Abstract
Despite advances in cancer immunotherapy, colorectal cancer patients exhibit limited therapeutic responses. Therefore, the exploration of strategies combining immunotherapy with adjuvant approaches to enhance adaptive immune responses is in demand. Here, we perform a customized in vivo CRISPR-Cas9 screen to target genes encoding membrane and secreted proteins in CRC mouse models with different immune characteristics. We observe that loss of membrane-bound transcription factor site-1 protease (MBTPS1) in tumor cells enhances antitumor immunity and potentiates anti-PD-1 therapy. Mechanistic studies reveal that tumor cell-intrinsic MBTPS1 competes with USP13 for binding to STAT1, thereby disrupting the USP13-dependent deubiquitination-mediated STAT1 stabilization. The upregulated STAT1-transcribed chemokines including CXCL9, CXCL10, and CXCL11, promote CXCR3+CD8+ T cell infiltration. Notably, the regulatory role of MBTPS1 in antitumor immunity operates independently of its classic function in cleaving membrane-bound transcription factors. Collectively, our results provide a theoretical basis for MBTPS1 as a potential immunotherapy target.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) remains a formidable challenge in the field of oncology, ranking third in incidence and second in mortality globally1. Immunotherapy, represented by immune checkpoint blockade (ICB) therapy, has achieved significant therapeutic effects in patients with solid tumors, including melanoma, renal cancer, and even with advanced metastatic cancers2. Pembrolizumab, an anti-PD-1 (αPD-1) monoclonal antibody, has been approved as the new standard of care for first-line treatment of colorectal cancer with mismatch repair (MMR)-deficient (dMMR) and microsatellite instability-high (MSI-H) phenotypes in which neoantigens are presented via MHC-I molecules, thus promoting T cell recognition and activation3. However, CRCs with dMMR and MSI-H phenotypes account for only a small proportion of all CRCs (approximately 15% of patients with CRC and 4% of patients with metastatic CRC)2,4. Consequently, it is crucial to explore potential targets for tumor-immune interactions and exploit novel combined immunotherapeutic strategies.
Membrane proteins and secreted proteins influence interactions between cancer cells and the immune system through various mechanisms, such as antigen presentation, immune checkpoint regulation, and cytokine and chemokine signaling regulation5,6,7. Moreover, proteins located in the intracellular membrane system, such as NLRP3 and STING located on the membranes of the endoplasmic reticulum and Golgi apparatus, also play significant roles in innate and adaptive immunity8. Consequently, understanding the functions and interactions of these proteins is crucial for elucidating the underlying mechanisms of immune evasion and immunotherapy failure in cancer patients, thus contributing to the discovery of new molecular targets that increase the efficacy of immunotherapy.
Clustered regularly interspaced short palindromic repeats (CRISPR) screening is a powerful tool for investigating a wide range of biological processes9. The use of genome-wide CRISPR-Cas9 screens has demonstrated potential as an effective and unbiased method for identifying novel cancer targets such as APMAP10 and SUSD611. However, the vast array of single-guide RNAs (sgRNAs) used for genome-wide screening may result in several issues, such as signal loss and insufficient specificity. Recently, CRISPR screens in various focused gene libraries have proven effective in identifying novel targets that can enhance the efficacy of immunotherapy. As demonstrated in previous studies, an epigenetic-focused screen pinpointed ASF1A as an immunotherapeutic target12, and a metabolism-centered screen revealed metabolic genes crucial for immune evasion in pancreatic cancer13. However, there is still a lack of systematic research and screening for membrane proteins and secreted proteins. To address this, a custom CRISPR knockout library to target murine membrane proteins and secreted proteins is constructed in the present study. Through in vivo screening of mouse colon cancer models under the pressure of competent host immunity, we identify membrane-bound transcription factor site 1 protease (MBTPS1) as an important regulator of tumor-infiltrating CD8+ T cells and the anti-PD-1 response.
MBTPS1 is a serine protease of the proprotein convertase subtilisin/kexin family14. Some studies have revealed that the inhibition of MBTPS1 effectively slows down the proliferation of certain tumor types, suggesting MBTPS1 as a potential therapeutic target for cancer15,16. However, most of these studies were limited to in vitro conditions and did not accurately simulate the tumor microenvironment (TME) in vivo. Moreover, the role of MBTPS1 in shaping the antitumor immune response within the tumor niche remains largely unexplored.
In this study, we reveal the function of MBTPS1 in cancer immunotherapy and identify it as a potential gene correlated with anti-PD-1 resistance. Tumor-intrinsic MBTPS1 mediates immune evasion by regulating the expression of signal transducer and activator of transcription 1 (STAT1) and its downstream key chemokines that promote the infiltration of CD8+ T cells. This finding differs from the classically known functions of MBTPS1, which involve the cleavage of certain membrane-bound transcription factors. Our findings suggest that MBTPS1 serves as a potential immunotherapy target in CRC.
Results
An in vivo CRISPR screen focused on membrane and secreted proteins identifies MBTPS1 as a potential immunotherapy target
To identify key gene targets whose loss enhances antitumor immunity, we first selected 4556 mouse membrane proteins and secreted proteins according to data from the Human Protein Atlas (HPA). Subsequently, we designed three sgRNAs for each gene (https://zlab.bio/guide-design-resources) and constructed a murine CRISPR-Cas9 knockout library (Supplementary Fig. 1a, b). We packaged the plasmid library into lentiviruses and infected MC38 mouse colon cancer cells with these lentiviruses. Then, we injected the pooled cells subcutaneously into 10 immunocompetent female C57 mice and 10 immunodeficient Rag1−/− mice (Fig. 1a). As expected, the two groups of mice exhibited differences in tumor growth. Rag1−/− mice lacking functional T and B lymphocytes had larger tumors than immunocompetent C57 mice (Fig. 1b, c). On day 14, we harvested the tumors, isolated genomic DNA and amplified the integrated sgRNA via two rounds of PCR for next-generation sequencing (Fig. 1a). Downregulated genes in the C57 group compared with the Rag1−/− group were identified according to the Model-based Analysis of Genome-wide CRISPR-Cas9 Knockout (MAGeCK) algorithm (Fig. 1d). We then analyzed the relevance of the clinical information of the top 10 genes using public databases (The genes Gm21498 and Olfr8 do not have corresponding human genes). In human colon adenocarcinoma (COAD), the expression of MBTPS1 was upregulated in tumor tissues (Fig. 1e, Supplementary Fig. 1c), and this upregulation predicted a shorter survival time (Fig. 1f). Given that tumor-infiltrating T lymphocytes (T-TILs) are the primary cells responsible for attacking and eliminating tumor cells, we further investigated the correlation between intratumoral T-TILs infiltration and the expression of these genes using The Cancer Genome Atlas (TCGA) database. We found that the expression of MBTPS1 was negatively correlated with the abundance of T-TILs across 32 cancer types including COAD (Fig. 1g, h, Supplementary Fig. 1d). Additionally, analysis in a cohort from Sun Yat-sen University Cancer Center (SYSUCC) confirmed that MBTPS1 was overexpressed in CRC tumor tissues compared with the matched normal tissues (Fig. 1i, j). Among the 20 types of cancer listed by the HPA, many types of cancer, including gastric cancer, colorectal cancer, and pancreatic cancer, also exhibit a high proportion of MBTPS1 positive staining (Supplementary Fig. 1e). Based on these results, we hypothesized that MBTPS1 may suppress tumor immunity, and its inhibition could potentially reverse tumor immune escape and improve immunotherapy efficacy.
a Strategy of in vivo CRISPR screen system. b Tumor growth curves for female C57 mice (6-8 weeks old) and female Rag1-/- mice (5-6 weeks old) in the CRISPR screen. c Tumor weights and tumor image of MC38 tumors for C57 mice and Rag1-/- mice in the CRISPR screen. Scale bar, 1 cm. d Plot of MAGeCK RRA score and rank of genes in the comparison between C57 mice and Rag1-/- mice. Top candidate genes are highlighted in color as marked. e The expression distribution of the top 10 genes in paired tumor tissues and normal tissues across 15 cancer types from TCGA. f Overall survival analysis based on the high or low expression of the top 10 genes across 32 cancer types from TCGA. g Correlation between MBTPS1 expression and tumor-infiltrating lymphocytes (TILs) in TCGA-COAD dataset. h Pearson correlation between tumor-infiltrating lymphocytes (TILs) proportion and MBTPS1 expression across 33 cancer types from TCGA. i, j Representative IHC staining images (i) and IHC staining scores (j) of MBTPS1 expression in paired primary tumor tissues (T) and adjacent normal tissues (N) of CRC patients from SYSUCC. Scale bar: 50 μm. n = 10 mice per group in b and c; n = 100 CRC tissue specimens in j. The data in b and c were presented as means ± SDs and the data in g and j were presented as box-and-whisker plots showing the median (centre line), 25th to 75th percentiles (box bounds), and minima to maxima (whiskers). Two-tailed unpaired Student’s t-test for b and c; two-tailed paired Student’s t-test for j; Wilcox test for e; Log-rank test for f; Pearson’s correlation analysis for g and h. Source data are provided as a Source Data file.
Loss of MBTPS1 inhibits tumor growth in immunocompetent mice and sensitizes tumors to immunotherapy
To determine whether the absence of MBTPS1 increases the susceptibility of tumor cells to antitumor immune response, we generated control (shVec) and Mbtps1-knockdown (shMbtps1) tumor cells using short hairpin RNA (shRNA) (Supplementary Fig. 2a) and compared their growth both in vitro and in vivo. We found that Mbtps1 knockdown did not affect the proliferation, colony formation, or apoptosis of tumor cells in vitro (Supplementary Fig. 2b–e). However, mice inoculated with Mbtps1-knockdown MC38, CT26 and E0771 cells exhibited significantly slower tumor growth and lower tumor weights than control groups in vivo (Fig. 2a–c, Supplementary Fig. 2f, g). Mbtps1 knockdown also significantly reduced the tumor weight in the cecal orthotopic xenograft model (Supplementary Fig. 2h). On the other hand, faster tumor growth was observed in mice inoculated with Mbtps1-overexpressing cells (Fig. 2d, e; Supplementary Fig. 2g and i–k). To further investigate the involvement of MBTPS1 in the immune system, we observed the growth of Mbtps1-knockdown tumors in Rag1−/− mice, which lack mature T and B cells (Fig. 2f, Supplementary Fig. 2l) and in BALB/c Nude mice, which lack mature T cells (Fig. 2g, Supplementary Fig. 2m). The results did not show any growth disadvantage in these two strains of immunodeficient mice, suggesting that the function of MBTPS1 depends on the existence of an intact immune system. To compare the proliferation and death rates of shVec and shMbtps1 cells in vivo, we performed IHC staining for Ki67 and cleaved caspase-3 on tumor tissues from Rag1−/− and C57 mice and found that MBTPS1 inhibition had no significant effect on the proliferation rate (% of Ki67 positive cells) but increased apoptosis (% of cleaved caspase-3 positive cells) of tumor cells in C57 mice (Supplementary Fig. 2n–p). Additionally, we conducted an in vivo competition assay using fluorescently labeled tumor cells to evaluate the growth of Mbtps1-knockdown cancer cells. Specifically, mCherry-labeled cells were mixed with GFP-labeled cells at a 1:1 ratio, and the mixture was implanted into C57 mice or immunodeficient NCG mice (Fig. 2h). Flow cytometric analysis demonstrated that the relative proportion of Mbtps1-knockdown cancer cells was significantly lower than that of the control cells 14 days after subcutaneous implantation in C57 mice (Fig. 2i and j, Supplementary Fig. 2q). However, the relative proportion was comparable in vitro or in NCG mice (Fig. 2i and j, Supplementary Fig. 2r). Collectively, these data indicated that targeting MBTPS1 in tumor cells enhanced antitumor immunity and that this effect was potentially mediated by mature T cells.
a Growth curves and weights of shVec/shMbtps1 MC38 tumors in female C57 mice (6-8 weeks old). b, c Growth curves of shVec/shMbtps1 CT26 tumors in female BALB/c mice (6-8 weeks old) and E0771 tumors in C57 mice. d, e Growth curves of OEVec/OEMbtps1 MC38 (d) and E0771 (e) tumors in C57 mice. f, g Growth curves of shVec/shMbtps1 MC38 tumors in female Rag1-/- mice (5-6 weeks old) (f) and female BALB/c Nude mice (5-6 weeks old) (g). h Schematic of in vivo competition assay (scale bar: 100 μm). i, j Ratio changes of E0771 (i) and MC38 (j) cells cultured in vitro or tansplanted in C57 mice. k, l Growth curves (k), weights and tumor image (l, scale bar: 1 cm) of shVec/shMbtps1 MC38 tumors in anti-PD-1 (5 mg/kg, i.p.)-treated C57 mice. m Survival of shVec/shMbtps1 MC38 tumor-bearing mice treated with anti-PD-1 (5 mg/kg, i.p.). n Growth curves of E0771 tumors in anti-PD-1 (5 mg/kg, i.p.)-treated C57 mice. o, p Growth curves of KPC (o) and B16 (p) tumors in anti-PD-1 (10 mg/kg, i.p.)-treated C57 mice. q, r Growth curves (q) and weights (r) of MC38 tumors in C57 mice treated with half-dose anti-PD-1 (2.5 mg/kg). s Survival of E0771-bearing mice with half-dose anti-PD-1 (2.5 mg/kg). n = 6 mice per group in a–g and n–r; n = 8 mice per group in i–l and s; n = 10 mice per group in m. Data are presented as means ± SDs. Two-tailed unpaired Student’s t-test for a–g, i and j. one-way ANOVA with Sidak’s multiple comparisons test for k, l and n–r; Kaplan-Meier analysis with the log-rank test for m and s. Source data are provided as a Source Data file.
