models in research: A review. Int. J. Surg. (Lond., Engl.) 72, 9–13 (2019).
Ni, L. et al. Atrial-Specific Gene Delivery Using an Adeno-Associated Viral Vector. Circulation Res. 124, 256–262 (2019).
Maass, A. H. Atrial-Specific Gene Transfer. Circulation Res. 124, 180–182 (2019).
Campbell, H. M. et al. Loss of SPEG Inhibitory Phosphorylation of Ryanodine Receptor Type-2 Promotes Atrial Fibrillation. Circulation 142, 1159–1172 (2020).
Xie, D. et al. Identification of an endogenous glutamatergic transmitter system controlling excitability and conductivity of atrial cardiomyocytes. Cell Res. 31, 951–964 (2021).
Tazmini, K. et al. Hypokalemia Promotes Arrhythmia by Distinct Mechanisms in Atrial and Ventricular Myocytes. Circulation Res. 126, 889–906 (2020).
Xie, D. et al. Memantine targets glutamate receptors in atrial cardiomyocytes to prevent and treat atrial fibrillation. Cell Discov. 8, 76 (2022).
Acknowledgements
This work was supported by the Programs of National Natural Science Foundation of China (82088101 and 81930013 to Y.-H.C.; 82070338, 82222008, and 82470330 to D.X.; 82400388 to X.S.); the National Key Research and Development Plan (2019YFA0801501 to Y.-H.C.); the Key Research Center Construction Project of Shanghai (2022ZZ01008 to Y.-H.C.); the Top-Level Clinical Discipline Project of Shanghai Pudong (PWYgf2021-01 and PWRd2020-09 to Y.S.); the Shanghai Key Clinical Specialty Project (shslczdzk06202 to Y.-H.C.); the Research Unit of Origin and Regulation of Heart Rhythm, Chinese Academy of Medical Sciences (2019RU045 to Y.-H.C.); and the National Key Clinical Specialty and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
X.S., B.S., Z.C., and H.G. designed the experiments, analyzed the data, and wrote the manuscript. X.S., H.G., G.W., and Z.Q. performed experiments including patch-clamp recordings, cell culture, and immunofluorescence. X.K., Y.C., and C.Z. conducted ECG recordings and optical mapping. H.X., Y.Y., and X.Z. performed western blot and MEA recordings. H.G. was responsible for atrial cardiomyocyte isolation and transfection. Y.L. revised the manuscript. Y.S., D.X., and Y.-H.C. conceived, supervised, and funded the project. All authors reviewed and revised the manuscript and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Somshuvra Mukhopadhyay, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, X., Shao, B., Chen, Z. et al. SNAP25-dependent membrane trafficking of the Kv1.5 channel regulates the onset of atrial fibrillation. Nat Commun 16, 3730 (2025). https://doi.org/10.1038/s41467-025-59096-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59096-4