Next, we confirmed whether MBTPS1 inhibition could potentiate ICB therapy. We observed that anti-PD-1 antibody treatment moderately slowed tumor growth. Moreover, the combination of Mbtps1 knockdown and anti-PD-1 treatment achieved the best efficacy, resulting in the smallest tumor volume and complete regression in 50% (4 of 8) of the tumors (Fig. 2k and l, Supplementary Fig. 3a). Kaplan-Meier survival analysis revealed that C57 mice bearing Mbtps1-knockdown tumors exhibited prolonged survival both with and without anti-PD-1 treatment (Fig. 2m). Similar results were observed in C57 mice bearing E0771 tumors (Fig. 2n, Supplementary Fig. 3b). In KPC and B16 tumors, neither anti-PD-1 treatment alone nor Mbtps1 knockdown alone affected tumor growth. However, the combination of Mbtps1 knockdown and anti-PD-1 treatment considerably decreased the tumor growth rate and weight (Fig. 2o and p, Supplementary Fig. 3c–f). Then, we reduced the dose of the anti-PD-1 antibody in the MC38 and E0771 tumor models to half of the original dose and noted that although the anti-PD-1 antibody alone was not particularly effective, the combination of MBTPS1 inhibition with the reduced anti-PD-1 antibody dose markedly reduced the tumor volume and extended the survival of the mice (Fig. 2q–s, Supplementary Fig. 3g). Taken together, these results revealed that MBTPS1 deficiency notably enhanced the antitumor effect of immunotherapy in vivo.
MBTPS1 depletion increases CD8+ T cell infiltration into the TME
To elucidate TME alterations caused by MBTPS1 loss, we performed single-cell RNA sequencing (scRNA-seq) on shVec and shMbtps1 MC38 tumors (Supplementary Fig. 4a). Transcriptomes were analyzed using the 10x Genomics droplet-based platform, revealing 12 major cell types: T cells, NK cells, B cells, monocytes, macrophages, dendritic cells (DCs), neutrophils, mast cells, fibroblasts, endothelial cells, pericytes, and cancer cells (Supplementary Fig. 4b, c). Among CD45+ immune cells, T cells were most significantly expanded in shMbtps1 tumors (Fig. 3a). Unsupervised clustering revealed seven distinct clusters representing various T cell populations, including naive CD8+ T (Tn) cells, effector CD8+ T (Teff) cells, exhausted CD8+ T (Tex) cells, proliferating exhausted CD8+ T (Tex-prolif) cells, CD4+ T helper (Th) cells, CD4+ T regulatory (Treg) cells, and γδ T (Tgd) cells (Supplementary Fig. 4d, Fig. 3b). Among the four clusters representing CD8+ T cells, effector CD8+ T cells exhibited an increased abundance in tumors from the Mbtps1-knockdown group compared with those from the control group (Fig. 3c). In addition, to identify which cell types express MBTPS1 in human and mouse tumors, we examined both the comprehensive single-cell atlas of human colorectal cancer from a previous study17 and the scRNA-seq data from our own study. The results demonstrated that MBTPS1 was highly expressed in malignant cells and fibroblasts (Supplementary Fig. 4e).
a Bar plots indicating the proportion of major cell lineages in each group. b Uniform manifold approximation and projection (UMAP) projection of 2340 lymphocytes from the shVec/shMbtps1 MC38 tumor groups, respectively. c Bar plots indicating the proportion of T cell lineages in each group. d, e The number and percentage of CD8+ T cells in shVec/shMbtps1 MC38 (d) or CT26 (e) tumors analyzed by flow cytometry. f, g The percentage of IFN-γ+CD8+ T cells in shVec/shMbtps1 MC38 (f) or CT26 (g) tumors analyzed by flow cytometry. h The percentage of Ki67+CD8+ T cells in shVec/shMbtps1 CT26 tumors analyzed by flow cytometry. i The number or percentage of CD4+ T cells in shVec/shMbtps1 MC38 or CT26 tumors analyzed by flow cytometry. j The percentage of NK cells in shVec or shMbtps1 MC38 tumors analyzed by flow cytometry. k Flow cytometric analysis of the percentage of CD8+ T cells and IFN-γ+CD8+ T cells in female C57 mice (6-8 weeks old) with shVec/shMbtps1 MC38 tumors treated with αPD-1 or isotype control. l The percentage and number of CD8+ T cells in OEVec or OEMbtps1 MC38 tumors analyzed by flow cytometry. m Statistical analysis of IHC stained CD8+ T cells in OEVec/OEMbtps1 E0771 tumors. n, o Representative IHC staining images (n) and statistical analysis (o) of CD8+ T cells in MC38 tumors for the indicated groups. Scale bar, 50 μm. p Growth curves of shVec/shMbtps1 MC38 tumors in C57 mice treated with CD8 neutralizing antibody or isotype control. n = 5 mice per group in d, f, (i, left) and j; n = 6 mice in e, g, h, (i, right), k–m, o and p. Data are presented as means ± SDs. Two-tailed unpaired Student’s t-test for d–j, l and m; one-way ANOVA with Sidak’s multiple comparisons test for k, o and p. Source data are provided as a Source Data file.
Furthermore, we compared the composition of immune cell subsets in the TME between control and Mbtps1-knockdown tumors using flow cytometry and found that Mbtps1 knockdown induced a significantly higher percentage and number of CD8+ T cells in MC38 and CT26 tumors (Fig. 3d, e). Moreover, the proportions of CD8+ T cells with cytotoxic capacity (IFN-γ positive) and proliferative capacity (Ki67 positive) were increased in the Mbtps1-knockdown groups (Fig. 3f–h). However, there were no significant changes in the number and function of CD4+ T cells, NK cells, B cells, dendritic cells, macrophages, or neutrophils (Fig. 3i and j, Supplementary Fig. 4f–k). Notably, the percentage of CD8+ T cells and the proportion of CD8+ T cells secreting IFN-γ reached the highest in the combination of Mbtps1 inhibition and anti-PD-1 therapy group (Fig. 3k). qPCR analysis revealed elevated Ifng, Gzmb, and Tnf mRNA expression in Mbtps1-knockdown group and the combined group (Supplementary Fig. 4l). On the other hand, Mbtps1-overexpressing tumors exhibited a lower percentage and number of CD8+ T cells (Fig. 3l). Slight increases in the number of CD8+ T cells in the tumor-draining lymph nodes and spleen were also observed (Supplementary Fig. 4m). Then, we confirmed the alterations in the infiltrating CD8+ T cells via immunohistochemical (IHC) staining of CD8 (Fig. 3m–o, Supplementary Fig. 4n). Depletion of CD8+ T cells via CD8 neutralizing antibody completely abolished the tumor growth inhibition mediated by Mbtps1 knockdown, suggesting the crucial role of CD8+ T cells in this process (Fig. 3p, Supplementary Fig. 4o–q). In summary, these observations revealed the pivotal role of MBTPS1 in shaping the immune landscape of the TME, mainly by influencing the infiltration of CD8+ T cells and ultimately inhibiting antitumor immunity.
MBTPS1 inhibits antitumor immunity by regulating chemokines with chemotactic effects on CD8+ T cells rather than through its classical functions
MBTPS1 and MBTPS2 usually act in a concerted manner to regulate the processing and activation of specific membrane-bound proteins, including SREBP-1, SREBP-2, and ATF6α14. These substrates undergo sequential cleavage by MBTPS1 and MBTPS2, resulting in the release of soluble N-terminal domains that translocate into the nucleus to activate transcription of downstream target genes. SREBP-1 and SREBP-2 are key transcription factors that regulate lipid metabolism and cholesterol metabolism, respectively18, while ATF6α is one of the key responders to endoplasmic reticulum stress19. We first verified the function of MBTPS2 and found that only the loss of MBTPS1 resulted in tumor growth inhibition, while MBTPS2 had no effect (Fig. 4a). Then, we explored the functions of the three aforementioned common substrates and found that only the knockdown of Srebf2 (encoding murine SREBP-2) led to tumor growth inhibition (Fig. 4b). Since SREBP-2 regulates cholesterol synthesis and cholesterol impairs antitumor immunity20, we hypothesized that SREBP-2 inhibition enhances immunity by reducing cholesterol synthesis. To verify this, we fed C57 mice a high-cholesterol diet (HCD) or a control diet (CD) for 7 weeks (Supplementary Fig. 5a) and observed that HCD feeding partially reversed the tumor growth inhibition caused by Srebp2 knockdown, but it did not alter the effect of Mbtps1 knockdown (Fig. 4c). To further confirm whether the tumor suppression is independent of SREBP-2, we constructed shMbtps1-OESrebf2 cells and found that overexpression of Srebf2 did not affect the tumor suppressive effect caused by Mbtps1 knockdown (Fig. 4d). These findings indicated that the regulation of antitumor immunity by MBTPS1 does not rely on its classical function of cleaving membrane-bound transcription factors.
a, b Growth curves of MC38 tumors with indicated gene knockdown in female C57 mice (6-8 weeks old). c Growth curves of shVec, shMbtps1 and shSrebf2 E0771 tumors in C57 mice subjected to CD or HCD feeding. d Growth curves of MC38 tumors from the indicated groups. e GSEA of chemokine pathway in shMbtps1 vs. shVec MC38 tumors. f qPCR analysis of Cxcl9, Cxcl10 and Cxcl11 mRNA in MC38 cells. g–i ELISA detection of CXCL10 in the culture medium. j qPCR analysis of Cxcl10 mRNA in tumors from the indicated groups. k Number of CFSE-labeled CD8+ T cells migrated into the lower chamber when co-incubated with culture medium of MC38 cells. l qPCR analysis of Cxcr3 mRNA of tumors from the indicated groups. m Percentage of CXCR3+ cells in CD8+ T cells in CT26 tumors. n, o Representative images of immunofluorescence assay showing the staining of CXCR3 (red), CD8 (green) and nuclei (blue) (n) and quantification of CXCR3+CD8+ T cells per high power field (o) in MC38 tumors. White arrows indicate CXCR3+CD8+ T cells. Scale bar, 50 μm. p Percentages of IFN-γ positive and Ki67 positive cells in CXCR3 positive/negative CD8+ T cells. q Growth curves of MC38 tumors in anti-PD-1 treated C57 mice. r ELISA detection of CXCL10 in the tumor interstitial fluid of MC38 tumors. s Growth curves of shVec/shMbtps1 MC38 tumors in CXCR3 neutralizing antibody treated C57 mice. t, u Representative images (t) and quantification (u) of IHC stained CD8+ T cells in MC38 tumors. Yellow arrows indicate CD8-positive cells. Scale bar: 50 μm. n = 6 mice per group in a–d, m, o and q–u; n = 5 mice per group in j and l; n = 18 mice per group in p. n = 3 biologically independent samples per group, representative of three independent experiments with similar results in f–i and k. Data are presented as means ± SDs. Two-tailed unpaired Student’s t-test for a, b, f–h, k, m and n; one-way ANOVA with Sidak’s multiple comparisons test for c, d, i, j, l, q-s and u; two-tailed paired Student’s t-test for p. Source data are provided as a Source Data file.
To further explore the mechanism by which Mbtps1 knockdown affects antitumor immunity, we performed bulk RNA-seq using subcutaneous tumor tissue. Gene set enrichment analysis (GSEA) revealed significant upregulation of the chemokine signaling pathway and cytokine-cytokine receptor interaction pathway in the Mbtps1-knockdown tumors (Fig. 4e, Supplementary Fig. 5b). Gene Ontology (GO) enrichment analysis also revealed the upregulation of pathways associated with cytokine production, and those related to leukocyte adhesion, migration, and activation (Supplementary Fig. 5c). Moreover, differential gene expression analysis revealed significant upregulation of genes encoding diverse chemokines and cytotoxic molecules in the Mbtps1-knockdown group. Notably, Cxcl9 and Cxcl10, which encode the CXCL9 and CXCL10 chemokines, respectively, were among the upregulated genes (Supplementary Fig. 5d). Previous studies have shown that CXCL9 and CXCL10, which are secreted by DCs, macrophages, and tumor cells, can attract CXCR3+CD8+ T cells from the circulation to the TME21. Analysis of scRNA-seq data revealed increases in Cxcl10 expression across several cell types, including cancer cells, macrophages, and fibroblasts (Supplementary Fig. 5e). Furthermore, to confirm the source of these chemokines, we conducted bulk RNA-seq using in vitro cultured Mbtps1-knockdown MC38 cells, which demonstrated an increase in Cxcl10 mRNA expression (Supplementary Fig. 5f). Using a Luminex assay, we quantified a panel of 31 chemokines in the supernatants of Mbtps1-knockdown cells and mouse tumor tissues. The results demonstrated elevations in the CXCL9 and CXCL10 levels (chemokines with levels below the detection threshold were not displayed) (Supplementary Fig. 5g–i). Then we performed qPCR assay and detected the increases in the Cxcl9, Cxcl10, and Cxcl11 mRNA levels in Mbtps1-knockdown tumor cells, and corresponding decreases in Mbtps1-overexpressing cells (Fig. 4f and Supplementary Fig. 5j). Notably, overexpression of Mbtps1 (S414A) with a mutated and inactivated serine protease enzyme activity site22 also suppressed the production of these chemokines (Supplementary Fig. 5j). Using ELISA, we confirmed the increase of CXCL9, CXCL10, and CXCL11 in the culture supernatants of MC38 cells with Mbtps1 knockdown (Fig. 4g, h, and Supplementary Fig. 5k), with CXCL10 showing the highest upregulation. Moreover, we also found that IFN-γ stimulation-induced CXCL10 expression was impaired upon Mbtps1 overexpression (Fig. 4i). Additionally, qPCR analysis revealed an upregulation of Cxcl10 mRNA levels in MC38 tumors with Mbtps1 knockdown, which were transplanted into C57 mice and subsequently subjected to anti-PD-1 treatment (Fig. 4j). The results of CD8+ T cell migration assay showed that Mbtps1 knockdown in MC38 cells increased the migration of CD8+ T cells from the upper chamber to the lower chamber, whereas Mbtps1 overexpression inhibited this migration (Fig. 4k). The CD8+ T cell cytotoxicity assay showed increased killing of Mbtps1-knockdown tumor cells and decreased killing of Mbtps1-overexpressing cells by T cells accordingly (Supplementary Fig. 5l). qPCR results revealed that Mbtps1 knockdown led to increased Cxcr3 mRNA expression in MC38 tumor and that anti-PD-1 treatment led to a further increase (Fig. 4l). Flow cytometry and immunofluorescence analyses confirmed that Mbtps1 knockdown increased both the proportion and number of CXCR3+ T cells infiltrating tumor tissues (Fig. 4m–o, Supplementary Fig. 5m). Additionally, CXCR3+CD8+ T cells expressed higher levels of IFN-γ and Ki67 compared with CXCR3-negative cells, revealing their stronger cytotoxic and proliferative capabilities (Fig. 4p). Then, we found that the effect induced by Mbtps1 knockdown was reversed by Cxcl10 knockdown (Fig. 4q). Furthermore, knockdown of Cxcl10 in tumor cells also reduced the elevated CXCL10 level in tumor interstitial fluid (Fig. 4r). The use of an CXCR3 neutralizing antibody reversed the tumor growth inhibition caused by Mbtps1 knockdown (Fig. 4s). This reversal was associated with a significant decrease in the infiltration of CD8+ T cells into tumors (Fig. 4t and u). Given these findings, we concluded that the loss of MBTPS1 induced the production of proinflammatory chemokines by tumor cells, resulting in increased recruitment of CXCR3+CD8+ T cells into the TME.
MBTPS1 suppresses the production of chemokines by reducing the expression of STAT1
Then, we performed in vitro experiments to explore the intrinsic mechanism underlying the upregulation of chemokines, including CXCL10, triggered by Mbtps1 knockdown. Epigenetic mechanisms regulate chemokine expression and anti-tumor immune modulation23,24,25. First, we examined H3K27me3, H3K4me2, and H3K79me2 modifications affecting Th1-type chemokines. Our results showed no changes in these methylation modifications after Mbtps1 knockdown (Supplementary Fig. 6a). Then, we overexpressed FLAG-tagged MBTPS1 in MC38 cells and then performed immunoprecipitation-mass spectrometry analysis to identify MBTPS1 interacting proteins. Interestingly, the results revealed a potential interaction between MBTPS1 and signal transducer and activator of transcription 1 STAT1 (Supplementary Fig. 6b). STAT1 is a key transcription factor that plays a crucial role in initiating the transcription of these chemokines in response to various stimuli26. Numerous studies have highlighted the importance of STAT1 in the regulation of CXCL10 production27. We also validated the binding of STAT1 to the promoter regions of Cxcl9, Cxcl10, and Cxcl11 using ChIP-PCR and ChIP-qPCR assays28,29,30 (Fig. 5a and b, Supplementary Fig. 6c). Subsequently, we performed immunoprecipitation (Co-IP) and detected that MBTPS1 interacted with STAT1 both endogenously and exogenously (Fig. 5c and d, Supplementary Fig. 6d). The results of the proximity ligation assay (PLA) further demonstrated the interaction between MBTPS1 and STAT1 (Fig. 5e). Then, we investigated the effect of MBTPS1 expression on pathways related to the activation and function of STAT1. In TCGA-COAD dataset, we observed a negative correlation between MBTPS1 expression and the response to the type I interferon pathway as well as the regulation of receptor signaling pathways via STAT1 (Supplementary Fig. 6e and f). Using a dual-luciferase reporter assay, we observed upregulated STAT1 promoter-driven luciferase (STAT1-luc) activity in 293T cells following Mbtps1 knockdown, suggesting an enhancement in the transcription of STAT1-mediated downstream genes (Fig. 5f). Results of ChIP-PCR and ChIP-qPCR showed that the knockdown of Mbtps1 increased the amount of Cxcl9, Cxcl10, and Cxcl11 promoter regions bound by STAT1, while the overexpression of Mbtps1 decreased this amount (Fig. 5g-i, Supplementary Fig. 6g). Then we detected increased STAT1 protein levels in Mbtps1-knockdown MC38 and E0771 tumor cells compared with the corresponding control cells (Fig. 6a). Accordingly, Mbtps1 overexpression significantly inhibited STAT1 expression and rescued the induction of STAT1 expression mediated by Mbtps1 knockdown (Fig. 6b). Overexpression of S414A-Mbtps1 also inhibited STAT1 expression, indicating that this inhibition was not dependent on the serine protease activity of MBTPS1 (Fig. 6c). Moreover, Mbtps1 knockdown enhanced IFN-γ-induced STAT1 expression and Y701 phosphorylation, whereas overexpression blocked these effects (Fig. 6d and e). The result of IHC staining also showed that the expression of STAT1 was elevated in Mbtps1-knockdown tumor tissues (Fig. 6f, Supplementary Fig. 6h). Subcellular fractionation and immunofluorescence confirmed that Mbtps1 knockdown increases STAT1 in both cytoplasm and nucleus (Supplementary Fig. 6i; Fig. 6g).
a, b Chromatin immunoprecipitation (ChIP)-PCR (a) and ChIP-qPCR (b) analysis of the interaction between STAT1 and the Cxcl9/10/11 promoter regions in MC38 cells. c Co-IP of endogenous STAT1 with endogenous MBTPS1 in MC38 and 293T cells. d Co-IP of FLAG-tagged MBTPS1 with endogenous STAT1 in MC38 and 293T cells. e Representative image of in situ PLA assay of anti-mouse FLAG and anti-rabbit STAT1 in MC38 cells transfected with Flag-MBTPS1. Scale bar: 10 μm. f Dual-luciferase reporter assay in MBTPS1-knockdown or control 293T cells transfected with the STAT1 promoter reporter. g, h ChIP-PCR (g) and ChIP-qPCR (h) analysis of the interaction between STAT1 and the Cxcl9/10/11 promoter regions in MC38 cells with Mbtps1 knockdown. i ChIP-qPCR analysis of the interaction between STAT1 and the Cxcl9/10/11 promoter regions in MC38 cells with Mbtps1 overexpression. n = 3 biologically independent samples per group, representative of three independent experiments with similar results in b, e, f, h and i. ChIP-PCR in a and g and IB experiments in c and d were repeated three times with similar results using biologically independent samples. Data are presented as means ± SDs. Two-tailed unpaired Student’s t-test for a, b, f, h, and i. Source data are provided as a Source Data file.
a IB detection of STAT1 expression in MC38 and E0771 cells after Mbtps1 knockdown. β-Actin was included as a loading control. b IB detection of STAT1 expression in MC38 or E0771 cells with Mbtps1 overexpression and Mbtps1 knockdown followed by its expression restoration. β-Actin was included as a loading control. c IB detection of STAT1 expression in MC38 or E0771 cells with overexpression of wild-type Mbtps1 or S414A Mbtps1. β-Actin was included as a loading control. d, e IB detection of STAT1 and phospho-STAT1 (Ser701) expression in MC38 cells with Mbtps1 knockdown (d) or overexpression (e) followed by IFN-γ stimulation (10 ng/ml, 24 h). β-Actin was included as a loading control. f Statistical analysis of IHC stained STAT1 in MC38 tumors with Mbtps1 knockdown. g Representative immunofluorescence staining images of STAT1 and analysis of its mean fluorescence intensity. Scale bar: 10 μm. h qPCR analysis of Cxcl10 expression in MC38 cells with indicated treatment. i qPCR analysis of Cxcl10 expression in E0771 cells with Mbtps1 knockdown followed by fludarabine (STAT1 inhibitor, 5 μM, 24 h) treatment. j, k Growth curves (j) and weight analysis (k) of E0771 tumors with Mbtps1 knockdown, Stat1 knockdown or Mbtps1/Stat1 double knockdown in female C57 mice (6-8 weeks old). l–n Flow cytometric analysis of the number of CD8+ T cells (l), IFN-γ+CD8+ T cells (m) and GZMB+CD8+ T cells (n) in E0771 tumors from the indicated groups. o, p Representative histogram image (o) and mean fluorescence intensity (MFI) (p) of H-2Kb on the cell surface after Mbtps1 knockdown. q MFI of H-2Kb after Mbtps1 and Stat1 knockdown analyzed by flow cytometry. n = 6 mice per group in j-n. n = 3 biologically independent samples per group, representative of three independent experiments with similar results in g, h, I and o–q. IB experiments in a–e were repeated three times with similar results using biologically independent samples. Data are presented as means ± SDs. Two-tailed unpaired Student’s t-test for f, g, and p; one-way ANOVA with Sidak’s multiple comparisons test for h–n and q. Source data are provided as a Source Data file.
To verify whether MBTPS1 regulates the expression of CXCL10 through STAT1, we inhibited the expression and function of STAT1 using a small interfering RNA (siRNA) and fludarabine (a STAT1 inhibitor). We detected that Stat1 knockdown suppressed Cxcl10 mRNA expression and reversed the Mbtps1 knockdown-induced increase (Fig. 6h). In addition, fludarabine treatment induced similar effects (Fig. 6i). In vivo experiments showed Stat1 knockdown reversed Mbtps1 knockdown-induced tumor growth inhibition and accelerated tumor progression (Fig. 6j and k). We also found that Stat1 knockdown reversed the increase in the number of CD8+ T cells caused by Mbtps1 knockdown (Fig. 6l). It also reversed the increase in the proportion and number of IFN-γ and GZMB expressing CD8+ T cells (Fig. 6m and n, Supplementary Fig. 6j and k). Given previous reports that STAT1 can upregulate MHC class I expression31,32, we detected the expression of the MHC class I molecule H-2Kb on the surface of MC38 cells. We found that knockdown of Mbtps1 increased the expression of H-2Kb (Fig. 6o and p) and Stat1 knockdown reversed its expression (Fig. 6q). This may explain the increased T-cell toxicity resulting from Mbtps1 knockdown.
Taken together, these results showed that inhibition of MBTPS1 increased STAT1 expression, leading to elevated expression of chemokines including CXCL10 that could attract CD8+ T cells into tumors.
MBTPS1 disrupts the stability of STAT1 by competing with USP13 for binding to STAT1
Then, we investigated the mechanism by which MBTPS1 affects STAT1 expression. qPCR analysis showed that MBTPS1 did not alter Stat1 mRNA levels (Supplementary Fig. 7a), suggesting post-transcriptional regulation of STAT1 protein stability by MBTPS1. As expected, cycloheximide (CHX) treatment to block protein synthesis resulted in a time-dependent reduction in the STAT1 expression, and Mbtps1 knockdown significantly attenuated the degradation of STAT1 (Fig. 7a, Supplementary Fig. 7b). Furthermore, treatment with the proteasome inhibitor MG132 blocked STAT1 degradation, which equilibrated the expression of STAT1 in shMbtps1 and shVec cells (Fig. 7b). Therefore, we hypothesized that MBTPS1 reduces STAT1 stability by binding to it, thereby influencing its degradation through the ubiquitin-proteasome pathway. Consistent with our hypothesis, Co-IP assay revealed that Mbtps1 overexpression resulted in a significantly higher ubiquitination level of STAT1 (Fig. 7c).
a, b IB detection of STAT1 in MC38 cells with Mbtps1 knockdown followed by CHX treatment (5 μg/ml) (a) and by MG132 treatment (10 μM, 6 h) (b). β-Actin was included as a loading control. c Co-IP analysis of the interaction between FLAG-STAT1 and endogenous ubiquitin in the indicated cells pretreated with MG132 (10 μM, 6 h). d Co-IP analysis of the interaction between FLAG-STAT1 and endogenous USP13 in MC38 and 293T cells. e Co-IP analysis of the interaction between FLAG-MBTPS1 and endogenous USP13 in MC38 cells. f Co-IP analysis of the interaction between FLAG-STAT1 and endogenous USP13 in MC38 and E0771 cells with Mbtps1 overexpression. g, h Co-IP analysis of USP13 (g) and MBTPS1 (h) interactions with wild-type or SH2 domain-deleted FLAG-STAT1 in MC38 cells. i Co-IP analysis of the interaction between FLAG-STAT1 and endogenous ubiquitin in MC38 and E0771 cells pretreated with MG132 (10 μM, 12 h) in the indicated groups. j IB analysis of STAT1 expression in E0771 cells overexpressing Mbtps1, Usp13, or both, followed by treatment with CHX (5 μg/ml). β-Actin was included as a loading control. k IB detection of STAT1 expression in E0771 cells with overexpressing Mbtps1 alone, overexpressing Usp13 alone, and overexpressing Mbtps1 and Usp13 simultaneously. β-Actin was included as a loading control. l, m Growth curves (l) and weight analysis (m) of E0771 tumors implanted in female C57 mice (6-8 weeks old) with Mbtps1 overexpression alone or both Mbtps1 and Usp13 overexpression. n Growth curves of MC38 tumors implanted in C57 mice with knockdown of Mbtps1 alone, knockdown of Usp13 alone, and simultaneous knockdown of both Mbtps1 and Usp13. n = 6 mice per group in l–n. IB experiments in a–k were repeated three times with similar results using biologically independent samples. Data are presented as means ± SDs. One-way ANOVA with Sidak’s multiple comparisons test for l–n. Source data are provided as a Source Data file.
Given the detected interaction between MBTPS1 and STAT1, we speculated that this potentially modulates the interaction between STAT1 and specific ubiquitin ligases (E3s) or deubiquitinases (DUBs), subsequently altering the ubiquitination status and degradation of STAT1. Then, we predicted the deubiquitinase-substrate interactions (DSIs) of STAT1 with UbiBrowser 2.0 (http://ubibrowser.ncpsb.org.cn) and found that USP13 is a DUB shared between humans and mice (Supplementary Fig. 7c). Co-IP assay confirmed the interaction between STAT1 and USP13 (Fig. 7d), but not between MBTPS1 and USP13 (Fig. 7e). To further confirm the USP13-dependent deubiquitination and stabilization of STAT1, we knocked down or overexpressed Usp13 and found that USP13 negatively regulates the ubiquitination level (Supplementary Fig. 7d). This finding was consistent with the findings of a recent study showing that USP13 deubiquitinates STAT133. To investigate the dependence of STAT1 on USP13 in vivo, we constructed OEStat1-shUsp13 MC38 cells and found that Stat1 overexpression suppressed tumor growth in MC38 cells, but this effect was abolished by Usp13 knockdown (Supplementary Fig. 7e and f). Moreover, we observed substantial attenuation of the interaction between STAT1 and USP13 upon overexpression of Mbtps1 (Fig. 7f). This finding indicated that MBTPS1 could competitively bind to STAT1, thereby interfering with the interaction between USP13 and STAT1. As a result, this competition impaired USP13-mediated STAT1 deubiquitination and stabilization. Based on the analysis via UbiBrowser 2.0, we hypothesized that USP13 may bind to the SH2 domain of STAT1. To confirm this, we expressed both full-length wild-type STAT1 (WT-STAT1) and a truncation mutant lacking the SH2 domain (ΔSH2-STAT1) and performed Co-IP assay. Results demonstrated that only WT-STAT1 was capable of interacting with USP13, whereas ΔSH2-STAT1 could not (Fig. 7g). This observation underscored the necessity of the SH2 domain of STAT1 for its interaction with USP13. Similarly, we found that the interaction between STAT1 and MBTPS1 was also dependent on the SH2 domain (Fig. 7h). Collectively, these observations suggested that both MBTPS1 and USP13 bind to the SH2 domain of STAT1, establishing a competitive relationship. Next, we investigated whether MBTPS1 reduces STAT1 stability in a USP13-dependent manner. We detected that overexpression of USP13 attenuated the ubiquitination of STAT1, reversing the MBTPS1-induced increase in STAT1 ubiquitination, reduction in stability, and subsequent decrease in STAT1 protein levels (Fig. 7i–k). It also reversed the inhibition of Cxcl9, Cxcl10, and Cxcl11 expression caused by Mbtps1 overexpression (Supplementary Fig. 7h). Results of animal experiments showed that Usp13 overexpression alleviated the increases in the tumor growth and weight triggered by Mbtps1 overexpression (Fig. 7l and m, Supplementary Fig. 7i). Likewise, we constructed cells with double-knockdown of Mbtps1 and Usp13 and obtained similar conclusions (Fig. 7n, Supplementary Fig. 7j–l). In summary, these results suggested that MBTPS1 decreased the stability of STAT1 by competing with USP13 for binding to STAT1. This competition subsequently interfered with the deubiquitination of STAT1 by USP13, promoting its degradation via the ubiquitin‒proteasome pathway.
MBTPS1 drives tumor development and serves as a marker of unfavorable prognosis
To assess the role of MBTPS1 in the occurrence and development of CRC, we generated mice with conditional intestinal-specific knockout of Mbtps1 (Mbtps1iKO mice). These mice were derived from animals harboring floxed alleles of Mbtps1 controlled by the pVillin-Cre-ERT2 driver (Supplementary Fig. 8a, b). Tamoxifen was administered for 5-day pre-azomethane (AOM) injection to induce specific knockout of the Mbtps1 gene in intestinal epithelial cells (Supplementary Fig. 8c). After three rounds of dextran sodium sulfate (DSS) treatment, we observed that conditional ablation of Mbtps1 in the AOM-DSS mouse model significantly reduced the number of intestinal adenomas and the overall tumor burden (Fig. 8a, Supplementary Fig. 8d). IHC staining revealed increased numbers of CD8+ T cells in the intestinal tumors of Mbtps1iKO mice (Fig. 8b). The increased expression of STAT1 and p-STAT1 was also demonstrated by IHC staining (Fig. 8c). Then, the intestinal tumor-bearing mice were randomly divided into 4 groups and treated with IgG control or PD-1 antibody for 2 weeks (Supplementary Fig. 8e). We found that treatment with PD-1 antibody further reduced the number of intestinal tumors in Mbtps1iKO mice (Fig. 8d–f). Flow cytometry analysis of intestinal tumors indicated that knockout of Mbtps1 in the intestine increased the percentage and number of infiltrating CD8+ T cells in the tumors (Fig. 8g, h), the proportion of CD8+ T cells secreting IFN-γ (Fig. 8i), as well as the infiltration of CXCR3+CD8+ T cells (Fig. 8j, k, Supplementary Fig. 8f). Furthermore, treatment with PD-1 antibody further enhanced the infiltration and function of CD8+ T cells (Fig. 8g–k). In addition, we generated WT-ApcMin/+ and Mbtps1iKO-ApcMin/+ mice by crossing Mbtps1iKO mice with ApcMin/+ mice. At 17 weeks of age in mice, we dissected their intestines to quantify the adenomas. Our results indicated that specific knockout of Mbtps1 in intestine suppressed adenoma formation in the ApcMin/+ mouse background (Fig. 8l).
a Representative images and quantification of intestinal tumors in female Mbtps1fl/fl, cre-/- (control) mice and Mbtps1fl/fl, cre+/- (Mbtps1iKO) mice (6-10 weeks old). Black arrows indicate intestinal tumors. Scale bar: 1 cm. b IHC staining of CD8 in intestinal tumors from control and Mbtps1iKO mice. Yellow arrows indicate CD8-positive cells. Scale bar: 1 mm (1×); 50 μm (40×). c IHC staining of STAT1 and p-STAT1 in intestinal tumors from control and Mbtps1iKO mice. Scale bar: 1 mm (1×); 50 μm (40×). d Representative images of colonoscopy and intestinal dissection of control and Mbtps1iKO mice. Arrows indicate intestinal tumors. e, f Number (e) and total surface area (f) of the intestinal tumors in anti-PD-1 treated control and Mbtps1iKO mice. g-k Percentage of CD8+ T cells (g), number of CD8+ T cells (h), percentages of IFN-γ+CD8+ T cells (i) and CXCR3+CD8+ T cells (j), and number of CXCR3+CD8+ T cells (k) in intestinal tumors from the indicated groups. l Number of intestinal tumors from female WT-ApcMin/+ and Mbtps1iKO-ApcMin/+ mice (17 weeks old at the endpoint). m OS and DFS based on MBTPS1 expression (SYSUCC cohort). n OS based on STAT1 expression (SYSUCC cohort). o IHC staining of the indicated markers in CRC tumor tissues from patients with high or low MBTPS1 levels. Yellow arrows indicate CD8-positive cells. Scale bar: 50 μm. p, q Percentages of tumor tissues exhibiting high or low expression of STAT1 and CD8 (p), as well as the percentage exhibiting either proficient mismatch repair (pMMR) or defective mismatch repair (dMMR) (q), was presented for the groups with low and high MBTPS1 expression, respectively. n = 6 mice per group in a–c and l; n = 8 mice per group in d–k. The data in a, b, e–l were presented as means ± SDs and the data in p, q were presented as the percentage of total samples. For a, b, and l data, two-tailed unpaired Student’s t-test. One-way ANOVA with Sidak’s multiple comparisons test for e–k data; Kaplan-Meier analysis with the log-rank test for m and n; two-sided Pearson’s correlation analysis and chi-square (and Fisher’s exact) test for p, q. Source data are provided as a Source Data file.
We subsequently investigated the clinical significance of MBTPS1 in cancer patients. We found that patients exhibiting high MBTPS1 expression displayed poorer overall survival (OS) and progression-free survival (PFS) in three independent immunotherapy cohorts (Supplementary Fig. 8g and h). The Tumor Immune Dysfunction and Exclusion (TIDE) score predicts the cancer immunotherapy response by assessing tumor immune dysfunction and TIL exclusion34. In these three cohorts, the expression of MBTPS1 was positively correlated with the TIDE score (Supplementary Fig. 8i). Additionally, MBTPS1 expression was positively correlated with the TIDE score across multiple cancer types, including stomach adenocarcinoma (STAD), esophageal carcinoma (ESCA), cutaneous melanoma (SKCM), and breast cancer (BRCA) (Supplementary Fig. 8j). These findings suggested that patients with lower MBTPS1 expression may exhibit a better response and achieve better outcomes when treated with immunotherapy. Additionally, analysis in a cohort from SYSUCC revealed that higher MBTPS1 expression was associated with both shorter OS and disease-free survival (DFS) in CRC patients (Fig. 8m). Multivariate analysis also indicated that MBTPS1 expression was an independent prognostic factor in CRC, suggesting MBTPS1 as a potential prognostic biomarker (Supplementary Data 1 and 2). In addition, higher expression of STAT1 and greater infiltration of CD8+ T cells were associated with a longer OS time as expected (Fig. 8n). A positive correlation between the expression of STAT1 and the infiltration of CD8+ T cells was also observed in our cohort (Supplementary Fig. 8k). More importantly, patients with high MBTPS1 expression were found to have lower STAT1 expression, p-STAT1 expression, and reduced infiltration of CD8+ T cells in tumors (Fig. 8p, Supplementary Fig. 8l). These findings indicated significant negative correlations between MBTPS1 expression and both STAT1 expression and CD8+ T cell infiltration. As for the MMR status, we found no significant correlation between MBTPS1 expression and the MMR status of the patients (Fig. 8q). In summary, our results demonstrated that MBTPS1 served as a prognostic marker, allowing the prediction of immunotherapy efficacy through its indication of CD8+ T cell infiltration within the TME.
Discussion
Immunotherapy, represented by ICB therapy, has rapidly emerged as a promising therapeutic approach for various solid tumors, including CRC. However, despite the potential of immunotherapy, most CRC patients are unresponsive to immunotherapy due to the immunosuppressive nature of the TME2,35. Therefore, there is an urgent need to identify new targets that can reprogram the immunosuppressive TME and enhance the efficacy of immunotherapy. To address this need, we established a CRISPR library targeting membrane proteins and secreted proteins. Through an in vivo loss-of-function screen, we aimed to discover genes that sensitize tumors to antitumor immune responses in host mice with competent immunity. Among the identified genes, we focused on MBTPS1 as a novel target with the potential to enhance antitumor immunity and ICB efficacy. Our investigation revealed a previously unknown role of MBTPS1 in controlling the immune evasion of cancer cells within tumors, and this role was independent of its cleavage of common substrates. Mechanistic studies further revealed STAT1 as a downstream effector of MBTPS1 that regulates the release of lymphocyte chemoattractants from cancer cells. Specifically, MBTPS1 was found to decrease the abundance of the STAT1 protein by disrupting its USP13-mediated ubiquitination, leading to decreased expression of chemokines within the TME. Blocking MBTPS1 was found to lead to increased infiltration of CD8+ T cells, increased sensitivity to anti-PD-1 treatment, and improved survival in mouse models. Additionally, we observed strong correlations among MBTPS1 expression, STAT1 expression, CD8+ T-cell infiltration, and clinical outcomes by analyses of human CRC samples. These findings support the hypothesis that MBTPS1 functions as a cancer-intrinsic immune suppressor, playing a crucial role in modulating CD8+ T-cell infiltration into tumors and suppressing the effects of immunotherapy.
Previous studies have shown that MBTPS1 plays an important role in tumor growth15,16. Numerous potential substrates of MBTPS1 have been identified and include SREBP-1, SREBP-2, and ATF6α. In addition, some proteins, including GlcNAc-1-phosphotransferase36 and (pro)Renin receptor37, can be cleaved by MBTPS1 alone. Liat H. et al.38 reported that the knockout of MBTPS1 inhibited the proliferation of CRC cells, primarily through suppression of SREBP-associated lipid metabolism. Despite the diverse functions of MBTPS1, no studies have confirmed its role in antitumor immunity or the response to immunotherapy. Here, we found that the loss of MBTPS1 significantly suppressed tumor growth and prolonged survival in immunocompetent mice, especially in mice treated with ICB. This finding suggests that the effects of MBTPS1 on tumor progression occur through TME reprogramming and immune response.
The mechanisms underlying immune resistance may be attributed to a low tumor mutation burden and the absence of immune cell infiltration39. In this study, by employing scRNA-seq and flow cytometric analyses, we profiled the immune microenvironment of tumors with MBTPS1 inhibition, revealing more robust infiltration of CD8+ T cells in these tumors. Hence, the loss of MBTPS1 increases the infiltration of CD8+ T cells, thereby enhancing anti-tumor immunity and the efficacy of immunotherapy.
Due to the diversity of protein structure and function, as well as the complexity of the internal environment of organisms, proteins can exhibit independent noncanonical functions during certain physiological processes40. For example, Li et al.41 revealed SKP1 phosphorylation as a regulatory switch that governs the transition between autophagy and unconventional secretion in a manner contingent upon the cellular nutrient status; this role is independent of the established role of SKP1 as an essential component of the family of SCF/CRL1 ubiquitin ligases. In this study, our findings suggested that MBTPS1 does not exert its suppressive effect on tumor immunity through its conventional substrates. This conclusion was supported by our observation that individual knockdown of Mbtps2, srebf1, or Atf6 did not exert an inhibitory effect on tumor growth. Although knocking down Srebf2 effectively suppressed tumor growth, feeding mice with a HCD abolished its tumor-suppressive effect. Notably, no such reversal was observed when MBTPS1 was knocked down.
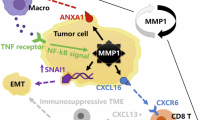
Access to the TME is essential for T cells to exert their therapeutic effects42. Thus, it is crucial to understand the factors mediating the entry of T cells into the tumor site. One of the main factors controlling this process is the production of chemokines, which guide leukocytes to extravasate into inflamed tissues, including the TME43. Among these chemokines, IFN-γ-induced CXCL9 and CXCL10 play pivotal roles44. The chemokines CXCL9, CXCL10, and CXCL11 are all ligands of the chemokine receptor CXCR3. These chemokines can be produced by cells in the TME and result in the recruitment of CD8+ T cells expressing CXCR3 from the circulation to the tumor bed45. As previously reported, effector CD8+ T cells cannot be recruited to tumors in Cxcl10−/− mice, demonstrating the necessity of this factor45. Moreover, efficient trafficking of cytotoxic T lymphocytes (CTLs) to metastatic sites in patients with melanoma was found to be strongly correlated with the expression of CXCL9 and CXCL10. CXCR3 is abundantly expressed on activated T cells and functions as a crucial regulator of their spatial distribution, migratory behavior, and overall function46. Tumor cells engineered to express ligands of CXCR3 can elicit an antitumor immune response. For example, low-dose radiotherapy (LDRT) enhances the sensitivity of tumors to dual PD-L1 and VEGFA blockade (DPVB) by facilitating the recruitment of stem-like progenitor exhausted CD8+ T (Tpex) cells from draining lymph nodes into tumors through the CXCL10/CXCR3 signaling axis47. Chow et al.48 demonstrated that the CXCR3 chemokine system is essential for the efficacy of anti-PD-1 therapy and could serve as an early biomarker of the response to ICB therapy. In this study, using bulk RNA-seq analysis and Luminex-based multicytokine detection, we revealed that inhibition of MBTPS1 caused elevated expression of the inflammatory cytokines CXCL9, CXCL10 and CXCL11 in tumor cells, which contributed to the recruitment of CXCR3+CD8+ T cells that in turn increased chemokine expression and T-cell inflammation via the secretion of IFN-γ. Besides, upregulation of CXCL10 was observed not only in tumor cells but also in some other cell types such as macrophages and neutrophils analyzed by scRNA-seq data. We speculate that the elevated CXCL10 levels in these cells may be part of an IFN-γ-induced positive feedback mechanism, and may reflect widespread immune activation in the immune microenvironment.
Although the mechanism underlying the release of CXCL10 from tumor cells are not fully understood, our study highlights the involvement of STAT1 in the transcriptional regulation of these chemokines. Specifically, we found an increase in STAT1 expression upon Mbtps1 knockdown in tumor cells. STAT1 is a crucial transcription factor that regulates the expression of CXCL9, CXCL10, and CXCL11, in response to interferon stimulation and plays a crucial role in the reguation of antitumor immunity27,49,50. Upon activation by cytokines or growth factors, STAT1 dimerizes and translocates to the nucleus, where it binds to specific DNA sequences in the promoter regions of its target genes51. Knockdown of STAT1 and its downstream target CXCL10, along with the use of CXCR3 neutralizing antibodies, can reverse the phenotypic change caused by Mbtps1 knockdown, indicating that the STAT1/CXCL10/CXCR3 axis is the key effector downstream of MBTPS1 in regulating the tumor immune microenvironment. Posttranslational modifications of STAT1, including phosphorylation52, ubiquitination53, and acetylation54, are important processes for regulating its expression, degradation, localization, and function. For instance, phosphorylation at Y701 is an essential step for the dimerization of STAT1, which subsequently facilitates its entry into the nucleus and promotes the transcription of its target genes52. Ubiquitination of STAT1 mediated by the E3 ligase RNF220 contributes significantly to STAT1 activation and innate immune responses53. In addition, OTUB2 deubiquitinates STAT1 to promote its phosphorylation and dimerization55. In this study, we detected an interaction between MBTPS1 and STAT1, which disrupted the USP13-dependent deubiquitination mediated STAT1 stabilization. Then, stabilized STAT1 translocates into the nucleus to initiate the transcription of downstream genes.
By analyzing scRNA-seq data of human colorectal cancer in previous study17, as well as the scRNA-seq data of mice in our study, we revealed high expression of the Mbtps1 in tumor cells and fibroblasts. The high expression of Mbtps1 in tumor cells offers potential for future discovery and development of specific drugs targeting the inhibition of MBTPS1 expression. On the other hand, fibroblasts, which are one of the components of the tumor stroma, can transform into cancer-associated fibroblasts (CAFs) when influenced by the TME56. CAFs have been shown to exert immunoregulatory effects by secreting immunosuppressive factors, pro-inflammatory cytokines, chemokines, and by remodeling the extracellular matrix57. Based on the previous study showing that CD248 expression can inhibit the expression and secretion of CXCL9/10 in CAFs, thereby suppressing CD8+ T cell infiltration58, we hypothesize that MBTPS1 in certain populations of fibroblasts may exert immunosuppressive effects by inhibiting the STAT1-CXCL10 signaling axis, which is similar to its role in tumor cells. However, given the numerous differences in the biological characteristics between tumor cells and fibroblasts, MBTPS1 may have distinct functions and roles in fibroblasts, which require further exploration.
In summary, we found that MBTPS1 inhibition in cancer cells promotes the expression of CXCL9, CXCL10 and CXCL11, especially CXCL10, by stabilizing STAT1 to recruit more CD8+ T cells to the tumor immune microenvironment (TIME), contributing to the suppression of tumor growth and an enhanced response to anti-PD-1 therapy. Our study has established the crucial role of MBTPS1 in the immune evasion of cancer cells and identified MBTPS1 as a potential therapeutic target for enhancing the response to immunotherapy in CRC patients. Thus, combining MBTPS1 inhibition with ICB therapy, for example, anti-PD-1 treatment, is a potential novel immunotherapeutic strategy for CRC patients.
Methods
Ethical statement
All patient samples used in the present study were obtained from the Sun Yat-sen University Cancer Center (SYSUCC) in Guangzhou, China. Prior to sample collection, written informed consent was obtained from each participant, ensuring compliance with the strict guidelines set forth by the Medical Ethics Committee of SYSUCC, as well as the principles enshrined in the Declaration of Helsinki. Additionally, the present study received approval from the Ethical Committee of SYSUCC (G2023-172-01). All animal studies were performed in strict accordance with the protocol approved by the Institutional Ethics Committee for Clinical Research and Animal Trials of SYSUCC (L025501202112016). The tumors that were transplanted were required to have a volume not exceeding 2,000 mm³ or to constitute no more than 10% of the body weight as permitted by the Institutional Ethics Committee for Clinical Research and Animal Trials of the SYSUCC.
Cell lines
The human embryonic kidney (HEK) 293 T cell line, mouse CT26 CRC cell line, and E0771 mouse breast cancer cell line was purchased from the American Type Culture Collection (ATCC, Rockville, USA). The MC38 mouse CRC cell line was purchased from iCell (Shanghai, China). The B16 mouse melanoma cell line was purchased from Pricella Life Science & Technology (Wuhan, China). The mouse pancreatic cell line was a kind gift from Prof. Jian Zheng (Sun Yat-sen University Cancer Center, Guangzhou). Cells were cultured in RPMI-1640 or DMEM (Gibco, Grand Island, USA) supplemented with 10% FBS (Wisent Inc., Quebec, Canada) and 1% penicillin/streptomycin (Gibco). Cells were characterized before use by short tandem repeat DNA fingerprinting (STR)-PCR DNA profiling and were determined to be free of mycoplasma contamination.
RNA isolation and qPCR analysis
Total RNA was extracted utilizing TRIzol reagent (Thermo Fisher Scientific, Carlsbad, USA) and subsequently reverse transcribed into cDNA using the Prime Script RT Master Mix Kit (TaKaRa, Tokyo, Japan). The obtained cDNAs were then subjected to quantitative PCR (qPCR) analysis using GoTaq qPCR Master Mix (Promega, Madison, USA) on a LightCycler 480 instrument (Roche Diagnostics, Switzerland). For normalization, Gapdh was used as the internal control gene, and the relative gene expression was analyzed using the 2−ΔCt or 2−ΔΔCt method. The primers utilized in this study were custom synthesized by TSINGKE Biological Technology (Guangzhou, China) and are listed in Supplementary Data 3.
Luminex multiplex chemokine assay
The Luminex multiplex chemokine assay was performed to detect the levels of 31 chemokines in the supernatants of mouse tumor cell cultures or the supernatants of mouse tumor tissue homogenates. After quantification of protein concentration using the BCA assay (Beyotime, Shanghai, China), the mouse chemokine detection kit (BIO-RAD, Hercules, USA) was employed. Sample processing, preparation of standards, plotting of standard curves, and other steps were carried out in accordance with the manufacturer’s instructions. The detection was conducted using the Luminex X-200 (Luminex, NASDAQ, USA), and the data were analyzed by Milliplex Analyst software.
CD8+ T cell migration and cytotoxicity
CD8+ T cells were isolated from the spleens of C57BL/6 J mice and cultured for 3-5 days in the presence of 10 ng/mL IL-2 (PeproTech, Rocky Hill, USA), 50 μM β-mercaptoethanol (Sigma Aldrich, Steinheim, Germany), 2.5 μg/ml anti-CD3, and 1 μg/ml anti-CD28 antibodies (PeproTech). Subsequently, cells were purified using a mouse CD8+ T cell isolation kit (StemCell, Vancouver, Canada) and labeled with CFSE (MedChemExpress, Monmouth Junction, USA) prior to coculture with tumor cells. For the CD8+ T cell migration assay, CFSE-labeled CD8+ T cells were placed in the upper chamber of a Transwell insert with a 5.0 μm pore size, allowing access to the lower chamber containing supernatant from MC38 cells. After 12 h, the number of CFSE-positive cells that migrated to the lower chamber was quantified by flow cytometry. For the CD8+ T cell cytotoxicity assay, CFSE-labeled CD8+ T cells were cocultured with MC38 cells at a 5:1 ratio. After 24 h of coculture, cells were harvested and stained with Annexin-V (KeyGEN, Nanjing, China). Cells that were positive for Annexin V staining and negative for CFSE staining (Annexin V+/CFSE-), as detected by flow cytometry, were considered cancer cells that had been killed by CD8+ T cells.
RNA interference, lentivirus, and plasmid transfection
The small interfering RNA (siRNA) targeting mouse Stat1 was synthesized by TSINGKE Biological Technology (Guangzhou, China), and cells were transfected using Lipofectamine RNAiMax (Invitrogen, Carlsbad, USA). Mouse Mbtps1 short hairpin RNA (shRNA), mouse Stat1 shRNA, mouse Srebf1 shRNA, mouse Srebf2 shRNA, mouse Atf6 shRNA, mouse Mbtps1 overexpression, and Usp13 overexpression constructs were obtained from PPL (Public Protein/Plasmid Library, China). To produce lentivirus, 4.45 μg of the psPAX2 packaging plasmid, 2.43 μg of the pMD2.G envelope vector, and 3.1 μg of the target plasmids were cotransfected into 293T cells cultured in T25 flasks using Lipofectamine 3000 Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. After 72 h, the medium containing the virus was collected and filtered through a 0.45 µm filter. Cells were infected with lentiviruses containing polybrene and selected with puromycin (Solarbio, Beijing, China) or hygromycin B (Solarbio) for 7 days. All the targeted sequences of the siRNAs and shRNAs are summarized in Supplementary Data 3. The N-terminal Flag-tagged Mbtps1 expression vectors for shRNA#1-resistant, N-terminal Flag-tagged Stat1, N-terminal Flag-tagged Stat1Δ573-670, and N-terminal HA-tagged Stat1 were cloned and inserted into the pCDNA3.1 vector by Savicred Biotechnology Co., Ltd. (Suzhou, China). The plasmids were transiently transfected into cells using Lipofectamine 3000 Reagent.
CRISPR-Cas9 knockout screen in vivo
The sgRNAs targeting 4556 genes of membrane and secreted proteins (4 sgRNAs per gene) were designed and synthesized by GENEWIZ (Suzhou, China). Subsequently, the sgRNA sequences were Gibson assembled with the linearized LentiCRISPR v2 vector as previously described by Zhang et al.59. The packaging of lentivirus using the CRISPR plasmid library followed the abovementioned procedures. For the in vivo CRISPR-Cas9 knockout screen, 8 × 107 MC38 cells were plated across 20 10-cm dishes to ensure comprehensive 1000-fold coverage of the sgRNAs. MC38 cells were then infected with the lentivirus library at a low multiplicity of infection (MOI = 0.3) to enhance the likelihood that most infected cells would receive only a single viral construct. At 48 h post-infection, the infected MC38 cells were subjected to a 7-day selection process with 5 μg/ml puromycin. This selection effectively separated the positively transduced cells from the uninfected cells, resulting in genome-edited cell pools. The selected cells (2 × 106 MC38 cells per mouse) were injected subcutaneously into the flank regions of female C57 or Rag1−/− mice. On Day 14, the tumors were surgically excised, weighed, and processed for genomic DNA extraction using the DNeasy Blood & Tissue Kit (Qiagen, Dusseldorf, Germany). DNA fragments containing the sgRNA sequences were amplified by 2-step PCR using NEBNext Ultra IIQ5 Master Mix (M0544L, New England Biolabs, Ipswich, USA), and the primers used are listed in Supplementary Data 3. Subsequently, the PCR products containing the sgRNA sequences were gel-extracted (Promega, Madison, USA) and sequenced by Novogene (Beijing, China). Identification of candidate genes was conducted through the use of the robust rank aggregation (RRA) score, which was assessed by MAGeCK60.
Immunohistochemistry (IHC) assay
The paraffin-embedded sections underwent initial deparaffinization and rehydration processes. Afterward, to neutralize endogenous peroxidase activity, the sections were treated with 3% hydrogen peroxide for 10 min. Antigen retrieval was then performed by applying sodium citrate buffer or EDTA for 10 min at subboiling temperatures in a pressure cooker. To prevent nonspecific binding, the samples were then blocked with 10% FBS for 1 h at room temperature, followed by overnight incubation with the primary antibody at 4 °C and a subsequent incubation for 1 h with a biotinylated secondary antibody at room temperature. Color development was facilitated by the Dako REAL™ EnVision™ Detection System (K5007, Copenhagen, Denmark), and counterstaining was achieved using Harris-modified hematoxylin. The stained sections were independently evaluated and scored based on staining intensity, which ranged from 0 (negative) to 3 (strong). The final score was determined by multiplying the staining intensity score by the percentage of positively stained cells. Finally, the sections were also stained with hematoxylin and eosin (H&E) according to standard staining procedures. Detailed information on the antibodies used is listed in Supplementary Data 4.
Immunoprecipitation (IP) assay and Chromatin immunoprecipitation (ChIP) assay
For IP assay, cells were collected and lysed using NP-40 lysis buffer (Beyotime) supplemented with a protease inhibitor cocktail (MedChemExpress) for 30 min on ice. Subsequently, the lysate was centrifuged at 12,000× g for 20 min. The clarified lysates were incubated overnight at 4 °C with an antibody and magnetic protein A/G beads (MedChemExpress). Normal immunoglobulin (IgG) served as a negative control. The beads were washed with NP-40 lysis buffer at least four times, and the proteins were eluted by boiling in 1× loading buffer at 100 °C for 15 min prior to western blot or LC/MS-MS analysis (Bioprofile Technology Company Ltd., Shanghai, China). The ChIP assay was performed using the Pierce Magnetic ChIP Kit (Thermo Scientific) following the manufacturer’s instructions. Briefly, or 4×106 MC38 cells, a final concentration of 1% formaldehyde was added into the culture medium and incubated for 10 minutes at room temperature. Subsequently, Glycine Solution (10×) was added to achieve a final concentration of 1× in the culture medium and left for 5 minutes at room temperature. The cross-linked cells were rinsed twice with ice-cold PBS and resuspended in 1 mL of PBS. Following centrifugation, the cell pellets were resuspended in 200 μL of membrane extraction buffer supplemented with 2 μL of protease inhibitors, and kept on ice for 10 minutes. After centrifugation, the nuclei were resuspended in 200 μL of MNase digestion buffer working solution. Then, 2 μL of diluted MNase was added, and the mixture was incubated in a 37 °C water bath for 15 minutes. Following centrifugation, the nuclei were resuspended in 100 μL of IP dilution buffer and subjected to sonication on ice. After centrifugation, the supernatant was transferred to 160 μL of IP dilution buffer. The chromatin was fragmented into 200–500 bp segments and verified using a 1.5% agarose gel. Subsequently, the fragmented DNA mixture underwent IP with either 2.5 μg of STAT1 antibody or normal rabbit IgG, overnight at 4 °C. After this, 20 μL of magnetic beads were added to each IP reaction and incubated for 2 hours at 4 °C. The beads were washed four times with IP wash buffer and then incubated in IP elution buffer at 65 °C for 30 minutes. Following the 65 °C incubation, the supernatant was supplemented with 6 μL of 5 M NaCl and 2 μL of 20 mg/mL proteinase K, and the mixture was heated in a 65 °C heat block for 1.5 hours. Following this, the DNA was purified, washed, and eluted for subsequent PCR and qPCR analysis. The primers used for the ChIP assay are listed in Supplementary Data 3.
Immunoblotting detection
Proteins were extracted using RIPA buffer (Beyotime), separated by SDS‒PAGE, and transferred onto PVDF membranes (Bio-Rad, Hercules, USA). The membranes were blocked using 5% milk (BD Biosciences, San Jose, USA) at room temperature for 1 h to minimize nonspecific binding and then incubated overnight at 4 °C with primary antibodies. Subsequently, the PVDF membranes were incubated with peroxidase-conjugated secondary antibodies at room temperature for 1 h. Finally, the protein bands were visualized using a SuperSignal West Femto Trial Kit (Thermo Fisher Scientific). Detailed information on the antibodies used is listed in Supplementary Data 4.
Animals
BALB/c mice (female, 6-8 weeks, Strain NO. 211), BALB/c Nude mice (female, 5-6 weeks, Strain NO. 401), and C57BL/6 J mice (female, 6-8 weeks, Strain NO. 213) were purchased from Beijing Vital River Laboratory (Beijing, China). B6-Rag1-KO mice (Rag1−/−, female, 5-6 weeks, Strain NO. T004753), NCG mice (female, 5-6 weeks, Strain NO. T001475) and APC-min mice (male and female, 8-12 weeks, Strain NO. T001457) were purchased from Gempharmatech (Jiangsu, China). Intestine-specific Mbtps1 knockout mice on the C57BL/6 J background (female, 6-10 weeks) were generated by crossing mice carrying a Mbtps1 exon 4-7 floxed allele (male and female, 8-12 weeks, Strain NO. T025387) with Villin-Cre-ERT2 mice (male and female, 8-12 weeks, Strain NO. T004829) purchased from Gempharmatech. Given that our experimental design excluded gender-related factors and preliminary findings indicated no difference in Mbtps1 knockdown phenotypes between male and female mice, we opted to use only female mice in subsequent experiments to maintain control over variables. All mice were housed in a temperature-controlled, specific pathogen-free (SPF) facility with a regular 12-h light-dark cycle, and euthanasia was carried out using carbon dioxide. The allocation of animals for the study was randomized through computer-generated random numbers and they were co-housed in the same environment. The number of animals per group for each animal experiment ranges from 5 to 10.
Subcutaneous tumor model
For the subcutaneous tumor mouse model, female BALB/c mice (6-8 weeks old), female C57BL/6 J (6-8 weeks old), female Rag1−/− (5-6 weeks old) and female NCG (5-6 weeks old) mice were used. After knocking down or overexpressing a specific gene in MC38, CT26, E0771, KPC, or B16 cells using lentivirus, both the modified cells and their control cells (1 × 106 cells suspended in 100 μL of PBS) were injected subcutaneously into the flanks of the corresponding mice. For anti-PD-1 treatment, IgG or αPD-1 was intraperitoneally injected once the tumor volume reached 100 mm3. MC38 and E0771 cells were injected at a dose of 5 mg/kg, while B16, CT26, and KPC cells were injected at a dose of 10 mg/kg (3 times, every 3 days). For mouse CD8+ or CXCR3+ cell neutralization, mouse CD8α antibody (Bioxcell) or mouse CXCR3 antibody (Bioxcell) was intraperitoneally injected once the tumor volume reached 100 mm3 at a dose of 100 μg per mouse (3 times, every 3 days). For experiments involving a high-cholesterol diet, mice were fed a 2.5% high-cholesterol diet (Dyets, Wixi, China) or a control diet for 4 weeks prior to subcutaneous tumor transplantation and were maintained on the respective diets throughout the entire experimental period. Every 3 days, the tumor size was measured with a caliper, and tumor volume was calculated using the following formula: volume (mm³) = 0.5 × (longer diameter × shorter diameter²). A tumor volume exceeding 2000 mm³ was considered the endpoint of the experiment. At the experimental endpoint, the tumor was dissected, and its weight was measured using a balance.
AOM/DSS-induced mouse CRC model
The establishment of AOM/DSS mouse model was based on the research of Neufert et al.61. To induce intestinal Mbtps1 deletion, tamoxifen (10 mg/kg, MedChemExpress) was intragastrically administered to 6- to 10-week-old female genetically engineered mice for 5 days. Subsequently, on Day 13, the mice were intraperitoneally injected with 10 mg/kg AOM (Sigma‒Aldrich, MO, USA). The mice were then treated with 2% DSS (MP Biomedicals, Irvine, USA) in the drinking water for 7 days, followed by a 14-day period of treatment with regular water. The cycle of DSS treatment was repeated three times in total. Additionally, to maintain the knockout efficiency of Mbtps1 in intestinal epithelial cells, tamoxifen was repeatedly administered twice during each period of regular water supply. Finally, the animals were euthanized on Day 100 for general inspection and statistical analysis of polyp number.
Sample processing for flow cytometry
For mouse tumor samples, subcutaneous tumors were collected on Day 14 after tumor cell injection. Immediately following euthanasia, freshly isolated tumor samples were surgically dissected into fine fragments and transferred to 5-mL Eppendorf tubes containing enzymes D, R, and A, which were obtained from the Mouse Tumor Dissociation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Subsequently, the samples were incubated at 37 °C with gentle rotation for 40 min. Following incubation, the samples were passed through 70-μm cell strainers (Biosharp, Beijing, China) and washed twice with MACS buffer to remove any residual debris. The supernatant was then aspirated, and the cell pellet was gently resuspended in 40% Percoll (Cytiva, Shanghai, China) for purification via gradient centrifugation. For tumor-draining lymph node samples, the DLNs were crushed in 6-cm dishes, passed through 40-μm cell strainers, and washed twice with MACS buffer. Similarly, for mouse spleen samples, the spleens were crushed in 6-cm dishes, passed through 40-μm cell strainers, and washed twice with MACS buffer. Subsequently, the cell pellets were resuspended in red blood cell lysis buffer (Biosharp, Beijing, China) and incubated for 5 min. For mouse blood dissociation, whole blood was collected into tubes containing heparin sodium. The blood was then lysed with red blood cell lysis buffer for 15-20 min at room temperature with gentle shaking, followed by neutralization with PBS. The samples were passed through 40-μm cell strainers and washed with MACS buffer. Finally, dissociated single-cell pellets from these samples were resuspended in sorting buffer (PBS containing 2% FBS) for further flow cytometry analysis.
In vivo flow cytometric analysis
As previously described, single cells were prepared from mouse tumor tissues, spleens, tumor-draining lymph nodes, and blood. The freshly isolated cells from mouse tissues were treated with a cell stimulation cocktail (Thermo Scientific) at 37 °C for 3 h, followed by staining with Zombie dye for 15 minutes at room temperature. The washed mouse tumor cells or splenocytes were then incubated with specific antibodies against mouse CD45, CD3, CD8, and/or CD4, and/or NK1.1, and/or CXCR3 for 30 min at 4 °C. Subsequently, the cells were washed, fixed, and permeabilized with a fixation buffer (BD Biosciences) overnight at 4 °C. Afterwards, the fixed cells were washed twice with 1× perm and wash buffer (BD Biosciences) and incubated with anti-mouse IFN-γ and/or Ki67 antibodies for 30 min at 4 °C. The cell pellets were then resuspended in sorting buffer to facilitate further flow cytometry analysis. To refine the fluorescence compensation settings for accurate multicolor flow cytometric analysis, compensation beads (Thermo Fisher Scientific) were incubated with the previously mentioned antibodies. Finally, cells were assessed using flow cytometry, and the collected data were analyzed with CytExpert software. Detailed information about the antibodies used is provided in Supplementary Data 4.
In vivo competition assay
Mbtps1-knockdown or control MC38 or E0771 cells were genetically modified to express either GFP or mCherry through lentiviral transduction, enabling the differentiation of cell populations. Subsequently, cells were mixed at a 1:1 ratio and cultured for at least two passages in vitro before being implanted into mice. On the day of tumor inoculation, the cell mixtures were analyzed by flow cytometry. At 14 days after tumor inoculation, the mice were euthanized for tumor collection. Single cells were isolated from mouse tumor tissues following the abovementioned protocol. The cell pellet was then stained with Zombie dye for 15 min at room temperature. Following staining, the tumor cells were washed and resuspended in ice-cold PBS containing 2% FBS, after which they were prepared for flow cytometry analysis. A CytoFLEX flow cytometry system (Beckman Coulter, Miami, FL, USA) was utilized to determine the final ratios of GFP- to mCherry-expressing cells.
Bulk RNA sequencing
We performed bulk RNA sequencing on MC38 cells transfected with shVec or shMbtps1, as well as shVec and shMbtps1 MC38 tumors transplanted into C57BL/6 J mice. DIATRE Biotechnology (Shanghai, China) performed the RNA sequencing. Briefly, total RNA was extracted from cells with TRIzol. The mRNA was isolated and fragmented using mRNA capture beads (VAHTS mRNA capture beads, N401). The resulting RNA samples were reverse-transcribed for library construction with the NEBNext VAHTS Universal V6 RNA-seq Library Prep Kit for Illumina, NR604. The libraries were purified by DNA clean beads (VAHTS DNA Clean Beads, N411) for size selection of 200-300 bp fragments, and they were analyzed on an Agilent 2100 Bioanalyzer (Agilent) to determine the fragment size distribution. The qualified libraries were sequenced on an Illumina Nova Seq 6000.
Bulk RNA-seq data processing
The FASTQ files were checked with FastQC (v. 0.11.9) for quality control. The adapters were removed from the raw reads with Cutadapt (v. 1.18) and mapped to the human reference genome (hg38) with STAR (v. 2.7.1a). SAM files were converted to Bam format using SAMtools (v. 1.3.1). Gene expression levels and differences were calculated using Cufflinks (v. 0.17.3) and HTseq-count (v. 0.13.5).
Single cell RNA sequencing
Tumor tissues were isolated from C57BL/6 J mice bearing either shVec or shMbtps1 MC38 cells. Using a single-cell 3’ Library and Gel Bead Kit V3.1 (10x Genomics, 1000121) and a Chromium Single-Cell G ChIP Kit (10x Genomics, 1000120), the cell suspension (300-600 living cells per microliter determined by Count Star) was loaded onto a Chromium single-cell controller (10x Genomics) to generate single-cell gel beads in the emulsion according to the manufacturer’s protocol. Captured cells were lysed, and the released RNA was barcoded through reverse transcription in individual GEMs. Reverse transcription was performed on an S1000TM Touch Thermal Cycler (Bio-Rad), using the following thermocycler protocol: 53 °C for 45 min, 85 °C for 5 min, and hold at 4 °C. cDNA was generated and then amplified, and its quality was assessed using an Agilent 4200 (CapitalBio Technology, Beijing). Subsequently, the libraries were sequenced utilizing an Illumina NovaSeq 6000 sequencer with a sequencing depth of at least 100,000 reads per cell with a paired-end 150 bp (PE150) reading strategy (performed by CapitalBio Technology, Beijing).
Single cell RNA-seq data processing
According to the standard pipeline and default parameters, sequencing reads were aligned to the mouse genome (mm10), and unique molecular identifiers (UMIs) were counted for each gene using Cell Ranger v7.0.0 (10x Genomics). The Seurat v4.3.0 package was utilized to generate the object and filter out cells of poor quality. Cell quality was assessed based on the following three metrics: (1) total UMI count per cell (library size) within the 2.5th and 95th percentile range; (2) number of detected genes within 2.5th and 95th percentile range; and (3) the library size and detected genes differed by less than 2.5 percentiles in their respective rankings. The DoubletFinder package was used to remove doublets within each sample. Dimension reduction and unsupervised clustering were performed according to the standard workflow in Seurat. To integrate cells from different samples into a shared space for unsupervised clustering, the Harmony algorithm in the Harmony v0.1.1 R package was employed for batch effect correction. For clustering and visualization, the FindClusters function in Seurat was used to obtain cell clusters at various resolutions, and the dimensionality of the data was reduced using uniform manifold approximation and projection (UMAP) implemented in the RunUMAP function with the following settings: reduction = ‘harmony’ and dims = 1:30. The area under the curve (AUC) for exhaustion and proliferation were computed using the AUCell R package. The five well-defined exhaustion markers included Pdcd1, Ctla4, Havcr2, Lag3, and Tigit. The known proliferation-related genes included Aurka, Bub1, Ccnb1, Ccnd1, Ccne1, Dek, E2f1, Fen1, Foxm1, Hmgb2, Mcm2, Mcm3, Mcm4, Mcm5, Mcm6, Mki67, Mybl2, Pcna, Plk1, Top2a, Tyms, and Zwint62.
Bioinformatics analysis of public cohorts and bulk RNA-seq data
Differential expression was estimated using DESeq2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses, as well as gene set enrichment analysis (GSEA), were performed using the clusterProfiler package63. Kaplan-Meier survival analysis was performed using the survminer R package, while log-rank tests and univariate Cox proportional hazards regression analysis were performed using the survival package. The xCell algorithm was used to evaluate the proportion of infiltrating immune cells64. The tumor immune dysfunction and exclusion (TIDE) online tool (http://tide.dfci.harvard.edu/) was used to predict potential immune checkpoint blockade responses. All analyses were implemented using R version 4.2.2, except for TIMER, which was accessed through its web interface (http://timer.comp-genomics.org/). A P value < 0.05 was considered to indicate statistical significance.
Statistical analysis
All experiments were conducted a minimum of three times, and the results are presented as the mean values with accompanying standard deviations (SDs). Student’s t-test was used to compare differences between two independent groups. For comparisons encompassing three or more groups, analysis of variance (ANOVA) coupled with Sidak’s multiple comparisons test was utilized. The Kaplan-Meier method and the log-rank test were used to estimate survival curves. Subsequently, univariate and multivariate Cox regression analyses were conducted to identify independent prognostic factors. Pearson’s correlation analysis and chi-square test were used to evaluate the relationships between two variables. Statistical analyses were performed using GraphPad Prism version 8.3.0 (La Jolla, CA, USA) and IBM SPSS Statistics version 21.0 (Armonk, NY, USA). All statistical tests were conducted in a two-tailed manner, and P values less than 0.05 were considered to indicate statistical significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The processed bulk RNA-seq and single-cell RNA-seq data reported in this study have been deposited in the Gene Expression Omnibus (GEO) database with accession number GSE262868 and GSE263524, and the protein mass spectrometry raw data have been uploaded to the PRIDE website (https://www.ebi.ac.uk/pride/, project accession: PXD053978). RNA-sequencing expression (level 3) profiles and corresponding clinical information from 33 types of cancer samples and corresponding normal samples were downloaded from the TCGA dataset (https://portal.gdc.cancer.gov/). The selection of membrane proteins and secreted proteins is based on the protein annotations provided by The Human Protein Atlas data (https://www.proteinatlas.org/humanproteome/tissue/secretome). Three immunotherapy cohorts were collected, including Liu65 (120 samples), Gide66 (73 samples) and Van Allen67 (40 samples). All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. Source data are provided with this paper.
Code availability
No custom code was generated in this study. Code used for all processing and analysis is available upon request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Weng, J. et al. Exploring immunotherapy in colorectal cancer. J. Hemat. Oncol. 15, 10.1186/s13045-022-01294-4 (2022).
André, T. et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Tolba, M. F. Revolutionizing the landscape of colorectal cancer treatment: The potential role of immune checkpoint inhibitors. Int. J. Cancer 147, 2996–3006 (2020).
Yang, K., Halima, A. & Chan, T. A. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat. Rev. Clin. Oncol. 20, 604–623 (2023).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Bhat, A. A. et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. (Lond., Engl.) 42, 689–715 (2022).
Harapas, C. R. et al. Organellar homeostasis and innate immune sensing. Nat. Rev. Immunol. 22, 535–549 (2022).
Chow, R. D. & Chen, S. Cancer CRISPR screens in vivo. Trends Cancer 4, 349–358 (2018).
Kamber, R. A. et al. Inter-cellular CRISPR screens reveal regulators of cancer cell phagocytosis. Nature 597, 549–554 (2021).
Chen, X. et al. A membrane-associated MHC-I inhibitory axis for cancer immune evasion. Cell 186, 3903–3920.e3921 (2023).
Li, F. et al. In Vivo Epigenetic CRISPR Screen Identifies Asf1a as an Immunotherapeutic target in kras-mutant lung adenocarcinoma. Cancer Discov. 10, 270–287 (2020).
Zhu, X. G. et al. Functional genomics in vivo reveal metabolic dependencies of pancreatic cancer cells. Cell Metab. 33, 211–221.e216 (2021).
Danyukova, T., Schöneck, K. & Pohl, S. Site-1 and site-2 proteases: A team of two in regulated proteolysis. Biochimica et. biophysica acta Mol. cell Res. 1869, 119138 (2022).
Wang, T. B. et al. SREBP1 site 1 protease inhibitor PF-429242 suppresses renal cell carcinoma cell growth. Cell death Dis. 12, 717 (2021).
Caruana, B. T., Skoric, A., Brown, A. J. & Lutze-Mann, L. H. Site-1 protease, a novel metabolic target for glioblastoma. Biochem Biophys. Res Commun. 490, 760–766 (2017).
Chu, X. et al. Integrative single-cell analysis of human colorectal cancer reveals patient stratification with distinct immune evasion mechanisms. Nat. Cancer 5, 1409–1426 (2024).
Engelking, L. J., Cantoria, M. J., Xu, Y. & Liang, G. Developmental and extrahepatic physiological functions of SREBP pathway genes in mice. Semin Cell Dev. Biol. 81, 98–109 (2018).
Dang, T. T. et al. Phosphorylation of EIF2S1 (eukaryotic translation initiation factor 2 subunit alpha) is indispensable for nuclear translocation of TFEB and TFE3 during ER stress. Autophagy 19, 2111–2142 (2023).
Gu, D. et al. Sterol regulatory element-binding protein 2 maintains glioblastoma stem cells by keeping the balance between cholesterol biosynthesis and uptake. Neuro Oncol. 25, 1578–1591 (2023).
Cappuyns, S. et al. PD-1(-) CD45RA(+) effector-memory CD8 T cells and CXCL10(+) macrophages are associated with response to atezolizumab plus bevacizumab in advanced hepatocellular carcinoma. Nat. Commun. 14, 7825 (2023).
Sakai, J. et al. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Mol. Cell 2, 505–514 (1998).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
Li, J. et al. Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J. Clin. Invest 130, 2712–2726 (2020).
Sheng, W. et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 174, 549–563.e519 (2018).
Bhoopathi, P. et al. Cytoplasmic-delivery of polyinosine-polycytidylic acid inhibits pancreatic cancer progression increasing survival by activating Stat1-CCL2-mediated immunity. J. Immunother. Cancer 11, e007624 (2023).
Shang, S. et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci. Transl. Med. 14, eabf0992 (2022).
Hiroi, M. & Ohmori, Y. The transcriptional coactivator CREB-binding protein cooperates with STAT1 and NF-kappa B for synergistic transcriptional activation of the CXC ligand 9/monokine induced by interferon-gamma gene. J. Biol. Chem. 278, 651–660 (2003).
Ohmori, Y. & Hamilton, T. A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 268, 6677–6688 (1993).
Tensen, C. P. et al. Genomic organization, sequence and transcriptional regulation of the human CXCL 11(1) gene. Biochimica et. biophysica acta 1446, 167–172 (1999).
Liang, Y. H. et al. Proteasome inhibitors restore the STAT1 pathway and enhance the expression of MHC class I on human colon cancer cells. J. Biomed. Sci. 28, 75 (2021).
Sénécal, V. et al. Production of IL-27 in multiple sclerosis lesions by astrocytes and myeloid cells: Modulation of local immune responses. Glia 64, 553–569 (2016).
Cheng, Q. et al. BRCC36 functions noncatalytically to promote antiviral response by maintaining STAT1 protein stability. Eur. J. Immunol. 51, 296–310 (2021).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Ganesh, K. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 16, 361–375 (2019).
Touré, B. B. et al. Biosynthesis and enzymatic characterization of human SKI-1/S1P and the processing of its inhibitory prosegment. J. Biol. Chem. 275, 2349–2358 (2000).
Nakagawa, T. et al. Site-1 protease is required for the generation of soluble (pro)renin receptor. J. Biochem 161, 369–379 (2017).
Hartal-Benishay, L. H. et al. MBTPS1 regulates proliferation of colorectal cancer primarily through its action on sterol regulatory element-binding proteins. Front Oncol. 12, 1004014 (2022).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e514 (2021).
Lv, L. & Lei, Q. Proteins moonlighting in tumor metabolism and epigenetics. Front Med 15, 383–403 (2021).
Li, J. et al. A noncanonical function of SKP1 regulates the switch between autophagy and unconventional secretion. Sci. Adv. 9, eadh1134 (2023).
Oliveira, G. & Wu, C. J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer 23, 295–316 (2023).
Chow, M. T. & Luster, A. D. Chemokines in cancer. Cancer Immunol. Res 2, 1125–1131 (2014).
Reschke, R. & Gajewski, T. F. CXCL9 and CXCL10 bring the heat to tumors. Sci. Immunol. 7, eabq6509 (2022).
Spranger, S., Dai, D., Horton, B. & Gajewski, T. F. Tumor-residing batf3 dendritic cells are required for effector t cell trafficking and adoptive t cell therapy. Cancer Cell 31, 711–723.e714 (2017).
Groom, J. R. & Luster, A. D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 89, 207–215 (2011).
Li, S. et al. Low-dose radiotherapy combined with dual PD-L1 and VEGFA blockade elicits antitumor response in hepatocellular carcinoma mediated by activated intratumoral CD8(+) exhausted-like T cells. Nat. Commun. 14, 7709 (2023).
Chow, M. T. et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of Anti-PD-1 Therapy. Immunity 50, 1498–1512.e1495 (2019).
Klement, J. D. et al. Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment. Cancer Cell 41, 620–636.e629 (2023).
Li, S. et al. Identification of key genes and functional pathway in radioresistance of non-small cell lung cancer. Cancer Manag. Res. 14, 2871–2884 (2022).
Agashe, R. P., Lippman, S. M. & Kurzrock, R. JAK: Not just another kinase. Mol. Cancer Ther. 21, 1757–1764 (2022).
Sadzak, I. et al. Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc. Natl Acad. Sci. USA 105, 8944–8949 (2008).
Guo, X. et al. RNF220 mediates K63-linked polyubiquitination of STAT1 and promotes host defense. Cell Death Differ. 28, 640–656 (2021).
Krämer, O. H. et al. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 23, 223–235 (2009).
Chang, W. et al. OTUB2 exerts tumor-suppressive roles via STAT1-mediated CALML3 activation and increased phosphatidylserine synthesis. Cell Rep. 41, 111561 (2022).
Ma, C. et al. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol. Cancer 22, 170 (2023).
Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 20, 131 (2021).
Gan, L. et al. Endosialin-positive CAFs promote hepatocellular carcinoma progression by suppressing CD8(+) T cell infiltration. J. Immunother. Cancer 12, e009111 (2024).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Neufert, C. et al. Inducible mouse models of colon cancer for the analysis of sporadic and inflammation-driven tumor progression and lymph node metastasis. Nat. Protoc. 16, 61–85 (2021).
Whitfield, M. L., George, L. K., Grant, G. D. & Perou, C. M. Common markers of proliferation. Nat. Rev. Cancer 6, 99–106 (2006).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov. (Camb.) 2, 100141 (2021).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Liu, D. et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med 25, 1916–1927 (2019).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/Anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e236 (2019).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Acknowledgements
This project was supported by the National Key R&D Program of China (2022YFA1105300), National Natural Science Foundation of China (82303306, 82273241, 82173128), Guangdong Basic and Applied Basic Research Foundation (2023B1515040030, 2023A1515012345) and the Sanming Project of Medicine in Shenzhen (SZSM202211017). We are grateful to Dr. Qi-nian Wu from the Pathology Department of Sun Yat-sen University Cancer Center for evaluating the MMR status based on the immunohistochemical results of the patient’s specimens.
Author information
Authors and Affiliations
Contributions
R.-H.X., K.L., H.-Q.J. and Y.-Y.W. designed the study. Y.-Y.W., and J.-F.L. carried out in vivo experiments and collected the data. Y.-Y.W. and W.-W.W. carried out in vitro experiments and collected the data. Y.-Y.W., Z.F., F.C. and Y.-X.C. performed the statistical analysis. Y.-Y.W. and Z.F. wrote the manuscript. Z.-L.Z., Z.-X.L. and H.S. contributed to discussion and data interpretation. K.L., X.-Y.G. and H.-Y.M. revised the manuscript. All authors have reviewed the manuscript and consented to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tao Shi and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, YY., Lin, JF., Wu, WW. et al. Inhibition of MBTPS1 enhances antitumor immunity and potentiates anti-PD-1 immunotherapy. Nat Commun 16, 4047 (2025). https://doi.org/10.1038/s41467-025-59193-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59193-4